Abstract
There is a growing need for cartilage defect grafts that are structurally adaptable to possess multifaceted functions to promote bone regeneration, sustain medication efficacy, and preferably remain injectable but solidify quickly upon injection. In this work, we developed an injectable multicomponent biomimetic gel (MBG) by integrating polyamidoamine dendrimer G3 (G3), mesoporous silica nanoparticles (MSN), and dendrimer-templated silver nanoparticles (G3-Ag) into a well-defined cross-linked network. MBGs composed of one particulate component (G3 alone), i.e., MBG-1, two particulate components (G3 and MSN-NH2), i.e., MBG-2, and three particulate components (G3, MSN-NH2, and G3-Ag), i.e., MBG-3 were prepared by inter-cross-linking dendrimeric and mesoporous silica nanoparticles with poly(ethylene glycol) diglycidyl ether (PEG-DGE, Mn=2000 g/mol) via the facile amine–epoxy click reaction. The water-soluble antibiotic isoniazid was loaded to the cross-linked PEG network, whereas the hydrophobic antibiotic rifampicin was encapsulated into mesoporous MSN particles. Our studies revealed that elasticity and mechanical strengths could be modulated and enhanced significantly with the inclusion of MSN and silver nanoparticles. Isoniazid was released rapidly while rifampicin was released over an extended period of time. In addition, MBGs showed injectability, high swelling capacity, structural stability and cytocompatibility. Taken together, MBGs have shown structural features that allow for the development of injectable gel grafts with the ability to promote cartilage defect repair and offer antibiotic medication benefits.
Keywords: hyperbranched polymer, dendrimer hydrogel, sustained release, biomimicry, bone joint tuberculosis
Graphical Abstract

INTRODUCTION
Osteoarticular diseases are usually accompanied with joint cartilage defects.1, 2 Cartilage tissue regeneration remains a clinical challenge because of the limited self-repairing ability of articular cartilage.3–5 A variety of biomaterials have been explored to develop tissue-engineered cartilage grafts with the ability to promote bone defect repair and regeneration.6, 7 An ideal cartilage scaffold is expected to be biocompatible and mechanically resilient, and to promote healing of joint bone defects resulting from pathological processes. Therapeutic, anti-inflammatory and antimicrobial functions are also needed of engineered bone grafts.8–15 For example, bone joint tuberculosis is a rare form of extrapulmonary tuberculosis.16, 17 The effective treatment of bone joint tuberculosis counts on the combination of medication and bone regeneration.18–21 Therefore, scaffolds that engage in medical therapy and bone regeneration simultaneously are most desired.22 Joint cartilage defects often have irregular shapes. In this scenario, in situ-forming or injectable scaffolds help fill irregular defects whereas preformed materials cannot.23–28 29 In addition, scaffolds are often required to supply cells and/or bioactive factors to facilitate bone defect repair and regeneration.27–30 Therefore, it has become an increasing need that cartilage bone defect grafts are preferably structurally adaptable to accommodate multifaceted needs including biocompatibility, mechanical strength, bone regeneration, injectability, and so on.29, 31
In this work, we prepared mesoporous silica nanoparticles (MSN) and dendrimer-templated silver nanoparticles (G3-Ag), and developed an injectable multicomponent biomimetic gel (MBG) by integrating dendrimer G3, MSN, and G3-Ag into a well-defined in-situ gelation system. We inter-cross-linked dendrimeric and mesoporous silica nanoparticles with poly(ethylene glycol) diglycidyl ether (PEG-DGE) by applying the facile amine–epoxy click reaction.32, 33 We tested tensile and rheological properties of MBGs. MBG possesses micronscale and nanoscale domains, thus providing multi-drug loading pathways and potential cell payload possibility. We explored the simultaneous delivery of drugs of distinct physiochemical properties by formulating two antibiotic drugs relating to tuberculosis therapy into MBG. The water-soluble antibiotic isoniazid was loaded to the cross-linked PEG network, whereas the hydrophobic antibiotic rifampicin was encapsulated into mesoporous MSN particles. We studied the dual drug release kinetics. The mechanical property of MBGs with reference to human joint cartilage was assessed. In addition, we tested their swelling capacity, structural stability and cytocompatibility. We would expect that this injectable multicomponent biomimetic gel has both tunable mechanical properties and multi-functionality to meet the complex requirements of cartilage regeneration.
RESULTS AND DISCUSSION
Upon mixing, PAMAM dendrimer G3 readily reacts with linear PEG-DGE in water to form a cross-linked network on the basis of the highly efficient amine–epoxy reaction (Figure 1a). In this study, we kept the molar ratio of dendrimer surface amine to epoxy of PEG-DGE at 1:1 in MBG-1 while varying G3 concentration (5, 10, and 20 wt%). We found that changing G3 concentration lead to dendrimer hydrogels (DH5, 10, and 20 corresponding to 5, 10, and 20 wt% G3 concentration) with different solidification times and morphological structures. Higher G3 concentration results in quick gelation. According to the assessment using the invert tube method, 5, 10, and 20 wt% of G3 result in different solidification times of 35, 83, and 190 min, respectively (Figure S1). SEM images show dense to loose wrinkle textures related to G3 concentrations used in preparation. The densest wrinkle intervals from DH20 are on the nano-to-submicron scale while the loosest ones from DH5 are ~30 μm (Figure S2). DH10 possesses a moderate morphological texture with average wrinkle intervals of around several micrometers (Figure 3a). In the preparation of multicomponent biomimetic gels, we selected 10 wt% G3 solution and the corresponding amount of PEG-DGE (1:1 surface amine to epoxy) as the primary formula because it only required a moderate solidification time to maintain the injectability and moderate physical property of the resulting hydrogel, referred to as MBG-1.
Figure 1.
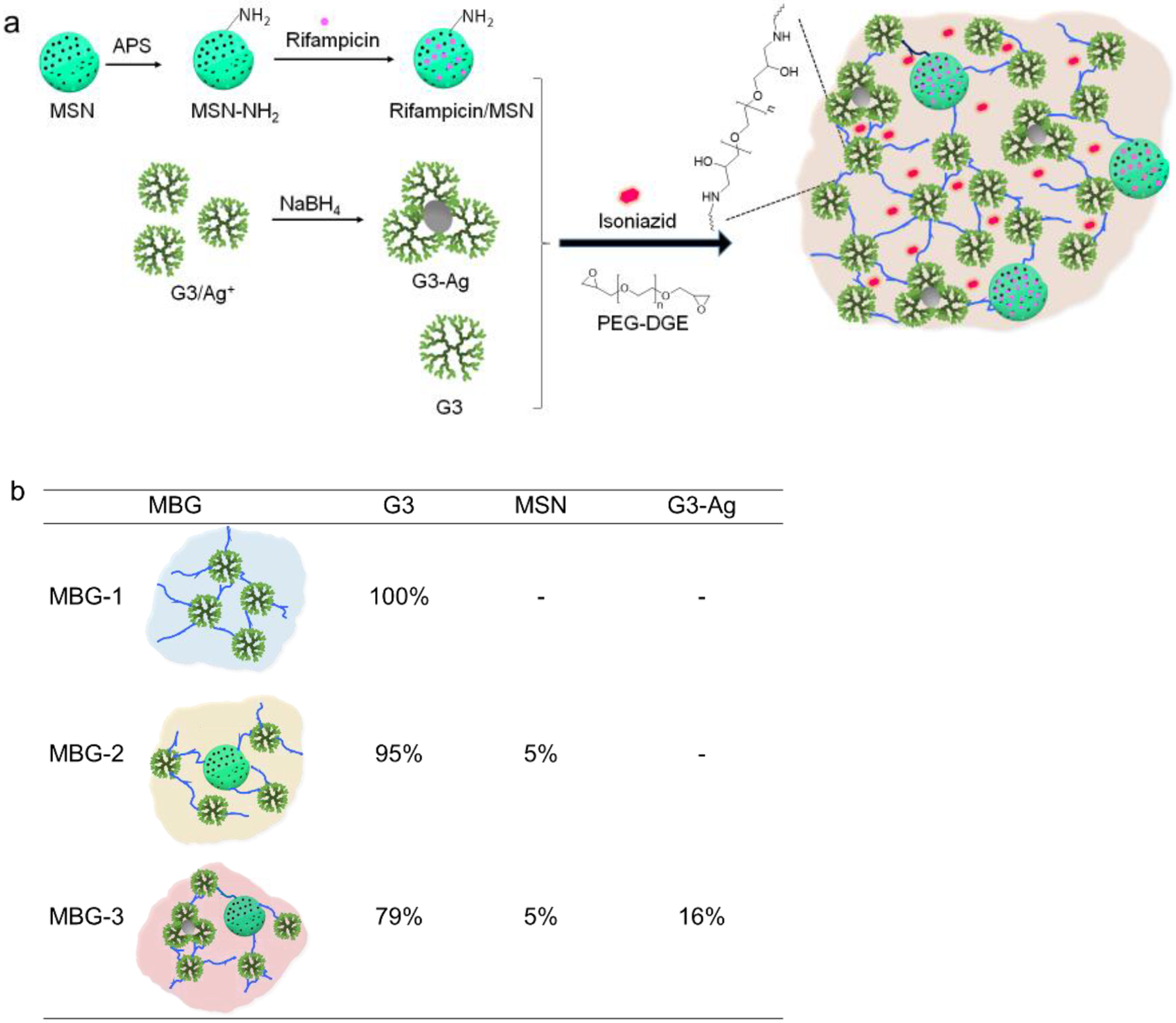
Preparation of MBGs. a) MBGS are composed of inter-crosslinked dendrimeric alone or with mesoporous silica nanoparticles and G3-Ag nanoparticle. b) Compositions of MBG-1, MBG-2, and MBG-3 and mass fractions of nanoparticles.
