Abstract
OBJECTIVE:
To estimate the absolute and relative risk of venous thromboembolism (VTE) among women who initiate depot medroxyprogesterone acetate (DMPA) immediately postpartum compared with those who do not initiate hormonal contraception.
METHODS:
The IBM MarketScan Commercial Claims and Encounters databases were used to identify delivery hospitalizations among women aged 15–44 years during 2005 through 2014. Diagnosis, procedure, and drug codes were used to identify contraception, VTE, and potential confounding chronic or pregnancy-related conditions. Women who initiated DMPA during days 0 through 7 postpartum were compared with women who did not initiate hormonal contraception during days 0 through 7 postpartum. Women were followed from date of delivery through 12 weeks postpartum for the occurrence of VTE, with censoring at hormonal contraception initiation or prescription, hysterectomy, sterilization, or inpatient death. The incidence rate of VTE and 95% CIs were calculated within each group and the incidence rate ratio was calculated comparing the two groups.
RESULTS:
The unadjusted VTE incidence rate through 12 weeks postpartum was 0.42/10,000 women-days in the immediate postpartum DMPA group (34 events among 11,159 women contributing 805,999 days of follow-up) and 0.15/10,000 women-days in the control group (3,107 events among 3,102,011 women contributing 206,180,811 days of follow-up). The incidence rate ratio for VTE was 2.87 (95% CI 2.05–4.03) among women in the immediate postpartum DMPA group compared with women in the control group, adjusting for age alone. After adjusting for age and pregnancy-related and chronic conditions, the adjusted incidence rate ratio for VTE was 1.94 (95% CI 1.38–2.72) among women in the immediate postpartum DMPA group compared with women in the control group.
CONCLUSION:
Initiation of DMPA immediately postpartum is associated with a low incidence but an increased relative risk of VTE compared with nonuse of hormonal contraception.
The immediate postpartum period is an ideal time to consider initiation of contraception because women have access to healthcare, are not pregnant, and may not follow up for postpartum visits. Initiating contraception immediately after delivery may reduce the risk of unintended pregnancies and short birth intervals, both of which have been associated with negative health outcomes.1–3 Depot medroxyprogesterone acetate (DMPA) is effective, well-tolerated and is generally considered safe for use by postpartum women, including those who are breastfeeding.4 In addition, it has the advantage of effectiveness for 12 weeks, allowing a bridge to long-acting or permanent methods for women who desire but are unable to receive those methods immediately postpartum because of logistical, financial, or other barriers.
An important concern for postpartum women is whether exposure to hormonal contraceptives might increase the risk of thrombosis. Depot medroxyprogesterone acetate has been associated with increased odds of venous thromboembolism (VTE) compared with nonuse, among the general population of women and women with certain risk factors for VTE, such as smoking, thrombogenic mutations, and diabetes.5–8 Because postpartum women are at elevated risk of VTE compared with nonpregnant, nonpostpartum women of reproductive age, particularly within the first week postpartum, any further elevation in risk with DMPA use is of potential concern.9,10 The objective of this study was to estimate the absolute and relative risk of VTE through 12 weeks postpartum among women who initiate DMPA immediately postpartum (up to 7 days postpartum) compared with those who do not initiate hormonal contraception.
METHODS
This analysis used the IBM MarketScan Commercial Claims and Encounters databases, which contain individual-level health care claims information from employers, health plans, and hospitals. The databases include linked information on health care services used in inpatient and outpatient settings as well as filled outpatient prescription drug claims. The databases undergo many steps to enhance data quality, including checking reasonableness of data against norms, checking validity of diagnosis and procedure codes against possible valid values and flagging improper coding for improvement.11 Because the data are deidentified, the Centers for Disease Control and Prevention deemed this study to be research not involving human subjects and therefore did not need review by an institutional review board.
