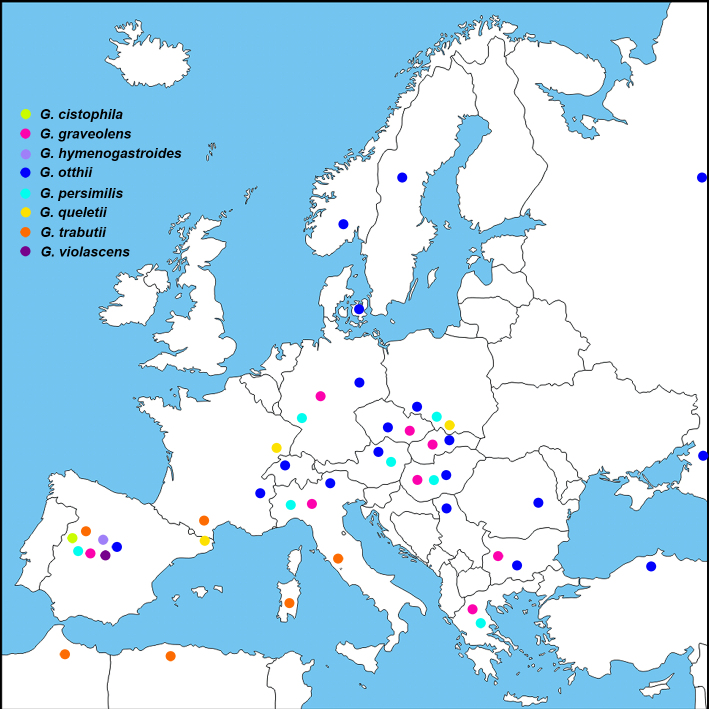
Abstract
Type material and additional collections of 11 taxa of Gautieria described in Europe and North Africa have been studied, namely G. dubia, G. graveolens, G. morchelliformis var. globispora, G. morchelliformis var. magnicellaris, G. morchelliformis var. morchelliformis, G. morchelliformis var. stenospora, G. otthii, G. pseudovestita, G. retirugosa, G. trabutii and G. villosa. At the same time, morphological and genetic studies on recent and herbarium collections from several European countries have been carried out. This enabled clarification of sections within Gautieria and differentiation of 28 taxa, of which 21 are new to science. However, the deeper relationships and nomenclature changes related to the phylogenetic position of the genus Gautieria within Gomphaceae will not be addressed in this study because they would require a more complete molecular analysis together with that of related genera, e.g., Gomphus, Turbinellus, and the four subgenera of Ramaria. In addition, a lectotype for G. villosa var. villosa and reference specimens for G. graveolens and G. morchelliformis var. morchelliformis are selected, and the new combination G. morchelliformis var. dubia is proposed. Detailed descriptions, macro- and microphotographs and distribution maps of all taxa are provided, as well as extensive information on their ecology, chorology and phylogeny. A key is included to facilitate identification of taxa.
Citation: Vidal JM, Cseh P, Merényi Z, et al. 2023. The genus Gautieria (Gomphales) in Europe and the Mediterranean Basin: a morphological and phylogenetic taxonomic revision. Persoonia 50: 48 –122. https://doi.org/10.3767/persoonia.2023.50.03.
Keywords: Europe, Gautieria, ITS-LSU phylogeny, Mediterranean Region, Ramaria, sequestrate fungi, taxonomy
INTRODUCTION
The genus Gautieria (nom. cons.) was erected by Vittadini (1831) in honour of the Italian physician and naturalist Giuseppe Gautier, with the description of two species G. morchelliformis and G. graveolens found under oaks in the region of Lombardy (Italy). Later, twelve new European and North African taxa were described or recombined in this genus, with the following chronology: Trog (1857) described G. otthii from a collection found in the region of Bern (Switzerland); Quélet (1878) described G. villosa from two collections found under conifers in the regions of Neuchâtel (Switzerland) and Thuringia (Germany), which was subsequently synonymized with G. morchelliformis by Hollós (1911) and later authors; Patouillard (1897) made the new combination G. trabutii for Hymenogaster trabutii (Chatin 1891), a species found under cedars in the region of Blida (Algeria); Fries (1909) described G. retirugosa from a collection found under conifers on the island of Gotland (Sweden); Velenovský (1922) created G. graveolens var. lacunosa for a collection found under firs near Prague (Czech Republic); Fischer (1938) proposed G. dubia for a collection found under firs near the city of Neuchâtel (Switzerland); Pilát (1953) described G. morchelliformis var. magnicellaris from a collection found under oaks in Central Bohemia (Czech Republic) and G. morchelliformis var. globispora from a collection found under conifers in the region of Košice (Slovakia), but those two varieties were invalidly published; Pilát (1958) published a monographic work of the genus Gautieria (in Czech) where he introduced G. morchelliformis var. stenospora, and provided a key for identification of all taxa (in Latin); Wichanský (1962) published G. morchelliformis var. microspora from a collection found under fir trees in the Czech Republic; and Malençon (1975) described G. pseudovestita from two collections found under cedars in the region of Rif (Morocco). Finally, Bougher & Castellano (1993) made the new combination G. citrina for Hymenogaster citrinus (Vittadini 1831).
The species of Gautieria most commonly cited in works devoted to the study of European hypogeous fungi are G. morchelliformis and G. graveolens, followed by G. otthii and more rarely G. trabutii, the rest of the species and varieties being virtually unknown until recently (see De Vries 1971, Rauschert 1975, Gross et al. 1980, 1982, Hintz & Winterhoff 1983, Hintz 1993, Montecchi & Lazzari 1993, Pegler et al. 1993, Montecchi & Sarasini 2000, Gori 2005). Species originally described from America, such as G. mexicana and G. pallida, have also been reported in Europe (see Soehner 1951, Pilát 1958, Rauschert 1975, Gross et al. 1980, Calonge et al. 1985b, 1996, Montecchi & Lazzari 1988, 1993, Calonge & Pasabán 1993, Vidal 1994), but most of these European identifications are shown to be incorrect in this study.
The genus Gautieria is characterised by having sequestrate basidiomata that lack a true peridium, commonly with a surface of morchelloid appearance exposing the external locules (Fig. 5f). The hymenophore has a coralloid development and consists of large hollow locules more or less radially arranged around a dendroid columella, which comes from a basal rhizomorph born within a white mycelial mass. Furthermore, the surface of basidiomata and the hymenophore react green in contact with FeSO4. Thus, the genus Gautieria shows a remarkable similarity with the coralloid genus Ramaria, especially when its development begins (Fig. 5g), so that a priori a possible phylogenetic relationship with this genus could be inferred. The spores are yellow-orange, non-amyloid, cyanophilous, elliptical to obovate or fusiform, and longitudinally costate (Fig. 5e, k, n). Zeller (1948) created the order Gautieriales and the family Gautieriaceae to place this genus because of the unique morphology, which was later adopted by Pilát (1958) and Jülich (1981, 1984). However, due to the similarity of the spores with those of the genus Boletellus, Castellano et al. (1989) transferred it to the family Strobilomycetaceae (Boletales). Subsequently, Bruns et al. (1998), in a preliminary phylogenetic study involving several representatives of the Basidiomycetes, placed it in the family Gomphaceae (Gomphales), alongside Kavinia, Ramaria and Gomphus. Humpert (1999) and Humpert et al. (2001) suggested that branched Gomphales are ancestral forms, and possibly the genus Gautieria is a sister group of Ramaria subg. Ramaria, a hypothesis confirmed by Hosaka et al. (2006) and Giachini et al. (2010) after sequencing new taxa and asserting that the genus Gautieria is monophyletic and nested with members of Ramaria subg. Ramaria. Currently, Ramaria is placed in the Gomphaceae, although older classifications place it in the Ramariaceae. Ramaria has been further subdivided into four subgenera (Christan & Hahn 2005), based on differences in spore ornamentation, substrate, and coralloid branching patterns of the basidiomata. The basidioma characteristics of Ramaria subg. Ramaria that exhibit shared, possibly homologous affinities with the genus Gautieria, are: coralloid to ramarioid growth pattern, green colour reaction to ferric sulphate, gloeoplerous hyphae (thromboplera), ampulliform septa, also referred to as trumpet-like inflated hyphae by Hahn & Christan (2002), acanthohyphae, paraphysoid hyphidia (pseudoparaphyses), striate spore ornamentation, with a variety of shapes of the hilar appendix and yellow to brown spore walls that have a dextrinoid and cyanophilic reaction. Both taxa are mycorrhizal and form mycelial mats. Basidiomata surfaces in some species exhibit a similar range of colour change when bruised or cut: yellow to ochre, pink to reddish or violaceous, and orangish to ochraceous brown. Spore sizes range considerably among species, and spore ornamentation, when present, ranges from weakly striate or lined, to prominently ribbed. Spore shapes are broadly elliptical to elliptico-fusiform. Similar conclusions were drawn by Agerer (1999), Palfner & Horak (2000) and Palfner (2001), who analyzed structural aspects of ectomycorrhizas, rhizomorphs and associated mycelium of the South American species Gautieria inapirae and found striking similarities in morphology of these structures with those of Gomphus, Kavinia and Ramaria. The most important shared features were: mat-like ectomycorrhizal morphology, slightly differentiated rhizomorphs (type C according to Agerer 2006) with ampullate hyphal enlargements at some septa, acanthohyphidia, roundish hyphal cells occasionally filled with yellow contents and the presence of amorphous yellow-opaque pigment-like material on hyphal surface.
Fig. 5.
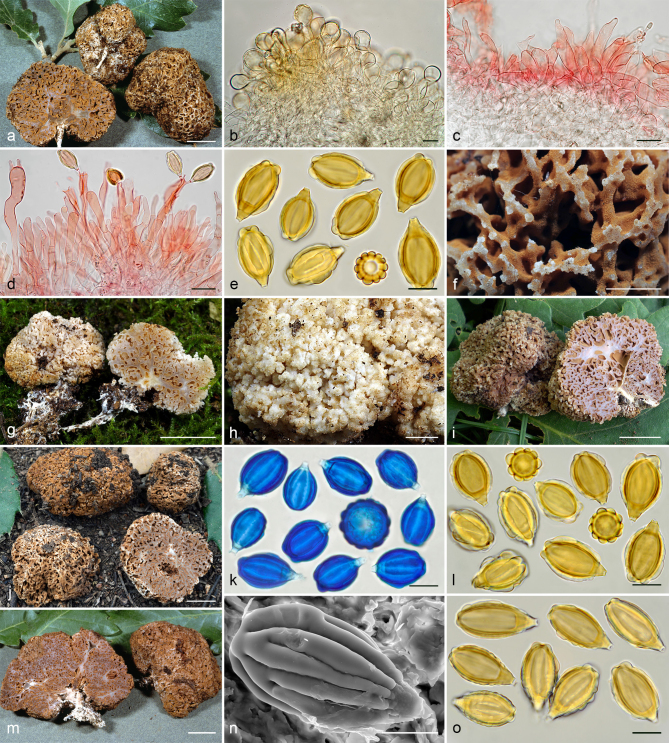
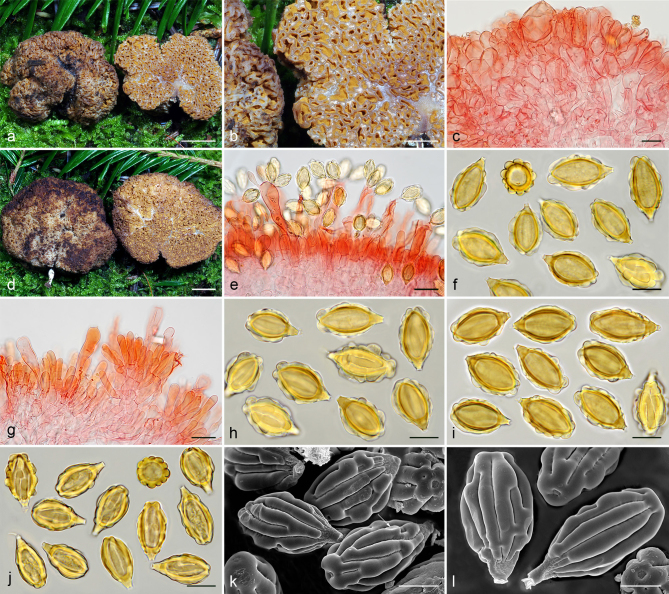
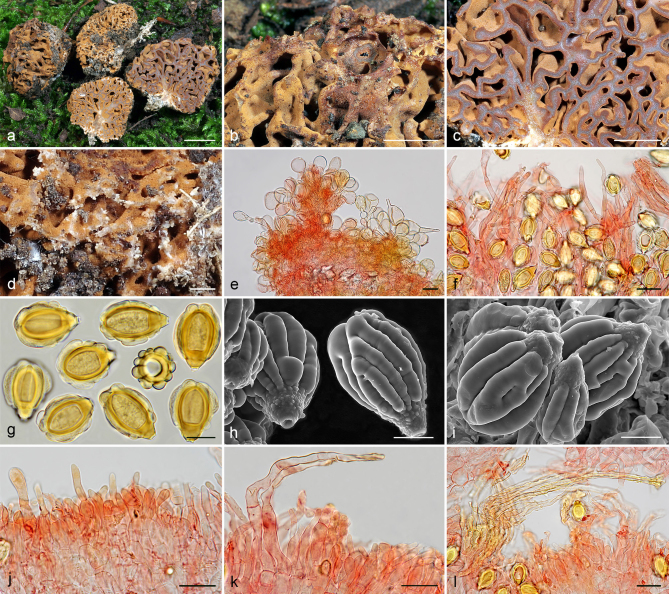
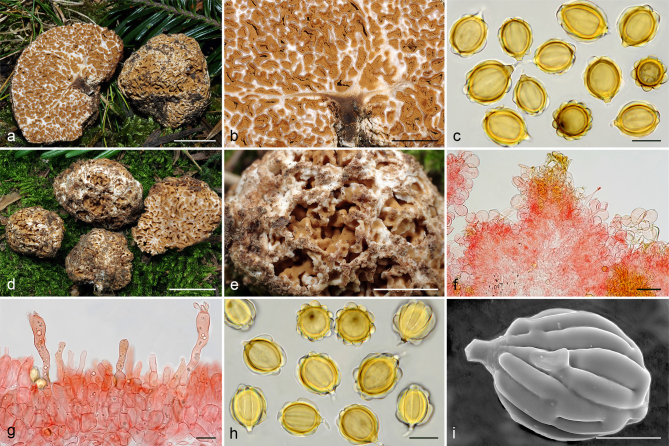
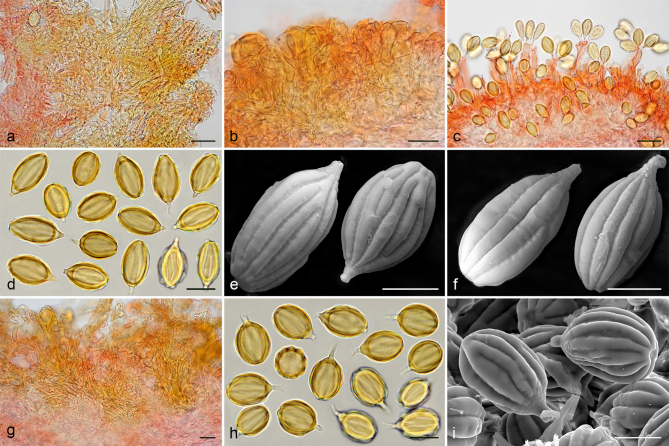
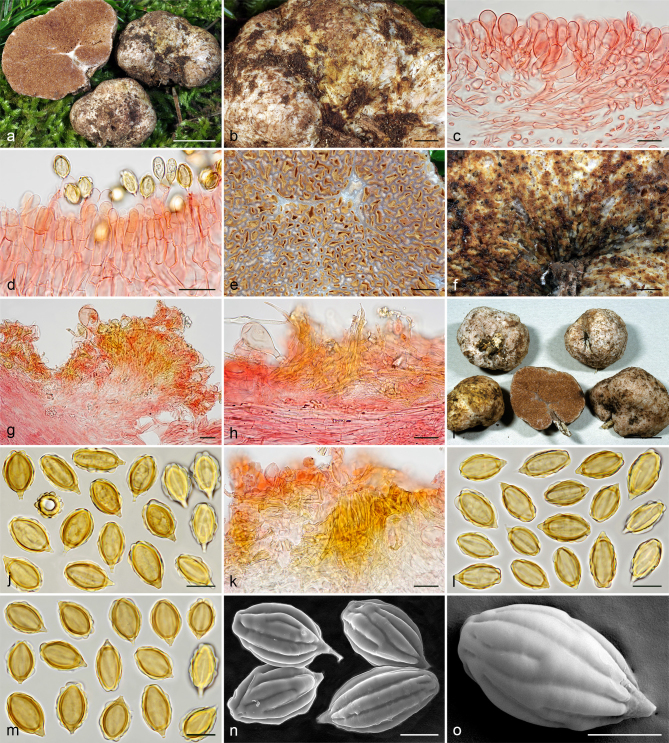
Gautieria morchelliformis var. morchelliformis. a–e. JMV20020721-1 (BCN, reference specimen). a. Basidiomata showing alveolate surface and coralloid hymenophore; b. cluster of exocystidia of pseudopellis; c. tips of paraphysoid cells; d. paraphysoid cells of young hymenium; e. spores in KOH. — f. JMV20200707. Detail of cristate ridges edge. — g–h. JMV20150421. g. Section of a very young basidioma showing a branched columella; h. detail of granulose surface. — i. VK1509. Basidiomata showing cristate ridges. — j. JMV20130709. Basidiomata showing morchelloid (anterior) and cerebriform surface (posterior). — k. VK2169. Spores in cotton blue showing a cyanophilous reaction. — l. W 342 (authentic material of G. morchelliformis). Spores in KOH. — m–n. JMV990612-8. m. Section of aggregate basidiomata showing compact hymenophore and independent columellae; n. SEM image of a spore. — o. MA-Fungi 58716. Spores with elongated morphology in KOH. — Scale bars: a, g, i–j, m = 1 cm; b–d = 20 µm; e, k–l, o = 10 µm; f = 2 mm; h = 1 mm; n = 5 µm. — Photos: a–h, l–m, o. J.M. Vidal; i. V. Kaounas; j. F. Rodríguez; k. A. Paz; n. UdG.
Therefore, the main objective of this study is to clarify the systematics of the genus Gautieria in Europe based on both molecular and morphological studies and other sources of information, such as chorology and ecology. All these data have allowed us to describe the new taxa found during this study and to clarify those already described, as well as to determine the different sections and phylogenetic clades within the genus Gautieria and to define their distinctive morphological characters.
MATERIAL AND METHODS
Fungal collections
Study of the European species of the genus Gautieria began in 2004, and has been carried out with the consultation of the type material and of relevant collections preserved in the public herbaria of B, BERN, BPI, BR, FH, H, IB, K, KRA (including the collection from the Tatra National Park, TPN), MA, M, MPU, NY, O, OSC, PAD, PC, PRM, S, UC, UPS, W and ZT (Thiers continuously updated), as well as recent collections made by the authors and collaborators. The holotypes and the collections of J.M. Vidal (JMV, including duplicates) and A. Paz (IC) are kept in the CeDocBiV of the University of Barcelona (BCN). The isotypes and the specimens of Z. Bratek (ZB), V. Erdei (EV), M. Misky (MIS), G. Pap (PAP), D. Pázmány (PÁZ) and I. Zagyva (ZI), as well as duplicates of sequenced material are kept in the herbarium of the Eötvös Loránd University of Budapest (ELTE). The collections of M. Slavova and B. Assyov, as well as other Bulgarian specimens received from correspondents, are deposited in the Mycological Collection of the Institute of Biodiversity and Ecosystem Research of the Bulgarian Academy of Sciences (SOMF). The collections of P. Chachuła, M. Kozak and P. Mleczko together with other Polish specimens provided by collaborators are deposited in the Herbarium of the Jagiellonian University in Kraków (KRA).
Morphological study
All taxa are fully described, except for the microscopic structure of mycelial hyphae and basal rhizomorphs. The colour of fresh basidiomata and the spore mass observed in the locules has been obtained from digital images and has been described following the Methuen colour code (Kornerup & Wanscher 1978). For each collection, the available data on location, collection place, altitude, habitat, substrate, date, collector, determiner, reviewer, and collection number are indicated. We use the term ‘calcareous soils’ to refer to basic soils that contain a large amount of calcium carbonate, and the term ‘siliceous soils’ to refer to acidic soils that contain a large amount of silica. When two or more collections from the same locality are reported, successive collections are preceded by the indication ‘ibid.’, indicating only the data that vary in relation to the first citation, such as the place of collection, altitude, habitat, date, collector or collection number. When there is more than one collection for the same place and date, the collection numbers are separated by a slash (/), for example, ZB2740/2744/2776. In total, some 900 herbarium samples have been studied with around 4500 reviewed specimens. Articles and examples cited in any nomenclatural comment refer to the current Shenzhen International Code of Nomenclature (Turland et al. 2018). For G. morchelliformis var. morchelliformis and G. graveolens we have selected a ‘reference specimen’ as the most representative specimen to interpret unambiguously these taxa according to our taxonomic concepts.
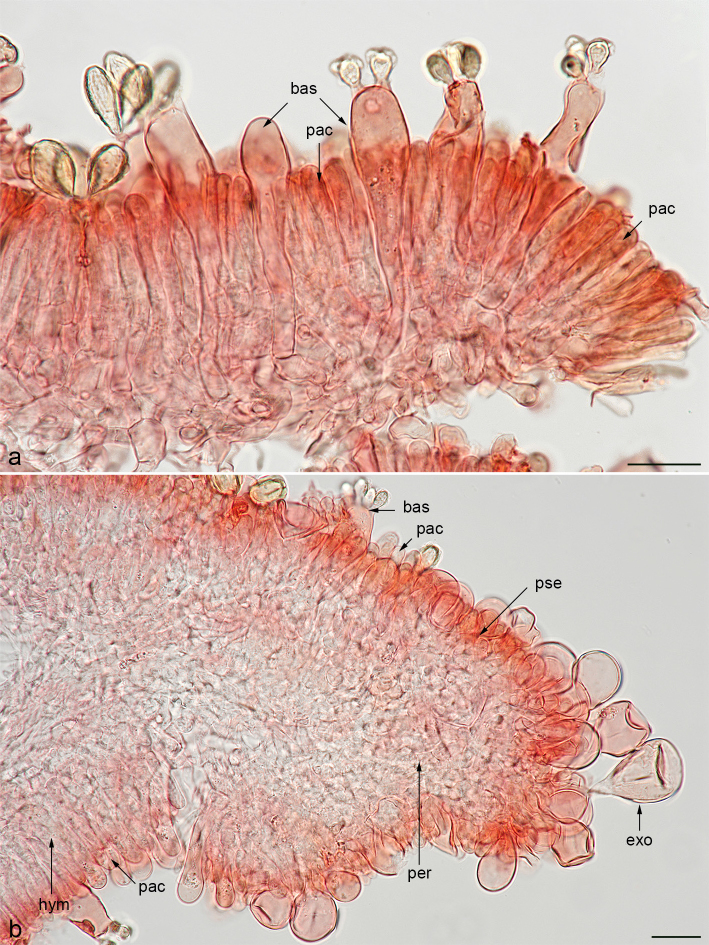
Since the genus Gautieria does not have a typical peridium, we must apply a specific terminology to name the different formations that protect the hymenophore. Most species have an alveolate basidiomata surface ornamented by prominent ribs or ‘ridges’ originating from the outer tramal plates. At the beginning of development, the surface of primordia is white and granulose, composed of sterile outer hymenial tramal plates; as primordia develop, these white granules remain as surface ridges in the form of rugosities or minute crests retaining their white. We name ‘cristae’ the minute crests that decorate the sterile edge of ridges (Fig. 5f). However, in some species, as a result of the fusion of the outlying tramal plates, a protective peridium-like covering can develop, which we name ‘pseudoperidium’ (Fig. 27b). We use the term ‘pseudopellis’ to refer to the cortical layer of the ridges and the pseudoperidium, originating from sterile hyphae of the hymenium, consisting of a hymeniderm mixed or not with hairs. We use the term ‘peritrama’ to refer to the tramal hyphae underlying the pseudopellis (Fig. 1b), which is the homologue of the typical hymenophoral trama. We name ‘exocystidia’ the leptocystidia-like sphaeropedunculate cells of the pseudopellis, considered sterile basidia by Malençon (1975: 299). We name ‘paraphysoid cells’ (Hawksworth et al. 1995, Vidal et al. 2019) the few differentiated sterile elements of the hymenium similar to paraphysis that are 0–3(–4)-septate and born at a higher level than the basidia (Fig. 1a, b), which were termed ‘paraphyses’ by Fitzpatrick (1913: 137) and Malençon (1975: 299). Some pseudoperidial species may develop a tomentose covering, which we name ‘trichotomentocutis’. This cortical layer has its origin in the differentiation of the sterile elements that line the pseudopellis and the exposed locules, consisting of the formation of tufts of long yellow thick-walled hairs, often being geliferous, or hyaline and thin-walled hyphae, often being crystalliferous, or a mixture of both (Fig. 15f–j, l–m).
Fig. 27.
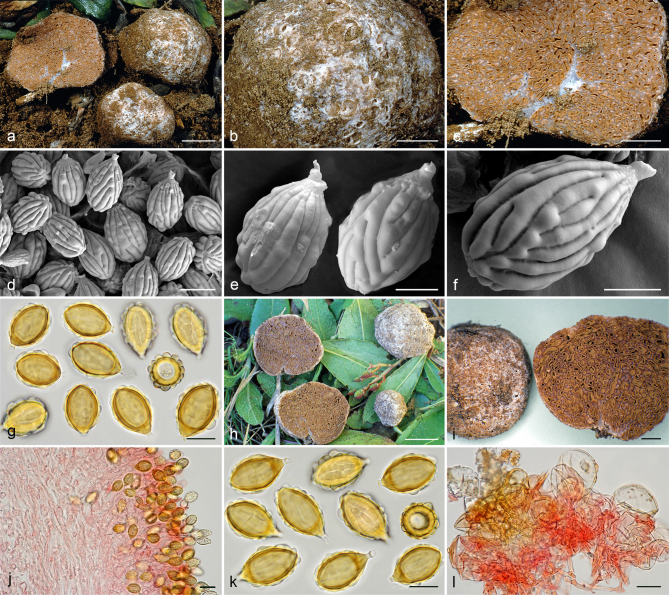
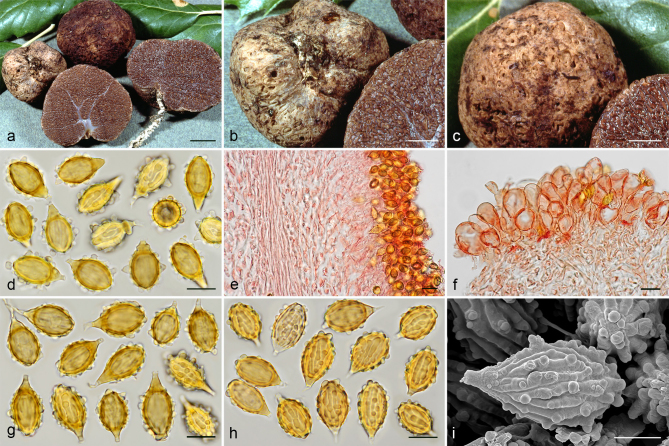
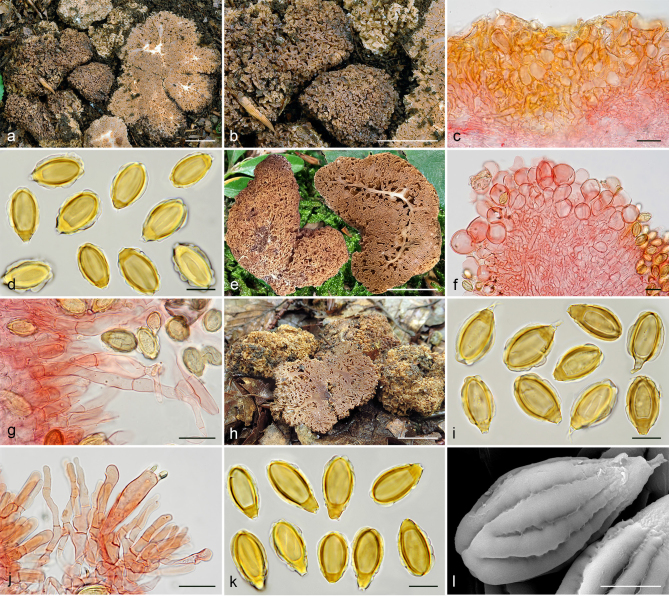
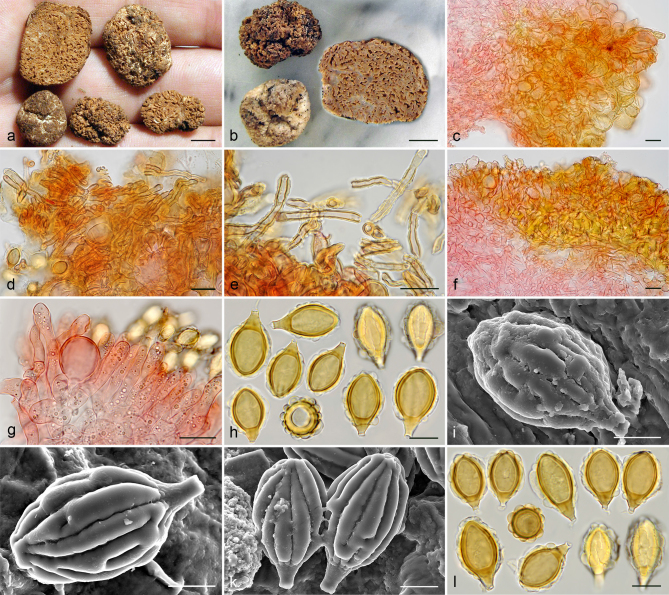
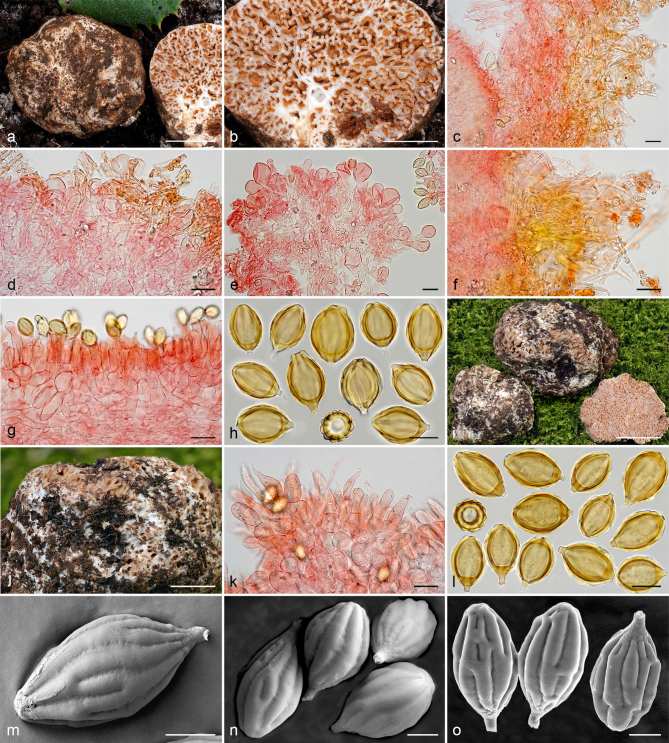
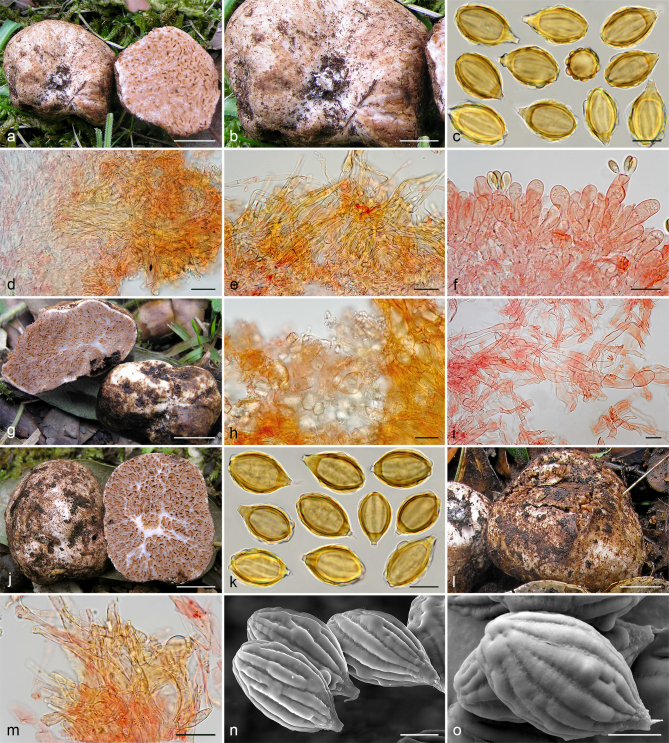
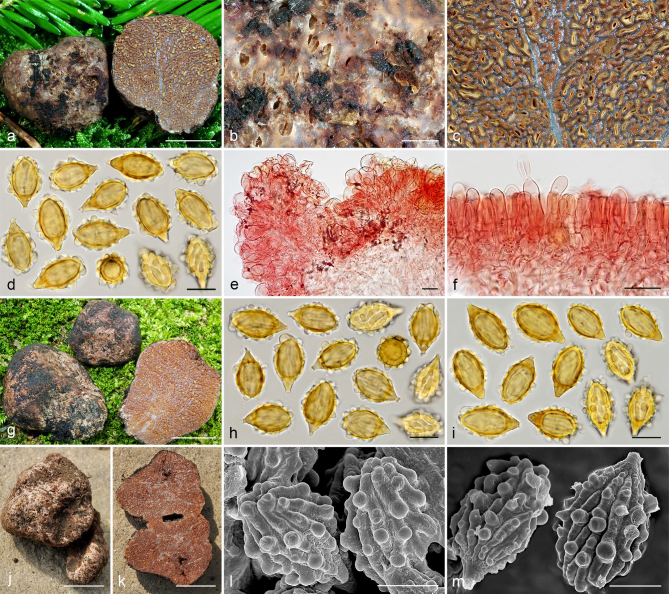
Gautieria cistophila. a–g. JMV960527-1 (BCN, holotype). a. Basidiomata; b. detail of foveate surface; c. tubular locules and slightly dendroid columella; d–f. SEM images of spores; g. spores in KOH. — h–k. MA-Fungi 33556. h. Basidiomata with furfuraceous surface; i. basidioma showing granular surface and gelatinized tramal plates; j. gelatinized subhymenium and hymenophoral trama, and collapsed hymenium; k. spores in KOH. — l. MA-Fungi 32260. Collapsed exocystidia with calcium oxalate crystals. — Scale bars: a, h = 1 cm; b–c = 5 mm; d, g, k = 10 µm; e–f = 5 µm; i = 2 mm; j, l = 20 µm. — Photos: a–c, g, j–l. J.M. Vidal; d–f. P. Mleczko, UJ; h–i. R. Mahiques.
Fig. 1.
a. Section of the hymenium of G. macrocoilia showing basidia and paraphysoid cells; b. section of a ridge of G. macrocoilia showing pseudopellis (sterile hymenium) and peritrama, and fertile hymenium and hymenophoral trama of an adjacent locule. — Abbreviations: bas = basidium; exo = exocystidium; hym = hymenium; pac = paraphysoid cell; per = peritrama; pse = pseudopellis. — Scale bar: 20 µm. — Photos: a–b. J.M. Vidal.
Fig. 15.
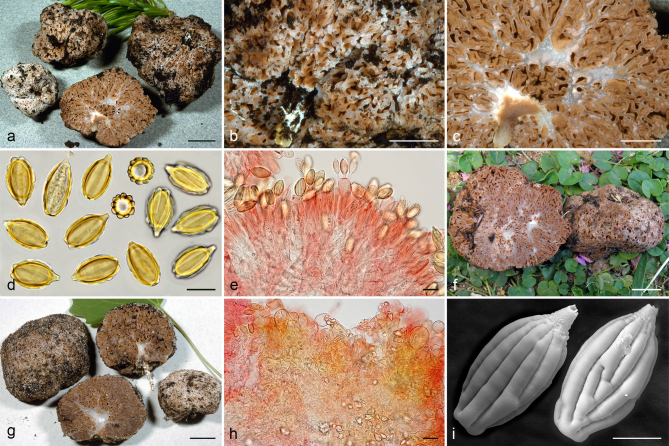
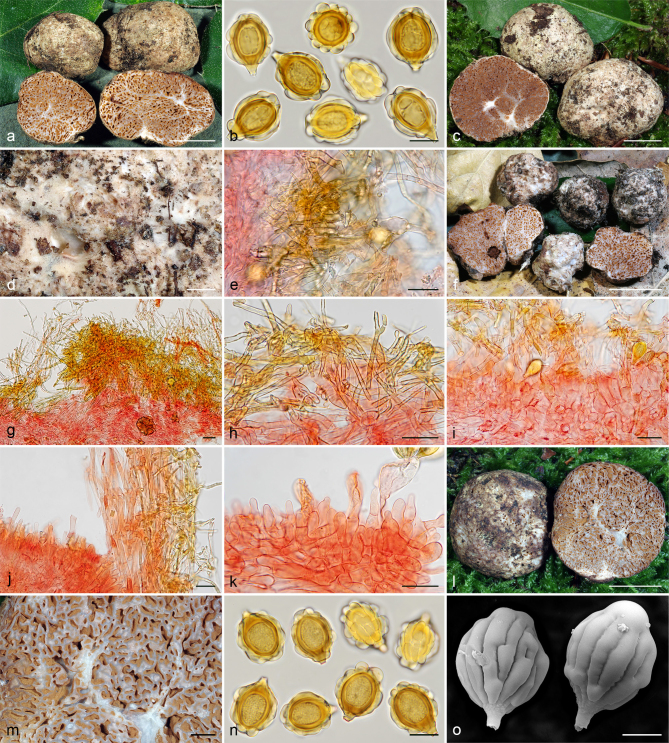
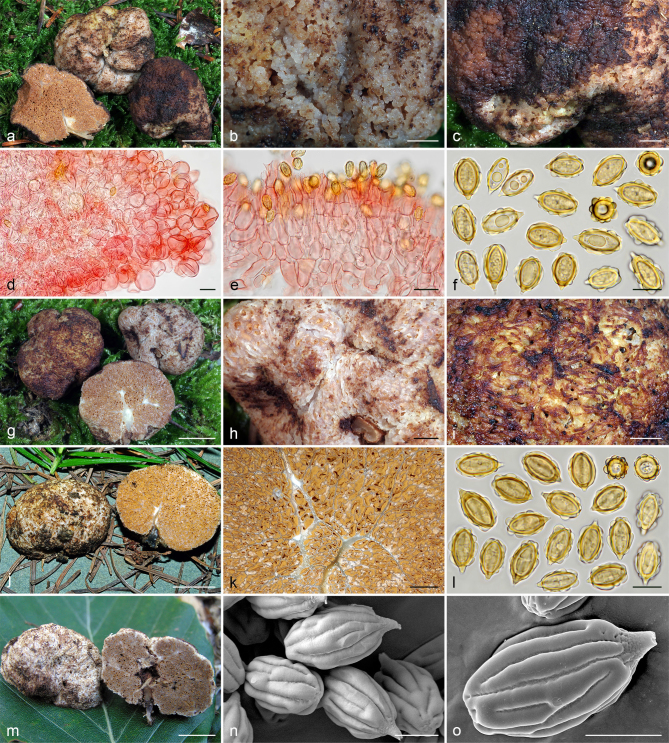
Gautieria hellenica. a–b. GK5779 (BCN JMV800569, holotype). a. Basidiomata; b. spores in KOH. — c. GK7147. Paraphysoid cells. — d–g. GK5594. d. Basidiomata; e. spores in KOH; f. hairy paraphysoid cells of an exposed locule; g. geliferous hairs of pseudopellis. — h–j. GK5778. h. Thin-walled hyphae of trichotomentocutis; i. cluster of exocystidia of pseudopellis and tuft of geliferous hairs of trichotomentocutis; j. crystalliferous hyphae of trichotomentocutis. — k–o. GK5608. k. Basidiomata; l. exocystidia of pseudopellis enclosed by the trichotomentocutis; m. crystalliferous hyphae of trichotomentocutis; n–o. SEM images of spores. — Scale bars: a, d, k = 1 cm; b, e = 10 µm; c, f–j, l–m = 20 µm; n–o = 5 µm. — Photos: a. D. Tabouras; b–c, e–j, l–m. J.M. Vidal; d, k. G. Konstantinidis; n. P. Mleczko, UJ; o. UdG.
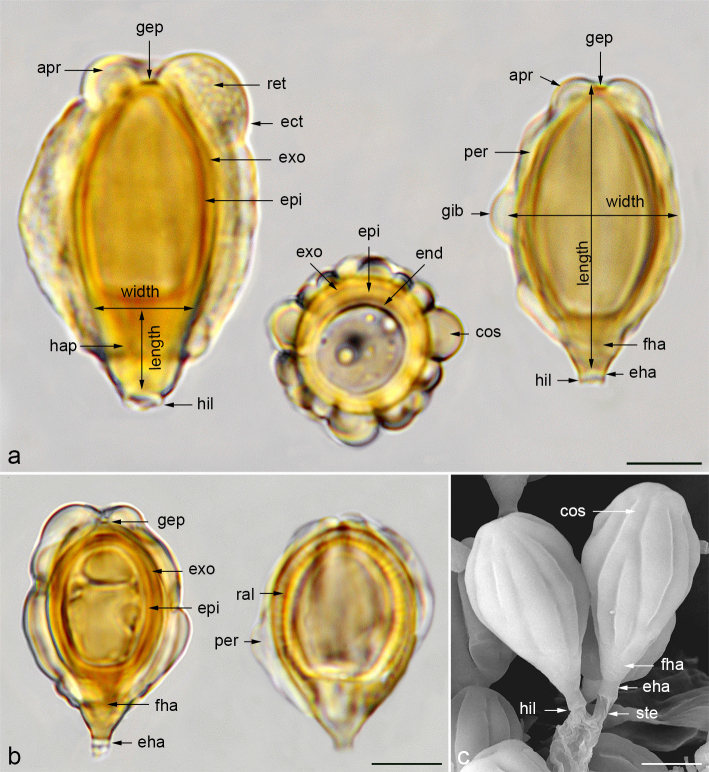
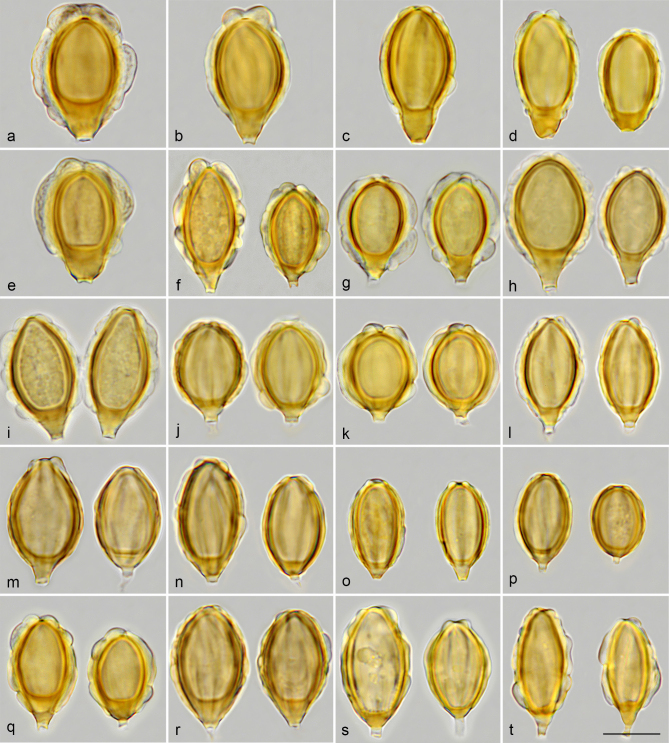
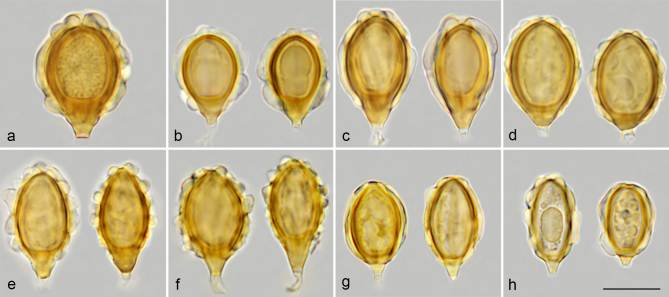
For spore morphology, we adopt the terminology of Perreau (1967) and Clémençon (2004) to name the wall structures seen with an optical microscope, and that of Hawksworth et al. (1995) to describe the shape of spores. We use the term ‘costae’ (Pegler et al. 1993) to refer to the ribs that decorate the spores. The costae are externally constituted by the ectosporium and internally by an inflated perisporium containing a three-dimensional coloured reticulum. This ‘perisporial reticulum’, which is not clearly distinguishable in all species, originates from the exosporium and provides turgidity to the costae (Fig. 2a). The perisporium is very swollen at the apex of the spore and has a deep depression around the germ pore giving rise to an ‘apical ring’. Often, the costae can have swollen zones, which we name ‘gibbosities’, and which may collapse when the spores dehydrate, as can be seen in an electron microscopy image of G. villosa var. villosa (Fig. 9k), except in G. queletii and G. trabutii where they remain rigid (Fig. 29i). In immature spores, dark radial lines can be observed inside the episporium and exosporium walls (Fig. 2b). These radial lines are denser areas that provide rigidity to the inner walls of immature spores. When the spores mature, the walls densify and darken without the dark lines being visible, but they continue to grow on the surface of the exosporium, giving rise to the perisporial reticulum.
Fig. 2.
a. Longitudinal section of a spore of G. macrocoilia (left) and G. villosa var. villosa (right) and cross section of a spore of G. violascens, indicating morphological parts and wall layers, and measurement of spores and hilar appendix applied in this work; b. mature spore of G. hymenogastroides (left) showing wall layers, germ pore, filled and empty hilar appendix, and immature spore of G. graveolens (right) showing developing perisporium and dense radial lines of episporium and exosporium; c. SEM image of two spores of G. violascens showing costae, sterigmata, and hilar appendix parts. — Abbreviations: apr = apical ring; cos = costa; ect = ectosporium; eha = empty end of hilar appendix; end = endosporium; epi = episporium; exo = exosporium; fha = filled hilar appendix; gep = germ pore; gib = gibbosity; hap = hilar appendix; hil = hilum; per = perisporium; ral = radial lines; ret = reticulum; ste = sterigma. — Scale bar: 5 µm. — Photos: a–b. J.M. Vidal; c. P. Mleczko, UJ.
Fig. 9.
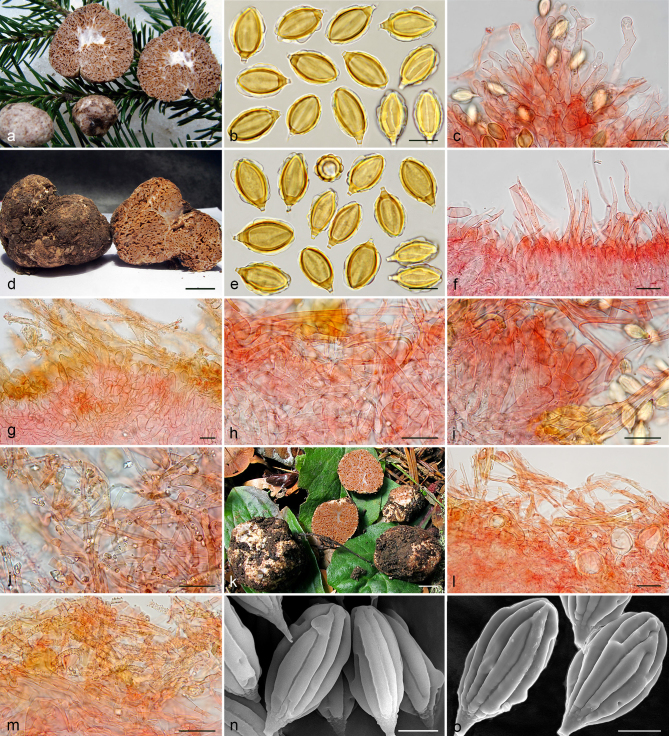
Gautieria villosa var. villosa. a–b. JMV20141014-12. a. Basidiomata showing compact ridges; b. hymenophore showing butter yellow locules and reddening of tramal plates and columella. — c. JMV20130605-2. Pseudopellis. — d–f. JMV20141014-26. d. Basidioma showing granular surface and intense reddening with handling; e. mature hymenium showing basidia and paraphysoid cells; f. spores in KOH. — g. MA-Fungi 2754 (M. Lawrynowicz as G. morchelliformis). Immature hymenium. — h. S F181882 (lectotype of G. villosa). Spores in KOH. — i. UPS F-550626 (E.Th. Fries as G. retirugosa). Spores in KOH. — j. UPS F-148988 (holotype of G. retirugosa). Spores in KOH, previously preserved in alcohol, causing a semi-opaque and non-inflatable perisporium. — k. KRA F-2012-3. SEM image of spores showing a collapsed gibbosity (arrow). — l. KRA F-2009-58. SEM image of spores. — Scale bars: a, d = 1 cm; b = 2 mm; c, e, g = 20 µm; f, h–j = 10 µm; k–l = 5 µm. — Photos: a–j. J.M. Vidal; k–l. P. Mleczko, UJ.
Fig. 29.
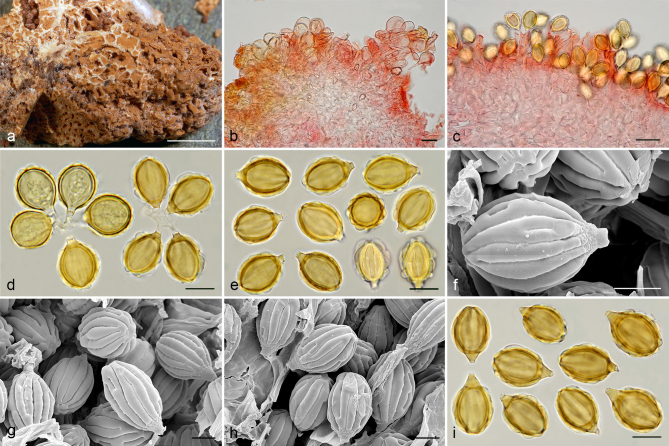
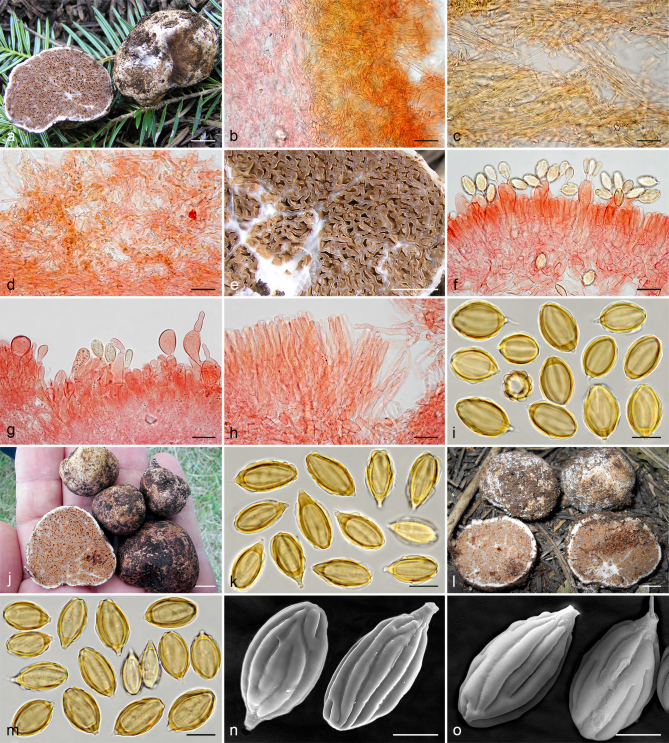
Gautieria trabutii. a–d. JMV990617-1. a. Basidiomata showing gelatinous hymenophore and dendroid columella; b–c. detail of foveate surface and gelatinized tramal plates; d. spores in KOH. — e–f. JMV20020523. e. Gelatinized subhymenium and hymenophoral trama, and collapsed hymenium; f. pseudopellis. — g. MA-Fungi 74767. Spores in KOH. — h. FH 301416 (authentic material of Hymenogaster trabutii). Spores in KOH. — i. JMV20020519-2. SEM image of spores. — Scale bars: a = 1 cm; b–c = 5 mm; d, g–h = 10 µm; e–f = 20 µm; i = 5 µm. — Photos: a–h. J.M. Vidal; i. UdG.
The microscopy was performed with the following microscopes equipped with plan apochromatic optics: Carl Zeiss Jena Jenaval with a DIC device, Leica DMRB with a P 1.40 OIL S1 condenser, both paired with Pentax K-20 Reflex cameras (14.6 Mpx), and Nikon Eclipse E800 attached to a Nikon D7100 Reflex camera (24.1 Mpx). In total, more than 10000 spore photographs and around 2000 photographs of hymenial and hyphal structures have been taken. Microscopic studies and microscopy images were performed with herbarium material rehydrated in 2 % KOH, and 1 % Congo red to stain the hyphal structures. Although KOH inflates the hyphal structures and slightly increases the size of the spores, we prefer its use in exsiccata because it allows better visualization of the hyphal and hymenial structures and especially the perisporium and other layers of the spore wall, all of them important characters for the identification of the different species of Gautieria. For spore comparisons, we have also made permanent slides in Hoyer’s medium of all collections. The spores have been measured in lateral view, including the perisporium and the filled hilar appendix (apicular plug), but excluding the gibbosities and the ‘empty-end’ of hilar appendix, which is cylindrical, empty and often colourless (Fig. 2a–b). At least 20 spores of each specimen studied were measured, indicating the minimum and maximum and the Q value. Measurements of spores and other elements were made from digital photographs with the help of ‘Mycometre VA’ software (G. Fannechère). Scanning electron microscopes (SEM) Hitachi S-4100 and Zeiss DSM960A (Microscopy Unit of the Technical Research Services of the University of Girona, UdG) and Hitachi S-4700 (Laboratory of Scanning Electron Microscopy and Microanalysis, Institute of Geological Sciences, Jagiellonian University in Kraków, UJ) were also used for spore imaging. Spore light microscopy images were digitally stacked with the help of ‘Helicon Focus’ (Helicon Soft Ltd.).
Molecular analysis
DNA extraction, amplification and sequencing
For DNA extraction, the internal tissue of fresh or dried basidiomata was carved out using a sterilized scalpel and tweezer. The procedure for the QIAGEN DNeasy Plant MiniKit and Geneaid Genomic DNA Mini Kit (Plant) DNA extraction kits were carried out according to the manufacturer’s instructions. In the polymerase chain reaction (PCR) the Internal Transcribed Spacer (ITS) and the Large Subunit (LSU) regions of the nuclear ribosomal RNA were amplified and sequenced. Due to the occasional intraspecific variability of the ITS (Smith et al. 2007a, Nilsson et al. 2008), we intended to support our studies by involving the LSU locus. The ITS region can be successfully amplified in the widest range of fungi, it is the most widespread barcoding marker to date, it is most likely to ensure correct species-level identification and classification in accordance with the preliminary species concepts. Its wide applicability is best approached by the LSU region (Schoch et al. 2012, Irinyi et al. 2015, Vu et al. 2019). During the work that lasted for more than a decade and a half, in addition to the continuously expanding sequence data, we did not have the opportunity to include additional genes due to the age and relative rarity of the herbaria materials, the arising methodological difficulties and the limitations of the available options. The PCR was implemented applying the primer pairs ITS1F (Gardes & Bruns 1993), ITS2, ITS3 and ITS4 (White et al. 1990) for the internal transcribed spacer region (ITS) of nuclear ribosomal DNA, and primers LR0R and LR5 (Vilgalys & Hester 1990) for the large ribosomal subunit (LSU region). Bioer Little Genius TC-25/H and Techne TC-312 devices were applied during the reactions. The temperature program was as follows for both loci: pre-denaturation: 94 °C for 4.5 min; 33 cycles of DNA denaturation: 94 °C for 30 s; annealing: 51 °C for 30 s; chain elongation: 72 °C for 45 s; and finally, a delay: 72 °C for 7 min. The amplified fragments were inspected by agarose gel electrophoresis, using 1 % Agarose gel made with TAE buffer (1×). The PCR products were cleaned of undesirable substances with the QIAGEN QIAquick PCR purification kit and sequenced with Sanger sequencing by BIOMI Ltd Biotechnology Service Provider (Gödöllő, Hungary).
Sequence analysis
The electropherograms of the sequences were viewed in the Finch TV 1.4.0 program. The sequences were edited manually with MEGA 7 software (Kumar et al. 2016). Both the obtained ITS and LSU Gautieria sequences (Table 1) and the publicly accessible ITS sequences (downloaded in November 2020 from the database of the National Center for Biotechnology Information, NCBI) were added to a FASTA file for the final alignment. Basic Local Alignment Tool (BLAST) was used to search for sequences related to ours and to find Ramaria sequences (ITS and LSU) to include as an outgroup. Sequences were aligned using the MAFFT L-INS-i algorithm (Katoh et al. 2009) in CIPRES Science Gateway (Miller et al. 2010). The online program ALTER (Glez-Peña et al. 2010) was used to convert the type and extension of the files between the different steps of the workflow. Model selection was performed with JModelTest (Darriba et al. 2012, Guindon & Gascuel 2003). Phylogenetic analyses were performed with the Maximum Likelihood (ML) method in the IQTree software (Nguyen et al. 2015, Trifinopoulos et al. 2016) using the IQ-TREE web server (http://iqtree.cibiv.univie.ac.at/). For Ultrafast bootstrap with 1000 bootstrap alignments, the TIM3+I+G substitution model was applied with a maximum of 1000 iterations and the value 0.99 for the minimum correlation coefficient. Visualisation of the phylogenetic tree was achieved with FigTree 1.4.3 (Rambaut 2017).
Table 1.
