Graphical abstract
Keywords: Trop2, β-catenin, Breast cancer, Metastasis, Lung colonization
Highlights
-
•
Trop2 played a vital role in metastasis by Trop2/β-catenin positive feedback loop.
-
•
Bruceine D inhibited cancer cells proliferation and metastasis by targeting Trop2.
-
•
Bruceine D suppressed Trop2-driven ECM-remodeling and EMT to reduce lung metastatic colonization in vivo.
-
•
Bruceine D blocked Trop2/β-catenin complex, inducing β-catenin degradation though ubiquitin.
Abstract
Introduction
Tumor-associated calcium signal transducer 2 (Trop2) has been used as a transport gate for cytotoxic agents into cells in antibody–drug conjugate designs because of its expression in a wide range of solid tumors. However, the specific role of Trop2 itself in breast cancer progression remains unclear and small molecules targeting Trop2 have not yet been reported.
Objectives
To screen small molecules targeting Trop2, and to reveal its pharmacological effects and the molecular mechanisms of action.
Methods
Small molecule targeting Trop2 was identified by cell membrane chromatography, and validated by cellular thermal shift assay and point mutation analyses. We investigated the pharmacological effects of Trop2 inhibitor using RNA-seq, human foreskin fibroblast (HFF)-derived extracellular matrix (ECM), Matrigel drop invasion assays, colony-forming assay, xenograft tumor model, 4T1 orthotopic metastasis model and 4T1 experimental metastasis model. The molecular mechanism was determined using immunoprecipitation, mass spectrometry, immunofluorescence, immunohistochemistry and Western blotting.
Results
Here we identified Bruceine D (BD) as the inhibitor of Trop2, and demonstrated anti-metastasis effects of BD in breast cancer. Notably, Lys307 and Glu310 residues of Trop2 acted as critical sites for BD binding. Mechanistically, BD suppressed Trop2-induced cancer metastasis by blocking the formation of Trop2/β-catenin positive loop, in which the Trop2/β-catenin complex prevented β-catenin from being degraded via the ubiquitin-proteosome pathway. Destabilized β-catenin caused by BD reduced nucleus translocation, leading to the reduction of transcription of Trop2, the reversal of epithelial-mesenchymal transition (EMT) process, and the inhibition of ECM remodeling, further inhibiting cancer metastasis. Additionally, the inhibitory effects of BD on lung metastatic colonization and the beneficial effects of BD on prolongation of survival were validated in 4T1 experimental metastasis model.
Conclusions
These results support the tumor-promoting role of Trop2 in breast cancer by stabilizing β-catenin in Trop2/β-catenin positive loop, and suggest Bruceine D as a promising candidate for Trop2 inhibition.
Introduction
Breast cancer became the most common diagnosed cancer replacing lung cancer, with approximately 2.3 million new cases and 0.69 million deaths in 2020[1]. Although recent research efforts have greatly improved the treatment of breast cancer, there is still a strong demand for new and specific therapeutic drug because of the complex and heterogeneous nature of breast cancer.
Metastasis occurs in 20–30% of patients with breast cancer after diagnosis and primary tumor treatment, and it is the leading cause of death in breast cancer[2], [3]. The risk of metastasis is subtype-dependent, and the patients with triple-negative breast cancer (TNBC) have higher metastasis potential than the patients with other breast cancer subtypes even in an early stage of cancer[2], [4], [5]. Due to the lack of three standard therapeutic targets, namely estrogen receptor, progesterone receptor and human epidermal growth factor receptor, chemotherapy still remains the main treatment option for TNBC[6], [7], and is followed by 5–10% low response rates and 2–3 months’ poor median progression-free survival under relapsed or refractory conditions[8]. Even if treated with (neo)adjuvant chemotherapy, most TNBC patients with metastasis relapse quickly[9], and only a small percentage survive >5 years[10]. Although immunotherapy has been at the forefront of cancer therapy approaches, the combination of immunotherapy and chemotherapy in TNBC remains limited with PD-L1 expression and weak improvement[11]. Therefore, new treatment strategies are necessary for breast cancer, especially for TNBC.
Tumor-associated calcium signal transducer 2 (Trop2) is a glycoprotein and encoded by the gene TACSTD2[12]. Trop2 is involved in cancer development through several mechanisms: cell growth through chimeric cyclin D1-Trop2 mRNA in various cancers[13], stem cell self-renewal via β-catenin signaling[14], and metastasis through β1 integrin-FAK signaling or PARP1 in prostate cancer[15], [16]. In breast cancer, Trop2 is overexpressed in tumor tissues compared to normal tissues[17], [18]. High Trop2 expression, accompanied by low E-cadherin expression in breast cancer, which is associated with cancer metastasis, suggesting that Trop2 may play a promotive role in epithelial-mesenchymal transition (EMT) [19].
Several antibodies against Trop2 have been reported and are found to exhibit considerable anti-cancer effects even when used alone without conjugation to cytotoxic compounds, thus indicating that Trop2 could be a promising antitumor drug target [20], [21]. Furthermore, Trop2 has been used as a transport gate for cytotoxic agents into cells in antibody–drug conjugate (ADC) designs because of its overexpression in a wide range of solid tumors, including colon, stomach, cervical, prostate, and breast cancers[17], [22], [23]. Sacituzumab govitecan-hziy (IMMU-132), an anti-Trop2 antibody (hRS7) conjugated with an inhibitor of topoisomerase I (SN-38), has been used for treating several cancers in clinic. Antibodies itself are generally restricted to possessing high specificity and affinity for targets on the cell surface. Small molecule agents can suppress tumor development by targeting a wide range of extracellular and intracellular proteins, and they are considered as an alternative approach for targeted cancer therapy[24]. Small molecule inhibitors targeting Trop2 have not yet been reported.
Bruceine D (BD) is a quassinoid compound, purified from the seeds of Brucea javanica, and used for the treatment of inflammation, malaria, and warts[25]. Recent studies have shown that BD has multiple biological actions, including restraining Parkinson's disease[26], treating ulcerative colitis[27], and preventing the progression of a wide variety of cancer types, such as lung cancer[28], hepatocellular carcinoma[29], pancreatic cancer[30], osteosarcoma[25], chronic myeloid leukemia[31], and breast cancer[32]. Although studies have reported the anticancer potential of BD, a connection between Trop2 and BD in breast cancer has not been explored. In this study, BD is identified as a Trop2 inhibitor, and BD exerts anti-tumor effects in breast cancer by depleting the Trop2/β-catenin complex which prevents β-catenin from being degraded via the ubiquitin-proteosome pathway.
Material and methods
Cell membrane chromatography (CMC)
Cells were collected and lysed with Tris-HCL solution. The lysate was broken by ultrasonic cell destructor (Ningbo Shanda Biotechnology Co., Ltd., China), and centrifuged (10 min, 1000 g,). The supernatant was harvested and centrifuged again (20 min, 12,000 g, 4 °C). Then the pellet was resuspended with saline, and mixed with 5 mg silica (pre-activated at 120 °C for 30 min) overnight to obtain Cell membrane stationary phase (CMSP). Subsequently, CMSP was packed into a column (10 mm × 2 mm, 5 μm) with PBS. CMC analysis was performed using LC-2010 A high-performance liquid chromatography (Shimadzu, Kyoto, Japan).
High-throughput transcriptome sequencing (RNA-seq) and data analyses
RNA was extracted by using Magzol Reagent (Magen, Guangzhou, China). Sequencing was performed on Illumina NovaSeq platform according to the manufacturer’s instructions. The DEGSeq R package (version 1.20.0) was used for the differential expression analysis between BD and the control. KEGG and Gene-ontology (GO) enrichment analysis were carried out using the GOseq R and KOBAS 3.0 package, respectively.
Cellular thermal shift assay (CETSA)
Cells were treated with BD for 4 h, trypsinized with trypsin (Solarbio, Beijing, China), collected by centrifugation. Cells were resuspended in PBS (Solarbio, Beijing, China), and heated for 4 min to 40, 45, 50, 55 or 60 °C. Then, cells were lysed by three cycles of freeze–thaw in liquid nitrogen. Subsequently, the samples were centrifuged (20 min, 17,000g), and added 5 × loading buffer to the supernatant, and then separated with western blotting [33], [34].
Xenograft tumor model
Female NCG mice (NOD/ShiltJGpt-Prkdcem26Cd52Il2rgem26Cd22/Gpt, 5 weeks old) were obtained from Gempharmatech Co., Ltd. (Jiangsu, China) and housed in Xi’an Jiaotong University Animal Center with SPF conditions. 2 × 106 MDA-MB-231 cells (Control, Vector, Trop2-OE) were injected subcutaneously into the right mammary fat pad of the mice. When tumor volumes were bigger than 100 mm3 (Tumor volume = length × width2 × 0.5), mice were randomized into control or treatment groups (n = 5 mice) which received either vehicle or 2.5 mg/kg BD via intraperitoneal injection daily for 21 days. Tumor volumes and body weights were measured daily. The mice were sacrificed at the end of treatment, tumors were removed, weighted, photographed and fixed in 4% paraformaldehyde for HE and immunohistochemistry staining. The lung, liver, spleen, kidney weights were determined and organ coefficients were calculated (organ weight/body weight). The tumor growth inhibition was calculated by the formula:
4T1 orthotopic metastasis model
2 × 106 4T1 cells (Wild type, Vector, Trop2-OE) were injected subcutaneously into the right mammary fat pad of the female BALB/c mice (5 weeks old). When tumor volumes were bigger than 100 mm3 (Tumor volume = length × width2 × 0.5), mice were randomized into control or treatment groups (n = 5 mice) which received either vehicle or 2.5 mg/kg BD via intraperitoneal injection daily for 14 days. Body weights were measured daily. The mice were sacrificed at the end of treatment, the tumors were removed, weighted and photographed. The tumor growth inhibition was calculated by above formula. The lungs were collected, weighted and fixed in Bouin’s fixative solution (Sbjbio, Nanjing, China) for counting and photographing the metastatic nodules. The liver, spleen, kidney weights were determined and organ coefficients were calculated (organ weight/body weight).
4T1 experimental metastasis model
For 4T1 experimental metastasis model, 1 × 106 4T1 cells were inoculated into the female BALB/c mice (5 weeks old) via tail intravenous injection. At different time points after inoculation, mice were grouped and administrated with either vehicle or BD daily at doses of 1.25 or 2.5 mg/kg via intraperitoneal injection. Body weights of mice were measured daily. Finally, the mice were sacrificed at the end of treatment, the lungs were collected and fixed in Bouin’s fixative solution for HE and immunohistochemistry staining as well as counting and photographing the metastatic nodules.
For mouse survival experiments, 1 × 106 4T1 cells were inoculated into the female BALB/c mice (5 weeks old) via tail intravenous injection (n = 10). Mice were grouped into control group and BD (2.5 mg/kg) group and the administration started from the same day of injection of 4T1 cells. The mice were weighed every day and sacrificed when presented weight loss (20–25%), lethargy, or other physical signs of illness. All mice that died underwent necropsy examination and confirmation of metastasis.
