Abstract
The trigeminal sensory nerve fiber branches supply afferent information from the skin and mucous membranes of the face and head and the oral cavity regarding information on temperature, touch, and pain. Under normal conditions, the trigeminal nerve serves to provide important information from nerve fibers and tissues using specialized receptors sensitive for irritant and painful stimuli. The current scientific consensus indicates that nerve endings responsible for chemical and thermal sensitivity of the skin and mucous membranes are the same nerves responsible for nociception. This “chemesthetic sense” allows many vertebrates to detect chemical agonists that induce sensations such as touch, burning, stinging, tingling, or changes in temperature. Research has been under way for many years to determine how exposure of the oral and/or nasal cavity to compounds that elicit pungent or irritant sensations can produce these sensations. In addition, these chemicals can alter other sensory information such as taste and smell to affect the flavor of foods and beverages. We now know that these ‘chemesthetic molecules’ are agonists of molecular receptors, which exist on primary afferent nerve fibers that innervate the orofacial area. However, under pathophysiologic conditions, over- or underexpression or activity of these receptors may lead to painful orotrigeminal syndromes. Some of these individual receptors are discussed in detail, including transient receptor potential channels and acid sensing ion channels, among others.
ANATOMY AND PHYSIOLOGY
The trigeminal nerves, stemming from the fifth and largest cranial nerve, form a very complex system of sensory and motor nerves that supply ascending and descending information from the central nervous system. The sensory nerve fiber branches, namely the mandibular, maxillary, and ophthalmic branches, supply afferent information from the skin, sinuses, mucous membranes, and oral cavity regarding information on temperature, touch, and pain. Each of the three trigeminal nerve branches cover roughly one-third of the craniofacial dermatome. The oral cavity is innervated by the maxillary and mandibular branches of the trigeminal nerve (Fig. 13.1). The maxillary branch receives input from the upper lip and teeth, and the hard and soft palate. The lower portion of the oral cavity, including the tongue, lower lip, teeth, and jaw, and many of the mucous membranes, send inputs via the mandibular branch. The mandibular branch also supplies the only motor output of the trigeminal nerves, responsible for jaw movements needed during mastication (e.g., pterygoid, masseter, and temporalis muscles) and swallowing (e.g., anterior belly of digastric and mylohyoid; van Eijden and Langenbach, 2017). The cell bodies of the trigeminal nerve branches are located in the trigeminal ganglia, on either side of the head near the temporal bones. The trigeminal nerve root provides a direct connection into the brainstem, where touch and pain information synapse into second order neurons in the main trigeminal nucleus and the spinal trigeminal nucleus, respectively (Fig. 13.2). Touch and pain information are both relayed via the trigeminothalamic tract (i.e., the trigeminal lemniscus) into the thalamus; however, pain information is also relayed by the anterior trigeminothalamic tract (Prasad and Galetta, 2007). A large portion of the touch and pain information eventually innervate the ventral posteromedial nucleus of the thalamus and finally culminate in the primary somatosensory cortex. In general, there is a medial to lateral representation of the eyes, nose, lips, tongue, and intraoral cavity in the somatosensory cortex (Penfield and Boldrey, 1937; McCarthy et al., 1993).
Fig. 13.1.
Nerves that innervate the orofacial region. Trigeminal branches including the maxillary nerve (V2) and the mandibular nerve (V3) contain cell bodies in the trigeminal ganglion located on either side of the head. The maxillary nerve (green) innervates the areas just below the eyes and the upper mouth. The mandibular nerve (blue) has both sensory and motor components. The sensory portion innervates the lower teeth, temporomandibular joint, the anterior two- thirds of the tongue, and the areas below the maxillary nerve. Portions of the facial nerve (7th cranial nerve, yellow) provides taste information from the anterior two-thirds of the tongue (i.e., chorda tympani) and also provides muscles control of facial expression.
Fig. 13.2.
Regions and nuclei of the brainstem involved in orotrigeminal and taste sensitivity. Brainstem regions from anterior to posterior: thalamus, midbrain, pons, and medulla oblongata (i.e., medulla). There are four trigeminal nerve nuclei that receive inputs from trigeminal primary afferent nerves (Fig. 13.1) and consist of three sensory and one motor nuclei. The trigeminal sensory nuclei receive orofacial sensory inputs and project to higher brain centers including the thalamus and include the mesencephalic nucleus (proprioception), principal/main nucleus (mechanoreception), and spinal nucleus (thermosensation and nociception). The trigeminal motor nucleus provides control for mastication. The nucleus of the solitary tract (NTS) receives input from several cranial nerves including those involved in the taste information, especially the chorda tympani (via the facial nerve). Nuclei are shown only on one-half of the brainstem for clarity.
ORIGINS OF CHEMESTHESIS
The senses of taste and smell provide us with a way to assess molecules in our environment and distinguish suitable from potentially dangerous foodstuffs. The basic design consists of specialized sensory cells, which can detect molecules that are smelled or tasted in the nose or mouth (Kandel et al., 2013). Because of their evolutionary importance, these systems are highly conserved across vertebrate species. In addition to taste and smell, there is also a “common chemical sense” (Parker, 1912) that allows vertebrate and invertebrate animals to detect chemical agonists that induce sensations such as changes in temperature, touch, burning, stinging, or tingling. It has been presumed for several decades that the nerve endings responsible for chemical and thermal sensitivity of the skin and mucous membranes were the same nerves responsible for nociception (Jones, 1954). In fact, the sensory system responsible for detecting chemical irritants is a part of the general somatic sensory system. This sensibility to chemical compounds is now called “chemesthesis” as it was coined several years ago and is believed to be important for detection of volatile compounds that could be damaging to an organism (Green, 2012). In particular, the oral cavity is sensitive to many chemical compounds found in food and spices, such as black pepper, chili peppers, mint, wasabi, mustard oil, carbonated and alcoholic beverages, and acidity from citrus fruits. Chemical exposure of the oral and even nasal cavity to these compounds elicits sensations described in terms of pungency, burning, freshness, tingling, sharpness, warmth, and cooling (Cliff and Heymann, 1992; Green, 2007; Ward, 2016). Research has been under way for many years to determine how different chemesthetic stimuli elicit different sensations. We now know that these “chemesthetic molecules” are agonists of molecular receptors that exist on primary afferent nerve fibers that innervate the mouth, lips, tooth pulp, etc. Some of these individual receptors are discussed in detail below.
TRP CHANNELS
Transient receptor potential (TRP) channels are tetramers that each have six transmembrane domains. These channels are distantly related, and not surprisingly, structurally similar to voltage gated sodium and calcium channels. Unlike their voltage gated cousins, TRP channels are nonselective cation channels (e.g., sodium and calcium permeability). TRP channels are expressed on trigeminal nerve terminals in addition to many tissue types innervated by trigeminal nerves (e.g., keratinocytes, tooth pulp, epithelial cells). TRP channels are also expressed in taste receptor cells and are involved in gustation (Roper and Chaudhari, 2017), but this chapter focuses on the subtypes involved in thermal sensitivity and chemesthesis.