Specimens used in molecular phylogenetic studies, GenBank accession codes and results of species delimitation tools applied to aligned ITS sequences. The last two columns contain the serial numbers of the groups (putative species) generated by the two programs (ABGD and ASAP), corresponding to the herbarium samples represented in the phylogenetic tree (Fig. 3). Individuals with the same serial number belong to the same group. In case the partitioning of the two programs is different, the one we find most relevant according to the topology of the tree is marked with an asterisk (*).
| Taxon name | Original identification | References | Country/State/Region | Host | Herbarium voucher1
** = holotype |
ITS GenBank acc. codes2 | LSU GenBank acc. codes2 | ABGD group | ASAP group |
|---|---|---|---|---|---|---|---|---|---|
| Gautieria sect. Gautieria — /GauGau-1 (morchelliformis) clade | |||||||||
| G. sp. | G. magnicellaris | Pena & Guevara (unpubl.) | Mexico, Bajío | 1716 ITCV | MK909905 | 4 | 5 | ||
| G. sp. | G. magnicellaris | Cázares et al. (1992), Bidartondo & Bruns (2002) | Mexico, Nuevo Leon | Quercus, Pinus | Cazares136MX | AF377058 | 4 | 5 | |
| G. macrocoilia | G. morchelliformis var. magnicellaris | Pilát (1953) | Czech Republic, Central Bohemia | Quercus | PRM 678964 | OK669118 | OK669119 | 28 | 3 |
| G. sp., G. retirugosa, G. graveolens | Soehner (1951: No. 2227) | Germany, Bavaria | Fagus | M 126222 | OL467181 | OL415561 | |||
| G. morchelliformis | Soehner (1951: No. 1622) | Germany, Bavaria | Fagus | M 126272 | OK669115 | OK669116 | 28 | 3 | |
| G. morchelliformis | Soehner (1951: No. 2257) | Germany, Bavaria | Fagus | M 126276 | OL467183 | ||||
| Spain, Catalonia | Corylus, Quercus | IC09121813 | OL467125 | ||||||
| Spain, Catalonia | Corylus, Fagus, Quercus | JMV970927-1** | OK669121 | 28 | 3 | ||||
| G. morchelliformis var. dubia | Poland, Lesser Poland | Abies | KRA F-2010-60 | OL467177 | OL415553 | ||||
| Poland, Lesser Poland | Abies, Picea | KRA F-2013-1 | OL467178 | OL415556 | |||||
| Poland, Lesser Poland | Abies, Fagus | KRA F-2013-93 | OK669088 | OK655679 | 2 | 2 | |||
| Poland, Lesser Poland | Abies, Fagus | KRA F-2014-168 | OL467179 | OL415558 | |||||
| Poland, Lesser Poland | Abies, Fagus | KRA F-2016-30 | OK669086 | OK655677 | 2 | 2 | |||
| Poland, Lesser Poland | Abies, Pinus, Fagus | KRA F-2017-27 | OL467180 | OL415560 | |||||
| Poland, Lesser Poland | Abies, Fagus | KRA F-2017-28 | OK669087 | OK655676 | 2 | 2 | |||
| Poland, Lesser Poland | Abies, Picea | TPN-19-0330 | OL467198 | OL415571 | |||||
| Spain, Catalonia | Abies, Pinus | JMV960713-9 | OK669093 | 2 | 2 | ||||
| G. morchelliformis | Vidal (1997) | Spain, Catalonia | Abies, Pinus | JMV960824-2 | OL467166 | ||||
| G. morchelliformis | Vidal (1997) | Spain, Catalonia | Abies, Pinus | JMV961003-3 | OL467167 | ||||
| G. morchelliformis | Vidal (1997) | Spain, Catalonia | Abies | JMV970905-13 | OK669089 | 2 | 2 | ||
| Spain, Catalonia | Abies, Pinus | JMV20020831-1 | OL467135 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20081014-1 | OL467142 | ||||||
| Spain, Catalonia | Abies | JMV20110920-3 | OK669096 | OK655684 | 2 | 2 | |||
| G. morchelliformis var. fageticola | Bulgaria, Kyustendil | Fagus | SOMF 30314 | OL415565 | |||||
| Bulgaria, Kyustendil | Fagus | SOMF 30315 | OL415566 | ||||||
| Bulgaria, Kyustendil | Fagus | SOMF 30340 | OL467197 | OL415570 | |||||
| Bulgaria, Kyustendil | Fagus | SOMF 30341 | OK669099 | OK662956 | 2 | 2 | |||
| Bulgaria, Pazardzik | Fagus, Abies | SOMF 30316 | OL467193 | OL415567 | |||||
| G. graveolens | Soehner (1951: No. 1079 ) | Germany, Bavaria | deciduous | M 126232 | OL415562 | ||||
| Greece, West Macedonia | Fagus | GK11859 | OK669108 | OK662986 | 2 | 2 | |||
| Greece, West Macedonia | Fagus | JMV800558** | OK669098 | OK662611 | 2 | 2 | |||
| Hungary, Borsod-Abaúj-Zemplén | Carpinus | ZB2482 | OK669107 | 2 | 2 | ||||
| Hungary, Heves | Fagus | ZB1237 | OL467204 | ||||||
| Hungary, Veszprém | Fagus | ZB1997 | OK669095 | 2 | 2 | ||||
| Romania, Harghita | Carpinus | ZB3547 | OK669094 | 2 | 2 | ||||
| Romania, Harghita-Covasna | deciduous | ZB2777 | OK669097 | 2 | 2 | ||||
| Romania, Harghita-Covasna | deciduous | ZB2779 | OK669100 | 2 | 2 | ||||
| G. morchelliformis var. intermedia | Spain, Catalonia | Castanea, Corylus | JMV20021110-1 | OK663116 | 2 | 2 | |||
| Spain, Catalonia | Castanea, Quercus | JMV20180726-3** | OK663108 | OK663115 | 2 | 2 | |||
| G. morchelliformis var. morchelliformis | Bulgaria, Blagoevgrad | Quercus | SOMF 30306 | OL467192 | OL415564 | ||||
| G. graveolens | Nedelin et al. (2016) | Bulgaria, Haskovo | Quercus | SOMF 29672 | MN044442 | ||||
| Bulgaria, Plovdiv | Carpinus, Corylus | GK6781 | OK669113 | OK663097 | 2 | 2 | |||
| Greece, Thessaly | Quercus | VK1508 | OL467201 | OL415575 | |||||
| Greece, West Macedonia | Quercus | GK5031 | OK669114 | OK663109 | 2 | 2 | |||
| Greece, West Macedonia | Quercus | GK7252 | OL467121 | OL415543 | |||||
| Greece, West Macedonia | Quercus | GK11338 | OK634024 | OK634030 | |||||
| G. morchelliformis var. morchelliformis | Greece, West Macedonia | Quercus | GK11862 | OK634023 | OK634022 | ||||
| Hungary, Baranya | Quercus, Corylus | ZB3576 | OK669110 | 2 | 2 | ||||
| Hungary, Borsod-Abaùj-Zemplén | deciduous | ZB2359 | OK642410 | ||||||
| Hungary, Borsod-Abaùj-Zemplén | Carpinus | ZB2587 | OL467212 | ||||||
| Hungary, Borsod-Abaùj-Zemplén | Carpinus | ZB2986 | OL467217 | ||||||
| Hungary, Heves | deciduous | ZB2368 | OK634027 | ||||||
| Hungary, Heves | Carpinus | ZI16 | OL351857 | ||||||
| Hungary, Heves | Quercus | ZI203 | OL351859 | ||||||
| Hungary, Heves | Carpinus | ZI239 | OL351860 | ||||||
| Hungary, Pest | Carpinus | EV00/4 | OK634021 | ||||||
| Hungary, Pest | Carpinus | ZB2018 | OK634029 | ||||||
| Hungary, Pest | Carpinus | ZB3272 | OL467218 | ||||||
| Hungary, Pest | Fagus | ZB3353 | OL467219 | ||||||
| Hungary, Pest | Quercus | ZB4147 | OL467224 | ||||||
| G. morchelliformis | Montecchi (in herb.) | Italy, Abruzzo | Fagus | MA-Fungi 35887 | OK669109 | 2 | 2 | ||
| G. graveolens | Rana et al. (2011) | Italy, Basilicata | Quercus, Carpinus | 1139 (M044) | FN666413 | ||||
| G. graveolens | Montecchi & Lazzari (1993) | Italy, Emilia-Romagna | Quercus | JMV800039 | OL467157 | ||||
| G. morchelliformis | Gori (2005) | Italy, Emilia-Romagna | Castanea, Quercus | ELG921208 | OL467117 | ||||
| Italy, Sardinia | Quercus | JMV800174 | OK634028 | ||||||
| Italy, Sardinia | Quercus | JMV800175 | OK642407 | ||||||
| Italy, Sardinia | Quercus | JMV800178 | OK642400 | ||||||
| Italy, Sardinia | Quercus | JMV800180 | OL467159 | ||||||
| G. morchelliformis | Gori (2005) | Italy, Tuscany | Castanea, Quercus, Ostrya | ELG20000108b | OL467115 | ||||
| G. morchelliformis | Gori et al. (2003), Gori (2005) | Italy, Tuscany | Quercus, Corylus | ELG20021123 | OL467116 | ||||
| Montenegro | Quercus | ZB2641 | OL467213 | ||||||
| Montenegro | Quercus | ZI129 | OK669111 | 2 | 2 | ||||
| Romania, Bihor | deciduous | ZB3870 | OL467223 | ||||||
| Romania, Harghita | deciduous | ZB1399 | OK642405 | ||||||
| Romania, Harghita-Covasna | deciduous | ZB2743 | OL467214 | ||||||
| Romania, Mure§ | Carpinus | ZB3017 | OK669112 | 2 | 2 | ||||
| Spain, Basque Country | Quercus | JMV20110701-1 | OL467152 | ||||||
| Spain, Castilla and Leon | Quercus | JC20070603 | OL467128 | ||||||
| Spain, Castilla and Leon | Quercus | JMV800471 | OL467160 | ||||||
| Spain, Castilla and Leon | Quercus | JMV800474 | OL467161 | ||||||
| Spain, Castilla and Leon | Quercus | JMV20010530-1 | OL467129 | ||||||
| Spain, Castilla and Leon | Quercus | JMV20020328-2 | OL467131 | ||||||
| Spain, Castilla and Leon | Quercus | JMV20030504-10 | OL467136 | ||||||
| Spain, Castilla and Leon | Quercus | PSS131001 | OK634026 | ||||||
| Spain, Catalonia | Quercus | JMV990612-8 | OL467172 | ||||||
| Spain, Catalonia | Quercus | JMV990630 | OL467174 | ||||||
| Spain, Catalonia | Quercus | JMV20020721-1 (ref. spec.) | OK669101 | 2 | 2 | ||||
| Spain, Catalonia | Quercus | JMV20110517-1 | OL467150 | ||||||
| Spain, Valencian Community | Quercus | FGA96653 | OL467118 | ||||||
| G. otthii | Calonge (in herb.) | Ukraine, Crimea | Fagus | MA-Fungi 58716 | OK634031 | 2 | 2 | ||
| /GauGau-2 (villosa) clade | |||||||||
| G. villosa var. inflata | Bulgaria, Kyustendil | Abies, Picea | SOMF 30321 | OL467195 | OL415569 | ||||
| G. morchelliformis | Lawrynowicz (in herb.) | France, Rhône-Alpes | Picea | MA-Fungi 5111 | OK999967 | 3 | 4 | ||
| G. morchelliformis | Bidartondo & Bruns (2002) | France, Rhône-Alpes | Picea | RioussetT4547 | AF377067 | ||||
| G. villosa var. inflata | G. graveolens | Osmundson et al. (2013) | Italy | Pinaceae | MCVE 16988 | JF908017 | |||
| G. graveolens | Sarasini (in herb.) | Italy, Trentino-Alto Adige | Abies | MS1068 | OK999968 | 3 | 4 | ||
| Poland, Lesser Poland | Abies, Picea | TPN-19-0123 | OL036691 | OL036693 | 3 | 4 | |||
| Poland, Świętokrzyskie | Abies | KRA F-2013-77 | OL415557 | ||||||
| Romania, Covasna | Picea | ZB2280 | OL467209 | ||||||
| Spain, Catalonia | Abies | JMV970905-8b | OL467170 | OL415550 | |||||
| Spain, Catalonia | Abies, Pinus | JMV970920-6 | OL467171 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20080930-2 | OL467139 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20081007-1 | OL467140 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20081014-9b | OL467143 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20081021-2 | OL467145 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20081021-5** | OK999965 | 3 | 4 | ||||
| Spain, Catalonia | Abies, Pinus | JMV20091006-2 | OL467147 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20091006-7 | OL467148 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20100720-4 | OL467149 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20110920-1 | OL467153 | ||||||
| G. villosa var. pilifera | Greece, Peloponnese | Abies | MG109 | OL036695 | OL036690 | 3 | 4 | ||
| Greece, Thessaly | Abies | JMV800561** | OL036694 | OL036692 | 3 | 4 | |||
| G. villosa var. villosa | G. morchelliformis | Moser (in herb.) | Austria, Carinthia | Pinaceae | IB 1985254a | OL467123 | OL415544 | ||
| Bulgaria, Sofia | Picea, Pinus | SOMF 30318 | OL467194 | OL415568 | |||||
| G. graveolens | Dreher (in herb.) | Germany, Bavaria | Fagus | M 126240 | OL467182 | ||||
| G. graveolens | Raidl (in herb.) | Germany, Bavaria | Picea | M 157765 | OK998517 | OK998518 | 3 | 4 | |
| G. graveolens | Sarasini (in herb.) | Italy, Lombardy | Picea | MS1006 | OL467189 | ||||
| G. morchelliformis | Moser (in herb.) | Italy, Trentino-Alto Adige | Picea | IB 19790029 | OL467122 | ||||
| Poland, Lesser Poland | Abies, Picea | KRA F-2009-58 | OL467176 | OL415552 | |||||
| Poland, Lesser Poland | Abies, Picea, Fagus | KRA F-2012-3 | OL415555 | ||||||
| Poland, Lesser Poland | Abies, Fagus | KRA F-2012-152 | OK669124 | OK669120 | 3 | 4 | |||
| Spain, Catalonia | Abies | JMV970905-8 | OL467169 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20020825-1 | OL467134 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20080923-5 | OL467138 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20090922-3 | OK669122 | 3 | 4 | ||||
| Spain, Catalonia | Abies, Pinus | JMV20141014-26 | OL467154 | OL415547 | |||||
| G. morchelliformis | Calonge et al. (1977) | Spain, Catalonia | Abies | MA-Fungi 2754 | OL467184 | ||||
| G. retirugosa | E.Th. Fries (in herb.) | Sweden, Gotland | Pinaceae | UPS F-550626 | OK669117 | 3 | 4 | ||
| G. morchelliformis | Schleiber & Suber (in herb.) | Switzerland, Neuchâtel | Pinaceae | UPS F-550645 | OL467199 | OL415572 | |||
| G. morchelliformis | Bidartondo & Bruns (2002) | Switzerland, Solothurn | Pinaceae | HH2222Swiss | AF377066 | 3 | 4 | ||
| G. xinjiangensis | G. xinjiangensis | Bau & Liu (2013) | China, Xinjiang | Picea | HMJAU6009** | JX860192 | 3 | 4 | |
| NR153450 | |||||||||
| /GauGau-3 (convoluta) clade | |||||||||
| G. convoluta var. convoluta | G. morchelliformis | Gori (in herb.) | Italy, Liguria | Abies, Fagus | ELG950600 | OL304003 | OL304004 | 29 | 6 |
| Poland, Lesser Poland | Abies, Pinus | KRA F-2008-259 | OL415551 | ||||||
| Poland, Lesser Poland | Picea | TPN-19-0476 | OL304005 | OL304002 | 29 | 6 | |||
| Spain, Aragon | Abies, Pinus, Fagus | IC01051502 | OL467124 | OL415545 | |||||
| Spain, Catalonia | Abies, Pinus | JMV20080923-3 | OL467137 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20141014-27** | OL304007 | OL304006 | 29 | 6 | |||
| G. convoluta var. petrakii | G. graveolens | Petrak (in herb.) | Czech Republic, Olomouc | M 126216** | OL304009 | 29 | 6 | ||
| /GauGau-4 (subglobispora) clade | |||||||||
| G. aff. subglobispora | G. sp., G. globospora | Stewart (1974), Bidartondo & Bruns (2002) | USA, Colorado | Pinaceae | HDT25707USA | AF377064 | 6 | 14 | |
| G. aff. subglobispora | G. sp., G. globospora | Stewart (1974), Bidartondo & Bruns (2002) | USA, California | Pinaceae | Gerry499USA | AF377065 | 6 | 14 | |
| G. subglobispora | G. morchelliformis | Eckblad (in herb.) | Norway, Oppland | Picea | O 152350 | OL304062 | OL304061 | 6 | 14 |
| Spain, Catalonia | Abies | JMV990626-12 | OL304059 | 6 | 14 | ||||
| Spain, Catalonia | Abies, Pinus | JMV20081021-1 | OL467144 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20081021-3** | OL304060 | 6 | 14 | ||||
| /GauGau-5 clade | |||||||||
| G. sp. | G. sp., G. magnicellaris | Bidartondo & Bruns (2002) | USA, California | deciduous | HS2305USA | AF377071 | 7 | 15 | |
| G. sp. | G. otthii | Bidartondo & Bruns (2002) | USA, California | deciduous | T7852-LindgrenUSA | AF377072 | 7 | 15 | |
| G. sp. | G. otthii | Bidartondo & Bruns (2002) | USA, California | deciduous | T8371USA | AF377073 | 7 | 15 | |
| /GauGau-6 (hellenica) clade | |||||||||
| G. sp. | G. sp., G. vestita | Bidartondo & Bruns (2002) | USA, California | Quercus | HS1831USA | AF377063 | 5 | 12 | |
| G. sp. | G. crispa | Smith et al. (2007b) | USA, California | Quercus | src627 | DQ974732 | 31 | 8 | |
| G. sp. | G. sp., G. chilensis | Bidartondo & Bruns (2002) | Mexico, Mexico State | OSC38736MX | AF377061 | 32 | 9 | ||
| G. sp. | G. sp., G. candida | Bidartondo & Bruns (2002) | USA, Oregon | Pseudotsuga | OSC48548OR | AF377060 | 32 | 9 | |
| G. sp. | G. sp. | Dunham et al. (2007) | USA, Oregon | Pseudotsuga | SD-37.2 | DQ365642 | 32 | 9 | |
| G. sp. | G. sp. | Frank & Southworth (unpubl.) | USA, Oregon | Abies | SOC1446 | JN022505 | 32 | 9 | |
| G. sp. | G. parksiana | Bidartondo & Bruns (2002) | USA, Califormia | Abies, Pinus | SNF236USA | AF377059 | 33 | 10 | |
| G. sp. | Uncultured Gomphaceae | Bergemann & Garbelotto (2006) | USA, California | Lithocarpus | D43 | DQ273388 | 34 | 11 | |
| G. sp. | Uncultured fungus | Taniguchi et al. (unpubl.) | USA, California | Quercus | JR101 | KC791118 | 34 | 11 | |
| G. hellenica | Greece, Central Greece | Abies | GK5594 | OL304014 | 35 | 13 | |||
| Greece, Thessaly | Abies | GK5608 | OL304013 | OL304015 | 35 | 13 | |||
| Greece, Thessaly | Abies | JMV800569** | OL304012 | ||||||
| G. iberica | Spain, Castilla and Leon | Quercus | JMV800472 | OL304008 | 30 | 7 | |||
| Spain, Castilla and Leon | Quercus | JMV800473 | OL304011 | 30 | 7 | ||||
| Spain, Castilla and Leon | Quercus | JMV800476** | OL304010 | 30 | 7 | ||||
| Spain, Castilla and Leon | Quercus | JMV20020202-1 | OL467130 | ||||||
| /GauGau-7 clade | |||||||||
| G. sp. | G. sp., G. chilensis | Bidartondo & Bruns (2002) | Mexico, Mexico State | Abies | Trappe3401 | AF377056 | 36 | 18 | |
| G. sp. | G. caudata | Bidartondo & Bruns (2002) | USA, California | Pinus, Quercus | OSC41323Ga-brielUSA | AF377057 | 36 | 18 | |
| G. sp. | G. sp. | Bidartondo & Bruns (2002) | USA, California | Pinaceae | SNF180CA | AF377068 | 36 | 18 | |
| /GauGau-8 (pityophila) clade | |||||||||
| G. pervestita | Greece, Central Greece | Abies | JMV800556** | OL310906 | OL310907 | 8 | 16 | ||
| Greece, Central Greece | Abies | VK880 | OL310904 | OL310908 | 8 | 16 | |||
| Greece, Epirus | Abies | GK5007 | OL310903 | OL310905 | 8 | 16 | |||
| Greece, Peloponnese | Abies | VK2959 | OL467202 | OL415576 | |||||
| G. pityophila | Greece, Thessaly | Abies | GK6359 | OL467119 | OL415541 | ||||
| Greece, Thessaly | Abies | VK2980 | OL310919 | OL310916 | 9 | 17 | |||
| G. mexicana | Montecchi (in herb.) | Italy, Emilia-Romagna | Pinus, Abies | AM1210 | OL310917 | 9 | 17 | ||
| G. otthii | Montecchi (in herb.) | Italy, Emilia-Romagna | Abies | AM1944 | OL467113 | ||||
| G. graveolens | Vidal et al. (1997) | Spain, Castilla and Leon | Pinus | JMV960407-4 | OL467163 | ||||
| G. graveolens | Vidal et al. (1997) | Spain, Castilla and Leon | Pinus | JMV960525-3 | OL310918 | 9 | 17 | ||
| G. otthii | Calonge et al. (1994) | Spain, Castilla and Leon | Pinus | MA-Fungi 32261 | OL467185 | ||||
| G. morchelliformis | Calonge et al. (1985a) | Spain, Castilla-La Mancha | Pinus | MA-Fungi 7922 | OL467188 | ||||
| G. graveolens | Vidal (1997) | Spain, Catalonia | Pinus, Abies | JMV960622-6** | OL310915 | 9 | 17 | ||
| G. graveolens | Vidal (1997) | Spain, Catalonia | Pinus, Abies | JMV960629-11 | OL467165 | ||||
| /GauGau-9 (confusa) clade | |||||||||
| G. aff. confusa | G. sp. | Matheny et al. (unpubl.) | USA, North Carolina | Tsuga | TENN071813 | MG773849 | 10 | 19 | |
| G. aff. confusa | G. sp. | Russell & Grootmyers (unpubl.) | USA, Ohio | Tsuga | 334885 | MK607602 | 10 | 19 | |
| G. confusa | Bulgaria, Kyustendil | Fagus | SOMF 30317 | OL311045 | OL311043 | 10 | 19 | ||
| Hungary, Heves | Fagus | ZB1732 | OL467206 | ||||||
| Hungary, Nógrád | ZB1821 | OL311048 | 10 | 19 | |||||
| Hungary, Nógrád | Pinus | ZB1823 | OL467208 | ||||||
| Poland, Lesser Poland | Abies, Fagus | KRA F-2017-17 | OL311044 | OL311047 | 10 | 19 | |||
| Spain, Castilla and Leon | Fagus | JMV800475 | OL467162 | ||||||
| G. morchelliformis | Calonge (in herb.) | Spain, Castilla and Leon | Quercus | MA-Fungi 39289 | OL467186 | ||||
| Spain, Catalonia | Abies, Pinus | JMV960622-11 | OL467164 | ||||||
| Spain, Catalonia | Fagus | JMV20081028-1 | OL467146 | ||||||
| Spain, Catalonia | Pseudotsuga | JMV20110621-8** | OL311046 | 10 | 19 | ||||
| G. obtexta | Greece, Central Greece | Quercus | VK1376 | OL311145 | OL311139 | 11 | 20 | ||
| Greece, Central Greece | Quercus | VK1395 | OL467200 | OL415573 | |||||
| Italy, Sardinia | Quercus | JMV800177 | OL467158 | OL415549 | |||||
| Italy, Sardinia | Quercus | JMV800179** | OL311140 | 11 | 20 | ||||
| /GauGau-10 (chilensis) clade | |||||||||
| G. sp. | G. sp. | Truong et al. (2017) | Argentina, Patagonia | Nothofagus | MES-1868 | KY462601 | 12 | 21 | |
| G. sp. | G. sp. | Smith & Caiafa (unpubl.) | Chile | Nothofagus | MES-3404 | MT366694 | 12 | 21 | |
| G. chilensis | G. sp. | Truong et al. (2017) | Argentina, Patagonia | Nothofagus | MES-1977 | KY462630 | 13 | 22 | |
| G. chilensis | Bidartondo & Bruns (2002) | Chile, Magallanes | Nothofagus | Halling5818 | AF377069 | 13 | 22 | ||
| /GauGau-11 (fusella) clade | |||||||||
| G. fenestrata | Bulgaria, Kyustendil | Fagus, Quercus | SOMF 30331 | OL314643 | OL314644 | 15 | 24 | ||
| Greece, Thessaly | Abies | GK6445 | OL467120 | OL415542 | |||||
| Greece, West Macedonia | Quercus | GK11861 | OL314642 | OL314645 | 15 | 24 | |||
| G. morchelliformis | Gori (2005) | Italy, Tuscany | Castanea, Quercus, Ostrya | ELG20000108 | OL314641 | 15 | 24 | ||
| G. morchelliformis | Gori (2005) | Italy, Tuscany | Fagus | ELG20000102 | OL467114 | ||||
| Spain, Catalonia | Fagus | JMV20110913-1** | OL314646 | 15 | 24 | ||||
| G. fusella | Bulgaria, Kyustendil | Fagus | SOMF 30328 | OL311143 | 14 | 23 | |||
| Bulgaria, Pazardzik | Picea, Abies, Fagus | SOMF 30329 | OL467196 | ||||||
| Greece, West Macedonia | Fagus | GK4998 | OL311144 | OL311141 | 14 | 23 | |||
| Hungary, Heves | Fagus | ZB5817 | OL467226 | ||||||
| Hungary, Pest | Quercus, Carpinus | ZB3223 | OL311142 | 14 | 23 | ||||
| Romania, Harghita | ZB1175 | OL467203 | |||||||
| Romania, Harghita | ZB1409 | OL467205 | |||||||
| Romania, Harghita-Covasna | ZB2740 | OL311149 | 14 | 23 | |||||
| Romania, Harghita-Covasna | ZB2744 | OL467215 | |||||||
| Romania, Harghita-Covasna | ZB2776 | OL467216 | |||||||
| Spain, Catalonia | Abies, Pinus | JMV961003-5 | OL467168 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20110425** | OL311138 | 14 | 23 | ||||
| Gautieria sect. Hymenogastroides | |||||||||
| G. hymenogastroides | Spain, Castilla and Leon | Quercus, Castanea | JC160320NR | OL314650 | OL314647 | 16 | 25 | ||
| Spain, Catalonia | Castanea, Quercus, Fagus | JMV20110720 | OL314649 | 16 | 25 | ||||
| Spain, Catalonia | Castanea, Quercus, Fagus | JMV20110811** | OL314648 | 16 | 25 | ||||
| Gautieria sect. Glutinosiglebae — /GauGlu-1 (graveolens) clade | |||||||||
| G. graveolens | Greece, Thessaly | Quercus, Castanea | VK1466 | OL415574 | |||||
| Greece, West Macedonia | Quercus | GK1228 | OL331103 | 26 | 39 | ||||
| G. graveolens | Hungary, Heves | Carpinus, Fagus | ZB2321 | OL331102 | 26 | 39 | |||
| Hungary, Heves | Carpinus, Quercus | ZB5822 | OL415578 | ||||||
| Slovakia | deciduous | sg9per165 | OL467191 | ||||||
| Spain, Castilla and Leon | Quercus | JC160529NR | OL331100 | OL330984 | 26 | 39 | |||
| Spain, Catalonia | Castanea | JMV20180712 (ref. spec.) | OL331101 | 26 | 39 | ||||
| Spain, Navarre | Fagus | PSS026005 | OL467190 | OL415563 | |||||
| G. violascens | Spain, Castilla and Leon | Quercus | JC120715BT | OL342571 | OL342573 | 27 | 40 | ||
| Spain, Catalonia | Quercus | JMV20170314** | OL342572 | ||||||
| /GauGlu-2 (trabutii) clade | |||||||||
| G. cistophila | Spain, Castilla and Leon | Cistus | JMV960527-1** | OL342777 | |||||
| G. morchelliformis | Calonge et al. (1994) | Spain, Castilla and Leon | Cistus | MA-Fungi 32260 | OL342775 | ||||
| G. trabutii | Mahiques et al. (1995) | Spain, Valencian Community | Arbutus, Cistus | MA-Fungi 33556 | OL342776 | ||||
| G. queletii | Poland, Lesser Poland | Abies, Picea, Fagus | KRA F-2011-77 | OL415554 | |||||
| Poland, Lesser Poland | Abies, Fagus | KRA F-2016-31 | OL342773 | ||||||
| Poland, Lesser Poland | Abies, Picea, Fagus | KRA F-2016-56 | OL342772 | ||||||
| Poland, Lesser Poland | Abies, Fagus | KRA F-2017-13 | OL415559 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20180823-1** | OL342774 | ||||||
| G. trabutii | Spain, Castilla and Leon | Quercus | JC70812BT | OL342780 | |||||
| G. trabutii | Calonge (in herb.) | Spain, Castilla-La Mancha | Cedrus | MA-Fungi 74767 | OL342779 | ||||
| Spain, Catalonia | Quercus | JMV20020519-2 | OL415546 | ||||||
| Spain, Catalonia | Quercus | JMV20020523 | OL342778 | ||||||
| Gautieria sect. Parvicellae — /GauParv-1 (otthii) clade | |||||||||
| G. aff. otthii | G. sp., G. rubescens | Bidartondo & Bruns (2002) | USA, Oregon | Pinus, Tsuga | OSC48138OR | AF377093 | 23 | 36 | |
| G. aff. otthii | G. monticola | Bidartondo & Bruns (2002) | USA, Washington | Pinaceae | H479 | AF377091 | 23 | 36 | |
| G. otthii | Hungary, Baranya | Picea | ZB443 | OL467225 | |||||
| Hungary, Heves | Picea | ZB2286 | OL467210 | ||||||
| Hungary, Nógrád | Pinaceae | ZB1822 | OL467207 | ||||||
| Hungary, Nógrád | Pinaceae | ZB2299 | OL467211 | ||||||
| Hungary, Pest | Picea | ZB1589 | OL331105 | 23 | 36 | ||||
| Hungary, Pest | Picea | ZB3543 | OL467220 | ||||||
| Hungary, Pest | Picea | ZB3548 | OL467221 | ||||||
| Hungary, Pest | Picea | ZB3569 | OL467222 | ||||||
| Hungary, Pest | Picea | ZI126 | OL351856 | ||||||
| Hungary, Pest | Picea | ZI183 | OL351858 | ||||||
| G. graveolens | Sarasini (in herb.) | Italy, Lombardy | Picea | MS0844 | OL331110 | 23 | 36 | ||
| Romania, Covasna | Picea | ZB2279 | OL331108 | 23 | 36 | ||||
| G. mexicana | Rubio (in herb.) | Spain, Castilla and Leon | Pinus | MA-Fungi 39619 | OL467187 | ||||
| Spain, Catalonia | Pinus | JMV990612-2 | OL331104 | 23 | 36 | ||||
| Spain, Catalonia | Abies | JMV990626-1 | OL467173 | ||||||
| Spain, Catalonia | Pinus | JMV990717-1 | OL467175 | ||||||
| Spain, Catalonia | Pinus | JMV20020615-5 | OL467132 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20020728-4 | OL467133 | ||||||
| Spain, Catalonia | Abies, Pinus | JMV20081007-3 | OL467141 | ||||||
| Spain, Community of Madrid | Pinus | IC11051908 | OL331109 | OL314783 | 23 | 36 | |||
| /GauParv-2 clade | |||||||||
| G. sp. | G. sp. | Garibay-Orijel et al. (unpubl.) | Mexico, Mexico State | GO-2009-208 | KC152099 | 22 | 35 | ||
| G. sp. | Fungal sp. | Bowman & Arnold (2018) | USA, Arizona | Pinus | ARIZ PM143E | MG761349 | 22 | 35 | |
| /GauParv-3 (persimilis) clade | |||||||||
| G. persimilis | G. graveolens var. otthii, G. mexicana | Soehner (1951: No. 1896) | Austria, Lower Austria | Picea | M 126241 | OL314149 | |||
| Germany, Lower Saxony | Fagus | ZB3549 | OL331107 | 20 | 33 | ||||
| Greece, Thessaly | Fagus | VK907 | OL415577 | ||||||
| Hungary, Pest | Fagus | ZB3136 | OL314150 | ||||||
| G. mexicana | Gori & Bernardini (1997) | Italy, Tuscany | Pseudotsuga | ELG940521-2 | OL331099 | 20 | 33 | ||
| Poland, Silesia | Fagus | KRA F-2013-4 | OL331106 | OL314779 | 20 | 33 | |||
| Spain, Castilla and Leon | Castanea | JC100613BT | OL467126 | ||||||
| Spain, Castilla and Leon | Quercus | JC110724BT | OL467127 | ||||||
| Spain, Catalonia | Pseudotsuga | JMV20110621-7 | OL467151 | ||||||
| Spain, Catalonia | Abies | JMV20180725-2 | OL467155 | ||||||
| Spain, Catalonia | Abies | JMV20180814-2 | OL467156 | OL415548 | |||||
| Spain, Catalonia | Abies | JMV20180904-2** | OL314151 | 20 | 33 | ||||
| G. sp. | Bidartondo & Bruns (2002) | Switzerland, Bern | Pinaceae | HH2221Swiss | AF377088 | ||||
| /GauParv-4 clade | |||||||||
| G. sp. | G. sp. | Bidartondo & Bruns (2002) | USA, California | Pinaceae | Blodgett29-22269CA | AF377095 | 21 | 34* | |
| G. sp. | G. sp. | Bidartondo & Bruns (2002) | USA, California | Pinaceae | StumpMdws-2273CA | AF377096 | 21 | 34* | |
| G. sp. | G. sp. | Bidartondo & Bruns (2002) | USA, California | Pinaceae | StumpMdws-2278CA | AF377097 | 21 | 34* | |
| G. sp. | G. sp. | Bidartondo & Bruns (2002) | USA, California | Pinaceae | GaddisCr2243CA | AF377098 | 37 | 34* | |
| /GauParv-5 (monticola) clade | |||||||||
| G. sp. | G. monticola | Bidartondo & Bruns (2002) | USA, California | Pinaceae | HS1997 | AF377076 | 17* | 26 | |
| G. sp. | G. sp. | Bidartondo & Bruns (2002) | USA, California | Pinaceae | Blodgett29-22272CA | AF377084 | 17* | 26 | |
| G. sp. | G. sp. | Bidartondo & Bruns (2002) | USA, Oregon | Pinaceae | Umpqua2206OR | AF377083 | 17* | 26 | |
| G. aff. monticola | G. monticola | Bidartondo & Bruns (2002) | USA, California | Pinaceae | SNF334CA | AF377087 | 17* | 27 | |
| G. aff. monticola | G. sp., G. fusispora | Bidartondo & Bruns (2002) | USA, Oregon | Pseudotsuga | T11164 | AF377077 | 17* | 29 | |
| G. monticola | G. monticola | Bidartondo & Bruns (2002) | USA, California | Pinaceae | SNF136CA | AF377079 | 17* | 28 | |
| /GauParv-6 clade | |||||||||
| G. sp. | G. sp. | Garibay-Orijel et al. (unpubl.) | Mexico, Mexico State | Pinaceae | GO-2009-198 | KC152097 | 18 | 30 | |
| G. sp. | Uncultured Gautieria | Baeza-Guzmán et al. (2017) | Mexico, Veracruz | Pinus | C2 | KU871236 | 18 | 30 | |
| /GauParv-7 clade | |||||||||
| G. sp. | G. sp. | Bidartondo & Bruns (2002) | USA, California | Pinaceae | Sequoia2287CA | AF377089 | 19* | 31 | |
| G. sp. | G. sp. | Izzo et al. (2005) | USA, California | Abies, Pinus | TK1621 | AY558751 | 19* | 32 | |
| /GauParv-8 clade | |||||||||
| G. sp. | G. sp. | Bidartondo & Bruns (2002) | USA, California | Pinaceae | Dinkey2409CA | AF377104 | 24 | 37 | |
| G. sp. | G. monticola | Bidartondo & Bruns (2002) | USA, California | Pinaceae | SNF95CA | AF377105 | 24 | 37 | |
| G. sp. | G. monticola | Bidartondo & Bruns (2002) | USA, California | Pinaceae | SNF346CA | AF377101 | 25 | 38 | |
| G. sp. | G. sp. | Bidartondo & Bruns (2002) | USA, California | Pinaceae | Dinkey2237CA | AF377102 | 25 | 38 | |
| Ramaria | |||||||||
| R. abietina | R. abietina | Berbeee & Lim (unpubl.) | Canada, British Columbia | UBC F16560 | FJ627035 | FJ627035 | 1 | 1 | |
| R. sp. | R. sp. | Knudson (unpubl.) | USA, Minnesota | AGK 066 | JQ408241 | JQ408241 | 1 | 1 | |
1 New collection sources: AM = A. Montecchi (pers. herb.); ELG = L. Gori (pers. herb.); EV = V. Erdei (ELTE); FGA = F Garda-Alonso (pers. herb.); GK = G. Konstantinidis (pers. herb.); IB = Herbarium of the University of Innsbruck; IC = A. Paz (BCN); JC = J. Cabero (pers. herb.); JMV = J.M. Vidal (BCN); KRA = Herbarium of the Jagiellonian University of Krakow; M = Herbarium of the Botanische Staatssammlung of Munich; MA = Herbarium of Cryptogamy of the Royal Botanic Garden of Madrid; MG = M. Gkilas (pers. herb.); MS = M. Sarasini (pers. herb.); O = Herbarium of the Natural History Museum of Oslo; PRM = Mycological Collection of the National Museum of Prague; PSS = F. Sainz (pers. herb.); sg = S. Glejdura (ELTE); SOMF = Mycological Collection of the Institute of Biodiversity and Ecosystem Research of the Bulgarian Academy of Sciences; TPN = Tatra National Park (KRA); UPS = Herbarium of the Museum of Evolution of Uppsala; VK = V. Kaounas (pers. herb.); ZB = Z. Bratek (ELTE); ZI = I. Zagyva (ELTE).
2 New sequences produced from this study are in bold.
Sequence boundary species test
Automatic Barcode Gap Discovery (ABGD) (Puillandre et al. 2012) on the web server (https://bioinfo.mnhn.fr/abi/public/abgd/abgdweb.html), and Assemble Species by Automatic Partitioning (ASAP) (Puillandre et al. 2021) (https://bioinfo.mnhn.fr/abi/public/asap/) was applied to estimate the number of species in the phylogeny. ITS sequences were utilised in the investigations, as the LSU dataset did not result in reliable partitions. The automatic ABGD process classifies sequences into groups and putative species based on gaps, which are detected via finding the boundary between intra- and interspecific divergence. ASAP creates ranges for the partitions considering the probability of panmixia (p-value) of a group created as a function of its pairwise differences within and between subgroups and also considering the relative width of the barcode gap (W ). The settings for ABGD were: Pmin 0.001, Pmax 0.1, 30 steps, relative gap width (X) 0.5, with the Kimura (K80) TS / TV 2.0 substitution model. In accordance with previous experiences (Puillandre et al. 2012), the partition with the value of the previous maximum divergence of intraspecific diversity (P) closest to 0.01 was chosen to represent putative species. For ASAP Kimura (K80) TS/TV 2.0 model, default settings were chosen for advanced options. The partition with p-value < 0.05 and with the lowest (best) ASAP score is considered the most relevant species hypothesis. The topology of the phylogenetic tree (Fig. 3) and the bootstrap values of the clades were also considered in the assessment of the species.
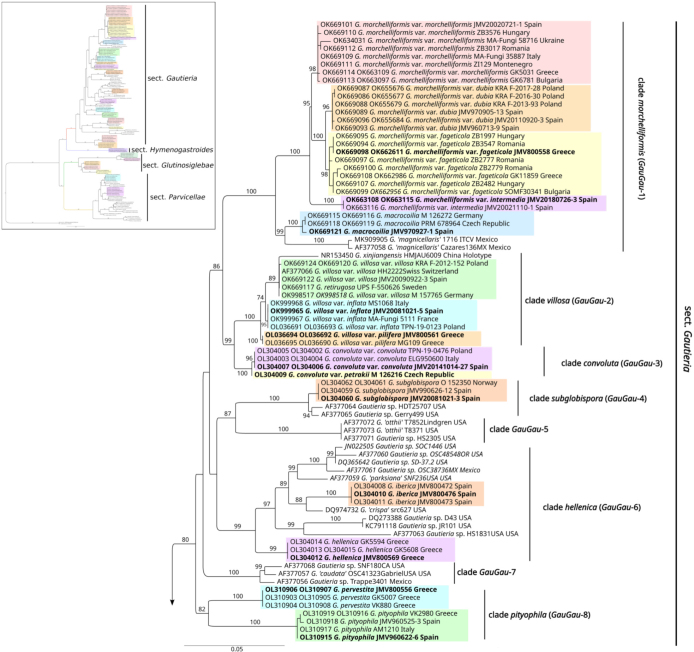
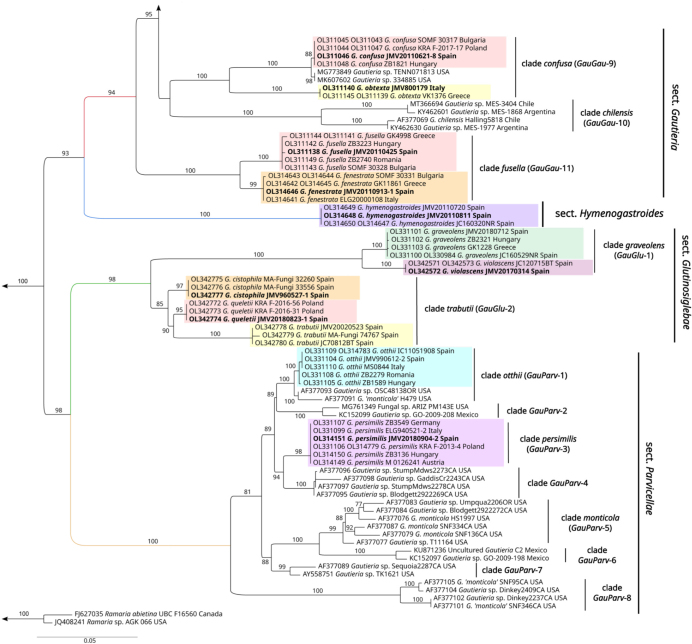
Fig. 3.
Maximum Likelihood (ML) phylogenetic tree of the currently described Gautieria taxa and related sequences from GenBank. Values of > 70 % for ML bootstrap are shown on the branches.
RESULTS
Molecular results
To build our phylogenetic tree we sequenced 243 European Gautieria samples obtaining 218 new ITS and 90 new LSUs to which we added 54 GenBank sequences, including 6 from European and 48 from extra-European taxa (Table 1). For the final phylogenetic tree, 88 ITS and 50 LSU sequences of new collections were included, 49 ITS sequences for Gautieria, and 2 ITS-LSU sequences for outgroup (Ramaria) from GenBank. Phylogenetic analysis based on ITS and LSU rDNA sequences represent four well supported sections: sect. Gautieria, sect. Hymenogastroides, sect. Glutinosiglebae and sect. Parvicellae (Fig. 3).
Gautieria sect. Gautieria: This section is represented in our phylogenetic tree by nineteen European taxa and several extra-European, most of them still pending description. Eleven main clades can be distinguished: clade morchelliformis (GauGau-1), clade villosa (GauGau-2), clade convoluta (GauGau-3), clade subglobispora (GauGau-4), clade GauGau-5, clade hellenica (GauGau-6), clade GauGau-7, clade pityophila (GauGau-8), clade confusa (GauGau-9), clade chilensis (GauGau-10) and clade fusella (GauGau-11).
Gautieria sect. Hymenogastroides: Represented only by G. hymenogastroides.
Gautieria sect. Glutinosiglebae: This section is represented in our phylogenetic tree by five European species, one of them also present in North Africa. Two main clades can be distinguished: clade graveolens (GauGlu-1) and clade trabutii (GauGlu-2).
Gautieria sect. Parvicellae: This section is represented in our phylogenetic tree by two European taxa and by numerous North American, most of which are pending description. Eight main clades can be distinguished, majority containing North American taxa: clade otthii (GauParv-1), clade GauParv-2, clade persimilis (GauParv-3), clade GauParv-4, clade monticola (GauParv-5), clade GauParv-6, clade GauParv-7 and clade GauParv-8.
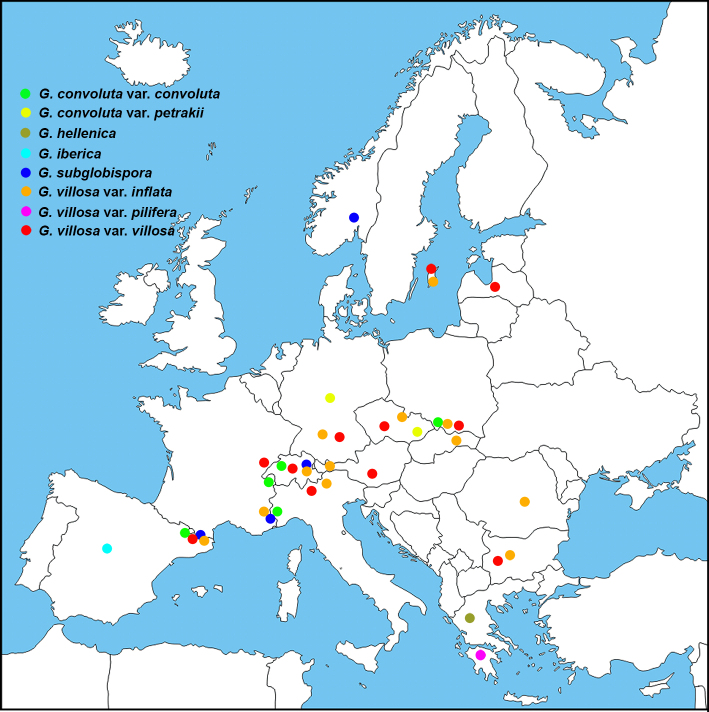
The composition and topology of the different clades, was not affected by the selection of the substitution model (although we used the one offered by the applied program). Each of the 21 clades representing the currently described species has a bootstrap value > 94 %, (18 of them have 100 % bootstrap support). Most of these clades show homogeneity or low intraspecific variability, unlike G. morchelliformis and G. villosa. The clades of the two above mentioned taxa show a division within the species resulting in subclades identified as varieties (G. morchelliformis var. dubia, G. morchelliformis var. fageticola, G. morchelliformis var. intermedia, G. morchelliformis var. morchelliformis, G. villosa var. inflata, G. villosa var. pilifera and G. villosa var. villosa). Among these subclades G. morchelliformis var. fageticola presents the greatest diversity, while the varieties of G. villosa are the most homogenous. It should be noted that the sequence NR153450 belonging to the Asian taxon G. xinjiangensis is part of the G. villosa clade (GauGau-2), although it has considerable differences with the European sequences of G. villosa. The results of Assemble Species by Automatic Partitioning (ASAP) and Automatic Barcode Gap Discovery (ABGD) are in agreement with the clades (Table 1) outlined in the phylogenetic tree (Fig. 3). The newly sequenced collections are marked with an asterisk symbol (*) in the paragraphs of material studied and the new sequences obtained are in bold in Table 1.
Species limits and ITS barcoding in European Gautieria
The Automatic Barcode Gap Discovery (ABGD) provided 37 groups as putative species in the examination of the aligned ITS sequences (Table 1). The varieties of G. morchelliformis constituted a group, as did the varieties of G. villosa and G. convoluta. Clades of currently recognised Gautieria species were separated as putative species: G. morchelliformis, G. macrocoilia, G. villosa, G. convoluta, G. subglobispora, G. iberica, G. hellenica, G. pervestita, G. pityophila, G. confusa, G. obtexta, G. fusella, G. fenestrata, G. hymenogastroides, G. graveolens, G. violascens, G. otthii and G. persimilis. The following GenBank sequences were in the same group as the currently published Gautieria species: AF377066 and NR153450 with G. villosa, AF377064 and AF377065 with G. subglobispora, MG773849 and MK607602 with G. confusa, and AF377091 and AF377093 with G. otthii. Other sequences were parts of different putative species. The ASAP output showed a very similar result, the limitation of the groups differed slightly in the case of clades formed only by previously published sequences (Table 1), in this case the number of groups is 40. Gautieria cistophila, G. queletii and G. trabutii due to the lack of ITS sequences were not included in the partitioning methods, they were instead evaluated by tree topology and branch support values as distinct species. The topology and bootstrap values of our phylogenetic tree (Fig. 3) and species delimitation tests (ABGD, ASAP) supports the species described above. Based on the topology and support values of phylogenetic tree branches, and the species hypotheses of the tools mentioned, the number of Gautieria species is 38 in our phylogenetic tree (Table 1), but 3 of them (sequences from the public database) are currently represented by a single sequence. In this study we present the sections and clades in the same order as in the phylogenetic tree.
TAXONOMY
Gautieria Vittad., Monogr. Tuberac.: 25. 1831 (nom. cons.); non Gautiera Raf., Med. Fl. 1: 202. 1828 (Ericaceae)
Synonyms. Ciliciocarpus Corda in Sturm, Deutschl. Fl., Abt. 3, Pilze Deutschl. 3, 11: 5. 1831.
Uslaria Nieuwl., Amer. Midl. Naturalist 4: 368. 1916.
Invalid name. Hydnospongos Wallr. ex Klotzsch, in A. Dietrich, Fl. Reg. Boruss. 7: No. 464. 1839. (nom. inval., Art. 36.1)
Etymology. In honour of the Italian physician Giuseppe Gautieri (1769–1833).
Type species. Gautieria morchelliformis Vittad. (‘morchellaeformis’) (see Dodge & Zeller 1934: 692, and Shenzhen Code, appendix III).
Basidiomata solitary or gregarious, hypogeous or emergent, 1–5(–8) cm wide, subglobose to tuberiform or lobate, sometimes coalescing, often reddening on rubbing, with a white basal rhizomorph, 1–2 mm thick. Surface usually ridged, with alveolate-reticulate appearance, exposing outlying locules, or pseudoperidial by fusion of ridges, with porate or foveate appearance, partially exposing outlying locules. Pseudoperidial species often developing a white, yellowish or brownish trichotomentocutis. Hymenophore coralloid, cartilaginous to gelatinous, becoming coriaceous in exsiccata. Locules hollow, 1–6(–15) × 0.3–2(–5) mm, labyrinthoid to irregularly rounded or tubular, pale yellow, light orange or brownish orange; cinnamon in exsiccata. Tramal plates 0.2–0.6(–1) mm thick, slightly to strongly gelatinized, greyish, often reddening when cut. Columella 1–3(–4.5) mm thick, dendroid to basal, gelatinized, white to grey; basal context darker. Odour pleasant, fruity to reminding of aromatic plants or Tuber-like, or nauseous, or alliaceous.
Spores orthotropic, pale yellow to greyish orange, cyanophilous, non-amyloid, variable in size and form, measuring 12–24 × 8–18 μm, being elliptical, fusiform, obovate, subglobose or pyriform, and provided with (7–)9–12(–16) straight to helicoidal costae, being entire or furcate, smooth or with gibbosities, an apical ring surrounding germ pore and a conico-truncate to acuminate hilar appendix, sometimes preserving remains of sterigma. Wall layers consisting of a thin coloured ectosporium, an inflated perisporium containing a three-dimensional coloured reticulum that the costae and apical ring originate from, a thick coloured exosporium and episporium, and a colourless endosporium (Fig. 2). Basidia clavate, 2–4-spored or 1–2-spored, soon collapsing. Cystidia absent in fertile hymenium. Paraphysoid cells abundant, often becoming long and hairy in mature hymenium, with the terminal cell clavate, cylindrical or acuminate, forming a trichotomentocutis in tomentose species. Hyphidia present in some species. Subhymenium consisting of chains of 3–5 cylindrical to subglobose elements. Hymenophoral trama formed by interwoven, cylindrical, septate hyphae, 2–7 µm diam, with some ampulliform septa, 8–14 µm wide, and often endocystidia-like terminal vesicles up to 30 µm wide. Hyphae slightly to strongly gelatinizing. Thromboplera present in all tramal tissues. Peritrama of ridges and pseudoperidium similar to hymenophoral trama, often with yellow pigment. Pseudopellis a hymeniderm made up of clusters of exocystidia circumscribed to the edge of ridges and the surface of pseudoperidium, sometimes mixed with geliferous hairs or, in tomentose species, made up of a trichotomentocutis mixed or not with exocystidia. Exocystidia usually sphaeropedunculate, 10–45 μm wide, sometimes papillate or mucronate, initially hyaline and thin-walled, then yellowish and thick-walled, collapsing at maturity (Fig. 1). Trichotomentocutis present in some pseudoperidial species, constituted by yellow thick-walled hairs and by hyaline thin-walled hyphae, 3–6 µm diam, becoming geliferous and crystalliferous. Acanthohyphae present in some species. Calcium oxalate crystals present in all tissues and in the surface of exocystidia. Clamp connections not found either in hymenium, pseudopellis or tramal tissues.
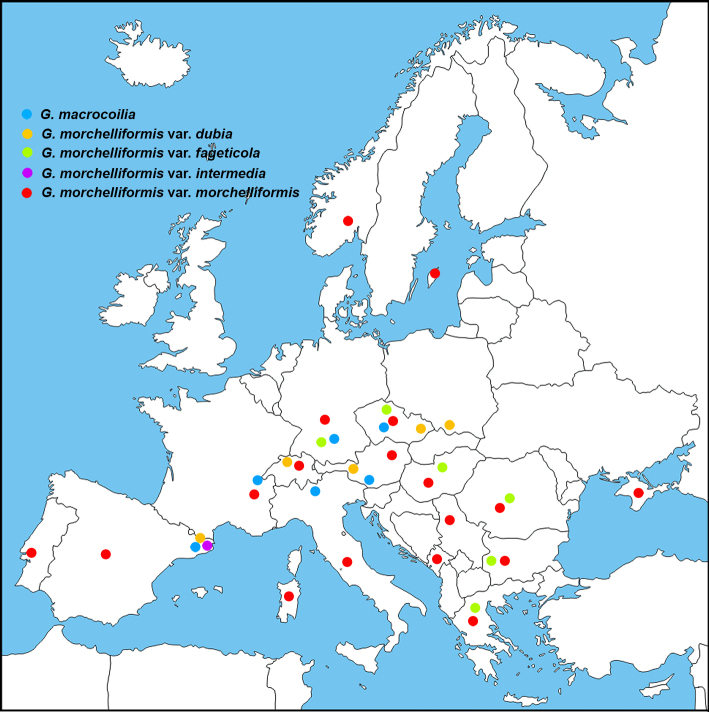
Habitat, Season & Distribution — Hypogeous or semi-hypogeous, in all types of soils. Linked to conifers and/or broadleaves, rarely to bushes. Found throughout the year, from the Mediterranean to the boreal region.
Notes — The genus Hydnospongos should be considered an invalid name, since Klotzsch (1839) does not accept the name Hydnospongos when considering H. morchellaeformis synonymous with G. morchellaeformis (see Art. 36.1, Ex. 1). The epithet ‘morchellaeformis’ does not comply with Rec. 60 G and consequently has been corrected to ‘morchelliformis’ in accordance with Art. 60.10. Nomenclatural changes arising from the phylogenetic placement of Gautieria species within Ramaria, and the subsequent change of all Ramaria names to Gautieria (older genus name) are not dealt with here.
Key to sections
1. Hyphae slightly gelatinizing, making hymenophore cartilaginous, non-gelatinous. Basidia 2–4-spored. Basidiomata ridged or pseudoperidial. Trichotomentocutis absent or present. Locules irregularly-shaped, labyrinthoid. Odour usually pleasant at maturity ............................. 2
1. Hyphae strongly gelatinizing, making hymenophore gelatinous or subgelatinous. Basidia 1–2-spored or 2–4-spored. Basidiomata pseudoperidial. Trichotomentocutis usually absent. Locules irregularly-shaped, narrowly elongated or tubular. Odour usually nauseating at maturity ......... 3
2. Basidiomata ridged or pseudoperidial. Pseudoperidium if present not foveate-porate and not turning purplish when rubbed. Trichotomentocutis if present not acquiring lemon yellow hues ................. Gautieria sect. Gautieria
2. Basidiomata pseudoperidial. Pseudoperidium foveate-porate turning purplish when rubbed. Trichotomentocutis acquiring lemon yellow hues .... Gautieria sect. Hymenogastroides
3. Basidia 1–2-spored. Hyphae usually promptly gelatinizing, making hymenophore gelatinous. Pseudoperidium foveate-porate, turning sanguineous or violaceous when bruised. Trichotomentocutis absent. Locules irregularly-shaped, narrowly elongated or tubular. Tramal plates usually thicker than locules ........... Gautieria sect. Glutinosiglebae
3. Basidia 2–4-spored. Hyphae late gelatinizing, making hymenophore subgelatinous. Pseudoperidium foveate-reticulate, turning lemon yellow with aging. Trichotomentocutis absent or undeveloped. Locules minute, irregularly-shaped. Tramal plates usually narrower than locules .......................................... Gautieria sect. Parvicellae
Gautieria Vittad. sect. Gautieria
Type species. Gautieria morchelliformis Vittad.
Basidiomata ridged or pseudoperidial. Pseudoperidium if present not foveate-porate and not turning purplish when rubbed. Trichotomentocutis absent or present, if present not acquiring lemon yellow hues. Locules irregularly-shaped, labyrinthoid. Hyphae subgelatinized, making hymenophore cartilaginous, non-gelatinous. Basidia 2–4-spored. Spores variable in shape, with a conico-truncate or acuminate hilar appendix. Odour pleasant, usually non-nauseous at maturity.
Key to species of section Gautieria
1. Ridges alveolate, alveolate-reticulate, convoluted or fused into a smooth pseudoperidium. Tramal plates rarely more than 0.6 mm thick, usually narrower than locules. Spores variable in shape, with a conico-truncate hilar appendix, usually lacking a sterigmal remnant ................ 2
1. Ridges venose or fused into a venose-reticulate pseudoperidium. Tramal plates reaching 1 mm thick, usually broader than locules. Spores tending to fusiform, with a rounded or pointed hilar appendix, provided with a sterigmal remnant ............................................ 19
2. Basidiomata lacking a trichotomentocutis ............ 3
2. Basidiomata covered by a trichotomentocutis ........ 15
3. Hilar appendix 4–8 μm wide at apex. Hilum 1.7–3 µm wide ........................................ 4
3. Hilar appendix 2.5–4.5(–5.5) μm wide at apex. Hilum 1–2.5 µm wide .................................. 8
4. Spore costae up to 2 µm high. Perisporial reticulum distinguishable .................................... 5
4. Spore costae up to 3 µm high. Perisporial reticulum inconspicuous . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 7
5. Spores 17–21 × 10–12 μm, elliptical to narrowly elliptical. — Basidiomata crisped, flabellate, white to light orange or greyish orange, turning reddish brown with rubbing. Under Carpinus, Fagus. Temperate (Central to South-Eastern Europe) ............ G. morchelliformis var. fageticola
5. Spores 19–24 × 12–14.5 μm, elliptical to obovate .... 6
6. Hilar appendix up to 1/4 of spore length. — Basidiomata alveolate-reticulate, white to melon yellow or brownish orange, turning reddish brown with age or rubbing. Hilar appendix up to 7 µm long × 6 μm wide. Under Abies, Picea, Pinus. Temperate (Central to South-Western Europe) .. ...................... G. morchelliformis var. dubia
6. Hilar appendix up to 1/5 of spore length. — Basidiomata alveolate-reticulate, white to carrot red, turning brownish red with rubbing. Hilar appendix up to 4.8 µm long × 5.8 μm wide. Under Carpinus, Castanea, Corylus, Fagus, Ostrya, Quercus. Mediterranean to temperate (Northern to Southern Europe) .... G. morchelliformis var. morchelliformis
7. Locules up to 1.5 mm wide. Spores 19–24 × 14.5–16.5 μm, broadly elliptical to obovate. — Basidiomata deeply alveolate, white to light orange, turning wine red with rubbing. Locules somewhat angular, up to 5 × 1.4 mm, light orange. Tramal plates up to 0.7 mm thick. Under Castanea, Corylus, Quercus. Mediterranean (Spain) ................... .................. G. morchelliformis var. intermedia
7. Locules up to 5 mm wide. Spores 19–24 × 14–17 μm, broadly elliptical to obovate. — Basidiomata deeply alveolate, crisped, white to melon yellow or chrome orange, turning raspberry red with rubbing. Locules somewhat angular, up to 15 × 5 mm, deep orange to chrome orange. Tramal plates up to 0.5 mm thick. Under Carpinus, Corylus, Fagus, Quercus, Tilia. Temperate (Central to Southern Europe) .................................. G. macrocoilia
8. Spores 15–18 × 12–15 µm, broadly elliptical to subglobose. Ridges flattened, forming white patches. — Basidiomata alveolate-reticulate, cerebrose, white to greyish orange, variegated, turning blood red with rubbing. Under Abies, Picea, Pinus. Boreal to temperate (Northern to Southern Europe) ........................ G. subglobispora
8. Spores ovate, elliptical or elliptico-fusiform. Ridges not forming white patches .......................... 9
9. Spores 16–19 × 8.5–10 μm, narrowly elliptical. — Basidiomata delicately alveolate-reticulate, white, slightly changing to pinkish with rubbing. Under Abies, Pinus. Temperate to Mediterranean (Southern Europe)........ G. pityophila
9. Spores wider and with other shapes . . . . . . . . . . . . . . 10
10. Ridges in young basidiomata crisped. Spores 16–19.5 × 11–13 µm, elliptical to broadly elliptical or broadly obovate. — Basidiomata granulose to alveolate-reticulate, white to melon yellow, turning deep red with rubbing. Under Abies, Fagus, Picea, Pseudotsuga, Quercus. Temperate (Central to Southern Europe) ................... G. confusa
10. Ridges in young basidiomata non-crisped ......... 11
11. Ridges convoluted to anfractuous or alveolate-reticulate ........................................... 12
11. Ridges densely convoluted ..................... 14
12. Spores 18–22.5 × 12–14 µm, broadly elliptical to pyriform. — Basidiomata anfractuous, rugose, white to light orange, reddening with rubbing. Presence of geliferous hairs in pseudopellis. Under Abies. Mediterranean (Greece) . . . ............................ G. villosa var. pilifera
12. Spores obovate or elliptico-fusiform .............. 13
13. Spores 16–19 × 12–13.5 µm, obovate. — Basidiomata markedly alveolate-reticulate, white to light orange, turning greyish magenta with rubbing. Under Abies, Larix, Picea, Pinus. Temperate (Northern to Southern Europe) ...... ............................. G. villosa var. inflata
13. Spores 18–22.5 × 10–13 µm, elliptico-fusiform to obovate. — Basidiomata convoluted to compactly alveolate-reticulate, white to light orange, turning pale red to greyish ruby with rubbing. Under Abies, Picea, Pinus. Temperate (Northern to Southern Europe) .... G. villosa var. villosa
14. Spores 20–24 × 12–14 µm, broadly elliptico-fusiform. — Basidiomata cerebriform, white to light orange, turning pale red to greyish ruby with rubbing. Under Abies, Picea. Temperate (Central to South-Western Europe) ........ ....................... G. convoluta var. convoluta
14. Spores 17–21 × 12–14 µm, broadly elliptical to subglobose. — Basidiomata as in var. convoluta. Temperate (Central Europe) .................. G. convoluta var. petrakii
15. Pseudoperidium with apertures. Pseudopellis presenting exocystidia ................................. 16
15. Pseudoperidium without apertures. Pseudopellis lacking exocystidia.................................. 18
16. Pseudoperidium up to 0.6 mm thick. Spores 14–18 × 8.5–10 µm, elliptical. — Basidiomata cottony-felted, white, turning reddish bistre with age or rubbing. Under Cedrus. Mediterranean (Morocco) ................ G. pseudovestita
16. Pseudoperidium up to 0.2 mm thick. Spores larger .. 17
17. Spores 17–20 × 9.5–11.5 μm, elliptico-fusiform. — Basidiomata cottony-felted, white, turning pale yellow to brown with age or rubbing. Under Abies. Mediterranean (Greece) .. ................................... G. hellenica
17. Spores 17–21 × 11.5–14.5 μm, ovate to ovato-fusiform. — Basidiomata cottony-felted, white, turning orange to reddish brown with age or rubbing. Under Quercus. Mediterranean (Spain) ............................... G. iberica
18. Pseudoperidium up to 0.3 mm thick. Spores 17.5–22.5 × 12–14.5 µm, ovato-fusiform. — Basidiomata cottony-felted, white, turning pale orange to dark brown with age or rubbing. Under Quercus. Mediterranean (Greece, Italy, Spain) ............................... G. obtexta
18. Pseudoperidium up to 1.5 mm thick or more. Spores 16–20 × 10–12.5 µm, elliptical to broadly elliptical. — Basidiomata cottony-felted, white, turning pale orange to brown with age or rubbing. Under Abies. Mediterranean (Greece) ..... ................................... G. pervestita
19. Ridges prominent, venose, surrounding broad exposed locules. Spores 16–21.5 × 10.5–12.5 μm, elliptical to broadly elliptico-fusiform. — Basidiomata white to buttercup yellow, turning deep brownish red with rubbing. Under Castanea, Fagus, Quercus. Temperate (Northern to Southern Europe) ........................ G. fenestrata
19. Ridges minute, compact, fusing into a venose-reticulate pseudoperidium. Spores 15–20 × 8–10 µm, fusiform. — Basidiomata white, often with yellow pruinosity, turning pastel red with age or rubbing. Presence of geliferous hairs in pseudopellis. Under Abies, Carpinus, Fagus, Pinus, Quercus. Temperate (Central to Southern Europe) ..... ..................................... G. fusella
Clade morchelliformis (GauGau-1)
Ridges alveolate-reticulate to deeply alveolate. Trichotomentocutis absent. Spores elliptical to obovate, reaching 23–26 × 14–17 μm. Costae slightly to very inflated, smooth or ornamented with broad gibbosities. Linked to broadleaf trees (Carpinus, Castanea, Corylus, Fagus, Ostrya, Quercus, Tilia), exceptionally with conifers (Abies, Picea, Pinus). Represented by G. macrocoilia, G. morchelliformis var. morchelliformis, G. morchelliformis var. dubia, G. morchelliformis var. fageticola, G. morchelliformis var. intermedia and an undescribed species from Mexico corresponding to the sequences AF377058 and MK909905.
