Abstract
Background
MicroRNA (miRNA)-21-5p participates in various biological processes, including cancer and autoimmune diseases. However, its role in the development of fibrosis in the in vivo model of systemic sclerosis (SSc) has not been reported. This study investigated the effects of miRNA-21a-5p overexpression and inhibition on SSc fibrosis using a bleomycin-induced SSc mouse model.
Methods
A murine SSc model was induced by subcutaneously injecting 100 μg bleomycin dissolved in 0.9% NaCl into C57BL/6 mice daily for 5 weeks. On days 14, 21, and 28 from the start of bleomycin injection, 100 μg pre-miRNA-21a-5p or anti-miRNA-21a-5p in 1 mL saline was hydrodynamically injected into the mice. Fibrosis analysis was conducted in lung and skin tissues of SSc mice using hematoxylin and eosin as well as Masson’s trichrome staining. Immunohistochemistry was used to examine the expression of inflammatory cytokines, phosphorylated signal transducer and activator of transcription-3 (STAT3) at Y705 or S727, and phosphatase and tensin homologue deleted on chromosome-10 (PTEN) in skin tissues of SSc mice.
Results
MiRNA-21a-5p overexpression promoted lung fibrosis in bleomycin-induced SSc mice, inducing infiltration of cells expressing TNF-α, IL-1β, IL-6, or IL-17, along with STAT3 phosphorylated cells in the lesional skin. Conversely, anti-miRNA-21a-5p injection improved fibrosis in the lung and skin tissues of SSc mice, reducing the infiltration of cells secreting inflammatory cytokines in the skin tissue. In particular, it decreased STAT3-phosphorylated cell infiltration at Y705 and increased the infiltration of PTEN-expressing cells in the skin tissue of SSc mice.
Conclusion
MiRNA-21a-5p promotes fibrosis in an in vivo murine SSc model, suggesting that its inhibition may be a therapeutic strategy for improving fibrosis in SSc.
Introduction
Systemic sclerosis (SSc) or scleroderma is a complex autoimmune connective tissue disease marked by uncontrolled fibroblast activation [1]. Bairkdar and colleagues performed a meta-analysis and reported that the overall pooled prevalence of SSc was 17.6 per 100,000 and the overall pooled incidence rate of SSc was 1.4 per 100,000 person-year [2]. Although the prevalence of SSc is relatively low, SSc has the highest mortality rate among all rheumatic diseases, with life loss of up to 11.3 years for women and 25.8 years for men [3]. SSc affects skin and multiple internal organ such as heart, lung, and kidney, but there is still no curative treatment for SSc [4].
While its cause remains unknown, chronic inflammation triggers sustained myofibroblast activation, leading to excessive deposition of extracellular matrix components, including collagen and glycoproteins, resulting in fibrosis and impaired cellular function [5, 6]. Transforming growth factor-β (TGF-β) is a potent profibrotic factor in SSc fibrosis [7]. Mothers against decapentaplegic homologue (SMAD)2 and SMAD3, downstream of TGF-β, promote fibrosis, while SMAD7, a negative regulator, can protect against TGF-β-mediated fibrosis [8].
MicroRNAs (miRNAs) are small non-coding RNAs (approximately 20–22 nucleotides) that regulate gene expression post-transcriptionally [9]. Recent observations highlight their involvement in biological processes, including cell proliferation, differentiation, and apoptosis [10, 11]. Aberrant expression of miRNAs is closely linked to the pathophysiology of diseases, suggesting that circulating miRNAs are potential biomarkers and therapeutic targets [12]. Studies have reported an altered miRNA profile in the serum of SSc patients, with differentially expressed miRNAs implicated in various fibrotic processes [13–15], indicating a potential role for miRNAs in SSc development. Based on the study of the miRNA profile that changes in disease conditions, the development of therapeutics using the form of miRNA mimics or anti-miRs is considered a promising treatment strategy. Several miRNA-based therapeutics for human diseases have reached the clinical development stage [16, 17].
Among extensively studied miRNAs, miRNA-21-5p is involved in multiple biological processes, including cancer, immune responses, and autoimmune diseases [18, 19]. Previous studies have demonstrated its role in regulating skin fibrosis, with elevated levels in the serum and skin tissue of SSc patients [20]. MiRNA-21-5p induction through TGF-β in fibroblasts promotes fibrosis by suppressing and regulating SMAD7, a negative regulator of TGF-β [21]. However, there are no reports on the effects of miRNA-21 overexpression or inhibition on SSc fibrosis in in vivo systems.
The objective of this study is to determine the effect of miRNA-21-5p on fibrosis development in SSc. Using a bleomycin-induced murine SSc model, we demonstrated for the first time that inhibiting miRNA-21a-5p improved fibrosis development by lowering signal transducer and activator of transcription 3 (STAT3) and increasing phosphatase and tensin homologue deleted on chromosome-10 (PTEN) in an in vivo system. These results suggest that miRNA-21 is a promising therapeutic target for SSc.
Materials and methods
Animals and treatment
We obtained 8-week-old male C57BL/6 mice from Jackson Laboratory (Bar Harbor, ME, USA). To create a murine model of SSc, mice received daily subcutaneous injections of 50 µg of bleomycin (BLM, HY-17565A, MedChemExpress) dissolved in 100 µL phosphate-buffered saline (PBS; CBP3071, Dynebio) for 2 weeks. On days 14, 21, and 28 from the start of bleomycin injection, mice were hydrodynamically injected with 100 µg of plasmids containing pre-miRNA-21a-5p (#PMIRH21PA-1) [22], negative control (pre-miR-NC), anti-sense miR against miRNA-21a-5p (miRZip-21 lentiviral vector expressing anti-sense miRNA-21a-5p, anti-miRNA-21a-5p, #mZIP21-PA-1) [23] or miRZip-scrambled hairpin vector (scrambled anti-miR, #mZIP000-PA-1) (System Bioscience Inc) in 1 mL saline (n = 5/group). #PMIRH21PA-1 is an expression vector containing the miR-21 precursor construct and #mZIP21-PA-1 represses miR-21. Each plasmid was hydrodynamically injected intravenously into the tail vein. The mice were sacrificed on day 33 from the start of bleomycin injection, using 2.5% isoflurane inhalation, followed by histologic analysis. The mice were housed under specific-pathogen-free conditions at the Institute of Medical Science of the Catholic University of Korea and provided standard mouse diet and water. All experimental procedures were approved by the Animal Research Ethics Committee of the Catholic University of Korea, adhering to National Institutes of Health guidelines (Permit number: 2017–0067–02).