We identified women ages 15–44 with delivery hospitalizations during 2005 through 2014 (Fig. 1). International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM), Diagnosis Related Group, and Current Procedural Terminology (CPT) codes were used to identify delivery hospitalizations, and the date of first report of delivery diagnoses or procedures was considered day 0 (Table 1). We included only women who were continuously enrolled from 12 months before through 12 weeks after delivery and who had information on outpatient pharmaceutical claims. For women who had multiple deliveries during the time period, only the first delivery was included. We excluded women with deliveries after September 30, 2014, to allow for 12 weeks of follow up for all women. We excluded women with ICD-9-CM codes indicating abortion, ectopic pregnancy, molar pregnancy, and other abnormal products of conception. We also excluded women who were prescribed anticoagulants during the 6 months before the delivery hospitalization because these women likely represent a group with a different baseline VTE risk, such as those with a previous VTE or thrombophilia. To assess whether we had identified all women with a previous VTE, we additionally examined women with VTE codes during the 12 months before delivery, and all of these women were prescribed anticoagulants during the 6 months before delivery hospitalization and were therefore excluded from analyses.
Fig. 1.
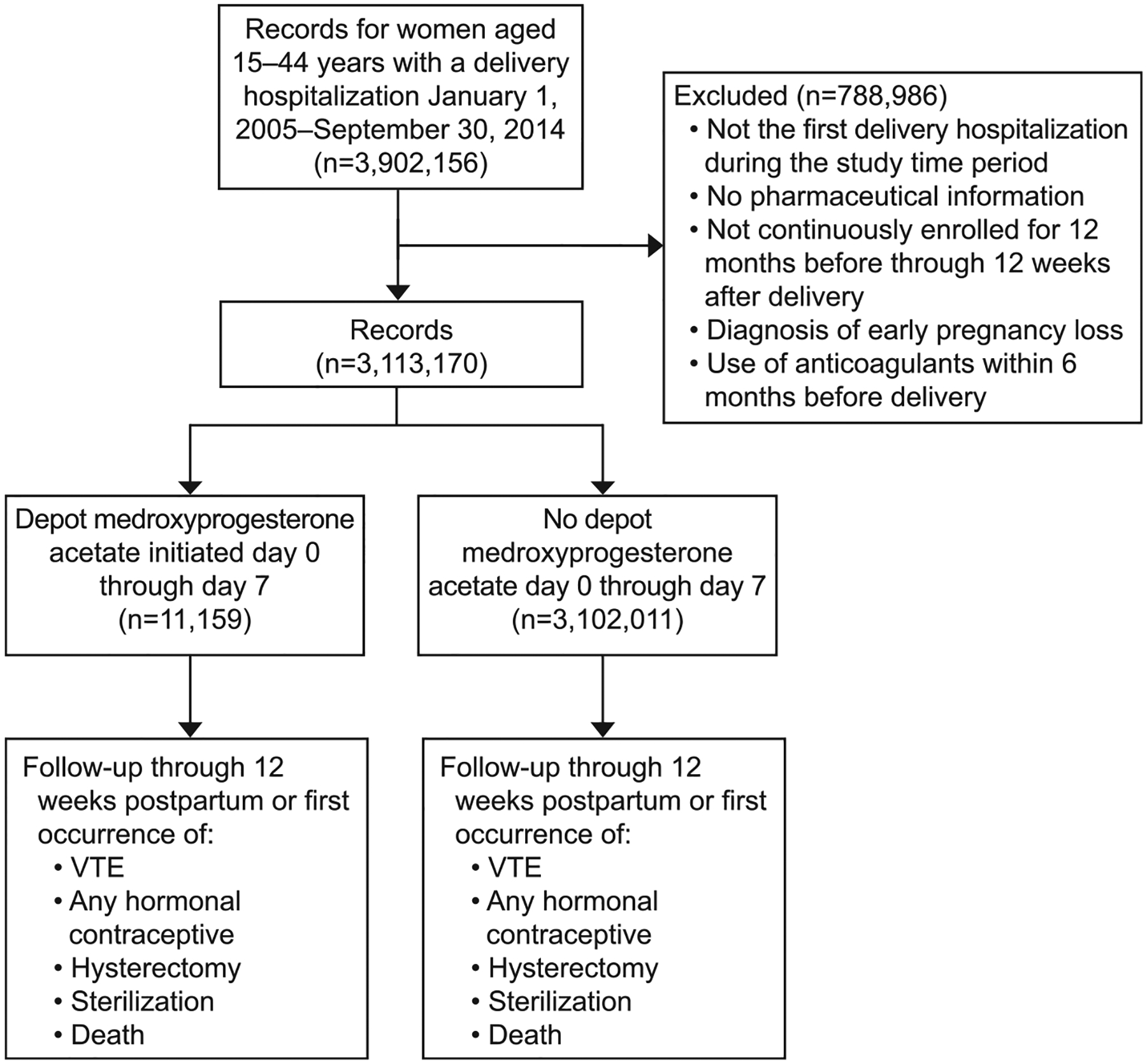
Categorization of potential participants by exclusion, exposure, and censoring event. VTE, venous thromboembolism.
