Abstract
The direct repeat (DR) sequences flanking the src gene in Rous sarcoma virus are essential posttranscriptional control elements; at least one copy of this sequence is necessary for cytoplasmic accumulation of unspliced viral RNA. These sequences promote Rev-independent human immunodeficiency virus type 1 expression, suggesting they act as constitutive transport elements (CTEs). To determine which regions of this sequence are critical for CTE function, mutations in the downstream DR were generated and tested in a viral deletion construct lacking src and the upstream DR. Two single-point mutations and three different clustered mutations caused substantial reductions in reverse transcriptase activity, Gag protein levels, and unspliced viral RNA in the cytoplasm. Three conserved regions of the CTE, including nucleotides 8844 to 8847, 8862 to 8864, and 8868 to 8870, were most sensitive to inactivation by mutagenesis.
All retroviruses need to transport their full-length, unspliced RNA from the nucleus to the cytoplasm, where it is translated to produce Gag and Gag-Pol polyproteins and is also encapsidated into virions (reviewed in references 3, 7, 8, 15, 19, and 28). The complex retroviruses contain accessory genes, which help regulate the nuclear export of their unspliced RNA, whereas simple retroviruses lack any accessory genes. The human immunodeficiency virus type 1 (HIV-1) rev gene encodes a regulatory protein, which binds to a specific viral RNA sequence and is involved in several aspects of HIV-1 RNA metabolism. Rev controls the nucleocytoplasmic export of intron-containing RNA (16, 21) and also contributes to stabilization of RNA in the nucleus and cytoplasm (14, 20), inhibition of splicing (12, 13, 18), and translation (2, 6, 9).
Certain simple retroviruses (Rous sarcoma virus [RSV], Mason-Pfizer monkey virus [MPMV], and the closely related simian retrovirus type 1 [SRV-1]) have cis-acting RNA elements located within the 3′ untranslated region of their genomes, which are necessary for cytoplasmic accumulation of unspliced viral RNA (5, 10, 11, 24, 32 [reviewed in reference 3). The MPMV element has been termed a constitutive transport element (CTE) (5). These viral RNA elements appear to be sites of interaction with cellular factors involved in nuclear export pathways (25, 30) and can confer Rev-independent expression when inserted into HIV-1 constructs (5, 10, 24, 32).
The cis-acting posttranscriptional control element from RSV is essential for efficient viral replication and localizes to either direct repeat (DR) sequence flanking the src gene (24). RSV contains two copies of this element, but the related avian leukosis viruses contain a single copy in the 3′ untranslated region of their RNA (24, 26). A single copy of either DR sequence promotes replication of Prague C (Pr-C) or Schmidt-Ruppin A (SR-A) RSV constructs (24, 27). While association of the RSV DR sequence with RNA nuclear export has not been demonstrated directly, the reduced cytoplasmic accumulation of unspliced RNA in the absence of both DRs may be a consequence of inhibition of export and a concomitant increase in RNA degradation. Similarly, instability of HIV-1 nuclear RNA in the absence of Rev has been observed in certain cell types (20). Hence, the RSV DR element, by analogy to the MPMV element, is likely to be a CTE. In Pr-C RSV, the 93-nucleotide (nt) upstream repeat was initially termed DR1, and the 101-nt downstream repeat was termed DR2 (26), and we have adopted this nomenclature (24). DR1 and DR2 are also called upstream and downstream dr1, respectively (31).
In this study, we determined the effect of mutations in different regions of the RSV downstream DR2 sequence on viral replication and CTE activity. Site-specific substitution mutations were generated in DR2 and characterized in an RSV viral clone lacking DR1 and the src gene. A number of viruses bearing substitution mutations showed reductions in reverse transcriptase (RT) activity, Gag protein levels, and unspliced RNA in the cytoplasm. Two adjacent single-point mutations and three clustered mutations abolished CTE activity.
Generation of RSV DR2 substitution mutations.
To begin to identify sequences necessary for function of the DR2 element, a series of 10 RSV DR2 substitution mutants were constructed by site-specific mutagenesis and cloned into the viral construct pPrCΔDR′. Deletion mutant pPrCΔDR (24), lacking both copies of the DR sequences and the src gene (Δ6897–8960), was modified to generate pPrCΔDR′, which contains a unique polylinker at the site of the deletion (23). When the minimal downstream DR2 sequence (nt 8791 to 8891) was inserted into this deletion construct, virus-associated RT activity was reduced about twofold compared to that for constructs containing additional 3′ flanking sequence (23). Therefore, the mutations in DR2 were generated by using a recombinatorial PCR protocol (17) involving fragments containing the minimal DR2 and flanking sequences (nt 8770 to 8925), which were inserted into the 3′ untranslated region of pPrCΔDR′ at unique NsiI and AscI sites in its polylinker (23).