Figure 3.
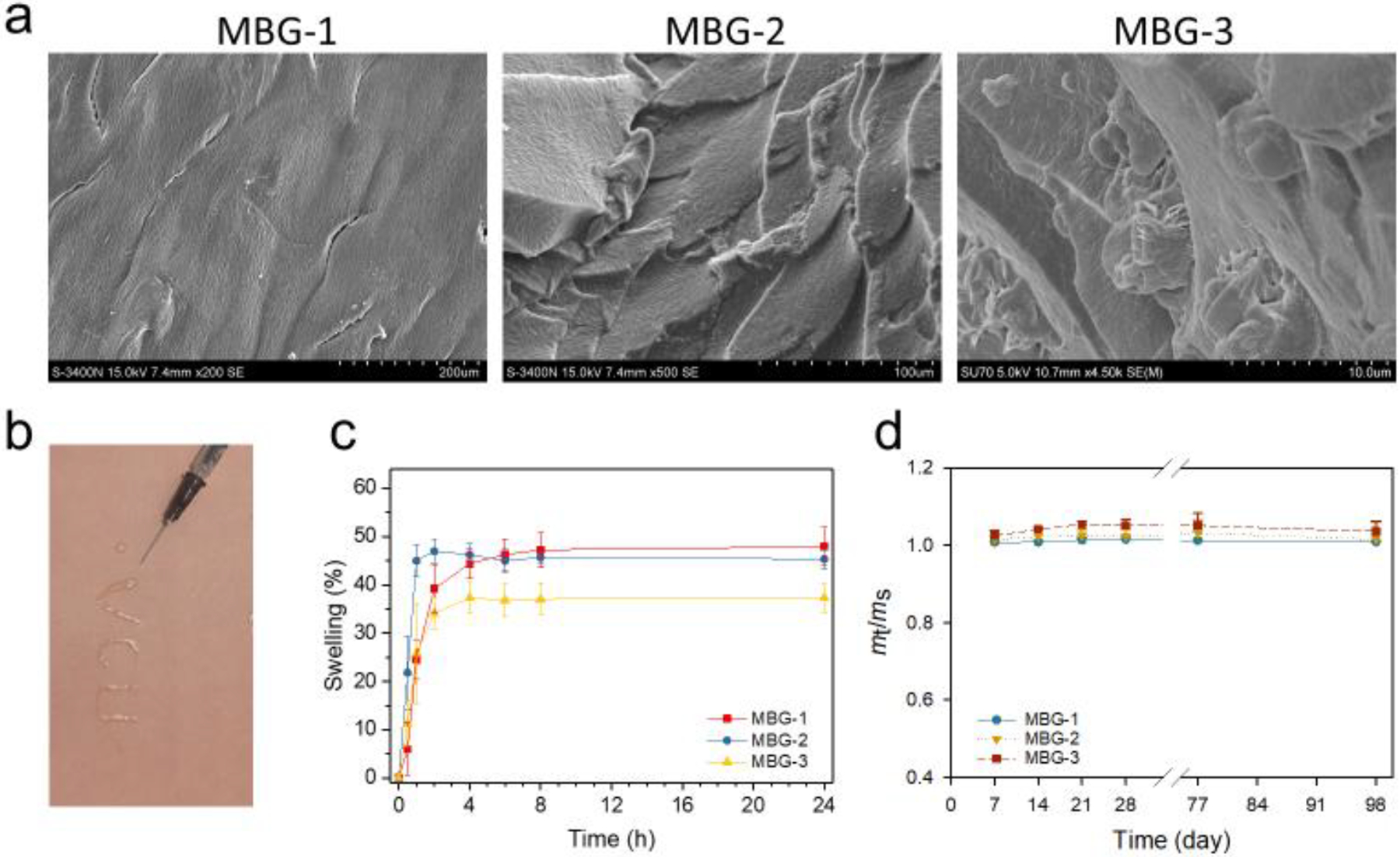
MBGs show classic gel morphologies, injectability, high swelling capacity and stability. a) SEM images. b) Injectability of MBG-1. c) Swelling kinetics of MBGs. d) Stability of MBGs incubated in PBS at 37 °C.
We followed the same protocol to make MBG-2 and MBG-3 by inter-crosslinking mesoporous silica nanoparticles (MSN) and dendrimer-silver nanoparticle (G3-Ag) with G3 (Figure 1b). We first prepared mesoporous silica nanoparticles (MSN) and then surface functionalized them with amines (MSN-NH2). We applied TEM and DLS to characterize the morphologies and size distributions of MSN and MSN-NH2. Both MSN and MSN-NH2 nanoparticles have exhibited mesoporous structures and a relatively uniform size (Figure 2a, b). According to the statistical analysis of the particles captured in TEM images, the particle sizes of MSN and MSN-NH2 were 104 nm and 105 nm, respectively, suggesting that surface modification did not alter the structure and size of MSN. In suspension, both MSN and MSN-NH2 underwent 3-fold and 4-fold size expansion. MSN-NH2 (417 nm) was found to be larger than MSN (318 nm) in suspension presumably because of their enhanced hydrophilicity. Because of the mesoporous characteristic of the MSN, amine-modification may not only occur in the outer surface of the particles, but also the surface of the pores. This significantly enhanced the hydrophilicity of MSN-NH2 and increased its hydrodynamic size. XPS was a highly sensitive surface analysis technology with the sample detecting depth about 2 nm. We used it to attain the functional surface compositions of MSN and MSN-NH2 from the C1s and N1s levels (Figure 2c). According to the N1s analysis of MSN-NH2, the appearance of a distinct peak at 399.9 eV confirms that amine groups had been successfully introduced into the surface of MSN-NH2. The TEM images of the formed G3-Ag discloses that the formation of dendrimer stabilized Ag NPs (Figure 2d). UV-vis spectroscopy confirms the formation of G3-Ag (Figure 2e). The appearance of the absorption peaks at 396 nm is attributed to the typical surface plasmon resonance (SPR) band of Ag NPs.34 G3-Ag in suspension was found to have a mean hydrodynamic size of 146 nm with a PDI of 0.167 (Figure 2f).
Figure 2.
