Key Points
-
•
More than half of the patients need transfusion support after CAR T-cell therapy; various factors may affect transfusion needs.
-
•
Transfused patients have a poorer outcome with both an increased lymphoma-related mortality and nonrelapse mortality.
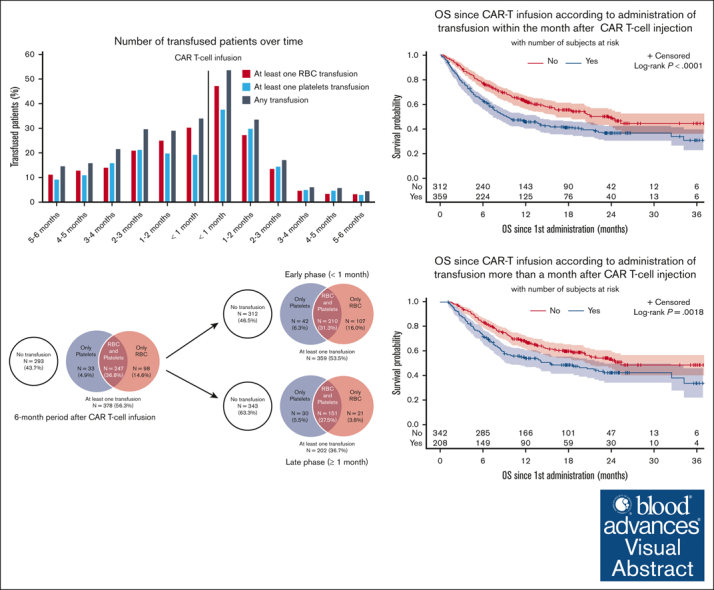
Visual Abstract
Abstract
Chimeric antigen receptor (CAR) T-cells targeting CD19 have been approved for the treatment of relapse/refractory large B-cell lymphoma. Hematotoxicity is the most frequent CAR T-cell–related adverse event. Transfusion support is a surrogate marker of severe cytopenias. Transfusion affects patients’ quality of life, presents specific toxicities, and is known to affect immunity through the so-called transfusion-related immunomodulation that may affect CAR T-cell efficacy. We analyzed data from 671 patients from the French DESCAR-T registry for whom exhaustive transfusion data were available. Overall, 401 (59.8%) and 378 (56.3%) patients received transfusion in the 6-month period before and after CAR T-cell infusion, respectively. The number of patients receiving transfusion and the mean number of transfused products increased during the 6-month period before CAR T-cell infusion, peaked during the first month after infusion (early phase), and decreased over time. Predictive factors for transfusion at the early phase were age >60 years, ECOG PS ≥2, treatment with axicabtagene ciloleucel, pre–CAR T-cell transfusions, and CAR-HEMATOTOX score ≥2. Predictive factors for late transfusion (between 1 and 6 months after infusion) were pre–CAR T-cell transfusions, CAR-HEMATOTOX score ≥2, ICANS ≥3 (for red blood cells [RBC] transfusion), and tocilizumab use (for platelets transfusion). Early transfusions and late platelets (but not RBC) transfusions were associated with a shorter progression-free survival and overall survival. Lymphoma-related mortality and nonrelapse mortality were both increased in the transfused population. Our data shed light on the mechanisms of early and late cytopenia and on the potential impact of transfusions on CAR T-cell efficacy and toxicity.
Introduction
Based on the impressive results in pivotal trials, 3 different chimeric antigen receptor (CAR) T-cell products targeting CD19 have been approved for the treatment of relapse or refractory (R/R) large B-cell lymphoma (LBCL): axicabtagene ciloleucel (axi-cel), tisagenlecleucel (tisa-cel), and lisocabtagene maraleucel.1, 2, 3, 4, 5 Initially, attention has been paid mostly on early and specific side effects such as cytokine release syndrome (CRS) and immune effector cell-associated neurotoxicity syndrome (ICANS).6,7 However, other complications have emerged with late follow-up, including hematotoxicity (also called immune effector cell-associated hemato-toxicity [ICAHT]), which is the most common long-term CAR T-cell–related adverse event in real-world studies.8,9 Grade ≥3 neutropenia, anemia, and thrombocytopenia as defined by the common terminology criteria of adverse events at any time after CAR T-cell infusion have been reported in 72% to 91%, 55% to 69%, and 28% to 62% of cases, respectively.9,10 Rejeski et al described 3 different patterns of hematopoietic reconstitution after CAR T-cell therapy: quick recovery, intermittent recovery, and aplastic phenotype.10 The second one is the most common pattern, characterized by a first recovery by week 3, followed by a second neutrophil and platelet dip observed later in month 2.9,10 Importantly, some patients present with cytopenia for months after treatment, potentially affecting outcome with severe infections or hemorrhages.11, 12, 13 The CAR-HEMATOTOX score, based on cytopenia and inflammatory markers (C-reactive protein and ferritin) before lymphodepletion chemotherapy helps to identify patients at risk of pronounced myelosuppression after CAR T-cell infusion and is predictive of a poorer outcome.10,11
A recent international survey performed by the European Hematology Association (EHA) and European society for Blood and Marrow Transplantation (EBMT) in 18 countries highlighted a strong heterogeneity in terms of grading and management of ICAHT14 and lead to the publication of experts’ recommendations but also underlined the lack of studies on this topic.15 Although growth factors are widely used,16, 17, 18, 19 transfusion support with packed red blood cells (RBC) or platelets is often needed to avoid symptomatic anemia or major bleeding. Transfusion represents a surrogate marker of hematologic toxicity. Transfusion needs directly affect patients’ quality of life and present specific adverse events such as iron overload, circulatory overload, or infections. Transfusions are also known to affect immunity through a phenomenon called transfusion-related immunomodulation20, 21, 22 and thus may affect CAR T-cell efficacy.
RBC or platelets transfusions have been reported in ∼55% to 66% of patients after CAR T-cell infusion,9,23 but little is known about the specific needs, complications, and outcomes in this population. The aim of this study is to describe the transfusion needs in patients receiving commercial anti-CD19 CAR T-cell therapy for LBCL in the real-world setting. Secondary objectives are to search for predictive factors associated with transfusion needs after CAR T-cell therapy and to examine the potential correlation between transfusion needs and CAR T-cell efficacy and toxicity.
Methods
Population and data collection
The DESCAR-T (Dispositif d’Enregistrement et de Suivi des CAR-T) registry is the French national registry designed by the Lymphoma Study Association/Lymphoma Academic Research Organization to collect data from patients treated with commercial CAR T cells outside of clinical trials. Patients are included in this registry if they are considered eligible for CAR T-cell therapy and if the indication for this treatment is validated by a multidisciplinary committee of an accredited center. Data regarding patients, tumor characteristics, CAR T-cell efficacy, and toxicity are prospectively completed by local investigators.
Etablissement Français du Sang is the French blood bank that coordinates collection, storage, and distribution of all blood-derived products. It is the only institution for such products at the national level and has an exhaustive database regarding transfusion. Because transfusion data were not registered in the DESCAR-T registry at the time of this study, we matched the Etablissement Français du Sang database with the DESCAR-T registry to get the precise number of received blood products for each patient, using common identifying data.
The inclusion criteria were: patients treated for R/R LBCL with commercial CD19 CAR T cells registered in the DESCAR-T database, presenting with at least a 6-month follow-up after infusion, and for whom exhaustive transfusion data were available. Patients were censored for transfusions at relapse, new treatment onset, or death. We distinguished transfusions received at the early phase, meaning during the first month after CAR T-cell infusion; and at the late phase, meaning beyond the first and until the sixth month.
