Abstract
Monocytes/macrophages (M/M) and CD4+ T cells are two important targets of human immunodeficiency virus (HIV) infection. Different strains of HIV-1 vary markedly in their abilities to infect cells belonging to the M/M lineage. Macrophagetropic (M-tropic) HIV-1 strains replicate well in primary lymphocytes as well as in primary macrophages; however, they generally infect T-cell lines poorly, if at all. Although promonocytic cell lines such as U937 have been used as in vitro models of HIV-1 infection of M/M, these cell lines are susceptible to certain T-cell-tropic (T-tropic) HIV-1 strains but are resistant to M-tropic HIV-1. In this study, we demonstrate that (i) certain U937 clones (“plus” clones), which are susceptible only to T-tropic HIV-1, become highly susceptible to M-tropic HIV-1 upon differentiation with retinoic acid (RA); (ii) other U937 clones (“minus” clones), which are resistant to both T- and M-tropic HIV-1, remain resistant to both viruses; and (iii) RA treatment induces expression of CCR5, a fusion/entry cofactor for M-tropic HIV-1 in both types of U937 clones, and yet enhances the fusogenicity of the plus clones, but not the minus clones, with M-tropic Env’s. These results indicate that the major restriction of M-tropic HIV-1 infection in promonocytic cells occurs at the fusion/entry level, that differentiation into macrophage-like phenotypes renders some of these cells highly susceptible to infection with M-tropic HIV-1, and that CD4 and CCR5 may not be the only determinants of fusion/entry of M-tropic HIV-1 in these cells.
Monocytes/macrophages (M/M) and CD4+ T cells are two important targets of human immunodeficiency virus (HIV) infection (17, 21). Different strains of HIV-1 vary markedly in their abilities to infect cells belonging to the M/M lineage (19, 31, 39). During primary infection most HIV-1 isolates are macrophagetropic (M-tropic) or dualtropic (43); however, during the course of HIV infection and especially as disease progresses, M-tropic viruses tend to become less prominent and are generally replaced by HIV-1 strains that have a broader coreceptor usage (10) and are referred to as T-cell-tropic (T-tropic) viruses (9, 37).
Promonocytic cell lines such as U937 and THP-1 have been used as in vitro models to investigate HIV-1 infection of M/M (reviewed in reference 7); however, these cells are actually resistant to M-tropic HIV-1 and susceptible to certain T-tropic HIV-1 strains, thereby differing markedly from their in vivo counterparts (38). Of note is the fact that treatment with certain differentiating agents may render these cell lines susceptible to M-tropic HIV-1 infection (20), although the mechanisms of acquisition of susceptibility remain unknown. We have previously demonstrated that certain subclones of U937 cells (“plus” clones) do but that others (“minus” clones) do not support replication of T-tropic HIV-1 (16), and that restriction of HIV-1 infection in minus clones occurs at the level of viral fusion/entry (24). In the present study, we investigate whether viral fusion/entry is also responsible for restriction of M-tropic HIV-1 infection in undifferentiated promonocytic cells and whether cells acquire susceptibility to M-tropic HIV-1 upon differentiation. We demonstrate that U937 plus clones become susceptible to M-tropic HIV-1 after treatment with retinoic acid (RA), an agent that is known to induce differentiation of promonocytic cells into cells with more mature phenotypes (32), and that RA treatment induces expression of CCR5, a major fusion/entry cofactor for M-tropic HIV-1, leading to increased fusogenicity with M-tropic Env.
(This research was conducted by M. Moriuchi in partial fulfillment of the requirements of the Ph.D. program of the Department of Microbiology at Howard University, Washington, D.C.)
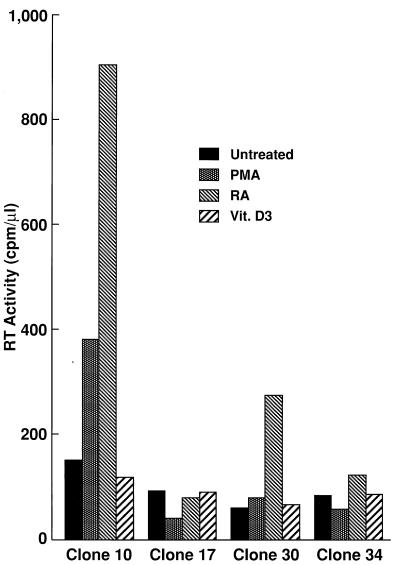
Effects of differentiating agents on the susceptibilities of U937 plus clones to M-tropic HIV-1 infection.
