Abstract
Objectives:
Alpha-amyrin (AA) is a pentacyclic triterpene that exhibits erratic gastrointestinal absorption and poor blood-brain barrier permeability. The study aims to isolate AA from the stem bark of Ficus benghalensis L. (Fb) (Moraceae), purify it, and formulate a nanoemulsion (NE) that may improve its bioavailability, characterization, and intranasal (IN) administration to Swiss albino mice to check its neurobehavioral effects in aluminum-induced neurotoxicity.
Materials and Methods:
AA was isolated from the stem bark of Fb by Soxhlet extraction, purified by analytical methods, prepared chitosan-decorated NE of the same, and characterized. It was then administered through IN route to aluminum-treated Swiss albino mice for 28 days to check its effect on neurobehavioral parameters.
Results:
IN delivery of chitosan-decorated AA, NE resulted in significant improvement in neurobehavioral parameters. It reduced the fall-off period in the rotarod test and the escape latency in the Morris water maze test, and animals showed improved learning and spatial memory in the elevated plus maze. The transfer latency of animals improved with treatment compared with the aluminum-induced groups, indicative of the neuroprotective role of the drug.
Conclusion:
IN administration of AA, NE isolated from the stem bark of Fb improved neurobehavioral parameters in aluminum-induced neurotoxicity in Swiss albino mice.
Keywords: Aluminium, alpha amyrin, nanoemulsion, intranasal, neurobehavioral
INTRODUCTION
Alpha-amyrin (AA) is a pentacyclic triterpene of the urbane group with an attractive pharmacological profile. The drug possesses bioavailability issues due to poor water solubility, whimsical gastrointestinal (GI) absorption, and poor blood-brain barrier (BBB) permeability, which restrains its usage as a drug. Plant-derived secondary metabolites are formidable moieties that are abundant in plant species from tropical rainforests.
Ficus benghalensis L. (Fb) (Moraceae) or the Great Indian banyan, a good source of phytocompounds, can be used to isolate triterpenes such as AA. It is found all over Indian rainforests. The root contains phytosterols, the leaves contain triterpenes, fridelin, and beta-sitosterol, and the bark is rich in bengalinoside, flavonoid glycoside, leucocyanidin, leucopelargonidin, AA, phenols, alkaloids, and tannins. Heartwood consists of alpha-taraxasterol and tiglic acid. Milky latex is used for wound healing, swelling, skin diseases, to treat vaginal diseases, and diabetes, as a uterine tonic, in diarrhea, nausea, vomiting, ulcers, irritable bowel syndrome (IBS), bleeding disorders, etc. Studies related to the antimicrobial, anti-arthritic, and wound-healing effects of Fb confirm the potential of the Banyan tree.1,2,3
Studies reveal that amyrins possess GI-protective action,4 anti-inflammatory activity, hepatoprotective,5 help to regulate blood glucose levels, and are useful against various cancer cell lines, including liver and breast colorectal cancers, as they induce cell death by apoptosis.6,7 The drug AA has also been reported to possess antihyperglycemic and hypolipidemic action,8 and it plays a role in modulating enzymatic, hormonal, and inflammatory responses.9 Although it has a potential, factors such as poor water solubility, extensive half-life, deplorable clearance, and wavering GI absorption evinced by AA impede its usage as a drug.10 The route through, which a drug is administered, plays a vital role in determining the bioavailability of a drug, especially when the candidate exhibits poor absorption through the GI route. To overcome this predicament, various approaches may be adopted, such as complexation of the drug moiety or conversion into salt form, preparing nanoformulations, etc.11 When a drug is supposed to target complex systems such as the central nervous system (CNS), the blood brain barrier (BBB) acts as a major barrier as it restricts and hinders the entry of xenobiotics. Various parameters related to the drug, such as acid dissociation constant, log P, lipophilicity, bioavailability, and first-pass metabolism, are important to ensure proper drug action on the system involved. The problems associated with drug solubility, poor oral bioavailability, poor GI absorption, etc., may be solved through the formulation of nanoparticles or any nanopreparation. The same, when administered through an alternate route such as an intranasal (IN) or intravenous route, would help to surpass the issues associated with GI absorption and first-pass metabolism.12 Such alternate routes improve the bioavailability of the drug and ensure full-fledged use of the drug moiety’s pharmacological potential.
Nanotechnology is a promising Promethean science that helps to meet the hurdles associated with absorption, distribution, metabolism, and excretion, and thereby attenuates bioavailability issues. Incorporation of a mucoadhesive polymer such as chitosan would help overcome mucociliary clearance13 and help to carry the IN administered drug moiety across the tight junctions of the BBB.14,15 The approach opted for here is through the formulation of a NE of the drug AA16 to be administered IN which would be carried to the brain17 through the olfactory and trigeminal nerve supply which links the nasal mucosa directly to the brain.18 This work aims at isolating AA from the stem bark of Fb and developing an AA-loaded chitosan NE suitable for IN delivery, targeting the brain, and studying its effect in altering neurobehavioral parameters in aluminum chloride-induced neurotoxicity.
MATERIALS AND METHODS
Chemicals and reagents
Extraction and isolation
AA standard (Sigma Aldrich, 98% pure), methanol, chloroform, petroleum ether, toluene, ethyl acetate, and formic acid (SDFCL Mumbai).
Thin layer chromatography (TLC): n-hexane, ethyl acetate (analytical grade), and iodine crystals (SDFCL Mumbai) instead of n-hexane, ethyl acetate (analytical grade), and iodine crystals.
