Abstract
Background
Chicken is one of the most numerous and widely distributed species around the world, and many studies support the multiple ancestral origins of domestic chickens. The research regarding the yellow skin phenotype in domestic chickens (regulated by BCO2) likely originating from the grey junglefowl serves as crucial evidence for demonstrating the multiple origins of chickens. However, beyond the BCO2 gene region, much remains unknown about the introgression from the grey junglefowl into domestic chickens. Therefore, in this study, based on whole-genome data of 149 samples including 4 species of wild junglefowls and 13 local domestic chicken breeds, we explored the introgression events from the grey junglefowl to domestic chickens.
Results
We successfully detected introgression regions besides BCO2, including two associated with growth trait (IGFBP2 and TKT), one associated with angiogenesis (TIMP3) and two members of the heat shock protein family (HSPB2 and CRYAB). Our findings suggest that the introgression from the grey junglefowl may impact the growth performance of chickens. Furthermore, we revealed introgression events from grey junglefowl at the BCO2 region in multiple domestic chicken breeds, indicating a phenomenon where the yellow skin phenotype likely underwent strong selection and was retained. Additionally, our haplotype analysis shed light on BCO2 introgression event from different sources of grey junglefowl into domestic chickens, possibly suggesting multiple genetic flows between the grey junglefowl and domestic chickens.
Conclusions
In summary, our findings provide evidences of the grey junglefowl contributing to the genetic diversity of domestic chickens, laying the foundation for a deeper understanding of the genetic composition within domestic chickens, and offering new perspectives on the impact of introgression on domestic chickens.
Supplementary Information
The online version contains supplementary material available at 10.1186/s40104-024-01006-7.
Keywords: BCO2, Domestic chickens, Grey junglefowl, Introgression
Background
As an essential poultry species closely associated with the origin and development of human agricultural civilization, chicken has been raised worldwide [1]. They are not only used for meat and egg production but also for ornamental and recreational purposes. And they have become the most numerous animals among livestock. Domestic chickens belong to the genus Gallus, which includes 4 diverse wild junglefowl species, the green junglefowl (G. varius), in Java and surrounding islands in Indonesia, the Ceylon junglefowl (G. lafayettii) in Sri Lanka, the grey junglefowl (G. sonneratii), native to Southern and Western India, and the red junglefowl (G. gallus), widely distributed across South Asia and Southeast Asia [2]. The red junglefowl further consists of 5 subspecies: Gallus gallus bankiva (G. g. bankiva) in Java, Gallus gallus murghi (G. g. murghi) in India, Kashmir, Nepal, Bangladesh, and Bhutan, Gallus gallus jabouillei (G. g. jabouillei) in North Vietnam and South China, Gallus gallus gallus (G. g. gallus) in Southeast Asia mainland (Thailand, Laos, Vietnam, and Cambodia), and Gallus gallus spadiceus (G. g. spadiceus) in Southwest China, Peninsular Malaysia, India, and Myanmar [1].
However, the issue of the domestic chickens’ origin has long been a contentious topic. Initially, Charles Darwin proposed that the red junglefowl was the ancestor of domestic chickens [3], a widely embraced viewpoint. With the advancement of molecular biology techniques, this perspective began to be challenged. Some researchers conducted haplotype analysis based on mitochondrial DNA sequences of domestic chickens and wild junglefowl species, which pointed toward a polyphyletic origin for domestic chickens, implying that domestic chickens may have genetic lineage from the other wild junglefowl species [4]. Later, upon the publication of the domestic chicken whole-genome sequence, Eriksson et al. used the yellow skin phenotype of domestic chickens as a point of entry, based on phylogenetic analysis, they further proposed that the yellow skin phenotype in domestic chickens likely originated from the grey junglefowl [5]. This study provides the first evidence suggesting the potential introduction of genetic lineage from the other wild junglefowl species during the domestication of chickens. Subsequently, studies by Wang et al. [6] and Lawal et al. [7] furtherly revealed that the red junglefowl is the primary wild ancestor of domestic chickens, and the other three junglefowl species also contribute to the genetic diversity of domestic chickens.
Actually, interspecies introgression and gene flow are frequently observed in the domestication of animals [8], serving as a crucial mechanism for introducing novel genetic variations [9]. Currently, in a variety of animals and plants, instances of introgression between wild and domesticated species are gradually being reported. Examples include introgression from markhor to goats [9], wild relatives to domestic sheep populations (argali to Bashibai, Asiatic mouflon to Grey Shiraz, European mouflon to Caucasian [10], argali to Tibetan sheep [11, 12]), European wild grapes to cultivated wine grapes[13], wild emmer to modern wheat [14] and so on.
Presently, the introgression event from the wild junglefowl to the genome of domestic chickens is still relatively limited. Regarding the introgression from the grey junglefowl into domestic chickens, the significant study is by Eriksson et al. [5], who demonstrated that the BCO2 (beta-carotene oxygenase 2) region, which regulates yellow skin phenotype, likely originated from the grey junglefowl. Lawal et al. further detected genomic regions introgressed by the grey junglefowl in local chicken populations from South Asia, Central Asia, and Africa [7]. However, whether domestic chickens from the other regions have experienced introgression and to what extent remains unexplored. Wang et al. [6] conducted a study using domestic chickens from various regions worldwide, further confirming the contribution of the grey junglefowl to the genetic diversity of domestic chickens. Still, they did not delve into detailed exploration of introgressed gene regions. In summary, there are many unknown introgression regions that require further investigation and exploration.
Thus, in the present study, we analyzed the genome sequence of 149 chickens including green junglefowl, grey junglefowl, Ceylon junglefowl, red junglefowl, and 13 domestic chicken breeds. We utilized genomic data to investigate the introgression from the grey junglefowl into domestic chickens, further exploring the introgression regions beyond the BCO2 gene region in the chicken genome. And finally, we identified that the introgression from the grey junglefowl could potentially impact the growth performance of domestic chickens. Additionally, we furtherly uncovered evidence of multiple genetic flows between the grey junglefowl and domestic chickens. Our findings offer novel insights into comprehending the genetic diversity and origins of domestic chickens.
Methods
Samples and sequencing
A total of 149 samples were used in this study, of which including 15 green junglefowl, 9 grey junglefowl, 10 Ceylon junglefowl, 16 red junglefowl, 10 Canada indigenous chickens, 4 India local chickens and 11 Chinese indigenous chicken breeds. The 11 Chinese chicken breeds comprise Beijing You chickens, Heilongjiang Lindian chickens, Jiangsu Liyang chickens, Hubei Yunyang Da chickens, Guangdong Xinghua chickens, Guangxi Dongzhong Dwarf chickens, Hainan Wenchang chickens, Xinjiang Hetian local chickens, Jiangxi local chickens, Shandong local chickens, and Taiwan local chickens. Among the samples used, 25 samples were sequenced for this study, while the remaining were downloaded from public databases (http://bigd.big.ac.cn/chickensd [6], DRA003951 [15], PRJNA432200 [7], PRJNA552030 [16], PRJNA720223 [17], SAMN14651083 [18], PRJNA800119 [19] ). The detail information of samples could be found in the Additional file 1: Table S1. For the samples generated in this study, we extracted the genomic DNA using the routine phenol-chloroform method and performed sequencing on Illumina HiSeq 2500 system.
