Abstract
Background
Non‐steroidal antiandrogens and castration are the main therapy options for advanced stages of prostate cancer. However, debate regarding the value of these treatment options continues.
Objectives
To assess the effects of non‐steroidal antiandrogen monotherapy compared with luteinising hormone–releasing hormone agonists or surgical castration monotherapy for treating advanced stages of prostate cancer.
Search methods
We searched the Cochrane Prostatic Diseases and Urologic Cancers Group Specialized Register (PROSTATE), the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Web of Science with Conference Proceedings, three trial registries and abstracts from three major conferences to 23 December 2013, together with reference lists, and contacted selected experts in the field and manufacturers.
Selection criteria
We included randomised controlled trials comparing non‐steroidal antiandrogen monotherapy with medical or surgical castration monotherapy for men in advanced stages of prostate cancer.
Data collection and analysis
One review author screened all titles and abstracts; only citations that were clearly irrelevant were excluded at this stage. Then, two review authors independently examined full‐text reports, identified relevant studies, assessed the eligibility of studies for inclusion, assessed trial quality and extracted data. We contacted the study authors to request additional information. We used Review Manager 5 for data synthesis and used the fixed‐effect model for heterogeneity less than 50%; we used the random‐effects model for substantial or considerable heterogeneity.
Main results
Eleven studies involving 3060 randomly assigned participants were included in this review. The quality of evidence is hampered by risk of bias. Use of non‐steroidal antiandrogens decreased overall survival (hazard ratio (HR) 1.24, 95% confidence interval (CI) 1.05 to 1.48, six studies, 2712 participants) and increased clinical progression (one year: risk ratio (RR) 1.25, 95% CI 1.08 to 1.45, five studies, 2067 participants; 70 weeks: RR 1.26, 95% CI 1.08 to 1.45, six studies, 2373 participants; two years: RR 1.14, 95% CI 1.04 to 1.25, three studies, 1336 participants), as well as treatment failure (one year: RR 1.19, 95% CI 1.02 to 1.38, four studies, 1539 participants; 70 weeks: RR 1.27, 95% CI 1.05 to 1.52, five studies, 1845 participants; two years: RR 1.14, 95% CI 1.05 to 1.24, two studies, 808 participants), compared with medical or surgical castration. The quality of evidence for overall survival, clinical progression and treatment failure was rated as moderate according to GRADE. Predefined subgroup analyses showed that use of non‐steroidal antiandrogens, compared with castration, was less favourable for overall survival, clinical progression (at one year, 70 weeks, two years) and treatment failure (at one year, 70 weeks, two years) in men with metastatic disease. Use of non‐steroidal antiandrogens also increased the risk for treatment discontinuation due to adverse events (RR 1.82, 95% CI 1.13 to 2.94, eight studies, 1559 participants), including events such as breast pain (RR 22.97, 95% CI 14.79 to 35.67, eight studies, 2670 participants), gynaecomastia (RR 8.43, 95% CI 3.19 to 22.28, nine studies, 2774 participants) and asthenia (RR 1.77, 95% CI 1.36 to 2.31, five studies, 2073 participants). The risk of other adverse events, such as hot flashes (RR 0.23, 95% CI 0.19 to 0.27, nine studies, 2774 participants), haemorrhage (RR 0.07, 95% CI 0.01 to 0.54, two studies, 546 participants), nocturia (RR 0.38, 95% CI 0.20 to 0.69, one study, 480 participants), fatigue (RR 0.52, 95% CI 0.31 to 0.88, one study, 51 participants), loss of sexual interest (RR 0.50, 95% CI 0.30 to 0.83, one study, 51 participants) and urinary frequency (RR 0.22, 95% CI 0.11 to 0.47, one study, 480 participants) was decreased when non‐steroidal antiandrogens were used. The quality of evidence for breast pain, gynaecomastia and hot flashes was rated as moderate according to GRADE. The effects of non‐steroidal antiandrogens on cancer‐specific survival and biochemical progression remained unclear.
Authors' conclusions
Currently available evidence suggests that use of non‐steroidal antiandrogen monotherapy compared with medical or surgical castration monotherapy for advanced prostate cancer is less effective in terms of overall survival, clinical progression, treatment failure and treatment discontinuation due to adverse events. Evidence quality was rated as moderate according to GRADE. Further research is likely to have an important impact on results for patients with advanced but non‐metastatic prostate cancer treated with non‐steroidal antiandrogen monotherapy. However, we believe that research is likely not necessary on non‐steroidal antiandrogen monotherapy for men with metastatic prostate cancer. Only high‐quality, randomised controlled trials with long‐term follow‐up should be conducted. If further research is planned to investigate biochemical progression, studies with standardised follow‐up schedules using measurements of prostate‐specific antigen based on current guidelines should be conducted.
Keywords: Humans; Male; Androgen Antagonists; Androgen Antagonists/adverse effects; Androgen Antagonists/therapeutic use; Anilides; Anilides/adverse effects; Anilides/therapeutic use; Antineoplastic Agents, Hormonal; Antineoplastic Agents, Hormonal/adverse effects; Antineoplastic Agents, Hormonal/therapeutic use; Disease Progression; Flutamide; Flutamide/adverse effects; Flutamide/therapeutic use; Gonadotropin‐Releasing Hormone; Gonadotropin‐Releasing Hormone/adverse effects; Gonadotropin‐Releasing Hormone/therapeutic use; Goserelin; Goserelin/adverse effects; Goserelin/therapeutic use; Leuprolide; Leuprolide/adverse effects; Leuprolide/therapeutic use; Medication Adherence; Medication Adherence/statistics & numerical data; Nitriles; Nitriles/adverse effects; Nitriles/therapeutic use; Orchiectomy; Orchiectomy/methods; Orchiectomy/mortality; Prostatic Neoplasms; Prostatic Neoplasms/mortality; Prostatic Neoplasms/pathology; Prostatic Neoplasms/therapy; Randomized Controlled Trials as Topic; Tosyl Compounds; Tosyl Compounds/adverse effects; Tosyl Compounds/therapeutic use; Triptorelin Pamoate; Triptorelin Pamoate/adverse effects; Triptorelin Pamoate/therapeutic use
Plain language summary
Androgen suppression monotherapy for treatment of advanced prostate cancer
Review question
We reviewed the evidence on the effects of androgen suppression monotherapies (non‐steroidal antiandrogens compared with medical or surgical castration monotherapy) in men with advanced prostate cancer.
Background
Prostate cancer is among the top six most lethal cancers, and treatment implies a high disease burden for patients. An advanced prostate cancer has spread outside the prostate gland or has metastasised to lymph nodes, bones and/or other areas. Currently no curative therapy for advanced prostate cancer is known, although androgen suppression therapy is commonly used to treat the disease at this stage. We wanted to discover the effects of androgen suppression monotherapies in the treatment of patients in advanced stages of prostate cancer.
Study characteristics
The evidence is current to December 2013. We included 11 studies involving 3060 randomly assigned participants at advanced stages of prostate cancer. The follow‐up period of participants ranged from six months to six years. In seven studies, authors reported possible conflicts of interest. In three studies, no conflicts of interest were declared. In one study, authors reported that they had received an educational grant from the sponsor, who had no role in any aspect of analysis or data interpretation.
Key results
Use of non‐steroidal antiandrogens decreased overall survival and increased clinical progression and treatment failure. Subgroup analyses showed that non‐steroidal antiandrogens, compared with castration, were less favourable for overall survival, for clinical progression and for treatment failure in men with metastatic disease. Participants receiving antiandrogens were also more likely to stop treatment as the result of side effects. The risk of suffering breast pain, enlargement of breast tissue or symptoms of physical weakness was also increased with non‐steroidal antiandrogens. The risks of feeling intense heat with sweating and rapid heartbeat and of bleeding, the need to get up in the night to urinate, loss of sexual interest, extreme tiredness and the need to urinate more often than usual were increased with castration. No difference was noted for other side effects. The effect of non‐steroidal antiandrogens on cancer‐specific survival and biochemical progression remained unclear.
Quality of the evidence
Included studies were poorly conducted, and the quality of evidence was rated as moderate. This means that further research is likely to have an important impact on our confidence in the accuracy of results.
Summary of findings
Summary of findings for the main comparison. Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy for advanced prostate cancer.
| Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy for advanced prostate cancer | ||||||
|
Patient or population: men with advanced prostate cancer
Settings: multi‐centre (9 studies) and single‐centre studies (2 studies) on outpatients
Intervention: non‐steroidal antiandrogen monotherapy Comparison: LHRH agonists or surgical castration monotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Hazard ratio/ Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Castration | Non‐steroidal antiandrogen | |||||
| Overall survival Follow‐up: median 1 to 6.3 years | 296 per 1000 | 353 per 1000 (308 to 405) | HR 1.24 (1.05 to 1.48) | 2712 (6 studies) | ⊕⊕⊕⊝ moderate1,6 | Overall survival was evaluated using the random‐effects model because of heterogeneity (I2 = 51%). Sensitivity analyses showed comparable results. Numbers of absolute risks relate to deaths |
| Clinical progression Follow‐up: median 70 weeks | 420 per 1000 | 529 per 1000 (453 to 608) | RR 1.26 (1.08 to 1.45) | 2373 (6 studies) | ⊕⊕⊕⊝ moderate2,6 | Clinical progression after median 70 weeks was evaluated using the random‐effects model because of heterogeneity (I2 = 64%). Sensitivity analyses showed comparable results. After imputation of event numbers: RR 1.43, 95% CI 1.19 to 1.73, I2 = 0%; fixed‐effect model |
| Treatment failure Follow‐up: median 70 weeks | 527 per 1000 | 669 per 1000 (553 to 801) | RR 1.27 (1.05 to 1.52) | 1845 (5 studies) | ⊕⊕⊕⊝ moderate3,6 | Treatment failure after median 70 weeks was evaluated using the random‐effects model because of heterogeneity (I2 = 81%). Sensitivity analyses showed comparable results. After imputation of event numbers: RR 1.21, 95% CI 1.09 to 1.35, I2 = 0%; fixed‐effect model |
| Breast pain Follow‐up: median 1 to 6.3 years | 17 per 1000 | 397 per 1000 (256 to 617) | RR 22.97 (14.79 to 35.67) | 2670 (8 studies) | ⊕⊕⊕⊝ moderate4 | Breast pain was evaluated using the fixed‐effect model (I2 = 0%) |
| Gynaecomastia Follow‐up: median 1 to 6.3 years | 44 per 1000 | 374 per 1000 (142 to 989) | RR 8.43 (3.19 to 22.28) | 2774 (9 studies) | ⊕⊕⊕⊝ moderate5,6 | Gynaecomastia was evaluated using the random‐effects model because of heterogeneity (I2 = 92%). Sensitivity analyses showed comparable results |
| Hot flashes Follow‐up: median 1 to 6.3 years | 451 per 1000 | 104 per 1000 (86 to 122) | RR 0.23 (0.19 to 0.27) | 2774 (9 studies) | ⊕⊕⊕⊝ moderate5 | Hot flashes were evaluated using the fixed‐effect model (I2 = 0%) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). LHRH: Luteinising hormone‐releasing hormone; CI: Confidence interval; HR: Hazard ratio; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded for study limitations (‐1): high risk of bias: 'allocation concealment' (Tyrrell 2006); unclear risk of bias: 'random sequence generation' (Study 0301; Study 0302; Study 0303; Study 306; Study 307); 'allocation concealment' (Study 0301;Study 0302; Study 0303; Study 306; Study 307); 'blinding of participants and personnel' (all included studies); 'other bias' (all included studies). 2Downgraded for study limitations (‐1): high risk of bias: 'blinding of participants and personnel' (all included studies); 'blinding of outcome assessment' (all included studies); 'incomplete outcome data' (Sciarra 2004a; Study 0301;Study 0302;Study 0303); 'selective reporting' (Sciarra 2004a); unclear risk of bias: 'random sequence generation' (all included studies); 'allocation concealment' (all included studies); 'other bias' (all included studies). 3Downgraded for study limitations (‐1): high risk of bias: 'blinding of participants and personnel' (all included studies); 'blinding of outcome assessment' (all included studies); 'incomplete outcome data' (Study 0301;Study 0302;Study 0303); unclear risk of bias: 'random sequence generation' (all included studies); 'allocation concealment' (all included studies); 'other bias' (all included studies). 4Downgraded for study limitations (‐1): high risk of bias: 'allocation concealment' (Tyrrell 2006); 'blinding of participants and personnel' (Sieber 2004;Study 0301;Study 0302;Study 0303; Study 306; Study 307; Tyrrell 2006); 'blinding of outcome assessment' (Sieber 2004; Study 0301; Study 0302; Study 0303; Study 306; Study 307; Tyrrell 2006); 'incomplete outcome data' (Study 0301; Study 0302; Study 0303); unclear risk of bias: 'random sequence generation' (Sieber 2004; Study 0301; Study 0302; Study 0303; Study 306; Study 307); 'allocation concealment' (Sieber 2004; Smith 2004; Study 0301; Study 0302; Study 0303; Study 306; Study 307); 'blinding of participants and personnel' (Smith 2004); 'blinding of outcome assessment' (Smith 2004); 'other bias' (all included studies). 5Downgraded for study limitations (‐1): high risk of bias: 'allocation concealment' (Tyrrell 2006); 'blinding of participants and personnel' (Boccon‐Gibod 1997; Sieber 2004; Study 0301; Study 0302; Study 0303; Study 306; Study 307; Tyrrell 2006); 'blinding of outcome assessment' (Boccon‐Gibod 1997; Sieber 2004; Study 0301; Study 0302; Study 0303; Study 306; Study 307; Tyrrell 2006); 'incomplete outcome data' (Study 0301; Study 0302; Study 0303); unclear risk of bias: 'random sequence generation' (Boccon‐Gibod 1997; Sieber 2004; Study 0301; Study 0302; Study 0303; Study 306; Study 307); 'allocation concealment' (Sieber 2004; Smith 2004; Study 0301; Study 0302; Study 0303; Study 306; Study 307); 'blinding of participants and personnel' (Smith 2004); 'blinding of outcome assessment' (Smith 2004); 'other bias' (all included studies). 6Heterogeneity was present but might be explained by subgroup or sensitivity analyses (see Effects of interventions; Quality of the evidence); therefore we did not downgrade for inconsistency.
Background
Description of the condition
Prostate cancer is a frequently occurring tumour that leads to 85,200 cancer deaths per year in Europe (Boyle 2005). Worldwide, tumours of this type are associated with significant morbidity and are among the top six most lethal cancers (Eheman 2012; GLOBOCAN 2012); therefore, optimising therapy for prostate cancer is crucial.
Prostate cancer is usually classified as localised disease that is limited to the prostate gland (localised, stage T1‐2, N0, M0) or more advanced disease that has spread locally outside the prostate gland (locally advanced, stage T3‐4, N0, M0), disseminated to regional lymph nodes (local to regionally advanced, stage T1‐4, N1, M0) or metastasised to bones and/or to other areas (advanced, stage T1‐4, N0‐1, M1). Localised and locally advanced prostate cancers are amenable to curative treatment. However, currently no curative therapy is known for patients at local to regionally advanced and advanced stages of prostate cancer. Androgen suppression therapy is usually recommended to treat patients at this stage of the disease (ASCO 2007; EAU 2013).
Description of the intervention
Several different approaches to androgen suppression monotherapy can be used at advanced stages of prostate cancer, including oestrogens, bilateral orchiectomy, luteinising hormone–releasing hormone (LHRH) agonists, LHRH antagonists, antiandrogens (non‐steroidal antiandrogens and steroidal antiandrogens) and 5‐alpha reductase inhibitors.
Oestrogens were among the first drugs used to treat patients at advanced stages of prostate cancer. They act through negative hormonal feedback. However, their side effects, even at low doses, are significantly greater than those observed with surgical castration. Therefore, their use is no longer recommended under current guidelines (ASCO 2007; EAU 2013).
Surgical castration removes the source of testicular androgen production and can be performed totally (bilateral orchiectomy) or by a subcapsular technique (preservation of tunica albuginea and epididymis). This intervention has been effectively used for decades, and current guidelines still consider it to be the 'gold standard' (EAU 2013). However, it is irreversible and might cause psychological distress.
LHRH agonists (e.g. leuprorelin, goserelin, buserelin, triptorelin) have been found to be as effective as surgical castration via orchiectomy, and no difference in overall survival has been reported among the different LHRH agonists (Seidenfeld 2000). These medications are recommended as standard initial treatment options for advanced stages of prostate cancer (ASCO 2007; EAU 2013).
LHRH antagonists are newer agents. They block hormonal effects at the pituitary gland. Whether they provide advantages over LHRH agonists has not yet been determined (EAU 2013).
Antiandrogens are classified as non‐steroidal (e.g. bicalutamide, flutamide, nilutamide) or steroidal antiandrogens (e.g. cyproterone acetate). Non‐steroidal antiandrogens are mentioned in current guidelines as an alternative to medical or surgical castration in selected patients with non‐metastatic prostate cancer (ASCO 2007; EAU 2013).
5‐alpha reductase inhibitors also have antiandrogenic activity. This form of androgen manipulation has a potential role in prevention and treatment of prostate cancer (Azzouni 2012). Antiandrogens combined with 5‐alpha reductase inhibitors for the treatment of biochemical disease recurrence after local therapy might be a therapeutic option (EAU 2013), but discussions on this topic are still controversial.
Oestrogens, LHRH antagonists, steroidal antiandrogens and 5‐alpha reductase inhibitors are not part of this review and will not be discussed further. This systematic review focuses on the effectiveness of non‐steroidal antiandrogens compared with LHRH agonists or surgical castration.
How the intervention might work
All treatment modalities that reduce androgen activity are referred to as androgen suppression therapy (EAU 2013). Androgen suppression therapy is usually recommended for patients with advanced prostate cancer to slow down progression and to increase the chance of survival (EAU 2013; Schmitt 1999). The androgen testosterone is essential for the growth of prostate cells; suppression of testosterone is therefore important in prostate cancer therapy. Testosterone is produced mainly in the testes but also to a lesser extent in the adrenal glands. The release of testosterone is regulated by the hypothalamic‐pituitary‐gonadal axis. Hypothalamic LHRH stimulates the pituitary gland to release luteinising hormone (LH) and follicle‐stimulating hormone (FSH). LH stimulates the testes to secrete testosterone. Testosterone is then converted to oestrogens, which contribute to negative feedback control of hypothalamic hormone secretion. This negative feedback in turn diminishes the secretion of LH, thereby reducing testicular testosterone production (Gibbs 1996; Huggins 2002).
Antiandrogens compete with testosterone and dihydrotestosterone at the receptor level in the prostate cell nucleus and thereby inhibit prostate cancer cell growth. Because non‐steroidal antiandrogens do not affect the pituitary gland and do not block the negative feedback mechanism, testosterone levels are not affected, but testosterone is still converted to oestrogens. This provides potential benefits for sexual function, but it also stimulates gynaecomastia (Iversen 2002).
Bilateral orchiectomy and LHRH agonists reduce testosterone to a castration level and have been used for decades. Surgical castration removes the source of testicular androgen production, which leads to a rapid reduction in testosterone. LHRH agonists stimulate the pituitary gland continuously, which leads to desensitisation of LH and testosterone secretion (medical castration). However, before the hormonal receptors are downregulated, LHRH agonists cause an initial stimulation of LH, FSH and thereby testosterone. This process is called 'testosterone flare' and can lead to potential exacerbations of clinical symptoms in metastatic disease by stimulating the growth of prostate cancer cells. Premedication with antiandrogens can be used for a few days before the start of LHRH agonist therapy to prevent flares (Gibbs 1996). However, castration therapies do not affect adrenal secretion of testosterone.
Why it is important to do this review
A systematic review published in 2000 concluded that survival rates might be lower with non‐steroidal antiandrogens than with medical or surgical castration (Seidenfeld 1999; Seidenfeld 2000). However, no update of the review has been performed, and no other current evaluation of this comparison has been published. Clinical practice guidelines on androgen suppression monotherapy for advanced stages of prostate cancer support antiandrogens for selected and motivated patients with low prostate‐specific antigen (PSA) (EAU 2013). Non‐steroidal antiandrogens have been argued to have fewer side effects (e.g. hot flashes), and they do not affect testosterone levels. This might offer potential benefits for sexual function. However, non‐steroidal antiandrogens have other side effects; testosterone is converted to oestrogens, and this stimulates gynaecomastia (Iversen 2002). Additionally, effectiveness has been challenged, and the debate concerning the value of different treatment options, especially the comparison between non‐steroidal antiandrogens and medical or surgical castration, continues. As current guidelines are based upon older literature, there is a need to revisit the topic to update our understanding in light of more recent data.
Objectives
To assess the effects of non‐steroidal antiandrogen monotherapy compared with luteinising hormone–releasing hormone agonists or surgical castration monotherapy for treating advanced stages of prostate cancer.
Methods
Criteria for considering studies for this review
Types of studies
We reviewed parallel‐group randomised controlled trials comparing non‐steroidal antiandrogens versus castration (surgical or medical) for advanced stages of prostate cancer.
Types of participants
Studies recruiting men at advanced stages of prostate cancer who had not received prior androgen suppression therapy were eligible. We included studies evaluating men with prostate cancer that had spread locally outside the prostate gland (locally advanced, T3‐4, N0, M0), to regional lymph nodes (local to regionally advanced, T1‐4, N1, M0), to the bones or to other areas (advanced, T1‐4, N0‐1, M1), or those who had recurrent disease after local therapy. No exclusions were based on age or ethnicity.
Types of interventions
For androgen suppression monotherapies, the following comparison was considered: non‐steroidal antiandrogen monotherapy versus medical or surgical castration monotherapy.
Medical castration and surgical castration are two different treatment options that are thought to be equally effective (EAU 2013; Seidenfeld 2000). For this reason, we decided to include randomised trials even if they did not differentiate between medical and surgical castration.
We defined medical castration monotherapy as androgen suppression therapy using LHRH agonists (e.g. leuprorelin, goserelin, buserelin, triptorelin).
Bilateral surgical castration included total and subcapsular techniques.
LHRH antagonists, oestrogen and steroidal antiandrogen monotherapies were not a topic of this review, and trials investigating these treatment options were not included in our analysis (see Description of the intervention). This review did not consider maximal androgen blockade (combination therapy of antiandrogens with medical or surgical castration). However, we did not exclude trials that used antiandrogens as short‐term flare protection for up to four weeks after medical castration (see Description of the intervention).
Types of outcome measures
Primary outcomes
Overall survival.
Secondary outcomes
Cancer‐specific survival (we assessed data for cancer‐specific mortality because data for cancer‐specific survival were not available).
Treatment discontinuation due to adverse events.
Clinical progression (time from random assignment to progression; determined by an increase in prostatic dimension, appearance of new or increase in existing bone or extraskeletal metastases confirmed by imaging or physical examination).
Biochemical progression (time from random assignment to progression; determined by an increase of more than 25% in serum PSA concentration from the nadir value on two determinations).
Treatment failure (determined by death; disease progression, i.e. an increase in prostatic dimensions, appearance of new or increase in existing bone or extraskeletal metastases confirmed by imaging or physical examination; addition of other systemic therapies for prostate cancer; loss to follow‐up; refusal to begin or continue with randomly assigned therapy; or discontinuation due to adverse events or for other reasons).
Adverse events, such as breast pain, pelvic pain, bone pain, back pain, headache, abdominal pain, general pain, gynaecomastia, constipation, diarrhoea, vomiting, cardiovascular events, hypertension, loss of sexual interest, asthenia, insomnia, hot flashes, night sweats, anaemia, hepatic enzyme increase, rash, pruritus, dyspnoea, infection, pharyngitis, arthritis, sinusitis, urinary tract infection, dizziness, haemorrhage, haematuria, nocturia, urinary frequency, urinary retention, oedema, anorexia, gastrointestinal disorders, loss of sexual function and lethargy, as well as serious adverse events (defined as adverse events causing death or events that are life threatening, require inpatient hospitalisation, result in persistent or significant disability/incapacity or require intervention to prevent permanent impairment or damage).
Search methods for identification of studies
Both electronic and manual searches were conducted.
Electronic searches
We searched the following electronic databases on 26 February 2013 and updated the search on 23 December 2013: Cochrane Prostatic Diseases and Urologic Cancers Group Specialized Register (PROSTATE; 23 December 2013); Cochrane Central Register of Controlled Trials (CENTRAL) 2013, Issue 12 (part of The Cochrane Library); Ovid MEDLINE, In‐Process & Other Non‐Indexed Citations, Daily (1946 to 23 December 2013); EMBASE via DIMDI (www.dimdi.de/static/en/index.html; 1947 to 23 December 2013); and Web of Science with Conference Proceedings (Thomson Reuters Web of Knowledge; 1945 to 23 December 2013). The search strategy was adapted for each electronic database. For the search strategies used by the review authors, see Appendix 1, Appendix 2, Appendix 3, Appendix 4 and Appendix 5. No language restriction was applied.
Searching other resources
The reference lists of all identified articles were screened to identify additional potentially relevant citations. We contacted selected experts in the field as well as manufacturers of non‐steroidal androgen suppression drugs to request information on unpublished studies. We searched all other resources on 26 February 2013 and updated the search on 23 December 2013.
We performed an electronic search of abstracts from three major conferences: the American Society of Clinical Oncology (ASCO; jco.ascopubs.org; 2004 to 23 December 2013), the European Association of Urology (EAU; www.uroweb.org; 2004 to 23 December 2013) and the American Urological Association (AUA; www.jurology.com/; 2008 to 23 December 2013). For keywords used to search meeting abstracts, see Appendix 6.
Additionally, we searched three trial registries for completed or ongoing studies: Current Controlled Trials (ISRCTN; www.controlled‐trials.com/; last searched 23 December 2013), ClinicalTrials.gov (www.clinicaltrials.gov/; last searched 23 December 2013) and the World Health Organization International Clinical Trials Registry Platform Search Portal (WHO ICTRP Search Portal; www.who.int/ictrp/en/; last searched 23 December 2013). For keywords used to search trial registries, see Appendix 7.
Data collection and analysis
Selection of studies
For the initial search, one review author (FK) screened all titles and abstracts of records identified by the search for relevance. Only records that were clearly irrelevant were excluded at this stage (e.g. animal/in vitro research and testing). Next, two review authors (FK, HG) independently examined the full‐text reports of the remaining records, identified relevant studies and assessed the eligibility of studies for inclusion. We resolved disagreements regarding study eligibility through discussion and consensus or, if necessary, with the help of a third review author (JM). We recorded details of excluded studies and the reasons for exclusion. One review author (FK) performed the search update, which included only records published since the time of our initial search (between 26 February 2013 and 23 December 2013). Few records were published since the time of our last search, and we retrieved no reference that fitted our inclusion criteria. Therefore we performed no full‐text screening.
Data extraction and management
In addition to details related to the quality (risk of bias) of the included studies, we extracted the following types of data.
Study characteristics: population characteristics, setting, detailed nature of the intervention, detailed nature of the comparator and outcomes, place of publication and date of publication. The key purpose of collecting these data was to explore the clinical heterogeneity of the included studies.
Results of the included studies: We extracted the results with respect to each of the main outcomes (see Types of outcome measures). We recorded the reasons why an included study did not contribute data on a particular outcome and considered the possibilities of selective reporting of the results of particular outcomes.
Two review authors (FK, HG) independently extracted data using a data extraction form based on the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). The review authors resolved disagreements by consensus or through discussion with a third review author (JM). In addition, when necessary, we contacted the original investigators.
Assessment of risk of bias in included studies
Two review authors (FK, HG) independently assessed all studies using our data extraction form and followed the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a) to assess the following domains as low risk of bias, unclear risk of bias or high risk of bias: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias.
We reviewed the assessments and discussed inconsistencies in the interpretation of information given and their significance for the selected studies. We resolved disagreements through discussion with a third review author (JM). In assessing the risk of bias, we did not automatically exclude any study as a result of an unclear or high risk of bias rating.
Measures of treatment effect
We analysed extracted data using Review Manager 5 (Review Manager 2012).
We extracted hazard ratios (HRs) with 95% confidence intervals (CIs) for time‐to‐event outcomes. If HRs were not given, we used indirect estimation methods (described by Parmar et al (Parmar 1998) and Williamson et al (Williamson 2002)) to calculate them. If we were unable to extract these data from the study reports or to receive the necessary information from the primary investigators, we alternatively used the proportions of participants with the respective outcomes measured at certain time points to calculate risk ratios (RRs) with 95% CIs.
We expressed results for binary outcomes as RRs with 95% CIs as measures of uncertainty.
Unit of analysis issues
Only randomised controlled trials were included; cluster‐randomised or cross‐over trials were excluded.
Dealing with missing data
We contacted the original investigators to request missing data. We analysed the data using an intention‐to‐treat (ITT) analysis. If we did not receive all required data, and if a substantial departure of people assigned to the intervention or control group was noted, we conducted best‐case and worst‐case scenarios, as proposed by Gamble 2005 and described briefly in the Cochrane Handbook for Systematic Reviews of Interventions (Section 16.2.2; Higgins 2011c), and presented the results as sensitivity analyses.
Assessment of heterogeneity
Statistical heterogeneity was examined by using the I2 statistic (Higgins 2002; Higgins 2003). Our definitions of the thresholds for interpretation of I2 are consistent with the definitions presented in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2008): 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% substantial heterogeneity; 75% to 100% considerable heterogeneity. Clinical heterogeneity was examined by performing subgroup analyses. For details, see Subgroup analysis and investigation of heterogeneity section.
Assessment of reporting biases
To minimise the impact of possible publication bias, we conducted electronic and manual searches of multiple databases, without imposing a language restriction, to identify published and unpublished studies. We performed a funnel plot asymmetry analysis to assess possible publication bias.
Data synthesis
For data synthesis, we used Review Manager 5 (Review Manager 2012), as provided by The Cochrane Collaboration. Meta‐analyses of the data from all contributing studies were conducted using a fixed‐effect model if I2 was less than 50%, and using a random‐effects model for substantial or considerable heterogeneity if I2 was greater than or equal to 50% (≥ 50%). We reported results from both models.
Subgroup analysis and investigation of heterogeneity
We explored the following potential sources of heterogeneity using subgroup analyses.
Disease stage: non‐metastatic (M0) versus metastatic (M1) disease.
Dose of non‐steroidal antiandrogen (e.g. bicalutamide 50 mg vs bicalutamide 150 mg).
We planned in advance to also evaluate a subgroup analysis regarding the effects of different control interventions (medical vs surgical castration). However, the largest included studies (Study 0302; Study 0303; Study 306; Study 307; Tyrrell 2006) permitted both control interventions but did not report results of subgroups. This involves 925 of the 1288 participants randomly assigned to the control groups (72%). We decided therefore not to evaluate subgroup analyses regarding the effects of different control interventions.
A current guideline mentioned that non‐steroidal antiandrogen monotherapy using bicalutamide at a dose of 150 mg daily for non‐metastatic prostate cancer might be an alternative to castration for selected patients (EAU 2013). A narrative review suggested that non‐steroidal antiandrogen monotherapy might be an established treatment option in patients with prostate cancer, but an unexplained trend towards decreased survival should prohibit their uncritical use (Wirth 2007). Therefore for the primary outcome of overall survival, we performed post hoc subgroup analyses regarding disease stage (non‐metastatic or metastatic disease) in combination with different doses of non‐steroidal antiandrogens (bicalutamide 50, 150, 450 or 600 mg daily; Analysis 1.1).
1.1. Analysis.
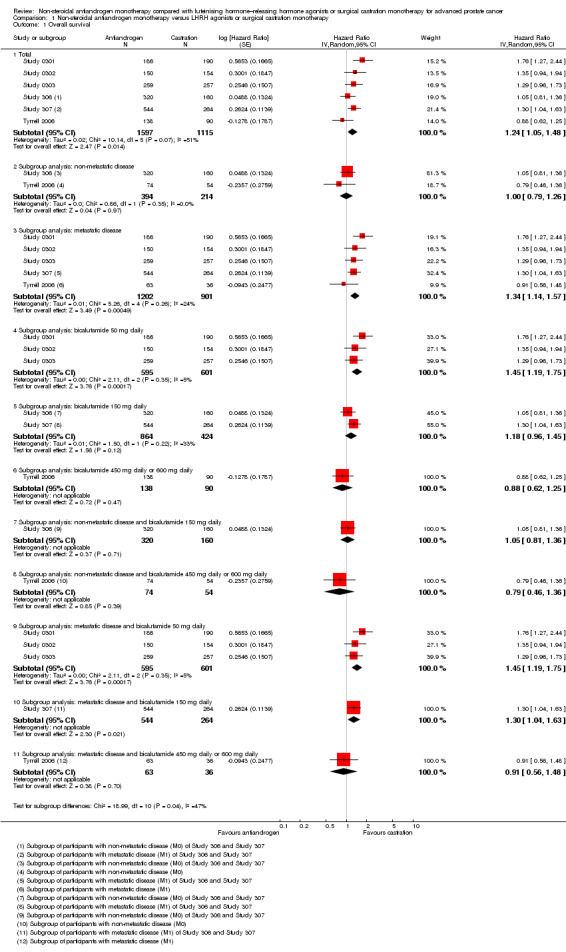
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 1 Overall survival.
In accordance with the recommendation of Higgins et al, we did not perform subgroup analyses if only a few studies were included in the meta‐analysis (Higgins 2004).
Sensitivity analysis
We performed sensitivity analyses to evaluate the effects of data imputations for best‐case and worst‐case scenarios (Analysis 1.5; Analysis 1.7; Analysis 1.9). Additionally, we investigated the robustness of results through sensitivity analyses when heterogeneity was substantial or considerable (I2 50% to 90% or 75% to 100%, respectively) by excluding smaller studies from the meta‐analysis (Analysis 1.1; Analysis 1.2; Analysis 1.4; Analysis 1.8; Analysis 1.17).
1.5. Analysis.
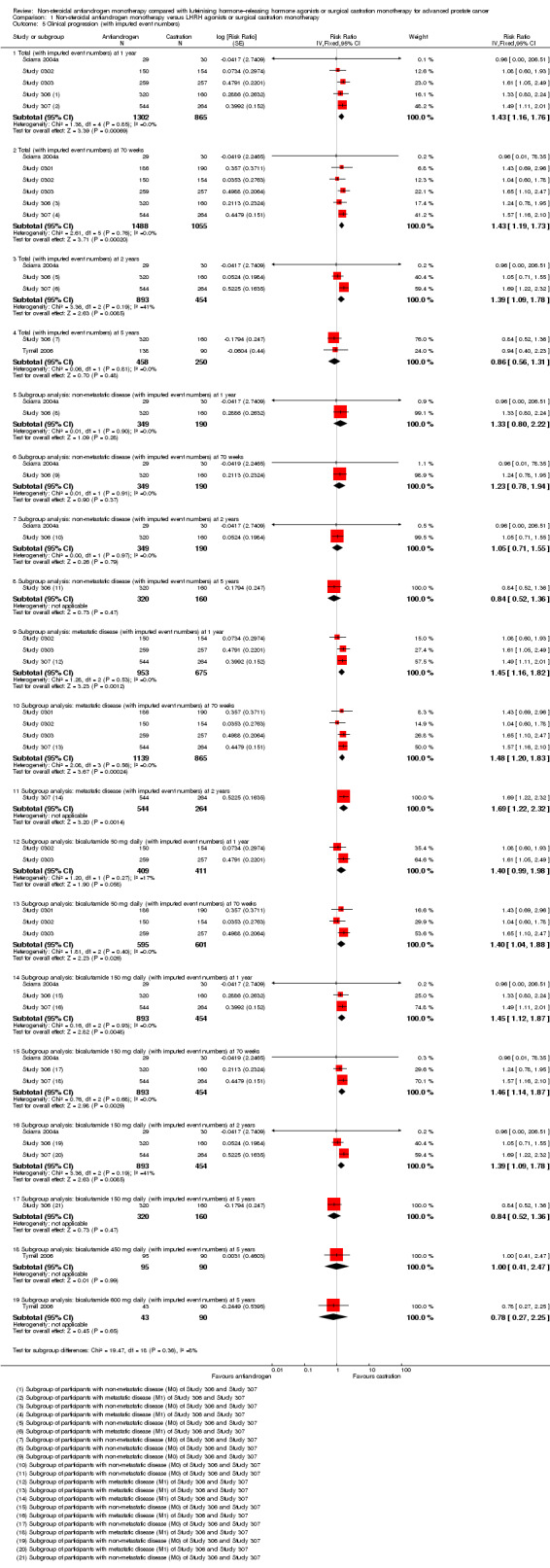
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 5 Clinical progression (with imputed event numbers).
1.7. Analysis.
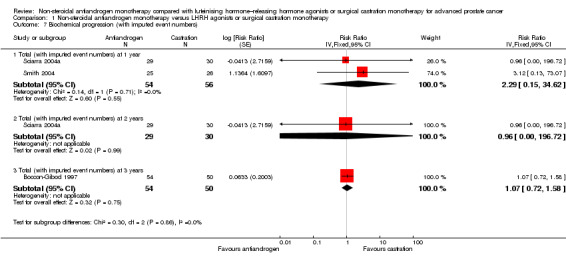
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 7 Biochemical progression (with imputed event numbers).
1.9. Analysis.
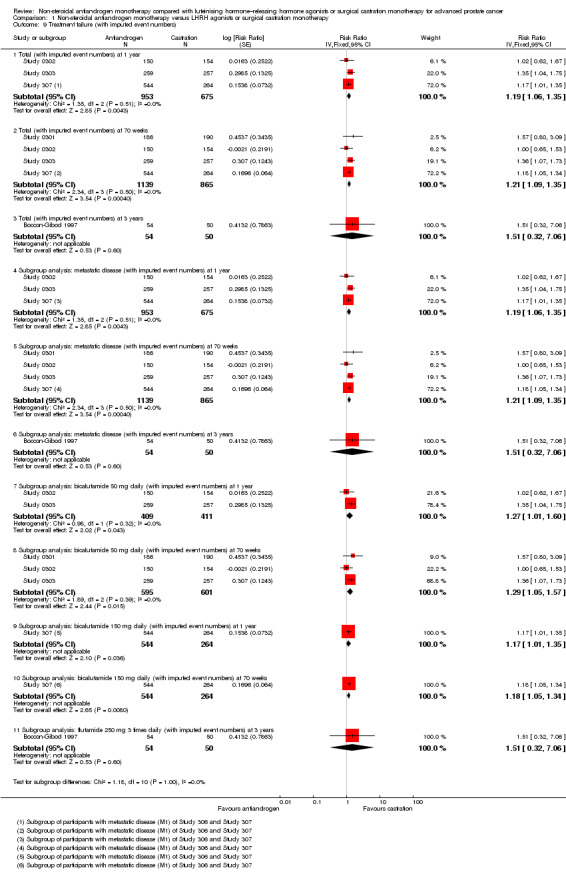
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 9 Treatment failure (with imputed event numbers).