Apparatus
Soxhlet apparatus, column chromatography: performed on silica gel (60-120 mesh, Thermofisher Scientific) and TLC plates, iodine chamber, glass chamber (Twin-trough).
Isolation of AA from Fb stem bark
Plant raw material collection, handling, and extraction
Fb stem bark gathered during January, 2022 from it is habitat in Bengaluru (India), authenticated by a taxonomist, and preserved as a herbarium (KCP-PCOG/FB/330/2021-22). The stem barks were segregated, followed by air drying and drying in an oven at 45 °C, and coarsely powdered. A total of 850 g of the powdered stem bark was subjected to exhaustive Soxhlet extraction with methanol-water (1:4) at 70 °C for about 2 hours and 3 washes. The final liquid extract was reduced using a rotary evaporator and was conserved in a glass container for further studies.19 The Institutional Animal Ethics Committee of Kruanidhi College of Pharmacy approved the protocol under the reference number: KCP/IAEC/PCOL/61/2020.
Isolation of AA
TLC was performed using the methanolic extract of Fb. A measure of the extract was mixed with little methanol and stowed and adsorbed on silica gel (grade 60-120 mm, 245 g). The extract was loaded into a silica gel column. The column was packed with petroleum ether, and the phytoconstituents were eluted first with petroleum ether (60-80 °C), followed by petroleum ether-chloroform (9:1, 1:1, 1:3, v/v), and finally with chloroform, chloroform-methanol (99:1, 98:2, 95:5, 9:1, 3:1, 1:1, 1:3, v/v), and methanol. Eluting the column with petroleum ether: chloroform (1:1) yielded colorless AA acetate crystals. AA was obtained by recrystallizing the same from acetone.19,20,21
TLC: The crystallized form of AA was then subjected to TLC [mobile phase: n-hexane: ethyl acetate (9:1)], infrared (IR) spectroscopy (ATR-IR), high performance liquid chromatography (HPLC) (mobile phase A- ammonium acetate 10 mm unadjusted, mobile phase B: acetonitrile: methanol (1:1), isocratic method: flow-1.2 mL/min; A-25% and B-75%, column temp: 35 °C, sample temperature: 15 °C) (HPLC waters 2695) and LC-MS/MS (LC-MS/MS condition: LC condition: solution A: 0.1% formic acid in water, solution B: acetonitrile MS grade, flow rate: 1.2 mL/min, column: Waters XBridge 50 x 4.6 mm 3.5 µ, C18, mode of elution: gradient, diluent: methanol, detector: ultraviolet-visible (UV-vis). LC-MS/MS -(MS quadrupole: capillary-3.45, cone-3.3, extractor-3, source temperature-110, desolvation temperature-500, gas flow-desolvation L/h-800 cone (L/h)-50, scan time-0.2 s). The spectroscopic analysis confirmed the presence of AA (purity: 98.37%) in Fb stem bark.
Preparation of AA-loaded chitosan-decorated NEs
Pre-formulation studies
The melting point of AA was determined using the Thiele tube method. Drug solubility in different solvents was assessed by the saturation shake flask method by dissolving the drug in solvents such as water, phosphate buffer, dimethyl sulfoxide (DMSO), and methanol. The λmax of AA was determined by preparing solutions of different concentrations and was then scanned from 200 to 400 nm using a UV-vis spectrophotometer.
Calibration curve of AA in methanol
A standard calibration curve of AA was made by drawing and making serial dilutions with AA stock solution, and the absorbance of the same was measured at 205 nm by the UV method with methanol as the blank.
Compatibility studies
Physicochemical characterization of AA NE formulation: [drug excipient compatibility study (ATR-IR analysis)]
The physicochemical interactions of AA with chitosan were studied using attenuated total reflectance-fourier transform infrared (FTIR) (Bruker ATR Alpha). Drugs and other ingredients (1:1) were stored in hermetically sealed glass vials at 40 °C and 75% relative humidity for one week. IR spectra of AA and the physical mixture were recorded using an ATR-IR (Bruker ATR alpha) instrument at wavelengths of 4400 cm-1 and 400 cm-1. This was done to check for the compatibility between the drug and other components to check for any interaction.
Formulation of the chitosan decorated NE
Chemicals and reagents
AA, low-molecular-weight chitosan [ICAR Central Institute of Fishery Technology (85% deacetylated)], sesame oil, Tween 80, poly ethylene glycol (PEG) 400 (Sigma-Aldrich), glacial acetic acid, deionized water.
Apparatus
Polytron high-speed homogenizer [Kinematica PolytronTM (PT-2100)], magnetic beads, stirrers, 0.2 m syringe filters, Horiba scientific particle size and zeta potential analyzer (Horiba SZ-100, Z-type, version 2.0), digital pH meter (Digisun electronics system), Brookfield viscometer (Brookfield Ametek), UV/Visible spectrophotometer (Shimadzu-1800).