Figure 1 depicts all of the mutations that were generated. Most of the sequences selected for mutagenesis were conserved between the seven DR sequences of avian retroviruses aligned previously (24). These included both the upstream and downstream DRs of Pr-C and SR-A strains of RSV, and the single CTE sequence in the 3′ noncoding region of the Rous-associated virus (RAV) types 2, 7, and 0. The conserved residues are underlined in Fig. 1.
FIG. 1.
Sites of substitution mutations on the DR2 RNA minimal sequence (RSV nt 8791 to 8891). Underlined sequences denote nucleotides conserved between the seven different DR sequences previously aligned (DR1 and DR2 of PrC and SR-A strains of RSV, and RAV-2, RAV-7, and RAV-0) (24). Mutations shown in boldface type resulted in large decreases in viral replication (decreases in RT activity of approximately 10-fold or more relative to that of the wild-type virus) (Table 1).
RSV DR2 mutants display various levels of virion-associated RT activity.
The effect of DR2 mutations on replication of a viral construct was initially tested by transfecting chicken embryo fibroblasts (CEFs) and performing RT assays on tissue culture supernatants 48 h later as described previously (24). Table 1 displays the results obtained for all the mutants as the percentage of PrC-DR2 (wild-type) RT activity.
TABLE 1.
Effect of RSV DR2 mutations on RT activity and total and cytoplasmic viral RNA levelsa
| Construct | % RTb | Effect on viral RNA level
|
|||
|---|---|---|---|---|---|
| Total
|
Cytoplasm
|
||||
| U/S ratioc | % Unspliced pPrC-DR2 RNAd | U/S ratio | % Unspliced pPrC-DR2 RNA | ||
| pPrC-DR2 | 100.0 | 1.8 ± 0.4 | 100.0 | 1.6 ± 0.2 | 100.0 |
| pPrCΔDR | 4.8 ± 0.9 | 0.5 ± 0.2 | 15.4 ± 0.9 | NDe | ND |
| 8825 | 74.4 ± 15.0 | 1.1 ± 0.2 | 35.4 ± 17.0 | ND | ND |
| 8825/28 | 55.2 ± 5.2 | 0.9 ± 0.1 | 29.1 ± 14.5 | ND | ND |
| 8836 | 83.0 ± 10.0 | ND | ND | ND | ND |
| 8838 | 67.1 ± 6.6 | ND | ND | ND | ND |
| 8844 | 8.0 ± 2.4 | 0.5 ± 0.1 | 23.6 ± 0.2 | 0.14 ± 0.04 | 9.8 ± 0.2 |
| G8844C | 52.0 ± 9.5 | 0.5 ± 0.2 | 48.3 ± 7.0 | 0.65 ± 0.15 | 61.9 ± 1.6 |
| G8845C | 39.0 ± 2.4 | 0.6 ± 0.1 | 55.1 ± 7.0 | 0.32 ± 0.04 | 47.6 ± 5.0 |
| 8851 | 65.7 ± 0.9 | 1.3 ± 0.2 | 38.2 ± 2.7 | ND | ND |
| 8855 | 86.4 ± 6.4 | 1.3 ± 0.1 | 39.1 ± 12.7 | ND | ND |
| 8862 | 12.0 ± 2.1 | 0.5 ± 0.1 | 20.9 ± 3.6 | 0.15 ± 0.06 | 13.6 ± 0.1 |
| G8862C | 7.9 ± 0.3 | 0.3 ± 0.1 | 27.5 ± 3.5 | 0.17 ± 0.05 | 14.3 ± 1.6 |
| G8863C | 3.6 ± 2.1 | 0.3 ± 0.2 | 29.6 ± 5.5 | 0.14 ± 0.01 | 14.3 ± 2.1 |
| 8868 | 7.1 ± 2.2 | 0.5 ± 0.1 | 26.4 ± 7.2 | 0.15 ± 0.04 | 10.2 ± 1.3 |
| 8873 | 93.3 ± 9.5 | 1.3 ± 0.1 | 70.9 ± 17.3 | ND | ND |
All values represent the mean ± standard deviation for at least three assays and a minimum of two separate transfections.
