Abstract
We have shown that rotavirus 2/6 viruslike particles composed of proteins VP2 and VP6 (2/6-VLPs) administered to mice intranasally with cholera toxin (CT) induced protection from rotavirus challenge, as measured by virus shedding. Since it is unclear if CT will be approved for human use, we evaluated the adjuvanticity of Escherichia coli heat-labile toxin (LT) and LT-R192G. Mice were inoculated intranasally with 10 μg of 2/6-VLPs combined with CT, LT, or LT-R192G. All three adjuvants induced equivalent geometric mean titers of rotavirus-specific serum antibody and intestinal immunoglobulin G (IgG). Mice inoculated with 2/6-VLPs with LT produced significantly higher titers of intestinal IgA than mice given CT as the adjuvant. All mice inoculated with 2/6-VLPs mixed with LT and LT-R192G were totally protected (100%) from rotavirus challenge, while mice inoculated with 2/6-VLPs mixed with CT showed a mean 91% protection from challenge. The availability of a safe, effective mucosal adjuvant such as LT-R192G will increase the practicality of administering recombinant vaccines mucosally.
Rotaviruses are the leading cause of viral gastroenteritis in young children worldwide, leading to more than 500,000 deaths each year in developing countries (10). Our laboratory is working on the development of a subunit vaccine for rotavirus. Viruslike particles (VLPs) are made by coinfecting insect cells with baculovirus recombinants that express rotavirus structural proteins; these proteins self-assemble into VLPs (3). We have previously shown that 10 μg of 2/6-VLPs composed of VP2 and VP6 (2/6-VLPs) administered intranasally with the mucosal adjuvant cholera toxin (CT) induced between 70 and 90% reduction in virus shedding (14). To assess the necessity of a mucosal adjuvant for protection, 2/6-VLPs were administered intranasally to mice at doses of 10, 50, and 100 μg without CT. Low levels of protection (20, 29, and 38%, respectively) from viral shedding were seen (14), indicating that a mucosal adjuvant is necessary to achieve high levels of protection from rotavirus challenge after intranasal administration of 2/6-VLPs. Although CT is a potent mucosal adjuvant, the holotoxin may not be approved for use in humans because the toxic dose is low; as little as 5 μg of purified CT is sufficient to induce significant diarrhea in volunteers (12). Efforts are under way to dissociate the toxic and adjuvant properties of CT (16).
Although limited, mild diarrhea in vaccinees may be tolerated when the perceived benefit of an oral vaccine is very high, there is a need for alternate mucosal adjuvants that do not cause side effects in the vaccinee. Escherichia coli heat-labile enterotoxin (LT) is similar to CT in having adjuvant activity (2, 5), but it may be less toxic to humans than CT (1). Unlike CT, LT is not secreted from bacteria or fully biologically active when first isolated from the cell. LT must undergo cleavage by proteases in the intestine for it to be toxic. This difference in the need for LT activation results in differences in the toxic dose of CT versus LT, with LT being less toxic (1). Although LT is less toxic, there is still a potential for administration of LT to induce diarrhea, limiting its benefits as a mucosal adjuvant. A mutant of the E. coli heat-labile toxin (LT-R192G) has been developed in an effort to separate the adjuvant and toxic properties of LT (4). The mutant encodes a glycine instead of an arginine at position 192 of the protein. This amino acid change eliminates the trypsin-sensitive cleavage site in the protein, thereby preventing cleavage of the A subunit, rendering the protein nontoxic at adjuvant-effective doses. In a randomized placebo-controlled, dose-escalating study in adult volunteers, 0 of 24 volunteers showed adverse reactions to single oral doses of 5, 25, or 50 μg of LT-R192G; at 100 μg of LT-R192G, 2 of 12 (16.7%) volunteers developed mild to moderate diarrhea which resolved after 24 h (15). Maximum antibody responses or antibody-secreting cells to labile toxin B subunit (LTB) were observed when administered with 25 μg of LT-R192G. Therefore, LT-R192G retains its adjuvant activity with greatly reduced toxicity when administered orally (4, 11, 15).