1.2. Analysis.
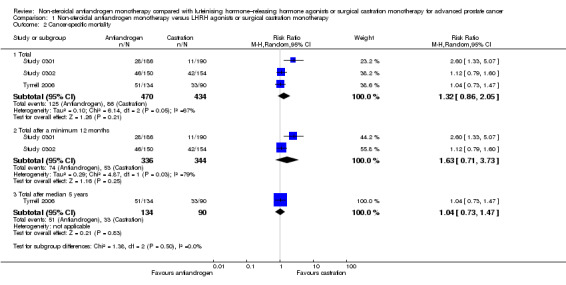
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 2 Cancer‐specific mortality.
1.4. Analysis.
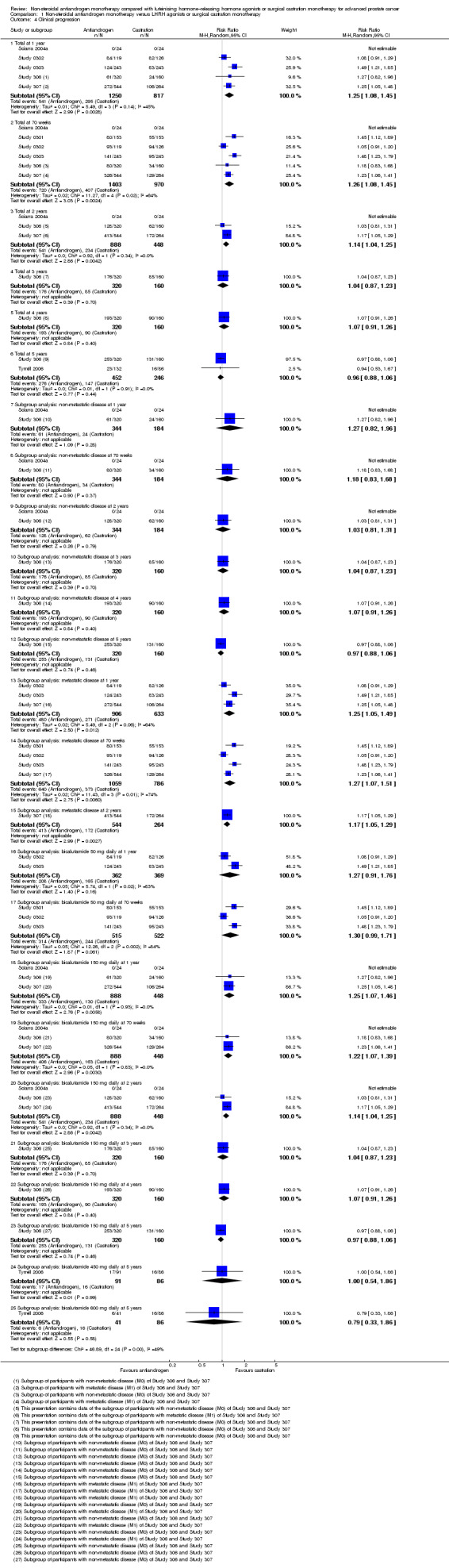
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 4 Clinical progression.
1.8. Analysis.
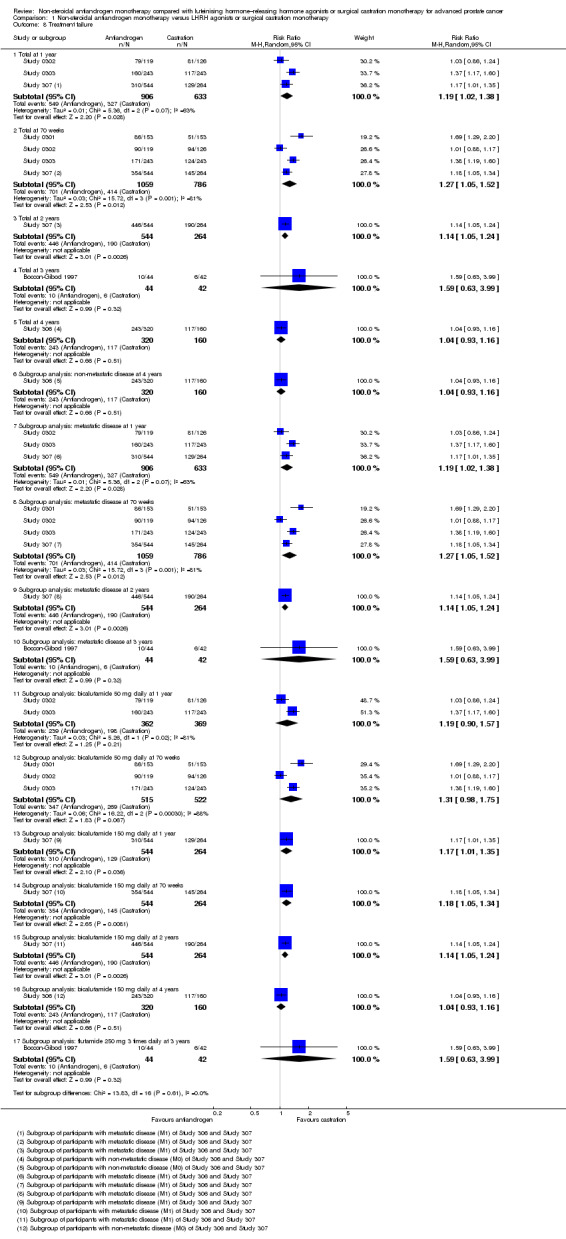
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 8 Treatment failure.
1.17. Analysis.
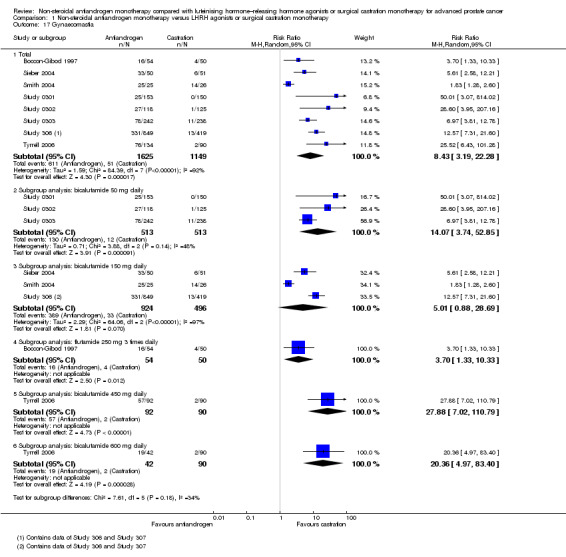
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 17 Gynaecomastia.
Summary of findings table
We summarised the findings in a summary of findings table (Table 1) in accordance with GRADE methodology (Guyatt 2011; Schünemann 2011).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
For details of the search results, see Figure 1. A total of 16 articles on 11 studies were finally included in the review. None of these studies was available in abstract form only. All included studies were published in English. We did not identify ongoing studies. We also did not identify further relevant studies through the search update.
1.
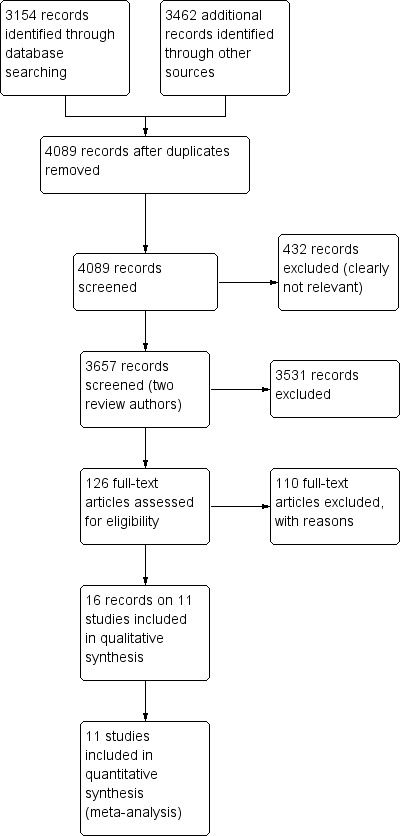
Study flow diagram (searched 26 February 2013; updated 23 December 2013).
Included studies
For details on the included studies, see Characteristics of included studies.
We included 11 studies that randomly assigned 3060 participants. All of the included studies fit our inclusion criteria and provided information on study population demographics. The type of non‐steroidal antiandrogen and the doses given varied among the included studies (flutamide 250 mg three times daily: Boccon‐Gibod 1997; bicalutamide 50 mg daily: Study 0301, Study 0302 and Study 0303; bicalutamide 150 mg daily: Dockery 2009, Sciarra 2004a, Sieber 2004, Smith 2004, Study 306 and Study 307; bicalutamide 450 mg daily and 600 mg daily: Tyrrell 2006). Two studies (Boccon‐Gibod 1997; Study 0301) used surgical castration, and four studies used medical castration (goserelin 10.8 mg three times monthly: Dockery 2009; triptorelin 3.75 mg monthly: Sciarra 2004a; leuprorelin 22.5 mg every three months: Smith 2004; drug not specified: Sieber 2004). In five studies, participants could choose between medical (using goserelin) and surgical castration (Study 0302; Study 0303; Study 306; Study 307; Tyrrell 2006). In two studies (Dockery 2009; Smith 2004), participants randomly assigned to castration also received a non‐steroidal antiandrogen for two (Dockery 2009) or four weeks (Smith 2004) to prevent a flare reaction. Four studies included participants with non‐metastatic prostate cancer (Dockery 2009; Sciarra 2004a; Sieber 2004; Smith 2004), and four studies included participants with metastatic prostate cancer (Boccon‐Gibod 1997; Study 0301; Study 0302; Study 0303). Three studies included participants with non‐metastatic or metastatic disease (Study 306; Study 307; Tyrrell 2006). The follow‐up period of participants ranged from six months (Dockery 2009) to six years (Study 306; Study 307).
In seven studies (Boccon‐Gibod 1997; Sieber 2004; Smith 2004; Study 0303; Study 306; Study 307; Tyrrell 2006), the trial authors reported possible conflicts of interest. In three studies (Sciarra 2004a; Study 0301; Study 0302), no conflicts of interest were declared. The authors of only one study (Dockery 2009) reported that they received an educational grant from the sponsor; however, they claimed that this sponsor had no role in any aspect of the study plan, protocol or analysis; data interpretation; or writing of the manuscript.
Excluded studies
Figure 1 and the table titled Characteristics of excluded studies provide information on the numbers of and reasons for exclusions from the review.
Risk of bias in included studies
We conducted a funnel plot asymmetry analysis for our primary outcome to assess potential publication bias (Figure 2). We found no indication of bias. However, the sensitivity of this analysis to assess publication bias might be low because fewer than 10 studies were included in the meta‐analyses performed. All studies were published in peer‐reviewed publications. For details on risk of bias, see Figure 3 and the table titled Characteristics of included studies.
2.
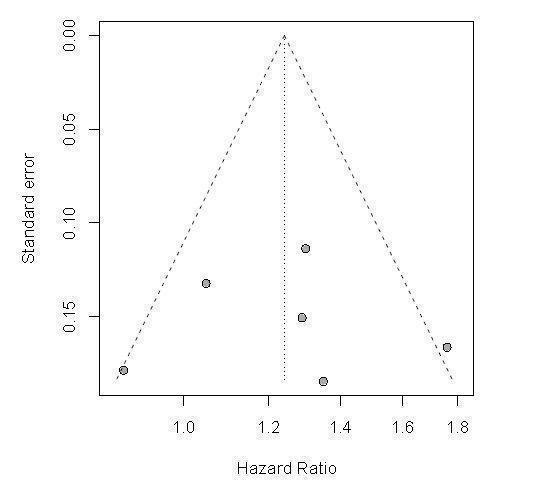
Funnel plot: Outcome: 1.1 Overall survival, 1.1.1 Total.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation
Three studies (Dockery 2009; Smith 2004; Tyrrell 2006) reported adequate sequence generation (low risk of bias). In all of the other studies, information on sequence generation was not reported or was insufficient to permit a judgement (unclear risk of bias).
Allocation concealment
Only one study (Boccon‐Gibod 1997) provided information indicating adequate allocation concealment using central random assignment (low risk of bias). One study (Tyrrell 2006) contained a high risk of bias because participant numbers were allocated sequentially as men entered the trial. No other studies reported information on allocation concealment (unclear risk of bias).
Blinding
We assessed risk of bias for blinding of participants and personnel and for blinding of outcome assessment on an outcome‐specific basis.
Blinding of participants and personnel
All included studies were open randomised trials that did not involve blinding of participants and/or personnel. Blinding was not feasible because of differences in the interventions, which included surgical therapy (orchiectomy), medical castration by injection (LHRH agonists) and oral medications (non‐steroidal antiandrogens).
Overall survival, cancer‐specific mortality, biochemical progression
We were uncertain to what extent outcomes such as overall survival, cancer‐specific mortality and biochemical progression were influenced by lack of blinding. We judged therefore that risk of bias regarding these outcomes for most of the included studies was unclear (Boccon‐Gibod 1997; Sciarra 2004a; Smith 2004; Study 0301; Study 0302; Study 0303; Study 306; Study 307; Tyrrell 2006). Two studies (Dockery 2009; Sieber 2004) did not assess these outcomes (unclear risk of bias).
Clinical progression, treatment failure, treatment discontinuation due to adverse events, adverse events
Outcomes such as clinical progression, treatment failure, treatment discontinuation due to adverse events and adverse events could be influenced by lack of blinding. These outcomes therefore present a high risk of bias in most of the included studies (Boccon‐Gibod 1997; Dockery 2009; Sciarra 2004a; Sieber 2004; Study 0301; Study 0302; Study 0303; Study 306; Study 307; Tyrrell 2006). Risk of bias was unclear for one study (Smith 2004). The original investigators responded that "subjects and study investigators were blinded to treatment assignment." However, the method of blinding bicalutamide 150 mg by mouth daily for 12 months compared with leuprorelin three‐month depot (22.5 mg intramuscularly every three months) for treatment discontinuation due to adverse events and adverse events remained unclear (unclear risk of bias).
Blinding of outcome assessment
Overall survival, cancer‐specific mortality, biochemical progression
In all studies, no blinding was provided or blinding was not reported. However, we judged that it was not likely that outcome assessments for overall survival, cancer‐specific mortality and biochemical progression were influenced by lack of blinding (low risk of bias). Two studies (Dockery 2009; Sieber 2004) did not assess these outcomes (unclear risk of bias).
Clinical progression, treatment failure, treatment discontinuation due to adverse events, adverse events
We judged that for most studies (Boccon‐Gibod 1997; Dockery 2009; Sciarra 2004a; Sieber 2004; Study 0301; Study 0302; Study 0303; Study 306; Study 307; Tyrrell 2006) it was likely that outcome assessments of clinical progression, treatment failure, treatment discontinuation due to adverse events and adverse events were influenced by lack of blinding. For one study (Smith 2004), the original investigators responded that blinding was performed ("subjects and study investigators were blinded to treatment assignment"). However, blinding of outcome assessments for treatment discontinuation due to adverse events and adverse events remained unclear (unclear risk of bias).
Incomplete outcome data
We assessed risk of bias for incomplete outcome data on an outcome‐specific basis.
Overall survival, cancer‐specific mortality
Five studies (Study 0301; Study 0302; Study 0303; Study 306; Study 307) were judged to report adequate information leading to low risk of attrition bias. In the study published by Tyrrell et al, the proportion of missing outcomes might not have had a clinically relevant impact on the intervention effect estimate, leading to low risk of bias (Tyrrell 2006). Boccon‐Gibod et al reported data on overall survival incompletely (Boccon‐Gibod 1997). Therefore risk of bias regarding overall survival was high. Four studies (Dockery 2009; Sciarra 2004a; Sieber 2004; Smith 2004) did not measure/report these outcomes (unclear risk of bias).
Treatment discontinuation due to adverse events, adverse events
Three studies (Boccon‐Gibod 1997; Study 306; Study 307) were judged to report adequate information leading to low risk of attrition bias. In four studies, the proportion of missing outcomes might not have had a clinically relevant impact on the intervention effect estimate, leading to low risk of bias (Dockery 2009; Sieber 2004; Smith 2004; Tyrrell 2006). One study (Sciarra 2004a) did not measure/report these outcomes (unclear risk of bias). Three studies (Study 0301; Study 0302; Study 0303) present high risk of attrition bias. These studies reported data on an 'as‐treated' analysis with a high rate of dropout from the intervention assigned at randomisation.
Clinical progression, biochemical progression
Two studies (Study 306; Study 307) were judged to report adequate information leading to low risk of attrition bias. Five studies (Boccon‐Gibod 1997; Sciarra 2004a; Study 0301; Study 0302; Study 0303) present high risk of attrition bias. These studies reported data on an 'as‐treated' analysis with a high rate of dropout from the intervention group. Two studies (Dockery 2009; Sieber 2004) did not measure/report these outcomes (unclear risk of bias). In two studies, the proportion of missing outcomes might not have had a clinically relevant impact on the intervention effect estimate, leading to low risk of bias (Smith 2004; Tyrrell 2006).
Treatment failure
Two studies (Study 306; Study 307) were judged to report adequate information, leading to low risk of attrition bias. Four studies (Boccon‐Gibod 1997; Study 0301; Study 0302; Study 0303) were judged as having high risk of attrition bias. These studies reported data on an 'as‐treated' analysis with a high rate of dropout from the intervention group. One study (Sciarra 2004a) provided an outcome definition for treatment failure in the report but did not report any data for this outcome (high risk of bias). Four studies (Dockery 2009; Sieber 2004; Smith 2004; Tyrrell 2006) did not measure/report this outcome (unclear risk of bias).
Selective reporting
Three studies (Boccon‐Gibod 1997; Dockery 2009; Sciarra 2004a) had a high risk of reporting bias. Boccon‐Gibod et al reported incomplete data on overall survival at 69 months. They reported only "identical" survival in both groups, which was irrespective of the second‐line treatment given (Boccon‐Gibod 1997). Thus, their study could not be entered into the meta‐analysis. Dockery et al reported data on treatment discontinuation due to adverse events but did not report any data concerning individual adverse events (Dockery 2009), and Sciarra et al did not report data on adverse events, treatment discontinuation due to adverse events or treatment failure (Sciarra 2004a). We expected that these outcomes would be reported for such studies. We did not identify study protocols with adequate information on primary or secondary outcomes for all other studies; however, published reports included all of the expected outcomes.
Other potential sources of bias
In seven studies (Boccon‐Gibod 1997; Sieber 2004; Smith 2004; Study 0303; Study 306; Study 307; Tyrrell 2006), the trial authors reported possible conflicts of interest. In three studies (Sciarra 2004a; Study 0301; Study 0302), no conflicts of interest were declared. The authors of only one study (Dockery 2009) reported that they received an educational grant from the sponsor; however, they claimed that this sponsor had no role in any aspect of the study plan, protocol or analysis; data interpretation; or writing of the manuscript. Potential conflicts of interest may exist in any study, but we believe that in itself, this is not a reason for high risk of bias. Therefore, the risk of bias remains unclear for all studies.
Effects of interventions
See: Table 1
Overall survival
Of the 11 included studies, six studies (Study 0301; Study 0302; Study 0303; Study 306; Study 307; Tyrrell 2006) involving 2712 randomly assigned participants measured overall survival. The quality of evidence for this outcome was moderate (Table 1). One study (Boccon‐Gibod 1997) reported incomplete data and therefore could not be entered into the meta‐analysis. Overall survival was significantly decreased when non‐steroidal antiandrogens were used as opposed to castration (HR 1.24, 95% CI 1.10 to 1.40, fixed‐effect model; not shown). A random‐effects model for heterogeneity (I2 = 51%) still revealed a significant result (HR 1.24, 95% CI 1.05 to 1.48, 2712 participants; Analysis 1.1). We performed a sensitivity analysis because heterogeneity was noted (I2 = 51%). After exclusion of the smallest study (Tyrrell 2006), results still showed significant differences with lower heterogeneity (HR 1.31, 95% CI 1.12 to 1.53, I2 = 33%; not shown).
Subgroup: disease stage
A meta‐analysis of three studies (Study 306; Study 307; Tyrrell 2006) on non‐metastatic disease showed no significant difference in overall survival between non‐steroidal antiandrogens and castration (HR 1.00, 95% CI 0.79 to 1.26, 608 participants; Analysis 1.1). However, a meta‐analysis of six studies (Study 0301; Study 0302; Study 0303; Study 306; Study 307; Tyrrell 2006) showed that overall survival was significantly decreased with non‐steroidal antiandrogens in participants with metastatic disease when compared with castration (HR 1.34, 95% CI 1.14 to 1.57, 2103 participants; Analysis 1.1).
Subgroup: dose of non‐steroidal antiandrogen
The non‐steroidal antiandrogen bicalutamide given in doses of 50 mg daily or 150 mg daily significantly decreased overall survival when compared with castration using the fixed‐effect model (bicalutamide 50 mg daily: HR 1.45, 95% CI 1.20 to 1.74, 1196 participants; bicalutamide 150 mg daily: HR 1.19, 95% CI 1.00 to 1.41, 1288 participants; not shown). However, the random‐effects model showed that bicalutamide 50 mg daily still significantly decreased overall survival (HR 1.45, 95% CI 1.19 to 1.75, 1196 participants), although the effect was compatible with benefit or harm when bicalutamide 150 mg daily was used (HR 1.18, 95% CI 0.96 to 1.45, 1288 participants; Analysis 1.1). No significant difference was noted between high‐dose bicalutamide (450 mg daily or 600 mg daily) and castration (HR 0.88, 95% CI 0.62 to 1.25, 228 participants; Analysis 1.1).
Subgroup (post hoc analysis): non‐metastatic disease and dose of non‐steroidal antiandrogen
No significant difference was found between non‐steroidal antiandrogens (bicalutamide 150 mg daily compared with 450 mg daily or 600 mg daily) and castration in participants with non‐metastatic prostate cancer (Analysis 1.1).
Subgroup (post hoc analysis): metastatic disease and dose of non‐steroidal antiandrogen
The non‐steroidal antiandrogen bicalutamide given in doses of 50 mg daily or 150 mg daily decreased overall survival in participants with metastatic disease when compared with castration (bicalutamide 50 mg daily: HR 1.45, 95% CI 1.19 to 1.75, 1196 participants; bicalutamide 150 mg daily: HR 1.30, 95% CI 1.04 to 1.63, 808 participants; Analysis 1.1). No significant difference was found between high‐dose bicalutamide (450 mg daily or 600 mg daily) and castration in participants with metastatic disease (HR 0.91, 95% CI 0.56 to 1.48, 99 participants; Analysis 1.1).
Cancer‐specific mortality
We presented data for cancer‐specific mortality in place of cancer‐specific survival based on availability of data in the included studies. Three studies (Study 0301; Study 0302; Tyrrell 2006) involving 904 randomly assigned participants provided data on cancer‐specific mortality. Non‐steroidal antiandrogens probably increased cancer‐specific mortality when compared with castration (RR 1.26, 95% CI 1.00 to 1.59, fixed‐effect model; not shown). However, this difference was no longer statistically significant when a random‐effects model was applied as the result of heterogeneity (I2 = 67%, RR 1.32, 95% CI 0.86 to 2.05, 904 participants; Analysis 1.2). We performed a sensitivity analysis because heterogeneity was noted (I2 = 67%). After the smallest study had been excluded (Tyrrell 2006), results were still comparable but heterogeneity was greater (RR 1.63, 95% CI 0.71 to 3.73, I2 = 79%; not shown). The included studies reported cancer‐specific mortality based on different follow‐up periods (Study 0301 and Study 0302: after a minimum 12 months of follow‐up; Tyrrell 2006: after a median of five years of follow‐up). Analysis of the different follow‐up periods showed that non‐steroidal antiandrogens might increase cancer‐specific mortality after a minimum of 12 months when compared with castration (RR 1.43, 95% CI 1.05 to 1.95, fixed‐effect model; not shown). However, this difference was no longer significant after a random‐effects model was applied because of heterogeneity (RR 1.63, 95% CI 0.71 to 3.73, 680 participants, I2 = 79%; Analysis 1.2). We performed a sensitivity analysis because heterogeneity was present (I2 = 79%). After the smaller of the two included studies had been excluded (Study 0302), results of Study 0301 showed a significant difference (RR 2.60, 95% CI 1.30 to 5.07; not shown). No difference was found between these therapies after a median of five years (RR 1.04, 95% CI 0.73 to 1.47, 224 participants; Analysis 1.2). The overall effect of non‐steroidal antiandrogens on cancer‐specific mortality and even more on cancer‐specific survival therefore remains unclear.
Subgroup: disease stage
We did not perform subgroup analyses because very few studies were included for this outcome for which results were reported after different follow‐up periods. The conduct and presentation of meta‐analyses therefore did not seem appropriate.
Subgroup: dose of non‐steroidal antiandrogen
We did not perform subgroup analyses because very few studies were included for this outcome for which results were reported after different follow‐up periods. The conduct and presentation of meta‐analyses therefore did not seem appropriate.
Treatment discontinuation due to adverse events
Eight studies (Boccon‐Gibod 1997; Dockery 2009; Sieber 2004; Smith 2004; Study 0301; Study 0302; Study 0303; Tyrrell 2006) involving 1559 randomly assigned participants reported data on treatment discontinuation due to adverse events. Non‐steroidal antiandrogens significantly increased the rate of withdrawal due to adverse events (RR 1.82, 95% CI 1.13 to 2.94, 1559 participants; Analysis 1.3).
1.3. Analysis.
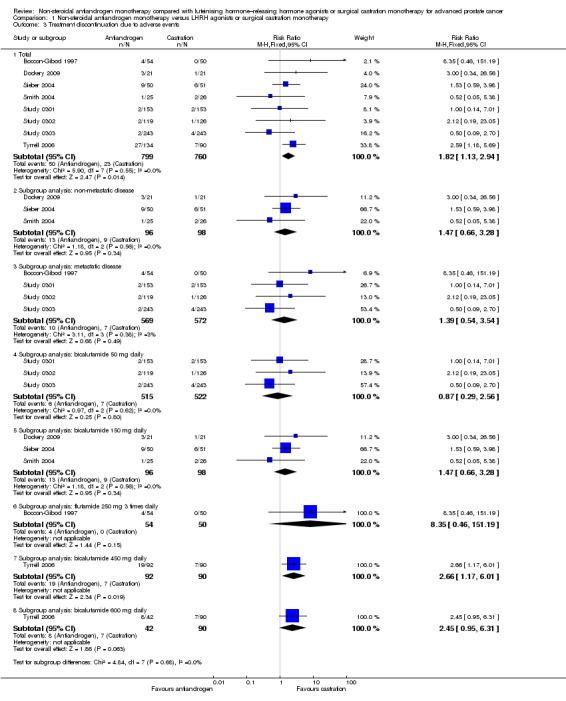
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 3 Treatment discontinuation due to adverse events.
Two studies (Study 306; Study 307) provided incomplete data on treatment discontinuation due to adverse events; thus, the data from these studies could not be included in the meta‐analysis. The trial authors reported that after 6.3 years, 4.1% of participants with non‐metastatic disease treated with bicalutamide (n = 314) were withdrawn; 1.3% of these withdrawals were due to breast pain and/or gynaecomastia (Study 306; Study 307). They reported no data for participants treated with castration. Two studies (Study 0301; Tyrrell 2006) did not specify the adverse events that led to discontinuation, and four studies (Sieber 2004; Study 0303; Study 306; Study 307) provided only partial information on adverse events. Smith et al reported that two participants in the leuprorelin group discontinued treatment early as the result of adverse events such as hot flashes and fatigue (Smith 2004). Additionally, treatment with bicalutamide was interrupted in one participant for three months because of elevated liver enzymes (Smith 2004). In the study conducted by Sieber et al, five of nine participants who withdrew from the study in the bicalutamide group discontinued treatment as the result of asthenia (Sieber 2004). In another study, four participants discontinued treatment because of adverse events; two participants withdrew because of impotence (one in each group for bicalutamide and castration) and two withdrew because of a skin reaction (both in the bicalutamide group) (Dockery 2009). In Study 0303, six participants discontinued treatment (three with rash and one with constipation), and in Study 0302, three participants withdrew from the study (in the group treated with bicalutamide, one withdrew because of gynaecomastia and back pain; in the group treated with castration, one withdrew because of severe hot flashes). Boccon‐Gibod et al reported that four participants discontinued therapy; two were suffering from nausea or vomiting, one reported diarrhoea and another showed an increase in hepatic enzymes before discontinuing therapy (Boccon‐Gibod 1997).
Subgroup: disease stage
The subgroup analysis included seven studies: three studies (Dockery 2009; Sieber 2004; Smith 2004) including participants with non‐metastatic disease, and four studies (Boccon‐Gibod 1997; Study 0301; Study 0302; Study 0303) including participants with metastatic disease. No significant difference was found between non‐steroidal antiandrogens and castration for participants with non‐metastatic (RR 1.47, 95% CI 0.66 to 3.28, 194 participants) or metastatic disease (RR 1.39, 95% CI 0.54 to 3.54, 1141 participants; Analysis 1.3). Data reported by Tyrrell et al could not be included into this analysis because they were not reported for subgroups of participants on the basis of disease stage (Tyrrell 2006).
Subgroup: dose of non‐steroidal antiandrogen
One study evaluated the non‐steroidal antiandrogen flutamide 250 mg three times daily (Boccon‐Gibod 1997), three studies evaluated the non‐steroidal antiandrogen bicalutamide 50 mg daily (Study 0301; Study 0302; Study 0303), three studies evaluated bicalutamide 150 mg daily (Dockery 2009; Sieber 2004; Smith 2004) and one study evaluated bicalutamide 450 mg daily and 600 mg daily (Tyrrell 2006). No significant differences were found for bicalutamide 50 mg daily, bicalutamide 150 mg daily or flutamide 250 mg three times daily (Analysis 1.3). However, the numbers of treatment discontinuations due to adverse events were significantly increased when bicalutamide 450 mg daily was used (RR 2.66, 95% CI 1.17 to 6.01, 182 participants). No significant differences were found between bicalutamide 600 mg daily and castration (RR 2.45, 95% CI 0.95 to 6.31, 132 participants; Analysis 1.3).
Clinical progression
Seven studies (Sciarra 2004a; Study 0301; Study 0302; Study 0303; Study 306; Study 307; Tyrrell 2006) involving 2591 randomly assigned participants were included in the meta‐analyses for clinical progression. For the definitions of clinical progression, see the Characteristics of included studies table. Two studies (Boccon‐Gibod 1997; Smith 2004) reported data on an outcome they referred to as “clinical progression.” However, we included the data in an analysis of biochemical progression because the definition provided in the reports was consistent with our previously established definition of biochemical progression. Non‐steroidal antiandrogens significantly increased clinical progression at one year, at 70 weeks and at two years when compared with castration, but no significant differences were found at three, four or five years when the fixed‐effect model was used (at one year: RR 1.27, 95% CI 1.14 to 1.41, 2067 participants; at 70 weeks: RR 1.27, 95% CI 1.16 to 1.38, 2373 participants; at two years: RR 1.13, 95% CI 1.03 to 1.24, 1336 participants; at three years: RR 1.04, 95% CI 0.87 to 1.23, 480 participants; at four years: RR 1.07, 95% CI 0.91 to 1.26, 480 participants; at five years: RR 0.96, 95% CI 0.87 to 1.06, 698 participants; not shown). The random‐effects model due to heterogeneity (I2 = 64%) at 70 weeks still showed comparable results (at one year: RR 1.25, 95% CI 1.08 to 1.45, 2067 participants; at 70 weeks: RR 1.26, 95% CI 1.08 to 1.45, 2373 participants; at two years: RR 1.14, 95% CI 1.04 to 1.25, 1336 participants; at three years: RR 1.04, 95% CI 0.87 to 1.23, 480 participants; at four years: RR 1.07, 95% CI 0.91 to 1.26, 480 participants; at five years: RR 0.96, 95% CI 0.88 to 1.06, 698 participants; Analysis 1.4). We performed a sensitivity analysis for clinical progression at 70 weeks because we noted heterogeneity (I2 = 64%). After the smallest study had been excluded (Study 0302), results still showed significant differences with lower heterogeneity (RR 1.33, 95% CI 1.19 to 1.48, I2 = 13%; not shown). Five studies (Sciarra 2004a; Study 0301; Study 0302; Study 0303; Tyrrell 2006) did not report ITT analysis data, but findings were summarised instead according to treatment received. An analysis that considered data imputations for the best‐case and worst‐case scenarios still showed significant results at one year, 70 weeks and two years but not at five years (Analysis 1.5). This analysis involved 2771 randomly assigned participants. The quality of evidence for clinical progression was moderate (Table 1).
Subgroup: disease stage
No significant differences were found between non‐steroidal antiandrogens and castration for participants with non‐metastatic disease at all evaluated time points (Analysis 1.4). An analysis considering data imputations for the best‐case and worst‐case scenarios showed comparable results (Analysis 1.5). Five studies were included in the subgroup analysis of participants with metastatic disease (Study 0301; Study 0302; Study 0303; Study 306; Study 307). Clinical progression at one year (RR 1.25, 95% CI 1.05 to 1.49, I2 = 64%, 1539 participants), at 70 weeks (RR 1.27, 95% CI 1.07 to 1.51, I2 = 74%, 1845 participants) and at two years (RR 1.17, 95% CI 1.05 to 1.29, 808 participants) increased with non‐steroidal antiandrogens when compared with castration in participants with metastatic disease (Analysis 1.4). We performed sensitivity analyses for clinical progression at one year and at 70 weeks because heterogeneity was present. After the smallest study had been excluded (Study 0302), results still showed significant differences with lower heterogeneity (at one year: RR 1.35, 95% CI 1.13 to 1.61, I2 = 42%; at 70 weeks: RR 1.35, 95% CI 1.18 to 1.55, I2 = 34%; not shown). The results remained significant after an analysis was performed by considering data imputations for best‐case and worst‐case scenarios (Analysis 1.5).
Subgroup: dose of non‐steroidal antiandrogen
The non‐steroidal antiandrogen bicalutamide at a dose of 50 mg daily showed no significant difference when compared with castration (at one year: RR 1.27, 95% CI 0.91 to 1.76, I2 = 83%, 731 participants; at 70 weeks: RR 1.30, 95% CI 0.99 to 1.71, 1037 participants, I2 = 84%; Analysis 1.4). We performed sensitivity analyses because heterogeneity was present. After the smallest study had been excluded (Study 0302), results showed significant differences with lower heterogeneity (at one year: RR 1.49, 95% CI 1.21 to 1.85; at 70 weeks: RR 1.47, 95% CI 1.26 to 1.72, I2 = 0%; not shown). The analysis considering data imputations for best‐case and worst‐case scenarios showed a significant increase in clinical progression with bicalutamide 50 mg daily at 70 weeks (RR 1.40, 95% CI 1.04 to 1.88, 1196 participants), but no difference was found at one year (Analysis 1.5). The non‐steroidal antiandrogen bicalutamide at a dose of 150 mg daily might increase clinical progression at one year (RR 1.25, 95% CI 1.07 to 1.46, 1336 participants), at 70 weeks (RR 1.22, 95% CI 1.07 to 1.39, 1336 participants) or at two years (RR 1.14, 95% CI 1.04 to 1.25, 1336 participants), but no differences were noted when compared with castration at three, four or five years (Analysis 1.4). An analysis considering data imputations for best‐case and worst‐case scenarios showed comparable results (Analysis 1.5). No significant differences were found between high‐dose bicalutamide (450 mg daily or 600 mg daily) and castration at five years.
Biochemical progression
Three studies (Boccon‐Gibod 1997; Sciarra 2004a; Smith 2004) involving 185 randomly assigned participants were included in the analysis of biochemical progression. For the definitions of biochemical progression in included studies, see the Characteristics of included studies table. The analysis considering data imputations for best‐case and worst‐case scenarios involved 214 randomly assigned participants. No significant differences were found between the non‐steroidal antiandrogen and castration groups at any of the evaluated time points (Analysis 1.6; Analysis 1.7). The study conducted by Smith et al was not designed to evaluate clinical cancer outcomes including clinical or biochemical progression (for details, see Characteristics of included studies). The overall effect on biochemical progression therefore remains unclear.
1.6. Analysis.
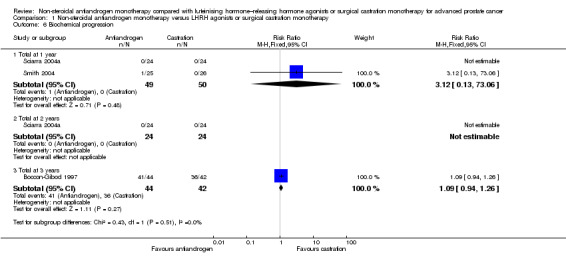
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 6 Biochemical progression.
Subgroup: disease stage
We did not perform subgroup analyses because very few studies were included for this outcome for which results were reported after different follow‐up periods. The conduct and presentation of meta‐analyses therefore did not seem appropriate.
Subgroup: dose of non‐steroidal antiandrogen
We did not perform subgroup analyses because very few studies were included for this outcome for which results were reported after different follow‐up periods. The conduct and presentation of meta‐analyses therefore did not seem appropriate.
Treatment failure
Six studies (Boccon‐Gibod 1997; Study 0301; Study 0302; Study 0303; Study 306; Study 307) involving 2411 randomly assigned participants reported data on treatment failure. For the definition of treatment failure, see the Characteristics of included studies table. Non‐steroidal antiandrogens increased treatment failure at one year, at 70 weeks and at two years, but no difference was found at three or four years (Analysis 1.8). The random‐effects model for heterogeneity revealed significant results (at one year: I2 = 63%, RR 1.19, 95% CI 1.02 to 1.38, 1539 participants; at 70 weeks: I2 = 81%, RR 1.27, 95% CI 1.05 to 1.52, 1845 participants; at two years: RR 1.14, 95% CI 1.05 to 1.24, 808 participants; Analysis 1.8). We performed sensitivity analyses because heterogeneity was present. After the smallest study had been excluded (Study 0302), results still showed significant differences with lower heterogeneity (at one year: RR 1.26, 95% CI 1.08 to 1.47, I2 = 53%; at 70 weeks: RR 1.36, 95% CI 1.14 to 1.62, I2 = 69%; not shown). An analysis considering data imputations for best‐case and worst‐case scenarios showed comparable results (Analysis 1.9). This analysis involved 2004 randomly assigned participants. The quality of evidence for treatment failure was moderate (Table 1).