RT results are expressed as a percentage of wild-type PrC-DR2 RT activity, and the results are an average of a minimum of three separate transfections.
U/S ratio, ratio of unspliced to spliced RSV RNA.
Unspliced RNA levels are normalized to RNA levels expressed from a cotransfected pmyc23 control plasmid and are expressed as a percentage of the wild-type PrC-DR2 RNA levels.
ND, not determined.
Viral mutants 8844, 8862, and 8868 exhibited markedly reduced RT levels, having an average of 8, 12, and 7% of the PrC-DR2 activity, respectively. The positions of the mutations in these mutants are shown in boldface in Fig. 1. In comparison, the PrCΔDR′ construct, which lacks both DR elements, had an average RT value equal to 5% of the PrC-DR2 activity in assays carried out in parallel. In prior studies with the DR deletion construct, no increase in RT activity was observed for up to 10 days after transfection (24).
Mutants 8836, 8855, and 8873 did not alter RT levels significantly from those of PrC-DR2, producing an average of 83, 86, and 93% activity, respectively. Viral mutants 8851 and 8838 had somewhat reduced RT activity of 66 and 67% compared to that of PrC-DR2. Although mutants 8825 and 8825/28 are in a highly conserved sequence (Fig. 1), they had only a moderate effect on viral replication. The 3-nt-substitution mutant 8825 produced about 74% RT activity, and the overlapping 7-nt mutant 8825/28 decreased activity only slightly further to 55% of wild-type levels.
Point mutations inactivate the CTE.
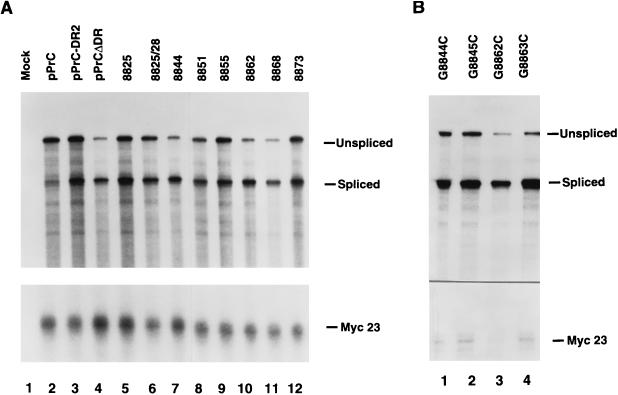
Since the regions defined by mutations 8844 and 8862 were critical for function, four single-point mutations were generated within these regions to further localize important nucleotides. Guanosine residues at nt 8844, 8845, 8862, and 8863 were changed to cytidines to generate mutants G8844C, G8845C, G8862C, and G8863C, respectively (Fig. 1). The results of RT activity produced 48 h after transfection for these mutants are shown in Table 1. G8844C and G8845C had RT levels of 52 and 39% of that of pPrC-DR2. In contrast, G8862C and G8863C mutants had dramatically reduced RT levels equivalent to 8 and 4% of that of pPrC-DR2, respectively. The latter mutants are highlighted in boldface type in Fig. 1.
RSV DR2 mutants produce low levels of Gag proteins.
To further characterize RSV constructs bearing mutations in DR2, the expression of Gag proteins was examined. Total cellular proteins from CEFs transfected with these viral constructs were harvested 48 h after transfection, and Western blots were prepared and probed with rabbit antiserum raised against avian myeloblastosis virus MA protein, obtained from D. P. Bolognesi, Duke University, as previously described (24). The cells were not treated to remove budding virus before lysis, so these lysates included associated viral particles. The processed Gag proteins observed are most likely present in such budding virus particles.
Similar to the results of the RT assays, mutants 8844, 8862, and 8868 had the greatest reduction in the levels of both Gag precursors and processed Gag proteins and exhibited phenotypes similar to that of the deletion-containing viral construct pPrCΔDR (Fig. 2). The other mutants tested showed smaller reductions in Gag protein levels. Among the three mutants exhibiting the greatest reduction in RT activity, processed Gag proteins were barely detectable, suggesting that little or no particle formation occurred; however, incompletely processed Gag precursors were detectable (Fig. 2). pPrCΔDR also exhibited reduced levels of Gag precursors and no detectable processed Gag proteins when total cellular proteins were isolated from transfected cells (Fig. 2). Of the other viral mutants tested, 8825/28 and 8851 had intermediate levels of Gag proteins, while the levels of 8825, 8855, and 8873 were similar to wild-type levels (Fig. 2). The relative ranking of mutants according to Gag protein levels correlated well with those based on levels of RT activity (Table 1).