In order to investigate whether differentiation into cells with M/M-like phenotypes modulates susceptibility of promonocytic cells to M-tropic HIV-1 infection, U937 clones were treated with either phorbol 12-myristate 13-phosphate (PMA), all trans RA, or 1α,25-dihydrovitamin D3 (Vit.D3), all of which are known to induce differentiation of U937 cells into cells with M/M-like phenotypes (18, 32, 33), for 7 days before infection with M- or T-tropic HIV-1. All these differentiating agents induced morphological changes as well as expression of differentiation-associated cell surface markers in each U937 clone (26). As shown in Fig. 1, plus clone 10 became highly susceptible to M-tropic HIV-1 after treatment with RA and, to a lesser extent, with PMA but not with Vit.D3; plus clone 30 became susceptible to M-tropic HIV-1 only after treatment with RA. The levels of M-tropic HIV-1 replication in these clones, judged by reverse transcription (RT) activity, were comparable to that in monocyte-derived macrophages (MDM) (26). In contrast, none of these differentiating agents rendered minus clones susceptible to M-tropic HIV-1. These results suggest that differentiation of cells belonging to the M/M lineage into cells with more mature phenotypes is critical for acquisition of susceptibility to M-tropic HIV-1; however, this phenomenon is not a consequence of differentiation in general, since the effect was not seen with every differentiation agent employed.
FIG. 1.
RA-differentiated U937 plus clones become highly susceptible to M-tropic HIV-1. U937 plus clones 10 and 30 and minus clones 17 and 34 were either untreated or treated with PMA (10−8 M; Sigma Chemical Co., St. Louis, Mo.), all trans RA (10−6 M; Sigma Chemical Co.), or Vit.D3 (10−6 M; a gift of M. Uskokovic, Hoffmann-La Roche, Inc., Nutley, N.J.) for 7 days and infected with ADA8 at an approximate multiplicity of infection of 0.05. ADA8 virus stocks were propagated by transfecting 293T cells with pAD8 (a gift of T. Theodore [41]), as described previously (24). Approximately half of the volume of each cell-free supernatant was collected every four days for RT assays (25), and peak RT titers on day 16 postinfection are shown. Experiments were repeated three times with similar results.
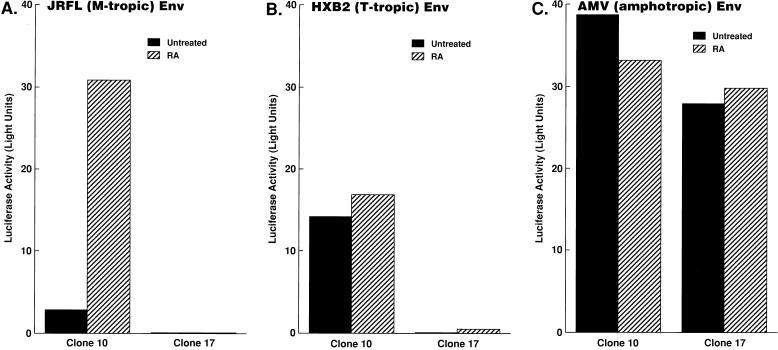
Treatment of U937 plus clones with RA renders these cells infectable and highly fusogenic with M-tropic HIV-1 Env’s.
In order to further investigate at which step(s) the replication of M-tropic HIV-1 is regulated in undifferentiated or differentiated U937 clones, representative clones were either untreated or treated with RA and infected with replication-incompetent, luciferase reporter viruses pseudotyped by Env from either M- or T-tropic HIV-1. Luciferase activity in the infected cell lysates reflects the ability of the virus to pass through early events of its replicative cycle (from binding, fusion/entry, uncoating, RT, and integration of proviral DNA into the host genome to early transcription). As expected, RA-differentiated U937 plus clone 10 efficiently supported infection with M-tropic Env-carrying virus, while undifferentiated plus clone 10 did not (Fig. 2A). Minus clone 17 did not support infection with M-tropic Env carrying virus even when it was treated with RA (Fig. 2A). As expected, plus clone 10, but not minus clone 17, exhibited a high level of luciferase activity after infection with a reporter virus carrying a T-tropic Env; RA treatment had little effect on infectivity (Fig. 2B). In contrast, a reporter virus carrying amphotropic murine leukemia virus (AMV) Env as a control was able to infect these clones at comparable levels (Fig. 2C). Since amphotropic viruses enter cells independently of cell surface receptors and coreceptors that are used by HIV-1 for entry (11), the results with the reporter virus carrying AMV Env suggest that the effects of the differentiating agent on the susceptibility of U937 clones to infection with HIV of various tropisms are mediated at the level of envelope interaction with the cell membrane.
FIG. 2.
Effects of RA treatment on susceptibility of U937 clones in single-round virus replication assays. Replication-incompetent, luciferase reporter virus NL4-3-Luc-R−E− was pseudotyped with Env from M-tropic HIV-1 JRFL (A), T-tropic HIV-1 HXB2 (B), or AMV (C), as described previously (11). The indicated U937 clones were treated as described in the legend to Fig. 1 and infected with the pseudotyped viruses, and luciferase activities in the infected cell lysates were measured. Experiments were repeated four times, and representative results are shown.