Histology
The skin tissues of mice were fixed in 10% neutral-buffered formalin (HT501320, Sigma) and embedded in paraffin. Sections (5 µm thick) were stained with hematoxylin and eosin (H&E) and Masson’s trichrome (MT), as described previously [24]. MT staining was conducted using ready–to-use kit (Trichrome Stain (Masson) Kit, HT15, Sigma-Aldrich). After deparaffinization and rehydration, the lung section slides were immersed in Bouin’s solution (HT 10132, Sigma-Aldrich) at 56 °C for 15 min. Subsequently, the slides were washed with tap water for 5 min. Next, the tissues were stained in Weigert’s hematoxylin for 5 min, and then washed again with tap water for 5 min. Then, the slides were stained in Biebrich scarlet-acid fuchsin for 5 min, rinsed in distilled water, incubated in phosphotungstic-phosphomolybdic acid for 5 min, dyed with aniline blue for 5 min, and fixed in 1% acetic acid for 2 min. Finally, the slides were washed in distilled water, dehydrated and mounted.
Histologic analysis
Dermal thickness and lung severity were scored as described previously [25–27]. Dermal thickness, which is the thickness of skin from the top of the granular layer to the junction between the dermis and subcutaneous fat, was measured. Fibrosis score was obtained by analyzing lung-cross section slides stained with H&E and MT. The extent of fibrotic lesions was scored as 0 (0–10%), 1 (10–25%), 2 (25–50%), 3 (50–75%), or 4 (75–100%). The severity of fibrosis was scored from 0 (normal lung) to 8 (total fibrosis), referring to the Ashcroft scoring system. The final fibrosis score was calculated by multiplying the extent of fibrotic lesions by the Ashcroft score [27].
Immunohistochemistry
Following deparaffinization, tissue Sects. 5 µm thick were subjected to antigen retrieval using proteinase K (Dako, #S3020) in Tris–EDTA buffer (Sigma-Aldrich, #93,283). To inhibit endogenous peroxidase activity, the sections were treated with 3% hydrogen peroxide (Samchun Chemicals, #H0300) in methyl alcohol (Duksan, #59). Then they were incubated overnight at 4 °C with primary antibodies against tumor necrosis factor-α (TNF-α, Abcam, #Ab6671), interleukin (IL)-1β (NOVUS #NB600–633), IL-6 (NOVUS, #NB600–1131), IL-17 (Abcam, #ab79056), STAT3 (Abcam, #ab68153), phosphorylated STAT3 at tyrosine 705 (Y705) (Abcam, #ab76315) or serine 727 (S727) (Abcam, #ab30647), and PTEN (R&D Systems, #AF847). After 2 h incubation at room temperature with the above antibodies, the sections were incubated for 30 min with horseradish peroxidase-conjugated secondary antibody. The final product was developed using DAB chromogen (Dako). Positive cells, identified based on dark-brown deposits in lymphocyte nuclei, were counted in 10 randomly selected high-power fields.
Statistical analysis
Data are expressed as mean ± standard deviation (± SD). Statistical significance was assessed using one-way analysis of variance (ANOVA) and Tukey’s test using GraphPad PRISM Version 5.0 (GraphPad Software, USA). Statistical significance was defined as p < 0.05.
Results
MiRNA-21a-5p accelerates lung fibrosis in the bleomycin-induced fibrosis model
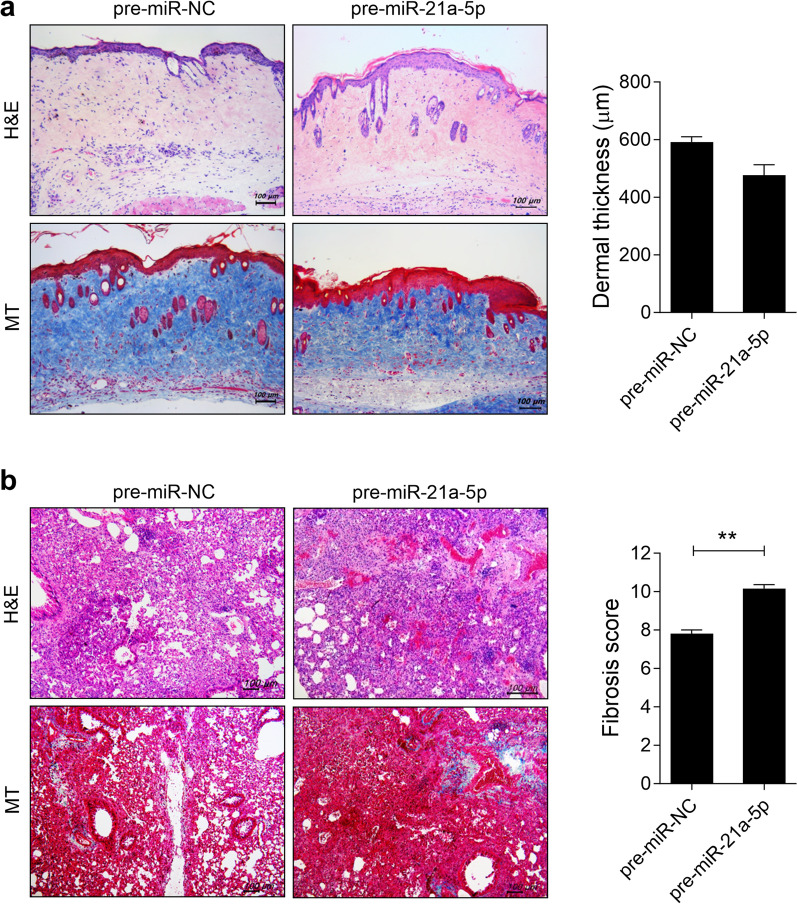
To investigate the pathological impact of miRNA-21a-5p on skin and lung fibrosis in vivo, we assessed fibrosis development through hydrodynamic injection of plasmids containing pre-miRNA-21a-5p, which is an expression vector containing the miR-21 precursor construct, or negative control (pre-miR-NC) into a bleomycin-induced SSc murine model. On days 14, 21, and 28 from the start of bleomycin injection, mice were hydrodynamically injected with 100 µg of plasmids containing pre-miRNA-21a-5p or pre-miR-NC in 1 mL saline (n = 5/group). Treatment with pre-miRNA-21a-5p did not alter the thickness of the dermal skin compared to BLM-induced SSc mice injected with pre-miR-NC (Fig. 1). However, pre-miRNA-21a-5p treatment promoted fibrosis and collagen deposition in the lungs of BLM-induced SSc mice. These findings suggest that miRNA-21a-5p may function as a pathogenic factor, potentially accelerating SSc progression.
