Abstract
To demonstrate that human immunodeficiency virus type 2 (HIV-2) mother-to-child transmission exists, HIV-2 isolates were obtained from both an asymptomatic mother (HIV-2 strain ARM), and her child (HIV-2 strain SAR), who had a diagnosis of AIDS. To determine their biological phenotype, primary isolates were used to infect various primary mononuclear cells and cell lines. HIV-2 ARM replicates in primary cells and Jurkat-tat, while HIV-2 SAR infects these cells plus SupT1, which led us to classify HIV-2 ARM as a slow/low virus and HIV-2 SAR as having an intermediate (slow/low-3) phenotype. Molecular analysis of the env region corresponding to gp125 was performed. Viral DNA was cloned, sequenced, and used to construct phylogenetic trees. The DNA sequence analysis demonstrated an overall nucleotide diversity of 7.6%. The results present evidence that the child’s strain is more virulent than the mother’s strain, which is in agreement with the immunodeficiency of the child. The phylogenetic trees that were constructed demonstrate that the two isolates cluster together, being closer to each other than to any other isolate described until now.
Heterosexual transmission is responsible for the majority of infections with human immunodeficiency virus (HIV), leading to the probability of perinatally acquired HIV infection (25). Although vertical transmission of HIV type 1 (HIV-1) is well documented (33), studies of HIV-2 vertical transmission have been much more limited, probably because of the later recognition of this second type of virus (7) and its more limited geographic distribution, mainly in West Africa (14). In Europe, reported cases of HIV-2 infection are rare, Portugal having the highest incidence, 5.7% of all HIV infections (19). First studies conducted in West Africa suggested that mother-to-child transmission of HIV-2 could be absent (3, 26). However, there is virological evidence suggesting that HIV-2 perinatal transmission may occur (8). Cross-sectional serological and clinical surveys have also demonstrated that pediatric HIV-2 infection seems to be extremely rare (11, 20). Recent epidemiological studies have confirmed that although HIV-2 vertical transmission exists, it is uncommon compared to that of HIV-1 (1, 8).
A HIV-2 vertical transmission study, in which a total of 47 children born to HIV-2-seropositive mothers are enrolled, has been conducted at Lisbon’s pediatric hospital. Here we report for the first time the isolation and characterization, on the biological and molecular levels, of two HIV-2 strains isolated from a mother-to-child transmission pair enrolled in this study in which perinatal transmission occurred.
The mother (infected with HIV-2 strain ARM), a 35-year-old female from Guinea-Bissau resident in Portugal since 1988, was probably infected by heterosexual contact, for there is no history of blood transfusion or drug addiction. She was diagnosed with HIV-2 infection during the second trimester of pregnancy and remained asymptomatic throughout gestation. The female child (infected with HIV-2 strain SAR), was born on 10/22/1992 in a Maternity Hospital in Lisbon by vaginal delivery and was bottle fed. At birth, neurological examination of the child was normal and there were no malformations. At 50 days of life, failure to thrive and increasing irritability were noted. Before 3 months of age, AIDS category C2 (6) was diagnosed when the child had Pneumocystis carinii pneumonia and serious HIV encephalopathy. The total lymphocyte count was 2,930/mm3, the CD4 count was 860/mm3 (29%), and the CD8 count was 470/mm3 (16%). Zidovudine therapy was started at 3 months of age.
Patients were tested by HIV-1 and HIV-2 enzyme-linked immunosorbent assays with confirmation by Western blot and synthetic peptide assays (ELAVIA I/II, NEW LAV-BLOT I/II, and Pepti LAV 1-2, all from Diagnostics Pasteur). Mother and infant sera reacted strongly in the enzyme-linked immunosorbent assay for HIV-2 and showed a complete reactivity profile for HIV-2 proteins on a Western blot. The Pepti LAV 1-2 analysis confirmed the HIV-2 seroreactivities (data not shown).
The fact that the mother described in our study is asymptomatic is interesting, for in other reports describing HIV-2 perinatal transmission, except in one epidemiological study (11), the majority of the mothers are symptomatic. To our knowledge, this is also the first case showing that neurological disease can be associated with an HIV-2-infected infant with a very early onset of symptoms. Other reports of HIV-2-related symptoms in pediatric populations have involved much older children, aged 20 months or more (17, 20).