Gautieria macrocoilia J.M. Vidal, A. Paz & States, sp. nov. — MycoBank MB 845105; Fig. 4, 32, 34, 35
Fig. 4.
Gautieria macrocoilia. a–e. JMV970927-1 (BCN, holotype). a–b. Mature basidiomata showing crisped surface and large locules; c. hymenophore showing locules of chrome orange colour; d. young hymenium showing basidia and paraphysoid cells; e. spores in KOH, showing the internal perisporial reticulum. — f. IC021220. Young basidiomata showing granulose surface and branched columella. — g–k. IC09121813. g. Mature basidiomata; h. detail of cristate ridges; i. cluster of exocystidia; j. paraphysoid cells of mature hymenium; k. spores in water. — l. M 126269 (E. Soehner as G. morchelliformis). SEM image of spores. — Scale bars: a–b, f–g = 1 cm; c = 5 mm; d, i–j = 20 µm; e, k–l = 10 µm; h = 1 mm. — Photos: a–e, i–j. J.M. Vidal; f–h, k. A. Paz; l. UdG.
Fig. 32.
Spores of Gautieria sect. Gautieria rehydrated with KOH 2 %. a. G. macrocoilia (JMV970927-1); b. G. morchelliformis var. morchelliformis (W 342); c. G. morchelliformis var. dubia (JMV960713-9); d. G. morchelliformis var. fageticola (ZB1237); e. G. morchelliformis var. intermedia (JMV20180726-3); G. villosa var. villosa (JMV20141014-26); g. G. villosa var. inflata (JMV20081021-5); h. G. villosa var. pilifera (JMV800561); i. G. convoluta var. convoluta (JMV20141014-27); j. G. convoluta var. petrakii (M 126216); k. G. subglobispora (JMV20081021-3); l. G. hellenica (GK5779); m. G. iberica (JMV800476); n. G. pervestita (VK422); o. G. pityophila (JMV960622-6); p. G. pseudovestita (MPU 312041); q. G. confusa (JMV20110621-8); r. G. obtexta (JMV800179); s. G. fenestrata (JMV20110913-1); t. G. fusella (JMV20110425). — Scale bar: 10 µm. — Photos: J.M. Vidal.
Fig. 34.
Spores of Gautieria rehydrated with water seen in relief by image coupling. a. G. macrocoilia (JMV970927-1); b1. G. morchelliformis var. morchelliformis (W 342); b2. G. morchelliformis var. dubia (JMV960713-9); b3. G. morchelliformis var. fageticola (ZB1237); b4. G. morchelliformis var. intermedia (JMV201807263); c1. G. villosa var. villosa (JMV20141014-26); c2. G. villosa var. inflata (JMV20081021-5); c3. G. villosa var. pilifera (JMV800561); d1. G. convoluta var. convoluta (JMV20141014-27); d2. G. convoluta var. petrakii (M 126216); e. G. subglobispora (JMV20081021-3); f. G. hellenica (GK5779); g. G. iberica (JMV800476); h. G. pervestita (VK422); i. G. pityophila (JMV960622-6); j. G. pseudovestita (MPU 312041); k. G. confusa (JMV20110621-8); l. G. obtexta (JMV800179); m. G. fenestrata (JMV20110913-1); n. G. fusella (JMV20110425); o. G. hymenogastroides (JMV20180814-1); p. G. graveolens (JMV20120612-1); q. G. violascens (JC110618NR); r. G. cistophila (JMV960527-1); s. G. queletii (JMV20180823-1); t. G. trabutii (JMV990617-1); u. G. otthii (JMV960713-1); v. G. persimilis (JMV20180904-2). — Scale bar: 10 µm. — Photos: A. Paz.
Fig. 35.
Distribution map of species of Gautieria sect. Gautieria clade morchelliformis (GauGau-1).
Invalid names. Gautieria morchelliformis var. magnicellaris Pilát, Sydowia 7: 10. 1953 (‘morchellaeformis’) (nom. nud., Art. 39.1).
Gautieria morchelliformis var. magnicellaris Pilát, Flora ČSR B1, Gasteromycetes: 745. 1958 (‘morchellaeformis’) (nom. inval. publ., Art. 40.1).
Gautieria magnicellaris Stewart & Trappe in Cázares et al., Mycologia 84, 3: 349. 1992 (nom. inval. publ., Art. 40.1).
Etymology. From Greek, makros = large, great + koilia = cavity, hole, by the large size of the hymenophoral locules. Equivalent Greek epithet replacing the invalidly published Latin magnicellaris.
Type. SPAIN, Catalonia, Girona, Vidrà, Ciuret, 1050 m, under Corylus avellana, Tilia platyphylla, Quercus pubescens and Fagus sylvatica, on calcareous soil, 27 Sept. 1997, J.M. Vidal (holo BCN JMV970927-1*; iso in ELTE).
Basidiomata ridged, 2–6.5 cm wide, subglobose, lobate or irregular, with a basal depression attached to a white basal rhizomorph, 1–1.5 mm thick, along with small strands and abundant mycelium. Surface of young basidiomata white and granulose as in G. morchelliformis. Mature basidiomata with the surface deeply alveolate, exposing colourful locules, with the ridges crisped, narrow and lamellar. Edge of ridges ornamented with prominent, white to yellow cristae. In old basidiomata, the cristae collapse and the edge of ridges become smooth and fertile, surface of basidiomata melon yellow to chrome orange (5A6–6A8), reddening to raspberry red (10D7) when handled. Locules 4–15 × 2–5 mm, very large, labyrinthoid to irregularly shaped, somewhat angular, deep orange (5A7) to chrome orange (6A8); cinnamon (6C6–D6) to orange-brown (6D7–D8) in exsiccata. Tramal plates thin, lamelliform, 0.3–0.5 mm thick, bluish grey, slightly reddish (9B4) when cut. Columella 0.6–1.5(–2.4) mm thick, well developed in young basidiomata, dendroid, then reduced and basal, greyish white to bluish grey; basal context brownish violet (11D6). Odour pleasant, of aromatic plants, similar to that of G. morchelliformis, and finally intensely fruity, like peach or pear, similar to that of Inocybe bongardii according to Pilát.
Spores 19–24(–26) × (13–)14–17(–18) μm, Q = 1.3–1.6, broadly elliptical to obovate, maize yellow (4A5–A6) in KOH (Fig. 32a, 34a). Episporium ovate. Costae 8–12, 1.6–3 μm high × 3–5 µm wide, very inflated, straight to helicoidal, entire or with few bifurcations, wrapping up to 3/4 of hilar appendix, smooth or slightly gibbose; apical ring distinct, 2–4 μm high; perisporial reticulum distinguishable, yellow-orange. Hilar appendix 4.5–6 µm long × 6–8 μm wide at apex, conico-truncate to rounded; empty-end 0.2–0.4 µm long; hilum 2–3 µm wide, without remnants of sterigma. Basidia 50–80 × 11–16 µm, clavate, 2–4-spored, soon collapsed. Paraphysoid cells in immature hymenium measuring 20–45 × 4–9 μm, 0–1-septate, with the terminal cell clavate, becoming long hairs in mature hymenium, 35–160 × 5–10 μm, 2–3-septate, with the terminal cell attenuate or acuminate. Subhymenium consisting of chains of cylindrical to subglobose elements, 8–15 µm diam. Hymenophoral trama formed by interwoven hyphae, 2–5 µm diam, with some ampulliform septa up to 12 µm wide. Thromboplera 1.5–5 µm diam. Peritrama of ridges prosenchymatous, consisting of densely interwoven hyphae 2–4 µm diam, with abundant yellow pigment. Pseudopellis a hymeniderm circumscribed to edge of ridges, formed by clusters of globose to subglobose or sphaeropedunculate exocystidia, 15–30 µm wide, long pedunculated, with a wall 1–1.5 µm thick, some of them constricted, papillate, with abundant incrustations of calcium oxalate.
Habitat, Season & Distribution — Gregarious, hypogeous or semi-hypogeous under plant debris, in montane broadleaf forests (Carpinus, Corylus, Fagus, Quercus, Tilia), on calcareous soils. From spring to autumn. Rare. Distributed in temperate regions, from 100 m altitude in Central Europe to over 1000 m in Southern Europe (Fig. 35).
Additional material studied. AUSTRIA, Carinthia, Klagenfurt-Land, Stemeritsch, Sattnitz Mountains, mixed forest of Fagus, Betula, Pinus and Picea, 22 Mar. 1969, M. Moser #69/9 as Gautieria sp. (IB 19690009). – CZECH REPUBLIC, Central Bohemia, Karlštejn, near Boubová, ‘in quercetis’, on calcareous soil, 27 July 1944, A. Pilát as G. morchelliformis var. magnicellaris (PRM 678964*; PC 92303, ex PRM; W 1781, ex PRM). – FRANCE, Rhône-Alpes, Savoie, Sept. 1905, ‘G. morchelliformis, dedit D. Bataille’ (PC 92640, herb. E. Boudier); from the Jura, sine dat., N. Patouillard as G. morchelliformis (PC 92636, herb. E. Boudier; BPI 711876, herb. C.G. Lloyd #46535, #08+53); Ain, Lépinay (Cras-sur-Reyssouze), Jura Mountains, under Quercus sp., 16 Aug. 1908, N. Patouillard as G. morchelliformis (FH 301417, herb. N. Patouillard; FH 301434, herb. C.W. Dodge, ex herb. N. Patouillard). – GERMANY, Bavaria, Bad Aibling to Rosenheim, under Fagus sylvatica, 21 Sept. 1941, E. Soehner #1622 as G. morchelliformis (M 126271/126272*; UPS F-550640); Grafrath, under Fagus sylvatica, 25 Oct. 1949, E. Soehner #2257 as G. morchelliformis (M 126276*); Landsberg am Lech, under Fagus sylvatica, June 1932, E. Soehner #1258 as G. morchelliformis (M 126266); Munich, Geiselgasteig-Grünwald, under Fagus sylvatica, 17 July 1919, E. Soehner #118/1380 as G. morchelliformis (M 126274); ibid., 19 July 1919, E. Soehner #119/1381 as G. morchelliformis (M 126275); ibid., 15 June 1920, E. Soehner #110 as Gautieria sp. (M 126229/126279); ibid., 2 Oct. 1920, E. Soehner #291 as Gautieria sp. (M 126290); ibid., 2 Oct. 1920, E. Soehner #293 as G. morchelliformis (M 126278); ibid., 28 Sept. 1921, E. Soehner #553 as G. morchelliformis (M 126277); ibid., 23 Sept. 1923, E. Soehner #815 as G. morchelliformis (M 126268); ibid., 1 Nov. 1923, E. Soehner #692 as Gautieria sp. (M 126289); ibid., 14 June 1943, E. Soehner #1922 as G. morchelliformis (M 126270); ibid., 21 Sept. 1948, E. Soehner #2227 as Gautieria sp. or G. retirugosa? (M 126222*; UPS F-550641); ibid., 21 Sept. 1948, E. Soehner #2228 as G. morchelliformis (M 126269); North Rhine-Westphalia, Münster, ‘mark Brandenburg aus Münster’, sine dat., W.G. Lasch, det. Dr. Milder as G. morchelliformis (B 700015006 /700015007); Saxony, near Dresden, ‘in querceto’, early Aug. 1860, T. Bail & W.G. Lasch as G. morchelliformis, Rabenhorst, Fungi Europaei #240, det. A. Pilát as G. morchelliformis var. magnicellaris (BR-MYCO 139385-93/139386-94; H 7017232; M 126251, herb. G. Niessl; M 126252, herb. E. Kayser; S F181877, ex herb. Sydow; S F181879/F181880, ex herb. H. Rehm; W 1780; W 366246, coll. Reichenbach fil.; ZT-Myc 3944). – ITALY, Piedmont, Vercelli, Serravalle Sesia, 23 Nov. 1901, O. Mattirolo as G. morchelliformis (FH 301419, herb. N. Patouillard); sine loc., sine dat., prof. Mattirolo as G. morchelliformis (BPI 712244, herb. C.G. Lloyd #46531); Trentino-Alto Adige, Trento, Arco, Braila, at foot of Stivo Mount, 500 m, 18 Mar. 1904, Diettrich as G. morchelliformis (S F181890); Trento, Ingenga, ‘pr. Magras, sub Corylus avellana’, summer 1881, G. Bresadola as G. morchelliformis (= G. villosa Quél.), det. L. Quélet as G. villosa (S F181874); ‘in coryletis’, sine dat., G. Bresadola as G. morchelliformis (= G. villosa Quél.) (FH 301418, herb. N. Patouillard); ‘in quercetis’, Aug. 1881, G. Bresadola as G. morchelliformis (UPS F-550638); ‘près de Magras, bois de noisetiers’, July 1882, G. Bresadola as G. villosa, C. Roumeguère, Fungi Gallici Exsiccati #2218, det. A. Pilát as G. morchelliformis var. magnicellaris (BR-MYCO 139387-95; NY 1952351; PC 92638); ‘Magras, Tirolia ital., July 1882, G. Bresadola as G. villosa’, det. A. Pilát as G. morchelliformis var. magnicellaris (S F181845, ex herb. Sydow); ‘in coryletis’, Oct. 1882, G. Bresadola as ‘G. villosa, non differt a G. morchelliformis Vittad.’ (UPS F-550639); ‘bosco in Ingenga, sotto Corylus’, autumn 1882, G. Bresadola as G. morchelliformis, det. L. Quélet as G. villosa, det. A. Pilát as G. morchelliformis var. magnicellaris (S F181846). – SPAIN, Catalonia, Girona, Sant Feliu de Pallarols, Bastons, 580 m, under Corylus avellana and Quercus pubescens, on calcareous soil, 9 Dec. 2018, A. Paz & C. Lavoise (IC09121813*); ibid., 22 Dec. 2019, A. Paz & C. Lavoise (IC22121901); ibid., 2 Dec. 2020, A. Paz, F. Rodríguez & C. Lavoise (IC02122004); Girona, Vidrà, Ciuret, 1050 m, under Corylus avellana, Tilia platyphylla, Quercus pubescens and Fagus sylvatica, on calcareous soil, 29 Sept. 2002, J.M. Vidal (JMV20020929-1).
Notes — Due to the great morphological similarities between ridged species, G. macrocoilia has been commonly identified as G. morchelliformis or as G. villosa. The first collection of this Gautieria was made in 1860 by the German botanists Theodor Bail and Wilhelm Gottfried Lasch in Saxony and labelled ‘Gautieria morchellaeformis Vittad., primus inveni fungum in Germania quidem rarissimum ineunte Augusto 1860 in querceto prope Driesen Cl. Laschio comite, Dr. Bail’ and later distributed to major European herbaria as duplicates of ‘Rabenhorst, Fungi Europaei Exsiccati No. 240’ (Rabenhorst 1861, Winter 1884). Later, Giacomo Bresadola found this species in 1881 and 1882 in Trentino, Italy, which he identified as G. morchelliformis or as G. villosa (Roumeguère 1882), Oreste Mattirolo found it in 1901 in Piedmont, Italy (Mattirolo 1935) and Narcisse Théophile Patouillard found it in 1908 in the French Jura (Patouillard 1914), both collections identified as G. morchelliformis. All these collections, which are kept in the herbaria of BPI, BR, FH, H, M, NY, PC, S, UPS, W and ZT, have been identified by us as belonging to G. macrocoilia, except Lloyd’s collection No. 46535 (08+53), which is composed of three Patouillard’s specimens: two preserved in Lloyd’s herbarium (BPI 711876) and the third in Zellers’s herbarium (NY 1952349). In these two sheets we have identified three different taxa: G. macrocoilia and G. villosa var. villosa in Lloyd’s sheet at BPI and G. fenestrata in Zeller’s sheet at NY.
The first description and illustration of this Gautieria was provided by Soehner (1951: 398, t. VII, f. 1–7) with an illustration of the spores and the hymenophore from his sample No. 2228 identified as G. morchelliformis (M 126269), from which we provide a SEM illustration (Fig. 4l). A Gautieria with identical characteristics was found on 27 July 1944 in Karlštejn (Czech Republic) by Albert Pilát. This collection consisting of a single specimen (PRM 678964) was described and illustrated by Pilát (1953) under the name G. morchelliformis var. magnicellaris, but unfortunately, this varietal name cannot be considered validly published since no Latin description was provided, nor was the combination of Stewart & Trappe in Cázares et al. (1992) based on Pilat’s 1953 publication. Pilát (1958), in his contribution to the Flora ČSR, revisited G. morchelliformis var. magnicellaris providing a new description and illustration of the specimen described in 1953, identifying with this variety specimens from the Corda herbarium (in PR herb.), from Roumeguère (Fungi Gallici Exsiccati No. 2218), from Rabenhorst (Fungi Europaei No. 240) and from Soehner (Bavaria, 21 Sept. 1941, in M herb.), and on page 745 provided a brief diagnosis in Latin (‘Sporae 20–25 (27) × 13–14 µm. Cellae magnae. Carposomata plerumque maiora, usque 4 × 5 cm. Bohemia: Karlštejn’), but without referring to it as var. nov. as it does with the other two varieties, so we do not interpret that it was intended to validate this taxon in this publication. To avoid nomenclatural confusion, we propose another epithet to name this species, but keeping the original meaning of the Latin epithet ‘magnicellaris’ proposed by Pilát, so we have chosen the analogous Greek epithet ‘macrocoilia’.
Gautieria macrocoilia is characterised by having: 1) surface crisped and deeply alveolate; 2) thin tramal plates; 3) large and angular locules of deep orange colour; and 4) large obovate spores ornamented with a highly inflated perisporium, featuring a clearly visible perisporial reticulum. A taxon with a similar spore morphology is G. morchelliformis var. intermedia, which differs macroscopically from G. macrocoilia by having non-crisped ridges, thicker tramal plates and smaller locules of light orange colour. Gautieria macrocoilia is a rare calcicolous species occurring in montane deciduous forests of temperate Europe. From this Gautieria we have studied 36 collections from 6 countries, of which we have successfully sequenced 6 (Table 1) with 3 sequences represented in our phylogenetic tree (Fig. 3), including Pilat’s original collection of G. morchelliformis var. magnicellaris (PRM 678964). The GenBank sequences identified as G. magnicellaris, specifically AF377058 (Bidartondo & Bruns 2002), which was obtained from the CZA136 collection of Cázares et al. (1992), and MK909905 (Pena & Guevara unpubl.), could represent a Mexican sister species of G. macrocoilia (Fig. 3).
Gautieria morchelliformis Vittad., Monogr. Tuberac.: 26. 1831, var. morchelliformis (‘morchellaeformis’) — Fig. 5, 32, 34, 35
Synonyms. Ciliciocarpus hypogaeus Corda in Sturm, Deutschl. Fl., Abt. 3, Pilze Deutschl. 3, 11: 5. 1831.
Uslaria morchelliformis (Vittad.) Nieuwl., Amer. Midl. Naturalist 4: 378. 1916 (‘morchellaeformis’).
Invalid name. Hydnospongos morchelliformis (Vittad.) Wallr. ex Klotzsch, in Dietrich, Fl. Reg. Boruss. 7: No. 464. 1839 (‘morchellaeformis’) (nom. inval., Art. 36.1).
Etymology. From the generic name Morchella = morel + formis = shape, due to the alveolate basidioma resembling a morel.
Type. Vittadini, Monogr. Tuberac.: t. III, f. VI, G–H. 1831 [icon] (lectotypus, hic designatus, MBT 10008567).
Authentic material studied. ITALY, Lombardy, ‘C. Vittadini misit’ (K(M) 69339, herb. C.E. Broome); ‘C. Vittadini dedit, ex herb. M.J. Berkeley’ (FH 79571, herb. C.W. Dodge); ‘Mediolanensi agro. Medialoni accepta ab ipso Vittadinio, mense maio 1845, Tulasne’ (PC 92115, herb. Tulasne); ‘Vittadini misit, ex herb. Tulasne’ (FH 79651, herb. C.W. Dodge); ‘Original v. Vittadini’ (W 342).
Basidiomata ridged, 1.5–5.5 cm wide, subglobose to irregular, often lobate and consisting of two or more aggregate or coalescing specimens, provided with a basal depression attached to one or more white basal rhizomorphs, 1.5–2 mm thick, and small filaments, all immersed in a dense mass of white mycelium. Immature basidiomata white to yellowish, granulose, externally and internally resembling a small Ramaria, with the exposed locules partially closed. As they mature, exposed locules gradually open and the surface becomes alveolate-reticulate, morchelloid in appearance, consisting of intertwined sinuous ridges that form a prominent reticulum with its edge surmounted by white cristae. Sometimes the ridges can be densely convoluted, giving the surface a cerebriform appearance. In old basidiomata the cristae collapse and the ridges become smooth and partially fertile, carrot red (6B6–B7), turning brownish red (10C6–D7) when rubbed. Locules 2.5–4.5 × 0.4–1.5 mm, labyrinthoid to irregularly shaped, sinuous, inordinate, light orange (5A5–A7) to greyish orange (6A6–B6); cinnamon (6C6–D6) in exsiccata. Tramal plates 0.4–0.7 mm thick, bluish grey, slightly reddening when cut. Occasionally, the tramal plates may be very thick and the locules tightly closed, making the hymenophore very compact. Columella 1–2.4 mm thick, dendroid, strongly branched, bluish grey; basal context reddish brown (9D6). Columellae of aggregate basidiomata independent or coalescent. Odour at first pleasant, fruity or of aromatic plants, finally very intense, of seafood or alliaceous.
Spores (18–)19–24(–27) × 12–14.5 μm, Q = 1.4–1.7(–2), elliptical to obovate, elongated in some collections, maize yellow with greenish hues (4A5–A6) in KOH (Fig. 32b, 34b1). Episporium narrowly ovate to ovate, basally rounded. Costae 10–12, 1.2–2 μm high × 2–4 µm wide, moderately inflated, straight or helicoidal, entire or with few bifurcations, wrapping up to 1/2 of hilar appendix, smooth or ornamented with low gibbosities; apical ring distinct, 1.5–3 µm high; perisporial reticulum almost indistinguishable. Hilar appendix 2.5–4.8 µm long × 4.2–5.8 μm wide at apex, conico-truncate; empty-end 0.1–0.8 µm long; hilum 1.7–2.4 µm wide, without remnants of sterigma. Basidia 50–80 × 10–14 µm, subclavate to clavate, 2 – 4-spored, soon collapsing. Paraphysoid cells 20 –75 × 3–9 μm, 0–3(–4)-septate, with the terminal cell cylindrical, clavate, attenuate or acuminate, becoming long hairs up to 120 µm in mature hymenium. Subhymenium consisting of chains of cylindrical to subglobose elements, 8–25 µm diam. Hymenophoral trama formed by loosely interwoven, cylindrical hyphae, 7–10 µm diam, containing large oily guttules, and some ampulliform septa up to 20 µm wide. Thromboplera 2–4 µm diam. Peritrama of ridges prosenchymatous consisting of densely interwoven hyphae, 2–4 µm diam, with abundant yellow pigment. Pseudopellis a hymeniderm circumscribed to edge of ridges, formed by clusters of sphaeropedunculate exocystidia, 15–35 μm wide, long pedunculate up to 40 µm, initially hyaline and thin-walled, then yellowish and thick-walled.
Habitat, Season & Distribution — Gregarious, hypogeous or semi-hypogeous under plant debris, from Mediterranean to montane broadleaf forests (Carpinus, Castanea, Corylus, Fagus, Ostrya, Quercus), with preference for calcareous soils. Almost throughout the year. Common. Widely distributed from Mediterranean to temperate regions, from sea level in Northern Europe to 1400 m altitude in Southern Europe (Fig. 35).
Additional material studied. AUSTRIA, Lower Austria, Wien-Umgebung, Purkersdorf, Gelber Berg, under Fagus sylvatica, Sept. 1912, F. von Höhnel as G. graveolens (FH 301426; M 126253); Wiener Neustadt, Waldschule, under Pinus sylvestris, July 1923, F. Winkler, comm. H. Huber (W 83, herb. Litschauer/Lohwag). – BULGARIA, Blagoevgrad, Kalimantsi, Southern Pirin Mountains, 300 m, under Quercus coccifera, on sandstone soil, 23 Dec. 2015, I. Gyonov (SOMF 30306*, dupl. JMV800731); Pernik, Zhedna, 845 m, under Carpinus betulus and Quercus sp., on sandstone soil, 10 July 2018, M. Slavova & B. Assyov (SOMF 30307, dupl. JMV800745); Plovdiv, between Yavrovo and Dobralak, Rhodope Mountains, 1000 m, under Carpinus betulus and Corylus avellana, on calcareous soil, 26 Sept. 2013, T. Chokova, comm. B. Assyov (GK6781*, dupl. JMV800571); Kuklen, Yavrovo, Rhodope Mountains, under Quercus petraea and Carpinus betulus, on calcareous soil, 16 Jan. 2014, T. Chokova (SOMF 30308, dupl. JMV800726); Shumen, Khan Krum, 200 m, under Quercus pubescens, on calcareous soil, 14 July 2017, I. Ivanov (SOMF 30309, dupl. JMV800737); ibid., under Tilia, Carpinus, Fraxinus and Crataegus, on calcareous soil, 3 July 2018, K. Krumov (SOMF 30310, dupl. JMV800743); Sofia, Kostinbrod, Beledie Khan, Western Stara Planina Mountains, 750 m, under Carpinus betulus, on calcareous soil, 31 July 2014, M. Slavova (SOMF 30311, dupl. JMV800727); Kostinbrod, Gradez, Western Stara Planina Mountains, 730 m, under Quercus sp., on calcareous soil, 26 June 2018, S. Nenov (SOMF 30312, dupl. JMV800742); Stara Zagora, Glavan, Liubimez, under Fraxinus, 9 June 2017, T. Chokova (SOMF 30313, dupl. JMV800735). – CZECH REPUBLIC, Central Bohemia, near Karlštejn, on calcareous soil, autumn 1831, A.C.J. Corda, det. A. Pilát as ‘G. morchelliformis specimen juvenile’ (holo PRM 155416, of Ciliciocarpus hypogaeus); Semice, near Lysá nad Labem, 29 May 1950, Ing. Lukavec, det. V. Vacek as G. graveolens (PRM 678928); Olomouc, Hranice (Mährisch Weisskirchen), Svrčow, in deciduous forest, Nov. 1923, F. Petrak #22536 as G. graveolens (W 29477); South Moravia, Súchov, under Quercus, Carpinus and Fagus, 8 July 1953, Kriz, det. A. Pilát (PRM 678955). – FRANCE, sine loc., sine dat., sine leg., as G. villosa, det. O. Mattirolo as G. morchelliformis (PC 92637, herb. E. Boudier); Franche-Comté, Doubs, Hérimoncourt, Jura Mountains, sine dat., L. Quélet as G. villosa (UPS s.n.); ibid., ‘bois feuillés’, sine dat., L. Quélet as G. villosa (FH 301444, herb. C.W. Dodge); Vosges, sine dat., L. Quélet (K(M) 170560, herb. C.B. Plowright); Rhône-Alpes, Ain, Oyonnax, Oct. 1971, mushroom exhibition, det. N. Suber (S F181887). – GERMANY, Baden-Württemberg, Wertheim am Main, Lindelbach, under Quercus robur, 29 May 1981, R.A. Hintz (M 126261); ibid., 6 June 1982, R.A. Hintz (M 126260); Bavaria, Arnstein, under Corylus and Quercus, 13 Nov. 1982, R.A. Hintz as G. morchelliformis var. globispora (M 126263); Dachau, under Quercus, 24 June 1944, E. Soehner #1992 as G. trabutii (M 126287); Gemünden am Main, under Corylus avellana, 22 Aug. 1982, R.A. Hintz (M 126265); ibid., 15 Oct. 1983, R.A. Hintz (M 126264); Karlstadt am Main, under Quercus and Corylus, 10 Nov. 1984, R.A. Hintz as G. dubia (M 126208); Thuringia, Nordhausen, sine dat., sine leg., det. A. Pilát (PRM 678952, probable specimen of Hydnospongos morchelliformis collected by C. Wallroth). – GREECE, Central Greece, Attica, Acharnes, Katsimidi, foot of Parnitha Mount, 600 m, under Quercus ilex and Pinus halepensis, on calcareous soil, 9 June 2009, V. Kaounas (VK897, dupl. JMV800540); Thessaly, Magnesia, Portaria, 750 m, under Quercus frainetto, on calcareous soil, 4 July 2010, V. Kaounas (VK1508*, dupl. JMV800551); ibid., 4 July 2010, V. Kaounas (VK1509, dupl. JMV800552); ibid., 2 May 2011, V. Kaounas (VK2169, dupl. JMV800544); ibid., 25 Apr. 2014, V. Kaounas (VK3390, dupl. JMV800612); West Macedonia, Grevena, Taxiarchis, 620 m, under Quercus sp. and Cistus sp., 21 May 2014, C. Plesiotis (GK7252*, dupl. JMV800717); Grevena, Rodia, 870 m, under Quercus spp., 10 Aug. 2018, G. Argyropoulos (GK11338*, dupl. JMV800817); Florina, Oxia, under Fagus sylvatica, 22 Nov. 2018, N. Tsilis (GK11860, dupl. JMV800819); Florina, Vrontero, under Quercus sp., 24 July 2018, N. Tsilis (GK11862*/11863, dupl. JMV800821/800822); Kozani, Rodochori, 770 m, under Quercus sp., 7 June 2010, T. Karagiannis (GK5031*, dupl. JMV800565). – HUNGARY, Baranya, near Pécs, c. 350 m, under Quercus sp., 30 June 2007, H.V. Tóthné (ZB3577, dupl. JMV800449); ibid., TV tower, 525 m, under Quercus sp. and Corylus avellana, 30 June 2007, H.V. Tóthné (ZB3576*, dupl. JMV800448); Borsod-Abaúj-Zemplén, Hoór Valley (Holló-tető), Bükk Mountains, c. 400 m, under Carpinus betulus among Tuber aestivum, 2 Jan. 2005, A. Bathó (ZB2986*, dupl. JMV800432); Bükkzsérc, Bükk Mountains, c. 350 m, 4 Sept. 2001, S. Vásárhelyi (ZB2359*, dupl. JMV800424); near Bükkszentkereszt, Lófőtisztás, Bükk Mountains, 600 m, under Carpinus betulus, 1 Dec. 2002, A. Bathó (ZB2587*, dupl. JMV800430); Budapest, Normafa, 450 m, under Carpinus betulus and Quercus petraea, 25 Aug. 1997, Z. Lukács (JMV800072); Heves, Bagolyirtás, Mátra Mountains, c. 800 m, under Carpinus betulus, 2 July 2007, I. Zagyva (ZI239*, dupl. JMV800450); ibid., 8 July 2007, A. Sági (ZI16*, dupl. JMV800452); Bátor, Bükk Mountains, c. 300 m, under Quercus sp., 10 Dec. 2007, Z. Nagy (ZI203*, dupl. JMV800454); near Felsőtárkány, Bükk Mountains, c. 350 m, 5 Sept. 2001, S. Vásárhelyi (ZB2368*, dupl. JMV800425); ibid., under Carpinus betulus and Quercus sp., Jan. 2002, S. Mészáros (ZB2464, dupl. JMV800426); near Parád, Sándor-rét, Mátra Mountains, c. 250 m, 2001, B. Estók (ZB2316, dupl. JMV800422); near Pásztó, Csór-rét, Mátra Mountains, 600 m, under Carpinus betulus, 8 July 2007, A. Sági (ZI15, dupl. JMV800451); near Szilvásvárad, Szalajka Valley, Bükk Mountains, c. 400 m, under Quercus petraea and Quercus cerris, 12 Nov. 1998, J. Szakács, L. Albert & I. Bagi (ZB1487, dupl. JMV800409); Nógrád, Litke, under Quercus, Carpinus and Fagus, 28 Aug. 1902, L. Hollós (UC 126168); Pest, Solymár, Csúcs-hegy, Budai Mountains, c. 350 m, under Carpinus betulus, 19 June 2000, V. Erdei (EV00/4*, dupl. JMV800417); Törökmező, Börzsöny Mountains, c. 200 m, under Carpinus betulus, 19 Mar. 2000, I. Bagi (ZB2018*, dupl. JMV800416); ibid., under Quercus sp., June 2009, K. Matyin (ZB4147*, dupl. JMV800459); Üröm, Csúcs-hegy, Budai Mountains, c. 350 m, under Fagus sylvatica, 18 Oct. 2006, I. Zagyva (ZB3353*, dupl. JMV800441); Szép-völgy, Budai Mountains, c. 300 m, Carici pilosae-Carpinetum/Vicio sparsiflorae-Quercetum, 1 July 2006, I. Zagyva (ZB3272*, dupl. JMV800439). – ITALY, Abruzzo, Teramo, Prati di Tivo, under Fagus sylvatica, 21 Sept. 1996, A. Montecchi (MA-Fungi 35887*); Apulia, Foggia, near Sant’Agata, 750 m, in mixed forest, 18 Oct. 2008, J. Szakács (ZB3910, dupl. JMV800457); Emilia-Romagna, Bologna, Porretta Ferone, Vigo Porretta, 1000 m, under Castanea sativa and Quercus cerris, 8 Dec. 1992, G. Bernardini, comm. L. Gori (ELG921208*, dupl. JMV800352); Reggio Emilia, Lamora, 600 m, under Quercus cerris, 28 Jan. 1993, A. Montecchi s.n. as G. graveolens (JMV800039*); Sardinia, Oristano, Laconi, Santa Sofia, 950 m, under Quercus ilex, on calcareous soil, 20 Dec. 1990, P. Fantini (JMV800178*); Oristano, Laconi, Scala Martini, 850 m, under Quercus ilex, on calcareous soil, 9 Dec. 2002, P. Fantini (JMV800180*); Oristano, Pau, Monte Arci-Is Lottus, 450 m, under Quercus ilex, on calcareous soil, 3 June 1993, P. Fantini (JMV800174*); ibid., 500 m, 10 July 1994, P. Fantini (JMV800175*); Tuscany, Lucca, Bagni di Lucca, Montefegatesi, 800 m, under Quercus cerris and Corylus avellana, 23 Nov. 2002, G. Bernardini & L. Gori (ELG20021123*, dupl. JMV800356); Lucca, Molazzana, Alpi di San Antonio, 900 m, under Castanea sativa, Quercus cerris and Ostrya carpinifolia, 8 Jan. 2000, G. Bernardini & L. Gori (ELG20000108b*, dupl. JM-V800355b). – MONTENEGRO, near Bar, near the sea, under Quercus sp., 27 Dec. 2002, V. Erdei (ZB2641*, dupl. JMV800431); near Podgorica, under Quercus pubescens, Jan. 2007, I. Bagi (ZI129*, dupl. JMV800444). – NORWAY, Oslo, Bygdøy, slope at Karljohan statue, 10 Aug. 1952, F.E. Eckblad, det. K.S. Bergsnov Hansen #4 (O 152355). – PORTUGAL, Setubal, Casais da Serra, Serra da Arrábida Nat. Park, c. 200 m, under Quercus coccifera and Quercus ilex, on calcareous soil, 26 May 1999, J.M. Vidal & F.D. Calonge (MA-Fungi 46932). – ROMANIA, Bihor, near Aleşd (Élesd), 230 m, 2008, A. Fekete (ZB3870*, dupl. JMV800456); Cluj, near Cluj-Napoca (Kolozsvár), Hoia Forest, c. 450 m, under Quercus spp. and Carpinus betulus, 15 July 1973, G. Pap (Pap17, Páz73/18, dupl. JMV800382/800383); ibid., Fáget Forest, 9 Aug. 1986, Z. Tőkés (Páz86/10, dupl. JMV800391); Harghita, near Cristuru Secuiesc (Székelykeresztúr), Sóskút Forest, c. 450 m, under Carpinus betulus, 1 Aug. 1980, M. Misky (Pap132, dupl. JMV800385); ibid., 2 Aug. 1980, M. Misky (Pap133, dupl. JMV800386); near Rugonfalva (Rugăneşti), c. 600 m, Aug. 1998, D. Váry (ZB1399*, dupl. JMV800406); ibid., in mixed forest, 2000, L. Szabó (ZB2043, dupl. JMV800418); Harghita-Covasna, sine loc., 1997, B. Pálfy (ZB2720/2743*, dupl. JMV800400/800402); sine loc., 1997, B. Pálfy (ZB2779b, dupl. JMV800405b); Mureş, near Kisgörgény (Gruişor), c. 400 m, mixed forest with Carpinus betulus, 9 July 2005, I. Bagi (ZB3017*, dupl. JMV800434). – SERBIA, Šumadija and Western Serbia, Valjevo, Gradac River Canyon, 200 m, under Quercus cerris and Quercus pubescens, Jan. 2007, I. Bagi (ZI128, dupl. JMV800443). – SPAIN, Andalusia, Córdoba, Priego de Córdoba, Sierras Subbéticas Nat. Park, under Quercus rotundifolia, on calcareous soil, 4 Apr. 1989, J. Gómez (JMV800100); Aragon, Zaragoza, Vera del Moncayo, Maderuela, under Quercus ilex and Quercus coccifera, 15 July 2000, F. García-Verdugo, det. F.D. Calonge (MA-Fungi 51189); Basque Country, Álava, Salinillas de Buradón, 650 m, under Quercus rotundifolia, 1 July 2011, F. Rodríguez (JMV20110701-1*); Castilla and Leon, Burgos, Pesquera de Ebro, 790 m, under Quercus rotundifolia, on calcareous soil, 6 June 2009, F. Sáinz & P.M. Pasabán (JMV800823); Burgos, Reinoso de Bureba, 830 m, under Quercus rotundifolia and Quercus faginea, on calcareous soil, 1 July 2017, F. Sáinz (PSS131001*, dupl. JMV800827); Segovia, Castrillo de Sepúlveda, 1100 m, under Quercus rotundifolia, on calcareous soil, 13 July 1998, F. García-Verdugo (JMV800471*); ibid., 28 Mar. 2003, F. García-Verdugo (JMV20030504-10*); Valladolid, Castromonte, Monasterio de La Santa Espina, 800 m, under Quercus faginea and Quercus rotundifolia, on calcareous soil, 10 June 2000, E.C.A., det. F.D. Calonge (MA-Fungi 47781); ibid., 30 Apr. 2001, P. Juste #1771 (JMV20010530-1*); ibid., 12 Feb. 2002, F. García-Verdugo (JMV800474*); ibid., 17 Feb. 2002, P. Juste & F. García-Verdugo (JMV20020328-2*); ibid., 3 June 2007, J. Cabero (JC20070603*, dupl. JMV800788); Catalonia, Girona, Aiguaviva, 150 m, under Quercus ilex and Quercus pubescens, 18 June 2012, F. Rodríguez (JMV20120618-1/20120618-2); Girona, Albanyà, pla de Sous, El Mont Massif, 950 m, under Quercus ilex, on calcareous soil, 7 July 2020, J.M. Vidal, A. Paz & C. Lavoise (JMV20200707); Girona, Campdevànol, Puig de Corones, Eastern Pyrenees, 1150 m, under Quercus pubescens, on calcareous soil, 21 July 2002, J.M. Vidal (JMV20020721-1*); Girona, Canet d’Adri, 200 m, under Quercus ilex, Quercus suber and Quercus pubescens, on calcareous soil, 23 June 2013, F. Rodríguez (JMV20130623); ibid., 28 Oct. 2014, F. Rodríguez (JMV20141028-6); ibid., 25 Nov. 2014, F. Rodríguez (JMV20141125-4); ibid., 21 Apr. 2015, F. Rodríguez (JMV20150421); ibid., 17 May 2015, F. Rodríguez (JMV20150517); ibid., 5 June 2018, F. Rodríguez (JMV20180605); ibid., 19 June 2018, F. Rodríguez (JMV20180619); Girona, Esponellà, Centenys, 255 m, under Quercus ilex, on siliceous soil, 28 June 2016, F. Rodríguez (JMV20160628-1); ibid., 5 July 2016, F. Rodríguez (JMV20160705-2); Girona, Gombrèn, Montgrony, Baga de La Molina Forest, Eastern Pyrenees, 1250 m, under Quercus pubescens and Corylus avellana, on calcareous soil, 19 July 2018, J.M. Vidal, A. Paz & C. Lavoise (JMV20180719-1); Girona, La Vall de Bianya, L’Hostalnou de Bianya, Puig Rodón, 470 m, under Quercus ilex and Quercus pubescens, 9 July 2013, F. Rodríguez (JMV20130709); ibid., 5 June 2014, F. Rodríguez (JMV201406052); ibid., Sant Salvador de Bianya, 610 m, under Quercus pubescens, Quercus ilex and Corylus avellana, on sandstone soil with calcium carbonate, 28 Jan. 2013, F. Rodríguez (JMV20130128); ibid., Sant Salvador de Bianya, Collet de Capsacosta, 860 m, under Quercus ilex, Quercus pubescens and Fagus sylvatica, on sandstone soil with calcium carbonate, 20 Aug. 2018, J.M. Vidal, A. Paz & C. Lavoise (JMV20180820-1); Girona, Les Planes d’Hostoles, Cogolls, 500 m, under Quercus ilex, on sandstone soil with calcium carbonate, 17 May 2011, F. Rodríguez (JMV20110517-1*); Girona, Les Planes d’Hostoles, crossroad to La Salut, 940 m, under Quercus pubescens and Fagus sylvatica, on sandstone soil with calcium carbonate, 2 July 2012, F. Rodríguez (JMV20120702-2); Girona, Sales de Llierca, 300 m, under Quercus ilex and Quercus pubescens, on calcareous soil, 12 June 1999, J.M. Vidal (JMV990612-8*); ibid., 22 June 2002, J.M. Vidal (JMV20020622-3); Girona, Sant Jaume de Llierca, under Quercus ilex, on calcareous soil, 15 June 2016, F. Rodríguez (JMV20160615); ibid., 28 June 2016, F. Rodríguez (JMV20160628-2); Girona, Sant Sadurní de l’Heura, 110 m, under Quercus ilex and Quercus suber, on sandstone soil with calcium carbonate, 30 June 1999, J.M. Vidal (JMV990630*); Girona, Susqueda, Turó d’Armadans, near Mas La Jaça, 1010 m, under Quercus pubescens and Fagus sylvatica, on sandstone soil with calcium carbonate, 4 July 2012, F. Rodríguez (JMV20120704-1); Girona, Vilablareix, 150 m, under Quercus ilex and Quercus pubescens, 16 Nov. 2012, F. Rodríguez (JMV20120611-1); Girona, Vidrà, Ciuret, 1050 m, under Corylus avellana, Tilia platyphylla, Quercus pubescens and Fagus sylvatica, growing mixed with G. macrocoilia, on calcareous soil, 27 Sept. 1997, J.M. Vidal (JMV970927-1b); Girona, Viladrau, Mas El Martí, Montseny Massif, 984 m, under Quercus ilex, on siliceous soil, 2 Aug. 2018, J.M. Vidal, A. Paz & C. Lavoise (JMV201808022); La Rioja, Logroño, Zarzosa, 1000 m, under Quercus sp., 17 June 1990, A. Caballero #1360, det. F.D. Calonge (MA-Fungi 26624); Valencian Community, Valencia, Bicorp, Hongarés, 820 m, under Quercus rotundifolia, Pinus halepensis and Juniperus oxycedrus, on calcareous soil, 11 Apr. 1996, F. García-Alonso as G. graveolens (FGA96653*, dupl. JMV800490); Valencia, Vallada, 620 m, under Quercus rotundifolia, 3 May 1995, F. García-Alonso (FGA95299, dupl. JMV800488); ibid., 16 May 1995, F. García-Alonso (FGA95313, dupl. JMV800489). – SWEDEN, Gotland, Hörsne, Mörby, under Quercus, 13 Aug. 1962, G. Eriksson (UPS F-550637); ibid., in clusters under Quercus, 14 Aug. 1962, G. Eriksson, conf. N. Suber (S F181885). – SWITZERLAND, Basel-Landschaft, near Arlesheim, footpath to Reichensteiner castle, under Fagus sylvatica, 16 Sept. 1956, C. Schwärzel #2 as G. dubia (ZT-Myc 3955); ibid., near Reichensteiner castle, under Fagus sylvatica, 21 June 1957, C. Schwärzel #53 as G. otthii (ZT-Myc 3935); Neuchâtel, Neuchâtel, 28 Sept. 1971, G. Schleiber & N. Suber (UPS F-550646). – UKRAINE, Crimea, Yalta, Silver Pavilion, Pendikul Mountain, 865 m, mixed forest with Fagus sylvatica, Acer sp. and Pinus sp., on calcareous soil, 24 Sept. 2003, D. Minter, det. F.D. Calonge as G. otthii (MA-Fungi 58716*).
Notes — Gautieria morchelliformis is the type of the genus Gautieria. It was described and illustrated by Vittadini (1831) in his Monographia Tuberacearum from specimens collected in the region of Pavia, Lombardy (Italy), also providing data on its ecology and phenology. Shortly after, in the same year, Corda (1831) found this species in Central Bohemia (Czech Republic) and published it as Ciliciocarpus hypogaeus. Corda (1831) mentioned Vittadini’s work several times (for instance on pages 5, 22, 31, 32 or 33) and therefore names in Vittadini’s work have priority. Next, Klotzsch (1839) provided new data, describing and illustrating a new collection of G. morchelliformis found in Thuringia (Germany) by C. Wallroth, who sent it to Klotzsch under the name Hydnospongos morchelliformis, which probably corresponds to the collection preserved in Prague herbarium with the number PRM 678952, but this name should be considered an invalid name, since Klotzsch (1839) does not accept the name Hydnospongos when considering H. morchellaeformis synonymous with G. morchellaeformis (see Art. 36.1, Ex. 1). Finally, Nieuwland (1916) recombined G. morchelliformis into the genus Uslaria. Gautieria morchelliformis was subsequently cited in many countries of Europe and in various habitats, e.g., Hazslinsky (1875) in Slovakia, Winter (1884), Soehner (1951) and Rauschert (1975) in Germany, Patouillard (1914) and Mattirolo (1938) in France, Mattirolo (1900, 1928, 1933) in Italy, Bucholtz (1902, 1903) in Latvia, Hollós (1911) in Hungary, Velenovský (1922) and Pilát (1958) in the Czech Republic, Italy and Switzerland, Mattirolo (1935) and Knapp (1941, 1958) in Switzerland, Hawker (1952, 1954) in the United Kingdom, Eriksson (1976) in Sweden, but those identifications were not always correct, many of them identified in this study as other taxa, namely G. confusa, G. fenestrata, G. macrocoilia, G. subglobispora, G. villosa var. inflata and G. villosa var. villosa. The Americans Dodge & Zeller (1934) studied the authentic material of G. morchelliformis preserved in the herbaria of Broome in Kew and of Tulasne in Paris, and proposed the Tulasne collection as a possible type. We studied this material (K(M) 69339, PC 92115), the duplicates of Kew and Paris preserved in Dodge’s herbarium in Farlow (FH 79571/79651), and other material preserved in the Vienna her-barium (W 342). Since none of those sheets refers to the location and date of the collection, we have chosen the specimen illustrated by Vittadini (1831: t. III, f. VI, H) as lectotype of G. morchelliformis var. morchelliformis, while we select our sequenced collection BCN JMV20020721-1 as a reference specimen and the most representative (Fig. 5a–e). Gautieria morchelliformis var. morchelliformis is characterised by having: 1) alveolate-reticulate surface with cristate ridges; 2) a branched columella; 3) greyish orange locules; and 4) elliptical to obovate spores of maize yellow colour, provided with entire, straight to helicoidal costae, a distinct apical ring and a broad hilar appendix. We consider this Gautieria the most abundant and widely distributed in Europe with ecology closely related to broadleaf trees, especially with Quercus.
We have also seen some collections with elongated spores, such as those of the French Jura determined by L. Quélet as G. villosa (FH 301444, K(M) 170560, PC 92637, UPS s.n.), or others from Hungary (UC 126168 ex Hollós, ZB2316, ZB2464, ZB2587) or from Ukraine (MA-Fungi 58716) (Fig. 5o), but our genetic studies on ZB2587 and MA-Fungi 58716 collections (Table 1) reveal a total identity with G. morchelliformis var. morchelliformis; therefore, we consider this morphology within its variability. From this taxon we have studied 144 collections from 17 countries, of which we have successfully sequenced 49 collections (Table 1) and represented 8 sequences in our phylogenetic tree (Fig. 3). We have not attempted to obtain genetic information from authentic material due to its age, but the spore comparison between our collections and those of Vittadini is in complete agreement (Fig. 5l). We also identify with G. morchelliformis var. morchelliformis the two GenBank sequences MN044442 and FN666413, identified as G. graveolens (Rana et al. 2011, Nedelin et al. 2016).
Gautieria morchelliformis var. dubia (E. Fisch.) J.M. Vidal & Merényi, comb. & stat. nov. — MycoBank MB 845108; Fig. 6, 32, 34, 35
Fig. 6.
Gautieria morchelliformis var. dubia. a–b. JMV960713-9. a. Aggregate basidiomata; b. spores in KOH showing the perisporial reticulum. — c. BERN (holotype of G. dubia). Spores in KOH, previously preserved in alcohol. — d. JMV20190806-3. Detail of cristate ridges. — e. JMV961003-3. Tips of paraphysoid cells emerging from hymenium. — f. W 1974-1420. (F. Petrak as G. morchelliformis). Spores in KOH. — g. JMV20141014-11. Detail of locules and branched columella. — h–k. JMV20110920-3. h. Mature basidiomata showing alveolate surface; i. spores in Hoyer; j–k. SEM images of spores. — l. KRA F-2017-28. SEM image of spores. — Scale bars: a, h = 1 cm; b–c, f, i = 10 µm; d, g = 2 mm; e = 20 µm; j–l = 5 µm. — Photos: a–i. J.M. Vidal; j–l. P. Mleczko, UJ.
Basionym. Gautieria dubia E. Fisch., Ber. Schweiz. Bot. Ges. 48: 44. 1938.
Etymology. From Latin, dubius = doubtful, indicating uncertainty about its relationship to similar species.
Type material studied. SWITZERLAND, Neuchâtel, near Neuchâtel, above Le Chanel, Forêt du Sapin, 19 Aug. 1914, C. Quinche, comm. E. Mayor (holo BERN s.n.).
Basidiomata ridged, 1.5–5.5 cm wide, subglobose to irregular or lobate, often consisting of two or more aggregate or coalescing specimens, provided with a basal depression attached to one or more white basal rhizomorphs, 1.5–2 mm thick. Young basidiomata white and granulose as in G. morchelliformis var. morchelliformis. Upon maturity the surface becomes alveolate-reticulate, melon yellow to brownish orange (5A6–6C8), exposing colourful locules, with edge of ridges ornamented with white cristae. Old basidiomata with surface deeply alveolate-reticulate and ridges becoming smooth and partially fertile, turning reddish brown (9D8) with aging or rubbing. Locules 1.5–4.5 × 0.4–2 mm, labyrinthoid to irregularly shaped, sinuous, inordinate, light orange (5A5–A7); cinnamon (6C5–D6) in exsiccata. Tramal plates 0.3–0.6 mm thick, white to greyish, turning pink (13A4) when cut. Columella 1–4 mm thick, dendroid, strongly branched, white to greyish; basal context violet brown (10E7). Columellae of aggregate basidiomata independent or coalescent. Odour at first of aromatic plants, finally very intense, of seafood or alliaceous, like that of G. morchelliformis var. morchelliformis.
Spores (18–)20–24.5 × 12–14.5 μm, Q = 1.5–1.8, elliptical to obovate, maize yellow with greenish hues (4A5–A7) in KOH (Fig. 32c, 34b2). Episporium narrowly ovate to ovate, basally truncate to rounded. Costae 10–12, 1–2 μm high × 1.7–4 µm wide, inflated, straight or helicoidal, entire or with few bifurcations, wrapping up to 1/2 of hilar appendix, ornamented with low gibbosities; apical ring distinct, 0.8–2.2 µm high; perisporial reticulum distinguishable. Hilar appendix 3.5–7 µm long × 4–6 μm wide at apex, conico-truncate; empty-end 0.2–0.4 µm long; hilum 1.7–2.2 µm wide, without remnants of sterigma. Basidia 40–55 × 12–15 µm, clavate, 2–4-spored, guttulate, soon collapsing. Paraphysoid cells short when young, 10–38 × 4–8 μm, 0–1-septate, with the terminal cell cylindrical or subclavate, becoming longer in mature hymenium, 50–100 × 4–8 μm, 0–3-septate, tortuous, difform, diverticulate, often with the terminal cell acute. Subhymenium constituted by chains of cylindrical elements, 5–12 µm diam, becoming subglobose near hymenophoral trama, measuring 14–20 µm diam. Hymenophoral trama formed by loosely interwoven, cylindrical hyphae, 3–8 µm diam, and some ampulliform septa up to 14 µm wide. Thromboplera 3–6 µm diam. Peritrama similar to the hymenophoral trama. Pseudopellis a hymeniderm circumscribed to edge of ridges, formed by clusters of sphaeropedunculate exocystidia, 16–30 μm wide, with peduncle up to 30 µm long, initially hyaline and thin-walled, then yellowish and thick-walled up to 1.5 µm. Calcium oxalate crystals present in tramal tissues and on surface of exocystidia.
Habitat, Season & Distribution — Gregarious, hypogeous or semi-hypogeous under needles, in montane and subalpine coniferous forests (Abies, Picea, Pinus), often accompanied by Fagus, preferably on calcareous soils. From summer to autumn. Common. Distributed in temperate regions, from 500 m altitude in Central Europe (Alps and Carpathians) to 1700 m in South-Western Europe (Pyrenees) (Fig. 35).
Additional material studied. AUSTRIA, Tyrol, Innsbruck, Solsteingebiet, Zirl, Ehnbachtal, July 1920, V. Litschauer as G. graveolens, det. A. Pilát as G. morchelliformis (W 4323, ex Dr. K. v. Keissler); ibid., in coniferous forest, 5 July 1920, V. Litschauer #1027 as G. graveolens (W 419); ibid., in moos cushion, 8 July 1920, V. Litschauer as G. graveolens, det. A. Pilát as G. morchelliformis (S F181891, herb. L. Romell). – CZECH REPUBLIC, Olomouc, Hranice (Mährisch Weisskirchen), Rybař-Thein, under Fagus sylvatica, Sept. 1928, F. Petrak #2474 (W 1420). – POLAND, Lesser Poland, near Czorsztyn, Poręba, Pieniny Nat. Park, 580 m, Abies alba forest, 13 Sept. 2010, P. Chachuła (KRA F-2010-60*, dupl. JMV800692); near Hałuszowa, by the Szkółka, Pieniny Nat. Park, 660 m, Abies-Fagus forest, 21 July 2017, P. Mleczko (KRA F-2017-11/F-2017-16, dupl. JMV800708/800710); near Krościenko nad Dunajcem, by the Bajków Groń, Pieniny Nat. Park, 560 m, Abies-Fagus forest, 25 Aug. 2013, P. Chachuła & R. Rutkowski (KRA F-2013-89/F-2013-91/ F-2013-92, dupl. JMV800697/800699/800700); ibid., Biały Potok, 550 m, Abies-Fagus forest, 23 July 2016, P. Chachuła (KRA F-2017-7, dupl. JMV800707); ibid., 600 m, 1 Oct. 2016, P. Chachuła (KRA F-2016-30*, dupl. JMV800766); ibid., Toporzyskowo, 600 m, Abies-Pinus-Fagus forest, 17 Aug. 2010, M. Kozak & P. Mleczko (KRA F-2010-62, dupl. JMV800694); ibid., 640 m, 25 Sept. 2013, P. Chachuła (KRA F-2013-97, dupl. JMV800703); ibid., Biały Potok Valley, 600 m, 28/30 Aug. 2017, P. Chachuła (KRA F-2017-27*, dupl. JMV800768); near Sromowce Niżne, by Podłaźce glade, Pieniny Nat. Park, 600 m, Abies-Fagus forest, 20 Oct. 2013, P. Chachuła (KRA F-2013-88, dupl. JMV800696); near Sromowce Wyżne, Głęboki Potok Valley, Pieniny Nat. Park, 540 m, Picea-Pinus forest, 10 Oct. 2017, P. Mleczko & P. Komur (KRA F-2017-19, dupl. JMV800712); near Krościenko nad Dunajcem, Łonny Potok Valley, Pieniny Nat. Park, 600 m, Abies-Fagus forest, 4 July 2013, P. Chachuła (KRA F-2013-93*, dupl. JMV800701); near Kościelisko, northern slope of the Hruby Regiel, Tatra Nat. Park, 980 m, Abieti-Piceetum, 15 Oct. 2019, M. Kozak & J. Brańka (TPN-19-0473, dupl. JMV800815); near Kościelisko, Lejowa Valley, Tatra Nat. Park, 960 m, Abieti-Piceetum, on calcareous soil, 25 Aug. 2019, M. Kozak & F. Karpowicz (TPN-19-0073/19-0095, dupl. JMV800797/800798); ibid., 970 m, 25 Aug. 2019, M. Kozak & F. Karpowicz (TPN-19-0096, dupl. JMV800799); ibid., Wielka Sucha Valley, 950 m, 24 Sept. 2019, F. Karpowicz & M. Kozak (TPN-19-0330*, dupl. JMV800810); ibid., 970 m, 24 Sept. 2019, F. Karpowicz & M. Kozak (TPN-19-0321, dupl. JMV800811); ibid., 1000 m, 24 Sept. 2019, F. Karpowicz & M. Kozak (TPN-19-0334, dupl. JMV800812); near Zakopane, Biały Stream Valley, Tatra Nat. Park, 920 m, Abieti-Piceetum, 2 Sept. 2019, F. Karpowicz (TPN-19-0013, dupl. JMV800796); ibid., Suchy Żleb, Tatra Nat. Park, 960 m, Abies-Picea-Fagus forest, 15 Aug. 2019, M. Kozak (TPN-19-0147, dupl. JMV800803); ibid., Strążyska Valley, 950 m, Abies-Fagus forest, 11 Aug. 2019, M. Kozak (TPN-19-0212, dupl. JMV800806); near Jaworki, Popradzki Landscape Park, Beskid Sądecki Mountains, 800 m, Abies-Fagus forest, 17 Sept. 2017, P. Chachuła (KRA F-2017-28*, dupl. JMV800769); near Koninki, Turbacz Stream valley, Gorce Nat. Park, 700 m, Abieti-Piceetum, 12 July 2013, M. Kozak, P. Mleczko & K. Kozak (KRA F-2013-1*, dupl. JMV800755); near Wołowiec, Magura Nat. Park, Beskid Niski Mountains, 520 m, Abies-Fagus forest, 4 Aug. 2014, P. Chachuła (KRA F-2014-168*, dupl. JMV800794). – SPAIN, Catalonia, Girona, Alp, Saltèguet Forest, Eastern Pyrenees, 1600 m, under Abies alba and Pinus uncinata, on schistose soil, 24 Aug. 1996, J.M. Vidal as G. morchelliformis (JMV960824-2*); ibid., 1700 m, under Abies alba, 20 Sept. 2011, J.M. Vidal & F. Rodríguez (JMV20110920-3*); Girona, Campelles, Baga de Campelles Forest, Eastern Pyrenees, 1500–1600 m, under Abies alba, Pinus uncinata and Pinus sylvestris, on calcareous soil, 13 July 1996, J.M. Vidal as G. morchelliformis (JMV960713-9*); ibid., 3 Oct. 1996, J.M. Vidal as G. morchelliformis (JMV961003-3*); ibid., 2 Aug. 2004, F. Rodríguez (JMV20040802); ibid., 23 Sept. 2008, F. Rodríguez (JMV20080923-1/20080923-2); ibid., 14 Oct. 2008, J.M. Vidal & F. Rodríguez (JMV20081014-1*/20081014-2); ibid., 24 Nov. 2009, F. Rodríguez (JMV20091124-2); ibid., 5 June 2013, F. Rodríguez (JMV20130605-2); ibid., 14 Oct. 2014, J.M. Vidal & F. Rodríguez (JMV20141014-13/20141014-22); ibid., 24 Oct. 2017, F. Rodríguez (JMV20171024-1); ibid., 27 June 2018, J.M. Vidal, A. Paz & C. Lavoise (JMV20180627-1); ibid., 6 Aug. 2019, J.M. Vidal, A. Paz & C. Lavoise (JMV20190806-3); Girona, Planoles, L’Avetar Forest, Eastern Pyrenees, 1600 m, under Abies alba and Pinus uncinata, on schistose soil, 31 Aug. 2002, J.M. Vidal (JMV20020831-1*); ibid., 14 Oct. 2014, J.M. Vidal & F. Rodríguez (JMV20141014-10/20141014-11); Lleida, Esterri d’Àneu, Mata de València Forest, Central Pyrenees, 1550 m, under Abies alba, on schistose soil, 5 Sept. 1997, J.M. Vidal & J. Vila (JMV970905-13*). – SWITZERLAND, Basel-Landschaft, Arlesheim, near Pfeffingen, SW Blauenberg, under Picea abies, near grasslands, 27 Sept. 1966, C. Schwärzel #78 as G. otthii (ZT-Myc 3933).