Statistical analysis
For the univariate analysis, continuous variables were analyzed by Wilcoxon test and categorical ones by Fisher exact test. Differences between groups were considered statistically significant for P values <.05. Variables with a significant P value were retained for the multivariate analysis. A stepwise selection of variables was used for each model in multivariate analysis. Estimates of survival were calculated according to the Kaplan-Meier method and compared using the log-rank test.
Ethical considerations
All patients or their representatives provided informed consent to noninterventional use of personal data before inclusion in the DESCAR-T registry. This study was approved by local ethics committee on 4 March 2022.
Results
Patients’ baseline characteristics
From August 2018 to September 2022, a total of 671 patients registered in the DESCAR-T registry treated in 19 different French centers met the eligibility criteria. Overall, 429 (63.9%) received axi-cel and 242 (36.1%) tisa-cel. Patients’ characteristics are presented in Table 1 (and supplemental Tables 3-6). At the time of CAR T-cell infusion, median age was 63 years (range, 18-82), 99 patients (18.4%) had an Eastern Cooperative Oncology Group performance status (ECOG PS) ≥2, and 204 (30.8%) presented a bulky disease (>5 cm). Median number of prior lines was 2 (range, 2-10), and 118 patients (17.6%) had received a prior autologous stem cell transplantation. Overall, 82.7% of patients received a bridging therapy, which consisted of systemic chemotherapy in 85.2% of cases. Before lymphodepletion, 126 patients (20.2%) presented at least 1 grade ≥3 cytopenia, and the CAR-HEMATOTOX score was high (≥2) in 383 patients (62.7%). Median follow-up was 18.2 months (95% confidence interval [CI], 17.7-19).
Table 1.
Patients’ baseline characteristics for the entire cohort, and a comparison between patients receiving at least 1 transfusion after CAR T-cell infusion vs no transfusion
| Entire cohort, N = 671 |
No transfusion, n = 293 |
At least 1 transfusion, n = 378 |
P value | ||||
|---|---|---|---|---|---|---|---|
| Histology | .179 | ||||||
| DLBCL, NOS | 457 | (68.1%) | 188 | (64.2%) | 269 | (71.2%) | |
| PMBL | 27 | (4.0%) | 14 | (4.8%) | 13 | (3.4%) | |
| HGBL | 34 | (5.1%) | 13 | (4.4%) | 21 | (5.6%) | |
| PCNSL | 1 | (0.1%) | 1 | (0.3%) | 0 | (0.0%) | |
| Transformed FL | 90 | (13.4%) | 47 | (16.0%) | 43 | (11.4%) | |
| Others∗ | 62 | (9.2%) | 30 | (10.2%) | 32 | (8.5%) | |
| Age at CAR T-cell infusion, y | |||||||
| Median (range) | 63.0 | (18-82) | 62.0 | (18-82) | 64.0 | (18-80) | .004 |
| >60 | 397 | (59.2%) | 159 | (54.3%) | 238 | (63.0%) | .027 |
| ECOG PS at CAR-T infusion | <.001 | ||||||
| 0-1 | 440 | (81.6%) | 222 | (89.5%) | 218 | (74.9%) | |
| ≥2 | 99 | (18.4%) | 26 | (10.5%) | 73 | (25.1%) | |
| Missing | 132 | 45 | 87 | ||||
| Number of prior lines | |||||||
| Median (range) | 2 | (2 - 10) | 2 | (2 – 9) | 3 | (2-10) | .082 |
| Refractory to first line | 302 | (45.0%) | 119 | (40.6%) | 183 | (48.4%) | .070 |
| Previous HSCT | |||||||
| Autologous | 118 | (17.6%) | 58 | (19.8%) | 60 | (15.9%) | .220 |
| Allogeneic | 8 | (1.2%) | 2 | (0.7%) | 6 | (1.6%) | .476 |
| Chemotherapy within 6 mo before CAR T-cell infusion (excluding bridge) | 503 | (75.0%) | 205 | (70.0%) | 298 | (78.8%) | .009 |
| Chemotherapy within 6 mo before CAR T-cell infusion (including bridge) | 618 | (92.1%) | 262 | (89.4%) | 356 | (94.2%) | .030 |
| HCT-CI score at CAR T-cell infusion | .982 | ||||||
| 1-2 | 237 | (88.8%) | 101 | (89.4%) | 136 | (88.3%) | |
| ≥3 | 30 | (11.2%) | 12 | (10.6%) | 18 | (11.7%) | |
| Missing | 404 | ||||||
| aaIPI at diagnostic | .009 | ||||||
| 0-1 | 230 | (38.1%) | 118 | (44.0%) | 112 | (33.3%) | |
| ≥2 | 374 | (61.9%) | 150 | (56.0%) | 224 | (66.7%) | |
| Missing | 67 | 25 | 42 | ||||
| Bulky disease (>5 cm) at CAR T-cell infusion | 204 | (30.8%) | 71 | (24.5%) | 133 | (35.7%) | .002 |
| Bridging therapy | 555 | (82.7%) | 236 | (80.5%) | 319 | (84.4%) | .217 |
| Type of bridging therapy | |||||||
| Chemotherapy | 469 | (84.5%) | 188 | (79.7%) | 281 | (88.1%) | .009 |
| Radiotherapy | 49 | (8.8%) | 21 | (8.9%) | 28 | (8.8%) | 1.000 |
| Corticosteroids | 49 | (8.8%) | 26 | (11.0%) | 23 | (7.2%) | .131 |
| Monoclonal antibody | 402 | (72.4%) | 167 | (70.8%) | 235 | (73.7%) | .501 |
| Other immunotherapy | 27 | (4.9%) | 9 | (3.8%) | 18 | (5.6%) | .425 |
| IMiD | 38 | (6.8%) | 18 | (7.6%) | 20 | (6.3%) | .611 |
| Other bridge† | 35 | (6.3%) | 14 | (5.9%) | 21 | (6.6%) | .860 |
| Type of CAR T-cell | <.001 | ||||||
| Tisa-cel | 242 | (36.1%) | 130 | (44.4%) | 112 | (29.6%) | |
| Axi-cel | 429 | (63.9%) | 163 | (55.6%) | 266 | (70.4%) | |
| CAR-HEMATOTOX score before lymphodepletion | <.001 | ||||||
| Low (0-1) | 228 | (37.3%) | 130 | (49.6%) | 98 | (28.1%) | |
| High (≥2) | 383 | (62.7%) | 132 | (50.4%) | 251 | (71.9%) | |
| Missing | 60 | 31 | 29 | ||||
| Cytopenia grade ≥3 before lymphodepletion | |||||||
| Anemia | 41 | (6.2%) | 10 | (3.5%) | 31 | (8.3%) | .014 |
| Thrombocytopenia | 41 | (6.2%) | 14 | (4.9%) | 27 | (7.3%) | .256 |
| Neutropenia | 64 | (10.4%) | 25 | (9.1%) | 39 | (11.4%) | .426 |
| Ferritin before lymphodepletion | <.001 | ||||||
| Median (range), μg/L | 555 | (5-27809) | 368 | (11.3-15209) | 804 | (5-27809) | |
| >UNL | 416 | (72.6%) | 151 | (59.4%) | 265 | (83.1%) | |
| Missing | 98 | 39 | 59 | ||||
aaIPI, age-adjusted international prognostic index; DLBCL, diffuse large B-cell lymphoma; FL, follicular lymphoma; HCT-CI, hematopoietic cell transplant comorbidity index; HGBL, high grade B-cell lymphoma; HSCT, hematopoietic stem cell transplantation; IMiD, immunomodulatory drug; NOS, not otherwise specified; PMBL, primary mediastinal B-cell lymphoma; PCNSL, primary central nervous system lymphoma; UNL, upper normal limit.
