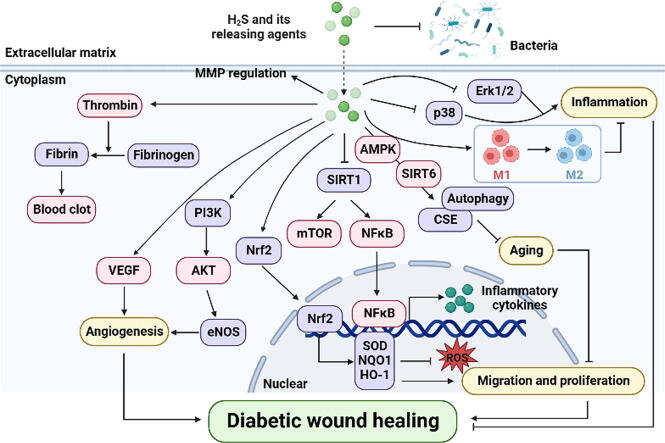
Graphical abstract
The role of hydrogen sulfide and its releasing agents in diabetic wound healing.
Keywords: Hydrogen sulfide, Diabetic wound healing, High glucose
Highlights
-
•
This mini-review briefly introduces the causes of diabetic wound healing and the direction of clinical treatment.
-
•
This mini-review provides reasons why hydrogen sulfide may be considered for the treatment of diabetic wound healing.
-
•
This mini-review discusses the role of hydrogen sulfide in various stages of diabetic wound healing.
-
•
This mini-review involves ideas to improve hydrogen sulfide availability to aid future developments.
-
•
The mini-review suggests that hydrogen sulfide is a new hope for improving diabetic wound healing.
Abstract
Background
Diabetes mellitus (DM) is a long-term metabolic disease accompanied by difficulties in wound healing placing a severe financial and physical burden on patients. As one of the important signal transduction molecules, both endogenous and exogenous hydrogen sulfide (H2S) was found to promote diabetic wound healing in recent studies. H2S at physiological concentrations can not only promote cell migration and adhesion functions, but also resist inflammation, oxidative stress and inappropriate remodeling of the extracellular matrix.
Aim of Review
The purpose of this review is to summarize current research on the function of H2S in diabetic wound healing at all stages, and propose future directions.
Key Scientific Concepts of Review
In this review, first, the various factors affecting wound healing under diabetic pathological conditions and the in vivo H2S generation pathway are briefly introduced. Second, how H2S may improve diabetic wound healing is categorized and described. Finally, we discuss the relevant H2S donors and new dosage forms, analyze and reveal the characteristics of many typical H2S donors, which may provide new ideas for the development of H2S-released agents to improve diabetic wound healing.
Introduction
Insufficient insulin secretion, either relative or absolute, is the hallmark of diabetes mellitus [1]. Minor wounds in diabetic patients might cause major health complications resulting in wounds that heal slowly or never heal. In some cases, they can even be fatal [2]. Insulin deficiency, hyperglycemia, hyperlipidemia, proinflammatory state, peripheral neuropathy, tissue hypoxia, impaired vascular and obesity in the pathological state of diabetes can affect the wound healing process [3]. Diabetic foot ulcer (DFU) is one of the most prevalent complications of diabetes-related delayed wound healing. The occurrence and development of DFU is associated with severe morbidity and mortality, as well as large economic consequences. Therefore, reducing the risk of poor wound healing in diabetes is a worldwide issue [4]. Diabetic wound healing is slowed by many factors and its treatment is comprehensive, existing treatment strategy is mainly to control blood glucose and symptomatic treatment [5], [6]. According to the characteristics of different stages in wound healing, vasodilator drugs (propranolol, etc.), drugs for the treatment of neuropathy (pregabalin, tramadol, etc.), anti-infection drugs (antibiotics, etc.), anti-oxidative stress drugs (probucol, etc.), and nutritional support therapy are widely used [7]. And now considerable dressings and bandages loaded with collagen, growth factors and natural active products have been extensively developed to inhibit the inflammatory response, promote angiogenesis, regulate collagen deposition, and further accelerate diabetic wound healing [8], [9], [10]. Research on the management of damaged wounds may focus on combination approaches in the future, robust studies are needed to identify agents that may play a role in different stages [11].
In previous studies, H2S was thought to be a toxic gas molecule or a byproduct of a metabolic pathway [12]. Increasing investigations in the past few years have revealed that H2S may be qualified as the third gasotransmitter involved in mediating the physiological and pathological functions of cells [13]. H2S is responsible for various functions such inhibiting inflammation, oxidant and tumors, regulating ion channel and protecting cardiovascular [12], [14]. The production of endogenous H2S depends on at least three enzyme systems involved in cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE) and 3-mercaptopyruvate sulfotransferase (3-MST), in addition to non-enzymatic pathways [15], [16]. All three enzymes have been shown to be present in endothelial cells (ECs) and are able to impact the angiogenic characteristics of ECs and respond to changes in angiogenic factors [17], [18]. Colorimetry, ion-selective electrodes, chromatographic methods and optical probes are commonly used for the determination of endogenous H2S [19]. Among them, fluorescent probes of optical probes are more promising and are expected to be used to monitor endogenous short-lived H2S, meeting the requirements of photostability, strong fluorescence signal, high sensitivity, high specificity and low cytotoxicity [20].
With the discovery of the physiological and pathological functions of H2S, its biological effects and pathophysiological significance have become a hot issue in the field of medical research, and considerable progress has been made. A large number of research results have shown that H2S could regulate many systemic diseases, and endogenous H2S level has been shown to be reduced in many chronic diseases such as myocardial infarction, non-alcoholic fatty liver disease and diabetes [21]. Several clinical studies have shown that endogenous H2S level is significantly reduced in vascular diseases [22]. The serum H2S level and H2S synthetase expression in patients were measured and their relationship with vascular indexes was investigated. The results indicate that endogenous H2S may be involved in vascular remodeling, and endogenous H2S production may be inhibited by inflammation and other factors [23], [24]. The low levels of H2S concentration in sulfur thermal water treatments was confirmed to enhance endogenous cytoprotective activity and promote wound healing in chronic wounds that are difficult to heal [18], [25].
In recent years, there has been a growth in the number of studies on H2S and diabetes. It is worth mentioning that in diabetic patients and animals, endogenous H2S levels and H2S synthase expression are decreased [26]. H2S can regulate hemostasis, promote cell migration and adhesion functions, it can also resist inflammation, oxidative stress and regulate extracellular matrix remodeling. H2S is a reducing substance, reducing or directly combining with the heme center of metalloprotein, and then directly or indirectly scavenging reactive oxygen species (ROS) [27]. Beyond that, Mustafa et al. (2011) first described the key molecular mechanism of H2S signaling, namely S-sulfhydration [28]. In this H2S-dependent post-translational modification, H2S promotes the conversion of reactive cysteine residues to more active persulfides (-SSH) [29]. Persulfide may be involved in various reactions such as sulfur-containing molecule biosynthesis, tRNA modification, mitochondrial energy metabolism, cellular antioxidation and inhibition of Toll-like receptor-mediated inflammation [30]. S-sulfhydration modification of specific proteins by H2S has been proven to have a significant impact on diabetes and its complications. H2S induces Keap1 S-sulfhydration which leads to the suppression of atherosclerosis in diabetes, and also ameliorates skeletal muscle atrophy in db/db mice. Moreover, S-sulfhydration of Muscle RING finger 1 (MuRF1) prevents cardiac structural damage in diabetic cardiomyopathy, S-sulfhydration of Hrd1 reduces lipid droplet accumulation in cardiac tissues of db/db mice, S-sulfhydration of SIRT1 attenuates diabetic renal lesions via inactivation of p65 NF-κB and STAT3 phosphorylation/acetylation [31], [32], [33], [34], [35].
Therefore, in order to generate ideas for future research and innovation of H2S in the field of diabetic wound healing management, this review will describe the function of H2S in the healing of diabetic wounds and discuss the methods for increasing the bioavailability of H2S.
Literature search strategy
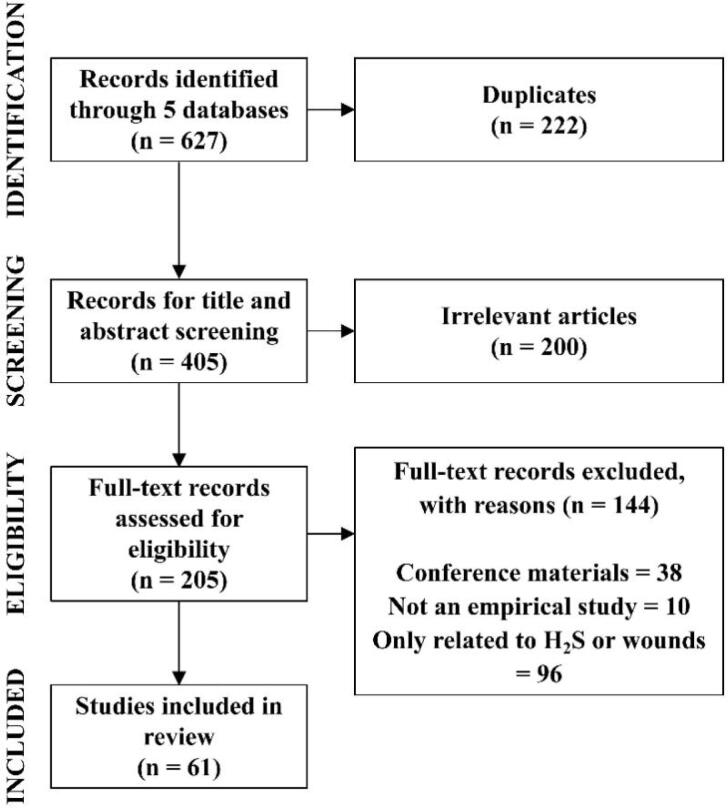
Five electronic databases, including Science Direct, PubMed, Web of Science, Wiley Online Library and ClinicalTrials.gov were searched from 2000 to 2023 for eligible studies. We performed a literature search for Web of Science with the following search strategy: TS = ((“hydrogen sulfide” or “H2S”) AND (“diabetic wound” or “wound”)). The strategies for other databases were similar but were adapted according to the guideline of the database. During the retrieval process, we conducted a manual search using references from the included literature to supplement the eligible studies. The duplicated literature was removed by Rayyan, and irrelevant articles were removed by browsing titles and abstracts. Finally, the conference materials, non-empirical research articles and articles related only to H2S or wounds were removed after full-text review. The screening process was shown in Fig. 1. In the end, 61 studies on the possible mechanisms of H2S accelerating wound healing were systematically summarized.