3D matrigel drop invasion assays
Cancer cells were transfected with plasmid and siRNA, and treated with BD for 48 h. Then, a 10-µL drop was prepared using complete medium, each containing 500 cells. 20 drops were incubated on the lid of 10 cm-dish for 48 h at 37 °C to generate spheroids. Spheroids were collected and settled for 10 min. 96-well plates were coated with 50 µL of Matrigel in each well and incubated to polymerize at 37 °C for 3 h. Then 30 µL spheroids were mixed with 150 µL Matrigel and seeded into Matrigel-coated 96-well plates (50 µL per well). After incubating for 30 min at 37 °C in incubator, 100 µL complete medium were supplemented into the well. After culturing 14 days, the 3D Matrigel drops were photographed, then fixed with methanol and stained with DAPI, and photographed again [14], [35].
HFF-derived ECM
0.2% gelatin (Solarbio, Beijing, China) was incubated at 37 °C for 1 h in the well containing a coverslip and cross-linked with 1% glutaraldehyde (Aladdin, Shanghai, China). The excess glutaraldehyde was permeabilized with 1 M glycine (Biofroxx, Guangzhou, China) for 20 min. Then, HFF cells (5 × 104) were seeded into gelatin-coated well and cultured in complete medium supplemented with 50 µg/mL ascorbic acid (Solarbio, Beijing, China) for 7–10 days. Subsequently, HFF cells were removed using extraction buffer (1 mL NH4OH, 250 µL 0.5 % Triton-X-100, 48.75 mL PBS). The remaining cell were removed with 20 μg/mL DNAse I (Solarbio, Beijing, China) [36], [37]. Breast cancer cells treated with BD or transfected with plasmids or siRNAs were seeded onto the extracted ECM, and incubated for 72 h at 37 °C, followed by immunofluorescence staining of Fibronectin 1 (FN1), COL1A2 and DAPI, and photographing with a laser confocal fluorescence microscope.
Ethics statement
The animal experiments were approved by the Biomedical Ethics Committee of Xi'an Jiaotong University Health Science Center (no. 2022-0053).
Statistical analysis
Data were showed as mean ± standard deviation (SD). GraphPad Prism Software (version 8) was used for statistical analysis. One-way analysis of variance (ANOVA) was used to compare statistical differences between different treatment groups, and two-way ANOVA was used to compare cell viability over concentration range and tumor volume over time between groups. Pearson correlation analysis was used to analyze the correlations. Survival analysis was performed using Kaplan-Meier survival analysis and the long-rank test.
Results
TACSTD2 was a critical gene associated with inhibitory effects of BD on breast cancer cell viability
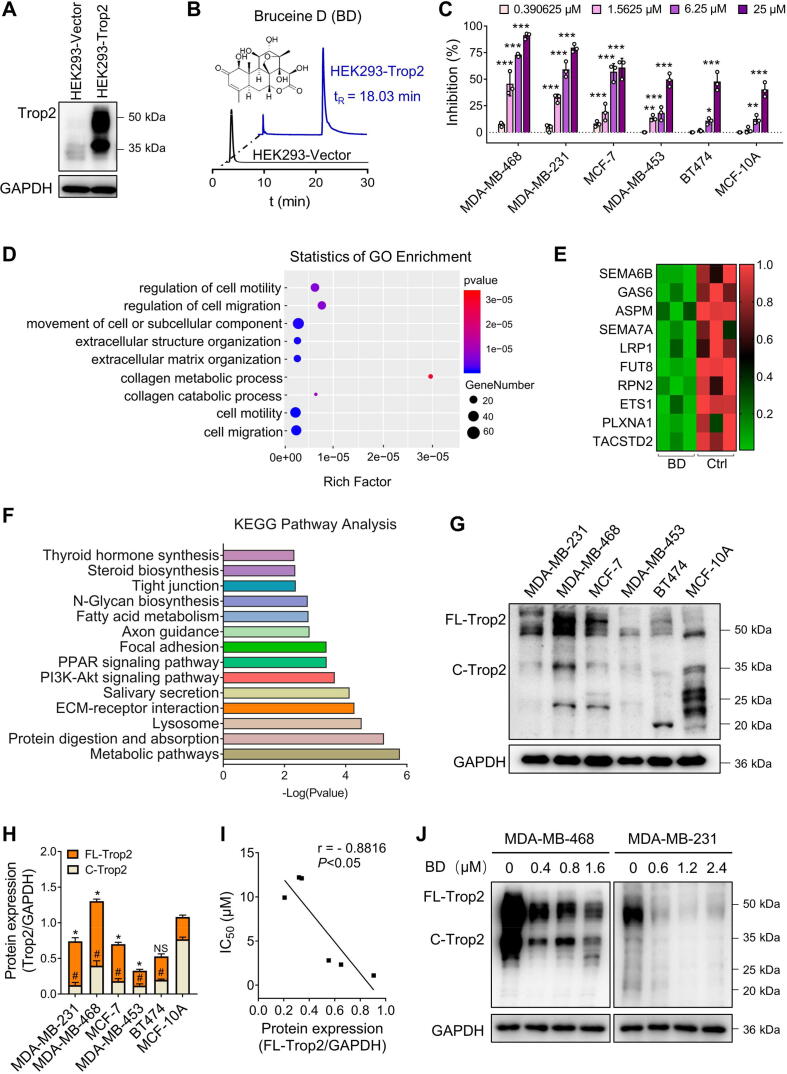
Cell membrane chromatography (CMC) has been used as a promising method for screening compounds or bioactive components that bind to specific receptors in complex systems. To identify the potent small molecule compounds targeting Trop2 (Gene name: TACSTD2), we constructed Trop2-overexpressed CMC to screen >30 compounds. HEK293-Trop2/CMC analysis suggested that BD retained on the HEK293-Trop2/CMC column rather than HEK293/CMC column, and the retention time of BD was the longest, which indicated better binding with Trop2 (Fig. 1A, 1B, S1).
Fig. 1.
TACSTD2 was a critical gene associated with inhibitory effects of Bruceine D (BD) on breast cancer cell viability. (A) Trop2 expression analysis in HEK293 cells transfected with Vector and plasmid. (B) The chromatograms of BD on the HEK293-Trop2/CMC column (blue) and HEK293/CMC column (blank). tR: the retention time of BD. flow rate: 0.2 mL/min; column temperature: 37 °C; mobile phase: 1 mM phosphate-buffer saline; pH 7.4; the detection wavelength was 248 nm. (C) Effects of BD on cell viability in MDA-MB-468, MDA-MB-231, MCF-7, MDA-MB-453, BT474, and MCF-10A cells. Cells were cultured with BD for 48 h and subjected to MTT assay. Data are presented as mean ± SD (n = 3), * P < 0.05, ** P < 0.01, *** P < 0.001 vs. the control. (D) GO biological process enrichment analysis of BD-downregulated genes, P < 0.05. (E) Heatmap of top 10 significant BD-downregulated genes associated with cell migration, P < 0.05. (F) Kyoto Encyclopedia of Genes and Genomes pathway analysis of BD-downregulated genes in RNA-seq, P < 0.05. (G) Protein levels of Trop2 in breast cancer cell lines and MCF-10A cells. (H) Quantification of (G), data are presented as mean ± SD (n = 3). *P < 0.05 vs the FL-Trop2 of MCF-10A cells; #P < 0.05 vs the C-Trop2 of MCF-10A cells; NS, P > 0.05, not significant. (I) Pearson’s correlation analysis between IC50 values and FL-Trop2 relative protein levels. (J) Protein levels of Trop2 in MDA-MB-468 and MDA-MB-231 cells exposed to BD for 48 h. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To investigate the effect of BD on cell viability of breast cancer cells, an MTT assay was performed after a 48-h treatment with BD. The results showed that BD preferentially inhibited the cell viability of MDA-MB-468, MDA-MB-231 and MCF-7 in a dose-dependent manner, with IC50 values of 1.092 µM, 2.328 µM, and 2.805 µM, respectively. IC50 values of MDA-MB-453, BT474, and MCF-10A were 9.919 µM, 12.100 µM, and 12.200 µM, respectively. However, BD at 25 µM displayed 87.80% and 79.20% cell viability inhibition in MDA-MB-468 and MDA-MB-231 cells, and only 60.47% inhibition in MCF-7 cells (Fig. 1C). These results suggested that BD preferentially inhibited cell viability of MDA-MB-468 and MDA-MB-231 cell lines.
Transcriptome sequencing (RNA-seq) of MDA-MB-231 cells was performed to identify transcripts of differentially expressed genes (DEGs) between BD-treated and control cells. RNA-seq showed that 118 genes were upregulated, and 184 genes were downregulated in the BD-treated cells (Fig. S2A). Gene-ontology (GO) analysis of the 184 down-regulated genes revealed significant enrichment of genes involved in biological processes of extracellular matrix organization, cell motility, and cell migration (GO:0030198, GO:0048870, GO:0016477), which were associated with cancer metastasis (Fig. 1D). KEGG analysis also showed that the pathways associated with tumor proliferation and metastasis were significantly downregulated in BD-treated cells, including the PI3K–Akt, focal adhesion, and ECM-receptor interaction pathways (Fig. 1F). Consistent with RNA-seq analysis, BD exerted inhibitory effects on cell migration, invasion and proliferation in MDA-MB-468 and MDA-MB-231 cells (Fig. S2B-F).
Among the top 10 genes correlated with the significantly enriched biological processes, (Fig. 1E), we verified that only 4 gene mRNA levels were downregulated in both MDA-MB-231 and MDA-MB-468 cells (Fig. S3A). Among these 4 genes, TACSTD2 was the only one that its protein expression level was also consistently downregulated in both MDA-MB-231 and MDA-MB-468 cells (Fig. S3B, Fig. 1J). To further validate this result, we tested the protein expression of Trop2 in human breast cancer cell lines and a normal human epithelial cell, MCF-10A. Interestingly, full-length Trop2 (FL-Trop2 ≥ 35 kDa) expression levels were higher in BD-sensitive cell lines, MDA-MB-231 and MDA-MB-468 compared with MCF-10A, while the cleaved Trop2 (C-Trop2 < 35 kDa) expression levels were lower in breast cancer cell lines than in MCF-10A (Fig. 1G, H). Correlation analysis showed a significantly negative correlation (r = − 0.8816) between FL-Trop2 expression levels and IC50 values (Fig. 1I). Meanwhile, Trop2 protein expression levels were decreased in a dose-dependent manner in BD-treated MDA-MB-468 and MDA-MB-231 cells (Fig. 1J). Altogether, these results suggested that BD might suppress breast cancer cell viability by downregulating the expression of Trop2.