Values in bold signify statistical significance (p<0.05).
Others include: T-cell/histiocyte-rich LBCL (n = 10), 3A-follicular lymphoma (n = 1), transformed marginal-zone lymphoma (n = 19), transformed chronic lymphocytic leukemia (n = 12), transformed Hodgkin (n = 8), DLBCL after PCNSL (n = 3), DLBCL leg type (n = 2), posttransplant lymphoproliferative disorder (n = 1), and “gray zone” meaning with features between DLBCL and classical Hodgkin lymphoma (n = 6).
Others include: intrathecal chemotherapy (n = 7), ibrutinib (n = 20), lenalidomide (n = 3), oral etoposide (n = 1), and missing (n=4).
Transfusion needs over time
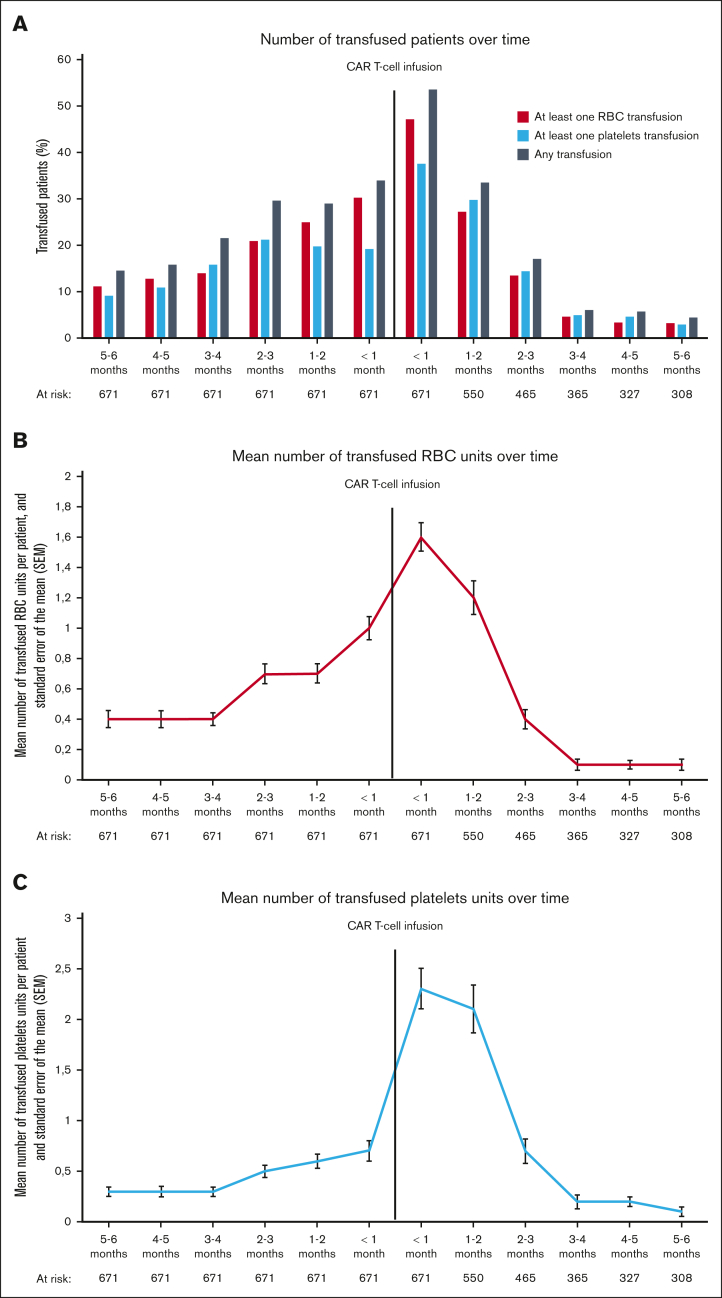
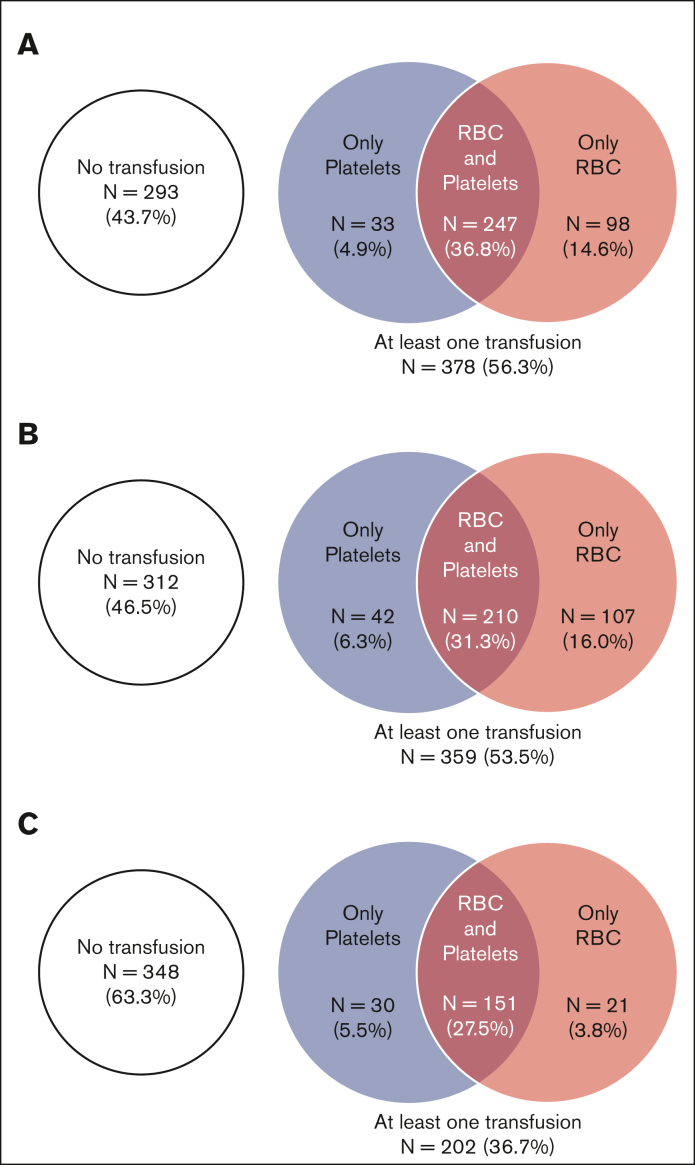
Overall, 401 patients (59.8%) were transfused in the 6-month period before CAR T-cell infusion: 357 (53.2%) received RBC, and 301 (44.9%) received platelets transfusion. The number of patients receiving transfusion increased month by month to reach a maximum of 228 patients (34.0%) within the month before treatment (30.3% requiring RBC and 19.2% platelets transfusion) (Figure 1A). The mean number of transfused RBC and platelets units per month in the 6-month period before CAR T-cell infusion also increased progressively to reach 1.0 (range, 0-15) and 0.7 (range, 0-29) during the month before infusion, respectively (Figures 1B,C). After CAR T-cell infusion, 378 patients (56.3%) received transfusion: 345 (51.4%) received RBC transfusion and 280 (41.7%) received platelets transfusion (Figure 2A). The highest transfusion needs were at the early phase (ie, within in the first month), with 359 patients (53.5%) requiring at least 1 transfusion, including 317 (47.2%) for RBC and 252 (37.6%) for platelets (Figure 2B). During the early phase, the mean number of transfused RBC and platelets units were 1.6 (range, 0-13) and 2.3 (range, 0-44), respectively. At the late phase (ie, between 1 and 6 months after infusion), 202 patients (36.7%) received transfusion, including 172 (31.3%) for RBC and 181 (32.9%) for platelets (Figure 2C). The mean number of transfused units decreased over time after CAR T-cell infusion. Beyond the third month, transfusion needs were very low with only 22 patients (6.0%) requiring at least 1 transfusion (4.7% for RBC and 4.9% for platelets).