Fig. 1.
The flowchart of literature search strategy in this review.
H2S in various phases of diabetic wound healing
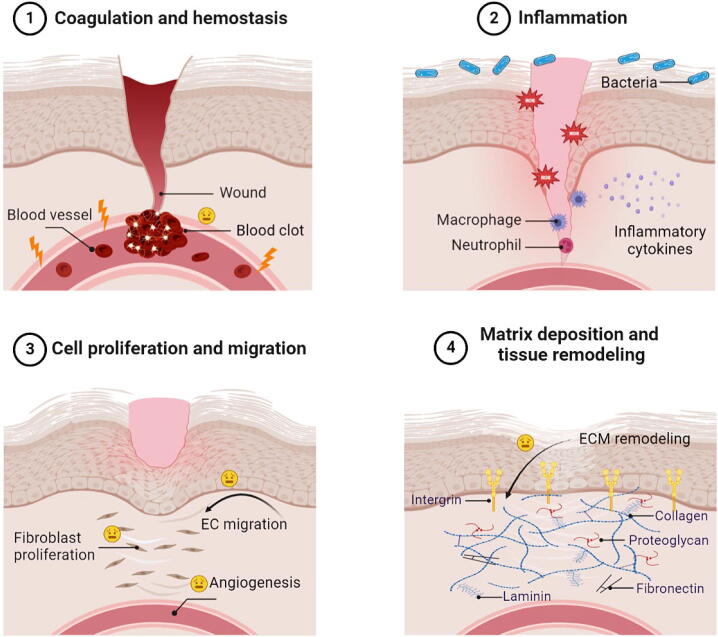
One of the most intricate processes in multicellular organisms is the healing of wounds, which involves numerous communications within and between multiple cells. Traditional classifications of the wound healing process include 4 overlapping classical stages: coagulation and homeostasis, inflammation, cell proliferation and migration, matrix deposition and tissue remodeling [36]. The whole process of wound healing involves the platelet activation, macrophages and macrophage polarization, as well as proliferation and migration of keratinocytes, fibroblasts and ECs. These cells also secrete growth factors and cytokines that affect different stages of wound healing [10].
The abnormal metabolism of diabetes patients can further complicate the process of wound healing, and can also cause chronic wound stasis. Vascular damage and blood transport disorders make it difficult for drugs to reach the wound through blood circulation [37]. Hyperglycemia can lead to hyperfibrinogenemia in diabetic patients, activating the coagulation cascade, thereby increasing thrombin formation and fibrinogen degradation products [38]. Local tissue edema, hypoxia, and high glucose environment are suitable for bacterial growth [2]. At the same time, pathologically high glucose levels decrease the chemotaxis and phagocytosis of inflammatory cells, leading to inflammation and oxidative stress. Ischemic tissue damage is a major ischemic vascular complication that occurs in diabetic patients with insufficient oxygen supplementation [26]. Cumulative studies have shown that impaired ECs function and angiogenic properties play a key role in diabetes-induced impaired angiogenesis [39]. It is not limited to the mutual influence of the above-mentioned factors, and the joint action leads to difficult healing of diabetic wounds. In addition, cells and cytokines have different roles in 4 wound healing stages, so the development of drugs aimed at promoting the healing of diabetic wounds needs to target different stages (Fig. 2).
Fig. 2.
Features of reduced wound healing in various stages of diabetes.
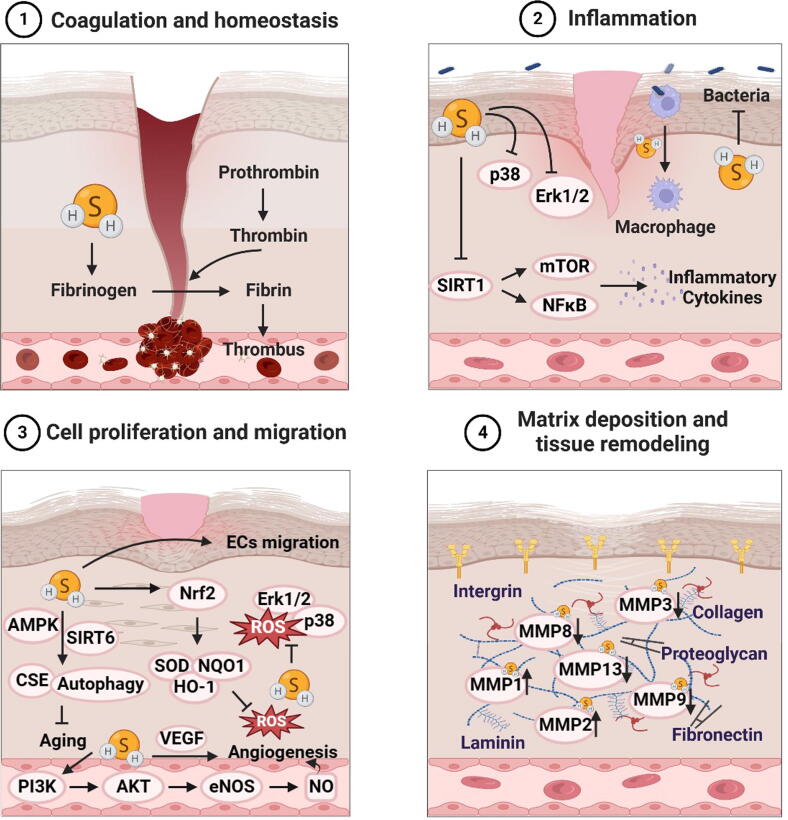
H2S in diabetic wound healing phase 1: coagulation and homeostasis
The presence of diabetes causes an imbalance between coagulation and fibrinolysis, resulting in a prothrombotic state [40]. A crucial aspect of the coagulation process involves converting of fibrinogen to fibrin, and higher fibrinogen levels have been observed in patients with DFU [41], [42]. In a study involving streptozotocin (STZ)-induced diabetic rats, it was observed that the administration of H2S hastened wound healing while also shortening both prothrombin time (PT) and thrombin time (TT). PT and TT of the diabetic rats treated with H2S were significantly improved. In contrast, serum fibrinogen levels were elevated in untreated diabetic rats, which were reduced after H2S treatment [43]. Similar results were found in patients with osteoarthritis and diabetes [44][[44], [45]]. Studies have shown that H2S can regulate thrombosis by interfering with platelet activation, H2S at physiological concentration may hinder the clustering of platelets caused by moderate stimulation, which may be attributed to the persulfuration of platelet-associated proteins and the antioxidant activity of H2S [45], [46], [47], [48]. Moreover, GYY4137 acts as a slow-releasing H2S donor which was found to regulate and enhance endogenous thrombolysis by diminishing platelet aggregation and disrupting platelet activation and adhesion molecule-mediated aggregation [49], [50]. Aged garlic extract slowly releases H2S to achieve physiological effects, which can inhibit platelet aggregation by inhibiting cAMP phosphodiesterase activity to increase cAMP levels [51].
H2S in diabetic wound healing phase 2: inflammation
After the hemostasis phase, platelets aggregate and release pro-inflammatory mediators to enter the inflammatory phase, where leukocytes enter the wound to destroy bacteria and remove debris. Macrophages continued the process of cleaning up debris while simultaneously producing growth factors that attract immune system cells to the wound [52], [53]. Monocytes-macrophages, macrophage phenotypic transition and reduced phagocytic ability play critical roles in anti-inflammatory, tissue debridement, and cell regulatory functions [54], [55]. H2S could reduce foamy macrophage numbers and promote macrophage phenotypes transformation [56]. In addition, there is now a wealth of data supporting the role of H2S in promoting inflammation resolution in diabetic wound healing. H2S treatment may abolish NETosis and neutrophil extracellular trap (NET) formation by blocking ROS mediated mitogen-activated protein kinase (MAPK) extracellular signal-regulated kinase (ERK) and p38 activation [57]. In a study, exogenous administration of sodium hydrosulfide (NaHS) to diabetic mice reduced neutrophil and macrophage infiltration, along with decreased interleukin-6 (IL-6) and tumor necrosis factor α (TNF-α) compared with untreated diabetic mice [58]. The administration of NaHS or S-adenosylmethionine (SAMe) could reverse the changes of Sirtuin 1 (SIRT1)-mammalian target of rapamycin (mTOR)/ nuclear factor kappa-B (NF-κB) signaling pathway induced by high glucose [59].
Antibacterial responses are often reduced in diabetic patients. The external structure of bacteria prevents the invasion of immune cells and antibiotics [60]. In addition, microbial diversity makes finding suitable and most effective treatments challenging [61]. Therefore, the development of novel dosage forms of antibacterial drugs that release H2S has become a research hotspot [62]. A polymersome wound dressing spray acts as a bacterial inhibitor and H2S generator can reduce by more than 50% for Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) at the concentrations of 60 and 80 μg/mL, respectively [63].