FIG. 2.
Viral DR2 mutants have diminished levels of Gag proteins. DR2 mutants within pPrCΔDR′ were transfected into CEFs, and total cellular proteins were harvested 48 h after transfection. Western blots of cellular proteins were probed with rabbit antiserum raised against avian myeloblastosis virus p19gag (MA). The larger-molecular-weight protein bands are incompletely processed Gag precursor proteins. MA, Gag matrix protein (p19).
RSV DR2 mutants exhibit reduced levels of unspliced RNA.
To further define the effects of the DR2 mutations on viral gene expression, viral RNA levels were quantified. Total cellular RNA from CEFs transfected with these viral constructs was harvested 48 h after transfection by the Ultraspec RNA isolation system (Biotecx Lab). Unspliced and spliced viral RNA levels were measured by an RNase protection assay with a riboprobe which spans the RSV 5′ splice site as described previously (1, 24). pMyc23 was cotransfected with the viral constructs as a control, and its expression was measured simultaneously with a specific riboprobe (22). The results of a representative RNase protection assay are depicted in Fig. 3. The radioactivity in each band was quantitated on an InstantImager (Packard), and the ratio of unspliced to spliced RNA for each construct tested is shown in Table 1. The level of unspliced viral RNA, normalized to the cotransfected myc23 control RNA, was also determined and presented as a percentage of that of wild-type PrC-DR2 RNA (Table 1).
FIG. 3.
RSV DR2 mutants have reduced levels of total unspliced RNA. RNA isolated from CEFs transfected with DR2 mutants was harvested 48 h after transfection, and viral RNA was probed in an RNase protection assay with a riboprobe which spans the viral 5′ splice site. The pMyc23 construct was cotransfected with the viral constructs as a control for RNA recovery and transfection efficiency. (A) Clustered substitution mutations. (B) Single-point mutations.
Mutants 8844, 8862, and 8868 exhibited a four- to fivefold reduction in their total unspliced RNA levels compared to that of wild-type PrC-DR2 (Fig. 3A, compare lanes 7, 10, and 11 with lane 3; Table 1) and a similar decrease in their unspliced/spliced RNA ratios (Table 1). These three mutants had an average unspliced/spliced RNA ratio of 0.5 compared to the ratio of 1.8 for pPrC-DR2. These three mutants had reduced levels of unspliced RNA similar to that of the proviral construct PrCΔDR, which lacks both DR elements (Fig. 3A, lane 4; Table 1). In accordance with previously reported results (24), PrCΔDR had an unspliced/spliced RNA ratio of 0.5.
Mutants 8851, 8855, and 8873 had intermediate unspliced/spliced RNA ratios of 1.3, while mutant 8825 had an average ratio of 1.1 (Fig. 3A; Table 1). These ratios are slightly lower than the pPrC-DR2 ratio of 1.8. However, these four mutants had levels of unspliced RNA adequate for viral replication, as demonstrated by the RT assay (Table 1) and gag gene expression (Fig. 2, lanes 8 and 10 to 12). Mutant 8825/28, which had nearly a twofold reduction in Gag protein (Fig. 2, compare lanes 3 and 9) and virion-associated RT activity (Table 1) compared to wild-type pPrC-DR2 also displayed a twofold reduction in the ratio of unspliced/spliced RNA (Fig. 3A, lane 6). As reported in Table 1, mutant 8825/28 had an unspliced/spliced RNA ratio of 0.9.
Viral RNAs produced by the point mutants were also analyzed by RNase protection of total cellular RNA, and the results are shown in Fig. 3B. Mutants containing point mutations at nt 8862 and 8863 showed greatly reduced levels of unspliced RNA (28 to 30% wild-type levels) (Fig. 3B, lanes 3 and 4; Table 1), while mutants 8844 and 8845 had less severe reductions (48 to 55% of the wild-type level) (Fig. 3B, lanes 1 and 2; Table 1). This is consistent with results from RT assays of these mutants (Table 1).
Reductions in unspliced viral RNA due to CTE mutations are detected in the cytoplasm.