Fig. 1.
MiRNA-21a-5p overexpression promotes skin and lung fibrosis in bleomycin-induced SSc mice. The SSc murine model was induced by subcutaneously injecting 100 μg bleomycin dissolved in 100 μL PBS into 8 week-old C57BL/6 mice daily for 5 weeks. On days 14, 21, and 28 from the start of bleomycin injection, plasmid containing of 100 μg pre-miRNA-21a-5p (n = 5) or pre-miR-NC as a control (n = 5) in 1 mL saline was hydrodynamically injected into the mice. The mice were sacrificed on day 33 from the start of bleomycin injection, and histologic analysis was performed. a, b Representative images of the skin stained with hematoxylin and eosin and Masson’s trichrome (MT) a and lung b tissues of bleomycin-induced mice injected with plasmid containing of pre-miRNA-21a-5p or pre-miR-NC. The graphs show the skin dermal thickness a and pulmonary fibrosis score b. Original magnification: 100 × . Scale bar: 100 µm
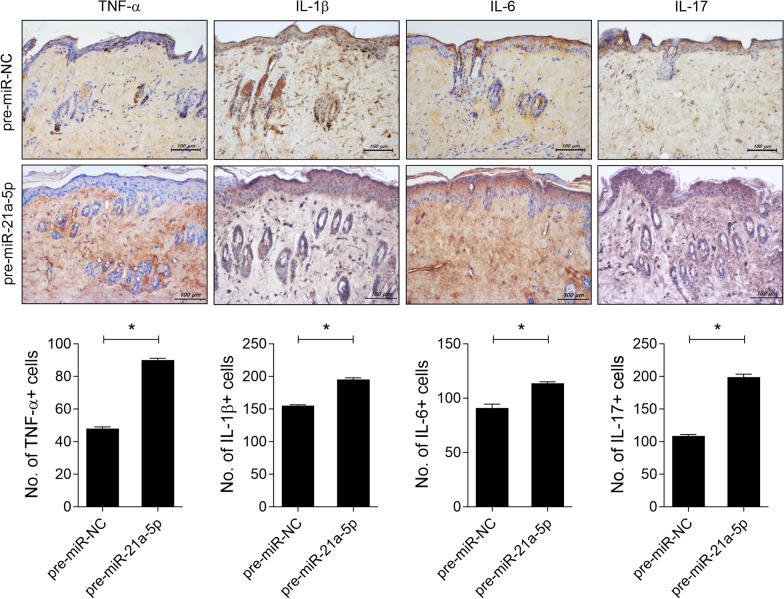
MiRNA-21a-5p increases the number of inflammatory cytokine-secreting cells
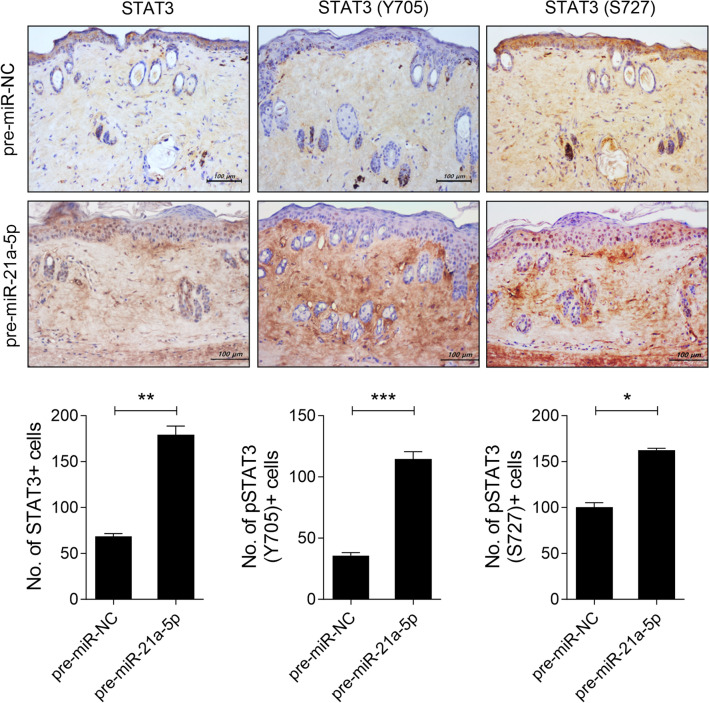
To assess the impact of miRNA-21a-5p on the infiltration of inflammatory cells in the skin of SSc mice, we conducted immunohistochemistry on the skin tissue of BLM-induced SSc mice injected with either plasmid containing pre-miRNA-21a-5p or pre-miR-NC. MiRNA-21a-5p overexpression led to increased infiltration of cells producing TNF-α, IL-1β, IL-6, or IL-17 compared to the control group (Fig. 2). These cytokines activate the STAT3 pathway [28–30], which then promote fibroblast activation and tissue fibrosis [31]. We further investigated the role of miRNA-21a-5p in STAT3 phosphorylation in the skin of SSc mice. Treatment of plasmid containing miRNA-21a-5p increased the number of STAT3-positive cells in the skin tissue of SSc mice, particularly those with STAT3 phosphorylation at tyrosine 705 and serine 727 compared to the control group (Fig. 3). These findings suggest that miRNA-21a-5p may contribute to tissue fibrosis in SSc by activating cells that secrete various inflammatory cytokines and promoting STAT3 phosphorylation in the skin tissue.