Biophysical analysis of the interaction between BD and Trop2
Biophysical analysis of the interaction between BD and Trop2 can provide more insight into the regulating mechanism of Trop2 by BD. Thus, we performed the CETSA to test whether BD physically bound to Trop2. BD increased the melting temperatures of Trop2 in MDA-MB-468 and MDA-MB-231 cells, indicating that physical interaction between BD and Trop2 enhanced the heat denaturation of Trop2 (Fig. 2A, 2B). Furthermore, molecular docking was performed to explore the binding mode of BD with Trop2. BD might form hydrogen bonds with Trop2 and hydrophobically interacted with the residues LYS302, LYS307, and GLU310 (Fig. 2C). Notably, hydrogen bond between the residue ARG301 and BD was observed in several conformers. To further elucidate the binding mode of BD and Trop2, we generated four Trop2 mutants by site-directed mutagenesis. The residues ARG301, LYS302, LYS307, and GLU310 of Trop2 in the binding pocket were mutated to alanine (R301A, K302A, K307A, E310A). MTT assay indicated that HEK293 cells transfected with K307A or E310A mutants exhibited weaker sensitivity to BD compared to those transfected with WT-Trop2, R301A, and K302A (Vector: IC50 = 7.64 µM, WT: IC50 = 1.77 µM, R301A: IC50 = 2.51 µM, K302A: IC50 = 4.90 µM, K307A: IC50 = 7.14 µM, E310A: IC50 = 7.43 µM) (Fig. 2D). Similar results were observed in western blotting, in which BD led to significant downregulation of Trop2 protein levels in HEK293 cells in the presence of WT-Trop2, R301A, and K302A, but not in K307A or E310A mutants (Fig. 2E). These results were further validated with CMC in which BD did not retain on the HEK293-Trop2/ K307A /CMC column and HEK293-Trop2/ E310A /CMC column (Fig. 2F). The similar results were observed in CETSA, in which BD did not affect the melting temperatures of Trop2 protein in HEK293 cells transfected with K307A or E310A mutants (Fig. 2G-I). Overall, we demonstrated that BD bound to Trop2, and the residues LYS307 and GLU310 of Trop2 were key sites for BD binding.
Fig. 2.
Biophysical analysis of the interaction between Bruceine D (BD) and Trop2. (A) Cellular Thermal Shift Assay (CETSA) was used to analyze the melting temperatures of Trop2. Cells were cultured with BD (MDA-MB-468: 0.8 μM; MDA-MB-231: 1.2 μM) for 4 h, after which cell was collected, heated, lysed and subjected to western blotting. (B) Quantification of (A), data are presented as mean ± SD, n = 3. * P < 0.05, ** P < 0.01, *** P < 0.001, NS, P > 0.05, not significant. (C) Binding pocket between Trop2 protein and BD using molecular docking analysis. (D) Effects of BD on cell viability in HEK293 cells transfected with Vector, WT-Trop2, and four mutations of predicted key interface residues on Trop2 (R301A, K302A, K307A, and E310A). Cells were cultured with BD for 48 h and subjected to MTT assays. (E) Effects of BD on Trop2 expression in HEK293 cells transfected with Vector, WT-Trop2, and four mutations. Cells were cultured with BD (Vector: 3.8 μM, WT-Trop2: 0.9 μM, R301A: 1.3 μM, K302A: 2.5 μM, K307A: 3.6 μM, E310A: 3.6 μM) for 48 h, and subjected to western blotting. (F) The chromatograms of BD on the CMC (cell membrane chromatography) column with four mutations of Trop2. tR: the retention time of BD. flow rate: 0.2 mL/min; column temperature: 37 °C; mobile phase: 1 mM phosphate-buffer saline; pH 7.4; the detection wavelength was 248 nm. (G) CETSA was used to analyze the melting temperatures of Trop2 in HEK293-Trop2/WT cells after treatment with 0.9 μM BD for 4 h. (H) Quantification of (G), data are presented as mean ± SD, n = 3. * P < 0.05, ** P < 0.01, NS, P > 0.05, not significant. (I) CETSA was used to analyze the melting temperatures of Trop2 in HEK293-Trop2/ K307A and HEK293-Trop2/ E310A cells following treatment with 3.6 μM BD for 4 h.
BD suppressed breast cancer proliferation in vitro and in vivo depending on Trop2 levels
To validate the association of the inhibitory effect of BD on cell viability with Trop2 expression, vector, Trop2 plasmid or siRNA was transfected into breast cancer cells (Fig. S4A). MTT assay was performed to measure cell viability and the results revealed that Trop2-knockdown (Trop2-KD) MDA-MB-468 and MDA-MB-231 cells with Trop2 siRNAs were virtually insensitive to BD (MDA-MB-468, 0.39–25 µM, MDA-MB-231, 0.78–25 µM) compared to the cells transfected with the Vector (Vector cells). However, Trop2-overexpressing (Trop2-OE) MDA-MB-468, MDA-MB-231 and MDA-MB-453 cells were more sensitive to BD (MDA-MB-468, 0.39–1.56 µM, MDA-MB-231, 0.78–3.13 µM, MDA-MB-453, 3.13–25 µM) compared to Vector cells (Fig. 3A-C). Similar results were seen in the colony-forming assay. The inhibitory effect of BD on colony formation was attenuated by the knockdown of Trop2 in MDA-MB-468 and MDA-MB-231 cells, whereas it was enhanced in Trop2-OE MDA-MB-468, MDA-MB-231 and MDA-MB-231 cells (Fig. 3D-I, S4B-D).
Fig. 3.
Bruceine D (BD) suppressed breast cancer proliferation in vitro and in vivo dependent on Trop2 levels. (A-C) Effects of BD on cell viability in Trop2-KD and Trop2-OE MDA-MB-468 (A), MDA-MB-231 cells (B), and Trop2-OE MDA-MB-453 cells (C). Cells were exposed to BD for 48 h and subjected to an MTT assay. Data are presented as mean ± SD, n = 3. * P < 0.05, ** P < 0.01, *** P < 0.001 vs. the Vector. (D-F) Colony formation assay in Vector, Trop2-KD and Trop2-OE MDA-MB-468 (D) and MDA-MB-231 cells (E) treated with 0.8 μM and 1.2 μM BD, and in Vector and Trop2-OE MDA-MB-453 cells (F) treated with 3 μM BD. (G-I) Quantification of (D), (E) and (F), respectively. Data are presented as mean ± SD, n = 3. ** P < 0.01, *** P < 0.001, NS, P > 0.05, not significant. (J) Tumor weight of different groups in an MDA-MB-231 xenograft model. Data are presented as mean ± SD, n = 5. ** P < 0.01, *** P < 0.01, NS, P > 0.05, not significant. (K) Tumor volume changes during the period of BD treatment. Data are presented as mean ± SD, n = 5. * P < 0.05, ** P < 0.01 vs. the Vector/Ctrl group. &&P < 0.01 vs. the Vector/Ctrl group. ##P < 0.01 vs. the Trop2-OE/Ctrl group. (L) Photographs of tumors in different groups (n = 5). (M) Immunohistochemical staining of Ki67 and Trop2 (× 400 magnification).
To evaluate the effect of BD on breast cancer cell proliferation in vivo, we used a xenograft model in immunodeficient NOD-Prkdc em26Cd52 Il2rg em26Cd22/Nju (NCG) mice, subcutaneously injected with wild-type MDA-MB-231 cells (Ctrl) and MDA-MB-231 cells transfected with vector (Vector) or Trop2 plasmid (Trop2-OE). Tumor-bearing mice were continuously treated with either vehicle or BD at 2.5 mg/kg for 21 days. Tumors in the Trop2-OE/Ctrl group grew much faster than in the Vector/Ctrl group, confirming that Trop2 promoted tumor growth in vivo (Fig. 3J-L). A significant inhibitory effect of BD was observed on tumor growth at a rate of 40.82% in the Vector/BD group and 70.81% in the Trop2-OE/BD group. Obvious inhibition of tumor volumes was noticed from day 11 to day 21 in the Vector/BD group and from day 7 to day 21 in the Trop-OE/BD group. These results indicated that BD yielded impressive benefits for tumors overexpressing Trop2 (Fig. 3J-L). These findings were confirmed in Ki67 staining reflecting cell proliferation ability. A higher percentage of Ki67-positive cells was observed in the Trop-OE group compared with the Vector group, while lower percentages of Ki67-positive cells were observed in the Vector/BD and Trop-OE/BD groups (Fig. 3M, S4E). Taken together, these data confirmed that BD displayed anti-proliferative effects in a Trop2-dependent manner, both in vitro and in vivo.
BD suppressed breast cancer metastasis by inhibiting Trop2-involved EMT and ECM remodeling
To investigate if the anti-metastatic effect of BD on breast cancer cells was associated with Trop2, we performed a migration assay and Matrigel invasion assay. BD distinctly suppressed the migration and invasion ability of MDA-MB-468 and MDA-MB-231 cells. The anti-metastatic effects of BD were weakened in Trop2-KD MDA-MB-468 and MDA-MB-231 cells while these effects were enhanced in Trop2-OE MDA-MB-468 and MDA-MB-231 cells (Fig. 4A, S5A). These findings were also observed in Matrigel drop invasion assay assessing the 3D-invasion ability of cancer cells, in which MDA-MB-468 cells had weak motility when grown in Matrigel. However, Trop2-OE MDA-MB-468 cells invaded through the border of the Matrigel droplet and migrated away. Trop2 knockdown in these cells or treatment with BD resulted in a strong decrease in invasion ability (Fig. 4B). These results suggested that Trop2 promoted an invasive phenotype in breast cancer cells.
Fig. 4.
Bruceine D (BD) suppressed breast cancer metastasis by inhibiting Trop2-involved EMT and ECM remodeling. (A) Effects of BD on migration and invasion abilities of MDA-MB-468 and MDA-MB-231 cells transfected with Vector, plasmid, siRNA. Cells were treated with BD (MDA-MB-468: 0.8 μM, MDA-MB-231: 1.2 μM) for 48 h, then cells were collected and subjected to migration assay and Matrigel invasion assay. Data are presented as mean ± SD, n = 3. *P < 0.05, **P < 0.01, ***P < 0.001, NS, P > 0.05, not significant. (B) Matrigel drop invasion assays to evaluate the 3D-invasion ability of Vector, Trop2-OE, and Trop2-KD MDA-MB-468 cells following treatment with 0.8 μM BD for 48 h. Upper row: the whole Matrigel drop; Lower row: the DAPI staining of the nucleus in the drop, the images were taken at × 200 magnification. (C) Confocal laser scanning microscopy images of HFF-derived ECM to observe deposition and assembly of fibronectin and collagen in Vector and Trop2-KD MDA-MB-468 cells (left) treated with 0.8 μM BD, as well as in Vector and Trop2-OE MDA-MB-231 cells (right) treated with 1.2 μM BD for 48 h. COL1A2: red, FN1: green, DAPI: blue. Scar bar: 75 μm. (D, E) Expression of protein associated with EMT (D) and ECM remodeling (E) in Vector and Trop2-KD MDA-MB-468 cells as well as in Vector and Trop2-OE MDA-MB-231 cells. MDA-MB-468 cells and MDA-MB-231 cells were exposed to 0.8 μM BD and 1.2 μM BD for 48 h, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
According to the results of RNA-seq, BD decreased the transcription levels of EMT-related genes, such as mesenchymal markers which included VIM and CDH2 (Fig. S5B). Similarly, VIM and N-cadherin (N-Ca) protein levels were decreased while the epithelial marker E-cadherin (E-Ca) was increased in BD-treated MDA-MB-468 and MDA-MB-231 cells (Fig. 4D, S5D). These results indicated that BD likely exerted anti-metastatic effects by reversing EMT.