H2S in diabetic wound healing phase 3: cell proliferation and migration
During the proliferation phase, fibroblasts are recruited from surrounding intact tissues, stimulate the migration of ECs and promote angiogenesis [64]. Activated fibroblasts are responsible for depositing collagen, secreting extracellular matrix and metalloproteinases, and providing adequate support for EC/epithelial cell adhesion, migration, growth and differentiation [65], [66]. A persistent high glucose environment in diabetic wounds leads to excessive superoxide production, which promotes oxidative stress and the production of advanced glycation end products (AGEs) [67], [68]. Modulation the level of ROS may be a prospective therapeutic method for the treatment of wounds [69].
The effects of ROS on fibroblast function are mainly reflected in inhibiting proliferation, migration and myofibroblast differentiation as well as inducing aging and apoptosis [70], [71]. H2S donors AP39 and AP123 can protect mouse fibroblasts against photoaging by increasing collagen levels and Nrf2 nuclear translocation [72]. In addition, NaHS can protect fibroblasts from hydrogen peroxide damage by regulating autophagy related proteins LC3, p62, and Beclin 1 [73]. In other ways, H2S inhibits high glucose-induced fibroblast senescence by triggering CSE and autophagy via the SIRT6/adenosine 5′-monophosphate-activated protein kinase (AMPK) signaling pathway [74]. After JK-1 (H2S sustained-release donor) treatment, fibroblasts L929 cells migrated to the site of the wound, and JK-1 dramatically improved the degree of wound closure, granulation tissue development and collagen deposition in mouse skin wounds [75].
As a signaling molecule, ROS mediates various biological reactions in ECs, including gene expression, cell proliferation, migration, angiogenesis, apoptosis and aging [76], [77]. The adverse effect of ROS production from ECs is a key factor for the dysfunction of wound healing in diabetes [78]. Studies have found that H2S can act as a direct scavenger of ROS or combat oxidative stress by up-regulating antioxidant-related pathways and enhancing the expression of antioxidant molecules including superoxide dismutase (SOD), HO-1, NADPH quinone dehydrogenase 1 (NQO1), catalase (CAT) and glutathione peroxidase (GSH-Px) [79], [80], [81]. Besides, NaHS (50 mol/L) could prevent ECs apoptosis induced by high glucose via upregulating SOD activity, reducing ROS generation and malondialdehyde levels, and downregulating Bax/Bcl-2 ratio [82]. NaSH reduces oxidative stress in diabetes by inhibiting NADPH oxidase isoforms, thereby restoring endogenous NO production and action in the vascular system [83]. Exogenous H2S protects ECs from HG-induced increased inflammation and ROS, autophagy, endoplasmic reticulum stress, and angiogenesis injured by activating the Nrf2-ROS-AMPK signaling pathway and PI3K/Akt/eNOS pathway [84], [85]. Furthermore, H2S inhibited oxidative stress and expression of ERK1/2 and p38, promoted ECs proliferation and migration and accelerated full-thickness wound healing in diabetic mice [86]. H2S also participates in mediating the proliferation and migration of ECs, thereby accelerating blood vessels formation, and takes part in regulating vasodilation and hemodynamics [87], [88]. ECs respond to pathophysiological stimuli by releasing vasodilator substances such as NO and vasoconstrictor substances including angiotensin II, endothelin-1, thromboxane A2, regulating vasodilator tone and organ perfusion [89]. Substantial evidence suggests that H2S promotes angiogenesis and wound healing in diabetic mice by increasing the vascular endothelial growth factor (VEGF), hypoxia-inducible factor (HIF-1α) and endothelin nictric oxide synthase (eNOS) transcription, promoting granulation tissue formation [43], [90]. H2S increases the level of miR-126-3p by down-regulating high-glucose-induced anti-angiogenic DNA methyltransferase 1 (DNMT1) protein levels and down-regulating methylation levels, thereby improving high-glucose impaired angiogenesis [91].
ROS can modify the cytoskeleton and cell junction related factors, thereby regulating the polarity, morphogenesis and functions of epithelial cells [92]. Keratinocyte damage and dysfunction is known to attribute to delayed wound healing by DM. In one study, H2S could protect HaCaT human keratinocytes from methylglyoxal (MGO) induced damage and behavioral disorders by inhibiting apoptosis, alleviating intracellular ROS content, increasing mitochondrial membrane potential, and ultimately promoting cell adhesion and migration [93]. Meanwhile, exogenous H2S inhibits NLRP3 inflammasome mediated inflammatory response by inhibiting high glucose induced ROS production in epithelial cells [94].
H2S in diabetic wound healing phase 4: matrix deposition and tissue remodeling
Inflammation, angiogenesis, granulation tissue remodeling, collagen deposition and other processes involved in diabetic wound healing all require the degradation of extracellular matrix (ECM), and matrix metalloproteinases (MMPs) are important proteins for degrading ECM. H2S and its donors have been widely demonstrated to combat adverse remodeling in diabetic nephropathy such as excessive collagen deposition, increased fibronectin and laminin expression, and decreased expression of elastin [95], [96]. H2S can regulate ECM by up-regulating the expression of PPARγ, down-regulating the expression of plasminogen activator inhibitor-1 (PAI-1) and transforming growth factor-β (TGF-β1), regulating the expression of MMPs family proteins, and inhibiting the mRNA expression of fibronectin and type IV collagen [97], [98], [99].
In brief, H2S regulates the coagulation process, resists inflammation and oxidative stress, and provides a prerequisite for diabetic wound healing. At the same time, H2S enhances the migration and proliferation of fibroblasts, ECs and epithelial cells in new tissues, and increases collagen synthesis and angiogenesis. During extracellular matrix remodeling, H2S regulates the activity of MMPs and promoting granulation tissue and scar formation. Thus, H2S contributes to all phases of diabetic wound healing (Fig. 3). At the same time, the level and action of endogenous H2S are affected by several factors. Angiotensin Ⅱ inhibits the production of endogenous H2S by increasing the level of ROS in vascular ECs and promoting the ubiquitination and degradation of CSE [100]. In addition, interactions between iron and H2S affect their respective metabolism and function. Excess iron produces ROS and non-enzymatically increases the production of H2S, whereas H2S reverses the excess iron and ROS [101]. There is also a reciprocal effect between CBS and CSE, the two major synthetase of H2S. CBS deficiency may up-regulate CSE expression by inducing Specific protein 1 (SP1), and CBS product cystathionine can also inhibit CSE synthesis of H2S [102], [103]. Exploring the effect of endogenous substances on the action and level of H2S is of key significance for H2S to play a role in accelerating diabetic wound healing in vivo.
Fig. 3.
The role of hydrogen sulfide in various stages of diabetic wound healing.
H2S and its releasing agents for better treatment outcomes
With the confirmation of the effect of H2S therapy, the development of H2S-based therapy depends on the ability to deliver H2S to the required location under the appropriate concentration and pharmacokinetics. H2S follows a two-step dissociation process with pKa values of approximately 6.88 and 19 (37 °C), respectively. In normal physiological conditions with a pH of 7.4, H2S exists primarily as neutral molecules of H2S and monoionized HS- [104], H2S and HS- can reach a dynamic equilibrium [105]. On the other hand, the dose - response curve of H2S is generally bell-shaped with a narrow therapeutic window [106], [107]. Furthermore, H2S exhibits a multitude of distinctive characteristics that pose a set of unique obstacles for the advancement of therapeutic methods. Generally, the most significant method of delivering exogenous gasotransmitters is the inhalation of gas directly, which avoids the pain and risk of infection from local delivery [108]. However, direct inhalation of gaseous H2S is limited by odor, irritation, toxicity, enhanced local concentrations and dosage control difficulties [109]. Thus, the development of H2S therapeutics still faces major challenges concerning the lack of controllability and targeting specificity.
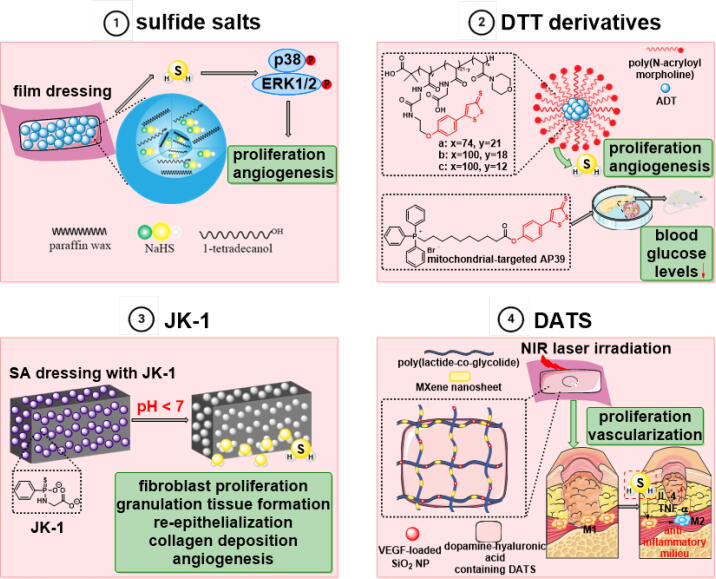
Increasing evidence suggests that H2S has preventive or therapeutic effects on diabetic wound healing, which highlight the application prospect of H2S-based treatment methods in the biomedical field. To investigate and comprehend the physiological functions of H2S, H2S donors are prescribed as the main source of exogenous H2S to compensate for the deficiency of endogenous H2S production, and numerous studies have demonstrated the therapeutic capacity of H2S donors in vitro and in vivo models. Although the characteristics required for H2S donors vary in different cases, some features are widely desirable. H2S donors should be stable, not produce or only produce harmless by-products when functioning, and have specific release mechanisms. Although donors that rapidly release H2S, such as NaHS and Na2S, have been widely used, the short half-life, rapid and uncontrolled release makes them unsuitable for the sustained donation of H2S [108]. The isothermal phase transition microparticles (NaHS@MPs) prepared by the emulsion method provide an in situ reservoir for the continuous release of exogenous H2S under physiological conditions that attenuate the high water instability of NaSH [86].
