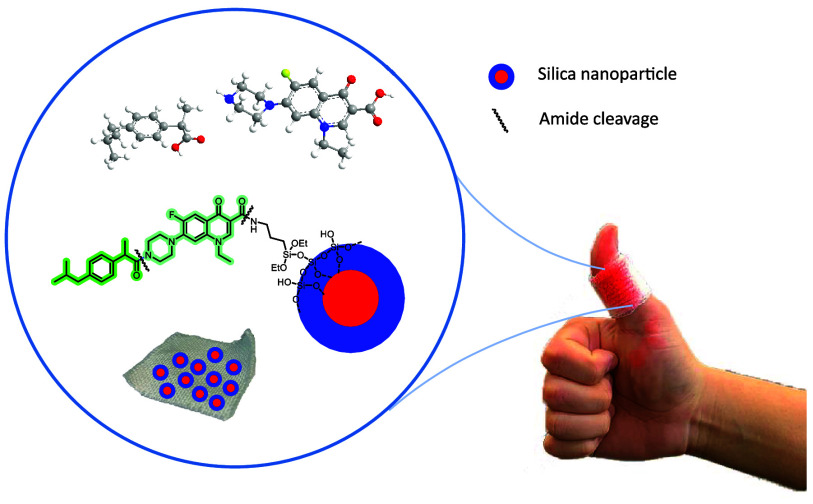
Abstract
Herein, we report the preparation of bifunctional silica nanoparticles by covalent attachment of both an anti-inflammatory drug (ibuprofen) and an antibiotic (levofloxacin or norfloxacin) through amide groups. We also describe the coating of cotton fabrics with silica nanoparticles containing both ibuprofen and norfloxacin moieties linked by amide groups by using a one-step coating procedure under ultrasonic conditions. The functionalized nanoparticles and cotton fabrics have been characterized using spectroscopic and microscopic techniques. The functionalized nanoparticles and textiles have been treated with model proteases for the in situ release of the drugs by the amide bond enzymatic cleavage. Topical dermal applications in medical bandages are expected, which favor wound healing.
Keywords: silica nanoparticles, anti-inflammatory, antimicrobial, cotton wound dressing, ibuprofen, norfloxacin, amide groups, enzymatic cleavage
1. Introduction
The microbial infection of wounds delays wound healing and increases patient discomfort, and it can also evolve into a more serious infection. Skin wounds requiring a long time to be repaired affect human health and may constitute a major clinical problem.1 The systemic administration of broad-spectrum antibiotics is the most common treatment for infected wounds. However, this indiscriminate use can affect skin microbiota and prompt the proliferation of multiresistant strains. Topical administration ensures the targeted release of antimicrobial agents at the infection site, minimizing the concentration needed to control the infection and consequently reducing systemic distribution and associated toxicity.2
The natural low-cost fabric cotton gauze is extensively used as a medical dressing in the clinical treatment of skin wounds due to its soft nature, moisture-absorbing ability, good biocompatibility, and excellent mechanical properties. However, due to its porous structure and the abundance of hydrophilic groups on its surface, it also creates an optimal environment for the growth and survival of a large variety of bacteria, which usually results in inflammation and excessive immune response and delays wound healing. Therefore, with increasing awareness of healthcare-associated infections, there is a strong demand to develop functional medical dressings endowed with a broad spectrum of anti-inflammatory and antibacterial activities to mitigate the risk of wound infection, facilitate wound healing, and expedite cicatrization.1−3
Since the 1990s, natural anti-inflammatory and antibacterial agents extracted from plants have drawn growing interest in medicine. Polyphenolic compounds, including gallic acid, are particularly well-known and have been widely used for numerous applications. Taking advantage of their excellent antibacterial, antioxidant, and anti-inflammatory properties, gallic acid has been encapsulated in cotton textiles functionalized with cyclodextrin–hydroxypropyl methyl cellulose-based hydrogel. The resulting composite wound dressing was capable of releasing gallic acid in vitro, showing enhanced biological properties.4 Following a different strategy, Liu’s group covalently immobilized plant-derived gallic acids on an amino-rich premodified cotton gauze (after treatment with (3-aminopropyl)triethoxysilane) by reacting their phenol groups through Schiff’s base formation.5 Other relevant examples of functional medical gauzes include a chitosan-based hydrogel encapsulating red cabbage extract (RCE), prepared and used in therapeutic pH-sensitive wound dressing. The preparation consisted of the reaction of RCE, methacrylic -chitosan, and N,N-methylene-bis-acrylamide in the presence of potassium persulfate. The reactive mixture was then cross-linked to a piece of gauze.6 El-Sayed and co-workers reported the microencapsulation of anti-inflammatory and antimicrobial-rich fractions from marine macroalgae and seagrass in sodium alginate or meypro gum. They used these extracts to finish cotton fabrics by dip coating technique.7
Nanomaterials based on the use of abundant natural compounds are appreciated in medicinal applications and have been also used in anti-inflammatory and antibacterial agents release.8 For instance, mesoporous silica SBA-15 was functionalized with 3-aminopropyltrimethoxysilane (NH2–SBA-15) and then added into a solution of poly(vinyl alcohol) and Curcumin. Nanofibers were then fabricated by electrospinning and studied for a skin wound healing application. Curcumin, a plant-derived phytochemical hydrophobic product, is recognized for its anti-inflammatory and antimicrobial properties. The incorporation of Curcumin into the highly porous and biocompatible amine-functionalized mesoporous silica improved its solubility, facilitating sustained drug release.9 In 2022, Zohoori’s group extracted the keratin of hedgehog spines and doped the extract with Harmaline and Ginkgo Biloba, and later, the mixture was electrospun on the cotton surface to produce multifunctional band-aid. These nanofibers exhibited anti-inflammatory and bactericidal effects.10 Nitric oxide-propelled nanomotors were prepared for endotoxin removal and bacterial biofilm elimination to treat infected burn wounds. First, silica layers were deposited on Fe3O4 nanoparticles (Fe3O4 NPs) using tetraethyl orthosilicate (TEOS) and 3-aminopropyl triethoxysilane. Then, a coupling reaction of residual amino groups with 3-mercaptopropionic acid gave rise to thiolated Fe3O4 NPs. These nanoparticles (NPs) were partially coated with polydopamine (PDA) layers. Polymyxin B (PMB) was conjugated on PDA, and then, NO donors were conjugated with –SH groups to build nitric oxide-propelled nanomotors.11