The first pivotal studies that implicated TRPV1 in pain and heat transduction were published almost 20 years ago (Caterina et al., 1997; Davis et al., 2000). TRPV1, known as the “capsaicin receptor,” is involved in much broader chemical responses to irritants including piperine, acids, endogenous cannabinoids, such as anandamide, and allyl isothiocyante found in mustard oil (Tominaga et al., 1998; Jordt et al., 2000; McNamara et al., 2005; Iwasaki et al., 2006; Ohta et al., 2007; Saunders et al., 2013). TRPV1 is expressed in the trigeminal ganglia and the spinal trigeminal nucleus and coexpressed with other neuropeptides implicated in pain transduction, including calcitonin gene-related peptide and substance P (SP) (Quartu et al., 2016). Capsaicin elicits a sensation of burning on the oral cavity, which sensitizes (i.e., increases intensity) when applied at short interstimulus intervals and desensitizes when the interstimulus interval increases beyond a few minutes (Green, 1986b, 1989, 1991b, 1996; Karrer and Bartoshuk, 1991; Cliff and Green, 1996). Capsaicin irritation is also known to cross-desensitize other trigeminal irritants, namely mustard oil, nicotine, sodium chloride, and eugenol among others, when applied first (Green, 1991a; Dessirier et al., 2000a,b, 2001a,b; Simons et al., 2003b; Klein et al., 2013). TRPV1 is thermally gated, as the channel’s opening probably greatly increases beyond a temperature of 43°C (Caterina et al., 1997). Not unexpectedly, lingual application of capsaicin on the tongue and oral cavity intensifies sensations of innocuous and noxious heat (Albin et al., 2008; Naganawa et al., 2015). Like many TRP channels, TRPV1 is sensitized by many inflammatory mediators, cytokines, and kinases (e.g., bradykinin, protein kinase A, prostaglandins). Increased TRPV1 expression and activity have been found in cases of oral disease, inflammation, or trigeminal nerve injury (Park et al., 2006; Yilmaz et al., 2007). Paradoxically, repeated stimulation of TRPV1 can cause desensitization of the channel, which is important when we consider the crucial role of TRPV1 (and other receptors, mentioned in the following text) in the physiologic perception of and reaction to the external environment. The repeated application of capsaicin also causes nerve fiber desensitization, and decreased sensitivity of noxious stimuli that follow (Koplas et al., 1997). Topical application of capsaicin and other analogues have become a common pharmaceutical strategy in over-the-counter balms and creams to relieve pain. High doses of capsaicin are known to cause strong calcium influx in neurons, inducing signaling cascades that lead to ablation of skin afferent terminals. This has become a useful strategy for the treatment of neuropathies that require several weeks or months of pain relief (see “Oral Pathophysiologies”). It is clear that TRPV1 expressing nociceptors contribute to pain and irritation in patients with trigeminal neuralgias, and future work is in progress to move TRPV1 targeting pharmaceuticals into the clinic.
TRPM8 was cloned only a few years after TRPV1, and its discovery was also pivotal for the neurophysiology community and our current understanding of cold transduction in mammals. TRPM8 is gated by temperatures below 26°C, and animals lacking TRPM8 have decreased sensitivity to cold temperatures (McKemy et al., 2002; Peier et al., 2002a; Bautista et al., 2007). Chemical agonists of TRPM8 include many naturally occurring products including menthol, eucalyptol and icilin, linalool, geraniol, and compounds commonly used in the food and cosmetics industry such as WS-3, Coolact P®, Cooling Agent 10, and PMD38 (Behrendt et al., 2004). In vitro studies suggest that when menthol or other TRPM8 agonists are applied, the threshold for TRPM8 activation shifts to a warmer temperature, which suggests that chemical agonists can potentiate the endogenous mechanism for thermal activation of TRPM8 (McKemy, 2007). High doses of TRPM8 agonists can elicit irritation in the oral cavity (e.g., burning, aching, and prickling), which desensitizes after repeated administration (Cliff and Green, 1994; Gwartney and Heymann, 1995; Cliff and Green, 1996). Similar to capsaicin’s activation of TRPV1, TRPM8 activation by chemical agonists can also cross-desensitize other oral irritants including cinnamaldehyde, capsaicin, and nicotine (Green and McAuliffe, 2000; Dessirier et al., 2001a; Zanotto et al., 2008; Klein et al., 2011), but not carbonation (Green, 1992; Wise and Bryant, 2014). TRPM8 agonists are used in oral products as analgesics because they reduce heat sensitivity and elevate heat pain thresholds (Green, 1986a, 2005) and provide an overall soothing cooling sensation. In the clinic, controlled hypothermia and cooling are widely used to suppress tissue damage and inflammation resulting from trauma or injury. It appears that cooling, and consequently TRPM8 activation, can attenuate tissue inflammation (Ramachandran et al., 2013). These data indicate that activation of nerve fibers expressing TRPM8 is a viable option to relieve trigeminal pain and irritation, especially under inflammatory conditions.
Similar to a number of TRP channels that have been implicated in temperature and irritant sensations, TRPA1 is implicated in noxious cold transduction (Story et al., 2003) as well as activation induced by pungent compounds found in mustard oil and wasabi (e.g., allyl isothiocynate), cinnamon (e.g., cinnamaldehyde), extra virgin olive oil (e.g., oleocanthal), garlic (e.g., allicin), and other environmental irritants (Bandell et al., 2004; Jordt et al., 2004; Bautista et al., 2005; Macpherson et al., 2007; Peyrot des Gachons et al., 2011). Agonists of TRPA1 induce irritation when applied orally or nasally, which is both self- and cross-desensitizing with other trigeminal irritants (Brand and Jacquot, 2002; Simons et al., 2003b; Klein et al., 2011). Application of mustard oil in particular to the skin induces intense burning, erythema, edema, and plasma extravasation, in addition to heat hyperalgesia and mechanical allodynia (Koltzenburg et al., 1992; Simons et al., 2003b; Albin et al., 2008). TRPA1 and TRPV1 have been shown to be coexpressed in some of the same sensory neurons, which is a common explanation behind the somewhat contradictory sensation of “burning” cold at very low temperatures (Kobayashi et al., 2005; Malin et al., 2011). Although TRPA1 has been implicated in cold transduction, application of cinnamaldehyde or mustard oil on the tongue does not enhance cold thresholds or cold pain sensitivity (Albin et al., 2008). It is interesting that TRPV1, TRPM8, and TRPA1 agonists can modulate each other’s effects on nociceptive perception and behaviors; hence it is easy to postulate that these agonists could be useful alone, or in combination, to modulate certain types of pain in humans. (Anderson et al., 2014).
TRPV3 and TRPV4 have also been implicated in heat transduction, although at much lower thresholds levels than TRPV1. TRPV3 and TRPV4 are highly expressed in keratinocytes, which presumably signal to primary afferent fibers through an ATP-signaling mechanism (Kida et al., 2012). TRPV3 is gated by temperatures >31°C and monoterpenoid chemical agonists from cloves (e.g., eugenol), thyme (e.g., thymol), and oregano (e.g., carvacrol) (Peier et al., 2002b; Smith et al., 2002; Xu et al., 2002). Eugenol and carvacrol both elicit sensations of warming and burning sensation on the tongue, and can increase sensitivity to heat and heat pain (Klein et al., 2013, 2014, 2015). TRPV4 is also activated by warm temperatures and changes in osmolarity (Mizuno et al., 2003), as are natural compounds such as bisandrographolide A from the South Indian plant Andrographis paniculata (Smith et al., 2006), apigenin from chamomile flowers (Ma et al., 2012b), 5,6-epoxyeicosatrienoic acid from the metabolism of arachidonic acids, and the endocannabinoid anandamide (Watanabe et al., 2003). Synthetic agonists such as phorbal esters and the commonly used preclinical inflammatory agent formalin are also agonists of TRPV4 (Klausen et al., 2009). TRPV4 is co-localized with TRPV3 and TRPV1 in human sensory nerve fibers and also appears to change expression after peripheral nerve injury (Facer et al., 2007), signifying that these channels are implicated in changes in pain sensitivity after damage or disease. Another related TRP channel in the vanilloid family, TRPV2, was originally believed to be an endogenous sensor of extreme heat (>52°C; Caterina et al., 1999), but this idea has since been questioned. Interestingly, TRPV2 agonists include Δ9-tetrahydrocannabinol and cannabidiols, which are found in the genus of the flowering plant, cannabis (Qin et al., 2008). Cannabinoids are also known to activate and desensitize TRPV1, TRPV3, and TRPV4 channels (De Petrocellis et al., 2012). This could partially explain the therapeutic and potential clinical validity of cannabinoids in trigeminal neuralgias (Liang et al., 2004; McDonough et al., 2014). To date, there are no clinically available pharmaceuticals targeting TRPV2, TRPV3, or TRPV4 channels, although research is still ongoing.
