Abstract
Environmental DNA (eDNA) water assays are beginning to be implemented for many important pathogens in confined aquaculture systems. Recirculating systems are rapidly being developed for fin fish aquaculture. Zebrafish (Danio rerio) are reared in these systems, and Pseudoloma neurophilia (Microsporidia) represents a serious challenge for zebrafish research facilities. Diagnosis of the pathogen has traditionally used histology or PCR of tissues with lethal sampling. However, with the development of a nonlethal assay to detect P. neurophilia in tank water, facilities will be able to integrate the assay into routine surveillance efforts to couple with their established protocols. Here, we first describe a modified protocol to extract and quantify parasite DNA from the environment for nonlethal detection of P. neurophilia in adult zebrafish populations. Using this modified assay, we then evaluated water samples from a longitudinal experimental infection study, targeting timepoints during initial infection. The parasite was detectable in the water immediately after initial exposure until week 4 post exposure (pe), when the parasite was undetectable until 7 weeks pe. After that time, the parasite was sporadically detected in the water for the 10-month study, likely correlating with the lifecycle of the parasite. Using water samples from the Zebrafish International Resource Center, we also validated the clinical relevance of the assay in a large zebrafish facility. The integration of this assay at ZIRC will significantly compliment surveillance and control efforts for the microsporidian parasite.
Keywords: Environmental DNA (eDNA), Digital PCR (dPCR), Zebrafish, Pathogens, Microsporidia
1. Introduction
Infectious diseases are one of the most serious constraints to the expansion and development of aquaculture (FAO, 2020). Directly transmissible pathogens are of particular concern with high-density systems such as recirculating aquaculture, and sensitive and early pathogen detection is important for control of these diseases. The zebrafish (Danio rerio) is a widely used biomedical model (Cassar et al., 2020; Phillips and Westerfield, 2020; Gomes and Mostowy, 2020) and is now the second most used vertebrate animal model in biomedical research (Kinter et al., 2021). Zebrafish are also an important model for fish biology and aquaculture, and they have also been utilized to study several important bacterial and viral diseases of aquaculture (Varela et al., 2017; Jørgensen, 2020),
As with other laboratory animals, a common threat to laboratory zebrafish and overall zebrafish facility operations is the presence of infectious diseases (Kent et al., 2020). Pseudoloma neurophilia, is an ongoing threat to the zebrafish model and continues to be the most prevalent pathogen found in zebrafish research facilities that utilize the diagnostic service at the Zebrafish International Resource Center (ZIRC) in Eugene, Oregon (Kent et al., 2020). This microsporidium is an obligate intracellular parasite that causes chronic infections in zebrafish, but also infects a broad range of fishes (Sanders et al., 2016). Infections by P. neurophilia are largely asymptomatic. However, a subset of an infected population may present with general clinical signs such as emaciation and skeletal deformities. Typical of microsporidia, the parasite is transmitted by ingestion of a resistant spore in the environment (Kent and Bishop-Stewart, 2003), while vertical transmission also occurs by release of spores from ovaries during spawning events or within eggs (Sanders et al., 2013). Most diagnostics for P. neurophilia have been based on examination of fish tissues, either via histology (Murray et al., 2011) or PCR methods on neural tissues (Whipps and Kent, 2006; Sanders and Kent, 2011; Crim et al., 2017; Miller et al., 2019; Schuster et al., 2022a). Furthermore, our recent study confirmed that PCR on whole fish was both more sensitive and detected infections earlier in infection than histology (Schuster et al., 2022b).
Sensitive, non-lethal diagnostics based on detection of environmental DNA (eDNA) in water are particularly useful with more confined systems with high fish to water ratios, such as zebrafish facilities or other recirculating systems that are increasingly expanding in food fish aquaculture. In addition, such tests provide an advantage of not having to handle or lethally sample valuable stocks that may be available only in small populations – e.g., brood stock or mutant lines. Several eDNA tests have recently been developed for fish pathogens in water (Hallett, 2006; Purcell et al., 2017; Rusch et al., 2018). Examples of eDNA water tests for fish parasites in captive fishes include Neobenedenia girellae (Agawa et al., 2016), Chilodonella hexasticha (Bastos Gomes et al., 2017), Ichthyophthirius multifiliis (Howell et al., 2019), Dactylogyrus spp. (Trujillo-González et al., 2019), and Neobenedenia girellae (Agawa et al., 2016). Moreover, Peters et al. (2018) developed a multi-plex assay to detect the salmon louse Lepeophtheirus salmonis and Paramoeba perurans. In the case of zebrafish parasites, an eDNA assay has recently been developed for the capillarid nematode Pseudocapillaria tomentosa. The assay for P. tomentosa was shown to effectively detect the pathogens in feces, detritus, and water samples (Norris et al., 2020).
With P. neurophilia, Sanders and Kent (2011) found that environmental screening of tank water or tank debris, resulted in occasional detection of the parasite in eggs and water during spawning events, but testing straight tank water on a flow-through system was not sufficiently sensitive for disease diagnosis. Likewise, Crim et al. (2017) and Miller et al. (2019) showed that PCR testing of detritus and water for P. neurophilia was inconsistent. We recently developed an improved eDNA test of P. neurophilia (Schuster et al., 2022a), coupling sonication and digital PCR (dPCR). We also showed that the assay was better for detection of P. neurophilia when tanks were static for 8 h compared to those with flowing water.
Previously, this assay was used on the Biorad droplet digital PCR (ddPCR) platform (Biorad, Hercules, CA), coupled with sonication by the Diagenode Bioruptor Pico (Diagenode, Denville, NJ) (Schuster et al., 2022a). In the present study we transitioned dPCR platforms to the Qiagen QIAcuity dPCR system (Qiagen, Hilden, Germany), coupled with a QSONICA sonicator and cuphorn accessory (QSONICA, Newtown, CT). After validation, we then evaluated water samples that were previously collected over a 10-month period following a low dose exposure of spores (Schuster et al., 2022b). Furthermore, water samples from the Zebrafish International Resource Center (ZIRC) (https://zebrafish.org/home/guide.php), Eugene, Oregon were evaluated. This facility has a history of prevalent P. neurophilia infections in certain stocks of fish in large recirculating systems (Murray et al., 2011; Murray et al., 2016). Here, we present data on the sensitivity and specificity with this modified format and initial field-testing of water samples from the ZIRC.