Quality control and variant calling
Raw reads were filtered by fastp [20]. And then, the filtered clean reads were mapped to the reference genome (Gallus gallus 6.0) using the Burrows-Wheeler Aligner (BWA) software [21] with default parameters. The aligned bam files were sorted using the Samtools (v.1.11) [22], and duplicated reads were marked with the “MarkDuplicates” module of Picard (https://broadinstitute.github.io/picard). Next, we performed SNP calling with the “Haplotypecaller” and “GenotypeGVCFs” parameter of the GATK [23]. Variants with “QUAL < 30.0 || QD < 5.0 || FS > 60.0 || MQ < 40.0 || MQRankSum < −12.5 || ReadPosRankSum < −8.0” were filtered using “VariantFiltration” function of GATK. Finally, 15,371,798 SNPS were obtained for the subsequent analysis.
Population structure analysis
To evaluate the population structure, we performed PCA (principal component analysis) using PLINK [24]. And then, we constructed a maximum likelihood (ML) tree implemented in SNPhylo [25] to reveal phylogenetic relationship. The ML tree was furtherly visualized using iTOL (https://itol.embl.de). Additionally, we used the Admixture software [26] to estimate the genetic composition of the populations by assuming the ancestral clusters (K) from 2 to 15. The final genetic structure and cluster results were visualized using Pophelper (http://pophelper.com) [27]. To mitigate the impact of LD on the population structure, we employed SNPs pruning with the "--indep-pairwise 50 5 0.05" option in PLINK.
Genomic introgression analysis
D-statistic method was employed in this study to detect introgression from the grey junglefowl to domestic chickens. D-statistic is also known as the ABBA-BABA test. Given a tree topology (((P1, P2), P3), O), the ABBA event indicates P2 shares more derived alleles with P3 (there are gene flow between P2 and P3), the BABA pattern indicates P1 shares more derived alleles with P3 (there are gene flow between P1 and P3). In our study, we used the green junglefowl as an outgroup, P1 is red junglefowl, P2 is a domestic chicken population, P3 is grey junglefowl. We performed D-statistic with Dsuite software [28]. First, we used Dtrios module to calculate D and f4-ratio statistics for all possible trios of populations. Subsequently, we used Dinvestigate module to evaluate the introgression level and locate the introgression region across the whole genome, using a sliding window contained 100 informative SNPs with a step of 20 SNPs. We considered the windows in the top 1% of the distribution of D values as the candidate introgression region. Then, we annotated genes in the related introgression region with the Biomart module of Ensembl (http://www.ensembl.org/biomart/martview).
Subsequently, we constructed the ML tree for the introgression genomic region using IQ-TREE [29] and visualized with online iTOL. And we compared the mean pairwise sequence divergence (dxy) between the introgressed domestic chickens and the grey junglefowl/the other domestic chicken using a 50-kb sliding window and a stepping of 10-kb [30].
In addition, to further examine the introgression event of yellow skin in domestic chickens, we constructed a haplotype network for BCO2 region using the POPART software [31] with default parameters.
Results
Population structure
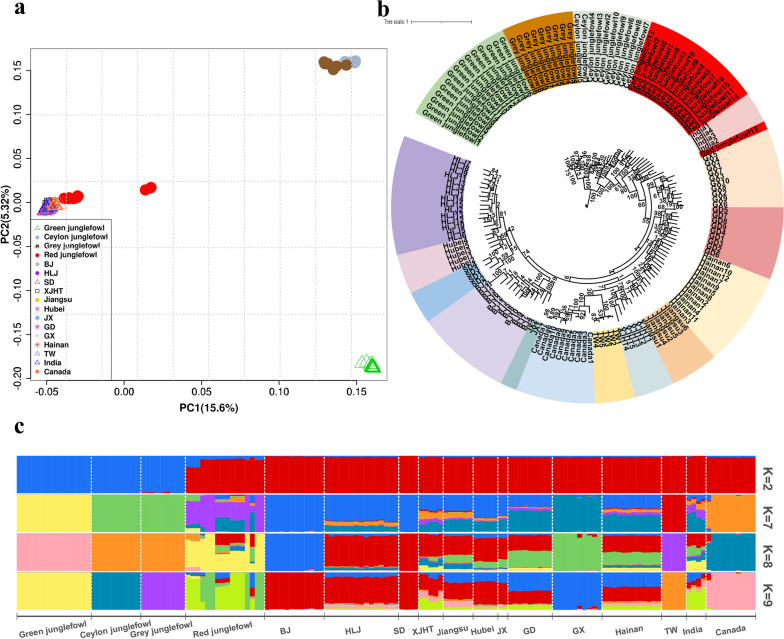
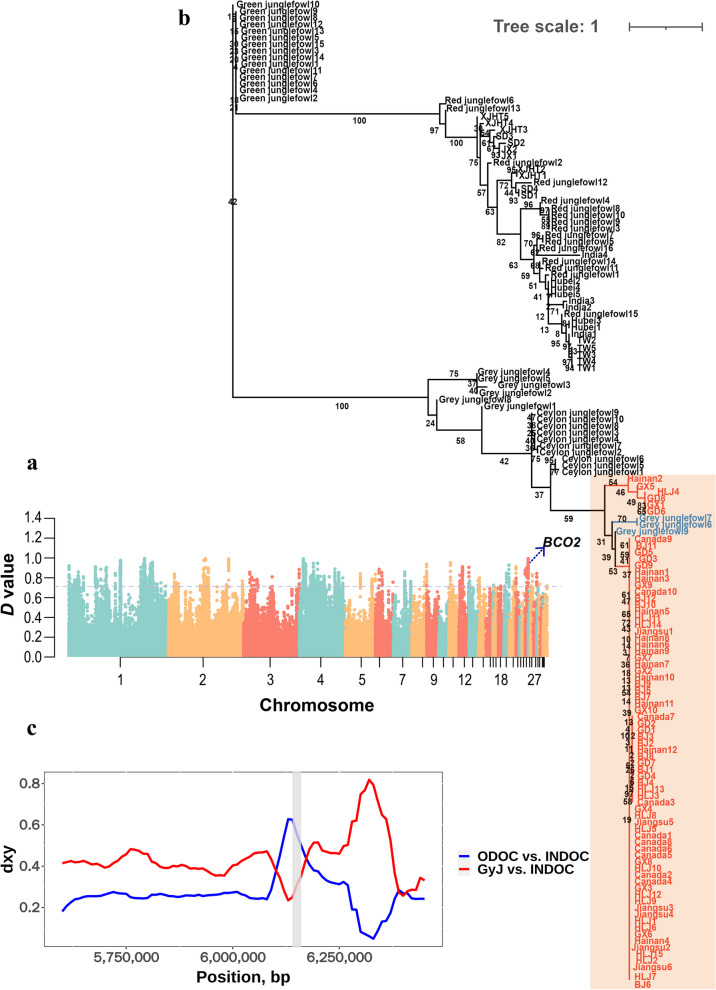
One hundred and forty-nine samples including 4 species of wild junglefowls and 13 local domestic chicken breeds were analyzed in the present study (Additional file 1: Table S1). After aligning the reads to the reference genome, 18.51 billion mapped reads were obtained, with an average sequencing depth of 15.5X per individual (Additional file 1: Table S2). Following variation calling and quality filtration, a total of 15.37 million SNPs were obtained for the subsequent analysis. First, we performed PCA to elucidate the genetic relationship among the populations in this study. In our PCA result, PC1 (the first principal component) could clearly separates 3 wild junglefowl species (green junglefowl, Ceylon junglefowl, and grey junglefowl) from domestic chickens, with domestic chickens exhibit a closer genetic clustering with the red junglefowl. Furthermore, we observed that the Ceylon junglefowl and grey junglefowl cluster together (Fig. 1a). Subsequently, a ML phylogenetic tree was constructed. The results show that domestic chickens could cluster according to the geographic origins and are closely clustered with the red junglefowl. And the Ceylon junglefowl and grey junglefowl were observed to cluster together (Fig. 1b). Additionally, we used Admixture to infer the population admixture proportions by assuming K from 2 to 15. When K = 2–8, we observed that the Ceylon junglefowl and grey junglefowl share common ancestral components, indicating a close relationship between these two junglefowl species. At K = 9, we found the separation of the Ceylon junglefowl and grey junglefowl into distinct clusters (Fig. 1c). In conclusion, all the aforementioned results support the sister-group relationship between the Ceylon junglefowl and grey junglefowl, as well as a closer relationship between the red junglefowl and domestic chickens.