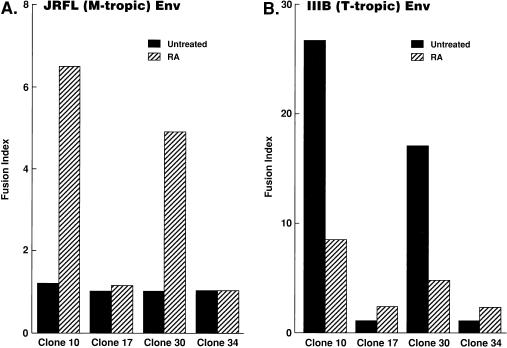
In order to investigate whether susceptibility or resistance of the U937 clones to HIV-1 is regulated at the level of viral fusion/entry, efficiency of cell-cell fusion mediated by HIV-1 Env was assayed for these clones. As described previously (24), plus clone 10 was highly fusogenic with cells expressing T-tropic HIV-1 Env (strain IIIB) but not with those expressing M-tropic HIV-1 Env (JRFL). Of note, RA treatment had dichotomous effects on M-tropic versus T-tropic HIV-mediated fusion in plus clones: RA treatment rendered the plus clones highly fusogenic with M-tropic HIV-1 Env (Fig. 3A); however, it reduced fusogenic activity with T-tropic HIV-1 Env (Fig. 3B). In contrast, fusogenic activities of minus clones 17 and 34 with either T-tropic or M-tropic Env were not markedly altered with RA treatment. These results suggest that viral fusion/entry is a critical step for acquisition of susceptibility of U937 plus clones to M-tropic HIV-1.
FIG. 3.
RA-differentiated U937 plus clones become highly fusogenic with M-tropic HIV-1 Env. The indicated U937 clones were treated as described in the legend for Fig. 1, infected with recombinant vaccinia virus vTF7-3 (expressing T7 RNA polymerase), and mixed with BSC-1 cells infected with vCB21R (encoding the lacZ gene driven by the T7 promoter) and either vCB16 (expressing the nonfusogenic mutant form of HIV-1 IIIB Env), vCB41 (expressing wild-type IIIB Env), or vCB28 (expressing HIV-1 JRFL Env), and the β-galactosidase activities in the cell lysates were measured, as described previously (15, 24, 29). Fusion index is the fold increase in optical density at 570 nm in vCB28 (A)- or vCB41 (B)-infected cell lysates relative to that in vCB16-infected cell lysates. Experiments were repeated three times with similar results.
Treatment of U937 clones with RA induces expression of CCR5, a coreceptor for M-tropic HIV-1.
In order to investigate whether cellular factors known to modulate viral fusion/entry are differentially expressed among the U937 clones and whether their expression is regulated upon differentiation of the cells, expression of CD4 (a receptor for HIV-1), CXCR4 (a fusion/entry cofactor for T-tropic HIV-1 [15]), or CCR5 (a fusion/entry cofactor for M-tropic HIV-1 [2, 5, 11–13]) was evaluated for representative plus clone 10 and minus clone 17 after stimulation with RA.
As previously reported (16, 28), both plus clone 10 (which is susceptible to T-tropic HIV-1) and minus clone 17 (which is resistant to T-tropic HIV-1) express CD4 (Table 1) as well as CXCR4 (Table 1; Fig. 4A, middle blots). RA treatment did not markedly alter CD4 expression in these clones (Table 1). In contrast, while RA treatment of clone 17 did not markedly influence CXCR4 expression, a 7-day treatment with RA of clone 10 decreased cell surface CXCR4 expression (Table 1). Furthermore, RA treatment of plus clone 10 and minus clone 17 induced expression of CCR5 mRNA (Fig. 4A, top blots) up to 3- to 10-fold (densitometrically judged by the intensity of the CCR5 mRNA bands relative to those of glyceraldehyde-3-phosphate dehydrogenase [26]). However, anti-hCCR5 monoclonal antibody (MAb) 2D7 stained only 2.0 and 3.9% of RA-differentiated clones 10 and 17, respectively, compared to less than 0.1% of either undifferentiated clone (Table 1), whereas the MAb stained 5 to 10% of primary CD4+ T cells (data not shown). These results suggest either that the level of cell surface CCR5 expression is very weak or that CCR5 expressed on the clones is conformationally different or associated with other cellular molecule(s), making the MAb inaccessible to its target epitope. In contrast to RA, other differentiating agents (Vit.D3 and PMA) had less pronounced effects on induction of CCR5 expression (Table 1).
TABLE 1.
Cell surface marker expression in U937 clonesa
| Clone | Treatment | % of staining of cells expressing:
|
||
|---|---|---|---|---|
| CCR5 | CXCR4 | CD4 | ||
| 10 | Untreated | <0.1 | 96.8 | 91.1 |
| RA | 2.0 | 65.0 | 90.8 | |
| Vit.D3 | 0.3 | 95.9 | 90.1 | |
| PMA | 0.5 | 21.9 | 90.8 | |
| 17 | Untreated | <0.1 | 89.4 | 98.7 |
| RA | 3.9 | 83.8 | 98.9 | |
| Vit.D3 | 0.9 | 97.3 | 97.7 | |
| PMA | 1.2 | 12.3 | 36.8 | |
Clones 10 (plus) and 17 (minus) were either untreated or treated with either RA, Vit.D3, or PMA for 7 days, stained with anti-hCCR5 MAb 2D7 (a gift of C. Mackay [4, 42]), anti-hCXCR4 MAb 12G5 (a gift of J. Hoxie [14]), or hCD4 MAb (Caltag Laboratories, San Francisco, Calif.), and analyzed on a CellQuest (Becton Dickinson Immunocytometry Systems, San Jose, Calif.). Background staining with isotype control was between 0.1 and 0.5% and was subtracted.