Silver nanoparticles, one of the most used commercial antibacterial nanomaterials, have found extensive application in cotton dressings to confer effective antiseptic properties. Silver nanoparticles embedded in zwitterionic poly(carboxybetaine-co-dopaminemethacrylamide) copolymer have been anchored onto cotton fabrics through interaction forces that include both covalent and noncovalent bonds, which prevented leaching. In vivo wound healing assay confirmed that these Ag nanoparticles effectively inhibit the wound infection and reduce the inflammatory response.12 Alisir’s group described the fabrication of poly(lactic acid) nanofibers embedded with a silver diclofenac complex with 2-methylimidazole. The unique combination of diclofenac, silver(I), and 2-methylimidazole in a single product ([Ag(mim)2](dicl)) accelerated the healing process by endowing the wounded skin with protection against inflammation, bacterial, and fungal infections.13
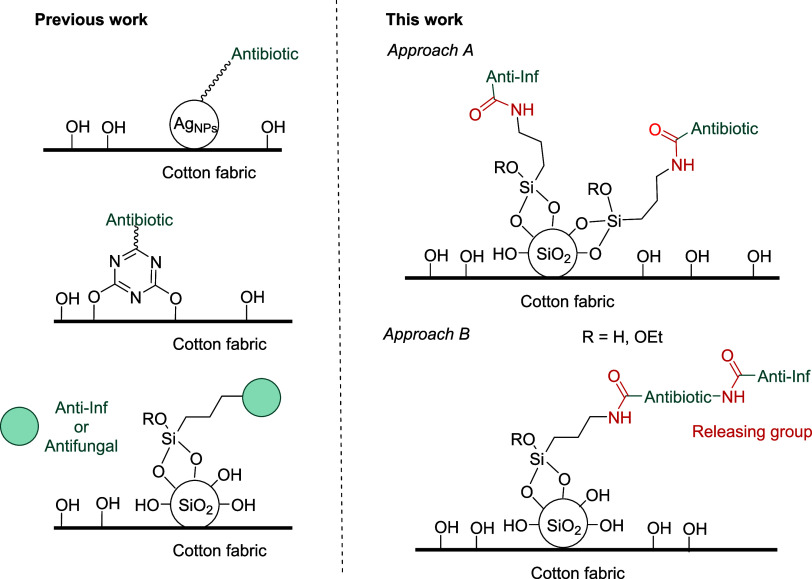
As part of our research program centered on the design and application of cotton fabrics for medical applications (Figure 1), in 2019, we prepared silver nanoparticles (Ag NPs) with appropriate modified antibiotics, which were applied on cotton fabrics.14 Later, we tethered antibiotic drugs through a triazine moiety onto the surface of cotton fabrics, thus minimizing the leaching of the bioactive molecule (Figure 1). The bactericidal activity of the functionalized fabrics was demonstrated, and of note, no release of the covalent-linked antibiotic was needed.15
Figure 1.
Previous work related to our program centered on the design and application of cotton fabrics for medical applications. This work: Approaches for the coating of cotton fabrics with anti-inflammatory and antibacterial silica nanoparticles.
Following our program, we then prepared anti-inflammatory cotton fabrics by direct covalent attachment of anti-inflammatory (anti-inf) silylated drugs derivatives (salicylic acid, ibuprofen and diclofenac) onto the surface of cotton fabrics through an amide group.16 In addition, as silica nanoparticles present significant advantages in biomedical applications, we synthesized functionalized silica nanoparticles using the same drug derivatives through an amide group and used them for the coating of textiles (Figure 1, bottom left).16 It should be noted that in most of the published studies for drug delivery, the cargo is encapsulated and physically adsorbed into the pores, while the covalent attachment of the drug to the surface of the nanoparticle is a less used strategy. During inflammation, many leukocytes exit the blood vessels and migrate toward the injured site (chemotaxis).17 As a result, the phagocytic activity and the release of proteases, peroxidases, and oxygen-reactive species are increased, bolstering the breakdown and elimination of the external agents. Thus, the protease activity in a wound may be high and has been measured using an appropriate substrate, usually consisting of a peptide linked with a fluorophore or chromophore moiety through an amide bond.18 The detection of the chromogenic or fluorogenic moiety is thus evidence supporting that proteases can cleave amide bonds.19,20 In fact, when these functionalized nanoparticles and textiles are treated with proteases and leukocytes of animal origin, an in situ release of the drug takes place by the selective enzymatic cleavage of the amide bond. In contrast to the case of antibiotics reported previously, the release of the anti-inflammatory is necessary for topical cutaneous applications.
Following an analogous approach, in 2022, classical antifungal bioactive molecules were also used with success in the functionalization of silica nanoparticles (Figure 1).21 We described the covalent connection of silylated derivatives of the topical antifungal agent miconazole onto silica nanoparticles. Employing grafting and co-condensation procedures, we synthesized functionalized mesoporous or dense nanoparticles. The coating of cotton fabrics with these antifungal-functionalized silica nanoparticles was conducted under ultrasonic conditions, resulting in notably high effectiveness toward Trichophyton mentagrophytes and Candida albicans.21
Pursuing our interest in the design and application of cotton fabrics for medical applications, we planned to elaborate bifunctional silica nanoparticles with both antibiotic and anti-inflammatory properties that could be used for coating cotton gauzes or strips toward wound healing applications. Thus, herein, we describe the preparation of silica nanoparticles covalently functionalized with carboxyl-containing antibiotics and carboxyl-containing nonsteroidal anti-inflammatory drugs through amide bonds (see approach A, Figure 1). In addition, we report the preparation of silica nanoparticles derived from a silylated monomer containing both antibiotic and anti-inflammatory moieties in the same organic backbone (see approach B, Figure 1). Finally, the modification of cotton fabrics with these bifunctional silica nanoparticles is described (from Approach B) by one-step coating. We expect potential topical applications in chronic skin wounds.
2. Results and Discussion
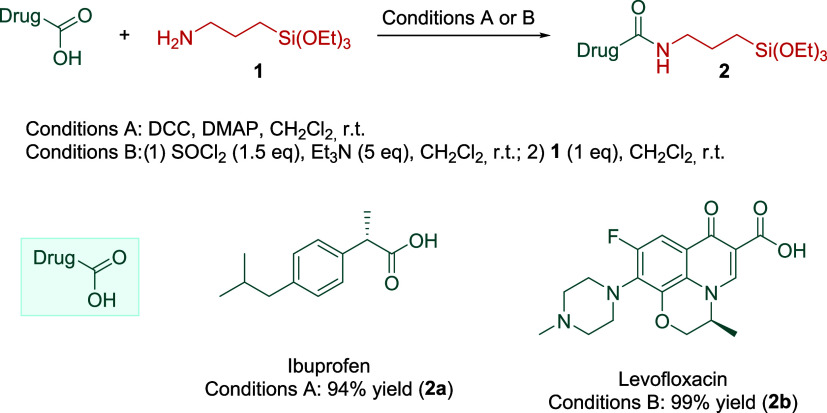
A topical nonsteroidal anti-inflammatory agent, ibuprofen, and common fluoroquinolone antibiotic drugs levofloxacin and norfloxacin were selected as model medicinally relevant molecules. A carboxylic acid group is the common moiety in all of the structures, which is necessary to form a covalent amide bond with the silylated linker (Scheme 1). In addition, the covalent grafting onto silica nanoparticles will prevent leaching of the bioactive molecule. On the other hand, the amide group is crucial for the drug release for cutaneous uses via enzymatic cleavage of the peptide bond.