Notes — In 1914, the mycologist E. Fischer received from Dr. E. Mayor a Gautieria found in the Alps in a fir forest near Neuchâtel (Switzerland), which he determined as ‘G. cf. graveolens’ (Fischer 1916). Later Fischer (1938) described it as a new species and assigned the epithet ‘dubia’, suggesting his continued doubt about its taxonomic placement. No mention was made of this taxon until the brief description by Pilát (1958), who, without seeing the holotype, believed that it was probably an abnormal stage of G. otthii, assuming that the preservative affected the dimensions of the spores. Vidal (1997) found this Gautieria in Spain and described it under the name G. morchelliformis. Although the Bernese holotype is preserved in alcohol, causing a greyish black coloration of the hymenophore and a reddish coloration of the spores, the size and shape of the microscopic structures remain essentially unchanged and allowed us to identify it with recent collections. Also, we have compared the basidiomata of our collections of G. dubia with those of G. morchelliformis var. morchelliformis and we have not observed any important differences, except in the melon yellow colour of locules of G. dubia, brighter than the greyish orange colour of G. morchelliformis var. morchelliformis. The shape of the spores is somewhat different in typical collections of G. dubia, as they usually have a prominent hilar appendix up to 1/4 of spore length (Fig. 6i). Regarding its ecology, the difference is more notable, because while G. morchelliformis var. morchelliformis is found inhabiting from Mediterranean to temperate broadleaf forests, G. dubia is closely linked to the montane and subalpine coniferous forests of Abies and Picea.
From this taxon we have studied 51 collections from 4 countries, of which we have successfully sequenced 15 collections (Table 1) and represented 6 sequences in our phylogenetic tree (Fig. 3). We have not tried to obtain genetic information from the holotype due to its age and conservation in alcohol, but the comparison of spores between our collections and that one is in perfect agreement (Fig. 6c). The morphological and ecological differences observed, which are supported by our genetic studies, lead us to consider G. dubia a variety of G. morchelliformis. Two genetically distinct populations can be distinguished in this taxon: a North-Eastern population located in the Carpathians, which has spores with intermediaries of G. morchelliformis var. fageticola, and a South-Western population located in the Pyrenees, which has more uniform spores and is consistent with the holotype of G. morchelliformis var. dubia found in the Alps.
Gautieria morchelliformis var. fageticola J.M. Vidal, Bratek, Konstantin., Slavova & Tsilis, var. nov. — MycoBank MB 845111; Fig. 7, 32, 34, 35
Fig. 7.
Gautieria morchelliformis var. fageticola. a–d. GK1653 (BCN JMV800558, holotype). a. Section of aggregate basidiomata; b. detail of crisped surface; c. pseudoparenchymatous pseudopellis of collapsed exocystidia embedded in orange pigment; d. spores in KOH. — e. GK11859. Two coalescing basidiomata. — f–g. SOMF 30340. f. Section of a ridge showing pseudopellis, peritrama and adjacent fertile hymenium; g. paraphysoid cells of mature hymenium. — h–i. SOMF 30314. h. Section of a basidioma with coalescing columellae; i. spores in KOH. — j. M 126232 (E. Soehner as G. graveolens). Basidia and paraphysoid cells of immature hymenium. — k. ZB1237. Spores in KOH. — l. ZB1997. SEM image of a spore. — Scale bars: a–b, e, h = 1 cm; c, f–g, j = 20 µm; d, i, k = 10 µm; l = 5 µm. — Photos: a–b. A. Papatsanis; c–d, f–g, i–k. J.M. Vidal; e. N. Tsilis; h. M. Slavova; l. P. Mleczko, UJ.
Etymology. From Latin, fagetum = beech-forest + -colus (from incola) = inhabitant of, for being mainly found in forests of Fagus sylvatica.
Type. GREECE, West Macedonia, Kastoria, Arrenes, Pefkofito, 1740 m, under Fagus sylvatica, 8 Aug. 2002, G. Konstantinidis (holo BCN JMV800558*; iso in herb. pers. G. Konstantinidis GK1653 and in ELTE).
Basidiomata ridged, 1.5–6 cm wide, subglobose to irregular or lobate, usually consisting of two or more aggregate or coalescing specimens, provided with a basal depression attached to one or more white basal rhizomorphs, 1.5–2 mm thick. Surface of young basidiomata white or yellowish, granulose, as in G. morchelliformis var. morchelliformis. Mature basidiomata with the surface crisped, flabellate, light orange to greyish orange (5A4–6B6), exposing colourful locules, with compressed, interwoven, sinuous, white cristate ridges, becoming reddish brown (9D8) with rubbing. Locules 2–6 × 0.6–2 mm, labyrinthoid, sinuous, somewhat angular, more or less radially arranged and elongated, largest near columella, light orange (5A4–6A5); cinnamon (6C6–D6) in exsiccata. Tramal plates 0.3–0.6 mm thick, greyish, changing to pinkish when cut. Columella 1.2–4.5 mm thick, dendroid, strongly branched, white to grey, turning greyish red (10C4) when exposed to air; basal context reddish brown (9D5). Columellae of aggregate basidiomata independent or coalescent. Odour initially pleasant, fungal, finally of chlorine or alliaceous.
Spores (15–)17–21(–22.5) × (9.5–)10–12(–13) μm, Q = 1.5–1.9, variable in size and shape, elliptical to narrowly elliptical, chrome yellow with greenish hues (3A7–A8) in KOH (Fig. 32d, 34b3). Episporium elliptical, basally truncate. Costae 9–11, 1–1.8 μm high × 2–4 µm wide, moderately inflated, straight or sometimes helicoidal, entire or with few bifurcations, wrapping 1/2 of hilar appendix, smooth or ornamented with low gibbosities; apical ring distinct, 1–1.8 µm high; perisporial reticulum almost indistinguishable. Hilar appendix 1.8–3.8 µm long × 4.3–6 μm wide at apex, very variable in shape, usually short and rounded but in some collections long and truncate; empty-end 0.3–0.6 µm long; hilum 1.8–2.4 µm wide, often with remnants of sterigma. Basidia 45–52 × 12–14 µm, clavate, 2–4-spored, filled with oily guttules and yellow pigment, soon collapsing and emptying the contents, agglutinating the spores on the surface of the hymenium. Paraphysoid cells 20–50 × 3–8 µm, 0–3-septate, straight to sinuous, sometimes furcate, with the terminal cell clavate or attenuate, becoming long hairs up to 120 µm in mature hymenium. Subhymenium consisting of chains of elements from cylindrical to subglobose, 12–20 µm diam. Hymenophoral trama of cylindrical, hyaline, loosely interwoven hyphae, 3–6 µm diam. Thromboplera 4–6 μm diam. Peritrama of ridges prosenchymatous, consisting of densely interwoven yellowish hyphae, 3–6 µm diam, with abundant yellow pigment. Pseudopellis a hymeniderm circumscribed to edge of ridges, formed by clusters of sphaeropedunculate exocystidia, 15–30 μm wide, often catenulate, initially hyaline and thin-walled, then yellow with a thickened wall, finally collapsed and embedded in orange pigment, with a pseudoparenchymatous appearance.
Habitat, Season & Distribution — Gregarious, hypogeous or semi-hypogeous under plant debris, in montane broadleaf forests (Carpinus, Fagus), preferably on calcareous soils. Almost throughout the year. Rare. Distributed in temperate regions of Central and South-Eastern Europe between 200–1750 m of altitude (Fig. 35).
Additional material studied. BULGARIA, Kyustendil, Osogovo Mountains, 1570 m, under Fagus sylvatica, on siliceous soil, 25 July 2018, M. Slavova (SOMF 30314*, dupl. JMV800748); ibid., 23 Oct. 2018, M. Slavova (SOMF 30315*/30340*/30341*, dupl. JMV800775/800776/800777); Pazardzik, Zigov Chark, Western Rhodope Mountains, 1100 m, under Fagus sylvatica and Abies alba, on siliceous soil, 31 Oct. 2018, A. Pishinkova (SOMF 30316*, dupl. JMV800778). – CZECH REPUBLIC, Pardubice, Květná, 1930, Tomášek, det. A. Pilát as ‘G. morchelliformis’ (W 1783, ex PRM; PC 92302, ex PRM). – GERMANY, Bavaria, Pfaffenhausen bei Mindelheim, in deciduous forest, Aug. 1928, E. Soehner #1079 as G. graveolens (M 126232*); Schliersee, Bodenschneid, Aug. 1898, C.O. Harz, det. Ade as ‘G. graveolens’ (M 126227, herb. E. Soehner #1190). – GREECE, West Macedonia, Florina, Oxia, under Fagus sylvatica, 20 Oct. 2018, N. Tsilis (GK11859*, dupl. JMV800818); ibid., 1000 m, under Carpinus betulus and Corylus avellana, 16 Oct. 2020, N. Tsilis (GK13105). – HUNGARY, Borsod-Abaúj-Zemplén, near Parasznya, Bükk Mountains, c. 250 m, in Carpinus betulus forest, Feb. 2002, K. Kemenesiné (ZB2482*, dupl. JMV800427); Heves, Gyalogút-bérc, Mátra Mountains, c. 400 m, in Luzulo nemorosae-Fagetum forest, 3 Aug. 1997, L. Albert (ZB1237*, dupl. JMV800397); Veszprém, near Eplény, Malom Valley, Bakony Mountains, c. 400 m, under Fagus sylvatica, 26 Feb. 1997, V. Erdei (ZB1997*, dupl. JMV800415). – ROMANIA, Cluj, Cluj-Napoca (Kolozsvár), near Szentjánoskút, Fáget Forest, c. 450 m, under Fagus sylvatica, 15 Aug. 1984, Z. Tőkés (Páz84/76, dupl. JMV800390); Harghita, near Cristuru Secuiesc (Székelykeresztúr), Zilad?, c. 450 m, under Carpinus betulus, 14 Aug. 1982, G. Pap (Pap179, dupl. JMV800387); ibid., Sóskút Forest, c. 450 m, under Carpinus betulus, 14 Aug. 1982, G. Pap 82/14 (ZB3547*, dupl. JMV800442); ibid., Dec. 1996, B. Pálfy (ZB1176, dupl. JMV800396); Rugonfalva (Rugăneşti), c. 600 m, 6 Sept. 1974, G. Pap (Mis74/1, dupl. JMV800384); Harghita-Covasna, sine loc., 1997, B. Pálfy (ZB2777*, dupl. JMV800405a); sine loc., 1997, B. Pálfy (ZB2779*).
Notes — Gautieria morchelliformis var. fageticola differs from G. morchelliformis var. morchelliformis by having: 1) crispulate ridges giving the basidiomata an appearance of a small Sparassis; 2) strongly branched and often coalescing columellae; and 3) smaller spores of a vivid yellow colour, in most collections presenting a short hilar appendix. From this taxon we have studied 21 collections from 6 countries, of which we have successfully sequenced 14 collections (Table 1) and represented 8 sequences in our phylogenetic tree (Fig. 3).
It is genetically very close to G. morchelliformis var. dubia and presents a great genetic diversity that is reflected in the diverse shape and size of the spores, as can be seen in the three illustrated spore collections (Fig. 7d, i, k). Collections with a predominance of spores with a long hilar appendix may be difficult to distinguish from G. morchelliformis var. dubia if their ecology and basidiomata morphology are unknown. While G. morchelliformis var. dubia is found in subalpine coniferous forests, G. morchelliformis var. fageticola occurs in the montane forests of Fagus and Carpinus of Central and South-Eastern Europe. In the coniferous forests of southern Poland that are abundant in Fagus, it is common to find specimens whose spores are typical of G. morchelliformis var. fageticola but which are genetically identified as G. morchelliformis var. dubia. The type collection (GK1653) was previously illustrated as G. morchelliformis by Konstantinidis (2006).
Gautieria morchelliformis var. intermedia J.M. Vidal & A. Paz, var. nov. — MycoBank MB 845112; Fig. 8, 32, 34, 35
Fig. 8.
Gautieria morchelliformis var. intermedia. a–i. JMV20180726-3 (BCN, holotype). a. Basidiomata showing deeply alveolate and coralloid surface; b. wine red maculae on bruised surface; c. hymenophore showing light orange locules and reddening of tramal plates; d. detail of cristae; e. clusters of exocystidia; f. paraphysoid cells of mature hymenium; g. spores in KOH, showing the internal perisporial reticulum; h–i. SEM images of spores. — j–l. JMV20021110-1. j. Paraphysoid cells of immature hymenium; k. transformation of paraphysoid cells of an exposed locule into hairs; l. tuft of hairs originating from paraphysoid cells. — Scale bars: a = 1 cm; b–c = 5 mm; d = 1 mm; e–f, j–l = 20 µm; g = 10 µm; h–i = 5 µm. — Photos: a–g, j–l. J.M. Vidal; h–i. UdG.
Etymology. From Latin, intermedius = intermediate, for its intermediate features between Gautieria morchelliformis var. morchelliformis and G. macrocoilia.
Type. SPAIN, Catalonia, Girona, La Cellera de Ter, Plantadís Forest, 780 m, under Castanea sativa and Quercus ilex, in the vicinity of Octaviania depauperata, on schistose soil, 26 July 2018, J.M. Vidal, A. Paz & C. Lavoise (holo BCN JMV20180726-3*; iso in ELTE).
Basidiomata ridged, 2–3.2 cm wide, subglobose to irregular, with a basal depression attached to a white basal rhizomorph, 1–1.5 mm thick, along with small strands and abundant mycelium. Surface of young basidiomata white and granulose as in G. morchelliformis var. morchelliformis. Mature basidiomata alveolate, exposing colourful locules, with thick ridges provided with a dentate or eroded edge ornamented with pronounced white cristae. In old basidiomata, the cristae collapse and the ridges become smooth and rounded, being the surface deeply alveolate with the same colour as the locules, turning wine red (11D8) in bruises or when handled. Locules large, 1.5–5 × 0.7–1.4 mm, labyrinthoid to irregularly shaped, somewhat angular, light orange (5A5–6A5); cinnamon (6C6–D6) in exsiccata. Tramal plates 0.4–0.7 mm thick, greyish, reddening to greyish red (11C6) when cut. Columella 1.5–3 mm thick, basal, undeveloped, greyish; basal context greyish red (11D5). Odour pleasant, of aromatic plants, similar to that of G. morchelliformis var. morchelliformis.
Spores 19–24 × 14.5–16.5 μm, Q = 1.3–1.5, broadly elliptical to obovate, maize yellow (4A5–A6) in KOH (Fig. 32e, 34b4). Episporium ovate, basally truncate. Costae 10–12, 1.5–3 μm high × 2–5 µm wide, very inflated, straight to helicoidal, entire or with few bifurcations, wrapping up to 3/4 of hilar appendix, smooth to gibbose; apical ring distinct, 1.5–3.5 μm high; perisporial reticulum distinguishable, yellow-orange. Hilar appendix 3–5 µm long × 5.5–7 μm wide at apex, conico-truncate to rounded; empty-end 0.2–0.4 µm long; hilum 2–2.7 µm wide, without remnants of sterigma. Basidia 60–80 × 8–14 µm, cylindric to clavate, 2–4-spored, with yellow content and guttules, which soon collapse into a yellow granular mass that agglutinates the spores. Paraphysoid cells 25–50 × 3–4.5 μm, 0–3-septate, straight to sinuous, with the terminal cell clavate or attenuate, becoming long hairs up to 150 × 9 µm in mature hymenium, with some furcations and anastomoses. Observed the transformation of paraphysoid cells into long hairs in the exposed locules at the base of the basidiomata and their aggregation into isolated tufts of thick-walled yellow hairs. Subhymenium consisting of chains of cylindrical to globose elements, 8–20 µm diam, with large oily guttules. Hymenophoral trama formed by interwoven hyphae, 1.5–4 µm diam, with some ampulliform septa and endocystidia-like terminal vesicles up to 12 µm wide. Thromboplera 4–7 μm diam. Peritrama of ridges prosenchymatous, consisting of densely interwoven hyphae, 2–4 µm diam, with abundant yellow pigment. Pseudopellis a hymeniderm circumscribed to edge of ridges, formed by clusters of globose to subglobose or sphaeropedunculate exocystidia, 13–25 µm wide, long pedunculated, with a wall 1–1.5 µm thick, some of them constricted, papillate, with abundant incrustations of calcium oxalate.
Habitat, Season & Distribution — Hypogeous or semi-hypogeous under plant debris, in montane broadleaf forests (Castanea, Corylus, Quercus), preferably on siliceous soils. From spring to autumn. Very rare and localized. Mediterranean species, found in Northern Spain in Catalonia (Fig. 35).
Additional material studied. SPAIN, Catalonia, La Cellera de Ter, Plantadís Forest, Les Guilleries Massif, 700 m, under Castanea sativa, with Quercus ilex and Corylus avellana, on schistose soil, 10 Nov. 2002, J.M. Vidal & F. Rodríguez (JMV20021110-1*).
Notes — Gautieria morchelliformis var. intermedia is characterised by having: 1) strongly cristate ridges; 2) thick tramal plates; 3) large locules of light orange colour; and 4) spores virtually identical in size and shape to those of G. macrocoilia, but with a truncate episporium. Given its morphological characteristics, we consider G. morchelliformis var. intermedia a Mediterranean taxon with intermediate features between those of G. morchelliformis var. morchelliformis and G. macrocoilia. Thus, due to its thick ridges and tramal plates and light orange locules, it would be more related to G. morchelliformis var. morchelliformis but, on the other hand, due to its large locules and spore shape, it would be more related to G. macrocoilia. Interesting is the finding of tufts of hairs at the base of the basidioma near the insertion of the basal rhizomorph (Fig. 8k–l). We have observed that these hairs have their origin in the growth of the paraphysoid cells that line the exposed locules and that they tend to grow in small tufts of identical morphology to those that originate the trichotomentocutis that covers the surface of the pseudoperidium of tomentose species. This predisposition to develop tufts of hairs and a subsequent trichotomentocutis has been observed particularly in Mediterranean species of different clades, which seems to indicate a climatic evolutionary trend within the genus Gautieria. From G. morchelliformis var. intermedia we only know the two sequenced Catalan collections (Fig. 3), both found in the same locality.
Clade villosa (GauGau-2)
Ridges alveolate-reticulate. Trichotomentocutis absent, but sometimes an incipient trichoderm may be observed. Spores variable in shape, elliptico-fusiform, broadly elliptical, obovate or pyriform, reaching 19–22.5 × 12–14 µm. Costae very inflated, ornamented with broad gibbosities. Linked to conifers (Abies, Larix, Picea, Pinus). Represented by G. villosa var. villosa, G. villosa var. inflata, G. villosa var. pilifera and the Chinese G. xinjiangensis corresponding to the sequence NR153450 (= JX860192).
Gautieria villosa Quél., Bull. Soc. Bot. France 25: 290, 1878, var. villosa — MycoBank MB 845149; Fig. 9, 32, 34, 36
Fig. 36.
Distribution map of species of Gautieria sect. Gautieria clades villosa, convoluta, subglobispora and hellenica (GauGau-2, 3, 4, 6).
Synonym. Gautieria retirugosa Th. Fr., Svensk Bot. Tidskr. 3: 271. 1909, syn. nov.
Etymology. From Latin, villus = hair, for Quélet regarding the hymenium of this species as velvety.
Type. SWITZERLAND, Neuchâtel, Corcelles, 9 Sept. 1877, P. Morthier (S F181882, ex herb. Sydow, lectotypus, hic designatus, MBT 10008522).
Basidiomata ridged, 1.5–6.5 cm wide, subglobose to lobate, provided with a basal cavity attached to a white basal rhizomorph, 1.5–2 mm thick. Surface white for a long time, initially granulose to rugose as in G. morchelliformis, then light orange (5A6), convoluted to compactly alveolate-reticulate, consisting of dense, sinuous, intricate ridges, ornamented with white cristae that surround small, narrow, irregular, colourful exposed locules. Ridges reddening to pale red or pink (12A3–A4) to greyish ruby (12D7) on handling. Locules 1.2–2.5 × 0.6–1.2 mm, labyrinthoid to irregularly rounded, inordinate, butter yellow to maize yellow (4A5–A6); brownish orange (6C6–C7) in exsiccata. Tramal plates 0.2–0.4 mm thick, greyish, becoming pink (12A4) with exposure to air. Columella 1.5–3 mm thick, dendroid, strongly branched, greyish; basal context greyish ruby (12E5). Odour at first pleasant, fruity or of aromatic plants, finally very intense, of seafood, similar to that of G. morchelliformis.
Spores (16.5–)18–22.5(–23) × 10–13 µm, Q = 1.5–2, elliptico-fusiform to obovate, light yellow with greenish hues (3A4–A5) in KOH (Fig. 32f, 34c1). Episporium narrowly ovate to subfusiform, often subpapillate. Costae 10–13, 1–2 µm high × 1.5–3.2 µm wide, moderately inflated, straight or sometimes helicoidal, entire, furcate or anastomosing, covering up to 1/2 of hilar appendix, ornamented with large gibbosities at bifurcations; apical ring distinct, 1.4–2 µm high; perisporial reticulum not always distinguishable. Hilar appendix 2.5–4 µm long × 3–4.5 µm wide at apex, conico-truncate; empty-end 0.2–0.6 µm long; hilum 1.2–1.9 µm wide, without remnants of sterigma. Basidia 45–70 × 7–15 µm, cylindrical to clavate, sinuous, 2–4-spored, with yellow granular content. Sclerobasidia present. Paraphysoid cells 14–35 × 3–9 μm, 0–1-septate, with the terminal cell cylindrical, clavate or attenuate. Subhymenium formed by chains of cylindrical to inflated elements, 8–14 µm wide. Hymenophoral trama consisting of interwoven hyphae 2–4 µm diam, with some ampulliform septa and endocystidia-like terminal vesicles, 8–12 µm wide. Thromboplera 2–7 µm diam. Peritrama of ridges similar to hymenophoral trama. Pseudopellis pseudoparenchymatous circumscribed to edge of ridges, formed by several layers of inflated cells, 10–20 µm diam, and pedicellate exocystidia, 20–40 µm wide, yellow, soon collapsed and gradually embedded in a yellow matrix.
Habitat, Season & Distribution — Solitary or gregarious, hypogeous or semi-hypogeous under needles, in montane and subalpine coniferous forests (Abies, Picea, Pinus), on calcareous or siliceous soils. From summer to autumn. Common. Distributed in temperate regions, from sea level in Northern Europe to 1750 m altitude in Southern Europe (Fig. 36).
Additional material studied. AUSTRIA, Carinthia, Sankt Veit an der Glan, Heft, 9 Oct. 1985, M. Moser #85/254a as G. morchelliformis (IB 1985254a*); Salzburg, Wagrain, trail to Ginau, in coniferous forest, 3 Aug. 1934, H. Lohwag as G. morchelliformis (W 418); Tyrol, sine dat., F. Lorinser, det. R. Raimann as G. graveolens, det. A. Pilát as ‘G. morchelliformis specimen atypical, smaller spores’ (W 5079); Vorarlberg, sine dat., G. Bresadola? as G. morchelliformis (S F181873); near Bludenz, Furkla Forest, ‘Fichtenwald’, 29 Aug. 1960, E. Horak #60/236 as G. morchelliformis (ZT-Myc 3945). – BULGARIA, Sofia, Ovnarsko, Malyovitsa area, Rila Mountains, 1220 m, under Picea abies and Pinus sylvestris, on siliceous soil, 5 Aug. 2018, M. Slavova (SOMF 30318*, dupl. JMV800750). – CZECH REPUBLIC, South Bohemia, Jindřichův Hradec, VII1919, Neuwirth, det. A. Pilát as G. morchelliformis (PC 92301, ex PRM); Lavička in Zvíkov, ‘inter Sambucus ebulus!’, 10 Aug. 1955, M. Svrček, det. A. Pilát as G. morchelliformis (PRM 678953); Tábor, ‘in silva ad flumen Lužnice’, 30 Aug. 1904, F. Bubák as G. morchelliformis (BPI 711872, herb. C.G. Lloyd #46533); ibid., ‘in silva ad Lužnice flumen’, Aug. 1904, F. Bubák #2589 as G. morchelliformis (FH 301423, herb. F. v. Höhnel); ibid., ‘in silva ad Lužnice’, sine dat., F. Bubák as G. morchelliformis (BPI 602474). – FRANCE, Franche-Comté, Jura, Abbévillers, sine dat., L. Quélet misit as ‘G. morillaeformis’ (UPS F-550644); Rhône-Alpes, from the Jura, sine dat., N. Patouillard as G. morchelliformis (BPI 711876, herb. C.G. Lloyd #46535, #08+53, pro parte, specimen that is part of Lloyd’s collection, identified here as G. macrocoilia). – GERMANY, Bavaria, Schäftlarn, under Fagus sylvatica,Aug. 1962, Dr. Dreher as G. graveolens (M 126240*); Starnberg, Wartameil, south of Herrsching, 540 m, garden with ‘Fichten’ and deciduous trees, 27 Jan. 2008, E. Facher, det. S. Raidl SR1371 as G. graveolens (M 157765*). – ITALY, Lombardy, Bergamo, Gromo, under Picea abies, 18 Sept. 1999, M. Sarasini as G. graveolens (MS1006*, dupl. JMV800183); Trentino-Alto Adige, Bolzano, Costantino, Alpe di Siusi, 800 m, under Picea abies, 28 Sept. 1985, A. Montecchi as G. otthii, comm. L. Gori (ELG850928, dupl. JMV800357); Trento, Levico, Forte Cima Verle, Passo di Vezzena, ‘Fichtenwald’, 26 May 1979, W. Mair, det. M. Moser #79/29 as G. morchelliformis (IB 19790029*); Trento, Mendola, ‘in silvis abiegnis’, Aug. 1902, G. Bresadola #644 as G. morchelliformis (M 126259, herb. S. Killermann); ibid., Aug. 1922, G. Bresadola as G. morchelliformis (BPI 602614). – LATVIA, Jūrmala, Kemeri Nat. Park (Kemmern), Sept. 1901, F. Bucholtz as G. morchelliformis, Fungi ross. coll. F. Bucholtz, det. A. Pilát as G. morchelliformis var. globispora (S F181883, ex herb. Sydow). – POLAND, Lesser Poland, near Hałuszowa, Lasek, Pieniny Nat. Park, 625 m, Abies-Fagus forest, 29 Aug. 2012, P. Chachuła (KRA F-2012-152*, dupl. JMV800695); near Zakopane, Boczań Mount, Tatra Nat. Park, 1250 m, Abieti-Piceetum, 1 Aug. 2009, M. Kozak (KRA F-2009-58*, dupl. JMV800752); near Zakopane, Spadowiec Valley, Tatra Nat. Park, 920 m, Fagus-Abies-Picea forest, 21 Aug. 2012, M. Kozak (KRA F-2012-3*, dupl. JMV800754); near Małe Ciche, Suchej Wody Valley, Tatra Nat. Park, 960 m, Abieti-Piceetum, 25 Sept. 2019, F. Karpowicz & M. Kozak (TPN-19-0320, dupl. JMV800809). – SPAIN, Aragon, Huesca, Jaca, Valle de Hecho, Central Pyrenees, under Fagus sylvatica, 25 Oct. 1999, D. Mitchell #NA-26, det. J. Trappe #25905 as G. graveolens (OSC 63447); Catalonia, Girona, Campelles, Pla dels Prats, Eastern Pyrenees, 1540 m, under Abies alba, Pinus sylvestris and Pinus uncinata, on calcareous soil, 5 June 2013, F. Rodríguez (JMV20130605-1); ibid., 14 Oct. 2014, J.M. Vidal & F. Rodríguez (JMV20141014-26*/20141014-28); Girona, Planoles, L’Avetar Forest, Eastern Pyrenees, 1650 m, under Abies alba and Pinus uncinata, on schistose soil, 25 Aug. 2002, J.M. Vidal & F. Rodríguez (JMV20020825-1*); ibid., 23 Sept. 2008, F. Rodríguez (JMV20080923-5*); ibid., 22 Sept. 2009, J.M. Vidal & F. Rodríguez (JMV20090922-3*); ibid., 14 Oct. 2014, J.M. Vidal & F. Rodríguez (JMV20141014-12); Lleida, Abella de la Conca, Coll de Bóixols, Central Pyrenees, 1200–1300 m, under Abies alba, 12 Oct. 1974, M. Sánchez-Montoya, det. M. Lawrynowicz as G. morchelliformis (MA-Fungi 2754*); Lleida, Espot, Aigüestortes i Estany de Sant Maurici Nat. Park, Central Pyrenees, 1750 m, under Abies alba, on siliceous soil, 5 Sept. 1997, J.M. Vidal & J. Vila (JMV970905-8*). – SWEDEN, Gotland, Arebäck, Suderbys, 28 Sept. 1950, E.Th. Fries as G. retirugosa (UPS F-550626*; O 152360, ex UPS); Vallstena, Alvena Park, under conifers, Aug. 1897, T. Vestergren (holo UPS F-148988, of G. retirugosa; FH 301445, herb. C.W. Dodge, iso). – SWITZERLAND, Grisons, Albula, Lenzerheide, above Igl Lai, 1500 m, ‘Fichtenwald’, 16 Sept. 2002, M. Kurs, det. E. Horak & P.-A. Moreau as G. graveolens (ZT-Myc 3949); Neuchâtel, Neuchâtel, 28 Nov. 1971, G. Schleiber & N. Suber as G. morchelliformis (UPS F-550645*); Solothurn, Gösgen, near Niedererlinsbach, under Abies alba, Aug. 1968, Busri, det. C. Schwärzel #51 as G. otthii (ZT-Myc 3929).
Notes — Gautieria villosa was briefly described and illustrated by Quélet (1878) from a collection made by the Swiss botanist Paul Morthier in the Jura region, and from another collection by the German botanist Johannes Kunze in the region of Thuringia. The epithet ‘villosa’ was applied by Quélet to this taxon after observing the hymenium under a binocular lens and interpreting the accumulation of spores on its surface as hairs. In our research on the different herbaria, we have only been able to trace a Swiss collection kept at the Sydow herbarium in Stockholm (S F181882) with the annotation ‘Gautieria villosa Quél., Corcelles pr. Neuchâtel, 9.9.1877, leg. P. Morthier, redivit as G. morchelliformis by Dr. A. Pilát, July 29, 1955’, which we select as lectotype. Gautieria villosa was an unrecognized taxon, considered synonymous with G. morchelliformis by Winter (1884) and later authors (Hollós 1911, Patouillard 1914, Zeller & Dodge 1918, Bataille 1923, Dodge & Zeller 1934, Pilát 1958, Jülich 1984, Pegler et al. 1993). Bucholtz found this Gautieria in Latvia (S F181883) and published it first under the name of G. graveolens (Bucholtz 1901) and later as G. morchelliformis (Bucholtz 1902, 1903). The subsequent collections of G. villosa made by Lucien Quélet in the French Jura region, preserved in FH, PC and UPS, have been identified by us as belonging to G. morchelliformis var. morchelliformis. Nor do we consider correct Quélet’s identification of the collections made by Giacomo Bresadola in the Italian Tyrol (Roumeguère 1882), which we have identified as belonging to G. macrocoilia. Instead, we identify as G. villosa var. villosa the collection made in Abbévillers and published by Quélet (1886) as ‘G. morillaeformis Vitt.’. The epithet ‘morillaeformis’ is here considered a wrong spelling for ‘morchellaeformis’ due to a Quélet’s lapsus calami. Thus, in most of the public herbaria consulted, G. villosa var. villosa has been determined as G. morchelliformis by sharing the ridged morphology of basidiomata, but G. villosa var. villosa has some differentiating macroscopic and microscopic characteristics, consisting of: 1) densely packed ridges; 2) pink oxidation of ridges and tramal plates with exposure to air; 3) yellowish orange locules; and 4) small elliptico-fusiform to obovate spores with a narrow hilar appendix. Gautieria villosa var. villosa occurs in montane and subalpine coniferous forests, often accompanied by G. convoluta and G. morchelliformis var. dubia.
A Gautieria with the same microscopy is G. retirugosa. This Gautieria was found under conifers in 1897 by the Swedish botanist Tycho Vestergren on the island of Gotland and described by Theodor Magnus Fries in a special publication focusing on Scandinavian truffles and truffle-like fungi (Fries 1909). The type collection (UPS F-148988) was preserved in alcohol and the perisporial wall was affected, making the inflated perisporium and the internal walls difficult to observe. A few years later, Thore Christian Elias Fries illustrated the type specimens and described them again (Fries 1922), and in 1950 new specimens of this Gautieria were found by E.Th. Fries near the type locality, keeping them as exsiccata (UPS F-550626), thus allowing us to observe the perisporium and spore walls and obtain DNA information, matching this genetic information with those of our collections (Fig. 3). Likewise, the comparative studies of the spores of the holotype of G. retirugosa and those of the later finding with those of the lectotype of G. villosa var. villosa show a complete coincidence in the shape and size of the spores, which is why we consider G. retirugosa conspecific with G. villosa var. villosa (Fig. 9h–j).
From this taxon we have studied 41 collections from 11 countries, from which we have successfully sequenced 17 collections (Table 1) and represented 5 sequences in our phylogenetic tree (Fig. 3), including the Swedish collection UPS F550626 of G. retirugosa. We have not tried to obtain genetic information from the lectotype of G. villosa var. villosa due to its age and condition, but the comparison of spores between our collections and that one is in perfect agreement (Fig. 9h). We also identify as G. villosa var. villosa the GenBank sequence AF377066 identified originally as G. morchelliformis (Bidartondo & Bruns 2002). Gautieria xinjiangensis, represented in our phylogenetic tree by the sequence NR153450 (= JX860192) (Fig. 3), could be an Asian variety considering that it is inside the clade. This collection was found in the Tianshan Mountains (Xinjiang, northwest China), growing semi-hypogeous under Picea schrenkiana. Bau & Liu (2013) describe it with clavate basidia of 20–30 × 7–10 µm, and with broadly elliptical to orbi-cular-ovate spores of (13–)15–19(–21) × (8–)8.5–10(–11) µm, Q = 1.8–2, provided with 9–11 costae and a hilar appendix of 2–3 × 1.5–2 µm. Macromorphologically it is similar to G. villosa var. villosa, but differs microscopically by its smaller basidia and its smaller elliptical spores provided with a narrower hilar appendix. Further Chinese collections are required to better determine relationships.
Gautieria villosa var. inflata J.M. Vidal, Fern. Rodr., Mleczko, Kozak & Slavova, var. nov. — MycoBank MB 845113; Fig. 10, 32, 34, 36
Fig. 10.
Gautieria villosa var. inflata. a–c. JMV20081021-5 (BCN, holotype). a–b. Basidiomata showing rugose surface; b. basidioma showing alveolate surface; c. spores in KOH. — d–f. JMV20190731-2. d. Detail of cristate ridges; e. hymenophore showing buttercup yellow locules and reddening of tramal plates; f. section of a crista showing clusters of exocystidia. — g. KRA F-2013-5. Basidia and paraphysoid cells. — h. S F181867 (G. Bresadola as G. morchelliformis). Spores in KOH. — i. PRM 678960 (A. Pilát as G. morchelliformis var. globispora). Spores in KOH. — j–k. PRM 678963 (A. Pilát as G. morchelliformis var. globispora). j. Spores in KOH; k. SEM image of spores. — l. KRA F-2013-2. SEM image of spores. — Scale bars: a–b = 1 cm; c, h–j = 10 µm; d = 2 mm; e = 5 mm; f–g = 20 µm; k–l = 5 µm. — Photos: a–j. J.M. Vidal; k–l. P. Mleczko, UJ.
Invalid names. Gautieria morchelliformis var. globispora Pilát, Sydowia 7: 12. 1953 (‘morchellaeformis’) (nom. nud., Art. 39.1).
Gautieria morchelliformis var. globispora Pilát, Flora ČSR B1, Gasteromycetes: 745. 1958 (‘morchellaeformis’) (nom. inval. publ., Art. 40.1).
Etymology. From Latin, inflatus = inflated, referring to the spores that are broad and ornamented with highly swollen costae.
Type. SPAIN, Catalonia, Girona, Setcases, Baga de Queràs Forest, Eastern Pyrenees, 1700 m, under Abies alba and Pinus uncinata, on siliceous soil, 21 Oct. 2008, J.M. Vidal & F. Rodríguez (holo BCN JMV20081021-5*; iso in ELTE).
Basidiomata ridged, 1.5–5 cm wide, globose to lobate, with a basal cavity attached to a white basal rhizomorph, 1–1.5 mm thick. Surface of young basidiomata white and granulose as in G. morchelliformis, then light orange (5A6), alveolate-reticulate, rugose, consisting of colourful exposed locules and of white, interwoven ridges, which form a prominent, strongly cristate reticulum. Ridges white and markedly cristate for a long time, contrasting with the colourful exposed locules, changing to greyish magenta (13D6) when handled. Locules 1.5–5 × 0.5–1.5 mm, labyrinthoid to irregularly rounded, inordinate, butter yellow to buttercup yellow (4A5–A7); melon yellow to reddish golden (5A6–6C7) in exsiccata. Tramal plates 0.2–0.5 mm thick, greyish, changing to purplish red (13A5) when cut. Columella 1–2.5 mm thick, dendroid, strongly branched, greyish, changing to purplish red when exposed to air; basal context dark magenta (13F6). Odour like G. morchelliformis, at first pleasant, fruity or of aromatic plants, finally very intense, of seafood.
Spores (15–)16–19(–20) × (11–)12–13.5(–14) µm, Q = 1.3–1.5, obovate, light yellow with greenish hues (3A5–A6) in KOH (Fig. 32g, 34c2). Episporium ovate. Costae 7–11, 1.5–3 µm high × 2–4 µm wide, very inflated, straight or sometimes helicoidal, entire, furcate or anastomosing, covering up to 3/4 of hilar appendix, smooth or with large gibbosities at bifurcations; apical ring distinct, 1.2–2.3 µm high; perisporial reticulum distinguishable, orange-yellow. Hilar appendix robust, 2.6–3.8 µm long × 3.9–4.6 µm wide at apex, conico-truncate; empty-end 0.2–0.8 µm long; hilum 1.4–1.8 µm wide, usually without remnants of sterigma. Basidia 45–70 × 12–13 µm, clavate, 2–4-spored. Paraphysoid cells 15–45 × 3–6 µm, 0–1-septate, with the terminal cell cylindrical, clavate or attenuate. Subhymenium consisting of a palisade of cylindrical elements, 6–13 µm diam. Hymenophoral trama of interwoven hyphae, 2–5 µm diam, with some ampulliform septa and endocystidia-like terminal vesicles, 8–12 µm wide. Thromboplera present. Peritrama of ridges of interwoven hyphae 4–6 µm diam, which grows to form erect irregular cristae. Pseudopellis pseudoparenchymatous circumscribed to edge of ridges, formed by chains of two or more globose to subglobose elements, ending in napiform exocystidia, 10–25 µm wide, hyaline to yellow, not collapsing completely at maturity.
Habitat, Season & Distribution — Gregarious, hypogeous or semi-hypogeous under needles, from lowland to subalpine coniferous forests (Abies, Larix, Picea, Pinus), preferably on siliceous soils. Almost throughout the year. Common. Widely distributed in temperate regions, from sea level in Northern Europe to 1800 m altitude in Southern Europe (Fig. 36).
Additional material studied. AUSTRIA, Upper-Austria, Scharnstein, ‘unter Fichte’, 4 Sept. 1973, Mader as G. graveolens (ZT-Myc 3950); Vorarlberg, Thüringerberg, off to Gassneralpe, ‘unter Fichte’, 4 Nov. 1975, E. Horak as G. retirugosa (ZT-Myc 3922). – BULGARIA, Kyustendil, Kirilova Polyana, Rila Nat. Park, 1310 m, under Picea abies, on siliceous soil, 21 Sept. 2016, B. Assyov (SOMF 30319, dupl. JMV800732); Ovnarsko, Malyovitsa area, Rila Mountains, 1500 m, under Picea abies, on siliceous soil, 22 July 2017, M. Slavova (SOMF 30320, dupl. JMV800738); Parangaliza, Rila Nat. Park, 1750 m, under Abies alba and Picea abies, on siliceous soil, 9 May 2018, M. Slavova (SOMF 30321*, dupl. JMV800741); Sofia, Borovets, Rila Mountains, 1400 m, under Abies alba and Fagus sylvatica, on siliceous soil, 13 Apr. 2018, M. Slavova (SOMF 30322, dupl. JMV800739); Sofia-City, Sofia, Vitosha Mountain, 1525 m, under Picea abies, on siliceous soil, 5 May 2017, M. Slavova (SOMF 30323, dupl. JMV800734); ibid., 1600 m, 30 July 2018, M. Slavova (SOMF 30324, dupl. JMV800771); ibid., 29 Aug. 2018, M. Slavova (SOMF 30325, dupl. JMV800772); ibid., 22 Sept. 2018, M. Slavova (SOMF 30326, dupl. JMV800774). – CZECH REPUBLIC, Vysočina, Chotĕboř, 19 Aug. 1915, C. Bayer as G. graveolens, det. A. Pilát as G. morchelliformis var. globispora (PRM 678960). – FRANCE, Provence-Alpes-Côte d’Azur, Saint-Martin-Vésubie, Le Boréon, Maritime Alps, 1725 m, under Abies alba, A. nebrodensis, Picea abies and Pinus sp., 29 Sept. 2011, A. Paz (IC29091101/29091102); Pyrénées-Orientales, Les Angles, Les Bouillouses, Eastern Pyrenees, 1800 m, under Picea, 25 Sept. 2008, A. Paz (IC25090803); Rhône-Alpes, Ain, Oyonnax, 8 Oct. 1977, from exhibition, det. N. Suber as G. morchelliformis (S F181889); Isère, Grande Chartreuse, 1200 m, under Picea and Fagus, Oct. 1971, L. Riousset, det. R.W. Dennis as G. morchelliformis (K(M) 170562); Isère, Col du Cucheron, Sierra de Chartreuse, 10 Oct. 1971, M. Lawrynowicz as G. morchelliformis (MA-Fungi 5111*); Savoie, Mèribel, Forêt du Dos des Branches, 1560 m, under Larix decidua, 18 Aug. 1993, P. Collin, comm. L. Gori as G. otthii (JMV800360); ibid., under Picea abies, 22 Aug. 1993, F. Petit as G. cf. otthii (PAM93082201, dupl. JMV800483). – GERMANY, Baden-Württemberg, Württemberg, 1882, leg. illegible, as G. morchelliformis (B 700015004, ex herb. Lettau); Bavaria, Engfurt bei Mühldorf, ‘Fichtenwald’, 23 Aug. 1922, E. Soehner #680 as G. graveolens (M 126230); Miesbach, ‘Nadelwald’, 1 Sept. 1885, A. Allescher as G. graveolens (M 126239); Munich, Isarauen (‘obere Isaranlagen’), 2 July 1920, E. Soehner #156 as G. graveolens (M 126238). – ITALY, Trentino-Alto Adige, Trento, Breguzzo, 1000 m, in mixed forest of Abies alba, 28 Aug. 1995, G. Campagnola, det. M. Sarasini as G. graveolens (MS1068*, dupl. JMV800182); Trento, Cavelonte, ‘in silvis abiegnis’, Aug. 1898, G. Bresadola as G. graveolens, P.A. Saccardo, Mycotheca Italica #427, det. A. Pilát as G. morchelliformis (PAD s.n., herb. P.A. Saccardo; B 700015012; BPI 602608; K(M) 170555; NY 1952384; PRM 678959; S F181869, ex herb. Sydow); ibid., Aug. 1898, G. Bresadola as G. morchelliformis (PRM 678946; S F181865); ibid., 1898, G. Bresadola as G. morchelliformis (S F181867); Trento, near Panevecchio, Lago Forte Buso, ‘Fichtenwald’, 17 Sept. 1986, M. Moser #86/237 as G. morchelliformis (IB 19860237). – POLAND, Lesser Poland, Ochotnica Górna, Duże Jaszcze Valley, Gorce Nat. Park, Fagus-Abies forest with Picea, 3 Aug. 2013, P. Mleczko (KRA F-2013-5, dupl. JMV800759); near Poręba Wielka, Turbacz Valley, Gorce Nat. Park, 690 m, Abieti-Piceetum with Fagus sylvatica, 29 July 2013, M. Kozak (KRA F-2013-2, dupl. JMV800756); near Poręba Wielka, Za Palacem Valley, Gorce Nat. Park, 810 m, Abieti-Piceetum, 18 Sept. 2014, M. Kozak & K. Kozak (KRA F-2014-57, dupl. JMV800764); near Kościelisko, Głębowiec Stream Valley, Tatra Nat. Park, 1040 m, Picea abies forest, on calcareous soil, 26 Sept. 2019, M. Kozak & F. Karpowicz (TPN-19-0306, dupl. JMV800808); near Kościelisko, northern slope of Hruby Regiel, Tatra Nat. Park, 970 m, Abieti-Piceetum, 15 Oct. 2019, M. Kozak & J. Brańka (TPN-19-0392, dupl. JMV800814); near Małe Ciche, Suchej Wody Valley, Tatra Nat. Park, 960 m, Abieti-Piceetum with Fagus sylvatica, 19 Aug. 2019, M. Kozak (TPN-19-0123*, dupl. JMV800801); near Kościelisko, Wielka Sucha Valley, Tatra Nat. Park, 1050 m, Abieti-Piceetum, on calcareous soil, 16 Aug. 2019, M. Kozak (TPN-19-0149, dupl. JMV800805); near Zubrzyca Górna, Babia Góra Nat. Park, Beskid Żywiecki Mountains, 950 m, Abies alba forest, 3 Nov. 2018, P. Chachuła (KRA F-2018-10, dupl. JMV800795); Świętokrzyskie, near Stąporków, Stara Góra, Garb Gielniowski Hills, 340 m, Abies alba forest, 3 Aug. 2013, P. Mleczko (KRA F-2013-6, dupl. JMV800760); ibid., 3 Aug. 2013, P. Mleczko & J. Kochanowski (KRA F-2013-77*, dupl. JMV800761). – ROMANIA, Covasna, near Ojdula (Ozsdola), under Picea abies, 3 Oct. 1998, B. Pálfy (ZB2280*, dupl. JMV800408). – SLOVAKIA, Košice, Spišské Vlachy, ‘in sylvis’, F.A. Hazslinsky as G. morchelliformis, det. A. Pilát as G. morchelliformis var. globispora (PRM 678963, herb. Kalchbrenner). – SPAIN, Aragon, Huesca, Bielsa, Torla, Turieto Alto, Valle de Ordesa, Central Pyrenees, 1360 m, under Abies alba, Pinus sylvestris and Fagus sylvatica, 1 May 2015, A. Paz (IC01051501); Catalonia, Girona, Alp, Saltèguet Forest, Eastern Pyrenees, 1600 m, under Abies alba and Pinus uncinata, on schistose soil, 22 June 1996, J.M. Vidal (JMV960622-10); ibid., 1700 m, 20 Sept. 2011, J.M. Vidal & F. Rodríguez (JMV20110920-1*); ibid., 1760 m, 31 July 2019, J.M. Vidal, A. Paz & C. Lavoise (JMV20190731-2); Girona, Campelles, Pla dels Prats, Eastern Pyrenees, 1550 m, under Abies alba and Pinus sylvestris, on siliceous soil, 14 Oct. 2008, J.M. Vidal & F. Rodríguez (JMV20081014-9b*); Girona, Setcases, Baga de Carboners Forest, Eastern Pyrenees, 1650 m, under Abies alba and Pinus uncinata, on siliceous soil, 20 Sept. 1997, J.M. Vidal (JMV970920-6*); ibid., 6 Oct. 2009, J.M. Vidal & F. Rodríguez (JMV20091006-7*); Girona, Setcases, Baga de Queràs Forest, Eastern Pyrenees, 1700 m, under Abies alba and Pinus uncinata, on siliceous soil, 30 Sept. 2008, F. Rodríguez (JMV20080930-2*); ibid., 7 Oct. 2008, F. Rodríguez (JMV20081007-1*); ibid., 21 Oct. 2008, J.M. Vidal & F. Rodríguez (JMV20081021-2*); ibid., 6 Oct. 2009, J.M. Vidal & F. Rodríguez (JMV20091006-2*); ibid., 20 July 2010, F. Rodríguez (JMV20100720-4*); ibid., 6 Sept. 2011, J.M. Vidal & F. Rodríguez (JMV20110906-1); ibid., 5 Oct. 2016, F. Rodríguez (JMV20161005); Lleida, Espot, Aigüestortes i Estany de Sant Maurici Nat. Park, Central Pyrenees, 1750 m, under Abies alba, on siliceous soil, 5 Sept. 1997, J.M. Vidal & J. Vila (JMV970905-8b*); ibid., 26 June 1999, J.M. Vidal & J. Vila (JMV990626-6). – SWEDEN, Gotland, Hörsne, Mörby, in mixed forest, 13 Aug. 1962, G. Eriksson as G. morchelliformis (UPS F-550636). – SWITZERLAND, Bern, near Bern, sine dat., G. Otth, det. J.G. Trog as G. morchelliformis (ZT-Myc 3946, ex BERN; ZT-Myc 3947, ex herb. L. Fischer); Grisons, Plessur, Arosa, 19 Aug. 1953, Rahm, det. A. Knapp #125b as G. graveolens (ZT-Myc 3948); Prättigau-Davos, Davos, under Picea abies, 17 Aug. 1975, I. Hohner, H. Hohner & D. Platz, det. C. Schwärzel #44 as G. otthii (ZT-Myc 3938).
Notes — Gautieria villosa var. inflata is morphologically very similar to G. villosa var. villosa, differing from it by having: 1) persistent white cristate ridges providing a rough touch on the surface of basidiomata; and 2) obovate spores with a highly inflated perisporium. In most of the public herbaria consulted, G. villosa var. inflata has been determined as G. morchelliformis or G. graveolens, as we can see in the collections found in Cavelonte by Bresadola, of which we illustrate the spores of the collection S F181867 (Fig. 10h). Another Gautieria that has the same spore shape is G. morchelliformis var. globispora (Fig. 10i–k). The original material used by Pilát to describe this variety was found by F.A. Hazslinsky under conifers in Slovakia near Spišské Vlachy and was initially published by Kalchbrenner (1865) as G. morchelliformis (PRM 678963). Later, Pilát (1953, 1958) considered this collection as a variety of G. morchelliformis characterised by having globose-elliptical spores with an average size of 18 × 13 µm. But this varietal name was invalidly published by Pilát (1953) as it lacked a Latin description, as well as by Pilát (1958) for not designating a holotype. As a result of the study of the rest of collections cited by Pilát (1958) (Chotěboř, leg. Bayer, PRM 678960; Mníšek, leg. Vacek, W 1785; Žarošice, leg. Vacek, PRM 678962; Clans, leg. Poirault, PC 92295; Kemmern, leg. Bucholtz, S F181883), only the collection PRM 678960 has been identified by us as belonging to G. villosa var. inflata, and the rest of the collections have been identified as belonging to other taxa, namely G. confusa (W 1785), G. graveolens (PRM 678962), G. subglobispora (PC 92295) and G. villosa var. villosa (S F181883).
With an acidophilic tendency, G. villosa var. inflata is common in montane and subalpine coniferous forests, usually accompanied by G. otthii. From this Gautieria we have studied 61 collections from 12 countries, of which we have successfully sequenced 17 collections (Table 1) and represented 4 sequences in our phylogenetic tree (Fig. 3). We also identify as G. villosa var. inflata the GenBank sequence AF377067 identified originally as G. morchelliformis (Bidartondo & Bruns 2002) and JF908017 identified originally as G. graveolens (Osmundson et al. 2013).
Gautieria villosa var. pilifera J.M. Vidal, Konstantin., Tabouras & Gkilas, var. nov. — MycoBank MB 845114; Fig. 11, 32, 34, 36
Fig. 11.
Gautieria villosa var. pilifera. a–k. GK3029 (BCN JMV800561, holotype). a–b. Basidiomata showing anfractuous-rugose surface; c. section of a crista showing clusters of exocystidia; d–e. trichodermial geliferous hairs of pseudopellis; f. collapsed pseudopellis and peritrama embedded in a yellow mucilaginous matrix; g. hymenium showing paraphysoid cells and an exocystidia-like element; h. spores in KOH; i–k. SEM images of spores. — l. MG109. Spores in KOH. — Scale bars: a–b = 1 cm; c–g = 20 µm; h, l = 10 µm; i–k = 5 µm. — Photos: a. G. Konstantinidis; b. D. Tabouras; c–h, l. J.M. Vidal; i–k. UdG.
Etymology. Fom Greek and Latin, πίλος, pilus = hair and φέρω, fero = to carry, because the presence of hairs in the surface of basidiomata.
Type. GREECE, Thessaly, Trikala, Pirra, Pindus Mountains, 1300 m, under Abies borisii-regis, 2 May 2008, D. Tabouras (holo BCN JMV800561*; iso in herb. pers. G. Konstantinidis GK3029 and in ELTE).
Basidiomata ridged, 1.5–2.5 cm wide, globose to applanate, with a basal cavity attached to a white basal rhizomorph, 0.8–1 mm thick. Surface initially granulose, white to yellowish, then light orange (5A6), rugose, anfractuous, formed of intricate, white cristate ridges, contrasting with the narrow and colourful exposed locules. Ridges becoming partially fertile in old basidiomata, reddening with handling. Locules 0.5–2 × 0.2–0.5 mm, labyrinthoid to irregularly shaped, irregular, sinuous, narrow, longer than wide, inordinate, yellowish orange (4A7–B7); caramel to cinnamon (6C5–D6) in exsiccata. Tramal plates 0.3–0.5 mm thick, greyish, slightly reddening when cut. Columella 0.8–1.4 mm thick, dendroid, strongly branched, greyish, slightly reddening when exposed to air. Odour of truffle.
Spores 18–22.5(–24.5) × 12–14(–15) µm, Q = 1.4–1.6, broadly elliptical to pyriform, slightly heterotropic, pale yellow with greenish hues (3A3–A4) in KOH (Fig. 32h, 34c3). Episporium ovate, sometimes subpapillate. Costae 11–13, 1–1.8 µm high × 2.3–3.6 µm wide, moderately inflated, straight or sometimes helicoidal, frequently furcate, covering up to 1/2 of hilar appendix, ornamented with rounded gibbosities; perisporium fragile, often corrugate and breaking down into lumps; apical ring distinct, 1–2 µm high; perisporial reticulum distinguishable, yellow coloured. Hilar appendix pronounced, 3.5 – 5.5 µm long × 3.5–5 µm wide at apex, conico-truncate; empty-end 0.3–1 µm long; hilum 1.4–2 µm wide, usually with remnants of sterigma. Basidia 35–55 × 8–14 µm, clavate, 2–4-spored, filled with guttules and yellow pigment, soon collapsing and emptying of the contents, which agglutinates the spores on the surface of hymenium. Sclerobasidia present. Paraphysoid cells 25–60 × 3–9 µm, 0–2-septate, straight to sinuous, with the terminal cell cylindrical, clavate or attenuate. Observed some exocystidia-like elements, 15–20 µm wide, in fertile hymenium. Subhymenium constituting a palisade of cylindrical to inflated elements, 5–14 µm diam. Hymenophoral trama of interwoven hyphae 3–7 µm diam, with some ampulliform septa 6–12 µm wide. Thromboplera 2–4 µm diam. Peritrama of ridges similar to hymenophoral trama. Pseudopellis pseudoparenchymatous to hymenidermial circumscribed to edge of ridges, consisting of several layers of inflated cells and yellow, sphaeropedunculate exocystidia, 18–36 µm wide, soon collapsing and embedded in a yellow matrix. Observed an incipient trichoderm of geliferous hairs mixed with exocystidia, isolated or in small clusters, sinuous, septate, often bifurcate, measuring 20–90 × 4–6 µm, thick-walled up to 1 µm, with an obtuse or subcapitate tip, gradually covered by a glutinous layer up to 5 µm thick.
Habitat, Season & Distribution — Gregarious, hypogeous, in montane forests of Abies borisii-regis and A. cephalonica, on calcareous soils. From winter to spring. Mediterranean variety, found in Greece at 1300 m altitude (Fig. 36).
Additional material studied. GREECE, Peloponnese, Arcadia, Alonistena, 1300 m, under Abies cephalonica, on calcareous soil, 15 Jan. 2015, M. Gkilas (MG109*, dupl. JMV800722).
Notes — Gautieria villosa var. pilifera shows great macroscopic similarities with G. villosa var. villosa, differing from it by: 1) anfractuous-rugose surface; 2) yellowish orange locules; 3) presence of geliferous hairs in pseudopellis; and 4) pyriform spores with a fragile perisporium, easily ruptured, and a pronounced hilar appendix. It is a calcicolous taxon living in forests of Bulgarian and Greek fir. We only know the two sequenced Greek collections (Fig. 3).
Clade convoluta (GauGau-3)
Ridges densely convoluted, compact, of cerebriform appearance. Trichotomentocutis absent. Spores broadly elliptico-fusiform, often papillate, reaching 21–24 × 14 µm. Costae very inflated, ornamented with broad gibbosities. Linked to conifers (Abies, Picea). Represented by G. convoluta var. convoluta and G. convoluta var. petrakii.
Gautieria convoluta J.M. Vidal, Fern. Rodr., A. Paz, Chachuła & Kozak, sp. nov. — MycoBank MB 845115
Etymology. From Latin, convolutus = convoluted, rolled around, due to the cerebriform aspect of the surface of basidiomata.
Type. SPAIN, Catalonia, Girona, Campelles, Pla dels Prats, Eastern Pyrenees, 1540 m, under Abies alba, Pinus sylvestris and Pinus uncinata, on calcareous soil, 14 Oct. 2014, J.M. Vidal & F. Rodríguez (holo BCN JMV20141014-27*; iso in ELTE).
Diagnosis — Basidiomata up to 5 cm wide, similar to G. morchelliformis, but with the surface cerebriform, the ridges densely convoluted, the locules more or less radially arranged, the columella usually a product of the fusion or coalescence of several specimens, and the spores broadly elliptico-fusiform, often papillate (broadly elliptical when immature), 20–24 × 12–14 µm, with 11–13 costae, 1.3–1.8 μm high, a gibbose and often corrugate perisporium and a pronounced hilar appendix. Found in temperate Europe under conifers (Abies, Picea).
Gautieria convoluta J.M. Vidal, Fern. Rodr., A. Paz, Chachuła & Kozak, var. convoluta — MycoBank MB 845150; Fig. 12, 32, 34, 36
Fig. 12.
Gautieria convoluta var. convoluta. a–b. JMV20141014-27 (BCN, holotype). Basidioma showing convoluted ridges, maize yellow locules, and pink oxidation of tramal plates; b. spores in KOH. — c. JMV20171024-2. Young basidia and paraphysoid cells. — d–e. JMV20180627-2. d. Section of aggregate basidiomata with strong columellae; e. detail of convoluted ridges. — f. IC21061901. Basidioma showing a branched columella. — g–h. JMV20190806-2. g. Semi-dried ridges showing rugose surface; h. section of a ridge showing pseudopellis, peritrama, hymenophoral trama and adjacent hymenium. — i. IC08071801. Spores in Melzer. — j. W 343 (holotype of G. morchelliformis var. stenospora). Spores in KOH. — k–l. KRA F-2008-259. k. Spores in KOH; l. SEM image of spores. — Scale bars: a, e = 5 mm; b, i–k = 10 µm; c = 20 µm; d, f = 1 cm; g = 2 mm; h = 50 µm; l = 5 µm. — Photos: a–e, g–h, j–k. J.M. Vidal; f, i. A. Paz; l. P. Mleczko, UJ.
Invalid name. Gautieria morchelliformis var. stenospora Pilát, Flora ČSR B1, Gasteromycetes: 745. 1958 (‘morchellaeformis’) (nom. inval. publ., Art. 40.1).
Basidiomata ridged, 1.5–5 cm wide, subglobose to lobate, often the product of the aggregation or coalescence of several specimens, with a small basal depression attached to a white basal rhizomorph, 1.5–2 mm thick. Surface of young basidiomata white and granulose as in G. morchelliformis. Mature basidiomata with the surface light orange (5A5–6A5), ridged, cerebriform, consisting of densely convoluted ridges, keeping the exposed locules occluded. Ridges initially white, rugose, cristate, becoming fertile and smooth, orange-yellow, turning pale red or pink (12A3–A4) to greyish ruby (12D6) with handling. Locules 2–5 × 0.5–2 mm, labyrinthoid to irregularly shaped, more or less radially arranged, sinuous, butter yellow to maize yellow (4A5–A6); brownish orange (6C6–C7) in exsiccata. Tramal plates 0.3–0.5 mm thick, greyish, turning pink (12A4) on exposure to air. Columella 1.5–3 mm thick, dendroid, strongly branched, usually with more than one columella basally fusing or coalescing, greyish, becoming pinkish on exposure to air; basal context greyish ruby (12E4). Odour at first pleasant, fruity or of aromatic plants, finally very intense of seafood, similar to that of G. morchelliformis.