Procedure
A spontaneous emulsification technique was utilized to prepare a chitosan-decorated NE of AA.22 The procedure was carried out at 25 °C. NEs were prepared by adding the organic phase (AA, sesame oil, and polyethylene glycol stirred continuously) to the aqueous phase (chitosan solution and Tween 80)23 followed by continuous stirring. Chitosan solution (2% w/v; low molecular weight, ~50 kDa) was prepared by dissolving chitosan in 100 mL of 1% glacial acetic acid and homogenizing at 2000 rpm for 24 hours. To 25 mL of chitosan solution, 2.5 mL of Tween 80 was added, blended well for 20 min, and homogenized at 2000 rpm. The oil phase consisting of AA mixed with 10 mL sesame oil and 5% polyethylene glycol was stirred for 1 h at high speed and added dropwise to the mixture of chitosan and Tween. The mixture was agitated for 60 min at room temperature with continuous stirring at 2000 rpm (Kinematica PolytronTM PT2100).24,25 The mixture was homogenized for approximately 2 min at 4000 rpm to produce NE with homogeneity and passed through a 0.2-micron syringe filter to reduce and homogenize the size of droplets. NE formed was collected and centrifuged, and the supernatant was collected to determine the percentage of drug content.
Evaluation of AA chitosan NE
The optimized AA-NE was investigated for 28 days under vigorous conditions as per International Council for Harmonisation (ICH) of Technical Requirements for Pharmaceuticals for Human Use guidelines to analyze it is thermodynamic constancy, followed by cycles of heating and cooling to observe the physical appearance, evidence of creaming or turbidity, etc., and was centrifuged for about 10 min at 4000 rpm for 10 min to check for any signs of instability.
The pH of the prepared chitosan NE was determined using a digital pH meter at room temperature. The globule size, size distribution, and zeta potential of AA-NE were determined using a Horiba scientific instrument.
The morphology and structural attributes of the prepared formulation were examined using a simple light microscope. The viscosity of the formulation was checked using a Brookfield viscometer at room temperature. All investigations were performed in triplicate.
The percentage of drug content was assessed using UV spectroscopy. A measured volume of the NE was centrifuged for 40 min at a speed of 15,000 rpm at 25 °C to separate the drug, which is separated in the supernatant, from the drug in the NE after dilution. The percentage drug content was calculated using the formula;
Surface morphology
Transmission electron microscopy (TEM) analysis was used to determine the morphological attributes of the AA-NE formulation and NE images were taken at various resolutions.25
Neurobehavioral studies
Apparatus used
Rolex digital rotarod apparatus, elevated plus maze, and Morris water maze were used. Swiss albino mice were procured from the animal house at Krupanidhi College of Pharmacy (in-house) after ethical clearance (KCP/IAEC/PCOL/61/2021). Animals were grouped into 4 groups with 6 animals in each group. Normal, positive control (AlCl3 100 mg/kg p.o.), treatment group 1 [10 mg/mL IN of AA-NE + AlCl3 (100 mg/kg p.o)], and treatment group 2 [20 mg/mL IN of AA-NE + AlCl3 (100 mg/kg p.o.)] for 28 days. Neurobehavioral tests such as the rotarod test, Morris water-maze test, and elevated plus-maze tests were performed on days 14 and 28 of the study.
Statistical analysis
Statistical significance of all the results was tested by comparing AA treatment groups with respective control. It was performed by one-way ANOVA ordinary measures followed by Dunnett’s comparison test where data are expressed as mean ± standard deviation (SD) (n= 6).
RESULTS
Isolation of AA from Fb stem bark
The Fb stem bark extract yielded a brownish mass (74 g, 11%) from which 0.457 g of the pure compound was isolated (0.609% yield).
Confirmation of AA using the analytical method
TLC shows AA in the sample compared with the standard (Figure 1). FT-IR of isolated AA compared with the standard confirms the purity of the isolated compound (Figure 2). LC-MS/MS results (Figures 3 and 4) confirmed the presence of AA with evident peaks at 426.61 and 218.72. Ions with mass-to-charge ratios of 218 and 426.6 were identified as AA. Isolated AA displays an m/z value of 426.61, similar to the standard AA of 426.7.26
Figure 1.

TLC of alpha-amyrin
TLC: Thin layer chromatography
Figure 2.
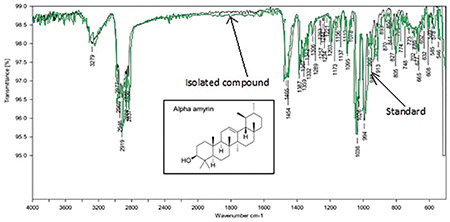
FT-IR of isolated AA compared to the standard
FT-IR: Fourier transform infrared, AA: Alpha-amyrin
Figure 3.
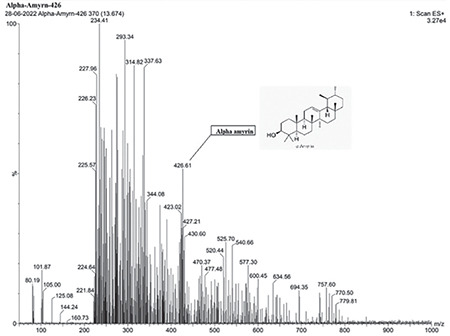
LC-MS/MS analysis of AA
AA: Alpha-amyrin
Figure 4.
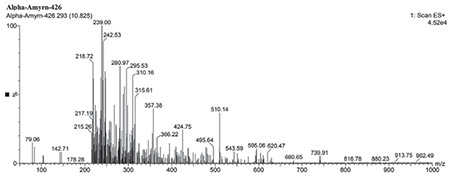
LC-MS/MS of isolated AA
AA: Alpha-amyrin
HPLC of AA
HPLC demonstrated that isolated AA, as well as standard, has retention at 5, confirming the purity of AA (Figure 5).
Figure 5.