2. Materials and methods
2.1. Improvement of assay
2.1.1. Microsporidian spore collection & purification
P. neurophilia spores were obtained from hindbrain and anterior spinal cords as described in Schuster et al. (2022a). The spore suspension was then quantified using a hemacytometer and diluted in diH2O as needed to obtain desired concentrations.
2.1.2. Transitioning assay to the Qiagen QIAcuity One platform
The digital PCR (dPCR) assay originally described in Schuster et al. (2022a) was performed on the Biorad Digital Droplet PCR (ddPCR) (Biorad, Hercules, California) platform and was transitioned to the Qiagen QIAcuity One (Qiagen, Hilden, Germany). dPCR was performed using DNA extracted from P. neurophilia spores collected from zebrafish tank water using a commercial Qiagen DNeasy Blood and Tissue Kit (Qiagen, Hilden, Germany) (Schuster et al., 2022a). The QIAcuity Probe PCR kit was used in combination with the forward and reverse P. neurophilia-specific primers Pn10F (5′ GTAATCGCGGGCTCACTAAG 3′) and Pn10R (5′ GCTCGCTCAGCCAAATAAAC 3′) and Probe (5′- 6-carboxyfluorescein (FAM)-ACACACCGCCCGTCGTTATCGAA – 3′-Black Hole Quencher 1 (BHQ1)) described in Sanders and Kent (2011). The QIAcuity Nanoplate 26 k 24-well plate was used for the analysis, which allows for a greater number of partitions per PCR reaction. Each reaction was composed of the following rations: 10 μL of Mastermix 4× (Qiagen), 3.6 μL of each primer, 1.8 μL probe (FAM), 21 μL nuclease-free water, and 5 μL extracted P. neurophilia DNA; total reaction volume = 45 μL. Individual reactions were then classified as positive (fluorescence present) or negative (no fluorescence) for each reaction using the Qiagen software suite analysis software (V. 1.1.3). Analyzing individual samples with this software, a detection threshold was set at 115 (RFU), a value that represents the fluorescence intensity that is used to distinguish positive from negative. The threshold was calibrated relative to a positive control of known P. neurophilia DNA concentration. Thresholding, as described in the QIAcuity One manual (Qiagen, 2021) was used consistently for every sample analyzed.
2.1.3. Optimizing assay on Qiagen QIAcuity One platform
Using the DNA extracts from the sonication time trial series in a previous study (Schuster et al., 2022a), we then analyzed the optimal dynamics for dPCR on the Qiagen QIAcuity One. Using the recommended concentrations from the Qiagen Quick-Start protocol for QIAcuity Probe PCR Kit, we adjusted the dPCR protocol, as the assay requirements differed from that of the Biorad ddPCR platform. Specifically, the overall reaction volume was increased from a 20 uL PCR reaction to 45 uL, as primer volumes were doubled, probe volume was increased, and a much larger volume of nuclease-free water was needed for the PCR reaction. The optimal PCR reaction recipe for P. neurophilia is provided above, but the cycling parameters were then assessed using the Qiagen standard priming profile: 2 min at 95 °C followed by 40 cycles of 15 s at 95 °C and 30 s at 60 °C. Image acquisition of all PCR wells is obtained through analyzing partitions that contain the target molecule inside, which will emit fluorescence light brighter than those without the target. For our assay, only the yellow channel was used, as this corresponds to the HEX fluorophores. The optimal DNA template input for each dPCR reaction was analyzed using a serial dilution series of 105, 104, 103, and 102 spores/L and a DNA template input of 5 and 10 μL (Supplementary data S2).
2.1.4. Optimization of sonication protocol
We optimized our sonication protocol and equipment to increase sensitivity. The Diagenode Bioruptor Pico (Diagenode, Denville, New Jersey) was used to disrupt spores in our first assay (Schuster et al., 2022a), as sonication was used to apply sound energy to agitate spores to release DNA for detection. However, this method was limited by sample volume and control of the amplitude of ultrasonic frequency, resulting in samples not being adequately disrupted. Thus, we changed the protocol to utilize the QSONICA sonicator ultrasonic processor (Part No. Q700) and cup horn accessory (Model #431C2) for disruption of spores in water samples, which allows more freedom to manipulate volume of samples and to allow for the freedom to fully control amplitude of ultrasonic frequency that samples were exposed to from 1 to 100%.
To determine the optimal sonication time required to adequately disrupt spores of P. neurophilia a time series experiment was performed using water (negative control tank water) spiked with 58,750 spores/L and processed using the water filtration and DNA extraction protocol described in Schuster et al. (2022a), with slight modifications. Ten filters were spiked at the same concentration and processed as described Schuster et al. (2022a), except samples for sonication were placed into 0.5 mL thin-walled PCR sample tubes (Brandtech, Part #781310) rather than the Diagenode bioruptor tubes. The samples were then hydrated in 100 uL PBS and placed in the cup horn accessory. The cuphorn was filled with water to the same level as the samples and the QSONICA was set at 50% frequency, with samples programmed to run for 1, 2, 5, 8, and 10 min using a 30 s on/off cycle. Duplicate filters were processed at each time interval. The samples were first evaluated using the qPCR assay from Sanders and Kent (2011) and then by dPCR (Table 1).
Table 1.