Spores (18.5–)20–24(–24.5) × 12–14 µm, Q = 1.5–1.8, variable in shape, usually broadly elliptico-fusiform and papillate, pale yellow with greenish hues (3A3–A4) in KOH (Fig. 32i, 34d1). Episporium narrowly ovate to ovate, apically acute or papillate. Costae 11–13, 1.3–1.8 µm high × 1.5–3.8 µm wide, very inflated, straight or sometimes helicoidal, mostly furcate or anastomosing, covering up to 1/2 of hilar appendix, ornamented with large gibbosities; perisporium fragile, often wrinkled, corrugate; apical ring distinct, 1.3–2 µm high; perisporial reticulum not always distinguishable. Hilar appendix pronounced, 2.5–4.5 µm long × 3.5–5.5 µm wide at apex, conico-truncate; empty-end 0.1–0.8 µm long; hilum 1.6–2.3 µm wide, without remnants of sterigma. Basidia 50–90 × 12–14 µm, clavate, 3–4-spored, with a yellow granular content. Sclerobasidia present. Paraphysoid cells 25–60 × 4–8 µm, 0–2-septate, tortuous, nodulous, often apically furcate, forming clusters irregularly distributed in hymenium, being abundant and longer, up to 100 µm or more, in exposed locules. Subhymenium consisting of cylindrical to subglobose elements, 8–20 µm wide. Hymenophoral trama of interwoven hyphae 3–6 µm diam, with some ampulliform septa and endocystidia-like terminal vesicles up to 12 µm wide. Thromboplera 2–6 µm diam. Peritrama of ridges prosenchymatous, of densely interwoven hyphae, 3–6 µm diam. Pseudopellis a hymeniderm or pseudoparenchymatous circumscribed to edge of ridges, formed by one or more layers of pedicellate exocystidia, 15–35 µm wide, fragile, thin-walled up to 0.5 µm, at first hyaline and then yellow.
Habitat, Season & Distribution — Solitary or gregarious, hypogeous, in montane and subalpine coniferous forests (Abies, Picea), preferably on calcareous soils. From spring to autumn. Rare. Distributed in temperate regions, from 500 m altitude in Central Europe (Alps and Western Carpathians) to 1700 m in South-Western Europe (Pyrenees) (Fig. 36).
Additional material studied. FRANCE, Rhône-Alpes, Savoie, sine dat., M. Bataille as G. villosa? (PC 92639, herb. E. Boudier). – ITALY, sine loc., sine dat., ‘Original v. Vittadini’, det. A. Pilát as G. morchelliformis var. stenospora (W 343); Liguria, Imperia, Montegrosso, Pian Latte, Le Salse, 1400 m, under Abies alba and Fagus sylvatica, June 1995, G. Baiano & M. Filippa, comm. L. Gori as G. morchelliformis (ELG950600*, dupl. JMV800353). – POLAND, Lesser Poland, near Kościelisko, Macicki Żleb Gully, Tatra Nat. Park, 1010 m, Galio-Piceetum, 15 Oct. 2019, M. Kozak & J. Brańka (TPN-19-0476*, dupl. JMV800816); near Krościenko nad Dunajcem, Toporzyskowo, Pieniny Nat. Park, 540 m, under Abies alba with Pinus sylvestris and Populus sp., 19 Aug. 2008, P. Chachuła (KRA F-2008-259*, dupl. JMV800691). – SPAIN, Aragon, Huesca, Bielsa, Parking of Pineta, Ordesa y Monte Perdido Nat. Park, Central Pyrenees, 1360 m, under Abies alba, Pinus sylvestris and Fagus sylvatica, 1 May 2015, A. Paz (IC01051502*); Catalonia, Girona, Campelles, Pla dels Prats, Eastern Pyrenees, 1540 m, under Abies alba, Pinus sylvestris and Pinus uncinata, on calcareous soil, 23 Sept. 2008, F. Rodríguez (JMV20080923-3*); ibid., 14 Oct. 2008, J.M. Vidal & F. Rodríguez (JMV20081014-9); ibid., 13 July 2010, F. Rodríguez (JMV20100713-4); ibid., 24 Oct. 2017, F. Rodríguez (JMV20171024-2); ibid., 27 June 2018, J.M. Vidal, A. Paz & C. Lavoise (JMV20180627-2); ibid., 8 July 2018, A. Paz & C. Lavoise (IC08071801); ibid., 21 June 2019, A. Paz & C. Lavoise (IC21061901); ibid., 6 Aug. 2019, J.M. Vidal, A. Paz & C. Lavoise (JMV20190806-2). – SWITZERLAND, Basel-Landschaft, Arlesheim, Pfeffingen, footpath to Blauen (ruined castle), under Picea abies, 15 June 1955, C. Schwärzel #54/55/56/57 as G. dubia (ZT-Myc 3956/3957/3958/3959).
Notes — Gautieria convoluta var. convoluta is distinguished by having: 1) densely convoluted ridges occluding exposed locules; 2) a strongly branched columella; and 3) elliptico-fusiform spores, often papillate, provided with a gibbose perisporium that wrinkles and a pronounced hilar appendix. Gautieria villosa var. villosa also has yellowish orange locules, pinkish oxidation of context, and also elliptico-fusiform spores, but they are smaller and perisporium does not wrinkle or break. Gautieria morchelliformis var. morchelliformis has spores of similar size, but they are elliptical, not papillate, and the costae are more or less smooth. Pilát (1958), after studying two specimens of G. morchelliformis devoid of locality and collection date, one from a Vittadini collection kept in the Vienna herbarium and the other from a Montagne collection kept in the Kew herbarium, on page 217 provides the average dimensions of spores of the Vittadini specimen (26 × 13 µm) while indicating that the Montagne specimen has similar spores (27 × 14 µm), and on page 745 provides a diagnosis in Latin mentioning only the Vittadini specimen (‘Sporae conspecte elongattae, fusoideoellipsoideae, 26 × 13 µm magnae. Unicum specimen Vittadinii in herbario W depositum, probabiliter in Italia boreali collectum est, var. stenospora Pilát v. n.’). But this variety was invalidly published because on page 217 he mentioned two specimens and the type was not indicated (Art. 40.1). We studied the specimen from Vienna described by Pilát (W 343) and observed subfusiform spores measuring (19–)20–24.5(–26) × 11–13.5 μm, Q = 1.6–2, which are ornamented with a wrinkled perisporium as in G. convoluta var. convoluta (Fig. 12j).
Gautieria convoluta var. convoluta is a rare calcicolous taxon occurring in montane and subalpine coniferous forests, often accompanied by G. morchelliformis var. dubia and G. villosa var. villosa. From this taxon we have studied 18 collections from 5 countries, of which we have successfully sequenced 6 collections (Table 1) and represented 3 sequences in our phylogenetic tree (Fig. 3).
Gautieria convoluta var. petrakii J.M. Vidal, var. nov. — MycoBank MB 845116; Fig. 13, 32, 34, 36
Fig. 13.
Gautieria convoluta var. petrakii. a–h. M 126216 (holotype). a. Basidioma; b. pseudopellis; c. subhymenium and collapsed hymenium; d–e. spores in KOH; f–h. SEM images of spores. — i. S F181875 (B. Auerswald as G. morchelliformis). Spores in KOH. — Scale bars: a = 2 mm; b–c = 20 µm; d–e, i = 10 µm; f–h = 5 µm. — Photos: a–e, i. J.M. Vidal; f–h. UdG.
Etymology. In honour of the Austrian-Czech mycologist Franz Petrak (1886–1973), collector of the type material.
Type. CZECH REPUBLIC, Olomouc, Hranice (Mährisch Weisskirchen), ‘Auf einer Waldwegböschung’, Sept. 1926, F. Petrak as G. graveolens (holo M 126216*, F. Petrak Pilzherbarium, Flora Moravica).
Basidiomata ridged, about to 3 cm wide, irregularly shaped, provided with a small basal depression attached to a white basal rhizomorph. Surface alveolate, cerebriform, consisting of densely convoluted ridges that occlude exposed locules. Ridges rugose, brownish orange, with edge ornamented with white cristae. Locules 1.5–2.5 × 0.6–0.8 mm, labyrinthoid to irregularly shaped, sinuous, cinnamon (6C6–D6). Tramal plates 0.3–0.4 mm thick, greyish. Columella dendroid, yellowish to greyish; basal context dark brown (6E6–F6). Odour unknown. (Description based on exsiccatum).
Spores (16–)17–21(–22) × (11.5–)12–14(–15) µm, Q = 1.2–1.4, broadly elliptical, subglobose when immature, light yellow with greenish hues (4A4–A5) in KOH (Fig. 32j, 34d2). Episporium ovate, apically obtuse. Costae 11–13, 0.7–1.5 µm high × 1.7–3.4 µm wide, moderately inflated, straight or sometimes helicoidal, entire or furcate, covering up to 1/4 of hilar appendix, ornamented with rounded gibbosities, often more inflated at spore base; apical ring distinct, 1–1.5 µm high; perisporial reticulum indistinguishable. Hilar appendix short, 1.5–2.8 µm long × 3.5–4.4 µm wide at apex, conico-truncate with a long cylindrical end; empty-end 0.5–1.2 µm long; hilum 1.7–2.5 µm wide, without remnants of sterigma. Basidia 60–70 × 12–14 µm, clavate, 3–4-spored. Paraphysoid cells 20–40 × 4–12 µm, 0–2-septate, with the terminal cell cylindrical or attenuate. Subhymenium pseudoparenchymatous, consisting of chains of inflated elements, 8–15 µm wide. Hymenophoral trama of interwoven hyphae 2.5–5 µm diam. Thromboplera 2–4 µm diam. Peritrama of ridges prosenchymatous, of densely interwoven hyphae, 3 – 6 µm diam. Pseudopellis a hymeniderm circumscribed to edge of ridges, formed by clusters of long pedicellate exocystidia, 15–30 µm wide, fragile, thin-walled up to 0.5 µm, at first hyaline and then yellow.
Additional material studied. GERMANY, Thuringia, Friedrichroda, Reinhardsbrunn, in forest, July 1867, B. Auerswald as G. morchelliformis (M 126257, herb. H. Wilms; M 126258, herb. G. Niessl; S F181875, herb. Sydow).
Notes — Gautieria convoluta var. petrakii is distinguished from G. convoluta var. convoluta by its broadly elliptical spores, being subglobose when immature, which have an usually short hilar appendix. Gautieria subglobispora differs by its basidiomata with flattened ridges forming white patches and by its spores with parallel costae and a more inflated perisporium. Of this variety we have only found Petrak’s collection from the Czech Republic, which has been successfully sequenced (Fig. 3), and the Auerswald collection from Germany, so its area of distribution is probably restricted to Central Europe (Fig. 36).
Clade subglobispora (GauGau-4)
Ridges flattened forming patches. Trichotomentocutis absent. Spores broadly elliptical to globose, reaching 18–22 × 12–15 µm. Costae moderately inflated, mostly entire, smooth or ornamented with broad gibbosities. Linked mainly to conifers (Abies, Picea, Pinus), but also to broadleaf trees and occasionally to shrubs (Arbutus). Represented by G. subglobispora and undescribed North American taxa corresponding to the sequences AF377064 and AF377065.
Gautieria subglobispora J.M. Vidal, sp. nov. — MycoBank MB 845117; Fig. 14, 32, 34, 36
Fig. 14.
Gautieria subglobispora. a–c. JMV20081021-3 (BCN, holotype). a. Basidiomata showing cerebrose surface and slightly branched columella; b. hymenophore showing filled locules; c. spores in KOH. — d–g. JMV20190731-3. d. Basidiomata showing flattened ridges and white patches; e. detail of rugose patches and ridges showing redness; f. section of a rugosity showing the pseudopellis and peritrama; g. hymenium showing young and mature basidia and some paraphysoid cells. — h. PC 92299 (A. Pilát as G. morchelliformis var. globispora). Spores in KOH. — i. JMV990626-12. SEM image of a spore. — Scale bars: a, d = 1 cm; b, e = 5 mm; c, h = 10 µm; f = 50 µm; g = 20 µm; i = 5 µm. — Photos: a–h. J.M. Vidal; i. UdG.
Etymology. From Latin, sub = nearly + globus = sphere + sporus = spore, in reference to its almost globose spores.
Type. SPAIN, Catalonia, Girona, Setcases, Baga de Queràs Forest, Eastern Pyrenees, 1700 m, under Abies alba and Pinus uncinata, on siliceous soil, 21 Oct. 2008, J.M. Vidal & F. Rodríguez (holo BCN JMV20081021-3*; iso in ELTE).
Basidiomata ridged, 1.5–3.5 cm wide, subglobose to reniform, with a basal cavity attached to a white basal rhizomorph, 1.5–2 mm thick. Surface closed in young basidiomata, white, granulose, consisting of conjoined, flattened, sinuous ridges, with its edge rugose, non-cristate. As they mature, the ridges separate and the surface opens irregularly, forming large white patches and exposing colourful locules. Finally, the surface appears alveolate-reticulate, cerebrose, greyish orange (5B6), variegated, turning blood red (10D8) when rubbed. Locules 1–3 × 0.3–1.5 mm, irregularly rounded to labyrinthoid, sinuous, inordinate, filled with spores at maturity, yellowish orange (5A4–A7); cinnamon (6D6–D7) in exsiccata. Tramal plates 0.2–0.4 mm thick, white to greyish, not changing when cut. Columella 0.5–1 mm thick, slightly dendroid, undeveloped, very small, white to brownish, not changing with exposure to air; basal context reddish brown (9E6). Odour pleasant, fruity, usually not very offensive at maturity.
Spores (14–)15–18(–19) × (11.5–)12–15(–15.5) µm, Q = 1.15–1.3, broadly elliptical to subglobose, light yellow with greenish hues (3A4–A5) in KOH (Fig. 32k, 34e). Episporium broadly ovate. Costae 9–13, 1.2–2.5 µm high × 1.5–4 µm wide, moderately inflated, straight or helicoidal, entire or furcate, mostly parallel, covering up to 1/2 of hilar appendix, smooth to strongly gibbose; apical ring distinct, 1.4–2.4 µm high; perisporial reticulum non-distinguishable. Hilar appendix short, 1.3–2.6 µm long × 3.2–4.6 µm wide at apex, conico-truncate to cylindrical; empty-end up to 0.5 µm long; hilum 1.1–1.8 µm wide, often with a sterigmal remnant. Basidia (30–)50–80(–95) × (12–)14–18(–20) µm, clavate, sinuous, 2–4-spored. Sclerobasidia present. Paraphysoid cells scarce 20–46 × 4–6.5 µm, 0–1-septate, with the terminal cell cylindrical or acuminate. Subhymenium consisting of chains of cylindrical to subglobose elements, 10–15(–20) µm wide, appearing pseudoparenchymatous or forming a palisade. Hymenophoral trama of interwoven hyphae, 2–5(–7) µm diam, intermixed with ampulliform septa. Thromboplera present. Peritrama of ridges similar to hymenophoral trama. Pseudopellis a hymeniderm circumscribed to edge of ridges and patches, consisting of clusters of hyaline, globose exocystidia, 8–40 µm wide, soon collapsed; the outermost exocystidia yellow and thick-walled, up to 1.5 µm.
Habitat, Season & Distribution — Gregarious, hypogeous or semi-hypogeous under needles, in subboreal and subalpine coniferous forests (Abies, Picea, Pinus), on siliceous soils. From summer to autumn. Rare. Distributed in boreal and temperate regions, from 200 m altitude in Northern Europe to 1800 m in Southern Europe (Fig. 36).
Additional material studied. FRANCE, Provence-Alpes-Côte d’Azur, Clans, Forêt Domaniale de Clans, Maritime Alps, 1500 m, under Abies alba and Picea abies, 17 Sept. 1913, G. Poirault as G. morchelliformis, det. A. Pilát as G. morchelliformis var. globispora (PC 92299; PRM 678961 ex PC). – NORWAY, Oppland, Gran, under Picea, 15 Aug. 1993, B. Raddum, det. F.E. Eckblad as G. morchelliformis (O 152350*). – SPAIN, Catalonia, Girona, Alp, Saltèguet Forest, Eastern Pyrenees, 1600 m, under Abies alba and Pinus uncinata, on schistose soil, 13 July 1996, J.M. Vidal as G. morchelliformis var. globispora (JMV960713-4); ibid., 1760 m, 31 July 2019, J.M. Vidal, A. Paz & C. Lavoise (JMV20190731-3); Girona, Setcases, Baga de Queràs Forest, Eastern Pyrenees, 1700 m, under Abies alba and Pinus uncinata, on siliceous soil, 7 Oct. 2008, F. Rodríguez (JMV20081007-2); ibid., 21 Oct. 2008, J.M. Vidal & F. Rodríguez (JMV20081021-1*/20081021-4a); ibid., 6 Oct. 2009, J.M. Vidal & F. Rodríguez (JMV20091006-1); ibid., 6 Sept. 2011, J.M. Vidal & F. Rodríguez (JMV20110906-2); Lleida, Espot, Aigüestortes i Estany de Sant Maurici Nat. Park, Central Pyrenees, 1400 m, 26 June 1999, under Abies alba, on siliceous soil, J.M. Vidal & J. Vila (JMV990626-12*). – SWITZERLAND, Grisons, Inn, Ftan, Lai da Pesch, Central Alps, 1780 m, under Picea and Larix, 19 Aug. 1992, Brunner & Egli as G. morchelliformis (ZT-Myc 3942).
Notes — Gautieria subglobispora is distinguished by having: 1) flattened ridges forming white patches; 2) yellowish orange locules; and 3) subglobose spores with inflated parallel costae and a minute hilar appendix. We have recognized this species in few herbarium collections, where it is identified as G. morchelliformis, such as the collection found by the French botanist Georges Poirault under Abies and Picea in the Maritime Alps (PC 92299, PRM 678961) and published by Pilát (1958) as G. morchelliformis var. globispora (Fig. 14h). With this same variety name, Vidal (1997) illustrated the spores of a Spanish collection found in the Pyrenees (JMV960713-4).
It is a rare silicicolous species living in subboreal and subalpine coniferous forests. From this Gautieria we have studied 12 collections from 4 countries, of which we have successfully sequenced 4 collections (Table 1) and represented 3 sequences in our phylogenetic tree (Fig. 3). The GenBank sequences AF377064 and AF377065 (Bidartondo & Bruns 2002), could represent two related North American taxa. A Chinese species having ridged basidiomata and globose spores is G. globispora. This species was found in Guan Lushan (Shanxi province) growing under Picea wilsonii, and has smaller spores, measuring (9–)10–12.5 × 8–11 µm (Tao et al. 1996, 1998), but it has not been possible to study the type material preserved in the HMAS herbarium to verify its relationship with G. subglobispora.
Clade GauGau-5
Represented by an undescribed North American species corresponding to the sequences AF377071 to AF377073.
Clade hellenica (GauGau-6)
Ridges fused into an irregular pseudoperidium. Trichotomentocutis undeveloped. Spores variable in shape, elliptico-fusiform, ovato-fusiform or ovate, reaching 20–21 × 11.5–14.5 µm. Costae slightly to moderately inflated, smooth or ornamented with broad gibbosities. Linked to conifers (Abies, Pinus, Pseudotsuga) or broadleaf trees (Lithocarpus, Quercus).
Represented by G. hellenica, G. iberica and several undescribed North American taxa corresponding to the sequences AF377059 to AF377061, AF377063, DQ273388, DQ365642, DQ974732, JN022505 and KC791118.
Gautieria hellenica J.M. Vidal, Tabouras, Konstantin. & Klisiari, sp. nov. — MycoBank MB 845118; Fig. 15, 32, 34, 36
Etymology. From Latin, hellenicus = native of Greece, for having been discovered in this country.
Type. GREECE, Thessaly, Trikala, Vlaha Elatis, 1150 m, under Abies borisiiregis, 26 Feb. 2010, D. Tabouras (holo BCN JMV800569*; iso in herb. pers. G. Konstantinidis GK5779 and in ELTE).
Basidiomata pseudoperidial, 2–4 cm wide, subglobose to tuberiform, with a little basal depression attached to a white basal rhizomorph, 1.5–2 mm thick. Surface white, cottony-felted, perforate, covered by an arachnoid tomentum that allows observation of the circumvolutions of the hymenophore and the exposed locules, becoming pale yellow (4A4) to brown (7D8) with age or when handled; greyish orange (5B5) with cinnamon (6D6) maculae in exsiccata. Ridges irregularly fused into a thin, porate, discontinuous pseudoperidium, 0.1–0.2 mm thick. Locules 1–3 × 0.2–0.6 mm, irregularly shaped, radially elongated, orange (5A6–A7); cinnamon (6C6–D7) in exsiccata. Tramal plates 0.3–0.6 mm thick, greyish, becoming greyish pink (11B3) when cut. Columella 0.5–3 mm thick, dendroid, basally well developed, irregularly branched, grey; basal context violet brown (10F4). Odour pleasant, initially fruity, then more intense, of caramel.
Spores (15–)17–20(–22) × (8.5–)9.5–11.5(–12) μm, Q = (1.4–) 1.5–1.9(–2.1), elliptico-fusiform, yellowish orange (4A6–A8) in KOH (Fig. 32l, 34f). Episporium ovato-fusiform. Costae 9–11, 0.8–1.7 μm high × 1.8–4 µm wide, moderately inflated, straight or sometimes helicoidal, entire or furcate, covering 1/2 of hilar appendix, ornamented with big gibbosities; apical ring inconspicuous, 0.8–1.4 μm high; perisporial reticulum indistinguishable. Hilar appendix 2.3–3.6 µm long × 2.9–4 μm wide at apex, conico-truncate; empty-end cylindrical, 0.5–1.1 µm long; hilum 0.8–1.5 μm wide, usually with sterigmal remnants. Basidia 30–45 × 8–14 µm, cylindrical to clavate, 3–4-spored. Paraphysoid cells 25–70 × 4–8 µm, 0–2-septate, with the terminal cell polymorphic, acuminate, mucronate, strangulated or subulate, becoming long hairs up to 150 µm or longer in exposed locules. Subhymenium consisting of chains of 3–4 cylindrical to subglobose elements, 8–16 µm diam. Hymenophoral trama of interwoven hyphae, 3–6 µm diam, with some ampulliform septa. Thromboplera 1.5–3 µm diam. Peritrama of interwoven hyphae, 3–6 µm diam, with some enlargements up to 16 µm wide, and with endocystidia-like terminal vesicles below the pseudopellis, 20 – 35 µm wide. Pseudopellis of irregular thickness and structure, consisting of a confused hymeniderm, from which originates a loose and intricate trichotomentocutis that gradually covers the ridges and external apertures. Trichotomentocutis constituted by hyaline, thin-walled hyphae, 2–4 µm diam, many of them crystalliferous, and tufts of yellow, thick-walled geliferous hairs, 3–6 µm diam with enlargements up to 20 µm wide, with walls 0.4–0.8 µm thick not staining with Congo red. Observed some isolated, clavate to globose exocystidia, 10–30 µm wide, mixed with hyphae of trichotomentocutis, or forming clusters mainly on the edge of ridges. Eventually, all elements of pseudopellis become embedded in a matrix of yellow pigment, constituting an intricate tissue with a prosenchymatous aspect. Calcium oxalate crystals present in all tissues, especially in outer hyphae of trichotomentocutis.
Habitat, Season & Distribution — Gregarious, hypogeous, in montane forests of Abies borisii-regis, on calcareous soils. From late winter to spring. Mediterranean species, known from Greece between 800–1300 m of altitude (Fig. 36).
Additional material studied. GREECE, Central Greece, Evritania, Krikello, 1300 m, under Abies borisii-regis, 25 Mar. 2011, G. Prountzopoulos & T. Tsantirtzioglou (GK5594*, dupl. JMV800566); Thessaly, Trikala, Pertouli, 1100 m, under Abies borisii-regis, 3 Apr. 2011, D. Klisiari (GK5608*, dupl. JMV800567); Trikala, Vlaha Elatis, 1200 m, under Abies borisii-regis, 20 Feb. 2010, D. Tabouras (GK5778, dupl. JMV800568); ibid., 1020 m, 13 Apr. 2014, D. Tabouras (GK7147, dupl. JMV800716).
Notes — Gautieria hellenica is characterised by having: 1) a loose trichotomentocutis gradually covering ridges and exposed locules; 2) ridges fusing into a porate pseudoperidium; 3) orange locules; 4) subulate paraphysoid cells; and 5) spores tending to fusiform, with an inflated perisporium. Unlike G. obtexta and G. pervestita, where the tomentum is dense and well-structured and the exocystidia are few or non-existent, in G. hellenica there are still abundant exocystidia in the pseudopellis mixed with various types of disordered hairs arranged to form a loose and intricate tomentum (Fig. 15i, l).
This species has an eastern Mediterranean distribution, so far only found in Greece in forests of Bulgarian fir. From this Gautieria we have studied 5 Greek collections, of which we have successfully sequenced the 3 collections represented in our phylogenetic tree (Fig. 3).
Gautieria iberica J.M. Vidal, Cabero, A. Paz & Faust. García, sp. nov. — MycoBank MB 845119; Fig. 16, 32, 34, 36
Fig. 16.
Gautieria iberica. a–h. JC80511BT (BCN JMV800476, holotype). a. Basidiomata; b. hymenophore showing deep orange locules and slightly dendroid columella; c. trichotomentocutis covering an exposed locule; d–e. hymenidermial pseudopellis; f. tuft of yellow thick-walled hairs of trichotomentocutis; g. hymenium showing some paraphysoid cells; h. spores in KOH. — i–n. IC24011506. i. Basidiomata; j. detail of exposed locules covered by an arachnoid tomentum; k. paraphysoid cells and two hyphidia of an exposed locule; l. spores in KOH, some of them with apicular plug in apical position; m–n. SEM images of spores. — o. JMV800473. SEM image of spores. — Scale bars: a, i = 1 cm; b, j = 5 mm; c–g, k = 20 µm; h, l = 10 µm; m–o = 5 µm. — Photos: a–b. J. Cabero; c–h, k–l. J.M. Vidal; i–j. A. Paz; m–n. P. Mleczko, UJ; o. UdG.
Etymology. From Latin, iberica = from Iberia, name by which the Romans knew the Iberian Peninsula (Spain and Portugal), in reference to the location of this species.
Type. SPAIN, Castilla and Leon, Burgos, Espinosa de Cervera, 1050 m, under Quercus rotundifolia and Quercus faginea, on calcareous soil, 11 May 2008, J. Cabero (holo BCN JMV800476*; iso in herb. pers. J. Cabero JC80511BT and in ELTE).
Basidiomata pseudoperidial, 3–5 cm wide, subglobose to irregular, with a basal protuberance attached to a white basal rhizomorph, 1.5–2 mm thick. Surface white, cottony-felted, covered by an arachnoid tomentum very fragile and evanescent when handled, turning orange (6A7) to reddish brown (8D7) when exposed to air or rubbed; greyish orange (5B4) in exsiccata. Ridges irregularly fused into a thin, porate, discontinuous pseudoperidium, 0.1–0.2 mm thick, presenting ridged parts, especially in young basidiomata, becoming gradually covered by a tomentum. Locules 1–2.5 × 0.2–0.5 mm, minute, labyrinthoid to irregularly shaped, inordinate, deep orange (6B8); from orange to cinnamon (5A6–6D7) in exsiccata. Tramal plates 0.4–0.6 mm thick, greyish, immutable when cut. Columella 1–1.5 mm thick, slightly dendroid, undeveloped, basal, white; basal context reddish brown (8E6). Odour mild when young, fruity, strong at maturity.
Spores (16–)17–21(–22) × (11–)11.5–14.5(–15.5) μm, Q = 1.3–1.6(–1.7), ovate to ovato-fusiform, greyish orange (5B5–B6) in KOH (Fig. 32m, 34g). Episporium ovate to broadly ovate, subpapillate. Costae 9–12, 0.5–1 μm high × 2–3 µm wide, slightly inflated, straight or sometimes helicoidal, mostly furcate or anastomosing, covering the hilar appendix except its empty end, ornamented with low gibbosities; apical ring inconspicuous, 0.2–1 μm high; perisporial reticulum indistinguishable. Hilar appendix 1.5–2.7 µm long × 4.6–6.6 μm wide at apex, conico-truncate to cylindrical; empty-end 0.6–1.2 µm long; hilum 1.3–2 μm wide, without sterigmal remnants or very short. Observed some spores with apicular plug in apical position (!) obtruding the germ pore. Basidia 20–45 × 6–11 µm, cylindrical to clavate, 3–4-spored. Sclerobasidia present. Paraphysoid cells 15–60 × 5–8 µm, 0–1-septate, with the terminal cell acuminate, mucronate or subulate. Exposed locules fertile or partially sterile, presenting exocystidia and long septate hyphidia. Subhymenium pseudoparenchymatous, consisting of chains of 3–4 globose elements, 15–28 µm diam. Hymenophoral trama of interwoven hyphae, 3–6 µm diam. Thromboplera present. Peritrama of ridges and pseudoperidium of interwoven hyphae, 2–7 µm diam, with some enlargements up to 16 µm wide. Pseudopellis a confused hymeniderm, which forms a discontinuous, irregular, loose, inordinate trichotomentocutis of variable thickness, progressively covering the surface of ridges and exposed locules. Pseudopellis of ridges a hymeniderm of chains of inflated hyphae, 10–18 µm wide, and clusters of exocystidia, 10–40 µm wide, sometimes provided with a long septate rostrum. Trichotomentocutis consisting of thin-walled hyphae, 2–6 µm diam, many of them crystalliferous, mixed with tufts of fasciculate and agglutinated, yellow, thick-walled hairs, 2–5 µm diam with frequent enlargements, with walls 0.4–0.8 µm thick not staining with Congo red. Eventually, all elements of pseudopellis become embedded in a matrix of yellow pigment, constituting a confused tissue with a prosenchymatous aspect. Calcium oxalate crystals present in all tissues, especially in outer hyphae of pseudopellis.
Habitat, Season & Distribution — Gregarious, hypogeous, in sclerophyllous forests of Quercus rotundifolia, on calcareous soils. From winter to spring. Mediterranean species, found in Spain between 600–1200 m of altitude (Fig. 36).
Additional material studied. SPAIN, Basque Country, Álava, Campezo, Orbiso, 600 m, under Quercus rotundifolia, on calcareous soil, 13 Dec. 2013, F. Sáinz & P.M. Pasabán (JMV800825); Castilla and Leon, Burgos, Covarrubias, 1000 m, under Quercus rotundifolia, 24 Jan. 2015, A. Paz (IC24011506); Valladolid, Aldealbar, La Fraila, 850 m, under Quercus rotundifolia, on calcareous soil, 2 Feb. 2002, F. García-Verdugo & P. Juste (JMV800473*/20020202-1*); Valladolid, Cogeces del Monte, 900 m, under Quercus rotundifolia, on calcareous soil, 30 Apr. 2001, F. García-Verdugo (JMV800472*); Valencian Community, Alicante, Alcoi, Carrascar de la Font Roja Forest, 1180 m, under Quercus rotundifolia, on calcareous soil, 16 Jan. 1997, F. García-Alonso (MES3060, dupl. JMV800615).
Notes — Gautieria iberica is characterised by having: 1) a loose trichotomentocutis gradually covering ridges and exposed locules; 2) ridges irregularly fusing into a thin pseudoperidium; 3) locules of deep orange colour; 4) subulate paraphysoid cells; and 5) ovate to ovato-fusiform spores, often papillate, provided with a slightly inflated perisporium. Morphologically it is very similar to G. hellenica, which has smaller spores of elliptico-fusiform shape.
Gautieria iberica has a western Mediterranean distribution and is so far only known from Spain under holm oaks (Quercus rotundifolia). From this Gautieria we have studied 7 Spanish collections, of which we have successfully sequenced 4 collections (Table 1) and represented 3 sequences in our phylogenetic tree (Fig. 3). The GenBank sequence DQ974732, identified as G. ‘crispa’ (Smith et al. 2007b), could represent a North American sister species of G. iberica.
Clade GauGau-7
Represented by three undescribed North American taxa corresponding to the sequences AF377056, AF377057 and AF377068. Linked to conifers.
Clade pityophila (GauGau-8)
Ridges delicately alveolate-reticulate or fused into a thick pseudoperidium. Trichotomentocutis absent or well-developed. Spores narrowly elliptical to broadly elliptical, reaching 16–20(–23) × 10–12.5 µm. Costae slightly inflated, usually parallel, entire and smooth. Linked to conifers (Abies, Cedrus, Pinus). Represented by G. pervestita, G. pityophila and maybe by the North African G. pseudovestita.
Gautieria pervestita J.M. Vidal, Kaounas, Gkilas & Setkos, sp. nov. — MycoBank MB 845120; Fig. 17, 32, 34, 37
Fig. 17.
Gautieria pervestita. a. VK422. Basidiomata showing tomentose surface and thick pseudoperidium. — b. MG74. Section of pseudoperidium showing peritrama and tufts of hairs of trichotomentocutis. — c–d. GK4702. c. Yellow thick-walled hairs of trichotomentocutis; d. crystalliferous hairs of trichotomentocutis. — e. GK2293. e. Section of hymenophore and pseudoperidium. — f–h. VK1306. f. Hymenium and subhymenium; g. basidia and exocystidia-like elements of an outlying locule; h. paraphysoid cells lining an outlying locule. — i. VK880. Spores in KOH. — j–k. GK5007. j. Basidiomata; k. spores in KOH. — l–o. VK3009 (BCN JMV800556, holotype). l. Basidiomata; m. spores in KOH; n–o. SEM images of spores. — Scale bars: a, j, l = 1 cm; b–d, f–h = 20 µm; e = 5 mm; i, k, m = 10 µm; n–o = 5 µm.― Photos: a, e, l. V. Kaounas; b–d, f–i, k, m. J.M. Vidal; j. G. Konstantinidis; n. UdG; o. P. Mleczko, UJ.
Fig. 37.
Distribution map of species of Gautieria sect. Gautieria clades pityophila, confusa and fusella (GauGau-8, 9, 11).
Etymology. From Latin, per- = throughout + vestitus = clothed, referring to the regular trichotomentocutis that covers the basidiomata and by its thick pseudoperidium.
Type. GREECE, Central Greece, Attica, Acharnes, Parnitha Mount, 1225 m, under Abies cephalonica, on calcareous soil, 27 June 2013, V. Kaounas (holo BCN JMV800556*; iso in herb. pers. V. Kaounas VK3009 and in ELTE).
Basidiomata pseudoperidial, 3–5 cm wide, subglobose to tuberiform, with a slight basal protuberance attached to a white basal rhizomorph, 1–2 mm thick, lacking rhizoids. Surface white, tomentose, cottony-felted, turning pale orange (5A2–A4) to brown (7E7) when handled; light orange (5A4–A6) with brownish orange maculae (6C6–C8) in exsiccata. Ridges completely fused into a very thick and regular pseudoperidium, 0.5–1.5(–2) mm thick, without openings or at most with rare perforations communicating with the underlying locules. Pseudoperidium pure white in section, contrasting with the colourful hymenophore, not changing in colour with exposure to air. Locules 2–4 × 0.3–1 mm, irregularly shaped, sinuous, narrow, inordinate, greyish orange (6B4); cinnamon (6C5–D6) in exsiccata. Tramal plates 0.5–1 mm thick, white to grey, immutable when cut. Columella 0.5–3 mm thick, dendroid, whitish to greyish; basal context violaceous brown (10F4). Odour at first mild and pleasant, finally strong and alliaceous.
Spores (14–)16–20(–21.5) × (9–)10–12.5(–13.5) µm, Q = (1.3–)1.5–1.8(–1.9), elliptical to broadly elliptical, maize yellow (4A6) in KOH (Fig. 32n, 34h); in collection GK5007: (16–)18–23 × 9–11 µm, Q = 1.7–2.3, narrowly elliptical to subfusiform. Episporium elliptico-ovate. Costae 10–12, 0.5–1.5 μm high × 1.8–3.5 µm wide, slightly inflated, parallel, furcate or anastomosed, straight to slightly helicoidal, covering 1/2 of hilar appendix, without gibbosities or very low; apical ring inconspicuous, 0.2–0.8 µm high; perisporial reticulum indistinguishable. Hilar appendix 1.7–2.8 µm long × 3.3–5 µm wide at apex, conico-truncate to rounded; empty-end cylindrical, 0.3–0.7 µm long; hilum 0.9–1.5 µm wide, usually with remnants of sterigma. Basidia 40–70 × 12–17 µm, clavate, 3–4-spored, promptly collapsing. Paraphysoid cells 15–35 × 3–7 µm, 0–1-septate, with the terminal cell clavate, becoming long hairs of 50–70 × 3–5 µm, 1–2-septate, in outlying locules. Subhymenium consisting of chains of 3–4 cylindrical to subglobose elements, 8–14 µm diam. Hymenophoral trama of interwoven cylindrical hyphae, 2–6 µm diam, often with ampulliform septa up to 10 µm wide and some endocystidia-like terminal vesicles, 15–18 µm wide. Thromboplera 3–7 µm diam. Peritrama very thick and regular, 100–200 µm, covering completely the hymenophore without any opening, formed by hyaline, thin-walled, interwoven hyphae, 4–8 µm diam, with frequent enlargements and endocystidia-like terminal vesicles, 12–20 µm wide, finally gelatinized and embedded in yellow pigment in a prosenchymatous tissue. Pseudopellis consisting of a regular and well-developed trichotomentocutis of yellow, thick-walled hairs, 3–6 µm diam, usually not staining with Congo red, grouped in tufts born perpendicular to the surface of context, becoming geliferous and crystalliferous, finally collapsing into a yellow prosenchymatous tissue. Exocystidia absent in pseudopellis, but exocystidia-like elements, 8–20 µm wide, crystalliferous, entire or septate, clavate, pyriform, constricted or lageniform, are present in outlying locules mixed with fertile basidia. Calcium oxalate crystals present in all tissues.
Habitat, Season & Distribution — Solitary or gregarious, hypogeous, in montane forests of Abies cephalonica and A. borisiiregis, on calcareous soils. Almost throughout the year. Mediterranean species, found in Greece between 600–1200 m of altitude (Fig. 37).
Additional material studied. GREECE, Central Greece, Attica, Acharnes, Parnitha Mount, 1100 m, under Abies cephalonica, on calcareous soil, 2 June 2007, V. Kaounas (GK2293, dupl. JMV800559); ibid., 28 June 2007, V. Kaounas (GK2405, dupl. JMV800560); ibid., 1150 m, 14 June 2008, V. Kaounas (VK422, dupl. JMV800539); ibid., 1225 m, 18 Jan. 2009, V. Kaounas (VK663, dupl. JMV800545); ibid., 1210 m, 15 May 2009, V. Kaounas (VK880*, dupl. JMV800546); ibid., 1180 m, 14 Aug. 2009, V. Kaounas (VK914, dupl. JMV800548); ibid., 1160 m, 23 Dec. 2009, V. Kaounas (VK1306, dupl. JMV800549); ibid., 1220 m, 31 Mar. 2011, V. Kaounas (VK2121, dupl. JMV800543); Epirus, Ioannina, Dilofo, 800 m, under Abies borisii-regis, 23 May 2010, G. Setkos (GK5007*, dupl. JMV800564); Peloponnese, Arcadia, Alonistena, 1300 m, under Abies cephalonica, on calcareous soil, 22 Oct. 2016, M. Gkilas (MG345, dupl. JMV800724); Arcadia, Elati, 1300 m, under Abies cephalonica, on calcareous soil, 16 Feb. 2013, M. Gkilas (MG17, dupl. JMV800718); ibid., 1400 m, 30 Mar. 2013, M. Gkilas (MG36, dupl. JMV800720); ibid., 1300 m, 20 Feb. 2014, M. Gkilas (MG74, dupl. JMV800721); Arcadia, Mainalo, 670 m, under Abies cephalonica, on calcareous soil, 13 Apr. 2013, M. Gkilas (VK2959*, dupl. JMV800554); Arcadia, Mainalo, Ski Center, c. 1550 m, under Abies cephalonica, on calcareous soil, 14 Aug. 2016, M. Gkilas (MG311, dupl. JMV800723); Arcadia, Vlacherna, Pigi, 1450 m, under Abies cephalonica, on calcareous soil, 10 Dec. 2016, I. Velissaris (VK4702, dupl. JMV800636).
Notes — Gautieria pervestita is characterised by having: 1) a well-developed trichotomentocutis of yellow hairs; 2) a thick pseudoperidium up to 2 mm, white in section; and 3) elliptical spores with a tendency to be broadly elliptical, with smooth costae. In this species, the tomentum that covers the basidiomata is very dense and completely devoid of exocystidia and the peritrama is very thick and continuous, so both structures could be considered as a true peridium (Fig. 17a–c). Gautieria obtexta also has a well-developed trichotomentocutis, but the pseudoperidium is thinner and spores are larger and broader.
Gautieria pervestita is a calcicolous species, so far only known from Greece, mainly under Greek fir. From this Gautieria we have studied 17 Greek collections, of which we have successfully sequenced 4 collections (Table 1) and represented by 3 sequences in our phylogenetic tree (Fig. 3). In our phylogenetic tree, the ridged species G. pityophila appears genetically related to G. pervestita, with which it shares the small elliptical spores ornamented with parallel costae and the ecology linked to conifers.
Gautieria pityophila J.M. Vidal, Faust. García, Fern.-García, Kaounas & Konstantin., sp. nov. — MycoBank MB 845121; Fig. 18, 32, 34, 37
Fig. 18.
Gautieria pityophila. a–d. JMV960622-6 (BCN, holotype). a. Basidiomata; b. detail of its thinly alveolate surface; c. dendroid columella and locules of caramel colour; d. spores in KOH. — e. MA-Fungi 7922. Hymenium. — f. VK2980. Basidioma showing porate surface. — g–i. JMV960525-3. g. Basidiomata; h. peritrama with calcium oxalate crystals and pseudopellis showing fragile exocystidia; i. SEM image of spores. — Scale bars: a, f–g = 1 cm; b–c = 5 mm; d = 10 µm; e, h = 20 µm; i = 5 µm. — Photos: a–e, g–h. J.M. Vidal; f. V. Kaounas; i. P. Mleczko, UJ.
Etymology. From Greek, pityos = pine + phila = friend of, for its habitat linked mainly to trees of genus Pinus.
Type. SPAIN, Catalonia, Girona, Alp, Saltèguet Forest, Eastern Pyrenees, 1600 m, under Pinus uncinata and Abies alba, on schistose soil, 22 June 1996, J.M. Vidal as G. graveolens (holo BCN JMV960622-6*; iso in ELTE).
Basidiomata ridged to pseudoperidial, 2–5 cm wide, globose to tuberiform, with a small basal cavity attached to a white basal rhizomorph, 1.5–2 mm thick, immersed in a white mass of mycelium and rhizoids. Surface delicately alveolate-reticulate, exposing colourful locules, with fragile, rugose to cristate ridges, white for a long time, slightly changing to pinkish with manipulation. In old basidiomata, the cristae collapse and ridges flattened and fused irregularly, constituting a rudimentary and incomplete, porate-alveolate pseudoperidium that maintains openings to the underlying locules. Locules 1.5–3 × 0.3–1 mm, labyrinthoid to irregularly shaped, more or less radially arranged, greyish orange (6B5–B7) to brownish orange or caramel (6C5–C6); cinnamon (6D6–D7) in exsiccata. Tramal plates 0.2–0.4 mm thick, grey, immutable when cut. Columella 0.8–4 mm thick, dendroid, strongly branched, white to greyish; basal context brown (7D8). Odour pleasant, as of a mix of various aromatic plants with components of thyme and mint.
Spores 16–19(–21.5) × 8.5–10 μm, Q = 1.7–2, narrowly elliptical, light yellow (3A4–4A5) in KOH (Fig. 32o, 34i). Episporium elliptical. Costae 9–13, 0.6–1.4 μm high × 1.4–2.6 µm wide, slightly inflated, straight, mostly entire and parallel, sometimes furcate, covering 1/2 of hilar appendix, without gibbosities or very low; apical ring distinct, 0.5–1.5 µm high; perisporial reticulum indistinguishable. Hilar appendix 1.2–2.4 µm long × 2.6–3.7 µm wide at apex, conico-truncate; empty-end 0.1–0.5 µm long; hilum 1.2–1.5 µm wide, without remnants of sterigma. Basidia 40–65 × 10–14 µm, clavate, 2–4-spored. Observed some sclerobasidia. Paraphysoid cells 20–40 × 4–6 µm, cylindrical, 0–1-septate. Subhymenium consisting of chains of cylindrical to inflated elements, 5–12 µm diam. Hymenophoral trama of interwoven, thin-walled, hyaline hyphae, 2–4 µm diam. Thromboplera present. Peritrama of ridges and pseudoperidial parts similar to hymenophoral trama, with abundant calcium oxalate crystals. Pseudopellis a hymeniderm, formed by clusters of napiform exocystidia, 20–40 µm wide, yellow, thin-walled and fragile, soon collapsing.
Habitat, Season & Distribution — Gregarious, hypogeous or semi-hypogeous under needles, in montane and subalpine coniferous forests (Abies, Pinus), on calcareous or siliceous soils. Almost throughout the year. Rare. Distributed in Mediterranean and temperate regions of Southern Europe between 600–1600 m of altitude (Fig. 37).
Additional material studied. GERMANY, Bavaria, Marquartstein, 29 June 1920, Dr. Schnegg, det. E. Soehner #152 as G. graveolens (M 126226). – GREECE, Peloponnese, Arcadia, Elati, 1400 m, under Abies cephalonica, on calcareous soil, 30 Mar. 2013, M. Gkilas (MG34, dupl. JMV800719); Arcadia, Vlacherna, Pigi, 1450 m, under Abies cephalonica, on calcareous soil, 10 Dec. 2016, I. Velissaris (VK4703, dupl. JMV800637); Thessaly, Trikala, Koziakas Mountain, 1000 m, under Abies borisii-regis and Fagus sylvatica, 19 Nov. 2012, K. Papadimitriou (GK6359*, dupl. JMV800572); Voiotia, Elikonas, 1200 m, under Abies cephalonica, 23 Apr. 2013, G. Prountzopoulos & T. Tsantirtzioglou (VK2980*, dupl. JMV800555). – ITALY, Emilia-Romagna, Reggio Emilia, Civago, Abetina Reale Forest, 1400 m, under Pinus spp. and Abies alba, 30 July 1993, A. Montecchi as G. mexicana (AM1210*, dupl. JMV800040); ibid., 1500 m, under Abies alba, 8 Aug. 1999, A. Montecchi as G. otthii (AM1944*, dupl. JMV800244). – SPAIN, Aragon, Teruel, Puertomingalvo, 1400 m, under Pinus sylvestris, 29 Apr. 2007, F. García-Alonso (FGA073633, dupl. JMV800491); Castilla and Leon, Burgos, Oña, 600 m, under Pinus pinaster, Quercus faginea and Juniperus communis, 28 Nov. 1992, T. Pérez-Jarauta, det. F.D. Calonge as G. otthii (MA-Fungi 32261*, dupl. JMV921128); Segovia, Cuéllar, La Corredera Forest, 820 m, under Pinus pinaster and Populus × canescens, on sandy soil, 30 Mar. 1996, F. García-Verdugo & J.C. Santos, det. F.D. Calonge as G. mexicana (MA-Fungi 35390; S F21939); ibid., 7 Apr. 1996, F. García-Verdugo & M. Tabarés, det. J.M. Vidal as G. graveolens (JMV960407-4*); ibid., 25 May 1996, F. García-Verdugo, J.M. Vidal & J. Vila as G. graveolens (JMV960525-3*); ibid., 4 Mar. 2000, F. García-Verdugo & E. Rubio (JMV20000304-1); ibid., 17 Feb. 2002, P. Juste (JMV20020328-1); Castilla-La Mancha, Cuenca, Buenache, c. 1100 m, under Pinus nigra, 9 May 1984, F.D. Calonge as G. morchelliformis (MA-Fungi 7922*); Catalonia, Girona, Campelles, Pla dels Prats, Eastern Pyrenees, 1540 m, under Pinus sylvestris and Abies alba, on calcareous soil, 29 June 1996, J.M. Vidal as G. graveolens (JMV960629-11*); ibid., 23 Nov. 2008, F. Rodríguez (JMV20080923-4); ibid., 27 June 2018, J.M. Vidal, A. Paz & C. Lavoise (JMV20180627-3); Valencian Community, Castelló de la Plana, Vistabella del Maestrat, 1200 m, under Pinus sylvestris and Quercus rotundifolia, 3 June 2007, F. García-Alonso (FGA073707, dupl. JMV800492); ibid., 9 Feb. 2008, F. García-Alonso (FGA083935, dupl. JMV800495).
Notes — Calonge et al. (1994) briefly described and illustrated the spores of a Gautieria found in Spain under Pinus pinaster, which they identified as G. otthii. Subsequently, Vidal (1997) and Vidal et al. (1997), studied several collections found under P. pinaster, P. sylvestris, P. uncinata and Abies alba, also from Spain, and provided a more detailed description and new microscopic images with the name of G. graveolens. The true G. graveolens, is in a completely separate and distinct clade in sect. Glutinosiglebae (Fig. 3). This Gautieria, which we name G. pityophila because of its clear affinity for conifers of the genus Pinus, is characterised by having: 1) a delicately alveolate surface that remains white for a long time; 2) ridges with a tendency to flatten and fuse to generate an incipient pseudoperidium; 3) caramel colour of locules; and 4) narrowly elliptical spores with slightly inflated parallel costae.
This species has a meridional distribution, being rare outside the Mediterranean basin. We have studied a total of 21 collections of this species, found in 4 countries, of which we have successfully sequenced 10 collections (Table 1) and represented 4 sequences in our phylogenetic tree (Fig. 3).
Gautieria pseudovestita Malençon, Rev. Mycol. 39: 298. 1975 — Fig. 19, 32, 34, 37
Fig. 19.
Gautieria pseudovestita. a–f. MPU 312041 (holotype). a. Gelatinised tufts of hairs of trichotomentocutis; b. collapsed clusters of exocystidia of pseudopellis; c. collapsed hymenium; d. spores in KOH; e–f. SEM images of spores. — g–i. MPU 312042 (paratype). g. Gelatinised tufts of hairs of trichotomentocutis mixed with some exocystidia; h. spores in KOH; i. SEM image of spores. — Scale bars: a–c, g = 20 µm; d, h = 10 µm; e–f, i = 5 µm. — Photos: a–d, g–h. J.M. Vidal; e–f. P. Mleczko, UJ; i. UdG.
Etymology. From Latin, pseudo- = false + vestitus = clothed, referring to the evanescent tomentum that covers the basidiomata.
Type material studied. MOROCCO, Taza-Al Hoceima-Taounate, Ketama, km. 161, near Llano Amarillo, Rif Mountains, c. 1500 m, under Cedrus atlantica, on siliceous soil, 9 Nov. 1957, G. Malençon #3247 as ‘Gautieria otthii var. pseudovestita nob.’ (holo MPU 312041); Jbel Dah Doh, near Ketama, Rif Mountains, c. 1800 m, under Cedrus atlantica, on siliceous soil, 16 Nov. 1957, G. Malençon #3285 as ‘Gautieria otthii var. pseudovestita nob.’ (MPU 312042, paratypus).
Basidiomata pseudoperidial, 3.8–5 cm wide, ovate to globoseflattened, weakly gibbose, basally provided with a little white basal rhizomorph lacking rhizoids. Surface white, irregularly cottony-felted, tomentose, with some pores communicating with the underlying locules, then argillaceous and reddish bistre when handled or dried; brownish orange (6C5) in exsiccata. Ridges fused into a perforate pseudoperidium, pure white in section, at least 600 µm thick. Locules up to 1.5 mm wide, rounded to elongated, sinuous, inordinate, radially arranged on the periphery, wider in the upper half of hymenophore, pale fulvous-brown (towards Sayal Brown Ridgw., according to Malençon). Tramal plates 150–225 µm thick, whitish. Columella dendroid, undeveloped, white. Odour not observed. (Description based on Malençon 1975 and herbarium exsiccata).
Spores in holotype: (13–)14–18(–19) × (7.5–)8.5–10(–11) µm, Q = (1.3–)1.4–2, elliptical (Fig. 32p, 34j); in paratype: 11.5–16(–17) × 9.5–12 µm, Q = 1.2–1.4(–1.5), broadly elliptical to subglobose; maize yellow (4A6) in KOH. Episporium elliptical to ovate. Costae 10–12, 0.5–1 μm high × 2.2–3.3 µm wide, slightly inflated, mostly parallel, sometimes furcate or anastomosed, straight to slightly helicoidal, covering 1/2 of hilar appendix, without gibbosities or very low; apical ring inconspicuous, 0.2–0.7 µm high; perisporial reticulum indistinguishable. Hilar appendix 1.6–2.9 µm long × 3–3.7 µm wide at apex, conico-truncate to rounded; empty-end cylindrical, 0.5–0.1.3 µm long; hilum 0.8–1 µm wide, with remnants of sterigma. Observed some spores with the apicular plug in apical position (!) obstructing the germ pore. Basidia 32–45 × 9–10 µm, cylindrical, 3–4-spored. Paraphysoid cells difficult to observe. Subhymenium consisting of chains of 3–4 cylindrical to subglobose elements. Hymenophoral trama consisting of subgelatinous hyphae, mostly 3–4 μm diam, forming a confusing trama, being 5–6 μm diam in tramal anastomoses where the structure is looser. Thromboplera 2–7 µm diam. Peritrama thick, composed of densely interwoven hyphae, 3–6 µm diam, with some enlargements up to 15 µm wide. Pseudopellis a confused hymeniderm consisting of clusters of exocystidia and a poorly developed trichotomentocutis composed of tufts of hairs born perpendicular to its surface. Exocystidia 15–30 µm wide, sphaeropedunculate to pyriform. Hairs yellow, thick-walled, 3–6 µm diam, usually not staining with Congo red, with enlargements 10–15 µm wide, becoming geliferous and crystalliferous. Finally, all structures collapse into a yellow prosenchymatous tissue. Calcium oxalate crystals present in all tissues.
Habitat, Season & Distribution — Solitary, semi-hypogeous among needles, in montane forests of Cedrus atlantica, on siliceous soils. In autumn. Mediterranean, so far only known from North Africa, in Morocco between 1500–1800 m altitude (Fig. 37).
Notes — Gautieria pseudovestita was first collected by G. Malençon in Morocco, in the mountainous region of Rif near Ketama, actually Issaguen, in a cedar forest. Malençon (1975) comments that a great contrast exists in this species between the white sterile cover of a distinct thickness and the colourful fertile inner mass, which suggests the existence of a differentiated peridium, but that in reality there is no continuity or organic separation between these parts. The hyphae of the trichotomentocutis observed by Malençon (1975: 296) were considered by him as possible vestiges of an ‘initial veil’. Despite the fact that type material is parasitized by fungi and the hymenium and some hyphal elements are collapsed, we have been able to rehydrate part of the microscopic structures and characterize this species by: 1) a pseudopellis having a trichotomentocutis and clusters of exocystidia (sterile basidia according to Malençon 1975: 299); 2) ridges fusing into a thick pseudoperidium, presenting some openings; and 3) elliptical spores with tendency to subglobose, ornamented with parallel costae. Attempts to obtain DNA sequences from the two type collections were unsuccessful, but considering its macro and micro features similar to those of G. pervestita, such as the presence of a trichotomentocutis and spores ornamented with parallel and smooth costae (Fig. 19e–f), we consider that G. pseudovestita would be part of the same clade. Gautieria pityophila also appears to be related to G. pseudovestita, as it has spores of the same size and shape and is also linked to conifers.
Gautieria pseudovestita is a silicicolous species hitherto only know from Morocco that lives under Atlas cedars. We have no evidence or new findings after the collections made by Malençon in 1957.
Clade confusa (GauGau-9)
Ridges crisped, irregularly alveolate-reticulate, or fused into a thin pseudoperidium. Trichotomentocutis absent or well-developed. Spores variable in shape, elliptical, broadly elliptical, broadly ovate or ovato-fusiform, reaching 19.5–22.5 × 13–14.5 µm. Costae ornamented with broad gibbosities. Linked to conifers (Abies, Picea, Pseudotsuga, Tsuga) or broadleaf trees (Fagus, Quercus). Represented by G. confusa, G. obtexta and a North American taxon related to G. confusa corresponding to the sequences MG773849 and MK607602.
Gautieria confusa J.M. Vidal, Fern. Rodr., L. Albert, Mleczko & Slavova, sp. nov. — MycoBank MB 845122; Fig. 20, 32, 34, 37
Fig. 20.
Gautieria confusa. a–d. JMV20110621-8 (BCN, holotype). a. Basidiomata showing granulose surface; b. detail of cristate ridges; c. section of a crista showing the pseudopellis and peritrama; d. spores in KOH. — e–f. JMV20131008. e. Young basidiomata showing crisped ridges and dendroid columella; f. detail of crisped ridges. — g–h. JMV20120604. g. Mature basidiomata showing alveolate-reticulate surface and dendroid columella; h. detail of melon yellow locules. — i. ZB1821. Hymenium showing basidia and paraphysoid cells. — j. ZB1823. Spores in KOH. — k–l. MA-Fungi 39289. SEM images of spores showing their high variability. — Scale bars: a, e, g = 1 cm; b, f, h = 5 mm; c, i = 20 µm; d, j = 10 µm; k–l = 5 µm. — Photos: a–b, e–f. F. Rodríguez; c–d, g–j. J.M. Vidal; k–l. P. Mleczko, UJ.
Etymology. From Latin, confusus = confused, because it is easy to confuse with other species, especially with G. morchelliformis.
Type. SPAIN, Catalonia, Girona, Viladrau, Mas El Martí, Montseny Massif, 980 m, Pseudotsuga menziesii plantation, 21 June 2011, J.M. Vidal & F. Rodríguez (holo BCN JMV20110621-8*; iso in ELTE).
Basidiomata ridged, 2–5 cm wide, globose to irregular, with a basal cavity attached to a white basal rhizomorph, 1.5–2 mm thick. Surface initially white to cream, with the ridges crisped, looking like a small bonfire cauliflower (Daleomyces phillipsii), then melon yellow, granulose, rugose, irregularly alveolate-reticulate, exposing colourful locules, with the ridges cristate, white. Ridges turning deep red (11C8) with handling. Locules 1.5–4.5 × 0.3–1 mm, labyrinthoid to irregularly shaped, inordinate, melon yellow (5A6); cinnamon (6D6–D8) in exsiccata. Tramal plates 0.2–0.4 mm thick, greyish, slightly pinkish when cut. Columella 0.5–2.5 mm thick, dendroid, strongly branched, greyish, not changing with exposure to air; basal context wine red (11D8). Odour strong, of seafood or alliaceous.
Spores (15.5–)16–19.5(–20) × 11–13 µm, Q = 1.3–1.7, very variable in shape, elliptical to broadly elliptical or broadly obovate, light yellow with greenish hues (3A5–A6) in KOH (Fig. 32q, 34k). Episporium narrowly ovate to ovate. Costae 9–12, 0.9–1.8 µm high × 2–3.7 µm wide, moderately to very inflated, straight or helicoidal, entire, furcate or anastomosing, covering 3/4 or entire hilar appendix, ornamented with large gibbosities; apical ring distinct, 1–2.8 µm high; perisporial reticulum distinguishable, orange-yellow. Hilar appendix 2.3–3.8 µm long × 4–5 µm wide at apex, conico-truncate to rounded due to a basal widening; empty-end 0.4–1 µm long; hilum 1.3–1.8 µm wide, mostly provided with a short sterigmal remnant 0.6–1.8 µm long. Basidia in early stages clavate or attenuate, measuring 18–45 × 6.5–14 µm; finally, cylindrical to clavate, 2–4-spored, measuring 40–60 × 8–15 µm. Sclerobasidia present. Paraphysoid cells 18–50 × 3–5 µm, 0–1-septate, sinuous, with the terminal cell cylindrical. Subhymenium pseudoparenchymatous, formed by several layers of subglobose cells, 10–18 µm diam. Hymenophoral trama of interwoven hyphae, 3–6 µm diam. Thromboplera present. Peritrama of ridges similar to hymenophoral trama. Pseudopellis a hymeniderm circumscribed to edge of ridges, formed by clusters of sphaeropedunculate exocystidia, 15–30 µm wide.
Habitat, Season & Distribution — Gregarious, hypogeous or semi-hypogeous under plant debris, from montane broadleaf (Fagus, Quercus) to subalpine coniferous forests (Abies, Picea, Pseudotsuga), preferably on siliceous soils. Almost throughout the year. Rare. Distributed in temperate regions, from 200 m altitude in Central Europe to 1700 m in Southern Europe (Fig. 37).