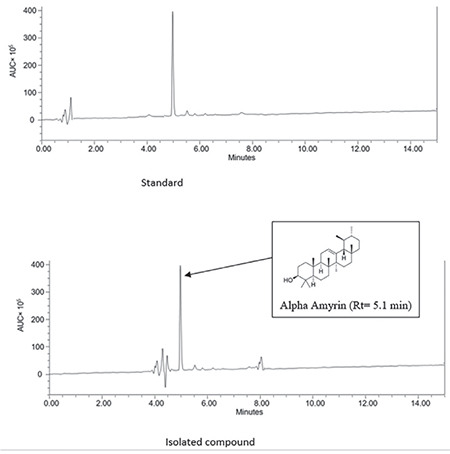
HPLC of isolated AA vs. standard
AA: Alpha-amyrin, HPLC: High performance liquid chromatography
Preparation of AA chitosan NE
Pre-formulation studies
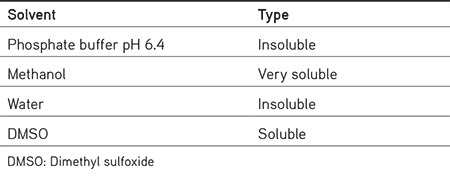
The melting point of AA was 186 °C. Solubility studies of AA in various solvents showed it is solubility (Table 1) in methanol and DMSO.
Table 1. Solubility of alpha amyrin in different solvents.
Calibration curve of AA
AA showed λmax at 205 nm, and this wavelength was chosen for analysis. Serial dilutions from a solution from a stock solution (10 mg of AA dissolved in 50 mL of methanol and sonicated) were prepared and analyzed using a UV spectrophotometer, which gave the calibration curve (Figure 6).
Figure 6.
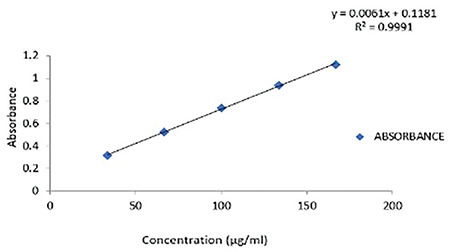
Calibration curve of AA
AA: Alpha-amyrin
Compatibility studies
FT-IR analysis
FT-IR studies of the drug and mixture of drug, polymer, and other components were performed to investigate the interaction at a wavelength between 4400 and 400 cm-1 (Figures 7 and 8). O-H stretching between 3550 and 3200 cm-1, C-H bending at 3550 and 3200 cm-1. C-H Bending at 1465. C=C bending at 995-985 cm-1. The mixture of AA with chitosan, tween, PEG, and sesame oil has all the characteristic peaks of AA, which confirms that the components are compatible (Figures 7 and 8).
Figure 7.
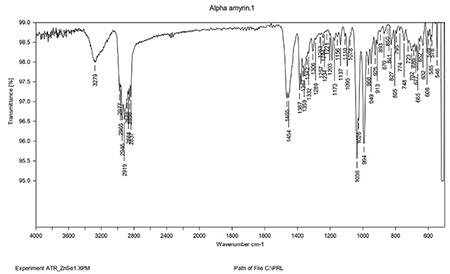
FT-IR of AA
FT-IR: Fourier transform infrared, AA: Alpha-amyrin
Figure 8.
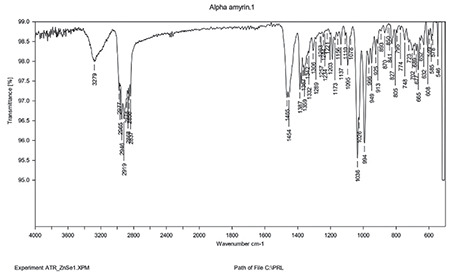
FT-IR of physical mixture
FT-IR: Fourier transform infrared, AA: Alpha-amyrin
Evaluation of the AA Chitosan NE
The NE was found to be thermodynamically stable. The physical appearance remained unaffected, and the preparation did not show a creaming effect or turbidity, when subjected to heating and cooling cycles upon centrifugation. It did not show phase separation and was stable.
Particle size (globule size), zeta potential, and polydispersity index (PDI) determination
The compositions of formulations F1 and F2 are given in Table 2, using the same components in both formulations at different ratios. The average size distribution of prepared NEs F2(a) and F2(b) was 57.9 nm and 63 nm, respectively, with PDI values of 0.4 and 0.9, which suggests the formation of nanosized formulations (Table 3). F1 exhibited a greater particle size, higher polydispersity index, and unstable zeta potential; hence, F1 was not studied further and F2 was selected for the rest of the analysis (Figures 9, 10, 11, 12).
Table 2. Formulation of alpha amyrin chitosan NE: Composition of formulation.
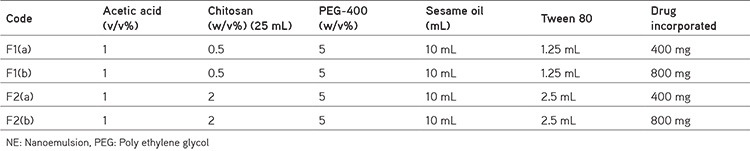
Table 3. Particle size (globule size), zeta potential, and PDI determination.
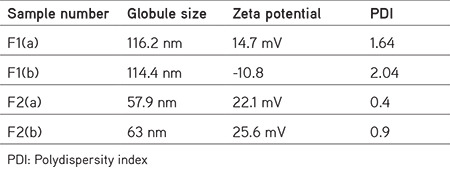
Figure 9.