Sonication time trials analyzed for the optimal detection of Pseudoloma neurophilia. Replicate spiked water filters at 58,750 spores/L were sonicated at 1, 2, 5, 8, and 10 min and validated using qPCR, with values reported as Ct values. Extracts from the sonication time trial were used to optimize dPCR parameters, in which replicate values are reported in copy numbers/μL. The Qiagen standard priming profile was employed (2 min at 95 ° C and 40 cycles of 95 °C for 15 s, then 60 °C for 30 s).
| Optimal Sonication Time (qPCR) | dPCR Validation | |||||||
|---|---|---|---|---|---|---|---|---|
| Filter 1 | Filter 2 | Filter 1 | Filter 2 | |||||
| Time | Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 |
| 1 min | 31.84 | 31.46 | 31.9 | 32.31 | 6.4 | 5 | 4.4 | 3.2 |
| 2 min | 30.54 | 30.95 | 30.52 | 30.84 | 7.7 | 9.9 | 11.7 | 7.9 |
| 5 min | 32.29 | 32.27 | 33.44 | 34.4 | 4.1 | 2.9 | 1 | 1.2 |
| 8 min | 30.63 | 30.22 | 29.57 | 29.51 | 10 | 8.4 | 32.1 | 33.7 |
| 10 min | 31.15 | 31.6 | 29.54 | 29.62 | 7.7 | 8.1 | 20.8 | 14.5 |
2.1.5. Comparison of protocols
To compare the water assay protocols, we set up another limit of detection experiment using inoculated water filters, as outlined in Schuster et al. (2022a). We tested concentrations of 45,000, 4,500, 450, 45, and 4.5 spores/L by inoculating 1 L of negative control water with the respective concentrations. Two replicate 1 L water samples were inoculated with the parasite at the respective concentration and filtered through a 0.45 μm Nalgene analytical test filter (Nalgene, Rochester, NY). Each of the filters were then processed using the Diagenode pico bioruptor and Biorad ddPCR protocol outlined in Schuster et al. (2022a). This was repeated for another set of filters to be processed using the QSONICA sonicator and Qiagen QIAcuity One dPCR protocol described here. The detection limit for both assays was compared (Supplementary data S1).
2.2. Analysis of water samples from experimental exposure
Weekly (up to week 9) and biweekly (weeks 9 to 45) water samples were collected from an experimental exposure of a large population of zebrafish over a duration of 10 months (Schuster et al., 2022b). The population consisted of 566 fish and was held in a 75 L tank. The goal for the experimental exposure was twofold, to track the progression of infection in a population using histopathology and tissues analyzed by qPCR, and to understand how detection in the water occurs over the course of infection. Thus, the large population size of the experimental study was to mimic a natural exposure with a low dose of the parasite and to ensure confidence in prevalence estimates at each timepoint throughout the duration of the study (Schuster et al., 2022b). The OSU vivarium is a flow-through system with a flow rate of 140 mL/min. The population was initially exposed a dose of 8000 spores/fish (60 spores/ mL). The tank was left static for 72 h, after which flow was returned to the tanks. As described in Schuster et al. (2022a), spores were extracted from infected populations, purified, and introduced directly into the tank. Infection was tracked throughout the study, as described in Schuster et al. (2022b). For each sample point, the tank was set to static by turning off water flow for 8 h before sampling, as we showed in previous studies that leaving the tanks static for 8 h increases the probability of detection compared to taking water samples from flow-through tanks (Schuster et al., 2022a). Following the recommended water filtration protocol described in Schuster et al. (2022a), the tank was thoroughly mixed, and two 1 L water samples were taken from the tank, processed individually through a 0.45 μm filter, resulting in two filters/sample point. The filters were initially placed in 100% ethanol and frozen at −20C. For analysis, the filters were placed uncapped into 15 mL conical tubes to dry overnight and then dissolved using 7 mL acetone/filter and processed using the updated sonication and dPCR protocol described above. Water samples were collected weekly and biweekly as described above. The same protocol was used for a negative control tank; a 16 L tank containing 100 fish not exposed to P. neurophilia spores.
2.3. Validation at the Zebrafish International Resource Center (ZIRC)
The modified protocol for the eDNA assay was then evaluated for its clinical relevance by testing several 75 L tanks of zebrafish at the ZIRC that were held on two large recirculating systems. 75 L tanks house 150–300 fish and receive post-filtration water at a rate of 184 L/h. The ZIRC uses reverse osmosis (RO) treated city water, with Instant Ocean added to restore salts and ions. The system is then filtered through bead filters and fluidized sand beds (Aquaneering Inc.) and treated with crushed Aragonite for denitrification. Water is gas equilibrated in trickle columns (Aquaneering Inc.) after filtration to ensure gas equilibrium and elimination of oxygen supersaturation. After both mechanical and biological filtration, effluent water is then exposed to ultraviolet disinfection (132 μWsec/cm2) to eliminate pathogens from filtered water before arriving to the aquaria (Varga, 2011).
The attending veterinarian, Dr. Katrina Murray, provided the water samples from infected tanks in which several of the tanks contained fish that were previously diagnosed as positive for P. neurophilia by histology or by a ZIRC in-house PCR test. Water was collected from the tanks after in-flowing water was turned off (i.e., static conditions) for 3 to 4 h and the tank water was mixed with debris scrubbed from tank surfaces. This time differed from the experimental study and was chosen based on facility dynamics and staff time constraints. Water was processed as described in Schuster et al. (2022a) and filters were placed in 100% ethanol and frozen at −80 °C until processed for DNA extraction. Water samples were analyzed using the modified sampling, sonication, extraction, and dPCR protocol described above. For 5 of the 12 tanks, 4 L of water were provided for analysis, while for the other 7, in addition to the negative controls, only 2 L of water was provided. Negative control water samples were collected from post-filtration and UV hoses before water entered the fish tanks. From eight of the tanks that were positive for P. neurophilia by histology, fish were also provided for analysis by whole-body PCR analysis, as described in Schuster et al. (2022b).
3. Results
3.1. Modification of sonication and validation of dPCR
The QSONICA manufacturers recommended a starting amplitude control of 50% and this was adequate for spore disruption at 8 min of total sonication, with 30 s on/off intervals, as determined by consistency of copy number/μL (Table 1). Thus, because there was adequate detection at every time interval at this frequency, we did not continue increasing amplitude frequency. Using qPCR to evaluate the sensitivity, it was shown that 8 min sonication provided the best and most consistent detection (Table 1).