Interestingly, GO term analysis and DEGs from RNA-seq data suggested that ECM might be altered in BD-treated cells. Therefore, we speculated that BD might inhibit ECM remodeling in breast cancer cells. We adopted a HFF cell-derived ECM system, to better recapitulate the formation and stabilization of ECM. The formation of thick cross-linked collagen fibers induced by breast cancer cells grown on HFF-derived ECM promoted tumor cell migration and invasion along collagen fibers. Decreased densities of both collagen and fibronectin, in addition to a clear disruption of the collagen fiber network in BD-treated cells, were observed via confocal microscopy (Fig. 4C, S5C). It was further proven that decreased protein levels of Fibronectin 1 (FN1), COL1A2, p-FAK (Y397) (the downstream signaling molecule of FN1 and COL1A2), and matrix metalloproteinases (MMPs) including MMP3, MMP9, and MMP7, were indeed detected in BD-treated MDA-MB-231 and MDA-MB-468 cells (Fig. 4E, S5D). Consistent with our prior observations, knockdown or overexpression of Trop2 would disfavor or favor the effect of BD in inhibiting collagen fibril cross-linking, respectively. Thus, these results demonstrated that Trop2 induced metastasis by promoting the EMT process and ECM remodeling in breast cancer cells, while BD elicited inhibitory roles in breast cancer cell metastasis by downregulating Trop2 expression.
To verify the above results in vivo, a 4T1 orthotopic metastasis model was established to evaluate the effects of BD on breast cancer spontaneous metastasis. We observed significant inhibitory effect of BD on tumor growth at a rate of 41.86% in the Vector/BD group and 65.04% in the Trop2-OE/BD group, which were consistent with the findings in MDA-MB-231 xenograft model (Fig. S6A, S6B). As shown in Fig. S6D, macroscopic lung metastases from the orthotopic mammary tumors were observed clearly, and significant more lung metastatic surface nodules were observed in Trop2-OE group than Vector group (Fig. S6C). As expected, BD showed a significant suppression of metastatic nodule at an inhibition rate of 67.81% in the Vector/BD group and 88.66% in the Trop2-OE/BD group (Fig. S6C). A significant decrease of lung coefficient was also observed in Vector/BD and Trop2-OE/BD groups (Fig. S6E). 4T1 tumor development led to splenomegaly due to increased infiltration of myeloid cells induced by tumor-derived secreted factors[38]. Splenomegaly was observed on tumor-bearing mice in this experiment and spleen coefficient was higher in Trop2-OE group than that in Vector group, the data presented in Fig. S6F demonstrated that BD led to a prominent reduction in spleen coefficient. Collectively, BD indeed suppressed breast cancer metastasis from the orthotopic mammary tumors.
BD decreased the Trop2/β-catenin complex and inhibited the formation of Trop2 /β-catenin positive feedback loop
Given the involvement of β-catenin in EMT progression and ECM remodeling, downregulated genes related to EMT and ECM after BD treatment, were correlated with β-catenin (Fig. S7A, S7B). For that matter, we hypothesized that a correlation might exist between β-catenin and Trop2. Co-Immunoprecipitation (Co-IP) experiments were used to identify the complex formation of Trop2 and β-catenin in MDA-MB-468 and MDA-MB-231 cells. Additionally, BD reduced the Trop2/β-catenin complex by decreasing the protein levels of both Trop2 and β-catenin (Fig. 5A). Mass spectrometry analysis of Co-IP samples was performed to further confirm the interaction between β-catenin with Trop2. Compared with anti-IgG group, 147 proteins were specifically pulled down by anti-Trop2 antibody in MDA-MB-468 cells, and 161 proteins in MDA-MB-231 cells. β-catenin protein was detected in both MDA-MB-468 and MDA-MB-231 cells with highest score of 323.31, suggesting the formation of β-catenin/Trop2 complex (Fig. 5B). Overall, the interaction between β-catenin and Trop2 was confirmed with Co-IP along with mass spectrometry. A significant positive correlation was also observed between Trop2 and β-catenin in tumor tissues from MDA-MB-231 xenograft model (r = 0.7273, P < 0.0001) (Fig. 5C, S7C). Similar results were found in GSE158309 database analysis (r = 0.36, P < 0.0001) (Fig. 5D). Based on these findings, it was necessary to investigate the mode of regulation between Trop2 and β-catenin. We found that knockdown or overexpression of Trop2 induced down- or up-regulation of β-catenin protein levels with no effect on β-catenin mRNA levels. These observations excluded the possibility that the change in β-catenin protein was due to transcription, indicating that Trop2 may affect the post-translational protein stabilization of β-catenin. (Fig. 5E, 5G). Inversely, knockdown or overexpression of β-catenin positively regulated the protein levels of Trop2 by directly affecting its transcriptional activity (Fig. 5H, 5I). Noticeably, the results showed that the decreased β-catenin protein level upon BD treatment alone was not due to transcription, which was consistent with the effects of Trop2 knockout, further demonstrating that Trop2 was the target of BD (Fig. 5E, 5G-I). Together, cross-regulation of Trop2 and β-catenin in breast cancer cells formed a positive feedback loop. Trop2/β-catenin complex could be a part of the positive feedback loop and BD induced its disruption through a Trop2-dependent manner.
Fig. 5.
Bruceine D (BD) decreased the Trop2/β-catenin complex and inhibited the formation of the Trop2/β-catenin positive feedback loop. (A) Co-Immunoprecipitation (Co-IP) of Trop2 and β-catenin in MDA-MB-468 and MDA-MB-231 cells following treatment with 0.8 μM and 1.2 μM BD for 48 h, respectively. (B) Mass spectrometry analysis of Co-IP samples conducted with anti-Trop2 antibody in MDA-MB-468 and MDA-MB-231 cells. Representative peptide sequence of the β-catenin protein (LVQNCLWTLR) was detected in MDA-MB-468 (left) and MDA-MB-231 cells (right). (C) Pearson’s correlation analysis between Trop2 and β-catenin in protein levels of tumor tissues obtained from the MDA-MB-231 xenograft model. (D) Pearson’s correlation analysis between Trop2 and β-catenin in mRNA levels of human breast cancer (GSE158309). (E) Protein levels of Trop2, β-catenin, and p-β-catenin in Vector, Trop2-KD MDA-MB-468 cells, and Vector, Trop2-OE MDA-MB-231 cells after treatment with BD (MDA-MB-468: 0.8 μM, MDA-MB-231: 1.2 μM) for 48 h. (F) Quantification of p-β-catenin in (E). Data are presented as mean ± SD, n = 3. NS, P > 0.05, not significant. (G) mRNA levels of β-catenin in Vector, Trop2-KD MDA-MB-468 cells (left) and Vector, Trop2-OE MDA-MB-231 cells (right) after treatment with BD (MDA-MB-468: 0.8 μM, MDA-MB-231: 1.2 μM) for 48 h. Data are presented as mean ± SD, n = 3. NS, P > 0.05, not significant. (H) Protein levels of Trop2, β-catenin in Vector, β-catenin-KD, and β-catenin-OE MDA-MB-468 cells following treatment with 0.8 μM BD for 48 h. (I) mRNA levels of TACSTD2 in Vector, β-catenin-KD, and β-catenin-OE MDA-MB-468 cells treated with 0.8 μM BD for 48 h. Data are presented as the mean ± SD, n = 3. **P < 0.01, ***P < 0.001, NS, P > 0.05, not significant.
BD disrupted the Trop2/β-catenin complex and led to the degradation of β-catenin via the ubiquitin-proteosome pathway in breast cancer
The decrease in β-catenin protein levels following the reduction of Trop2 after BD treatment was due to post-translational protein stabilization rather than transcription. Therefore, we investigated the protein half-life of β-catenin upon control of Trop2 in the presence of cycloheximide to determine whether Trop2 affected the stability of β-catenin. Knockdown of Trop2 decreased the half-life of β-catenin from 12 h to 6 h in MDA-MB-468 cells, whereas overexpression of Trop2 extended it from 12 h to over 15 h in MDA-MB-231 cells (Fig. 6A-C). These data indicated that the Trop2/β-catenin complex contributed to the stability of β-catenin and BD disrupted β-catenin stability by reducing the Trop2/β-catenin complex.
Fig. 6.
Bruceine D (BD) disrupted the Trop2/β-catenin complex and led to the degradation of β-catenin via the ubiquitin-proteosome pathway in breast cancer. (A) Effects of knockdown or overexpression of Trop2 on β-catenin stability in MDA-MB-231 and MDA-MB-468 cells in the presence of 25 μg/mL cycloheximide (CHX) at different time points. (B, C) Quantification of (A). Data are presented as mean ± SD, n = 3. (D, E) Protein levels of β-catenin in BD-treated MDA-MB-468 (BD, 0.8 μM) and MDA-MB-231 cells (BD, 1.2 μM) in the presence of 0.1 μM MG132 (D) or 20 μM CQ (E). (F) Co-Immunoprecipitation of Trop2 and β-catenin in nucleus (Nuc) and cytoplasm (Cyto) in BD-treated MDA-MB-468, respectively. (G) Distribution of Trop2 and β-catenin in the nucleus (Nuc) and cytoplasm (Cyto) in BD-treated MDA-MB-468 (BD, 0.8 μM) and MDA-MB-231 cells (BD, 1.2 μM). Histone H3 serves as a nucleus protein marker, GAPDH as a cytosolic protein marker. (H) Immunofluorescence co-staining of Trop2 and β-catenin to detect the effects of BD on their expression and distribution in MDA-MB-468 and MDA-MB-231 cells. Trop2 (green), β-catenin (red) and DAPI (blue) images were taken at × 200 magnification. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The ubiquitin–proteasome pathway has been previously reported to be the main mechanism for controlling the degradation of β-catenin. However, the presence of β-catenin/LC3 complex may result in the degradation of β-catenin in an autophagy-dependent manner[39]. To verify which pathway exerted the major role in BD-treated cells, we utilized MG132 (the inhibitor of proteasome) and chloroquine (CQ, the inhibitor of lysosomal acidification) to block proteasome and autophagy degradation, respectively. The results showed that MG132, but not CQ, reversed the pro-degradative effects of BD on β-catenin in MDA-MB-468 and MDA-MB-231 cells, proving that BD induced β-catenin degradation via ubiquitin-proteosome pathway (Fig. 6D, E). Moreover, western blotting unexpectedly showed a decrease in LC3 (an autophagosome marker) and an increase in P62 (an autophagic cargoes marker) in BD treatment cells, indicating the inhibitory effect of BD on autophagy (Fig. S8A). The ubiquitination of β-catenin can occur dependent or independent to phosphorylation. As shown in Fig. 5E and F, changes of phospho-β-catenin upon BD and Trop2-manipulated treatment were paralleled with the corresponding total β-catenin levels, proving that ubiquitylation of β-catenin upon BD treatment was independent of phosphorylation of β-catenin. Overall, BD disrupted β-catenin stability by the ubiquitin-proteosome pathway in a phosphorylation-independent manner.