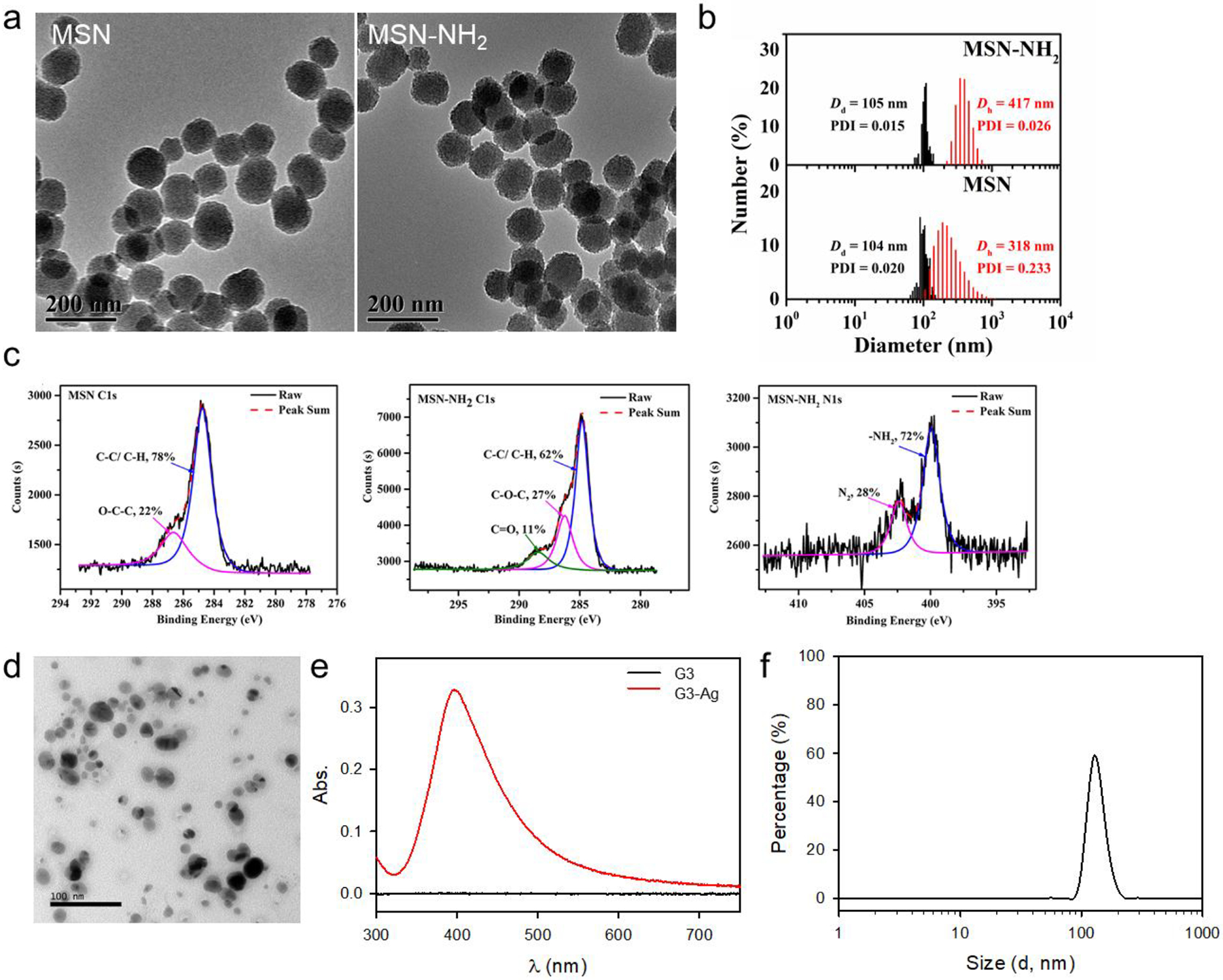
Characterization of MSN and amine-functionalized MSN (MSN-NH2). a) TEM images of MSN and MSN-NH2. b) Size distributions of MSN and MSN-NH2 by DLS (red) and statistical results by TEM (black). c) XPS analysis of MSN and MSN-NH2. d) TEM image of G3-Ag. e) UV-Vis spectra of G3 and G3-Ag showing the plasma resonance absorption of G3-Ag at 396 nm. f) Size distribution of G3-Ag by DLS.
Figure 3a shows the morphology differences among MBG-1, 2, and 3. Compared to MBG-1, the introduction of MSNs to MBG, i.e., MBG-2, has no significant effect on gel morphology except for a slight decrease on texture uniformity. The morphology of MBG-3 appears to be more isotropic and rougher. The difference of morphologies among MBG-1, 2, and 3 might because of the size variance between G3, MSN and G3-Ag. Injectability of MBGs was demonstrated (Figure 3b). Hydrated MBGs swell rapidly and reach equilibrium within 2 h of incubation in PBS and reach an equilibrium at ~4 h. The swelling ratios of MBG-1, 2, and 3 are 48±4%, 45±2%, and 37±3%, respectively (Figure 3c). Further statistical analysis revealed that MBG-1 and MBG-2 have similar swelling capacity but MBG-3 has a lower swelling capacity (p=0.02 vs. MBG-1, MBG-2). As discussed above, the hydrophilicity and mesoporous feature of MSN-NH2 account for the equivalent swelling behavior of MBG-2 with MBG-1. The lowered swelling capacity of MBG-3 might because of the incorporation of inorganic silver nanoparticles. All the three MBGs have high structural stability as none of them experienced detectable mass losses in 14 weeks (Figure 3d). This moderate swelling property and the negligible degradation in a certain period provide the basis of sustained drug release of this biomimetic gel as bone regeneration scaffold.
Due to the multidrug-resistance and complexity of infections of tuberculosis, a multi-drug regimen is needed.35, 36 Isoniazid is an antibiotic and has long been used for the clinical treatment of tuberculosis.37, 38 Rifampicin is an antibiotic used to treat several types of bacterial infections, including tuberculosis.37 In a clinical regimen, isoniazid is usually used in combination with rifampicin or other medicines for the treatment of tuberculosis to avoid antibiotic resistance if used alone.39 In this study, we loaded isoniazid and rifampicin to the different domains of MBG-3. The water-soluble isoniazid is loaded into the cross-linked PEG network of MBG-3. The hydrophobic rifampicin is loaded in the mesoporous structures of MSNs. As shown in Figure 4b, isoniazid experienced a burst release. Approximately 50% of the loaded isoniazid was released in the first 4 h, concurrent with the swelling kinetics, confirming the release of isoniazid is diffusion-controlled (Figure 4a). Approximately 15% more of the isoniazid was slowly released in the following 48 h. In contrast, the release of rifampicin was significantly prolonged. The complete release of rifampicin needs to diffuse from the mesoporous of MSN to the network structure first and then escape from the cross-linking dendritic network, which lead to a quite the (Figure 4a). About 68% of rifampicin was continuously released over a course of nine days following zero-order release kinetics. MBG-3 provides the burst release of isoniazid and the prolonged release of rifampicin. The complementary release kinetics enabled by the platform allow for fine tuning of drug release and meeting the demand for long-term drug treatment during the bone defect regeneration.
Figure 4.

MBGs enable dual drug release kinetics. a) Schematic illustration of drug release: Ⅰ, burst release of isoniazid; Ⅱ, sustained release of rifampicin. b) Drug release of isoniazid and rifampicin from MBG-3 (n = 3).
The cytotoxicity of MBG-3 was investigated both in NIH3T3 fibroblast cells and MG63 human osteosarcoma cells. MBG-3 is highly cytocompatible. It does not cause toxicity effects up to 0.27 mg/mL (equivalent to 80 μg/mL of G3) on NIH3T3 cells (Figure 5a). Even when the concentration of MBG-3 increased by ten-fold (2.7 mg/mL), more than 70% of the NIH3T3 cells remained viable. There is no toxicity exhibited on MG63 cells even when the MBG-3 is up to 5.3 mg/mL (Figure 5b). This highly cytocompatibility endows this biomimetic gel suitability as cartilage regeneration scaffold. It is also promising to load cells in-situ in the future study. An antimicrobial property against E. coli ER2738 was shown on all the three gels benefits from both polyamidoamine dendrimers and silver nanoparticles (Figure S6). MBG-3 shows a slightly better antimicrobial efficacy owing to the introduction of silver nanoparticles.40, 41
Figure 5.