3.2. Comparison of sonication efficiency
The comparison between the two methods showed that the modified method described above provides a more sensitive assay than our previous format (Schuster et al., 2022a) and could detect two logs lower concentration of target DNA (Fig. 1; Table S1). Two replicate PCR reactions were conducted with each filter, which differed from our previous reports of executing the reactions in triplicate. The limit of detection using the updated sonication and dPCR protocol resulted in positive detections as low as 4.5 spores/L, but only one of the two replicate filters were positive at 45 and 4.5. spores/L (Fig. 1). Intra-filter PCR replicates were similar for all samples. For samples scored as negative, both replicate filters were negative.
Fig. 1.
Sensitivity with filters spiked with Pseudoloma neurophilia spores. Two replicate filters were processed for each concentration; Two PCR reactions were conducted for each filter. Dotted bars with the solid circles represent filter 1 and the grey bars with solid circles represent filter 2. The solid circles represent the PCR technical replicates for each sample.
3.3. Detection of P. neurophilia in water from an experimentally infected tank
P. neurophilia was detected in tank water throughout the duration of the 10-month longitudinal analysis (Fig. 2). Spanning the entire sampling period– 16 of 27 time points were positive. Reported copy numbers/L were low, ranging from about 0.5–3 copies/L. At each sample point, two L were taken from each tank; thus, of the sample dates that were positive, there were 11 instances when only 1 L sample was positive, while the other was negative. During initial infection, which was 0–11 weeks post exposure (pe), the parasite was detected in 8 out of 10 sampling points and the highest copy numbers detected occurred at 1 week during the initial inoculation period. The parasite was detected in 9 of 17 time points during active infections, in which fish were positive by histology (13–45 weeks post-exposure) as described in Schuster et al., 2022b. All the negative control filters collected from a tank of fish without infections were negative.
Fig. 2.
Water samples were collected over a 10-month experimental exposure. Samples were collected weekly until wk. 9, when the samples were then taken every other week. At each timepoint, 2 filters were taken, thus the grey bars represent filter A, while the black bars represent filter B. A zero (0) indicates that the filter did not have positive detection and the color of each (0) corresponds to either Filter A (grey) or Filter B (black). Active infections are defined here as when fish were positive by histology, weeks 13–45 post exposure as described in Schuster et al. (2022b).
3.4. Validation of water test at the ZIRC
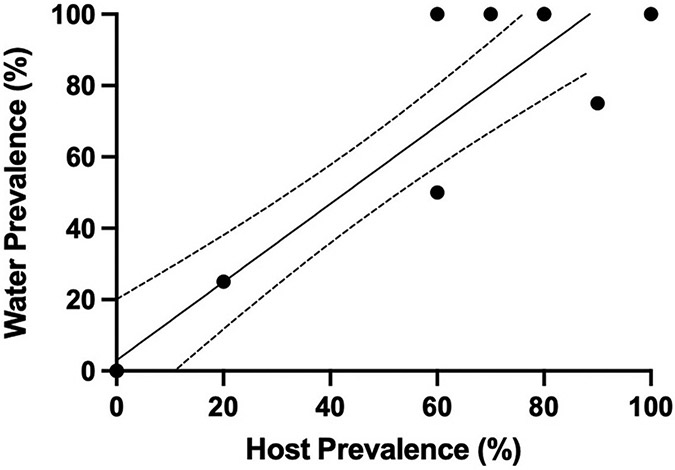
P. neurophilia DNA was detected in water from all seven tanks that were confirmed as positive based on fish examinations (histology or PCR), with copy numbers ranging from 0.86 to 28 copies/L (Table 2). In contrast, P. neurophilia was not detected in the water samples from the four tanks holding fish that were diagnosed as negative by in-house PCR diagnostics at ZIRC (U, V, W, and X). Moreover, post-filtration and UV system water was negative, and our laboratory water control was also consistently negative. The laboratory water, in this case, is dechlorinated city water that flows into the vivarium, and is taken directly from a negative control tank. The most pronounced detection occurred in tank Z, with copy numbers ranging from 15 to 28 copies/L. Tanks M and AA, are examples of infected tanks, where only one of the 1 L water samples was positive. All tanks with fish older than 250 dpf, were positive by histology and had the greatest detection in water (N, Q, R, Y, and Z), which was in contrast to ZIRC tanks consisting of younger populations (Table 2). In contrast, no parasite DNA was detected in the water in the tanks with younger populations (128 dpf), except for sample group M. Likewise, detection of the infection by histology or neural tissue PCR with these younger fish was consistently negative. When the tanks had higher copy numbers, all PCR replicates, and technical replicates had higher copy numbers, while when the assay was operating at its lower end of detection, parasite DNA was minimal (0.86 copies/L in one replicate in post-filtration water) (Table 2). As seen in Schuster et al. (2022a), there was positive correlation with the amount of parasite in tank water and the prevalence of infection in the fish tank population (Fig. 3; Table 2) (Simple linear regression; R2 = 0.88).
Table 2.
Validation of water samples from the Zebrafish International Resource Center (ZIRC) using eDNA assay for Pseudoloma neurophilia. For every filter, triplicate PCR reactions were executed; 2 of the 3 reactions must result in a positive detection to be deemed positive. Negative control samples include ZIRC samples (con O and con P), which are post-filtration water from two recirculating systems: System A and B. Con T = laboratory water. Dpf = days post fertilization. * Indicates that the filter was deemed positive for detection of the parasite. Estimates of the prevalence of infection was determined based on ZIRC histology or tissue PCR results. Letters (column 1) are the designations for tanks that were sampled. PCR values are reported as copy number/L.