Scheme 1. Preparation of Silylated Derivatives 2a and 2b.
The synthesis of silylated derivatives 2a–b was done following two different methodologies indicated in Scheme 1. Ibuprofen was mixed with N,N′-dicyclohexylcarbodiimide (DCC), (triethoxysilyl)propylamine, and a catalytic amount of 4-(dimethylamino)pyridine (DMAP) in CH2Cl2 at room temperature affording 2a in 50% yield. In the case of the silylated derivative of levofloxacin, we obtained product 2b via acyl chloride synthesis followed by the addition of a stoichiometric amount of (triethoxysilyl)propylamine in 99% yield.
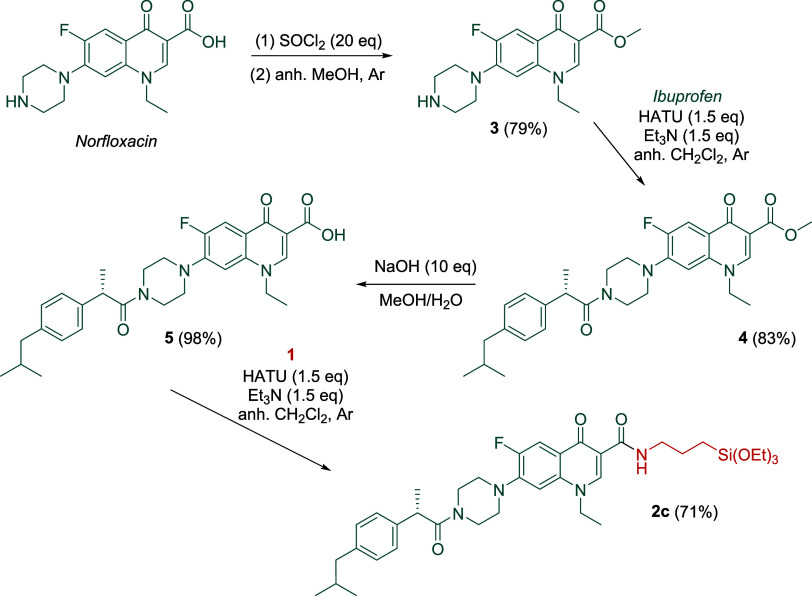
On the other hand, derivative 2c was synthesized following the steps summarized in Scheme 2. In this approach B (Figure 1), norfloxacin was selected due to the nucleophilic secondary amine present in the piperazine ring, which allows covalent linking of it to the anti-inflammatory ibuprofen derivative via an amide bond (step 2, Scheme 2). First, the norfloxacin methyl ester 3 was prepared in 79% yield through the treatment of its acyl chloride derivative. Then, ibuprofen was tethered to 3 using hexafluorophosphate azabenzotriazole tetramethyl uronium (HATU) as a coupling reagent in the presence of triethylamine to form 4 in 83% yield. After basic ester hydrolysis, the coupling between acid 5 and silylated amine 1 was carried out with HATU, yielding the silylated norfloxacin derivative 2c in 71% yield. Thus, compound 2c was synthesized in 4 steps with a 46% overall yield.
Scheme 2. Preparation of Silylated Derivative 2c.
Then, the covalent connection of antibiotics and anti-inflammatory drugs to silica nanoparticles by sol–gel condensation methods was conducted. Materials SiO2@Ibu + Leflox and SiO2@Norflox-Ibu (see Scheme 3 for the structure of these nanomaterials) were prepared by co-condensation of silylated precursors 2a–c with tetraethyl orthosilicate (TEOS) using a 28% ammonium hydroxide-ethanol solution as a promoter (Scheme 3 and Table 1). For the synthesis of SiO2@Ibu + Leflox, different molar ratios TEOS/2a/2b/EtOH/NH3/H2O were used, maintaining a ratio TEOS/2a + 2b (20:1). First, a 50:50 ratio of silylated drug derivatives 2a:2b was assayed, obtaining modified silica nanoparticles that exhibited a low ζ-potential value, which was indicative of low stability (Table 1, entry 1). In addition, transmission electron microscopy (TEM) images showed a high level of nanoparticle aggregation, presumably due to the high concentration of the 2b. Thus, the molar ratio of 2a:2b was increased to 75:25. In this case, TEOS (16.97 mmol), the silylated ibuprofen derivative 2a (0.63 mmol), and the silylated levofloxacin derivative 2b (0.21 mmol) were dissolved in absolute EtOH. Then, an ammonium hydroxide-ethanol solution was added. As shown in Table 1 (entry 2), the higher ζ-potential for the new sample indicated better stability. Then, we centered our attention on approach B (Figure 1) using the same procedure for the sol–gel process but now using the monosilylated monomer 2c. Mixtures of TEOS/2c/EtOH/NH3/H2O were used at three different ratios TEOS/2c (20:1, 30:1, and 40:1). The functionalized silica NPs showed high negative values of ζ-potential, from −65.5 to −72.3 mV, in agreement with residual deprotonated silanol groups and no protonation of the amino group, and indicative of high stability (Table 1, entries 3–5).
Scheme 3. Preparation of Functionalized Silica Nanoparticles.