Correlation of viral phenotype with clinical status and transmission.
Peripheral blood was collected from both subjects on the 27th day after delivery. Follow-up child samples were also obtained at ages of 5, 8, and 12 months. Peripheral blood mononuclear cells (PBMC) were cocultured with phytohemagglutinin-stimulated PBMC from healthy blood donors and monitored by reverse transcriptase (RT) activity (13) and syncytium formation assays every 3 to 4 days. Peak RT activity was reached very rapidly, i.e., day 4 of culture for HIV-2 SAR and day 6 for HIV-2 ARM, with values of 35,000 and 15,000 cpm/ml, respectively; no visible cytopathic effects were seen. Virus isolation was achieved for all samples obtained from the infant, which fulfills the criteria of HIV diagnosis in children born to HIV-seropositive mothers (6).
Virus tropism/phenotype studies were done by infecting a variety of primary cells and cell lines: CEM, Hut78, SupT1, Jurkat-tat, and U-937, an established monocytic cell line. Monocyte-derived macrophages (MDM) were prepared from phytohemagglutinin-stimulated PBMC by a modification of the plastic adherence technique (10), and peripheral blood lymphocytes (PBL), a T-lymphocyte-enriched cell fraction, were obtained as the resultant nonadherent fraction. Both isolates replicated well in PBMC and PBL, showing formation of a few small syncytia, but failed to do so in MDM (Table 1). Of the established cell lines, only Jurkat-tat and SupT1 were susceptible to infection. HIV-2 SAR Jurkat-tat cultures yielded a very high RT titer (6 × 106 cpm/ml), exhibiting extensive cytopathic effects, such as ballooning degeneration and large syncytia; on the other hand, HIV-2 SAR also infected SupT1 cells but gave significantly lower RT yields and no syncytia were observed. The only cell line that HIV-2 ARM could infect, but without visible cytopathic effects, was Jurkat-tat (RT titer of 1.5 × 106 cpm/ml), which is unique, as it allows the replication of most poorly replicating viruses (4). With these characteristics, the mother’s virus, HIV-2 ARM, can be classified as belonging to the slow/low or non-syncytium-inducing type (9), suggesting that this type of virus can be involved in HIV-2 vertical transmission, and HIV-2 SAR can be classified as an intermediate (slow/low-3 or syncytium-inducing [SI]) variant. Therefore, no apparent correlation seems to exist between syncytium induction and vertical transmission of HIV-2, which is in agreement with the results of other studies regarding HIV-1 phenotype and the increased risk of vertical transmission (15). The finding that the mother’s slow/low variant gave rise to an intermediate virus in the child is also uncommon. Scarlatti and coworkers (27) described the contrary: mothers with rapid/high viruses transmitted slow/low variants to their children, but mothers with slow/low viruses only transmitted viruses of the same phenotype. The possible selection of an intermediate SI variant could be a sign of transmission upon delivery, for transmission during gestation implies passage of the virus through the placenta, which is enriched in macrophages that might select against SI variants which replicate poorly in MDM (28). Selective tropism for macrophages might be an important factor in transmission of HIV via transplacental infection (18). A more recent report stated that all primary isolates from transmitting mothers and their infants replicated in MDM (24), while others have presented evidence that the ability of HIV to replicate efficiently in PBMC and infect human T-cell lines is associated with transmission from mother to child (15, 27). Our results are in agreement with the findings of the latter studies, for neither the mother’s nor the infant’s virus productively infected MDM. On the other hand, it is possible that due to the immunosuppression present in the child, an SI variant that could have represented a minor phenotype in the mother emerged, becoming the predominant one in the child.
TABLE 1.
Cellular host range of HIV-2 strains ARM and SARa
| Cells | Host range/CPE
|
|
|---|---|---|
| HIV-2 ARM | HIV-2 SAR | |
| Peripheral blood cells | ||
| PBMC | ++/+ | ++/+ |
| MDM | −/− | −/− |
| PBL | ++/+ | ++/+ |
| T-cell lines | ||
| CEM | −/− | −/− |
| HUT78 | −/− | −/− |
| Jurkat-tat III | ++/− | +++/+ |
| SupT1 | −/− | ++/− |
| U937 monocytes | −/− | −/− |
All results are means of three different experiments. Host range was determined by virus replication as assessed by RT activity in culture supernatants. Results were considered positive when values were at least five times the background level. +++, >2 × 106 cpm/ml; ++, 1 × 106 to 2 × 106 cpm/ml; −, <1 × 104 cpm/ml. Cytopathic effect (CPE) scoring was based on the presence (+) or absence (−) of syncytium formation.