ACID SENSING ION CHANNELS AND OTHER PROTON SENSITIVE CHANNELS
Acid from citrus fruits or fermentation of carbohydrates (e.g., acetic acid) can elicit irritation in the oral cavity. Carbonated sodas also contain acid (e.g., phosphoric acid in dark colas), but can also elicit an effervescent sensation in the mouth. The tingling sensation of carbonation is actually due to the acidification of tissue and peripheral nerve endings. This acidification occurs when CO2 is hydrated to carbonic acid through the ubiquitous enzyme carbonic anhydrase present in the oral cavity (Komai and Bryant, 1993; Simons et al., 1999). Protons can activate acid sensing ion channels (ASICs), namely ASIC1, ASIC2, and ASIC3, which are expressed in the peripheral and central nervous systems and are activated by extracellular protons (Sluka et al., 2009). ASIC3 is also activated by the endogenous arginine metabolite, agmatine (Li et al., 2010), an inhibitor of nitric oxidase synthase, which is potentially involved in neuropathic pain. Protons can also activate or inhibit hyperpolarization-activated cyclic nucleotide-gated (HCN) channels (Wahl-Schott and Biel, 2009), two pore domain K+ channels (2P; Ma et al., 2012a), and polycystic-kidney disease-like channels (PKDL). PKD1L3 and PKD2L1 are in the TRP channel family, but are predominately found in taste receptor cells, where they are believed to be involved in sour taste transduction (Ishimaru et al., 2006). PKD1L3/PKD2L1 are also weakly expressed in the human trigeminal ganglion (Flegel et al., 2015), but their function in the trigeminal nervous system is not known at this time. Other TRP channels are also sensitive to extracellular protons, including TRPV1 and TRPV4 (Suzuki et al., 2003). There are multiple channel types that can be modulated by acids, and this amount of molecular redundancy indicates that detection of pH levels is especially important in the oral cavity.
MECHANICAL SENSITIVITY
In addition to pain sensitivity, the oral cavity is exquisitely sensitive to both innocuous and noxious mechanical stimuli. In the somatosensory cortex, a neurologic “map” can be drawn of areas and proportions of the human body that correspond to the sensory function of touch. This “map” is often referred to as the human sensory homunculus, and the results resemble a human figure that has a disproportionately huge face, especially the tongue and lips. Mechanotransduction is the conversion of mechanical forces into a biological signal (e.g., action potentials from nerve fibers). The large area of the cortex dedicated to trigeminal somatosensory input suggests that mechanotransduction is a fundamental physiologic process an organism needs to reveal and monitor its environment. Somewhat recently, mechanosensitive Piezo channels were found in periodontal ligament cells and trigeminal ganglia (Bron et al., 2014; Jin et al., 2015). Piezo 1 and Piezo 2 are very large proteins that have multiple (>30) transmembrane domains. Piezo 2, in particular, participates in the detection of light touch in both primary afferents and Merkel cells, in addition to noxious stimuli (Bagriantsev et al., 2014). Potassium two pore domain channel subfamily K (KCNK) channels are also implicated in mechanotransduction in human sensory neurons. Of the 18 KCNK channel subtypes, KCNK12 (i.e., THIK-2), KCNK3 (i.e., TASK-1), and KCNK1 (i.e., TWIK-1) appear to have the highest expression in human trigeminal ganglia (Flegel et al., 2015). KNCK channels are modulated by various chemicals that affect pain signaling. KCNK12 is inhibited by halothane, KCNK3 is inhibited by anandamide and activated by halothane, while KCNK1 is inhibited by extracellular protons. KCNK3 is also inhibited by the molecule hydroxy-α-sanshool, found in popular and pungent Sichuan peppercorns native to East Asia (Bautista et al., 2008). The pharmacologic knowledge of Piezo and KNCK channels is still very limited, but their role in mechanotransduction may be an important tool for treatment of facial allodynia in the future.
OROTRIGEMINAL ITCH
Itch is defined as an unpleasant sensation leading to the desire to scratch. An itching sensation in the oral cavity can be caused by nerve damage, allergies, or infections. Damage to any of the cranial nerves can cause pruritus if the nerve is recurrently compacted or damaged or degraded by disease. Trigeminal trophic syndrome is a neuropathic itch of the throat, mouth, jaw, or ears that is typically caused by ablation of the trigeminal nerves or ganglia (Oaklander, 2011). When the oral itch is caused by allergies, it is often referred to as oral allergy syndrome. Oral allergy syndrome is believed to occur when proteins in raw foods mimic the allergenic proteins that are similar to common seasonal allergens, such as grass, mold, or pollen. These oral allergens can cause itching and tingling in and around the mouth, tongue, and throat (Muluk and Cingi, 2018). Oral itch can also be caused by a yeast infection in the mouth, otherwise known as oral candidiasis (a.k.a., oral thrush). Oral thrush can also cause a burning sensation in addition to a decreased sensitivity to taste (Dreizen, 1984). Infection of herpes simplex virus can cause the outbreak of cold sores (a.k.a., fever blisters), which are usually painful during an active blister formation, but can cause itching and tingling just prior to a sore appearing on the mouth or lips. The molecular biology and mechanisms of cellular itch transduction has expanded greatly within the last 10 years, and potentially some current leads being pursued for cutaneous itch on other parts of the body could be promising candidates for orofacial itch conditions in the future (Carstens and Akiyama, 2014).
TRIGEMINAL AND GUSTATORY NERVE CIRCUITRY AND INTERACTIONS
Running anatomically close to the mandibular nerve, gustatory (i.e., taste) information enters the central nervous system through the glossopharyngeal, vagus, and facial nerves (i.e., cranial nerves 9, 10, and 7, respectively). Unlike the trigeminal nerves, nerves involved in taste sensation enter the central nervous system and synapse in the nucleus of the solitary tract (Bradley and Grabauskas, 1998). Taste information finally terminates in the gustatory cortex, which contains two substructures: the anterior insular cortex and the frontal operculum. Although the taste and trigeminal pathways are largely separated beyond the level of second order neurons, there is evidence that thermal and chemical sensitivity communicated by the trigeminal nerves can alter the perceived quality of taste. The perception of flavor is achieved by the integration of sensory information from taste and smell, as well as trigeminal input regarding temperature, texture, and pungency. These separate modalities are integrated into the central nervous system, which is an important process that significantly impacts food and beverage choices, including consumption rate (Hutchings et al., 2017). Clearly there is overlap between these modalities; the question remains where this integration actually occurs in the nervous system. Although some channels involved in chemesthesis are also expressed on taste receptor cells, it is unknown (1) if there is a direct involvement of taste receptor cells in the peripheral nervous system with trigeminal nerve fibers and (2) if so, how this interaction would affect sensitivity to either taste or temperature (Roper, 2014).