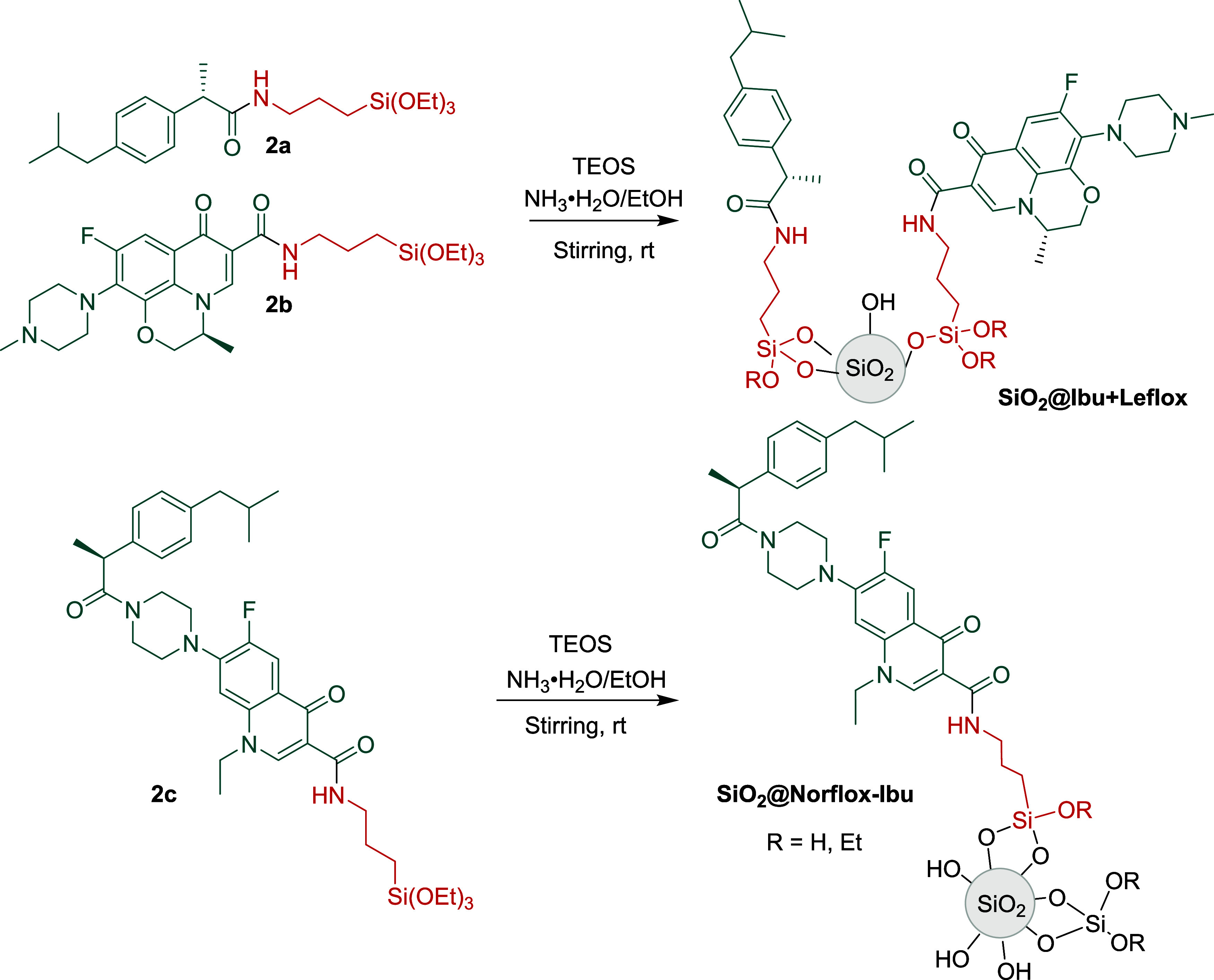
Table 1. Characterization Data of Functionalized Silica Nanoparticles.
| particle
size (nm) |
||||
|---|---|---|---|---|
| material | drug loading (mmol/g)a | TEM | DLSb | ζ-potential (mV) |
| SiO2@Ibu + Leflox (20:0.5:0.5) | 0.23:0.23 | / | 2733 | 5.7 |
| SiO2@Ibu + Leflox (20:0.75:0.25) | 0.075:0.025 | 317 ± 22 | 426 | –41.0 |
| SiO2@Norflox-Ibu (20:1) | 0.15 | 686 ± 32 | 836 | –65.5 |
| SiO2@Norflox-Ibu (30:1) | 0.13 | 540 ± 30 | 658 | –69.9 |
| SiO2@Norflox-Ibu (40:1) | 0.15 | 389 ± 22 | 486 | –72.3 |
Estimated from the N elemental analysis.
Hydrodynamic diameters.
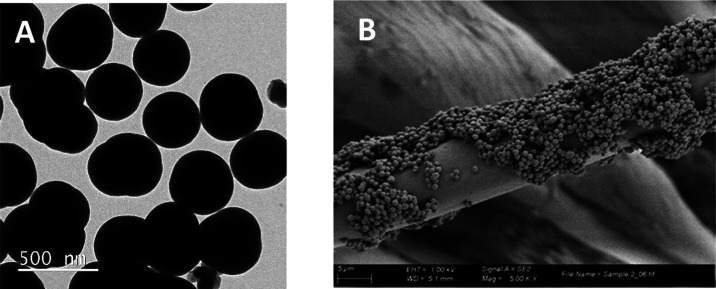
The TEM analysis of materials SiO2@Ibu + Leflox and SiO2@Norflox-Ibu confirmed the nanometric size and features of the functionalized nanoparticles, showing dense spherical morphologies with sizes from 317 to 686 nm (see Figure 2A for a representative image of SiO2@Norflox-Ibu (TEOS/2c 40:1); see also SI). Dynamic light scattering measurements (DLS) were consistent with the size observed via transmission electron microscopy (TEM) for the corresponding dried nanoparticles, considering the likely adsorption of water molecules onto the nanoparticle surface (Table 1).
Figure 2.
(A) TEM of SiO2@Norflox-Ibu (TEOS/2c 40:1). (B) SEM of Fabric-SiO2@Norflox-Ibu (TEOS/2c 40:1).