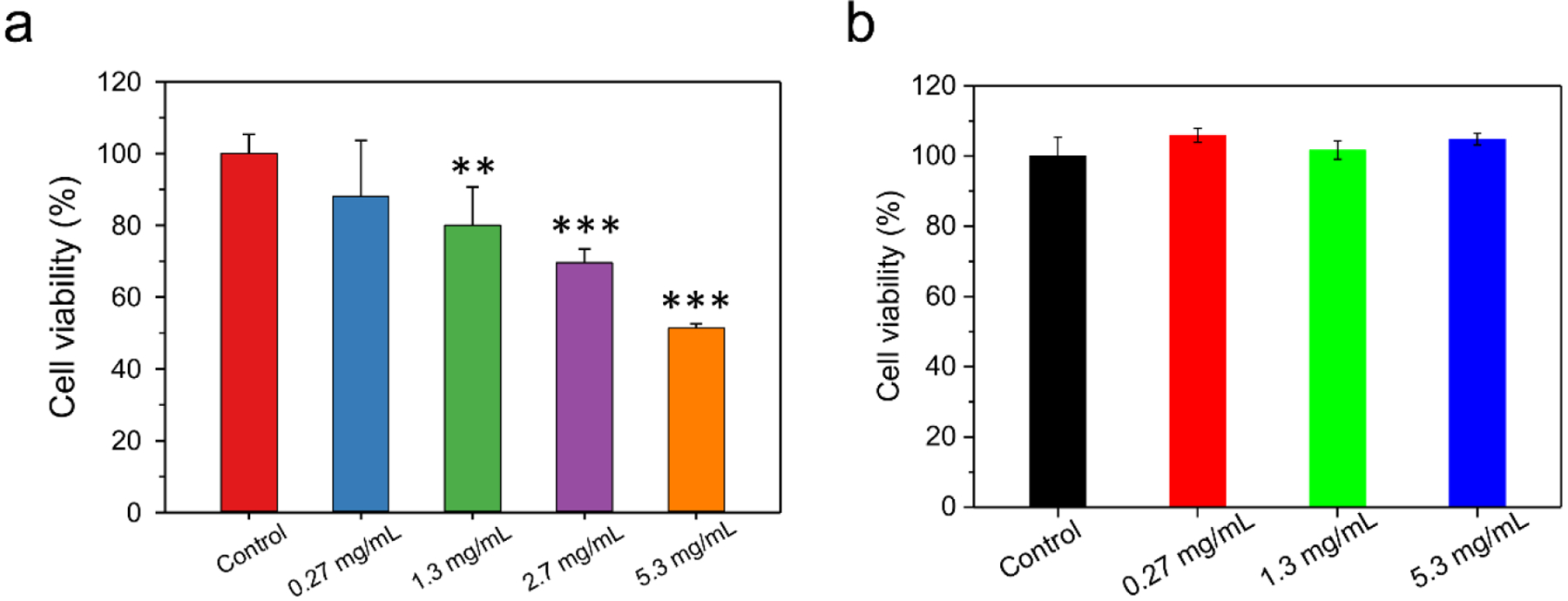
MBGs and exhibit high cytocompatibility on a) NIH3T3 cells, and b) MG63 cells. n = 4, ** indicates p < 0.01 and *** indicates p < 0.001.
The nanoparticulate components and compositions have shown significant influences on the mechanical properties of MBGs. We performed oscillatory frequency sweep tests on MBGs to further understand their three-dimensional structures. Before the frequency sweep, an amplitude sweep was carried out to determine the linear viscoelastic region (Figure 6a). As shown in Figure 6b, G′ is higher than G″ for all the three types of samples. Furthermore, G′ is frequency-independent over the measured frequency range of 0.1–100 rad/s. The frequency-independency of G′ and the feature of G′ > G″ are typically viscoelastic behaviors of a hydrogel.42 MBG-3 shows a slightly higher G′ than MBG-1 and MBG-2. The effects of MSN and G3-Ag on shear performance are insignificant. The time sweep of MBG-1 (Figure S3b) showed a gelation time of 5050 s, which indicates that this formulation is suitable to be used as an injectable scaffold. Introducing MSNs and Ag NPs does not affect the gelation kinetics (Figure S3c, d). We also characterized the frequency sweep of the MBG-3 samples after 14 weeks of immersion in PBS at 37 °C (Figure 6c). Although its mass loss was negligible, the storage modulus decreased from 38 kPa to 26 kPa. The reason for the decreasing storage modulus is likely to be the swelling after long-time incubation in PBS.
Figure 6.
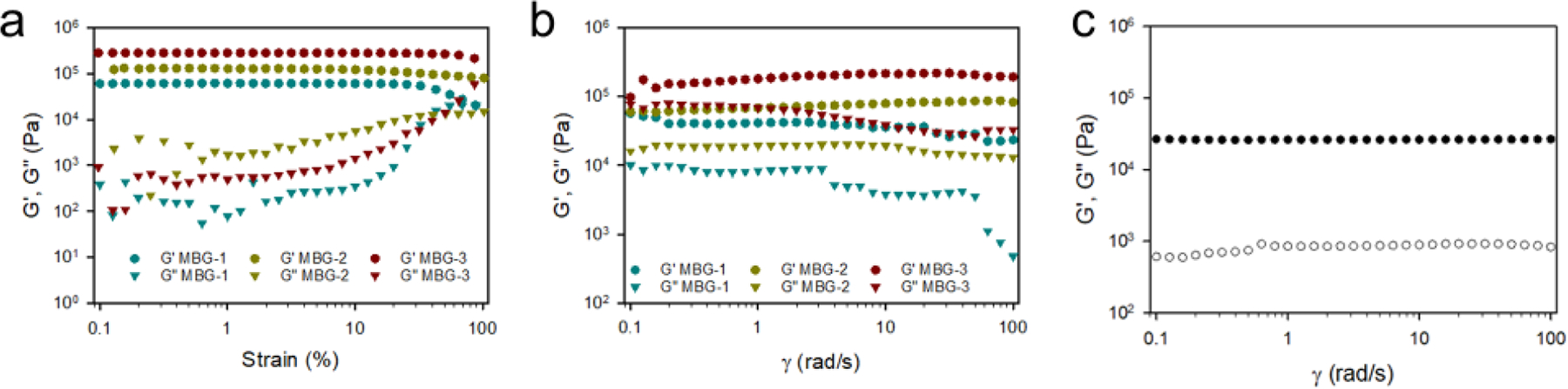
a) Rheological amplitude sweep tests on MBGs. b) Rheological frequency sweep tests on MBGs. c) Frequency sweep of MBG-3 after 14 weeks of incubation in pH7.4 PBS at 37 °C.
More impressively, we observed the dramatically enhanced mechanical properties of MBGs upon inclusion of MSNs and G3-Ag. MBG-1 displays a typical tensile brittle fracture curve with a weak Ec value of 360 ± 11 kPa and εb of 18 ± 2% (Figure 7a). With the presence of MSNs, MBG-2 shows enhanced mechanical properties in terms of both strength and toughness. In contrast to MBG-1, its Ec increases to 469 ± 8 kPa and εb increases to 25 ± 3%. By further incorporating G3-Ag into the network, MBG-3 shows the highest Ec (2.1 ± 0.2 MPa) and εb (56 ± 6%). The micro-morphologies of tensile fracture surfaces were shown as Figure 7b–d. The fracture surface of MBG-1 is smooth, while those of MBG-2 and 3 showed rough and fine microstructures. Especially for MBG-3, there was microstructures dispersed uniformly on the fracture surface at the scale of several microns, which indicates the toughness of tensile breaking. The interaction between nanoparticles and polymer chains and the uniform distribution of nanoparticles in the cross-linked network are the keys for the enhancement of tensile modulus.43, 44 The amine terminated MSNs participate the cross-linking reaction thus leads to a more uniform and stable dispersion in the final network compared to physical doping. In addition, the interaction between MSNs and polymer chains, e. t. entanglement, is also strengthened. Ag NPs surrounded by dendrimers further enhanced the organic-inorganic interaction. Bone tissue consists of a multi-scale layered structure from nanometers to centimeters and composes of organic (mainly collagen) and inorganic (mainly nano-hydroxyapatite) components. Besides the organic/inorganic features, the introducing of MSNs and G3-Ag leads to a hierarchically structured MBG-3 possessing nanoscale to micron-scale domains. The tensile moduli of human knee joint cartilage are between 1.0–15 MPa.45 The nano-hybrid scaffold developed by us meets the mechanical requirements for joint cartilage scaffold. Furthermore, it has high adaptability and is tunable to have different mechanical strengths and drug release kinetics. Our future studies will test whether it provides a biomimetic structure that closely mimics native bone architecture to support cellular behaviors such as proliferation, differentiation, and organization, collectively contributing to the generation of functional bone tissues. In addition, we noticed MBG-3 has more than 10-fold higher electrical conductivity than MBG-1 and MBG-2 owing to the presence of silver particles (Table S1).