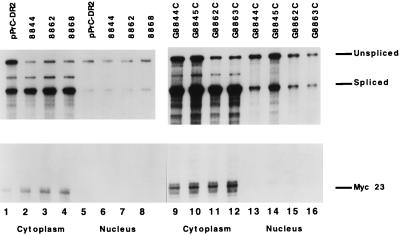
RSV DR2 mutants, which displayed significantly reduced levels of Gag proteins and low levels of virion-associated RT activity, also had significantly reduced levels of unspliced viral RNA. To determine the subcellular levels of unspliced RNA for the three RSV DR2 clustered mutants that inactivate viral replication and for the four single-point mutants, constructs were transfected into CEFs, and cytoplasmic and nuclear RNA fractions were obtained by a citric acid cell fractionation method (4, 24). Viral RNA was detected by RNase protection assays (Fig. 4), and the levels of unspliced RNA were normalized to those of RNA expressed from a cotransfected pmyc23 control plasmid. The normalized RNA levels were expressed as a percentage of wild-type PrC-DR2 RNA in Table 1. Mutants 8844, G8862C, G8863C, 8862, and 8868 had significant reductions in cytoplasmic, unspliced RNA levels equivalent to 10, 14, 14, 14, and 10% of PrC-DR2 levels, respectively (Fig. 4; Table 1). These mutants also produced little or no processed Gag proteins (Fig. 2) or RT activity (Table 1). In contrast, point mutants G8844C and G8845C had 62 and 48% of PrC-DR2 cytoplasmic RNA levels, respectively, and RT activity equivalent to 52 and 39% of PrC-DR2 RT activity, respectively (Fig. 4; Table 1).
FIG. 4.
RSV DR2 mutants have reduced levels of unspliced RNA in the cytoplasm. Viral constructs were transfected into CEFs with the pMyc23 control plasmid. Cells were fractionated into nuclear and cytoplasmic fractions, and RNA was detected by RNase protection assays as described in the legend to Fig. 3.
Cumulative results of these data suggest that correlations exist between the relative levels of Gag proteins, RT activity, and the levels of cytoplasmic, unspliced RSV RNA for these viral constructs containing mutations in DR2. In contrast, the levels of unspliced RNA within the nucleus were similar for wild-type pPrC-DR2 and the different pPrC-DR2 mutants (Fig. 4). However, the phenotypes of RSV DR2 mutants which displayed the greatest reduction in viral expression were most similar to that observed with the deletion mutant pPrCΔDR, which lacks both DRs and the intervening src gene. Gag proteins and RT activity were barely detectable for these mutants, and their total unspliced RNA levels were reduced four- to fivefold compared to that of wild-type RSV DR2. Previously we found an approximately 10-fold decrease in the cytoplasmic accumulation of unspliced RNA with the pPrCΔDR construct (24). These RSV DR2 mutants also displayed a similar reduction in the cytoplasmic levels of unspliced RNA (Fig. 4; Table 1).
Sequence comparison of critical CTE sequences of RSV and MPMV or SRV-1.
Selective mutagenesis of specific regions of RSV DR2 showed that one critical region for CTE function is within the conserved sequence that includes nt 8862 to 8870. Four different viral mutants with mutations in this region, which altered nt 8862, 8863, 8862 to 8864, or 8868 to 8870, exhibited a greater than 10-fold reduction in RT activity and a similar decrease in unspliced cytoplasmic RNA levels (Table 1). These mutations fall within a 13-nt sequence (nt 8860 to 8872), which is the longest sequence stretch that is conserved between six different upstream and downstream DR sequences (24) (Fig. 1). However, the Pr-C upstream DR1 sequence has an additional A inserted adjacent to the A at nt 8868, which does not appear to affect its CTE activity (24). A second important region was designated by the mutation of nt 8844 to 8847, which is also within a highly conserved sequence. However, point mutations at nt 8844 and 8845 within the latter sequence were less inactivating than the 4-nt-substitution mutation. In contrast, mutations in other conserved sequences (for example, 8825, 8838, or 8855) caused less than twofold reductions in viral replication, assayed by RT activity. It is possible that the inactivating mutations may designate regions of the CTE that are the site of interaction with cellular factors involved in CTE function.