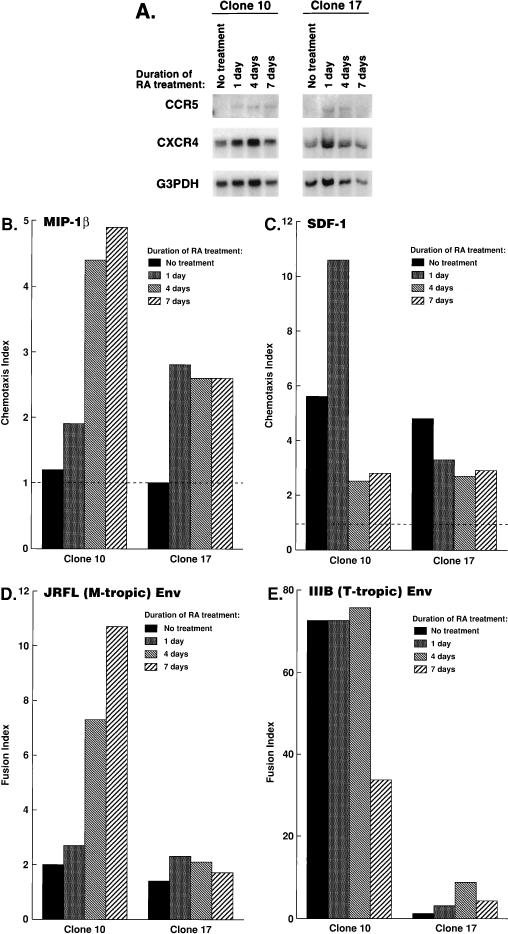
FIG. 4.
Expression and functional properties of HIV-1 coreceptors in U937 clones upon differentiation. Plus clone 10 and minus clone 17 were treated with RA for the indicated periods. The same cell samples were subjected to Northern blot analysis (A), chemotaxis assays (B and C), and fusion assays (D and E). (A) Total cellular RNAs from the indicated clones were analyzed by Northern blotting, as described previously (28). The same blot was repeatedly hybridized with probes specific to CCR5 (top), CXCR4 (middle), and GAPDH (bottom) genes, which were prepared as described previously (25). (B and C) Chemotactic responsiveness of plus clone 10 or minus clone 17 to either MIP-1β (B) or SDF-1α (C) was determined by chemotaxis assays, as described previously (24). The chemokines were purchased from R&D Systems (Minneapolis, Minn.). Chemotaxis index is the fold increase in migrated cells relative to that in the absence of chemokine. The values at optimal concentrations of the chemokine in a range between 100 ng/ml and 2.5 μg/ml are shown. (D and E) Cells were infected with vTF7-3 and mixed with BSC-1 cells infected with vCB21R and either vCB16 (nonfusogenic mutant of IIIB Env), vCB41 (IIIB Env), or vCB28 (JRFL Env), and the β-galactosidase activities in the cell lysates were measured. Fusion index was calculated as described in the legend to Fig. 3.
In order to demonstrate functional expression of CCR5 and CXCR4 in the U937 clones, their responsiveness to MIP-1β (specific to CCR5 [8, 34, 35]) or SDF-1 (specific to CXCR4 [3, 30]) was measured in chemotaxis assays. Neither plus clone 10 nor minus clone 17 migrated in response to MIP-1β when either clone was untreated; however, after differentiation with RA, both clones became responsive to MIP-1β (Fig. 4B), in agreement with CCR5 mRNA expression after RA treatment (Fig. 4A, top blots). In contrast, responsiveness of clone 10 to SDF-1 was modestly decreased with RA treatment (Fig. 4C), in agreement with decreased levels of cell surface CXCR4 expression after RA treatment (Table 1), while the chemotactic responsiveness of clone 17 to SDF-1 was not markedly changed with RA treatment (Fig. 4C). Although responsiveness to SDF-1 of clone 10 was modestly increased shortly after RA treatment in some experiments (Fig. 4C, day 1), that increase was not consistently reproducible (data not shown).
In order to demonstrate whether the kinetics of CCR5 expression correlate well with fusogenicity of the cells with Env’s from M-tropic HIV-1, fusion assays were performed with the same samples used for Northern blot analysis and flow cytometry analysis described above. While expression of CCR5 mRNA was clearly induced in clone 10 as early as day 1 after stimulation (Fig. 4A, top blots), fusogenic activity with Env from M-tropic HIV-1 JRFL was gradually increased over the 7 days of the experimental period (Fig. 4D). Therefore, levels of CCR5 expression may not be the only determinant of fusogenic activity of the clone with an M-tropic HIV-1 Env. Fusogenic activity of clone 17 with an M-tropic HIV-1 Env was not beyond the background activity throughout the experimental period, despite the fact that CCR5 expression was also induced in this clone (Fig. 4A). In contrast, 7 days of RA treatment of clone 10 reduced its fusogenic activity with T-tropic Env (Fig. 4E), in agreement with reduced expression of CXCR4 after the 7-day treatment with RA (Table 1).