Figure 7.
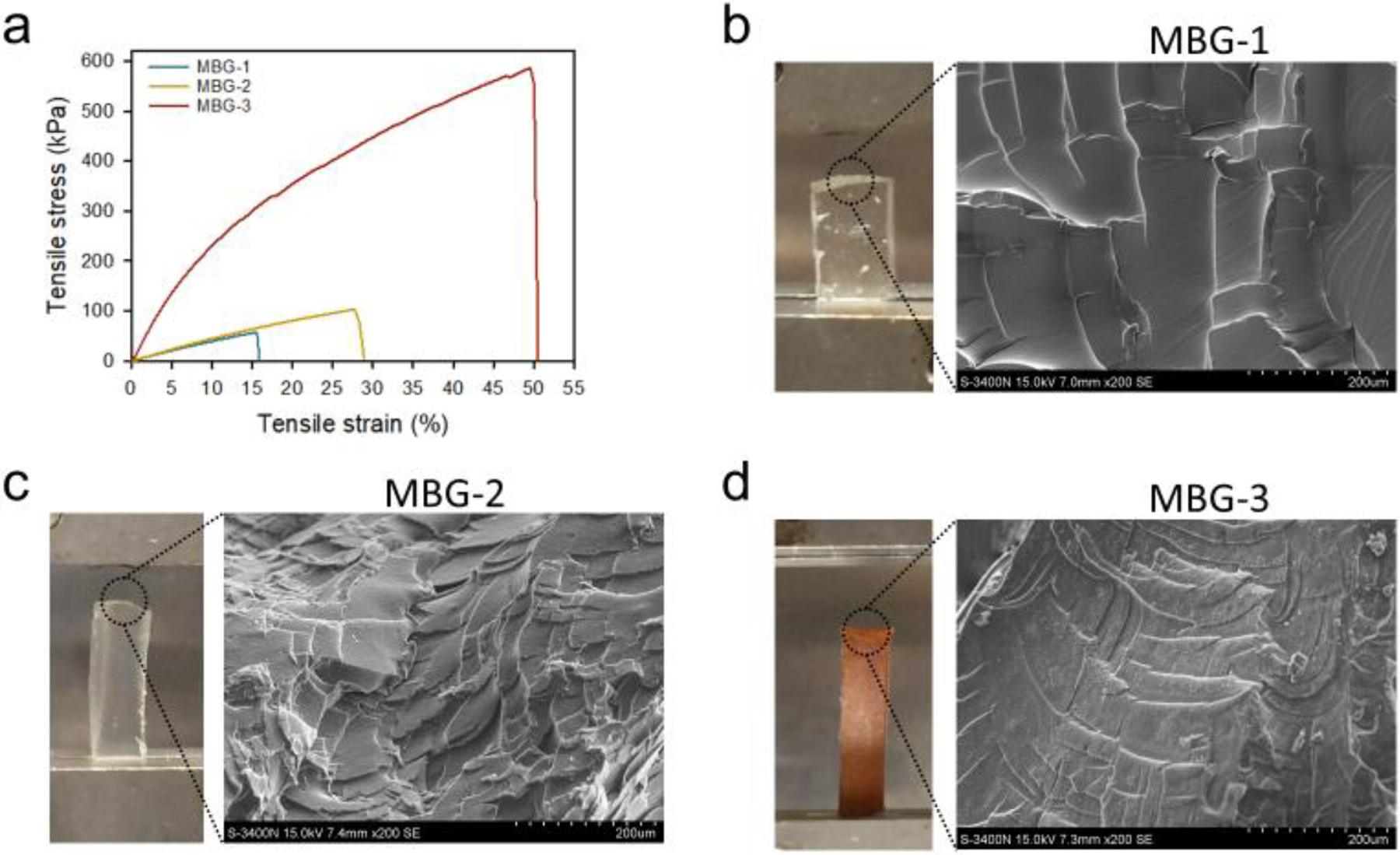
a) Tensile stress-strain curves of MBGs. b, c, d) Pictures showing MBGs samples at break of tensile test (left) and SEM images of microstructures on fracture surfaces of tensile samples of MBGs (right).
CONCLUSIONS
In this work, we successfully developed a multicomponent biomimetic gel (MBG), in which nanoparticulate components including PAMAM dendrimer G3, MSN and G3-Ag are inter-crosslinked with PEG-DGE on the basis of the highly efficient amine–epoxy reaction. Our studies revealed that the elasticity and mechanical strengths can be modulated and enhanced significantly with the inclusion of MSN and silver nanoparticles. MBG-3 allows for the simultaneous delivery of drugs of distinct physiochemical properties. We observed that the two drugs can be released following significantly different release kinetics. Isoniazid was released rapidly while rifampicin was released over an extended period of time. In addition, MBGs showed high swelling capacity, structural stability and cytocompatibility. Taken together, MBGs have shown structural features that allow for the development of injectable gel grafts with the ability to promote cartilage defect repair and offer antibiotic medication benefits.
MATERIALS AND METHODS
Materials.
EDA-core PAMAM dendrimer generation 3 (G3) was purchased from Dendritech (Midland, MI, USA). Poly(ethylene glycol) diglycidyl ether (PEG-DGE, Mn = 2 000 g/mol), sodium borohydride (NaBH4), 3-aminopropyltriethoxysilane (APS), silver nitrate (AgNO3), hexadecyltrimethylammonium bromide (CTAB), tetraethyl orthosilicate (TEOS), isoniazid, rifampicin, and cell proliferation reagent WST-1 were purchased from Sigma-Aldrich. Acetonitrile (HPLC grade), water (HPLC grade), trifluoroacetate (TFA), sodium hydroxide (NaOH), phosphate buffered saline (PBS) were purchased from Fisher Scientific.
Characterization.
Transmission Electron Microscopy (TEM).
The visualized images of MSN and MSN-NH2 were imaged under a JEM-2200FS microscope with an acceleration voltage of 120 kV. The images of dendrimer/silver nanoparticle complex (G3-Ag) were obtained by JEM-1400 Plus TEM with an acceleration voltage of 120 kV. Samples were prepared by drop-coating and air-drying nanoparticle solutions on carbon-coated copper grids.
Dynamic Light Scattering (DLS) Measurements.
The hydrodynamic sizes of MSN and MSN-NH2 were measured on Zetasizer Nano-ZS90 (Malvern Instruments, U.K.). All samples were measured at 25 °C with 632.8 nm laser light set at a scattering angle of 90°. The hydrodynamic size G3-Ag particles was obtained on ALV/CGS-3 Compact Goniometer System (Germany). The sample was measured at 25 °C with 632.8 nm laser light set at a scattering angle of 90°. Each measurement was repeated at least three times.
X-Ray Photoelectron Spectroscopy (XPS).
X-ray photoelectron spectroscopy was used to detect the element binding energy on the surface of MSN nanoparticles. The data were obtained with an ESCALab220i-XL electron spectrometer from VG Scientific using 300W AlKα radiation. The base pressure was about 3×10−9 mbar. The binding energies were referenced to the C1s line at 284.8 eV from adventitious carbon.
Scanning Electron Microscopy (SEM).
The morphologies of lyophilized hydrogels were measured by a field emission SEM microscope (Hitachi FE-SEM Su-70, Japan) with an acceleration voltage of 5 kV or SEM (Hitachi S-3400N, Japan) with an acceleration voltage of 15 kV.