Many early human psychophysical experiments have often suggested that oral trigeminal input can suppress or enhance some taste qualities (Lawless and Stevens, 1984). In fact, even heating the tongue can stimulate the sensation of sweetness, whereas cooling the tongue below 15°C can evoke sensations of sourness or saltiness (Cruz and Green, 2000). Specifically, the interaction between heat sensation and sweetness can be explained by TRPM5 expression in taste receptor cells, as their currents increase steeply at temperatures between 15 and 35°C (Talavera et al., 2005). The relationship between perception of other tastants and thermal stimulation are not as clear. In healthy adults, taste recognition thresholds for sweet, sour, salt, and bitter were heightened after holding an ice cube in the mouth for 1 min (Fujiyama and Toda, 2017). Conversely, pretreatment of the tongue with capsaicin has been shown to increase thresholds (decrease sensitivity) to sweetness, bitterness, sourness, and umami (Karrer and Bartoshuk, 1995; Simons et al., 2002), which could be explained by the ability of capsaicin to reduce responsiveness of NTS neurons during gustatory stimulation (Simons et al., 2003a). An alternative explanation could be a direct effect of thermal or chemical modulation of taste receptor cells in the periphery, e.g., currents from cells expressing PKD1L3/PKD2L1 are inhibited by capsaicin application (Ishii et al., 2012). Trigeminal input has also been implicated in modulating the perception of odor. Genetic variants of TRPA1 are known to affect pain sensitivity in humans; however, they can also affect the perception of various odorants (Schütz et al., 2014). Together, all of these data suggest that perception of taste and smell may involve a trigeminal component. Future treatment options for dysgeusia could be corrected by stimulating or blocking channels/receptors on orotrigeminal nerve fibers.
The idea that somehow tastants (or odorants) can modulate trigeminal irritant sensations has also been investigated. Oral rinsing with citric acid and sucrose has been reported to decrease the burning sensation from capsaicin and piperine (Stevens and Lawless, 1986). There is also some evidence that taste information can modulate the severity of trigeminal neuropathies. Ligation or dysfunction of the chorda tympani is believed to contribute to burning mouth syndrome (see below) (Eliav et al., 2007). The mechanism for this inhibitory action has not been fully fleshed out. There is some evidence that lingual application of salt or glutamate can inhibit trigeminal second order neuron sensitivity to noxious cold or electrical stimulation, but transection of the chorda tympani does not decrease sensitivity of these neurons to nociceptive inputs (Boucher et al., 2013). There is even less evidence for modulation of trigeminal irritation from odorant stimulation; however, compounds found in rose oil (i.e., geraniol), vanilla beans (i.e., vanillin), and laundry soap (i.e., helional) have been found to activate TRP channels and inhibit KCNK3 and KCNK9 in vitro (Lübbert et al., 2013). The tingling and pungent qualities of hydroxyl-α-sanshool have also been found to be decreased after exposure to oral sucrose, but not after salt or glutamate (Zhang et al., 2017). Consuming spicy or pungent food is highly desirable for most individuals; however, flavor profile can be greatly affected by input from tastants and odors that somehow modulate trigeminal activity. This is an area of research that could be greatly expanded for clinical and commercial motives.
OROTRIGEMINAL PATHOPHYSIOLOGY
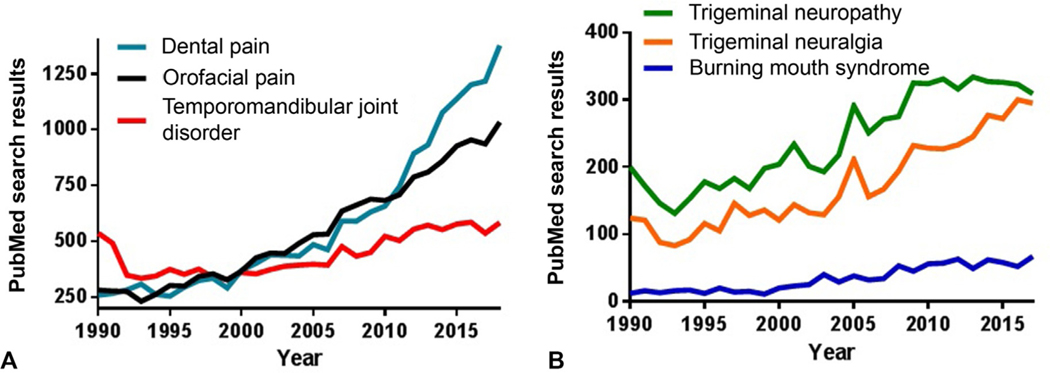
Orofacial pain is a blanket term covering any pain that is felt in the mouth, teeth, lips, cheek, jaws, and/or other parts of the face. Although most orofacial pain is believed to result from dental causes, other causes and reports include periodontal, oral ulceration, burning mouth syndrome, trigeminal neuralgia/trigeminal neuropathy, temporomandibular disorder (TMD), and oral cancer (Fig. 13.3). Orofacial pain is a common symptom of numerous diseases, of which there are many causes, and consists of at least four categories: pain due to muscle injury (i.e., musculoskeletal pain), pain due to tissue inflammation, pain due to interaction between nerves and blood vessels (i.e., neurovascular pain), and pain due to nerve damage (i.e., neuropathic pain) (Romero-Reyes and Uyanik, 2014). A quick review of the current literature suggests that most orofacial pain studies are clinical in nature (Fig. 13.4), which is not surprising since preclinical animal models in this field are limited. A few animal models in the trigeminal system have been reported; the infraorbital nerve (ION) or inferior alveolar nerve (IAN) injury models are common because these models demonstrate mechanical allodynia and heat hyperalgesia, as reported in the patient population. For this review, we highlight orofacial pain in and around the mouth, although nonoral facial pain such as headache (including migraine) and sinusitis are commonly reported and are important clinical topics for discussion on another forum.
Fig. 13.3.
Search results from PubMed (https://www.ncbi.nlm.nih.gov/pubmed/) containing a list of publications corresponding to search queries related to orotrigeminal research areas/topics from 1990 until 2018. PubMed is a primary source of biomedical literature from the United States National Library of Medicine database, which is composed of life science journals and online books. (A) “Orofacial pain” search results contain many entries related to clinical conditions and lists almost 15,000 entries since 1990. The most common returns include conditions such as “dental pain” and “temporomandibular joint disorder.” (B) Other clinical orofacial conditions are not as widely reported.
Fig. 13.4.
The large majority of recent literature on orofacial syndromes consists of clinical data regarding management and treatment programs. Word cloud of research article titles listed in PubMed regarding the search term, “orofacial.” The inclusionary criteria include publications from 1997 to 2018. The world cloud gives context about the most important or prevalent ideas/topics in a field. Titles of articles are weighted visually corresponding to their frequency of article title appearance. Frequency lists provided by wordclouds.com. The top 50 words are presented.
Many chemoreceptors are expressed in mammalian trigeminal ganglia (see earlier), and most of these receptors/channels are implicated in at least one orofacial pain syndrome. The symptoms of these pathophysiologies including burning, stinging, heat hyperalgesia, loss of taste, etc., are similar to those sensations elicited by irritant chemicals and channel/receptor stimulation of the trigeminal nerves. This leads us to suspect that pain and dysesthesias seen during disease or nerve injury are due to activation or depression of these same trigeminal channels/receptors. Some examples have been outlined below.