Fig. 1.
Population structure analysis of samples in this study. a. PCA plot of samples in this study. The abbreviation of domestic chickens could be found in Additional file 1: Table S1. b. Phylogenetic tree of the samples in this study. c. Admixture results for K = 2, K = 7, K = 8 and K = 9
Introgression analysis
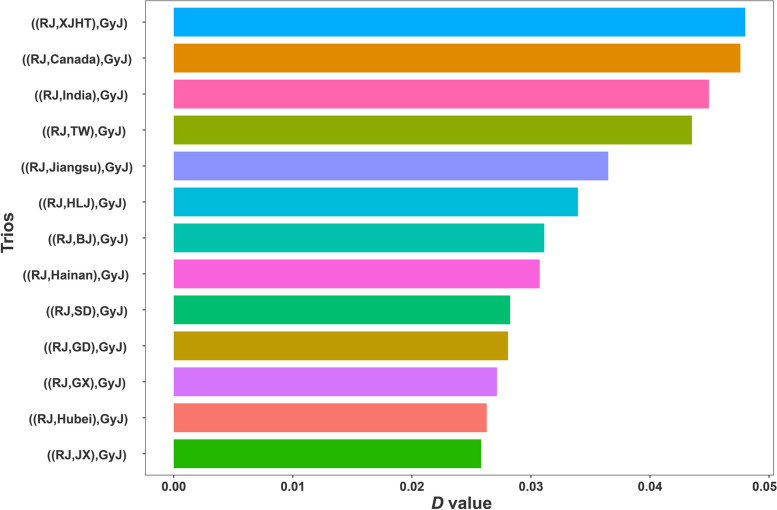
To investigate the introgression events from the grey junglefowl to domestic chickens, we calculated the D-statistics for each combination of domestic chicken populations and grey junglefowl. The result of D-statistics showed that all domestic chicken populations in this study exhibited significant introgression (Z > 3, P < 0.001), and the fraction of introgression ranging from 1.28% to 2.43% (Fig. 2 and Additional file 1: Table S3). We identified ((RJ, XJHT), GyJ) and ((RJ, Canada), GyJ) having the most significant D value and f4-ratio, indicating a relative high introgression level from the grey junglefowl to XJHT and Canada indigenous chickens, at 2.39% and 2.43%, respectively. Consequently, following that, we separately studied the genomic regions shared between the grey junglefowl and local chickens from XJHT, as well as between the grey junglefowl and chickens from Canada.
Fig. 2.
The result of D-statistics for introgression between the grey junglefowl and domestic chickens. The RJ is red junglefowl, the GyJ is grey junglefowl, and the remaining abbreviation of domestic chickens could be found in Additional file 1: Table S1
Introgression from grey junglefowl to XJHT indigenous chickens
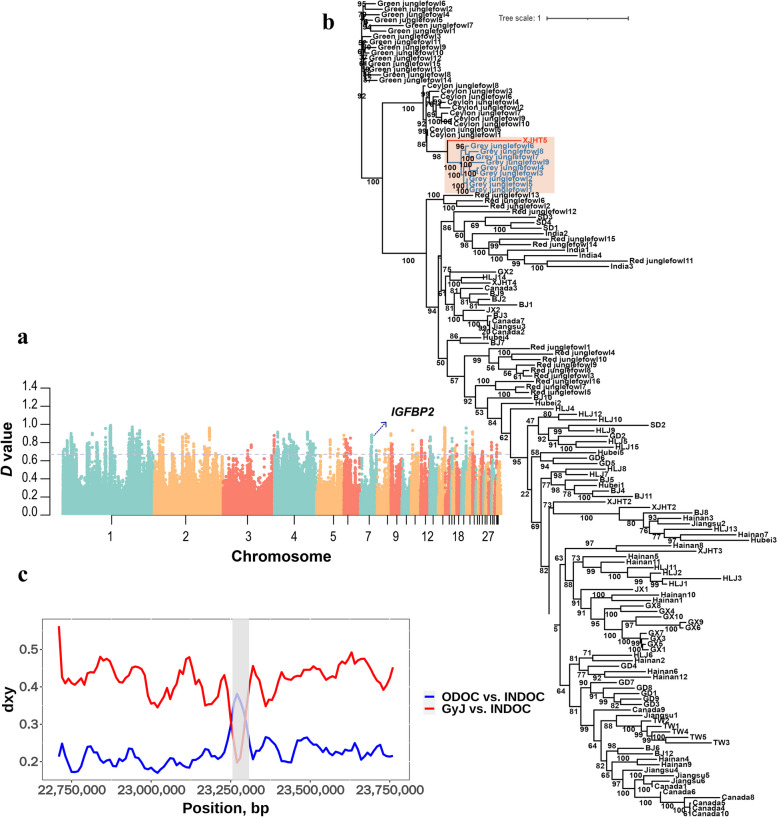
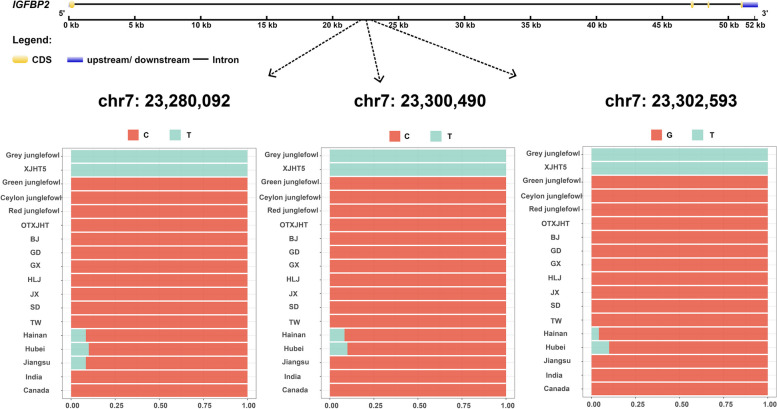
We further detected 173 candidate introgression genes from the grey junglefowl to XJHT indigenous chickens (Fig. 3a and Additional file 1: Table S4). In particular, we identified two genes related to the development of growth and angiogenesis including IGFBP2 (insulin like growth factor binding protein 2) and TIMP3 (TIMP metallopeptidase inhibitor 3). Taking IGFBP2 as an example, we first constructed an ML tree for this gene region. In the ML tree, we noticed that XJHT5 clustered with the grey junglefowl, while the remaining XJHT local chickens clustered together with the other domestic chickens (Fig. 3b and Table 1). Moreover, for this region, we observed a remarkably reduced dxy between XJHT5 and the grey junglefowl, in contrast to the significantly increased dxy in XJHT5 versus the other XJHT local chickens (Fig. 3c). To further understand the introgression event in the IGFBP2 region, we conducted a study on the gene variation. We found 3 SNPs were exhibiting homozygous mutations in both grey junglefowl and the introgressed XJHT5 individual, while these mutations were either absent or have extremely low allele frequencies in the other junglefowl and domestic chickens (Fig. 4). The result reconfirmed the introgression event at the IGFBP2 gene region. Additionally, we also performed ML tree construction and dxy comparison in the TIMP3 related to angiogenesis. The result was consistent with those above, and further supported the introgression from the grey junglefowl to XJHT chickens (Additional file 2: Fig. S1 and Table 1).