The ubiquitination of β-catenin can occur in the nucleus and cytoplasm. It was not currently known where Trop2/β-catenin complex was located. We observed that Trop2/β-catenin complex was present in both nucleus and cytoplasm to maintain β-catenin stability (Fig. 6F). Moreover, TCF4 was the most common coregulator of β-catenin. TCF4 interacted with β-catenin to regulate a wide range of gene expression at the transcriptional level[40]. Although BD had no effect on the expression of TCF4, the TCF4/β-catenin complex still was decreased in BD-treated MDA-MB-468 cells because of the degradation of β-catenin caused by BD (Fig. S8B). It is well known that the nucleus translocation of endogenous β-catenin was the hallmark of its activation. Therefore, we tested the cytoplasmic and nucleus quantities of β-catenin in BD-treated MDA-MB-231 and MDA-MB-468 cells. Western blotting showed that the quantities of β-catenin in both the cytoplasm and nucleus were decreased in BD-treated cells (Fig. 6G). Immunofluorescence analysis showed similar results, in which it was clear that BD decreased immunofluorescence intensities of both nucleus and cytoplasmic β-catenin (Fig. 6H). These results suggested that the inhibitory effect of BD on β-catenin translocation to the nucleus was due to the reduction of total β-catenin protein. Notably, Trop2 was detected in both the cytoplasm and nucleus, and co-localization of Trop2 and β-catenin was observed in both the cytoplasm and nucleus in immunofluorescence staining (Fig. 6G, 6H, S10, S11). These findings strongly suggested that the Trop2/β-catenin complex was present in both the nucleus and cytoplasm to protect β-catenin from being degraded.
In summary, these results indicated that BD destabilized β-catenin via the reduction of the Trop2/β-catenin complex which played an important role in preventing the degradation of β-catenin via the ubiquitin-proteosome pathway. Subsequently, it resulted in decreased nucleus translocation of β-catenin in breast cancer cells.
β-catenin was key factor for Trop2-mediated breast cancer cells metastasis
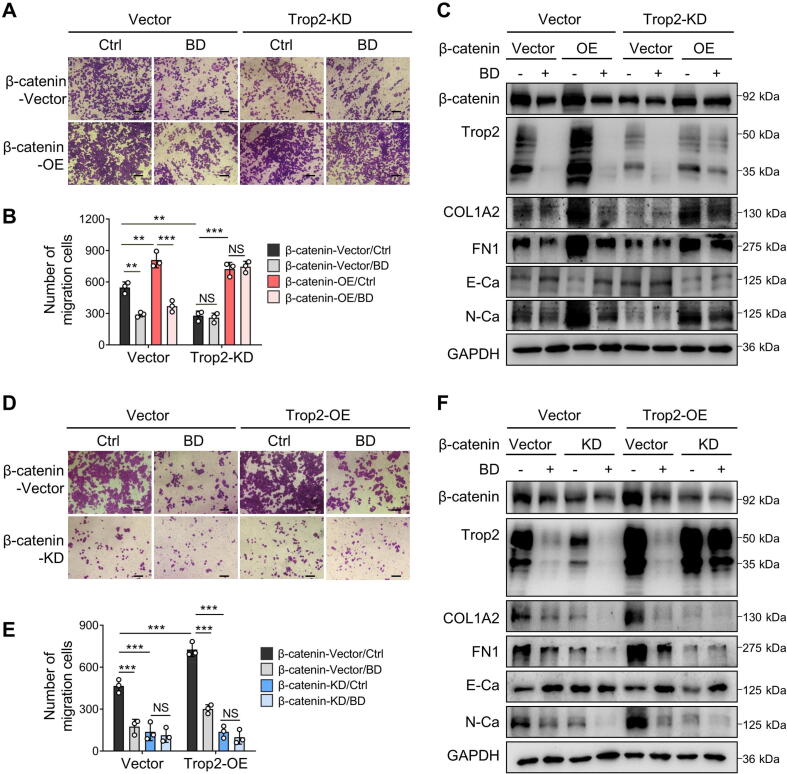
To evaluate the role of β-catenin in EMT and ECM-remodeling in Trop2-driven breast cancer cells, β-catenin was overexpressed in Trop2-KD cells and knocked down in Trop2-OE cells. β-catenin overexpression restored the migration ability of Trop2-KD cells. Conversely, β-catenin knockdown significantly counteracted the pro-migration effects of Trop2 in Trop2-OE cells (Fig. 7A, B, D, E). As validated by western blotting, β-catenin positively regulated the expression of N-Ca, FN1, and COL1A2, accompanied by negatively regulating the epithelial marker E-Ca. Subsequently, overexpression or knockdown of β-catenin reversed the effects of Trop2 knockdown or overexpression on these proteins (Fig. 7C, F). Consistent with the aforementioned results, the inhibitory role of BD on cell migration was even more pronounced in the presence of the Trop2, and reciprocally, BD displayed little inhibition in Trop2-KD cells (Fig. 7A-F). Taken together, these data suggested that the promotive role of Trop2 in cell migration was attributed to β-catenin, while the inhibitory effects of BD on the migration depended on Trop2 expression levels.
Fig. 7.
β-catenin was key factor for Trop2-mediated breast cancer cells metastasis. (A) Migration assay to observe the effects of β-catenin overexpression on migration ability in Vector and Trop-KD MDA-MB-468 cells with or without 0.8 μM BD after 48 h. The images were taken at × 100 magnification. (B) Quantification of (A), data are presented as mean ± SD, n = 3. **P < 0.01, ***P < 0.001, NS, P > 0.05, not significant. (C) Effects of β-catenin overexpression on the protein levels of Trop2, β-catenin, COL1A2, FN1, E-Ca and N-Ca in Vector and Trop-KD MDA-MB-468 cells treated with or without 0.8 μM BD for 48 h. (D) Migration assay to observe the effects of β-catenin knockdown on migration ability in Vector and Trop-OE MDA-MB-468 cells treated with or without 0.8 μM BD for 48 h. The images were taken at × 100 magnification. (E) Quantification of (D), data are presented as mean ± SD, n = 3. ***P < 0.001, NS, P > 0.05, not significant. (F) Effects of β-catenin knockdown on the protein levels of Trop2, β-catenin, COL1A2, FN1, E-Ca and N-Ca in Vector and Trop-OE MDA-MB-468 cells treated with or without 0.8 μM BD for 48 h.
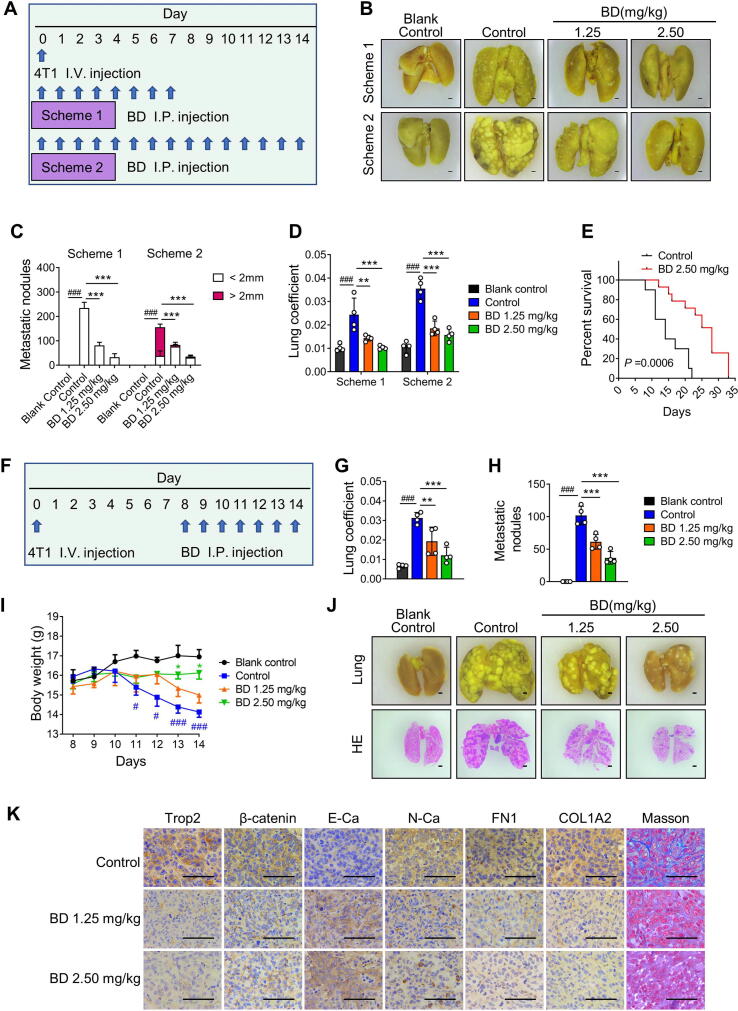
BD suppressed metastatic lung colonization and prolonged in vivo survival
The 4T1 tumor model is a suitable experimental animal model for human TNBC, and the Trop2 protein expression level in 4T1 cells was similar with MDA-MB-231 cells (Fig. S9A). To evaluate whether BD inhibited the cancer cell colonization on target tissues, 4T1 experimental metastasis model was performed by injecting 4T1 cells into mice through the tail vein, and we monitored lung colonies at different periods after injecting BD or vehicle (Fig. 8A). On Day 7 of Scheme 1, cancer cells fully colonized the lungs and grew in the control group, while few cancer colonies were observed in BD-treated groups, especially in the 2.50 mg/kg BD group. On Day 14 of Scheme 2, colonized cancer cells became larger nodules in the control group, and most of them were >2 mm. However, there were much fewer nodules with smaller sizes in the 1.25 mg/kg BD and 2.5 mg/kg BD groups (Fig. 8B-D, S9B-D). These results suggested that BD strongly inhibited 4T1 cell colonization. Survival analysis was used to further evaluate the therapeutic effects of BD. Prolonged survival for 4T1 experimental metastasis model mice was observed with daily 2.5 mg/kg BD administration (Fig. 8E). These results suggested that BD effectively inhibited 4T1 cells colonization and growth on lung.
Fig. 8.