While no extensive region of sequence similarity was observed between the RSV and MPMV or SRV-1 CTEs, we found that all three contained an ACG within regions shown to be important for CTE activity. In RSV, the ACG is present at nt 8868 to 8870, and its mutagenesis inactivated viral replication. In MPMV and SRV-1 CTEs, an ACG is present as part of both internal loops of the secondary structure models (11, 29). Mutagenesis of either the A or the C in the loops was found to inactivate the CTE (11, 29). It will be interesting to determine the secondary structure of the RSV DR sequences and to localize these inactivating mutations on that structure.
Acknowledgments
This work was supported by Public Health Service grants CA48746 and GM48327 to K.L.B. R.A.O. was supported by NIH Predoctoral Training grant 52T32G07231.
K.L.B. thanks Marc Sitbon and Marylène Mougel for discussions.
REFERENCES
- 1.Arrigo S, Beemon K. Regulation of Rous sarcoma virus RNA splicing and stability. Mol Cell Biol. 1988;8:4858–4867. doi: 10.1128/mcb.8.11.4858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arrigo S J, Chen I S Y. Rev is necessary for translation but not cytoplasmic accumulation of HIV-1 vif, vpr, and env/vpu 2 RNAs. Gene Dev. 1991;5:808–819. doi: 10.1101/gad.5.5.808. [DOI] [PubMed] [Google Scholar]
- 3.Banks, J. D., K. L. Beemon, and M. L. Linial. RNA regulatory elements in the genomes of simple retroviruses. Semin. Virol., in press.
- 4.Barker G F, Beemon K. Nonsense codons within the Rous sarcoma virus gag gene decrease the stability of unspliced viral RNA. Mol Cell Biol. 1991;11:2760–2768. doi: 10.1128/mcb.11.5.2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bray M, Prasad S, Dubay J W, Hunter E, Jeang K, Rekosh D, Hammarskjold M L. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc Natl Acad Sci USA. 1994;91:1256–1260. doi: 10.1073/pnas.91.4.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cochrane A W, Jones K S, Beidas S, Dillon P J, Skalka A M, Rosen C A. Identification and characterization of intragenic sequences which repress human immunodeficiency virus structural gene expression. J Virol. 1991;65:5305–5313. doi: 10.1128/jvi.65.10.5305-5313.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffin J M. Retroviridae: the viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lipincott-Raven Publishers; 1996. pp. 1767–1847. [Google Scholar]
- 8.Cullen, B. M. 1995. Regulation of HIV gene expression. AIDS 9(Suppl. A):S19–S32. [PubMed]
- 9.D’Agostino D M, Felber B K, Harrison J E, Pavlakis G N. The Rev protein of human immunodeficiency virus type 1 promotes polysomal association and translation of gag/pol and vpu/env mRNAs. Mol Cell Biol. 1992;12:1375–1386. doi: 10.1128/mcb.12.3.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ernst R K, Bray M, Rekosh D, Hammarskjöld M L. A structured retroviral RNA element that mediates nucleocytoplasmic export of intron-containing RNA. Mol Cell Biol. 1997;17:135–144. doi: 10.1128/mcb.17.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst R K, Bray M, Rekosh D, Hammarskjold M L. Secondary structure and mutational analysis of the Mason-Pfizer monkey virus RNA constitutive transport element. RNA. 1997;3:210–222. [PMC free article] [PubMed] [Google Scholar]
- 12.Feinberg M B, Jarrett R F, Aldovini A, Gallo R C, Wong-Staal F. HTLV-III expression and production involve complex regulation at the levels of splicing and translation of viral RNA. Cell. 1986;46:807–817. doi: 10.1016/0092-8674(86)90062-0. [DOI] [PubMed] [Google Scholar]
- 13.Felber B K, Drysdale C M, Pavlakis G N. Feedback regulation of human immunodeficiency virus type 1 expression by the Rev protein. J Virol. 1990;64:3734–3741. doi: 10.1128/jvi.64.8.3734-3741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felber B K, Hadzopoulou-Cladaras M, Cladaras C, Copeland T, Pavlakis G N. Rev protein of human immunodeficiency virus type 1 affects the stability and transport of the viral mRNA. Proc Natl Acad Sci USA. 1989;86:1496–1499. doi: 10.1073/pnas.86.5.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Felber, B. K., and G. N. Pavlakis. 1993. Molecular biology of HIV-1: positive and negative regulatory elements important for virus expression. AIDS 7(Suppl.):S51–S62. [PubMed]