Figure 1.
Evolution of transfusion needs overtime. (A) represents the percentage of patients who received transfusion; (B) represents the mean number of transfused RBC units with standard error of the mean; and (C) represents the mean number of transfused platelets units with standard error of the mean.
Figure 2.
Venn diagrams representing transfusion needs for RBCs and platelets after CAR T-cell infusion. (A) in the 6-month period after CAR-T infusion, (B) in the early phase (<1 months), and (C) in the late phase (≥1 and until 6 months).
Predictive factors of transfusion after CAR T-cell infusion
We defined 4 different groups based on the time between CAR-T infusion and transfusion (early <1 month vs late ≥1 month and until 6 months) and the type of transfused product (RBC vs platelets). In a univariate analysis comparing 23 factors between the transfused and nontransfused populations, 18 of them were found significantly different in at least 1 group (supplemental Table 1).
Those 18 variables were integrated into a multivariate model (Table 2). Factors significantly associated with early RBC transfusion were: age >60 years at infusion (adjusted odds ratio [aOR], 2.06; 95% CI, 1.38-3.07; P = .0004), at least 1 transfusion before CAR T-cell therapy (aOR, 9.71; 95% CI, 6.37-14.71; P < .0001), high (≥2) CAR-HEMATOTOX score (aOR, 2.01; 95% CI, 1.34-3.01; P = .0007), treatment with axi-cel vs tisa-cel (aOR, 2.02; 95% CI, 1.34-3.05; P = .0008), and ECOG PS ≥2 at infusion (aOR, 2.86; 95% CI, 1.62-5.04; P = .0004). Factors significantly associated with early platelets transfusion were: age >60 years at infusion (aOR, 1.61; 95% CI, 1.09-2.38; P = .0175), at least 1 transfusion before CAR T-cell therapy (aOR, 7.35; 95% CI, 4.74-11.49; P < .0001), high (≥2) CAR-HEMATOTOX score (aOR, 2.42; 95% CI, 1.60-3.66; P < .0001), treatment with axi-cel vs tisa-cel (aOR, 2.12; 95% CI, 1.40-3.19; P = .0004), and ECOG PS ≥2 at infusion (aOR, 2.28; 95% CI, 1.35-3.87; P = .0028). Factors significantly associated with late RBC transfusion were: at least 1 transfusion before CAR T-cell therapy (aOR, 5.43; 95% CI, 3.37-8.85; P < .0001), high (≥2) CAR-HEMATOTOX score (aOR, 1.98; 95% CI, 1.27-3.09; P = .0026), and ICANS grade ≥3 (aOR, 3.01; 95% CI, 1.62-5.59; P = .0005). Factors significantly associated with late platelets transfusion were: at least 1 transfusion before CAR T-cell therapy (aOR, 7.75; 95% CI, 4.65-12.99; P < .0001), high (≥2) CAR-HEMATOTOX score (aOR, 2.15; 95% CI, 1.37-3.37; P = .0008), and tocilizumab use (aOR, 1.85; 95% CI, 1.15-2.98; P = .0118).
Table 2.
Predictive factors of early and late transfusions after CAR T-cell therapy
| A | Transfusion in the early phase (<1 month) |
|||
|---|---|---|---|---|
| RBC |
Platelets |
|||
| aOR (95% CI) | P value | aOR (95% CI) | P value | |
| Age >60 y at infusion | 2.06 (1.38-3.07) | .0004 | 1.61 (1.09-2.38) | .0175 |
| ≥1 transfusion (RBC or platelets) within 6 mo before CAR T-cell therapy | 9.71 (6.37-14.71) | <.0001 | 7.35 (4.74-11.49) | <.0001 |
| High (≥2) CAR-HEMATOTOX score | 2.01 (1.34-3.01) | .0007 | 2.42 (1.60-3.66) | <.0001 |
| Treatment with axi-cel (vs tisa-cel) | 2.02 (1.34-3.05) | .0008 | 2.12 (1.40-3.19) | .0004 |
| ECOG PS ≥2 at infusion | 2.86 (1.62-5.04) | .0004 | 2.28 (1.35-3.87) | .0028 |
| B | Transfusion in the late phase (≥1 mo) |
|||
|---|---|---|---|---|
| RBC |
Platelets |
|||
| aOR (95% CI) | P value | aOR (95% CI) | P value | |
| ≥1 transfusion (RBC or platelets) within 6 mo before CAR T-cell therapy | 5.43 (3.37-8.85) | <.0001 | 7.75 (4.65-12.99) | <.0001 |
| High (≥2) CAR-HEMATOTOX score | 1.98 (1.27-3.09) | .0026 | 2.15 (1.37-3.37) | .0008 |
| ICANS grade ≥3 | 3.01 (1.62-5.59) | .0005 | NS | |
| Tocilizumab use | NS | 1.85 (1.15-2.98) | .0118 | |
The table presents a multivariate analysis.
Factors tested in the multivariate analysis but not predictive were age-adjusted international prognostic index (aaIPI) a diagnostic, refractoriness to firstline treatment, prior autologous stem cell transplantation, chemotherapy within 6 months before CAR T-cell therapy, chemotherapy as bridging therapy, bulky disease (>5 cm) at infusion, CRS grade ≥3, corticosteroids use after CAR T-cell infusion, intensive care unit (ICU) admission after CAR T-cell infusion, erythropoietin (EPO) use after CAR T-cell infusion, and granulocyte-colony stimulating factor (G-CSF) use after CAR T-cell infusion.
NS, non-significant.