| Tank | ZIRC Stock (System) |
Age (dpf) |
No. of Fish in Tanks |
ZIRC Diagnostic Prevalence |
WB-qPCR Prevalence |
Filter | dPCR result (copy #/L) Replicate 1 |
dPCR result (copy #/L) Replicate 2 |
dPCRresult (copy #/L) Replicate 3 |
|---|---|---|---|---|---|---|---|---|---|
| M | ZS 11236 (A) | 105 | 316 | N/A | 2/10 | 1 | 0 | 0 | 0 |
| 2* | 0.86 | 0.86 | 0 | ||||||
| 3 | 0 | 0 | 0.86 | ||||||
| 4 | 0 | 0 | 0 | ||||||
| N | ZS 11015 (B) | 251 | 279 | 1/5 histo | 8/10 | 1* | 12.92 | 20 | 12.08 |
| 2* | 6.88 | 12.06 | 7.76 | ||||||
| 3* | 7.76 | 2.58 | 7.76 | ||||||
| 4* | 11.46 | 7.74 | 6.02 | ||||||
| Q | ZS 11229 (A) | 253 | 255 | 6/11 histo | 8/10 | 1* | 5.16 | 6.04 | 3.46 |
| 2* | 1.72 | 1.72 | 0.86 | ||||||
| 3* | 1.72 | 2.58 | 1.72 | ||||||
| 4* | 0.88 | 0 | 0.86 | ||||||
| R | ZS 11229 (A) | 253 | 253 | 1/11 histo | 6/10 | 1* | 0.86 | 2.58 | 3.94 |
| 2* | 2.58 | 0 | 2.58 | ||||||
| 3* | 6.02 | 7.76 | 3.44 | ||||||
| 4* | 0 | 0.86 | 0.86 | ||||||
| S | ZS 11229 (A) | 253 | 291 | 7/11 histo | 9/10 | 1* | 3.44 | 0.86 | 0 |
| 2* | 0 | 0.86 | 0.86 | ||||||
| 3 | 0 | 0 | 0.86 | ||||||
| 4* | 0.88 | 4.3 | 0.86 | ||||||
| U | ZS 11403 (A) | 128 | 235 | 0/11 PCR | – | 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | ||||||
| V | ZS 11403 (A) | 128 | 259 | 0/11 PCR | – | 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | ||||||
| W | ZS 11403 (A) | 128 | 287 | 0/11 PCR | – | 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | ||||||
| X | ZS 11403 (A) | 128 | 285 | 0/11 PCR | – | 1 | 0 | 0.86 | 0 |
| 2 | 0 | 0.86 | 0 | ||||||
| Y | ZS 11522 (A) | 266 | 260 | 9/11 histo | 10/10 | 1* | 12.06 | 2.6 | 8.66 |
| 2* | 8.62 | 3.44 | 6.9 | ||||||
| Z | ZS 11522 (A) | 266 | 170 | 7/11 histo | 7/10 | 1* | 19.82 | 24 | 18.1 |
| 2* | 15.86 | 28 | 24 | ||||||
| AA | ZS 11497 (B) | 281 | 229 | 4/5 histo | 6/10 | 1* | 0.86 | 0 | 0.86 |
| 2 | 0 | 0 | 0 | ||||||
| CONO | System B post filtration | – | – | – | – | 1 | 0 | 0.086 | 0 |
| 2 | 0 | 0 | 0 | ||||||
| CONP | System A post filtration | – | – | – | – | 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | ||||||
| CONT | System A post filtration | – | – | – | – | 1 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 |
Fig. 3.
Simple linear regression showing detection in tank water increases as prevalence in tank population increases at the Zebrafish International Resource Center (ZIRC). Individual fish were evaluated by either PCR or histology. Line of best fit (Y = 1.096*X + 0.03041) with 95% confidence intervals (dashed lines) (R2 = 0.88).
4. Discussion
Previous studies have defined eDNA as DNA that is not necessarily obtained from the target organism itself, however with the detection of pathogens in water, often whole organisms are detected and the same technologies are used (Sieber et al., 2020). As with other protistan and myxozoan parasites, in the case of P. neurophilia, DNA may come from intact spores that is released after sonication, as well as from developmental (pre-sporgonic) stages inside of sloughed host cells or from defective spores that have misfired leaving the sporoplasm to lyse and release DNA freely in the environment. Therefore, the assay presented and applied in this study importantly highlights the potential for detection of pathogen DNA from various life stages using environmental-based assays in aquaculture systems.
Adapting our eDNA assay to the Qiagen QIAcuity platform, coupled with the change in sonication equipment, resulted in an improved diagnostic assay for the detection of P. neurophilia in water, compared to our previously developed protocol (Schuster et al., 2022a). Our modified test resulted in approximately two logs improvement, likely due to the improved sonication efficiency with the QSONICA protocol compared to the Diagenode Bioruptor Pico sonication protocol as used in Schuster et al. (2022a). The assay had a detection limit down to 4.5 spores/L, an improvement from the previous 77.5 spores/L. Furthermore, in the longitudinal laboratory study, the parasite DNA was detected throughout the duration of the study by PCR in whole body samples as early as 4 days post exposure (Schuster et al., 2022b), coinciding with early detection of the parasite in water (Fig. 2). This finding reveals key dynamics about the detection of the parasite on a population level, shedding light on the progression of infection in adult zebrafish populations.
Water samples typically showed low copy numbers, usually on the lower end of the detection limit. We are confident that the samples with multiple low copy numbers were positive as negative control samples were always negative. Using the water samples from Schuster et al. (2022b), parasite detection in tank water was highest during the initial inoculation (week 1), which likely represented residual spores from the original inoculum of 60,000 spores/L. Whereas whole body PCR was also able to detect the parasite shortly after exposure, only 12% of the fish were positive by PCR before active infection (> week 11, at which time fish were positive by histology) (Schuster et al., 2022b). Hence, without the non-lethal eDNA assay, detection still would have required sampling a large percentage of fish from a population to detect the initial and early timepoints in infection. This is particularly important if populations are small or very valuable, such as certain mutant lines of zebrafish or valuable brood stock for certain aquaculture species.
When combining the whole-body analysis by qPCR (WB-qPCR), histology data (Schuster et al., 2022b), and the eDNA results of the longitudinal analysis reported here, detection in the water was prevalent during initial inoculation up to week 4, then there was no detection until week 7 post exposure. By this time, the tank water volume had turned over multiple times and spores free in the environment from the initial exposure were minimal. It was not until 3 mo later that spores were detected in the spinal cord by histology. At this same time, there was a large increase in detection by WB-qPCR and histology. From 27 to 92 dpe, although the parasite was visualized in histological sections, a few fish showed mild inflammation in the spinal cord without observable spores, which may have indicated early infections (Schuster et al., 2022b; Sanders and Kent, 2014).