This article reports studies to determine whether the immunogenicity and protective efficacy of rotavirus 2/6-VLPs administered intranasally are retained by coadministration of the nontoxic mutant-labile toxin, LT-R192G.
Rotavirus VLPs administered mucosally with CT, LT, and LT-R192G are immunogenic.
The 2/6-VLPs were prepared in insect cells, purified, and characterized as previously described (3). VLPs were composed of bovine Rf VP2 and SA11 VP6. Electron microscopic analysis of the particles showed the 2/6-VLPs were intact and properly formed (data not shown). Coomassie blue staining and Western blot analysis of the proteins in the VLPs confirmed the presence in the expressed particles of each of the expected structural proteins; no additional proteins were present (data not shown).
Virus antibody-free adult female CD-1 (22 to 24 g) mice were obtained from Charles River Laboratories (Portage, Mich.). Mice were confirmed to be free of rotavirus antibody by enzyme-linked immunosorbent assay (ELISA) prior to vaccination. We compared the effects of the different adjuvants on the serologic antibody response, the intestinal immunoglobulin A (IgA) and intestinal IgG antibody response, and on protection from rotavirus challenge. Groups (n = 10) of adult mice (>30 days of age) were vaccinated intranasally, as previously described (14), with 10 μg of 2/6-VLPs mixed with either 5 μg of CT (Sigma St. Louis, Mo.), 10 μg of LT (1), or 10 μg of LT-R192G (4) or with phosphate-buffered saline (PBS) on 0, 14, and 45 days postinoculation. The choice of dose for each adjuvant was the optimum dose described in the literature for each adjuvant.
Antibody responses were evaluated in serum and fecal samples collected 1 month after the third vaccination. Fecal samples collected from individual mice were processed and stored as previously described (8, 14). Measurement of total antirotavirus antibody in serum and antirotavirus IgA and IgG in intestinal samples was performed as previously described (14). Antibody titers were defined as the reciprocal of the highest dilution giving a net optical density (OD) value (OD with SA11 minus OD with 0.5% Blotto) above 0.1. All control mice, inoculated with PBS, remained negative for rotavirus antibody until challenge.
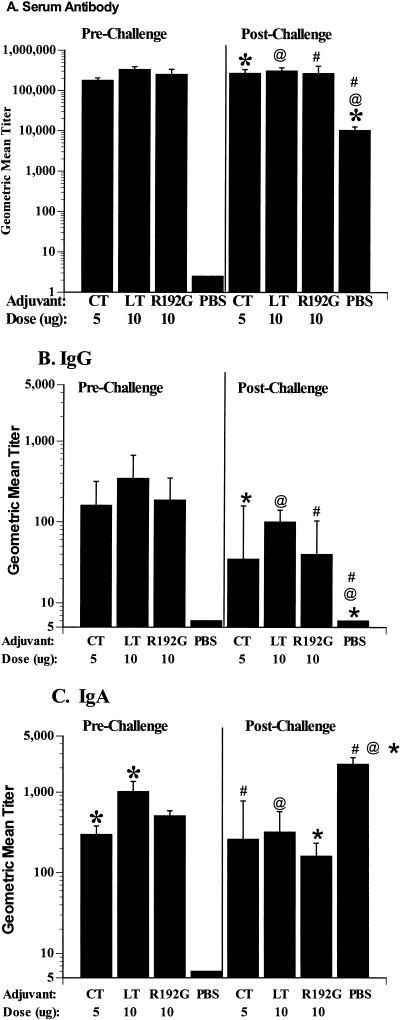
The choice of adjuvant did not affect the serologic or intestinal IgG immune response to 2/6-VLPs (Fig. 1A and B). All groups of mice inoculated with 2/6-VLPs had high, but equivalent, rotavirus-specific serum antibody geometric mean titer (GMT) responses regardless of the adjuvant used (range, 51,200 to 819,200) (P > 0.05, Kruskal-Wallis) (Fig. 1A). Twenty-eight of the 30 mice inoculated with 2/6-VLPs had intestinal IgG responses (Fig. 1B) regardless of the adjuvant used (range, 10 to 2,560), and the GMTs did not vary significantly between the groups (P > 0.05, Kruskal-Wallis).