Subgroup: disease stage
The subgroup analysis for non‐metastatic prostate cancer included two studies (Study 306; Study 307) and showed no significant differences between non‐steroidal antiandrogens and castration at four years (RR 1.04, 95% CI 0.93 to 1.16, 480 participants; Analysis 1.8). For participants with metastatic prostate cancer, non‐steroidal antiandrogens increased treatment failure at one year (RR 1.19, 95% CI 1.02 to 1.38, I2 = 63%, 1539 participants), at 70 weeks (RR 1.27, 95% CI 1.05 to 1.52, I2 = 81%, 1845 participants) and at two years (RR 1.14, 95% CI 1.05 to 1.24, 808 participants). We performed sensitivity analyses for treatment failure at one year and at 70 weeks because heterogeneity was present. After the smallest study had been excluded (Study 0302), results still showed significant differences with lower heterogeneity (at one year: RR 1.26, 95% CI 1.08 to 1.47, I2 = 53%; at 70 weeks: RR 1.36, 95% CI 1.14 to 1.62, I2 = 69%; not shown). No significant difference was found at three years (Analysis 1.8). An analysis considering data imputations for best‐case and worst‐case scenarios revealed comparable results (Analysis 1.9).
Subgroup: dose of non‐steroidal antiandrogen
No significant differences were found between the non‐steroidal antiandrogen bicalutamide at a dose of 50 mg daily and castration at any of the time points assessed using the random‐effects model for heterogeneity (Analysis 1.8). However, the analysis considering data imputations for best‐case and worst‐case scenarios showed that without heterogeneity, bicalutamide at 50 mg daily significantly increased treatment failure at one year and at 70 weeks (Analysis 1.9). Additionally, the non‐steroidal antiandrogen bicalutamide at a dose of 150 mg daily significantly increased treatment failure at one year (RR 1.17, 95% CI 1.01 to 1.35, 808 participants), at 70 weeks (RR 1.18, 95% CI 1.05 to 1.34, 808 participants) and at two years (RR 1.14, 95% CI 1.05 to 1.24, 808 participants). No difference was found at four years (Analysis 1.8; Analysis 1.9). One study (Boccon‐Gibod 1997) assessed the non‐steroidal antiandrogen flutamide at a dose of 250 mg three times daily compared with castration and showed no significant differences at three years (Analysis 1.8; Analysis 1.9).
Adverse events
Nine studies (Boccon‐Gibod 1997; Sieber 2004; Smith 2004; Study 0301; Study 0302; Study 0303; Study 306; Study 307; Tyrrell 2006) reported data on adverse events associated with treatment with non‐steroidal antiandrogens compared with castration.
Non‐steroidal antiandrogens were associated with a significantly increased occurrence of breast pain (RR 22.97, 95% CI 14.79 to 35.67, 2670 participants; Analysis 1.10). Subgroup analyses showed that this was also evident for bicalutamide at a dose of 50 mg, 150 mg, 450 mg or 600 mg daily (Analysis 1.10).
1.10. Analysis.
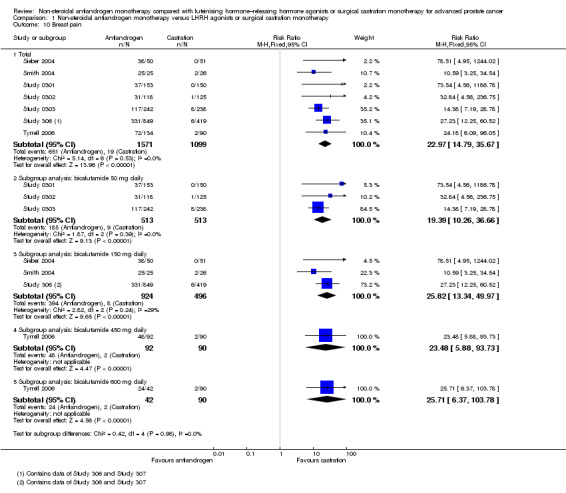
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 10 Breast pain.
The risk of suffering gynaecomastia was increased with non‐steroidal antiandrogens (RR 8.43, 95% CI 3.19 to 22.28, 2774 participants; Analysis 1.17). We performed a sensitivity analysis because considerable heterogeneity was noted (I2 = 92%). After the smallest study had been excluded (Smith 2004), results still showed significant differences with lower heterogeneity (RR 9.34, 95% CI 5.43 to 16.05, I2 = 53%; not shown). Subgroup analyses showed that gynaecomastia occurred more often with bicalutamide 50 mg daily (RR 14.07, 95% CI 3.74 to 52.85), flutamide 250 mg three times daily (RR 3.70, 95% CI 1.33 to 10.33), bicalutamide 450 mg daily (RR 27.88, 95% CI 7.02 to 110.79) and bicalutamide 600 mg daily (RR 20.36, 95% CI 4.97 to 83.40). However, no significant difference was found between bicalutamide 150 mg daily and castration. We performed a sensitivity analysis because heterogeneity (I2 = 97%) was present for this comparison. After the smallest study had been excluded (Smith 2004), a significant increase in gynaecomastia with reduced heterogeneity was found with bicalutamide 150 mg daily (RR 8.79, 95% CI 3.88 to 18.94, I2 = 67%; not shown).
The occurrence of asthenia was significantly increased when non‐steroidal antiandrogens were used compared with castration (RR 1.77, 95% CI 1.36 to 2.31, 2073 participants; Analysis 1.23). Subgroup analyses showed higher incidences of asthenia with bicalutamide 50 mg, 150 mg and 450 mg daily (Analysis 1.23). No significant difference was found between bicalutamide 600 mg daily and castration (RR 2.45, 95% CI 0.95 to 6.31, 132 participants; Analysis 1.23).
1.23. Analysis.
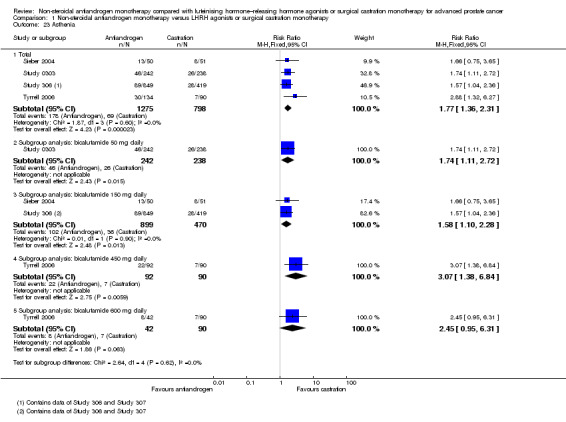
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 23 Asthenia.
No differences in the risk of suffering arthralgia were found between non‐steroidal antiandrogens and castration in overall analysis and subgroup analysis for bicalutamide 600 mg daily (Analysis 1.46). However, the occurrence of arthralgia was significantly increased with the non‐steroidal antiandrogen bicalutamide at a dose of 450 mg daily compared with castration (RR 1.96, 95% CI 1.01 to 3.80, 182 participants; Analysis 1.46).
1.46. Analysis.
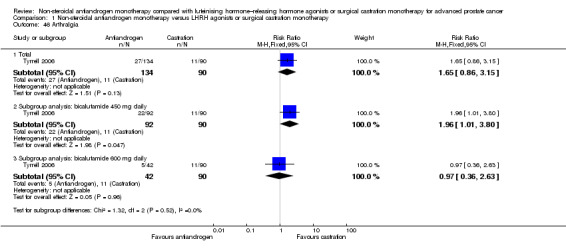
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 46 Arthralgia.
One small study (Smith 2004) of participants receiving bicalutamide 150 mg daily showed that non‐steroidal antiandrogens might preserve sexual interest compared with castration (RR 0.50, 95% CI 0.30 to 0.83, 51 participants; Analysis 1.22).
1.22. Analysis.
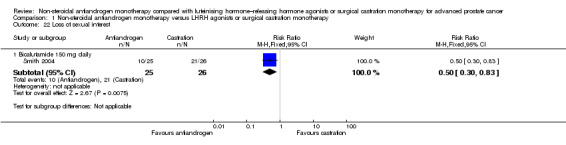
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 22 Loss of sexual interest.
Risk of hot flashes (RR 0.23, 95% CI 0.19 to 0.27, 2774 participants; Analysis 1.25), haemorrhage (RR 0.07, 95% CI 0.01 to 0.54, 546 participants; Analysis 1.38), nocturia (RR 0.38, 95% CI 0.20 to 0.69, 480 participants; Analysis 1.40), urinary frequency (RR 0.22, 95% CI 0.11 to 0.47, 480 participants; Analysis 1.41) and occurrence of fatigue (RR 0.52, 95% CI 0.31 to 0.88, 51 participants; Analysis 1.49) was decreased with non‐steroidal antiandrogens compared with castration. These significant differences were also evident for all subgroup analyses regarding the different doses of non‐steroidal antiandrogens.
1.25. Analysis.
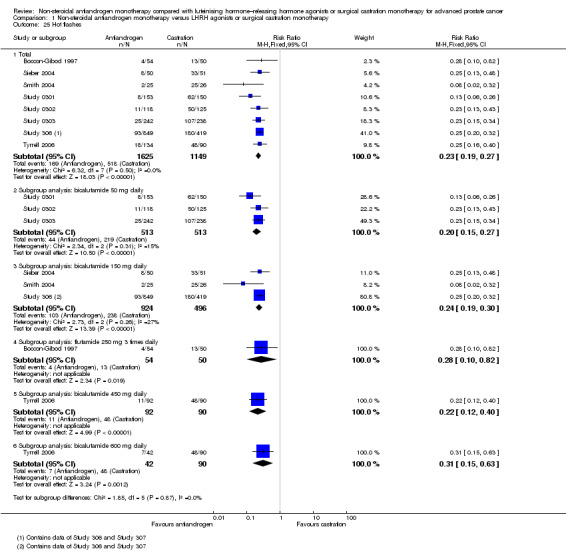
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 25 Hot flashes.
1.38. Analysis.
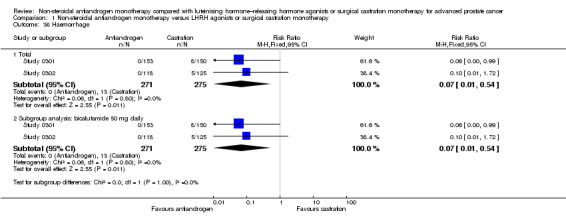
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 38 Haemorrhage.
1.40. Analysis.
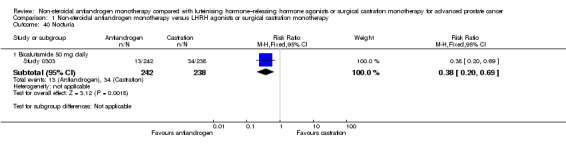
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 40 Nocturia.
1.41. Analysis.
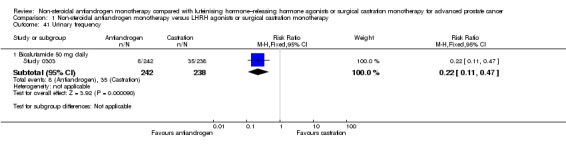
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 41 Urinary frequency.
1.49. Analysis.
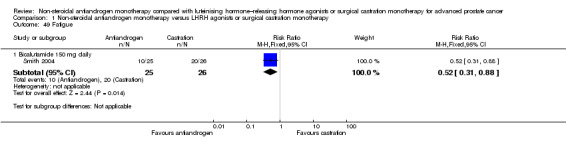
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 49 Fatigue.
The overall risk to suffer night sweats was decreased with non‐steroidal antiandrogens compared with castration (RR 0.29, 95% CI 0.17 to 0.49, 1571 participants; Analysis 1.26). However, although a significant difference was noted in the subgroup of participants treated with bicalutamide 150 mg daily (RR 0.26, 95% CI 0.14 to 0.49, 1268 participants), this finding was not evident for participants treated with bicalutamide 50 mg daily (RR 0.36, 95% CI 0.12 to 1.09, 303 participants).
1.26. Analysis.
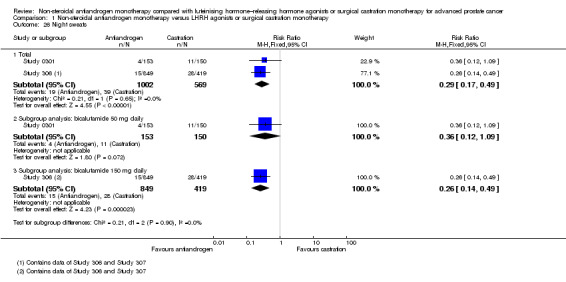
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 26 Night sweats.
Infection occurred less frequently with bicalutamide at 50 mg daily but showed no significant difference for overall analysis or bicalutamide at 150 mg daily when compared with castration (Analysis 1.32).
1.32. Analysis.
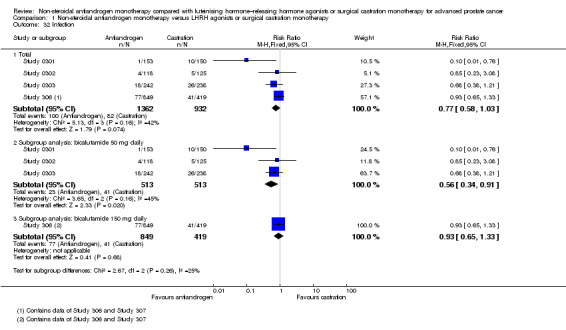
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 32 Infection.
The occurrence of peripheral oedema was significantly decreased for bicalutamide at 50 mg daily (RR 0.42, 95% CI 0.21 to 0.82, 480 participants); however, we found no statistically significant difference for bicalutamide at 150 mg daily compared with castration and in overall analysis (Analysis 1.43).
1.43. Analysis.
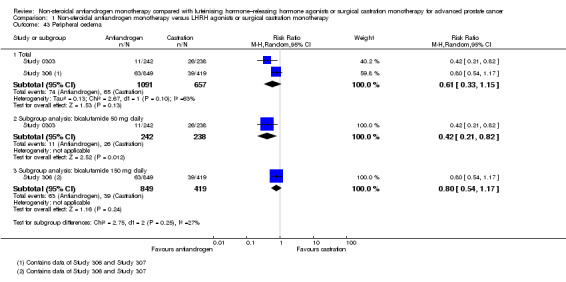
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 43 Peripheral oedema.
We found an increased occurrence of constipation (Analysis 1.18) and a decreased risk of anaemia (Analysis 1.27) with higher doses of non‐steroidal antiandrogens compared with castration.
1.18. Analysis.
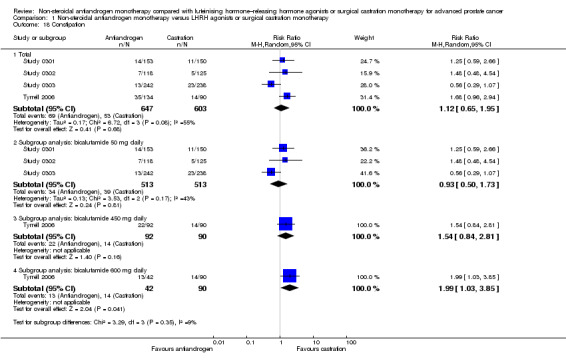
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 18 Constipation.
1.27. Analysis.
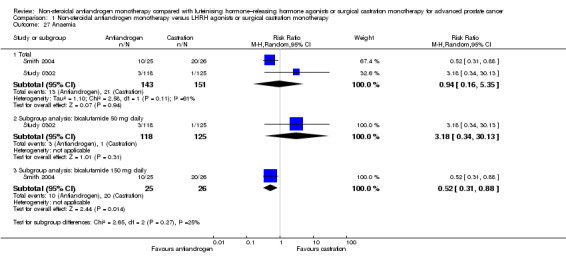
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 27 Anaemia.
No significant difference between non‐steroidal antiandrogens and castration was noted for occurrence of haematuria (Analysis 1.39). However, results of the meta‐analysis including two studies (Study 0303; Study 306) show considerable heterogeneity (I2 = 97%). Subgroup analyses showed a significantly decreased risk with bicalutamide 50 mg daily but an increased risk with bicalutamide 150 mg daily to suffer haematuria when compared with castration (bicalutamide 50 mg daily: RR 0.41, 95% CI 0.26 to 0.67, 480 participants; bicalutamide 150 mg daily: RR 3.49, 95% CI 2.01 to 6.05, 474 participants; Analysis 1.39).
1.39. Analysis.
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 39 Haematuria.
Conflicting results were found for the occurrence of vomiting because events in both groups were very rare (Analysis 1.20).
1.20. Analysis.
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 20 Vomiting.
We identified no statistically significant differences for the following adverse events when we compared non‐steroidal antiandrogens with castration: pelvic pain (Analysis 1.11), bone pain (Analysis 1.12), back pain (Analysis 1.13), headache (Analysis 1.14), abdominal pain (Analysis 1.15), general pain (Analysis 1.16), gastralgia (Analysis 1.47), diarrhoea (Analysis 1.19), hypertension (Analysis 1.21), nausea (Analysis 1.48), insomnia (Analysis 1.24), hepatic enzyme increase (Analysis 1.28), rash (Analysis 1.29), pruritus (Analysis 1.30), dyspnoea (Analysis 1.31), pharyngitis (Analysis 1.33), arthritis (Analysis 1.34), sinusitis (Analysis 1.35), urinary tract infection (Analysis 1.36), dizziness (Analysis 1.37), urinary retention (Analysis 1.42), anorexia (Analysis 1.44), loss of sexual function (Analysis 1.45), dry skin (Analysis 1.50), aggravation reaction (Analysis 1.51) and serious adverse events (Analysis 1.52).
1.11. Analysis.
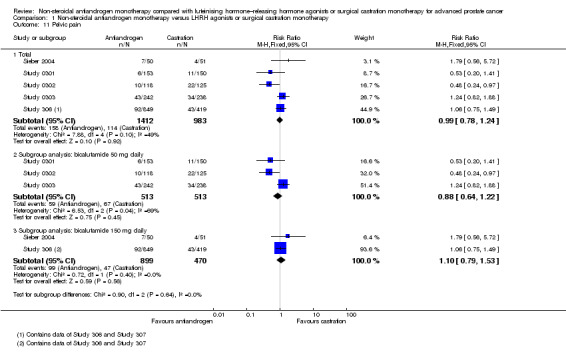
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 11 Pelvic pain.
1.12. Analysis.
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 12 Bone pain.
1.13. Analysis.
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 13 Back pain.
1.14. Analysis.
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 14 Headache.
1.15. Analysis.
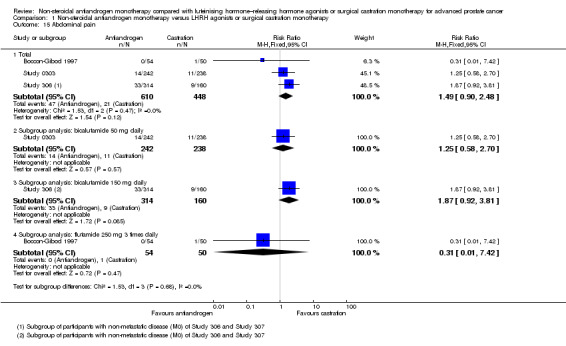
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 15 Abdominal pain.
1.16. Analysis.
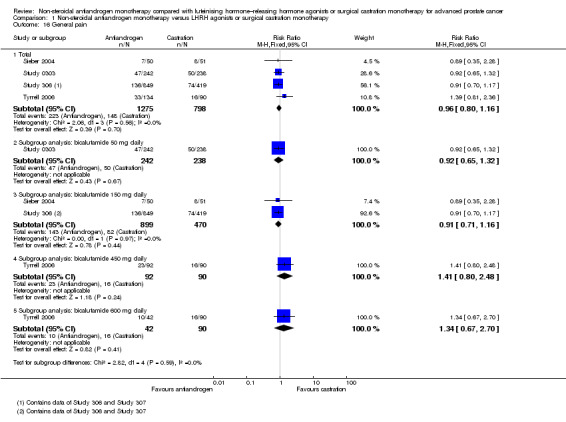
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 16 General pain.
1.47. Analysis.
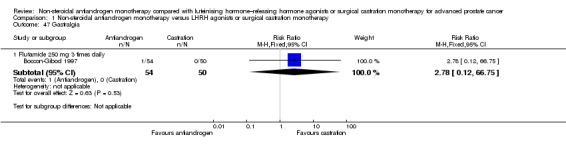
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 47 Gastralgia.
1.19. Analysis.
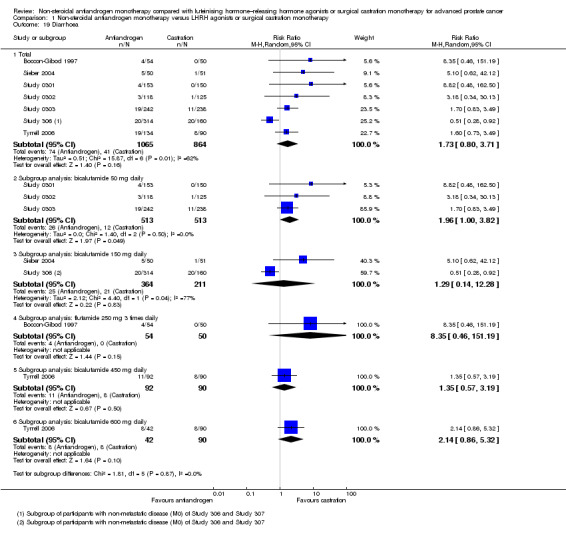
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 19 Diarrhoea.
1.21. Analysis.
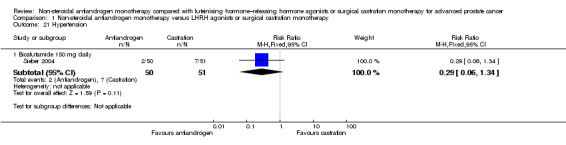
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 21 Hypertension.
1.48. Analysis.
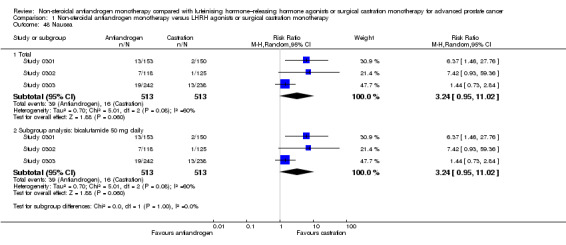
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 48 Nausea.
1.24. Analysis.
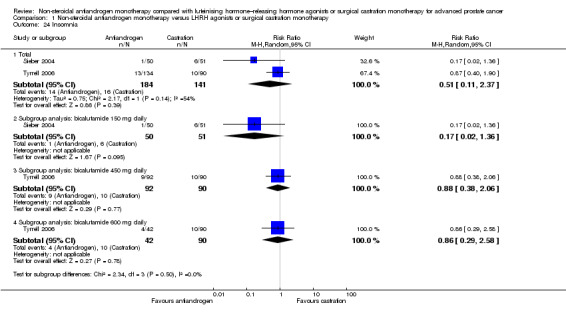
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 24 Insomnia.
1.28. Analysis.
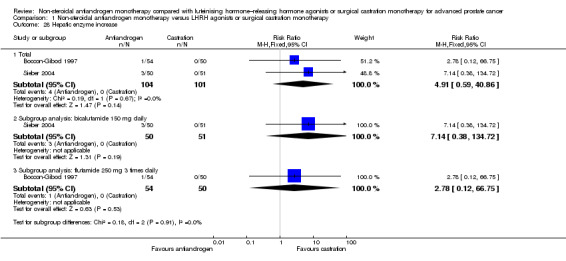
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 28 Hepatic enzyme increase.
1.29. Analysis.
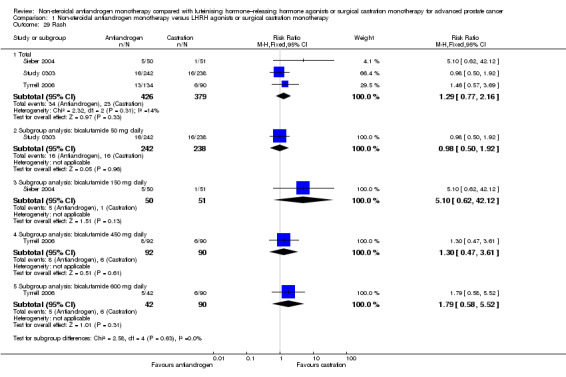
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 29 Rash.
1.30. Analysis.
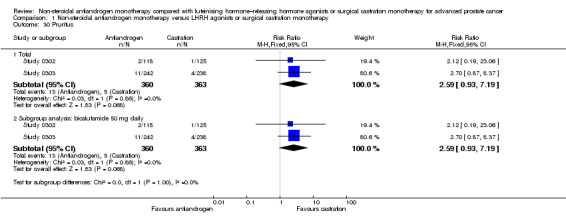
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 30 Pruritus.
1.31. Analysis.
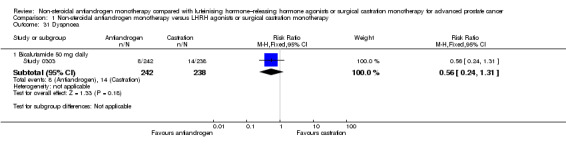
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 31 Dyspnoea.
1.33. Analysis.
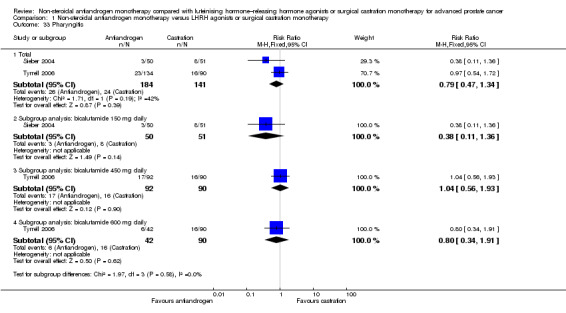
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 33 Pharyngitis.
1.34. Analysis.
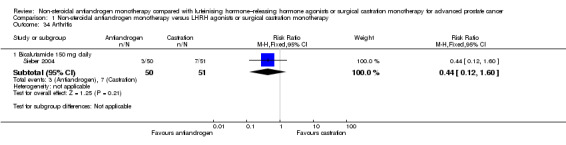
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 34 Arthritis.
1.35. Analysis.
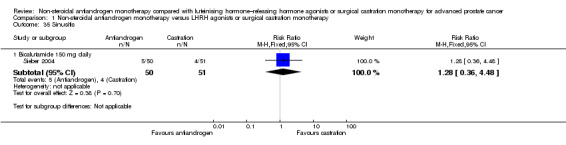
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 35 Sinusitis.
1.36. Analysis.
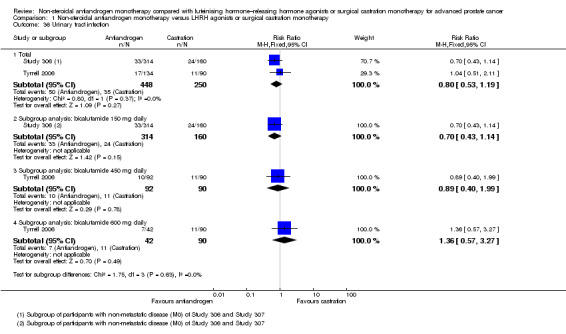
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 36 Urinary tract infection.
1.37. Analysis.
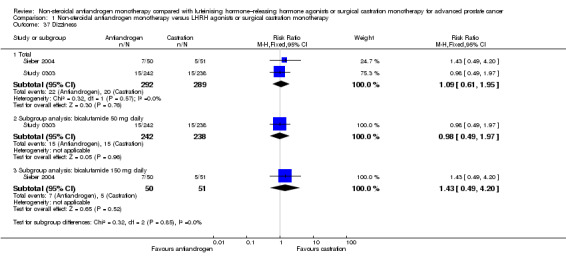
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 37 Dizziness.
1.42. Analysis.
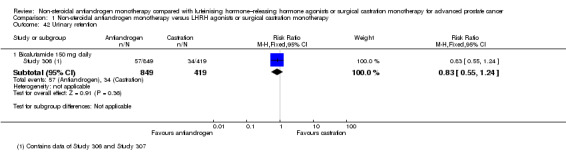
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 42 Urinary retention.
1.44. Analysis.
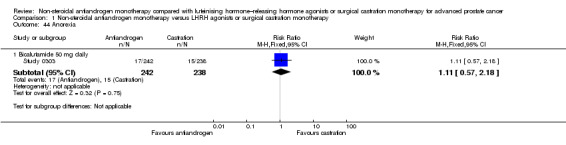
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 44 Anorexia.
1.45. Analysis.
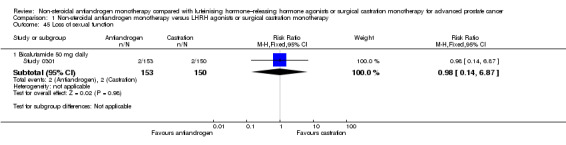
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 45 Loss of sexual function.
1.50. Analysis.
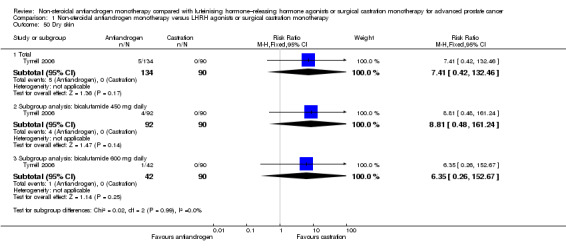
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 50 Dry skin.
1.51. Analysis.
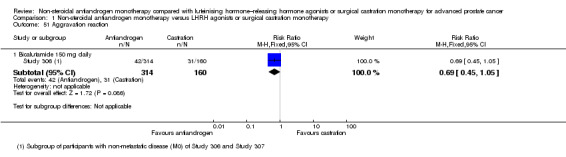
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 51 Aggravation reaction.
1.52. Analysis.
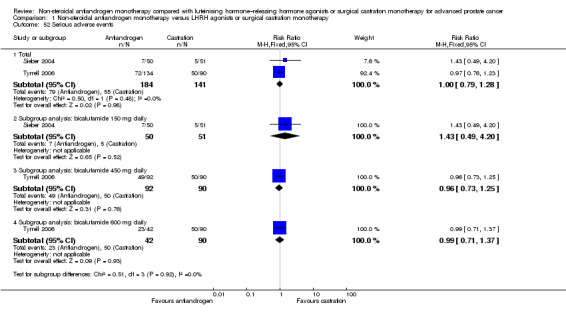
Comparison 1 Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy, Outcome 52 Serious adverse events.
No study reported data on the predefined outcomes of cardiovascular events, gastrointestinal disorders and lethargy.
The quality of evidence for breast pain, gynaecomastia and hot flashes was moderate (Table 1).
Discussion
Summary of main results
Eleven studies were included. The quality of the evidence for overall survival, clinical progression, treatment failure, breast pain, gynaecomastia and hot flashes was moderate (Table 1). Non‐steroidal antiandrogens significantly decreased overall survival and increased clinical progression as well as treatment failure. Subgroup analyses showed that non‐steroidal antiandrogens, compared with castration, were consistently less favourable for overall survival, clinical progression and treatment failure in men with metastatic disease. Additionally, less favourable effects were seen with the non‐steroidal antiandrogen bicalutamide 50 mg daily for overall survival, clinical progression with imputed event numbers at 70 weeks and treatment failure with imputed event numbers at 70 weeks, as well as for the non‐steroidal antiandrogen bicalutamide 150 mg daily for clinical progression and treatment failure at one year, 70 weeks and two years, when compared with castration. Non‐steroidal antiandrogens also increased the risk for treatment discontinuation due to adverse events and increased the risk of breast pain, gynaecomastia and asthenia. The risk of other adverse events, such as hot flashes, fatigue, loss of sexual interest, haemorrhage, nocturia and urinary frequency, was significantly increased with castration. Effects of non‐steroidal antiandrogens on cancer‐specific survival and biochemical progression remained unclear.
Overall completeness and applicability of evidence
The included studies examined clinically important populations that were representative of patients seen in routine clinical practice. These participants and assessed interventions directly conformed to the review question. However, several points must be considered regarding the applicability of evidence.
The included studies provided data on our predefined outcomes. However, four studies (Dockery 2009; Sciarra 2004a; Sieber 2004; Smith 2004) did not address the review question directly and instead assessed primary outcomes that were not relevant to the review question, such as bone mineral density (Sieber 2004; Smith 2004), arterial stiffness (Dockery 2009), metabolic changes (Sieber 2004; Smith 2004) and markers of neuroendocrine differentiation (Sciarra 2004a). However, these studies also reported data on adverse events and/or progression and therefore were included in the review.
Only three studies were evaluated for biochemical progression. However, this outcome was defined by the authors in two of the three studies (Boccon‐Gibod 1997; Smith 2004) as clinical progression. In accordance with our predetermined definition, we classified these reported outcomes as biochemical progression because a PSA measurement was used for the definition of this outcome. The definition of biochemical progression varied among the included studies. The results of this outcome assessment should be interpreted carefully.
The two largest included studies (Study 306; Study 307) assessed participants with non‐metastatic and metastatic prostate cancer. PSA values were measured for participants with non‐metastatic disease only. For participants in the non‐steroidal antiandrogen group, these values ranged between 0.1 and 7691 ng/mL (median 69.2 ng/mL, mean 173.2 ng/mL). These rather high PSA values might no longer represent populations with non‐metastatic disease and might lead to bias in the evaluations.
We included seven studies (Boccon‐Gibod 1997; Study 0301; Study 0302; Study 0303; Study 306; Study 307; Tyrrell 2006) that assessed men treated with surgical castration. This therapy is suggested as a potential alternative to medical castration (Abrahamsson 2005; ASCO 2004; ASCO 2007; Seidenfeld 1999; Seidenfeld 2000). However, surgical castration could lead to potential psychological strain, and the resulting adverse events are only partially treatable. Nyman et al suggested that when patients can choose between different androgen suppression therapy options (non‐steroidal antiandrogens and medical or surgical castration) and receive comprehensive information about the treatment, nearly all patients are satisfied with their choice after three months of treatment (Nyman 2005). However, it should be mentioned that the clinical heterogeneity of these treatments might lead to bias in our results regarding treatment discontinuation due to adverse events because reversal of surgical castration is not possible.
Quality of the evidence
Most of the included studies reported insufficient information on sequence generation and allocation concealment.
No study performed blinding of participants and personnel because different therapy options were included (surgical castration, oral medication and injection therapy). Opinions vary as to whether lack of blinding has a relevant impact on outcomes such as overall survival, cancer‐specific mortality and biochemical progression. It might be conceivable that these outcomes are influenced by lack of blinding. Therefore we judged that risk of bias for most of the included studies is unclear regarding overall survival, cancer‐specific mortality and biochemical progression. Outcomes such as clinical progression, treatment failure, treatment discontinuation due to adverse events and adverse events could be influenced by lack of blinding, presenting a high risk of bias in most of the included studies. Therefore the effects of intervention may have been overestimated (Als‐Nielsen 2004; Pildal 2007; Wood 2008).
In all studies, no blinding of outcome assessment was performed or blinding was not reported or underlying methodology remained unclear. However, this type of blinding would have been feasible and could have been expected in all studies. We suggest that it is not likely that outcome assessments for overall survival, cancer‐specific mortality and biochemical progression are influenced by lack of blinding. For outcomes such as clinical progression, treatment failure, treatment discontinuation due to adverse events and adverse events, this lack of blinding might, however, introduce detection bias due to potentially overestimated intervention effects (Pildal 2007).
The results of five studies (Boccon‐Gibod 1997; Sciarra 2004a; Study 0301; Study 0302; Study 0303) were based on data for which risk for incomplete outcomes was high. A risk of bias is present because per‐protocol analyses may lead to overestimated effects (Akl 2012; Meerpohl 2010; Porta 2007; Schulz 1996; Tierney 2005; Wood 2004). We performed sensitivity analyses based on best‐/worst‐case scenarios for outcomes such as treatment failure (Analysis 1.9), biochemical progression (Analysis 1.7) and clinical progression (Analysis 1.5) with imputations of missing data to minimise this bias because ITT analyses are regarded as the preferred way to estimate the effects of interventions in randomised controlled trials (Newell 1992).
Possible conflicts of interest should be considered for all of the included studies because the trial authors reported a possible conflict of interest or provided no disclosure statement. Conflicts of interest are common in the field of urology (Hampson 2012; Ramm 2012). Conflicts of interest may introduce a risk of bias because studies funded by industry have been shown to be more likely to report positive results than studies with other funding sources (Gøtzsche 2006; Okike 2007; Shah 2005). However, the risk of bias remains unclear because lack of a disclosure statement in itself is not an indicator of bias. Peer reviewers and journal editors may require conflict of interest disclosures at any step of the peer review process without providing a summary statement on the issue (Meerpohl 2010).
Non‐steroidal antiandrogens were assessed using subgroup analyses regarding disease stage. The two largest included studies (Study 306; Study 307) recruited participants with metastatic or non‐metastatic prostate cancer. However, participants with metastatic disease withdrew from the study early (after 100 weeks) and were excluded from further evaluations. We did not examine the protocols for these studies; it is therefore unclear whether the subgroup analyses were predefined. Post hoc subgroup analyses are common and might introduce a risk that differences in effect sizes across subgroups could produce statistically significant differences (Sun 2009; Sun 2012; Wang 2010). The influence of subgroup effects might be low (Sun 2012; Wang 2010) and should be interpreted carefully.
Overall, we identified several methodological limitations during our assessment of risk of bias, leading to downgrading of the quality of evidence for study limitations (section Characteristics of included studies; Figure 3). No indication of publication bias was found by funnel plot asymmetry analysis for our primary outcome, and we believe that risk of publication bias might be low (Risk of bias in included studies; Figure 2). Heterogeneity was noted for overall survival, clinical progression, treatment failure and gynaecomastia. However, this heterogeneity might be explained by subgroup or sensitivity analyses (see Effects of interventions); we believe that it should not be required that the quality of the evidence should be downgraded for inconsistency. Additionally, we believe that it might not be necessary to downgrade the quality of the evidence because of imprecision. The quality of the body of evidence for outcomes such as overall survival, clinical progression, treatment failure, breast pain, gynaecomastia and hot flashes was therefore rated as moderate (Table 1).