Similar to sulfide salts, H2S generation from Lawesson’s reagent (LR) is also rapid and uncontrolled, as well as poorly water-soluble. For ameliorating the above limitations, one of the most widely used GYY4137, a slow-releasing H2S donor with good water solubility under physiological conditions, was developed on the basis of LR [110]. DTT is a class of H2S donors triggered by hydrolysis, and ADT-OH is the result of demethylation of the compound ADT, which is widely utilized to convert established drug molecules into H2S donor [111]. AP39, a DTT derivative, is a triphenylphosphonium derivatized anethole dithiolethione and mitochondria-targeted H2S donor [112]. AP39 is effective and safer in hyperglycemic injury of endothelial cells at 1000-fold lower concentrations than Na2S [113].
The pH-response JK-1 is a phosphorothioate based H2S donor and protonation induced intramolecular cyclization greatly accelerates H2S release at acidic pH. JK-1 was added into the alginate sponge obtained by crosslinking sodium alginate (SA) with Ca2+ to prepare a functional dressing (SA/JK-1) with H2S release property. In comparison to the JK-1 in solution, the SA sponge’s porous structure caused the gradual release of H2S from the JK-1 and attenuate the loss of H2S in the system. Both in vitro and in vivo studies have shown that SA/JK-1 not only has good cytocompatibility, but also improves fibroblasts proliferation and migration, and increases the formation of granulation tissue, re-epithelialization and angiogenesis during wound healing [75].
Over the last two decades, research on dietary organosulfur compounds has received increasing attention. Among them, garlic polysulfides and isothiocyanates deriving from the Brassicaceae have been considered as H2S donors with important pharmacological and nutritional value [114]. Furthermore, materials such as antioxidant-antibiotic polymer vesicles and antibacterial hydrogel dressings have been developed for the treatment of diabetic wounds [115], [116]. A NIR-responsive band-aid with core (MXene-loaded nanofibers)-shell (dopamine-hyaluronic acid hydrogel) structure encapsulating VEGF and H2S donor (diallyl trisulfide, DATS) was prepared. The sustained release of DATS achieved continuous H2S production, which induces the polarization of macrophages into an M2 phenotype, inhibits the excessive inflammatory response of wounds, facilitates the proliferation of skin cells, and promotes wound healing [117] (Fig. 4).
Fig. 4.
H2S and its releasing agents.
Nanomaterials have the advantages of controlled release, high efficiency, and low toxicity [18], [63]. There are various substances conjugated with different anti-oxidative nanomaterials which are highly active in diabetic wound healing process [118], [119], [120]. For instance, cerium oxide nanoparticle (CNP) acts as a free radical scavenger conjugated with miR146a inhibiting the pro-inflammatory NF-κB pathway, which has a synergistic effect in regulating oxidative stress and inflammation, ultimately accelerating the healing process of diabetic wounds. CNP-miR146a inhibited the expression of proinflammatory cytokines IL6, TRAF6 and CXCL2, reduced the number of CD45 + and NOX2 + cells, and increased the expression of collagen type 1 and CD31, as well as dermal thickness [121], [122], [123]. Zinc sulfide nanoparticles (ZNS NPs) is a new type of nanomaterials that generate H2S in an acidic microenvironment, regulate redox homeostasis and promote fibroblast viability [124]. The released Zn2+ can synergize with H2S to depolarize the bacterial cell membrane, resulting in antibacterial and re-epithelialization enhancing activities of ZNS NPs [125]. The ideal gas release therapy should be biocompatible, released in a sustained and controlled manner, and maintaining a good wound environment [18]. The controlled release of H2S from H2S donors is triggered by cysteine, which can regulate the release rate of H2S and improve its stability [126], [127]. Different dosage forms and administration methods of hydrogen sulfide and its donors have their own characteristics (Table 1). Due to the special gas form of H2S, compared with injections, patches and gels, the preparation of H2S into sprays can allow gas exchange, which is conducive to wound healing. And it can reduce the risk of contamination, making it easier to remove from wounds and thereby reducing toxicity [63], [128]. The advancement of nanotechnology has made it possible to rapidly develop stimulus responsive nanomaterials for precise gas therapy [129]. It is important to be cautious about utilizing inhaled H2S for therapeutic purposes since it can lead to harmful side effects when present in high quantities, possibly by hindering the mitochondrial respiration process [130].
Table 1.
Comparison of several H2S donor preparations in different dosage forms.
| Dosage form | Active ingredient | Advantage | Disadvantages or adverse reactions | Reference |
|---|---|---|---|---|
| Microparticles | NaHS | Improved water stability and readily removed from wound bed. | A lack of a successful method for encapsulating effectively. | [86] |
| Hydrogels | JK-1 | Absorbed wound exudate, controlled H2S release and improved biocompatibility. | Deformation caused by swelling of the spongy matrix may lead to wound tears. | [75] |
| Nano-fibrous | JK-1 | Controlled H2S release and good cyto-compatibility. | Hard to pinpoint how H2S contribute to various phases of the wound healing process. | [128] |
| Fibers and hydrogels | DATS | High photothermal conversion efficiency. | Fibrous materials are often affected by the expansion of the fibrous interface, and hydrogels are easy to exchange with cells or tissues. | [117] |
| Microfibers | NSHD1 | Prolonged the release time and achieved a tunable release curve. | Biothiols to trigger H2S release, potentially leading to unpredictable and unstable H2S release. | [131] |
| Spray | SATO | Substance can disperse in water and maintain its colloidal stability when diluted. | [63] |
In addition to exogenous H2S supplementation, the use of certain drugs to increase endogenous H2S levels or activation of H2S producing enzymes can also increase overall H2S levels. The level of endogenous H2S and the enzymatic activity of CSE in macrophages were significantly increased after fluvastatin treatment [132]. Metformin, a commonly used oral antidiabetic drug, has been shown to increase H2S tissue concentration in brain, heart, kidney and liver of mice and regulate CSE expression [133], [134]. In addition, both CBS agonist S-adenosyl-L-methionine (SAM) and NaHS increased H2S levels and inhibited intracerebral haemorrhage (ICH) - induced neutrophil infiltration and NLRP3 inflammasome activation [135]. Besides drugs that affect H2S levels and H2S synthase agonists, the mRNA levels of H2S-synthetase CSE and 3-MST were significantly increased in aged animals undergoing exercise training, ultimately leading to the restoration of endogenous H2S production to the levels observed in adults [136].
Conclusion and perspective
The above evidences suggest that endogenous or exogenous H2S can accelerate various phases of wound healing in diabetic pathological conditions. Some important questions in the field of H2S remain unanswered, which will require the joint efforts of researchers in disciplines such as medicinal chemistry, pharmacology, pharmaceutics, and biology. A major concern is to identify the therapeutic window of H2S for a given target to deepen understanding of how H2S releases from its donors and following byproducts, signaling mechanism molecules and biological functions. The application of endogenous or exogenous H2S and its related pathways can provide new ideas for improving poor diabetic wound healing, and adjust the release rate and target of H2S by changing the dosage form and release mode. However, many issues of H2S in diabetic wound healing remain to be studied. H2S plays a major role in which stage of diabetic wound healing, and the S-sulfhydrated modification of specific proteins in related pathways remains to be explored. In addition, known drugs such as non-steroidal anti-inflammatory drugs (aspirin, diclofenac, naproxen, etc.) and derivatives of sildenafil combined with H2S have been demonstrated to have both the efficacy of the parent drug itself and the biological effects of H2S, which can attenuate the toxic side effects of the parent drug to some extent. In the future, it is expected that H2S will be connected to known drugs used for wound healing to constitute derivatives with better efficacy, and the specific mechanism of how they will play a biological role needs to be further studied.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This paper was financially supported by Foundation of Liaoning Educational Committee (LJKZ0932), National Natural Science Foundation of China (81803603), Project funded by China Postdoctoral Science Foundation (202017M621161, 2018T110462), Lianyungang Postdoctoral Research Funding Program (LYG20210017), Excellent Young Scholars Program of Shenyang Pharmaceutical University (YQ202114), Liao Ning Revitalization Talents Program (XLYC2007095) and Natural Science Foundation of Liao Ning Province (2022-YQ-17), Shenyang Young and Middle-aged Scientific and Technological Innovation Talents Support Project (RC220420).
Biographies

Xinyi Shi is currently a graduate student in pharmacology at Shenyang Pharmaceutical University. Her research direction is the effect of natural product and hydrogen sulfide derivatives on macrophages in diabetic wound healing.

Haonan Li obtained his Ph.D. degree in natural product chemistry in 2022 from the Shenyang Pharmaceutical University. At present, as a postdoctor, he continues to study the antitumor activity of natural product derivatives and hydrogen sulfide donors.

Fengrui Guo is currently a graduate student in natural product chemistry at Shenyang Pharmaceutical University. Her research direction is the mechanisms of natural product and hydrogen sulfide derivatives protecting endothelial cells in diabetic wound healing.

Dahong Li received his Ph.D. degree in 2013 under the supervision of Prof. Jinyi Xu from China Pharmaceutical University. Then he joined the group of professor Huiming Hua in Shenyang Pharmaceutical University after graduation. He is a professor now and the main research directions are the discovery of lead compounds from natural sources and the pharmacochemical biology based on specific skeleton and activity of natural molecules.

Dr. Fanxing Xu received his Ph.D. degree in biochemical engineering from Dalian University of Technology in 2014. Then he joined Shenyang Pharmaceutical University as a lecturer and was promoted to associate professor in 2018. His current research interests focus on the pathophysiology and treatment of diabetes and its complications.