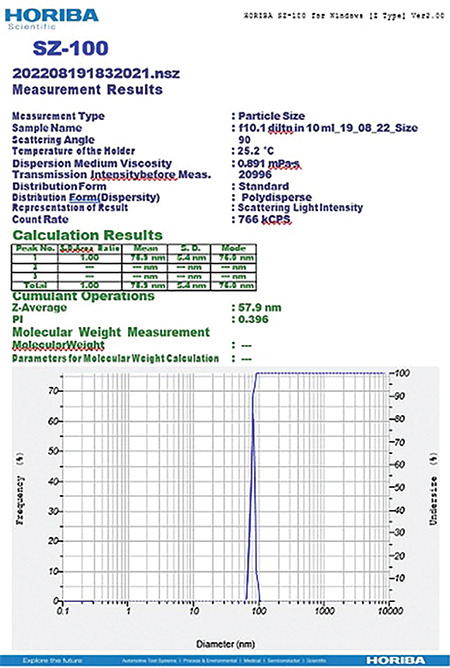
Globule size of the NE F2 (a)
Figure 10.
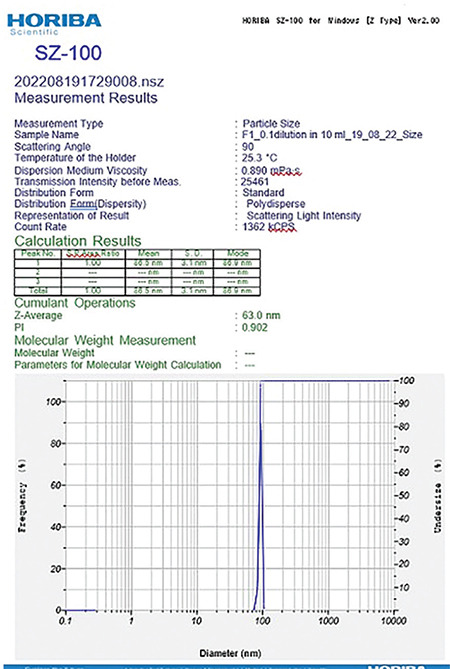
Globule size of the NE F2 (b)
Figure 11.
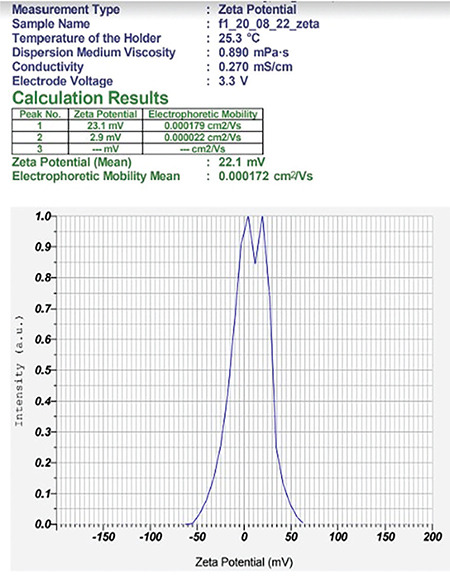
Zeta potential of the NE F2 (a)
Figure 12.
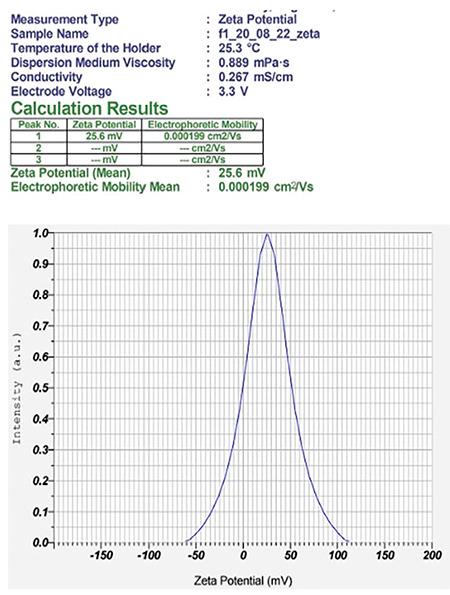
Zeta potential of the NE F2 (b)
The pH and viscosity of NE are depicted in Table 4. NE was found to have a percentage drug content of 98%.
Table 4. pH and viscosity of chitosan NE F2.

Surface morphology by TEM analysis
TEM images indicated that the average particle size of the NE F2 was between 50 and 100 nm. The particle size of the chitosan NE abides by the literature (Figure 13).
Figure 13.
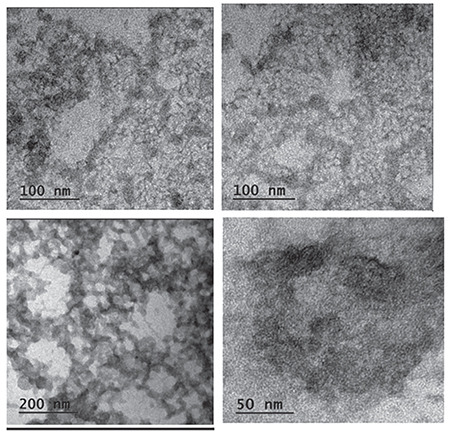
TEM images of AA, NEs F2(a) and F2 (b)
TEM: Transmission electron microscopy, AA: Alpha-amyrin
Neurobehavioral studies
Rotarod test
The fall-off time of animals using the rotarod apparatus was measured on days 14 and 28.
Statistical significance of the results of the rotarod test was established by collating treatment groups with the respective positive control group by applying one-way ANOVA ordinary measures followed by Dunnett’s test. The data are expressed as mean ± SD (n= 6), and ap < 0.001 when in contrast with the normal group. b, cp < 0.01 & when in contrast with the positive control group (Figure 14).
