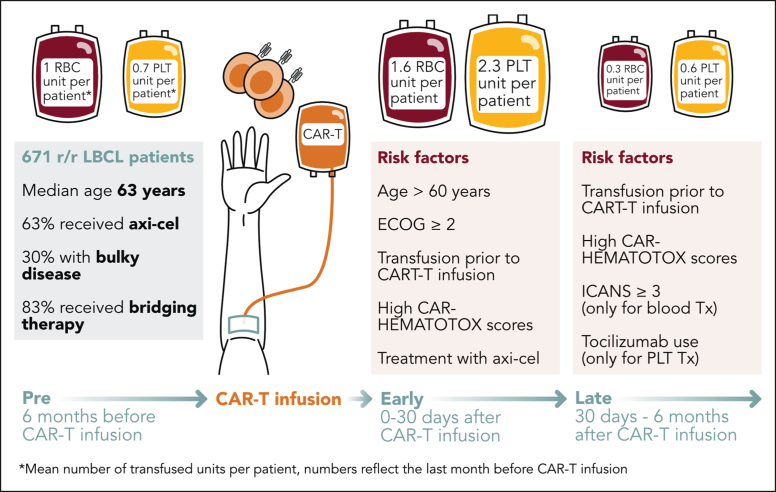
In this issue of Blood Advances, Vic et al1 report on transfusion support after CD19-directed chimeric antigen receptor (CAR) T-cell therapy for large B-cell lymphoma (LBCL). To collect patient-level data on transfusion use and timing, the authors matched 671 patients with LBCL from the French commercial CAR-T national registry (DESCAR-T) with the French blood bank (EFS), thereby obtaining, to the best of our knowledge, the most comprehensive data set on this subject to date. They first provide a descriptive characterization of post–CAR-T transfusion needs and perform a multivariate analysis to identify risk factors associated with red blood cell (RBC) and platelet transfusions after CAR-T. Next, they examine the associations between transfusion requirements and clinical outcomes. The authors are to be lauded for this extensive effort, which adds to the literature of CAR-T–associated hematological toxicity, now recognized as immune effector cell-associated hematotoxicity (ICAHT), which is the most frequently encountered side effect of CAR-T therapy.2
The authors divided the transfusion timeline into 3 distinct phases: a pre–CAR-T phase spanning the 6 months before CAR-T infusion; an early phase (day 0-30); and a late phase (day 30-180). In the pre–CAR-T phase, transfusion needs incrementally increased over time until reaching their peak in the month immediately preceding CAR-T infusion (one-third of patients). Approximately half of all patients were transfused in the early phase, representing the highest transfusion peak over the entire study timeframe (see figure). One-third of patients were transfused with RBCs or platelets in the late phase, predominantly in the second and third month.
Timeline of transfusion needs following CD19 CAR-T. Study schema summarizes the transfusion requirements for patients with LBCL treated with CAR-T therapy based on the 3 phases: pre- and post–CAR-T transfusion (early and late). Axi-cel, axicabtagene ciloleucel; ECOG, Eastern Cooperative Oncology Group. Professional illustration by Somersault18:24.
The high rate of pre–CAR-T transfusion needs (especially in the last month before infusion) is likely driven by the cytotoxic bridging therapy received by the vast majority of patients (>80%) and the possibility of LBCL bone marrow involvement in the setting of recurrent and/or progressive disease. The high transfusion requirements after CAR-T infusion are of particular interest. Although early transfusion needs can be understood as a lingering effect of bridging chemotherapy and/or driven by the lymphodepletion regimen, prolonged anemia and thrombocytopenia are biologically distinct and less elucidated. Overall, the underlying mechanisms are likely multifactorial. The association between high baseline CAR-HEMATOTOX scores and transfusion needs strongly suggests that baseline systemic inflammation and poor marrow reserve play a key role.3,4 Furthermore, recent studies have shed light on the importance of immune dysregulation characterized by hallmarks of macrophage activation (eg, elevations of IL-15, IL-18, and MCP-1), expression of inhibitory T-cell checkpoint ligands, and clonal expansion of IFN-γ expressing CD8+ T cells, which collectively contribute to delayed hematopoietic recovery.5,6
On multivariate analysis, the authors identified prior transfusions, older age (>60 years), poor functional status, high CAR-HEMATOTOX scores, and use of CD28z costimulated CAR-T products (axicabtagene ciloleucel > tisagenlecleucel) as key predictors of both early RBC and platelet transfusions. Despite higher baseline serum ferritin levels in the transfusion cohort, the authors surprisingly did not identify an association between cytokine release syndrome (CRS) and transfusion requirements, in contrast to previous reporting by Juluri et al, which linked high-grade CRS and the associated cytokine patterns to ICAHT (especially IL-6 elevations).7 In light of these results, future translational efforts should ask the question whether CRS-related inflammatory mediators merely compound preexisting immune dysregulation (both systemically and locally within the bone marrow niche) or independently facilitate myelosuppression.