Thus, fusogenicity of clone 10 with either M-tropic or T-tropic Env correlates with the level of expression of CCR5 or CXCR4, respectively; however, induction of CCR5 or the presence of CXCR4 did not confer fusogenicity to clone 17 with M-tropic or T-tropic Env, respectively, suggesting that cell-type specific modification of HIV coreceptors or association with another cellular molecule(s) may affect coreceptor functions.
M-tropic HIV-1 infection in RA-differentiated U937 plus clones is inhibited by ligands for CCR5.
In order to demonstrate whether CCR5 serves as the major coreceptor for M-tropic HIV-1 in RA-differentiated U937 plus clones, we infected plus clone 10 with M-tropic HIV-1 in the presence or absence of ligands for CCR5.
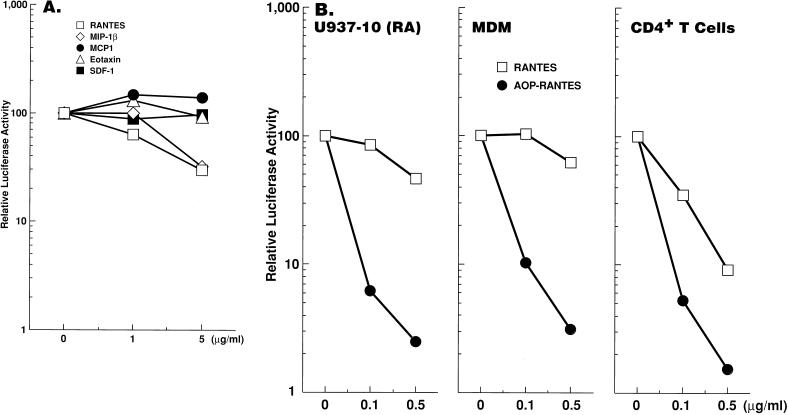
In single-round virus replication assays, RANTES and MIP-1β, natural ligands for CCR5, reduced infectivity of M-tropic Env-carrying virus (70% reduction at 5 μg/ml) while pretreatment of the RA-differentiated clone 10 with MCP-1, eotaxin, or SDF-1, which are not ligands for CCR5, did not suppress infection with the virus (Fig. 5A), suggesting that CCR5 plays a critical role in M-tropic HIV-1 infection in the differentiated plus clone. Modest (up to 50 to 60%) increases of HIV-1 infectivity in the presence of MCP-1 were observed in several experiments; however, those increases were not consistently reproducible (26). Of note is the fact that antiviral effects of RANTES or MIP-1β (6) were more pronounced in primary CD4+ T cells than in the plus clone or primary macrophages, while AOP-RANTES, a modified chemokine which has potent anti-HIV activity in both T cells and macrophages (40), efficiently suppressed M-tropic HIV-1 infection in all cell types tested (Fig. 5B; data not shown). Limited sensitivity of the U937 clone to antiviral activity of the natural chemokines is probably analogous to that of primary macrophages (11, 25, 36, 40).
FIG. 5.
Ligands for CCR5 inhibit M-tropic HIV-1 infection of RA-differentiated clone 10. Clone 10 differentiated with RA for 7 days, MDM, and primary CD4+ T cells were either untreated or treated with the indicated chemokine at the indicated concentrations for 1 h before and throughout infection with NL4-3-Luc-R−E− virus pseudotyped by M-tropic HIV-1 JRFL Env. AOP-RANTES was kindly provided by R. Offord (40), and other chemokines were purchased from R&D Systems. Results were representative of four (A) or three (B) independent experiments.
In this study, we have demonstrated that in undifferentiated promonocytic U937 cells, M-tropic HIV-1 infection is blocked at the level of viral fusion/entry and that differentiation into cells with more mature phenotypes renders certain U937 clones (plus clones) susceptible to M-tropic HIV-1 infection, probably by regulating expression of a cellular factor(s) that modulates viral fusion/entry. Induction of fusogenic activity with M-tropic HIV-1 Env was also reported for another monocytic cell line, THP-1, which was treated with RA (1). Northern blot analysis clearly demonstrated induction of mRNA for CCR5 (a fusion/entry cofactor for M-tropic HIV-1) after RA differentiation, and we have recently demonstrated that RA induction of CCR5 expression in U937 cells occurs at the promoter level (26, 27); the functional expression of CCR5 was confirmed by chemotaxis assays. Receptor competition studies using MIP-1β, RANTES, and AOP-RANTES (ligands for CCR5) also indicated that CCR5 is critical for M-tropic HIV-1 entry into these cells. The difficulty in detecting CCR5 expression by flow cytometry may reflect very low levels of expression on the cell surface or may suggest that CCR5 that is expressed on the clone (and possibly on M/M) is modified or associated with another cellular molecule(s), resulting in relatively poor accessibility of the MAb (2D7) to its epitope.