FIG. 1.
Serum and intestinal antibody responses after intranasal immunization with 2/6-VLPs particles. Total (IgA, IgG, and IgM) serum (A) and intestinal IgG (B) and IgA (C) antirotavirus antibodies were measured in mice after three inoculations (72 days postinoculation) (left panels) with 2/6-VLPs administered intranasally with CT, LT, or LT-R192G, as indicated along the x axis, and 23 days postchallenge with ECwt (right panels). Antibody titers were measured for individual mice by ELISA, and results are plotted as the GMTs of the groups (n = 10 per group). Error bars represent one standard error of the mean. Significant differences in GMTs (P < 0.05), measured by Mann-Whitney U, within a panel are indicated (∗, @, and #).
The choice of adjuvant did affect the rotavirus-specific intestinal IgA responses (Fig. 1C). All mice inoculated intranasally with 2/6-VLPs had detectable rotavirus-specific intestinal IgA regardless of the adjuvant used. However, mice inoculated with 2/6-VLPs mixed with LT had extremely high IgA titers (range, 320 to 2,560) that were significantly higher than those of mice inoculated with 2/6-VLPs mixed with CT (range, 80 to 640) (P = 0.029, Mann-Whitney U). Mice inoculated with 2/6-VLPs with LT-R192G had an IgA GMT that was intermediate between those of groups receiving CT or LT (range, 80 to 1,280) but was not significantly different from that of either group (P > 0.05, Kruskal-Wallis).
2/6-VLPs administered intranasally with LT and LT-R192G induced significantly higher protection against rotavirus challenge than CT.
One month following the third vaccination, mice were challenged with 10 50% shedding doses (SD50) of wild-type murine virus (ECwt), obtained from Harry Greenberg (Stanford University Medical School, Palo Alto, Calif.) (7), following oral administration of 40 μl of 5% bicarbonate buffer. Protection from infection was determined (Fig. 2). Fecal samples from individual mice were collected daily for 11 days, starting on the day of challenge. Fecal samples were processed and stored as previously described (14). The level of viral antigen in fecal samples was measured by ELISA as previously described (14). Percent reduction in shedding was calculated for each animal, and the mean percent reduction was calculated for each vaccine group.
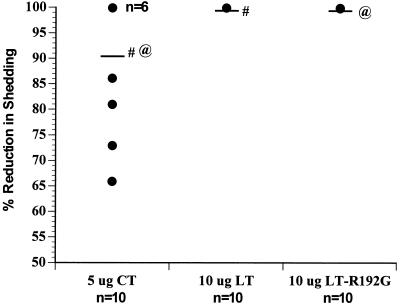
FIG. 2.
Protection from rotavirus challenge in mice receiving three intranasal inoculations of 2/6-VLPs. 2/6-VLPs were administered intranasally with CT, LT, or LT-R192G, as indicated along the x axis. Mice were challenged at 73 days postinoculation with 10 SD50 of ECwt rotavirus. Individual stool samples were collected daily and quantitated for levels of rotavirus antigen by ELISA. Virus shedding curves were plotted, and the area under the curve was calculated for each animal along with the mean for the group. Results are plotted as percent reduction in antigen shedding for each individual animal (•) (n = 10 per group) as well as the mean for each group (—), calculated by comparing the mean area under the curve of each vaccine group to the mean area under the curve of the control group (1 − mean area of vaccine group/mean area of control group). The number of mice with overlapping levels of protection is indicated on the graph (n = 6). Significant differences in protection, measured by the Mann Whitney U, are indicated (# and @).