Fig. 3.
Introgression at the IGFBP2 gene region. a. Manhattan plot of D values between XJHT local chickens and grey junglefowl. The dashed line indicates the significance threshold (top 1% of the distribution of D values). b. ML tree constructed with the IGFBP2 gene sequence. The introgression event was highlighted with orange color, the introgressed XJHT individual and the grey junglefowl were highlighted with red and blue color, respectively. c. Mean pairwise sequence divergence at the IGFBP2 gene region between the introgressed XJHT local chickens (INDOC) and either grey junglefowl (GyJ) or remaining non-introgressed XJHT local chickens (ODOC), represents by red and blue lines, respectively. The shaded area represents the IGFBP2 gene region
Table 1.
The detail information of 6 introgressed genes
| Gene name | Chromosomal position | Breeds shown introgression | Number of introgressed animals | IDs of introgressed animals |
|---|---|---|---|---|
| TIMP3 | 1:53,094,947–53,127,580 | XJHT | 1 | XJHT4 |
| IGFBP2 | 7:23,255,548–23,307,791 | XJHT | 1 | XJHT5 |
| TKT | 12:7,348,689–7,371,332 | Canada | 4 | Canada2, Canada3, Canada8, Canada9 |
| BCO2 | 24:6,140,068–6,160,788 | Hainan, GX, BJ, Canada, HLJ, GD, Jiangsu | 74 | Hainan1, Hainan2, Hainan3, Hainan4, Hainan5, Hainan6, Hainan7, Hainan8, Hainan9, Hainan10, Hainan11, Hainan12, GX1, GX2, GX3, GX4, GX5, GX6, GX7, GX8, GX9, GX10, BJ1, BJ2, BJ3, BJ4, BJ5, BJ6, BJ7, BJ8, BJ9, BJ10, BJ11, BJ12, Canada1, Canada2, Canada3, Canada4, Canada5, Canada6, Canada7, Canada8, Canada9, Canada10, HLJ1, HLJ2, HLJ3, HLJ4, HLJ5, HLJ6, HLJ7, HLJ8, HLJ9, HLJ10, HLJ11, HLJ12, HLJ13, HLJ14, HLJ15, GD1, GD2, GD3, GD4, GD5, GD6, GD7, GD8, GD9, Jiangsu1, Jiangsu2, Jiangsu3, Jiangsu4, Jiangsu5, Jiangsu6 |
| HSPB2 | 24:6,227,008–6,259,713 | Hainan, GX, SD, BJ, Canada, HLJ | 14 | Hainan6, Hainan10, GX1, GX9, GX10, BJ6, BJ11, SD1, Canada4, Canada9, Canada10, HLJ2, HLJ4, HLJ6 |
| CRYAB | 24:6,239,995–6,246,232 | Hainan, GX, SD, BJ, Canada, HLJ | 14 | Hainan6, Hainan10, GX1, GX9, GX10, BJ6, BJ11, SD1, Canada4, Canada9, Canada10, HLJ2, HLJ4, HLJ6 |
Fig. 4.
Allele frequency distribution of 3 SNPs (chr7: 23,280,092, chr7: 23,300,490 and chr7: 23,302,593) within IGFBP2 in the wild junglefowl, introgressed XJHT individual (XJHT5), non-introgressed XJHT chickens (OTXJHT), and the remaining domestic chickens
Introgression from grey junglefowl to Canada indigenous chickens
In Canada local chickens, a 20 kb region (within the range of 6.14–6.16 Mb) on chromosome 24 exhibited a significant signal of admixture with the grey junglefowl (Fig. 5a and Additional file 1: Table S5). This region contains BCO2 gene, which regulates the yellow skin phenotype. We applied the same methods to study the BCO2 gene as mentioned for IGFBP2. In the ML tree, we found that some domestic chickens clustered together with the grey junglefowl, while the rest of the domestic chickens formed a separate cluster with the red junglefowl (Fig. 5b and Table 1), consistent with the previous studies [7]. Notably, the chickens clustering with the grey junglefowl included not only Canada indigenous chickens but also those from BJ, GD, GX, HLJ, Hainan, and Jiangsu. To validate the results observed from the phylogenetic tree, we further examined the regions originating from the grey junglefowl in the genomes of these domestic chicken populations (Additional file 1: Table S6–S11). The results confirmed that BCO2 was commonly identified as a candidate introgression region in these populations, supporting the findings from the ML tree. Furthermore, the result of dxy indicated that within this region, the dxy between the introgressed individuals and the grey junglefowl was significantly decreased, while the dxy between the introgressed individuals and the other domestic chickens was significantly increased (Fig. 5c). Additionally, we also identified one gene related to growth regulation (TKT (transketolase)) and two heat shock protein members (CRYAB (crystallin alpha B), HSPB2 (heat shock protein family B)). ML tree and dxy results of these gene regions all indicate admixture between the grey junglefowl and Canada indigenous chickens (detail see Table 1, Additional file 3: Fig. S2 and Additional file 4: Fig. S3).
Fig. 5.