Tensile Property Measurements.
The tensile stress-strain curves of hydrated DHs were obtained on a TA instruments RSA-III dynamic mechanical analyzer (DMA). The rectangle samples with defined dimensions were subjected to tensile tests until fracture at the rate of 0.05 mm/s. The tensile modulus (E) was calculated from the slope of the linear section of the stress-strain curve, and the elongation at break (εb) was taken as the strain when the sample breaks.
Rheological Measurements.
The rheological test was performed on hydrated samples (20 mm diameter disks) by a Discovery Hybrid Rheometer (HR-3, TA Instruments). An amplitude sweep was performed to confirm that all the measurements were conducted within the linear viscoelastic region. Oscillatory frequency sweeps were then carried out with a constant strain of 1% in the frequency region of 0.1–100 rad/s. For DH20, DH10 (MBG-1), MBG-2, and MBG-3, a small-amplitude dynamic oscillatory time sweep was conducted to examine the evolution of storage modulus (G′) and loss modulus (G″) and determine the sol-to-gel transition of the hydrogel solutions. During the small-amplitude dynamic oscillatory time sweep, the angular frequency was set to be 1 rad/s, and the strain was kept constant as 1%.
Ultraviolet-Visible Spectroscopy (UV-Vis).
The UV-Vis spectra of isoniazid and rifampicin were determined by using Evolution 201 UV-Visible spectrophotometer (Thermo Scientific).
High-Performance Liquid Chromatography (HPLC).
The standard curve and drug release of isoniazid and rifampicin were determined by using an HPLC (Waters) system equipped with the Waters 2487 dual λ absorbance detector. Acetonitrile/water/TFA 1/1/0.05 (volume ratio) was used as the mobile phase. The eluents were monitored at 255 and 334 nm for isoniazid and rifampicin, respectively.
Synthesis of mesoporous silica nanoparticles (MSNs).
MSNs were prepared following the reported procedure.46, 47 Briefly, 50 mL of CTAB aqueous solution (2 mg/mL) and 350 μL of 2 M NaOH solution were added to a round-bottom flask and mixed under vigorously stirring. The flask was immersed in an oil bath and maintained at 70 °C. TEOS (0.5 mL) was added slowly to the flask. The color of the reaction mixture turned from original white to bluish tint. Ethyl acetate (0.5 mL) was then added to the flask, and the mixture solution was gently stirred for 2 h. Afterwards, the flask was taken out of the oil bath and allowed to cool down to room temperature, yielding white precipitate, i.e., MSNs, on the bottom of the flask. The precipitate was collected following centrifugation and washed with water (2 times × 50 mL/time) and ethanal (2 times × 50 mL/time). The washed precipitate was re-suspended in ethanol-hydrochloric acid (25:1, v/v) (approximately 0.01 mg/mL) and stirred under reflux overnight. The mixture was cooled to room temperature and then centrifuged to collect the precipitate. The same process was repeated one more time to completely remove CTAB from MSNs.
To introduce amine groups to the surface of MSNs, the obtained MSNs (1 mg) were suspended in 10 mL of ethanol, and the suspension was heated under reflux. APS (40 μL) was added to the suspension and reflux was continued for 24 h. Upon cooling to room temperature, amino-functionalized MSNs precipitated out of the mixture. They were collected via centrifugation and washing with ethanol two times (2 × 50 mL).
Preparation of dendrimer templated-silver nanoparticle complex (G3-Ag).
PAMAM dendrimer-templated silver nanoparticles were prepared following a reported method with minor modifications.48–52 Briefly, a methanol solution of AgNO3 (0.1 M, 1 mL) was added to a methanol solution of G3 (4.8 mM, 10 mL) and stirred vigorously. After 30 min, 4 mL of ice-cold 0.5 M NaBH4 aqueous solution was added to the G3/Ag+ mixture. The color of the solution immediately turned brown upon NaBH4 addition, and the reaction was continued for 2 h in the dark. Excess reactants were removed via 2-day dialysis at room temperature in a dialysis tubing (MWCO 7000 Da) against deionized (DI). The dendrimer-silver nanoparticles were obtained after freeze-drying.
Preparation of MBGs.
MBGs composed of inter-crosslinked one particulate component (G3 alone), i.e., MBG-1, two particulate components (G3 and MSN-NH2), i.e., MBG-2, and three particulate components (G3, MSN-NH2, and G3-Ag), i.e., MBG-3 were prepared. G3 alone, with MSN-NH2, or with MSN-NH2 and G3-Ag, was mixed with appropriate amounts of PEG-DGE according to Figure 1b. For each gel preparation, the multicomponent mixture in 2 mL of PBS was vortexed for 30 s and then cast into a Teflon mold (14 mm L × 6.9 mm W × 3 mm D) for solidification.
Swelling and stability of MBGs.
Each hydrogel was immersed in 2 mL of PBS (pH = 7.4) and incubated at 37 °C. The swollen hydrogel was gently taken out at different time intervals, blot-dried, and weighed. The measurement period was up to 14 weeks in order to reach the maximum absorption and monitor possible degradation. Swelling ratio was calculated with the formula: Swelling ratio (%) = (mt − m0)/m0 × 100, where mt represents the mass of the swollen sample, and m0 represents the mass of the dry sample. The weight at 24 h was taken as the swelling equilibrium weight and the initial weight (ms) for monitoring the stability. The ratio of mt/ms represents the weight change and stability of hydrogel.
Study of dual drug release kinetics.
To prepare MBG-3 carrying rifampicin and isoniazid, rifampicin-encapsulating MSN was prepared first. Briefly, 50 mg of rifampicin and 50 mg of MSN-NH2 were mixed in 50 mL of PBS, sonicated for 60 min, and then allowed to stand still for 3 h. Rifampicin-encapsulating MSN particles were collected via centrifugation (4500 rpm, 5 min). The amount of rifampicin loaded into MSN particles (310 μg rifampicin/1 mg of MSN) was determined indirectly by measuring nonencapsulated rifampicin in the supernatant with HPLC. Following the MBG preparation method, 7.7 mg isoniazid was dissolved in the final volume of 2 mL PBS containing the same particle mass fractions as MBG-3 and cast into Teflon mold for solidification. The drug-loaded MBG-3 was incubated in 40 mL of PBS at 37 °C. At predetermined time intervals up to 11 days, 1 mL of the release medium was withdrawn, and the amount of drug released into the medium was analyzed with HPLC. After each sampling, 1 mL of fresh PBS was immediately added to maintain a constant volume and sink condition. The experiments were performed in triplicate.
Cytotoxicity assessment.
Prior to the test, freeze-dried MBG-3 was sterilized with 70% ethanol for 30 min, washed with PBS three times, and air dried. NIH3T3 fibroblast cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) formulated with the addition of 10% fetal bovine serum (FBS) at 37 °C in 95% air/5% CO2. MG63 (ATCC CRL-1427) were cultured in EMEM medium supplemented with 10% FBS, streptomycin (100 U/mL) and penicillin (100 U/mL) at 37 °C in 95% air/5% CO2. NIH3T3 cells or MG63 cells were seeded in a 12-well plate at a density of 1 × 105 cells/well and cultured overnight. MBG-3 of different masses were placed on Corning® Transwell® polycarbonate membrane cell culture inserts. The inserts were introduced to the wells, and the samples were submerged in the cell culture medium (6 mL per well). After incubation for 48 h at 37 °C in 5% CO2, cell viability (n = 4) was assessed using the WST-1 assay.
Bacterial inhibition study.
The inhibition against E. coli ER2738 of biomimetic gels was evaluated. E. coli ER2738 bacterial was first mixed with 5 mL of culture media (an aqueous solution of trypsin, yeast extract and NaCl) and cultured for 4.5 h at 37 °C. The resultant mixture (300 μL) was mixed with 5 mL of agar complex (agar, trypsin, yeast extract, NaCl and MgCl2) and transferred to 90 mm diameter petri dishes which was pre-coated with a payer of agar. Lyophilized MBG-1, 2 and 3 gels were punched to disks with 6 mm diameter and sterilized under UV irradiation for 1 h followed by hydration in sterile PBS (pH 7.4, 1×). The hydrated gel disks were placed on top of agar plates and placed in an incubator at 37 °C for 36 h. Zones of bacterial growth inhibition were measured.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported, in part, by the National Institutes of Health (R01EY024072)(HY) and Sichuan University faculty startup funds (JW).