Potential biases in the review process
Limitations of the review at the study or outcome level
All studies were published in peer‐reviewed publications. However, results might be hampered by several limitations. As discussed above, included participants might no longer represent the contemporary population (see Overall completeness and applicability of evidence). Additionally, only two included studies (Sciarra 2004a; Smith 2004) measured PSA as a marker for clinical or biochemical progression. Nowadays, PSA plays an important role in the follow‐up of patients with prostate cancer and in early detection of disease progression. This certainly has an effect on clinical and biochemical progression and might be important for outcomes such as overall and cancer‐specific mortality.
Limitations of the review at the review level
We followed the recommendations outlined in the Cochrane Handbook for Systematic Reviews of Interventions to minimise potential biases (Higgins 2011a). We performed an extensive literature search and contacted selected experts in the field, as well as manufacturers of non‐steroidal androgen suppression drugs, to request information on unpublished studies. Therefore it is not likely that relevant studies were overlooked. Two review authors independently assessed the information given in the reports of included studies and contacted the investigators of the identified studies to request supplemental data. This review therefore assessed the best evidence available from published randomised controlled trials. Unfortunately, even after contacting the primary investigators, we received no additional data.
Limitations of the review related to detection of serious and/or rare adverse events
We considered only randomised controlled trials for inclusion in this review. However, for evaluation of serious and/or rare adverse events, it is also necessary to consider non‐randomised studies such as controlled clinical trials, cohort studies and case‐control studies. Observational studies often utilise large databases and likely or possibly show greater external validity when compared with data from randomised controlled trials (Gartlehner 2008). Additionally, data on adverse events from randomised controlled trials could underestimate rare but serious adverse events as the result of small sample size and might be susceptible to bias due to the inclusion of highly selected participants (Chou 2010).
Agreements and disagreements with other studies or reviews
We identified that overall survival was significantly decreased with non‐steroidal antiandrogens when compared with castration. This is consistent with the findings of other studies. A systematic review published in 2000 by Seidenfeld et al already suggested that overall survival might be lower when non‐steroidal antiandrogens are used (Seidenfeld 1999; Seidenfeld 2000). However, no update was performed of the review published by Seidenfeld et al, and no other systematic review evaluating overall or cancer‐specific survival was published comparing non‐steroidal antiandrogens with medical or surgical castration. A narrative review suggested that non‐steroidal antiandrogen monotherapy is an established treatment option in men with prostate cancer, but that an unexplained trend towards increased mortality should prohibit their uncritical use (Wirth 2007). The Early Prostate Cancer program investigated the effect of bicalutamide 150 mg daily compared with placebo. It showed that bicalutamide might delay clinical progression, but that for overall survival, it provided an advantage only when combined with external beam radiotherapy for locally advanced prostate cancer (EPC program; Wirth 2008).
Non‐steroidal antiandrogens are thought to provide advantages such as oral application and the potential preservation of libido, potency and muscle mass or bone mineral density when compared with castration (EAU 2013; Daniell 1997; Prezioso 2007; Sciarra 2004b; Sieber 2004; Smith 2002; Smith 2004; Study 306; Study 307; Wadhwa 2011). However, adverse events should be considered. This review suggests that the occurrence of adverse events such as breast pain, gynaecomastia and asthenia is increased with non‐steroidal antiandrogens. Breast events were the most common adverse events in recent reports of treatment with non‐steroidal antiandrogens (Boccardo 1999; Boccardo 2002; EPC program; Kotake 1996b; Lunglmayr 1995; Raina 2007; Tyrrell 1994; Wadhwa 2011), and hot flashes occurred in approximately 30% to 40% of these participants (Boccardo 1999; EPC program; Lunglmayr 1995). Results of studies evaluating non‐steroidal antiandrogens assume that the incidence of adverse events ranges between 40% and 74% (EPC program; Kotake 1996b; Raina 2007). Castration, on the other hand, increased adverse events such as hot flashes, haemorrhage, nocturia, urinary frequency, fatigue and loss of sexual interest, as indicated by this review. Side effects that have an impact on physiological and psychological health should be considered when androgen suppression therapies are prescribed because these effects can interfere with compliance as soon as the patient notices symptoms; thus, these patients might require additional treatment (Kunath 2012).
Non‐steroidal antiandrogens lead to an increased rate of treatment discontinuation compared with castration because of their associated adverse events. This finding is consistent with the results of the systematic review published by Seidenfeld et al, as well as other studies, and accounts for 4% to 10% of patients receiving non‐steroidal antiandrogens (EPC program; Seidenfeld 2000; Study 306; Study 307; Tyrrell 1994). The main reasons for discontinuing treatment were elevated liver enzymes (Boccon‐Gibod 1997; Smith 2004; Tyrrell 1994) and breast events (EPC program).
We found no data on the impact of androgen suppression therapy on cardiovascular events. However, androgen suppression might adversely affect cardiovascular risk (Dockery 2002; Dockery 2009), and men with existing cardiovascular disease might have increased mortality (Efstathiou 2009). However, adjuvant castration does not appear to increase cardiovascular mortality in men with advanced prostate cancer who do not have notable cardiovascular risk (Efstathiou 2008; Nguyen 2011).
In the included studies, bicalutamide was the most frequently assessed non‐steroidal antiandrogen. Only one study (Boccon‐Gibod 1997) with a small sample size evaluated flutamide; we identified no studies evaluating nilutamide. This observation is consistent with common prescribing practices. A recent study evaluated men registered in the National Prostate Cancer Register of Sweden to determine the prescribing patterns of therapy with bicalutamide (Grundmark 2012). Of the 58,143 patients with prostate cancer registered in the National Prostate Cancer Register of Sweden, 4.4% (n = 2558) were treated with non‐steroidal antiandrogens and 1406 received bicalutamide monotherapy. Of these, 79% received a dosage of 150 mg per day. The prescription of other antiandrogens was very rare (n = 88) (Grundmark 2012).
We did not include studies that compared non‐steroidal antiandrogens with placebo. Evidence from large randomised controlled trials suggests that non‐steroidal antiandrogens (bicalutamide at 150 mg daily) given as an adjuvant to radiotherapy significantly improve progression‐free survival compared with radiotherapy alone. However, these studies showed no significant differences in overall survival after 9.7 years (EPC program).
Authors' conclusions
Implications for practice.
Based on our assessment of the best available evidence, use of non‐steroidal antiandrogen monotherapy rather than medical or surgical castration monotherapy is less effective for treating men with advanced prostate cancer with respect to overall survival, clinical progression, treatment failure and treatment discontinuation due to adverse events. Some of the variation in study results may be attributable to disease stage, as subgroup analyses showed that these effects were more pronounced in men with metastatic disease. Additionally, subgroup analyses by dose showed less favourable effects regarding the non‐steroidal antiandrogen bicalutamide 50 mg daily for overall survival, clinical progression with imputed event numbers at 70 weeks and treatment failure with imputed event numbers at 70 weeks, as well as for the non‐steroidal antiandrogen bicalutamide 150 mg daily for clinical progression and treatment failure at one year, 70 weeks and two years compared with castration. However, subgroup analyses could be confounded because their results are observational in nature and contain greater uncertainty. Adverse events should be considered in both non‐steroidal antiandrogen and castration therapies.
Implications for research.
The quality of evidence according to GRADE is only moderate. However, we believe that further research on non‐steroidal antiandrogen monotherapy is likely not necessary for the subgroup of men with metastatic prostate cancer. Further research is likely to have an important impact on results for the subgroup of patients with advanced but non‐metastatic prostate cancer treated with non‐steroidal antiandrogen monotherapy. Only high‐quality, randomised controlled trials with long‐term follow‐up should be conducted. If further research is planned to investigate biochemical progression, studies with standardised follow‐up schedules using measurements of PSA based on current guidelines should be conducted.
Notes
For the next update of this review, we will adapt our search strategy to the recommendations of the trial search co‐ordinators of the Cochrane Prostatic Diseases and Urologic Cancers Group. We will add the following search terms to our search strategy: "antiandrogen*", "anti androgen*", "testectomy", and "orchidectomy". Addtionally, we will search EMBASE without The 'SAME SENT' adjacency operator (e.g. change in search strategy from '(nilutamid* or nilandron* or anandron* or bicalutamid* or casodex* or casudex*)/same sent' to '(nilutamid* or nilandron* or anandron* or bicalutamid* or casodex* or casudex*).' We will also prespecify outcomes for summary of findings tables to minimise bias.
Acknowledgements
We thank the peer reviewers for their valuable comments, which helped us to improve the protocol and the review. We would like to acknowledge the editing services of American Journal Experts (http://www.aje.com/).
Appendices
Appendix 1. PROSTATE register search strategy
| #1 | SR‐Prostate |
| #2 | MeSH descriptor: [Prostatic Neoplasms] explode all trees |
| #3 | (prostat* near (cancer* or tumo* or neoplas* or carcinom* or malign*)) |
| #4 | #2 or #3 |
| #5 | MeSH descriptor: [Androgen Antagonists] explode all trees |
| #6 | MeSH descriptor: [Flutamide], this term only |
| #7 | (androgen* near antagonist*) or nilutamid* or nilandron* or anandron* or (RU next 23908*) or bicalutamid* or casodex* or casudex* or (ICI next 176334*) or fluta* or niftolid* or chimax* or cytamid* or eulexin* or drogenil* or euflex* or fluken* or flulem* or flumid* or flutamid* or flutexin* or fugerel* or grisetin* or oncosal* or prostacur* or prostica* or SCH13521* or (SCH next 13521*) or prostogenat* or testotard* or apimid* |
| #8 | #5 or #6 or #7 |
| #9 | #1 and #4 and #8 |
Appendix 2. CENTRAL search strategy
| #1 | MeSH descriptor: [Prostatic Neoplasms] explode all trees |
| #2 | (prostat* near (cancer* or tumo* or neoplas* or carcinom* or malign*)) |
| #3 | (#1 OR #2) |
| #4 | MeSH descriptor: [Castration], this term only |
| #5 | MeSH descriptor: [Orchiectomy] explode all trees |
| #6 | (orchiectom* or (surg* near castrat*)) |
| #7 | (#4 OR #5 OR #6) |
| #8 | MeSH descriptor: [Androgen Antagonists] explode all trees |
| #9 | MeSH descriptor: [Flutamide], this term only |
| #10 | (androgen* near antagonist*) or nilutamid* or nilandron* or anandron* or (RU next 23908*) or bicalutamid* or casodex* or casudex* or (ICI next 176334*) or fluta* or niftolid* or chimax* or cytamid* or eulexin* or drogenil* or euflex* or fluken* or flulem* or flumid* or flutamid* or flutexin* or fugerel* or grisetin* or oncosal* or prostacur* or prostica* or SCH13521* or (SCH next 13521*) or prostogenat* or testotard* or apimid* |
| #11 | (#8 OR #9 OR #10) |
| #12 | MeSH descriptor: [Gonadotropin‐Releasing Hormone] explode all trees |
| #13 | (LHRH next agonist*) or (LH next RH next agonist*) |
| #14 | luteinizing next hormone next releasing hormone* |
| #15 | gonadotropin next releasing next hormone* |
| #16 | (gnrh next agonist*) or (gn next rh next agonist*) |
| #17 | gonadorelin* or leuprolid* or leuprorelin* or enanton* or lupron* or eligard* or (TAP next 144*) or TAP144* or (A next 43818*) or A43818* or goserelin* or ICI118630* or (ICI next 118630*) or zoladex* or buserelin* or receptal* or bigonist* or tiloryth* or profact* or suprecur* or suprefact* or HOE766* or (HOE next 766*) or triptorelin* or Wy42462* or (Wy next 42462*) or decapeptyl* or trelstar* or trimestral* or AY25650* or (AY next 25650*) or CL118532* or (CL next 118532*) |
| #18 | MeSH descriptor: [Leuprolide] explode all trees |
| #19 | MeSH descriptor: [Goserelin] explode all trees |
| #20 | MeSH descriptor: [Buserelin] explode all trees |
| #21 | MeSH descriptor: [Triptorelin] explode all trees |
| #22 | (#12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21) |
| #23 | (#7 OR #22) |
| #24 | (#11 AND #23) |
| #25 | (#3 AND #24) |
Appendix 3. MEDLINE (Ovid) search strategy
| #1 | Prostatic Neoplasms/ |
| #2 | (prostat* adj3 (cancer* or tumo* or neoplas* or carcinom* or malign*)).tw. |
| #3 | 1 or 2 |
| #4 | castration/ or orchiectomy/ |
| #5 | (orchiectom* or (surg* adj3 castrat*)).tw. |
| #6 | 4 or 5 |
| #7 | exp Androgen Antagonists/ |
| #8 | (androgen* adj3 antagonist*).mp. |
| #9 | (nilutamid* or nilandron* or anandron* or RU 23908*).mp. |
| #10 | (bicalutamid* or Casodex* or Casudex* or ICI 176334*).mp. |
| #11 | (flutamid* or fluta* or niftolid* or chimax* or cytamid* or eulexin* or drogenil* or euflex* or fluken* or flulem* or flumid* or flutexin* or fugerel* or grisetin* or oncosal* or prostacur* or prostica* or SCH13521* or SCH 13521* or prostogenat* or restotard* or apimid*).mp. |
| #12 | Flutamide/ |
| #13 | 7 or 8 or 9 or 10 or 11 or 12 |
| #14 | exp Gonadotropin‐Releasing Hormone/ |
| #15 | (LHRH agonist* or LH RH agonist*).tw. |
| #16 | luteinizing hormone releasing hormone*.mp. |
| #17 | Gonadotropin Releasing Hormone*.mp. |
| #18 | (gnrh agonist* or gn rh agonist*).mp. |
| #19 | gonadorelin*.mp. |
| #20 | (leuprolid* or leuprorelin* or enanton* or lupron* or eligard* or TAP 144* or TAP144* or A 43818* or A43818*).mp. |
| #21 | (goserelin* or ICI118630* or ICI 118630* or zoladex*).mp. |
| #22 | (buserelin* or receptal* or bigonist* or tiloryth* or profact* or suprecur* or suprefact* or HOE766* or HOE 766*).mp. |
| #23 | (triptorelin* or Wy42462* or Wy 42462* or decapeptyl* or trelstar* or trimestral* or AY25650* or AY 25650* or CL118532* or CL 118532*).mp. |
| #24 | buserelin/ or goserelin/ or triptorelin pamoate/ |
| #25 | Leuprolide/ |
| #26 | 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 |
| #27 | randomized controlled trial.pt. |
| #28 | controlled clinical trial.pt. |
| #29 | random*.ab. |
| #30 | clinical trials as topic.sh. |
| #31 | trial.ti. |
| #32 | 27 or 28 or 29 or 30 or 31 |
| #33 | exp animals/ not humans.sh. |
| #34 | 32 not 33 |
| #35 | 6 or 26 |
| #36 | 13 and 35 |
| #37 | 3 and 36 |
| #38 | 34 and 37 |
| #39 | remove duplicates from 38 |
| #40 | 39 not retracted publication.pt. |
Appendix 4. EMBASE (DIMDI) search strategy
| #1 | EM74 |
| #2 | CT=("PROSTATE TUMOR"; "PROSTATE CANCER"; "PROSTATE ADENOCARCINOMA"; "PROSTATE CARCINOMA") |
| #3 | (prostat* and (cancer* or tumo* or neoplas* or carcinom* or malign*))/same sent |
| #4 | 2 OR 3 |
| #5 | CT=("CONTROLLED CLINICAL TRIAL"; "RANDOMIZED CONTROLLED TRIAL") |
| #6 | CT="RANDOMIZATION" |
| #7 | CT="DOUBLE BLIND PROCEDURE" |
| #8 | CT="SINGLE BLIND PROCEDURE" |
| #9 | CT="PROSPECTIVE STUDY" |
| #10 | RANDOM* |
| #11 | ((SINGL* OR DOUBL*) AND (BLIND* OR MASK*))/SAME SENT |
| #12 | (CONTROLLED AND TRIAL)/SAME SENT |
| #13 | ti=trial |
| #14 | groups |
| #15 | 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 OR 13 OR 14 |
| #16 | CT="ORCHIECTOMY" |
| #17 | CT="CASTRATION" |
| #18 | (orchiectom* or (surg* and castrat*))/same sent |
| #19 | 16 OR 17 OR 18 |
| #20 | CT="ANTIANDROGEN" |
| #21 | CT="BICALUTAMIDE" |
| #22 | CT="NILUTAMIDE" |
| #23 | CT="FLUTAMIDE" |
| #24 | (androgen* and antagonist*)/same sent |
| #25 | (nilutamid* or nilandron* or anandron* or bicalutamid* or casodex* or casudex*)/same sent |
| #26 | (flutamid* or fluta* or niftolid* or chimax* or cytamid* or eulexin* or drogenil* or euflex* or fluken* or flulem* or flumid* or flutexin* or fugerel* or grisetin* or oncosal* or prostacur* or prostica* or prostogenat* or testotard* or apimid*)/same sent |
| #27 | 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 |
| #28 | CT="GONADORELIN" |
| #29 | CT="LEUPRORELIN" |
| #30 | CT="GOSERELIN" |
| #31 | CT="BUSERELIN" |
| #32 | CT="TRIPTORELIN" |
| #33 | ((LHRH and agonist*) or (LH RH and agonist*))/same sent |
| #34 | ((luteinizing hormone releasing hormone*) or (gonadotropin releasing hormone*))/same sent |
| #35 | ((GnRH and agonist*) or (gn rh and agonist*))/same sent |
| #36 | (gonadorelin* or leuprolid* or leuprorelin* or enanton* or lupron* or eligard* or goserelin* or zoladex* or buserelin* or receptal* or bigonist* or tiloryth* or profact* or suprecur* or suprefact*)/same sent |
| #37 | (triptorelin* or decapeptyl* or trelstar* or trimestral*)/same sent |
| #38 | 28 OR 29 OR 30 OR 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 |
| #39 | 19 OR 38 |
| #40 | 4 AND 15 AND 27 AND 39 |
| #41 | su=medline |
| #42 | 40 NOT 41 |
Appendix 5. Web of Science search strategy
| #1 | ts=(prostat* same (cancer* or tumo* or neoplas* or carcinom* or malign*)) |
| #2 | ts=(orchiectom* or (surg* same castrat*)) |
| #3 | ts=(Androgen* same Antagonist*) |
| #4 | ts=(nilutamid* or nilandron* or anandron* or "RU 23908*") |
| #5 | ts=(bicalutamid* or casodex or casudex or "ICI 176334") |
| #6 | ts=(fluta* or niftolid* or chimax* or cytamid* or eulexin* or drogenil* or euflex* or fluken* or flulem* or flumid* or flutamid* or flutexin* or fugerel* or grisetin* or oncosal* or prostacur* or prostica* or SCH13521* or "SCH 13521" or prostogenat* or testotard* or apimid*) |
| #7 | #3 OR #4 OR #5 OR #6 |
| #8 | ts=(Gonadotropin same Releasing same Hormone*) |
| #9 | ts=((LHRH same agonist*) or (LH same RH same agonist*)) |
| #10 | ts=(luteinizing same hormone same releasing same hormone*) |
| #11 | ts=(gonadorelin* or (gnrh same agonist*) or (gn same rh same agonist*)) |
| #12 | ts=(leuprolid* or leuprorelin* or enanton* or lupron* or eligard* or "TAP 144" or TAP144* or "A 43818" or A43818* or goserelin* or ICI118630* or (ICI same 118630*) or zoladex* or buserelin* or receptal* or bigonist* or tiloryth* or profact* or suprecur* or suprefact* or HOE766* or "HOE 766" or triptorelin* or Wy42462* or "Wy 42462" or decapeptyl* or trelstar* or trimestral* or AY25650* or "AY 25650" or CL118532* or "CL 118532") |
| #13 | #8 OR #9 OR #10 OR #11 OR #12 |
| #14 | #2 OR #13 |
| #15 | #14 AND #7 |
| #16 | #15 AND #1 |
| #17 | ts=(rando* or (controlled same trial) or (clinical same trial) or (double* same blind*) or (singl* same blind*)) or ti=trial |
| #18 | #16 AND #17 |
Appendix 6. Keywords used to search meeting abstracts
| bicalutamide | casodex | nilutamide | apimid | flutamide |
| nilandron | anandron | casudex | niftolid | chimax |
| cytamid | eulexin | drogenil | euflex | fluken |
| flulem | flumid | flutexin | fugerel | grisetin |
| oncosal | prostacur | prostica | SCH‐13521 | prostogenat |
Appendix 7. Keywords used to search trial registries
| bicalutamide | casodex | nilutamide | apimid | flutamide |
| nilandron | anandron | casudex | niftolid | chimax |
| cytamid | eulexin | drogenil | euflex | fluken |
| flulem | flumid | flutexin | fugerel | grisetin |
| oncosal | prostacur | prostica | SCH‐13521 | prostogenat |
Data and analyses
Comparison 1. Non‐steroidal antiandrogen monotherapy versus LHRH agonists or surgical castration monotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 6 | Hazard Ratio (Random, 95% CI) | Subtotals only | |
| 1.1 Total | 6 | 2712 | Hazard Ratio (Random, 95% CI) | 1.24 [1.05, 1.48] |
| 1.2 Subgroup analysis: non‐metastatic disease | 2 | 608 | Hazard Ratio (Random, 95% CI) | 1.00 [0.79, 1.26] |
| 1.3 Subgroup analysis: metastatic disease | 5 | 2103 | Hazard Ratio (Random, 95% CI) | 1.34 [1.14, 1.57] |
| 1.4 Subgroup analysis: bicalutamide 50 mg daily | 3 | 1196 | Hazard Ratio (Random, 95% CI) | 1.45 [1.19, 1.75] |
| 1.5 Subgroup analysis: bicalutamide 150 mg daily | 2 | 1288 | Hazard Ratio (Random, 95% CI) | 1.18 [0.96, 1.45] |
| 1.6 Subgroup analysis: bicalutamide 450 mg daily or 600 mg daily | 1 | 228 | Hazard Ratio (Random, 95% CI) | 0.88 [0.62, 1.25] |
| 1.7 Subgroup analysis: non‐metastatic disease and bicalutamide 150 mg daily | 1 | 480 | Hazard Ratio (Random, 95% CI) | 1.05 [0.81, 1.36] |
| 1.8 Subgroup analysis: non‐metastatic disease and bicalutamide 450 mg daily or 600 mg daily | 1 | 128 | Hazard Ratio (Random, 95% CI) | 0.79 [0.46, 1.36] |
| 1.9 Subgroup analysis: metastatic disease and bicalutamide 50 mg daily | 3 | 1196 | Hazard Ratio (Random, 95% CI) | 1.45 [1.19, 1.75] |
| 1.10 Subgroup analysis: metastatic disease and bicalutamide 150 mg daily | 1 | 808 | Hazard Ratio (Random, 95% CI) | 1.30 [1.04, 1.63] |
| 1.11 Subgroup analysis: metastatic disease and bicalutamide 450 mg daily or 600 mg daily | 1 | 99 | Hazard Ratio (Random, 95% CI) | 0.91 [0.56, 1.48] |
| 2 Cancer‐specific mortality | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Total | 3 | 904 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.86, 2.05] |
| 2.2 Total after a minimum 12 months | 2 | 680 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.71, 3.73] |
| 2.3 Total after median 5 years | 1 | 224 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.73, 1.47] |
| 3 Treatment discontinuation due to adverse events | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Total | 8 | 1559 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.13, 2.94] |
| 3.2 Subgroup analysis: non‐metastatic disease | 3 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.66, 3.28] |
| 3.3 Subgroup analysis: metastatic disease | 4 | 1141 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.54, 3.54] |
| 3.4 Subgroup analysis: bicalutamide 50 mg daily | 3 | 1037 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.29, 2.56] |
| 3.5 Subgroup analysis: bicalutamide 150 mg daily | 3 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.66, 3.28] |
| 3.6 Subgroup analysis: flutamide 250 mg 3 times daily | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.35 [0.46, 151.19] |
| 3.7 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.17, 6.01] |
| 3.8 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [0.95, 6.31] |
| 4 Clinical progression | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 4.1 Total at 1 year | 5 | 2067 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.08, 1.45] |
| 4.2 Total at 70 weeks | 6 | 2373 | Risk Ratio (M‐H, Random, 95% CI) | 1.26 [1.08, 1.45] |
| 4.3 Total at 2 years | 3 | 1336 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.04, 1.25] |
| 4.4 Total at 3 years | 1 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.87, 1.23] |
| 4.5 Total at 4 years | 1 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.91, 1.26] |
| 4.6 Total at 5 years | 2 | 698 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.88, 1.06] |
| 4.7 Subgroup analysis: non‐metastatic disease at 1 year | 2 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.82, 1.96] |
| 4.8 Subgroup analysis: non‐metastatic disease at 70 weeks | 2 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [0.83, 1.68] |
| 4.9 Subgroup analysis: non‐metastatic disease at 2 years | 2 | 528 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.81, 1.31] |
| 4.10 Subgroup analysis: non‐metastatic disease at 3 years | 1 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.87, 1.23] |
| 4.11 Subgroup analysis: non‐metastatic disease at 4 years | 1 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.91, 1.26] |
| 4.12 Subgroup analysis: non‐metastatic disease at 5 years | 1 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.06] |
| 4.13 Subgroup analysis: metastatic disease at 1 year | 3 | 1539 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.05, 1.49] |
| 4.14 Subgroup analysis: metastatic disease at 70 weeks | 4 | 1845 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.07, 1.51] |
| 4.15 Subgroup analysis: metastatic disease at 2 years | 1 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.05, 1.29] |
| 4.16 Subgroup analysis: bicalutamide 50 mg daily at 1 year | 2 | 731 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [0.91, 1.76] |
| 4.17 Subgroup analysis: bicalutamide 50 mg daily at 70 weeks | 3 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [0.99, 1.71] |
| 4.18 Subgroup analysis: bicalutamide 150 mg daily at 1 year | 3 | 1336 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [1.07, 1.46] |
| 4.19 Subgroup analysis: bicalutamide 150 mg daily at 70 weeks | 3 | 1336 | Risk Ratio (M‐H, Random, 95% CI) | 1.22 [1.07, 1.39] |
| 4.20 Subgroup analysis: bicalutamide 150 mg daily at 2 years | 3 | 1336 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.04, 1.25] |
| 4.21 Subgroup analysis: bicalutamide 150 mg daily at 3 years | 1 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.87, 1.23] |
| 4.22 Subgroup analysis: bicalutamide 150 mg daily at 4 years | 1 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.91, 1.26] |
| 4.23 Subgroup analysis: bicalutamide 150 mg daily at 5 years | 1 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.97 [0.88, 1.06] |
| 4.24 Subgroup analysis: bicalutamide 450 mg daily at 5 years | 1 | 177 | Risk Ratio (M‐H, Random, 95% CI) | 1.00 [0.54, 1.86] |
| 4.25 Subgroup analysis: bicalutamide 600 mg daily at 5 years | 1 | 127 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.33, 1.86] |
| 5 Clinical progression (with imputed event numbers) | 7 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 5.1 Total (with imputed event numbers) at 1 year | 5 | 2167 | Risk Ratio (Fixed, 95% CI) | 1.43 [1.16, 1.76] |
| 5.2 Total (with imputed event numbers) at 70 weeks | 6 | 2543 | Risk Ratio (Fixed, 95% CI) | 1.43 [1.19, 1.73] |
| 5.3 Total (with imputed event numbers) at 2 years | 3 | 1347 | Risk Ratio (Fixed, 95% CI) | 1.39 [1.09, 1.78] |
| 5.4 Total (with imputed event numbers) at 5 years | 2 | 708 | Risk Ratio (Fixed, 95% CI) | 0.86 [0.56, 1.31] |
| 5.5 Subgroup analysis: non‐metastatic disease (with imputed event numbers) at 1 year | 2 | 539 | Risk Ratio (Fixed, 95% CI) | 1.33 [0.80, 2.22] |
| 5.6 Subgroup analysis: non‐metastatic disease (with imputed event numbers) at 70 weeks | 2 | 539 | Risk Ratio (Fixed, 95% CI) | 1.23 [0.78, 1.94] |
| 5.7 Subgroup analysis: non‐metastatic disease (with imputed event numbers) at 2 years | 2 | 539 | Risk Ratio (Fixed, 95% CI) | 1.05 [0.71, 1.55] |
| 5.8 Subgroup analysis: non‐metastatic disease (with imputed event numbers) at 5 years | 1 | 480 | Risk Ratio (Fixed, 95% CI) | 0.84 [0.52, 1.36] |
| 5.9 Subgroup analysis: metastatic disease (with imputed event numbers) at 1 year | 3 | 1628 | Risk Ratio (Fixed, 95% CI) | 1.45 [1.16, 1.82] |
| 5.10 Subgroup analysis: metastatic disease (with imputed event numbers) at 70 weeks | 4 | 2004 | Risk Ratio (Fixed, 95% CI) | 1.48 [1.20, 1.83] |
| 5.11 Subgroup analysis: metastatic disease (with imputed event numbers) at 2 years | 1 | 808 | Risk Ratio (Fixed, 95% CI) | 1.69 [1.22, 2.32] |
| 5.12 Subgroup analysis: bicalutamide 50 mg daily (with imputed event numbers) at 1 year | 2 | 820 | Risk Ratio (Fixed, 95% CI) | 1.40 [0.99, 1.98] |
| 5.13 Subgroup analysis: bicalutamide 50 mg daily (with imputed event numbers) at 70 weeks | 3 | 1196 | Risk Ratio (Fixed, 95% CI) | 1.40 [1.04, 1.88] |
| 5.14 Subgroup analysis: bicalutamide 150 mg daily (with imputed event numbers) at 1 year | 3 | 1347 | Risk Ratio (Fixed, 95% CI) | 1.45 [1.12, 1.87] |
| 5.15 Subgroup analysis: bicalutamide 150 mg daily (with imputed event numbers) at 70 weeks | 3 | 1347 | Risk Ratio (Fixed, 95% CI) | 1.46 [1.14, 1.87] |
| 5.16 Subgroup analysis: bicalutamide 150 mg daily (with imputed event numbers) at 2 years | 3 | 1347 | Risk Ratio (Fixed, 95% CI) | 1.39 [1.09, 1.78] |
| 5.17 Subgroup analysis: bicalutamide 150 mg daily (with imputed event numbers) at 5 years | 1 | 480 | Risk Ratio (Fixed, 95% CI) | 0.84 [0.52, 1.36] |
| 5.18 Subgroup analysis: bicalutamide 450 mg daily (with imputed event numbers) at 5 years | 1 | 185 | Risk Ratio (Fixed, 95% CI) | 1.00 [0.41, 2.47] |
| 5.19 Subgroup analysis: bicalutamide 600 mg daily (with imputed event numbers) at 5 years | 1 | 133 | Risk Ratio (Fixed, 95% CI) | 0.78 [0.27, 2.25] |
| 6 Biochemical progression | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Total at 1 year | 2 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.12 [0.13, 73.06] |
| 6.2 Total at 2 years | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Total at 3 years | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.94, 1.26] |
| 7 Biochemical progression (with imputed event numbers) | 3 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 7.1 Total (with imputed event numbers) at 1 year | 2 | 110 | Risk Ratio (Fixed, 95% CI) | 2.29 [0.15, 34.62] |
| 7.2 Total (with imputed event numbers) at 2 years | 1 | 59 | Risk Ratio (Fixed, 95% CI) | 0.96 [0.00, 196.72] |
| 7.3 Total (with imputed event numbers) at 3 years | 1 | 104 | Risk Ratio (Fixed, 95% CI) | 1.07 [0.72, 1.58] |
| 8 Treatment failure | 6 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 8.1 Total at 1 year | 3 | 1539 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.02, 1.38] |
| 8.2 Total at 70 weeks | 4 | 1845 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.05, 1.52] |
| 8.3 Total at 2 years | 1 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.05, 1.24] |
| 8.4 Total at 3 years | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.63, 3.99] |
| 8.5 Total at 4 years | 1 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.93, 1.16] |
| 8.6 Subgroup analysis: non‐metastatic disease at 4 years | 1 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.93, 1.16] |
| 8.7 Subgroup analysis: metastatic disease at 1 year | 3 | 1539 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [1.02, 1.38] |
| 8.8 Subgroup analysis: metastatic disease at 70 weeks | 4 | 1845 | Risk Ratio (M‐H, Random, 95% CI) | 1.27 [1.05, 1.52] |
| 8.9 Subgroup analysis: metastatic disease at 2 years | 1 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.05, 1.24] |
| 8.10 Subgroup analysis: metastatic disease at 3 years | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.63, 3.99] |
| 8.11 Subgroup analysis: bicalutamide 50 mg daily at 1 year | 2 | 731 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.90, 1.57] |
| 8.12 Subgroup analysis: bicalutamide 50 mg daily at 70 weeks | 3 | 1037 | Risk Ratio (M‐H, Random, 95% CI) | 1.31 [0.98, 1.75] |
| 8.13 Subgroup analysis: bicalutamide 150 mg daily at 1 year | 1 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [1.01, 1.35] |
| 8.14 Subgroup analysis: bicalutamide 150 mg daily at 70 weeks | 1 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 1.18 [1.05, 1.34] |
| 8.15 Subgroup analysis: bicalutamide 150 mg daily at 2 years | 1 | 808 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [1.05, 1.24] |
| 8.16 Subgroup analysis: bicalutamide 150 mg daily at 4 years | 1 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [0.93, 1.16] |
| 8.17 Subgroup analysis: flutamide 250 mg 3 times daily at 3 years | 1 | 86 | Risk Ratio (M‐H, Random, 95% CI) | 1.59 [0.63, 3.99] |
| 9 Treatment failure (with imputed event numbers) | 5 | Risk Ratio (Fixed, 95% CI) | Subtotals only | |
| 9.1 Total (with imputed event numbers) at 1 year | 3 | 1628 | Risk Ratio (Fixed, 95% CI) | 1.19 [1.06, 1.35] |
| 9.2 Total (with imputed event numbers) at 70 weeks | 4 | 2004 | Risk Ratio (Fixed, 95% CI) | 1.21 [1.09, 1.35] |
| 9.3 Total (with imputed event numbers) at 3 years | 1 | 104 | Risk Ratio (Fixed, 95% CI) | 1.51 [0.32, 7.06] |
| 9.4 Subgroup analysis: metastatic disease (with imputed event numbers) at 1 year | 3 | 1628 | Risk Ratio (Fixed, 95% CI) | 1.19 [1.06, 1.35] |
| 9.5 Subgroup analysis: metastatic disease (with imputed event numbers) at 70 weeks | 4 | 2004 | Risk Ratio (Fixed, 95% CI) | 1.21 [1.09, 1.35] |
| 9.6 Subgroup analysis: metastatic disease (with imputed event numbers) at 3 years | 1 | 104 | Risk Ratio (Fixed, 95% CI) | 1.51 [0.32, 7.06] |
| 9.7 Subgroup analysis: bicalutamide 50 mg daily (with imputed event numbers) at 1 year | 2 | 820 | Risk Ratio (Fixed, 95% CI) | 1.27 [1.01, 1.60] |
| 9.8 Subgroup analysis: bicalutamide 50 mg daily (with imputed event numbers) at 70 weeks | 3 | 1196 | Risk Ratio (Fixed, 95% CI) | 1.29 [1.05, 1.57] |
| 9.9 Subgroup analysis: bicalutamide 150 mg daily (with imputed event numbers) at 1 year | 1 | 808 | Risk Ratio (Fixed, 95% CI) | 1.17 [1.01, 1.35] |
| 9.10 Subgroup analysis: bicalutamide 150 mg daily (with imputed event numbers) at 70 weeks | 1 | 808 | Risk Ratio (Fixed, 95% CI) | 1.18 [1.05, 1.34] |
| 9.11 Subgroup analysis: flutamide 250 mg 3 times daily (with imputed event numbers) at 3 years | 1 | 104 | Risk Ratio (Fixed, 95% CI) | 1.51 [0.32, 7.06] |
| 10 Breast pain | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 Total | 7 | 2670 | Risk Ratio (M‐H, Fixed, 95% CI) | 22.97 [14.79, 35.67] |
| 10.2 Subgroup analysis: bicalutamide 50 mg daily | 3 | 1026 | Risk Ratio (M‐H, Fixed, 95% CI) | 19.39 [10.26, 36.66] |
| 10.3 Subgroup analysis: bicalutamide 150 mg daily | 3 | 1420 | Risk Ratio (M‐H, Fixed, 95% CI) | 25.82 [13.34, 49.97] |
| 10.4 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 23.48 [5.88, 93.73] |
| 10.5 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 25.71 [6.37, 103.78] |
| 11 Pelvic pain | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Total | 5 | 2395 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.78, 1.24] |
| 11.2 Subgroup analysis: bicalutamide 50 mg daily | 3 | 1026 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.64, 1.22] |
| 11.3 Subgroup analysis: bicalutamide 150 mg daily | 2 | 1369 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.79, 1.53] |
| 12 Bone pain | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Bicalutamide 50 mg daily | 1 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.68, 1.72] |
| 13 Back pain | 5 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13.1 Total | 5 | 1351 | Risk Ratio (M‐H, Random, 95% CI) | 1.06 [0.70, 1.61] |
| 13.2 Subgroup analysis: bicalutamide 50 mg daily | 3 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.54, 1.94] |
| 13.3 Subgroup analysis: bicalutamide 150 mg daily | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.54, 7.71] |
| 13.4 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.59, 1.80] |
| 13.5 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.29, 1.57] |
| 14 Headache | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Total | 2 | 584 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.24, 1.15] |
| 14.2 Subgroup analysis: bicalutamide 50 mg daily | 1 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.20, 1.05] |
| 14.3 Subgroup analysis: flutamide 250 mg 3 times daily | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [0.12, 66.75] |
| 15 Abdominal pain | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Total | 3 | 1058 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.90, 2.48] |
| 15.2 Subgroup analysis: bicalutamide 50 mg daily | 1 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.58, 2.70] |
| 15.3 Subgroup analysis: bicalutamide 150 mg daily | 1 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.92, 3.81] |
| 15.4 Subgroup analysis: flutamide 250 mg 3 times daily | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.01, 7.42] |
| 16 General pain | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Total | 4 | 2073 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.80, 1.16] |
| 16.2 Subgroup analysis: bicalutamide 50 mg daily | 1 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.65, 1.32] |
| 16.3 Subgroup analysis: bicalutamide 150 mg daily | 2 | 1369 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.71, 1.16] |
| 16.4 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.41 [0.80, 2.48] |
| 16.5 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.34 [0.67, 2.70] |
| 17 Gynaecomastia | 8 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 17.1 Total | 8 | 2774 | Risk Ratio (M‐H, Random, 95% CI) | 8.43 [3.19, 22.28] |
| 17.2 Subgroup analysis: bicalutamide 50 mg daily | 3 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 14.07 [3.74, 52.85] |
| 17.3 Subgroup analysis: bicalutamide 150 mg daily | 3 | 1420 | Risk Ratio (M‐H, Random, 95% CI) | 5.01 [0.88, 28.69] |
| 17.4 Subgroup analysis: flutamide 250 mg 3 times daily | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 3.70 [1.33, 10.33] |
| 17.5 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 27.88 [7.02, 110.79] |
| 17.6 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 20.36 [4.97, 83.40] |
| 18 Constipation | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 18.1 Total | 4 | 1250 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.65, 1.95] |
| 18.2 Subgroup analysis: bicalutamide 50 mg daily | 3 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.50, 1.73] |
| 18.3 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 1.54 [0.84, 2.81] |
| 18.4 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 1.99 [1.03, 3.85] |
| 19 Diarrhoea | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 19.1 Total | 7 | 1929 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.80, 3.71] |
| 19.2 Subgroup analysis: bicalutamide 50 mg daily | 3 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 1.96 [1.00, 3.82] |
| 19.3 Subgroup analysis: bicalutamide 150 mg daily | 2 | 575 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.14, 12.28] |
| 19.4 Subgroup analysis: flutamide 250 mg 3 times daily | 1 | 104 | Risk Ratio (M‐H, Random, 95% CI) | 8.35 [0.46, 151.19] |
| 19.5 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.57, 3.19] |
| 19.6 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 2.14 [0.86, 5.32] |
| 20 Vomiting | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20.1 Total | 3 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.88 [1.38, 10.87] |
| 20.2 Subgroup analysis: bicalutamide 50 mg daily | 2 | 546 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.99, 9.35] |
| 20.3 Subgroup analysis: flutamide 250 mg 3 times daily | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.2 [0.58, 179.88] |
| 21 Hypertension | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 21.1 Bicalutamide 150 mg daily | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.34] |
| 22 Loss of sexual interest | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 22.1 Bicalutamide 150 mg daily | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.30, 0.83] |
| 23 Asthenia | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 Total | 4 | 2073 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.36, 2.31] |
| 23.2 Subgroup analysis: bicalutamide 50 mg daily | 1 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.11, 2.72] |
| 23.3 Subgroup analysis: bicalutamide 150 mg daily | 2 | 1369 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.10, 2.28] |
| 23.4 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.07 [1.38, 6.84] |
| 23.5 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.45 [0.95, 6.31] |
| 24 Insomnia | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 24.1 Total | 2 | 325 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.11, 2.37] |
| 24.2 Subgroup analysis: bicalutamide 150 mg daily | 1 | 101 | Risk Ratio (M‐H, Random, 95% CI) | 0.17 [0.02, 1.36] |
| 24.3 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Random, 95% CI) | 0.88 [0.38, 2.06] |
| 24.4 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.29, 2.58] |
| 25 Hot flashes | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 25.1 Total | 8 | 2774 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.19, 0.27] |
| 25.2 Subgroup analysis: bicalutamide 50 mg daily | 3 | 1026 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.15, 0.27] |
| 25.3 Subgroup analysis: bicalutamide 150 mg daily | 3 | 1420 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.19, 0.30] |
| 25.4 Subgroup analysis: flutamide 250 mg 3 times daily | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.10, 0.82] |
| 25.5 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.12, 0.40] |
| 25.6 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.15, 0.63] |
| 26 Night sweats | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 26.1 Total | 2 | 1571 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.17, 0.49] |
| 26.2 Subgroup analysis: bicalutamide 50 mg daily | 1 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.12, 1.09] |
| 26.3 Subgroup analysis: bicalutamide 150 mg daily | 1 | 1268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.14, 0.49] |
| 27 Anaemia | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 27.1 Total | 2 | 294 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.16, 5.35] |
| 27.2 Subgroup analysis: bicalutamide 50 mg daily | 1 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 3.18 [0.34, 30.13] |
| 27.3 Subgroup analysis: bicalutamide 150 mg daily | 1 | 51 | Risk Ratio (M‐H, Random, 95% CI) | 0.52 [0.31, 0.88] |
| 28 Hepatic enzyme increase | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 28.1 Total | 2 | 205 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.91 [0.59, 40.86] |
| 28.2 Subgroup analysis: bicalutamide 150 mg daily | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.14 [0.38, 134.72] |
| 28.3 Subgroup analysis: flutamide 250 mg 3 times daily | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [0.12, 66.75] |
| 29 Rash | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 29.1 Total | 3 | 805 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.77, 2.16] |
| 29.2 Subgroup analysis: bicalutamide 50 mg daily | 1 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.50, 1.92] |
| 29.3 Subgroup analysis: bicalutamide 150 mg daily | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.10 [0.62, 42.12] |
| 29.4 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.47, 3.61] |
| 29.5 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.79 [0.58, 5.52] |
| 30 Pruritus | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 30.1 Total | 2 | 723 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.59 [0.93, 7.19] |
| 30.2 Subgroup analysis: bicalutamide 50 mg daily | 2 | 723 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.59 [0.93, 7.19] |
| 31 Dyspnoea | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 31.1 Bicalutamide 50 mg daily | 1 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.24, 1.31] |
| 32 Infection | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 32.1 Total | 4 | 2294 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.58, 1.03] |
| 32.2 Subgroup analysis: bicalutamide 50 mg daily | 3 | 1026 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.34, 0.91] |
| 32.3 Subgroup analysis: bicalutamide 150 mg daily | 1 | 1268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.65, 1.33] |
| 33 Pharyngitis | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 33.1 Total | 2 | 325 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.47, 1.34] |
| 33.2 Subgroup analysis: bicalutamide 150 mg daily | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.11, 1.36] |
| 33.3 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.56, 1.93] |
| 33.4 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.34, 1.91] |
| 34 Arthritis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 34.1 Bicalutamide 150 mg daily | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.12, 1.60] |
| 35 Sinusitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 35.1 Bicalutamide 150 mg daily | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.36, 4.48] |
| 36 Urinary tract infection | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 36.1 Total | 2 | 698 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.53, 1.19] |
| 36.2 Subgroup analysis: bicalutamide 150 mg daily | 1 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.43, 1.14] |
| 36.3 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.40, 1.99] |
| 36.4 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.57, 3.27] |
| 37 Dizziness | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 37.1 Total | 2 | 581 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.61, 1.95] |
| 37.2 Subgroup analysis: bicalutamide 50 mg daily | 1 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.49, 1.97] |
| 37.3 Subgroup analysis: bicalutamide 150 mg daily | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.49, 4.20] |
| 38 Haemorrhage | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 38.1 Total | 2 | 546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.54] |
| 38.2 Subgroup analysis: bicalutamide 50 mg daily | 2 | 546 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.07 [0.01, 0.54] |
| 39 Haematuria | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 39.1 Total | 2 | 954 | Risk Ratio (M‐H, Random, 95% CI) | 1.20 [0.14, 9.87] |
| 39.2 Subgroup analysis: bicalutamide 50 mg daily | 1 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.26, 0.67] |
| 39.3 Subgroup analysis: bicalutamide 150 mg daily | 1 | 474 | Risk Ratio (M‐H, Random, 95% CI) | 3.49 [2.01, 6.05] |
| 40 Nocturia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 40.1 Bicalutamide 50 mg daily | 1 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.20, 0.69] |
| 41 Urinary frequency | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 41.1 Bicalutamide 50 mg daily | 1 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.22 [0.11, 0.47] |
| 42 Urinary retention | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 42.1 Bicalutamide 150 mg daily | 1 | 1268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.55, 1.24] |
| 43 Peripheral oedema | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 43.1 Total | 2 | 1748 | Risk Ratio (M‐H, Random, 95% CI) | 0.61 [0.33, 1.15] |
| 43.2 Subgroup analysis: bicalutamide 50 mg daily | 1 | 480 | Risk Ratio (M‐H, Random, 95% CI) | 0.42 [0.21, 0.82] |
| 43.3 Subgroup analysis: bicalutamide 150 mg daily | 1 | 1268 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.54, 1.17] |
| 44 Anorexia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 44.1 Bicalutamide 50 mg daily | 1 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.57, 2.18] |
| 45 Loss of sexual function | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 45.1 Bicalutamide 50 mg daily | 1 | 303 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.14, 6.87] |
| 46 Arthralgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 46.1 Total | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [0.86, 3.15] |
| 46.2 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.96 [1.01, 3.80] |
| 46.3 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.36, 2.63] |
| 47 Gastralgia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 47.1 Flutamide 250 mg 3 times daily | 1 | 104 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [0.12, 66.75] |
| 48 Nausea | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 48.1 Total | 3 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 3.24 [0.95, 11.02] |
| 48.2 Subgroup analysis: bicalutamide 50 mg daily | 3 | 1026 | Risk Ratio (M‐H, Random, 95% CI) | 3.24 [0.95, 11.02] |
| 49 Fatigue | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 49.1 Bicalutamide 150 mg daily | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.31, 0.88] |
| 50 Dry skin | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 50.1 Total | 1 | 224 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.41 [0.42, 132.46] |
| 50.2 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.81 [0.48, 161.24] |
| 50.3 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.35 [0.26, 152.67] |
| 51 Aggravation reaction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 51.1 Bicalutamide 150 mg daily | 1 | 474 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.45, 1.05] |
| 52 Serious adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 52.1 Total | 2 | 325 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.79, 1.28] |
| 52.2 Subgroup analysis: bicalutamide 150 mg daily | 1 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [0.49, 4.20] |
| 52.3 Subgroup analysis: bicalutamide 450 mg daily | 1 | 182 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.73, 1.25] |
| 52.4 Subgroup analysis: bicalutamide 600 mg daily | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.71, 1.37] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Boccon‐Gibod 1997.