Additional material studied. AUSTRIA, Tyrol, Innsbruck, Schlotthof bei Hötting, ‘unter jungfichte’, 14 Sept. 1952, W. Gams, det. M. Moser #52/42 as G. morchelliformis (M 126255); sine loc., Nov. 1950, W. Gams, det. M. Moser #50/133 as G. morchelliformis (M 126256). – BULGARIA, Kyustendil, Osogovo Mountains, 1650 m, under Fagus sylvatica, on siliceous soil, 14 Apr. 2017, M. Slavova (SOMF 30317*, dupl. JMV800733); ibid., 17 Apr. 2018, M. Slavova (SOMF 30342, dupl. JMV800740). – CZECH REPUBLIC, Central Bohemia, Kačina, near Čáslav, under Quercus, 1855, Peyl, det. A. Pilát as G. morchelliformis (W 1784, ex PRM); Strašice, Brdy Mountains, J. Velenovský, det. A. Pilát as G. morchelliformis (W 1786, ex PRM); Liberec, Mníšek, Oct. 1937, V. Vacek, det. A. Pilát as ‘G. morchelliformis fo. tending to var. globispora’ (W 1785, ex PRM). – GERMANY, Baden-Württemberg, Wiesental, Dossenbach (Friburg), near Schopfheim, under Picea abies, 7 Aug. 1955, C. Schwärzel #52 as G. otthii (ZT-Myc 3930); ibid., under Abies alba, 17 Sept. 1955, C. Schwärzel #69 as G. otthii (ZT-Myc 3931); Bavaria, Bad Wörishofen (Wörishofen), Fichtelgebirge, Abies-Picea forest, Aug. 1916, S. Killermann as G. graveolens (M 126267; M 126237, herb. E. Soehner #133/1395); ibid., 25 Aug. 1916, S. Killermann as G. morchelliformis (M 126273); Erharting bei Mühldorf, ‘Fichtenwald’, 27 July 1921, E. Soehner #615 as G. graveolens (M 126224); ibid., 20 July 1922, E. Soehner #500 as G. graveolens (M 126221); ibid., ‘waldparzelle Eiskeller’, 29 July 1924, E. Soehner #866 as G. graveolens (M 126223; FH 301440, herb. C.W. Dodge). – HUNGARY, Heves, near Parádsasvár, Mátra Mountains, 200 m, under Fagus sylvatica, 3 Sept. 1999, L. Albert (ZB1732*, dupl. JMV800411); Nógrád, near Bátonyterenye, Mátra Mountains, c. 250 m, under Pinus sp., 10 Oct. 1999, S. Csikós (ZB1823*, dupl. JMV800414); near Keszeg, Cserhát Mountains, c. 300 m, Oct. 1999, sine leg. (ZB1821*, dupl. JMV800412); near Kisterenye, Mátra Mountains, c. 250 m, under Quercus cerris and Quercus rubra, 27 May 2005, B. Dima, L. Albert & M. Németh (ZB3000, dupl. JMV800433). – ITALY, Piedmont, sine dat., O. Mattirolo as G. morchelliformis (PRM 678942); Trentino-Alto Adige, Trento, Vetriolo Terme, 700 m, in deciduous forest, 15 Sept. 1986, A. Montecchi as G. otthii, comm. L. Gori (ELG860915, dupl. JMV800358). – POLAND, Lesser Poland, near Hałuszowa, about the Szkółka, Pieniny Nat. Park, 660 m, Abies-Fagus forest, 21 July 2017, P. Mleczko & P. Komur (KRA F-2017-17*, dupl. JMV800711). – SPAIN, Castilla and Leon, Salamanca, El Cabaco, under Quercus pyrenaica, 25 Oct. 1997, B. Marcos, det. F.D. Calonge as G. morchelliformis (MA-Fungi 39289*); Segovia, Riofrío de Riaza, Hayedo de La Pedrosa, under Fagus sylvatica, on siliceous soil, 29 Sept. 2011, F. García-Verdugo (JMV800475*); Catalonia, Girona, Alp, Saltèguet Forest, Eastern Pyrenees, 1600 m, under Abies alba and Pinus uncinata, on schistose soil, 19 June 1993, J.M. Vidal (JMV930619-1b); ibid., 22 June 1996, J.M. Vidal (JMV960622-11*); Girona, Sant Joan de les Abadesses, Castella Forest, 1000 m, under Fagus sylvatica, on siliceous soil, 28 Oct. 2008, J.M. Vidal & F. Rodríguez (JMV20081028-1*); Girona, Viladrau, Camí de la Font de Viladrau, Montseny Massif, 900 m, under Fagus sylvatica, on siliceous soil, 4 June 2012, F. Rodríguez (JMV20120604); ibid., 8 Oct. 2013, F. Rodríguez (JMV20131008); Girona, Viladrau, Mas El Martí, Montseny Massif, 984 m, Pseudotsuga menziesii plantation, 10 June 2013, F. Rodríguez (JMV20130610); Valencian Community, Castelló de la Plana, Vistabella del Maestrat, 1400 m, under Quercus pyrenaica, 25 Jan. 2008, F. García-Alonso (FGA083915, dupl. JMV800494). – SWITZERLAND, Basel-Landschaft, Basel-Stadt, Basel, 1921, A. Knapp #106b as G. morchelliformis (ZT-Myc 3943); Solothurn, Dornach to Gempen, under Fagus sylvatica, 23 Oct. 1955, C. Schwärzel #29 as G. otthii (ZT-Myc 3934); Zürich, near Zürich, ‘in silvis abietis’, Aug. 1880, G. Winter as G. graveolens, Fungi Helvetici, suppl. 66 (FH 301442b, two specimens mixed with the Winter’s collection #66 identified here as G. otthii; H 7017229, herb. P.A. Karsten); Zürich, sine dat., G. Winter as G. morchelliformis (S F181876, ex herb. Sydow).
Notes — Gautieria confusa is characterised by having: 1) young basidiomata looking like a bonfire cauliflower; 2) yellowish orange locules; and 3) small gibbose spores with an ovate episporium and usually a rounded hilar appendix that is mostly enveloped by the perisporium. Due to its basidiomata morphology, this species appears often determined as G. morchelliformis in the different herbaria consulted or, due to its small spores, as G. graveolens or G. otthii. Gautieria morchelliformis var. fageticola and G. villosa var. villosa have spores of similar size, but in the former the hilar appendix, which also tends to be rounded, is wider and the episporium is elliptical, and in the latter the hilar appendix is narrower and the episporium is subfusiform.
Gautieria confusa is a fairly rare species with a variable ecology, inhabiting from montane broadleaf forests to subalpine coniferous forests. From this Gautieria we have studied 35 collections from 9 countries, of which we have successfully sequenced 10 collections (Table 1) and represented 4 sequences in our phylogenetic tree (Fig. 3). The GenBank sequences MG773849 (Matheny et al. unpubl.) and MK607602 (Russell & Grootmyers unpubl.) could represent a North American variety of G. confusa.
Gautieria obtexta J.M. Vidal, P. Fantini & Kaounas, sp. nov. — MycoBank MB 845123; Fig. 21, 32, 34, 37
Fig. 21.
Gautieria obtexta. a–e. JMV800179 (BCN, holotype). a. Basal view of a basidioma and transversal section of hymenophore; b. detail of surface and cracking of pseudoperidium; c. spores in KOH; d. section of pseudoperidium showing hyphae of peritrama and tufts of hairs of trichotomentocutis; e. yellow thick-walled hairs of trichotomentocutis. — f. JMV800176. Hymenium. — g–i. VK1395. g. Basidiomata showing the white context of pseudoperidium and the dark brown bruised surface; h. hairs of trichotomentocutis mixed with some exocystidia and crystals of calcium oxalate; i. hyphae of peritrama. — j–k. VK1376. j. Basidiomata; k. spores in KOH. — l–o. JMV800177. l. Mature basidioma showing cracked pseudoperidium; m. fascicle of hairs of trichotomentocutis showing inflated hyphae; n–o. SEM images of spores. — Scale bars: a, g, j = 1 cm; b, l = 5 mm; c, k = 10 µm; d–f, h–i, m = 20 µm; n–o = 5 µm. — Photos: a–b. l.P. Fantini; c–f, h–i, k, m. J.M. Vidal; g, j. V. Kaounas; n. UdG; o. P. Mleczko, UJ.
Etymology. From Latin, obtextus = wrapped, in reference to the thin membrane covering the basidiomata.
Type. ITALY, Sardinia, Oristano, Laconi, Funtana Gioia, 800 m, under Quercus ilex, on calcareous soil, 20 Feb. 2002, P. Fantini (holo BCN JMV800179*; iso in ELTE).
Basidiomata pseudoperidial, 1.5–4 cm wide, subglobose to tuberiform, with a small basal depression attached to a white basal rhizomorph, 1.5–2 mm thick. Surface white, cottony-felted, covered by a regular tomentum, turning pale orange (5A3) to dark brown (6F6) when exposed to air or rubbed; greyish orange (5B4) in exsiccata. Ridges fused into a thin, membranous, regular pseudoperidium, white in section, 0.15–0.3 mm thick, cracking at maturity and exposing the hymenophore. Locules 1–3 × 0.2–0.6 mm, irregularly shaped, sinuous, more or less radially elongated but inordinate, greyish orange (5B6); cinnamon (6C6–D7) in exsiccata. Tramal plates 0.3–0.5 mm thick, greyish, slightly reddening to pale red (11A3) when cut. Columella 0.7–2.5 mm thick, slightly dendroid, whitish to greyish; basal context brownish. Odour at first fruity and finally intense, similar to that of Tuber rufum.
Spores 17.5–22.5 × 12–14.5 µm, Q = 1.4–1.7, ovato-fusiform, reddish yellow (4A7–A8) in KOH (Fig. 32r, 34l). Episporium ovate, red to brownish red in section (9B7–C8). Costae 9–12, 0.5–1 μm high × 2–3 µm wide, slightly inflated, straight or sometimes helicoidal, mostly furcate or anastomosing, covering the hilar appendix except its empty end, ornamented with low gibbosities; apical ring inconspicuous, 0.2–1 μm high; perisporial reticulum indistinguishable. Hilar appendix 3–4.5 µm long × 4.5–6 μm wide at apex, conico-truncate; empty-end 0.3–0.8 µm long; hilum 1.2–1.8 μm wide, usually without sterigmal remnants. Basidia 45–85(–100) × 10–15 µm, clavate, 3–4-spored. Sclerobasidia abundant. Paraphysoid cells 20–45(–80) × 3–5(–7) µm, 0–1-septate, abundant in exposed locules. Sometimes also observed little groups of exocystidia-like elements. Subhymenium consisting of chains of 3–4 cylindrical to subglobose elements, 6–15 µm diam. Hymenophoral trama of interwoven hyphae, 3–6 µm diam, with ampulliform septa up to 10 µm wide. Thromboplera present. Peritrama of densely interwoven hyphae, 2–7 µm diam, with some ampulliform septa up to 14 µm wide, and some endocystidia-like terminal vesicles, 25–35 µm wide. Pseudopellis consisting of a regular trichotomentocutis of dense tufts of yellow, thick-walled hairs, 3–6 µm diam, born perpendicular to the surface of context, mixed with inflated hyphae, 7–20 µm wide, usually not staining with Congo red, becoming geliferous and crystalliferous, finally collapsing into a yellow prosenchymatous tissue. Scattered intermingled exocystidia up to 20 µm wide can be observed. Calcium oxalate crystals present in all tissues.
Habitat, Season & Distribution — Gregarious, hypogeous, in sclerophyllous woods of Quercus ilex, on calcareous soils. From autumn to spring. Rare. Mediterranean species, known from Greece and Italy between 200–800 m of altitude (Fig. 37).
Additional material studied. GREECE, Central Greece, Attica, Katsimidi, foot of Parnitha Mount, 600 m, under Quercus ilex and Pinus halepensis, on calcareous soil, 11 Feb. 2010, V. Kaounas (VK1376*, dupl. JMV800541); ibid., 615 m, 20 Feb. 2010, V. Kaounas (VK1395*, dupl. JMV800550). – ITALY, Sardinia, Oristano, Palmas Arborea, Monte Arci, Fundu Ureu, 400 m, under Quercus ilex, on basaltic soil, 20 Apr. 1997, P. Fantini (JMV800176); ibid., 13 Feb. 2002, P. Fantini (JMV800177*); Tuscany, Siena, Granaio, Castellina in Chianti, 220 m, under Quercus ilex, Cupressus sempervirens and Arbutus unedo, 5 Nov. 1996, L. Gori as G. otthii (JMV800362).
Notes — Gautieria obtexta is characterised by having: 1) a well-developed trichotomentocutis of yellow hairs; 2) ridges fusing into a thin and regular pseudoperidium up to 0.3 mm thick; and 3) ovato-fusiform spores. In morphological comparisons between the elements that make up the hymenium and the pseudopellis, we have observed that the sterile elements of the hymenium (paraphysoid cells) of tomentose species are transformed into different types of hairs existing on the edge of the ridges and the pseudoperidial surface. This transformation into hairs is very noticeable in this species, which grow dense clusters of hairs (Fig. 21d–e).
This species is distributed in eastern Mediterranean region, living in basic soils under holm oaks (Quercus ilex), in the same habitat as G. morchelliformis var. morchelliformis, with which it can coexist. From this Gautieria we have studied 6 collections from Greece and Italy, of which we have successfully sequenced 4 collections (Table 1) and represented 2 sequences in our phylogenetic tree (Fig. 3).
Clade chilensis (GauGau-10)
Characterised by having ridged basidiomata and elliptical or fusiform spores with slightly inflated parallel costae. Represented by G. chilensis and other undescribed South American taxa corresponding to the sequences AF377069, KY462601, KY462630 and MT366694. Linked to Nothofagus.
Clade fusella (GauGau-11)
Ridges venose or fused into a venose-reticulate pseudoperidium. Trichotomentocutis absent, but sometimes an incipient trichoderm may be observed. Tramal plates usually broader than locules, reaching 1 mm in thickness. Spores fusiform, reaching 19–21.5 × 10–12.5 µm, with a rounded or acuminate hilar appendix, with a sterigmal remnant. Costae usually inflated and gibbose. Linked to broadleaf trees (Carpinus, Castanea, Fagus, Nothofagus, Quercus) or conifers (Abies, Picea, Pinus). Represented by G. fenestrata and G. fusella.
Gautieria fenestrata J.M. Vidal, Cabero, Papadimitriou & Slavova, sp. nov. — MycoBank MB 845124; Fig. 22, 32, 34, 37
Fig. 22.
Gautieria fenestrata. a–f. JMV20110913-1 (BCN, holotype). a. Basidiomata showing broadly alveolate surface; b. detail of venose ridges; c. hymenophore showing buttercup yellow locules and reddening of tramal plates; d. section of a ridge showing the pseudopellis and adjacent fertile hymenium; e. detail of developing pseudopellis containing some fertile basidia; f. spores in KOH. — g. JC080511BT. Basidiomata with buttercup yellow coloration. — h. K(M) 59218 (L.E. Hawker as G. morchelliformis). Spores in KOH. — i. JMV800355a. SEM image of spores. — Scale bars: a, g = 1 cm; b–c = 5 mm; d–e = 20 µm; f, h = 10 µm; i = 5 µm. — Photos: a–f, h. J.M. Vidal; g. J. Cabero; i. P. Mleczko, UJ.
Etymology. From Latin, fenestra = window, in reference to the large openings on the surface of the basidiomata.
Type. SPAIN, Catalonia, Girona, La Vall de Bianya, Sant Salvador de Bianya, Coll de Capsacosta, 900 m, under Fagus sylvatica, on sandstone soil with calcium carbonate, 13 Sept. 2011, J.M. Vidal & F. Rodríguez (holo BCN JMV20110913-1*; iso in ELTE).
Basidiomata ridged, 2–4.5 cm wide, globose to irregular, gibbose, with a small basal depression attached to a white basal rhizomorph, 1–1.5 mm thick, immersed in a dense mass of white mycelium that covers the base of the basidiomata. Surface initially white, granulose, then cerebrose and finally venose, broadly alveolate, buttercup yellow (4A7), with smooth or granulose, rounded and protruding white ridges that tend to flatten, which surround broad exposed locules. Ridges turning deep brownish red (10D7) when rubbed. Locules 1–4 × 0.3–1.5 (–2) mm, labyrinthoid to irregularly shaped, more or less radially elongated, inordinate, sinuous, usually with a narrow lumen, buttercup yellow (4A7); cinnamon (5D6–6D7) in exsiccata. Tramal plates 0.3–0.7(–1) mm thick, usually broader than locules, white, staining pale violet (15A3–B3), dull red or brownish red (10C3–D6) when cut. Columella 0.3–1.5 mm thick, undeveloped, basal, white; basal context violet brown (10E6). Odour pleasant of aromatic plants, similar to that of G. morchelliformis.
Spores (15–)16–21.5(–23) × (10–)10.5–12.5(–14) μm, Q = 1.4–1.7(–2), elliptical to broadly elliptico-fusiform, yellowish orange (4A6–A8) in KOH (Fig. 32s, 34m). Episporium broadly elliptico-fusiform to ovate. Costae (8–)10–13, 0.6–1.2 μm high × 2–4 µm wide, slightly inflated, straight or sometimes helicoidal, mostly entire and parallel, sometimes furcate, covering the entire hilar appendix, ornamented with low gibbosities; apical ring distinct, 1–2 μm high; perisporial reticulum indistinguishable. Hilar appendix short, 1–2.5 µm long × 2.3–4 μm wide at apex, rounded; empty-end cylindrical, 0.3–0.9 µm long; hilum 1–1.5 μm wide, retaining a sterigmal remnant, 1–4 µm long. Basidia 35–45 × 8–12 µm, clavate, 2–4-spored. Paraphysoid cells 15–45 × 8–10 µm, 0–1-septate, with the terminal cell clavate. Subhymenium composed of chains of 3–4 cylindrical elements, 5–10 µm diam, densely compacted. Hymenophoral trama of interwoven hyphae, 2–5 µm diam, with some ampulliform septa, 8–10 µm wide, and endocystidia-like terminal vesicles up to 18 µm wide. Thromboplera abundant, 2–4 µm diam, with enlargements in septa up to 11 µm wide. Peritrama of ridges of compact interwoven hyphae, 2–3 µm diam, with some enlargements. Pseudopellis a hymeniderm circumscribed to edge of ridges, formed by clavate to sphaeropedunculate exocystidia, 20–25(–30) µm wide, hyaline to yellow, with a wall up to 1.5 µm thick, sometimes with a mucronate apex.
Habitat, Season & Distribution — Gregarious, hypogeous or semi-hypogeous under plant debris, from montane broadleaf forests (Castanea, Fagus, Quercus) to subalpine coniferous forests (Abies), on calcareous or siliceous soils. Almost throughout the year. Rare. Distributed in temperate and Mediterranean regions, from sea level in Northern Europe to 1000 m altitude in Southern Europe (Fig. 37).
Additional material studied. BULGARIA, Kyustendil, Giueshevo, Osogovo Mountains, 1200 m, under Fagus sylvatica and Quercus sp., on siliceous soil, 26 July 2018, M. Slavova (SOMF 30331*, dupl. JMV800749). – FRANCE, Provence-Alpes-Côte d’Azur, Saint-Vallier-de-Thiey, near cemetery, Maritime Alps, 750 m, under Abies alba, 8 Feb. 2013, A. Paz (IC08021306); Rhône-Alpes, from the Jura, sine dat., N. Patouillard as G. morchelliformis (NY 1952349, herb. S.M. Zeller #1533, ex C.G. Lloyd #08+53, pro parte, specimen that is part of Lloyd’s collection, identified here as G. macrocoilia). – GERMANY, Bavaria, Munich, Geiselgasteig, under Fagus sylvatica, 15 June 1920, E. Soehner #109 as G. graveolens (M 126218); Munich, Grünwald, under Fagus sylvatica, 23 Sept. 1923, E. Soehner #816 as G. graveolens (M 126228); ibid., 14 June 1943, E. Soehner #1921 as G. graveolens (M 126225). – GREECE, Thessaly, Trikala, Koziakas Mountain, 1050 m, under Abies borisii-regis, 21 Dec. 2011, K. Papadimitriou (GK6445*, dupl. JMV800570); West Macedonia, Florina, Vatochori, under Quercus sp. and Populus sp., 30 Oct. 2018, N. Tsilis (GK11861*, dupl. JMV800820). – HUNGARY, Heves, Bagolyirtás, Mátra Mountains, c. 800 m, under Carpinus betulus, growing mixed with G. morchelliformis, 2 July 2007, I. Zagyva (ZI239b, dupl. JMV800450b). – ITALY, Tuscany, Lucca, Molazzana, Alpi di San Antonio, 1200 m, under Castanea sativa, Quercus cerris and Ostrya carpinifolia, 8 Jan. 2000, G. Bernardini & L. Gori as G. morchelliformis (ELG20000108*, dupl. JMV800355a); Lucca, Vergemoli, 900 m, under Fagus sylvatica, 2 Jan. 2000, G. Bernardini & L. Gori as G. morchelliformis (ELG20000102*, dupl. JMV800354). – NORWAY, Oslo, Bygdøy, slope at Oscarshall, 23 Sept. 1952, M. Lange & F.E. Eckblad as G. graveolens, det. K.S. Bergsnov Hansen #5 as G. morchelliformis (O 152357); Bygdøy to Oscarhall, 6 Oct. 1953, O. Skifte, J. Stordal & F.E. Eckblad, det. K.S. Bergsnov Hansen #6 as G. morchelliformis (O 152356); ibid., 9 Oct. 1956, R.Y. Berg & F.E. Eckblad, det. K.S. Bergsnov Hansen #7 as G. morchelliformis (O 152351). – SPAIN, Castilla and Leon, Palencia, Vado, 1000 m, under Fagus sylvatica and Quercus rotundifolia, 22 Sept. 2007, J. Cabero (JC70922BT, dupl. JMV800477). – SWITZERLAND, Bern, Spiez, Faulenseewald, under Fagus sylvatica, November, J.G. Trog as G. morchelliformis (FH 301432, herb. C.W. Dodge). – UNITED KINGDOM, England, West Gloucestershire, Wotton-under-Edge, Westrige Wood, Brackenbury Path, under Fagus sylvatica, 7 Sept. 1949, L.E. Hawker H14 as G. morchelliformis (K(M) 59220); ibid., 14 May 1950, L.E. Hawker H110 as G. morchelliformis (K(M) 59221); ibid., Brackenbury Ring, 5 Aug. 1953, J. Fraymouth #201, det. L.E. Hawker H757 as G. morchelliformis (K(M) 59217); ibid., 5 Aug. 1953, L.E. Hawker H728 as G. morchelliformis (K(M) 59218).
Notes — Gautieria fenestrata is characterised by having: 1) white venose ridges surrounding broad exposed locules; 2) tramal plates turning violaceous or brownish red when cut; 3) buttercup yellow locules; and 4) broadly elliptico-fusiform spores with a short rounded hilar appendix, retaining a sterigmal remnant. Gautieria fusella also has appendiculate spores, but they are smaller and narrower, and the pseudopellis has a pseudoparenchymatous structure. Gautieria fenestrata is a rare but widespread species that, like G. fusella, can be found in montane deciduous and subalpine coniferous forests. Between 1949 and 1953, Lilian Edith Hawker collected in England a Gautieria that she identified and described as G. morchelliformis (Hawker 1952). Hawker (1954), in a special work on British hypogeous fungi, provided a further description of this species and good microscopic representations of the mature hymenium, the sterile surface of the ridges, and the adjacent hymenium of an exposed locule. Pegler et al. (1993), after studying the collections made by Hawker, provided a new description, illustrations and SEM images of spores, again under the name G. morchelliformis. We have studied the spores of these collections and have observed a total identity with G. fenestrata (Fig. 22h). From this Gautieria we have studied 21 collections from 10 countries, of which we have successfully sequenced 6 collections (Table 1) and represented 4 sequences in our phylogenetic tree (Fig. 3).
Gautieria fusella J.M. Vidal, Bratek, Slavova, Chachuła, Kozak & Konstantin., sp. nov. — MycoBank MB 845125; Fig. 23, 32, 34, 37
Fig. 23.
Gautieria fusella. a–c. JMV20110425 (BCN, holotype). a. Basidiomata; b. detail of compact ridges; c. spores in KOH. — d. SOMF 30329. Basidiomata showing venose-reticulate surface and semi-dried hymenophore showing cinnamon locules. — e–f. TPN-19-0148. e. Pseudoparenchymatous pseudopellis; f. trichodermial geliferous hairs of pseudopellis — g–h. SOMF 30328. g. Basidiomata showing yellow flocculosities and compact hymenophore; h. detail of thick tramal plates and obliterated locules. — i. ZB2744. Pseudopellis. — j–k. SOMF 30327. j. Young basidiomata showing yellow flocculosities and pointed caudal appendages; k. young hymenium. — l. ZB2740. Spores in Hoyer. — m–n. KRA F-2010-61. SEM images of spores showing remnants of sterigmata. — Gautieria caudata. o. NY 780825 (holotype of Hymenogaster caudatus). Spores in KOH previously preserved in alcohol. — Scale bars: a, d, g, j = 1 cm; b, h = 5 mm; c, l, o = 10 µm; e–f, i, k = 20 µm; m–n = 5 µm. — Photos: a–b. F. Rodríguez; c, e–f, i, k–l, o. J.M. Vidal; d, g–h, j. M. Slavova; m–n. P. Mleczko, UJ.
Etymology. From Latin, fusus = spindle + the diminutive suffix -ella, in reference to the tapered shape and small size of the spores.
Type. SPAIN, Catalonia, Girona, Campelles, Baga de Campelles Forest, Eastern Pyrenees, 1500 m, under Abies alba and Pinus sylvestris, 25 Apr. 2011, F. Rodríguez (holo BCN JMV20110425*; iso in ELTE).
Basidiomata ridged to pseudoperidial, 1.5–5 cm wide, subglobose to tuberiform, with a small basal depression attached to a robust white basal rhizomorph, 2 mm thick. When basidiomata are uprooted, the end of basal rhizomorph often remains attached to its base in the form of a pointed caudal appendage. Surface initially white, consisting of minute ridges, compactly convoluted, without exposing the peripheral locules, being often covered by an evanescent pastel yellow (2A4) pruinosity. At maturity, ridges fuse forming a venose-reticulate pseudoperidium, 0.2–0.5 mm thick, reddening to pastel red (9B5) with handling. Locules 0.75–1.5 × 0.1–0.5 mm, labyrinthoid to irregularly shaped, narrow, almost obliterated, inordinate, initially pastel yellow (3A3–A4) and then pale orange (5A3–A4); cinnamon (6D6) in exsiccata. Tramal plates very thick, 0.4–1 mm, broader than locules, greyish white, turning pale red (9A3) when cut. Columella 0.2–1 mm thick, dendroid, basally well developed, sometimes finely branched to the surface, grey; basal context reddish brown (9D8). Odour at first fruity, then alliaceous, very intense and nauseous, reminiscent of G. otthii.
Spores (13–)15–20(–21) × (7–)8–10(–11) µm, Q = (1.6–)1.7–2.2(–2.3), narrowly elliptico-fusiform, yellowish orange (4A6–A8) in KOH (Fig. 32t, 34n). Episporium subfusiform, basally rounded to truncate. Costae 7–9(–11), straight or helicoidal, entire or furcate, 0.8–2 µm high × 2–3 µm wide, moderately to very inflated, covering up to 1/4 of hilar appendix and occasionally constituting a visible peri-hilar ring, usually ornamented with large gibbosities; apical ring inconspicuous, 0.5–1 μm high; perisporial reticulum indistinguishable. Hilar appendix 1–3.4 µm long × 2.3–3.5 μm wide at apex, subacute; empty-end 0.3–1 µm long; hilum 0.7–1.4 µm wide, usually with a sterigmal remnant, 1–4 µm long. Basidia 25–45 × 7–11 µm, cylindrical to clavate, 2–4-spored. Paraphysoid cells 10–30 × 3–7 µm, 0–2-septate, with the terminal cell cylindrical to clavate. Subhymenium pseudoparenchymatous, consisting of chains of 2–3 inflated elements, 10–13 µm diam. Hymenophoral trama of interwoven hyphae, 3–7 µm diam, with ampulliform septa, 7–12 µm wide, and endocystidia-like terminal vesicles up to 25 µm wide. Thromboplera abundant, 2–5 µm diam, yellow, with enlargements up to 10 µm wide. Peritrama of ridges and pseudoperidium up to 200 µm thick, consisting of interwoven hyphae, 2–3 µm diam, with some enlargements and endocystidia-like terminal vesicles up to 20 µm wide, more abundant near surface. Pseudopellis pseudoparenchymatous, 80–180 µm thick, formed by several layers of globose to sphaeropedunculate exocystidia, 10–35 µm wide, and isolated or small clusters of geliferous hairs, 10–100 × 2–4 μm, tortuous, yellow and thick walled up to 1 µm, constituting an incipient trichoderm. The outermost exocystidia are yellow and thick-walled up to 2 µm, disintegrating to generate yellow flocculosities.
Habitat, Season & Distribution — Gregarious, hypogeous or semi-hypogeous under plant debris, from montane broadleaf (Carpinus, Fagus, Quercus) to subalpine coniferous forests (Abies, Picea, Pinus), on calcareous or siliceous soils. Almost throughout the year. Rare. Distributed in temperate regions, from 300 m altitude in Central Europe to 1600 m in Southern Europe (Fig. 37).
Additional material studied. BULGARIA, Kyustendil, near Hotel Trite Buki, Osogovo Mountains, 1570 m, under Fagus sylvatica, 11 Sept. 2014, M. Slavova (SOMF 30327, dupl. JMV800729); ibid., 9 Nov. 2014, M. Slavova (SOMF 30328*, dupl. JMV800613); Pazardzik, Zigov Chark, Western Rhodope Mountains, 1100 m, under Picea abies, Abies alba and Fagus sylvatica, on siliceous soil, 3 Nov. 2018, M. Slavova (SOMF 30329*/30343, dupl. JMV800780/800781); Sofia-City, Sofia, Vitosha Mountain, 1525 m, under Fagus sylvatica, on siliceous soil, 31 Mar. 2019, M. Slavova (SOMF 30330, dupl. JMV800792). – GERMANY, Baden-Württemberg, Beuron, under Fagus sylvatica, 20 Aug. 1969, H. Steinmann, det. G. Gross #318 as G. otthii (M 126281); Bavaria, Munich, Grünwald, in deciduous forest, 2 Oct. 1920, E. Soehner #292 as G. graveolens (M 126217); Rhineland-Palatinate, near Oestrich (Nassau), Oelberg Mount, ‘in pinetis’, autumn 1894, Fuckel as Rhizopogon luteolus, herb. Barbey-Boissier #2152, Fungi Rhenani #1250, pro parte (W 11135, specimen that is part of the Barbey-Boissier collection, identified here as G. otthii). – GREECE, West Macedonia, Grevena, Smixi, Vasilitsa Mountain, 1300 m, under Fagus sylvatica, 9 May 2010, D. Klisiari (GK4998*, dupl. JMV800563). – HUNGARY, Borsod-Abaúj-Zemplén, Szőlősardó, Putnoki Mountains, c. 300 m, mixed forest with Carpinus betulus, 10 Jan. 2001, I. Kiss, I. Mészáros, L. Albert, A. Bathó & Z. Bratek (ZB2185, dupl. JMV800419); Heves, Gyöngyös, Mátraháza, Viktória source, Mátra Mountains, 650 m, under Fagus sylvatica, 3 Sept. 2020, Z. Bratek (ZB5817*); Pest, near Pilisszentkereszt, Pilis Mountains, c. 350 m, under Quercus sp. and Carpinus betulus, 22 Mar. 2006, P. Patonai (ZB3223*, dupl. JMV800437). – POLAND, Lesser Poland, near Barnowiec, Popradzki Landscape Park, Beskid Sądecki Mountains, 680 m, Fagus-Abies-Picea forest, 14 Oct. 2012, P. Chachuła (KRA F-2014-166, dupl. JMV800765); near Konina, Za Palacem Valley, Gorce Mountains, 740 m, Abieti-Piceetum with scarce Fagus sylvatica, 6 Sept. 2014, M. Kozak (KRA F-2014-33, dupl. JMV800763); near Czorsztyn, Poręba, Pieniny Nat. Park, 580 m, Abies alba forest, 13 Sept. 2010, P. Chachuła (KRA F-2010-61, dupl. JMV800693); ibid., 610 m, Abies alba forest, 25 Aug. 2013, P. Chachuła (KRA F-2013-90, dupl. JMV800698); near Krościenko nad Dunajcem, by the Bajków Groń, Pieniny Nat. Park, 560 m, Abies-Fagus forest, 25 Aug. 2013, P. Chachuła & R. Rutkowski (KRA F-2013-95/F-2013-98, dupl. JMV800702/800704); near Kościelisko, Wielka Sucha Valley, Tatra Nat. Park, 1010 m, Abieti-Piceetum, on calcareous soil, 16 Aug. 2019, M. Kozak (TPN-19-0148, dupl. JMV800804). – ROMANIA, Harghita, near Rugonfalva (Rugăneşti), c. 600 m, Dec. 1996, B. Pálfy (ZB1175*, dupl. JMV800395); ibid., 12 Mar. 1997, B. Pálfy (ZB1409*, dupl. JMV800399); ibid., Nov. 1997, D. Váry (ZB1317, dupl. JMV800398); Harghita-Covasna, sine loc., 1997, B. Pálfy (ZB2740*/2744*/2776*, dupl. JMV800401/800403/800404). – SPAIN, Aragon, Huesca, Bielsa, El Cornato, Valle de Pineta, Ordesa y Monte Perdido Nat. Park, Central Pyrenees, 1170 m, under Abies alba, 2 May 2015, A. Paz (IC02051501); Catalonia, Girona, Campelles, Baga de Campelles Forest, Eastern Pyrenees, 1450 m, under Abies alba and Pinus sylvestris, on siliceous soil, 3 Oct. 1996, J.M. Vidal (JMV961003-5*). – TURKEY, Erdek, 2 km NE Ocaklar, Kapidağ Yarimadasi, 400 m, 10 Apr. 1971, E. Uruni, det. J. Schiffler as G. morchelliformis (ZT-Myc 3941).
Type material of Gautieria caudata studied. USA, California, Marin Co., Mill Valley, under sequoias and oaks, April, H.W. Harkness #240 (holo NY 780825, herb. S.M. Zeller, ex herb. W.R. Dudley, of Hymenogaster caudatus).
Notes — Gautieria fusella is well characterised by: 1) a yellow pruinosity on the surface of basidiomata, produced by the disintegration of the outer pseudopellis; 2) compact ridges that fuse to form a venose-reticulate pseudoperidium; 3) compact hymenophore consisting of thick tramal plates and obliterated locules; and 4) small fusiform spores with a long subacute hilar appendix retaining a remnant of sterigma. It is a rare species, but widespread in Europe, which can be found from montane broadleaf forests to subalpine coniferous forests. From this Gautieria we have studied 29 collections from 8 countries, of which we have successfully sequenced 12 collections (Table 1) and represented 5 sequences in our phylogenetic tree (Fig. 3).
A North American Gautieria with similar macroscopic and microscopic features is G. caudata. This species was found by H.W. Harkness in California (USA), under Sequoia and Quercus. Harkness (1899) briefly describes it as Hymenogaster caudatus after observing at the base of the basidiomata a remnant of basal rhizomorph that has the shape of a pointed caudal appendage. Subsequently, this species was redescribed and recombined into the genus Gautieria by Dodge & Zeller (1934), indicating a thickness of the tramal plates wider than the cavities. These distinctive macroscopic features of G. caudata, consisting of the pointed caudal appendage and thick tramal plates, are also present in the studied specimens of G. fusella. Although the holotype of G. caudata (NY 780825) is immature and has been affected by preservation in alcohol, some microscopic features that are also present in G. fusella can be observed: 1) pseudoparenchymatous structure of the pseudopellis; and 2) narrowly elliptico-fusiform spores provided with a long subacute hilar appendix, which retains traces of sterigma (Fig. 23o). However, due to the poor condition of the type material of G. caudata, no attempt was made to obtain DNA sequences to determine its genetic relationship with G. fusella.
Gautieria sect. Hymenogastroides J.M. Vidal, sect. nov. — MycoBank MB 845134
Etymology. From the epithet of the type species.
Type species. Gautieria hymenogastroides J.M. Vidal, Fern. Rodr., Cabero, Sáinz & Pasabán
Basidiomata pseudoperidial. Pseudoperidium foveate-porate, becoming purplish when rubbed. Trichotomentocutis well-developed, acquiring lemon yellow hues. Locules irregularly shaped. Tramal plates subgelatinized. Hyphae slightly gelatinizing, making hymenophore cartilaginous, non-gelatinous. Basidia 2–4-spored. Spores broadly elliptical to subglobose, ornamented with broad gibbosities. Odour pleasant. Only a single representative is known for this section.
Gautieria hymenogastroides J.M. Vidal, Fern. Rodr., Cabero, Sáinz & Pasabán, sp. nov. — MycoBank MB 845126; Fig. 24, 33, 34, 38
Fig. 24.
Gautieria hymenogastroides. a–b. JMV20110811 (BCN, holotype). a. Basidiomata showing a dendroid columella; b. spores in KOH. — c–e. JMV20180802-4. c. Basidiomata showing yellow and pink hues; d. detail of tomentose surface; e. tufts of thick-walled hairs of trichotomentocutis. — f–k. JMV20140708-5. f. Basidiomata morphologically similar to a Hymenogaster; g. trichotomentocutis; h. hairs of trichotomentocutis; i. hymenidermial pseudopellis originating the hairs of trichotomentocutis; j. trichotomentocutis covering an exposed locule; k. basidia and paraphysoid cells of an outlying locule. — l–n. JMV20180814-1. l. Brushed basidioma showing foveate-porate pseudoperidium with purplish oxidation; m. hymenophore showing melon yellow locules; n. spores in KOH. — o. JC160320NR. SEM image of spores. — Scale bars: a, c, f, l = 1 cm; b, n = 10 µm; d, m = 2 mm; e, g–k = 20 µm; o = 5 µm. — Photos: a–n. J.M. Vidal; o. P. Mleczko, UJ.
Fig. 33.
Spores of Gautieria sect. Hymenogastroides, sect. Glutinosiglebae and sect. Parvicellae rehydrated with KOH 2 %. a. G. hymenogastroides (JMV20180814-1); b. G. graveolens (JMV20120612-1); c. G. violascens (JC110618NR); d. G. cistophila (JMV960527-1); e. G. queletii (JMV20180823-1); f. G. trabutii (MA-Fungi 74767); g. G. otthii (JMV960713-1); h. G. persimilis (JMV20180904-2). — Scale bar: 10 µm. — Photos: J.M. Vidal.
Fig. 38.
Distribution map of species of Gautieria sect. Hymenogastroides, sect. Glutinosiglebae and sect. Parvicellae.
Etymology. From the generic name Hymenogaster + the Greek suffix -oides = resembling, due to its macromorphological similarity with a Hymenogaster fungus.
Type. SPAIN, Catalonia, Girona, Viladrau, Camí de la Font de Viladrau, Montseny Massif, 900 m, under Castanea sativa, Quercus ilex, Quercus pubescens and Fagus sylvatica, on siliceous soil, 11 Aug. 2011, F. Rodríguez (holo BCN JMV20110811*; iso in ELTE).
Basidiomata pseudoperidial, 1–2.8 cm wide, subglobose to tuberiform, with a slight basal protuberance attached to a fragile yellowish basal rhizomorph, 0.4–1 mm thick. Surface lanuginose, tomentose, initially white and later acquiring lemon yellow hues (2A3) with pinkish (9A2) maculae; brownish orange (5B4–C4) in exsiccata. Ridges flattened, white, irregularly fused into a foveate-porate pseudoperidium, 0.1–0.2 mm thick, turning greyish magenta (13D5–14D5) when bruised. Locules 0.5–2 × 0.1–0.4 mm, minute, irregularly shaped to radially elongated, narrow, sinuous, inordinate, melon yellow (5A5–A6); intense cinnamon (6D7–D8) in exsiccata. Tramal plates 0.2–0.3 mm thick, white to greyish, becoming greyish pink (12B3) when cut. Columella 0.2–1 mm thick, dendroid, undeveloped, slightly branched to the surface, white; basal context reddish brown (8E6). Odour pleasant when young, fruity, and of seafood when mature.
Spores (18–)19–22.5(–23) × (13–)14–18 µm, Q = 1.2–1.5, broadly elliptical to subglobose, maize yellow (4A5–A6) in KOH (Fig. 33a, 34o). Episporium ovate to broadly ovate. Costae 11–14, 1.2–2.2 µm high × 2.7–6 µm wide, very inflated and refringent, straight, mostly furcate, covering 1/2 of hilar appendix and sometimes developing a peri-hilar ring, ornamented with broad gibbosities; apical ring prominent, 2.3–4.2 µm high; perisporial reticulum barely distinguishable. Hilar appendix 3.5–5.5 µm long × 5–7 µm wide at apex, conico-truncate; empty-end 0.3–1 µm long; hilum 1.4–2 µm wide, without remnants of sterigma or very short. Basidia 40–50 × 12–15 µm, clavate, 2–4-spored. Paraphysoid cells abundant in outlying locules, 20–45 × 2–10 µm, 0–2-septate, difform, sinuous, with polymorphic terminal cell, cylindrical, clavate, pyriform, papillate or attenuate. Subhymenium consisting of chains of cylindrical to subglobose elements, 7–12 µm diam. Hymenophoral trama prosenchymatous, consisting of interwoven, guttulate, slightly gelatinized hyphae, 2–6 µm diam, with abundant ampulliform septa, 5–9 µm wide. Thromboplera sparse, 2–3 µm diam. Peritrama similar to hymenophoral trama, staining with Congo red. Pseudopellis a confused hymeniderm giving rise to a dense trichotomentocutis of long, thick-walled hairs, some of them becoming geliferous. Hairs 2–5 µm diam, vivid yellow to orange, isolated or in tufts, with walls up to 1.5 µm thick and some enlargements at septa up to 8 µm wide. Exocystidia lacking in pseudopellis. Calcium oxalate crystals present.
Habitat, Season & Distribution — Gregarious, hypogeous, in montane broadleaf forests (Castanea, Fagus, Quercus), on siliceous soils. Almost throughout the year. Mediterranean, found in Northern Spain between 500–1100 m of altitude (Fig. 38).
Additional material studied. SPAIN, Castilla and Leon, Zamora, Galende, Llanes de Sanabria, 1080 m, under Quercus pyrenaica and Castanea sativa, on siliceous soil, 16 Aug. 2015, J. Cabero (JC140816NR, dupl. JMV800783); Zamora, Palacios de Sanabria, El Remesal, 1010 m, under Quercus pyrenaica,on siliceous soil, 4 Jan. 2015, J. Cabero(JC150104NR, dupl. JMV800784); ibid., 1000 m, under Quercus pyrenaica and Castanea sativa, 20 Mar. 2016, J. Cabero (JC160320NR*, dupl. JMV800785); Catalonia, Barcelona, Seva, Sobrevià, Montseny Massif, 780 m, under Quercus ilex, on siliceous soil, 20 Feb. 2018, F. Rodríguez (JMV20180220); Girona, Riells de Montseny, Can Pere Arnau, Montseny Massif, 520 m, under Quercus suber and Quercus ilex, on siliceous soil, 8 July 2014, J.M. Vidal & F. Rodríguez (JMV201407085); Girona, Sant Hilari Sacalm, Torre de Vilavecchia, Les Guilleries Massif, 880 m, under Quercus ilex, on siliceous soil, 30 July 2018, F. Rodríguez (JMV20180730-9); Girona, Viladrau, Camí de la Font de Viladrau, Montseny Massif, 890 m, under Castanea sativa, Quercus ilex, Quercus pubescens and Fagus sylvatica, on siliceous soil, 20 July 2011, F. Rodríguez (JMV20110720*); ibid., 8 Nov. 2011, F. Rodríguez (JMV20111108); ibid., 12 Dec. 2017, F. Rodríguez (JMV20171212); ibid., 2 July 2018, F. Rodríguez (JMV20180702); ibid., 11 July 2018, F. Rodríguez (JMV20180711); ibid., 25 July 2018, F. Rodríguez (JMV20180725-4); ibid., 31 July 2018, F. Rodríguez (JMV20180731-2); ibid., 2 Aug. 2018, J.M. Vidal, A. Paz & C. Lavoise (JMV20180802-4); ibid., 14 Aug. 2018, F. Rodríguez (JMV20180814-1); Navarre, Leiza, 630 m, under Quercus petraea, on siliceous soil, 30 Dec. 2013, F. Sáinz & P.M. Pasabán (JMV800826).
Notes — Gautieria hymenogastroides is characterised by having: 1) a tomentose surface acquiring lemon yellow hues; 2) a foveate-porate pseudoperidium with a purplish oxidation when rubbed; 3) a non-gelatinous hymenophore; 4) locules of melon yellow colour; 5) absence of exocystidia; 6) basidia 2–4-spored; and 7) subglobose spores provided with large gibbosities. This is a highly evolved species that presents a mixture of features between those of Gautieria sect. Gautieria and Gautieria sect. Glutinosiglebae. With its developed trichotomentocutis that covers the basidiomata, its slightly gelatinizing hyphae, and its 2–4-spored basidia, it seems to be related to the tomentose species of Gautieria sect. Gautieria, but observing its spore morphology (Fig. 24b) and its foveate-porate pseudoperidium becoming purplish (Fig. 24l), it seems to be more related to Gautieria sect. Glutinosiglebae, especially with G. violascens.
This Gautieria, due to its morphology, the non-gelatinous hymenophore, and being deeply buried in the ground, may easily be mistaken with a Hymenogaster. It is a rare species, hitherto only known from Northern Spain. From this Gautieria we have studied 17 Spanish collections, of which we have successfully sequenced the 3 collections represented in our phylogenetic tree (Fig. 3).
Gautieria sect. Glutinosiglebae J.M. Vidal, sect. nov. — MycoBank MB 845133
Etymology. From Latin, glutinosus = sticky + gleba = gleba, due to its strongly gelatinized hymenophore.
Type species. Gautieria graveolens Vittad.
Basidiomata pseudoperidial. Pseudoperidium foveate-porate, becoming sanguineous or violaceous when rubbed. Trichotomentocutis absent. Locules irregularly-shaped, narrowly elongated or tubular. Tramal plates strongly gelatinized, usually thicker than locules. Hyphae usually rapidly gelatinizing, making hymenophore gelatinous. Basidia 1–2-spored. Odour usually strong at maturity, pleasant or unpleasant.
Key to species of section Glutinosiglebae
1. Locules irregularly shaped to narrowly elongated. Spore costae 9–13 in number, up to 1.6 µm high, ornamented with broad gibbosities ............................... 2
1. Locules tubular. Spore costae 12–16(–20) in number, up to 0.8 µm high, ornamented with rounded gibbosities or tubercles ..................................... 3
2. Pseudoperidium turning wine red. Spores 15–17.5 × 10.5–12.5 µm, obovate. — Basidiomata furfuraceous, cerebriform to subreticulate-porate, white. Under Carpinus, Castanea, Fagus, Quercus. Mediterranean to temperate (Central to Southern Europe) .................... G. graveolens
2. Pseudoperidium turning violaceous. Spores 16.5–20.5 × 11.5–14 µm, pyriform. — Basidiomata furfuraceous, smooth-porate, white. Under Quercus. Mediterranean (Spain) ... ................................... G. violascens
3. Costae gibbosities rounded up to 1.5 µm high. Spores 17–22 × 12.5–14 μm, broadly elliptical to obovate. — Basidiomata granulose to foveate, white, not changing of colour with age or rubbing. Under Arbutus, Cistus. Mediterranean (Spain) .................................... G. cistophila
3. Costae gibbosities forming tubercles up to 3.3 µm high . 4
4. Pseudoperidium with prominent veins. Spores 15–19 × 9–10.5 µm, elliptical, obovate or pyriform. — Basidiomata pruinose, foveate-porate to venose-reticulate, yellowish grey to greyish orange, changing to dark reddish brown with rubbing. Under Cedrus, Pinus, Pseudotsuga, Quercus. Mediterranean (Western Mediterranean Basin) ........ G. trabutii
4. Pseudoperidium lacking prominent veins. Spores 16.5–20 × 9.5–11 µm, elliptical to obovate. — Basidiomata pruinose, foveate-porate, whitish to orange white, changing to violet brown on wounds or with rubbing. Under Abies, Picea. Temperate (Central to South-Western Europe) .... G. queletii
Clade graveolens (GauGlu-1)
Locules irregularly shaped to narrowly elongated. Spores obovate to pyriform, reaching 17.5–20.5 × 12.5–14 µm, ornamented with broad gibbosities. Costae 9–13, up to 1.6 µm high. Linked to broadleaf trees (Carpinus, Castanea, Fagus, Quercus). Represented by G. graveolens and G. violascens.
Gautieria graveolens Vittad., Monogr. Tuberac.: 27. 1831 — Fig. 25, 33, 34, 38
Fig. 25.
Gautieria graveolens. a–f. JMV20120612-1. a. Basidiomata showing flattened ridges; b. cerebriform surface of young basidiomata showing wine red maculae; c. hymenophore showing pale orange locules and strongly gelatinization of columella and tramal plates; d. pseudopellis and peritrama; e. gelatinized subhymenium and hymenophoral trama, and collapsed hymenium; f. spores in KOH. — g. VK1466. Young basidiomata with a well-developed columella and radial arrangement of locules and ridges. — h. JMV20180712 (BCN, reference specimen). Basidioma with flattened ridges and gelatinized tramal plates. — i. W 344 (authentic material of G. graveolens). Spores in KOH. — j–l. JMV20120715. j. Basidioma showing thin ramifications of columella and subreticulate surface; k–l. SEM images of spores — Scale bars: a, g–h, j = 1 cm; b = 5 mm; c = 2 mm; d–e = 20 µm; f, i = 10 µm; k–l = 5 µm. — Photos: a–f, i. J.M. Vidal; g. V. Kaounas; h, j. F. Rodríguez; k–l. UdG.
Etymology. From Latin, gravis = strong + olens = odoriferous, due to its offensive odour.
Type. Vittadini, Monogr. Tuberac.: t. IV, f. XIII, F–G. 1831 [icon.] (lecto, hic designatus, MBT 10008568).
Authentic material studied. ITALY, Lombardy, ‘type’ (FH 301420, herb. N. Patouillard); ‘comm. C. Vittadini, authentic material’ (K(M) 170553, herb. M.J. Berkeley); ‘Mediol. 1844, herb. Vittad.!’ (PAD s.n., herb. A. Balsamo); ‘ex agro Mediolanensis, ex A. Balsamo’ (PC 92118, herb. De Notaris); ‘ex A. Balsamo, comm. De Notaris’ (PC 92117, coll. Desmazières 1863, n° 8); ‘specimina Vittadinii ex Italia boreali, cum tipo in Musei Caesar Palat. Vindobonensis, A. Pilát comparavit’ (PRM 678926, herb. A.C.J. Corda, Icon. Fung. VI, p. 34, Taf. VII, f. 63); ‘Original v. Vittadini’ (W 344).
Basidiomata pseudoperidial, 1.5–4 cm wide, subglobose to tuberiform, or lobate by fusion of several specimens, with a basal depression attached to a white basal rhizomorph, 1–1.5 mm thick, easily detachable. Surface initially cerebriform, consisting of white, furfuraceous, sinuous, flattened ridges, arranged radially at the base of basidiomata, reddening strongly when handled, acquiring a wine red (11D8) to greyish magenta (12D5–13D6) colour. Among them, minute openings can be observed in the form of pits or sinuous grooves that communicate with the underlying locules. At maturity, the ridges close and fuse giving rise to a rugose, subreticulate, porate pseudoperidium, 0.25–0.5 mm thick. Locules 0.5–2 × 0.15–0.6 mm, minute, irregularly shaped, radially elongated, progressively closed, becoming narrow and sinuous, tubular, light yellow to light orange (4A5–5A4); cinnamon (5C6–6D6) in exsiccata. Tramal plates 0.2–0.7 mm thick, grey, intensely reddening when cut. Columella 0.25–1 mm thick, dendroid, strongly branched towards the surface, grey; basal context greyish violet (18E7). Odour strong and unpleasant, foetid.
Spores (14.5–)15–17.5(–19) × (10–)10.5–12.5(–13) µm, Q = 1.3–1.5, obovate, maize yellow (4A5–A6) in KOH (Fig. 33b, 34p). Episporium broadly elliptical. Costae 9–12, 0.8–1.4 µm high × 2.5–4(–5) µm wide, very inflated, straight, entire or furcate, covering 2/3 of hilar appendix, ornamented with broad gibbosities; apical ring distinct, 1.3–2 µm high; perisporial reticulum distinguishable. Hilar appendix 2–4 µm long × 3–5 µm wide at apex, conico-truncate; empty-end 0.5–1 µm long; hilum 1–1.6 µm wide, usually provided with a short remnant of sterigma. Basidia 40–50 × 8–10 µm, clavate, 1–2-spored. Paraphysoid cells 20–55 × 4–8 µm, 0–2-septate, with the terminal cell cylindrical, attenuate or papillate. Subhymenium consisting of chains of cylindrical to inflated elements, 5–8 µm diam, strongly gelatinized. Hymenophoral trama of interwoven hyphae, 2–5 µm diam, strongly gelatinized, with some ampulliform septa and endocystidia-like terminal vesicles up to 16 µm wide. Thromboplera present. Peritrama prosenchymatous, consisting of densely interwoven hyphae, 2–5 µm diam, strongly gelatinized. Pseudopellis a hymeniderm formed by dense clusters of hyaline to yellow globose exocystidia, 12–32 µm wide. Calcium oxalate crystals present.
Habitat, Season & Distribution — Gregarious, hypogeous or semi-hypogeous under plant debris, in Mediterranean and montane broadleaf forests (Carpinus, Castanea, Fagus, Quercus), preferably on siliceous soils. From spring to summer. Rare. Distributed in Mediterranean and temperate regions, from 200 m altitude in Central Europe to 1000 m in Southern Europe (Fig. 38).
Additional material studied. BULGARIA, Pernik, Zemen, Jablyano, 745 m, under Quercus frainetto and Quercus cerris, on sandy soil, 10 July 2018, M. Slavova & B. Assyov (SOMF 30332, dupl. JMV800746). – CZECH REPUBLIC, South Moravia, Žarošice, ‘in querceto’, 27 Aug. 1944, V. Vacek as G. graveolens, det. A. Pilát as G. morchelliformis var. globispora (PRM 678962). – GERMANY, Hesse, around Marburg, under Fagus sylvatica, 1889, R. Hesse (UPS F-550642). – GREECE, Thessaly, Magnesia, Pouri, 230 m, under Quercus ilex and Castanea sativa, 23 Apr. 2010, V. Kaounas (VK1466*, dupl. JMV800542); ibid., 4 July 2010, V. Kaounas (VK1510, dupl. JMV800553); ibid., 25 Apr. 2014, V. Kaounas (VK3345, dupl. JMV800610); Magnesia, Zagora, 900 m, under Fagus sylvatica, 25 Apr. 2014, V. Kaounas (VK3389, dupl. JMV800611); West Macedonia, Grevena, Trikorfo, 1000 m, under Quercus sp. and Cistus sp., 10 June 2000, G. Konstantinidis (GK1228*, dupl. JMV800557). – HUNGARY, Hajdú-Bihar, Vekerd, 90 m, under Quercus robur, 3 June 2007, A. Sági (ZI63, dupl. JMV800445); Heves, sine loc., Mátra Mountains, c. 800 m, 8 July 2007, I. Zagyva (ZI205, dupl. JMV800453); Gyöngyös, Mátraháza, Gyökeresrét, Mátra Mountains, 550 m, Carpineto-Quercetum, 3 Sept. 2020, Z. Bratek (ZB5822*); near Felsőtárkány, Bükk Mountains, c. 350 m, under Carpinus betulus and Fagus sylvatica, Aug. 2001, S. Vásárhelyi (ZB2321*, dupl. JMV800423); Vas, near Szatta, 230 m, under Carpinus betulus and Quercus sp., 3 July 1995, N. Bodonyi & Z. Bratek (ZB480, dupl. JMV800394). – ITALY, Tuscany, Lucca, Careggine, 800 m, under Castanea sativa and Betula pendula, on siliceous soil, 6 July 2002, G. Bernardini & L. Gori as G. otthii (ELG20020706, dupl. JMV800351). – SLOVAKIA, sine loc., sine dat., S. Glejdura (sg9per165*). – SPAIN, Castilla and Leon, Zamora, Quintana de Sanabria, Cobreros, 1000 m, under Quercus pyrenaica and Castanea sativa, on siliceous soil, 18 July 2010, J. Cabero (JC100718BT, dupl. JMV800789); Zamora, Palacios de Sanabria, El Remesal, 954 m, under Quercus pyrenaica, on siliceous soil, 29 May 2016, J. Cabero (JC160529NR*, dupl. JMV800786); Catalonia, Girona, Amer, El Colomer, Les Guilleries Massif, 425 m, under Castanea sativa, on siliceous soil, 12 July 2018, F. Rodríguez (JMV20180712*); Girona, Cruïlles, Puig d’Arques, Castanyeda d’en Genoher, Les Gavarres Massif, 500 m, under Castanea sativa, on schistose soil, 12 June 2012, J.M. Vidal & F. Rodríguez (JMV20120612-1); Girona, Les Planes d’Hostoles, crossroad to La Salut, 940 m, under Quercus pubescens and Fagus sylvatica, 2 July 2012, F. Rodríguez (JMV20120702-1); Girona, Quart, Sant Mateu de Montnegre, Les Gavarres Massif, 350 m, under Quercus ilex and Quercus suber, on schistose soil, 7 June 2014, F. Rodríguez (JMV20140607-2); Girona, Sant Hilari Sacalm, Boscos de Reixac, Les Guilleries Massif, 800 m, under Castanea sativa and Corylus avellana, on siliceous soil, 4 June 2014, F. Rodríguez (JMV20140604-1); Girona, Santa Coloma de Farners, Sant Miquel de Cladells, Les Guilleries Massif, 450 m, under Quercus suber, on siliceous soil, 10 July 2014, F. Rodríguez (JMV20140710); ibid., 25 June 2018, F. Rodríguez (JMV20180625); Girona, Susqueda, road to El Far, 1045 m, under Fagus sylvatica, 15 July 2012, F. Rodríguez (JMV20120715); Girona, Susqueda, Turó d’Armadans, near Mas La Jaça, 990 m, under Fagus sylvatica, 4 July 2012, F. Rodríguez (JMV20120704-2); Girona, Viladrau, Camí de la Font de Viladrau, Montseny Massif, 900 m, under Fagus sylvatica, Castanea sativa and Quercus ilex, on siliceous soil, 19 June 2012, F. Rodríguez (JMV20120619-1); ibid., 13 June 2018, F. Rodríguez (JMV20180613); ibid., 31 July 2018, F. Rodríguez (JMV20180731-1); Navarre, Ezkurra, 800 m, under Fagus sylvatica, 16 July 2019, F. Sáinz & P.M. Pasabán (PSS026005*, dupl. JMV800828).
Notes — Gautieria graveolens was described and illustrated by Vittadini (1831) in his Monographia Tuberacearum, based on specimens collected in oak woods in the region of Pavia (Lombardy, Italy), also providing data on its ecology and phenology. Tulasne & Tulasne (1851) noted a spore size of 16 × 8–9 µm from an exsiccatum received from Vittadini, and Corda in Zobel (1854) included a new description and illustration, also from a duplicate of Vittadini’s specimen (PRM 678926). As a result, later mycologists identified the small-spored species with foetid odour as belonging to G. graveolens, such as Soehner (1951), whose collections belong mostly to G. confusa, but also to G. fenestrata, G. macrocoilia, G. morchelliformis var. fageticola, G. villosa var. inflata and G. otthii. Presumed original material of G. graveolens determined by Vittadini is preserved in several European and North American herbaria (FH, K, PAD, PC, PRM, TO, W), but none of them indicates the location and date of collection, making it impossible to select a lectotype. Dodge & Zeller (1934) selected as types the sheets preserved in the herbaria of Saccardo in Padova (PAD) and of Tulasne in Paris (PC). Pilát (1958) selected as a type the Vittadini sheet kept in the herbarium of Vienna (W) and as cotypes the ones of Paris (PC) and Prague (PRM). In this work, we select the image of Vittadini (1831: t. IV, f. XIII, F) as a lectotype, while we appoint our sequenced collection BCN JMV20180712 as a reference specimen and the most representative for G. graveolens (Fig. 25h).
Gautieria graveolens is characterised by having: 1) intense reddening in young basidiomata; 2) flattened ridges, fusing into a subreticulate-porate pseudoperidium; 3) light yellow to light orange locules; 4) strong gelatinization of tramal plates and columella; and 5) obovate spores. It is a rather rare species that occurs in temperate and Mediterranean deciduous forests. From this species we have studied 37 collections from 8 countries, of which we have successfully sequenced 8 collections (Table 1) and represented 4 sequences in our phylogenetic tree (Fig. 3). We have not attempted to obtain genetic information from authentic material due to its age, but the spore comparison between our collections and those of Vittadini is in complete agreement (Fig. 25i). The sequenced Greek collection GK1228 was previously illustrated by Konstantinidis (2006).
Gautieria violascens J.M. Vidal, Cabero & Fern. Rodr., sp. nov. — MycoBank MB 845128; Fig. 26, 33, 34, 38
Fig. 26.
Gautieria violascens. a–d. JMV20170314 (BCN, holotype). a. Basidioma showing violaceous oxidation and gelatinous hymenophore; b. detail of striations around basal rhizomorph attachment; c. gelatinized subhymenium and hymenophoral trama, and collapsed hymenium; d. spores in KOH. — e–f. JMV20150421. e. Basidioma showing smooth-porate pseudoperidium; f. violaceous oxidation of hymenophore when exposed to air. — g. JMV20070619. Pseudopellis. — h. JC120715BT. Section of a basidioma showing a basal columella. — i. JC120624BT. Detail of basal striation of a basidioma. — j–l. JC110618NR. j. Spores in KOH; k–l. SEM images of spores. — Scale bars: a, e, h = 1 cm; b = 5 mm; c, g = 20 µm; d, j = 10 µm; f–i = 2 mm; k–l = 5 µm. — Photos: a–b, e–f. F. Rodríguez; c–d, g, j. J.M. Vidal; h–i. J. Cabero; k. UdG; l. P. Mleczko, UJ.
Etymology. From Latin, violaceus = violet + the suffix -ascens = tending to, due to the tendency of the context to turn violet with exposure to air.
Type. SPAIN, Catalonia, Girona, Santa Coloma de Farners, Sant Miquel de Cladells, Les Guilleries Massif, 450 m, under Quercus suber, on siliceous soil, 14 Mar. 2017, F. Rodríguez (holo BCN JMV20170314*; iso in ELTE).
Basidiomata pseudoperidial, 1.5–4 cm wide, globose to tuberiform, striate radially at base, with a slight basal depression attached to a white branched basal rhizomorph, 1–1.5 mm thick, easily detached. Surface furfuraceous, originated by fusion of ridges into a smooth, porate pseudoperidium, 0.15–0.25 mm thick, initially white, becoming violaceous (18A4) when handled or with exposure to air, acquiring reddish brown (8E6) maculae. Locules 0.5–2 × 0.15–0.6 mm, minute, narrow, radially elongated, tubular, sinuous, pastel red (7A4–A5); cinnamon (6D6–D7) in exsiccata. Tramal plates 0.2–0.7 mm thick, white, becoming violaceous (18A4) with exposure to air. Columella 0.25–1 mm thick, undeveloped, basal, white to greyish; basal context deep violet (18D8). Odour strong but pleasant, like peach or apricot jam.