Bruceine D (BD) suppressed metastatic lung colonization and prolonged survival. (A) A procedure of tail vein injection (i.v.) of 4T1 tumor cells and intraperitoneal injection (i.p.) of BD or vehicle daily starting on day 0. There were 2 schemes, mice were sacrificed on day 7 (Scheme 1) or Day 14 (Scheme 2). (B) Representative images of lung tissues fixed with Bouin’s solution in Scheme 1 and Scheme 2 (×4.5 magnification). (C, D) Metastatic nodules (C) and lung coefficient (D) in the Scheme 1 and Scheme 2. Data are presented as mean ± SD, n = 4. **P < 0.01, ***P < 0.001 vs the control group. ###P < 0.001, control group vs blank control group. (E) Kaplan-Meier survival curve for control and BD-treated mice in 4T1 experimental metastasis model, n = 10. (F) Procedure for tail vein injection (i.v.) of 4T1 tumor cells at day 0, and intraperitoneal injection (i.p.) of BD or vehicle from days 8 to 14. (G-I) Lung coefficient (G), metastatic nodules (H) and body weight (I) in different treatment groups. Data are presented as mean ± SD, n = 4. *P < 0.05, **P < 0.01, ***P < 0.001 vs the control group. #P < 0.05, ###P < 0.001, control group vs blank control group. (J) Representative images of lung tissues fixed with Bouin’s solution (upper row) and stained with HE (lower row). The images were taken at × 4.5 magnification. (K) Representative images of immunohistochemical staining of Trop2, β-catenin, E-Ca, N-Ca, FN1 and COL1A2, and Masson staining in lung metastatic nodules (×400 magnification).
To further confirm the inhibitory effects of BD on cancer cells growth on lung, the mice received BD via intraperitoneal injection from days 8 to 14, because it took 7 days for 4T1 cells to fully colonized the lungs and grew (Fig. 8F). The lung weight, lung coefficient, and metastatic nodules were lower in the BD groups than the control group. Remarkably, weight loss of body was aggravated in the control group as of day 11, while significant remission was observed in the 2.5 mg/kg BD group (Fig. 8G-J, S9E). Immunohistochemistry analysis of metastatic nodules showed that BD downregulated the levels of Trop2, β-catenin, N-Ca, FN1, and COL1A2, while it upregulated the levels of E-Ca. Moreover, we confirmed decreased fibrillar collagen in BD groups compared to the control by Masson staining (Fig. 8K). Taken together, BD suppressed 4T1 cell colonization and metastasis, prolonging survival in vivo.
Discussion
Metastasis and resistance are among the two main obstacles to effective breast cancer chemotherapy[41] and thus, novel therapeutic options are urgently needed. Recent studies have recognized Trop2 as a novel target for ADCs development, however, small molecule inhibitors targeting it have not yet been reported. In this study, we found that BD was an effective Trop2 inhibitor, which exhibited excellent inhibitory effects on the growth and metastasis of breast cancer in a Trop2-dependent manner. Mechanistically, the Trop2/β-catenin complex stabilized β-catenin, which in turn contributed to the transcription of Trop2 and metastasis-related genes. Thus, a positive feedback loop was formed to promote the metastatic behavior of breast cancer. In addition, BD disrupted the formation of the Trop2/β-catenin positive feedback loop by reducing the Trop2/β-catenin complex, exerting anti-metastatic effects. Further optimization of BD is expected to develop clinical drug candidates for breast cancer therapy.
The function of Trop2 in the pathological processes of breast cancer remains elusive. High expression of Trop2 was associated with decreased survival in breast cancer[42], and upregulation of Trop2 promoted ER-positive MCF-7 cell growth[17]. However, some evidences suggested the opposite role of Trop2 in breast cancer. Zimmers et al. showed that knockdown of Trop2 enhanced proliferation in ER-positive MCF-7 cells[43]. In another report, Remsik et al. showed that Trop2 loss in basal-like breast cancer cells increased cell migration[44]. Such conflicting results may be due to the involvement of Trop2 in complex signaling pathways. Despite functional studies reveal both oncogenic and tumor suppressive roles of Trop2 in different cancers, of note, it has been reported in most of the studies that Trop2 functions as an oncogene in breast cancer. Trop2 is expressed in 80% of TNBC patients and associated with lymph node status, metastasis, and tumor grade, as well as poor prognosis in breast cancer[45]. In fact, Trop2 has been validated as an ideal target for cancer therapy, Trop2 targeted therapeutics such as Sacituzumab govitecan-hziy, an antibody-drug conjugate (ADC), has been approved by FDA for patients with metastatic TNBC. Although Trop2 is usually used as a transport gate for cytotoxic agents into cells in ADC design, Trop2 itself is a potential drug target, which has been validated by anti-Trop2 antibodies alone[20], [21]. Here, we demonstrated that overexpression of Trop2 significantly contributed to breast cancer cell proliferation, migration, invasion, cross-linking of collagen fiber in vitro, and tumor growth in MDA-MB-231 xenograft model as well as metastasis in the 4T1 metastatic model. In addition, knockdown of Trop2 resulted in the opposite effect, highlighting the pro-oncogenic role of Trop2 in breast cancer. In this study, trop2 was selected as a treatment target for breast cancer, and we found that BD exhibited inhibitory effects on breast cancer by downregulating the expression of Trop2 and regulating its related signaling pathway, which suggested that BD might be a potential small molecule inhibitor of Trop2.
It was reported that, in addition to topoisomerase-I E418K mutation and subsequent frameshift mutation, Trop2 T256R missense mutation conferred IMMU-132 resistance via defective plasma membrane localization and reduced cell-surface binding by hRS7[8]. BD bound to Lys307 and Glu310 residues of Trop2, Trop2 T256R missense mutation might not affect the pharmacodynamic effects of BD, which indicated that BD might be potential in treatment of the patients with breast cancer resistance to IMMU-132. So, BD was worthy of investigation and development for further clinical application.
We evaluated the safety profile of BD according to the in vivo and in vitro data. As shown in Fig. 1, in vitro experiments confirmed that MCF-10A cell was much less sensitive to BD than other cancer cells, IC50 value in MCF-10A was 12.20 µM, which was about 11.17, 5.24 and 4.35 times higher than that in MDA-MB-468, MDA-MB-231 and MCF-7 cells respectively. This result showed that BD inhibited cell viability in many breast cancer cell lines with less toxicity in normal cell. In xenograft model of immunodeficient NCG mice, BD at 2.5 mg/kg exhibited obvious inhibition of tumor growth but had no significant effect on organ coefficients (Supplementary Fig. 4F). In 4T1 orthotopic metastasis mice model, BD at 2.5 mg/kg could reverse splenomegaly induced by tumor, but had no significant effect on liver and kidney coefficient (Fig. S6). In 4T1 experimental metastasis model, BD at the dose of 2.5 mg/kg prolonged survival and showed significant remission in weight loss of body (Fig. 8). These results showed that BD had no obvious toxicity towards the major organs of mice after continuous administration. In acute toxicity test, healthy mice were treated intraperitoneally with BD at single dose of 10 mg/kg or 5 mg/kg, respectively, 40% of mice administered with 10 mg/kg BD died and other mice were observed mild acute toxicities and resolved spontaneously, no significant acute toxicity was observed in mice with 5 mg/kg BD. Taken together, BD can be considered a safe therapeutic agent since the amount of drug required to achieve a therapeutic effect is significantly lower than the amount of drug causing toxicity.
Trop2 is a single-pass transmembrane protein composed of an extracellular domain (1–274 aa), a transmembrane domain (275–297 aa) and an intracellular domain (298–323 aa). It can be cleaved via multiple proteolytic enzymes at multiple different sites[15], [18], [46]. This may explain the observation of multiple bands of Trop2 in breast cancer cell lines and MCF-10A cells in this study. Although a variety of Trop2 cleavage forms have been reported, the role of Trop2 cleavage in cancer progression remains controversial. Some studies showed that Trop2 cleavage contributed to cell self-renewal in prostate cancer[15] and cell growth and metastasis in breast cancer[18]. However, recent study showed that high expression levels of membrane Trop2 were associated with poor prognosis, whereas increased cytoplasmic Trop2 levels suggested increased survival[47]. In the present study, cleaved-Trop2 was detected in both breast cancer cell lines and normal human epithelial cell line MCF-10A, but more cleaved-Trop2 were observed in MCF-10A cells. Interestingly, we found that cleaved-Trop2 could only be detected in the nucleus rather than cytoplasm in MDA-MB-468 and MDA-MB-231 cells. This phenomenon is worth further investigation. In addition, the inhibitory effects of BD on cell viability positively correlated with FL-Trop2 but not cleaved-Trop2. These findings suggested that the binding pocket of Trop2 and BD depended on the intact conformation of Trop2, whereas the Trop2 cleavage resulted in its disruption.
The activation of invasion and metastasis is one of the hallmarks of cancer[48], and a main cause of death in cancer patients[49]. EMT process and ECM remodeling are necessary steps in the early stage of cancer metastasis[50]. During the invasion and metastasis process, activated EMT procedures cause loss of cell-to-cell contact and increase cell motility and migration to the surrounding matrix. Excessive deposition and remodeling of ECM result in increase of ECM stiffness and activation of integrin-FAK signaling, followed by cancer cell migration along the ECM fibers[51], [52]. This study revealed that Trop2 contributed to the EMT process of breast cancer through positive regulation of the mesenchymal markers N-Ca, VIM, and negative regulation of E-cadherin. Moreover, Trop2 enhanced ECM-related gene expressions of FN1 and COL1A2, which aggravated the deposition and cross-linking of fibronectin and collagen as well as the expression of high levels of MMPs. As a result, this phenomenon led to the activation of downstream p-FAK (Y397), followed by migration along the ECM fibers. BD strongly suppressed Trop2-driven metastatic behavior of breast cancer, and reversed the EMT phenotype and ECM remodeling, indicating the excellent anti-metastasis and anti-invasion effects of BD on breast cancer. Additionally, some studies have shown that the EMT program and ECM stiffness induced resistance to chemotherapy drugs such as paclitaxel and doxorubicin, and targeted therapeutic drugs including lapatinib and vemurafenib[53]. Given the inhibitory effects of BD on EMT program and ECM remodeling, a combination of BD with chemotherapeutic agents certainly deserves further investigation in the future.
Metastasis is a multistep biological process in vivo, including local invasion, then passage through the blood and lymphatic systems followed by distant tissues, and finally forming small nodules of cancer cells; the final step known as colonization[48]. Although EMT is preconditioned to escape from the primary tumor, EMT may be deleterious for early colonization because cancer cells need MET (mesenchymal-epithelial transition) to gain epithelial characteristics required to grow[54], [55]. Some findings signaled that reversion of EMT may be counter-productive and enhanced the metastatic colonization when patients already have circulating cancer cells[56]. However, some studies reported that colonization is the last rate-limiting step of metastasis and requires a large number of cellular and molecular events to complete and MET alone was not sufficient for colonization[56]. Furthermore, recent research showed that MET was not essential for metastasis to the lung[57]. Therefore, to evaluate whether the reversal effect of BD on EMT was promoted under tumor colonization, the 4T1 experimental metastasis model was established. In one model, BD was administered after 4T1 cells had been injected for 7 days to assess its effects on proliferation and metastasis. In another model, the mice were injected with 4T1 cells, followed by BD administration to access the effects of BD on metastatic lung colonization. These data suggested BD-treated groups in both models showed fewer and smaller metastatic nodules in the lung with low toxicity compared with the control group. Therefore, prolonged survival of mice occurred, indicating that BD exhibited excellent anti-proliferation, anti-metastasis, and anti-colonization effects. BD appeared to hinder both early steps of migration and invasion, and late steps of metastatic colonization, which demonstrated the value of BD in suppressing the metastatic phenotype of breast cancer.