| Methods |
Start date, end date of recruitment: April 1989 to June 1991 Follow‐up period: minimum follow‐up of 36 months Design: randomised controlled trial |
|
| Participants |
Population/Inclusion criteria: newly diagnosed hormone‐naive metastatic prostate cancer; histologically proven prostate cancer with documented metastatic disease beyond pelvic lymph nodes, no prior hormonal manipulation, chemotherapy or radiation therapy outside of the primary tumour, normal liver function test, ECOG performance status 0 to 2 and absence of any previous malignant disease except skin cancer Setting: multi‐centre (7 institutions) Geographical location: France Exclusion criteria: not reported Total number randomly assigned: 104 Baseline imbalances: balanced Number of participants with non‐metastatic disease: 0 Number of participants with metastatic disease: 104 Age (mean ± SD): intervention: 72.3 ± 8.5 years; control: 73.8 ± 8 years PSA (mean ± SD): intervention: 666 ± 961 ng/mL; control: 681 ± 1073 ng/mL Subgroup measured: no |
|
| Interventions |
Intervention Description/Timing: flutamide 250 mg 3 times daily; tablets were taken after each meal Number randomly assigned to this group: 54 Control Description/Timing: orchiectomy (formal or subcapsular orchiectomy at the discretion of each urologist) Number randomly assigned to this group: 50 |
|
| Outcomes |
Overall survival Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: minimum follow‐up of 36 months; at 69 months Time points reported: at 69 months Number of participants randomly assigned: intervention: 54; control: 50 Number of participants in evaluation for ITT: unclear Cancer‐specific mortality Outcome not reported/measured Treatment discontinuation due to adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: minimum follow‐up of 36 months Time points reported: minimum follow‐up of 36 months Number of participants randomly assigned: intervention: 54; control: 50 Number of participants in evaluation for ITT: intervention: 54; control: 50 Clinical progression Not measured/reported Biochemical progression Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: minimum follow‐up of 36 months Time points reported: minimum follow‐up of 36 months Outcome definition in report: defined by an increase in serum PSA > 50% of its nadir value confirmed over 2 months or a PSA rise > 50% over the nadir in association with another objective parameter (newly proven metastasis) Number of participants randomly assigned: intervention: 54; control: 50 Number of participants in evaluation for ITT: intervention 44; control: 42 Note: This outcome was named as clinical progression by the primary investigators. However, because of the outcome definition reported, we classified it as biochemical progression. The primary investigators reported that at progression, treatment was left to the discretion of the urologist. Participants treated by orchiectomy could receive flutamide or another antiandrogen. Participants receiving flutamide were treated with orchiectomy or medical castration using an LHRH agonist. Continuation of flutamide was done at the urologist's discretion. Also investigators reported no significant differences whether the progression‐free survival plot concerned only follow‐up participants or all included participants Treatment failure Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: minimum follow‐up of 36 months Time points reported: minimum follow‐up of 36 months Outcome definition in report: not reported Number of participants randomly assigned: intervention: 54; control: 50 Number of participants in evaluation for ITT: intervention: 44; control: 42 Adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: minimum follow‐up of 36 months Time points reported: minimum follow‐up of 36 months Number of participants randomly assigned: intervention: 54; control: 50 Number of participants in evaluation for ITT: intervention: 54; control: 50 Other outcomes reported Serum testosterone, salvage therapy by second‐line flutamide |
|
| Notes | Sexual function was not assessable because of advanced age of participants. Study authors reported no data on overall survival at 69 months but noted an "identical" survival, irrespective of second‐line treatment. They reported no definition of hepatic enzyme increase Study funding source: not reported Possible conflict of interest: Co‐author is a member of Schering‐Plough |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Participants centrally randomly assigned after signing the informed consent form |
| Blinding of participants and personnel (performance bias) overall survival, cancer‐specific mortality, biochemical progression | Unclear risk | No blinding for overall survival and biochemical progression. Blinding was not possible because of different interventions provided. It might be conceivable that outcomes such as overall survival, cancer‐specific mortality or biochemical progression are influenced by lack of blinding. We finally judge that risk of bias regarding these outcomes is unclear |
| Blinding of participants and personnel (performance bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for treatment discontinuation due to adverse events, adverse events and treatment failure. Blinding was not possible because of different interventions provided. We judge that outcomes such as treatment discontinuation due to adverse events, adverse events and treatment failure are likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) overall survival, cancer‐specific mortality, biochemical progression | Low risk | No blinding for overall survival and biochemical progression. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. However, we judge that it is not likely that outcome assessment for overall survival and biochemical progression are influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for treatment discontinuation due to adverse events, adverse events and treatment failure. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. We judge that it is likely that outcome assessment for clinical progression, treatment failure, adverse events and treatment discontinuation due to adverse events is influenced by lack of blinding |
| Incomplete outcome data (attrition bias) overall survival, cancer‐specific mortality | High risk | Data on overall survival were reported incompletely (Boccon‐Gibod et al reported only "identical" survival in both groups) |
| Incomplete outcome data (attrition bias) treatment discontinuation due to adverse events, adverse events | Low risk | Intention‐to‐treat analysis for adverse events and treatment discontinuation due to adverse events |
| Incomplete outcome data (attrition bias) clinical progression, biochemical progression | High risk | 'As‐treated' analysis on biochemical progression done with substantial departure of the intervention received from that assigned at randomisation |
| Incomplete outcome data (attrition bias) treatment failure | High risk | 'As‐treated' analysis on treatment failure done with substantial departure of the intervention received from that assigned at randomisation |
| Selective reporting (reporting bias) | High risk | Data on overall survival were reported incompletely and could not be entered into the meta‐analysis |
| Other bias | Unclear risk | Unclear risk for conflict of interest |
Dockery 2009.
| Methods |
Start date, end date of recruitment: unclear Follow‐up period: All participants had atrial stiffness measures at baseline and at 12 and 24 weeks Design: randomised controlled trial |
|
| Participants |
Population/Inclusion criteria: men with localised prostate cancer who were deemed as requiring hormonal treatment by local urology or oncology services Setting: single centre Geographical location: UK (London) Exclusion criteria: metastatic cancer, atrial fibrillation, severe hepatic renal or cardiac failure, any acute illness and any recent (in the preceding 12 months) hormone treatment Total number randomly assigned: 43 Baseline imbalances: balanced Number of participants with non‐metastatic disease: 42 Number of participants with metastatic disease: 0 Age (mean ± SD): intervention: 71.1 ± 6.1 years; control: 71.3 ± 6.6 years PSA: not reported Subgroup measured: no |
|
| Interventions |
Intervention Description/Timing: bicalutamide 150 mg once daily for 6 months Number randomly assigned to this group: 21 (1 participant excluded) Control Description/Timing: goserelin depot injection 10.8 mg 3 times monthly (1 injection preceded by 2 weeks of flutamide, an androgen receptor blocker, 250 mg 3 times daily, as per standard guidelines) Number randomly assigned to this group: 21 |
|
| Outcomes |
Overall survival Outcome not measured/reported Cancer‐specific mortality Outcome not measured/reported Treatment discontinuation due to adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: at 6 months Time points reported: at 3 months Number of participants randomly assigned: intervention: 22; control: 21 Number of participants in evaluation for ITT: intervention: 21; control: 21 Clinical progression Outcome not measured/reported Biochemical progression Outcome not measured/reported Treatment failure Outcome not measured/reported Adverse events Outcome not measured/reported Other outcomes reported Metabolic parameters, carotid‐femoral and carotid‐radial pulse wave velocity, blood pressure |
|
| Notes |
Study funding source: educational grant from AstraZeneca Possible conflict of interest: Sponsor had no role in any aspect of the study plan, conduct or analysis; interpretation of data; or writing of manuscript |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated and was balanced for every 6 participants |
| Allocation concealment (selection bias) | Unclear risk | Randomly allocated; no other statement reported |
| Blinding of participants and personnel (performance bias) overall survival, cancer‐specific mortality, biochemical progression | Unclear risk | Not measured/reported |
| Blinding of participants and personnel (performance bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for treatment discontinuation due to adverse events. Blinding was not possible because of different interventions provided. We judge that the outcome of treatment discontinuation due to adverse events is likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) overall survival, cancer‐specific mortality, biochemical progression | Unclear risk | Not measured/reported |
| Blinding of outcome assessment (detection bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for treatment discontinuation due to adverse events. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. We judge that it is likely that outcome assessment for treatment discontinuation due to adverse events is influenced by lack of blinding |
| Incomplete outcome data (attrition bias) overall survival, cancer‐specific mortality | Unclear risk | Not measured/reported |
| Incomplete outcome data (attrition bias) treatment discontinuation due to adverse events, adverse events | Low risk | One man in intervention arm dropped out after his baseline visit, leaving 42 participants available for analysis. The proportion of missing outcomes compared with observed event risk is not enough to induce clinically relevant bias in intervention effect estimate |
| Incomplete outcome data (attrition bias) clinical progression, biochemical progression | Unclear risk | Not measured/reported |
| Incomplete outcome data (attrition bias) treatment failure | Unclear risk | Not measured/reported |
| Selective reporting (reporting bias) | High risk | Only adverse events leading to discontinuation reported; no other adverse events reported |
| Other bias | Unclear risk | Unclear risk for conflict of interest |
Sciarra 2004a.
| Methods |
Start date, end date of recruitment: between December 1998 and January 2001 Follow‐up period: After radical retropubic prostatectomy, when serum PSA levels exceeded 0.2 ng/mL, PSA determinations were repeated every 2 weeks. Participants entered the study once their PSA level progressed, defined as 3 or more consecutive elevated levels (greater than 0.4 ng/mL) Serum CgA levels were analysed at baseline (PSA progression) and at 1, 3, 6, 12, 18 and 24 months after random assignment. Total PSA levels were measured every 4 weeks for the first 12 months of treatment and every 8 weeks thereafter Design: randomised controlled trial |
|
| Participants |
Population/Inclusion criteria: Men with pT3pN0M0 prostate cancer and biochemical progression (defined as PSA level greater than 0.4 ng/mL) within 12 months of radical retropubic prostatectomy were enrolled. Other inclusion criteria were histologically proven prostate cancer; no preoperative hormonal therapy or radiotherapy; radical retropubic prostatectomy with regional lymphadenectomy performed at our institution; and negative surgical margins Setting: single centre Geographical location: Italy Exclusion criteria: not reported Total number randomly assigned: 186 men with clinically localised prostate cancer underwent radical retropubic prostatectomy and bilateral pelvic lymphadenectomy at our institution; 59 fulfilled inclusion criteria and agreed to random assignment. Only the 48 men who concluded and successfully responded to the first 24 months of treatment were analysed Baseline imbalances: balanced Number of participants with non‐metastatic disease: 48 Number of participants with metastatic disease: 0 Age (mean ± SD): intervention: 64.2 ± 3.7 years (range 54 to 70 years); control: 64.8 ± 3.5 years (range 56 to 70 years) PSA (mean ± SD): PSA at baseline (progression after prostatectomy) in non‐steroidal antiandrogen group: 1.12 ± 0.32 ng/mL (median 1.0 ng/mL; range 0.60 to 1.80 ng/mL); PSA at baseline (progression after prostatectomy) in castration group: 1.05 ± 0.30 ng/mL (median 1.0 ng/mL; range 0.60 to 1.60 ng/mL) Subgroup measured: no |
|
| Interventions |
Intervention Description/Timing: bicalutamide 150 mg daily Number randomly assigned to this group: unclear (analysed and reported: 24) Control Description/Timing: triptorelin 3.75 mg monthly Number randomly assigned to this group: unclear (analysed and reported: 24) |
|
| Outcomes |
Overall survival Outcome not measured/reported Cancer‐specific mortality Outcome not measured/reported Treatment discontinuation due to adverse events Outcome not measured/reported Clinical progression Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: at 24 months Time points reported: at 24 months Outcome definition in report: Treatment was considered to have failed when the PSA level increased to greater than 0.4 ng/mL or at clinical progression. Biopsy of the urethrovesical anastomosis, abdominal‐pelvic magnetic resonance imaging and a total body bone scan were performed 12 and 24 months after random assignment or at PSA progression Number of participants randomly assigned: intervention: unclear; control: unclear Number of participants in evaluation for ITT: intervention: 24; control: 24 Biochemical progression Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: at 24 months Time points reported: at 24 months Outcome definition in report: Treatment was considered to have failed when the PSA level increased to greater than 0.4 ng/mL or at clinical progression. Biopsy of the urethrovesical anastomosis, abdominal‐pelvic magnetic resonance imaging and a total body bone scan were performed 12 and 24 months after random assignment or at PSA progression Number of participants randomly assigned: intervention: unclear; control: unclear Number of participants in evaluation for ITT: intervention: 24; control: 24 Treatment failure (outcome measured but not reported) Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: at 24 months Time points reported: at 24 months Outcome definition in report: Treatment was considered to have failed when the PSA level increased to greater than 0.4 ng/mL or at clinical progression. Biopsy of the urethrovesical anastomosis, abdominal‐pelvic magnetic resonance imaging and a total body bone scan were performed 12 and 24 months after random assignment or at PSA progression Number of participants randomly assigned: intervention: unclear; control: unclear Number of participants in evaluation for ITT: intervention: 24; control: 24 Adverse events Outcome not measured/reported Other outcomes reported Serum CgA levels |
|
| Notes | During the first 24 months of follow‐up, no participant showed evidence of clinical progression, and the PSA serum levels remained at 0.4 ng/mL or less Study funding source: unclear Possible conflict of interest: unclear |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) overall survival, cancer‐specific mortality, biochemical progression | Unclear risk | No blinding for biochemical progression. Blinding was not possible because of different interventions provided. It might be conceivable that biochemical progression is influenced by lack of blinding. We finally judge that risk of bias regarding this outcome is unclear |
| Blinding of participants and personnel (performance bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for clinical progression and treatment failure. Blinding was not possible because of different interventions provided. We judge that outcomes such as clinical progression and treatment failure are likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) overall survival, cancer‐specific mortality, biochemical progression | Low risk | No blinding for biochemical progression. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. However, we judge that it is not likely that outcome assessment for biochemical progression is influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for clinical progression and treatment failure. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. We judge that it is likely that outcome assessment for clinical progression and treatment failure is influenced by lack of blinding |
| Incomplete outcome data (attrition bias) overall survival, cancer‐specific mortality | Unclear risk | Not measured/reported |
| Incomplete outcome data (attrition bias) treatment discontinuation due to adverse events, adverse events | Unclear risk | Not measured/reported |
| Incomplete outcome data (attrition bias) clinical progression, biochemical progression | High risk | 'As‐treated' analysis on clinical/biochemical progression done with substantial departure of the intervention received from that assigned at randomisation (59 men fulfilled the inclusion criteria and agreed to random assignment; only the 48 participants who concluded and successfully responded to the first 24 months of treatment were analysed) |
| Incomplete outcome data (attrition bias) treatment failure | High risk | Outcome measured but not reported |
| Selective reporting (reporting bias) | High risk | Study report fails to include results for a key outcome that would be expected to have been reported for such a study (no data on adverse events, treatment discontinuation due to adverse events or treatment failure reported). No protocol available |
| Other bias | Unclear risk | Unclear risk for conflict of interest |
Sieber 2004.
| Methods |
Start date, end date of recruitment: not reported Follow‐up period: Subsequent assessments were obtained within 7 days of weeks 24, 48, 72 and 96 Design: randomised controlled trial |
|
| Participants |
Population/Inclusion criteria: Study population consisted of men with histologically confirmed prostate cancer with no distant metastases (T1–T4, Nx, M0) for whom immediate androgen deprivation was indicated Inclusion criteria were an Eastern Cooperative Oncology Group performance status of 0 or 1; life expectancy greater than 6 months; no history or presence of metabolic bone disease, renal failure, malabsorption, rheumatoid arthritis, recent fracture or any other condition known to affect bone metabolism; no hormone therapy within the previous 6 months, no concomitant treatment with any drugs known to affect calcium or vitamin D metabolism and no treatment with systemic steroids. Participants were also required to have a baseline testosterone level greater than the lower limit of normal (194 ng/dL or greater), a calcium level less than the upper limit of normal (10.6 mg/dL or less), a thyroid‐stimulating hormone level within normal limits (0.4 to 10 mg/mL) and a bone mineral density (BMD) within 2 standard deviations of age‐matched controls Setting: multi‐centre (11 locations) Geographical location: United States Exclusion criteria: not reported Total number randomly assigned: 103 Baseline imbalances: balanced Number of participants with non‐metastatic disease: 103 Number of participants with metastatic disease: 0 Age: intervention: mean 74.6 years (range 53 to 87 years); control: mean 75.2 years (range 61 to 90 years) PSA: not reported Subgroup measured: no |
|
| Interventions |
Intervention Description/Timing: bicalutamide 150 mg once daily for 96 weeks Number randomly assigned to this group: 51 Control Description/Timing: medical castration with an LHRH agonist for 96 weeks (drug not specified) Number randomly assigned to this group: 52 |
|
| Outcomes |
Overall survival Outcome not measured/reported Cancer‐specific mortality Outcome not measured/reported Treatment discontinuation due to adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: at 96 weeks Time points reported: at 96 weeks Number of participants randomly assigned: intervention: 51; control: 52 Number of participants in evaluation for ITT: intervention: 50; control: 51 Clinical progression Outcome not measured/reported Biochemical progression Outcome not measured/reported Treatment failure Outcome not measured/reported Adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: at 96 weeks Time points reported: at 96 weeks Number of participants randomly assigned: intervention: 51; control: 52 Number of participants in evaluation for ITT: intervention: 50; control: 51 Other outcomes reported Primary efficacy end points were mean percentage change in lumbar spine BMD, hip BMD and fat‐free mass (FFM) from baseline to 96 weeks. Secondary efficacy end points included mean percentage change in lumbar spine (L2 to L4) BMD, hip BMD and FFM from baseline to 24, 48 and 72 weeks; and mean change from baseline in serum lipid levels of HDL, LDL, total and very low‐density lipoprotein (VLDL) cholesterol and triglycerides. Lumbar spine BMD, hip BMD and FFM were assessed by dual‐energy x‐ray absorptiometry within 6 weeks before random assignment |
|
| Notes |
Study funding source: supported by a research grant from AstraZeneca Possible conflict of interest: financial interest and/or other relationship with AstraZeneca. Two authors had financial interest and/or another relationship with AstraZeneca |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement (eligible participants were randomly assigned in a 1:1 ratio to receive bicalutamide 150 mg once daily or medical castration with an LHRH analogue for 96 weeks) |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) overall survival, cancer‐specific mortality, biochemical progression | Unclear risk | Not measured/reported |
| Blinding of participants and personnel (performance bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for treatment discontinuation due to adverse events and adverse events. Blinding was not possible because of different interventions provided. We judge that outcomes such as treatment discontinuation due to adverse events and adverse events are likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) overall survival, cancer‐specific mortality, biochemical progression | Unclear risk | Not measured/reported |
| Blinding of outcome assessment (detection bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for treatment discontinuation due to adverse events and adverse events. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. We judge that it is likely that outcome assessment for treatment discontinuation due to adverse events and adverse events are influenced by lack of blinding |
| Incomplete outcome data (attrition bias) overall survival, cancer‐specific mortality | Unclear risk | Not measured/reported |
| Incomplete outcome data (attrition bias) treatment discontinuation due to adverse events, adverse events | Low risk | Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups. The proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate (2 participants, 1 in each treatment group, who did not receive randomly assigned therapy and were excluded from the analysis of adverse events). For analyses of all efficacy variables, a modified per‐protocol data set was used. Participants were included according to the treatment received but, unlike in a true per‐protocol data set, participants with major protocol violations were not excluded |
| Incomplete outcome data (attrition bias) clinical progression, biochemical progression | Unclear risk | Not measured/reported |
| Incomplete outcome data (attrition bias) treatment failure | Unclear risk | Not measured/reported |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but it is clear that the published reports include all expected outcomes |
| Other bias | Unclear risk | Unclear risk for conflict of interest |
Smith 2004.
| Methods |
Start date, end date of recruitment: Study participants were recruited between May 2000 and May 2002 Follow‐up period: treatment time: 12 months. Participants were evaluated at baseline and at 3, 6, 9 and 12 months Design: randomised controlled trial |
|
| Participants |
Population/Inclusion criteria: Participants had locally advanced, lymph node–positive or recurrent prostate cancer. Men with prior neoadjuvant or adjuvant hormone therapy were included if the interval between completion of treatment and study entry was longer than 1 year; 10 men (5 men in each group) had received neoadjuvant or adjuvant treatment with a gonadotropin‐releasing hormone agonist Setting: single‐centre trial Geographical location: United States Exclusion criteria: Men with bone metastases by radionuclide bone scan were excluded. Men with Karnofsky performance status less than 90, history of hypogonadism, history of growth hormone or anabolic steroid use, Paget’s disease, hyperthyroidism, Cushing’s disease, hyperprolactinaemia, chronic liver disease, corrected serum calcium 8.4 mg/dL or 10.6 mg/dL or serum creatinine concentration 2.0 mg/dL (177 mol/L) were also excluded. Men were excluded if they had received bisphosphonate, calcitonin or glucocorticoid therapy, or suppressive doses of thyroxine, within 1 year Total number randomly assigned: 52 Baseline imbalances: unclear: balanced for data reported; data for staging not reported Number of participants with non‐metastatic disease: 51 (52 men were randomly assigned to leuprorelin monotherapy or bicalutamide monotherapy. Fifty‐one men completed the baseline evaluation and initiated study treatment; 51 participants completed the study. All 51 participants are included in the analyses, including 3 men who discontinued treatment early) Number of participants with metastatic disease: 0 Age: intervention: mean 63 ± 8 years; control: mean 65 ± 10 years PSA (mean ± SD): intervention: 158 ± 670 ng/mL; control: 40 ± 119 ng/mL Subgroup measured: no |
|
| Interventions |
Intervention Description/Timing: bicalutamide 150 mg by mouth daily for 12 months Number randomly assigned to this group: 25 Control Description/Timing: Leuprorelin 3‐month depot (Lupron Depot; TAP Pharmaceuticals Inc, Deerfield, Illinois; 22.5 mg intramuscularly every 3 months). Men assigned to leuprorelin treatment also received bicalutamide (50 mg by mouth daily) for 1 month to prevent the potential disease flare associated with initial leuprorelin administration Number randomly assigned to this group: 26 |
|
| Outcomes |
Overall survival Outcome not measured/reported Cancer‐specific mortality Outcome not measured/reported Treatment discontinuation due to adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: at 12 months Time points reported: at 12 months Number of participants randomly assigned: intervention: 25; control: 26 Number of participants in evaluation for ITT: intervention: 25; control: 26 Clinical progression Outcome not measured/reported Biochemical progression Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: at 12 months Time points reported: at 12 months Outcome definition in report: Disease progression was defined as new metastatic disease or a greater than 25% increase in serum PSA concentration from nadir value on 2 determinations and 5 ng/mL absolute increase in PSA Number of participants randomly assigned: intervention: 25; control: 26 Number of participants in evaluation for ITT: intervention: 25; control: 26 Note: This outcome was named as clinical progression by the study authors. However, because of the outcome definition reported, we classified it as biochemical progression Treatment failure Outcome not measured/reported Adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: at 12 months Time points reported: at 12 months Number of participants randomly assigned: intervention: 25; control: 26 Number of participants in evaluation for ITT: intervention: 25; control: 26 Other outcomes reported Primary study end points were percentage change in bone mineral density in posterior‐anterior lumbar spine and percentage change in thigh muscle area from baseline to 12 months |
|
| Notes | Email response: "Subjects and study investigators were blinded to treatment assignment. The main outcomes for this 1‐year study were bone mineral density and body composition and the study was NOT designed to evaluate clinical cancer outcomes including time to progression" Study funding source: supported by research award from AstraZeneca PLC Possible conflict of interest: Study authors indicated no potential conflicts of interest |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) overall survival, cancer‐specific mortality, biochemical progression | Unclear risk | Email response: "Subjects and study investigators were blinded to treatment assignment." However, the method of blinding bicalutamide 150 mg by mouth daily for 12 months and leuprorelin 3‐month depot (22.5 mg intramuscularly every 3 months) for biochemical progression remains unclear |
| Blinding of participants and personnel (performance bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | Unclear risk | Email response: "Subjects and study investigators were blinded to treatment assignment." However, the method of blinding bicalutamide 150 mg by mouth daily for 12 months compared with leuprorelin 3‐month depot (22.5 mg intramuscularly every 3 months) for treatment discontinuation due to adverse events and adverse events remains unclear |
| Blinding of outcome assessment (detection bias) overall survival, cancer‐specific mortality, biochemical progression | Low risk | Not reported. However, we judge that it is not likely that outcome assessment for biochemical progression is influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | Unclear risk | Not reported. However, original investigators responded that blinding was performed ("Subjects and study investigators were blinded to treatment assignment"). However, blinding of outcome assessment for treatment discontinuation due to adverse events and adverse events remains unclear |
| Incomplete outcome data (attrition bias) overall survival, cancer‐specific mortality | Unclear risk | Not measured/reported |
| Incomplete outcome data (attrition bias) treatment discontinuation due to adverse events, adverse events | Low risk | Proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate (52 men were randomly assigned to leuprorelin monotherapy or bicalutamide monotherapy. Fifty‐one men completed baseline evaluation and initiated study treatment; 51 participants completed the study. All 51 participants are included in the analyses, including 3 men who discontinued treatment early) |
| Incomplete outcome data (attrition bias) clinical progression, biochemical progression | Low risk | Proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate (52 men were randomly assigned to leuprorelin monotherapy or bicalutamide monotherapy. Fifty‐one men completed the baseline evaluation and initiated study treatment; 51 participants completed the study. All 51 participants are included in the analyses, including 3 men who discontinued treatment early) |
| Incomplete outcome data (attrition bias) treatment failure | Unclear risk | Not measured/reported |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but it is clear that published reports include all expected outcomes |
| Other bias | Unclear risk | Unclear risk for conflict of interest |
Study 0301.