- 16.Fischer U, Huber J, Boelens W C, Mattaj I W, Luhrmann R. The HIV-1 Rev activation domain is a nuclear export signal that accesses an export pathway used by specific cellular RNAs. Cell. 1995;82:475–483. doi: 10.1016/0092-8674(95)90436-0. [DOI] [PubMed] [Google Scholar]
- 17.Higuchi R. Using PCR to engineer DNA. In: Erlich H A, editor. PCR technology: principles and applications for DNA amplification. New York, N.Y: Stockton Press; 1990. pp. 61–70. [Google Scholar]
- 18.Kjems J, Sharp P A. The basic domain of Rev from human immunodeficiency virus type 1 specifically blocks the entry of U4/U6 · U5 small nuclear ribonucleoprotein in splicesome assembly. J Virol. 1993;67:4769–4776. doi: 10.1128/jvi.67.8.4769-4776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luciw P. Human immunodeficiency viruses and their replication. In: Fields B N, Knipe D M, Howley P M, editors. Fields virology. 3rd ed. Philadelphia, Pa: Lipincott-Raven Publishers; 1996. pp. 1881–1952. [Google Scholar]
- 20.Malim M H, Cullen B R. Rev and the fate of pre-mRNA in the nucleus: implications for the regulation of RNA processing in eukaryotes. Mol Cell Biol. 1993;13:6180–6189. doi: 10.1128/mcb.13.10.6180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malim M H, Hauber J, Le S V, Maizel J V, Cullen B R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature (London) 1989;338:254–275. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- 22.McNally M, Gontarek R, Beemon K. Characterization of Rous sarcoma virus intronic sequences that negatively regulate splicing. Virology. 1991;185:99–108. doi: 10.1016/0042-6822(91)90758-4. [DOI] [PubMed] [Google Scholar]
- 23.Ogert R A. Avian retroviral RNA export. Ph.D. thesis. Baltimore, Md: Johns Hopkins University; 1997. [Google Scholar]
- 24.Ogert R A, Lee L H, Beemon K L. Avian retroviral RNA element promotes unspliced RNA accumulation in the cytoplasm. J Virol. 1996;70:3834–3843. doi: 10.1128/jvi.70.6.3834-3843.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saavedra C, Felber B, Izaurralde E. The simian retrovirus-1 constitutive transport element, unlike the HIV-1 RRE, uses factors required for cellular mRNA export. Curr Biol. 1997;7:619–628. doi: 10.1016/s0960-9822(06)00288-0. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz D E, Tizard R, Gilbert W. Nucleotide sequence of Rous sarcoma virus. Cell. 1983;32:853–869. doi: 10.1016/0092-8674(83)90071-5. [DOI] [PubMed] [Google Scholar]
- 27.Sorge J, Ricci W, Hughes S H. cis-Acting RNA packaging locus in the 115-nucleotide direct repeat of Rous sarcoma virus. J Virol. 1983;48:667–675. doi: 10.1128/jvi.48.3.667-675.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stoltzfus C M. Synthesis and processing of avian sarcoma retrovirus RNA. Adv Virus Res. 1988;35:1–37. doi: 10.1016/s0065-3527(08)60707-1. [DOI] [PubMed] [Google Scholar]
- 29.Tabernero C, Zolotukhin A, Valentin A, Pavlakis G N, Felber B K. The posttranscriptional control element of the simian retrovirus type 1 forms an extensive RNA secondary structure necessary for its function. J Virol. 1996;70:5998–6011. doi: 10.1128/jvi.70.9.5998-6011.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang H, Gaietta G M, Fischer W H, Ellisman M H, Wong-Staal F. A cellular cofactor for the constitutive transport element of type D retrovirus. Science. 1997;276:1412–1415. doi: 10.1126/science.276.5317.1412. [DOI] [PubMed] [Google Scholar]
- 31.Van Beveren C, Coffin J, Hughes S. Appendixes. In: Weiss R, Teich N, Varmus H, Coffin J, editors. RNA tumor viruses. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1985. pp. 578–600. [Google Scholar]
- 32.Zolotukhin A S, Valentin A, Pavlakis G N, Felber B K. Continuous propagation of RRE(−) and Rev(−)RRE(−) human immunodeficiency virus type 1 molecular clones containing a cis-acting element of simian retrovirus type 1 in human peripheral blood lymphocytes. J Virol. 1994;68:7944–7952. doi: 10.1128/jvi.68.12.7944-7952.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]