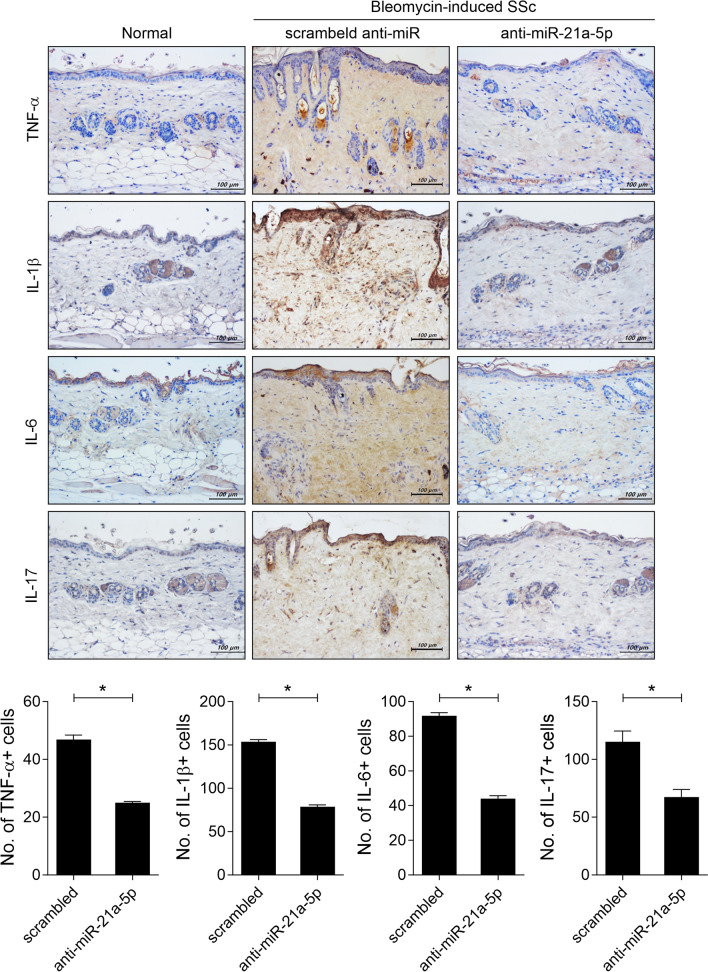
Fig. 2.
MiRNA-21a-5p treatment enhances inflammatory cell infiltration into the skin of bleomycin mice. On days 14, 21, and 28 from the start of bleomycin injection, plasmid containing of 100 μg pre-miRNA-21a-5p (n = 5) or pre-miR-NC as a control (n = 5) in 1 mL saline was hydrodynamically injected into the mice. The mice were sacrificed on day 33 from the start of bleomycin injection, and immunohistochemistry was performed on the skin tissue. Sections of skin tissue were stained with antibodies against TNF-α, IL-β, IL-6, and IL-17. The graphs present the number of antibody-positive cells (mean ± SD, n = 5/group). Original magnification: 200 × . Scale bar: 100 µm
Fig. 3.
MiRNA-21a-5p increases the number of STAT3-phosphorylated cells in the skin of bleomycin mice. On days 14, 21, and 28 from the start of bleomycin injection, plasmid containing of pre-miRNA-21a-5p (n = 5) or pre-miR-NC as a control (n = 5) was hydrodynamically injected into the mice. The mice were sacrificed on day 33 from the start of bleomycin injection, and skin tissue sections were stained with antibodies against STAT3, phosphorylated STAT3 at Y705, or S727. The graphs present the number of antibody-positive cells (mean ± SD, n = 5/group). Original magnification: 200 × . Scale bar: 100 µm
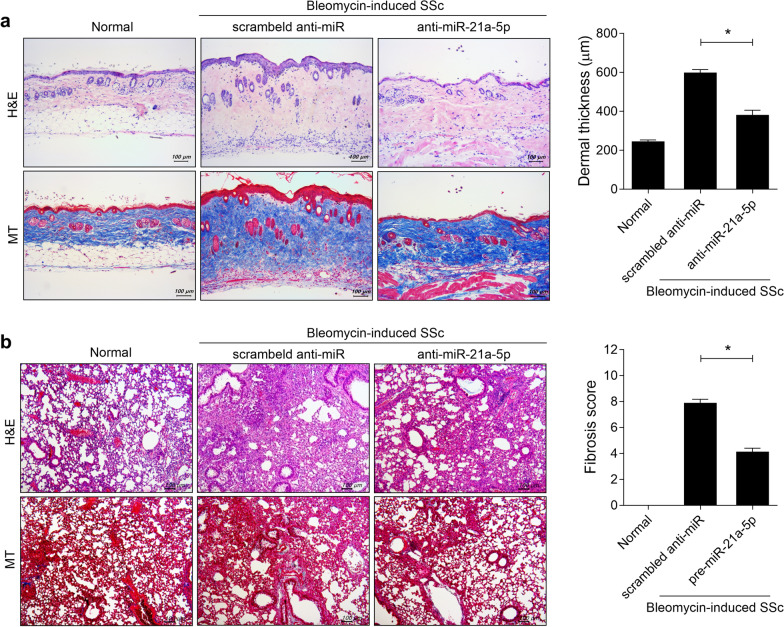
MiRNA-21a-5p inhibition attenuates bleomycin-induced dermal fibrosis in mice
To evaluate the impact of miRNA-21a-5p blockade on skin fibrosis in vivo, we compared the bleomycin-induced scleroderma model treated with plasmid containing of anti-sense miR against miRNA-21a-5p (miRZip-21 lentiviral vector expressing anti-sense miRNA-21a-5p, anti-miRNA-21) to that treated with miRZip-scrambled hairpin vector (scrambled anti-miR). On days 14, 21, and 28 from the start of bleomycin injection, mice were hydrodynamically injected with 100 µg of anti-miRNA-21a-5p or scrambled anti-miR in 1 mL saline (n = 5/group). Hydrodynamic injection of anti-miRNA-21a-5p resulted in reduced skin dermal thickness and alleviated collagen deposition compared to the group injected with scrambled anti-miR (Fig. 4). Moreover, miRNA-21a-5p inhibition led to an improvement in fibrosis development and collagen deposition in the lungs of BLM-induced SSc mice. These findings suggest that miRNA-21a-5p blockade can mitigate SSc progression.
Fig. 4.