Correlation between transfusion needs and outcome
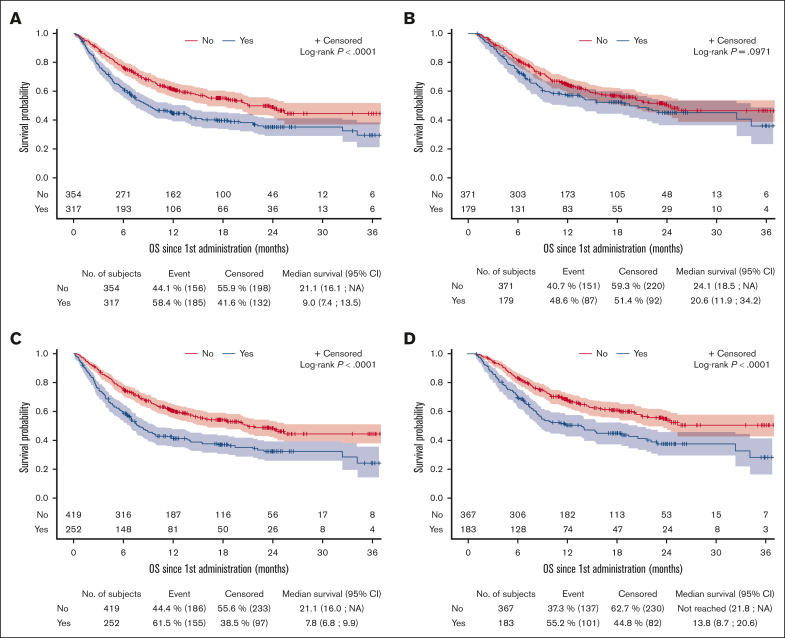
The best overall response rate (ORR) for the entire cohort was 71.8%, including 54.1% of patients with a complete response (CR) and 17.7% with a partial response (PR). Neither ORR nor CR were statistically different between patients who received transfusion and nontransfused patients, at early and late phase after CAR T-cell infusion (supplemental Figure 1). However, early transfusions were associated with a shorter progression-free survival (PFS) for RBC (median PFS, 3.1 vs 6.0 months; P = .0042) and platelets (median PFS, 3.1 vs 5.8 months; P = .0054). Late platelets transfusion also affected PFS (median PFS, 5.6 vs 12.0 months; P = .0072), whereas late RBC transfusion did not (median PFS, 8.8 vs 8.5 months; P = .7199) (Table 3 and supplemental Figure 2). Similarly, early transfusions were associated with a shorter overall survival (OS) for RBC (median OS, 9.0 vs 21.1 months; P < .0001) and platelets (median OS, 7.8 vs 21.1 months; P < .0001). Late platelets transfusion also affected OS (median OS, 13.8 months vs not reached; P < .0001), whereas late RBC transfusion did not (median OS, 20.6 vs 24.1 months; P = .0971) (Figure 3 and Table 3).
Table 3.
Survival outcomes including median PFS and OS for early and late transfusions, as well as NRM and LRM in the different populations
| A | Median PFS (months) |
Median OS (months) |
||||
|---|---|---|---|---|---|---|
| Transfused | Not transfused | P value | Transfused | Not transfused | P value | |
| Early RBC (<1 mo) | 3.1 | 6.0 | .0042 | 9.0 | 21.1 | <.0001 |
| Early platelets (<1 mo) | 3.1 | 5.8 | .0054 | 7.8 | 21.1 | <.0001 |
| Late RBC (≥ 1 mo) | 8.8 | 8.5 | .7199 | 20.6 | 24.1 | .0971 |
| Late platelets (≥ 1 mo) | 5.6 | 12.0 | .0072 | 13.8 | Not reached | <.0001 |
| B | NRM (%) |
LRM (%) |
||||
|---|---|---|---|---|---|---|
| Transfused | Not transfused | P value | Transfused | Not transfused | P value | |
| Early RBC (< 1 mo) | 12.3 | 8.2 | .095 | 44.8 | 35.3 | .014 |
| Early platelets (< 1 mo) | 14.3 | 7.6 | .008 | 46.0 | 36.0 | .012 |
| Late RBC (≥ 1 mo) | 14.0 | 6.9 | .010 | 34.3 | 32.5 | .697 |
| Late platelets (≥ 1 mo) | 14.9 | 6.2 | .001 | 38.7 | 30.4 | .054 |
LRM, lymphoma-related mortality.
Values in bold signify statistical significance (p<0.05).
Figure 3.
OS after CAR T-cell infusion for patients who received transfusion and those who did not. (A) RBC transfusion at the early phase, (B) RBC transfusion at the late phase, (C) platelets transfusion at the early phase, (D) platelets transfusion at the late phase.
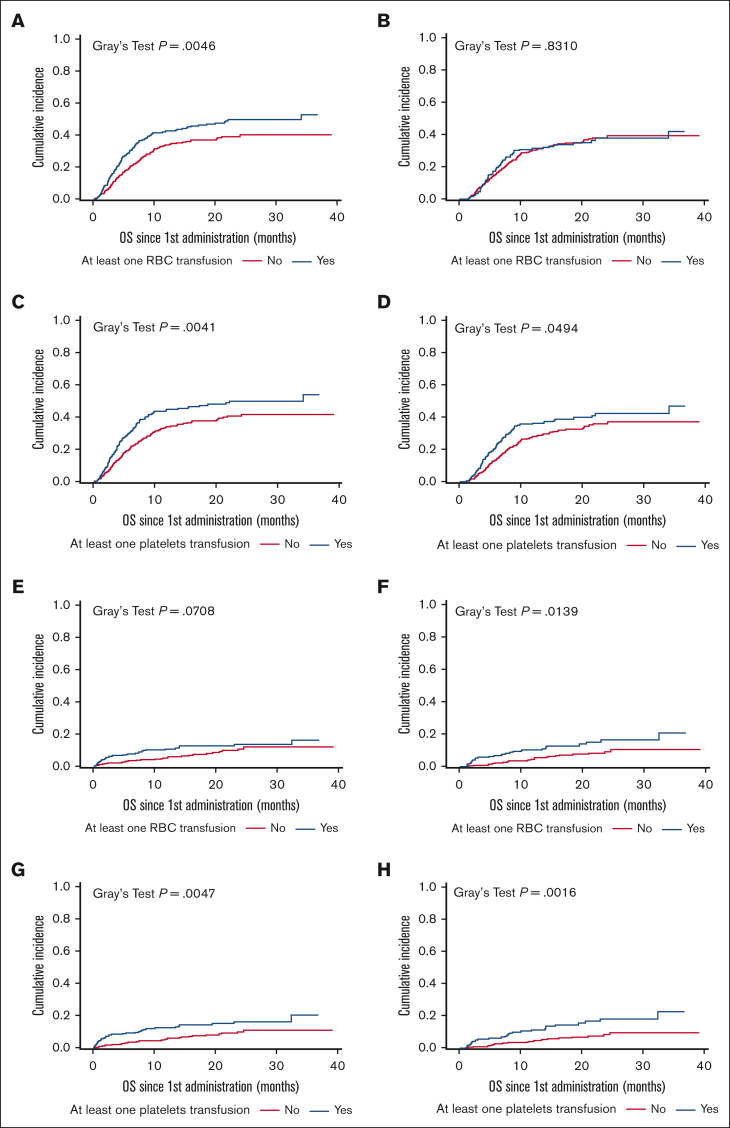
In the entire study population, 345 patients (51.4%) died: 267 (39.8%) from disease progression or relapse, referred to as lymphoma-related mortality; 68 (10.1%) from nonrelapse mortality (NRM), defined as deaths due to toxicity (8.3%), concurrent illness (0.6%), or other reason (1.2%); and 10 (1.5%) from unknown reasons (supplemental Table 2). Cumulative incidence of lymphoma-related mortality was significantly higher for patients receiving early RBC and/or platelets transfusion and late platelets transfusion but not late RBC transfusion (Figure 4 and Table 3). Cumulative incidence of NRM was significantly higher for patients receiving early and late platelet transfusion and late (but not early) RBC transfusion. Indeed, NRM was ∼2 times higher for patients receiving early RBC transfusion (12.3% vs 8.2%; P = .095), early platelets transfusion (14.3% vs 7.6%; P = .008), late RBC transfusion (14.0% vs 6.9%; P = .010), and late platelets transfusion (14.9% vs 6.2%; P = .001) (Figure 4 and Table 3).