Contributor Information
Dahong Li, Email: lidahong0203@163.com.
Fanxing Xu, Email: fanxing0011@163.com.
References
- 1.Chatterjee S., Khunti K., Davies M.J. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 2.Boateng J., Catanzano O. Advanced therapeutic dressings for effective wound healing—a review. J Pharm Sci. 2015;104(11):3653–3680. doi: 10.1002/jps.24610. [DOI] [PubMed] [Google Scholar]
- 3.Laitiff A., Teoh S., Das S. Wound healing in diabetes mellitus: traditional treatment modalities. La Clin Ter. 2010;161(4):359–364. [PubMed] [Google Scholar]
- 4.Lazzarini P., Pacella R.E., Armstrong D., Van Netten J. Diabetes-related lower-extremity complications are a leading cause of the global burden of disability. Diabet Med. 2018;35(9):1297–1299. doi: 10.1111/dme.13680. [DOI] [PubMed] [Google Scholar]
- 5.Wang H., Xu Z., Zhao M., Liu G., Wu J. Advances of hydrogel dressings in diabetic wounds. Biomater Sci. 2021;9(5):1530–1546. doi: 10.1039/d0bm01747g. [DOI] [PubMed] [Google Scholar]
- 6.Kavitha K.V., Tiwari S., Purandare V.B., Khedkar S., Bhosale S.S., Unnikrishnan A.G. Choice of wound care in diabetic foot ulcer: a practical approach. World J Diabetes. 2014;5(4):546–556. doi: 10.4239/wjd.v5.i4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan S., Chalotra R., Rathi A., Saini M., Deol S., Lard M., et al. Current approaches in healing of wounds in diabetes and diabetic foot ulcers. Curr Bioact Compd. 2023;19(3):18. [Google Scholar]
- 8.Yang L., Zhang L., Hu J., Wang W., Liu X. Promote anti-inflammatory and angiogenesis using a hyaluronic acid-based hydrogel with miRNA-laden nanoparticles for chronic diabetic wound treatment. Int J Biol Macromol. 2021;166:166–178. doi: 10.1016/j.ijbiomac.2020.10.129. [DOI] [PubMed] [Google Scholar]
- 9.Park K.H., Kwon J.B., Park J.H., Shin J.C., Han S.H., Lee J.W. Collagen dressing in the treatment of diabetic foot ulcer: a prospective, randomized, placebo-controlled, single-center study. Diabetes Res Clin Pract. 2019;156 doi: 10.1016/j.diabres.2019.107861. [DOI] [PubMed] [Google Scholar]
- 10.Matoori S, Veves A, Mooney DJ. Advanced bandages for diabetic wound healing. Sci Transl Med 2021;13(585):eabe4839. [DOI] [PubMed]
- 11.Patel S., Srivastava S., Singh M.R., Singh D. Mechanistic insight into diabetic wounds: pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed Pharmacother. 2019;112 doi: 10.1016/j.biopha.2019.108615. [DOI] [PubMed] [Google Scholar]
- 12.Kimura H. Hydrogen sulfide (H2S) and polysulfide (H2Sn) signaling: the first 25 years. Biomolecules. 2021;11(6):896. doi: 10.3390/biom11060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv B., Chen S., Tang C., Jin H., Du J., Huang Y. Hydrogen sulfide and vascular regulation – an update. J Adv Res. 2020;27:85–97. doi: 10.1016/j.jare.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang R. The gasotransmitter role of hydrogen sulfide. Antioxid Redox Signal. 2003;5(4):493–501. doi: 10.1089/152308603768295249. [DOI] [PubMed] [Google Scholar]
- 15.Bełtowski J. Synthesis, metabolism, and signaling mechanisms of hydrogen sulfide: an overview. Vascul Eff Hydrogen Sulfide. 2019;2007:1–8. doi: 10.1007/978-1-4939-9528-8_1. [DOI] [PubMed] [Google Scholar]
- 16.Dilek N., Papapetropoulos A., Toliver-Kinsky T., Szabo C. Hydrogen sulfide: an endogenous regulator of the immune system. Pharmacol Res. 2020;161 doi: 10.1016/j.phrs.2020.105119. [DOI] [PubMed] [Google Scholar]
- 17.Katsouda A., Bibli S.-I., Pyriochou A., Szabo C., Papapetropoulos A. Regulation and role of endogenously produced hydrogen sulfide in angiogenesis. Pharmacol Res. 2016;113:175–185. doi: 10.1016/j.phrs.2016.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinto R.V., Carvalho S., Antunes F., Pires J., Pinto M.L. Emerging nitric oxide and hydrogen sulfide releasing carriers for skin wound healing therapy. ChemMedChem. 2021;17(1) doi: 10.1002/cmdc.202100429. [DOI] [PubMed] [Google Scholar]
- 19.Luo Y., Zhu C., Du D., Lin Y. A review of optical probes based on nanomaterials for the detection of hydrogen sulfide in biosystems. Anal Chim Acta. 2019;1061:1–12. doi: 10.1016/j.aca.2019.02.045. [DOI] [PubMed] [Google Scholar]
- 20.Ibrahim H., Serag A., Farag M.A. Emerging analytical tools for the detection of the third gasotransmitter H2S, a comprehensive review. J Adv Res. 2020;27:137–153. doi: 10.1016/j.jare.2020.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang N., Liu Y., Li T., Tuo Q. Role of hydrogen sulfide in chronic diseases. DNA Cell Biol. 2020;39(2):187–196. doi: 10.1089/dna.2019.5067. [DOI] [PubMed] [Google Scholar]
- 22.van den Born JC, Hammes H-P, Greffrath W, van Goor H, Hillebrands J-L, null n. Gasotransmitters in vascular complications of diabetes. Diabetes 2016;65(2):331–45. [DOI] [PubMed]
- 23.Liao Y.-X., Wang X.-H., Bai Y., Lin F., Li M.-X., Mi W.-J., et al. Relationship between endogenous hydrogen sulfide and pulmonary vascular indexes on high-resolution computed tomography in patients with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2021;16:2279–2289. doi: 10.2147/COPD.S314349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wiest E.F., Rios L., Friedrichsen D., Silaski G., Mowry C., Shekarriz R., et al. Abstract P131: transdermal detection of low concentrations of hydrogen sulfide. Hypertension. 2018;72 [Google Scholar]
- 25.Davinelli S, Bassetto F, Vitale M, Scapagnini G. Chapter 10 - Thermal waters and the hormetic effects of hydrogen sulfide on inflammatory arthritis and wound healing. In: Rattan SIS, Kyriazis M, editors. Sci Hormesis Health Longevity; 2019. p. 121–6.
- 26.Cheng Z., Kishore R. Potential role of hydrogen sulfide in diabetes-impaired angiogenesis and ischemic tissue repair. Redox Biol. 2020;37 doi: 10.1016/j.redox.2020.101704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murphy B., Bhattacharya R., Mukherjee P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019;33(12):13098–13125. doi: 10.1096/fj.201901304R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mustafa A.K., Sikka G., Gazi S.K., Steppan J., Jung S.M., Bhunia A.K., et al. Hydrogen sulfide as endothelium-derived hyperpolarizing factor sulfhydrates potassium channels. Circ Res. 2011;109(11):1259–1268. doi: 10.1161/CIRCRESAHA.111.240242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paul B.D., Snyder S.H. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol. 2012;13(8):499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 30.Zhang T., Tsutsuki H., Ono K., Akaike T., Sawa T. Antioxidative and anti-inflammatory actions of reactive cysteine persulfides. J Clin Biochem Nutr. 2020;68(1):5–8. doi: 10.3164/jcbn.20-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie L., Gu Y., Wen M., Zhao S., Wang W., Ma Y., et al. Hydrogen sulfide induces keap1 S-sulfhydration and suppresses diabetes-accelerated atherosclerosis via Nrf2 activation. Diabetes. 2016;65(10):3171–3184. doi: 10.2337/db16-0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lu F., Lu B., Zhang L., Wen J., Wang M., Zhang S., et al. Hydrogen sulphide ameliorating skeletal muscle atrophy in db/db mice via Muscle RING finger 1 S-sulfhydration. J Cell Mol Med. 2020;24(16):9362–9377. doi: 10.1111/jcmm.15587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y., Zhang L., Lu B., Wen J., Wang M., Zhang S., et al. Hydrogen sulphide reduced the accumulation of lipid droplets in cardiac tissues of db/db mice via Hrd1 S-sulfhydration. J Cell Mol Med. 2021;25(19):9154–9167. doi: 10.1111/jcmm.16781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun X., Zhao D., Lu F., Peng S., Yu M., Liu N., et al. Hydrogen sulfide regulates muscle RING finger-1 protein S-sulfhydration at Cys44 to prevent cardiac structural damage in diabetic cardiomyopathy. Br J Pharmacol. 2019;177(4):836–856. doi: 10.1111/bph.14601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun H.-J., Xiong S.-P., Cao X., Cao L., Zhu M.-Y., Wu Z.-Y., et al. Polysulfide-mediated sulfhydration of SIRT1 prevents diabetic nephropathy by suppressing phosphorylation and acetylation of p65 NF-κB and STAT3. Redox Biol. 2020;38 doi: 10.1016/j.redox.2020.101813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abazari M., Ghaffari A., Rashidzadeh H., Badeleh S.M., Maleki Y. A systematic review on classification, identification, and healing process of burn wound healing. Int J Low Extr Wound. 2022;21(1):18–30. doi: 10.1177/1534734620924857. [DOI] [PubMed] [Google Scholar]
- 37.Lemkes B.A., Hermanides J., DeVries J.H., Holleman F., Meijers J.C., Hoekstra J.B. Hyperglycemia: a prothrombotic factor? J Thromb Haemost. 2010;8(8):1663–1669. doi: 10.1111/j.1538-7836.2010.03910.x. [DOI] [PubMed] [Google Scholar]