In contrast to U937 plus clones, induction of CCR5 expression in other U937 clones (minus clones) did not confer susceptibility to M-tropic HIV-1 infection. These cells are also resistant to T-tropic HIV-1 infection despite the fact that they express CD4 and CXCR4 (24) (Table 1). The resistance to HIV-1 of minus clones occurs, at least in part, at the level of fusion/entry (24) (Fig. 4). However, since these cells efficiently allowed entry of virus carrying amphotropic Env (Fig. 3C), an intrinsic defect in the ability to fuse in general does not seem to be responsible for their resistance to HIV-1. Possible explanations for a defect in HIV entry despite adequate receptor and coreceptor expression include the possibility that cell-type-specific modification and/or association of HIV coreceptors with other cell surface molecule(s) may alter their coreceptor function.
Differentiation of M/M lineage cells may have dichotomous effects on fusogenicity of cells with either M-tropic or T-tropic Env. Upon differentiation with RA, cell surface CXCR4 expression in plus clone 10 was downregulated, resulting in less fusogenic activity with T-tropic Env, whereas the same treatment induced CCR5 expression and conferred fusogenic activity with M-tropic Env. Downregulation of cell surface CXCR4 expression upon differentiation has also been reported for MDM (22). We are currently investigating what cellular events that occur during monocytic differentiation are critical for the regulation of expression of HIV-1 fusion/entry cofactors and other cellular factors involved in viral fusion/entry.
Cells of the M/M lineage are known to vary in their susceptibilities to HIV-1 infection. Macrophages in skin, lung, lymph nodes, or brain harbor HIV-1, while HIV-1 is rarely detected in Kupfer cells in the liver (23). Various factors (i.e., growth factors, cytokines, or extracellular matrix) at the sites of terminal differentiation may differentially influence phenotypic features of cells belonging to the M/M system in vivo, and the in vitro heterogeneity observed in the present study among the U937 subclones may partially reflect such heterogeneity in their in vivo counterparts. Our in vitro U937 subclone model may provide a useful experimental system to investigate which cellular factor(s) is critical for HIV-1 infection of cells of the M/M system and how expression of this factor(s) is regulated upon differentiation of cells into more mature phenotypes.
Acknowledgments
H.M. and M.M. contributed equally to this project.
We thank G. Franzoso, U. Siebenlist, T. Theodore, N. Landau, E. Berger, J. Hoxie, C. Mackay, R. Offord, and K. Uskokovic for providing materials; J. Weddle for graphic work; and P. Walsh for editorial assistance.
REFERENCES
- 1.Alkhatib G, Broder C C, Berger E A. Cell type-specific fusion cofactors determine human immunodeficiency virus type 1 tropism for T-cell lines versus primary macrophages. J Virol. 1996;70:5487–5494. doi: 10.1128/jvi.70.8.5487-5494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alkhatib G, Combadiere C, Broder C C, Feng Y, Kennedy P E, Murphy P M, Berger E A. CC CKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. doi: 10.1126/science.272.5270.1955. [DOI] [PubMed] [Google Scholar]
- 3.Bleul C C, Farzan M, Choe H, Parolin C, Clark-Lewis I, Sodroski J, Springer T A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature. 1996;382:829–833. doi: 10.1038/382829a0. [DOI] [PubMed] [Google Scholar]
- 4.Bleul C C, Wu L, Hoxie J A, Springer T A, Mackay C R. The HIV coreceptors CXCR4 and CCR5 are differentially expressed and regulated on human T lymphocytes. Proc Natl Acad Sci USA. 1997;94:1925–1930. doi: 10.1073/pnas.94.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choe H, Farzan M, Sun Y, Sullivan N, Rollins B, Ponath P D, Wu L, Mackay C R, LaRosa G, Newman W, Gerard N, Gerard C, Sodroski J. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell. 1996;85:1135–1148. doi: 10.1016/s0092-8674(00)81313-6. [DOI] [PubMed] [Google Scholar]
- 6.Cocchi F, DeVico A L, Garzino-Demo A, Arya S K, Gallo R C, Lusso P. Identification of RANTES, MIP-1α, and MIP-1β as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]
- 7.Collman R. Human immunodeficiency virus type 1 tropism for human macrophages. Pathobiology. 1992;60:213–218. doi: 10.1159/000163725. [DOI] [PubMed] [Google Scholar]
- 8.Combadiere C, Ahuja S K, Tiffany H L, Murphy P M. Cloning and functional expression of CC CKR5, human monocyte CC chemokine receptor selective for MIP-1α, MIP-1β, and RANTES. J Leukocyte Biol. 1996;60:147–152. doi: 10.1002/jlb.60.1.147. [DOI] [PubMed] [Google Scholar]