Tepper. Postpartum Thrombosis and Injectable Contraception. Obstet Gynecol 2019.
Table 1.
Codes Used to Idenify Delivery Hospitalizations, Initiation of Depot Medroxyprogesterone Acetate, and Venous Thromboembolism
| Code Type | Code | Description |
|---|---|---|
| Deliveries | ||
| ICD-9-CM diagnosis codes | V27.x | Outcome of delivery (all) |
| 650 | Normal delivery | |
| 669.7x | Cesarean delivery | |
| ICD-9-CM procedure codes | 74.0, 74.1, 74.2, 74.4, 74.99, 669.7x | Cesarean delivery |
| 72.0, 72.1, 72.21, 72.29, 72.31, 72.39 | Low, mid, high forceps operation | |
| 72.4 | Forceps rotation of fetal head | |
| 72.6 | Forceps application to aftercoming head | |
| 72.51, 72.52, 72.53, 72.54 | Breech extraction | |
| 72.71, 72.79 | Vacuum extraction | |
| 72.8 | Other specified instrumental delivery | |
| 72.9 | Unspecified instrumental delivery | |
| 73.22 | Internal and combined version with extraction | |
| 73.59 | Other manually assisted delivery | |
| 73.6 | Episiotomy | |
| CPT codes | 59514 | Cesarean delivery only, no postpartum care |
| 59620 | Cesarean delivery only, after attempted vaginal delivery | |
| 59409 | Vaginal delivery only (with or without episiotomy, forceps, or both), no postpartum care | |
| 59612 | Vaginal delivery only, after previous cesarean delivery (with or without episiotomy, forceps, or both) | |
| DRG codes | 2006 or before (370–375); after 2006 (765–768, 774,775) | Outcome of delivery (all) |
| DMPA | ||
| ICD-9-CM diagnosis codes | V25.02 | Initiation of other contraceptive method |
| V25.04 | Family planning | |
| V25.09 | Counseling | |
| V25.40 | Contraceptive surveillance, unspecified | |
| V25.49 | Surveillance other contraceptives | |
| V25.8 | Other contraceptive management | |
| V25.9 | Unspecified contraceptive management | |
| ICD-9 procedure codes | 99.24 | Injection of other hormone |
| CPT codes | 90772 | Therapeutic, prophylactic or diagnostic injection (specify substance or drug); subcutaneous or intramuscular |
| 96372 | Therapeutic, prophylactic, or diagnostic injection (specify substance or drug); subcutaneous or intramuscular | |
| HCPCS codes | J1050, J1051, J1055 | Medroxyprogesterone acetate |
| NDC codes | 00009074630, 00009074631, 00009074634, 00009074635, 00009470901, 00009470913, 00009737601, 00009737602, 00009737603, 00009737604, 00009737607, 00009737611, 00403504318, 00703680101, 00703680104, 00703681121, 23490585401, 54569370100, 54569490400, 54569552700, 54569561600, 54569621900, 54868361300, 54868410000, 54868410001, 54868525700, 55045350501, 55045394001, 59762453701, 59762453702, 59762453801, 59762453802, 59762453809 | Medroxyprogesterone acetate |
| VTE | ||
| ICD-9 diagnosis codes | 671.3x, 671.4x, 671.5x, 671.9x, 451.1, 451.2, 451.9, 451.81, 453.1, 453.2, 453.40–453.42, 453.8, 453.9 | DVT |
| 673.2x, 673.8x, 415.1 | Pulmonary embolism | |
| AHFS therapeutic class | 39 | Anticoagulants |
| 44 | Thrombolytic agents, not elsewhere classified |
ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; CPT, Current Procedural Terminology; DRG, Diagnosis Related Group; DMPA, depot medroxyprogesterone acetate; HCPCS, Healthcare Common Procedure Coding System; NDC, National Drug Code; VTE, venous thromboembolism; DVT, deep vein thrombosis; AHFS, American Hospital Formulary Service.