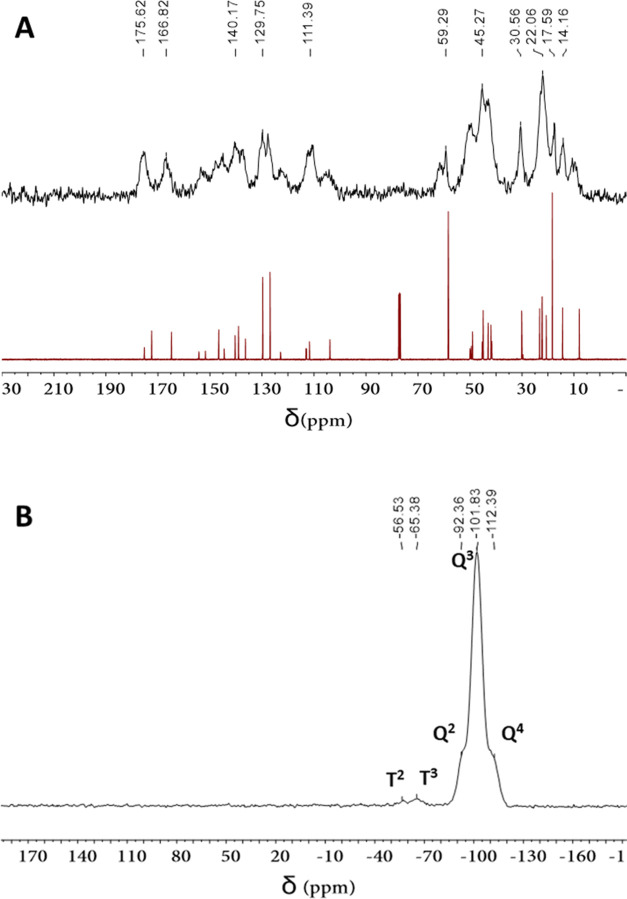
The presence of the organic moiety in the functionalized NPs was confirmed by solid-state 29Si and 13C nuclear magnetic resonance (NMR) spectra. Thus, a clear match between the 13C NMR spectrum of 2c in solution and that of SiO2@Norflox-Ibu (TEOS/2c 20:1) in the solid state is observed, supporting the integrity of the organic framework (Figure 3A). The 29Si cross-polarization magic-angle-spinning nuclear magnetic resonance (CP MAS NMR) spectrum of SiO2@Norflox-Ibu (TEOS/2c 40:1) showed two groups of chemical shifts: T units (−56.5 and −65.4 ppm) derived from organosilane 2c and Q units (−92.4, −101.8, and −112.4 ppm) derived from TEOS (Figure 3B). The presence of T signals is also an indication that the Si–C bond of the precursors was maintained during the sol–gel co-condensation process.
Figure 3.
(A) 13C CP MAS NMR spectrum of SiO2@Norflox-Ibu (TEOS/2c 20:1) and 13C NMR spectrum of 2c. (B) 29Si CP MAS NMR spectrum of SiO2@Norflox-Ibu (TEOS/2c 40:1).
The drug content in the NPs was inferred from the nitrogen elemental analysis (Table 1). For the materials SiO2@Ibu + Leflox, the given values are based on the initial amounts of silylated monomers and the estimation that 2a and 2b have the same condensation rate. For the nanomaterial SiO2@Ibu + Leflox (20:0.75:0.25), the drug content found was quite low (Table 1, entry 2). The materials SiO2@Norflox-Ibu contain both drug moieties in molecule 2c, and thus, the values correspond to the whole organic moiety in the materials.
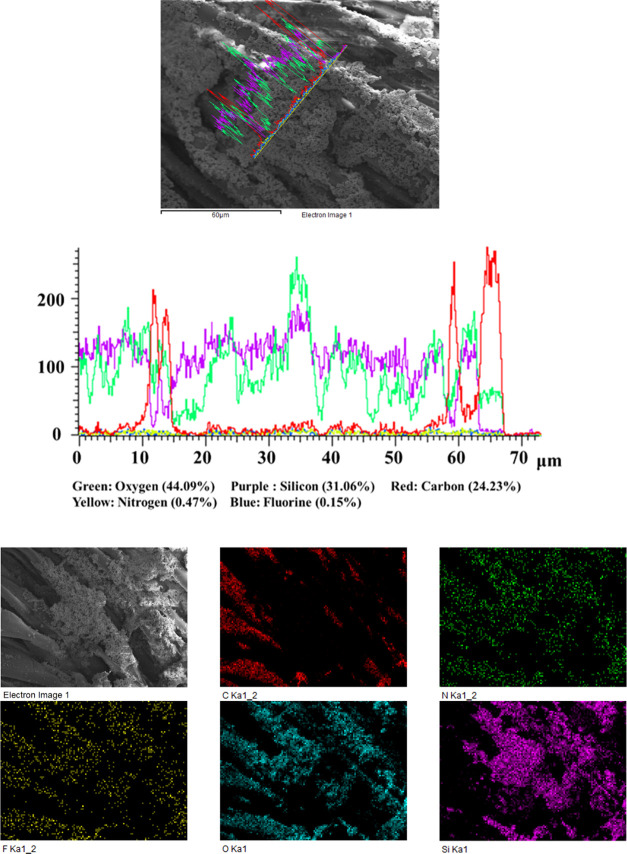
With the drug-functionalized silica NPs in hand, we proceeded with the loading of cotton fabrics with these nanoparticles by the one-step coating method. As we have mentioned, for SiO2@Ibu + Leflox, a low proportion of antibiotic (ratio Ibu/Leflox 75:25) was required to afford stable silica nanoparticles, and the total amount of drugs in the final nanomaterial was quite low according to the elemental analysis. On the other hand, the precise amount of ibuprofen vs levofloxacin in the silica matrix cannot be estimated without assuming the same reactivity in sol–gel process for both 2a and 2b. For these reasons, we decided to perform the coating only with SiO2@Norflox-Ibu materials. As depicted in Table 1, the drug loading in the nanoparticles did not significantly vary with the molar ratio TEOS/2c. For that reason, the coating solution was obtained by hydrolysis and co-condensation of TEOS with organosilane 2c (molar ratio 40:1) in aqueous ammonia and ethanol under stirring. Without any further isolation, the obtained milky solution was ultrasonicated for 30 min, producing a homogeneous suspension. A piece of cotton was immersed in this suspension, and the whole system was ultrasonicated for an additional half an hour. Then, after removal of the cotton fabric from the solution, it was washed with distilled water and dried at 120 °C for 1 h. By scanning electron microscopy (SEM) analysis, the presence of abundant silica nanoparticles was observed on the surface of cotton fabrics loaded with SiO2@Norflox-Ibu (TEOS/2c 40:1) by one-step coating (Figure 2B). The textile piece gained 10 mg after coating with NPs according to gravimetric analysis. The chemical composition of the surface of the modified fabrics was analyzed by energy-dispersive X-ray spectroscopy (EDX) (Figure 4). As expected, the EDX analysis exhibited peaks for the elements C, O, N, Si, and F. The peaks corresponding to C, N, and F indicate the presence of the organic moiety in silica nanoparticles.
Figure 4.
EDX linear scanning and element mapping of Fabric-SiO2@Norflox-Ibu (TEOS/2c 40:1).