- 9.Connor R I, Ho D D. Human immunodeficiency virus type 1 variants with increased replicative capacity develop during the asymptomatic stage before disease progression. J Virol. 1994;68:4400–4408. doi: 10.1128/jvi.68.7.4400-4408.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor R I, Sheridan K E, Ceradini D, Choe S, Landau N R. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J Exp Med. 1997;185:621–628. doi: 10.1084/jem.185.4.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng D, Liu R, Ellmeier W, Choe S, Unutmaz D, Burkhart M, DiMarzio P, Marmon S, Sutton R E, Hill C M, Davis C B, Peiper S C, Schall T J, Littman D R, Landau N R. Identification of a major co-receptor for primary isolates of HIV-1. Nature. 1996;381:661–666. doi: 10.1038/381661a0. [DOI] [PubMed] [Google Scholar]
- 12.Doranz B J, Rucker J, Yi Y, Smyth R J, Samson M, Peiper S C, Parmentier M, Collman R G, Dom R W. A dual-tropic primary HIV-1 isolate that uses Fusin and the β-chemokine receptors CKR-5, CKR-3, and CKR-2b as fusion cofactors. Cell. 1996;85:1149–1158. doi: 10.1016/s0092-8674(00)81314-8. [DOI] [PubMed] [Google Scholar]
- 13.Dragic T, Litwin V, Allaway G P, Martin S R, Huang Y, Nagashima K A, Cayanan C, Maddon P J, Koup R A, Moore J P, Paxton W A. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature. 1996;381:667–673. doi: 10.1038/381667a0. [DOI] [PubMed] [Google Scholar]
- 14.Endres M J, Clapham P R, Marsh M, Ahuja M, Davis Turner J, McKnight A, Thomas J F, Stoebenau-Haggarty B, Choe S, Vance P J, Wells T N C, Power C A, Sutterwala S S, Doms R W, Lindau N R, Hoxie J A. CD4-independent infection by HIV-2 is mediated by fusin/CXCR4. Cell. 1996;87:745–756. doi: 10.1016/s0092-8674(00)81393-8. [DOI] [PubMed] [Google Scholar]
- 15.Feng Y, Broder C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 16.Franzoso G, Biswas P, Poli G, Carlson L M, Brown K D, Tomita-Yamaguchi M, Fauci A S, Siebenlist U. A family of serine proteases expressed exclusively in myelo-monocytic cells specifically processes the nuclear factor-κB subunit p65 in vitro and may impair human immunodeficiency virus replication in these cells. J Exp Med. 1994;180:1445–1456. doi: 10.1084/jem.180.4.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gartner S, Markovitz P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 18.Goletti D, Kinter A L, Biswas P, Bende S M, Poli G, Fauci A S. Effect of cellular differentiation on cytokine-induced expression of human immunodeficiency virus in chronically infected promonocytic cells: dissociation of cellular differentiation and viral expression. J Virol. 1995;69:2540–2546. doi: 10.1128/jvi.69.4.2540-2546.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang S S, Boyle T J, Lyerly H K, Cullen B R. Identification of the envelope V3 loop as the primary determinant of cell tropism in HIV-1. Science. 1991;253:71–74. doi: 10.1126/science.1905842. [DOI] [PubMed] [Google Scholar]
- 20.Kitano K, Baldwin G C, Raines M A, Golde D W. Differentiating agents facilitate infection of myeloid leukemic cell lines by monocytotropic HIV-1 strains. Blood. 1990;10:1980–1988. [PubMed] [Google Scholar]
- 21.Koenig S, Gendelman J M, Orenstein J M, Dal Canto M C, Pezeshkpour G D, Yungbluth M, Janotta F, Akasamit A, Martin M A, Fauci A S. Detection of AIDS virus in macrophages in brain tissues from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 22.McKnight A, Wilkinson D, Simmons G, Talbot S, Picard L, Ahuja M, Marsh M, Hoxie J A, Clapham P R. Inhibition of human immunodeficiency virus fusion by a monoclonal antibody to a coreceptor (CXCR4) is both cell type and virus strain dependent. J Virol. 1997;71:1692–1696. doi: 10.1128/jvi.71.2.1692-1696.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meltzer M S, Hoover D L, Finbloom D S, Turpin J A, Kalter D C, Friedman R M, Moyer M P, Naya P, Gendelman H E. Mononuclear phagocytes in the pathogenesis of human immunodeficiency virus disease. In: Lopez-Berestein G, Klostergaard J, editors. Mononuclear phagocytes in cell biology. Boca Raton, Fla: CRC Press, Inc.; 1992. pp. 147–175. [Google Scholar]
- 24.Moriuchi H, Moriuchi M, Arthos J, Hoxie J, Fauci A S. Promonocytic U937 subclones expressing CD4 and CXCR4 are resistant to infection with and cell-to-cell fusion by T-cell-tropic human immunodeficiency virus type 1. J Virol. 1997;71:9664–9671. doi: 10.1128/jvi.71.12.9664-9671.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moriuchi H, Moriuchi M, Combadiere C, Murphy P M, Fauci A S. CD8+ T cell-derived soluble factor(s), but not β-chemokines RANTES, MIP-1α, and MIP-1β, suppress HIV-1 replication in monocyte/macrophages. Proc Natl Acad Sci USA. 1996;93:15341–15435. doi: 10.1073/pnas.93.26.15341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moriuchi, H., M. Moriuchi, and A. S. Fauci. Unpublished data.