Introgression at the BCO2 gene region. a. Manhattan plot of D values between Canada local chickens and grey junglefowl. The dashed line indicates the significance threshold (top 1% of the distribution of D values). b. ML tree constructed with the BCO2 gene sequence. The introgression event was highlighted with orange color, the introgressed chickens and the grey junglefowl were highlighted with red and blue color, respectively. c. Mean pairwise sequence divergence at the BCO2 gene region between the introgressed individuals (INDOC) and either grey junglefowl (GyJ) or remaining non-introgressed domestic chickens (ODOC), represents by red and blue lines, respectively. The shaded area represents the BCO2 gene region
Exploring the origin of yellow skin phenotype in domestic chickens
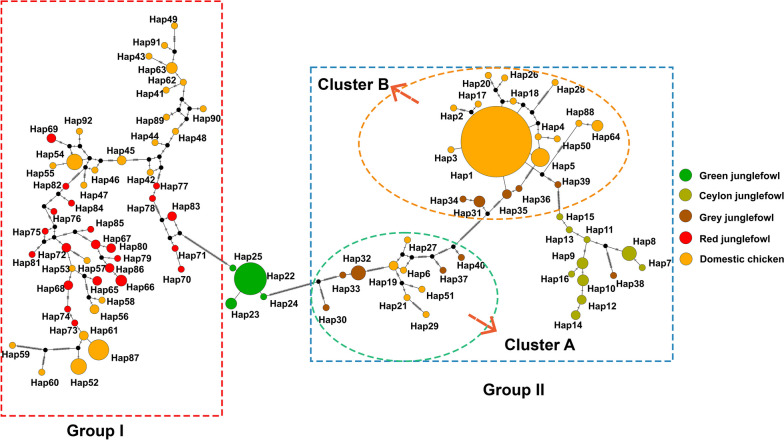
To further explore the origin of the yellow skin phenotype in domestic chickens, we constructed a haplotype network using the BCO2 gene region. Our results show that the entire haplotype network can be divided into two parts including group I and group II, with the green junglefowl haplotype as the dividing point, highlighted by red and blue frames, respectively. The group I consists of the red junglefowl and some domestic chickens, while the group II consists of the grey junglefowl, Ceylon junglefowl, and the remaining domestic chickens (Fig. 6). The clustering results and pattern of the haplotype network were consistent with that observed in the ML tree (Fig. 5b).
Fig. 6.
Haplotype network constructed with the BCO2 gene region. Two different cluster group including group I and group II were indicated by red and blue frames, respectively. And further haplotype clustering part of domestic chickens and grey junglefowl were indicated by green and yellow circles, respectively
Additionally, we found that the haplotype clustering of domestic chickens and grey junglefowl can be further divided into two parts including cluster A and cluster B, highlighted by green and yellow circles, respectively. In either part, it is evident that these domestic chickens’ haplotypes are closer to the grey junglefowl, supporting the yellow skin phenotype in domestic chickens actually originated from the grey junglefowl. Furthermore, we observed that in these two distinct clusters, cluster A is closer to the green junglefowl, while cluster B is relatively more distant. Moreover, we clearly observed Hap1 in the cluster B as being the main haplotype in the introgressed domestic chickens.
Discussion
Using genomic data from 4 wild junglefowl species and 13 indigenous chicken populations from China, India, and Canada, we systematically investigated the introgression events from the grey junglefowl into domestic chickens, and uncovered additional genomic introgression regions in the chicken genome beyond BCO2, providing valuable insights into the contribution of grey junglefowl to the genetic diversity of domestic chickens.
Our phylogenetic and population structure analysis results support a closer genetic relationship between the red junglefowl and domestic chickens. Additionally, we observed in PCA and the phylogenetic tree that the grey junglefowl and Ceylon junglefowl are genetically closer, and admixture analysis further indicates a shared ancestral component within these two junglefowl species. Therefore, our results suggest a sister-group relationship between the grey junglefowl and Ceylon junglefowl. These findings are consistent with the previous analyses based on whole-genome data [6, 7, 16].
Moreover, we confirmed the introgression events from the grey junglefowl into domestic chickens by D-statistics analysis. We detected certain genes related to growth regulation, including IGFBP2 and TKT. IGFBP2 is a member of the IGFBP family and further influences the growth and development of animals by regulating the biological activity of IGF1 [32]. Researches by Tapanainen et al. [33] and Solomon et al. [34] found that overexpression of IGFBP2 results in fetal growth restriction and leads to a significant decrease in mouse body weight. In domestic chickens, previous studies also indicated its significance as a candidate gene affecting chicken growth traits [32, 35, 36]. TKT is an essential enzyme in the pentose phosphate pathway (PPP), and is involved in growth regulation. Existing research has demonstrated that a deficiency of TKT could lead to short stature and development delay [37]. In this study, we utilized genomic data to confirm the introgression events at the IGFBP2 and TKT gene region from the grey junglefowl into domestic chickens. However, the functional consequences of this introgression of growth-related genes remain unclear. Therefore, further research and investigation are necessary to uncover the phenotypic differences between the introgressed individuals and the non-introgressed domestic chickens. This will contribute to better understanding the consequences of genetic introgression.
Notably, we simultaneously identified a significant introgression signal (chr24: 6,140,068–6,160,788) from the grey junglefowl into multiple chicken populations, which includes the BCO2 gene. The BCO2 gene plays a crucial role in the degradation of skin carotenoids [38]. Multiple studies have confirmed that this gene is a key regulator of the yellow skin phenotype in domestic chickens [5, 38–41]. Taking market demand into consideration, we believe that the phenomenon identified in this study, where the BCO2 introgression event was found in multiple domestic chicken breeds, may be attributed to the preference for yellow-skinned domestic chickens among people. This preference has likely led to intense selection on this region, ultimately resulting in its retention. Furthermore, we delved into the detail of yellow skin introgression event in domestic chickens by constructing haplotype networks. From the haplotype networks, we observed two distinct clusters include domestic chickens and grey junglefowl. We believe this may reflect the different introgression sources at BCO2 gene region. In these two distinct clusters, cluster A is closer to the green junglefowl, while cluster B is relatively more distant (Fig. 6). Therefore, we suggest that this may indicate a difference of early and recent introgression event, and possibly reflect multiple genetic flow events between the grey junglefowl and domestic chickens. We can also clearly observe that Hap 1 haplotype in cluster B is the predominant haplotype in the introgressed domestic chickens, which may indicate that this haplotype has undergone widespread dissemination and retention within the domestic chicken population. In summary, these results provide a new insight into the yellow skin phenotype introgression events from the grey junglefowl to the domestic chicken.
Additionally, we also identified two members of the small heat shock protein family, including CRYAB and HSPB2, which have been introgressed in many domestic chickens. They play crucial roles in cellular defense mechanisms when organisms are exposed to high-temperature environments [42] and may be involved in the high-altitude adaptation of animals [43, 44]. These two genes are located within the significantly introgressed region on chromosome 24, downstream of BCO2, spanning from 0.07 Mb to 0.08 Mb. In fact, the introgression event of BCO2 has been detected in multiple chicken populations due to strong selection on the yellow skin phenotype in domestic chickens [45]. Considering the close physical position of these two genes to BCO2, we suggest that the introgression events of CRYAB and HSPB2 are likely a result of a hitchhiking effect.