This population of fish was inoculated with a spore suspension at a concentration of 8000 spores/fish (56 spores/mL), which is considerably lower than previous experimental exposures (e.g., 10,000 spores/ fish) (Ferguson et al., 2007; Ramsay et al., 2009; Cali et al., 2012; Sanders et al., 2013). Of note, as evident in the water results with these lighter infections, the assay at times is operating at its lower limits of detection. Thus, repeat sampling of tanks would result in a higher confidence of detection, as the sensitivity of this assay is considerably increased with additional subsequent sampling (Schuster et al., 2022a). In fact, many Specific Pathogen Free programs rely on repeat testing to increase confidence in negative results (Murray et al., 2022). The possibility of false positives exists; however, the analytical capacity of the assay is reported to be high, with a high positive predictive value (Schuster et al., 2022a).
Our study included examination of water samples from a large zebrafish facility (ZIRC). This facility has large recirculating systems in which effluent water is treated with mechanical and biologic filtration, followed by ultraviolet irradiation, before it is returned to aquaria with fish (Varga, 2011; Murray et al., 2016). Water that was subjected to the filtration and disinfection process was used for the control water in the ZIRC validation and was found to be negative for P. neurophilia. However, one PCR replicate showed a copy number of 0.086 copies/L, but this is likely a result of a small amount of parasite DNA occurring in the water after filtration and UV irradiation. The lack of positivity in the post-filtered water is consistent with sentinel fish observations at ZIRC in which fish exposed to prefiltered water from many tanks were consistently positive for P. neurophilia by histology, whereas sentinel fish receiving post-filtered water were always negative (Murray et al., 2011; https://zebrafish.org/wiki/health/health_reports/start). The two large systems at ZIRC process very large amounts of water through their recirculating systems, and we estimate that the 75 L tanks used in this study received about 4.0 L/min/tank. For the experiment in our vivarium at Oregon State University (OSU), the experimental tank’s flow rate was only 0.4 L/min (Schuster et al., 2022b). Hence, the water turnover (replacement) rate in ZIRC tanks was estimated to turnover 2.5 times every hour compared to the OSU study occurring once every 9 h (roughly 18 turnovers/week), assuming 100% turnover. To mitigate the large difference in turnover rates, tanks at ZIRC were left static for 3–4 h before water sampling. The differences in detection and system dynamics highlight the need to optimize the use of this assay for individual facilities. Halting water flow in a tank for many hours will increase the concentration of spores present in the water and hence enhance detection (Schuster et al., 2022a). As evident in the ZIRC samples, in some cases, it may also be of benefit to increase the amount of water that is filtered and filter 4 L of water rather than only 2.
With the ZIRC samples, we analyzed water from tanks in which 4 L were sampled and other tanks where only 2 L were sampled. Within the former, group M had only one positive filter. Of note, the fish in group M were approximately 3 months old, and the copy numbers and trends observed in this group were very similar to the copy numbers observed in the longitudinal laboratory experiment. In this tank, the copy numbers were generally low (Fig. 2). ZIRC diagnostic data was not available for this specific tank, however fish samples were provided for this population, and a low prevalence of infection in the population was detected by whole-body qPCR. Given the age of the fish, we suspect the prevalence of infection was low at this time and would have continued to increase over time, as seen in Schuster et al. (2022a). In contrast, detection was highest in tanks with the oldest fish. Thus, these results, in combination with the finding that detection is correlated with time after exposure (Ramsay et al., 2009; Schuster et al., 2022b), suggest a positive correlation with increased detection and the age of fish.
Comparing results from the validation with ZIRC samples and the experimental transmission studies (Schuster et al., 2022b), findings suggest that spores are frequently and sporadically present, but often in low numbers in water. The dynamics of detection are correlated to days post exposure (Schuster et al., 2022b), which suggests that the detection of the parasite is dependent not only on the system dynamics (flow-through systems compared to static), but also on the lifecycle of the intracellular parasite. Thus, consistent with our understanding of the life cycle, P. neurophilia spores are probably only shed in high numbers from live, infected fish during spawning and at chronic states of infection through urine and feces (Sanders et al., 2013). As was described in Schuster et al. (2022a), prevalence of the parasite in the water increased as prevalence in fish tissues increased. This was consistent with the observations in the ZIRC samples, where generally, tank populations with a higher estimated prevalence of infection resulted in the most consistent detection in the water.
Collectively, many of the water samples screened for this study had relatively low copy numbers and this is reflective of the low amount of parasite in the water. Detection by whole-body qPCR, revealed positive detections throughout the entire 10-month duration reported in Schuster et al. (2022b). However, during initial infection (up to 8 weeks pe), infections were minimal and ranged from 1 to 4 fish positive out of 18 at a given sample point. At this same time, the fish were 3–4 months of age, and the trends in detection, age, and copy numbers reported appear very similar to sample M provided by ZIRC. This highlights important aspects about the progression of the infection in large populations of zebrafish and the dynamics regarding detection. Because the parasite is often detected in low copy numbers in water prior to active infections, consistent and regular sampling of tanks should be implemented into surveillance efforts.
Lastly, the results presented here extend beyond zebrafish facilities. Bastos Gomes et al. (2017) used an eDNA test for Chilodonella hexasticha coupled with water quality parameters to predict outbreaks of this parasite in barramundi, Lates calcarife. This study and others (Bastos Gomes et al., 2017; Peters et al., 2018) highlight the potential for eDNA surveillance of important pathogens of aquaculture to provide sensitive early detection of pathogens and to pinpoint their locations in confined aquatic systems. This approach may also help to decrease the amount of lethal sampling needed to maintain specific-pathogen free or high health facilities (Murray et al., 2022).