Spores (16–)16.5–20.5(–21.5) × (10.5–)11.5–14(–16) µm, the larger ones predominating, Q = 1.35–1.6, pyriform, maize yellow (4A5–A7) in KOH (Fig. 33c, 34q). Episporium ovate to broadly elliptical. Costae 10–13, 1–1.6 µm high × 2–4 µm wide, very inflated, straight, entire or furcate, covering 1/3 of hilar appendix, ornamented with broad gibbosities; apical ring distinct, 1–2 µm high; perisporial reticulum barely distinguishable. Hilar appendix robust, 3–6 µm long × 5–7 µm wide at apex, conico-truncate; empty-end 0.2–1 µm long; hilum 1.2–2.2 µm wide, without sterigmal remnant or very short. Basidia 35–55 × 7–9 µm, clavate, 1–2-spored. Paraphysoid cells 15–60 × 3–6 µm, 0–2-septate, with the terminal cell cylindrical. Subhymenium consisting of chains of gelatinized, cylindrical to subglobose elements, 7–20 µm diam. Hymenophoral trama of gelatinized interwoven hyphae, 2–7 µm diam, with ampulliform septa up to 10 µm wide and some endocystidia-like vesicles up to 20 µm wide. Thromboplera present, 4 µm diam. Peritrama consisting of densely interwoven gelatinized hyphae, 2–4 µm diam, with some enlargements and endocystidia-like terminal vesicles up to 14 µm wide. Pseudopellis a hymeniderm formed by a single layer of hyaline to yellow exocystidia, 10–20 µm wide. Calcium oxalate crystals present.
Habitat, Season & Distribution — Solitary or gregarious, deeply hypogeous, in sclerophyllous forests of Quercus, on siliceous soils. Almost throughout the year. Mediterranean species, found in Spain between 400–1000 m of altitude (Fig. 38).
Additional material studied. SPAIN, Aragon, Zaragoza, Aguaron, 700 m, under Quercus rotundifolia and Cistus sp., on siliceous soil, 19 June 2007, F. Serrano (JMV20070619); Castilla and Leon, Soria, Añavieja, 1000 m, under Quercus rotundifolia and Cistus ladanifer, on siliceous soil, 23 Dec. 1997, I. Miramón, det. F.D. Calonge as G. otthii (MA-Fungi 47186); Zamora, Toro, Monte La Reina, 700 m, under Quercus rotundifolia, on sandy soil, 18 June 2011, J. Cabero (JC110618NR, dupl. JMV800478); ibid., 24 June 2012 (JC120624BT, dupl. JMV800829); ibid., 15 July 2012, J. Cabero (JC120715BT*, dupl. JMV800782); Catalonia, Girona, Santa Coloma de Farners, Sant Miquel de Cladells, Les Guilleries Massif, 450 m, under Quercus suber, on siliceous soil, 21 Apr. 2015, F. Rodríguez (JMV20150421).
Notes — Distinctive features of G. violascens include: 1) a smooth-porate pseudoperidium already present in young basidiomata; 2) violaceous oxidation of basidiomata when exposed to air; 3) pastel red locules; and 4) pyriform spores with a pronounced hilar appendix. Gautieria graveolens is a similar species, differentiated by having a subreticulate-porate pseudoperidium that becomes wine red when exposed to air, and obovate spores with a less pronounced hilar appendix. Gautieria violascens has a meridional distribution and is found exclusively on siliceous soils, under holm oaks (Quercus rotundifolia) and cork oaks (Q. suber), being so far only known from the Iberian Peninsula. From this Gautieria we have studied 7 Spanish collections, of which we have successfully sequenced the 2 collections represented in our phylogenetic tree (Fig. 3).
Clade trabutii (GauGlu-2)
Locules tubular. Spores variable in shape, elliptical, broadly elliptical, obovate or pyriform, reaching 19–22 × 10.5–14 µm, ornamented with rounded gibbosities or tubercles. Costae 12–16(–20), up to 0.8 µm high. Linked to shrubs (Arbutus, Cistus), broadleaf trees (Quercus) and conifers (Abies, Cedrus, Picea, Pinus, Pseudotsuga). Represented by G. cistophila, G. queletii and G. trabutii, the latter also found in North Africa.
Gautieria cistophila J.M. Vidal, Pérez-Jar., Mahiques & A. Paz, sp. nov. — MycoBank MB 845129; Fig. 27, 33, 34, 38
Etymology. From de generic name Cistus = rock rose + philus = friend of, due to its association with plants of the genus Cistus.
Type. SPAIN, Castilla and Leon, León, Santa María del Monte del Condado, in a thicket of Cistus laurifolius, on siliceous soil, 27 May 1996, J.M. Vidal, T. Pérez-Jarauta & J. Vila (holo BCN JMV960527-1*; iso in ELTE).
Basidiomata pseudoperidial, 1.5–3.5 cm wide, globose to subglobose, furfuraceous, with a slight basal depression attached to a white basal rhizomorph, 1.5–2 mm thick. Surface in young basidiomata granulose, porate, consisting of white, small, dense and intricate ridges. When basidiomata develop, the ridges fuse to progressively become a membranous, pruinose, foveate, pseudoperidium, 0.15–0.35 mm thick, pure white for a long time, without noticeable colour change when handled, but pink hues may appear in wet weather. Locules 0.5–3.5 × 0.2–0.8 mm, irregularly shaped to tubular, radially elongated, sinuous, light orange to greyish orange (5A4–B6); cinnamon (6C5–D7) in exsiccata. Tramal plates 0.2–0.4 mm thick, grey, immutable or slightly violaceous when cut. Columella 0.5–2 mm thick, slightly dendroid, basal, grey; basal context greyish. Odour first mild, and intense at maturity.
Spores (16.5–)17–22(–23.5) × 12.5–14(–14.5) μm, Q = 1.3–1.7, broadly elliptical to obovate, maize yellow (4A5–A6) in KOH (Fig. 33d, 34r). Episporium ovate. Costae 13–16(–20), 0.4–0.8 µm high × 1.5–4 µm wide, slightly inflated, straight, mostly furcate and anastomosing, covering 2/3 of hilar appendix, ornamented with rounded gibbosities, 0.5–1.5 µm high, 4–8 arranged along each costa, majority at furcations; apical ring inconspicuous, 0.6–1.4 µm high; perisporial reticulum indistinguishable. Hilar appendix 0.8–3.5(–4.5) µm long × 4–6 µm wide at apex, rounded, oblong; empty-end 0.3–1.6 µm long; hilum 1.5–1.9 µm wide, usually provided with a short remnant of sterigma. Basidia 40–50 × 10–12 µm, cylindrical, 1–2-spored. Paraphysoid cells 18–45 × 4–7 µm, 0–1-septate, with the terminal cell clavate or attenuate. Subhymenium consisting of chains of gelatinized, cylindrical to inflated hyphae, 8–18 µm diam. Hymenophoral trama of interwoven, guttulate, gelatinized hyphae, 2–6 µm diam, with ampulliform septa up to 10 µm wide and some endocystidia-like terminal vesicles up to 22 µm wide. Thromboplera sparse, 2–3 µm diam. Peritrama similar to hymenophoral trama. Pseudopellis a hymeniderm formed by a single layer of dense clusters of hyaline to yellow, fragile, globose exocystidia, 18–32 µm wide. Calcium oxalate crystals present.
Habitat, Season & Distribution — Gregarious, hypogeous or semi-hypogeous, in Arbutus and Cistus scrubland, on siliceous soils. From winter to spring. Mediterranean species, so far only known from Spain between 600–1000 m altitude (Fig. 38).
Additional material studied. SPAIN, Castilla and Leon, León, Castrillino, 950 m, under Cistus populifolius, on siliceous soil, 16 Mar. 1993, T. Pérez-Jarauta, det. F.D. Calonge as G. morchelliformis (MA-Fungi 32260*, dupl. JMV930316); Extremadura, Cáceres, Jarandilla de la Vera, 600 m, under Cistus ladanifer, on siliceous soil, 20 May 2012, A. Paz (IC20051204); Valencian Community, Valencia, Barx, Pla de Suros, 250 m, under Arbutus unedo with Pistacia lentiscus and Cistus salviifolius, on siliceous soil, 8 Jan. 1994, R. Mahiques MES2219 as G. trabutii (MA-Fungi 33556*).
Notes — Distinctive morphological features of G. cistophila include: 1) a white granulose to foveate pseudoperidium, not reddening when handled; and 2) gibbose spores with a large number of costae (up to 20). This interesting species was found for the first time growing in a Cistus populifolius scrubland in northern Spain, in the Leonese region, which was published by Calonge et al. (1994) as G. morchelliformis. Another collection was retrieved in a mixed scrubland of Arbutus unedo, Pistacia and Cistus in eastern Spain, in Valencian Community, being briefly described and illustrated by Mahiques et al. (1995) as G. trabutii. In 1996 we had the opportunity to visit the Leon localities kindly accompanied by T. Pérez-Jarauta, and located a group of basidiomata, growing hypogeous under the mantle of leaf litter in the middle of a dense formation of Cistus laurifolius shrubs. Recently, this species has also been found in Extremadura (western Spain), in a Cistus ladanifer scrubland. Its mycorrhizal association with Arbutus unedo is not confirmed, but Kennedy et al. (2012) found in North America a mycorrhizal association of an undescribed Gautieria with Arbutus menziesii, corresponding to the GenBank JQ393051 sequence. A similar mycorrhizal association of hypogeous basidiomycetes with Cistaceae plants was recently mentioned by Vidal et al. (2019) for three Mediterranean angiocarpic members of Russulaceae (Lactarius subgiennensis found in Cyprus and L. giennensis and Russula andaluciana found in Spain). Other interesting fungal associations with Cistaceae plants can be found in Loizides (2016). From this Gautieria we have studied 4 Spanish collections, of which we have successfully sequenced the 3 collections represented in our phylogenetic tree (Fig. 3).
Gautieria queletii J.M. Vidal, Kozak, Mleczko, Karpowicz, Fern. Rodr. & A. Paz, sp. nov. — MycoBank MB 845130; Fig. 28, 33, 34, 38
Fig. 28.
Gautieria queletii. a–d. JMV20180823-1 (BCN, holotype). a. Basidioma showing a dendroid columella; b. detail of foveate-porate pseudoperidium; c. tubular locules and strongly gelatinized tramal plates and columella; d. spores in KOH. — e–f. KRA F-2014-29. e. Clusters of exocystidia of pseudopellis; f. immature hymenium. — g. JMV20110906-3. Basidiomata. — h. UPS F-550643 (L. Quélet as G. graveolens). Spores in KOH. — i. KRA F-2016-56. Spores in KOH. — j–k. KRA F-2013-3. Basidiomata. — l–m. KRA F-2011-77. SEM images of spores. — Scale bars: a, g, j–k = 1 cm; b–c = 2 mm; d, h–i = 10 µm; e–f = 20 µm; l–m = 5 µm. — Photos: a–f, h–i. J.M. Vidal; g. F. Rodríguez; j–k. M. Kozak; l–m. P. Mleczko, UJ.
Etymology. In honour of the French mycologist and naturalist Lucien Quélet (1832–1899), the first collector of this species.
Type. SPAIN, Catalonia, Girona, Setcases, Baga de Queràs Forest, Eastern Pyrenees, 1700 m, under Abies alba and Pinus uncinata, on siliceous soil, 23 Aug. 2018, J.M. Vidal & A. Paz (holo BCN JMV20180823-1*; iso in ELTE).
Basidiomata pseudoperidial, 1.5–3 cm wide, irregular, tuberiform, often deformed due to the growth of several crowded specimens, provided with a basal protuberance attached to a white basal rhizomorph, 1.5–2 mm thick. Surface pruinose, whitish to orange white (5A2–6A2), changing to brownish red to violet brown (10D8–E8) on wounds or when handled, transparent with moisture, allowing the circumvolutions of the underlying tramal plates to be observed, consisting of flattened ridges, which fuse to form a smooth, thin, foveate-porate pseudoperidium, 0.15–0.2 mm thick, becoming radially venose at base of basidiomata. Locules 1–2.5 × 0.15–0.3 mm, minute, radially elongated, tubular, sinuous, pale yellow to light orange (4A3–5A4); light orange to greyish orange or light brown (5A4–B5–D5) in exsiccata. Tramal plates 0.15–0.25 mm thick, greyish, becoming brownish red (8C8) on exposure to air. Columella 0.2–2.5 mm thick, dendroid, branched to the surface, greyish; basal context reddish brown (8E8). Odour strong, alliaceous, reminiscent of G. otthii.
Spores (16–)16.5–20(–21) × 9.5–11(–12) µm, Q = 1.6–2, variable in shape, elliptical to obovate, pallid, butter yellow to maize yellow (4A5–A6) in KOH (Fig. 33e, 34s). Episporium elliptical to ovate. Costae (11–)13–14, 0.3–0.6 µm high × 1.8–2.7 µm wide, slightly inflated, straight, mostly furcate and anastomosing, covering up to 1/2 of hilar appendix, strongly ornamented with hyaline, rigid but fragile, rounded to acute tubercles 0.8–3.3 µm high × 1.7–4.2 µm wide, 4–8 arranged along each costa; apical ring tuberculous, 0.4–1 µm high; perisporial reticulum indistinguishable. Hilar appendix 2.5–4.8 µm long × 3.6–5 µm wide at apex, conico-truncate; empty-end 0.1–0.5 µm long; hilum 1–1.5 µm wide, without remnants of sterigma or very short. Basidia 35–50 × 8–12 µm, cylindrical, 1–2-spored. Paraphysoid cells 25–60 × 3–6 µm, 0–2-septate, with the terminal cell cylindrical or clavate. Subhymenium consisting of long chains of cylindrical elements, 6–10 µm diam, strongly gelatinized, becoming globose near trama. Hymenophoral trama formed by perpendicular bundles of hyphae, 2–4 µm diam, strongly gelatinized, presenting ampulliform septa, 5–10 µm wide, and some endocystidia-like terminal vesicles. Thromboplera present, abundant in peritrama. Peritrama 30–60 µm thick, prosenchymatous, yellowish in mature specimens, formed by densely interwoven, strongly gelatinized hyaline hyphae, 1–4 µm diam. Pseudopellis a hymeniderm composed of clusters of hyaline to yellow, fragile, sphaeropedunculate exocystidia, 15–30 µm wide, often grouped in pseudoparenchymatous accumulations. Emerging thick-walled hairs, 30–100(–200) µm long × 2–4 µm wide, with a yellow wall and an obtuse tip, were also observed, sometimes grouped in small bundles, finally turning brown, geliferous and thick-walled up to 2 µm. Nodulose and tortuous hyphae were also observed among them. At last, all structures are embedded in a brown gelatinous mass, becoming indistinguishable. Calcium oxalate crystals present in trama and surface of exocystidia.
Habitat, Season & Distribution — Gregarious, hypogeous, in montane and subalpine coniferous forests (Abies, Picea), preferably on calcareous soils. From spring to autumn. Rare. Distributed in temperate regions, from 500 m altitude in Central Europe (Western Carpathians) to 1700 m in South-Western Europe (Pyrenees) (Fig. 38).
Additional material studied. FRANCE, Franche-Comté, ‘Jura Septentrionel, Montagne du Lomont, bois de conifères’, L. Quélet as G. graveolens (UPS F-550643); ‘Lomont de Porrentruy, sapinières’, 22 July 1880, L. Quélet as G. graveolens (PC 92296). – POLAND, Lesser Poland, Ochotnica Górna, Duże Jaszcze Valley, Gorce Mountains, 800 m, Fagus-Abies forest, on sandstone soil with calcium carbonate, 10 Aug. 2016, P. Mleczko (KRA F-2016-31*, dupl. JMV800767); near Konina, Z Pod Figurek Valley, Gorce Mountains, 830 m, Abies alba forest with scarce Picea abies and Fagus sylvatica, 31 May 2014, M. Kozak (KRA F-2014-29, dupl. 800762); near Poręba Wielka, Olszowy Stream Valley, Gorce Mountains, 690 m, Abieti-Piceetum with scarce Fagus sylvatica, on sandstone soil with calcium carbonate, 27 July 2013, M. Kozak (KRA F-2013-3, dupl. JMV800757); near Berest, Beskid Niski Mountains, 610 m, mixed forest of Abies, Picea, Fagus and Tilia, 31 Aug. 2011, M. Kozak & P. Mleczko (KRA F-2011-77*, dupl. JMV800753); near Czorsztyn, by Barbarzyna Glade, Pieniny Nat. Park, 560 m, Fagus-Abies forest, 18 July 2016, P. Chachuła (KRA F-2016-90, dupl. JMV800706); near Hałuszowa, by the Szkółka, Pieniny Nat. Park, 670 m, Abies-Fagus forest with Picea abies and Corylus avellana, 31 July 2016, P. Komur (KRA F-2016-56*, dupl. JMV800705); ibid., 660 m, Abies-Fagus forest with Corylus avellana, 21 July 2017, P. Mleczko (KRA F-2017-13*, dupl. JMV800709); near Kuźnice, Tatra Nat. Park, 1030 m, Galio-Piceetum, on calcareous soil, 26 July 2019, M. Kozak (TPN-19-0241, dupl. JMV800807); near Małe Ciche, Wierch Poroniec Mount, Tatra Nat. Park, 1060 m, Abies-Picea-Fagus forest, 19 July 2019, F. Karpowicz, M. Kozak & P. Mleczko (TPN-19-0387, dupl. JMV800813); near Kościelisko, Mała Sucha Valley, Tatra Nat. Park, 1020 m, Abieti-Piceetum, on calcareous soil, 16 Aug. 2019, M. Kozak (TPN-19-0144, dupl. JMV800802); near Zakopane, northern slope of Mały Kopieniec Mount, Tatra Nat. Park, 940 m, Abieti-Piceetum, on calcareous soil, 23 Aug. 2019, F. Karpowicz (TPN-19-0111, dupl. JMV800800). – SPAIN, Catalonia, Girona, Setcases, Baga de Queràs Forest, Eastern Pyrenees, 1700 m, under Abies alba and Pinus uncinata, on siliceous soil, 6 Sept. 2011, J.M. Vidal & F. Rodríguez (JMV20110906-3).
Notes — Quélet (1879), in his ninth contribution on the fungi of the Jura and the Vosges, briefly described a Gautieria found in the fir forests of the French Jura mountains that he identified as G. graveolens, and which was later identified as G. trabutii by Dodge & Zeller (1934) after seeing an Uppsala exsiccatum. We have consulted Quélet’s herbarium material preserved in Uppsala (UPS F-550643) and in Paris (PC 92296), and initially we also identified Quélet’s collection as belonging to G. trabutii, but its habitat and northern distribution so different from G. trabutii made us suspect its incorrect identification. Recently, we have found 13 new collections of this Gautieria in montane and subalpine fir forests of southern Poland and northern Spain, which were also identified as G. trabutii. Of these, we have successfully sequenced 5 collections (Table 1) with the representation of 3 sequences in our phylogenetic tree (Fig. 3). We have not attempted to obtain genetic information from the Quélet material due to its age, but spore comparisons with our collections are in complete agreement (Fig. 28h). The genetic studies have identified our specimens as a sister species of G. trabutii, which we name G. queletii in honour of the French naturalist Lucien Quélet, differing from G. trabutii by having: 1) smaller basidiomata; 2) absence of pronounced reticulations on the upper surface of basidiomata; 3) thinner pseudoperidium that becomes transparent with moisture; 4) lighter and brighter locules; and 5) paler spores provided with broader gibbosities and shorter hilar appendix. Another differentiating character of G. queletii with respect to G. trabutii is its ecology. While G. trabutii is linked to broadleaf or coniferous trees in the Mediterranean region and shows a trend towards siliceous soils, G. queletii is linked to montane and subalpine conifers with an affinity for calcareous soils.
Gautieria trabutii (Chatin) Pat., Bull. Soc. Mycol. France 13: 203. 1897 — Fig. 29, 33, 34, 38
Basionym. Hymenogaster trabutii Chatin, Bull. Soc. Bot. France 38: 64. 1891.
Etymology. In honour of the French botanist and physician Louis Charles Trabut (1853–1929), collector of the type material.
Authentic material studied. ALGERIA, ‘Blida’ (FH 301416, herb. N. Patouillard); ‘Sidi abd el Kader, sous les cèdres, specimen original don. Dr. M. Trabut’ (FH 301415, herb. N. Patouillard); ‘Sidi abd el Kader près de Blida, sous des cèdres à 1640 m, Trabut, com. O. Mattirolo’ (FH 301447, herb. C.W. Dodge #2086); ‘à Sidi abd el Kader, Blida, Trabut, ex herb. N. Patouillard’ (FH 301448, herb. C.W. Dodge); ‘part du type, N. Patouillard’ (BPI 712247, herb. C.G. Lloyd #46539).
Basidiomata pseudoperidial, 2.5–5 cm wide, subglobose to tuberiform, often deformed due to the growth of several crowded specimens, provided with a basal depression or protuberance attached to a white basal rhizomorph, 1.5–2 mm thick. Surface pruinose, initially whitish, then yellowish grey (5A2–A3) to greyish orange (6B4), changing intensely to dark reddish brown (9D8–E8) with aging or when handled, opaque with humidity, consisting of flattened ridges that fuse forming foveate-porate pseudoperidium, 0.2–0.5 mm thick, pronouncedly venose-reticulate, becoming the veins and foveae radially elongated at base of basidiomata. Locules 1–4 × 0.2–0.4 mm, minute, radially elongated, tubular, sinuous, light yellow to greyish orange (5A4–6B4); cinnamon (6C6–D6) in exsiccata. Tramal plates 0.2–0.3 mm thick, greyish, becoming dark reddish brown (9E8) on exposure to air. Columella 0.5–3 mm thick, dendroid, branched to the surface, greyish; basal context reddish brown (8E8). Odour strong, of seaweeds or of seafood.
Spores 15–19(– 22) × 9–10.5 µm, Q = 1.6 – 2, variable in shape, elliptical, obovate or pyriform, highlighted, maize yellow to deep yellow (4A6–A8) in KOH (Fig. 33f, 34t). Episporium ovate. Costae (12–)14–16, 0.5–0.7 µm high × 1.4–2.4 µm wide, slightly inflated, straight, mostly furcate and anastomosing, covering up to 1/2 of hilar appendix, strongly ornamented with yellowish, rigid but fragile, rounded to acute tubercles 0.5–3 µm high × 1.5–3 µm wide, 4–8 arranged along each costa; apical ring tuberculous, 0.4–0.6 µm high; perisporial reticulum indistinguishable. Hilar appendix 3–6 µm long × 4–5 µm wide at apex, conico-truncate; empty-end 0.2–0.8 µm long; hilum 0.8–1.8 µm wide, without remnants of sterigma or very short. Basidia 35–45 × 8–10 µm, cylindrical, 1–2-spored. Paraphysoid cells 10–40 × 2.5–6 µm, 0–2-septate, with the terminal cell cylindrical or clavate. Subhymenium consisting of long chains of cylindrical to inflated elements, 6–14 µm diam, strongly gelatinized, becoming globose near trama. Hymenophoral trama formed by perpendicular bundles of hyphae, 1.5–5 µm diam, strongly gelatinized, presenting ampulliform septa up to 10 µm wide and endocystidia-like terminal vesicles. Thromboplera present, more abundant in peritrama. Peritrama 60–80 µm thick, prosenchymatous, brown in mature specimens, formed by densely interwoven, strongly gelatinized yellow hyphae, 2–3 µm diam. Pseudopellis a hymeniderm of clusters of hyaline to yellow, fragile, sphaeropedunculate exocystidia, 10–30 µm wide, often grouped in pseudoparenchymatous accumulations. Isolated hairs 30–100 µm long × 2–5 µm wide, capitate and yellow walled, can be observed, later becoming brown, geliferous and thick walled up to 2 µm. In some specimens, the hairs are abundant and arranged in dense clusters. Observed also tortuous and nodulose hyphae among them. Finally, all structures are embedded in a brown gelatinous mass, becoming indistinguishable. Calcium oxalate crystals present in trama and surface of exocystidia.
Habitat, Season & Distribution — Gregarious, hypogeous or semi-hypogeous under plant debris, in Mediterranean and montane coniferous and broadleaf forests (Cedrus, Pinus, Pseudotsuga, Quercus), on siliceous soils. From spring to autumn. Rare. Distributed in the western Mediterranean basin, from 100 m altitude in Southern Europe to 2000 m in North Africa (Fig. 38).
Additional material studied. FRANCE, Pyrénées-Orientales, near Perpignan, under Quercus ilex, 21 May 1980, L. Riousset (OSC, herb. J. Trappe #6118). – ITALY, Sardinia, Cagliari, under Quercus ilex and Quercus suber, 19 Nov. 1983, C. Lavorato (ZT-Myc 3921, herb. E. Horak #1840); Oristano, Laconi, 800 m, under Quercus ilex and Quercus pubescens, 22 June 1994, A. Lasio, G. Gregori & M. Puxeddu, comm. A. Montecchi (AM1261, dupl. JMV800242); Trentino-Alto Adige, sine loc., ‘in quercetis’, Oct. 1882, G. Bresadola as G. graveolens (UPS F-550651); Tuscany, Lucca, Arsina, 100 m, under Pinus pinaster, Quercus cerris and Quercus pubescens, 4 July 1992, G. Bernardini & L. Gori (ELG920704-3, dupl. JMV800167); ibid., 12 July 1992, G. Bernardini & L. Gori (ELG920712-2, dupl. JMV800168); Lucca, Tubbiano, Brancoleria, 700 m, Pseudotsuga menziesii plantation, 23 June 1991, M. Balli, G. Bernardini & L. Gori (ELG910623, dupl. JMV800166). – SPAIN, Basque Country, Guipúzcoa, Anoeta, 150 m, under Quercus robur, on calcareous soil, 14 May 2013, P.M. Pasabán (JMV800824); Castilla and Leon, Zamora, Quintana de Sanabria, Cobreros, 1040 m, under Quercus pyrenaica, on siliceous soil, 12 Aug. 2007, J. Cabero (JC70812BT*, dupl. JMV800791); Castilla-La Mancha, Ciudad Real, Fuencaliente, Fuente del Almirez, 780 m, in humus of Cedrus sp. and Cistus sp., 8 May 2007, F. Prieto & A. González, det. F.D. Calonge (MA-Fungi 74767*); Catalonia, Girona, Aiguaviva, 150 m, under Quercus ilex and Quercus pubescens, on siliceous soil, 30 June 2018, A. Paz (IC30061806); Girona, Palafrugell, Font de la Teula, Les Gavarres Massif, 150 m, under Quercus suber, on siliceous soil, 19 May 2002, J.M. Batlle (JMV20020519-2*); Girona, Sant Sadurní de l’Heura, Can Cavarroques, Les Gavarres Massif, 220 m, under Quercus suber and Quercus ilex, on siliceous soil, 17 June 1999, J.M. Vidal (JMV990617-1); ibid., 23 May 2002, J.M. Vidal (JMV20020523*).
Notes — Gautieria trabutii was collected in Algeria by Louis Charles Trabut, professor of natural history at the medical college in Algiers, in a cedar forest (Cedrus atlantica) at Sidi-Abd-el-Kader above ‘Blidah’, in the vicinity of Sidi-Ahmed-El-Kebir, now Chréa National Park. This collection was sent to Adolphe Chatin, who proposed it as a member of the genus Hymenogaster describing it in a single sentence (Chatin 1891). Later, Patouillard (1897) provided a more detailed description and an illustration, and transferred it to the genus Gautieria. Authentic material is preserved in Dodge’s and Patouillard’s herbaria at FH and in Lloyd’s herbarium at BPI. Gautieria trabutii was found again under Cedrus atlantica in Morocco by Malençon (1937), who described it in detail in his publication on hypogeous fungi of North Africa (Malençon 1975), interpreting spore tubercles as exudations and stating that in Morocco it is a common species strictly linked to cedars. Martín et al. (1996) offer an excellent treatment of its spore ornamentation after the study of type material and other collections found under Cedrus in Morocco and under Quercus in France and Spain, proving that G. trabutii is not strictly linked to cedars as Malençon believed. This species was found also in Italy by Gregori & Puxeddu (1995) and by Gori & Bernardini (1997) who found it also under Pseudotsuga, and again in Spain by Vidal (2003) and Cabero (2008), under Quercus. The German citation by Soehner (1951) is incorrect and corresponds to G. morchelliformis var. morchelliformis, as we have verified by studying the herbarium material. This species shares the tuberculate spore ornamentation with G. queletii, but differs from it by its ecology under Mediterranean broadleaves and conifers, with a clearly affinity with siliceous substrates. From this species we have studied 19 collections from 4 countries, of which we have successfully sequenced 4 collections (Table 1) and represented 3 sequences in our phylogenetic tree (Fig. 3). We have not attempted to obtain genetic information from authentic Algerian material due to its age, but the spore comparison between our collections and those made by Trabut is in complete agreement (Fig. 29h).
Gautieria sect. Parvicellae J.M. Vidal & States, sect. nov. — MycoBank MB 845132
Etymology. From Latin, parvus = small + cella = cavity, due to the small size of the hymenophore locules.
Type species. Gautieria otthii Trog.
Basidiomata pseudoperidial. Pseudoperidium foveate-reticulate, becoming lemon yellow with aging. Trichotomentocutis absent or undeveloped. Locules minute, irregularly-shaped. Tramal plates up to 0.3 mm thick, usually narrower than locules. Hyphae late gelatinizing, making hymenophore subgelatinous. Basidia 2–4-spored. Spores elliptical to ovate, small with a minute hilar appendix, usually lacking a sterigmal remnant. Odour usually alliaceous, nauseating. Linked to broadleaf trees (Castanea, Fagus, Quercus) or conifers (Abies, Larix, Picea, Pinus, Pseudotsuga).
Key to species of section Parvicellae
1. Pseudopellis with an undeveloped trichotomentocutis. Spores (13.5–)15–18 × (8–)8.5–10 µm, elliptical to ovate. — Basidiomata reticulate-foveate, porate, white, appearing lemon yellow maculae with age and changing to brownish orange with rubbing. Foveae progressively obliterated by an arachnoid trichotomentocutis. Under Abies, Larix, Picea, Pinus. Boreal to temperate (Northern to Southern Europe) ............................................. G. otthii
1. Pseudopellis lacking a trichotomentocutis. Spores 12.5–15.5 × 8–9.5 µm, elliptical. — Basidiomata granulose to reticulate-porate, white with some lemon-yellow maculae, changing to greyish ruby with age or rubbing. Under Abies, Castanea, Fagus, Pseudotsuga, Quercus. Temperate (Central to Southern Europe) ................ G. persimilis
Clade otthii (GauParv-1)
Pseudoperidium reticulate-foveate, porate. Trichotomentocutis undeveloped, obliterating the foveae in mature basidiomata. Spores elliptical to ovate, reaching 18 × 10 µm. Linked to conifers (Abies, Larix, Picea, Pinus). Represented by G. otthii and two North American taxa corresponding to the sequences AF377091 and AF377093.
Gautieria otthii Trog, Mitth. Naturf. Ges. Bern.: 43. 1857 — Fig. 30, 33, 34, 38
Fig. 30.
Gautieria otthii. a–d. JMV20180705-1. a. Basidiomata; b. detail of foveate-reticulate surface; c. hymenidermial pseudopellis (sterile hymenium) of a fovea; d. young hymenium showing paraphysoid cells. — e. JMV20180823-2. Subgelatinized tramal plates and light orange locules. — f. JMV20190731-1. Basal ridges radially arranged showing brownish orange maculae. — g–h. JMV20110920-2. g. Erected cluster of exocystidia of pseudopellis; h. pseudopellis showing a loose trichotomentocutis with scattered exocystidia, and underlying peritrama. — i–j. JMV960713-1. i. Basidiomata showing white surface with lemon yellow maculae; j. spores in KOH. — k–l. ZT-Myc 3923 (holotype of G. otthii ). k. Yellow thick-walled hairs of trichotomentocutis mixed with collapsed exocystidia; l. spores in KOH. — m. K(M) 170554 (G. Winter as G. graveolens). Spores in KOH. — n–o. ZB1589. SEM images of spores. — Scale bars: a, i = 1 cm; b, e–f = 2 mm; c–d, g–h, k = 20 µm; j, l–m = 10 µm; n–o = 5 µm. — Photos: a–m. J.M. Vidal; n. UdG; o. P. Mleczko, UJ.
Synonyms. Gautieria graveolens var. otthii (Trog) Zeller & C.W. Dodge, Ann. Missouri Bot. Gard. 21: 696. 1934.
Gautieria morchelliformis var. microspora Wichanský, Mykol. Sborn. 39: 67. 1962 (‘morchellaeformis’), syn. nov.
Invalid names. Gautieria otthii forma costata Pilát, Flora ČSR B1, Gasteromycetes: 746. 1958. (nom. inval. publ., Art. 40.1).
Gautieria sciurea T.C.E. Fr., in sched. (nom. inval. publ., Art. 32.1a).
Gautieria microcoilia Pilát, in sched. (nom. inval. publ., Art. 32.1a).
Gautieria microcoilia forma subpallida Pilát, in sched. (nom. inval. publ., Art. 32.1a).
Etymology. In honour of the Swiss mycologist Gustav Heinrich Otth (1806–1874), collector of the type material.
Type. SWITZERLAND, Bern, Steffisburg, on the Hardlisberg, c. 800 m, June 1855, G. Otth, ‘G. otthii mihi’, rev. A. Pilát holo ‘G. graveolens auct. non Vittad., nec Corda, nec Tulasne!’ (holo ZT-Myc 3923, herb. Trog, ex herb. Bernense); Hardlisberg, June, ‘G. otthii mihi typus’, rev. O. Mattirolo as G. graveolens (iso ZT-Myc 3923bis, herb. Trog, ex herb. Bernense); bei Steffisburg, sine dat., G. Otth, ‘G. otthii Trog inedit’ (iso ZT-Myc 3924, herb. L. Fischer).
Basidiomata pseudoperidial, 1.5–4(–5) cm wide, subgloboseflattened to tuberiform, basally lobed with an omphaloid depression attached to a white basal rhizomorph, 1.5–2 mm thick. Surface consisting of flattened ridges that fuse and anastomose forming a rugose, reticulate-foveate pseudoperidium, 0.15–0.3 mm thick, arranged radially at base of basidiomata, with small irregular openings that communicate with the underlying locules, which are obliterated in mature basidiomata by the growth of a white arachnoid trichotomentocutis; surface initially white, appearing lemon yellow (3A7) maculae with aging, turning brownish orange (7C7–D7) with handling, more intensely at the edge of ridges. Locules 0.25–2 × 0.1–0.5 mm, minute, irregularly shaped, radially elongated, narrow, sinuous, light orange (5A4–A5); light orange to cinnamon (5A5–6D7) in exsiccata. Tramal plates 0.15–0.25 mm thick, greyish, immutable when cut. Columella 0.2–3.5 mm thick, dendroid, strongly branched to the surface, greyish; basal context greyish ruby (12E6). Odour at first fruity, then unpleasant, alliaceous, very intense and nauseating.
Spores variable in size and shape, (14–)15–18(–19) × (8–) 8.5–10(–11) µm, Q = 1.5–1.8(–2), elliptical to ovate, light yellow (3A4–A5) in KOH (Fig. 33g, 34u). Collections with smaller spores, 13.5–17 × 8–10 µm, have also been observed. Episporium elliptical to ovate. Costae 9–11, 0.5–1.3 µm high × 1–2.3 µm wide, moderately inflated, straight, entire or furcate, covering up to 1/2 of hilar appendix, ornamented with low gibbosities; apical ring distinct, 1–1.7 µm high; perisporial reticulum indistinguishable. Hilar appendix small, 1.4–2.8 µm long × 2.6–3.6 µm wide at apex, conico-truncate to subacute; empty-end 0.2–0.8 µm long; hilum 0.6–1 µm wide, usually without remnants of sterigma or very short. Basidia 35–50 × 7–11 µm, cylindro-clavate, 2–4-spored. Paraphysoid cells 15–32 × 4–7 µm, 0–2-septate, with the terminal cell cylindrical or clavate. Subhymenium consisting of chains of cylindrical to inflated elements, 4–13 µm diam. Hymenophoral trama of parallel gelatinized hyphae, 2–4 µm diam, with some ampulliform septa up to 18 µm wide, and endocystidia-like terminal vesicles up to 30 µm wide. Thromboplera present. Peritrama of parallel gelatinized hyphae, 3–6 µm diam, with some enlargements up to 12 µm wide. Pseudopellis an irregular hymeniderm of scattered or dense clusters of hyaline to yellow exocystidia, 10–25 µm wide, mixed with a loose, undeveloped trichotomentocutis of tufts of yellow, geliferous, thick-walled hairs, 4–6 µm diam and walls up to 1.5 µm thick, which join in fascicles that progressively covers the surface of basidiomata and the openings of pseudoperidium. Foveae lined with clavate to sphaeropedunculate exocystidia, 8–16 µm wide, constituting a hymeniderm. Observed some acanthohyphae. Calcium oxalate crystals present in all tissues.
Habitat, Season & Distribution — Gregarious, hypogeous or semi-hypogeous under needles, in montane and subalpine coniferous forests (Abies, Larix, Picea, Pinus), on siliceous soils. From spring to autumn. Common. Distributed in boreal and temperate regions, from sea level in Northern Europe to 1700 m altitude in Southern Europe (Fig. 38).
Additional material studied. AUSTRIA, Lower Austria, Zwettl, Kleinschönau, sine dat., V. Schiffner as G. graveolens (W 422, herb. Litschauer/Lohwag); Styria, Liezen, Hoher Trett, 26 July 1931, H. Lohwag as G. graveolens (W 423); Tyrol, Imst, Jerzens, Pitztal, ‘Fichtenwald’, 31 Aug. 2007, mushroom exhibition of Jenbach, sine leg., det. M. Kirchmair as G. graveolens (IB 20070503); Innsbruck, near Igls, 900 m, June 1903, A. Schumacher as G. graveolens, det. A. Pilát as G. microcoilia (S F181859); Mutters, upper Nockhof, coniferous forest, 20 July 1946, V. Vareschi, det. M. Moser #46 as G. graveolens (M 126212); Upper Austria, Rohrbach, Böhmerwald, ‘gegen den Grünberger’, 25 Oct. 1918, V. Schiffner as G. graveolens (W 84, herb. Litschauer/Lohwag). – BULGARIA, Pazardzik, Atoluka, Brazigovo, Western Rhodope Mountains, 1450 m, under Picea abies and Abies alba, on siliceous soil, 4 July 2018, T. Georgiev (SOMF 30333, dupl. JMV800744); Belovo, between Sestrimo and Belmeken, Rila Mountains, 1250 m, under Abies alba, Picea abies, Fagus sylvatica and Corylus avellana, on siliceous soil, 2 Sept. 2014, G. Kunev (SOMF 30334, dupl. JMV800728); Zigov Chark, Western Rhodope Mountains, 1100 m, under Picea abies, Abies alba and Fagus sylvatica, on siliceous soil, 3 Nov. 2018, M. Slavova (SOMF 30335, dupl. JMV800779); Smoljan, Chepelare, Central Rhodope Mountains, under Picea abies, 26 June 2017, S. Mihailova (SOMF 30336, dupl. JMV800736); Sofia-City, Sofia, Vitosha Mountain, under Picea abies, on siliceous soil, 19 July 2018, M. Slavova (SOMF 30337, dupl. JMV800747); ibid., 22 Sept. 2018, M. Slavova (SOMF 30338, dupl. JMV800773). – CZECH REPUBLIC, Central Bohemia, Černošice, near Dobřichovice, ‘in picetis’, 8 July 1944, A. Pilát as G. graveolens, det. A. Pilát (PRM 678972; W 1775, ex PRM); Kamýk, near Vltavou, ‘in picetis’, June 1961, A. Pilát (PRM 600649); Karlštejn, ‘in picetis’, 29 June 1946, M. Svrček, det. A. Pilát as G. microcoilia (W 1782, ex PRM); Mnichovice, Aug. 1922, J. Velenovský as G. graveolens, det. A. Pilát (PRM 678979); ibid., ‘Budíkov’ ‘in picetis’, June 1935, J. Velenovský, det. A. Pilát as G. microcoilia forma subpallida cotypus (W 1777, ex PRM); Nová Ves pod Pleší, ‘sanatorium’, 25 July 1916, L. Trikn, det. F. Bubák as G. graveolens (BPI 602604); Sedlčany, 26 July 1903, J. Rmondil, det. F. Bubák as G. graveolens (BPI 602605); Strašice, near Brdy, sine dat., J. Velenovský as G. graveolens, det. A. Pilát (PRM 678967); Třebotov, near Černošice, ‘in nemora’, 8 May 1918, J. Velenovský as G. graveolens, det. A. Pilát (PRM 678978); Vysoký Chlumec to Sedlčany, 24 Aug. 1903, J. Rmondil, det. F. Bubák as G. graveolens (FH 301425, herb. F. v. Höhnel); ibid., 28 Aug. 1904, F. Bubák as G. graveolens (BPI 712241, Lloyd Mus. #05859, herb. C.G. Lloyd #19426, ex herb. F. Bubák; NY 1952373, herb. Zeller #1534, ex Lloyd Mus.); Liberec, Dubá, ‘in silva mixta’, 11 July 1965, E. Strahaivuá, det. A. Pilát (PRM 604582); Olomouc, Hranice (Mährisch Weisskirchen), in forest, Oct. 1921, F. Petrak #70 as Gautieria sp. (W 9911); Vilémov, near Litovel, under Picea, Pinus and Larix, 19 June 1955, J. Kupha, det. A. Pilát as G. microcoilia (PRM 678932; W 1776, ex PRM); Prague, Prague, J. Velenovský as G. graveolens, det. A. Pilát (PRM 678984); South Moravia, Brno, Hádyberg, in forest, July 1925, J. Hruby as Rhizopogon virescens, F. Petrak, Flora Bohemiae et Moraviae exsiccata #2195, det. A. Pilát (M 126262; PRM 483527; S F181860); Lomnička, near Střibro, Eichelschlag Forest, under Picea and Pinus, 24 June 1954, F. Kotlaba, det. A. Pilát as G. microcoilia (PRM 678930; W 1779, ex PRM); Vyškov-Dĕdice, 27 June 1951, Lange, det. A. Pilát as G. graveolens (PRM 678927); Vysočina, Netín-Velké Meziříčí, in coniferous forest, Aug. 1924, R. Picbauer as G. graveolens (S F57145). – DENMARK, Capital Region of Denmark, Ravnholm n. Ørholm, J. Koch (OSC s.n.). – FRANCE, Provence-Alpes-Côte d’Azur, Clans, Forêt Domaniale de Clans, Maritime Alps, 1500 m, under Abies alba and Picea abies, 17 Sept. 1913, G. Poirault as G. graveolens (PC 92295); Rhône-Alpes, Savoie, Forêt du Mâcot-la-Plagne, 1650 m, under Picea abies, on siliceous soil, 3 July 1996, P.-A. Moreau, det. L. Riousset as G. mexicana (PAM96070306, dupl. JMV800484). – GERMANY, Baden-Württemberg, Wiesenthal, Dossenbach (Friburg), under Picea abies, 7 Aug. 1955, C. Schwärzel #94 (ZT-Myc 3932); Bavaria, Garmisch-Partenkirchen, on the Wamberger ridge, 1100 m, ‘Fichtenwald’, 23 June 1917, J. Zametzer, det. S. Killermann as G. graveolens (M 126234; M 126248, also containing Hysterangium coriaceum and Lactarius borzianus); Haüsen, Simonsberg, ‘Fichtenwald’, 21 Aug. 1920, E. Soehner #252 as G. graveolens (M 126220); Lichtenfels, Buch am Forst, 10 June 1981, Engel-Gross #2620 (M 307396); Lorenzen, Fichtelgebirge, young ‘Fichtenwald’, 15 June 1916, S. Killermann as G. graveolens (M 126233; M 126219, herb. E. Soehner #1216); Pfaffenhausen, road to Oberrieden, Community Forest, in coniferous forest, 27 July 1936, E. Soehner #1519 as Hysterangium rubricatum (M 126288); Rothenburg ob der Tauber, Fichtelgebirge, mixed forest, Oct. 1939, S. Killermann as G. graveolens (M 126235); North Rhine-Westphalia, Münster, acc. 1 Apr. 1925, O. Brefeld P392 as G. graveolens (B 700015008); Rhineland-Palatinate, near Oestrich (Nassau), Oelberg Mount, ‘in pinetis’, autumn 1894, Fuckel as Rhizopogon luteolus, herb. Barbey-Boissier #2152, Fungi Rhenani #1250 (S F57152; UPS s.n., mixed with Rhizopogon sp.); Saxony, Elsterberg, ‘Fichtenwald’, 28 June 1936, prof. J. Arno, det. E. Soehner #1515 as G. graveolens (M 126214); Saxony-Anhalt, ‘Neckendorf bei Eisleben, unter den Tannen, in sandigen boden’, 29 May 1872, J. Kunze as Hymenogaster vulgaris, herb. Barbey-Boissier #2154, herb. Fuckel 1894 (NY 1952364; UC s.n.; UPS s.n.); ‘Eisleben, unter Fichtenwaldern’, 29 May 1872, J. Kunze as G. graveolens, herb. P. Magnus, det. A. Pilát as G. microcoilia (FH 301430; K(M) 170556, herb. C.B. Plowright; PC 92297/92298; S F181855); ‘pr. Eisleben, Neckendorfer Tannen, in pinetis umbrosis Picea excelsae’, Aug. 1873, J. Kunze as G. graveolens, Rabenhorst, Fungi Europaei #1731, det. A. Pilát as G. microcoilia (BPI 602485; BR-MYCO 139382-90; H 7017233; M 126245; K(M) 170557; NY 1952362, Ellis collection; S F57161, ex herb. Sydow; S F119186, ex herb. H. Rehm; W 1774; ZT-Myc 3953); ‘Eisleben, Bischofrode Hofe’, Sept. 1873, G. Winter #8 as G. morchelliformis (B 700015010; BPI 602615; K(M) 170561, ex herb. C.B. Plowright; M 126244, herb. G. Niessl; W 346484/355017, coll. Reichenbach fil.); ‘Eisleben, in sylvis abietinis’, May 1874, G. Winter as G. graveolens, De Thümen, Mycotheca Universalis #12, det. A. Pilát as G. microcoilia (B 700015011; BPI 602607; BR-MYCO 139383-91; FH 301424; H 7017231; K(M) 170554; M 126246; NY 1952363, Ellis collection; NY 1952367, herb. L.M. Underwood; NY 1952366, ex herb. W. Welch; S F57167, ex herb. Sydow; W 1773; ZT-Myc 3952); ‘bei Eisleben, unter Picea excelsa’, June 1875, J. Kunze as G. graveolens (BPI 602602, herb. F. Bubák, ex herb. P. Magnus; BR-MYCO 139384-92); ‘pr. Eisleben, Neckendorfer Tannen, in pinetis umbrosis Picea excelsae’, mid-June 1875, J. Kunze as G. graveolens, Fungi Selecti Exsiccati #14, det. A. Pilát as G. microcoilia (BPI 602490; BPI 711884, herb. C.G. Lloyd #19427; BPI 712242, herb. C.G. Lloyd #19428; BPI 602489, herb. W.H. Long; H 7017228, herb. P.A. Karsten; K(M) 170551/170552, ex M.C. Cooke; M 126247; NY 1952416; PAD s.n., herb. P.A. Saccardo; S F57165, ex herb. Sydow; S F119190, ex herb. H. Rehm); ‘Eisleben, aus den Fichtenwaldern’, June 1878, J. Kunze as G. morchelliformis, det. A. Pilát as G. microcoilia (B 700015005; W 365896, coll. Reichenbach fil.); Thuringia, Friedrichroda, Reinhardsbrunn, July 1867, B. Auerswald as G. morchelliformis, det. A. Pilát as G. microcoilia (S F57168, ex herb. Sydow). – HUNGARY, Baranya, near Abaliget, Mecsek Mountains, c. 300 m, under Picea abies, 1 Nov. 1994, Z. Lukács & Z. Bratek (ZB443*, dupl. JMV800393); Borsod-Abaúj-Zemplén, sine loc., Bükk Mountains, 1984, G. Répási (ZB332, dupl. JMV800389); Heves, near Parád-Parádsasvár, Mátra Mountains, c. 350 m, under Picea abies, 17 June 2001, I. Kiss (ZB2286*, dupl. JMV800420); Nógrád, near Keszeg, Cserhát Mountains, c. 300 m, Oct. 1999, sine leg. (ZB1822*, dupl. JMV800413); near Nagybátony, Mátra Mountains, c. 250 m, under Picea abies, 20 Aug. 2006, I. Zagyva, A. Bathó & Z. Bratek (ZB3345, dupl. JMV800440); near Nagyoroszi, Börzsöny Mountains, c. 300 m, July 2001, I. Újlaczki (ZB2299*, dupl. JMV800421); Pest, Kóspallag, Nagyhideghegy, Börzsöny Mountains, c. 850 m, under Picea abies, 29 Aug. 2008, I. Bagi (ZI183*, dupl. JMV800455); near Pilisszentkereszt, Pilis Mountains, c. 350 m, under Picea abies, June 1999, I. Meuser (ZB1589*, dupl. JMV800410); near Szokolya, Királyrét, Börzsöny Mountains, c. 350 m, under Picea abies, 5 Oct. 2002, B.E. Prutkayné (ZB2559, dupl. JMV800428); ibid., Oct. 2002, F. Pál-Fám (ZB3548*, dupl. JMV800429); ibid., 31 July 2005, I. Zagyva (ZB3022, dupl. JMV800435); ibid., 15 June 2006, I. Bagi (ZB3543*, dupl. JMV800438); ibid., 24 June 2007, I. Zagyva (ZI126*, dupl. JMV800446); ibid., 24 June 2007, D. Zagyva (ZB3569*, dupl. JMV800447). – ITALY, Lombardy, Sondrio, Chiesa in Valmalenco, under Picea abies, on siliceous soil, 13 June 1997, C. Piuri, det. M. Sarasini as G. graveolens (MS0844*, dupl. JMV800184); Piedmont, Asti, San Grato, Valle d’Aosta, sine dat., O. Mattirolo as G. graveolens (OSC s.n.); Trentino-Alto Adige, Bolzano, Renon, Kematen, under Pinus, 9 Oct. 2000, K. Soop (S F42752); Trento, Bellamonte, ‘in silvis abiegnis’, Aug. 1898, G. Bresadola as G. graveolens, det. A Pilát as G. microcoilia (S F181856); Trento, Cavelonte, ‘in silvis abiegnis’, Aug. 1898, G. Bresadola #643 as G. graveolens (M 126213, herb. S. Killermann); ibid., Aug. 1921, G. Bresadola as G. graveolens (BPI 602606); Trento, Mendola, Passo Mendola, Aug. 1903, G. Bresadola & Murrill as G. graveolens (NY 1952365); Trento, Varena, ‘in silvis abiegnis’, July 1914, G. bresadola as G. graveolens, det. A Pilát as G. microcoilia (S F57164); Tuscany, Florencia, Vallombrosa Forest, 22 June 1902, O. Mattirolo as G. graveolens (FH 301422, herb. Patouillard). – NORWAY, Buskerud, Ringerike, Vik, under conifers, 2 Sept. 1969, G. Gulden #802/69 as G. cf. morchelliformis (O 152359); Oppland, Lunner, under Picea, 24 July 1982, T.E. Brandrud #163 as Gautieria sp. (O 152358); Telemark, Bamble, ‘ved Krabberød gard vest for Stathello’, under Picea, 15 Oct. 1952, F.E. Eckblad, det. K.S. Berjsnov Hansen #3a as G. morchelliformis (O 152353); Notodden, Bolkesjö, Gransherad, under Picea, 17 Sept. 1964, K. Kvavik as Hymenogaster vulgaris, det. K.S. Berjsnov Hansen as G. morchelliformis (O 152349). – POLAND, Lower Silesia, Żdanów (Herzogswalde), near Srebrna Góra (Silberberg), 24 June 1919, prof. Buchs, det. E. Soehner #1172 as G. graveolens (M 126215). – ROMANIA, Covasna, near Ojdula (Ozsdola), under Picea abies, 3 Oct. 1998, B. Pálfy (ZB2279*, dupl. JMV800407). – RUSSIA, Caucasus, Nothnagel, G. Gross 626 (OSC s.n.); Tyumen, Tobolsk, near Marajskoye (Kurgan), under Pinus sylvestris, 1898, Skalosuboff, comm. W. Franzschel, det. E. Fischer? as G. graveolens (ZT-Myc 3954). – SERBIA, Vojvodina, Novi Sad, Fruška Gora Nat. Park, Eugenweg, July 1926, A. Hofer, det. H. Lohwag as G. graveolens (W 421). – SLOVAKIA, Prešov, Levoča (‘Leutschau’), ‘sub humus coniferarum’, July 1899, V. Greschik as G. graveolens, det. A Pilát as G. microcoilia (S F181858). – SPAIN, Aragon, Huesca, Benasque, Cerler, Central Pyrenees, 1600 m, under Quercus pyrenaica, 24 July 2009, F. García-Alonso (FGA094313, dupl. JMV800496); Teruel, Puerto de Orihuela, 1650 m, under Pinus sylvestris, 5 June 1988, F.D. Calonge as G. mexicana (MA-Fungi 29406); Basque Country, Álava, Zárate, Zuia, 800 m, under Pinus radiata, on siliceous soil, Aug. 1984, V. Irigoyen, det. F.D. Calonge as G. mexicana (MA-Fungi 9946); Castilla and Leon, Palencia, Guardo, 1200 m, under Pinus sylvestris, 9 May 1998, E. Rubio as G. mexicana (MA-Fungi 39619*, dupl. JMV980509); Palencia, Villalba de Guardo, 1100 m, under Pinus sylvestris, 21 May 2009, J. Martínez (IC21050932); Segovia, La Granja de San Ildefonso, 1991, P. Juste, det. F.D. Calonge as G. mexicana (S F22958); Segovia, Puerto de Navacerrada, under Pinus sylvestris, 15 July 1995, F. García-Verdugo #48, det. F.D. Calonge as G. otthii (MA-Fungi 35391, dupl. JMV950715); Catalonia, Girona, Alp, Saltèguet Forest, Eastern Pyrenees, 1650 m, under Abies alba and Pinus uncinata, on schistose soil, 19 June 1993, J.M. Vidal as G. mexicana (JMV930619-1a); ibid., 22 June 1996, J.M. Vidal (JMV960622-7); ibid., 13 July 1996, J.M. Vidal (JMV960713-1); ibid., 24 Aug. 1996, J.M. Vidal (JMV960824-1); ibid., 1750 m, 20 Sept. 2011, J.M. Vidal & F. Rodríguez (JMV20110920-2); ibid., 1760 m, 31 July 2019, J.M. Vidal, A. Paz & C. Lavoise (JMV20190731-1); Girona, Campelles, Coll d’Arola, Eastern Pyrenees, 1650 m, under Pinus uncinata, on siliceous soil, 15 June 2002, J.M. Vidal (JMV20020615-5*); Girona, Setcases, Baga de Carboners Forest, Eastern Pyrenees, 1650 m, under Abies alba and Pinus uncinata, on siliceous soil, 28 July 2002, J.M. Vidal (JMV20020728-4*); Girona, Setcases, Baga de Queràs Forest, Eastern Pyrenees, 1700 m, under Abies alba and Pinus uncinata, on siliceous soil, 7 Oct. 2008, F. Rodríguez (JMV20081007-3*); ibid., growing in the vicinity of G. subglobispora, 1 Oct. 2008, J.M. Vidal & F. Rodríguez (JMV20081021-4b); ibid., 23 Aug. 2018, J.M. Vidal & A. Paz (JMV20180823-2); Lleida, Alt Àneu, Bonabé Forest, Central Pyrenees, 1550 m, under Abies alba, on siliceous soil, 26 June 1999, J.M. Vidal & J. Vila (JMV990626-1*); Lleida, Lles de Cerdanya, Cap de Rec Forest, Eastern Pyrenees, 1900 m, under Pinus uncinata, on siliceous soil, 12 June 1999, J.M. Vidal (JMV990612-2*); ibid., 17 July 1999, J.M. Vidal (JMV990717-1*); Lleida, Riu de Cerdanya, La Mata Negra Forest, Eastern Pyrenees, 1780 m, under Abies alba, on siliceous soil, 5 July 2018, J.M. Vidal, A. Paz, C. Lavoise, L. Sánchez & J. Bometón (JMV20180705-1); Community of Madrid, Madrid, Rascafría, El Brezal Forest, 1125 m, under Pinus sylvestris and Quercus pyrenaica, on siliceous soil, 11 May 2019, A. Paz & C. Lavoise (IC11051908*); Valencian Community, Castelló de la Plana, Vistabella del Maestrat, 1280 m, under Pinus sylvestris and Quercus pyrenaica, 24 May 2003, F. García-Alonso (FGA032275, dupl. JMV800487); ibid., 7 July 2007, F. García-Alonso (FGA073758, dupl. JMV800493). – SWEDEN, Jämtland, Fårskinnsberget, under Picea and Pinus, 22 July 1950, L.E. Hawker H138 as G. graveolens (K(M) 170558); 6 km NE Klövsjö, 15 July 1981, R.A. Hintz (S F57160); Östersund, Odensala, 22 July 1950, M. Lange #3262 as G. graveolens (K(M) 170559); ibid., 22 July 1950, M. Lange #3263 as G. graveolens (UPS F-550634); Södermanland, Lena, Årby Forest, 17 June 1936, S. Malmborg as G. graveolens (UPS F-550633); Uppsala, Älvkarleby, Laxön, Aug. 1891, A. Zethelius as G. graveolens (UPS F-550632); Fristaden, between Graneberg and Flottsund, under Pinus sylvestris, 13 July 1903, E.A. Fries as G. graveolens (UPS F-550630); Uppsala, Granebergs Skogen, under Abies, 29 July 1900, E.A. Fries as G. graveolens? (UPS F-550629); ibid., 1901, T.M. Fries as G. graveolens (O 88621, ex UPS; UPS F-550631); ibid., 20 July 1902, T.C.E. Fries as G. graveolens (UPS F-550625); ibid., 20 July 1902, T.C.E. Fries as G. sciurea n. sp. (?) (UPS F-550635); ibid., 20 July 1905, E.A. Fries as G. graveolens (UPS F-550628); Västerbotten, Umeå, Ersmark-Tavelán, under conifers, 19 Aug. 1980, J. Nitare as G. graveolens (S F57147). – SWITZERLAND, Grisons, Prättigau-Davos, Davos, under Picea abies, 17 Aug. 1975, I. Hohner, H. Hohner & D. Platz, det. C. Schwärzel #64/25/82/6/83/63/45 (ZT-Myc 3925/3926/3927/3928/3936/ 3937/3939); Zürich, near Zürich, ‘in silvis abietis’, Aug. 1880, G. Winter as G. graveolens, Fungi Helvetici, suppl. 66 (FH 301442a, one slice; NY 1952372, Ellis collection). – TURKEY, Çankiri, Ilgaz Daği Nat. Park, 1800 m, under Abies bornmuelleriana, July/Aug. 1931, A. Pilát as G. microcoilia (W 1778, ex PRM).
Notes — Due to the nauseating odour and small spores of G. otthii, there is a long history of confusion regarding its identity and perceived synonymy with G. graveolens. It is also noteworthy that of the twenty or more taxonomic treatments of Gautieria, only Tulasne, Hollós, Mattirolo and Pilát examined and compared their specimens to the original collections. The German mycologists Johannes Kunze and Georg Winter identified as G. graveolens several collections of G. otthii made between 1872 and 1875 in the coniferous forests near Eisleben (Saxony-Anhalt, Germany), and duplicates of these exsiccata labelled as ‘Gautieria graveolens Vittad.’ (Fungi Europaei Exsiccati No. 1731, Rabenhorst 1874; Mycotheca Universalis No. 12, De Thümen 1875; Fungi Selecti Exsiccati No. 14, Kunze 1876) were later distributed to major European and American herbaria, adding to the confusion between those two species (see Fig. 30m, spores from Winter’s collection K(M) 170554). Thus, G. otthii is a long-neglected species, often identified as G. graveolens in the literature (e.g., Winter 1884, Hesse 1891, Mattirolo 1900, 1903, 1928, 1933, 1935, 1936, 1938, Bucholtz 1901, 1902, 1903, Fries 1909, 1922, Hollós 1911, Velenovský 1922, Pilát 1937, Lange & Hawker 1951) and in most herbaria consulted.
Gautieria otthii was collected in June 1855 by the Swiss mycologist Gustav Heinrich Otth on Mount Hardlisberg above Steffisburg and published by his colleague Jakob Gabriel Trog in 1857 with the following collection data ‘147. Gautieria otthii Trog. Auf dem Hardlisberg. Otth.’ followed by a brief description, highlighting its unpleasant odour, but without providing spore size, but only their ovoid shape (Trog 1857). We have not tried to obtain genetic information from the type material due to its age, but we have studied the holotype and isotypes of G. otthii preserved in BERN and, despite being in poor condition, they are still fully representative, having elliptical to ovate spores measuring (13–)14.5–19(–19.5) × (8–)9–10(–11) µm, Q = 1.5–2, and remains of trichotomentocutis in the pseudopellis, both morphological characters coinciding with those of our recent collections (Fig. 30k–l).