MiRNA-21a-5p inhibition promotes skin and lung fibrosis in bleomycin-induced SSc mice. On days 14, 21, and 28 from the start of bleomycin injection, 100 μg miRZip-scrambled hairpin vector (scrambled anti-miR) as a control (n = 5) or anti-sense miR against miRNA-21a-5p (anti-miRNA-21a-5p), which inhibits miR-21a-5p (n = 5) in 1 mL saline was hydrodynamically injected into the mice. The mice were sacrificed on day 33 from the start of bleomycin injection, and histologic analysis was performed. a, b Representative images of the skin stained with hematoxylin and eosin and Masson’s trichrome (MT) a and lung tissue b of bleomycin-induced mice injected with scrambled anti-miR or anti-miRNA-21a-5p. The graphs show the skin dermal thickness a and pulmonary fibrosis score b. Original magnification: 100 × . Scale bar: 100 µm
MiRNA-21a-5p suppression reduces infiltration of cells secreting inflammatory cytokines in lesional skin
To determine whether miRNA-21a-5p inhibition could reduce the infiltration of cells secreting inflammatory cytokines in the lesional skin of SSc mice, we investigated the expression of inflammatory cytokines in the skin tissue of BLM-induced SSc mice treated with anti-miRNA-21. MiRNA-21a-5p inhibition through plasmid containing of anti-miRNA-21a-5p treatment lowered the infiltration of cells secreting TNF-α, IL-β, IL-6, or IL-17 in the lesional skin tissue of SSc mice (Fig. 5).
Fig. 5.
MiRNA-21a-5p inhibition decreases inflammatory cell infiltration into the lesional skin of bleomycin-induced SSc mice. On days 14, 21, and 28 from the start of bleomycin injection, plasmid containing of 100 μg scrambled anti-miR (n = 5) or anti-miRNA-21a-5p (n = 5) in 1 mL saline was hydrodynamically injected into the mice. The mice were sacrificed on day 33 from the start of bleomycin injection, and skin tissue sections were immunohistochemically stained for TNF-α, IL-β, IL-6, and IL-17. The graphs present the number of antibody-positive cells (mean ± SD, n = 5/group). Original magnification: 200 × . Scale bar: 100 µm
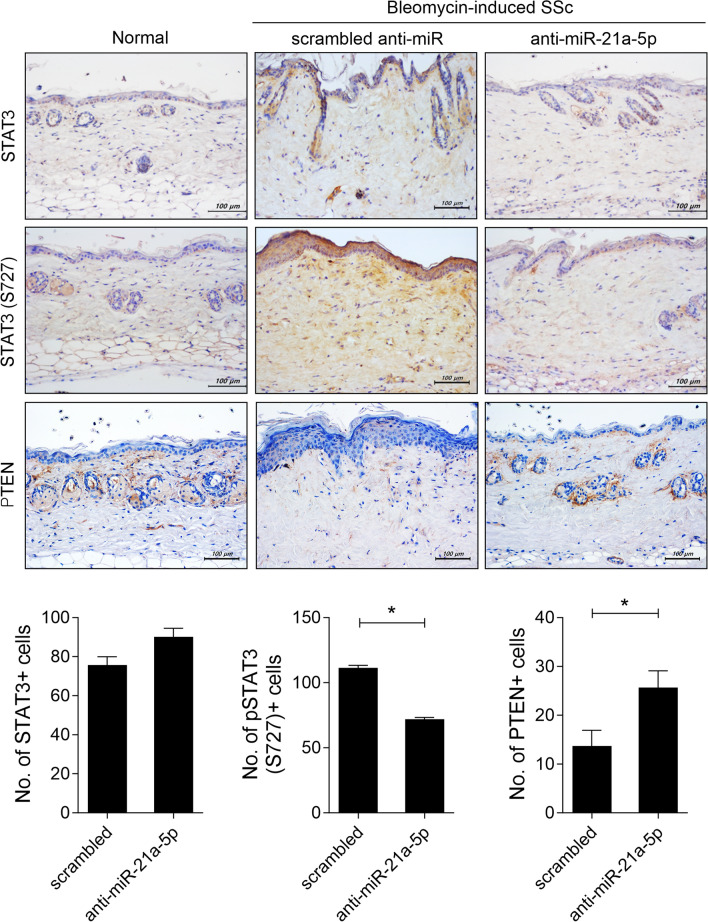
MiRNA-21a-5p inhibition reduces STAT3-phosphorylated cell invasion and increases the number of PTEN-positive cells in the skin tissue of BLM-induced SSc mice
Subsequently, we examined whether blocking miRNA-21a-5p affected phosphorylated STAT3-positive cell infiltration within lesional skin in BLM-induced SSc mice. Compared to the control group injected with scrambled anti-miR, there were no differences in the infiltration of STAT3-positive cells. However, the number of phosphorylated STAT3-positive cells was significantly reduced in the skin tissue of mice injected with anti-miRNA-21a-5p (Fig. 6). Previous studies have reported that PTEN knockout in fibroblasts increases collagen deposition [32], and PTEN levels are low, with an expression negatively correlated with the miRNA-21-5p level in human keloid fibroblasts [33]. Therefore, we investigated whether miRNA-21a-5p inhibition affects PTEN level in the skin of SSc mice. Anti-miRNA-21a-5p treatment increased PTEN-positive cell infiltration into the lesional skin of SSc mice (Fig. 6). These results suggest that controlling the action of miRNA-21-5p may lead to SSc improvement.
Fig. 6.