The phenotypes of the mother’s and child’s viruses correlate with their respective disease statuses and support reports associating more-virulent SI strains with quicker progression to AIDS in both HIV-1- and HIV-2-infected individuals (2, 31).
To analyze the size and antigenic reactivity profiles of viral proteins, radioimmunoprecipitation-polyacrylamide gel electrophoretic assays were performed with anti-HIV-1 and anti-HIV-2 human serum pools and cells infected with HIV-2 strains ARM, SAR, and ROD. For HIV-2 strains ARM and SAR, env gene-encoded glycoproteins gp140, gp125, and gp41 were detected with human anti-HIV-2 serum, as were gag-encoded proteins p55 and p26. The molecular weights of the HIV-2 strain ARM and SAR proteins were similar to each other and also to those of HIV-2 strain ROD proteins (results not shown).
Molecular analysis of HIV-2 env gene fragments derived from the mother-infant pair.
To characterize HIV-2 strains ARM and SAR at the genetic level, a nested PCR technique was used to amplify a region of approximately 1,150 bp situated in the env gene region encoding the carboxy-terminal part of V1 up to and including the C5 region of gp125. The first round of amplification was performed with previously described primers A1 and NT5 (30). The nested primers were PAT2 (CTGT TGGAATTCAACCCAAGAACCCTAGCAC) and PAT1 (CCATGCGCGTCGACAGACAAACTGCTCAGGA) (positions 6579 to 6610 and 7719 to 7689, respectively in HIV-2 ROD), containing EcoRI and SalI restriction sites (underlined nucleotides). These fragments were cloned into M13 mp18/mp19 vectors and sequenced by using the gene-walking method.
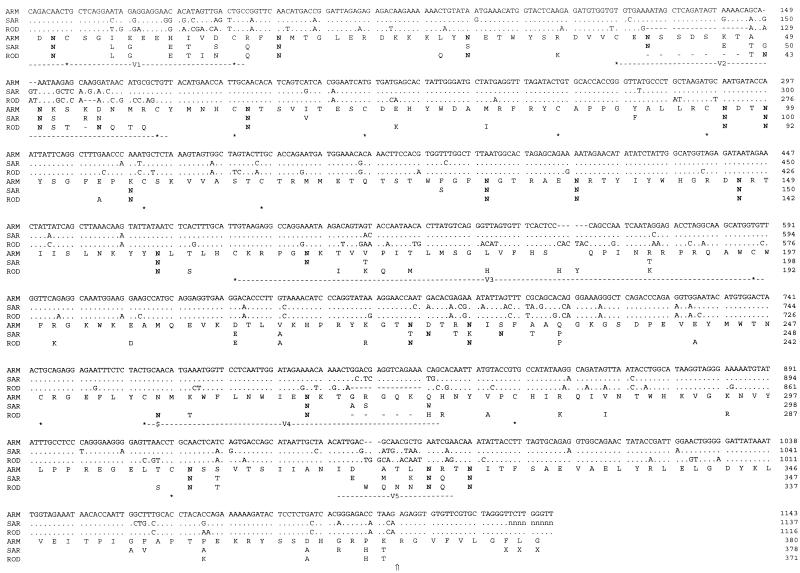
Figure 1 gives alignments of the nucleotide and translation products for HIV-2 strains ARM and SAR with prototype HIV-2 strain ROD. The percentage of nucleotide diversity between ARM and SAR over the sequenced region was 7.6%. This translates into 89% sequence homology at the amino acid level, which is 2.4 to 2.9% higher than that for the most closely related HIV-2 CBL23 glycoprotein and at least 11% higher than that for the HIV-2B and simian immunodeficiency virus (SIV) sequences in the database (22). In common with other HIV-2 isolates, the glycoproteins of HIV-2 ARM and SAR show alternating variable and constant domains organized around a conserved core of 16 cysteine residues over the region analyzed (22). Both sequences contain 19 potential N-linked glycosylation sites, 16 of which are conserved in position and 2 of which are shifted by one or two amino acids, and ARM contains one at residue 31 while SAR has one at residue 107 (Fig. 1). Notably, both viruses lack the glycosylation site (marked in Fig. 1) at the start of the CD4-binding domain, which is conserved in all other HIV-2A isolates reported to date (22). The absence of this glycosylation site is unusual and may be of some importance, as its removal has been associated with a decrease in the CD4-binding capacity of gp125 (21).