Footnotes
Supporting Information
Solidification time, rheological time sweep, amplitude and frequency sweep tests of DH20, DH10, and DH5; SEM images of DH20 and DH5; UV-vis spectra rifampincin and isoniazid; antibacterial activity and electric conductivity of MBG-1, 2, and 3.
REFERENCES
- 1.Deng J; She R; Huang W; Dong Z; Mo G; Liu B A Silk Fibroin/Chitosan Scaffold in Combination with Bone Marrow-Derived Mesenchymal Stem Cells to Repair Cartilage Defects in the Rabbit Knee. J. Mater. Sci. Mater. Med 2013, 24, 2037–2046. [DOI] [PubMed] [Google Scholar]
- 2.Zhu Y; Yuan M; Meng HY; Wang AY; Guo QY; Wang Y; Peng J Basic Science and Clinical Application of Platelet-Rich Plasma for Cartilage Defects and Osteoarthritis: A Review. Osteoarthritis Cartilage 2013, 21, 1627–1637. [DOI] [PubMed] [Google Scholar]
- 3.Bicho D; Ajami S; Liu C; Reis RL; Oliveira JM Peptide-Biofunctionalization of Biomaterials for Osteochondral Tissue Regeneration in Early Stage Osteoarthritis: Challenges and Opportunities. J. Mater. Chem. B 2019, 7, 1027–1044. [DOI] [PubMed] [Google Scholar]
- 4.Keller L; Pijnenburg L; Idoux-Gillet Y; Bornert F; Benameur L; Tabrizian M; Auvray P; Rosset P; Gonzalo-Daganzo RM; Barrena EG; Gentile L; Benkirane-Jessel N Preclinical Safety Study of A Combined Therapeutic Bone Wound Dressing for Osteoarticular Regeneration. Nat. Commun 2019, 10, 2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moura C; Santos-Richa R; Franco S; Malca C; Galhano C; Henriques M; Morouco P A Brief Review on Processes for Cartilage Repair. Appl. Mech. Mater 2019, 890, 229–236. [Google Scholar]
- 6.Drosse I; Volkmer E; Capanna R; De Biase P; Mutschler W; Schieker M Tissue Engineering for Bone Defect Healing: An Update on A Multi-Component Approach. Injury 2008, 39, S9–S20. [DOI] [PubMed] [Google Scholar]
- 7.Liao J; Wang B; Huang Y; Qu Y; Peng G; Qian Z Injectable Alginate Hydrogel Cross-Linked by Calcium Gluconate-Loaded Porous Microspheres for Cartilage Tissue Engineering. ACS Omega 2017, 2, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ahsan SM; Thomas M; Reddy KK; Sooraparaju SG; Asthana A; Bhatnagar I Chitosan as Biomaterial in Drug Delivery and Tissue Engineering. Int. J. Biol. Macromol 2018, 110, 97–109. [DOI] [PubMed] [Google Scholar]
- 9.Dang W; Li T; Li B; Ma H; Zhai D; Wang X; Chang J; Xiao Y; Wang J; Wu C A Bifunctional Scaffold with CuFeSe2 Nanocrystals for Tumor Therapy and Bone Reconstruction. Biomaterials 2018, 160, 92–106. [DOI] [PubMed] [Google Scholar]
- 10.Du L; Yang S; Li W; Li H; Feng S; Zeng R; Yu B; Xiao L; Nie HY; Tu M Scaffold Composed of Porous Vancomycin-Loaded Poly(lactide-co-glycolide) Microspheres: A Controlled-Release Drug Delivery System with Shape-Memory Effect. Mat. Sci. Eng. C 2017, 78, 1172–1178. [DOI] [PubMed] [Google Scholar]
- 11.Li R; Pang Z; He H; Lee S; Qin J; Wu J; Pang L; Wang J; Yang VC Drug Depot-Anchoring Hydrogel: A Self-Assembling Scaffold for Localized Drug Release and Enhanced Stem Cell Differentiation. J. Controlled Release 2017, 261, 234–245. [DOI] [PubMed] [Google Scholar]
- 12.Quade M; Schumacher M; Bernhardt A; Lode A; Kampschulte M; Vob A; Simon P; Uckermann O; Kirsch M; Gelinsky M Strontium-Modification of Porous Scaffolds from Mineralized Collagen for Potential Use in Bone Defect Therapy. Mater. Sci. Eng. C Mater. Biol. Appl 2018, 84, 159–167. [DOI] [PubMed] [Google Scholar]
- 13.Salerno A; Verdolotti L; Raucci MG; Saurina J; Domingo C; Lamanna R; Lozzino V; Lavorgna M Hybrid Gelatin-Based Porous Materials with A Tunable Multiscale Morphology for Tissue Engineering and Drug Delivery. Eur. Polym. J 2018, 99, 230–239. [Google Scholar]
- 14.Trombetta R; Inzana JA; Schwarz EM; Kates SL; Awad HA 3D Printing of Calcium Phosphate Ceramics for Bone Tissue Engineering and Drug Delivery. Ann. Biomed. Eng 2017, 45, 23–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang YG; Zhu YJ; Chen F; Sun TW A Novel Composite Scaffold Comprising Ultralong Hydroxyapatite Microtubes and Chitosan: Preparation and Application in Drug Delivery. J. Mater. Chem. B 2017, 5, 3898–3906. [DOI] [PubMed] [Google Scholar]
- 16.Enarson DA; Fujii M; Nakielna EM; Grzybowski S Bone and Joint Tuberculosis: A Continuing Problem. Can. Med. Assoc. J 1979, 120, 139–145. [PMC free article] [PubMed] [Google Scholar]
- 17.Shaikh NS; Sawarkar SP Targeting Approaches for Effective Therapeutics of Bone Tuberculosis. J. Pharm. Microb 2017, 3, 1–13. [Google Scholar]
- 18.Alarçin E; Lee TY; Karuthedom S; Mohammadi M; Brennan MA; Lee DH; Marrella A; Zhang J; Syla D; Zhang YS; Khademhosseini A; Jang HL Injectable Shear-Thinning Hydrogels for Delivering Osteogenic and Angiogenic Cells and Growth Factors. Biomater. Sci 2018, 6, 1604–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dai T; Wang C; Wang Y; Xu W; Hu J; Cheng Y A Nanocomposite Hydrogel with Potent and Broad-Spectrum Antibacterial Activity. ACS Appl. Mater. Interfaces 2018, 10, 15163–15173. [DOI] [PubMed] [Google Scholar]
- 20.Gao W; Vecchio D; Li J; Zhang Q; Fu V; Li J; Thamphiwatana S; Lu D; Zhang L Hydrogel Containing Nanoparticle-Stabilized Liposomes for Topical Antimicrobial Delivery. ACS Nano 2014, 8, 2900–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu M; Li K; Zhu Y; Zhang J; Ye X 3D-Printed Hierarchical Scaffold for Localized Isoniazid/Rifampin Drug Delivery and Osteoarticular Tuberculosis Therapy. Acta Biomater. 2015, 16, 145–155. [DOI] [PubMed] [Google Scholar]
- 22.Huang D; Li D; Wang T; Shen H; Zhao P; Liu B; You Y; Ma Y; Yang F; Wu D; Wang S Isoniazid Conjugated Poly(lactide-co-glycolide): Long-Term Controlled Drug Release and Tissue Regeneration for Bone Tuberculosis Therapy. Biomaterials 2015, 52, 417–425. [DOI] [PubMed] [Google Scholar]
- 23.Chang B; Ahuja N; Ma C; Liu X Injectable Scaffolds: Preparation and Application in Dental and Craniofacial Regeneration. Mater. Sci. Eng. R Rep 2017, 111, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van Houdt CIA; Cardoso DA; van Oirschot BAJA; Ulrich DJ; Jansen JA; Leeuwenburgh SCG; van den Beucken JJJP Porous Titanium Scaffolds with Injectable Hyaluronic Acid–DBM Gel for Bone Substitution in A Rat Critical-Sized Calvarial Defect Model. J. Tissue Eng. Regen. Med 2016, 11, 2537–2548. [DOI] [PubMed] [Google Scholar]