The anti-inflammatory drug must be released from the fabric to be in contact with the damaged area and locally modulate the inflammation of the wounds.16 On the contrary, we have previously demonstrated that some fluoroquinolone derivatives covalently linked to the surface of a cotton fabric exhibited excellent antimicrobial activity for S. aureus being able to reduce preformed Staphylococcus aureus biofilms. Some experiments were carried out to demonstrate that these covalently attached microbicidal fluoroquinolone derivatives do not leach from the fabric surface, and thus, they do not contribute to the development of resistance.15
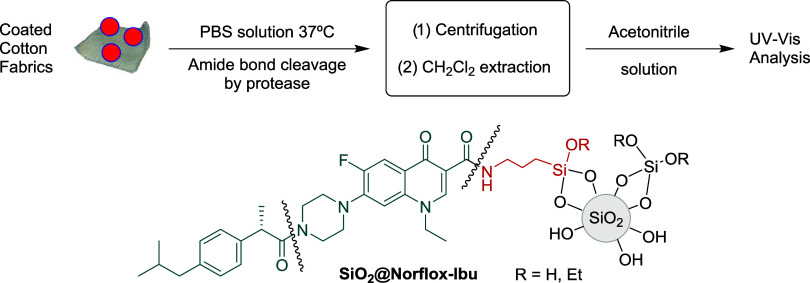
With the SiO2@Norflox-Ibu (TEOS/2c 40:1) and Fabric-SiO2@Norflox-Ibu (TEOS/2c 40:1) in hand, the release of the bioactive components was studied, assaying proteases such as trypsin, papain, and proteinase K. These enzymes would produce the cleavage of the amide bonds (Figure 5), giving rise to ibuprofen and norfloxacin release. First, the detachment from nanoparticles SiO2@Norflox-Ibu (TEOS/2c 40:1, 20 mg) was tested (Table 2, entries 1–3). The experiments were carried out in phosphate-buffered saline (PBS) buffer (pH = 7.4, 0.2 M, 2 mL) at 37 °C under stirring for 24 h. The nanoparticles were separated by centrifugation, and the supernatant was extracted with dichloromethane. After the removal of the organic solvent, the residue was dissolved in acetonitrile. The resulting solution was analyzed by ultraviolet–visible (UV–vis) spectroscopy, and the amount of released drugs could be quantified by analyzing the intensity of the absorption peaks at λ = 220 nm for ibuprofen and λ = 285 nm for norfloxacin according to the corresponding calibration curves. The percentage of release was increased by performing a 48 h treatment (Table 2, entry 4). However, the low solubility of norfloxacin at neutral pH made the quantitative measurement inaccurate. As an alternative, we centered our attention on the qualitative detection of norfloxacin by changing the post-treatment analysis. Thus, 2 mL of glacial acetic acid was added to the mixture after protease treatment. After 30 min stirring, the mixture was centrifugated at 12,000 rpm. The supernatant was separated, diluted with water to 10 mL, analyzed by UV–vis, and compared with an acidic aqueous solution of commercial norfloxacin (see the Supporting Information). Finally, we treated a 3 cm × 3 cm cotton piece of Fabric-SiO2@Norflox-Ibu (TEOS/2c 40:1) with papain in PBS buffer (pH = 7.4, 0.2 M, 5 mL) at 37 °C under stirring for 48 h (Table 2, entry 5). Moreover, the supernatants of papain-treated SiO2@Norflox-Ibu (TEOS/2c 40:1) and Fabric-SiO2@Norflox-Ibu (TEOS/2c 40:1) were used to test the inhibitory effects of released norfloxacin on the growth of S. Aureus. Indeed, a small area of inhibition of bacterial growth could be observed (Supporting Information). Thus, this is an indication that norfloxacin has been released from the modified silica nanoparticles and fabrics upon enzymatic hydrolysis of the amide bond.
Figure 5.
Procedure for the drug-release experiment.
Table 2. Release of Drugs from Nanoparticles and Coated Fabrics.
| release
(%) |
|||||
|---|---|---|---|---|---|
| entry | sample | protease | time (h) | norfloxacin | ibuprofen |
| 1a | SiO2@Norflox-Ibu (TEOS/2c 40:1) | trypsin | 24 | 0.12 ± 0.01 | 4.5 ± 0.3 |
| 2a | SiO2@Norflox-Ibu (TEOS/2c 40:1) | papain | 24 | 0.26 ± 0.05 | 7.1 ± 0.4 |
| 3a | SiO2@Norflox-Ibu (TEOS/2c 40:1) | proteinase K | 24 | 0.15 ± 0.05 | 5.8 ± 0.5 |
| 4a | SiO2@Norflox-Ibu (TEOS/2c 40:1) | papain | 48 | c | 11.1 ± 0.2 |
| 5b | Fabric-SiO2@Norflox-Ibu (TEOS/2c 40:1) | papain | 48 | c | 43.5 ± 2.5 |
Experiments with 20 mg of NPs and 4 × 10–4 mmol of protease.
Experiment with 10 mg of NPs in the coating and 1 × 10–3 mmol of papain.
Qualitative detection. See text.
3. Conclusions
Herein, we have presented the preparation of silica nanoparticles covalently functionalized with both a carboxyl-containing antibiotic (levofloxacin) and a nonsteroidal anti-inflammatory drug (ibuprofen) through amide bonds (SiO2@Ibu + Leflox). We also synthesized silica nanoparticles derived from a monosilylated monomer bearing both carboxyl-containing antibiotic (norfloxacin) and anti-inflammatory moieties (ibuprofen) in the same molecule, linked by amide bonds (SiO2@Norflox-Ibu). All of these nanoparticles were obtained by sol–gel methodologies under basic conditions and by co-condensation of the corresponding monomers with tetraethoxysilane. These dense nanoparticles were characterized by transmission electron microscopy, dynamic light scattering, ζ-potential, solid-state 29Si and 13C NMR spectroscopy, and elemental analysis. Of note, cotton fabrics have been coated with drug-functionalized nanoparticles SiO2@Norflox-Ibu by a one-step procedure under ultrasonic conditions. The modified textiles Fabric-SiO2@Norflox-Ibu have been characterized by scanning electron microscopy, energy-dispersive X-ray spectroscopy, and element mapping. The corresponding drugs were released in situ through enzymatic cleavage of the amide bonds in SiO2@Norflox-Ibu and Fabric-SiO2@Norflox-Ibu by treatment with proteases (UV–vis analysis).
In contrast to some sophisticated procedures, our method is operationally simple. Ibuprofen and norfloxacin are classical drugs on the market that have been widely used, and therefore, all toxicity parameters have been thoroughly studied. Of note, in this study, their antimicrobial and anti-inflammatory properties are not compromised after their incorporation into the composite network because the enzymatic release does not alter their initial structure. On the other hand, silica-based nanoparticles have received special attention as a biocompatible form of silica.