FIG. 1.
Alignment of HIV-2 strain ARM, SAR, and ROD nucleotide and translation product sequences spanning the gp125 region. For the nucleotide sequences, dashes indicate gaps introduced to improve the alignment, dots show agreement with the ARM sequence, and the letter n represents nucleotides not resolved in the SAR sequence. For amino acid alignments, residues which differ from the ARM sequence are shown, dashes mark deletions compared to the ARM sequence, and X indicates an amino acid that cannot be assigned in the SAR translation. The locations of asparagine residues (N) which form part of a potential N-linked glycosylation sequon are in boldface type, and $ marks the position of the deleted sequon in the ARM and SAR sequences. The positions of conserved cysteines are indicated (asterisks), as are the locations of the variable domains (V1 to V5) and the cleavage site between gp125 and gp41 (⇑).
Overall, compared to isolate-specific and consensus sequences in the database (22), HIV-2 ARM and SAR show unusually long V2 regions (17 and 18 amino acids, respectively, between the demarking cysteines), which strengthen their close epidemiological relationship, and high levels of nonconservative amino acid substitutions (73% for HIV-2 ARM and 56% for HIV-2 SAR).
In the env gene, it has been shown that there is a divergence of 0.5 to 10% within mother-infant pairs while the divergence is higher in unlinked pairs at 8 to 20% (16). HIV-1 sequences of epidemiologically unlinked mothers and infants have been reported to differ by 10 to 17.3% in the V3 loop region and by 0.5 to 6.1% for epidemiologically linked pairs (32); in the case reported here, the two viruses present a total divergence of 7.6% and the V3 sequences differ by 2.9%, both values which are typical of epidemiologically linked individuals.
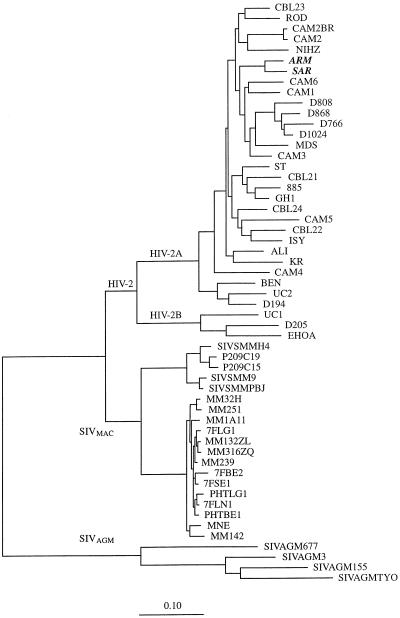
To determine the evolutionary relationships of the newly identified HIV-2 isolates, phylogenetic trees were constructed. Within the genetic data environment (29), the ARM and SAR env gene translation products were aligned with those of HIV-2 and SIV, as reported in the Los Alamos database (22), by using CLUSTAL V (12). The nucleotide sequences of all of the viruses were then aligned on the basis of this protein alignment. Positions in the alignment where gaps had been introduced were cut out, as were positions where ambiguous nucleotides occurred. On the basis of an alignment of the env nucleotide sequences, phylogenetic analysis was performed on the resultant nucleic acid alignments by the maximum-likelihood method by using the program fastDNAml (23). As shown in Fig. 2, the viruses from the mother and infant cluster together, being more related to each other than to any of the other viruses described to date. They also belong to subtype A, which is the predominant HIV-2 subtype in Guinea-Bissau (5) and worldwide (22).
FIG. 2.