- 25.Jia L; Danna C; Huaiqing L Injectable Composite of Calcium Alginate Hydrogel and Adipose-Derived Stem Cells to Remediate Bone Defect. IOP Conf. Ser.: Mater. Sci. Eng 2018, 423, 012182. [Google Scholar]
- 26.Liu M; Zeng X; Ma C; Yi H; Ali Z; Mou X; Li S; Deng Y; He N Injectable Hydrogels for Cartilage and Bone Tissue Engineering. Bone Res. 2017, 5, 17014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo Z; Pan J; Sun Y; Zhang S; Yang Y; Liu H; Li Y; Xu X; Sui Y; Wei S Injectable 3D Porous Micro-Scaffolds with a Bio-Engine for Cell Transplantation and Tissue Regeneration. Adv. Funct. Mater 2018, 28, 1804335. [Google Scholar]
- 28.Whitely M; Cereceres S; Dhavalikar P; Salhadar K; Wilems T; Smith B; Mikos A; Cosgriff-Hernandez E Improved in Situ Seeding of 3D Printed Scaffolds Using Cell-Releasing Hydrogels. Biomaterials 2018, 185, 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guyot C; Lerouge S Can We Achieve the Perfect Injectable Scaffold for Cell Therapy? Future Sci. OA 2018, 4, FSO284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang Q; Zou Y; Arno MC; Chen S; Wang T; Gao J; Dove AP; Du J Hydrogel Scaffolds for Differentiation of Adipose-Derived Stem Cells. Chem. Soc. Rev 2017, 46, 6255–6275. [DOI] [PubMed] [Google Scholar]
- 31.O’Brien FJ Biomaterials & Scaffolds for Tissue Engineering. Mater. Today 2011, 14, 88–95. [Google Scholar]
- 32.Cano M; de la Cueva-Méndez G Self-Assembly of A Superparamagnetic Raspberry-like Silica/Iron Oxide Nanocomposite Using Epoxy–Amine Coupling Chemistry. Chem. Commun 2015, 51, 3620–3622. [DOI] [PubMed] [Google Scholar]
- 33.Li D; Bu Y; Zhang L; Wang X; Yang Y; Zhuang Y; Yang F; Shen H; Wu D Facile Construction of pH- and Redox-Responsive Micelles from a Biodegradable Poly(β-hydroxyl amine) for Drug Delivery. Biomacromolecules 2016, 17, 291–300. [DOI] [PubMed] [Google Scholar]
- 34.Amendola V; Bakr OM; Stellacci F A Study of the Surface Plasmon Resonance of Silver Nanoparticles by the Discrete Dipole Approximation Method: Effect of Shape, Size, Structure, and Assembly. Plasmonics 2010, 5, 85–97. [Google Scholar]
- 35.De Maio F; Palmieri V; De Spirito M; Delogu G; Papi M Carbon Nanomaterials: A New Way against Tuberculosis. Expert Rev. Med. Dev 2019, 16, 863–875. [DOI] [PubMed] [Google Scholar]
- 36.Nyang’wa BT; Berry C; Fielding K; Nunn AJ Multidrug-Resistant Tuberculosis. The Lancet 2019, 394, 298–299. [DOI] [PubMed] [Google Scholar]
- 37.Gangadharam PRJ Isoniazid, Rifampin, and Hepatotoxicity. Am. Rev. Respir. Dis 1986, 133, 963–965. [DOI] [PubMed] [Google Scholar]
- 38.Timmins GS; Deretic V Mechanisms of Action of Isoniazid. Mol. Microbiol 2006, 62, 1220–1227. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Y; Heym B; Allen B; Young D; Cole S The Catalase-Peroxidase Gene and Isoniazid Resistance of Mycobacterium Tuberculosis. Nature 1992, 358, 591–593. [DOI] [PubMed] [Google Scholar]
- 40.Calabretta MK; Kumar A; McDermott AM; Cai C Antibacterial Activities of Poly(amidoamine) Dendrimers Terminated with Amino and Poly(ethylene glycol) Groups. Biomacromolecules 2007, 8, 1807–1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balogh L; Swanson DR; Tomalia DA; Hagnauer GL; McManus AT Dendrimer-Silver Complexes and Nanocomposites as Antimicrobial Agents. Nano Lett. 2001, 1, 18–21. [Google Scholar]
- 42.Zuidema JM; Rivet CJ; Gilbert RJ; Morrison FA A Protocol for Rheological Characterization of Hydrogels for Tissue Engineering Strategies. J. Biomed. Mater. Res., Part B 2014, 102, 1063–1073. [DOI] [PubMed] [Google Scholar]
- 43.Jafari A; Hassanajili S; Karimi MB; Emami A; Ghaffari A; Azarpira N Effect of Organic/Inorganic Nanoparticles on Performance of Polyurethane Nanocomposites for Potential Wound Dressing Applications. J. Mech. Behav. Biomed. Mater 2018, 88, 395–405. [DOI] [PubMed] [Google Scholar]
- 44.Li J; Shen Z; Li H; Xu L; Song H; Guan G; Liu G Reinforced Properties of Polybenzoxazine-based Nanocomposites with Siloxane Benzoxazine-Modified Halloysite Nanotubes. J. Appl. Polym. Sci 2019, 136, 47882. [Google Scholar]
- 45.Akizuki S; Mow VC; Pita JC; Howell DS; Manicourt DH Tensile Properties of Human Knee Joint Cartilage: I. Influence of Ionic Conditions, Weight Bearing, and Fibrillation on the Tensile Modulus. J. Orthop. Res 1986, 4, 379–392. [DOI] [PubMed] [Google Scholar]
- 46.Luo GF; Chen WH; Liu Y; Lei Q; Zhuo RX; Zhang XZ Multifunctional Enveloped Mesoporous Silica Nanoparticles for Subcellular Co-Delivery of Drug and Therapeutic Peptide. Sci. Rep 2014, 4, 6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y; Zhi Z; Jiang T; Zhang J; Wang Z; Wang S Spherical Mesoporous Silica Nanoparticles for Loading and Release of the Poorly Water-Soluble Drug Telmisartan. J. Controlled Release 2010, 145, 257–263. [DOI] [PubMed] [Google Scholar]
- 48.Dang G; Shi Y; Fu Z; Yang W Polymer Particles with Dendrimer@SiO2–Ag Hierarchical Shell and Their Application in Catalytic Column. J. Colloid Interface Sci 2012, 369, 170–178. [DOI] [PubMed] [Google Scholar]
- 49.He Y; Xie S; Yang X; Yuan R; Chai Y Electrochemical Peptide Biosensor Based on in Situ Silver Deposition for Detection of Prostate Specific Antigen. ACS Appl. Mater. Interfaces 2015, 7, 13360–13366. [DOI] [PubMed] [Google Scholar]
- 50.Matai I; Sachdev A; Gopinath P Multicomponent 5-Fluorouracil Loaded PAMAM Stabilized-Silver Nanocomposites Ssynergistically Induce Apoptosis in Human Cancer Cells. Biomater. Sci 2015, 3, 457–468. [DOI] [PubMed] [Google Scholar]
- 51.Wang X; Wang H; Wang Y; Yu X; Zhang S; Zhang Q; Cheng Y A Facile Strategy to Prepare Dendrimer-stabilized Gold Nanorods with Sub-10-nm Size for Efficient Photothermal Cancer Therapy. Sci. Rep 2016, 6, 22764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang YZ; Hao N; Feng QM; Shi HW; Xu JJ; Chen HY A Ratiometric Electrochemiluminescence Detection for Cancer Cells Using g-C3N4 Nanosheets and Ag–PAMAM–Luminol Nanocomposites. Biosens. Bioelectron 2016, 77, 76–82. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