β-catenin stabilization is known to increase its nucleus levels, and constitutive activation of β-catenin results in tumorigenesis. In our study, we found that β-catenin bound to Trop2 to form the Trop2/β-catenin complex, contributing to β-catenin stability which was mainly affected by the ubiquitin–proteasome pathway. However, a recent study showed that autophagy also contributed to β-catenin degradation[39]. Consistent with these reports, we found that the two pathways destabilized β-catenin, however, Trop2/β-catenin complex in the cytoplasm and nucleus blocked the ubiquitin–proteasome pathway but not the autophagic pathway. Furthermore, BD was found to inhibit autophagy by downregulating the expression of LC3-I and LC3-II, followed by upregulation of P62 levels in breast cancer cells. Owing to the protective role of autophagy in survival, chemotherapy resistance, stem cell maintenance, and metastasis in breast cancer[58], clinical trials are currently exploring the treatment effect of inhibiting autophagy through chloroquine or hydroxychloroquine combined with chemotherapy in a variety of cancers including breast cancer[59]. These findings suggest that further experiments are necessary to investigate the exact mechanism whereby BD affects autophagy, which can provide new insight into cancer treatment in the future.
Our data confirmed that the pro-metastatic effect of Trop2 was β-catenin-dependent. Trop2 positively regulated β-catenin protein expression and β-catenin positively regulated Trop2 transcription. Thus, they composed a positive reciprocal regulation loop. Trop2/β-catenin complex participated in the positive loop by preventing β-catenin degradation via the ubiquitin-proteosome pathway in breast cancer. Stoyanova et al. demonstrated that Trop2 could be cleaved into the extracellular domain and the intracellular domain. The intracellular domain of Trop2 was released from the membrane and accumulated in the nucleus, and the extracellular domain of Trop2 was scarcely detectable in the nucleus of prostate cancer cells. The co-localization of nucleus β-catenin and nucleus intracellular domain of Trop2 promoted self-renewal and the transformation activity in human prostate cancer [15]. However, we detected the presence of FL-Trop2 in the nucleus of breast cancer cells by cell fractionation assay (Fig. 6G). To visualize directly the distribution of intracellular and extracellular domain of Trop2, cells were immunolabelled with two anti-Trop2 antibodies, recognizing the fragments containing 138–215 aa of Trop2 (No. 27360–1-AP, Proteintech) and 300–323 aa of Trop2 (No. 214488, Abcam) respectively, then imaged with a laser scanning confocal microscope. The fragments containing 138–215 aa and 300–323 aa of Trop2 were observed in nucleus and cytoplasm, validating the presence of FL-Trop2 in the nucleus (Fig. S10 and Fig. S11). The finding that β-catenin bound to different fragments of Trop2, and the complex played different roles in different cancers, was certainly an interesting phenomenon and deserved further investigation.
Conclusion
In summary, we found that BD, a natural compound, restricted breast cancer tumor growth and metastasis, and impeded lung metastatic colonization by targeting Trop2. BD bound to residues Lys307 and Glu310 of Trop2, and then depleted the Trop2/β-catenin complex, causing β-catenin degradation. Thus, β-catenin nucleus translocation decreased, thereby reducing the transcription of Trop2 and metastasis-related genes. Overall, our results provide valuable novel insights into breast cancer therapy.
Compliance with Ethics Requirements
All Institutional and National Guidelines for the care and use of animals (fisheries) were followed.
CRediT authorship contribution statement
Wenjuan Tang: Conceptualization, Investigation, Validation, Methodology, Data curation, Visualization, Writing – original draft. Yu Hu: Data curation, Validation, Methodology. Kaihui Tu: Data curation, Validation, Methodology. Zhengyan Gong: Validation, Methodology. Man Zhu: Methodology, Resources, Formal analysis. Tianfeng Yang: Methodology, Resources, Formal analysis. Ammar Sarwar: Investigation, Writing – review & editing. Bingling Dai: Investigation, Methodology, Supervision. Dongdong Zhang: Investigation, Methodology, Supervision. Yingzhuan Zhan: Funding acquisition, Resources, Project administration, Data curation, Conceptualization, Writing – review & editing, Supervision. Yanmin Zhang: Funding acquisition, Resources, Project administration, Data curation, Conceptualization, Writing – review & editing, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This study was supported by the Shaanxi Province Science and Technology Development Plan Project (No. 2022ZDLSF05-05, China), the Project of Shaanxi Provincial Administration of Traditional Chinese Medicine (No. 2021-03-ZZ-002, China), Natural Science Basic Research Plan of Shaanxi Province in China (No. 2021JM-030, China), and Shaanxi Province Science Fund for Distinguished Young Scholars (2023-JC-JQ-59).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jare.2023.05.012.
Contributor Information
Yingzhuan Zhan, Email: zyzlt2009@xjtu.edu.cn.
Yanmin Zhang, Email: zhang2008@xjtu.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Waks A.G., Winer E.P. Breast cancer treatment. JAMA. 2019;321(3):316. doi: 10.1001/jama.2018.20751. [DOI] [PubMed] [Google Scholar]
- 3.Liang Y., Zhang H., Song X., Yang Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin Cancer Biol. 2020;60:14–27. doi: 10.1016/j.semcancer.2019.08.012. [DOI] [PubMed] [Google Scholar]
- 4.Metzger-Filho O., Tutt A., de Azambuja E., Saini K.S., Viale G., Loi S., et al. Dissecting the heterogeneity of triple-negative breast cancer. J Clin Oncol. 2012;30(15):1879–1887. doi: 10.1200/JCO.2011.38.2010. [DOI] [PubMed] [Google Scholar]
- 5.Hwang S.Y., Park S., Kwon Y. Recent therapeutic trends and promising targets in triple negative breast cancer. Pharmacol Ther. 2019;199:30–57. doi: 10.1016/j.pharmthera.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 6.Bianchini G., De Angelis C., Licata L., Gianni L. Treatment landscape of triple-negative breast cancer - expanded options, evolving needs. Nat Rev Clin Oncol. 2022;19(2):91–113. doi: 10.1038/s41571-021-00565-2. [DOI] [PubMed] [Google Scholar]
- 7.Gradishar W.J., Anderson B.O., Abraham J., Aft R., Agnese D., Allison K.H., et al. Breast cancer, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2020;18(4):452–478. doi: 10.6004/jnccn.2020.0016. [DOI] [PubMed] [Google Scholar]
- 8.Coates J.T., Sun S., Leshchiner I., Thimmiah N., Martin E.E., McLoughlin D., et al. Parallel genomic alterations of antigen and payload targets mediate polyclonal acquired clinical resistance to sacituzumab govitecan in triple-negative breast cancer. Cancer Discov. 2021;11(10):2436–2445. doi: 10.1158/2159-8290.CD-21-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berrada N., Delaloge S., André F. Treatment of triple-negative metastatic breast cancer: toward individualized targeted treatments or chemosensitization? Ann Oncol. 2010;21:vii30–vii35. doi: 10.1093/annonc/mdq279. [DOI] [PubMed] [Google Scholar]
- 10.Amith S.R., Fliegel L. Na(+)/h(+) exchanger-mediated hydrogen ion extrusion as a carcinogenic signal in triple-negative breast cancer etiopathogenesis and prospects for its inhibition in therapeutics. Semin Cancer Biol. 2017;43:35–41. doi: 10.1016/j.semcancer.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Franzoi M.A., Romano E., Piccart M. Immunotherapy for early breast cancer: Too soon, too superficial, or just right? Ann Oncol. 2021;32(3):323–336. doi: 10.1016/j.annonc.2020.11.022. [DOI] [PubMed] [Google Scholar]
- 12.Shvartsur A., Bonavida B. Trop2 and its overexpression in cancers: Regulation and clinical/therapeutic implications. Genes Cancer. 2015;6(3–4):84–105. doi: 10.18632/genesandcancer.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guerra E., Trerotola M., Dell’ Arciprete R., Bonasera V., Palombo B., El-Sewedy T. A bicistronic Cyclin D1-trop2 mrna chimera demonstrates a novel oncogenic mechanism in human cancer. Cancer Res. 2008;68(19):8113–8121. doi: 10.1158/0008-5472.CAN-07-6135. [DOI] [PubMed] [Google Scholar]
- 14.Hsu E.-C., Rice M.A., Bermudez A., Marques F.J.G., Aslan M., Liu S., et al. Trop2 is a driver of metastatic prostate cancer with neuroendocrine phenotype viaPARP1. Proc Natl Acad Sci U S A. 2020;117(4):2032–2042. doi: 10.1073/pnas.1905384117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stoyanova T., Goldstein A.S., Cai H., Drake J.M., Huang J., Witte O.N. Regulated proteolysis of trop2 drives epithelial hyperplasia and stem cell self-renewal via beta-catenin signaling. Genes Dev. 2012;26(20):2271–2285. doi: 10.1101/gad.196451.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trerotola M., Jernigan D.L., Liu Q., Siddiqui J., Fatatis A., Languino L.R. Trop-2 promotes prostate cancer metastasis by modulating beta(1) integrin functions. Cancer Res. 2013;73(10):3155–3167. doi: 10.1158/0008-5472.CAN-12-3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trerotola M., Cantanelli P., Guerra E., Tripaldi R., Aloisi A.L., Bonasera V., et al. Upregulation of trop-2 quantitatively stimulates human cancer growth. Oncogene. 2013;32(2):222–233. doi: 10.1038/onc.2012.36. [DOI] [PubMed] [Google Scholar]
- 18.Trerotola M., Guerra E., Ali Z., Aloisi A.L., Ceci M., Simeone P., et al. Trop-2 cleavage by ADAM10 is an activator switch for cancer growth and metastasis. Neoplasia. 2021;23(4):415–428. doi: 10.1016/j.neo.2021.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao W., Kuai X., Zhou X., Jia L., Wang J., Yang X., et al. Trop2 is a potential biomarker for the promotion of EMT in human breast cancer. Oncol Rep. 2018;40(2):759–766. doi: 10.3892/or.2018.6496. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka T., Ohishi T., Asano T., Takei J., Nanamiya R., Hosono H., et al. An anti-trop2 monoclonal antibody TRMAB-6 exerts antitumor activity in breast cancer mouse xenograft models. Oncol Rep. 2021;46(1):132. doi: 10.3892/or.2021.8083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin H., Zhang H., Wang J., Lu M., Zheng F., Wang C., et al. A novel human fab antibody for trop2 inhibits breast cancer growth in vitro and in vivo. Int J Cancer. 2014;134(5):1239–1249. doi: 10.1002/ijc.28451. [DOI] [PubMed] [Google Scholar]