Topical applications for medical gauzes provided with antibiotic and anti-inflammatory properties to prevent infection and accelerate wound healing in chronic cutaneous wounds are expected.
4. Experimental Section
4.1. General Procedure for the Preparation of SiO2@Ibu + Leflox
TEOS (2.08 g, 10.0 mmol), the silylated anti-inflammatory ibuprofen 2a (0.25 mmol), and the silylated antibiotic levofloxacin 2b (0.25 mmol) were dissolved in 25 mL of absolute EtOH. Then, an ethanol solution of ammonium hydroxide (6 mL of 28% NH3·H2O in 25 mL EtOH) was added. The resulting mixture was magnetically stirred (1400 rpm) at rt for 12 h, after which the nanoparticles were isolated by centrifugation (13,500 rpm) at rt and washed with ethanol until neutral pH was reached. The resulting solid was then sequentially washed with Mili-Q water and 96% ethanol and dried under a vacuum for several hours. For other molar ratio details, see the SI.
4.2. General Procedure for the Preparation of SiO2@Norflox-Ibu
TEOS (2.08 g, 10.0 mmol) and the silylated derivative 2c (0.5, 0.33, and 0.25 mmol) were dissolved in 25 mL of absolute EtOH. Then, an ethanol solution of ammonium hydroxide was added (6 mL of 28% NH3 H2O in 25 mL of EtOH). The mixture was magnetically stirred (1400 rpm) at rt for 12 h. The working-up procedure was the same as described in the previous paragraph. We obtained SiO2@Norflox-Ibu with different ratios of TEOS and silylated drug 2c. For other molar ratio details, see the SI.
4.3. Procedure for the Preparation of Cotton Fabrics Coated with SiO2@Norflox-Ibu (Fabric-SiO2@Norflox-Ibu 40:1)
TEOS (2.08 g, 10.0 mmol) and the silylated derivative 2c (0.25 mmol) were dissolved in 25 mL of absolute EtOH. Then, an ethanol solution of ammonium hydroxide was added (6 mL of 28% NH3 H2O in 25 mL of EtOH). The mixture was magnetically stirred (1400 rpm) at rt for 12 h. Without any further isolation, the obtained milky solution was ultrasonicated for 30 min to produce a homogeneous suspension in which a piece of clean cotton fabric (3 cm × 3 cm) was immersed. The whole system was ultrasonicated for an additional 30 min. Then, the cotton textile was removed from the solution, washed with distilled water, and finally dried at 120 °C for 1 h.
4.4. Treatment of Functionalized Silica Nanoparticle SiO2@Norflox-Ibu (40:1) with Proteases for Quantitative Analysis by UV–vis
The functionalized silica nanoparticles SiO2@Norflox-Ibu (40:1) (20 mg) were dispersed in phosphate-buffered saline (PBS, pH 7.4) (2 mL) in an Eppendorf tube. Subsequently, the corresponding protease (see Table 2) was added, and the mixture was gently stirred at 37 °C for the given time (orbital shaker). The concentration of protease was 0.2 mM. Following incubation, the nanoparticles were removed by centrifugation, and the supernatant was extracted with dichloromethane (10 × 3 mL). The solvent was then evaporated under vacuum from the combined organic phases. The resulting residue was dissolved in acetonitrile, and the solution was analyzed by UV–vis (ibuprofen λ = 220 nm̧, norfloxacin λ = 285 nm). See page S33 in the SI.
4.5. Treatment of Functionalized Silica Nanoparticle SiO2@Norflox-Ibu (40:1) with Proteases for Qualitative Detection of Norfloxacin
The functionalized silica nanoparticles SiO2@Norflox-Ibu (40:1) (20 mg) were dispersed in phosphate-buffered saline (PBS) (2 mL) in an Eppendorf tube, the corresponding protease was added, and the mixture was gently stirred at 37 °C for 48 h (orbital shaker). The concentration of protease was 0.2 mM. Glacial acetic acid (2 mL) was added, and the mixture was stirred for 30 min. The nanoparticles were separated by centrifugation, the supernatant was diluted to 10 mL, and the aqueous solution was analyzed by UV–vis. The commercial norfloxacin aqueous solution (0.24 mmol/L) was prepared with the same amount of glacial acetic acid and was analyzed by UV–vis (λ = 285 nm). See page S32 in the SI.
4.6. Treatment of Cotton Fabrics Coated with Functionalized Silica Nanoparticles with Proteases
A piece of Fabric-SiO2@Norflox-Ibu (40:1) (3 cm × 3 cm) was cut into small pieces and dispersed in phosphate-buffered saline (PBS) (5 mL) in an Eppendorf tube, the corresponding protease was added, and the mixture was gently stirred at 37 °C for 48 h (orbital shaker). The concentration of protease was 0.2 mM. After the removal of cotton fabrics, the supernatant was extracted with dichloromethane (25 × 3 mL). The solvent was then evaporated under vacuum from the combined organic phases. The resulting residue was dissolved in acetonitrile, and the solution was analyzed by UV–vis.
Acknowledgments
We gratefully acknowledge support for this work under grants RTI2018-097853–B-I00, PID2021-124916NB-I00, RED2022-134287-T, RYC2019-027423-I, and PID2021-128496OB-I00 from the Ministerio de Ciencia, Innovación y Universidades (MICINN) (Spain); SGR2017-0465, and SGR2021-00064 from AGAUR-Generalitat de Catalunya; China Scholarship Council (CSC) (predoctoral scholarship to M.L., No. 202006560005).
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsami.4c00383.
General considerations, the general procedure for the preparation of 2a–c as well as spectroscopic data, and characterization data of functionalized silica nanoparticles and cotton fabrics (PDF)
Author Contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Ministerio de Ciencia, Innovación y Universidades (MICINN) of Spain (Projects RTI2018–097853–B-I00, PID2021–124916NB-I00, RED2022–134287-T, RYC2019–027423-I, and PID2021–128496OB-I00). AGAUR-Generalitat de Catalunya (Projects SGR2017–0465 and SGR2021–00064). China Scholarship Council (CSC) (predoctoral scholarship to Ming Liu, CSC No. 202006560005).
The authors declare no competing financial interest.