Conclusion
Based on whole-genome data of wild junglefowl and domestic chickens, we identified introgression events from the grey junglefowl to domestic chickens in regions beyond BCO2. These introgression events have the potential to impact the growth performance. Furthermore, by conducting a detailed haplotype analysis of the BCO2 region, we believe that the yellow skin phenotype likely underwent strong selection and was retained. And we uncovered evidence of multiple genetic flow between the grey junglefowl and domestic chickens. Therefore, our research is of significant importance in understanding the contribution of grey junglefowl to the genetic diversity of domestic chickens.
Supplementary Information
Additional file 1: Table S1. Summary of samples included in this study. Table S2. Summary of genome sequencing and mapping statistic. Table S3. Results of D statistics. Table S4. Significant introgression region between the grey junglefowl and XJHT local chickens. Table S5. Significant introgression region between the grey junglefowl and Canada local chickens. Table S6. Significant introgression region between the grey junglefowl and BJ local chickens. Table S7. Significant introgression region between the grey junglefowl and GD local chickens. Table S8. Significant introgression region between the grey junglefowl and GX local chickens. Table S9. Significant introgression region between the grey junglefowl and HLJ local chickens. Table S10. Significant introgression region between the grey junglefowl and Hainan local chickens. Table S11. Significant introgression region between the grey junglefowl and Jiangsu local chickens. Table S12. Significant introgression region between the grey junglefowl and SD local chickens.
Additional file 2: Fig. S1. Introgression at the TIMP3 gene region. a. Manhattan plot of D values between XJHT local chickens and grey junglefowl. The dashed line indicates the significance threshold (top 1% of the distribution of D values). b. ML tree constructed with the TIMP3 gene sequence. The introgressed event was highlighted with orange color, the introgressed XJHT individual and the grey junglefowl were highlighted with red and blue color, respectively. c. Mean pairwise sequence divergence at the TIMP3 gene region between the introgressed XJHT local chickens (INDOC) and either grey junglefowl (GyJ) or remaining non-introgressed XJHT local chickens (ODOC), represents by red and blue lines, respectively. The shaded area represents the TIMP3 gene region.
Additional file 3: Fig. S2. Introgression at the TKT gene region. a. Manhattan plot of D values between Canada local chickens and grey junglefowl. The dashed line indicates the significance threshold (top 1% of the distribution of D values). b. ML tree constructed with the TKT gene sequence. The introgressed event was highlighted with orange color, the introgressed Canada individuals and the grey junglefowl were highlighted with red and blue color, respectively. c. Mean pairwise sequence divergence at the TKT gene region between the introgressed Canada local chickens (INDOC) and either grey junglefowl (GyJ) or remaining non-introgressed Canada local chickens (ODOC), represents by red and blue lines, respectively. The shaded area represents the TKT gene region.
Additional file 4: Fig. S3. Introgression at the CRYAB and HSPB2 gene region. a. Manhattan plot of D values between Canada local chickens and grey junglefowl. The dashed line indicates the significance threshold (top 1% of the distribution of D values). b. ML tree constructed with the CRYAB gene sequence. The introgressed event was highlighted with orange color, the introgressed individuals and the grey junglefowl were highlighted with red and blue color, respectively. c. ML tree constructed with the HSPB2 gene sequence. The introgressed event was highlighted with orange color, the introgressed individuals and the grey junglefowl were highlighted with red and blue color, respectively. d. Mean pairwise sequence divergence at the CRYAB gene region between the introgressed individuals (INDOC) and either grey junglefowl (GyJ) or remaining non-introgressed domestic chickens (ODOC), represents by red and blue lines, respectively. The shaded area represents the CRYAB gene region. e. Mean pairwise sequence divergence at the HSPB2 gene region between the introgressed individuals (INDOC) and either grey junglefowl (GyJ) or remaining non-introgressed domestic chickens (ODOC), represents by red and blue lines, respectively. The shaded area represents the HSPB2 gene region.
Acknowledgements
We gratefully acknowledge our colleagues in the Poultry Team at the National Engineering Laboratory for Animal Breeding of China Agricultural University, and High-performance Computing Platform of China Agricultural University.
Abbreviations
- BCO2
Beta-carotene oxygenase 2
- CRYAB
Heat shock protein family B
- dxy
Mean pairwise sequence divergence
- HSPB2
Heat shock protein family B
- IGFBP2
Insulin like growth factor binding protein 2
- ML
Maximum likelihood
- PCA
Principal component analysis
- PC1
The first principal component
- TKT
Transketolase
Authors' contributions
LQ and ZN conceived and designed the experiment. XRZ performed bioinformatics analyses. JW, XYZ, JZ, TZ and XRZ performed the experiments and interpreted the result data. HW, WY, GC, WX, YL and CQ contributed resources and funding. LQ and XRZ led the manuscript writing. All authors read and approved the final manuscript.
Funding
This work was supported by the earmarked fund for the Beijing Agriculture Innovation Consortium (BAIC06-2023-G01), open project of Xinjiang Production & Construction Corps Key Laboratory of Protection and Utilization of Biological Resources in Tarim Basin (BRZD2104), and Fuyang Normal University Provincial and Ministerial Open Platform Fund (FSKFKT026D).
Availability of data and materials
The whole genome resequencing data of Guangxi Dongzhong Dwarf chickens, Hainan Wenchang chickens and 2 Beijing You chickens are available under the NCBI accession numbers of PRJNA871052 and PRJNA724749. The data for the other domestic chickens, green junglefowl, Ceylon junglefowl, grey junglefowl and red junglefowl were obtained from previous studies [6, 7, 15–19].
Declarations
Ethics approval and consent to participate
This study conformed to protocols approved by the Laboratory Animal Welfare and Animal Experimentation Ethics Review Committee of China Agricultural University (No. XK622).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lawal RA, Hanotte O. Domestic chicken diversity: Origin, distribution, and adaptation. Anim Genet. 2021;52(4):385–94. doi: 10.1111/age.13091. [DOI] [PubMed] [Google Scholar]
- 2.Clark W, Johnsgard P. The pheasants of the world: Biology and natural history. J Wildl Manage. 2001;65:164. doi: 10.2307/3803290. [DOI] [Google Scholar]
- 3.Darwin C. The variation of animals and plants under domestication. 2nd ed. London: John Murray; 1868.