Trigeminal neuralgia is caused by sensory nerve root compaction or compression and is characterized by sudden onset of stabbing pain attacks. Trigeminal neuropathy, either painful or nonpainful, is associated with trigeminal nerve injury due to lesion or systemic disease, which leads to burning and aching chronic pain. Trigeminal neuropathic pain includes two types: typical (type 1) trigeminal neuralgia, characterized by agonizing and abrupt pain generated by mechanical hyperalgesia, and atypical (type 2) trigeminal neuralgia, associated with ongoing low intensity pain with interruptions of sharp painful bursts (Zakrzewska and Linskey, 2014). TRPV1 has been shown to change its expression profile under neuropathic conditions. For example, transection of the IAN nerve in rats, results in mechanical hyperalgesia. Interestingly, the level of TRPV1-expressing regenerated trigeminal ganglion cells was significantly higher in the rats that did not display neuropathic pain symptoms versus those with clear hyperalgesia after IAN transection (Zakir et al., 2012). Recently it was found that currents from HCN channels can have a decisive role in determining the resting membrane potential of peripheral nerves. These channels have been implicated in a variety of neurologic disorders, and modulation of HCN channel gating properties could be used to diagnose or treat dysfunction of sensory and motor neurons in neuropathy (Weerasinghe et al., 2017).
Burning mouth syndrome is a complex condition that is often described as a burning sensation on the lips and in the oral mucosa that typically affects peri- and postmenopausal women (Aravindhan et al., 2014). The pain can be moderate to severe, and is often accompanied by taste alterations and dry mouth. It is currently believed that burning mouth syndrome is due to nerve damage, placing this disease in the same category as trigeminal neuropathy (Lauria et al., 2005). Human tongue biopsies from burning mouth syndrome patients indicate that TRPV1 is upregulated, but overall nerve fiber density is decreased (Yilmaz et al., 2007). Since TRPV1 has been implicated in various neuropathies in rodent and human models, it is not surprising that TRPV1 antagonists can reverse heat hyperalgesia in a mouse model with burning mouth syndrome (Shinoda et al., 2015). Topical application of capsaicin and/or oral rinses containing capsaicin have been shown to be effective in the treatment of burning mouth syndrome and other trigeminal neuropathies (Epstein and Marcoe, 1994; Silvestre et al., 2012).
During nerve injury, there can also be a release of inflammatory mediators from damaged nerve fibers and surrounding unaffected nerve fibers. Inflammatory pain is caused by activation of peripheral nerves and central sensitization through tissue release of various mediators, such as bradykinin, prostaglandins, calcitonin gene related peptide, and SP. During cell damage, protons are generated, which induce acidification of the surrounding tissue that also triggers depolarization of peripheral nociceptors. In a mouse model of ION constriction, TRPA1 genetic deletion and application of a TRPA1 antagonist decreased pain-related behaviors (Trevisan et al., 2016). Increased TRPA1 channel expression on keratinocytes and macrophages has been reported in patients with chronic oral inflammatory disease and oral lichen planus, compared to healthy controls (Kun et al., 2017). The mRNA expression of HCN channels was significantly increased in trigeminal ganglia of rats after a complete Freund’s adjuvant (CFA) model of tooth pulp inflammation (Cho et al., 2015). In a formalin rat model of orofacial inflammation, ASIC1, ASIC2, and ASIC3 trigeminal ganglion currents were increased, and ASIC3 protein was found to be upregulated (Fu et al., 2016).
Temporomandibular joint disorder (TMD) is a highly prevalent musculoskeletal disorder in the general population characterized by jaw aching and general facial pain during mastication. Treatment of TMD is especially tricky due to its therapy-refractoriness and unknown pathogenesis in most patients. TRPV1 and TRPA1 are expressed in nociceptors that innervate muscle, and are believed to contribute to mechanical hyperalgesia seen in rodent models with masseter muscle CFA inflammation (Chung et al., 2016). In a similar TMD model, mouse pain behavioral scores improved after pharmacologic inhibition of TRPV1 and TRPA1, although the bite force was not improved (Wang et al., 2018). In addition, mice with the TRPV4 genetic deletion have improved bite force and evoked pain behavioral scores compared to wild type mice (Chen et al., 2013). These data collectively suggest that pharmaceuticals that target TRP channels could be an important therapy for relieving TMD pain and improving jaw function.
The orotrigeminal nervous system has many distinct functions, including motor output and sensory inputs into the central nervous system. Under normal conditions, the trigeminal nerve serves to provide information on the external surroundings and internal feedback from nerve fibers and tissues using specialized receptors that are cued for irritant and painful stimuli. Under pathophysiologic conditions, over- or underexpression or activity of these channels/receptors are thought to contribute to painful syndromes that can last for several years. With our current understanding of these molecular receptors, nerve fiber and intracellular pathways of the trigeminal nervous system, we are on our way to a better understanding of the etiology of trigeminal disease and future treatment options for many patients.
References
- Albin KC, Carstens MI, Carstens E (2008). Modulation of oral heat and cold pain by irritant chemicals. Chem Senses 33: 3–15. [DOI] [PubMed] [Google Scholar]
- Anderson EM, Jenkins AC, Caudle RM et al. (2014). The effects of a co-application of menthol and capsaicin on nociceptive behaviors of the rat on the operant orofacial pain assessment device. PLoS One 9: e89137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravindhan R, Vidyalakshmi S, Kumar MS et al. (2014). Burning mouth syndrome: a review on its diagnostic and therapeutic approach. J Pharm Bioallied Sci 6 (Suppl. 1): S21–S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagriantsev SN, Gracheva EO, Gallagher PG (2014). Piezo proteins: regulators of mechanosensation and other cellular processes. J Biol Chem 289: 31673–31681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW et al. (2004). Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41: 849–857. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A et al. (2005). Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci U S A 102: 12248–12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista DM, Siemens J, Glazer JM et al. (2007). The menthol receptor TRPM8 is the principal detector of environmental cold. Nature 448: 204–208. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Sigal YM, Milstein AD et al. (2008). Pungent agents from Szechuan peppers excite sensory neurons by inhibiting two-pore potassium channels. Nat Neurosci 11: 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrendt HJ, Germann T, Gillen C et al. (2004). Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br J Pharmacol 141: 737–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boucher Y, Felizardo R, Klein AH et al. (2013). Gustatory modulation of the responses of trigeminal subnucleus caudalis neurons to noxious stimulation of the tongue in rats. Eur J Neurosci 38: 2812–2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RM, Grabauskas G (1998). Neural circuits for taste. Excitation, inhibition, and synaptic plasticity in the rostral gustatory zone of the nucleus of the solitary tract. Ann N Y Acad Sci 855: 467–474. [DOI] [PubMed] [Google Scholar]