| Methods |
Start date, end date of recruitment: June 1990 to February 1992 Follow‐up period: follow‐up of a minimum of 12 months; mean duration for all 376 participants was 67 weeks for intervention and 75 weeks for control (for all analyses except survival, a data cutoff point was used when a minimum follow‐up of 3 months was reached for the first 306 participants; for the analyses of survival, information from all 376 participants was used with a minimum follow‐up of 12 months) Design: randomised controlled trial |
|
| Participants |
Population/Inclusion criteria: histologically or cytologically diagnosed prostate cancer with evaluable distant metastases and suitable for orchiectomy Setting: multi‐centre Geographical location: Denmark, Norway, Sweden Exclusion criteria: Men who had received or were receiving systemic treatment for prostate cancer (including radiotherapy, antiandrogen, oestrogen, LHRH analogue, ketoconazole or cytotoxic therapy) or who had previously received radiotherapy to the prostate within 3 months of entry into the study were excluded. Other exclusion criteria were history or presence of an invasive malignancy within the past 5 years (other than prostate cancer or squamous/basal cell carcinoma of the skin), an ECOG performance score of 3 or 4, a bilirubin value 1.26 times the upper limit of the reference range or greater and any severe concomitant medical condition that would limit participation in the study. No other treatment for prostate cancer or drugs that could affect sex hormone status were allowed during the follow‐up period Total number randomly assigned: 376 Baseline imbalances: balanced Number of participants with non‐metastatic disease: 0 Number of participants with metastatic disease: 376 Age: intervention: mean 71.5 years (range 54 to 80 years); control: mean 70.3 years (range 49 to 85 years) PSA: not reported Subgroup measured: no |
|
| Interventions |
Intervention Description/Timing: bicalutamide 50 mg once daily Number randomly assigned to this group: 186 Control Description/Timing: bilateral orchiectomy Number randomly assigned to this group: 190 |
|
| Outcomes |
Overall survival Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: For analyses of survival, information from all 376 participants was used, with a minimum follow‐up of 12 months Time points reported: For analyses of survival, information from all 376 participants was used, with a minimum follow‐up of 12 months Number of participants randomly assigned: intervention: 186; control: 190 Number of participants in evaluation for ITT: intervention: 186; control: 190 Cancer‐specific mortality Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: For analyses of survival, information from all 376 participants was used, with a minimum follow‐up of 12 months Time points reported: For analyses of survival, information from all 376 participants was used, with a minimum follow‐up of 12 months Number of participants randomly assigned: intervention: 186; control: 190 Number of participants in evaluation for ITT: intervention: 186; control: 190 Treatment discontinuation due to adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: minimum follow‐up of 3 months Time points reported: mean duration for all 376 participants was 67 weeks for intervention and 75 weeks for control Number of participants randomly assigned: intervention: 186; control: 190 Number of participants in evaluation for ITT: intervention: 153; control: 153 Clinical progression Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: minimum follow‐up of 3 months Time points reported: mean duration for all 376 participants was 67 weeks for intervention and 75 weeks for control Outcome definition in report: an increase in prostatic dimensions (product of 2 greatest perpendicular diameters) by 50% or more (1 initial diameter at least 3 cm) compared with the minimum dimensions recorded during the study; appearance of any new or worsening of existing bone metastases on x‐ray or isotope bone scan; appearance of any new extraskeletal metastasis or an increase in dimensions (by 25% or more) of any existing extraskeletal metastasis compared with the minimum dimensions recorded during the study; death occurring before evidence of objective progression Number of participants randomly assigned: intervention: 186; control: 190 Number of participants in evaluation for ITT: intervention: 153; control: 153 Biochemical progression Outcome not measured/reported Treatment failure Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: minimum follow‐up of 3 months Time points reported: Mean duration for all 376 participants was 67 weeks for intervention and 75 weeks for control Outcome definition in report: death (in the absence of disease progression); objective disease progression; addition of any recognised systemic treatment for prostate cancer before objective progression; patient lost to follow‐up; cessation of therapy because of adverse event, at the discretion of the investigator or at the request of the participant (applicable only to participants receiving bicalutamide) Number of participants randomly assigned: intervention: 186; control: 190 Number of participants in evaluation for ITT: intervention: 153; control: 153 Adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: minimum follow‐up of 3 months Time points reported: mean duration for all 376 participants was 67 weeks for intervention and 75 weeks for control Number of participants randomly assigned: intervention: 186; control: 190 Number of participants in evaluation for ITT: intervention: 153; control: 150 (3 refused surgery) Other outcomes reported Subjective response, quality of life |
|
| Notes |
Study funding source: not reported Possible conflict of interest: not reported; the assistance of a member of Zeneca in preparation of the manuscript was gratefully acknowledged |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) overall survival, cancer‐specific mortality, biochemical progression | Unclear risk | No blinding for overall survival and cancer‐specific mortality. Blinding was not possible because of different interventions provided. It might be conceivable that outcomes such as overall survival and cancer‐specific mortality are influenced by lack of blinding. We finally judge that risk of bias regarding these outcomes is unclear |
| Blinding of participants and personnel (performance bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for clinical progression, treatment discontinuation due to adverse events, adverse events and treatment failure. Blinding was not possible because of different interventions provided. We judge that outcomes such as clinical progression, treatment discontinuation due to adverse events, adverse events and treatment failure are likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) overall survival, cancer‐specific mortality, biochemical progression | Low risk | No blinding for overall survival and cancer‐specific mortality. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. However, we judge that it is not likely that outcome assessment for overall survival and cancer‐specific mortality is influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for clinical progression, treatment discontinuation due to adverse events, adverse events and treatment failure. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. We judge that it is likely that outcome assessment for clinical progression, treatment failure, adverse events and treatment discontinuation due to adverse events is influenced by lack of blinding |
| Incomplete outcome data (attrition bias) overall survival, cancer‐specific mortality | Low risk | Intention‐to‐treat analysis and therefore low risk of bias for incomplete outcome data for overall survival |
| Incomplete outcome data (attrition bias) treatment discontinuation due to adverse events, adverse events | High risk | 'As‐treated' analysis on adverse events done with substantial departure of the intervention received from that assigned at randomisation (participants included who had at least 3 months of follow‐up) |
| Incomplete outcome data (attrition bias) clinical progression, biochemical progression | High risk | 'As‐treated' analysis on clinical progression done with substantial departure of the intervention received from that assigned at randomisation (participants included who had at least 3 months of follow‐up) |
| Incomplete outcome data (attrition bias) treatment failure | High risk | 'As‐treated' analysis on treatment failure done with substantial departure of the intervention received from that assigned at randomisation (participants included who had at least 3 months of follow‐up) |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but it is clear that published reports include all expected outcomes |
| Other bias | Unclear risk | Unclear risk for conflict of interest |
Study 0302.
| Methods |
Start date, end date of recruitment: May 1990 to December 1991 Follow‐up period: study closed in September 1993; median duration of treatment at the time of data cutoff for analysis was 35.3 weeks and 37.7 weeks for participants in the bicalutamide and castration groups, respectively Design: randomised controlled trial |
|
| Participants |
Population/Inclusion criteria: histologically or cytologically confirmed prostate cancer, presence of evaluable metastatic disease and fitness for orchiectomy Setting: multi‐centre (3 countries) Geographical location: United Kingdom, The Netherlands, Austria Exclusion criteria: Men who had received or who were receiving systemic therapy for prostate cancer were excluded. Those who had received radiotherapy to the prostate gland within 3 months of entry of the study and those men with a history in the previous 5 years of invasive malignancy (other than prostate cancer or squamous/basal cell carcinoma of the skin) were also excluded, as were patients with an ECOG performance score of 3 or 4. Patients with a bilirubin value greater than 1.26 times the upper limit of the reference range were also excluded Total number randomly assigned: 304 Baseline imbalances: data presented only for subgroup (characteristics of participants who completed 3 months of treatment; 245 participants instead of 304) Number of participants with non‐metastatic disease: intervention: 0; control: 1 Number of participants with metastatic disease: intervention: 119; control: 125 Age (mean ± SD): intervention: 71.9 ± 8.2 years; control: 72.1 ± 7.8 years PSA: not reported Subgroup measured: no |
|
| Interventions |
Intervention Description/Timing: bicalutamide 50 mg once daily Number randomly assigned to this group: 150 Control Description/Timing: Participants were offered a choice between Zoladex (goserelin 3.6 mg subcutaneous every 28 days) and surgical bilateral orchiectomy (complete or subcapsular); goserelin: 60, orchiectomy: 65 (126 participants included in 'as‐treated' analysis; 1 participant excluded; distribution of participants receiving medical or surgical castration for ITT analysis not reported) Number randomly assigned to this group: 154 |
|
| Outcomes |
Overall survival Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: median follow‐up 61 weeks for intervention and 66 weeks for control Time points reported: median follow‐up 61 weeks for intervention and 66 weeks for control Number of participants randomly assigned: intervention: 150; control: 154 Number of participants in evaluation for ITT: intervention: 150; control: 154 Cancer‐specific mortality Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: median follow‐up 61 weeks for intervention and 66 weeks for control Time points reported: median follow‐up 61 weeks for intervention and 66 weeks for control Number of participants randomly assigned: intervention: 150; control: 154 Number of participants in evaluation for ITT: intervention: 150; control: 154 Treatment discontinuation due to adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: median follow‐up 61 weeks for intervention and 66 weeks for control Time points reported: median follow‐up 61 weeks for intervention and 66 weeks for control Number of participants randomly assigned: intervention: 150; control: 154 Number of participants in evaluation for ITT: intervention: 119; control: 126 Clinical progression Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: median follow‐up 61 weeks for intervention and 66 weeks for control Time points reported: median follow‐up 35.3 weeks for intervention and 37.7 weeks for control Outcome definition in report: any of the following defined objective progressions: increase in prostatic dimensions (product of 2 greatest perpendicular diameters) by 50% or more compared with minimum dimensions recorded during study; appearance of any new or worsening of existing bone metastasis on x‐ray or isotope bone scan; appearance of any new extraskeletal metastasis or increase in dimensions (by 25% or more) of any existing extraskeletal metastasis compared with minimum dimensions recorded during the study; death occurred before evidence of objective progression Number of participants randomly assigned: intervention: 150; control: 154 Number of participants in evaluation for ITT: intervention: 119; control: 126 Biochemical progression Outcome not measured/reported Treatment failure Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: median follow‐up 61 weeks for intervention and 66 weeks for control Time points reported: median follow‐up 35.3 weeks for intervention and 37.7 weeks for control Outcome definition in report: earliest occurrence of any of the following defined treatment failures: death (in the absence of progression); objective disease progression; addition of any recognised systemic treatment for prostate cancer before objective progression; participants lost to follow‐up; cessation of therapy because of adverse events, at the discretion of the investigator or at the request of the participant (applicable only to participants in the bicalutamide group) Number of participants randomly assigned: intervention: 150; control: 154 Number of participants in evaluation for ITT: intervention: 119; control: 126 Adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: median follow‐up 61 weeks for intervention and 66 weeks for control Time points reported: median follow‐up 35.3 weeks for intervention and 37.7 weeks for control Number of participants randomly assigned: intervention: 150; control: 154 Number of participants in evaluation for ITT: intervention: 118; control: 125 Other outcomes reported PSA levels, subjective response, quality of life |
|
| Notes |
Study funding source: not reported Possible conflict of interest: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) overall survival, cancer‐specific mortality, biochemical progression | Unclear risk | No blinding for overall survival and cancer‐specific mortality. Blinding was not possible because of different interventions provided. It might be conceivable that outcomes such as overall survival and cancer‐specific mortality are influenced by lack of blinding. We finally judge that risk of bias regarding these outcomes is unclear |
| Blinding of participants and personnel (performance bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for clinical progression, treatment discontinuation due to adverse events, adverse events and treatment failure. Blinding was not possible because of different interventions provided. We judge that outcomes such as clinical progression, treatment discontinuation due to adverse events, adverse events and treatment failure are likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) overall survival, cancer‐specific mortality, biochemical progression | Low risk | No blinding for overall survival and cancer‐specific mortality. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. However, we judge that it is not likely that outcome assessment for overall survival and cancer‐specific mortality is influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for clinical progression, treatment discontinuation due to adverse events, adverse events and treatment failure. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. We judge that it is likely that outcome assessment for clinical progression, treatment discontinuation due to adverse events, adverse events and treatment failure is influenced by lack of blinding |
| Incomplete outcome data (attrition bias) overall survival, cancer‐specific mortality | Low risk | Intention‐to‐treat analysis and therefore low risk of bias for incomplete outcome data for overall survival (once randomly assigned, all participants were followed for survival regardless of reason for discontinuation) |
| Incomplete outcome data (attrition bias) treatment discontinuation due to adverse events, adverse events | High risk | 'As‐treated' analysis for treatment discontinuation due to adverse events and adverse events done with substantial departure of the intervention received from that assigned at randomisation (efficacy and tolerability analysis was restricted to 245 participants who completed 3 months of treatment) |
| Incomplete outcome data (attrition bias) clinical progression, biochemical progression | High risk | 'As‐treated' analysis for clinical progression done with substantial departure of the intervention received from that assigned at randomisation (efficacy and tolerability analysis was restricted to 245 participants who completed 3 months of treatment) |
| Incomplete outcome data (attrition bias) treatment failure | High risk | 'As‐treated' analysis for treatment failure done with substantial departure of the intervention received from that assigned at randomisation (efficacy and tolerability analysis was restricted to 245 participants who completed 3 months of treatment) |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but it is clear that published reports include all expected outcomes |
| Other bias | Unclear risk | Unclear risk for conflict of interest |
Study 0303.
| Methods |
Start date, end date of recruitment: March 1990 to October 1991 Follow‐up period: All randomly assigned participants who reached treatment failure endpoints or completed at least 3 months of follow‐up as of November 1991 (n = 486) were evaluated. Mean time on randomly assigned therapy was 39 weeks for the bicalutamide treatment group and 42 weeks for the castration group Design: randomised controlled trial |
|
| Participants |
Population/Inclusion criteria: men with untreated stage D2 prostate cancer; confirmation of metastatic disease was based on results of scintigraphy, radiography or computed tomography Setting: multi‐centre (31 locations) Geographical location: United States Exclusion criteria: Men physically unfit to undergo orchiectomy were excluded before random assignment Total number randomly assigned: 516 Baseline imbalances: balanced Number of participants with non‐metastatic disease: 516 Number of participants with metastatic disease: 0 Age: intervention: median 70 years; control: median 71 years PSA: intervention: median 187.3 µg/L; control: median 147.3 µg/L Subgroup measured: no |
|
| Interventions |
Intervention Description/Timing: bicalutamide 50 mg daily Number randomly assigned to this group: 259 Control Description/Timing: Participants randomly assigned to undergo castration chose the method of castration: bilateral orchiectomy or a depot injection of the LHRH analogue goserelin acetate (Zoladex, Zeneca Pharmaceuticals) every 28 days Number randomly assigned to this group: 257 |
|
| Outcomes |
Overall survival Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: unclear Time points reported: 39 weeks for bicalutamide group and 42 weeks for castration group; survival analysis approximately 1 year later was based on data collected through December 1992 (n = 516) Number of participants randomly assigned: intervention: 259; control: 257 Number of participants in evaluation for ITT: intervention: 259; control: 257 Cancer‐specific mortality Outcome not measured/reported Treatment discontinuation due to adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: unclear Time points reported: 39 weeks for bicalutamide group and 42 weeks for castration group Number of participants randomly assigned: intervention: 259; control: 257 Number of participants in evaluation for ITT: intervention: 243; control: 243 Clinical progression Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: unclear Time points reported: 39 weeks for bicalutamide group and 42 weeks for castration group Outcome definition in report: an increase of 50% or more in prostatic dimensions compared with the minimum dimensions recorded during the trial, provided that 1 perpendicular diameter was at least 3 cm; 1 or more new or worsening bone metastases; or 1 or more new extraskeletal metastases or an increase in linear dimensions of at least 25% in any existing measurable extraskeletal metastasis. Increases in serum PSA alone were not considered evidence of progression. Findings from digital rectal examination and changes in acid phosphatase concentrations were not used as tumour markers Number of participants randomly assigned: intervention: 259; control: 257 Number of participants in evaluation for ITT: intervention: 243; control: 243 Biochemical progression Outcome not measured/reported Treatment failure Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: unclear Time points reported: 39 weeks for bicalutamide group and 42 weeks for castration group Outcome definition in report: time from random assignment to treatment failure (death from any cause; objective progression; addition of any recognised systematic treatment; any adverse event leading to discontinuation; discontinuation due to discretion of investigator or request of participant; loss to follow‐up) Number of participants randomly assigned: intervention: 259; control: 257 Number of participants in evaluation for ITT: intervention: 243; control: 243 Adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: no Time points measured: unclear Time points reported: 39 weeks for bicalutamide group and 42 weeks for castration group Number of participants randomly assigned: intervention: 259; control: 257 Number of participants in evaluation for ITT: intervention: 242; control: 238 Other outcomes reported Subjective response, quality of life |
|
| Notes | Prostate‐specific antigen (PSA) was measured before treatment began, every 3 months thereafter and at time of progression. Increases in serum PSA alone were not considered evidence of progression Study funding source: Trial was supported by a grant from Zeneca Pharmaceuticals, Zeneca Inc Possible conflict of interest: Co‐author is a member of Zeneca Pharmaceuticals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) overall survival, cancer‐specific mortality, biochemical progression | Unclear risk | No blinding for overall survival. Blinding was not possible because of different interventions provided. It might be conceivable that overall survival is influenced by lack of blinding. We finally judge that risk of bias regarding this outcome is unclear |
| Blinding of participants and personnel (performance bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for clinical progression, treatment discontinuation due to adverse events, adverse events and treatment failure. Blinding was not possible because of different interventions provided. We judge that outcomes such as clinical progression, treatment discontinuation due to adverse events, adverse events and treatment failure are likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) overall survival, cancer‐specific mortality, biochemical progression | Low risk | No blinding for overall survival. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. However, we judge that it is not likely that overall survival is influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for clinical progression, treatment discontinuation due to adverse events, adverse events and treatment failure. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. We judge that it is likely that outcome assessment for clinical progression, treatment failure, adverse events and treatment discontinuation due to adverse events is influenced by lack of blinding |
| Incomplete outcome data (attrition bias) overall survival, cancer‐specific mortality | Low risk | Intention‐to‐treat analysis and therefore low risk of bias for incomplete outcome data for overall survival (once randomly assigned, all participants were followed for survival regardless of reason for discontinuation) |
| Incomplete outcome data (attrition bias) treatment discontinuation due to adverse events, adverse events | High risk | 'As‐treated' analysis for treatment discontinuation due to adverse events and adverse events done with substantial departure of the intervention received from that assigned at randomisation (efficacy and tolerability analysis was restricted to 486 participants who completed 3 months of treatment) |
| Incomplete outcome data (attrition bias) clinical progression, biochemical progression | High risk | 'As‐treated' analysis for clinical progression done with substantial departure of the intervention received from that assigned at randomisation (efficacy and tolerability analysis was restricted to 486 participants who completed 3 months of treatment) |
| Incomplete outcome data (attrition bias) treatment failure | High risk | 'As‐treated' analysis for treatment failure done with substantial departure of the intervention received from that assigned at randomisation (efficacy and tolerability analysis was restricted to 486 participants who completed 3 months of treatment) |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but it is clear that published reports include all expected outcomes |
| Other bias | Unclear risk | Unclear risk for conflict of interest |
Study 306.
| Methods | Study 306 and Study 307 are 2 individual randomised controlled trials, but with very similar methodology. These studies were planned for pooled analysis, and publications report mostly combined data analysis. We present therefore a summary of the 2 studies Start date, end date of recruitment: January 1992 to June 1993 Follow‐up period: non‐metastatic participants: 6.3 years; metastatic participants: 100 weeks Design: randomised controlled trial Trial registration number of study 307: ISRCTN44967321 |
|
| Participants |
Population/Inclusion criteria: PSA > 20 ng/mL and T3/4 M0 or M1; men with histologically or cytologically diagnosed stage who were considered candidates for palliative hormonal management; metastatic status was assessed for bone metastases by bone scan or x‐ray and for non‐skeletal metastases by x‐ray, computed tomography scan, clinical examination, ultrasound or other relevant test Setting: multi‐centre Geographical location: Study 306: Denmark, Norway, Finland, Sweden; Study 307: UK, South Africa, Austria, The Netherlands, Spain, Australia, Germany, Belgium, Italy Exclusion criteria: previous systemic therapy for prostate cancer; radiation therapy in previous 3 months; invasive malignancy in previous 5 years; ECOG 3 to 4; serum bilirubin ≥ 1.26 times the upper reference range Total number randomly assigned: 1450 (a total of 1453 men were recruited for the 2 studies (Study 306 and Study 307), of whom 1450 received their randomly assigned treatment (bicalutamide 100 mg daily: 165 participants; bicalutamide 150 mg daily: 862 participants; castration: 423 participants); of bicalutamide 150 mg and castration: 480 metastatic participants, 805 non‐metastatic participants) Baseline imbalances: balanced Number of participants with non‐metastatic disease: 480 Number of participants with metastatic disease: 805 Age: intervention: Study 306: mean 71.0 years (range 47 to 88 years); Study 307: mean 72.2 years (range 49 to 92 years); control: Study 306: mean 72.4 years (range 46 to 94 years); Study 307: mean 73.4 years (range 50 to 93 years) PSA: reported only for non‐metastatic participants: intervention (n = 320): range 0.1 to 7691 ng/mL, median 69.2 ng/mL, mean 173.2 ng/mL; control (n = 160): range 8.6 to 6267.7 ng/mL, median 65.3 ng/mL, mean 194.5 ng/mL Subgroup measured: non‐metastatic and metastatic participants (metastatic participants later withdrawn from study after 100 weeks—they received standard therapy; participants with non‐metastatic disease continued); data reported only for bicalutamide 150 mg versus castration |
|
| Interventions |
Intervention Description/Timing: bicalutamide 100 mg or 150 mg daily Number randomly assigned to this group: 864; data reported only for bicalutamide 150 mg versus castration Note: "Initially patients were randomised on a 2:2:1 basis to receive either bicalutamide 100 mg daily, bicalutamide 150 mg daily or castration. The dose of bicalutamide was blinded. After 3 months the higher dose of bicalutamide showed a greater fall in PSA. This dose was selected for further investigation. Patients were then randomised to either bicalutamide 150 mg daily or castration on a 2:1 basis." Investigators presented no data for bicalutamide 100 mg daily Control Description/Timing: bilateral orchiectomy or goserelin acetate 3.6 mg every 28 days at the discretion of participants and investigators Number randomly assigned to this group: 424 |
|
| Outcomes |
Overall survival Comparison: non‐steroidal antiandrogen versus castration Subgroup: non‐metastatic and metastatic participants; data reported only for bicalutamide 150 mg versus castration Time points measured: Clinical visits were scheduled at 4, 8 and 12 weeks after random assignment and at 12‐week intervals thereafter until death occurred Time points reported: non‐metastatic and metastatic participants after 100 weeks; non‐metastatic participants after median follow‐up of 202 to 205 weeks and 6.3 years Number of participants randomly assigned: intervention: non‐metastatic participants: 320, metastatic participants: 544; control: non‐metastatic participants: 160, metastatic participants: 264 Number of participants in evaluation for ITT: intervention: non‐metastatic participants: 320, metastatic participants: 544; control: non‐metastatic participants: 160, metastatic participants: 264 Cancer‐specific mortality Outcome not measured/reported Treatment discontinuation due to adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: reported only for non‐metastatic participants (data reported only for bicalutamide 150 mg vs castration) Time points measured: Clinical visits were scheduled at 4, 8 and 12 weeks after randomisation and at 12‐week intervals thereafter until death occurred Time points reported: at 6.3 years Number of participants randomly assigned: intervention: 320; control: 160 Number of participants in evaluation for ITT: intervention: 314; control: 160 Clinical progression Comparison: non‐steroidal antiandrogen versus castration Subgroup: non‐metastatic and metastatic participants (data reported only for bicalutamide 150 mg vs castration) Time points measured: Clinical visits were scheduled at 4, 8 and 12 weeks after randomisation and at 12‐week intervals thereafter until death occurred Time points reported: metastatic participants after 12 months and 24 months; non‐metastatic and metastatic participants after 100 weeks; non‐metastatic participants after median follow‐up of 202 to 205 weeks and 6.3 years Outcome definition in report: time of random assignment to 50% or greater increase in prostatic dimension (product of the 2 largest perpendicular dimensions) from the minimum recorded during the trial, bony metastases on x‐ray or isotope bone scan or appearance of extraskeletal metastases Number of participants randomly assigned: intervention: non‐metastatic participants: 320, metastatic participants: 544; control: non‐metastatic participants: 160, metastatic participants: 264 Number of participants in evaluation for ITT: intervention: non‐metastatic participants: 320, metastatic participants: 544; control: non‐metastatic participants: 160, metastatic participants: 264 Biochemical progression Outcome not measured/reported Treatment failure Comparison: non‐steroidal antiandrogen versus castration Subgroup: non‐metastatic and metastatic participants (data reported only for bicalutamide 150 mg vs castration) Time points measured: Clinical visits were scheduled at 4, 8 and 12 weeks after random assignment and at 12‐week intervals thereafter until death occurred Time points reported: median 202 to 205 weeks Outcome definition in report: death from any cause; objective progression; addition of other systemic therapy for prostate cancer; loss to follow‐up; refusal to take or continue with randomly assigned therapy; cessation due to adverse events or other reasons (bicalutamide and medical castration only) Number of participants randomly assigned: intervention: non‐metastatic participants: 320, metastatic participants: 544; control: non‐metastatic participants: 160, metastatic participants: 264 Number of participants in evaluation for ITT: intervention: non‐metastatic participants: 320, metastatic participants: 544; control: non‐metastatic participants: 160, metastatic participants: 264 Adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: non‐metastatic and metastatic participants (data reported only for bicalutamide 150 mg vs castration) Time points measured: Clinical visits were scheduled at 4, 8 and 12 weeks after random assignment and at 12‐week intervals thereafter until death occurred Time points reported: after 100 weeks, at 202 to 205 weeks and after 6.3 years Number of participants randomly assigned: intervention: 320, control: 160 Number of participants in evaluation for ITT: intervention: 314; control: 160 Other outcomes reported Subjective response, quality of life, bone mineral density (evaluated in only 29 non‐metastatic participants) |
|
| Notes |
Study funding source: not reported Possible conflict of interest: Seven trial authors had a financial interest and/or relationship with AstraZeneca; co‐author is a member of sponsor (AstraZeneca) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) overall survival, cancer‐specific mortality, biochemical progression | Unclear risk | No blinding for overall survival. Blinding was not possible because of different interventions provided. It might be conceivable that overall survival is influenced by lack of blinding. We finally judge that risk of bias regarding this outcome is unclear Note: Comparison of bicalutamide 100 mg daily versus 150 mg daily was blinded, but participants receiving bicalutamide 100 mg daily discontinued |
| Blinding of participants and personnel (performance bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for clinical progression, treatment discontinuation due to adverse events, adverse events and treatment failure. Blinding was not possible because of different interventions provided. We judge that outcomes such as clinical progression, treatment discontinuation due to adverse events, adverse events and treatment failure are likely to be influenced by lack of blinding Note: Comparison of bicalutamide 100 mg daily versus 150 mg daily was blinded, but participants receiving bicalutamide 100 mg daily discontinued |
| Blinding of outcome assessment (detection bias) overall survival, cancer‐specific mortality, biochemical progression | Low risk | No blinding for overall survival. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. However, we judge that it is not likely that outcome assessment for overall survival is influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. We judge that it is likely that outcome assessment for clinical progression, treatment failure, adverse events and treatment discontinuation due to adverse events is influenced by lack of blinding |
| Incomplete outcome data (attrition bias) overall survival, cancer‐specific mortality | Low risk | Data are reported on intention‐to‐treat |
| Incomplete outcome data (attrition bias) treatment discontinuation due to adverse events, adverse events | Low risk | Data are reported on intention‐to‐treat. Proportion of missing outcomes for treatment discontinuation due to adverse events and adverse events data compared with observed event risk were not enough to have a clinically relevant impact on the intervention effect estimate |
| Incomplete outcome data (attrition bias) clinical progression, biochemical progression | Low risk | Data are reported on intention‐to‐treat |
| Incomplete outcome data (attrition bias) treatment failure | Low risk | Data are reported on intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but it is clear that published reports include all expected outcomes |
| Other bias | Unclear risk | Unclear risk for conflict of interest Note: A protocol interim analysis after a median of approximately 100 weeks revealed a statistically significant qualitative interaction between randomly assigned treatment group and stage of disease at entry (M0 and M1); consequently, data from these subgroups were analysed separately. At that time, the M1 data were considered mature (43% deaths, combined trials) and indicated a significant survival advantage of 6 weeks for participants treated by castration. In accordance with DMSC advice, the M1 participants were withdrawn from the trial, and no further data for this subgroup are presented here. The M0 data, at that time, were considered immature (13% of cases resulting in death, combined trials), and so these participants remained in the trial |
Study 307.
| Methods | Study 306 and Study 307 are individual randomised controlled trials, but with very similar methodology. These studies were planned for pooled analysis, and publications report mostly combined data analysis. We present therefore a summary of the 2 studies Start date, end date of recruitment: January 1992 to June 1993 Follow‐up period: non‐metastatic participants: 6.3 years; metastatic participants: 100 weeks Design: randomised controlled trial Trial registration number of study 307: ISRCTN44967321 |
|
| Participants |
Population/Inclusion criteria: PSA > 20 ng/mL and T3/4 M0 or M1; men with histologically or cytologically diagnosed stage who were considered candidates for palliative hormonal management; metastatic status was assessed for bone metastases by bone scan or x‐ray and for non‐skeletal metastases by x‐ray, computed tomography scan, clinical examination, ultrasound or other relevant test Setting: multi‐centre Geographical location: Study 306: Denmark, Norway, Finland, Sweden; Study 307: UK, South Africa, Austria, The Netherlands, Spain, Australia, Germany, Belgium, Italy Exclusion criteria: previous systematic therapy for prostate cancer; radiation therapy in previous 3 months; invasive malignancy in previous 5 years; ECOG 3 to 4; serum bilirubin ≥ 1.26 times the upper reference range Total number randomly assigned: 1450 (a total of 1453 men were recruited for the 2 studies (Study 306 and Study 307), of whom 1450 received their randomly assigned treatment (bicalutamide 100 mg daily: 165 participants; bicalutamide 150 mg daily: 862 participants; castration: 423 participants); of bicalutamide 150 mg and castration: 480 metastatic participants, 805 non‐metastatic participants) Baseline imbalances: balanced Number of participants with non‐metastatic disease: 480 Number of participants with metastatic disease: 805 Age: intervention: Study 306: mean 71.0 years (range 47 to 88 years), Study 307: mean 72.2 years (range 49 to 92 years); control: Study 306: mean 72.4 years (range 46 to 94 years), Study 307: mean 73.4 years (range 50 to 93 years) PSA: reported only for non‐metastatic participants: intervention (n = 320): range 0.1 to 7691 ng/mL, median 69.2 ng/mL, mean 173.2 ng/mL; control (n = 160): range 8.6 to 6267.7 ng/mL, median 65.3 ng/mL, mean 194.5 ng/mL Subgroup measured: non‐metastatic and metastatic participants (metastatic participants later withdrawn from study after 100 weeks—they received standard therapy; participants with non‐metastatic disease continued); data reported only for bicalutamide 150 mg versus castration |
|
| Interventions |
Intervention Description/Timing: bicalutamide 100 mg daily or 150 mg daily Number randomly assigned to this group: 864; data reported only for bicalutamide 150 mg versus castration Note: "Initially patients were randomised on a 2:2:1 basis to receive either bicalutamide 100 mg daily, bicalutamide 150 mg daily or castration. The dose of bicalutamide was blinded. After 3 months the higher dose of bicalutamide showed a greater fall in PSA. This dose was selected for further investigation. Patients were then randomised to either bicalutamide 150 mg daily or castration on a 2:1 basis." Investigators presented no data for bicalutamide 100 mg daily Control Description/Timing: bilateral orchiectomy or goserelin acetate 3.6 mg every 28 days at the discretion of participants and investigators Number randomly assigned to this group: 424 |
|
| Outcomes |
Overall survival Comparison: non‐steroidal antiandrogen versus castration Subgroup: non‐metastatic and metastatic participants; data reported only for bicalutamide 150 mg versus castration Time points measured: Clinical visits were scheduled at 4, 8 and 12 weeks after random assignment and at 12‐week intervals thereafter until death occurred Time points reported: non‐metastatic and metastatic participants after 100 weeks; non‐metastatic participants after median follow‐up of 202 to 205 weeks and 6.3 years Number of participants randomly assigned: intervention: non‐metastatic participants: 320, metastatic participants: 544; control: non‐metastatic participants: 160, metastatic participants: 264 Number of participants in evaluation for ITT: intervention: non‐metastatic participants: 320, metastatic participants: 544; control: non‐metastatic participants: 160, metastatic participants: 264 Cancer‐specific mortality Outcome not measured/reported Treatment discontinuation due to adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: reported only for non‐metastatic participants (data reported only for bicalutamide 150 mg vs castration) Time points measured: Clinical visits were scheduled at 4, 8 and 12 weeks after random assignment and at 12‐week intervals thereafter until death occurred Time points reported: at 6.3 years Number of participants randomly assigned: intervention: 320; control: 160 Number of participants in evaluation for ITT: intervention: 314; control: 160 Clinical progression Comparison: non‐steroidal antiandrogen versus castration Subgroup: non‐metastatic and metastatic participants (data reported only for bicalutamide 150 mg vs castration) Time points measured: Clinical visits were scheduled at 4, 8 and 12 weeks after random assignment and at 12‐week intervals thereafter until death occurred Time points reported: metastatic participants after 12 months and 24 months; non‐metastatic and metastatic participants after 100 weeks; non‐metastatic participants after median follow‐up of 202 to 205 weeks and 6.3 years Outcome definition in report: time of random assignment to 50% or greater increase in prostatic dimension (product of the 2 largest perpendicular dimensions) from the minimum recorded during the trial, bony metastases on x‐ray or isotope bone scan or appearance of extraskeletal metastases Number of participants randomly assigned: intervention: non‐metastatic participants: 320, metastatic participants: 544; control: non‐metastatic participants: 160, metastatic participants: 264 Number of participants in evaluation for ITT: intervention: non‐metastatic participants: 320, metastatic participants: 544; control: non‐metastatic participants: 160, metastatic participants: 264 Biochemical progression Outcome not measured/reported Treatment failure Comparison: non‐steroidal antiandrogen versus castration Subgroup: non‐metastatic and metastatic participants (data reported only for bicalutamide 150 mg vs castration) Time points measured: Clinical visits were scheduled at 4, 8 and 12 weeks after random assignment and at 12‐week intervals thereafter until death occurred Time points reported: median 202 to 205 weeks Outcome definition in report: death from any cause; objective progression; addition of other systemic therapy for prostate cancer; loss to follow‐up; refusal to take or continue with randomly assigned therapy; cessation due to adverse events or other reasons (bicalutamide and medical castration only) Number of participants randomly assigned: intervention: non‐metastatic participants: 320; metastatic participants: 544; control: non‐metastatic participants: 160; metastatic participants: 264 Number of participants in evaluation for ITT: intervention: non‐metastatic participants: 320; metastatic participants: 544; control: non‐metastatic participants: 160; metastatic participants: 264 Adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: non‐metastatic and metastatic participants (data reported only for bicalutamide 150 mg vs castration) Time points measured: Clinical visits were scheduled at 4, 8 and 12 weeks after random assignment and at 12‐week intervals thereafter until death occurred Time points reported: after 100 weeks, at 202 to 205 weeks and after 6.3 years Number of participants randomly assigned: intervention: 320; control: 160 Number of participants in evaluation for ITT: intervention: 314; control: 160 Other outcomes reported Subjective response, quality of life, bone mineral density (evaluated in only 29 non‐metastatic participants) |
|
| Notes |
Study funding source: not reported Possible conflict of interest: Seven study authors had a financial interest and/or relationship with AstraZeneca; co‐author is a member of sponsor (AstraZeneca) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) overall survival, cancer‐specific mortality, biochemical progression | Unclear risk | No blinding for overall survival. Blinding was not possible because of different interventions provided. It might be conceivable that overall survival is influenced by lack of blinding. We finally judge that risk of bias regarding this outcome is unclear Note: Comparison of bicalutamide 100 mg daily versus 150 mg daily was blinded, but participants receiving bicalutamide 100 mg daily discontinued |
| Blinding of participants and personnel (performance bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for clinical progression, treatment discontinuation due to adverse events, adverse events and treatment failure. Blinding was not possible because of different interventions provided. We judge that outcomes such as clinical progression, treatment discontinuation due to adverse events, adverse events and treatment failure are likely to be influenced by lack of blinding Note: Comparison of bicalutamide 100 mg daily versus 150 mg daily was blinded, but participants receiving bicalutamide 100 mg daily discontinued |
| Blinding of outcome assessment (detection bias) overall survival, cancer‐specific mortality, biochemical progression | Low risk | No blinding for overall survival. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. However, we judge that it is not likely that overall survival is influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for clinical progression, treatment discontinuation due to adverse events, adverse events and treatment failure. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. We judge that it is likely that outcome assessment for clinical progression, treatment failure, adverse events and treatment discontinuation due to adverse events is influenced by lack of blinding |
| Incomplete outcome data (attrition bias) overall survival, cancer‐specific mortality | Low risk | Data are reported on intention‐to‐treat |
| Incomplete outcome data (attrition bias) treatment discontinuation due to adverse events, adverse events | Low risk | Data are reported on intention‐to‐treat. Proportions of missing outcomes for safety data compared with observed event risk were not enough to have a clinically relevant impact on the intervention effect estimate |
| Incomplete outcome data (attrition bias) clinical progression, biochemical progression | Low risk | Data are reported on intention‐to‐treat |
| Incomplete outcome data (attrition bias) treatment failure | Low risk | Data are reported on intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Study protocol is not available, but it is clear that published reports include all expected outcomes |
| Other bias | Unclear risk | Unclear risk for conflict of interest Note: A protocol interim analysis after a median of approximately 100 weeks revealed a statistically significant qualitative interaction between randomly assigned treatment group and stage of disease at entry (M0 and M1); consequently, data from these subgroups were analysed separately. At that time, the M1 data were considered mature (43% deaths, combined trials) and indicated a significant survival advantage of 6 weeks for participants treated by castration. In accordance with DMSC advice, M1 participants were withdrawn from the trial, and no further data for this subgroup are presented here. The M0 data, at that time, were considered immature (13% of cases resulting in death, combined trials), and so these participants remained in the trial |
Tyrrell 2006.