- 4.Nishibori M, Shimogiri T, Hayashi T, Yasue H. Molecular evidence for hybridization of species in the genus gallus except for gallus varius. Anim Genet. 2005;36(5):367–75. doi: 10.1111/j.1365-2052.2005.01318.x. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson J, Larson G, Gunnarsson U, Bed'hom B, Tixier-Boichard M, Strömstedt L, et al. Identification of the yellow skin gene reveals a hybrid origin of the domestic chicken. PLoS Genet. 2008;4(2):e1000010. doi: 10.1371/journal.pgen.1000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M-S, Thakur M, Peng M-S, Jiang Y, Frantz LAF, Li M, et al. 863 genomes reveal the origin and domestication of chicken. Cell Res. 2020;30(8):693–701. doi: 10.1038/s41422-020-0349-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawal RA, Martin SH, Vanmechelen K, Vereijken A, Silva P, Al-Atiyat RM, et al. The wild species genome ancestry of domestic chickens. BMC Biol. 2020;18(1):13. doi: 10.1186/s12915-020-0738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frantz LAF, Schraiber JG, Madsen O, Megens H-J, Cagan A, Bosse M, et al. Evidence of long-term gene flow and selection during domestication from analyses of eurasian wild and domestic pig genomes. Nat Genet. 2015;47(10):1141–8. doi: 10.1038/ng.3394. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Wu Y, Chen B, Cai Y, Guo J, Leonard AS, et al. Markhor-derived introgression of a genomic region encompassing papss2 confers high-altitude adaptability in tibetan goats. Mol Biol Evol. 2022;39(12):msac253. doi: 10.1093/molbev/msac253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cao Y-H, Xu S-S, Shen M, Chen Z-H, Gao L, Lv F-H, et al. Historical introgression from wild relatives enhanced climatic adaptation and resistance to pneumonia in sheep. Mol Biol Evol. 2021;38(3):838–55. doi: 10.1093/molbev/msaa236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hu X-J, Yang J, Xie X-L, Lv F-H, Cao Y-H, Li W-R, et al. The genome landscape of tibetan sheep reveals adaptive introgression from argali and the history of early human settlements on the qinghai–tibetan plateau. Mol Biol Evol. 2019;36(2):283–303. doi: 10.1093/molbev/msy208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lv F-H, Cao Y-H, Liu G-J, Luo L-Y, Lu R, Liu M-J, et al. Whole-genome resequencing of worldwide wild and domestic sheep elucidates genetic diversity, introgression, and agronomically important loci. Mol Biol Evol. 2022;39(2):msab353. doi: 10.1093/molbev/msab353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao H, Liu Z, Wang N, Long Q, Cao S, Huang G, et al. Adaptive and maladaptive introgression in grapevine domestication. Proc Natl Acad Sci USA. 2023;120(24):e2222041120. doi: 10.1073/pnas.2222041120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He F, Pasam R, Shi F, Kant S, Keeble-Gagnere G, Kay P, et al. Exome sequencing highlights the role of wild-relative introgression in shaping the adaptive landscape of the wheat genome. Nat Genet. 2019;51(5):896–904. doi: 10.1038/s41588-019-0382-2. [DOI] [PubMed] [Google Scholar]
- 15.Lawal RA, Al-Atiyat RM, Aljumaah RS, Silva P, Mwacharo JM, Hanotte O. Whole-genome resequencing of red junglefowl and indigenous village chicken reveal new insights on the genome dynamics of the species. Front Genet. 2018;9:264. doi: 10.3389/fgene.2018.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariadassou M, Suez M, Sathyakumar S, Vignal A, Arca M, Nicolas P, et al. Unraveling the history of the genus gallus through whole genome sequencing. Mol Phylogenet Evol. 2021;158:107044. doi: 10.1016/j.ympev.2020.107044. [DOI] [PubMed] [Google Scholar]
- 17.Xu NY, Si W, Li M, Gong M, Larivière JM, Nanaei HA, et al. Genome-wide scan for selective footprints and genes related to cold tolerance in chantecler chickens. Zool Res. 2021;42(6):710–20. doi: 10.24272/j.issn.2095-8137.2021.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo W, Luo C, Wang M, Guo L, Chen X, Li Z, et al. Genome diversity of chinese indigenous chicken and the selective signatures in chinese gamecock chicken. Sci Rep. 2020;10(1):14532. doi: 10.1038/s41598-020-71421-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shi S, Shao D, Yang L, Liang Q, Han W, Xue Q, et al. Whole genome analyses reveal novel genes associated with chicken adaptation to tropical and frigid environments. J Adv Res. 2023;47:13–25. doi: 10.1016/j.jare.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S, Zhou Y, Chen Y, Gu J. Fastp: An ultra-fast all-in-one fastq preprocessor. Bioinformatics. 2018;34(17):i884–i90. doi: 10.1093/bioinformatics/bty560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li H, Durbin R. Fast and accurate long-read alignment with burrows–wheeler transform. Bioinformatics. 2010;26(5):589–95. doi: 10.1093/bioinformatics/btp698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Danecek P, Bonfield JK, Liddle J, Marshall J, Ohan V, Pollard MO, et al. Twelve years of samtools and bcftool. GigaScience. 2021;10(2):giab008. doi: 10.1093/gigascience/giab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, et al. The genome analysis toolkit: A mapreduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20(9):1297–303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MAR, Bender D, et al. Plink: A tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81(3):559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee T-H, Guo H, Wang X, Kim C, Paterson AH. Snphylo: A pipeline to construct a phylogenetic tree from huge snp data. BMC Genomics. 2014;15(1):162. doi: 10.1186/1471-2164-15-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alexander DH, Novembre J, Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 2009;19(9):1655–64. doi: 10.1101/gr.094052.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Francis RM. Pophelper: An r package and web app to analyse and visualize population structure. Mol Ecol Resour. 2017;17(1):27–32. doi: 10.1111/1755-0998.12509. [DOI] [PubMed] [Google Scholar]
- 28.Malinsky M, Matschiner M, Svardal H. Dsuite - fast d-statistics and related admixture evidence from vcf files. Mol Ecol Resour. 2021;21(2):584–95. doi: 10.1111/1755-0998.13265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. Iq-tree: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol Biol Evol. 2015;32(1):268–74. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Martin SH, Davey JW, Jiggins CD. Evaluating the use of abba-baba statistics to locate introgressed loci. Mol Biol Evol. 2015;32(1):244–57. doi: 10.1093/molbev/msu269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Leigh JW, Bryant D. Popart: Full-feature software for haplotype network construction. Meth Ecol Evol. 2015;6(9):1110–6. doi: 10.1111/2041-210X.12410. [DOI] [Google Scholar]
- 32.Lei MM, Nie QH, Peng X, Zhang DX, Zhang XQ. Single nucleotide polymorphisms of the chicken insulin-like factor binding protein 2 gene associated with chicken growth and carcass traits. Poult Sci. 2005;84(8):1191–8. doi: 10.1093/ps/84.8.1191. [DOI] [PubMed] [Google Scholar]
- 33.Tapanainen PJ, Bang P, Wilson K, Unterman TG, Vreman HJ, Rosenfeld RG. Maternal hypoxia as a model for intrauterine growth retardation: Effects on insulin-like growth factors and their binding proteins. Pediatr Res. 1994;36(2):152–8. doi: 10.1203/00006450-199408000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Solomon A, Grueterich M, Li DQ, Meller D, Lee SB, Tseng SC. Overexpression of insulin-like growth factor-binding protein-2 in pterygium body fibroblasts. Invest Ophthalmol Vis Sci. 2003;44(2):573–80. doi: 10.1167/iovs.01-1185. [DOI] [PubMed] [Google Scholar]