Figure 14.
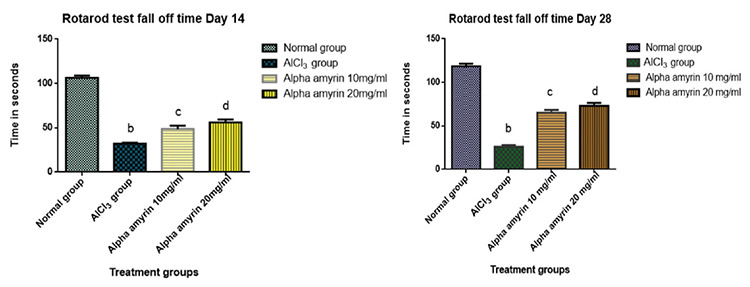
Estimation of fall-off period using the rotarod test
The positive control group (AlCl3) animals exhibited a significant reduction in fall-off period, motor coordination, and balance, when compared with the normal group on days 14 and 28. The experimental animals under AlCl3 (100 mg/kg p.o.) induced oxidative stress exhibited significant improvement in the fall period, when treated with AA at a dose of 10 mg/mL IN and at a dose of 20 mg/mL IN compared with the positive control group. The results suggested that AA treatment improved aluminum chloride-induced impairment in balance and coordination in animals at doses of 10 and 20 mg/mL IN, respectively.
Morris water maze test
Escape latency, spatial memory and learning in animals were tested using the Morris water maze. The statistical significance of the results of the Morris water maze test was determined by collating treatment groups with the respective positive control group by applying one-way ANOVA ordinary measures followed by Dunnett’s test. The data are expressed as mean ± SD (n= 6), and a,bp < 0.001 and significant in contrast to the positive control (AlCl3) group. Treatment with AA at doses of 10 mg/mL and 20 mg/mL IN significantly reduced escape latency, indicating that AA at both doses helps to improve learning and spatial memory in animals despite AlCl3 treatment (Figure 15).
Figure 15.
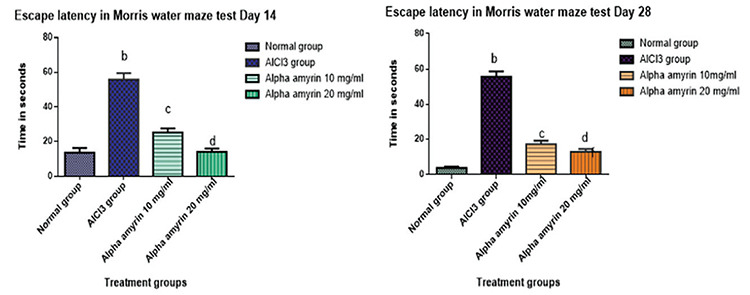
Estimation of escape latency using the Morris water maze test
Elevated plus maze test
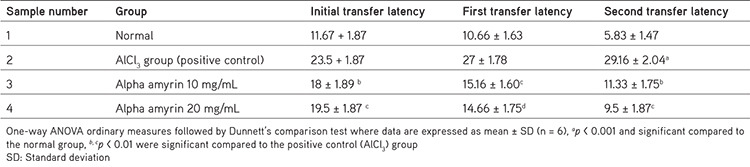
Statistical significance of the initial, first, and second transfer latency of animals in the elevated plus maze test was established by collating treatment groups with the respective positive control group by applying one-way ANOVA ordinary measures followed by Dunnett’s test. Here the initial transfer latency data are revealed as mean ± SD (n= 6), ap < 0.001 and significant concerning the normal group, b, cp < 0.01 were significant concerning the positive control (AlCl3) group (Table 5). The first transfer latency data are expressed as mean ± SD (n= 6), p < 0.01 and significant compared to the positive control (AlCl3) group, and data related to the second transfer latency are expressed as mean ± SD (n= 6), and ap < 0.001 when compared to the normal group and b, cp < 0.01 compared to positive control (AlCl3) group (Table 5).
Table 5. Transfer latency of animals (initial, first, and second transfer latency) in the elevated plus maze.
DISCUSSION
The investigation involved the isolation of AA from Fb, devising a chitosan-decorated NE of AA, characterization and evaluation of its effect on neurobehavioral parameters after IN administration to aluminum chloride administered Swiss albino mice. AA was isolated from the methanolic extract of Fb stem bark in colorless crystals by silica gel column chromatography. The obtained was subjected to TLC, FT-IR, HPLC, and LC-MS/MS. The spectroscopic analysis confirmed the presence of AA (purity: 98.37%) in the Fb bark.
Chitosan-decorated NE of AA for IN administration was prepared and characterized, and it showed the desired size range, zeta potential, PDI, percentage drug content, etc. Upon treatment, animals showed significant improvement in neurobehavioral parameters in the AA treatment groups compared with the groups with aluminum chloride-induced neurotoxicity. The amount of chitosan plays a role in the size and proper coating of the globule. An optimum amount of chitosan resulted in F2 in particles with an appropriate globule size below 100 nm with zeta potential and PDI. The viscosity of the nasal preparation is essential as it should have optimum viscosity and should remain in the nasal cavity, resisting mucociliary clearance and increasing the residence time. This attribute was also confirmed by the incorporation of chitosan in the preparation. The optimum pH for the IN formulation is 4.5-6.5, and formulation F2 abides the same.26
AlCl3 induced impairment in motor coordination and balance compared with the normal group, which reduced the fall-off time on both days 14 and 28. The experimental animals under AlCl3 (100 mg/kg p.o) induced oxidative stress exhibited improvement in the fall period when treated with AA administered IN at doses of 10 mg/mL and 20 mg/mL, respectively, with a better effect on day 28. The results suggest that AA treatment improved aluminum chloride-induced impairment in balance and coordination in animals at doses of 10 and 20 mg/mL IN, respectively. The results are contrary to the results of a study where amyrin (30 mg/kg), given i.p. 30 min prior, could not alter the motor response of the animals. The probable reason is thought to be due to the effect of the NE form of AA administered through an IN route that allowed direct action of the drug on centers for coordination and balance in the brain.