Contraceptive methods were identified using ICD-9-CM, CPT, National Drug Code or Healthcare Common Procedure Coding System codes. The exposed group was comprised of women who initiated DMPA within the first 7 days postpartum. Because National Drug Code and Healthcare Common Procedure Coding System codes were not available for the inpatient setting, DMPA initiation in the hospital required ICD-9-CM procedure code 99.24 (injection of other hormone) plus an ICD-9-CM diagnosis code indicating contraceptive management (Table 1). The control group of interest was women using nonhormonal contraception (ie, copper intrauterine device) or no contraceptives in the immediate postpartum period.
Our outcome of interest was VTE, including deep vein thrombosis (DVT) and pulmonary embolism (PE) as identified by ICD-9-CM codes (Table 1). The date of VTE diagnosis was assigned as the date of first reported DVT or PE code after delivery. For VTEs identified in outpatient claims only, we required a diagnosis code plus a filled anticoagulant prescription claim, in an attempt to minimize misclassification of women who were assessed for VTE but ultimately ruled out.12,13 For inpatient VTEs, we required diagnosis codes only because diagnoses are assigned at the time of hospital discharge and are therefore likely to be confirmed diagnoses.13
Women were followed through 12 weeks postpartum, or were censored at the earliest occurrence of VTE, hormonal contraception initiation or prescription (including DMPA after 7 days postpartum), female sterilization, hysterectomy, or inpatient death. Sterilization and hysterectomy were identified using ICD-9 or CPT codes if they appeared once in either inpatient or outpatient claims.
We considered age and certain chronic medical conditions that have been found to be significant risk factors for VTE during pregnancy as potential confounders.14,15 Age at delivery was categorized into five groups (15–24, 25–29, 30–34, 35–39, and 40–44 years). Chronic medical conditions were identified by ICD-9-CM codes during the 12 months before delivery and included pregestational diabetes, chronic hypertension, obesity, and smoking. Cardiovascular conditions, cerebrovascular disorders, and heart failure were identified by ICD-9-CM codes reported during 12 months before delivery through 12 weeks postpartum, given that these conditions could be pre-existing or pregnancy-related. Outpatient diagnoses were considered valid only if the codes appeared two or more times at least 30 days apart. Inpatient diagnoses were considered valid even if they appeared only once.
We also considered several pregnancy-related conditions and complications as potential confounders, including cesarean delivery, gestational hypertension, preeclampsia, eclampsia, multiple birth, anemia, antepartum hemorrhage, postpartum hemorrhage, postpartum infection, intrauterine fetal demise, blood transfusion, pulmonary edema, adult respiratory distress syndrome, disseminated intravascular coagulation, obstetric shock, sepsis, ventilation, and peripartum cardiomyopathy. These conditions were identified by ICD-9-CM codes or CPT codes reported within 40 weeks before delivery through 12 weeks postpartum. Outpatient diagnoses were considered valid only if the codes appeared two or more times. Outpatient procedures (transfusion or ventilation) were considered valid if reported only once. Inpatient diagnoses were considered valid if they appeared only once.