- Brand G, Jacquot L (2002). Sensitization and desensitization to allyl isothiocyanate (mustard oil) in the nasal cavity. Chem Senses 27: 593–598. [DOI] [PubMed] [Google Scholar]
- Bron R, Wood RJ, Brock JA et al. (2014). Piezo2 expression in corneal afferent neurons. J Comp Neurol 522: 2967–2979. [DOI] [PubMed] [Google Scholar]
- Carstens E, Akiyama T (2014). Itch: mechanisms and treatment, CRC Press/Taylor & Francis, Boca Raton, FL. [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M et al. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389: 816–824. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M et al. (1999). A capsaicin-receptor homologue with a high threshold for noxious heat. Nature 398: 436–441. [DOI] [PubMed] [Google Scholar]
- Chen Y, Williams SH, McNulty AL et al. (2013). Temporomandibular joint pain: a critical role for Trpv4 in the trigeminal ganglion. Pain 154: 1295–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YS, Kim YS, Moozhayil SJ et al. (2015). The expression of hyperpolarization-activated cyclic nucleotide-gated channel 1 (HCN1) and HCN2 in the rat trigeminal ganglion, sensory root, and dental pulp. Neuroscience 291: 15–25. [DOI] [PubMed] [Google Scholar]
- Chung MK, Park J, Asgar J et al. (2016). Transcriptome analysis of trigeminal ganglia following masseter muscle inflammation in rats. Mol Pain 12. 10.1177/1744806916668526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliff MA, Green BG (1994). Sensory irritation and coolness produced by menthol: evidence for selective desensitization of irritation. Physiol Behav 56: 1021–1029. [DOI] [PubMed] [Google Scholar]
- Cliff MA, Green BG (1996). Sensitization and desensitization to capsaicin and menthol in the oral cavity: interactions and individual differences. Physiol Behav 59: 487–494. [DOI] [PubMed] [Google Scholar]
- Cliff M, Heymann H (1992). Descriptive analysis of oral pungency. J Sens Stud 7: 279–290. [Google Scholar]
- Cruz A, Green BG (2000). Thermal stimulation of taste. Nature 403: 889–892. [DOI] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ et al. (2000). Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405: 183–187. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Orlando P, Moriello AS et al. (2012). Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastro-intestinal inflammation. Acta Physiol (Oxf ) 204: 255–266. [DOI] [PubMed] [Google Scholar]
- Peyrot des Gachons C, Uchida K, Bryant B et al. (2011). Unusual pungency from extra-virgin olive oil is attributable to restricted spatial expression of the receptor of oleocanthal. J Neurosci 31: 999–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessirier JM, O’Mahony M, Iodi-Carstens M et al. (2000a). Sensory properties of citric acid: psychophysical evidence for sensitization, self-desensitization, cross-desensitization and cross-stimulus-induced recovery following capsaicin. Chem Senses 25: 769–780. [DOI] [PubMed] [Google Scholar]
- Dessirier JM, Simons CT, Sudo M et al. (2000b). Sensitization, desensitization and stimulus-induced recovery of trigeminal neuronal responses to oral capsaicin and nicotine. J Neurophysiol 84: 1851–1862. [DOI] [PubMed] [Google Scholar]
- Dessirier JM, O’Mahony M, Carstens E (2001a). Oral irritant properties of menthol: sensitizing and desensitizing effects of repeated application and cross-desensitization to nicotine. Physiol Behav 73: 25–36. [DOI] [PubMed] [Google Scholar]
- Dessirier JM, O’Mahony M, Iodi-Carstens M et al. (2001b). Oral irritation by sodium chloride: sensitization, self-desensitization, and cross-sensitization to capsaicin. Physiol Behav 72: 317–324. [DOI] [PubMed] [Google Scholar]
- Dreizen S (1984). Oral candidiasis. Am J Med 77: 28–33. [PubMed] [Google Scholar]
- Eliav E, Kamran B, Schaham R et al. (2007). Evidence of chorda tympani dysfunction in patients with burning mouth syndrome. J Am Dent Assoc 138: 628–633. [DOI] [PubMed] [Google Scholar]
- Epstein JB, Marcoe JH (1994). Topical application of capsaicin for treatment of oral neuropathic pain and trigeminal neuralgia. Oral Surg Oral Med Oral Pathol 77: 135–140. [DOI] [PubMed] [Google Scholar]
- Facer P, Casula MA, Smith GD et al. (2007). Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol 7: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flegel C, Schöbel N, Altmüller J et al. (2015). RNA-Seq analysis of human trigeminal and dorsal root ganglia with a focus on chemoreceptors. PLoS One 10: e0128951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu H, Fang P, Zhou HY et al. (2016). Acid-sensing ion channels in trigeminal ganglion neurons innervating the orofacial region contribute to orofacial inflammatory pain. Clin Exp Pharmacol Physiol 43: 193–202. [DOI] [PubMed] [Google Scholar]
- Fujiyama R, Toda K (2017). Functional effects of cold stimulation on taste perception in humans. Odontology 105: 275–282. [DOI] [PubMed] [Google Scholar]
- Green BG (1986a). Menthol inhibits the perception of warmth. Physiol Behav 38: 833–838. [DOI] [PubMed] [Google Scholar]
- Green BG (1986b). Sensory interactions between capsaicin and temperature in the oral cavity. Chem Senses 11: 371–382. [Google Scholar]
- Green BG (1989). Capsaicin sensitization and desensitization on the tongue produced by brief exposures to a low concentration. Neurosci Lett 107: 173–178. [DOI] [PubMed] [Google Scholar]
- Green BG (1991a). Capsaicin cross-desensitization on the tongue: psychophysical evidence that oral chemical irritation is mediated by more than one sensory pathway. Chem Senses 16: 675–689. [Google Scholar]
- Green BG (1991b). Temporal characteristics of capsaicin sensitization and desensitization on the tongue. Physiol Behav 49: 501–505. [DOI] [PubMed] [Google Scholar]
- Green BG (1992). The effects of temperature and concentration on the perceived intensity and quality of carbonation. Chem Senses 17: 435–450. [Google Scholar]
- Green BG (1996). Rapid recovery from capsaicin desensitization during recurrent stimulation. Pain 68: 245–253. [DOI] [PubMed] [Google Scholar]
- Green BG (2005). Lingual heat and cold sensitivity following exposure to capsaicin or menthol. Chem Senses 30: 201–202. [DOI] [PubMed] [Google Scholar]
- Green BG (2007). Oral chemesthesis: an integral component of flavour. In: Taylor AJ, Roberts DD (Eds.), Flavor perception, Blackwell Publishing Ltd, Oxford, UK, pp. 151–165. [Google Scholar]
- Green BG (2012). Chemesthesis and the chemical senses as components of a “chemofensor complex”. Chem Senses 37: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG, McAuliffe BL (2000). Menthol desensitization of capsaicin irritation. Evidence of a short-term anti-nociceptive effect. Physiol Behav 68: 631–639. [DOI] [PubMed] [Google Scholar]
- Gwartney E, Heymann H (1995). The temporal perception of menthol. J Sens Stud 10: 393–400. [Google Scholar]
- Hutchings SC, Horner KM, Dible VA et al. (2017). Modification of aftertaste with a menthol mouthwash reduces food wanting, liking, and ad libitum intake of potato crisps. Appetite 108: 57–67. [DOI] [PubMed] [Google Scholar]
- Ishii S, Kurokawa A, Kishi M et al. (2012). The response of PKD1L3/PKD2L1 to acid stimuli is inhibited by capsaicin and its pungent analogs. FEBS J 279: 1857–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru Y, Inada H, Kubota M et al. (2006). Transient receptor potential family members PKD1L3 and PKD2L1 form a candidate sour taste receptor. Proc Natl Acad Sci U S A 103: 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasaki Y, Morita A, Iwasawa T et al. (2006). A nonpungent component of steamed ginger—[10]-shogaol—increases adrenaline secretion via the activation of TRPV1. Nutr Neurosci 9: 169–178. [DOI] [PubMed] [Google Scholar]