Figure 4.
Cumulative incidence of lymphoma-related mortality and NRM after CAR T-cell infusion for patients who received transfusion and those who did not. (A) LRM for RBC transfusion at the early phase. (B) LRM for RBC transfusion at the late phase. (C) LRM for platelets transfusion at the early phase. (D) LRM for platelets transfusion at the late phase. (E) NRM for RBC transfusion at the early phase. (F) NRM for RBC transfusion at the late phase. (G) NRM for platelets transfusion at the early phase. (H) NRM for platelets transfusion at the late phase. LRM, lymphoma-related mortality.
Discussion
To our knowledge, this cohort is the largest to provide an accurate description of transfusion needs in patients with R/R LBCL receiving CD19 CAR T-cell therapy. In the 6-month period before CAR T-cell infusion, the number of patients requiring transfusion and the mean number of transfused units increased progressively. Deep anemia and thrombocytopenia in this period may be explained by cytotoxic treatments administered as salvage and/or bridging therapy but also by the disease itself that may affect hematopoiesis due to an inflammatory environment or a bone marrow infiltration.24 After CAR T-cell infusion, the highest transfusion needs peaked during the first month with 53.5% of patients requiring at least 1 transfusion (RBC or platelets). Beyond the first month, transfusion needs decreased over time with only 6% of patients requiring at least 1 RBC or platelet transfusion per month after month 3, which is less than before treatment at any time and may reflect a good disease control. This timeline is consistent with the description of cytopenias’ evolution after CAR T-cell infusion reported in the literature.1, 2, 3,23,25,26 Of note, the type of transfusion products slightly differed with time, with most patients requiring RBC during the first month (47.2% vs 37.6% for platelets) and most patients requiring platelets beyond month 1 (32.9% vs 31.3% for RBC), which is also consistent with previous studies showing that anemia was more linked to the lymphodepleting chemotherapy and recovered earlier than thrombocytopenia that was characterized by a second dip mostly occurring in the second month.10
We tried to assess risk factors associated with transfusion after CAR T-cell therapy. In previous studies, clear differences have been shown between early (usually defined as occurring within the first month after CAR T-cell infusion) and late (occurring beyond the first month) cytopenias, in terms of frequency, pathophysiology, and prognostic value.9,11,23,27,28 Early cytopenias are mostly explained by bridging and lymphodepleting chemotherapies, acute CAR T-cell toxicities such as CRS/ICANS, infections, and less frequently immune effector cell-associated hemophagocytic lymphohistiocytosis-like syndrome.29 Delayed cytopenias are less understood, with a suggested role of persistent inflammation, poor marrow reserve, CAR T-cell costimulatory domain, and immune-driven suppression of the hematopoietic stem cells mediated by oligoclonal T cells or stromal cell-derived factor 1.9,10,23,27,30 European recommendations differentiated between early (≤30 days) and late ICAHT (>30 days) for grading and treatment.15 In our study, we dichotomized our analysis using this 1-month cutoff. In multivariate analysis, 2 factors were associated with RBC and platelets transfusion, regardless of the time after CAR T-cell treatment: a high CAR-HEMATOTOX score (≥2) and the history of at least 1 transfusion before CAR T-cell infusion. Our study therefore confirms the ability of the CAR-HEMATOTOX score to predict profound and protracted cytopenia and highlights the correlation between pre– and post–CAR T-cell transfusion needs. Age >60 years, ECOG PS ≥2, and the CAR T-cell type were found to be specifically linked to early transfusions. Being older is not known to be a risk factor of high-grade ICAHT, but a higher rate of cardiac and pulmonary comorbidities in this population, combined with a more frequent antithrombotic therapy, may increase the transfusion thresholds in this frailer population.31,32 The association between a poor functional status and higher treatment-related toxicity has been assessed in-depth in many studies, and our correlation between ECOG PS and early transfusion reinforces this observation.33 The CD28 CAR costimulatory domain, compared with 4-1BB, has already been demonstrated to increase CAR T-cell toxicity, including ICAHT.34,35 Focusing on late transfusion events, they seemed to be linked to early inflammatory adverse events because severe ICANS and tocilizumab use (reflecting clinically relevant CRS) increased the risk of RBC and platelets transfusion, respectively. The impact of CRS and ICANS on cytopenias remains controversial in the literature, mostly because the end points differed between studies.9,10,23,36 Therefore, we could not compare directly our study with others. Our data suggest that acute toxicities influence late but not early transfusion needs. Tocilizumab’s impact may reflect the presence of clinically relevant CRS, but attention should be paid on its proper effect because some studies reported an increased risk of neutropenia when used to treat other conditions, such as rheumatic polyarthritis, or in a prophylactic use for other T-cell immunotherapies.37,38
Finally, in our study, transfusion needs correlated with clinical outcome. Although no significant difference was found in terms of best ORR or CR between patients receiving transfusion and those who did not, early transfusions (RBC or platelets) and late platelets transfusion negatively affected PFS and OS, with a higher mortality rate related both to lymphoma progression and CAR T-cell toxicity. Several reasons may explain this difference. First, the transfused and nontransfused populations presented some baseline differences, notably in terms of performance status, age-adjusted international prognostic index, and bulky disease (only for early RBC transfusions) that may be confounding factors affecting relapse and survival.39,40 Second, Rejeski et al showed recently that an aplastic phenotype after CAR T-cell infusion, defined as continuous severe neutropenia >14 days, is associated with limited CAR T-cell expansion, immune dysregulation with higher markers of T-cell suppression, endothelial dysfunction, inflammatory cytokines and macrophage activation, and eventually a poorer PFS and OS.11,41 Because neutrophils and platelets usually display a similar course after CAR T-cell infusion,10 patients receiving late platelet transfusion may overlap with the aplastic phenotype population and thus share the same outcomes. On the contrary, RBC recovery does not overlap platelets and neutrophils kinetic. Thus, the RBC-transfused population may represent a distinct population. Third, there is growing evidence that RBC and platelets transfusion, by the means of allogenic antigen-presenting cells, soluble mediators, and extracellular vesicles, may mitigate the recipient’s immune system, notably with a decreased T-cell proliferation and a shift toward a Th-2–secreting phenotype.42 This phenomenon corresponds to the so-called “transfusion-related immunomodulation,” which has been reported to induce tolerance and immune suppression. Transfusions have demonstrated a positive impact on solid organ transplant outcome, a negative one on cancer dissemination and relapse, and on RR to checkpoint inhibitors in solid cancers.20,22,43 Transfusion may thus impair CAR T-cell fitness and lead to an increased relapse rate, although this hypothesis needs to be further explored. Finally, prolonged cytopenia can predispose for significant infectious complications, which is the main driver of NRM.12,44 We were not able to analyze the association between transfusion needs and the severity/duration of neutropenia because of missing data, but we found a significant association between both transfusion types and the use of granulocyte-colony stimulating factor in the univariate analysis, suggesting that the patients receiving transfusion display a multilineage aplastic phenotype. Our data showed a 2-fold increased NRM in the transfused population, mostly related to infectious and hemorrhagic complications, highlighting the need to monitor closely these patients.