The percent distributions of risk factors for women in the DMPA group and women in the control group were calculated and compared using a Chi-square test. Incidence rates of VTE through 12 weeks postpartum for women in the immediate postpartum DMPA and control groups were calculated as the number of VTEs divided by women-days of follow up. The denominator of women-days was used to account for different lengths of follow-up contributed by women who were censored before 12 weeks postpartum. A Poisson regression model was used to estimate the incidence rate ratio and 95% CI for VTE among women exposed to immediate postpartum DMPA compared with women in the control group. The incidence rate ratio was adjusted for age group alone (ages 15–24, 25–29, 30–34, 35–39, and 40–44) and additionally for age group and risk factors that were found to be associated with DMPA use in our bivariate analyses and with VTE risk in previous studies.10,16,17
A subgroup analysis was conducted among women less than 35 years of age and without any of the considered risk factors to assess idiopathic VTE among low-risk women. SAS 9.4 was used for all analyses. Analyses were independently conducted by two coauthors and any differences were adjudicated by discussion.
RESULTS
After applying exclusions, there were 11,159 women who initiated DMPA during the first 7 days postpartum and 3,102,011 women who did not (Table 2). Women who initiated immediate postpartum DMPA were younger than women in the control group; the proportion of women ages 15–24 was 29% in the immediate postpartum DMPA group and 17% in the control group (P<.001). Women who initiated immediate postpartum DMPA were more likely than women in the control group to have pregestational diabetes (2.6% vs 2.0%), to have chronic hypertension (5.8% vs 4.1%), to be obese (3.4% vs 2.5%), and to be smokers (3.6% vs 1.6%) (P<.001 for all comparisons). Women in the DMPA group were less likely than women in the control group to have experienced cesarean delivery (10.3% vs 14.7%) and were more likely to have experienced pregnancy or postpartum complications including hypertensive disorders (ie, gestational hypertension, preeclampsia, or eclampsia) (16.2% vs 11.7%), multiple birth (5.2% vs 2.7%), anemia (16.7% vs 11.2%), antepartum hemorrhage (9.0% vs 4.6%), postpartum hemorrhage (4.4% vs 2.4%), postpartum infection (1.8% vs 0.7%), intrauterine fetal demise (0.9% vs 0.4%), and other severe complications (2.5% vs 1.6%) (P<.001 for all comparisons).
Table 2.
Characteristics of Women With Delivery Hospitalizations During 2005–2014, Stratified by Receipt of DMPA During Postpartum Days 0 Through 7
| Condition* | DMPA Group | Control Group |
|---|---|---|
| Total | 11,159 | 3,102,011 |
| Age group (y) | ||
| 15–24 | 3,231 (29.0) | 532,743 (17.2) |
| 25–29 | 3,439 (30.8) | 937,164 (30.2) |
| 30–34 | 2,881 (25.8) | 1,015,684 (32.7) |
| 35–39 | 1,304 (11.7) | 505,292 (16.3) |
| 40–44 | 304 (2.7) | 111,128 (3.6) |
| Chronic conditions | ||
| Pregestational diabetes | 288 (2.6) | 60,731 (2.0) |
| Chronic hypertension | 649 (5.8) | 127,481 (4.1) |
| Obesity | 375 (3.4) | 75,911 (2.5) |
| Smoking | 399 (3.6) | 49,319 (1.6) |
| Pregnancy or postpartum conditions or complications | ||
| Mode of delivery | ||
| Vaginal | 10,011 (89.7) | 2,645,680 (85.3) |
| Cesarean | 1,148 (10.3) | 456,331 (14.7) |
| Gestational hypertension, preeclampsia, eclampsia | 1,812 (16.2) | 361,647 (11.7) |
| Multiple birth | 581 (5.2) | 83,317 (2.7) |
| Anemia | 1,859 (16.7) | 345,988 (11.2) |
| Antepartum hemorrhage | 1,002 (9.0) | 143,388 (4.6) |
| Postpartum hemorrhage | 489 (4.4) | 73,978 (2.4) |
| Postpartum infection | 205 (1.8) | 21,054 (0.7) |
| Intrauterine fetal demise | 98 (0.9) | 12,726 (0.4) |
| Other severe complications† | 277 (2.5) | 50,159 (1.6) |
DMPA, depot medroxyprogesterone acetate.
Data are n (%).
Chi-square P<.001 for difference in all conditions between DMPA group and control group.