MiRNA-21a-5p inhibition prevented STAT3-phosphorylated cell invasion and increased the number of PTEN-positive cells in the skin tissue of bleomycin-induced SSc mice. On days 14, 21, and 28 from the start of bleomycin injection, plasmid containing of scrambled anti-miR (n = 5) or anti-miRNA-21a-5p (n = 5) was hydrodynamically injected into the mice. The mice were sacrificed on day 33 from the start of bleomycin injection, and skin tissue sections were stained with antibodies against STAT3, phosphorylated STAT3 at S727, and PTEN. The graphs present the number of antibody-positive cells (mean ± SD, n = 5/group). Original magnification: 200 × . Scale bar: 100 µm
Discussion
The present study demonstrated that miRNA-21a-5p inhibition ameliorated fibrosis by reducing phosphorylated STAT3-positive cell infiltration and increasing PTEN-positive cell infiltration in the lesional skin of SSc mice. In the skin of BLM-induced SSc mice, miRNA-21a-5p overexpression increased the infiltration of cells expressing inflammatory factors, including TNF-α, IL-1β, IL-6, and IL-17, as well as STAT3 phosphorylated cells. Conversely, miRNA-21a-5p inhibition through plasmid containing anti-miRNA-21a-5p treatment reduced the number of inflammatory cytokine-positive cells in the skin tissue of BLM-induced SSc mice. Moreover, miRNA-21a-5p inhibition decreased STAT3-phosphorylated cell infiltration and increased the infiltration of cells positive for PTEN, a regulator of collagen deposition [32], in the skin tissue of SSc mice. Based on the elevated miRNA-21a-5p expression in the SSc mouse model and its reported promotion of fibrogenic skin activation [21], our findings suggest that blocking miRNA-21-5p could be a therapeutic strategy for alleviating SSc development.
MiRNAs play a pivotal role in the pathogenesis of tissue fibrosis in SSc, influencing ECM synthesis and TGF-β signaling [9]. In particular, dysregulated miRNA expression associated with SSc fibrosis has been reported in the skin tissue of diffuse cutaneous SSc patients. These miRNAs regulate the expression of fibrosis-related target genes, including SMAD3 and SMAD7 [34]. Among the significantly increased miRNAs in SSc, miRNA-21-5p has been extensively investigated for its potential role in skin fibrosis. Elevated miRNA-21-5p levels have been reported in the serum of SSc patients [20], and TGF-β stimulation in skin fibroblasts increases miRNA-21-5p, which negatively regulates SMAD7, exhibiting profibrogenic effects [21]. MiRNA-21-5p overexpression in SSc fibroblasts increases the mRNA and protein levels of the anti-apoptotic gene Bcl2, while inhibition of miRNA-21-5p induces apoptosis in apoptosis-resistant SSc fibroblasts [35]. Despite substantial evidence suggesting a pathogenic role for miRNA-21-5p in progressive fibrosis in SSc, the efficacy of miRNA-21-5p has not been investigated in an in vivo system of SSc. The present study demonstrated that miRNA-21a-5p overexpression promotes inflammatory cell infiltration and exacerbates fibrosis in lesional skin of BLM-induced SSc mice. Conversely, miRNA-211-5p inhibition through plasmid containing anti-miRNA-21a-5p injection alleviated fibrosis in both lungs and skin, reducing inflammatory cell infiltration in skin tissue. In particular, anti-miRNA-21a-5p plasmid treatment increased PTEN expression, which negatively regulates collagen deposition in the lesional skin [32]. TGF-β upregulates miRNA-21-5p and decreases PTEN expression in fibroblasts, and miRNA-21-5p inhibition restores the decreased PTEN expression caused by TGF-β [36, 37]. Although the molecular mechanism by which miRNA-21a-5p inhibition affects PTEN expression requires further investigation, our findings suggest that increased PTEN through miRNA-21a-5p inhibition may improve fibrosis by inhibiting collagen deposition.
STAT3 serves as a central integrator of TGF-β-mediated profibrotic effects. Increased STAT3 phosphorylation is observed in dermal fibroblasts of SSc patients, and TGF-β stimulation significantly increases STAT3 phosphorylation in healthy dermal fibroblasts [38, 39]. STAT3 inhibition through JAK2 inhibition or small molecule inhibitors reduces the activation of dermal fibroblasts and increase in collagen levels induced by TGF-β [38, 39]. TGF-β-mediated STAT3 phosphorylation is influenced not only by JAK2, but also by JNK, SRC, and c-ABL kinases in fibroblasts. Fibroblast-specific knockout of STAT3 alleviates the development of bleomycin-induced skin fibrosis [31]. The profibrotic cytokine IL-6 induces collagen-1 synthesis by STAT3-dependent TGF-β-Smad3 activation through Gremlin, a bone morphogenetic protein antagonist [40]. Previous studies have demonstrated that miRNA-21-5p overexpression promotes cardiac fibrosis through STAT3 signaling [41] while STAT3 directly activates transcription of miRNA-21-5p, and increased miRNA-21-5p expression suppresses PTEN during cellular transformation [42]. Our findings demonstrate that miRNA-21a-5p overexpression promotes STAT3 phosphorylation in the skin tissues of SSc mice, while miRNA-21a-5p inhibition downregulates STAT3 phosphorylation. Although no studies have investigated the relationship between miRNA-21a-5p and STAT3 in lesional skin of SSc, our findings suggest that miRNA-21a-5p may act on STAT3-mediated fibrosis in SSc.
Our results were obtained by examining the levels of inflammation-related factors in the skin of mice that overexpressed or suppressed miR-21a-5p using immunohistochemistry. There is a need to confirm the results using various experimental techniques. To clarify the potential therapeutic target of miR-21 in SSc, further studies are needed to determine the mechanism of action of miR-21 in target cells. To this end, it is necessary to analyze cellular mRNA profile and cell phenotypic changes to determine in specific cells. To gain more insight, it is also necessary to investigate related signaling pathways by performing multi-omics analysis on specific cells in the skin of mice that overexpress or suppress miR-21a-5p.
Conclusion
Our findings demonstrate that miRNA-21a-5p exacerbates fibrosis by promoting STAT3 phosphorylation and the expression of inflammation-related factors in an in vivo SSc mouse model. Furthermore, this was the first study to demonstrate that miRNA-21a-5p inhibition controls SSc fibrosis through STAT3 inhibition and PTEN upregulation. These findings highlight the potential of targeting miRNA-21 as a therapeutic strategy to alleviate SSc development.
Acknowledgements
None.
Author contributions
JSP participated in the data interpretation and writing the manuscript. JSP, CK, JC, HYJ, YMM and HK carried out the experiments and analyzed data. EKL, MLC and SHP conceived and designed the study, interpreted the data, and made critical revisions of the manuscript for important intellectual content. All authors read and approved the final manuscript.
Funding
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (Grant Number: HI20C1496) and by Korea Drug Development Fund funded by Ministry of Science and ICT, Ministry of Trade, Industry, and Energy, and Ministry of Health and Welfare (RS-2023-00217274, Republic of Korea) and by Basic Science Research Program through the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. RS-2023-00208207) and by the Ministry of Education (Grant Number 2021R1I1A1A01050939).