Supplementary Material
References
- Pierce G. F.; Mutsoe T. A. Pharmacologic enhancement of wound healing. Annu. Rev. Med. 1995, 46, 467–481. 10.1146/annurev.med.46.1.467. [DOI] [PubMed] [Google Scholar]
- Miraftab M.Wound Care Materials: An Overview. In Medical and Healthcare Textiles; Anand S. C.; Kennedy J. F.; Miraftab M.; Rajendran S., Eds.; Woodhead, 2010; pp 193–197. [Google Scholar]
- Chen S.; Li A.; Wang Y.; Zhang Y.; Liu X.; Ye Z.; Gao S.; Xu H.; Deng L.; Dong A.; Zhang J. Janus polyurethane sponge as an antibiofouling, antibacterial, and exudate-managing dressing for accelerated wound healing. Acta Biomater. 2023, 171, 428–439. 10.1016/j.actbio.2023.09.015. [DOI] [PubMed] [Google Scholar]
- Pinho E.; Calhelha R. C.; Ferreira I. C. F. R.; Soares G. Cotton-hydrogel composite for improved wound healing: Antimicrobial activity and anti-inflammatory evaluation—Part 2. Polym. Adv. Technol. 2019, 30, 863–871. 10.1002/pat.4519. [DOI] [Google Scholar]
- Lang S.; Chen C.; Xiang J.; Liu Y.; Li K.; Hu Q.; Liu G. Facile and robust antibacterial functionalization of medical cotton gauze with gallic acids to accelerate wound healing. Ind. Eng. Chem. Res. 2021, 60, 10225–10234. 10.1021/acs.iecr.1c01833. [DOI] [Google Scholar]
- Arafa A. A.; Nada A. A.; Ibrahim A. Y.; Sajkiewicz P.; Zahran M. K.; Hakeim O. A. Preparation and characterization of smart therapeutic pH-sensitive wound dressing from red cabbage extract and chitosan hydrogel. Int. J. Biol. Macromol. 2021, 182, 1820–1831. 10.1016/j.ijbiomac.2021.05.167. [DOI] [PubMed] [Google Scholar]
- El-Rafie H. M.; El-Rafie M. H.; AbdElsalamc H. M.; El-Sayed W. A. Antibacterial and anti-inflammatory finishing of cotton by microencapsulation using three marine organisms. Int. J. Biol. Macromol. 2016, 86, 59–64. 10.1016/j.ijbiomac.2016.01.039. [DOI] [PubMed] [Google Scholar]
- Granados A.; Pleixats R.; Vallribera A. Recent advances on antimicrobial and anti-inflammatory cotton fabrics containing nanostructures. Molecules 2021, 26, 3008 10.3390/molecules26103008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathinavel S.; Indrakumar J.; Korrapati P. S.; Dharmalingam S. Synthesis and fabrication of amine functionalized SBA-15 incorporated PVA/Curcumin nanofiber for skin wound healing application. Colloids Surf., A 2022, 637, 128185 10.1016/j.colsurfa.2021.128185. [DOI] [Google Scholar]
- Monavari M.; Zohoori S.; Davodiroknabadi A. Anti-inflammatory and bactericidal effect of keratin/harmaline/ginkgo biloba electrospun nano fibres as band aid. Micro Nano Lett. 2022, 17, 210–215. 10.1049/mna2.12125. [DOI] [Google Scholar]
- Peng J.; Xie S.; Huang K.; Ran P.; Wei J.; Zhang Z.; Li X. J. Nitric oxide-propelled nanomotors for bacterial biofilm elimination and endotoxin removal to treat infected burn wounds. J. Mater. Chem. B 2022, 10, 4189–4202. 10.1039/D2TB00555G. [DOI] [PubMed] [Google Scholar]
- Xiang J.; Zhu R.; Lang S.; Yan H.; Liu G.; Peng B. Mussel-inspired immobilization of zwitterionic silver nanoparticles toward antibacterial cotton gauze for promoting wound healing. Chem. Eng. J. 2021, 409, 128291 10.1016/j.cej.2020.128291. [DOI] [Google Scholar]
- Alisir S. H.; Ozdemir N.; Burgaz E.; Dege N.; Canavar Y. E. Fabrication and Antimicrobial Activity of Poly(lactic acid) Nanofibers Containing Firstly Synthesized Silver Diclofenac Complex with (2-methylimidazole) for Wound Dressing Applications. Fibers Polym. 2021, 22, 2738–2749. 10.1007/s12221-021-0166-z. [DOI] [Google Scholar]
- Montagut A. M.; Granados A.; Ballesteros A.; Pleixats R.; Llagostera M.; Cortés P.; Sebastián R. M.; Vallribera A. Antibiotic protected silver nanoparticles for microbicidal cotton. Tetrahedron 2019, 75, 102–108. 10.1016/j.tet.2018.11.035. [DOI] [Google Scholar]
- Montagut A. M.; Granados A.; Lazurko C.; El-Khoury A.; Suuronen E. J.; Alarcon E. I.; Sebastián R. M.; Vallribera A. Triazine Mediated Covalent Antibiotic Grafting on Cotton Fabrics as a Modular Approach for Developing Antimicrobial Barriers. Cellulose 2019, 26, 7495–7505. 10.1007/s10570-019-02584-w. [DOI] [Google Scholar]
- Li H.; Granados A.; Fernández E.; Pleixats R.; Vallribera A. Anti-inflammatory cotton fabrics and silica nanoparticles with potential topical medical applications. ACS Appl. Mater. Interfaces 2020, 12, 25658–25675. 10.1021/acsami.0c06629. [DOI] [PubMed] [Google Scholar]
- Cañedo-Dorantes L.; Cañedo-Ayala M. Skin Acute Wound Healing: A Comprehensive Review. Int. J. Inflammation 2019, 2019, 3706315 10.1155/2019/3706315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox S. W.; Cho K.; Eley B. M.; Smith R. E. A simple, combined fluorogenic and chromogenic method for the assay of proteases in gingival crevicular fluid. J. Periodontal Res. 1990, 25, 164–171. 10.1111/j.1600-0765.1990.tb01039.x. [DOI] [PubMed] [Google Scholar]
- Bendicho S.; Martí G.; Hernández T.; Martín O. Determination of proteolytic activity in different milk systems. Food Chem. 2002, 79, 245–249. 10.1016/S0308-8146(02)00126-7. [DOI] [Google Scholar]
- Lottenberg R.; Christensen U.; Jackson C. M.; Coleman P. L. Assay of coagulation proteases using peptide chromogenic and fluorogenic substrates. Methods Enzymol. 1981, 80, 341–361. 10.1016/S0076-6879(81)80030-4. [DOI] [PubMed] [Google Scholar]
- Liu M.; Granados A.; Reyes-Mesa D.; Arosemena-Angulo E. L.; Calvo-Torras M. A.; Pleixats R.; Vallribera A. Silica nanostructures against fungal growth: design and preparation of antifungal cotton fabrics. Cellulose 2022, 29, 8889–8905. 10.1007/s10570-022-04726-z. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.