Includes: cardiovascular conditions, cerebrovascular disorders, heart failure, blood transfusion, pulmonary edema, adult respiratory distress syndrome, disseminated intravascular coagulation, obstetric shock, sepsis, ventilation, and peripartum cardiomyopathy.
Venous thromboembolism occurred among 34 women (contributing 805,999 days of follow-up) in the immediate postpartum DMPA group and 3,107 women (contributing 206,180,811 days of follow-up) in the control group (Table 3). The unadjusted VTE incidence rate through 12 weeks postpartum was 0.42/10,000 women-days (95% CI 0.30–0.59) for women in the immediate postpartum DMPA group and 0.15/10,000 women-days (95% CI 0.15–0.16) for women in the control group. When adjusting for age, the incidence rate ratio for VTE was 2.87 (95% CI 2.05–4.03) among women in the immediate postpartum DMPA group compared with women in the control group. After adjusting for age and all other considered risk factors, the incidence rate ratio for VTE was 1.94 (95% CI 1.38–2.72).
Table 3.
Risk of VTE Through 12 Weeks Postpartum Among Women Who Received DMPA During Postpartum Week 1 Compared With Nonusers, 2005–2014
| Group | Total No. of Women | Total No. of VTE | Total No. of Follow-up Days | VTE Incidence Rate/10,000 Women-Days (95% CI) | Incidence Rate Ratio (95% CI), Partially Adjusted* | Incidence Rate Ratio (95% CI), Fully Adjusted† |
|---|---|---|---|---|---|---|
| Immediate postpartum DMPA group | 11,159 | 34 | 805,999 | 0.42 (0.30–0.59) | 2.87 (2.05–4.03) | 1.94 (1.38–2.72) |
| Control group | 3,102,011 | 3,107 | 206,180,811 | 0.15 (0.15–0.16) | Ref | Ref |
VTE, venous thromboembolism; DMPA, depot medroxyprogesterone acetate; Ref, reference.
Data are n, incidence rate (95% CI), or incidence rate ratio (95% CI).
Adjusted for age group (15–24, 25–29, 30–34, 35–39, and 40–44 years).
Adjusted for age group, pregestational diabetes, chronic hypertension, obesity, smoking, cesarean delivery, gestational hypertension, preeclampsia, eclampsia, multiple birth, anemia, antepartum hemorrhage, postpartum hemorrhage, postpartum infection, intrauterine fetal demise, and other severe complications.
In a subgroup analysis including women younger than 35 years without any of the considered risk factors, the VTE incidence rate was 0.15/10,000 women-days (95% CI 0.06–0.37) for women in the immediate postpartum DMPA group and 0.06/10,000 women-days (95% CI 0.05–0.06) for women in the control group. The age-adjusted incidence rate ratio of VTE for women in the immediate postpartum DMPA group compared with women in the control group was 2.82 (95% CI 1.17–6.81).
DISCUSSION
This analysis found that initiation of DMPA in the first week postpartum was associated with an increased incidence rate ratio for VTE relative to nonuse of hormonal methods. The subgroup analysis among women without VTE risk factors demonstrated similar results. Although we found an increased incidence rate ratio, it is important to note the absolute incidence rate for women in the immediate postpartum DMPA group was low (0.42/10,000 women-days), and was even lower when examining younger women without risk factors (0.15/10,000 women-days).
Our findings are similar to studies looking at VTE risk among women in the general population using DMPA (odds ratios 2.2–3.0 compared with nonhormonal users).5,6,8 Although little is known about risk of VTE with DMPA use in the immediate postpartum period, a few studies have examined VTE risk with DMPA use among women with other VTE risk factors.18 These studies have found increased odds of VTE among women with factor V Leiden mutation, diabetes, or who smoke.5–7 However, the small numbers and wide CIs in those studies limit interpretation, and it is unclear whether those results can be extrapolated to postpartum women. Little is known about VTE risk with use of other hormonal methods during the postpartum period. Although estrogen-containing methods are generally not recommended,4 progestin-only methods other than DMPA have not been found to increase VTE risk.18
The mechanisms by which DMPA could potentially affect thrombosis are not well understood. Depot medroxyprogesterone acetate has been found to have varying effects on coagulation parameters; however, clinical effects are not clear.19,20 In addition, it is not clear whether biological effects of progestins might interact with mechanisms related to postpartum thrombosis.