- Jin Y, Li J, Wang Y et al. (2015). Functional role of mechanosensitive ion channel Piezo1 in human periodontal ligament cells. Angle Orthod 85: 87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MH (1954). A study of the ‘common chemical sense’. Am J Psychol 67: 696–699. [PubMed] [Google Scholar]
- Jordt SE, Tominaga M, Julius D (2000). Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc Natl Acad Sci U S A 97: 8134–8139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH et al. (2004). Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature 427: 260–265. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM et al. (2013). Smell and taste: the chemical senses. In: Anne Sydor A, Lebowitz H (Eds.), Principles of neural science, fifth ed. McGraw-Hill; Education, 32: Smell and Taste: The Chemical Senses, 625–647. [Google Scholar]
- Karrer T, Bartoshuk L (1991). Capsaicin desensitization and recovery on the human tongue. Physiol Behav 49: 757–764. [DOI] [PubMed] [Google Scholar]
- Karrer T, Bartoshuk L (1995). Effects of capsaicin desensitization on taste in humans. Physiol Behav 57: 421–429. [DOI] [PubMed] [Google Scholar]
- Kida N, Sokabe T, Kashio M et al. (2012). Importance of transient receptor potential vanilloid 4 (TRPV4) in epidermal barrier function in human skin keratinocytes. Pflugers Arch 463: 715–725. [DOI] [PubMed] [Google Scholar]
- Klausen TK, Pagani A, Minassi A et al. (2009). Modulation of the transient receptor potential vanilloid channel TRPV4 by 4alpha-phorbol esters: a structure-activity study. J Med Chem 52: 2933–2939. [DOI] [PubMed] [Google Scholar]
- Klein AH, Carstens MI, Zanotto KL et al. (2011). Self- and cross-desensitization of oral irritation by menthol and cinnamaldehyde (CA) via peripheral interactions at trigeminal sensory neurons. Chem Senses 36: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AH, Carstens MI, Carstens E (2013). Eugenol and carvacrol induce temporally desensitizing patterns of oral irritation and enhance innocuous warmth and noxious heat sensation on the tongue. Pain 154: 2078–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AH, Joe CL, Davoodi A et al. (2014). Eugenol and carvacrol excite first- and second-order trigeminal neurons and enhance their heat-evoked responses. Neuroscience 271: 45–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AH, Trannyguen M, Joe CL et al. (2015). Thermosensitive transient receptor potential (TRP) channel agonists and their role in mechanical, thermal and nociceptive sensations as assessed using animal models. Chemosens Percept 8: 96–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K et al. (2005). Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol 493: 596–606. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Lundberg LE, Torebjörk HE (1992). Dynamic and static components of mechanical hyperalgesia in human hairy skin. Pain 51: 207–219. [DOI] [PubMed] [Google Scholar]
- Komai M, Bryant BP (1993). Acetazolamide specifically inhibits lingual trigeminal nerve responses to carbon dioxide. Brain Res 612: 122–129. [DOI] [PubMed] [Google Scholar]
- Koplas PA, Rosenberg RL, Oxford GS (1997). The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. J Neurosci 17: 3525–3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kun J, Perkecz A, Knie L et al. (2017). TRPA1 receptor is upregulated in human oral lichen planus. Oral Dis 23: 189–198. [DOI] [PubMed] [Google Scholar]
- Lauria G, Majorana A, Borgna M et al. (2005). Trigeminal small-fiber sensory neuropathy causes burning mouth syndrome. Pain 115: 332–337. [DOI] [PubMed] [Google Scholar]
- Lawless H, Stevens DA (1984). Effects of oral chemical irritation on taste. Physiol Behav 32: 995–998. [DOI] [PubMed] [Google Scholar]
- Li WG, Yu Y, Zhang ZD et al. (2010). ASIC3 channels integrate agmatine and multiple inflammatory signals through the nonproton ligand sensing domain. Mol Pain 6: 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YC, Huang CC, Hsu KS (2004). Therapeutic potential of cannabinoids in trigeminal neuralgia. Curr Drug Targets CNS Neurol Disord 3: 507–514. [DOI] [PubMed] [Google Scholar]
- Lübbert M, Kyereme J, Schöbel N et al. (2013). Transient receptor potential channels encode volatile chemicals sensed by rat trigeminal ganglion neurons. PLoS One 8 e77998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Zhang X, Zhou M et al. (2012a). Acid-sensitive TWIK and TASK two-pore domain potassium channels change ion selectivity and become permeable to sodium in extracellular acidification. J Biol Chem 287: 37145–37153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, He D, Ru X et al. (2012b). Apigenin, a plant-derived flavone, activates transient receptor potential vanilloid 4 cation channel. Br J Pharmacol 166: 349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson LJ, Dubin AE, Evans MJ et al. (2007). Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature 445: 541–545. [DOI] [PubMed] [Google Scholar]
- Malin S, Molliver D, Christianson JA et al. (2011). TRPV1 and TRPA1 function and modulation are target tissue dependent. J Neurosci 31: 10516–10528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy G, Allison T, Spencer DD (1993). Localization of the face area of human sensorimotor cortex by intracranial recording of somatosensory evoked potentials. J Neurosurg 79: 874–884. [DOI] [PubMed] [Google Scholar]
- McDonough P, McKenna JP, McCreary C et al. (2014). Neuropathic orofacial pain: cannabinoids as a therapeutic avenue. Int J Biochem Cell Biol 55: 72–78. [DOI] [PubMed] [Google Scholar]
- McKemy DD (2007). TRPM8: the cold the menthol receptor. In: Liedtke WB, Heller S (Eds.), TRP ion channel function in sensory transduction and cellular signaling cascades, CRC Press/Taylor & Francis, Boca Raton, FL: p. 13. [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D (2002). Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature 416: 52–58. [DOI] [PubMed] [Google Scholar]
- McNamara FN, Randall A, Gunthorpe MJ (2005). Effects of piperine, the pungent component of black pepper, at the human vanilloid receptor (TRPV1). Br J Pharmacol 144: 781–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno A, Matsumoto N, Imai M et al. (2003). Impaired osmotic sensation in mice lacking TRPV4. Am J Physiol Cell Physiol 285: C96–101. [DOI] [PubMed] [Google Scholar]
- Muluk NB, Cingi C (2018). Oral allergy syndrome. Am J Rhinol Allergy 32: 27–30. [DOI] [PubMed] [Google Scholar]
- Naganawa T, Baad-Hansen L, Ando T et al. (2015). Influence of topical application of capsaicin, menthol and local anesthetics on intraoral somatosensory sensitivity in healthy subjects: temporal and spatial aspects. Exp Brain Res 233: 1189–1199. [DOI] [PubMed] [Google Scholar]
- Oaklander AL (2011). Neuropathic itch. Semin Cutan Med Surg 30: 87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T, Imagawa T, Ito S (2007). Novel agonistic action of mustard oil on recombinant and endogenous porcine transient receptor potential V1 (pTRPV1) channels. Biochem Pharmacol 73: 1646–1656. [DOI] [PubMed] [Google Scholar]
- Park CK, Kim MS, Fang Z et al. (2006). Functional expression of thermo-transient receptor potential channels in dental primary afferent neurons: implication for tooth pain. J Biol Chem 281: 17304–17311. [DOI] [PubMed] [Google Scholar]