All mice inoculated intranasally with 2/6-VLPs mixed with LT or LT-R192G were totally protected from challenge; there was no detectable virus shedding. The reduction in virus shedding in mice inoculated with 2/6-VLPs mixed with CT ranged from 66% to 100%, with mean protection for the group being 91%; this was significantly lower protection than that achieved using LT or LT-R192G (P = 0.013, Mann Whitney U).
Following challenge with murine rotavirus, all mice previously mock inoculated with PBS developed serum antibody and intestinal IgA but no intestinal IgG (Fig. 1). Mice inoculated with PBS and then challenged with ECwt murine rotavirus had equivalent levels of serum antibody and intestinal IgA but lower levels of intestinal IgG compared to mice inoculated intranasally with 2/6-VLPs with any adjuvant prior to challenge. After challenge, significant elevations in serum antibody and intestinal IgA GMTs were only observed in mice inoculated with PBS (P = 0.04, Wilcoxon signed ranks test). Significant decreases in intestinal IgG following challenge were only observed in mice inoculated with LT-R192G (P = 0.018, Wilcoxon signed ranks test). Following challenge, mice inoculated with PBS had significantly lower serum antibody and intestinal IgG GMTs than mice inoculated with VLPs with any adjuvant (P = 0.001 and P = 0.001, respectively, Kruskal-Wallis) but higher intestinal IgA titers (P = 0.007, Kruskal-Wallis).
The majority of human pathogens enter the body through interactions with mucosal surfaces. Therefore, the development of vaccines that induce effective mucosal immune responses is vital. We have recently shown that nonreplicating rotaviruslike particles administered orally or intranasally can protect mice against a live rotavirus challenge (14). Significantly higher levels of protection were achieved when the mucosal adjuvant CT was used in conjunction with the VLPs, showing the necessity of an effective mucosal adjuvant. CT can cause diarrhea in humans at very low doses and therefore cannot be considered for use as an adjuvant in humans. Other, potentially safer adjuvants have been developed; these include LT, mutants of CT, and mutants of LT. In this study, we compared the adjuvanticities of CT, LT, and mutant LT-R192G when administered intranasally with rotavirus 2/6- VLPs. Although ≥91% protective efficacy was achieved with 2/6-VLPs in all adjuvants, 2/6-VLPs administered with LT or the nontoxic mutant LT-R192G induced higher levels of protection against rotavirus challenge than did CT. However, the increased protective efficacy of VLPs with LT or LT-R192G may be due to the higher dose of LT or LT-R192G (10 μg) compared to that of CT (5 μg) or to the induction of different immune responses by the different adjuvants (16). Of note is the high level of protective efficacy achieved with the mutant LT-R192G, which was at least equivalent to the protection obtained with native CT and LT, indicating that LT-R192G is an effective mucosal adjuvant.
A number of mutants of LT and CT have been developed principally for use as homologous vaccines to prevent E. coli-induced diarrhea (4, 6, 9, 13, 16). Some of these mutant toxins have been examined to determine if they retain their ability to function as mucosal adjuvants. The two most widely studied are LT(S63K) (6) and LT-R192G (4). LT(S63K) is an active-site mutant and has been shown to lack in vitro ADP-ribosyltransferase and enterotoxicity and is completely devoid of activity in the mouse Y-1 adrenal tumor cell assay (a surrogate indicator for induction of cyclic AMP). LT-R192G is a protease site mutant and has been shown to lack in vitro ADP-ribosyltransferase and enterotoxicity, but LT-R192G retains a basal level of activity in the mouse Y-1 adrenal tumor cell assay. Importantly for the development of mucosal vaccines, LT-R192G retains the adjuvanticity of the native molecule. Both intranasal immunization of rotavirus VLPs and oral immunization of inactivated influenza virus with LT-R192G induced protection against live-virus challenge (11).
Nonreplicating VLPs administered mucosally can induce protection against rotavirus challenge. Nonreplicating vaccines are safer than replicating vaccines but are generally less immunogenic; therefore safe, mucosal adjuvants are needed. Mutants of CT and LT have been developed and shown to have adjuvant activity. Our results show that equivalent or better responses, either antibody or protection from rotavirus challenge, can be achieved with a nontoxic mutant of LT (LT-R192G). Mutants of LT and CT need to be tested further in animals and humans for safety and adjuvanticity but offer promising alternatives.