Another striking finding of this study was the remarkably shorter overall survival and increased rate of disease progression and relapse in the transfusion group, particularly during the early phase. However, these data should be interpreted in the context of the significant disease burden carried by the patients in the transfusion group. For example, patients who needed transfusions were older (63% aged >60 years), had poor performance status (25% with ECOG ≥2), more than one-third displayed bulky disease, and 84% received systemic bridging therapy to control their disease during CAR-T manufacturing. As a result, transfusion needs may simply represent a surrogate marker for higher risk disease features. Additionally, the authors also postulated that transfusions may negatively affect the recipient’s immune system through various mechanisms, potentially impairing CAR T-cell function in a phenomenon called transfusion-related immunomodulation, although this would require further mechanistic exploration in the context of CAR-T.1 Intriguingly, the transfusion group also demonstrated an approximately twofold increase in the rate of nonrelapse mortality (NRM) compared to the control group. This was particularly evident for early transfusions, suggesting these patients also developed concurrent severe ICAHT with profound neutropenia. Although nonrelapse-related causes of death were not captured in this analysis for each group separately, one may speculate that the higher NRM rate in patients receiving transfusions may reflect an increase in fatal infections due to severe hematotoxicity.8,9 In line with this, we recently demonstrated that severe ICAHT was associated with increased transfusion requirements, a higher rate of severe infections, and increased infection-driven NRM.10
One limitation of the study is the lack of longitudinal assessment of neutrophil counts, which would provide an important insight on different interlineage interactions and the overall kinetics of hematological recovery. Another limitation relates to the absence of important correlative measurements, including assessment of serum cytokine levels and CAR T-cell expansion, which could further enrich the mechanistic evaluation and guide future targeted therapeutic approaches. Similarly, data on bone marrow infiltration were lacking in this study, an important metric to assess how infiltrative disease may contribute to transfusion needs. Designing prospective studies to thoroughly characterize multilineage hematological toxicity in the context of CAR-T, evaluate transfusion requirements and growth factor usage, and eventually establish transfusion thresholds (liberal vs restrictive), is imperative and will diminish the limitations of this analysis that arise from its retrospective nature.
With this study, Vic et al have raised awareness of the high transfusion burden carried by patients who receive CAR-T. Transfusion needs can complicate the patient’s clinical course, impair patient’s quality of life due to prolonged hospital stays, result in overutilization of clinical resources, and can potentially limit subsequent therapy at relapse. Expanding our understanding of hematotoxicity in the context of CAR-T is crucial and will eventually lead to refinement of our current management approaches for patient populations at high risk.
Conflict-of-interest disclosure: K.R. reports research funding, consultancy, honoraria, and travel support from Kite/Gilead; honoraria from Novartis; consultancy and honoraria from BMS/Celgene; and travel support from Pierre-Fabre. M.A. declares no competing financial interests.
References
- 1.Vic S, Thibert J-B, Bachy E, et al. Transfusion needs after CAR T-cell therapy for large B-cell lymphoma: predictive factors and outcome. A DESCAR-T study. Blood Adv. 2024;8(6):1573–1585. doi: 10.1182/bloodadvances.2023011727. [DOI] [PubMed] [Google Scholar]
- 2.Rejeski K, Subklewe M, Aljurf M, et al. Immune effector cell-associated hematotoxicity: EHA/EBMT consensus grading and best practice recommendations. Blood. 2023;142(10):865–877. doi: 10.1182/blood.2023020578. [DOI] [PubMed] [Google Scholar]
- 3.Jain T, Knezevic A, Pennisi M, et al. Hematopoietic recovery in patients receiving chimeric antigen receptor T-cell therapy for hematologic malignancies. Blood Adv. 2020;4(15):3776–3787. doi: 10.1182/bloodadvances.2020002509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rejeski K, Perez A, Sesques P, et al. CAR-HEMATOTOX: a model for CAR T-cell-related hematologic toxicity in relapsed/refractory large B-cell lymphoma. Blood. 2021;138(24):2499–2513. doi: 10.1182/blood.2020010543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rejeski K, Perez A, Iacoboni G, et al. Severe hematotoxicity after CD19 CAR-T therapy is associated with suppressive immune dysregulation and limited CAR-T expansion. Sci Adv. 2023;9(38) doi: 10.1126/sciadv.adg3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strati P, Li X, Deng Q, et al. Prolonged cytopenia following CD19 CAR T cell therapy is linked with bone marrow infiltration of clonally expanded IFNγ-expressing CD8 T cells. Cell Rep Med. 2023;4(8) doi: 10.1016/j.xcrm.2023.101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juluri KR, Wu QV, Voutsinas J, et al. Severe cytokine release syndrome is associated with hematologic toxicity following CD19 CAR T-cell therapy. Blood Adv. 2022;6(7):2055–2068. doi: 10.1182/bloodadvances.2020004142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cordas dos Santos DM, Tix T, Rejeski K. Infections drive non-relapse mortality following CAR-T therapy across disease entities and CAR products - a meta-analysis of clinical trials and real-world studies. Blood. 2023;142(suppl 1) 1064-1064. [Google Scholar]
- 9.Lemoine J, Bachy E, Cartron G, et al. Non-relapse mortality after CAR T-cell therapy for large B-cell lymphoma: a LYSA Study from the DESCAR-T Registry. Blood Adv. 2023;7(21):6589–6598. doi: 10.1182/bloodadvances.2023010624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rejeski K, Wang Y, Hansen DK, et al. Applying the EHA/EBMT grading for ICAHT after CAR-T: comparative incidence and association with infections and mortality. Blood Adv. Published online 4 January 2024 doi: 10.1182/bloodadvances.2023011767. [DOI] [PubMed] [Google Scholar]