Although our study is strengthened by the large size of the MarketScan database, which allows for assessment of relatively rare events such as VTEs, the use of administrative data has several limitations. First, without access to drug codes in the inpatient setting, we identified DMPA initiated in the hospital using less specific diagnosis and procedure codes which may have led to misclassification of DMPA use. The frequency of DMPA initiation immediately postpartum in our study was lower than in other populations in the United States, although these studies included women who were largely minority, low income, and Medicaid users.21,22 Second, because we were unable to verify VTE diagnoses with medical records, it is likely that some misclassification of VTEs occurred; however, we do not expect misclassification would be differential between groups. We attempted to minimize misclassification by requiring anticoagulation prescriptions for VTEs diagnosed in outpatient settings. Finally, there may have been misclassification of the factors that we controlled for in the analysis, such as obesity and smoking, and we were unable to examine certain factors unavailable in this administrative dataset such as race, ethnicity, immobility, and other demographic and socioeconomic characteristics; both limitations may have led to residual confounding. In a study of hospital discharge data, the validity of diagnostic codes for certain chronic conditions such as obesity, diabetes, and hypertension, was variable; however, the validity of pregnancy-related comorbidities, such as cesarean delivery, multiple gestation, hemorrhage, infection, and preeclampsia, was high.23 The higher prevalence of most of the chronic conditions and pregnancy complications (except cesarean delivery) for women in the DMPA group indicates that this group might have been at higher risk for VTE regardless of contraceptive use. This may reflect preferential prescribing of DMPA (for example, over combined hormonal methods) for women at risk of VTE. Although we adjusted for the risk factors in the analysis, residual confounding may be present.
In conclusion, the increased relative risk of VTE with initiation of DMPA immediately postpartum observed in this analysis needs further study using additional data sources and different study populations. When counseling women about risks and benefits of postpartum contraception, it is important to discuss the low absolute risk of VTE even with use of DMPA and that the VTE risk associated with pregnancy is likely higher than any potential small risk associated with contraception. In addition, it is important to consider risks associated with short-interval pregnancies when discussing whether to initiate contraception immediately compared with delayed postpartum. Future research including comparison of risk with different hormonal contraceptive methods and risk throughout the postpartum period may help clarify this issue.
Footnotes
The findings and conclusions in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Zhu BP. Effect of interpregnancy interval on birth outcomes: findings from three recent US studies. Int J Gynaecol Obstet 2005;89(suppl 1):S25–33. [DOI] [PubMed] [Google Scholar]
- 2.Gipson JD, Koenig MA, Hindin MJ. The effects of unintended pregnancy on infant, child, and parental health: a review of the literature. Stud Fam Plann 2008;39:18–38. [DOI] [PubMed] [Google Scholar]
- 3.Bujold E, Gauthier RJ. Risk of uterine rupture associated with an interdelivery interval between 18 and 24 months. Obstet Gynecol 2010;115:1003–6. [DOI] [PubMed] [Google Scholar]
- 4.Curtis KM, Tepper NK, Jatlaoui TC, Berry-Bibee E, Horton LG, Zapata LB, et al. U.S. Medical eligibility criteria for contraceptive use, 2016. MMWR Recomm Rep 2016;65:1–103. [DOI] [PubMed] [Google Scholar]
- 5.Cardiovascular disease and use of oral and injectable progestogen-only contraceptives and combined injectable contraceptives. Results of an international, multicenter, case-control study. World Health Organization Collaborative Study of Cardiovascular Disease and Steroid Hormone Contraception. Contraception 1998;57:315–24. [PubMed] [Google Scholar]