- 38.Li Z., Qi C., Jia Z., Zhen R., Ren L., Jia Y., et al. The correlation between estimated glucose disposal rate and coagulation indexes in type 2 diabetes mellitus. Diabetes Metab Syndr Ob. 2022;15:2643–2652. doi: 10.2147/DMSO.S371457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng Z., Shen X., Jiang X., Shan H., Cimini M., Fang P., et al. Hyperhomocysteinemia potentiates diabetes-impaired EDHF-induced vascular relaxation: role of insufficient hydrogen sulfide. Redox Biol. 2018;16:215–225. doi: 10.1016/j.redox.2018.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li X., Weber N.C., Cohn D.M., Hollmann M.W., DeVries J.H., Hermanides J., et al. Effects of hyperglycemia and diabetes mellitus on coagulation and hemostasis. J Clin Med. 2021;10(11):2419. doi: 10.3390/jcm10112419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Q.-F., Cao D., Ye T.-T., Deng H.-H., Zhu H. Peripheral arterial disease in type 2 diabetes is associated with an increase in fibrinogen levels. Int J Endocrinol. 2018:3709534. doi: 10.1155/2018/3709534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Elkhalifa H., Mustafa R., El-Zubair H., Salah M., Sadig O., Nimer A. Plasma fibrinogen level in sudanese patients with Type2 diabetes mellitus. J Hypertens. 2018;36:E103. [Google Scholar]
- 43.Wang G., Li W., Chen Q., Jiang Y., Lu X., Zhao X. Hydrogen sulfide accelerates wound healing in diabetic rats. Int J Clin Exp Pathol. 2015;8(5):5097–5104. [PMC free article] [PubMed] [Google Scholar]
- 44.Piñeiro-Ramil M., Burguera E.F., Hermida-Gómez T., Caramés B., Oreiro-Villar N., Meijide-Faílde R., et al. Reduced levels of H2S in diabetes-associated osteoarthritis are linked to hyperglycaemia, Nrf-2/HO-1 signalling downregulation and chondrocyte dysfunction. Antioxidants. 2022;11(4):628. doi: 10.3390/antiox11040628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Grambow E., Leppin C., Leppin K., Kundt G., Klar E., Frank M., et al. The effects of hydrogen sulfide on platelet-leukocyte aggregation and microvascular thrombolysis. Platelets. 2016;28(5):509–517. doi: 10.1080/09537104.2016.1235693. [DOI] [PubMed] [Google Scholar]
- 46.Olas B. Hydrogen sulfide in hemostasis: Friend or foe? Chem Biol Interact. 2014;217:49–56. doi: 10.1016/j.cbi.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Olas B. Gasomediators (·NO, CO, and H2S) and their role in hemostasis and thrombosis. Clin Chim Acta. 2015;445:115–121. doi: 10.1016/j.cca.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 48.Morel A., Malinowska J., Olas B. Antioxidative properties of hydrogen sulfide may involve in its antiadhesive action on blood platelets. Clin Biochem. 2012;45(18):1678–1682. doi: 10.1016/j.clinbiochem.2012.08.025. [DOI] [PubMed] [Google Scholar]
- 49.Grambow E., Klee G., Klar E., Vollmar B. The slow releasing hydrogen sulfide donor GYY4137 reduces neointima formation upon FeCl3 injury of the carotid artery in mice. Clin Hemorheol Microcirc. 2020;75(4):409–417. doi: 10.3233/CH-190747. [DOI] [PubMed] [Google Scholar]
- 50.Grambow E., Mueller-Graf F., Delyagina E., Frank M., Kuhla A., Vollmar B. Effect of the hydrogen sulfide donor GYY4137 on platelet activation and microvascular thrombus formation in mice. Platelets. 2014;25(3):166–174. doi: 10.3109/09537104.2013.786823. [DOI] [PubMed] [Google Scholar]
- 51.Allison G.L., Lowe G.M., Rahman K. Aged garlic extract inhibits platelet activation by increasing intracellular cAMP and reducing the interaction of GPIIb/IIIa receptor with fibrinogen. Life Sci. 2012;91(25–26):1275–1280. doi: 10.1016/j.lfs.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 52.Rehak L., Giurato L., Meloni M., Panunzi A., Manti G.M., Uccioli L. The immune-centric revolution in the diabetic foot: monocytes and lymphocytes role in wound healing and tissue regeneration—a narrative review. J Clin Med. 2022;11(3):889. doi: 10.3390/jcm11030889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eming S.A., Martin P., Tomic-Canic M. Wound repair and regeneration: mechanisms, signaling, and translation. Sci Transl Med. 2014;6(265) doi: 10.1126/scitranslmed.3009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mirza R., DiPietro L.A., Koh T.J. Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol. 2009;175(6):2454–2462. doi: 10.2353/ajpath.2009.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lucas T., Waisman A., Ranjan R., Roes J., Krieg T., Müller W., et al. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184(7):3964–3977. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 56.Farrugia G., Szurszewski J.H. Carbon monoxide, hydrogen sulfide, and nitric oxide as signaling molecules in the gastrointestinal tract. Gastroenterology. 2014;147(2):303–313. doi: 10.1053/j.gastro.2014.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang C.-T., Chen L., Chen W.-L., Li N., Chen M.-J., Li X., et al. Hydrogen sulfide primes diabetic wound to close through inhibition of NETosis. Mol Cell Endocrinol. 2019;480:74–82. doi: 10.1016/j.mce.2018.10.013. [DOI] [PubMed] [Google Scholar]
- 58.Zhao H., Lu S., Chai J., Zhang Y., Ma X., Chen J., et al. Hydrogen sulfide improves diabetic wound healing in ob/ob mice via attenuating inflammation. J Diabetes Complications. 2017;31(9):1363–1369. doi: 10.1016/j.jdiacomp.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 59.Li X., Yu P., Yu Y., Xu T., Liu J., Cheng Y., et al. Hydrogen sulfide ameliorates high glucose-induced pro-inflammation factors in HT-22 cells: Involvement of SIRT1-mTOR/NF-κB signaling pathway. Int Immunopharmacol. 2021;95 doi: 10.1016/j.intimp.2021.107545. [DOI] [PubMed] [Google Scholar]
- 60.Caldwell M.D. Bacteria and antibiotics in wound healing. Surg Clin North Am. 2020;100(4):757–776. doi: 10.1016/j.suc.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Łobocka M., Dąbrowska K., Górski A. Engineered bacteriophage therapeutics: rationale, challenges and future. BioDrugs. 2021;35(3):255–280. doi: 10.1007/s40259-021-00480-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jiang S., Cui C., Bai W., Wang W., Ren E., Xiao H., et al. Shape-controlled silver nanoplates colored fabric with tunable colors, photothermal antibacterial and colorimetric detection of hydrogen sulfide. J Colloid Interface Sci. 2022;626:1051–1061. doi: 10.1016/j.jcis.2022.07.011. [DOI] [PubMed] [Google Scholar]
- 63.Liu D., Liao Y., Cornel E.J., Lv M., Wu T., Zhang X., et al. Polymersome wound dressing spray capable of bacterial inhibition and H2S generation for complete diabetic wound healing. Chem Mater. 2021;33(20):7972–7985. [Google Scholar]
- 64.Li J., Zhang Y.P., Kirsner R.S. Angiogenesis in wound repair: angiogenic growth factors and the extracellular matrix. Microsc Res Tech. 2003;60(1):107–114. doi: 10.1002/jemt.10249. [DOI] [PubMed] [Google Scholar]
- 65.Landén N.X., Li D., Ståhle M. Transition from inflammation to proliferation: a critical step during wound healing. Cell Mol Life Sci. 2016;73(20):3861–3885. doi: 10.1007/s00018-016-2268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kay E.J., Koulouras G., Zanivan S. Regulation of extracellular matrix production in activated fibroblasts: roles of amino acid metabolism in collagen synthesis. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.719922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Li Q., Xia S., Yin Y., Guo Y., Chen F., Jin P. miR-5591-5p regulates the effect of ADSCs in repairing diabetic wound via targeting AGEs/AGER/JNK signaling axis. Cell Death Dis. 2018;9(5):566. doi: 10.1038/s41419-018-0615-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giacco F., Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi: 10.1161/CIRCRESAHA.110.223545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu T., Lu Y., Zhan R., Qian W., Luo G. Nanomaterials and nanomaterials-based drug delivery to promote cutaneous wound healing. Adv Drug Deliv Rev. 2022;193 doi: 10.1016/j.addr.2022.114670. [DOI] [PubMed] [Google Scholar]
- 70.Kunkemoeller B., Kyriakides T.R. Redox signaling in diabetic wound healing regulates extracellular matrix deposition. Antioxid Redox Signal. 2017;27(12):823–838. doi: 10.1089/ars.2017.7263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu P., Wang Z., Sun X., Chen X., Zeng S., Chen L., et al. Hydrogen-rich medium protects human skin fibroblasts from high glucose or mannitol induced oxidative damage. Biochem Biophys Res Commun. 2011;409(2):350–355. doi: 10.1016/j.bbrc.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 72.Panich U., Lohakul J., Whiteman M. The novel mitochondria-targeted hydrogen sulfide donors AP39 and AP123 exert anti-photoaging effects on primary dermal fibroblasts and mouse skin exposed to UVA in association with activation of Nrf2-mediated antioxidant response. Free Radic Biol Med. 2018;128:S126. [Google Scholar]