Availability of data and materials
All data are available in the manuscript or upon request to the authors.
Declarations
Ethics approval and consent to participate
The mice were housed under specific-pathogen-free conditions at the Institute of Medical Science of the Catholic University of Korea and provided standard mouse diet and water. All experimental procedures were approved by the Animal Research Ethics Committee of the Catholic University of Korea, adhering to National Institutes of Health guidelines (Permit number: 2017–0067–02).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Eun Kyung Lee, Mi-La Cho and Sung-Hwan Park have contributed equally to this work.
Contributor Information
Eun Kyung Lee, Email: leeek@catholic.ac.kr.
Mi-La Cho, Email: iammila@catholic.ac.kr.
Sung-Hwan Park, Email: rapark@catholic.ac.kr.
References
- 1.Gabrielli A, Avvedimento EV, Krieg T. Scleroderma. N Engl J Med. 2009;360:1989–2003. doi: 10.1056/NEJMra0806188. [DOI] [PubMed] [Google Scholar]
- 2.Bairkdar M, Rossides M, Westerlind H, Hesselstrand R, Arkema EV, Holmqvist M. Incidence and prevalence of systemic sclerosis globally: a comprehensive systematic review and meta-analysis. Rheumatology. 2021;60:3121–3133. doi: 10.1093/rheumatology/keab190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morrisroe K, Stevens W, Sahhar J, Ngian GS, Ferdowsi N, Hansen D, Patel S, Hill CL, Roddy J, Walker J, et al. The clinical and economic burden of systemic sclerosis related interstitial lung disease. Rheumatology. 2020;59:1878–1888. doi: 10.1093/rheumatology/kez532. [DOI] [PubMed] [Google Scholar]
- 4.Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390:1685–1699. doi: 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 5.Pattanaik D, Brown M, Postlethwaite BC, Postlethwaite AE. Pathogenesis of systemic sclerosis. Front Immunol. 2015;6:272. doi: 10.3389/fimmu.2015.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allanore Y, Simms R, Distler O, Trojanowska M, Pope J, Denton CP, Varga J. Systemic sclerosis. Nat Rev Dis Primers. 2015;1:15002. doi: 10.1038/nrdp.2015.2. [DOI] [PubMed] [Google Scholar]
- 7.Lafyatis R. Transforming growth factor beta–at the centre of systemic sclerosis. Nat Rev Rheumatol. 2014;10:706–719. doi: 10.1038/nrrheum.2014.137. [DOI] [PubMed] [Google Scholar]
- 8.Hu HH, Chen DQ, Wang YN, Feng YL, Cao G, Vaziri ND, Zhao YY. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem Biol Interact. 2018;292:76–83. doi: 10.1016/j.cbi.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 9.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. doi: 10.1038/nature02871. [DOI] [PubMed] [Google Scholar]
- 11.Seeley JJ, Baker RG, Mohamed G, Bruns T, Hayden MS, Deshmukh SD, Freedberg DE, Ghosh S. Induction of innate immune memory via microRNA targeting of chromatin remodelling factors. Nature. 2018;559:114–119. doi: 10.1038/s41586-018-0253-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alevizos I, Illei GG. MicroRNAs as biomarkers in rheumatic diseases. Nat Rev Rheumatol. 2010;6:391–398. doi: 10.1038/nrrheum.2010.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broen JC, Radstake TR, Rossato M. The role of genetics and epigenetics in the pathogenesis of systemic sclerosis. Nat Rev Rheumatol. 2014;10:671–681. doi: 10.1038/nrrheum.2014.128. [DOI] [PubMed] [Google Scholar]
- 14.Affandi AJ, Radstake TR, Marut W. Update on biomarkers in systemic sclerosis: tools for diagnosis and treatment. Semin Immunopathol. 2015;37:475–487. doi: 10.1007/s00281-015-0506-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang L, Wu H, Zhao M, Lu Q. Meta-analysis of differentially expressed microRNAs in systemic sclerosis. Int J Rheum Dis. 2020;23:1297–1304. doi: 10.1111/1756-185X.13924. [DOI] [PubMed] [Google Scholar]
- 16.Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 17.Ho PTB, Clark IM, Le LTT. MicroRNA-based diagnosis and therapy. Int J Mol Sci. 2022;23:7167. doi: 10.3390/ijms23137167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garchow BG, Bartulos Encinas O, Leung YT, Tsao PY, Eisenberg RA, Caricchio R, Obad S, Petri A, Kauppinen S, Kiriakidou M. Silencing of microRNA-21 in vivo ameliorates autoimmune splenomegaly in lupus mice. EMBO Mol Med. 2011;3:605–615. doi: 10.1002/emmm.201100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xie J, Wu W, Zheng L, Lin X, Tai Y, Wang Y, Wang L. Roles of MicroRNA-21 in skin wound healing: a comprehensive review. Front Pharmacol. 2022;13:828627. doi: 10.3389/fphar.2022.828627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou B, Zuo XX, Li YS, Gao SM, Dai XD, Zhu HL, Luo H. Integration of microRNA and mRNA expression profiles in the skin of systemic sclerosis patients. Sci Rep. 2017;7:42899. doi: 10.1038/srep42899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu H, Luo H, Li Y, Zhou Y, Jiang Y, Chai J, Xiao X, You Y, Zuo X. MicroRNA-21 in scleroderma fibrosis and its function in TGF-beta-regulated fibrosis-related genes expression. J Clin Immunol. 2013;33:1100–1109. doi: 10.1007/s10875-013-9896-z. [DOI] [PubMed] [Google Scholar]