| Methods |
Start date, end date of recruitment: between November 1994 and September 1996 Follow‐up period: Mean duration of treatment in months was 27.6 (range 1.9 to 75.6) in the bicalutamide 300 mg group, 30.0 (range 2.0 to 78.7) in the 450 mg group and 29.2 (range 0.5 to 73.5) in the 600 mg group. Mean duration of treatment in the castration group was 33.5 (range 1.0 to 78.2) months; median follow‐up: 5 years Design: randomised controlled trial Trial registration number: ISRCTN16559899 |
|
| Participants |
Population/Inclusion criteria: Men with histologically or cytologically confirmed M0 (T3 or T4) or M1 prostate cancer were included in the trial, provided they had a PSA level ≥ 20 ng/mL, a life expectancy greater than 3 months and evaluable disease and were considered fit enough to receive any of the randomly assigned treatments Setting: multi‐centre Geographical location: conducted at 26 centres across Belgium, Denmark, Finland, France, Norway, Portugal, Spain, Sweden and the UK Exclusion criteria: any previous or concurrent systemic therapy for prostate cancer; previous radiotherapy to the prostate within 3 months before trial entry Total number randomly assigned: 248 Baseline imbalances: balanced Number of participants with non‐metastatic disease: 139 Number of participants with metastatic disease: 109 Age: bicalutamide 300 mg daily: mean 69.5 years (range 46 to 82 years); bicalutamide 450 mg daily: mean 71.2 years (range 52 to 89 years); bicalutamide 600 mg daily: mean 72.1 years (range 56 to 84 years); castration: mean 71.3 years (range 51 to 86 years) PSA: bicalutamide 300 mg daily: mean 76.5 ng/mL (range 21 to 545 ng/mL); bicalutamide 450 mg daily: mean 105.9 ng/mL (range 20 to 7008 ng/mL); bicalutamide 600 mg daily: mean 89.4 ng/mL (range 19 to 965 ng/mL); castration: mean 91.2 ng/mL (range 16 to 2000 ng/mL) Subgroup measured: Results are presented for the overall participant population and by disease stage (M0 or M1); however data for these subgroups were reported only for overall survival |
|
| Interventions |
Intervention Description/Timing: bicalutamide 300 mg daily or 450 mg daily or 600 mg daily Number randomly assigned to this group: 21 participants received bicalutamide 300 mg daily, 95 participants received bicalutamide 450 mg daily and 43 participants received bicalutamide 600 mg daily Note: "Patients were initially recruited into a non‐randomised phase to assess the tolerability of oral bicalutamide 300 mg (two 150 mg tablets) given once daily. Tolerance was assessed in these patients after 6 weeks of treatment and, if acceptable, future patients were randomised on 1:1 to either bicalutamide 450 mg (three 150 mg tablets) or castration (bilateral orchidectomy or goserelin acetate 3.6 mg depot every 28 days). If, after 6 weeks, tolerance at the 450 mg dose was acceptable, further patients were to be randomised at 1:1:1 to bicalutamide 450 mg, bicalutamide 600 mg (four 150 mg tablets) or castration. However, if tolerance at the 450 mg dose was unacceptable, future patients were to be randomised to bicalutamide 300 mg or castration. If the 600 mg dose was acceptable (after 6 weeks of follow‐up), recruitment to the bicalutamide 450 mg dose was ended and all future patients entering the study were to be randomised at 1:1:1 to bicalutamide 600 mg, bicalutamide 750 mg (5 150 mg tablets) or castration. If tolerance at the 600 mg dose was unacceptable, new patients were to be randomised to bicalutamide 450 mg or castration. If tolerability at the 750 mg dose was acceptable, a further and final dose escalation to 900 mg (six 150 mg tablets) was to be done. Recruitment of 20 patients per treatment group was planned for each dose‐escalation stage"; "As patients who received bicalutamide at the 300 mg dose were not randomised, it was considered inappropriate to provide any comparisons with the other treatments" Control Description/Timing: goserelin acetate or bilateral orchidectomy Number randomly assigned to this group: Of 90 participants randomly assigned to castration, 82 received goserelin acetate and 8 underwent bilateral orchiectomy |
|
| Outcomes |
Overall survival Comparison: non‐steroidal antiandrogen versus castration Subgroup: non‐metastatic and metastatic participants Time points measured: median follow‐up of 5 years Time points reported: median follow‐up of 5 years Number of participants randomly assigned: intervention: bicalutamide 450 mg daily: 95; bicalutamide 600 mg daily: 43; control: 90 (non‐metastatic disease: bicalutamide 450/600 mg daily: 74, castration: 54; metastatic disease: bicalutamide 450/600 mg daily: 63; castration: 36) Number of participants in evaluation for ITT: intervention: bicalutamide 450 mg daily: 92; bicalutamide 600 mg daily: 42; control: 90 Cancer‐specific mortality Comparison: non‐steroidal antiandrogen versus castration Subgroup: non‐metastatic and metastatic participants (data reported only for combined population) Time points measured: median follow‐up of 5 years Time points reported: median follow‐up of 5 years Number of participants randomly assigned: intervention: bicalutamide 450 mg daily: 95; bicalutamide 600 mg daily: 43; control: 90 Number of participants in evaluation for ITT: intervention: bicalutamide 450 mg daily: 92; bicalutamide 600 mg daily: 42; control: 90 Treatment discontinuation due to adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: non‐metastatic and metastatic participants (data reported only for combined population) Time points measured: median follow‐up of 5 years Time points reported: median follow‐up of 5 years Number of participants randomly assigned: intervention: bicalutamide 450 mg daily: 95; bicalutamide 600 mg daily: 43; control: 90 Number of participants in evaluation for ITT: intervention: bicalutamide 450 mg daily: 92; bicalutamide 600 mg daily: 42; control: 90 Clinical progression Comparison: non‐steroidal antiandrogen versus castration Subgroup: non‐metastatic and metastatic participants (data reported only for combined population) Time points measured: median follow‐up of 5 years Time points reported: median follow‐up of 5 years Outcome definition in report: Objective evidence for disease progression was considered to include any of the following: ≥ 50% increase in prostatic dimensions compared with the minimum dimensions recorded during the trial (provided 1 of the dimensions was > 3 cm at the time of assessment); the appearance of new or worsening of existing bone metastases on x‐ray or isotopic bone scan; or the appearance of new extraskeletal metastases or a ≥ 25% increase in the dimensions of any existing extraskeletal metastases compared with the minimum dimensions recorded during the trial Number of participants randomly assigned: intervention: bicalutamide 450 mg daily: 95; bicalutamide 600 mg daily: 43; control: 90 Number of participants in evaluation for ITT: intervention: bicalutamide 450 mg daily: 92; bicalutamide 600 mg daily: 42; control: 90 Biochemical progression Outcome not measured/reported Treatment failure Outcome not measured/reported Adverse events Comparison: non‐steroidal antiandrogen versus castration Subgroup: non‐metastatic and metastatic participants (data reported only for combined population) Time points measured: median follow‐up of 5 years Time points reported: median follow‐up of 5 years Number of participants randomly assigned: intervention: bicalutamide 450 mg daily: 95; bicalutamide 600 mg daily: 43; control: 90 Number of participants in evaluation for ITT: intervention: bicalutamide 450 mg daily: 92; bicalutamide 600 mg daily: 42; control: 90 Other outcomes reported Pharmacokinetic, change in serum PSA level from baseline after 12 weeks of treatment |
|
| Notes |
Study funding source: AstraZeneca Possible conflict of interest: Five study investigators were funded by sponsor; author is a paid consultant to sponsor; another author is an employee of sponsor; editorial support was provided; financial assistance for this support was provided by AstraZeneca |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomly assigned treatment was assigned according to a scheme prepared by AstraZeneca using appropriate computer software. Random assignment was co‐ordinated in the UK by the Clinical Trials Unit, Queen Elizabeth Hospital, Birmingham; in Sweden, Denmark, Finland and Norway by the Randomisation Bureau, Danish Cancer Society, Aarhus, Denmark; and for all other centres by the local AstraZeneca Pharma Office |
| Allocation concealment (selection bias) | High risk | Participant numbers were allocated sequentially by entry as participants entered the trial |
| Blinding of participants and personnel (performance bias) overall survival, cancer‐specific mortality, biochemical progression | Unclear risk | No blinding for overall survival and cancer‐specific mortality. Blinding was not possible because of different interventions provided. It might be conceivable that outcomes such as overall survival and cancer‐specific mortality are influenced by lack of blinding. We finally judge that risk of bias regarding these outcomes is unclear |
| Blinding of participants and personnel (performance bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding for clinical progression, treatment discontinuation due to adverse events and adverse events. Blinding was not possible because of different interventions provided. We judge that outcomes such as clinical progression, treatment discontinuation due to adverse events and adverse events are likely to be influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) overall survival, cancer‐specific mortality, biochemical progression | Low risk | No blinding for overall survival and cancer‐specific mortality. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. However, we judge that it is not likely that outcome assessment for overall survival and cancer‐specific mortality is influenced by lack of blinding |
| Blinding of outcome assessment (detection bias) treatment discontinuation due to adverse events, clinical progression, treatment failure, adverse events | High risk | No blinding. Blinding was not possible because of different interventions provided, and study authors reported insufficient information only. We judge that it is likely that outcome assessment for clinical progression, treatment failure, adverse events and treatment discontinuation due to adverse events is influenced by lack of blinding |
| Incomplete outcome data (attrition bias) overall survival, cancer‐specific mortality | Low risk | Proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate (all participants were evaluable except for 3 in the bicalutamide 450 mg group and 1 in the bicalutamide 600 mg group, who received no study treatment) |
| Incomplete outcome data (attrition bias) treatment discontinuation due to adverse events, adverse events | Low risk | Proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate (all participants were evaluable except for 3 in the bicalutamide 450 mg group and 1 in the bicalutamide 600 mg group, who received no study treatment) |
| Incomplete outcome data (attrition bias) clinical progression, biochemical progression | Low risk | Proportion of missing outcomes compared with observed event risk was not enough to have a clinically relevant impact on the intervention effect estimate (all participants were evaluable except for 3 in the bicalutamide 450 mg group and 1 in the bicalutamide 600 mg group, who received no study treatment) |
| Incomplete outcome data (attrition bias) treatment failure | Unclear risk | Not measured/reported |
| Selective reporting (reporting bias) | Low risk | Study protocol was not available, but it was clear that published reports included all expected outcomes |
| Other bias | Unclear risk | Unclear risk for conflict of interest Note: Allocation of bicalutamide 300 mg was not randomly assigned in this trial, and random assignment to the higher doses was done in 2 sections. Consequently, although the similar demographics of the treatment groups offer reassurance as to the validity of the results, the efficacy data should still be interpreted with some caution. Analysis of time to death was one of the endpoints not specified in the protocol that were analysed retrospectively to compare the effect of high doses (450 mg to 600 mg) of bicalutamide or castration on survival rates of participants with advanced prostate cancer |
BMD, bone mineral density; CgA, chromogranin A; DMSC, Data Management Sub‐Committee; ECOG, Eastern Cooperative Oncology Group; FFM, fat‐free mass; HDL, high‐density lipoprotein; ITT, intention‐to‐treat; LDL, low‐density lipoprotein; LHRH, luteinising hormone–releasing hormone; PSA, prostate‐specific antigen; SD, standard deviation; VLDL, very low‐density lipoprotein; x‐ray, x‐radiation.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akaza 1993 | Comparison of other androgen suppression therapies |
| Akaza 2003 | Comparison of other androgen suppression therapies |
| Alberts 2006 | Patients not fitting to inclusion criteria. Comparison of other androgen suppression therapies |
| Aso 1993a | Comparison of other androgen suppression therapies |
| Aso 1993b | Comparison of other androgen suppression therapies |
| Auvinen 2004 | Different topic |
| Ayub 1990 | Comparison of other androgen suppression therapies |
| Blackledge 1996 | Not a randomised controlled study (review article) |
| Boccardo 2002 | Comparison of other androgen suppression therapies |
| Bono 2007 | Not a randomised controlled study. Patients not fitting to inclusion criteria |
| Bruun 1996 | Comparison of other androgen suppression therapies |
| Chang 1996 | Comparison of other androgen suppression therapies |
| Chatelain 1994 | Comparison of other androgen suppression therapies |
| Cockshott 1990 | No comparison with control group |
| Colquhoun 2012 | Different topic |
| Dawson 1997 | Comparison of other androgen suppression therapies |
| Decensi 2007 | Comparison of bicalutamide versus no treatment |
| Ekwueme 2012 | Not a randomised controlled study |
| EPC program | Comparison of bicalutamide versus placebo |
| Eri 1993 | Patients not fitting to inclusion criteria. Comparison of bicalutamide versus placebo |
| Eri 2001 | Comparison of bicalutamide versus placebo. Patients not fitting to inclusion criteria |
| Festuccia 2007 | Different topic. Comparison of other androgen suppression therapies |
| Gravina 2007 | Different topic |
| Henderson 2003 | Not a randomised controlled study. Different topic |
| Iida 2011 | Comparison of other androgen suppression therapies. Not a randomised controlled study |
| Jacobo 1976 | Comparison of other androgen suppression therapies |
| Johansson 1988 | Comparison of other androgen suppression therapies |
| Jones 1994 | Not a randomised controlled study |
| Kaisary 1994 | Not a randomised controlled study |
| Kariakin 2001 | No comparison with control group |
| Kasimis 2000 | Not a randomised controlled study |
| Kotake 1996a | Not a randomised controlled study |
| Kotake 1996b | Comparison of different doses of bicalutamide (no comparison with castration) |
| Kulkarni 2003 | Comparison of other androgen suppression therapies |
| Lazzaro 2007 | Different topic |
| Lee 2009 | Comparison of other androgen suppression therapies. Randomised cross‐over study. Patients not fitting to inclusion criteria |
| Lee 2010 | Comparison of other androgen suppression therapies. Randomised cross‐over study. Patients not fitting to inclusion criteria |
| Lehmusvaara 2012 | Not a randomised controlled study. Different topic |
| Lin 2011 | Comparison of other androgen suppression therapies |
| Lissoni 2002 | No comparison with control group |
| Loran 2001 | Comparison of other androgen suppression therapies. Not a randomised controlled study. Patients not fitting to inclusion criteria |
| Lund 1988 | Comparison of other androgen suppression therapies |
| Lunglmayr 1995 | No comparison with control group |
| McGivern 2011 | Different topic. Not a randomised controlled study |
| McGivern 2012 | Different topic. Not a randomised controlled study |
| Migliari 1991 | Not a randomised controlled study |
| Migliari 1992 | No comparison with control group |
| Motofei 2011 | Not a randomised controlled study |
| Murphy 2004 | Comparison of different doses of flutamide. No comparison with castration |
| Newling 1989 | Not a randomised controlled study |
| Noldus 1996 | Comparison of other androgen suppression therapies |
| Nyman 2005 | Not a randomised controlled study. Different topic |
| Oosterlinck 1996 | Comparison of other androgen suppression therapies. Not a randomised controlled study |
| Ostri 1991 | Comparison of other androgen suppression therapies |
| Petit 2008 | Not a randomised controlled study |
| Prezioso 2007 | Not a randomised controlled study |
| Raina 2007 | Not a randomised controlled study |
| Raynaud 1988 | Not a randomised controlled study |
| Scattoni 2006 | Comparison of bicalutamide versus no treatment |
| Scher 1997 | No comparison with control group. Patients not fitting to inclusion criteria |
| Schröder 2000 | Comparison of other androgen suppression therapies |
| Schröder 2004 | Comparison of other androgen suppression therapies |
| Sciarra 2004b | Not a randomised controlled study |
| See 2004 | Not a randomised controlled study |
| Shipley 2011 | Comparison of bicalutamide versus placebo |
| Smith 2003 | Not a randomised controlled study |
| Soloway 1996 | No comparison with control group |
| Thorpe 1996 | Comparison of other androgen suppression therapies |
| Thrasher 2000 | Comparison of other androgen suppression therapies |
| Tyrrell 1998 | No comparison with castration |
| Verhelst 1994 | No comparison with control group |
| Wadhwa 2009 | Not a randomised controlled study |
| Wadhwa 2011 | Not a randomised controlled study |
| Wirth 2004 | Comparison of flutamide versus no treatment |
| Yoshimura 2003 | Not a randomised controlled study. Different topic |
| Zanardi 2009 | Patients not fitting to inclusion criteria. Not a randomised controlled study |
| Zhigang 2008 | Patients not fitting to inclusion criteria. Comparison of flutamide with placebo |
Differences between protocol and review
We revised the title to clarify that non‐steroidal antiandrogen monotherapy was compared with luteinising hormone–releasing hormone agonists or surgical castration monotherapy. Consequently, we also changed the objective section from 'To determine the effects of non‐steroidal antiandrogen monotherapy compared to surgical/medical castration monotherapy for advanced stages of prostate cancer' to 'To assess the effects of non‐steroidal antiandrogen monotherapy compared with luteinising hormone–releasing hormone agonists or surgical castration monotherapy for treating advanced stages of prostate cancer' for consistency with the review title.
For clarification, we specified the information regarding assessment of heterogeneity and data synthesis.
We revised the section Assessment of heterogeneity for consistency with the thresholds for interpretation of I2 presented in the Cochane Handbook for Systematic Reviews of Interventions (Deeks 2008). It now reads: 'Statistical heterogeneity was examined by using the I2 statistic (Higgins 2002; Higgins 2003). Our definitions of the thresholds for interpretation of I2 are consistent with the definitions presented in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2008): 0% to 40% might not be important; 30% to 60% may represent moderate heterogeneity; 50% to 90% substantial heterogeneity; 75% to 100% considerable heterogeneity. Clinical heterogeneity was examined by performing subgroup analyses. For details, see Subgroup analysis and investigation of heterogeneity section.'
We revised the section Data synthesis to clarify the I2 value threshold for which the random‐effects model was used. It now reads: 'For data synthesis, we used Review Manager 5 (Review Manager 2012), as provided by The Cochrane Collaboration. Meta‐analyses of the data from all contributing studies were conducted using a fixed‐effect model if I2 was less than 50%, and using a random‐effects model for substantial or considerable heterogeneity if I2 was greater than or equal to 50% (≥ 50%). We reported results from both models.'
We changed designations but not definitions of the following outcomes: 'discontinuation due to adverse events,' 'time to clinical progression,' 'time to biochemical progression' and 'time to treatment failure.' These are now: 'treatment discontinuation due to adverse events,' 'clinical progression,' 'biochemical progression' and 'treatment failure,' respectively.
We adapted our review to the MECIR recommendations. The names of the risk of bias domains "randomisation, concealment of allocation, blinding (of patients, personnel and outcome assessors), incomplete outcome data, selective outcome reporting, and other sources of bias" have been changed to "random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting and other sources of bias," for consistency with the updated risk of bias tool included in the 2011 update of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
We planned in advance to evaluate subgroup analyses regarding the effects of different control interventions (medical vs surgical castration). However, the largest included studies permitted both control interventions but did not report results of subgroups. This involves 925 of the 1288 participants randomly assigned to control groups (72%). We therefore abstained from subgroup analyses regarding the effects of different control interventions.
A current guideline mentioned that non‐steroidal antiandrogen monotherapy using bicalutamide with a dose of 150 mg daily for non‐metastatic prostate cancer might be an alternative to castration for selected patients (EAU 2013). A narrative review suggested that non‐steroidal antiandrogen monotherapy might be an established treatment option in patients with prostate cancer, but an unexplained trend towards decreased survival should prohibit their uncritical use (Wirth 2007). Therefore for the primary outcome of overall survival, we performed post hoc planned subgroup analyses regarding non‐metastatic or metastatic disease in combination with different doses of non‐steroidal antiandrogens.
We revised the name of our subgroup analysis to allow assessment of other doses. It now reads: 'dose of non‐steroidal antiandrogen' (formerly: 'bicalutamide 50 mg (milligrams) versus bicalutamide 150 mg').
We revised the section Sensitivity analysis to be more precise. It now reads: 'We performed sensitivity analyses to evaluate the effects of data imputations for best‐case and worst‐case scenarios (Analysis 1.5; Analysis 1.7; Analysis 1.9). Additionally, we investigated the robustness of results through sensitivity analyses when heterogeneity was substantial or considerable (I2 50% to 90% or 75% to 100%, respectively) by excluding smaller studies from the meta‐analysis (Analysis 1.1; Analysis 1.2; Analysis 1.4; Analysis 1.8; Analysis 1.17).'
We revised the section Measures of treatment effect. It now reads: 'We analysed extracted data using Review Manager 5 (Review Manager 2012). We extracted hazard ratios (HRs) with 95% confidence intervals (CIs) for time‐to‐event outcomes. If HRs were not given, we used indirect estimation methods (described by Parmar et al (Parmar 1998) and Williamson et al (Williamson 2002)) to calculate them. If we were unable to extract these data from the study reports or to receive the necessary information from the primary investigators, we alternatively used the proportions of participants with the respective outcomes measured at certain time points to calculate risk ratios (RRs) with 95% CIs. We expressed results for binary outcomes as RRs with 95% CIs as measures of uncertainty' (formerly: '[...] If we will be unable to either extract these data from the study reports or receive the necessary information from the primary investigators, we will use as an alternative the proportions of participants with the respective outcomes measured at certain time points (i.e. six months, then 12‐monthly intervals) to calculate risk ratios (RRs). If outcome data are presented for other time periods, we will give consideration to examine these as well [...]').
We extended the definition of 'cancer‐specific survival' with a statement on cancer‐specific mortality because of data availability in the included studies.
Contributions of authors
Frank Kunath: correspondence, design and coordination of the review, search for trials, selection of studies for inclusion, extraction of data, assessment of risk of bias, entry of data, data analysis, interpretation of data analyses and final approval.
Henrik R Grobe: search for trials, selection of studies for inclusion, data collection and data management.
Gerta Rücker: statistical advice and methodological support, general advice on the review and final approval.
Edith Motschall: trial search co‐ordinator, advice on search strategy, general advice on the review and final approval.
Gerd Antes: methodological support, general advice on the review and final approval.
Philipp Dahm: methodological support, urological/clinical advice on the review and final approval.
Bernd Wullich: urological/clinical advice on the review and final approval.
Joerg J Meerpohl: data collection, interpretation of data, methodological support, general advice, involvement in writing the review and final approval.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Deutsche Gesellschaft für Urologie e.V., Germany.
- The review was supported by a Ferdinand Eisenberger grant of the Deutsche Gesellschaft für Urologie (German Society of Urology; grant ID KuF1/FE‐10).
Declarations of interest
This review was supported by a Ferdinand Eisenberger grant of the Deutsche Gesellschaft für Urologie (German Society of Urology; grant ID KuF1/FE‐10).
New
References
References to studies included in this review
Boccon‐Gibod 1997 {published data only}
- Boccon‐Gibod L, Fournier G, Bottet P, Marechal JM, Guiter J, Rischman P, et al. Flutamide versus orchidectomy in the treatment of metastatic prostate carcinoma. European Urology 1997;32(4):391‐5; discussion 5‐6. [PubMed] [Google Scholar]
Dockery 2009 {published data only}
- Dockery F, Bulpitt CJ, Agarwal S, Vernon C, Nihoyannopoulos P, Kemp M, et al. Anti‐androgens increase N‐Terminal pro‐BNP in men with prostate cancer. Clinical Endocrinology 2008;68:59‐65. [DOI] [PubMed] [Google Scholar]
- Dockery F, Bulpitt CJ, Agarwal S, Vernon C, Rajkumar C. Effect of androgen suppression compared with androgen receptor blockade on arterial stiffness in men with prostate cancer. Journal of Andrology 2009;30(4):410‐5. [DOI] [PubMed] [Google Scholar]
Sciarra 2004a {published data only}
- Sciarra A, Silverio F. Effect of nonsteroidal antiandrogen monotherapy versus castration therapy on neuroendocrine differentiation in prostate carcinoma. Urology 2004;63(3):523‐7. [DOI] [PubMed] [Google Scholar]
Sieber 2004 {published data only}
- Sieber PR, Keiller DL, Kahnoski RJ, Gallo J, McFadden S. Bicalutamide 150 mg maintains bone mineral density during monotherapy for localized or locally advanced prostate cancer. Journal of Urology 2004;171(6 Pt 1):2272‐6. [DOI] [PubMed] [Google Scholar]
Smith 2004 {published data only}
- Pirl WF, Greer JA, Goode M, Smith MR. Prospective study of depression and fatigue in men with advanced prostate cancer receiving hormone therapy. Psycho‐oncology 2008;17(2):148‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Goode M, Zietman AL, McGovern FJ, Lee H, Finkelstein JS. Bicalutamide monotherapy versus leuprolide monotherapy for prostate cancer: effects on bone mineral density and body composition. Journal of Clinical Oncology 2004;22(13):2546‐53. [DOI] [PubMed] [Google Scholar]
Study 0301 {published data only}
- Bales GT, Chodak GW. A controlled trial of bicalutamide versus castration in patients with advanced prostate cancer. Urology 1996;47(1A Suppl):38‐43; discussion 48‐53. [DOI] [PubMed] [Google Scholar]
- Iversen P, Tveter K, Varenhorst E. Randomised study of casodex 50 mg monotherapy vs orchidectomy in the treatment of metastatic prostate cancer. Scandinavian Journal of Urology and Nephrology 1996;30(2):93‐8. [DOI] [PubMed] [Google Scholar]
Study 0302 {published data only}
- Bales GT, Chodak GW. A controlled trial of bicalutamide versus castration in patients with advanced prostate cancer. Urology 1996;47(1A Suppl):38‐43; discussion 48‐53. [DOI] [PubMed] [Google Scholar]
- Kaisary AV, Tyrrell CJ, Beacock C, Lunglmayr G, Debruyne F. A randomized comparison of monotherapy with Casodex 50 mg daily and castration in the treatment of metastatic prostate carcinoma. European Urology 1995;28(3):215‐22. [DOI] [PubMed] [Google Scholar]
Study 0303 {published data only}
- Bales GT, Chodak GW. A controlled trial of bicalutamide versus castration in patients with advanced prostate cancer. Urology 1996;47(1A Suppl):38‐43; discussion 48‐53. [DOI] [PubMed] [Google Scholar]
- Chodak G, Sharifi R, Kasimis B, Block NL, Macramalla E, Kennealey GT. Single‐agent therapy with bicalutamide: a comparison with medical or surgical castration in the treatment of advanced prostate carcinoma. Urology 1995;46(6):849‐55. [DOI] [PubMed] [Google Scholar]
Study 306 {published data only}
- Iversen P, Tyrrell CJ, Kaisary AV, Anderson JB, Baert L, Tammela T, et al. Casodex (bicalutamide) 150‐mg monotherapy compared with castration in patients with previously untreated nonmetastatic prostate cancer: results from two multicenter randomized trials at a median follow‐up of 4 years. Urology 1998;51(3):389‐96. [DOI] [PubMed] [Google Scholar]
- Iversen P, Tyrrell CJ, Kaisary AV, Anderson JB, Poppel H, Tammela TL, et al. Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup. Journal of Urology 2000;164(5):1579‐82. [PubMed] [Google Scholar]
- Tyrrell CJ, Blake GM, Iversen P, Kaisary AV, Melezinek I. The non‐steroidal antiandrogen, bicalutamide ('Casodex'), may preserve bone mineral density as compared with castration: results of a preliminary study. World Journal of Urology 2003;21(1):37‐42. [DOI] [PubMed] [Google Scholar]
- Tyrrell CJ, Kaisary AV, Iversen P, Anderson JB, Baert L, Tammela T, et al. A randomised comparison of 'Casodex' (bicalutamide) 150 mg monotherapy versus castration in the treatment of metastatic and locally advanced prostate cancer. European Urology 1998;33(5):447‐56. [DOI] [PubMed] [Google Scholar]
Study 307 {published data only}
- Iversen P, Tyrrell CJ, Kaisary AV, Anderson JB, Baert L, Tammela T, et al. Casodex (bicalutamide) 150‐mg monotherapy compared with castration in patients with previously untreated nonmetastatic prostate cancer: results from two multicenter randomized trials at a median follow‐up of 4 years. Urology 1998;51(3):389‐96. [DOI] [PubMed] [Google Scholar]
- Iversen P, Tyrrell CJ, Kaisary AV, Anderson JB, Poppel H, Tammela TL, et al. Bicalutamide monotherapy compared with castration in patients with nonmetastatic locally advanced prostate cancer: 6.3 years of followup. Journal of Urology 2000;164(5):1579‐82. [PubMed] [Google Scholar]
- Tyrrell CJ, Blake GM, Iversen P, Kaisary AV, Melezinek I. The non‐steroidal antiandrogen, bicalutamide ('Casodex'), may preserve bone mineral density as compared with castration: results of a preliminary study. World Journal of Urology 2003;21(1):37‐42. [DOI] [PubMed] [Google Scholar]
- Tyrrell CJ, Kaisary AV, Iversen P, Anderson JB, Baert L, Tammela T, et al. A randomised comparison of 'Casodex' (bicalutamide) 150 mg monotherapy versus castration in the treatment of metastatic and locally advanced prostate cancer. European Urology 1998;33(5):447‐56. [DOI] [PubMed] [Google Scholar]
Tyrrell 2006 {published data only}
- Tyrrell CJ, Iversen P, Tammela T, Anderson J, Bjork T, Kaisary AV, et al. Tolerability, efficacy and pharmacokinetics of bicalutamide 300 mg, 450 mg or 600 mg as monotherapy for patients with locally advanced or metastatic prostate cancer, compared with castration. BJU International 2005;96(3):563‐72. Erratum in: BJU International 2006;98(3):572. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Akaza 1993 {published data only}
- Akaza H, Usami M, Kotake T, Koiso K, Aso Y. A randomized phase II trial of flutamide vs chlormadinone acetate in previously untreated advanced prostatic cancer. The Japan Flutamide Study Group. Japanese Journal of Clinical Oncology 1993;23(3):178‐85. [PubMed] [Google Scholar]
Akaza 2003 {published data only}
- Akaza H, Homma Y, Okada K, Yokoyama M, Usami M, Hirao Y, et al. A prospective and randomized study of primary hormonal therapy for patients with localized or locally advanced prostate cancer unsuitable for radical prostatectomy: results of the 5‐year follow‐up. BJU International 2003;91(1):33‐6. [DOI] [PubMed] [Google Scholar]
Alberts 2006 {published data only}
- Alberts SR, Novotny PJ, Sloan JA, Danella J, Bostwick DG, Sebo TJ, et al. Flutamide in men with prostatic intraepithelial neoplasia: a randomized, placebo‐controlled chemoprevention trial. American Journal of Therapeutics 2006;13(4):291‐7. [DOI] [PubMed] [Google Scholar]
Aso 1993a {published data only}
- Aso Y, Akaza H, Koiso K, Kumamoto Y, Kawai T, Origasa S, et al. Phase I study of flutamide, a nonsteroidal antiandrogen, in patients with prostatic cancer. Hinyokika Kiyo. Acta Urologica Japonica 1993;39(4):381‐9. [PubMed] [Google Scholar]
Aso 1993b {published data only}
- Aso Y, Akaza H, Kameyama S, Koiso K, Koyanagi T, Kawai T, et al. Clinical evaluation of flutamide, a pure antiandrogen, in prostatic cancer phase II dose‐finding study. Hinyokika Kiyo. Acta Urologica Japonica 1993;39(4):391‐403. [PubMed] [Google Scholar]
Auvinen 2004 {published data only}
- Auvinen A, Hakama M, Ala‐Opas M, Vornanen T, Leppilahti M, Salminen P, et al. A randomized trial of choice of treatment in prostate cancer: the effect of intervention on the treatment chosen. BJU International 2004;93(1):52‐6; discussion 6. [DOI] [PubMed] [Google Scholar]
Ayub 1990 {published data only}
- Ayub M, Levell MJ. Suppression of plasma androgens by the antiandrogen flutamide in prostatic cancer patients treated with Zoladex, a GnRH analogue. Clinical Endocrinology 1990;32(3):329‐39. [DOI] [PubMed] [Google Scholar]
Blackledge 1996 {published data only}
- Blackledge GR. High‐dose bicalutamide monotherapy for the treatment of prostate cancer. Urology 1996;47(1A Suppl):44‐7; discussion 8‐53. [DOI] [PubMed] [Google Scholar]
Boccardo 2002 {published data only}
- Boccardo F, Barichello M, Battaglia M, Carmignani G, Comeri G, Ferraris V, et al. Bicalutamide monotherapy versus flutamide plus goserelin in prostate cancer: updated results of a multicentric trial. European Urology 2002;42(5):481‐90. [DOI] [PubMed] [Google Scholar]
- Boccardo F, Rubagotti A, Borichello M, Battaglia M, Carmignani G, Comeri G, et al. Bicalutamide monotherapy versus flutamide plus goserelin in prostate cancer patients: results of an Italian Prostate Cancer Project study. Journal of Clinical Oncology 1999;17(7):2027‐38. [DOI] [PubMed] [Google Scholar]
Bono 2007 {published data only}
- Bono AV, Mazzucchelli R, Ferrari I, Lopez‐Beltran A, Galosi AB, Cheng L, et al. Bicalutamide 50 mg monotherapy in patients with isolated high‐grade PIN: findings in repeat biopsies at 6 months. Journal of Clinical Pathology 2007;60(4):443‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bruun 1996 {published data only}
- Bruun E, Frimodt‐Møller C. The effect of buserelin versus conventional antiandrogenic treatment in patients with T2‐4NXM1 prostatic cancer. A prospective, randomized multicentre phase III trial. Scandinavian Journal of Urology and Nephrology 1996;30(4):291‐7. [DOI] [PubMed] [Google Scholar]
Chang 1996 {published data only}
- Chang A, Yeap B, Davis T, Blum R, Hahn R, Khanna O, et al. Double‐blind, randomized study of primary hormonal treatment of stage D2 prostate carcinoma: flutamide versus diethylstilbestrol. Journal of Clinical Oncology 1996;14(8):2250‐7. [DOI] [PubMed] [Google Scholar]
Chatelain 1994 {published data only}
- Chatelain C, Rousseau V, Cosaert J. French multicentre trial comparing Casodex (ICI 176,334) monotherapy with castration plus nilutamide in metastatic prostate cancer: a preliminary report. European Urology 1994;26 Suppl 1:10‐4. [DOI] [PubMed] [Google Scholar]
Cockshott 1990 {published data only}
- Cockshott ID, Cooper KJ, Sweetmore DS, Blacklock NJ, Denis L. The pharmacokinetics of Casodex in prostate cancer patients after single and during multiple dosing. European Urology 1990;18 Suppl 3:10‐7. [DOI] [PubMed] [Google Scholar]
Colquhoun 2012 {published data only}
- Colquhoun AJ, Venier NA, Vandersluis AD, Besla R, Sugar LM, Kiss A, et al. Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer and Prostatic Diseases 2012;15(4):346‐52. [DOI] [PubMed] [Google Scholar]
Dawson 1997 {published data only}
- Dawson NA, Figg WD, Cooper MR, Sartor O, Bergan RC, Senderowicz AM, et al. Phase II trial of suramin, leuprolide, and flutamide in previously untreated metastatic prostate cancer. Journal of Clinical Oncology 1997;15(4):1470‐7. [DOI] [PubMed] [Google Scholar]
Decensi 2007 {published data only}
- Decensi A, Zanardi S, Puntoni M, Bandelloni R, Branchi D, Argusti A, et al. Phase I‐II trial of weekly bicalutamide in men with high PSA and negative biopsy. Journal of Clinical Oncology 2007;25(18 Suppl):1500. [Google Scholar]
- Zanardi S, Puntoni M, Maffezzini M, Bandelloni R, Mori M, Argusti A, et al. Phase I‐II trial of weekly bicalutamide in men with elevated prostate‐specific antigen and negative prostate biopsies. Cancer Prevention Research (Philadelphia, Pa.) 2009;2(4):377‐84. [DOI] [PubMed] [Google Scholar]
Ekwueme 2012 {published data only}
- Ekwueme K, Parr N. Bicalutamide monotherapy in osteoporotic men requiring hormone manipulation for advanced prostate cancer: 12 year outcomes from a 'step up' regime. Journal of Urology 2012;187(4 Suppl):e383. [Google Scholar]
EPC program {published data only}
- Fourcade RO, Richaud P, Brune D, Colombel P, Sarramon JP, Fournier G, et al. Effect of bicalutamide 150 mg, after 3 years of median follow‐up, in non‐metastatic prostatic cancer [Effet du bicalutamide 150 mg, à trois ans de suivi médian, dans le cancer de la prostate non métastatique]. Progres en Urologie 2003;13(3):430‐9. [PubMed] [Google Scholar]
- Fourcade RO, Richaud P, Coloby P, Malavaud B, Group des investigateuers français du programme EPC. Role of 150 mg bicalutamide in the treatment of prostate cancer: 3rd analysis of the EPC (early prostate cancer) program [Le bicalutamide 150 mg dans le traitement du cancer de la prostate localement avancé : des résultats à la pratique : Place du bicalutamide 150 mg dans le traitement du cancer de la prostate : hème analyse du programme EPC (Early Prostate Cancer)]. Progres en Urologie 2007;17(4 Suppl 1):891‐910. [PubMed] [Google Scholar]
- Iversen P, Johansson J‐E, Lodding P, Lukkarinen O, Lundmo PI, Klarskov P, et al. Efficacy and tolerability of bicalutamide in early non‐metastatic prostate cancer: Latest findings from the Scandinavian Prostatic Cancer Group Study No 6 (SPCG‐6) of the early prostate cancer programme. Meeting abstract of the 21st annual European Association of Urology Congress. 2006. www.uroweb.org/index.php?id=108&AID=9715.
- Iversen P, Johansson JE, Lodding P, Kylmala T, Lundmo P, Klarskov P, et al. Bicalutamide 150 mg in addition to standard care for patients with early non‐metastatic prostate cancer: updated results from the Scandinavian Prostate Cancer Period Group‐6 Study after a median follow‐up period of 7.1 years. Scandinavian Journal of Urology and Nephrology 2006;40(6):441‐52. [DOI] [PubMed] [Google Scholar]
- Iversen P, Johansson JE, Lodding P, Lukkarinen O, Lundmo P, Klarskov P, et al. Bicalutamide (150 mg) versus placebo as immediate therapy alone or as adjuvant to therapy with curative intent for early nonmetastatic prostate cancer: 5.3 year median followup from the Scandinavian Prostate Cancer Group Study Number 6. Journal of Urology 2004;172(5 Pt 1):1871‐6. [DOI] [PubMed] [Google Scholar]
- Iversen P, McLeod D, See W, Wirth M, Morris T, Morris C, et al. Bicalutamide (‘Casodex’) 150 mg in addition to watchful waiting in patients with early non‐metastatic prostate cancer: updated analysis at a median 5.4 years’ follow‐up. Meeting abstract of the XIXth annual European Association of Urology Congress. 2004. www.uroweb.org/index.php?id=108&AID=758.
- Iversen P, McLeod DG, See WA, Morris T, Armstrong J, Wirth MP. Antiandrogen monotherapy in patients with localized or locally advanced prostate cancer: final results from the bicalutamide Early Prostate Cancer programme at a median follow‐up of 9.7 years. BJU International 2010;105(8):1074‐81. [DOI] [PubMed] [Google Scholar]
- Iversen P, Roder MA. The Early Prostate Cancer program: bicalutamide in nonmetastatic prostate cancer. Expert Review of Anticancer Therapy 2008;8(3):361‐9. [DOI] [PubMed] [Google Scholar]
- Iversen P, Tammela TLJ, Vaage S, Lukkarinen O, Lodding P, Bull‐Njaa T, et al. A randomised comparison of bicalutamide ('Casodex') 150 mg versus placebo as immediate therapy either alone or as adjuvant to standard care for early non‐metastatic prostate cancer ‐ First report from the Scandinavian Prostatic Cancer Group Study No. 6. European Urology 2002;42(3):204‐11. [DOI] [PubMed] [Google Scholar]
- Iversen P, Wirth MP, See WA II, McLeod DG, Morris T, Armstrong J, et al. The influence of nodal status on progression outcomes in patients with prostate cancer: data from the Early Prostate Cancer program at 7.4 years. Journal of Clinical Oncology 2006;24(18 Suppl):4628. [Google Scholar]
- Iversen P, Wirth MP, See WA, McLeod DG, Klimberg I, Gleason D, et al. Is the efficacy of hormonal therapy affected by lymph node status? Data from the bicalutamide (Casodex) Early Prostate Cancer program. Urology 2004;63(5):928‐33. [DOI] [PubMed] [Google Scholar]
- McLeod D, Iversen P, See W, Wirth M, Carroll K, Morris C, et al. Bicalutamide ('Casodex') 150 mg as adjuvant to radiotherapy significantly improves progression‐free survival in early non‐metastatic prostate cancer: results from the bicalutamide early prostate cancer programme after a median 5.4 years' follow‐up. Meeting abstract of the XIXth European Association of Urology Congress. 2004. www.uroweb.org/index.php?id=108&AID=752.