5. Conclusions
The optimized protocol for detection of P. neurophilia in water provides a valuable alternative or complement to lethal sampling for analysis by histology or whole-body PCR. Coupling this assay with other surveillance efforts, would aid in positively identifying infections early, before active infections are present, and to confirm that a stock remains P. neurophilia free. Moreover, continuing to include histological analysis in surveillance efforts provides valuable information on the pathologic changes associated with the infection and allows for detection of other pathogens and lesions. The ZIRC samples validate the clinical assessment of this assay. Implementation of the assay into routine surveillance at research facilities would be of great benefit for the zebrafish research community and we are moving forward with implementation of this test to track and minimize the infection at ZIRC.
Supplementary Material
Acknowledgements
This study was supported by the National Institutes of Health under the Research Supplements to promote Diversity in health-related research (NIH ORIP 3 R24 OD010998). We are grateful to the Zebra-fish International Resource Center (ZIRC) for providing us with consistent supply of infected subject animals for investigation. The ZIRC is support by a grant from the National Institutes of Health, Office of Research Infrastructure Programs in collaboration with the Eunice Kennedy Shriver National Institute of Child Health and Development (P40 OD011021). We thank the Sinnhuber Aquatic Resource Center at Oregon State University for providing pathogen free fish. All work, aside from samples obtained from the Zebrafish International Resource Center (ZIRC), was completed at Oregon State University, a land grant university, located on the traditional homelands of the Kalapuya people, who are now absorbed into the Grande Ronde and Siletz Nations.
Footnotes
Ethics approval and consent to participate
This study was conducted following the 2012 international guidelines for Biomedical Research Involving Animal (Council for International Organizations of Medical sciences, http://www.cioms.ch). The care and use of laboratory animals were in full compliance with these guidelines as well as with the approval of the Institutional Animal Care and Use Committee at Oregon State University and the University of Oregon (Zebrafish International Resource Center).
CRediT authorship contribution statement
Corbin J. Schuster: Data curation, Conceptualization, Methodology, Formal analysis, Visualization, Writing - original draft, Writing - review & editing. Katrina N. Murray: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Justin L. Sanders: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. Michael L. Kent: Conceptualization, Methodology, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that there are no known competing financial interests nor personal relationships that could have influenced the work reported in this study.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.aquaculture.2022.739044.
Data availability statement
All data analyzed and presented for this study was generated from raw dPCR and qPCR values and is presented in full in this manuscript.
Data availability
Data will be made available on request.
References
- Agawa Y, Tani K, Yamamoto S, Hirano C, Shirakashi S, 2016. Development of a quantitative polymerase chain reaction assay for the detection of skin fluke Neobenedenia girellae larvae from environmental water. Fish. Sci 82, 827–833. 10.1007/s12562-016-1016-6. [DOI] [Google Scholar]
- Bastos Gomes G, Hutson KS, Domingos JA, Chung C, Hayward S, Miller TL, Jerry DR, 2017. Use of environmental DNA (eDNA) and water quality data to predict protozoan parasites outbreaks in fish farms. Aquaculture. 479, 467–473. 10.1016/j.aquaculture.2017.06.021. [DOI] [Google Scholar]
- Cali A, Kent M, Sanders J, Pau C, Takvorian PM, 2012. Development, ultrastructural pathology, and taxonomic revision of the microsporidial genus, Pseudoloma and its type species Pseudoloma neurophilia, in skeletal muscle and nervous tissue of experimentally infected zebrafish Danio rerio. J. Eukaryot. Microbiol 59, 40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassar S, Adatto I, Freeman JL, Gamse JT, Iturria I, Lawrence C, Muriana A, Peterson R, Van Cruchten S, Zon LI, 2020. Use of zebrafish in drug discovery toxicology. Chem. Res. Toxicol 33, 95–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crim MJ, Lawrence C, Livingston RS, Rakitin A, Hurley SJ, Riley LK, 2017. Comparison of antemortem and environmental samples for zebrafish health monitoring and quarantine. J. Am. Assoc. Lab. Anim. Sci 56, 412–424. [PMC free article] [PubMed] [Google Scholar]
- FAO, 2020. The State of World Fisheries and Aquaculture 2020. Sustainability in Action. Rome 10.4060/ca9229en. [DOI] [Google Scholar]
- Ferguson JA, Watral V, Schwindt AR, Kent ML, 2007. Spores of two fish microsporidia (Pseudoloma neurophilia and Glugea anomala) are highly resistant to chlorine. Dis. Aquat. Org 76, 205–214. [DOI] [PubMed] [Google Scholar]
- Gomes MC, Mostowy S, 2020. The case for modeling human infection in zebrafish. Trends Microbiol. 28, 10–18. [DOI] [PubMed] [Google Scholar]
- Hallett SL, 2006. Application of a real-time PCR assay to detect and quantify the myxozoan parasite Ceratomyxa shasta in river water samples. Dis. Aquat. Org 71, 109–118. [DOI] [PubMed] [Google Scholar]
- Howell CK, Atkinson SD, Bartholomew JL, Hallett SL, 2019. Development and application of a qPCR assay targeting Ichthyophthirius multifiliis in environmental water samples. Dis. Aquat. Org 134, 43–55. [DOI] [PubMed] [Google Scholar]
- Jørgensen LV, 2020. Zebrafish as a Model for Fish Diseases in Aquaculture. Pathogens 9 (8). 10.3390/pathogens9080609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent ML, Bishop-Stewart JK, 2003. Transmission and tissue distribution of Pseudoloma neurophilia (Microsporidia) of zebrafish, Danio rerio (Hamilton). J. Fish Dis 26, 423–426. 10.1046/j.1365-2761.2003.00467.x. [DOI] [PubMed] [Google Scholar]