Acknowledgments
We thank Sue Crawford and Andrea Bertolotti-Ciarlet for production of the VLPs and Juan Alvarado for assistance in processing of fecal samples.
REFERENCES
- 1.Clements J D, Finkelstein R A. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect Immun. 1979;24:760–769. doi: 10.1128/iai.24.3.760-769.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clements J D, Hartzog N M, Lyon F L. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 3.Crawford S E, Labbe M, Cohen J, Burroughs M H, Zhou Y, Estes M K. Characterization of virus-like particles produced by the expression of rotavirus capsid proteins in insect cells. J Virol. 1994;68:5945–5952. doi: 10.1128/jvi.68.9.5945-5952.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dickinson B L, Clements J D. Use of Escherichia coli heat-labile enterotoxin as an oral adjuvant. In: Kiyono H, Ogra P L, McGhee J R, editors. Mucosal vaccines. San Diego, Calif: Academic Press, Inc.; 1996. pp. 73–87. [Google Scholar]
- 6.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domenghini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng N, Burns J W, Bracy L, Greenberg H B. Comparison of mucosal and systemic humoral immune responses and subsequent protection in mice orally inoculated with a homologous or a heterologous rotavirus. J Virol. 1994;68:7766–7773. doi: 10.1128/jvi.68.12.7766-7773.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franco M A, Greenberg H B. Role of B cells and cytotoxic T lymphocytes in clearance of and immunity to rotavirus infection in mice. J Virol. 1995;69:7800–7806. doi: 10.1128/jvi.69.12.7800-7806.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giannelli V, Fontana M R, Giuliani M M, Guangcai D, Rappuoli R, Pizza M. Protease susceptibility and toxicity of heat-labile enterotoxins with a mutation in the active site or in the protease-sensitive loop. Infect Immun. 1997;65:331–334. doi: 10.1128/iai.65.1.331-334.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Institute of Medicine. Diseases of importance in developing countries. Vol. 2. Washington, D.C: National Academy Press; 1986. New vaccine development. Establishing priorities; pp. 308–318. [PubMed] [Google Scholar]
- 11.Katz J M, Lu X, Galphin J C, Clements J D. Heat labile enterotoxin from Escherichia coli as an adjuvant for oral influenza vaccination. In: Brown L E, Hampson A W, Webster R G, editors. Options for the control of influenza. Vol. 3. New York, N.Y: Elsevier Science; 1996. pp. 292–297. [Google Scholar]
- 12.Levine M M, Kaper J B, Black R E, Clements M L. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol Rev. 1983;47:510–550. doi: 10.1128/mr.47.4.510-550.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lobet Y, Cluff C W, Cieplak W. Effect of site-directed mutagenic alterations on ADP-ribosyltransferase activity of the A subunit of Escherichia coli heat-labile enterotoxin. Infect Immun. 1991;59:2870–2879. doi: 10.1128/iai.59.9.2870-2879.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Neal C M, Crawford S E, Estes M K, Conner M E. Rotavirus virus-like particles administered mucosally induce protective immunity. J Virol. 1997;71:8707–8717. doi: 10.1128/jvi.71.11.8707-8717.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oplinger M L, Baqar S, Trofa A F, Clements J D, Gibbs P, Pazzaglia G, Bourgeois A L, Scott D A. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Safety and immunogenicity in volunteers of a new candidate oral mucosal adjuvant, LT(R192G), abstr. G-10; p. 193. [Google Scholar]
- 16.Yamamoto S, Takeda Y, Yamamoto M, Kurazono H, Imaoka K, Yamamoto M, Fujihashi K, Noda M, Kiyono H, McGhee J R. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrhoeagenicity but retain adjuvanticity. J Exp Med. 1997;185:1203–1210. doi: 10.1084/jem.185.7.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]