- 6.Bergendal A, Persson I, Odeberg J, Sundström A, Holmström M, Schulman S, et al. Association of venous thromboembolism with hormonal contraception and thrombophilic genotypes. Obstet Gynecol 2014;124:600–9. [DOI] [PubMed] [Google Scholar]
- 7.O’Brien SH, Koch T, Vesely SK, Schwarz EB. Hormonal contraception and risk of thromboembolism in women with diabetes. Diabetes Care 2017;40:233–8. [DOI] [PubMed] [Google Scholar]
- 8.van Hylckama Vlieg A, Helmerhorst FM, Rosendaal FR. The risk of deep venous thrombosis associated with injectable depot-medroxyprogesterone acetate contraceptives or a levonorgestrel intrauterine device. Arterioscl Throm Vas 2010;30:2297–300. [DOI] [PubMed] [Google Scholar]
- 9.Jackson E, Curtis KM, Gaffield ME. Risk of venous thromboembolism during the postpartum period: a systematic review. Obstet Gynecol 2011;117:691–703. [DOI] [PubMed] [Google Scholar]
- 10.Tepper NK, Boulet SL, Whiteman MK, Monsour M, Marchbanks PA, Hooper WC, et al. Postpartum venous thromboembolism: incidence and risk factors. Obstet Gynecol 2014;123:987–96. [DOI] [PubMed] [Google Scholar]
- 11.Truven Health Marketscan Research Databases. Commercial claims and encounters Medicare Supplemental, data year 2014 edition. Ann Arbor (MI): Truven Health Analytics; 2015. [Google Scholar]
- 12.Tamariz L, Harkins T, Nair V. A systematic review of validated methods for identifying venous thromboembolism using administrative and claims data. Pharmacoepidemiol Drug Saf 2012;21(suppl 1):154–62. [DOI] [PubMed] [Google Scholar]
- 13.Fang MC, Fan D, Sung SH, Witt DM, Schmelzer JR, Steinhubl SR, et al. Validity of using inpatient and outpatient administrative codes to identify acute venous thromboembolism: the CVRN VTE study. Med Care 2017;55:e137–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.James AH. Pregnancy-associated thrombosis. Hematology Am Soc Hematol Educ Program 2009:277–85. [DOI] [PubMed] [Google Scholar]
- 15.Sultan AA, Tata LJ, West J, Fiaschi L, Fleming KM, Nelson-Piercy C, et al. Risk factors for first venous thromboembolism around pregnancy: a population-based cohort study from the United Kingdom. Blood 2013;121:3953–61. [DOI] [PubMed] [Google Scholar]
- 16.Sultan AA, West J, Grainge MJ, Riley RD, Tata LJ, Stephansson O, et al. Development and validation of risk prediction model for venous thromboembolism in postpartum women: multinational cohort study. BMJ 2016;355:i6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.James AH, Jamison MG, Brancazio LR, Myers ER. Venous thromboembolism during pregnancy and the postpartum period: incidence, risk factors, and mortality. Am J Obstet Gynecol 2006;194:1311–5. [DOI] [PubMed] [Google Scholar]
- 18.Tepper NK, Whiteman MK, Marchbanks PA, James AH, Curtis KM. Progestin-only contraception and thromboembolism: a systematic review. Contraception 2016;94:678–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhl H Effects of progestogens on haemostasis. Maturitas 1996;24:1–19. [DOI] [PubMed] [Google Scholar]
- 20.Melhado-Kimura V, Bizzacchi JMA, Quaino SKP, Montalvao S, Bahamondes L, Fernandes A. Effect of the injectable contraceptive depot-medroxyprogesterone acetate on coagulation parameters in new users. J Obstet Gynaecol Res 2017;43: 1054–60. [DOI] [PubMed] [Google Scholar]
- 21.Dozier AM, Nelson A, Brownell EA, Howard CR, Lawrence RA. Patterns of postpartum depot medroxyprogesterone administration among low-income mothers. J Womens Health 2014;23:224–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh RH, Rogers RG, Leeman L, Borders N, Highfill J, Espey E. Postpartum contraceptive choices among ethnically diverse women in New Mexico. Contraception 2014;89:512–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yasmeen S, Romano PS, Schembri ME, Keyzer JM, Gilbert WM. Accuracy of obstetric diagnoses and procedures in hospital discharge data. Am J Obstet Gynecol 2006;194:992–1001. [DOI] [PubMed] [Google Scholar]