- 22.Cardillo T.M., Govindan S.V., Sharkey R.M., Trisal P., Arrojo R., Liu D., et al. Sacituzumab govitecan (immu-132), an anti-trop-2/sn-38 antibody-drug conjugate: Characterization and efficacy in pancreatic, gastric, and other cancers. Bioconjug Chem. 2015;26(5):919–931. doi: 10.1021/acs.bioconjchem.5b00223. [DOI] [PubMed] [Google Scholar]
- 23.Huang H., Groth J., Sossey-Alaoui K., Hawthorn L., Beall S., Geradts J. Aberrant expression of novel and previously described cell membrane markers in human breast cancer cell lines and tumors. Clin Cancer Res. 2005;11(12):4357–4364. doi: 10.1158/1078-0432.CCR-04-2107. [DOI] [PubMed] [Google Scholar]
- 24.Liao S., Wang B., Zeng R., Bao H., Chen X., Dixit R., et al. Recent advances in trophoblast cell-surface antigen 2 targeted therapy for solid tumors. Drug Dev Res. 2021;82(8):1096–1110. doi: 10.1002/ddr.21870. [DOI] [PubMed] [Google Scholar]
- 25.Wang S., Hu H., Zhong B., Shi D., Qing X., Cheng C., et al. Bruceine d inhibits tumor growth and stem cell-like traits of osteosarcoma through inhibition of stat3 signaling pathway. Cancer Med. 2019;8(17):7345–7358. doi: 10.1002/cam4.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang Y., Kong F., Ding Q., Cai Y., Hao Y., Tang B. Bruceine d elevates nrf2 activation to restrain parkinson's disease in mice through suppressing oxidative stress and inflammatory response. Biochem Biophys Res Commun. 2020;526(4):1013–1020. doi: 10.1016/j.bbrc.2020.03.097. [DOI] [PubMed] [Google Scholar]
- 27.Dou Y.X., Zhou J.T., Wang T.T., Huang Y.F., Chen V.P., Xie Y.L., et al. Self-nanoemulsifying drug delivery system of bruceine d: A new approach for anti-ulcerative colitis. Int J Nanomedicine. 2018;13:5887–5907. doi: 10.2147/IJN.S174146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan J., Ren D., Wang J., Liu X., Zhang H., Wu M., et al. Bruceine d induces lung cancer cell apoptosis and autophagy via the ros/mapk signaling pathway in vitro and in vivo. Cell Death Dis. 2020;11(2):126. doi: 10.1038/s41419-020-2317-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang R., Zhang L., Jin J., Zhou Y., Zhang H., Lv C., et al. Bruceine D inhibits Hif-1alpha-mediated glucose metabolism in hepatocellular carcinoma by blocking ICAT/beta-catenin interaction. Acta Pharm Sin B. 2021;11(11):3481–3492. doi: 10.1016/j.apsb.2021.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu L., Lin Z.X., Leung P.S., Chen L.H., Zhao M., Liang J. Involvement of the mitochondrial pathway in bruceine D-induced apoptosis in capan-2 human pancreatic adenocarcinoma cells. Int J Mol Med. 2012;30(1):93–99. doi: 10.3892/ijmm.2012.980. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J.Y., Lin M.T., Tung H.Y., Tang S.L., Yi T., Zhang Y.Z., et al. Bruceine D induces apoptosis in human chronic myeloid leukemia K562 cells via mitochondrial pathway. Am J Cancer Res. 2016;6(4):819–826. [PMC free article] [PubMed] [Google Scholar]
- 32.Luo C., Wang Y., Wei C., Chen Y., Ji Z. The anti-migration and anti-invasion effects of bruceine d in human triple-negative breast cancer MDA-MB-231 cells. Exp Ther Med. 2020;19(1):273–279. doi: 10.3892/etm.2019.8187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dai J., Liang K., Zhao S., Jia W., Liu Y., Wu H., et al. Chemoproteomics reveals baicalin activates hepatic cpt1 to ameliorate diet-induced obesity and hepatic steatosis. Proc Natl Acad Sci U S A. 2018;115(26):E5896–E5905. doi: 10.1073/pnas.1801745115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alshareef A., Zhang H.-F., Huang Y.-H., Wu C., Zhang J.D., Wang P., et al. The use of cellular thermal shift assay (CETSA) to study crizotinib resistance in ALK-expressing human cancers. Sci Rep. 2016;6(1) doi: 10.1038/srep33710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berens E.B., Holy J.M., Riegel A.T., Wellstein A. A cancer cell spheroid assay to assess invasion in a 3D setting. J Vis Exp. 2015;105:e53409. doi: 10.3791/53409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kaukonen R., Jacquemet G., Hamidi H., Ivaska J. Cell-derived matrices for studying cell proliferation and directional migration in a complex 3D microenvironment. Nat Protoc. 2017;12(11):2376–2390. doi: 10.1038/nprot.2017.107. [DOI] [PubMed] [Google Scholar]
- 37.Saatci O., Kaymak A., Raza U., Ersan P.G., Akbulut O., Banister C.E., et al. Targeting lysyl oxidase (lox) overcomes chemotherapy resistance in triple negative breast cancer. Nat Commun. 2020;11(1):2416. doi: 10.1038/s41467-020-16199-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baram T., Erlichman N., Dadiani M., Balint-Lahat N., Pavlovski A., Meshel T., et al. Chemotherapy shifts the balance in favor of cd8+ tnfr2+ tils in triple-negative breast tumors. Cells. 2021;10(6):1429. doi: 10.3390/cells10061429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petherick K.J., Williams A.C., Lane J.D., Ordonez-Moran P., Huelsken J., Collard T.J., et al. Autolysosomal beta-catenin degradation regulates wnt-autophagy-p62 crosstalk. EMBO J. 2013;32(13):1903–1916. doi: 10.1038/emboj.2013.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang K., Zhang J.-X., Han L., You Y.-P., Jiang T., Pu P.-Y., et al. Microrna roles in beta-catenin pathway. Mol Cancer. 2010;9(1):252. doi: 10.1186/1476-4598-9-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu G., Chong R.A., Yang Q., Wei Y., Blanco M.A., Li F., et al. Mtdh activation by 8q22 genomic gain promotes chemoresistance and metastasis of poor-prognosis breast cancer. Cancer Cell. 2009;15(1):9–20. doi: 10.1016/j.ccr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Györffy B., Lanczky A., Eklund A.C., Denkert C., Budczies J., Li Q., et al. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123(3):725–731. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 43.Zimmers S.M., Browne E.P., Williams K.E., Jawale R.M., Otis C.N., Schneider S.S., et al. Trop2 methylation and expression in tamoxifen-resistant breast cancer. Cancer Cell Int. 2018;18(1):94. doi: 10.1186/s12935-018-0589-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Remšík J., Binó L., Kahounová Z., Kharaishvili G., Šimečková Š., Fedr R., et al. Trop-2 plasticity is controlled by epithelial-to-mesenchymal transition. Carcinogenesis. 2018;39(11):1411–1418. doi: 10.1093/carcin/bgy095. [DOI] [PubMed] [Google Scholar]
- 45.Jeon Y., Jo U., Hong J., Gong G., Lee H.J. Trophoblast cell-surface antigen 2 (trop2) expression in triple-negative breast cancer. BMC Cancer. 2022;22(1):1014. doi: 10.1186/s12885-022-10076-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kamble P.R., Rane S., Breed A.A., Joseph S., Mahale S.D., Pathak B.R. Proteolytic cleavage of trop2 at arg87 is mediated by matriptase and regulated by val194. FEBS Lett. 2020;594(19):3156–3169. doi: 10.1002/1873-3468.13899. [DOI] [PubMed] [Google Scholar]
- 47.Ambrogi F., Fornili M., Boracchi P., Trerotola M., Relli V., Simeone P., et al. Trop-2 is a determinant of breast cancer survival. PLoS One. 2014;9(5):e96993. doi: 10.1371/journal.pone.0096993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hanahan D., Weinberg R.A. Hallmarks of cancer: The next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 49.Redig A.J., McAllister S.S. Breast cancer as a systemic disease: A view of metastasis. J Intern Med. 2013;274(2):113–126. doi: 10.1111/joim.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mashouri L., Yousefi H., Aref A.R., Ahadi A.M., Molaei F., Alahari S.K. Exosomes: Composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18(1):75. doi: 10.1186/s12943-019-0991-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yuzhalin A.E., Gordon-Weeks A.N., Tognoli M.L., Jones K., Markelc B., Konietzny R., et al. Colorectal cancer liver metastatic growth depends on pad4-driven citrullination of the extracellular matrix. Nat Commun. 2018;9(1):4783. doi: 10.1038/s41467-018-07306-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fattet L., Jung H.Y., Matsumoto M.W., Aubol B.E., Kumar A., Adams J.A., et al. Matrix rigidity controls epithelial-mesenchymal plasticity and tumor metastasis via a mechanoresponsive EPHA2/LYN complex. Dev Cell. 2020;54(3):302–316 e7. doi: 10.1016/j.devcel.2020.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang Y., Zhang H., Wang J., Liu Y., Luo T., Hua H. Targeting extracellular matrix stiffness and mechanotransducers to improve cancer therapy. J Hematol Oncol. 2022;15(1):34. doi: 10.1186/s13045-022-01252-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prakash V., Carson B.B., Feenstra J.M., Dass R.A., Sekyrova P., Hoshino A., et al. Ribosome biogenesis during cell cycle arrest fuels EMT in development and disease. Nat Commun. 2019;10(1):2110. doi: 10.1038/s41467-019-10100-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tam W.L., Lu H., Buikhuisen J., Soh B.S., Lim E., Reinhardt F., et al. Protein kinase c alpha is a central signaling node and therapeutic target for breast cancer stem cells. Cancer Cell. 2013;24(3):347–364. doi: 10.1016/j.ccr.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tsai J.H., Donaher J.L., Murphy D.A., Chau S., Yang J. Spatiotemporal regulation of epithelial-mesenchymal transition is essential for squamous cell carcinoma metastasis. Cancer Cell. 2012;22(6):725–736. doi: 10.1016/j.ccr.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Reichert M., Bakir B., Moreira L., Pitarresi J.R., Feldmann K., Simon L., et al. Regulation of epithelial plasticity determines metastatic organotropism in pancreatic cancer. Dev Cell. 2018;45(6):696 e8–711 e8. doi: 10.1016/j.devcel.2018.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi D.S., Blanco E., Kim Y.-S., Rodriguez A.A., Zhao H., Huang T.-M., et al. Chloroquine eliminates cancer stem cells through deregulation of Jak2 and DNMT1. Stem Cells. 2014;32(9):2309–2323. doi: 10.1002/stem.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chung S.J., Nagaraju G.P., Nagalingam A., Muniraj N., Kuppusamy P., Walker A., et al. ADIPOQ/adiponectin induces cytotoxic autophagy in breast cancer cells through STK11/LKB1-mediated activation of the AMPK-ULK1 axis. Autophagy. 2017;13(8):1386–1403. doi: 10.1080/15548627.2017.1332565. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.