In the Morris water maze, normal animals showed shorter escape latency on days 14 and 28 in a dose-dependent manner, and the results were quite good as they expressed good learning and spatial memory as they identified the cues quickly and showed shorter escape latency in the positive control group. AA at doses of 10 mg/mL and 20 mg/mL IN significantly reduced escape latency, and the animals could find the hidden platform very quickly, no matter from which quadrant they started. A supportive study involving the administration of amyrin-rich bombax ceiba extract showed a good effect in animals as it increased the escape latency in animals.27 In the case of the elevated plus maze test, the total entries made by the animals to the open arm increased in AA-treated groups (results not given here), indicative of its anti-anxiety and antidepressant potential28 and its a role in the improvement of retention memory in treated animals compared with the induced group. This indicates that AA at both doses helps improve learning and spatial memory in AA-treated animals despite AlCl3 treatment.
The results convey that AA brought about significant improvement in learning, spatial memory, retention memory, motor coordination, balance, etc., in aluminum chloride-induced oxidative stress.
Acknowledgments
We would like to thank ICAR-Central Institute of Fisheries Technology for providing a sample of low-molecular-weight chitosan and Poornayu Research Lab, Bengaluru for the analytical part of the study.
Footnotes
Ethics
Ethics Committee Approval: The Institutional Animal Ethics Committee of Kruanidhi College of Pharmacy approved the protocol under the reference number: (KCP/IAEC/PCOL/61/2020).
Informed Consent: Not required.
Authorship Contributions
Surgical and Medical Practices: R.B., Concept: R.S.V., K.D., Design: R.S.V., K.D., Data Collection or Processing: R.B., Analysis or Interpretation: R.B., Literature Search: R.B., Writing: R.B.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declare that they have no financial interests that could have influenced the research or its interpretation. This study was completely a self-funded work.
References
- 1.Banyan Tree: Ficus benghalensis: uses, research, remedies, side effects. [Internet] https://www.easyayurveda.com/2017/05/22/banyan-tree:ficus-benghalensis.
- 2.Murugesu S, Selamat J, Perumal V. Phytochemistry, pharmacological properties, and recent applications of Ficus benghalensis and Ficus religiosa. Plants (Basel). 2021;10:2749. doi: 10.3390/plants10122749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Etratkhah Z, Ebrahimi SES, Dehaghi NK, Seifalizadeh Y. Antioxidant activity and phytochemical screening of Ficus benghalensis aerial roots fractions. J Rep Pharm Sci. 2019;8:24–27. [Google Scholar]
- 4.Vyas TK, Babbar AK, Sharma RK, Singh S, Misra A. Preliminary brain-targeting studies on intranasal mucoadhesive microemulsions of sumatriptan. AAPS PharmSciTech. 2006;7:49–57. doi: 10.1208/pt070108. [DOI] [PubMed] [Google Scholar]
- 5.Oliveira FA, Chaves MH, Almeida FR, Lima RC Jr, Silva RM, Maia JL, Brito GA, Santos FA, Rao VS. Protective effect of alpha- and beta-amyrin, a triterpene mixture from Protium heptaphyllum (Aubl.) March. trunk wood resin, against acetaminophen-induced liver injury in mice. J Ethnopharmacol. 2005;98:103–108. doi: 10.1016/j.jep.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 6.Barros FW, Bandeira PN, Lima DJ, Meira AS, de Farias SS, Albuquerque MR, dos Santos HS, Lemos TL, de Morais MO, Costa-Lotufo LV, Pessoa Cdo Ó. Amyrin esters induce cell death by apoptosis in HL-60 leukemia cells. Bioorg Med Chem. 2011;19:1268–1276. doi: 10.1016/j.bmc.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Mishra T, Arya RK, Meena S, Joshi P, Pal M, Meena B, Upreti DK, Rana TS, Datta D. Isolation, characterization and anticancer potential of cytotoxic triterpenes from Betula utilis bark. PLoS One. 2016;11:e0159430. doi: 10.1371/journal.pone.0159430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos FA, Frota JT, Arruda BR, de Melo TS, da Silva AA, Brito GA, Chaves MH, Rao VS. Antihyperglycemic and hypolipidemic effects of α, β-amyrin, a triterpenoid mixture from Protium heptaphyllum in mice. Lipids Health Dis. 2012;11:98. doi: 10.1186/1476-511X-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carvalho KM, de Melo TS, de Melo KM, Quinderé AL, de Oliveira FT, Viana AF, Nunes PI, Quetz JD, Viana DA, da Silva AA, Havt A, Fonseca SG, Chaves MH, Rao VS, Santos FA. Amyrins from Protium heptaphyllum reduce high-fat diet-induced obesity in mice via modulation of enzymatic, hormonal and inflammatory responses. Planta Med. 2017;83:285–291. doi: 10.1055/s-0042-114222. [DOI] [PubMed] [Google Scholar]