- 35.Hosnedlova B, Vernerova K, Kizek R, Bozzi R, Kadlec J, Curn V, et al. Associations between IGF1, IGFBP2 and TGFß3 genes polymorphisms and growth performance of broiler chicken lines. Animals (Basel). 2020;10(5):800. [DOI] [PMC free article] [PubMed]
- 36.Li ZH, Li H, Zhang H, Wang SZ, Wang QG, Wang YX. Identification of a single nucleotide polymorphism of the insulin-like growth factor binding protein 2 gene and its association with growth and body composition traits in the chicken. J Anim Sci. 2006;84(11):2902–6. doi: 10.2527/jas.2006-144. [DOI] [PubMed] [Google Scholar]
- 37.Boyle L, Wamelink MMC, Salomons GS, Roos B, Pop A, Dauber A, et al. Mutations in tkt are the cause of a syndrome including short stature, developmental delay, and congenital heart defects. Am J Hum Genet. 2016;98(6):1235–42. doi: 10.1016/j.ajhg.2016.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang Y, Gan S, Luo C, Liu S, Ma J, Luo W, et al. Variations in BCO2 coding sequence causing a difference in carotenoid concentration in the skin of chinese indigenous chicken. Genes. 2023;14(3):671. [DOI] [PMC free article] [PubMed]
- 39.Kong FL, Chen S-Y, Ran JS, Yang C, Jiang XS, Lan D, et al. The identification of snps in bcdo2 gene for skin color in chinese indigenous chicken. Revista Brasileira de Ciência Avícola. 2017;19:393–8. doi: 10.1590/1806-9061-2016-0397. [DOI] [Google Scholar]
- 40.Sun J, Chen T, Zhu M, Wang R, Huang Y, Wei Q, et al. Whole-genome sequencing revealed genetic diversity and selection of guangxi indigenous chickens. PLoS One. 2022;17(3):e0250392. doi: 10.1371/journal.pone.0250392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang X, Otecko NO, Peng M, Weng Z, Li W, Chen J, et al. Genome-wide genetic structure and selection signatures for color in 10 traditional chinese yellow-feathered chicken breeds. BMC Genomics. 2020;21(1):316. doi: 10.1186/s12864-020-6736-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sieck GC. Molecular biology of thermoregulation. J Appl Physiol (1985) 2002;92(4):1365–6. doi: 10.1152/japplphysiol.00003.2002. [DOI] [PubMed] [Google Scholar]
- 43.Murphy S, Ohlendieck K. Proteomic profiling of the hspb chaperonome: Mass spectrometric identification of small heat shock proteins in stressed skeletal muscles. J Integr OMICS. 2015;5:186. doi: 10.5584/jiomics.v5i1.186. [DOI] [Google Scholar]
- 44.Zhang B, Chamba Y, Shang P, Wang Z, Ma J, Wang L, et al. Comparative transcriptomic and proteomic analyses provide insights into the key genes involved in high-altitude adaptation in the tibetan pig. Sci Rep. 2017;7(1):3654. doi: 10.1038/s41598-017-03976-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Girdland Flink L, Allen R, Barnett R, Malmström H, Peters J, Eriksson J, et al. Establishing the validity of domestication genes using DNA from ancient chickens. Proc Natl Acad Sci USA. 2014;111(17):6184–9. doi: 10.1073/pnas.1308939110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. Summary of samples included in this study. Table S2. Summary of genome sequencing and mapping statistic. Table S3. Results of D statistics. Table S4. Significant introgression region between the grey junglefowl and XJHT local chickens. Table S5. Significant introgression region between the grey junglefowl and Canada local chickens. Table S6. Significant introgression region between the grey junglefowl and BJ local chickens. Table S7. Significant introgression region between the grey junglefowl and GD local chickens. Table S8. Significant introgression region between the grey junglefowl and GX local chickens. Table S9. Significant introgression region between the grey junglefowl and HLJ local chickens. Table S10. Significant introgression region between the grey junglefowl and Hainan local chickens. Table S11. Significant introgression region between the grey junglefowl and Jiangsu local chickens. Table S12. Significant introgression region between the grey junglefowl and SD local chickens.
Additional file 2: Fig. S1. Introgression at the TIMP3 gene region. a. Manhattan plot of D values between XJHT local chickens and grey junglefowl. The dashed line indicates the significance threshold (top 1% of the distribution of D values). b. ML tree constructed with the TIMP3 gene sequence. The introgressed event was highlighted with orange color, the introgressed XJHT individual and the grey junglefowl were highlighted with red and blue color, respectively. c. Mean pairwise sequence divergence at the TIMP3 gene region between the introgressed XJHT local chickens (INDOC) and either grey junglefowl (GyJ) or remaining non-introgressed XJHT local chickens (ODOC), represents by red and blue lines, respectively. The shaded area represents the TIMP3 gene region.
Additional file 3: Fig. S2. Introgression at the TKT gene region. a. Manhattan plot of D values between Canada local chickens and grey junglefowl. The dashed line indicates the significance threshold (top 1% of the distribution of D values). b. ML tree constructed with the TKT gene sequence. The introgressed event was highlighted with orange color, the introgressed Canada individuals and the grey junglefowl were highlighted with red and blue color, respectively. c. Mean pairwise sequence divergence at the TKT gene region between the introgressed Canada local chickens (INDOC) and either grey junglefowl (GyJ) or remaining non-introgressed Canada local chickens (ODOC), represents by red and blue lines, respectively. The shaded area represents the TKT gene region.
Additional file 4: Fig. S3. Introgression at the CRYAB and HSPB2 gene region. a. Manhattan plot of D values between Canada local chickens and grey junglefowl. The dashed line indicates the significance threshold (top 1% of the distribution of D values). b. ML tree constructed with the CRYAB gene sequence. The introgressed event was highlighted with orange color, the introgressed individuals and the grey junglefowl were highlighted with red and blue color, respectively. c. ML tree constructed with the HSPB2 gene sequence. The introgressed event was highlighted with orange color, the introgressed individuals and the grey junglefowl were highlighted with red and blue color, respectively. d. Mean pairwise sequence divergence at the CRYAB gene region between the introgressed individuals (INDOC) and either grey junglefowl (GyJ) or remaining non-introgressed domestic chickens (ODOC), represents by red and blue lines, respectively. The shaded area represents the CRYAB gene region. e. Mean pairwise sequence divergence at the HSPB2 gene region between the introgressed individuals (INDOC) and either grey junglefowl (GyJ) or remaining non-introgressed domestic chickens (ODOC), represents by red and blue lines, respectively. The shaded area represents the HSPB2 gene region.
Data Availability Statement
The whole genome resequencing data of Guangxi Dongzhong Dwarf chickens, Hainan Wenchang chickens and 2 Beijing You chickens are available under the NCBI accession numbers of PRJNA871052 and PRJNA724749. The data for the other domestic chickens, green junglefowl, Ceylon junglefowl, grey junglefowl and red junglefowl were obtained from previous studies [6, 7, 15–19].