- Kent ML, Sanders JL, Spagnoli S, Al-Samarrie CE, Murray KN, 2020. Review of diseases and health management in zebrafish Danio rerio (Hamilton 1822) in research facilities. J. Fish Dis 43, 637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinter LB, DeHaven R, Johnson DK, DeGeorge JJ, 2021. A brief history of use of animals in biomedical research and perspective on non-animal alternatives. ILAR J. Ilab020 10.1093/ilar/ilab020. [DOI] [PubMed] [Google Scholar]
- Miller M, Sabrautzki S, Beyerlein A, Brielmeier M, 2019. Combining fish and environmental PCR for diagnostics of diseased laboratory zebrafish in recirculating systems. PLoS One 14, e0222360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KN, Dreska M, Nasiadka A, Rinne M, Matthews JL, Carmichael C, Westerfield M, 2011. Transmission, diagnosis, and recommendations for control of Pseudoloma neurophilia infections in laboratory zebrafish (Danio rerio) facilities. Comp. Med 61, 322–329. [PMC free article] [PubMed] [Google Scholar]
- Murray KN, Varga ZM, Kent ML, 2016. Biosecurity and health monitoring at the zebrafish international resource center. Zebrafish. 13 (Suppl. 1), S30–S38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KN, Clark TS, Kebus MJ, Kent ML, 2022. Specific pathogen free – a review of strategies in agriculture, aquaculture, and laboratory mammals and how they inform new recommendations for laboratory zebrafish. Res. Vet. Sci 142, 78–93. 10.1016/j.rvsc.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris L, Lawler N, Hunkapiller A, Mulrooney DM, Kent ML, Sanders JL, 2020. Detection of the parasitic nematode, Pseudocapillaria tomentosa, in zebrafish tissues and environmental DNA in research aquaria. J. Fish Dis 43, 1087–1095. 10.1111/jfd.13220. [DOI] [PubMed] [Google Scholar]
- Peters L, Spatharis S, Dario MA, Dwyer T, Roca IJT, Kintner A, Kanstad-Hanssen Ø, Llewellyn MS, Praebel K, 2018. Environmental DNA: a new low-cost monitoring tool for pathogens in salmonid aquaculture. Front. Microbiol 9, 3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JB, Westerfield M, 2020. Chapter 47 - zebrafish as a model to understand human genetic diseases. In: Cartner SC, Eisen JS, Farmer SC, Guillemin KJ, Kent ML, Sanders BR (Eds.), American College of Laboratory Animal Medicine. Academic Press, pp. 619–626. [Google Scholar]
- Purcell MK, Powers RL, Besijn BL, Hershberger PK, 2017. Detection of Nanophyetus salmincola in water, snails, and fish tissues by quantitative polymerase chain reaction. J. Aquat. Anim. Health 29, 189–198. 10.1080/08997659.2017.1365780. [DOI] [PubMed] [Google Scholar]
- Qiagen, 2021. QIAcuity User manual, https://www.qiagen.com/us/products/instruments-and-automation/pcr-instruments/qiacuity-digital-pcr-system/ (Accessed 22 June 2021).
- Ramsay JM, Watral V, Schreck CB, Kent ML, 2009. Pseudoloma neurophilia infections in zebrafish Danio rerio: effects of stress on survival, growth, and reproduction. Dis. Aquat. Org 88, 69–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch JC, Hansen H, Strand DA, Markussen T, Hytterød S, Vrålstad T, 2018. Catching the fish with the worm: a case study on eDNA detection of the monogenean parasite Gyrodactylus salaris and two of its hosts, Atlantic salmon (Salmo salar) and rainbow trout (Oncorhynchus mykiss). Parasit. Vectors 11, 333. 10.1186/sl3071-018-2916-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JL, Kent ML, 2011. Development of a sensitive assay for the detection of Pseudoloma neurophilia in laboratory populations of the zebrafish Danio rerio. Dis. Aquat. Org 96, 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JL, Kent ML, 2014. The zebrafish as a model for microsporidiosis. In: Microsporidia, pp. 357–370. 10.1002/9781118395264.ch14. [DOI] [Google Scholar]
- Sanders JL, Watral V, Clarkson K, Kent ML, 2013. Verification of intraovum transmission of a microsporidium of vertebrates: Pseudoloma neurophilia infecting the zebrafish, Danio rerio. PLoS One 8, e76064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JL, Watral V, Stidworthy MF, Kent ML, 2016. Expansion of the known host range of the microsporidium, Pseudoloma neurophilia. Zebrafish 13 (Suppl. 1), S102–S106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CJ, Kent ML, Peterson JT, Sanders JL, 2022a. Multi-state occupancy model estimates probability of detection of an aquatic parasite using environmental dna: Pseudoloma neurophilia in zebrafish aquaria. J. Parasitol 108, 527–538. 10.1645/22-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CJ, Kreul TG, Al-Samarrie CE, Peterson JT, Sanders JL, Kent ML, 2022b. Progression of infection and detection of Pseudoloma neurophilia in zebrafish Danio rerio Hamilton by PCR and histology. J. Fish Dis 45, 1463–1475. 10.1111/jfd.13675. [DOI] [PubMed] [Google Scholar]
- Sieber N, Haiti kainen H, Vorburger C, 2020. Validation of an eDNA-based method for the detection of wildlife pathogens in water. Diseases of Aquatic Organisms 141, 171–184. 10.3354/dao03524. [DOI] [PubMed] [Google Scholar]
- Trujillo-González A, Edmunds RC, Becker JA, Hutson KS, 2019. Parasite detection in the ornamental fish trade using environmental DNA. Sci. Rep 9, 5173. 10.1038/s41598-019-41517-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela M, Figueras A, Novoa B, 2017. Modelling viral infections using zebrafish: innate immune response and antiviral research. Antivir. Res 139, 59–68. 10.1016/j.antiviral.2016.12.013. [DOI] [PubMed] [Google Scholar]
- Varga ZM, 2011. Chapter 24 - aquaculture and husbandry at the zebrafish international resource center. In: Detrich HW, Westerfield M, Zon CB (Eds.), The Zebrafish: Genetics, Genomics and Informatics, vol. 104. Academic Press, pp. 453–478. [DOI] [PubMed] [Google Scholar]
- Whipps CM, Kent ML, 2006. Polymerase chain reaction detection of Pseudoloma neurophilia, a common microsporidian of zebrafish (Danio rerio) reared in research laboratories. J. Am. Assoc. Lab. Anim. Sci 45, 36–39. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data analyzed and presented for this study was generated from raw dPCR and qPCR values and is presented in full in this manuscript.
Data will be made available on request.