- 10.Ching J, Lin HS, Tan CH, Koh HL. Quantification of α- and β-amyrin in rat plasma by gas chromatography-mass spectrometry: application to preclinical pharmacokinetic study. J Mass Spectrom. 2011;46:457–464. doi: 10.1002/jms.1912. [DOI] [PubMed] [Google Scholar]
- 11.Ferreira RG, Silva Júnior WF, Veiga Junior VF, Lima ÁA, Lima ES. Physicochemical characterization and biological activities of the triterpenic mixture α, β-amyrenone. Molecules. 2017;22:298. doi: 10.3390/molecules22020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thakker A, Shanbag P. A randomized controlled trial of intranasalmidazolam versus intravenous-diazepam for acute childhood seizures. J Neurol. 2013;260:470–474. doi: 10.1007/s00415-012-6659-3. [DOI] [PubMed] [Google Scholar]
- 13.Cui F, Qian F, Yin C. Preparation and characterization of mucoadhesive polymer-coated nano-particles. Int J Pharm. 2006;316:154–161. doi: 10.1016/j.ijpharm.2006.02.031. [DOI] [PubMed] [Google Scholar]
- 14.Fessi H, Puisieux F, Devissaguet JP, Ammoury N, Benita S. Nano-capsule formation by interfacial polymer deposition following solvent displacement. Int J Pharm. 1989;55:1–4. [Google Scholar]
- 15.Md S, Khan RA, Mustafa G, Chuttani K, Baboota S, Sahni JK, Ali J. Bromocriptine loaded chitosan nano-particles intended for direct nose to brain delivery: pharmacodynamic, pharmacokinetic and scintigraphy study in mice model. Eur J Pharm Sci. 2013;48:393–405. doi: 10.1016/j.ejps.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Rodrigues IV, Seibert JB, Carneiro SP, de Souza GH, dos Santos OD, Lopes NP. Preparation and in vitro evaluation of α and β-amyrins loaded nano-emulsions. Curr Pharm Biotechnol. 2013;14:1235–1241. doi: 10.2174/1389201015666140317121647. [DOI] [PubMed] [Google Scholar]
- 17.Al-Ghananeem AM, Saeed H, Florence R, Yokel RA, Malkawi AH. Intranasal drug delivery of didanosine-loaded chitosan nano-particles for brain targeting; an attractive route against infections caused by AIDS viruses. J Drug Target. 2010;18:381–388. doi: 10.3109/10611860903483396. [DOI] [PubMed] [Google Scholar]
- 18.Fazil M, Md S, Haque S, Kumar M, Baboota S, Sahni JK, Ali J. Development and evaluation of rivastigmine loaded chitosan nano-particles for brain targeting. Eur J Pharm Sci. 2012;47:6–15. doi: 10.1016/j.ejps.2012.04.013. [DOI] [PubMed] [Google Scholar]
- 19.Naquvi KJ, Ali M, Ahamad J. Two new phytosterols from the stem bark of Ficus bengalensis L. J Saudi Chem Soc. 2015;19:650–654. [Google Scholar]
- 20.Ali M, Ravinder E, Ramachandran R. New ursane-type triterpenic esters from the stem bark of Thevatia peruviana. Pharmazie. 2000;55:385–389. [PubMed] [Google Scholar]
- 21.Aragão GF, Carneiro LM, Junior AP, Vieira LC, Bandeira PN, Lemos TL, Viana GS. A possible mechanism for anxiolytic and antidepressant effects of alpha- and beta-amyrin from Protium heptaphyllum (Aubl.) March. Pharmacol Biochem Behav. 2006;85:827–834. doi: 10.1016/j.pbb.2006.11.019. [DOI] [PubMed] [Google Scholar]
- 22.Kandav G, Singh SK. Review of nano-emulsion formulation and characterization techniques. Indian J Pharm Sci. 2018;80:781–789. [Google Scholar]
- 23.Kumar K, Singh L, Mishra S, Singh VK. Preparation and optimization of nano-emulsion formulations of antihypertensive drug carvedilol. EJMCM. 2018;5:282–290. [Google Scholar]
- 24.Khan RU, Shah SU, Rashid SA, Naseem F, Shah KU, Farid A, Hakeem KR, Kamli MR, Althubaiti EH, Alamoudi SA. Lornoxicam-loaded chitosan-decorated nano-emulsion: preparation and in vitro evaluation for enhanced transdermal delivery. Polymers (Basel). 2022;14:1922. doi: 10.3390/polym14091922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Akrawi SH, Gorain B, Nair AB, Choudhury H, Pandey M, Shah JN, Venugopala KN. Development and optimization of naringenin-loaded chitosan-coated nano-emulsion for topical therapy in wound healing. Pharmaceutics. 2020;12:893. doi: 10.3390/pharmaceutics12090893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pharma | Lovis 2000 M/ME and pH ME: viscosity and pH of nasal sprays or eye drops. Anton Paar. [Internet] https://www.anton-paar.com.
- 27.Mostafa MN. β-amyrin rich Bombax ceiba leaf extract with potential neuroprotective activity against scopolamine-induced memory impairment in rats. Rec Nat Prod. 2018;12:480–492. [Google Scholar]
- 28.Kun X, Zuhua G. Amyrin exerts potent anxiolytic and antidepressant effects via mechanisms involving monoamine oxidase and γ-aminobutyric acid in mouse hippocampus. Trop J Pharm Res. 2019;18:1673–1681. [Google Scholar]