- 73.Feng A., Ling C., Xin-Duo L., Bing W., San-Wu W., Yu Z., et al. Hydrogen sulfide protects human cardiac fibroblasts against H2O2-induced injury through regulating autophagy-related proteins. Cell Transplant. 2018;27(8):1222–1234. doi: 10.1177/0963689718779361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Y., Liu M., Song X., Zheng X., Yi J., Liu D., et al. Exogenous hydrogen sulfide ameliorates diabetic myocardial fibrosis by inhibiting cell aging through SIRT6/AMPK autophagy. Front Pharmacol. 2020;11:1150. doi: 10.3389/fphar.2020.01150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhao X., Liu L., An T., Xian M., Luckanagul J.A., Su Z., et al. A hydrogen sulfide-releasing alginate dressing for effective wound healing. Acta Biomater. 2020;104:85–94. doi: 10.1016/j.actbio.2019.12.032. [DOI] [PubMed] [Google Scholar]
- 76.Frey R.S., Ushio-Fukai M., Malik A.B. NADPH oxidase-dependent signaling in endothelial cells: role in physiology and pathophysiology. Antioxid Redox Signal. 2009;11(4):791–810. doi: 10.1089/ars.2008.2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xiong Y, Chu X, Yu T, Knoedler S, Schroeter A, Lu L, et al. Reactive oxygen species-scavenging nanosystems in the treatment of diabetic wounds. Adv Healthc Mater 2023:e2300779. [DOI] [PubMed]
- 78.Nensat C., Songjang W., Tohtong R., Suthiphongchai T., Phimsen S., Rattanasinganchan P., et al. Porcine placenta extract improves high-glucose-induced angiogenesis impairment. BMC Complement Med Ther. 2021;21(1):66. doi: 10.1186/s12906-021-03243-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bai L., Qi Y., Chen S., Wang J., Tang C., Du J., et al. Angiotensin II downregulates vascular endothelial cell hydrogen sulfide production by enhancing cystathionine γ-lyase degradation through ROS-activated ubiquitination pathway. Biochem Biophys Res Commun. 2019;514(3):907–912. doi: 10.1016/j.bbrc.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 80.Wang P., Chen F., Wang W., Zhang X.-D. Hydrogen sulfide attenuates high glucose-induced human retinal pigment epithelial cell inflammation by inhibiting ROS formation and NLRP3 inflammasome activation. Mediators Inflamm. 2019 doi: 10.1155/2019/8908960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun W.-H., Liu F., Chen Y., Zhu Y.-C. Hydrogen sulfide decreases the levels of ROS by inhibiting mitochondrial complex IV and increasing SOD activities in cardiomyocytes under ischemia/reperfusion. Biochem Biophys Res Commun. 2012;421(2):164–169. doi: 10.1016/j.bbrc.2012.03.121. [DOI] [PubMed] [Google Scholar]
- 82.Guan Q., Zhang Y., Yu C., Liu Y., Gao L., Zhao J. Hydrogen sulfide protects against high-glucose–induced apoptosis in endothelial cells. J Cardiovasc Pharmacol. 2012;59(2):188–193. doi: 10.1097/FJC.0b013e31823b4915. [DOI] [PubMed] [Google Scholar]
- 83.Ng H.H., Yildiz G.S., Ku J.M., Miller A.A., Woodman O.L., Hart J.L. Chronic NaHS treatment decreases oxidative stress and improves endothelial function in diabetic mice. Diab Vasc Dis Res. 2017;14(3):246–253. doi: 10.1177/1479164117692766. [DOI] [PubMed] [Google Scholar]
- 84.Lin F., Yang Y., Wei S., Huang X., Peng Z., Ke X., et al. Hydrogen sulfide protects against high glucose-induced human umbilical vein endothelial cell injury through activating PI3K/Akt/eNOS pathway. Drug Des Devel Ther. 2020;14:621–633. doi: 10.2147/DDDT.S242521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu J., Wu J., Sun A., Sun Y., Yu X., Liu N., et al. Hydrogen sulfide decreases high glucose/palmitate-induced autophagy in endothelial cells by the Nrf2-ROS-AMPK signaling pathway. Cell Biosci. 2016;6:33. doi: 10.1186/s13578-016-0099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lin W.-C., Huang C.-C., Lin S.-J., Li M.-J., Chang Y., Lin Y.-J., et al. In situ depot comprising phase-change materials that can sustainably release a gasotransmitter H2S to treat diabetic wounds. Biomaterials. 2017;145:1–8. doi: 10.1016/j.biomaterials.2017.08.023. [DOI] [PubMed] [Google Scholar]
- 87.Li L., Whiteman M., Guan Y.Y., Neo K.L., Cheng Y., Lee S.W., et al. Characterization of a novel, water-soluble hydrogen sulfide–releasing molecule (GYY4137) new insights into the biology of hydrogen sulfide. Circulation. 2008;117(18):2351–2360. doi: 10.1161/CIRCULATIONAHA.107.753467. [DOI] [PubMed] [Google Scholar]
- 88.Coletta C., Papapetropoulos A., Erdelyi K., Olah G., Módis K., Panopoulos P., et al. Hydrogen sulfide and nitric oxide are mutually dependent in the regulation of angiogenesis and endothelium-dependent vasorelaxation. Proc Natl Acad Sci. 2012;109(23):9161–9166. doi: 10.1073/pnas.1202916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Peng Z., Shu B., Zhang Y., Wang M. Endothelial response to pathophysiological stress. Arterioscler Thromb Vasc Biol. 2019;39(11):e233–e243. doi: 10.1161/ATVBAHA.119.312580. [DOI] [PubMed] [Google Scholar]
- 90.Wang G.-G., Li W. Hydrogen sulfide improves vessel formation of the ischemic adductor muscle and wound healing in diabetic db/db mice. Iran J Basic Med Sci. 2019;22(10):1192–1197. doi: 10.22038/ijbms.2019.36551.8709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Xue W.-L., Chen R.-Q., Zhang Q.-Q., Li X.-H., Cao L., Li M.-Y., et al. Hydrogen sulfide rescues high glucose-induced migration dysfunction in HUVECs by upregulating miR-126-3p. Am J Physiol Cell Physiol. 2020;318(5):C857–C869. doi: 10.1152/ajpcell.00406.2019. [DOI] [PubMed] [Google Scholar]
- 92.Hebbar S., Knust E. Reactive oxygen species (ROS) constitute an additional player in regulating epithelial development. Bioessays. 2021;43(8):e2100096. doi: 10.1002/bies.202100096. [DOI] [PubMed] [Google Scholar]
- 93.Yang C.-T., Zhao Y., Xian M., Li J.-H., Dong Q., Bai H.-B., et al. A novel controllable hydrogen sulfide-releasing molecule protects human skin keratinocytes against methylglyoxal-induced injury and dysfunction. Cell Physiol Biochem. 2014;34(4):1304–1317. doi: 10.1159/000366339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhao H., Liu H., Yang Y., Wang H. The role of H2S regulating NLRP3 inflammasome in diabetes. Int J Mol Sci. 2022;23(9):4818. doi: 10.3390/ijms23094818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Juin S.K., Pushpakumar S., Sen U. GYY4137 regulates extracellular matrix turnover in the diabetic kidney by modulating retinoid X receptor signaling. Biomolecules. 2021;11(10):1477. doi: 10.3390/biom11101477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dugbartey G.J., Alornyo K.K., Diaba D.E., Adams I. Activation of renal cse/h2s pathway by alpha-Lipoic acid protects against histological and functional changes in the diabetic kidney. Biomed Pharmacother. 2022;153 doi: 10.1016/j.biopha.2022.113386. [DOI] [PubMed] [Google Scholar]
- 97.Kundu S., Pushpakumar S.B., Tyagi A., Coley D., Sen U. Hydrogen sulfide deficiency and diabetic renal remodeling: role of matrix metalloproteinase-9. Am J Physiol Endocrinol Metab. 2013;304(12):E1365–E1378. doi: 10.1152/ajpendo.00604.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li Y., Li L., Zeng O., Liu J.M., Yang J. H2S improves renal fibrosis in STZ-induced diabetic rats by ameliorating TGF-β 1 expression. Ren Fail. 2017;39(1):265–272. doi: 10.1080/0886022X.2016.1257433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sen U., Rodriguez W.E., Tyagi N., Kumar M., Kundu S., Tyagi S.C. Ciglitazone, a PPARγ agonist, ameliorates diabetic nephropathy in part through homocysteine clearance. Am J Physiol Endocrinol Metab. 2008;295(5):E1205–E1212. doi: 10.1152/ajpendo.90534.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bai L., Qi Y., Chen S., Wang J., Tang C., Du J., et al. Angiotensin II downregulates vascular endothelial cell hydrogen sulfide production by enhancing cystathionine gamma-lyase degradation through ROS-activated ubiquitination pathway. Biochem Biophys Res Commun. 2019;514(3):907–912. doi: 10.1016/j.bbrc.2019.05.021. [DOI] [PubMed] [Google Scholar]
- 101.Arif H.M., Qian Z.M., Wang R. Signaling integration of hydrogen sulfide and iron on cellular functions. Antioxid Redox Signal. 2022;36(4–6):275–293. doi: 10.1089/ars.2021.0203. [DOI] [PubMed] [Google Scholar]
- 102.Kabil O., Yadav V., Banerjee R. Heme-dependent metabolite switching regulates H2S synthesis in response to endoplasmic reticulum (ER) stress. J Biol Chem. 2016;291(32):16418–16423. doi: 10.1074/jbc.C116.742213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Nandi S.S., Mishra P.K. H2S and homocysteine control a novel feedback regulation of cystathionine beta synthase and cystathionine gamma lyase in cardiomyocytes. Sci Rep. 2017;7(1):3639. doi: 10.1038/s41598-017-03776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hughes M., Centelles M., Moore K. Making and working with hydrogen sulfide: the chemistry and generation of hydrogen sulfide in vitro and its measurement in vivo: a review. Free Radic Biol Med. 2009;47(10):1346–1353. doi: 10.1016/j.freeradbiomed.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 105.Furne J., Springfield J., Koenig T., DeMaster E., Levitt M. Oxidation of hydrogen sulfide and methanethiol to thiosulfate by rat tissues: a specialized function of the colonic mucosa. Biochem Pharmacol. 2001;62(2):255–259. doi: 10.1016/s0006-2952(01)00657-8. [DOI] [PubMed] [Google Scholar]