- 22.Vandewalle V, Essaghir A, Bollaert E, Lenglez S, Graux C, Schoemans H, Saussoy P, Michaux L, Valk PJM, Demoulin JB, Havelange V. miR-15a-5p and miR-21-5p contribute to chemoresistance in cytogenetically normal acute myeloid leukaemia by targeting PDCD4, ARL2 and BTG2. J Cell Mol Med. 2021;25:575–585. doi: 10.1111/jcmm.16110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morente-Lopez M, Mato-Basalo R, Lucio-Gallego S, Silva-Fernandez L, Gonzalez-Rodriguez A, De Toro FJ, Fafian-Labora JA, Arufe MC. Therapy free of cells vs human mesenchymal stem cells from umbilical cord stroma to treat the inflammation in OA. Cell Mol Life Sci. 2022;79:557. doi: 10.1007/s00018-022-04580-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moon J, Lee SY, Choi JW, Lee AR, Yoo JH, Moon SJ, Park SH, Cho ML. Metformin ameliorates scleroderma via inhibiting Th17 cells and reducing mTOR-STAT3 signaling in skin fibroblasts. J Transl Med. 2021;19:192. doi: 10.1186/s12967-021-02860-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okamoto Y, Hasegawa M, Matsushita T, Hamaguchi Y, Huu DL, Iwakura Y, Fujimoto M, Takehara K. Potential roles of interleukin-17A in the development of skin fibrosis in mice. Arthr Rheum. 2012;64:3726–3735. doi: 10.1002/art.34643. [DOI] [PubMed] [Google Scholar]
- 26.Yoshizaki A, Iwata Y, Komura K, Ogawa F, Hara T, Muroi E, Takenaka M, Shimizu K, Hasegawa M, Fujimoto M, et al. CD19 regulates skin and lung fibrosis via toll-like receptor signaling in a model of bleomycin-induced scleroderma. Am J Pathol. 2008;172:1650–1663. doi: 10.2353/ajpath.2008.071049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Park JS, Park HJ, Park YS, Lee SM, Yim JJ, Yoo CG, Han SK, Kim YW. Clinical significance of mTOR, ZEB1, ROCK1 expression in lung tissues of pulmonary fibrosis patients. BMC Pulm Med. 2014;14:168. doi: 10.1186/1471-2466-14-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mori T, Miyamoto T, Yoshida H, Asakawa M, Kawasumi M, Kobayashi T, Morioka H, Chiba K, Toyama Y, Yoshimura A. IL-1beta and TNFalpha-initiated IL-6-STAT3 pathway is critical in mediating inflammatory cytokines and RANKL expression in inflammatory arthritis. Int Immunol. 2011;23:701–712. doi: 10.1093/intimm/dxr077. [DOI] [PubMed] [Google Scholar]
- 29.De Simone V, Franze E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, Sica GS, Sileri P, MacDonald TT, Pallone F, et al. Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34:3493–3503. doi: 10.1038/onc.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Yi T, Kortylewski M, Pardoll DM, Zeng D, Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J Exp Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chakraborty D, Sumova B, Mallano T, Chen CW, Distler A, Bergmann C, Ludolph I, Horch RE, Gelse K, Ramming A, et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun. 2017;8:1130. doi: 10.1038/s41467-017-01236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones CE, Sharick JT, Colbert SE, Shukla VC, Zent JM, Ostrowski MC, Ghadiali SN, Sizemore ST, Leight JL. Pten regulates collagen fibrillogenesis by fibroblasts through SPARC. PLoS ONE. 2021;16:e0245653. doi: 10.1371/journal.pone.0245653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Y, Wang X, Yang D, Xiao Z, Chen X. MicroRNA-21 affects proliferation and apoptosis by regulating expression of PTEN in human keloid fibroblasts. Plast Reconstr Surg. 2014;134:561e–573e. doi: 10.1097/PRS.0000000000000577. [DOI] [PubMed] [Google Scholar]
- 34.Zhu H, Li Y, Qu S, Luo H, Zhou Y, Wang Y, Zhao H, You Y, Xiao X, Zuo X. MicroRNA expression abnormalities in limited cutaneous scleroderma and diffuse cutaneous scleroderma. J Clin Immunol. 2012;32:514–522. doi: 10.1007/s10875-011-9647-y. [DOI] [PubMed] [Google Scholar]
- 35.Jafarinejad-Farsangi S, Farazmand A, Gharibdoost F, Karimizadeh E, Noorbakhsh F, Faridani H, Mahmoudi M, Jamshidi AR. Inhibition of MicroRNA-21 induces apoptosis in dermal fibroblasts of patients with systemic sclerosis. Int J Dermatol. 2016;55:1259–1267. doi: 10.1111/ijd.13308. [DOI] [PubMed] [Google Scholar]
- 36.Liu Y, Li Y, Li N, Teng W, Wang M, Zhang Y, Xiao Z. TGF-beta1 promotes scar fibroblasts proliferation and transdifferentiation via up-regulating MicroRNA-21. Sci Rep. 2016;6:32231. doi: 10.1038/srep32231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Qiu Y, Shi NW, Zhao JN, Wang YC, Jiang H, Qian HB. microRNA-21 mediates the TGF-beta1-induced migration of keratinocytes via targeting PTEN. Eur Rev Med Pharmacol Sci. 2016;20:3748–3759. [PubMed] [Google Scholar]
- 38.Pedroza M, To S, Assassi S, Wu M, Tweardy D, Agarwal SK. Role of STAT3 in skin fibrosis and transforming growth factor beta signalling. Rheumatology. 2018;57:1838–1850. doi: 10.1093/rheumatology/kex347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dees C, Tomcik M, Palumbo-Zerr K, Distler A, Beyer C, Lang V, Horn A, Zerr P, Zwerina J, Gelse K, et al. JAK-2 as a novel mediator of the profibrotic effects of transforming growth factor beta in systemic sclerosis. Arthritis Rheum. 2012;64:3006–3015. doi: 10.1002/art.34500. [DOI] [PubMed] [Google Scholar]
- 40.O'Reilly S, Ciechomska M, Cant R, van Laar JM. Interleukin-6 (IL-6) trans signaling drives a STAT3-dependent pathway that leads to hyperactive transforming growth factor-beta (TGF-beta) signaling promoting SMAD3 activation and fibrosis via Gremlin protein. J Biol Chem. 2014;289:9952–9960. doi: 10.1074/jbc.M113.545822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao W, Shi P, Ge JJ. miR-21 enhances cardiac fibrotic remodeling and fibroblast proliferation via CADM1/STAT3 pathway. BMC Cardiovasc Disord. 2017;17:88. doi: 10.1186/s12872-017-0520-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493–506. doi: 10.1016/j.molcel.2010.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the manuscript or upon request to the authors.