Our study presents several limitations. Due to its retrospective nature, some data are missing, especially regarding baseline cytopenia and inflammatory markers that did not allow for us to calculate the CAR-HEMATOTOX score in ∼10% of the patients. Data regarding bone marrow infiltration and duration of neutropenia were only available in very few cases. Finally, data from the DESCAR-T registry mostly include routine laboratory tests, so we were not able to analyze CAR T-cell expansion and causes of relapse that could have helped us to better understand the association between transfusion and outcome.
Conclusion
Our study provides an accurate description of transfusion needs in patients with LBCL treated with CD19 CAR T-cell therapy. We identified risk factors associated with early and late transfusion needs after CAR T-cell infusion, mainly CAR-HEMATOTOX score, pre–CAR T-cell transfusion needs, high-grade ICANS, and tocilizumab use. Finally, our study sheds light on the potential impact of transfusions on CAR T-cell efficacy and toxicity. Our results may help inform the management of patients treated with CAR T-cell and support strategies that reduce transfusion needs after CAR T-cell therapy.
Conflict-of-interest disclosure: E.B. received honoraria from Kite/Gilead, Bristol Myers Squibb (BMS), Novartis, Pfizer, Incyte, and ADC Therapeutics; personal fees from Kite/Gilead, BMS, Novartis, and Pfizer; and research funding from Amgen and BMS. G.C. received honoraria from Sanofi, AbbVie, Takeda, Roche, Janssen, Celgene, Novartis, and Incyte; consulting fees from Roche and Celgene-BMS; served on scientific advisory boards for Onwards Therapeutics, MedxCell, EmerCell, and MabQi. T.G. received honoraria from Pfizer, Takeda, and Gilead Sciences; served on consulting or advisory role for Takeda and Gilead Sciences; received travel or accommodations fees from Roche and Pfizer. F.M. provided consultancy and advisory boards for Novartis and Gilead. K.B. provided consultancy for and received honoraria or travel fees from Kite/Gilead and Takeda. O.C. received honoraria from Roche, Takeda, Kite/Gilead, MSD, BMS, Amgen, AbbVie, and Janssen; provided advisory/consultancy for Roche, Takeda, Kite/Gilead, MSD, and BMS; received research grant/funding (paid to institution) from Roche, Takeda, and Kite/Gilead; received travel/accommodations/expenses fees from Roche, Takeda, Kite/Gilead, MSD, BMS, Amgen, AbbVie, and Janssen. S.C. disclosed conflict-of-interest with Novartis and Kite/Gilead. C.C.-L. received honoraria from Kite/Gilead. S.G. received honoraria from Gilead, Novartis, Incyte, and Roche. M.L. received honoraria from Alexion, BMS Celgene, Gilead, Iqone, MSD, Novartis, Pfizer, Sanofi, and Sobi. L.D.L.R. received travel/accommodation fees from Takeda and Kite/Gilead; and provided consultancy for Kite/Gilead. R.H. received honoraria from Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda, and Roche; and consultancy fee from Kite/Gilead, Novartis, BMS/Celgene, ADC Therapeutics, Incyte, and Miltenyi. The remaining authors declare no competing financial interests.
Acknowledgments
The authors thank the patients and their families, all the investigators and their staff involved in data collection and analyses, the Etablissement Français du Sang for the data collection regarding transfusions, and the LYSARC for study organization and support.
Authorship
Contribution: S.V., J-B.T., and R.H. conceived, designed the study, and wrote the article; M.G. performed statistical analysis; and all authors provided study material and patients, collected and assembled data, provided final approval for the article, and are accountable for all aspects of the work.
Footnotes
S.V. and J.-B.T. contributed equally to this study.
The data sets analyzed during this study are available upon reasonable request from the corresponding author, Roch Houot (roch.houot@chu-rennes.fr).
The full-text version of this article contains a data supplement.
Supplementary Material
References
- 1.Neelapu SS, Locke FL, Bartlett NL, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med. 2019;380(1):45–56. doi: 10.1056/NEJMoa1804980. [DOI] [PubMed] [Google Scholar]
- 3.Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet. 2020;396(10254):839–852. doi: 10.1016/S0140-6736(20)31366-0. [DOI] [PubMed] [Google Scholar]
- 4.Locke FL, Miklos DB, Jacobson CA, et al. Axicabtagene ciloleucel as second-line therapy for large B-cell lymphoma. N Engl J Med. 2022;386(7):640–654. doi: 10.1056/NEJMoa2116133. [DOI] [PubMed] [Google Scholar]
- 5.Kamdar M, Solomon SR, Arnason J, et al. Lisocabtagene maraleucel versus standard of care with salvage chemotherapy followed by autologous stem cell transplantation as second-line treatment in patients with relapsed or refractory large B-cell lymphoma (TRANSFORM): results from an interim analysis of an open-label, randomised, phase 3 trial. Lancet. 2022;399(10343):2294–2308. doi: 10.1016/S0140-6736(22)00662-6. [DOI] [PubMed] [Google Scholar]
- 6.Lee DW, Santomasso BD, Locke FL, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and neurologic toxicity associated with immune effector cells. Biol Blood Marrow Transplant. 2019;25(4):625–638. doi: 10.1016/j.bbmt.2018.12.758. [DOI] [PubMed] [Google Scholar]
- 7.Neelapu SS, Tummala S, Kebriaei P, et al. Chimeric antigen receptor T-cell therapy — assessment and management of toxicities. Nat Rev Clin Oncol. 2018;15(1):47–62. doi: 10.1038/nrclinonc.2017.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wudhikarn K, Pennisi M, Garcia-Recio M, et al. DLBCL patients treated with CD19 CAR T cells experience a high burden of organ toxicities but low nonrelapse mortality. Blood Adv. 2020;4(13):3024–3033. doi: 10.1182/bloodadvances.2020001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fried S, Avigdor A, Bielorai B, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone Marrow Transplant. 2019;54(10):1643–1650. doi: 10.1038/s41409-019-0487-3. [DOI] [PubMed] [Google Scholar]
- 10.Rejeski K, Perez A, Sesques P, et al. CAR-HEMATOTOX: a model for CAR T-cell–related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood. 2021;138(24):2499–2513. doi: 10.1182/blood.2020010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rejeski K, Perez A, Iacoboni G, et al. The CAR-HEMATOTOX risk-stratifies patients for severe infections and disease progression after CD19 CAR-T in R/R LBCL. J Immunother Cancer. 2022;10(5) doi: 10.1136/jitc-2021-004475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hill JA, Li D, Hay KA, et al. Infectious complications of CD19-targeted chimeric antigen receptor–modified T-cell immunotherapy. Blood. 2018;131(1):121–130. doi: 10.1182/blood-2017-07-793760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnsrud A, Craig J, Baird J, et al. Incidence and risk factors associated with bleeding and thrombosis following chimeric antigen receptor T-cell therapy. Blood Adv. 2021;5(21):4465–4475. doi: 10.1182/bloodadvances.2021004716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rejeski K, Greco R, Onida F, et al. An international survey on grading, diagnosis, and management of Immune Effector Cell-Associated Hematotoxicity (ICAHT) following CAR T-cell therapy on behalf of the EBMT and EHA. HemaSphere. 2023;7(5) doi: 10.1097/HS9.0000000000000889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rejeski K, Subklewe M, Aljurf M, et al. Immune effector cell–associated hematotoxicity: EHA/EBMT consensus grading and best practice recommendations. Blood. 2023;142(10):865–877. doi: 10.1182/blood.2023020578. [DOI] [PubMed] [Google Scholar]