Zeller & Dodge (1918) considered G. otthii a doubtful species. Later, Dodge & Zeller (1934) consulted the holotype and considered G. otthii a variety of G. graveolens, despite significant morphological differences. The wide variability in spore size of G. otthii convinced Soehner (1951) to consider collections with comparatively smaller spores as separate species. Soehner, after receiving American specimens of G. mexicana and G. pallida from C.W. Dodge, assigned his small-spored collections to G. mexicana (No. 152, 292, 1216, 1515 and 1896) and G. pallida (No. 252). However, as we have verified when studying Soehner’s herbarium, three collections correspond to G. otthii (No. 252, 1216 and 1515) and another three to G. fusella (No. 292), G. persimilis (No. 1896) and G. pityophila (No. 152). Pilát (1958), in his study of the genus Gautieria for Flora ČSR, identified a small-spored collection found by Zvára in Radotín in October 1915 as belonging to G. mexicana (spores 15–16 × 9 µm according to Pilát) and, at the same time, identified his herbarium samples previously labelled G. microcoilia as G. otthii. In the same work, he assigned the name G. otthii forma costata to a Velenovský’s collection found in Mnichovice in June 1935, which was kept in PR herbarium with No. 153807. We have studied a duplicate of this collection preserved in Wien her-barium (W 1777) labelled ‘Gautieria microcoilia forma subpallida Pilát cotypus’ and we have observed typical spores of G. otthii. In the T.C.E. Fries herbarium at UPS (No. F-550635), G. otthii appears labelled under the name ‘Gautieria sciurea n. sp. (?)’. However, none of those names, G. microcoilia, G. microcoilia forma subpallida, G. otthii forma costata and G. sciurea, were ever validated by their authors. Until recently, many European mycologists continued to assign their small-spored collections of G. otthii to G. mexicana (Rauschert 1975, Gross et al. 1980, Calonge et al. 1985b, 1996, Montecchi & Lazzari 1988, 1993, Calonge & Pasabán 1993, Vidal 1994), but to date no specimens corresponding to the holotype of G. mexicana have been found outside the Americas. Wichanský (1962) validly published G. morchelliformis var. microspora found in July 1961 by Mrs. Marta Pospíšilová in the forests near Nové Město nad Metují (Hradec Králové Region, Czech Republic), but although the specimen on which the description was based has not been located, the data provided by the author (spores measuring 14.5–18.5 × 8.5–10.5 µm, habitat under fir trees and unpleasant odour) allow us to identify this species as G. otthii.
Distinctive features of G. otthii include: 1) presence of a loose trichotomentocutis of thick-walled hyphae; 2) reticulate-foveate pseudoperidium with lemon yellow maculae; 3) lower degree of gelatinization of tramal plates that not redden when exposed to air; and 4) small elliptical spores with a slightly developed perisporium and a subacute hilar appendix. Specimens with very small spores are difficult to differentiate from G. persimilis if the structure of the pseudopellis is not studied. While G. graveolens is a rare species linked to broadleaf trees, especially oaks, G. otthii is very common in montane and subalpine coniferous forests, with numerous records in western Europe from Spain to Scandinavia, and also in Central and Southern Europe in the Sudetes, Carpathians and Balkans. The occurrence of G. otthii in Asia and the Middle East has also been confirmed with scattered collections from eastern Russia, Siberia and Turkey. From this species we have studied 135 collections from 18 countries, of which we have successfully sequenced 20 collections (Table 1) and represented 5 sequences in our phylogenetic tree (Fig. 3). The GenBank sequences AF377091 and AF377093 (Bidartondo & Bruns 2002) could represent North American varieties of G. otthii.
Clade GauParv-2
Represented by two undescribed North American taxa corresponding to the sequences KC152099 and MG761349.
Clade persimilis (GauParv-3)
Pseudoperidium granulose to reticulate-porate. Trichotomentocutis absent. Spores elliptical, reaching 15.5 × 9.5 µm. Linked to conifers (Abies, Pinus, Picea, Pseudotsuga) and broadleaf trees (Castanea, Fagus, Quercus). Represented by G. persimilis.
Gautieria persimilis J.M. Vidal, Fern. Rodr., Cabero, Bratek, Mleczko & Kaounas, sp. nov. — MycoBank MB 845131; Fig. 31, 33, 34, 38
Fig. 31.
Gautieria persimilis. a–f. JMV20180904-2 (BCN, holotype). a. Basidiomata showing granular (left) and grumose (right) surface; b. detail of granulose surface; c. detail of grumose surface showing reddish brown maculae; d. chains of globose exocystidia constituting a granule; e. subhymenium and collapsed hymenium; f. spores in KOH. — g–i. JMV20180814-2. g. Basidiomata; h. detail of ridges fusing into a porate pseudoperidium; i. detail of reticulate-porate surface showing greyish ruby maculae. — j. JMV20110621-7. Basidioma showing lemon yellow maculae. — k. JMV20180709-1. Detail of hymenophore showing subgelatinized tramal plates and pale orange locules. — l. ZB3136. Spores in KOH. — m. VK907. Basidioma showing granular surface. — n. JMV20180730-8. SEM image of spores. — o. KRA F-2013-04. SEM image of a spore. — Scale bars: a, g, j, m = 1 cm; b–c, h–i, k = 2 mm; d–e = 20 µm; f, l = 10 µm; n–o = 5 µm. — Photos: a–l. J.M. Vidal; m. V. Kaounas; n–o. P. Mleczko, UJ.
Etymology. From Latin, per = very + similis = similar, for its resemblance to Gautieria otthii.
Type. SPAIN, Catalonia, Girona, Sant Hilari Sacalm, Torre de Vilavecchia, Les Guilleries Massif, 900 m, Abies × masjoannis plantation, on siliceous soil, 4 Sept. 2018, J.M. Vidal & F. Rodríguez (holo BCN JMV20180904-2*; iso in ELTE).
Basidiomata pseudoperidial, 2–5 cm wide, subglobose to tuberiform, basally lobed and with an omphaloid depression attached to a white basal rhizomorph, 1.5–2 mm thick. Surface of young basidiomata granulose to minutely ridged, white with some lemon yellow (3A4) maculae. On maturing, granules and ridges fuse forming a grumose or reticulate-porate pseudoperidium, 0.2–0.5 mm thick, with minute apertures communicating with underlying locules. Surface turning reddish brown (9D5) to greyish ruby (12D6) with aging or when handled. Locules 1.25–3 × 0.2–0.7 mm, minute, rounded to irregularly shaped, radially elongated, sinuous, narrow, more or less arranged radially, light orange (5A4–A5); light orange to cinnamon (5A5–6D7) in exsiccata. Tramal plates 0.15–0.25 mm thick, grey, slightly reddening when cut. Columella 0.2–2 mm thick, dendroid, strongly branched to the surface, grey; basal context dark ruby (12F4). Odour fruity, then alliaceous, very intense and nauseous as in G. otthii.
Spores (12–)12.5–15.5(–16.5) × (7.5–)8–9.5(–10) µm, Q = 1.5–1.8(–1.9), elliptical, light yellow (3A4–A5) in KOH (Fig. 33h, 34v). Episporium elliptical. Costae 8–12, 0.8–1.6 µm high × 1.8–2.8 µm wide, very inflated, straight, mostly furcate or anastomosing, covering up to 1/2 of hilar appendix, ornamented with large gibbosities; apical ring distinct, 0.7–1.5 µm high; perisporial reticulum indistinguishable. Hilar appendix small, 1.3–2.3 µm long × 2.3–3.2 µm wide at apex, conico-truncate to subacute; empty-end 0.3–0.9 µm long; hilum 0.4–0.8 µm wide, usually without remnants of sterigma. Basidia 35–50 × 8–10 µm, clavate, 2–4-spored. Paraphysoid cells 12–40 × 4–7 µm, 0–1-septate, sinuous, with the terminal cell cylindrical or attenuate. Subhymenium consisting of chains of cylindrical to inflated elements, 5–16 µm diam. Hymenophoral trama of parallel gelatinized hyphae, 2–5 µm diam, with some ampulliform septa up to 12 µm wide, and endocystidia-like terminal vesicles up to 14 µm wide. Thromboplera present. Peritrama consisting of a single layer of hyaline, gelatinized, thin-walled hyphae, 2–4 µm diam. Pseudopellis a hymeniderm formed by large erected clusters of chains of hyaline to yellow exocystidia, 15–35(–45) µm wide, thick walled up to 2 µm, collapsing at maturity. Trichotomentocutis of yellow, thick-walled hyphae absent. Calcium oxalate crystals present in all tissues.
Habitat, Season & Distribution — Gregarious, hypogeous or semi-hypogeous under plant debris, in montane broadleaf forests (Castanea, Fagus, Quercus) and in plantations of conifers (Abies, Pseudotsuga), on siliceous soils. From spring to autumn. Rare. Distributed in temperate regions, from 400 m altitude in Central Europe to 1000 m in Southern Europe (Fig. 38).
Additional material studied. AUSTRIA, Lower Austria, Neunkirchen, between Feistritz and Kirchberg, 700 m, ‘Fichten’, on gneiss soil, 24 June 1937, M. Jakob, det. E. Soehner #1896 as G. graveolens var. otthii (B 700015009; M 126241*); Tulln, Riederberg, under Fagus sylvatica, 5 July 1925, Cernohnski, det. H. Lohwag as G. graveolens (W 420). – GERMANY, Lower Saxony, near Salzgitter, under Fagus sylvatica, sine dat., sine leg. (ZB3549*, dupl. JMV800458); Saarland, Völklingen, under Fagus sylvatica, 17 June 1969, H. Derbsch, det. G. Gross #289 as G. otthii (M 126282). – GREECE, Thessaly, Magnesia, Zagora, 1070 m, under Fagus sylvatica, 30 July 2009, V. Kaounas (VK907*, dupl. JMV800547). – HUNGARY, Pest, Pilisszentlászló-Visegrád, Pilis Mountains, c. 450 m, under Fagus sylvatica, July 2005, T. Tóbler (ZB3136*, dupl. JMV800436). – ITALY, Emilia-Romagna, Parma, Tarsogno, 700 m, mixed forest, 2 Oct. 1987, A. Montecchi as G. mexicana, comm. L. Gori (ELG871002, dupl. JMV800359); Tuscany, Pistoia, Torri, 800 m, Pseudotsuga menziesii plantation, 21 May 1994, M. & D. Antonini, comm. L. Gori as G. mexicana (ELG940521-2*, dupl. JMV800361). – POLAND, Silesia, Giebło, Częstochowa Upland, 400 m, Fagus sylvatica forest, 2 Aug. 2013, R. Rutkowski & P. Mleczko (KRA F-2013-4*, dupl. JMV800758). – SPAIN, Castilla and Leon, Zamora, Quintana de Sanabria, Cobreros, 1000 m, under Castanea sativa, on siliceous soil, 13 June 2010, J. Cabero (JC100613BT*, dupl. JMV800790); ibid., under Quercus pyrenaica, 24 July 2011, J. Cabero (JC110724BT*, dupl. JMV800479); Catalonia, Girona, Espinelves, crossroad to Viladrau, foot of Montseny Massif, 740 m, Abies alba and Abies × masjoannis plantation, on siliceous soil, 25 May 2018, A. Paz (JMV20180525-3); ibid., 25 July 2018, F. Rodríguez (JMV20180725-1/20180725-2*); ibid., 14 Aug. 2018, F. Rodríguez (JMV20180814-2*); Girona, Sant Hilari Sacalm, Torre de Vilavecchia, Les Guilleries Massif, 900 m, Abies × masjoannis plantation, on siliceous soil, 10 June 2018, F. Rodríguez (JMV20180610); ibid., 18 June 2018, F. Rodríguez (JMV20180618); ibid., 9 July 2018, F. Rodríguez (JMV20180709-1); ibid., 30 July 2018, F. Rodríguez (JMV20180730-8); Girona, Viladrau, Mas El Martí, Montseny Massif, 985 m, Pseudotsuga menziesii plantation, on siliceous soil, 21 June 2011, J.M. Vidal & F. Rodríguez (JMV20110621-7*); ibid., 28 May 2012, F. Rodríguez (JMV20120528-1); ibid., 26 June 2018, F. Rodríguez (JMV20180626).
Notes — Gautieria persimilis has been confused with G. graveolens and G. otthii, but it is with the latter that it has more similarities. It can be distinguished from G. otthii by having: 1) absence of a trichotomentocutis; 2) granulose ridges that fuse to form a grumose or reticulate-porate pseudoperidium, turning greyish ruby when rubbed; and 3) smaller elliptical spores ornamented with large gibbosities. This species has a wider ecological range than G. otthii, and can be found both in coniferous forests, where it is quite rare, as well as in broadleaf forests, where it is more common, mainly in beech forests. From this Gautieria we have studied 23 collections from 7 countries, of which we have successfully sequenced 12 collections (Table 1) and represented 6 sequences in our phylogenetic tree (Fig. 3). We also identify as G. persimilis the GenBank sequence AF377088 (Bidartondo & Bruns 2002). The GenBank sequences AF377095 to AF377098 (Bidartondo & Bruns 2002) could represent a North American related species.
Clade GauParv-4
Represented by undescribed North American taxa corresponding to the sequences AF377095 to AF377098. Linked to conifers and broadleaf trees.
Clade monticola (GauParv-5)
Represented by G. monticola and some undescribed North American taxa corresponding to the sequences AF377076, AF377077, AF377079, AF377083, AF377084 and AF377087.
Clade GauParv-6
Represented by two undescribed North American taxa corresponding to the sequences KC152097 and KU871236.
Clade GauParv-7
Represented by two undescribed North American species corresponding to the sequences AF377089 and AY558751.
Clade GauParv-8
Represented by two undescribed North American species corresponding to the sequences AF377101, AF377102, AF377104 and AF377105.
DISCUSSION
Our work focuses on the relationship among the sequestrate Gomphales species found in the genus Gautieria. This research aimed to clarify the diversity and identity of European species of this hypogeous or semi-hypogeous genus. Considering the related literature, it can be observed that among the scattered polyphyletic clades of Ramaria, various morphologies of basidiomata are present, such as resupinate-odontoid (Kavinia, Ramaricium), agaricoid stipitate (Gloeocantharellus), pileate (Gomphus, Turbinellus), club-shaped (Clavariadelphus), pileate and also ramarioid (Phaeoclavulina) (Humpert et al. 2001, Giachini et al. 2010, Wannathes et al. 2018, González-Ávila et al. 2020). The monophyly of the genus Ramaria is not supported in multigene analyses and the morphology of the coralloid sporocarp is suggested to be the ancestral one of the Gomphales, while the other types (resupinate, club-shaped, pileate and sequestrate) are its derivatives (Humpert et al. 2001, Giachini et al. 2010). The genus Gautieria forms a monophyletic clade with its closely related sister group Ramaria subg. Ramaria (Humpert et al. 2001, Giachini et al. 2010, González-Ávila et al. 2020). The assessment of the taxa belonging to Gomphales and the relevance of the unification under the name Ramaria for some clades and Gautieria for others or, conversely, revision of the nomenclature of separate genus names for separate Ramaria clades will require the investigation of many taxa (e.g., Turbinellus and Gloeocantharellus) along with Ramaria. Even the investigation of the separation at the generic level of the two monophyletic taxa Gautieria and Ramaria subg. Ramaria needs other loci to involve in a comprehensive phylogenetic analysis, which goes far beyond our purpose. While it could be supported that these clades be unified under the earlier name Gautieria, we choose not to do this based on our new data, instead suggesting further work is needed on other taxa in the Gomphales.
Ecology and distribution of European Gautieria
With the large number of collections examined and species determined, we can state that most European Gautieria taxa are associated with a rather restricted range of bioclimatic regions, vegetation types, mycorrhizal partners and soil types, and only a few have broader ecological requirements. Some are only linked with Pinaceae trees: in montane and subalpine coniferous forests, G. villosa var. inflata and G. otthii are common on siliceous soils, and to a lesser extent G. pityophila and G. subglobispora, and on limestone soils G. morchelliformis var. dubia and G. villosa var. villosa, and to a lesser extend G. convoluta and G. queletii. Some of them have also been found in boreal forests, notably G. subglobispora and G. otthii. The taxa found in Mediterranean coniferous forests are few, namely G. pityophila and G. trabutii, some with a limited distribution, namely G. pervestita and G. villosa var. pilifera only known from Greece and G. pseudovestita from Morocco.
Other taxa live exclusively associated with Betulaceae and Fagaceae such as G. morchelliformis var. morchelliformis, G. morchelliformis var. fageticola, G. macrocoilia and G. graveolens. Some have a broader ecology and may also be found under conifers, such as G. confusa, G. fenestrata, G. fusella and G. persimilis. In Mediterranean sclerophyllous forests we find a great variety of Gautieria: on calcareous soils G. morchelliformis var. morchelliformis, G. iberica and G. obtexta, and on siliceous soils G. morchelliformis var. intermedia, G. hymenogastroides, G. graveolens, G. violascens and G. trabutii. A species to be highlighted is G. cistophila, which occurs on the Iberian Peninsula associated with plants of genus Cistus (Cistaceae) and maybe also with Arbutus (Ericaceae).
Section Gautieria, where the most obvious diversification takes place, has several taxa, restricted geographically and/or by host. In clade /morchelliformis, the polymorphic G. morchelliformis is of particular interest, containing four distinct phylogenetic lineages, treated here as varieties. They differ by their preference for particular host trees and also by their geographic distribution pattern. Of these G. morchelliformis var. fageticola seems to occur in Eastern and Central Europe and links usually with trees of genus Fagus and apparently more rarely with Carpinus. Gautieria morchelliformis var. intermedia is so far exclusively known from the Iberian Peninsula and most probably linked to Fagaceae. The remaining two varieties, G. morchelliformis var. morchelliformis and G. morchelliformis var. dubia are apparently more widespread and with somewhat overlapping ranges in Europe, but occurring with different host trees, the former being linked to broadleaf trees and the latter occurring with conifers. Moreover, G. morchelliformis var. dubia seems to exhibit characteristic Pyrenean – Alpine – Carpathian distribution, matching closely the range of the southern lineage of the European spruce (Picea abies) as presented in Tsuda et al. (2016), while G. morchelliformis var. morchelliformis has a wide distribution, ranging from the sclerophyllous forests of Southern Spain to the deciduous forests of Northern Europe. Such genetic differentiation and contrasting distribution pattern are also seen in the three varieties of G. villosa that are part of the clade /villosa, where G. villosa var. pilifera is notable for its limited geographic range confined to Greece and its occurrence with Mediterranean species of Abies. The remaining two varieties, G. villosa var. villosa and G. villosa var. inflata are more widespread in Europe and linked to a variety of conifers, thus suggesting that G. villosa var. pilifera may be the product of ongoing speciation process, which could be linked to the evolution of Abies populations in the Balkan Peninsula, following the disruption of the range of the Tertiary progenitor (Linares 2011). This is likely corroborated by the existence of other lineages with similar geographic and host patterns.
In clades /hellenica and /confusa, G. iberica and G. obtexta draw attention. These are associated with oaks of the Quercus ilex group and their known distribution apparently coincides with the ranges of their hosts, the vicariant species Quercus rotundifolia, which is restricted to the Iberian Peninsula, and Quercus ilex s.str., which main range is in the Central and the Eastern Mediterranean (López de Heredia et al. 2007, Simeone et al. 2013).
Among the conifer-associated species with Mediterranean distribution such restricted pattern is also known in G. hellenica and G. pervestita, which nest in the clades /hellenica and /pityophila, respectively. Both of them occur with species of Balkan firs, G. pervestita with Abies borisii-regis and Abies cephalonica, and G. hellenica with Abies borisii-regis. The two species are so far known only from Greek collections. Notably, two species of hypogeous ascomycetes were recently described from Greece with one of those host trees, namely Abies cephalonica. These are Barssia hellenica (now accommodated in Balsamia) and Genea cephalonicae (Kaounas et al. 2015, 2016). The former was later found in Turkey with another Mediterranean fir (Abies cilicica) (Uzun et al. 2018). It is notable that it has been hypothesized that the ancestor of Abies cephalonica existed on what is now the Balkans since between the Miocene and Pliocene, along with the ancestor of Abies cilicica in adjacent areas, nowadays Anatolia, and that their areas seem to have become effectively isolated towards the Late Pliocene (Linares 2011). As far as all four mentioned hypogeous species seem to be geographically restricted, they could represent results of parallel evolution with that of their host trees. In contrast, the clade /fusella which is basal to sect. Gautieria contains members with diverse hosts and is widespread E to W and S to N Europe.
The position of the sect. Glutinosiglebae, where G. cistophila belongs, suggests this host-shift to Cistus could have taken place relatively early in the evolution of the European Gautieria. It is not the only sequestrate species known to occur with Cistaceae. Among the basidiomycetes such is Pisolithus calongei, which is also known as strict associate of Cistus (Martín et al. 2013). It has been hypothesised that Cistus may act as substitute host in burnt areas, where the original host is extinct (Hernández-Rodríguez et al. 2013, Loizides 2016). Such events could possibly trigger or facilitate host-shift and further specialization and speciation. Sequestrate Russulaceae also offer examples of Cistus associations of species with restricted distribution in different parts of the Mediterranean Basin (Vidal et al. 2019). It is notable that basal sections Hymenogastroides and Glutinosiglebae include predominantly Mediterranean species, with the exception of G. graveolens, which is more widespread, but still not known so far from Northern Europe. This, along with the existence in the other sections of a number of taxa with restricted distribution in the Mediterranean Basin outlines the role of this geographic domain for the evolution and the diversification of the genus in Europe.
The members of sect. Parvicellae seem to be widespread from S to N and W to E Europe, such as G. otthii which is found from the montane forests of Central Spain to the boreal forests of Norway and Sweden (also found in Eastern Russia, Siberia and Turkey). Such a widespread taxon, as also occurs with G. morchelliformis var. morchelliformis, seems a suitable model and could be the target of future phylogeographic studies, possibly to highlight different aspects of historical migration and evolution within the genus Gautieria.
Most temperate taxa are ridged or pseudoperidial and lack a trichotomentocutis, the taxa with a trichotomentocutis being typical for Mediterranean forests, namely G. pervestita, G. pseudovestita, G. hellenica, G. iberica, G. obtexta and G. hymenogastroides. This feature is, however, not present in all species with Mediterranean distribution, e.g., it is not seen in those in sect. Glutinosiglebae. The presence of this peculiarity in several not necessarily closely related phylogenetic lineages implies that this character may have evolved more than once in the evolution of the European species and its acquisition may be a response to the peculiar climatic conditions of the Mediterranean Basin. The growth of Gautieria is slow and the basidiomata can be present in the substrate for several months, especially those that have a hypogeous development, as long as the humidity conditions are constant and favour their prolonged development. Temperate taxa are more abundant in summer and autumn, and Mediterranean taxa are more abundant in autumn, winter and spring.
EXCLUDED TAXA
Gautieria citrina (Vittad.) Bougher & Castellano, Mycologia 85: 275. 1993
Basionym. Hymenogaster citrinus Vittad., Monogr. Tuberac.: 21. 1831.
Bougher & Castellano (1993) transferred Hymenogaster citrinus to Gautieria based on ribbed spore ornamentation, but this combination is not justified because, apart from having different genetics, spore ornamentation lacks typical costae and apical ring, and internal ornamentation is spiny and not reticulated.
Gautieria graveolens var. lacunosa Velen., České Houby 4–5: 799. 1922
Velenovský (1922) published this variety from a collection found under fir trees on a hill near Radotín (Prague, Czech Republic), describing it as abundant, up to 5 cm wide, pale, odourless and with obovoid spores, 24–26 µm long. Pilát (1958) studied a Czech collection stored in preservative liquid in PRC herbarium, consisting of three specimens collected by Zvára at Radotín in October 1915 and identified by Velenovský as G. lacunosa. Pilát described them as 20–28 mm wide, paler than G. otthii, and with elliptical spores, measuring 15–16 × 9 µm. Pilát also studied a collection of G. lacunosa from Velenovský and found typical specimens of G. otthii, which he identified as G. mexicana. We could not locate the Velenovský’s specimens, but only the PRM 678935 collection labelled ‘Flora Bohemica, Radotín, ex coll. PRC, ut G. lacunosa Vel., leg. & det. Velenovský 1918, rev. A. Pilát as G. mexicana’, consisting of two black-and-white photographs. Therefore, due to discrepancies between the spore data provided by Velenovský and Pilát, and the impossibility of studying Velenovský’s original material from Radotín, we consider this variety to be a doubtful taxon.
Acknowledgments
We are grateful to Antoni Sánchez-Cuxart, curator of the BCN herbarium (Universitat de Barcelona, CeDocBiV, Barcelona), and Lluís Vilar, curator of the UdG herbarium (Universitat de Girona, Girona), for their help in the request of loans. We extend our sincere thanks to the directors and curators of the following herbaria: Robert Vogt (Freie Universität Berlin, Botanischer Garten und Botanisches Museum, Berlin, B), Beatrice Senn-Irlet and Steffen Boch (Universität Bern, Botanischer Garten, Bern, BERN), A.Y. Rossman and Shannon Dominik (U.S. National Fungus Collections, Systematic Mycology and Microbiology Laboratory, Beltsville, BPI), Ann Bogaerts (Plantentuin Meise, Meise, BR), Emily W. Wood and Genevieve Lewis-Gentry (Harvard University, Farlow Herbarium, Cambridge, FH), Pertti Salo (University of Helsinki, Luomus Finnish Museum of Natural History, Helsinki, H), Regina Kuhnert (Universität Innsbruck, Innsbruck, IB, mycological collection transferred to IBF), Angela D. Bond (Royal Botanic Gardens, Mycological Collection, Kew, K), Dagmar Triebel (Botanische Staatssammlung, München, M), Margarita Dueñas (Real Jardín Botánico, Herbario de Criptogamia, Madrid, MA), Caroline Loup (Université de Montpellier, Service du Patrimoine Historique, Montpellier, MPU), Barbara M. Thiers and Ellen Diane Bloch (New York Botanical Garden, Steere Herbarium, New York, NY), Katriina Bendiksen (Universitetet i Oslo, Naturhistorisk Museum, Oslo, O), Richard R. Halse (Oregon State University, Corvallis, OSC), Rossella Marcucci (Università di Padova, Museo Botanico, Padova, PAD), Bart Buyck and Edith Bury (Muséum National d’Histoire Naturelle, Herbier Cryptogamique, Paris, PC), Jan Holec (National Museum, Mycological Collection, Praha, PRM), Anna-Lena Andeberg (Naturhistoriska Riksmuseet, Stockholm, S), Isabelle Tavares and Ana Penny (University of California, The University Herbarium, Berkeley, UC), Barbro Lidfeld-Rahm and Monica Myrdal (Uppsala University, The Museum of Evolution, Uppsala, UPS), Uwe Passauer and Anton Igersheim (Naturhistorisches Museum, Wien, W), and Egon Horak and Reinhard Berndt (Universität Zürich, Zürcher Herbarien, Zürich, ZT).
The authors wish to express their sincere gratitude to all collectors and collaborators of this project for kindly providing collections, images or valuable data: László Albert, Daniele Antonini, Massimo Antonini, George Argyropoulos, István Bagi, Giorgio Baiano, Maurizio Balli, Attila Bathó, Giampaolo Bernardini, N. Bodonyi, Jakub Brańka, Giuseppe Campagnola, Teodora Chokova, Pierre Collin, Sándorné Csikós, Bálint Dima, Vajk Erdei, Bertalan Estók, A. Fekete, M. Filippa, Todor Georgiev, Michalis Gkilas†, Stanislav Glejdura, Lamberto Gori†, Gianluigi Gregori, Ilia Gyonov, Ivan Ivanov, Pablo Juste, Theodoros Karagiannis, Filip Karpowicz, K. Kemenesiné, I. Kiss, Despina Klisiari, J. Kochanowski, Patryk Komur, Katarzyna Kozak, Kalin Krumov, Georgi Kunev, Antonio Lasio, Claude Lavoise, Zoltán Lukács, Jesús Martínez, Károly Matyin, I. Mészáros, S. Mészáros, I. Meuser, Slaviana Mihailova, M. Misky, Amer Montecchi, Pierre-Arthur Moreau, Zoltán T. Nagy, M. Németh, Stefan Nenov, Ferenc Pál-Fám, Béla Pálfy, G. Pap, Agorastos Papatsanis, Kostas Papadimitriou, Pedro María Pasabán, P. Patonai, D. Pázmány, Tomás Pérez-Jarauta, François Petit, Anna Pishinkova, Carlo Piuri, Christos Plesiotis, George Prountzopoulos, Bartha E. Prutkayné, Michele Puxeddu, G. Répási, Ryszard Rutkowski, Ági Sági, Francisco Sáinz, Mario Sarasini, Francisco Serrano, George Setkos, L. Szabó, J. Szakács, Manuel Tabarés, Dimitris Tabouras, T. Tóbler, Z. Tőkés, H.Veronika Tóthné, Tilda Tsantirtzioglou, Nikos Tsilis, Istvánné Újlaczki, D. Váry, S. Vásárhelyi, Ilias Velissaris, Jordi Vila, D. Zagyva and István Zagyva.
The authors also present their thanks to Luis Parra for his contribution to safeguarding against possible nomenclature errors, to P.P. Daniëls for solving doubts about hyphal structures, to Helena Velayos of the Library of Real Jardín Botánico de Madrid for her help in digitizing the type image of G. villosa, and to Carles Roqué and the Unitat de Microscòpia dels Serveis Tècnics de Recerca of the Universitat de Girona (STR, UdG) and to Anna Łatkiewicz (Laboratory of Scanning Electron Microscopy and Microanalysis, Institute of Geological Sciences, Jagiellonian University in Kraków) for their invaluable help with the SEM imaging. Boris Assyov would like to acknowledge the support of the project ‘Phylogeny, distribution and sustainable use of fungi’. Piotr Mleczko and Maciej Kozak acknowledge financial support from the Polish National Science Center within the project ‘Hypogeous Basidiomycota of Poland against their occurrence in Central Europe’ (N N305 304540) and from the Forest Fund of the State Forests – National Forest Holding within the project ‘Diversity and distribution of hypogeous fungi in the Tatra National Park’ (2019). Last but not least, the authors also wish to express their appreciation to Steven L. Miller and Ákos Kund Orczán for their participation in the sequencing work.
Declaration on conflict of interest
The authors declare that there is no conflict of interest.
REFERENCES
- Agerer R. 1999. Never change a functionally successful principle. The evolution of Boletales s.1. (Hymenomycetes, Basidiomycota) as seen from below-ground features. Sendtnera 6: 5–91. [Google Scholar]
- Agerer R. 2006. Fungal relationships and structural identity of their ectomycorrhizae. Mycological Progress 5: 67–107. [Google Scholar]
- Baeza-Guzmán Y, Medel-Ortiz R, Garibay-Orijel R. 2017. Caracterización morfológica y genética de los hongos ectomicorrízicos asociados a bosques de Pinus hartwegii en el Parque Nacional Cofre de Perote, Veracruz. Revista Mexicana de Biodiversidad 88, 1: 41–48. [Google Scholar]
- Bataille F. 1923. Flore analytique et descriptive des Hyménogastracées d’Europe. Bulletin Trimestriel de la Société Mycologique de France 39: 157–196. [Google Scholar]
- Bau T, Liu Y. 2013. A new species of Gautieria from China. Mycotaxon 123: 289–292. [Google Scholar]
- Bergemann SE, Garbelotto M. 2006. High diversity of fungi recovered from the roots of mature tanoak (Lithocarpus densiflorus) in northern California. Canadian Journal of Botany 84, 9: 1380–1394. [Google Scholar]
- Bidartondo MI, Bruns TD. 2002. Fine-level mycorrhizal specificity in the Monotropoideae (Ericaceae): specificity for fungal species groups. Molecular Ecology 11, 3: 557–569. [DOI] [PubMed] [Google Scholar]
- Bougher N, Castellano MA. 1993. Delimitation of Hymenogaster sensu stricto and four new segregate genera. Mycologia 85, 2: 273–293. [Google Scholar]
- Bowman EA, Arnold AE. 2018. Distributions of ectomycorrhizal and foliar endophytic fungal communities associated with Pinus ponderosa along a spatially constrained elevation gradient. American Journal of Botany 105, 4: 687–699. [DOI] [PubMed] [Google Scholar]
- Bruns TD, Szaro TM, Gardes M. et al. 1998. A sequence database for the identification of ectomycorrhizal basidiomycetes by phylogenetic analysis. Molecular Ecology 7, 3: 257–272. [Google Scholar]
- Bucholtz F. 1901. Hypogaeen aus Russland. Hedwigia 40: 304–322. [Google Scholar]
- Bucholtz F. 1902. Materiali k morfologii i systematike podzemnich gribow (Tuberaceae et Gasteromycetes). Riga. [Google Scholar]
- Bucholtz F. 1903. Zur Morphologie und Systematik der Fungi hypogaei. Annales Mycologici 1, 2: 152–174, taf. IV–V. [Google Scholar]
- Cabero J. 2008. Aportaciones al conocimiento de las especies de hongos hipogeos en la provincia de Zamora. Boletín Micológico de la FAMCAL 3: 13–30. [Google Scholar]
- Calonge FD, De La Torre M, Lawrynowicz M. 1977. Contribución al estudio de los hongos hipogeos de España. Anales del Instituto Botánico A. J. Cavanilles 34, 1: 15–31. [Google Scholar]
- Calonge FD, Juste P, García F. et al. 1996. Nuevos datos sobre los hongos hipogeos de España. VII. Genea hispidula, novedad para el catálogo. Boletín de la Sociedad Micológica de Madrid 21: 325–332. [Google Scholar]
- Calonge FD, Pasabán PM. 1993. Nuevos datos sobre los hongos hipogeos de España. V: Registro de nueve citas nuevas. Boletín de la Sociedad Micológica de Madrid 18: 41–58. [Google Scholar]
- Calonge FD, Pérez-Jarauta T, Terrón A. et al. 1994. Nuevos datos sobre los hongos hipogeos de España. VI. Gautieria otthii e Hysterangium cistophilum, novedades para el catálogo. Boletín de la Sociedad Micológica de Madrid 19: 165–173. [Google Scholar]
- Calonge FD, Rocabruna A, Tabarés M. 1985a. Nuevos datos sobre los hongos hipogeos de España. Boletín de la Sociedad Micológica Castellana 9: 45–54. [Google Scholar]
- Calonge FD, Rocabruna A, Tabarés M. et al. 1985b. Nuevos datos sobre los hongos hipogeos de España. II. Géneros Balsamia, Delastria y Genea, novedades para el catálogo español. Butlletí de la Societat Catalana de Micologia 9: 57–64. [Google Scholar]
- Castellano MA, Trappe JM, Maser Z. et al. 1989. Key to spores of the genera of hypogeous fungi of North Temperate Forests. Mad River Press, Eureka, USA. [Google Scholar]
- Cázares E, García J, Castillo J. et al. 1992. Hypogeous fungi from Northern Mexico. Mycologia 84, 3: 341–359. [Google Scholar]
- Chatin A. 1891. Contribution à l’histoire naturelle de la Truffe. Bulletin de la Société Botanique de France 38: 54–64. [Google Scholar]
- Christan J, Hahn C. 2005. Zur Systematic der Gattung Ramaria (Basidiomycota, Gomphales). Zeitschrift für Mykologie 71, 1: 7–42. [Google Scholar]
- Clémençon H. 2004. Cytology and Plectology of the Hymenomycetes. Bibliotheca Mycologica 199. Cramer, Berlin-Stuttgart, Germany. [Google Scholar]
- Corda ACJ. 1831. Ciliciocarpus hypogaeus: 5-6, pl. 3. In: Sturm J. Deutschlands Flora in Abbildungen nach der Natur mit Beschreibungen. Die Pilze Deutschlands. III Abt., 11 heft: 1–32, pl. 1–16. Nürnberg, Germany. [Google Scholar]
- Darriba D, Taboada GL, Doallo R. et al. 2012. jModelTest 2: more models, new heuristics and parallel computing. Nature Methods 9, 8: 772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Thümen F. 1875. Mycotheca Universalis. Centuria I. No. 1–100. Klosterneuburg. [Google Scholar]
- De Vries GA. 1971. De Fungi van Nederland 3. Hypogaea. Wetenschappelijke Mededelingen van de Koninklijke Nederlandse Natuurhistorische Vereniging 88: 1–64. [Google Scholar]
- Dodge CW, Zeller SM. 1934. Hymenogaster and related genera. Annals of the Missouri Botanical Garden 21: 625–708, pl. 17–18. [Google Scholar]
- Dunham SM, Larsson KH, Spatafora JW. 2007. Species richness and community composition of mat-forming ectomycorrhizal fungi in old- and second-growth Douglas-fir forests of the HJ Andrews Experimental Forest, Oregon, USA. Mycorrhiza 17, 8: 633–645. [DOI] [PubMed] [Google Scholar]
- Eriksson G. 1976. Anteckningar om svampfynd på Gotland, Sverige. Friesia 11, 2: 126–134. [Google Scholar]
- Fischer E. 1916. Pilzen (inkl. Flechten). Berichte der Schweizerischen Botanischen Gesellschaft 24–25: 50–79. [Google Scholar]
- Fischer E. 1938. Hypogaeen-Studien. 2. Über eine neue schweizerische Gautieria-Spezies. Berichte der Schweizerischen Botanischen Gesellschaft 48: 43–44. [Google Scholar]
- Fitzpatrick HM. 1913. A comparative study of the development of the fruit body in Phallogaster, Hysterangium, and Gautieria. Annales Mycologici 11: 119–149, pl. IV–VII. [Google Scholar]
- Fries TM. 1909. Skandinaviens Tryfflar och tryffelliknande svampar. Svensk Botanisk Tidskrift 3, 3: 223–300. [Google Scholar]
- Fries TCE. 1922. Sveriges Gasteromyceter. Arkiv für Botanik 17, 9: 1–63. [Google Scholar]
- Gardes M, Bruns TD. 1993. ITS primers with enhanced specificity for basidiomycetes application to the identification of mycorrhizae and rusts. Molecular Ecology 2, 2: 113–118. [DOI] [PubMed] [Google Scholar]
- Giachini AJ, Hosaka K, Nouhra E. et al. 2010. Phylogenetic relationships of the Gomphales based on nuc-25S-rDNA, mit-12S-rDNA, and mit-atp6-DNA combined sequences. Fungal Biology 114, 2–3: 224–234. [DOI] [PubMed] [Google Scholar]
- Glez-Peña D, Gómez-Blanco D, Reboiro-Jato M. et al. 2010. ALTER: pro-gram-oriented format conversion of DNA and protein alignments. Nucleic Acids Research 38 (Web Server issue): W14–W18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Ávila A, Martínez-González CR, Alvarado-Sizzo H. et al. 2020. Three new combinations in Gloeocantharellus (Gomphales, Agaricomycetes) from Mexico based on molecular evidence. Phytotaxa 447, 1: 42–50. [Google Scholar]
- Gori L. 2005. Funghi Ipogei della Lucchesia, di altre province italiane e dall’stero. Maria Pacini Fazzi Editore, Lucca, Italia. [Google Scholar]
- Gori L, Bernardini G. 1997. Primo contributo al censimento dei funghi ipogei della Lucchesia e di alcune provincie vicine della regione Toscana con annotazioni ecologiche, geologiche, metereologiche e bibliografiche. Atti delle 4e Giornate della Confederazione Europea di Micologia Mediterranea CEMM (a.e.) - Poggibonsi, novembre 1996: 36–73. [Google Scholar]
- Gori L, Bernardini G, Poggiani V. 2003. Censimento dei funghi ipogei della Lucchesia, provincia della regione Toscana (Italia), aggiornato al Gennaio 2002. Pagine di Micologia 19: 43–50. [Google Scholar]
- Gregori G, Puxeddu M. 1995. Ancora sui funghi ipogei della Sardegna. Micologia Italiana 24, 2: 3–10. [Google Scholar]
- Gross G, Runge A, Winterhoff W. et al. 1980. Bauchpilze (Gasteromycetes s.l.) in der Bundesrepublik Deutschland und Westberlin. Beihefte zur Zeitschrift für Mykologie 2: 1–220. [Google Scholar]
- Gross G, Runge A, Winterhoff W. et al. 1982. Erster Nachtrag zu ʻBauchpilze (Gasteromycetes s.l.) in der Bundesrepublik Deutschland und Westberlinʼ. Zeitschrift für Mykologie 49, 1: 5–18. [Google Scholar]
- Guindon S, Gascuel O. 2003. A simple, fast and accurate algorithm to estimate large phylogenies by maximum-likelihood. Systematic Biology 52, 5: 696–704. [DOI] [PubMed] [Google Scholar]
- Hahn C, Christan J. 2002. Ramaria chocoënsis sp. nov., a gomphoid member of Ramaria sect. Dendrocladium from Colombia, El Chocó, with special regards to rhizomorph anatomy. Mycological Progress 1, 4: 383–398. [Google Scholar]
- Harkness HW. 1899. Californian hypogaeous fungi. Proceedings of the California Academy of Sciences, Third Series, 1, 8: 241–292, pl. XLII–XLV. [Google Scholar]
- Hawker LE. 1952. Hypogeous Fungi. II. A new variety of Hydnangium carneum Wallr. from North Wales. III. Three new British records: Gautieria morchellaeformis Vitt., Hymenogaster hessei Soehner, and Elaphomyces aculeatus Vitt. Transactions of the British Mycological Society 35, 4: 279–284. [Google Scholar]
- Hawker LE. 1954. British hypogeous fungi. Philosophical Transactions of the Royal Society London, Series B, 237: 429–546. [Google Scholar]
- Hawksworth DL, Kirk PM, Sutton BC. et al. 1995. Ainsworth & Bisbyʼs dictionary of the fungi. 8th edition. CAB International, Oxon, UK. [Google Scholar]
- Hazslinsky FA. 1875. Beiträge zur Kenntniss der ungarischen Pilzflora. III. Fungi hypogaei. Verhandlungen der K. K. Zoologisch-Botanischen Gesellschaft in Wien 25: 63–68, taf. III. [Google Scholar]
- Hernández-Rodríguez M, Oria-de-Rueda JA, Martín-Pinto P. 2013. Post-fire fungal succession in a Mediterranean ecosystem dominated by Cistus ladanifer L. Forest Ecology and Management 289: 48–57. [Google Scholar]
- Hesse R. 1891. Die Hypogaeen Deutschlands. I. Die Hymenogastreen. Ludw. Hofstetter, Halle a S, Germany. [Google Scholar]
- Hintz RA. 1993. Hypogäen in Mainfranken (4 und Schluß): - Zusammenstellung der Funde von 1980–1989. Zeitschrift für Mykologie 59, 1: 51–76. [Google Scholar]
- Hintz RA, Winterhoff W. 1983. Seltene Hypogäen in Mainfranken (MTB 6223 Wertheim). Zeitschrift für Mykologie 49, 1: 51–60. [Google Scholar]
- Hollós L. 1911. Magyarország földalatti gombái (Fungi hypogaei Hungariae). A.M.T., Budapest, Hungary. [Google Scholar]
- Hosaka K, Bates ST, Beever RE. et al. 2006. Molecular phylogenetics of the gomphoid-phalloid fungi with an establishment of the new subclass Phallomycetidae and two new orders. Mycologia 98, 6: 949–959. [DOI] [PubMed] [Google Scholar]
- Humpert AJ. 1999. Systematics of the genus Ramaria inferred from nuclear large subunit and mitochondrial small subunit DNA sequences. PhD Dissertation. Oregon State University, Corvallis. [Google Scholar]
- Humpert AJ, Muench EL, Giachini AJ. et al. 2001. Molecular phylogenetics of Ramaria and related genera: evidence from nuclear large subunit and mitochondrial small subunit rDNA sequences. Mycologia 93, 3: 465–477. [Google Scholar]
- Irinyi L, Serena C, Garcia-Hermoso D. et al. 2015. International Society of Human and Animal Mycology (ISHAM)-ITS reference DNA barcoding database – the quality controlled standard tool for routine identification of human and animal pathogenic fungi. Medical Mycology 53, 4: 313–337. [DOI] [PubMed] [Google Scholar]
- Izzo AD, Meyer M, Trappe JM. et al. 2005. Hypogeous ectomycorrhizal fungal species on roots and in small mammal diet in a mixed-conifer forest. Forest Science 51, 3: 243–254. [Google Scholar]
- Jülich W. 1981. Higher taxa of basidiomycetes. In: Bibliotheca Mycologica 85. Cramer, Vaduz, Germany. [Google Scholar]
- Jülich W. 1984. Die Nichtblätterpilze, Gallertpilze und Bauchpilze (Aphyllophorales, Heterobasidiomycetes, Gastromycetes). In: Gams H. Kleine Kryptogamenflora II b/1, Basidiomyceten. Gustav Fischer, Stuttgart, Germany. [Google Scholar]
- Kalchbrenner K. 1865. A szepesi gombák jegyzéke. Mathematikai és Természettudományi Közlemények 3: 192-319, 2 tabs. [Google Scholar]
- Kaounas V, Agnello C, Alvarado P. 2016. Genea cephalonicae sp. nov. (Ascomycota, Pezizales), a new hypogeous species from Greece. Ascomycete.org 8, 3: 105–110. [Google Scholar]
- Kaounas V, Agnello C, Alvarado P. et al. 2015. Barssia hellenica sp. nov. (Ascomycota, Pezizales), a new hypogeous species from Greece. Ascomycete.org 7, 5: 213–219. [Google Scholar]
- Katoh K, Asimenos G, Toh H. 2009. Multiple alignment of DNA sequences with MAFFT. In: Posada D. (eds), Bioinformatics for DNA sequence analysis. Methods in Molecular Biology (Methods and Protocols) 537: 39–64. Humana Press. [DOI] [PubMed] [Google Scholar]
- Kennedy PG, Smith DP, Horton TR. et al. 2012. Arbutus menziesii (Ericaceae) facilitates regeneration dynamics in mixed evergreen forests by promoting mycorrhizal fungal diversity and host connectivity. American Journal of Botany 99, 10: 1691–1701. [DOI] [PubMed] [Google Scholar]
- Klotzsch F. 1839. Gautieria morchellaeformis Vittadini. Morchelförmig Gautierie. Abb. 464. In: Dietrich A. Flora Regni Borussici, Band VII. Ludwig Ochmigke, Berlin, Germany. [Google Scholar]
- Knapp A. 1941. Die Hypogaeen um Basel. Schweizerische Zeitschrift für Pilzkunde 18, 11: 161–164. [Google Scholar]
- Knapp A. 1958. Die europaïschen Hypogaeen-Gattungen und ihre Gattungstypen. II. Familie Hysterangiaceae. Schweizerische Zeitschrift für Pilzkunde 35, 6: 129–143. [Google Scholar]
- Konstantinidis G. 2006. 1000 mushrooms of Western Makedonia (in Greek). The Mushroom Friends Society of Western Makedonia, Kastoria, Greece. [Google Scholar]
- Kornerup A, Wanscher JH. 1978. Methuen Handbook of Colour. 3 ed. Eyre Methuen, London, UK. [Google Scholar]
- Kumar S, Stecher G, Tamura K. 2016. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Molecular Biology and Evolution 33, 7: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunze J. 1876. Fungi Selecti Exsiccati. Centuria 1. No. 1–100. [Google Scholar]
- Lange M, Hawker LE. 1951. Some hypogeal Gasteromycetes from Jämtland, Sweden, and adjacent districts of Norway. Svensk Botanisk Tidskrift 45, 4: 591–596. [Google Scholar]
- Linares JC. 2011. Biogeography and evolution of Abies (Pinaceae) in the Mediterranean basin: the roles of long-term climatic change and glacial refugia. Journal of Biogeography 38, 4: 619–630. [Google Scholar]
- Loizides M. 2016. Macromycetes within Cistaceae-dominated ecosystems in Cyprus. Mycotaxon 131: 255–256. [Google Scholar]
- López de Heredia U, Jiménez P, Collada C. et al. 2007. Multi-marker phylogeny of three evergreen oaks reveals vicariant patterns in the Western Mediterranean. Taxon 56, 4: 1209–1220. [Google Scholar]
- Mahiques R, García-Alonso F, Conca T. 1995. Hipogeus de la Vall d’Albaida i zones limítrofes (València). Butlletí de la Societat Micològica Valenciana 1: 53–89. [Google Scholar]
- Malençon G. 1937. Cent champignons nouveaux pour la Flore Mycologique Marocaine. Bulletin de la Société des Sciences Naturelles du Maroc 17, 1: 1–10. [Google Scholar]
- Malençon G. 1975. Champignons hypogés du Nord de l’Afrique II. Basidiomycetes. Revue de Mycologie, Paris 39, 4: 279–306. [Google Scholar]
- Martín MP, Demoulin V, Llistosella J. 1996. Gautieria trabutii (Gasteromycetes), nueva cita para la Península Ibérica. Anales del Jardín Botánico de Madrid 54: 84–88. [Google Scholar]
- Martín MP, Durán F, Phosri C. et al. 2013. A new species of Pisolithus from Spain. Mycotaxon 124, 1: 149–154. [Google Scholar]
- Mattirolo O. 1900. Elenco dei funghi hypogaei raccolti nelle Foreste di Vallombrosa negli anni 1899–1900. Malpighia 14: 247–270. [Google Scholar]
- Mattirolo O. 1903. I. funghi ipogei italiani raccolti da O. Beccari, L. Caldesi, A. Carestia, V. Cesati, P.A. Saccardo. Memorie della Reale Accademia delle Scienze di Torino, Ser. Seconda, 53: 331–366. [Google Scholar]
- Mattirolo O. 1928. Secondo elenco dei funghi hypogaei raccolti nelle Foreste di Vallombrosa (1900–1926). Nuovo Giornale Botanico Italiano 34: 1343–1358. [Google Scholar]
- Mattirolo O. 1933. I. funghi ipogei della Campania, del Lazio e del Molise raccolti dal compianto prof. Carlo Campbell. Nuovo Giornale Botanico Italiano, N. Ser. 40: 313–326. [Google Scholar]
- Mattirolo O. 1935. Catalogo ragionato dei Funghi Ipogei raccolti nel Canton Ticino e nelle provincie italiane confinanti. Beiträge zur Kryptogamenflora der Schweiz 8, 2: 1-53, 2 tab. [Google Scholar]
- Mattirolo O. 1936. Funghi ipogei delle Alpi occidentale, con speciale riguardo a quelli delle valle di Susa, Aosta, Pinerolo, ecc. Annali della Reale Accademia dʼAgricoltura di Torino 79: 190–191. [Google Scholar]
- Mattirolo O. 1938. Glanures hydnologiques françaises. Bulletin Trimestriel de la Société Mycologique de France 54: 23–28. [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. 2010. Creating the CIPRES science gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE), 14 Nov. 2010, New Orleans, LA: 1–8. [Google Scholar]
- Montecchi A, Lazzari G. 1988. Invito allo studio dei Funghi Ipogei IV – I Gasteromiceti (II Parte). Rivista di Micologia AMB 31, 1–2: 77–92. [Google Scholar]
- Montecchi A, Lazzari G. 1993. Atlante fotografico di Funghi Ipogei. Associazione Micologica Bresadola, Fondazione Centro Studi Micologici, Trento-Vicenza, Italy. [Google Scholar]
- Montecchi A, Sarasini M. 2000. Funghi Ipogei d’Europa. Associazione Micologica Bresadola, Fondazione Centro Studi Micologici, Trento-Vicenza, Italy. [Google Scholar]
- Nedelin T, Gyosheva M, Kostov K. et al. 2016. New records and data on hypogeous ectomycorrhizal fungi in Bulgaria. Forestry Ideas 22, 2 (52): 113–126. [Google Scholar]
- Nguyen LT, Schmidt HA, Von Haeseler A. et al. 2015. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution 32, 1: 268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwland JA. 1916. Critical notes of new and old genera of plants. VII. The American Midland Naturalist 4: 374–386. [Google Scholar]
- Nilsson RH, Kristiansson E, Ryberg M. et al. 2008. Intraspecific ITS variability in the kingdom Fungi as expressed in the international sequence databases and its implications for molecular species identification. Evolutionary Bioinformatics Online 4: 193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osmundson TW, Robert VA, Schoch CL. et al. 2013. Filling gaps in biodiversity knowledge for macrofungi: Contributions and assessment of an herbar-ium collection DNA barcode sequencing project. PloS ONE 8, 4: e62419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfner G. 2001. Taxonomische Studien an Ektomykorrizen aus den Notho-fagus-Wäldern Mittelsüdchiles (Estudios taxonómicos en ectomicorrizas de los bosques de Nothofagus del Centro sur de Chile). Bibliotheca Mycologica 190: 1–243. [Google Scholar]
- Palfner G, Horak E. 2000. Gautieria inapire sp. nov., a new hypogeous species from Nothofagus forest in southern Central Chile. Sydowia 53, 1: 140–151. [Google Scholar]
- Patouillard N. 1897. Additions au Catalogue des Champignons de la Tunisie. Bulletin Trimestriel de la Société Mycologique de France 13: 197–216. [Google Scholar]
- Patouillard N. 1914. Contribution à la flore mycologique du Jura. Bulletin Trimestriel de la Société Mycologique de France 30: 347–354. [Google Scholar]
- Pegler DN, Spooner BM, Young TWK. 1993. British truffles. A revision of British hypogeous fungi. Royal Botanic Gardens, Kew, UK. [Google Scholar]
- Perreau J. 1967. Recherches sur la différentiation et la structure de la paroi sporale chez les Homobasidiomycètes à spores ornées. Annales des Sciences Naturelles, Botanique, 12 ème série, 8: 639–746, 36 f., 2 t., 15 pl. [Google Scholar]
- Pilát A. 1937. Additamenta ad floram Asiae Minoris Hymenomycetum et Gasteromycetum. Pars Quarta (1). Bulletin Trimestriel de la Société Mycologique de France 53: 253–264, pl. VII–VIII. [Google Scholar]
- Pilát A. 1953. Über eine neue Varietät von Gautieria morchellaeformis Vitt. Sydowia 7, 1–4: 8–13. [Google Scholar]
- Pilát A. 1958. IV. Gautieriales: 209-225. In: Pilát A. Flora ČSR B1, Gasteromycetes. Československé Akademie Věd, Prague, Czech Republic. [Google Scholar]
- Puillandre N, Brouillet S, Achaz G. 2021. ASAP: assemble species by automatic partitioning. Molecular Ecology Resources 21, 2: 609–620. [DOI] [PubMed] [Google Scholar]
- Puillandre N, Lambert A, Brouillet S. et al. 2012. ABGD: automatic barcode gap discovery for primary species delimitation. Molecular Ecology 21, 8: 1864–1877. [DOI] [PubMed] [Google Scholar]
- Quélet L. 1878. Quelques espèces nouvelles de champignons. Bulletin de la Société Botanique de France 25: 287–292. [Google Scholar]
- Quélet L. 1879. Champignons récemment observés en Normandie, aux environs de Paris et de la Rochelle, en Alsace, en Suisse et dans les montagnes du Jura et des Vosges (IXe suppl. des Champignons du Jura et des Vosges). Bulletin de la Société des Amis des Sciences Naturelles de Rouen, Sér. 2, 15e année: 151-184, 195 + pl. I–III. [Google Scholar]
- Quélet L. 1886. Enchiridion Fungorum in Europa media et praesertim in Gallia Vigentium. Lutetiae, Octavius Doin, France. [Google Scholar]
- Rabenhorst L. 1861. Fungi Europaei Exsiccati. (Klotzschii herbarii vivi mycologici continuatio) Editio nova. Series secunda. Centuria III. Botanische Zeitung 19, 16: 101–104. [Google Scholar]
- Rabenhorst L. 1874. Fungi Europaei. Centuria XVIII. No. 1701–1800. Hedwigia 1: 40–48. [Google Scholar]
- Rambaut A. 2017. FigTree-version 1.4.3, a graphical viewer of phylogenetic trees. http://tree.bio.ed.ac.uk/software/figtree/.
- Rana GL, Signore SF, Baglivo A. et al. 2011. Aggiornamento delle conoscenze sui funghi ipogei lucani e pugliesi. Micologia e Vegetazione Mediterranea 26, 2: 151–168. [Google Scholar]
- Rauschert R. 1975. Die Gattung Gautieria (Gasteromycetes) in der DDR. Hercynia, N. F. 12, 2: 217–227. [Google Scholar]
- Roumeguère C. 1882. Fungi Gallici Exsiccati. Centuria XXIII. No. 2201–2300. Revue Mycologique 4, 16: 214–224. [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S. et al. 2012. Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences 109, 16: 6241–6246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone MC, Piredda R, Papini A. et al. 2013. Application of plastid and nuclear markers to DNA barcoding of Euro-Mediterranean oaks (Quercus, Fagaceae): problems, prospects and phylogenetic implications. Botanical Journal of the Linnean Society 172, 4: 478–499. [Google Scholar]
- Smith ME, Douhan GW, Rizzo DM. 2007a. Intra-specific and intra-sporocarp ITS variation of ectomycorrhizal fungi as assessed by rDNA sequencing of sporocarps and pooled ectomycorrhizal roots from a Quercus woodland. Mycorrhiza 18: 15–22. [DOI] [PubMed] [Google Scholar]
- Smith ME, Douhan GW, Rizzo DM. 2007b. Ectomycorrhizal community structure in a xeric Quercus woodland based on rDNA sequence analysis of sporocarps and pooled roots. New Phytologist 174, 4: 847–863. [DOI] [PubMed] [Google Scholar]
- Soehner E. 1951. Bayerische Gautieria-Arten. Sydowia 5: 396–406. [Google Scholar]
- Stewart EL. 1974. The genus Gautieria Vitt. (Hymenogastrales-Basidiomy-cetes). PhD dissertation. Oregon State University, Corvallis. [Google Scholar]
- Tao K, Chang M, Liu B. 1996. New species and new records of hypogeous fungi from China V. Journal of Shanxi University 19, 1: 82–87. [Google Scholar]
- Tao K, Liu B, Chang M. 1998. Gautieriales: 69-75. In: Liu B. Flora Fungorum Sinicorum, Vol. 7, Hymenogastrales, Melanogastrales et Gautieriales. Science Press, Beijing, Xina. [Google Scholar]
- Thiers B. Continuously updated. Index Herbariorum: A global directory of public herbaria and associated staff. New York Botanical Garden’s Virtual Herbarium. http://sweetgum.nybg.org/science/ih/. [Google Scholar]
- Trifinopoulos J, Nguyen LT, Von Haeseler A. et al. 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Research 44, W1: W232–W235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trog JG. 1857. Dritter Nachtrag zu den in No. 15-23 der Mittheilungen enthaltenen Verzeichnisse schweizerischer Schwämme. Mittheilungen der Naturforschenden Gesellschaft in Bern, 1857: 25–47. [Google Scholar]
- Truong C, Mujic AB, Healy R. et al. 2017. How to know the fungi: combining field inventories and DNA-barcoding to document fungal diversity. New Phytologist 214, 3: 913–919. [DOI] [PubMed] [Google Scholar]
- Tsuda Y, Chen J, Stocks M. et al. 2016. The extent and meaning of hybridization and introgression between Siberian spruce (Picea obovata) and Norway spruce (Picea abies): cryptic refugia as stepping stones to the west? Molecular Ecology 25, 12: 2773–2789. [DOI] [PubMed] [Google Scholar]
- Tulasne L-R, Tulasne C. 1851. Fungi hypogaei. Histoire et monographie des champignons hypogés. Klincksieck, Paris, France. [Google Scholar]
- Turland NJ, Wiersema JH, Barrie FR. et al. 2018. International Code of Nomenclature for algae, fungi, and plants (Shenzhen Code) adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017. Regnum Vegetabile 159. Glashütten, Koeltz Botanical Books. [Google Scholar]
- Uzun Y, Yakar S, Karacan IH. et al. 2018. New additions to the Turkish Pezizales. Turkish Journal of Botany 42, 3: 335–345. [Google Scholar]
- Velenovský J. 1920–1922. České houby. 5 parts. Nákladem České Botanické Společnosti, Prague, Czech Republic. [Google Scholar]
- Vidal JM. 1994. Algunos hongos hipogeos interesantes para la micoflora catalana. Butlletí de la Societat Catalana de Micologia 16–17: 221–248. [Google Scholar]
- Vidal JM. 1997. Algunos hongos hipogeos nuevos o poco citados de Cataluña (Zygomycotina, Ascomycotina y Basidiomycotina). Revista Catalana de Micologia 20: 25–62. [Google Scholar]
- Vidal JM. 2003. Gautieria trabutii (Chatin) Pat. Bolets de Catalunya 22: lám. 1066. [Google Scholar]
- Vidal JM, Alvarado P, Loizides M. et al. 2019. A phylogenetic and taxonomic revision of sequestrate Russulaceae in Mediterranean and temperate Europe. Persoonia 42: 127–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal JM, Vila J, García F. et al. 1997. Algunos hongos hipogeos de Castilla-León (España): Youngiomyces multiplex y Genea thaxteri, primeras citas para Europa. Revista Catalana de Micologia 20: 85–98. [Google Scholar]
- Vilgalys R, Hester M. 1990. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. Journal of Bacteriology 172, 1: 4238–4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittadini C. 1831. Monographia Tuberacearum. Typographia F. Rusconi, Milano, Italy. [Google Scholar]
- Vu D, Groenewald M, De Vries M. et al. 2019. Large-scale generation and analysis of filamentous fungal DNA barcodes boosts coverage for kingdom fungi and reveals thresholds for fungal species and higher taxon delimitation. Studies in Mycology 92: 135–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wannathes N, Kaewketsri R, Suwannarach N. et al. 2018. Phaeoclavulina pseudozippelii sp. nov. (Gomphales, Basidiomycota) from Northern Thailand. Phytotaxa 362: 211–219. [Google Scholar]
- White TJ, Bruns TD, Lee S. et al. 1990. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: PCR protocols: A guide to methods and applications, Academic Press Inc, Part 3: 315–322. [Google Scholar]
- Wichanský E. 1962. Bedla stydlivá. Mykologický sborník (Časopis československých houbařů) 39, 5–6: 67. [Google Scholar]
- Winter G. 1884. Die Pilze: 1-921. In: Rabenhorst L. Kryptogamen-Flora von Deutschland, Oesterreich und der Schweiz. 1 Band. 2 Aufl. Eduard Kummer, Leipzig, Germany. [Google Scholar]
- Zeller SM. 1948. Notes on certain Gasteromycetes, including two new Orders. Mycologia 40, 6: 639–668. [PubMed] [Google Scholar]
- Zeller SM, Dodge CW. 1918. Gautieria in North America. Annals of the Missouri Botanical Garden 5: 133–142. [Google Scholar]
- Zobel JB. 1854. Abbildungen von Pilzen und Schwæmmen. In: Corda ACJ. Icones Fungorum 6. Ehrlich, Pragae, Czech Republic. [Google Scholar]