- 27.Moriuchi H, Moriuchi M, Fauci A S. Cloning and analysis of the promoter region of CCR5, a coreceptor for HIV-1 entry. J Immunol. 1997;159:5441–5449. [PubMed] [Google Scholar]
- 28.Moriuchi H, Moriuchi M, Smith H A, Straus S E, Cohen J I. Varicella-zoster virus open reading frame 61 protein is functionally homologous to herpes simplex virus type 1 ICP0. J Virol. 1992;66:7303–7308. doi: 10.1128/jvi.66.12.7303-7308.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nussbaum O, Broder C C, Berger E A. Fusogenic mechanisms of enveloped-virus glycoproteins analyzed by a novel recombinant vaccinia virus-based assay quantitating cell fusion-dependent reporter gene activation. J Virol. 1994;68:5411–5422. doi: 10.1128/jvi.68.9.5411-5422.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J-L, Arenzana-Seisdedos F, Schwartz O, Heard J-M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien W, Koyanagi Y, Namazie A, Zho J-Q, Diagne A, Idler K, Zack J, Chen I S Y. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature. 1990;348:69–73. doi: 10.1038/348069a0. [DOI] [PubMed] [Google Scholar]
- 32.Olsson I L, Breitman T R. Induction of differentiation of the human histocytic lymphoma cell line U-937 by retinoic acid and cyclic adenosine 3′:5′-monophosphatate-inducing agents. Cancer Res. 1983;42:3924–3927. [PubMed] [Google Scholar]
- 33.Olsson I L, Gullberg U, Ivhed I, Nilsson K. Induction of differentiation of human histiocytic lymphoma cell line U-937 by 1α,25-dihydroxychelocalciferol. Cancer Res. 1982;43:5862–5867. [PubMed] [Google Scholar]
- 34.Raport C J, Gosling J, Schweickart V L, Gray P W, Charo I F. Molecular cloning and functional characterization of a novel human CC chemokine receptor (CCR5) for RANTES, MIP-1α, and MIP-1β. J Biol Chem. 1996;271:17161–17166. doi: 10.1074/jbc.271.29.17161. [DOI] [PubMed] [Google Scholar]
- 35.Samson M, Labbe O, Mollereau C, Vassart G, Parmentier M. Molecular cloning and functional expression of a new human CC-chemokine receptor gene. Biochemistry. 1996;35:3362–3367. doi: 10.1021/bi952950g. [DOI] [PubMed] [Google Scholar]
- 36.Schmidtmayerova H, Sherry B, Bukrinsky M. Chemokines and HIV replication. Nature. 1996;382:767. doi: 10.1038/382767a0. [DOI] [PubMed] [Google Scholar]
- 37.Schuitemaker H, Koot M, Kootsra N A, Dercksen M W, de Goede R E, van Steenwijk R P, Lange J M, Schattenkerk J K, Miedema F. Biological phenotype of human immunodeficiency virus type 1 clones at different stages of infection: progression of disease is associated with a shift from monocytotropic to T-cell-tropic virus population. J Virol. 1992;66:1354–1360. doi: 10.1128/jvi.66.3.1354-1360.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schuitemaker H, Koot M, Groenink M, de Goede R E, Miedema F, Tersmette M. Differential tropism of clinical HIV-1 isolates for primary monocytes and promonocytic cell lines. AIDS Res Hum Retroviruses. 1992;8:1679–1682. doi: 10.1089/aid.1992.8.1679. [DOI] [PubMed] [Google Scholar]
- 39.Shioda T, Levy J A, Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991;349:167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- 40.Simmons G, Clapham P R, Picard L, Offord R E, Rosenkilde M M, Schwarts T W, Buser R, Wells T N C, Proudfoot A E I. Potent inhibition of HIV-1 infectivity in macrophages and lymphocytes by a novel CCR5 antagonist. Science. 1997;276:276–279. doi: 10.1126/science.276.5310.276. [DOI] [PubMed] [Google Scholar]
- 41.Theodore T S, Englund G, Buckler-White A, Buckler C E, Martin M A, Peden K W. Construction and characterization of a stable full-length macrophage-tropic HIV type 1 molecular clone that directs the production of high titers of progeny virions. AIDS Res Hum Retroviruses. 1996;12:191–194. doi: 10.1089/aid.1996.12.191. [DOI] [PubMed] [Google Scholar]
- 42.Wu L, Paxton W A, Kassam N, Ruffing N, Rottman J B, Sullivan N, Choe H, Sodroski J, Newman W, Koup R A, Mackay C R. CCR5 levels and expression pattern correlate with infectability by macrophage-tropic HIV-1, in vitro. J Exp Med. 1997;185:1681–1691. doi: 10.1084/jem.185.9.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu T, Mo H, Wang N, Nam D S, Cao Y, Koup R A, Ho D D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993;261:1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]