- Parker GH (1912). The relation of smell, taste, and the common chemical sense in vertebrates. J Acad Natl Sci Phila 2: 221–234. [Google Scholar]
- Peier AM, Moqrich A, Hergarden AC et al. (2002a). A TRP channel that senses cold stimuli and menthol. Cell 108: 705–715. [DOI] [PubMed] [Google Scholar]
- Peier AM, Reeve AJ, Andersson DA et al. (2002b). A heat-sensitive TRP channel expressed in keratinocytes. Science 296: 2046–2049. [DOI] [PubMed] [Google Scholar]
- Penfield W, Boldrey E (1937). Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain 60: 389–443. [Google Scholar]
- Prasad S, Galetta S (2007). The trigeminal nerve. In: Goetz CG (Ed.), Textbook of clinical neurology, Elsevier, Philadelphia, PA, pp. 165–183, Chapter 10. [Google Scholar]
- Qin N, Neeper MP, Liu Y et al. (2008). TRPV2 is activated by cannabidiol and mediates CGRP release in cultured rat dorsal root ganglion neurons. J Neurosci 28: 6231–6238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartu M, Serra MP, Boi M et al. (2016). TRPV1 receptor in the human trigeminal ganglion and spinal nucleus: immunohistochemical localization and comparison with the neuropeptides CGRP and SP. J Anat 229: 755–767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran R, Hyun E, Zhao L et al. (2013). TRPM8 activation attenuates inflammatory responses in mouse models of colitis. Proc Natl Acad Sci U S A 110: 7476–7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero-Reyes M, Uyanik JM (2014). Orofacial pain management: current perspectives. J Pain Res 7: 99–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD (2014). TRPs in taste and chemesthesis. Handb Exp Pharmacol 223: 827–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roper SD, Chaudhari N (2017). Taste buds: cells, signals and synapses. Nat Rev Neurosci 18: 485–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders CJ, Li WY, Patel TD et al. (2013). Dissecting the role of TRPV1 in detecting multiple trigeminal irritants in three behavioral assays for sensory irritation. F1000Res 2: 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schütz M, Oertel BG, Heimann D et al. (2014). Consequences of a human TRPA1 genetic variant on the perception of nociceptive and olfactory stimuli. PLoS One 9: e95592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinoda M, Takeda M, Honda K et al. (2015). Involvement of peripheral artemin signaling in tongue pain: possible mechanism in burning mouth syndrome. Pain 156: 2528–2537. [DOI] [PubMed] [Google Scholar]
- Silvestre FJ, Silvestre-Rangil J, Tamarit-Santafé C et al. (2012). Application of a capsaicin rinse in the treatment of burning mouth syndrome. Med Oral Patol Oral Cir Bucal 17: e1–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons CT, Dessirier JM, Carstens MI et al. (1999). Neurobiological and psychophysical mechanisms underlying the oral sensation produced by carbonated water. J Neurosci 19: 8134–8144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons CT, O’Mahony M, Carstens E (2002). Taste suppression following lingual capsaicin pre-treatment in humans. Chem Senses 27: 353–365. [DOI] [PubMed] [Google Scholar]
- Simons CT, Boucher Y, Carstens E (2003a). Suppression of central taste transmission by oral capsaicin. J Neurosci 23: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons CT, Carstens MI, Carstens E (2003b). Oral irritation by mustard oil: self-desensitization and cross-desensitization with capsaicin. Chem Senses 28: 459–465. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Winter OC, Wemmie JA (2009). Acid-sensing ion channels: a new target for pain and CNS diseases. Curr Opin Drug Discov Devel 12: 693–704. [PMC free article] [PubMed] [Google Scholar]
- Smith GD, Gunthorpe MJ, Kelsell RE et al. (2002). TRPV3 is a temperature-sensitive vanilloid receptor-like protein. Nature 418: 186–190. [DOI] [PubMed] [Google Scholar]
- Smith PL, Maloney KN, Pothen RG et al. (2006). Bisandrographolide from Andrographis paniculata activates TRPV4 channels. J Biol Chem 281: 29897–29904. [DOI] [PubMed] [Google Scholar]
- Stevens DA, Lawless HT (1986). Putting out the fire: effects of tastants on oral chemical irritation. Percept Psychophys 39: 346–350. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ et al. (2003). ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell 112: 819–829. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Mizuno A, Kodaira K et al. (2003). Impaired pressure sensation in mice lacking TRPV4. J Biol Chem 278: 22664–22668. [DOI] [PubMed] [Google Scholar]
- Talavera K, Yasumatsu K, Voets T et al. (2005). Heat activation of TRPM5 underlies thermal sensitivity of sweet taste. Nature 438: 1022–1025. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB et al. (1998). The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531–543. [DOI] [PubMed] [Google Scholar]
- Trevisan G, Benemei S, Materazzi S et al. (2016). TRPA1 mediates trigeminal neuropathic pain in mice downstream of monocytes/macrophages and oxidative stress. Brain 139 (Pt. 5): 1361–1377. [DOI] [PubMed] [Google Scholar]
- van Eijden TMGJ, Langenbach GEJ (2017). Anatomy of the trigeminal nerve. In: Baart J, Brand H (Eds.), Local anaesthesia in dentistry. Springer, Cham, pp. 19–36. [Google Scholar]
- Wahl-Schott C, Biel M (2009). HCN channels: structure, cellular regulation and physiological function. Cell Mol Life Sci 66: 470–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Brigoli B, Lim J et al. (2018). Roles of TRPV1 and TRPA1 in spontaneous pain from inflamed masseter muscle. Neuroscience 384: 290–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C (2016). Some like it hot! Sensory analysis of products containing chemesthetic compounds. In: McDonald ST, Bolliet DA, Hayes JE (Eds.), Chemesthesis: chemical touch in food and eating. John Wiley & Sons, Ltd, Sussex, UK, pp. 166–183. [Google Scholar]
- Watanabe H, Vriens J, Prenen J et al. (2003). Anandamide and arachidonic acid use epoxyeicosatrienoic acids to activate TRPV4 channels. Nature 424: 434–438. [DOI] [PubMed] [Google Scholar]
- Weerasinghe D, Menon P, Vucic S (2017). Hyperpolarization-activated cyclic-nucleotide-gated channels potentially modulate axonal excitability at different thresholds. J Neurophysiol 118: 3044–3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise PM, Bryant B (2014). The effect of temperature and menthol on carbonation bite. Chem Senses 39: 571–582. [DOI] [PubMed] [Google Scholar]
- Xu H, Ramsey IS, Kotecha SA et al. (2002). TRPV3 is a calcium-permeable temperature-sensitive cation channel. Nature 418: 181–186. [DOI] [PubMed] [Google Scholar]
- Yilmaz Z, Renton T, Yiangou Y et al. (2007). Burning mouth syndrome as a trigeminal small fibre neuropathy: increased heat and capsaicin receptor TRPV1 in nerve fibres correlates with pain score. J Clin Neurosci 14: 864–871. [DOI] [PubMed] [Google Scholar]
- Zakir HM, Mostafeezur RM, Suzuki A et al. (2012). Expression of TRPV1 channels after nerve injury provides an essential delivery tool for neuropathic pain attenuation. PLoS One 7: e44023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrzewska JM, Linskey ME (2014). Trigeminal neuralgia. BMJ 348: g474. [DOI] [PubMed] [Google Scholar]
- Zanotto KL, Iodi Carstens M, Carstens E (2008). Cross-desensitization of responses of rat trigeminal subnucleus caudalis neurons to cinnamaldehyde and menthol. Neurosci Lett 430: 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Shi B, Wang H et al. (2017). Pungency evaluation of hydroxyl-sanshool compounds after dissolution in taste carriers per time-related characteristics. Chem Senses 42: 575–584. [DOI] [PubMed] [Google Scholar]