- 106.Stein A., Bailey S.M. Redox biology of hydrogen sulfide: implications for physiology, pathophysiology, and pharmacology. Redox Biol. 2013;1(1):32–39. doi: 10.1016/j.redox.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hellmich M.R., Szabo C. Hydrogen sulfide and cancer. Handb Exp Pharmacol. 2015;230:233–241. doi: 10.1007/978-3-319-18144-8_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Powell C.R., Dillon K.M., Matson J.B. A review of hydrogen sulfide (H2S) donors: chemistry and potential therapeutic applications. Biochem Pharmacol. 2018;149:110–123. doi: 10.1016/j.bcp.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zheng Y., Yu B., De La Cruz L.K., Roy Choudhury M., Anifowose A., Wang B. Toward hydrogen sulfide based therapeutics: critical drug delivery and developability issues. Med Res Rev. 2018;38(1):57–100. doi: 10.1002/med.21433. [DOI] [PubMed] [Google Scholar]
- 110.Sone K., Mori A., Sakamoto K., Nakahara T. GYY4137, an extended-release hydrogen sulfide donor, reduces NMDA-induced neuronal injury in the murine retina. Biol Pharm Bull. 2018;41(4):657–660. doi: 10.1248/bpb.b17-01032. [DOI] [PubMed] [Google Scholar]
- 111.Zheng Y., Ji X., Ji K., Wang B. Hydrogen sulfide prodrugs—a review. Acta Pharm Sin B. 2015;5(5):367–377. doi: 10.1016/j.apsb.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Szczesny B., Módis K., Yanagi K., Coletta C., Le Trionnaire S., Perry A., et al. AP39, a novel mitochondria-targeted hydrogen sulfide donor, stimulates cellular bioenergetics, exerts cytoprotective effects and protects against the loss of mitochondrial DNA integrity in oxidatively stressed endothelial cells in vitro. Nitric Oxide. 2014;41:120–130. doi: 10.1016/j.niox.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gerő D., Torregrossa R., Perry A., Waters A., Le-Trionnaire S., Whatmore J.L., et al. The novel mitochondria-targeted hydrogen sulfide (H2S) donors AP123 and AP39 protect against hyperglycemic injury in microvascular endothelial cells in vitro. Pharmacol Res. 2016;113:186–198. doi: 10.1016/j.phrs.2016.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Munteanu C., Rotariu M., Turnea M., Dogaru G., Popescu C., Spînu A., et al. Recent advances in molecular research on hydrogen sulfide (H2S) role in diabetes mellitus (DM)—a systematic review. Int J Mol Sci. 2022;23(12):6720. doi: 10.3390/ijms23126720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang T., Li Y., Cornel E.J., Li C., Du J. Combined antioxidant-antibiotic treatment for effectively healing infected diabetic wounds based on polymer vesicles. ACS Nano. 2021;15(5):9027–9038. doi: 10.1021/acsnano.1c02102. [DOI] [PubMed] [Google Scholar]
- 116.Yang N., Zhu M., Xu G., Liu N., Yu C. A near-infrared light-responsive multifunctional nanocomposite hydrogel for efficient and synergistic antibacterial wound therapy and healing promotion. J Mater Chem B. 2020;8(17):3908–3917. doi: 10.1039/d0tb00361a. [DOI] [PubMed] [Google Scholar]
- 117.Jin L., Guo X., Gao D., Liu Y., Ni J., Zhang Z., et al. An NIR photothermal-responsive hybrid hydrogel for enhanced wound healing. Bioact Mater. 2022;16:162–172. doi: 10.1016/j.bioactmat.2022.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bai Q., Han K., Dong K., Zheng C., Zhang Y., Long Q., et al. Potential applications of nanomaterials and technology for diabetic wound healing. Int J Nanomedicine. 2020;15:9717–9743. doi: 10.2147/IJN.S276001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Renuka R.R., Julius A., Yoganandham S.T., Umapathy D., Ramadoss R., Samrot A.V., et al. Diverse nanocomposites as a potential dressing for diabetic wound healing. Front Endocrinol. 2023;13:1074568. doi: 10.3389/fendo.2022.1074568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Singh S.S., Behera S.K., Rai S., Tripathy S.K., Chakrabortty S., Mishra A. A critical review on nanomaterial based therapeutics for diabetic wound healing. Biotechnol Genet Eng Rev. 2022:1–35. doi: 10.1080/02648725.2022.2161732. [DOI] [PubMed] [Google Scholar]
- 121.Dewberry L.C., Niemiec S.M., Hilton S.A., Louiselle A.E., Singh S., Sakthivel T.S., et al. Cerium oxide nanoparticle conjugation to microRNA-146a mechanism of correction for impaired diabetic wound healing. Nanomedicine. 2021;40 doi: 10.1016/j.nano.2021.102483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sener G., Hilton S.A., Osmond M.J., Zgheib C., Newsom J.P., Dewberry L., et al. Injectable, self-healable zwitterionic cryogels with sustained microRNA - cerium oxide nanoparticle release promote accelerated wound healing. Acta Biomater. 2020;101:262–272. doi: 10.1016/j.actbio.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 123.Zgheib C., Hilton S.A., Dewberry L.C., Hodges M.M., Ghatak S., Xu J., et al. Use of cerium oxide nanoparticles conjugated with MicroRNA-146a to correct the diabetic wound healing impairment. J Am Coll Surg. 2019;228(1):107–115. doi: 10.1016/j.jamcollsurg.2018.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Han B., Fang W.H., Zhao S., Yang Z., Hoang B.X. Zinc sulfide nanoparticles improve skin regeneration. Nanomedicine. 2020;29 doi: 10.1016/j.nano.2020.102263. [DOI] [PubMed] [Google Scholar]
- 125.Zhang Y., Yue T., Gu W., Liu A., Cheng M., Zheng H., et al. pH-responsive hierarchical H2S-releasing nano-disinfectant with deep-penetrating and anti-inflammatory properties for synergistically enhanced eradication of bacterial biofilms and wound infection. J Nanobiotechnology. 2022;20(1):55. doi: 10.1186/s12951-022-01262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kang X., Huang H., Jiang C., Cheng L., Sang Y., Cai X., et al. Cysteine-activated small-molecule H2Se donors inspired by synthetic H2S donors. J Am Chem Soc. 2022;144(9):3957–3967. doi: 10.1021/jacs.1c12006. [DOI] [PubMed] [Google Scholar]
- 127.Zhao Y., Wang H., Xian M. Cysteine-activated hydrogen sulfide (H2S) donors. J Am Chem Soc. 2011;133(1):15–17. doi: 10.1021/ja1085723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wu J., Li Y., He C., Kang J., Ye J., Xiao Z., et al. Novel H2S releasing nanofibrous coating for in vivo dermal wound regeneration. ACS Appl Mater Interfaces. 2016;8(41):27474–27481. doi: 10.1021/acsami.6b06466. [DOI] [PubMed] [Google Scholar]
- 129.Ding H., Chang J., He F., Gai S., Yang P. Hydrogen sulfide: an emerging precision strategy for gas therapy. Adv Healthc Mater. 2022;11(4):e2101984. doi: 10.1002/adhm.202101984. [DOI] [PubMed] [Google Scholar]
- 130.Wagner K., Georgieff M., Asfar P., Calzia E., Knöferl M.W., Radermacher P. Of mice and men (and sheep, swine etc.): the intriguing hemodynamic and metabolic effects of hydrogen sulfide (H2S) Crit Care. 2011;15(2):146. doi: 10.1186/cc10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Feng S., Zhao Y., Xian M., Wang Q. Biological thiols-triggered hydrogen sulfide releasing microfibers for tissue engineering applications. Acta Biomater. 2015;27:205–213. doi: 10.1016/j.actbio.2015.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Zhang L.-L., Zhu H.-K., Zhao C.-C., Gu X.-F. A near-infrared fluorescent probe for monitoring fluvastatin-stimulated endogenous H2S production. Chin Chem Lett. 2017;28(2):218–221. [Google Scholar]
- 133.Ma X., Jiang Z., Wang Z., Zhang Z. Administration of metformin alleviates atherosclerosis by promoting H2S production via regulating CSE expression. J Cell Physiol. 2020;235(3):2102–2112. doi: 10.1002/jcp.29112. [DOI] [PubMed] [Google Scholar]
- 134.Wiliński B., Wiliński J., Somogyi E., Piotrowska J., Opoka W. Metformin raises hydrogen sulfide tissue concentrations in various mouse organs. Pharmacol Rep. 2013;65(3):737–742. doi: 10.1016/s1734-1140(13)71053-3. [DOI] [PubMed] [Google Scholar]
- 135.Zhao H., Pan P., Yang Y., Ge H., Chen W., Qu J., et al. Endogenous hydrogen sulphide attenuates NLRP3 inflammasome-mediated neuroinflammation by suppressing the P2X7 receptor after intracerebral haemorrhage in rats. J Neuroinflammation. 2017;14(1):163. doi: 10.1186/s12974-017-0940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Strutynska N., Strutynskyi R., Mys L., Luchkova A., Korkach Y., Goshovska Y., et al. Exercise restores endogenous H2S synthesis and mitochondrial function in the heart of old rats. Eur J Clin Invest. 2022;52(12):e13829. doi: 10.1111/eci.13829. [DOI] [PubMed] [Google Scholar]