- McLeod DG, Iversen P, See WA, Morris T, Armstrong J, Wirth MP. Bicalutamide 150 mg plus standard care vs standard care alone for early prostate cancer. BJU International 2006;97(2):247‐54. [DOI] [PubMed] [Google Scholar]
- McLeod DG, See WA, Klimberg I, Gleason D, Chodak G, Montie J, et al. The bicalutamide 150 mg early prostate cancer program: findings of the North American trial at 7.7‐year median followup. Journal of Urology 2006;176(1):75‐80. [DOI] [PubMed] [Google Scholar]
- See W, Iversen P, Wirth M, McLeod D, Garside L, Morris T. Immediate treatment with bicalutamide 150 mg as adjuvant therapy significantly reduces the risk of PSA progression in early prostate cancer. European Urology 2003;44(5):512‐8. [DOI] [PubMed] [Google Scholar]
- See WA, McLeod D, Iversen P, Wirth M. The bicalutamide Early Prostate Cancer Program: demography. Urologic Oncology 2001;6(2):43‐7. [DOI] [PubMed] [Google Scholar]
- See WA, McLeod DG, Iversen P, Wirth MP, Armstrong J, Navani S. Effect of bicalutamide 150 mg on PSA progression in M0 prostate cancer: results from Early Prostate Cancer program. Journal of Clinical Oncology 2006;24(18 Suppl):4624. [Google Scholar]
- See WA, Tyrrell CJ. The addition of bicalutamide 150 mg to radiotherapy significantly improves overall survival in men with locally advanced prostate cancer. Journal of Cancer Research and Clinical Oncology 2006;132 Suppl 1:S7‐16. [DOI] [PubMed] [Google Scholar]
- See WA, Wirth MP, McLeod DG, Iversen P, Klimberg I, Gleason D, et al. Bicalutamide as immediate therapy either alone or as adjuvant to standard care of patients with localized or locally advanced prostate cancer: first analysis of the Early Prostate Cancer Program. Journal of Urology 2002;168(2):429‐35. [PubMed] [Google Scholar]
- Tyrrell C. Immediate treatment with bicalutamide, 150 mg/d, following radiotherapy in localized or locally advanced prostate cancer. Reviews in Urology 2004;6 Suppl 2:S29‐36. [PMC free article] [PubMed] [Google Scholar]
- Tyrrell CJ, Payne H, See WA, McLeod DG, Wirth MP, Iversen P, et al. Bicalutamide ('Casodex') 150 mg as adjuvant to radiotherapy in patients with localised or locally advanced prostate cancer: results from the randomised Early Prostate Cancer Programme. Radiotherapy and Oncology 2005;76(1):4‐10. [DOI] [PubMed] [Google Scholar]
- Wirth M, Hakenberg O, Froehner M. Adjuvant hormonal treatment ‐ the Bicalutamide Early Prostate Cancer Program. Frontiers of Radiation Therapy and Oncology 2008;41:39‐48. [DOI] [PubMed] [Google Scholar]
- Wirth M, Iversen P, McLeod D, See W, Morris C, Morris T, et al. Bicalutamide (‘Casodex’) 150 mg as adjuvant to radical prostatectomy significantly increases progression‐free survival in patients with early non‐metastatic prostate cancer: analysis at a median follow‐up of 5.4 years. Meeting abstract of the XIXth European Association of Urology Congress. 2004. www.uroweb.org/index.php?id=108&AID=560.
- Wirth M, Tyrrell C, Delaere K, Sanchez‐Chapado M, Ramon J, Wallace D, et al. Adjuvant therapy with bicalutamide 150 mg versus standard care alone: third analysis results from Trial 24 of the Early Prostate Cancer programme. Meeting abstract of the XIXth annual European Association of Urology Congress. 2004. www.uroweb.org/index.php?id=108&AID=9672.
- Wirth M, Tyrrell C, Delaere K, Sanchez‐Chapado M, Ramon J, Wallace DM, et al. Bicalutamide ('Casodex') 150 mg in addition to standard care in patients with nonmetastatic prostate cancer: updated results from a randomised double‐blind phase III study (median follow‐up 5.1 y) in the early prostate cancer programme. Prostate Cancer and Prostatic Diseases 2005;8(2):194‐200. [DOI] [PubMed] [Google Scholar]
- Wirth M, Tyrrell C, Delaere K, Sanchez‐Chapado M, Ramon J, Wallace DM, et al. Bicalutamide (Casodex) 150 mg plus standard care in early non‐metastatic prostate cancer: results from Early Prostate Cancer Trial 24 at a median 7 years' follow‐up. Prostate Cancer and Prostatic Diseases 2007;10(1):87‐93. [DOI] [PubMed] [Google Scholar]
- Wirth M, Tyrrell C, Wallace M, Delaere KP, Sánchez‐Chapado M, Ramon J, et al. Bicalutamide (Casodex) 150 mg as immediate therapy in patients with localized or locally advanced prostate cancer significantly reduces the risk of disease progression. Urology 2001;58(2):146‐51. [DOI] [PubMed] [Google Scholar]
- Wirth MP, Froehner M. Adjuvant hormonal treatment for prostate cancer: the Bicalutamide Early Prostate Cancer Program. Oncology 2003;65 Suppl 1:1‐4. [DOI] [PubMed] [Google Scholar]
- Wirth MP, See WA, McLeod DG, Iversen P, Morris T, Armstrong J. Delaying metastatic disease progression in locally advanced disease ‐ results from the Early Prostate Cancer program at a median follow‐up of 7.4 years. Journal of Clinical Oncology 2006;24(18 Suppl):4629. [Google Scholar]
- Wirth MP, See WA, McLeod DG, Iversen P, Morris T, Carroll K. Bicalutamide 150 mg in addition to standard care in patients with localized or locally advanced prostate cancer: results from the second analysis of the Early Prostate Cancer program at median followup of 5.4 years. Journal of Urology 2004;172(5 Pt 1):1865‐70. [DOI] [PubMed] [Google Scholar]
Eri 1993 {published data only}
- Eri LM, Tveter KJ. A prospective, placebo‐controlled study of the antiandrogen Casodex as treatment for patients with benign prostatic hyperplasia. Journal of Urology 1993;150(1):90‐4. [DOI] [PubMed] [Google Scholar]
Eri 2001 {published data only}
- Eri LM, Svindland A, Tveter KJ. The effect of bicalutamide on prostate histology. Prostate 2001;46(4):275‐80. [DOI] [PubMed] [Google Scholar]
Festuccia 2007 {published data only}
- Festuccia C, Gravina GL, Muzi P, Pomante R, Ventura L, Ricevuto E, et al. In vitro and in vivo effects of bicalutamide on the expression of TrkA and P75 neurotrophin receptors in prostate carcinoma. Prostate 2007;67(12):1255‐64. [DOI] [PubMed] [Google Scholar]
Gravina 2007 {published data only}
- Gravina GL, Festuccia C, Galatioto GP, Muzi P, Angelucci A, Ronchi P, et al. Surgical and biologic outcomes after neoadjuvant bicalutamide treatment in prostate cancer. Urology 2007;70(4):728‐33. [DOI] [PubMed] [Google Scholar]
Henderson 2003 {published data only}
- Henderson A, Langley SEM, Laing RW. Is bicalutamide equivalent to goserelin for prostate volume reduction before radiation therapy? A prospective, observational study. Clinical Oncology 2003;15(6):318‐21. [DOI] [PubMed] [Google Scholar]
Iida 2011 {published data only}
- Iida K. Monotherapy versus combined androgen blockade for advanced/metastatic prostate cancer. Gan to Kagaku Ryoho. Cancer & Chemotherapy 2011;38(13):2553‐7. [PubMed] [Google Scholar]
Jacobo 1976 {published data only}
- Jacobo E, Schmidt JD, Weinstein SH, Flocks RH. Comparison of flutamide (SCH‐13521) and diethylstilbestrol in untreated advanced prostatic cancer. Urology 1976;8(3):231‐3. [DOI] [PubMed] [Google Scholar]
Johansson 1988 {published data only}
- Johansson J‐E, Lingårdh G, Andersson S‐O, Zador G, Beckman K‐W. Clinical evaluation of flutamide and estramustine as initial treatment of metastatic carcinoma of prostate. Urology 1987;29(1):55‐9. [DOI] [PubMed] [Google Scholar]
- Johansson JE, Andersson SO, Beckman KW, Zador G. Clinical evaluation with long‐term follow‐up of flutamide and estramustine as initial treatment of metastatic carcinoma of the prostate. American Journal of Clinical Oncology 1988;11 Suppl 2:S183‐6. [DOI] [PubMed] [Google Scholar]
Jones 1994 {published data only}
- Jones HB, Betton GR, Bowdler AL, McFarquhar RL, Middleton BJ, Lunglmayr G. Pathological and morphometric assessment of testicular parameters in patients with metastatic prostate cancer following treatment with either the antiandrogen Casodex (ZM176,334) or bilateral orchidectomy. Urological Research 1994;22(3):191‐5. [DOI] [PubMed] [Google Scholar]
Kaisary 1994 {published data only}
- Kaisary AV. Current clinical studies with a new nonsteroidal antiandrogen, Casodex. Prostate. Supplement 1994;5:27‐33. [DOI] [PubMed] [Google Scholar]
Kariakin 2001 {published data only}
- Kariakin OB, Sviridova TV, Tsodikova LB, Grishin GN, Sergeeva TN, Perekhrest MA, et al. Changes in prostate‐specific antigen in casodex (bicalutamide) monotherapy in a dose 150 mg/day given to patients with locally advanced and/or advanced prostatic cancer. Urologiia (Moscow, Russia : 1999) 2001;4:26‐9. [PubMed] [Google Scholar]
Kasimis 2000 {published data only}
- Kasimis B, Wilding G, Kreis W, Feuerman M, Chang V, Hwang S, et al. Survival of patients who had salvage castration after failure on bicalutamide monotherapy for stage (D‐2) prostate cancer. Cancer Investigation 2000;18(7):602‐8. [DOI] [PubMed] [Google Scholar]
Kotake 1996a {published data only}
- Kotake T, Usami M, Isaka S, Shimazaki J, Oishi K, Yoshida O, et al. Phase I study of bicalutamide (Casodex), a nonsteroidal antiandrogen in patients with prostatic cancer. Hinyokika Kiyo. Acta Urologica Japonica 1996;42(2):143‐53. [PubMed] [Google Scholar]
Kotake 1996b {published data only}
- Kotake T, Usami M, Isaka S, Shimazaki J, Nakano E, Okuyama A, et al. Clinical early phase II study of bicalutamide (Casodex) in patients with prostatic cancer. Hinyokika Kiyo. Acta Urologica Japonica 1996;42(2):157‐68. [PubMed] [Google Scholar]
Kulkarni 2003 {published data only}
- Kulkarni JN, Gupta R. Early report of randomized double blind clinical trial of hormonal therapy of carcinoma of prostate (CAP) stage D2. Indian Journal of Urology 2003;19(2):135‐9. [Google Scholar]
Lazzaro 2007 {published data only}
- Lazzaro C, Bartoletti R, Guazzoni G, Orestano F, Pappagallo GL, Prezioso D, et al. Economic evaluation of different hormonal therapies for prostate cancer. Final results from the Quality of Life Antiandrogen Blockade Italian Observational Study (QuABIOS). Archivio Italiano di Urologia, Andrologia 2007;79(3):104‐7. [PubMed] [Google Scholar]
Lee 2009 {published data only}
- Lee S, Chung YJ, Kim BH, Shim JH, Yoon SH, Shin SG, et al. Comparative pharmacokinetic evaluation of two formulations of bicalutamide 50‐mg tablets: an open‐label, randomized‐sequence, single‐dose, two‐period crossover study in healthy Korean male volunteers. Clinical Therapeutics 2009;31(12):3000‐8. [DOI] [PubMed] [Google Scholar]
Lee 2010 {published data only}
- Lee S, Yoon SH, Cho JY, Shin SG, Jang IJ, Yu KS. Relative bioavailability and tolerability of two formulations of bicalutamide 50‐mg tablets: a randomized‐sequence, open‐label, two‐period crossover study in healthy Korean male subjects. Clinical Therapeutics 2010;32(14):2496‐501. [DOI] [PubMed] [Google Scholar]
Lehmusvaara 2012 {published data only}
- Lehmusvaara S, Erkkila T, Urbanucci A, Waltering K, Seppala J, Larjo A, et al. Chemical castration and anti‐androgens induce differential gene expression in prostate cancer. Journal of Pathology 2012;227(3):336‐45. [DOI] [PubMed] [Google Scholar]
Lin 2011 {published data only}
- Lin YH, Chen CL, Hou CP, Chang PL, Tsui KH. A comparison of androgen deprivation therapy versus surgical castration for patients with advanced prostatic carcinoma. Acta Pharmacologica Sinica 2011;32(4):537‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lissoni 2002 {published data only}
- Lissoni P, Malugani F, Casu M, Bukovec R, Egardi R, Bordin V, et al. Effect of bicalutamide therapy on prolactin response to L‐dopa in metastatic prostate cancer patients. Neuro Endocrinology Letters 2002;23(1):61‐3. [PubMed] [Google Scholar]
Loran 2001 {published data only}
- Loran OB, Pushkar D, Kosko D, Bernikov AN. Bicalutamide monotherapy of patients with disseminated forms of prostatic cancer. Urologiia (Moscow, Russia : 1999) 2001;3:26‐8. [PubMed] [Google Scholar]
Lund 1988 {published data only}
- Lund F, Rasmussen F. Flutamide versus stilboestrol in the management of advanced prostatic cancer. A controlled prospective study. British Journal of Urology 1988;61(2):140‐2. [DOI] [PubMed] [Google Scholar]
Lunglmayr 1995 {published data only}
- Lunglmayr G. Efficacy and tolerability of Casodex in patients with advanced prostate cancer. Anti‐cancer Drugs 1995;6(4):508‐13. [DOI] [PubMed] [Google Scholar]
McGivern 2011 {published data only}
- McGivern U, Mitchell DM, O'Hare J, Corey G, O'Sullivan JM. How does neoadjuvant bicalutamide 150 mg monotherapy compare to luteinising hormone‐releasing hormone agonist (LHRHa) therapy in localized prostate cancer treated with radical prostatectomy? A case‐matched comparison of PSA kinetics and biochemical outcome. Journal of Clinical Oncology 2011;29(7 Suppl):146. [Google Scholar]
McGivern 2012 {published data only}
- McGivern U, Mitchell DM, McDowell C, O'Hare J, Corey G, O'Sullivan JM. Neoadjuvant hormone therapy for radical prostate radiotherapy: bicalutamide monotherapy vs. luteinizing hormone‐releasing hormone agonist monotherapy: a single‐institution matched‐pair analysis. Clinical Genitourinary Cancer 2012;10(3):190‐5. [DOI] [PubMed] [Google Scholar]
Migliari 1991 {published data only}
- Migliari R, Muscas G, Melis M, Garau M, Sorgia M, Scarpa M, et al. Monitoring of erection function in patients with prostatic carcinoma treated with Casodex. Archivio Italiano di Urologia, Nefrologia, Andrologia [Urological, Nephrological, and Andrological Sciences] 1991;63(1):155‐61. [PubMed] [Google Scholar]
Migliari 1992 {published data only}
- Migliari R, Muscas G, Usai E. Effect of Casodex on sleep‐related erections in patients with advanced prostate cancer. Journal of Urology 1992;148(2 Pt 1):338‐41. [DOI] [PubMed] [Google Scholar]
Motofei 2011 {published data only}
- Motofei IG, Rowland DL, Popa F, Kreienkamp D, Paunica S. Preliminary study with bicalutamide in heterosexual and homosexual patients with prostate cancer: a possible implication of androgens in male homosexual arousal. BJU International 2011;108(1):110‐5. [DOI] [PubMed] [Google Scholar]
Murphy 2004 {published data only}
- Murphy JC, Srinivas S, Terris MK. Flutamide administration at 500 mg daily has similar effects on serum testosterone to 750 mg daily. Journal of Andrology 2004;25(4):630‐4. [DOI] [PubMed] [Google Scholar]
Newling 1989 {published data only}
- Newling DW. The use of flutamide as monotherapy in the treatment of advanced prostate cancer. Progress in Clinical and Biological Research 1989;303:117‐21. [PubMed] [Google Scholar]
Noldus 1996 {published data only}
- Noldus J, Ferrari M, Prestigicomo A, Stamey TA. Effect of flutamide and flutamide plus castration on prostate size in patients with previously untreated prostate cancer. Urology 1996;47(5):713‐8. [DOI] [PubMed] [Google Scholar]
Nyman 2005 {published data only}
- Nyman CR, Andersen JT, Lodding P, Sandin T, Varenhorst E. The patient's choice of androgen‐deprivation therapy in locally advanced prostate cancer: bicalutamide, a gonadotrophin‐releasing hormone analogue or orchidectomy. BJU International 2005;96(7):1014‐8. [DOI] [PubMed] [Google Scholar]
Oosterlinck 1996 {published data only}
- Oosterlinck W, Casselman J, Mattelaer J, Velthoven R, Kurjatkin O, Schulman C. Tolerability and safety of flutamide in monotherapy, with orchiectomy or with LHRH‐a in advanced prostate cancer patients ‐ a Belgian multicenter study of 905 patients. European Urology 1996;30(4):458‐63. [DOI] [PubMed] [Google Scholar]
Ostri 1991 {published data only}
- Ostri P, Bonnesen T, Nilsson T, Frimodt‐Moller C. Treatment of symptomatic metastatic prostatic cancer with cyproterone acetate versus orchiectomy: a prospective randomized trial. Urologia Internationalis 1991;46(2):167‐71. [DOI] [PubMed] [Google Scholar]
Petit 2008 {published data only}
- Petit JH, Gluck C, Kiger WS 3rd, Henry DL, Karasiewicz C, Talcott J, et al. Bicalutamide alone prior to brachytherapy achieves cytoreduction that is similar to luteinizing hormone‐releasing hormone analogues with less patient‐reported morbidity. Urologic Oncology 2008;26(4):372‐7. [DOI] [PubMed] [Google Scholar]
Prezioso 2007 {published data only}
- Prezioso D, Bartoletti R, Cecchi M, Cicalese V, Cunico SC, Damiano R, et al. Quality of life evaluation by the EORTC QLQ‐C30 questionnaire in patients treated with hormonal treatment in Italy. A QuABIOS group study. Archivio Italiano di Urologia, Andrologia 2007;79(3):99‐103. [PubMed] [Google Scholar]
Raina 2007 {published data only}
- Raina R, Pahalajani G, Agarwal A, Zippe C. Long‐term effectiveness of luteinizing hormone‐releasing hormone agonist or antiandrogen monotherapy in elderly men with localized prostate cancer (T1‐2): a retrospective study. Asian Journal of Andrology 2007;9(2):253‐8. [DOI] [PubMed] [Google Scholar]
Raynaud 1988 {published data only}
- Raynaud JP. Antiandrogens in combination with LH‐RH agonists in prostate cancer. American Journal of Clinical Oncology 1988;11 Suppl 2:S132‐47. [DOI] [PubMed] [Google Scholar]
Scattoni 2006 {published data only}
- Scattoni V, Montironi R, Mazzucchelli R, Freschi M, Nava L, Losa A, et al. Pathological changes of high‐grade prostatic intraepithelial neoplasia and prostate cancer after monotherapy with bicalutamide 150 mg. BJU International 2006;98(1):54‐8. [DOI] [PubMed] [Google Scholar]
Scher 1997 {published data only}
- Scher HI, Liebertz C, Kelly WK, Mazumdar M, Brett C, Schwartz L, et al. Bicalutamide for advanced prostate cancer: the natural versus treated history of disease. Journal of Clinical Oncology 1997;15(8):2928‐38. [DOI] [PubMed] [Google Scholar]
Schröder 2000 {published data only}
- Schröder FH, Collette L, Reijke TM, Whelan P, members of the EORTC Genitourinary Group. Prostate cancer treated by anti‐androgens: is sexual function preserved?. British Journal of Cancer 2000;82(2):283‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schröder 2004 {published data only}
- Schröder FH, Whelan P, Reijke TM, Kurth KH, Pavone‐Macaluso J, Mattelaer J, et al. Metastatic prostate cancer treated by flutamide versus cyproterone acetate. Final analysis of the "European Organization for Research and Treatment of Cancer" (EORTC) Protocol 30892. European Urology 2004;45(4):457‐64. [DOI] [PubMed] [Google Scholar]
Sciarra 2004b {published data only}
- Sciarra A, Cardi A, Silverio F. Antiandrogen monotherapy: recommendations for the treatment of prostate cancer. Urologia Internationalis 2004;72(2):91‐8. [DOI] [PubMed] [Google Scholar]
See 2004 {published data only}
- See WA. Bicalutamide adjuvant to radical prostatectomy. Reviews in Urology 2004;6 Suppl 2:S20‐8. [PMC free article] [PubMed] [Google Scholar]
Shipley 2011 {published data only}
- Shipley WU, Hunt D, Lukka HR, Major P, Heney NM, Grignon D, et al. Initial report of RTOG 9601, a phase III trial in prostate cancer: effect of anti‐androgen therapy (AAT) with bicalutamide during and after radiation therapy (RT) on freedom from progression and incidence of metastatic disease in patients following radical prostatectomy (RP) with pT2‐3,N0 disease and elevated PSA levels. Journal of Clinical Oncology 2011;29(7 Suppl):1. [Google Scholar]
Smith 2003 {published data only}
- Smith MR, Fallon MA, Goode MJ. Cross‐sectional study of bone turnover during bicalutamide monotherapy for prostate cancer. Urology 2003;61(1):127‐31. [DOI] [PubMed] [Google Scholar]
Soloway 1996 {published data only}
- Soloway MS, Schellhammer PF, Smith JA Jr, Chodak GW, Vogelzang NJ, Kennealey GT. Bicalutamide in the treatment of advanced prostatic carcinoma: a phase II non‐comparative multicenter trial evaluating safety, efficacy and long‐term endocrine effects of monotherapy. Journal of Urology 1995;154(6):2110‐4. [DOI] [PubMed] [Google Scholar]
- Soloway MS, Schellhammer PF, Smith JA, Chodak GW, Kennealey GT. Bicalutamide in the treatment of advanced prostatic carcinoma: a phase II multicenter trial. Urology 1996;47(1 Suppl 1):33‐7; discussion 48‐53. [DOI] [PubMed] [Google Scholar]
Thorpe 1996 {published data only}
- Thorpe SC, Azmatullah S, Fellows GJ, Gingell JC, O'Boyle PJ. A prospective, randomised study to compare goserelin acetate (Zoladex) versus cyproterone acetate (Cyprostat) versus a combination of the two in the treatment of metastatic prostatic carcinoma. European Urology 1996;29(1):47‐54. [DOI] [PubMed] [Google Scholar]
Thrasher 2000 {published data only}
- Thrasher JB, Deeths J, Bennett C, Iyer P, Dineen MK, Zhai S, et al. Comparative study of the clinical efficacy of two dosing regimens of flutamide. Molecular Urology 2000;4(3):259‐63; discussion 265. [PubMed] [Google Scholar]
Tyrrell 1998 {published data only}
- Tyrrell CJ, Denis L, Newling D, Soloway M, Channer K, Cockshott ID, et al. Casodex 10‐200 mg daily, used as monotherapy for the treatment of patients with advanced prostate cancer. European Urology 1998;33(1):39‐53. [DOI] [PubMed] [Google Scholar]
Verhelst 1994 {published data only}
- Verhelst J, Denis L, Vliet P, Poppel H, Braeckman J, Cangh P, et al. Endocrine profiles during administration of the new non‐steroidal anti‐androgen Casodex in prostate cancer. Clinical Endocrinology 1994;41(4):525‐30. [DOI] [PubMed] [Google Scholar]
Wadhwa 2009 {published data only}
- Wadhwa VK, Weston R, Mistry R, Parr NJ. Long‐term changes in bone mineral density and predicted fracture risk in patients receiving androgen‐deprivation therapy for prostate cancer, with stratification of treatment based on presenting values. BJU International 2009;104(6):800‐5. [DOI] [PubMed] [Google Scholar]
Wadhwa 2011 {published data only}
- Wadhwa V, Weston R, Parr NJ. Bicalutamide monotherapy preserves bone mineral density, muscle strength and has significant health‐related quality of life benefits for osteoporotic men with prostate cancer. BJU International 2011;107(12):1923‐9. [DOI] [PubMed] [Google Scholar]
Wirth 2004 {published data only}
- Wirth MP, Weissbach L, Marx FJ, Heckl W, Jellinghaus W, Riedmiller H, et al. Prospective randomized trial comparing flutamide as adjuvant treatment versus observation after radical prostatectomy for locally advanced, lymph node‐negative prostate cancer. European Urology 2004;45(3):267‐70; discussion 270. [DOI] [PubMed] [Google Scholar]
Yoshimura 2003 {published data only}
- Yoshimura K, Sumiyoshi Y, Hashimura T, Ueda T, Kamiryo Y, Yamamoto A, et al. Neoadjuvant flutamide monotherapy for locally confined prostate cancer. International Journal of Urology 2003;10(4):190‐5. [DOI] [PubMed] [Google Scholar]
Zanardi 2009 {published data only}
- Zanardi S, Puntoni M, Maffezzini M, Bandelloni R, Mori M, Argusti A, et al. Phase I‐II trial of weekly bicalutamide in men with elevated prostate‐specific antigen and negative prostate biopsies. Cancer Prevention Research (Philadelphia, Pa.) 2009;2(4):377‐84. [DOI] [PubMed] [Google Scholar]
Zhigang 2008 {published data only}
- Zhigang Z, Wenlu S. Flutamide reduced prostate cancer development and prostate stem cell antigen mRNA expression in high grade prostatic intraepithelial neoplasia. International Journal of Cancer. Journal International du Cancer 2008;122(4):864‐70. [DOI] [PubMed] [Google Scholar]
Additional references
Abrahamsson 2005
- Abrahamsson PA, Anderson J, Boccon‐Gibod L, Schulman C, Studer UE, Wirth M. Risks and benefits of hormonal manipulation as monotherapy or adjuvant treatment in localised prostate cancer. European Urology 2005;48(6):900‐5. [DOI] [PubMed] [Google Scholar]
Akl 2012
- Akl EA, Briel M, You JJ, Sun X, Johnston BC, Busse JW, et al. Potential impact on estimated treatment effects of information lost to follow‐up in randomised controlled trials (LOST‐IT): systematic review. BMJ (Clinical Research Ed.) 2012;18(344):e2809. [DOI] [PubMed] [Google Scholar]
Als‐Nielsen 2004
- Als‐Nielsen B, Gluud LL, Gluud C. Methodological quality and treatment effects in randomized trials: a review of six empirical studies. Meeting abstract of the 12th Cochrane Colloquium, Ottawa (Canada). 2004. www.cochrane.org/events/colloquia.
ASCO 2004
- Loblaw DA, Mendelson DS, Talcott JA, Virgo KS, Somerfield MR, Ben‐Josef E, et al. American Society of Clinical Oncology recommendations for the initial hormonal management of androgen‐sensitive metastatic, recurrent, or progressive prostate cancer. Journal of Clinical Oncology 2004;22(14):2927‐41. [DOI] [PubMed] [Google Scholar]
ASCO 2007
- Loblaw DA, Virgo KS, Nam R, Somerfield MR, Ben‐Josef E, Mendelson DS, et al. Initial hormonal management of androgen‐sensitive metastatic, recurrent, or progressive prostate cancer: 2007 update of an American Society of Clinical Oncology practice guideline. Journal of Clinical Oncology 2007;25(12):1596‐605. [DOI] [PubMed] [Google Scholar]
Azzouni 2012
- Azzouni F, Mohler J. Role of 5α‐reductase inhibitors in prostate cancer prevention and treatment. Urology 2012;79(6):1197‐205. [DOI] [PubMed] [Google Scholar]
Boccardo 1999
- Boccardo F, Rubagotti A, Barichello M, Battaglia M, Carmignani G, Comeri G, et al. Bicalutamide monotherapy versus flutamide plus goserelin in prostate cancer patients: results of an Italian Prostate Cancer Project study. Journal of Clinical Oncology 1999;17(7):2027‐38. [DOI] [PubMed] [Google Scholar]
Boyle 2005
- Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Annals of Oncology 2005;16(3):481‐8. [DOI] [PubMed] [Google Scholar]
Chou 2010
- Chou R, Aronson N, Atkins D, Ismaila AS, Santaguida P, Smith DH, et al. AHRQ series paper 4: assessing harms when comparing medical interventions: AHRQ and the effective health‐care program. Journal of Clinical Epidemiology 2010;63(5):502‐12. [DOI] [PubMed] [Google Scholar]
Daniell 1997
- Daniell HW. Osteoporosis after orchiectomy for prostate cancer. Journal of Urology 1997;157(2):439‐44. [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008:243‐96. [Google Scholar]
Dockery 2002
- Dockery F, Bulpitt CJ, Agarwal S, Rajkumar C. Testosterone suppression in men with prostate cancer is associated with increased arterial stiffness. Aging Male 2002;5(4):216‐22. [PubMed] [Google Scholar]
EAU 2013
- Heidenreich A, Bastian PJ, Bellmunt J, Bolla M, Joniau S, Mason MD, et al. Guidelines on prostate cancer. European Association of Urology; 2013. www.uroweb.org/gls/pdf/09_Prostate_Cancer_LR.pdf (accessed 30 August 2013).
Efstathiou 2008
- Efstathiou JA, Bae K, Shipley WU, Hanks GE, Pilepich MV, Sandler HM, et al. Cardiovascular mortality and duration of androgen deprivation for locally advanced prostate cancer: analysis of RTOG 92‐02. European Urology 2008;54(4):816‐23. [DOI] [PubMed] [Google Scholar]
Efstathiou 2009
- Efstathiou JA, Bae K, Shipley WU, Hanks GE, Pilepich MV, Sandler HM, et al. Cardiovascular mortality after androgen deprivation therapy for locally advanced prostate cancer: RTOG 85‐31. Journal of Clinical Oncology 2009;27(1):92‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eheman 2012
- Eheman C, Henley SJ, Ballard‐Barbash R, Jacobs EJ, Schymura MJ, Noone A‐M, et al. Annual Report to the Nation on the status of cancer, 1975‐2008, featuring cancers associated with excess weight and lack of sufficient physical activity. Cancer 2012;118(9):2338‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gamble 2005
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58(6):579‐88. [DOI] [PubMed] [Google Scholar]
Gartlehner 2008
- Gartlehner G. Assessment of adverse effects and applicability ‐ two areas not (yet) covered adequately in Cochrane reports. Zeitschrift fur Evidenz, Fortbildung und Qualitat Im Gesundheitswesen 2008;102(8):497‐502. [DOI] [PubMed] [Google Scholar]
Gibbs 1996
- Gibbs SJ, Plowman PN. Androgen deprivation and antagonism in the treatment of advanced prostatic carcinoma. Clinical Oncology 1996;8(6):346‐52. [DOI] [PubMed] [Google Scholar]
GLOBOCAN 2012
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. http://globocan.iarc.fr (accessed 8 April 2014).
Grundmark 2012
- Grundmark B, Garmo H, Zethelius B, Stattin P, Lambe M, Holmberg L. Anti‐androgen prescribing patterns, patient treatment adherence and influencing factors; results from the nationwide PCBaSe Sweden. European Journal of Clinical Pharmacology 2012;68(12):1619‐30. [DOI] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Gøtzsche 2006
- Gøtzsche PC, Hróbjartsson A, Johansen HK, Haahr MT, Altman DG, Chan AW. Constraints on publication rights in industry‐initiated clinical trials. JAMA 2006;295(14):1645‐6. [DOI] [PubMed] [Google Scholar]
Hampson 2012
- Hampson LA, Montie JE. Conflict of interest in urology. Journal of Urology 2012;187(6):1971‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2004
- Higgins JPT, Thompson SG. Controlling the risk of spurious findings from meta‐regression. Statistics in Medicine 2004;23(11):1663‐82. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Huggins 2002
- Huggins C, Hodges CV. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Journal of Urology 2002;167(2 Pt 2):948‐51. [PubMed] [Google Scholar]
Iversen 2002
- Iversen P. Antiandrogen monotherapy: indications and results. Urology 2002;60(3 Suppl 1):64‐71. [DOI] [PubMed] [Google Scholar]
Kunath 2012
- Kunath F, Keck B, Antes G, Wullich B, Meerpohl JJ. Tamoxifen for the management of breast events induced by non‐steroidal antiandrogens in patients with prostate cancer: a systematic review. BMC Medicine 2012;10:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meerpohl 2010
- Meerpohl JJ, Wolff RF, Niemeyer CM, Antes G, Elm E. Editorial policies of pediatric journals: survey of instructions for authors. Archives of Pediatrics & Adolescent Medicine 2010;164(3):268‐72. [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
Nguyen 2011
- Nguyen PL, Je Y, Schutz FAB, Hoffman KE, Hu JC, Parekh A, et al. Association of androgen deprivation therapy with cardiovascular death in patients with prostate cancer: a meta‐analysis of randomized trials. JAMA 2011;306(21):2359‐66. [DOI] [PubMed] [Google Scholar]
Okike 2007
- Okike K, Kocher MS, Mehlman CT, Bhandari M. Conflict of interest in orthopaedic research. An association between findings and funding in scientific presentations. Journal of Bone and Joint Surgery. American Volume 2007;89(3):608‐13. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Pildal 2007
- Pildal J, Hróbjartsson A, Jørgensen KJ, Hilden J, Altman DG, Gøtzsche PC. Impact of allocation concealment on conclusions drawn from meta‐analyses of randomized trials. International Journal of Epidemiology 2007;36(4):847‐57. [DOI] [PubMed] [Google Scholar]
Porta 2007
- Porta N, Bonet C, Cobo E. Discordance between reported intention‐to‐treat and per protocol analyses. Journal of Clinical Epidemiology 2007;60(7):663‐9. [DOI] [PubMed] [Google Scholar]
Ramm 2012
- Ramm O, Brubaker L. Conflicts‐of‐interest disclosures at the 2010 AUGS Scientific Meeting. Female Pelvic Medicine & Reconstructive Surgery 2012;18(2):79‐81. [DOI] [PubMed] [Google Scholar]
Review Manager 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schmitt 1999
- Schmitt B, Bennett C, Seidenfeld J, Samson D, Wilt TJ. Maximal androgen blockade for advanced prostate cancer. Cochrane Database of Systematic Reviews 1999, Issue 2. [DOI: 10.1002/14651858.CD001526] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1996
- Schulz KF, Grimes DA, Altman DG, Hayes RJ. Blinding and exclusions after allocation in randomised controlled trials: survey of published parallel group trials in obstetrics and gynaecology. BMJ (Clinical Research Ed.) 1996;312(7033):742‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Seidenfeld 1999
- Seidenfeld J, Samson DJ, Aronson N, Albertson PC, Bayoumi AM, Bennett C, et al. Relative effectiveness and cost‐effectiveness of methods of androgen suppression in the treatment of advanced prostate cancer. Evidence Report/technology Assessment (Summary) 1999; Vol. 4:1‐246. [PMC free article] [PubMed]
Seidenfeld 2000
- Seidenfeld J, Samson DJ, Hasselblad V, Aronson N, Albertsen PC, Bennett CL, et al. Single‐therapy androgen suppression in men with advanced prostate cancer: a systematic review and meta‐analysis. Annals of Internal Medicine 2000;132(7):566‐77. [DOI] [PubMed] [Google Scholar]
Shah 2005
- Shah RV, Albert TJ, Bruegel‐Sanchez V, Vaccaro AR, Hilibrand AS, Grauer JN. Industry support and correlation to study outcome for papers published in Spine. Spine 2005;30(9):1099‐104. [DOI] [PubMed] [Google Scholar]
Smith 2002
- Smith MR. Osteoporosis during androgen deprivation therapy for prostate cancer. Urology 2002;60(3 Suppl 1):79‐85. [PubMed] [Google Scholar]
Sun 2009
- Sun X, Briel M, Busse JW, Akl EA, You JJ, Mejza F, et al. Subgroup Analysis of Trials Is Rarely Easy (SATIRE): a study protocol for a systematic review to characterize the analysis, reporting, and claim of subgroup effects in randomized trials. Trials 2009;10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sun 2012
- Sun X, Briel M, Busse JW, You JJ, Akl EA, Mejza F, et al. Credibility of claims of subgroup effects in randomised controlled trials: systematic review. BMJ (Clinical Research Ed.) 2012;344:e1553. [DOI] [PubMed] [Google Scholar]
Tierney 2005
- Tierney JF, Stewart LA. Investigating patient exclusion bias in meta‐analysis. International Journal of Epidemiology 2005;34(1):79‐87. [DOI] [PubMed] [Google Scholar]
Tyrrell 1994
- Tyrrell CJ. Tolerability and quality of life aspects with the anti‐androgen Casodex (ICI 176,334) as monotherapy for prostate cancer. International Casodex Investigators. European Urology 1994;26 Suppl 1:15‐9. [DOI] [PubMed] [Google Scholar]
Wang 2010
- Wang SS, Ou YC, Cheng CL, Dahm P. Evidence‐based urology in practice: when to believe a subgroup analysis?. BJU International 2010;105(2):162‐4. [DOI] [PubMed] [Google Scholar]
Williamson 2002
- Williamson PR, Smith CT, Hutton JL, Marson AG. Aggregate data meta‐analysis with time‐to‐event outcomes. Statistics in Medicine 2002;21(22):3337‐51. [DOI] [PubMed] [Google Scholar]
Wirth 2007
- Wirth MP, Hakenberg OW, Froehner M. Antiandrogens in the treatment of prostate cancer. European Urology 2007;51(2):306‐14. [DOI] [PubMed] [Google Scholar]
Wirth 2008
- Wirth MP, Hakenberg OW, Froehner M. Adjuvant hormonal treatment ‐ the bicalutamide early prostate cancer program. Frontiers of Radiation Therapy and Oncology 2008;41:39‐48. [DOI] [PubMed] [Google Scholar]
Wood 2004
- Wood AM, White IR, Thompson SG. A review of published randomized controlled trials in major medical journals. Clinical Trials (London, England) 2004;1(4):368‐76. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed.) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]