- 16.Galli E, Allain V, Di Blasi R, et al. G-CSF does not worsen toxicities and efficacy of CAR-T cells in refractory/relapsed B-cell lymphoma. Bone Marrow Transplant. 2020;55(12):2347–2349. doi: 10.1038/s41409-020-01006-x. [DOI] [PubMed] [Google Scholar]
- 17.Liévin R, Di Blasi R, Morin F, et al. Effect of early granulocyte-colony-stimulating factor administration in the prevention of febrile neutropenia and impact on toxicity and efficacy of anti-CD19 CAR-T in patients with relapsed/refractory B-cell lymphoma. Bone Marrow Transplant. 2022;57(3):431–439. doi: 10.1038/s41409-021-01526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drillet G, Lhomme F, De Guibert S, Manson G, Houot R. Prolonged thrombocytopenia after CAR T-cell therapy: the role of thrombopoietin receptor agonists. Blood Adv. 2023;7(4):537–540. doi: 10.1182/bloodadvances.2022008066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beyar-Katz O, Perry C, On YB, et al. Thrombopoietin receptor agonist for treating bone marrow aplasia following anti-CD19 CAR-T cells—single-center experience. Ann Hematol. 2022;101(8):1769–1776. doi: 10.1007/s00277-022-04889-6. [DOI] [PubMed] [Google Scholar]
- 20.Remy KE, Hall MW, Cholette J, et al. Mechanisms of red blood cell transfusion-related immunomodulation: MECHANISMS OF RBC TRIM. Transfusion. 2018;58(3):804–815. doi: 10.1111/trf.14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maouia A, Rebetz J, Kapur R, Semple JW. The immune nature of platelets revisited. Transfus Med Rev. 2020;34(4):209–220. doi: 10.1016/j.tmrv.2020.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sut C, Tariket S, Aubron C, et al. The non-hemostatic aspects of transfused platelets. Front Med. 2018;5:42. doi: 10.3389/fmed.2018.00042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain T, Knezevic A, Pennisi M, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4(15):3776–3787. doi: 10.1182/bloodadvances.2020002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gnanaraj J, Parnes A, Francis CW, Go RS, Takemoto CM, Hashmi SK. Approach to pancytopenia: diagnostic algorithm for clinical hematologists. Blood Rev. 2018;32(5):361–367. doi: 10.1016/j.blre.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Schuster SJ, Tam CS, Borchmann P, et al. Long-term clinical outcomes of tisagenlecleucel in patients with relapsed or refractory aggressive B-cell lymphomas (JULIET): a multicentre, open-label, single-arm, phase 2 study. Lancet Oncol. 2021;22(10):1403–1415. doi: 10.1016/S1470-2045(21)00375-2. [DOI] [PubMed] [Google Scholar]
- 26.Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019;20(1):31–42. doi: 10.1016/S1470-2045(18)30864-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jain T, Olson TS, Locke FL. How I treat cytopenias after CAR T-cell therapy. Blood. 2023;141(20):2460–2469. doi: 10.1182/blood.2022017415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou J, Zhang Y, Shan M, et al. Cytopenia after chimeric antigen receptor T cell immunotherapy in relapsed or refractory lymphoma. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.997589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hashmi H, Bachmeier C, Chavez JC, et al. Haemophagocytic lymphohistiocytosis has variable time to onset following CD19 chimeric antigen receptor T cell therapy. Br J Haematol. 2019;187(2):e35–e38. doi: 10.1111/bjh.16155. [DOI] [PubMed] [Google Scholar]
- 30.Rejeski K, Wu Z, Blumenberg V, et al. Oligoclonal T-cell expansion in a patient with bone marrow failure after CD19 CAR-T therapy for Richter-transformed DLBCL. Blood. 2022;140(20):2175–2179. doi: 10.1182/blood.2022017015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Simon GI, Craswell A, Thom O, Chew MS, Anstey CM, Fung YL. Impacts of aging on anemia tolerance, transfusion thresholds, and patient blood management. Transfus Med Rev. 2019;33(3):154–161. doi: 10.1016/j.tmrv.2019.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Lund J, Saunders CL, Edwards D, Mant J. Anticoagulation trends in adults aged 65 years and over with atrial fibrillation: a cohort study. Open Heart. 2021;8(2) doi: 10.1136/openhrt-2021-001737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh H, Gong Y, Roy P, et al. FDA analysis of ECOG performance status and safety outcomes. J Clin Orthod. 2020;38(15_suppl):12024. [Google Scholar]
- 34.Bachy E, Le Gouill S, Di Blasi R, et al. A real-world comparison of tisagenlecleucel and axicabtagene ciloleucel CAR T cells in relapsed or refractory diffuse large B cell lymphoma. Nat Med. 2022;28(10):2145–2154. doi: 10.1038/s41591-022-01969-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xia Y, Zhang J, Li J, et al. Cytopenias following anti-CD19 chimeric antigen receptor (CAR) T cell therapy: a systematic analysis for contributing factors. Ann Med. 2022;54(1):2951–2965. doi: 10.1080/07853890.2022.2136748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Juluri KR, Wu QV, Voutsinas J, et al. Severe cytokine release syndrome is associated with hematologic toxicity following CD19 CAR T-cell therapy. Blood Adv. 2022;6(7):2055–2068. doi: 10.1182/bloodadvances.2020004142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim YE, Ahn SM, Oh JS, et al. Clinical significance of tocilizumab-related neutropenia in patients with rheumatoid arthritis. Joint Bone Spine. 2023;90(3) doi: 10.1016/j.jbspin.2022.105510. [DOI] [PubMed] [Google Scholar]
- 38.Trudel S, Bahlis NJ, Spencer A, et al. Pretreatment with tocilizumab prior to the CD3 bispecific cevostamab in patients with relapsed/refractory multiple myeloma (RRMM) showed a marked reduction in cytokine release syndrome incidence and severity. Blood. 2022;140(Supplement 1):1363–1365. [Google Scholar]
- 39.Locke FL, Rossi JM, Neelapu SS, et al. Tumor burden, inflammation, and product attributes determine outcomes of axicabtagene ciloleucel in large B-cell lymphoma. Blood Adv. 2020;4(19):4898–4911. doi: 10.1182/bloodadvances.2020002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vercellino L, Di Blasi R, Kanoun S, et al. Predictive factors of early progression after CAR T-cell therapy in relapsed/refractory diffuse large B-cell lymphoma. Blood Adv. 2020;4(22):5607–5615. doi: 10.1182/bloodadvances.2020003001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rejeski K, Perez A, Iacoboni G, et al. Severe hematotoxicity after CD19 CAR-T therapy is associated with suppressive immune dysregulation and limited CAR-T expansion. Sci Adv. 2023;9(38) doi: 10.1126/sciadv.adg3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vamvakas EC, Blajchman MA. Transfusion-related immunomodulation (TRIM): An update. Blood Rev. 2007;21(6):327–348. doi: 10.1016/j.blre.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 43.Mispelbaum R, Hattenhauer ST, Brossart P, Heine A. Red blood cell transfusions impact response rates to immunotherapy in patients with solid malignant tumors. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.976011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nastoupil LJ, Jain MD, Feng L, et al. Standard-of-care axicabtagene ciloleucel for relapsed or refractory large B-cell lymphoma: results from the US lymphoma CAR T Consortium. J Clin Orthod. 2020;38(27):3119–3128. doi: 10.1200/JCO.19.02104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.