Phylogenetic relationships of HIV-2 strains ARM and SAR to other HIV-2 and SIV strains. The highest-likelihood tree is shown for alignments based on the coding regions spanning V2 to the carboxy terminus of gp120, encompassing 973 nucleotides of HIV-2 and SIV. One hundred unrooted trees were generated. The scale bar represents 10 substitutions per 100 nucleotides.
Our findings, linked to the first well-documented case of mother-to-child HIV-2 transmission, support the importance of the study of these viruses and their implications for HIV infection generally. From the characteristics of the case described here, it seems reasonable to suggest that the properties of the transmitted virus are crucial for efficient transmission. The intermediate phenotype of HIV-2 strain ARM that gave rise to more-virulent strain SAR in the infant might explain why there was efficient transmission in the case studied here. Further studies are needed to understand better the mechanisms of HIV-2 vertical transmission, why it occurs so rarely, and what its consequences are for infant survival.
Nucleotide sequence accession numbers.
The nucleotide sequences corresponding to the partial envelope sequences of HIV-2 strains ARM and SAR have been submitted to the GenBank database and assigned accession no. AJ001162 and AJ001163, respectively.
Acknowledgments
Patricia Cavaco-Silva is on a grant from Junta Nacional de Investigação Científica e Tecnológica. This work was supported in part by Comissão Nacional de Luta contra a SIDA (Portuguese Ministry of Health).
REFERENCES
- 1.Adjorlolo-Johnson G, De Cock K M, Ekpini E, Vetter K M, Sibailly T, Brattegaard K, Yavo D, Doorly R, Whitaker J P, Kestens L, Ou C Y, George J R, Gayle H D. Prospective comparison of mother-to-child transmission of HIV-1 and HIV-2 in Abidjan, Ivory Coast. JAMA. 1994;272:462–466. [PubMed] [Google Scholar]
- 2.Albert J, Nauclér A, Böttiger B, Broliden P A, Albino P, Ouattara S A, Björkegren C, Valentin A, Biberfeld G, Fenyö E M. Replicate capacity of HIV-2, like HIV-1, correlates with severity of immunodeficiency. AIDS. 1990;4:291–295. doi: 10.1097/00002030-199004000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Andreasson P A, Dias F, Nauclér A, Andersson S, Biberfeld G. A prospective study of vertical transmission of HIV-2 in Bissau, Guinea-Bissau. AIDS. 1993;7:989–993. [PubMed] [Google Scholar]
- 4.Asjö B, Albert J, Chiodi F, Fenyö E M. Improved tissue culture technique for production of poorly replicating human immunodeficiency virus isolates. J Virol Methods. 1988;19:191–196. doi: 10.1016/0166-0934(88)90013-4. [DOI] [PubMed] [Google Scholar]
- 5.Boeri E, Giri A, Lillo F, Ferrari G, Varnier O E, Ferro A, Sabbatani S, Saxinger W C, Franchini G. In vivo genetic variability of the human immunodeficiency virus type 2 V3 region. J Virol. 1992;66:4546–4550. doi: 10.1128/jvi.66.7.4546-4550.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. Revised classification system for human immunodeficiency virus infection in children less than 13 years of age 1994. Morbid Mortal Weekly Rep. 1994;43:1–10. [Google Scholar]
- 7.Clavel F, Guétard D, Brun-Vézinet F, Chamaret S, Rey M-A, Santos-Ferreira M O, Laurent A G, Dauguet C, Katlama C, Rouzioux C, Klatzmann D, Champalimaud J L, Montagnier L. Isolation of a new human retrovirus from West African patients with AIDS. Science. 1986;233:343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- 8.De Cock K M, Zadi F, Adjorlolo G, Diallo M O, Sassa-Morokro M, Ekpini E, Sibailly T, Doorly R, Batter V, Brattegaard K, Gayle H. Retrospective study of maternal HIV-1 and HIV-2 infections and child survival in Abidjan, Côte d’Ivoire. Br Med J. 1994;308:441–443. doi: 10.1136/bmj.308.6926.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fenyö E M, Morfeldt-Manson L, Chiodi F, Lind A, Von Gegerfelt A, Albert J, Olausson E, Asjö B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988;62:4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gartner S, Markovits P, Markowitz D M, Kaplan M H, Gallo R C, Popovic M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 11.Gayle H D, Gnaore E, Adjorlolo G, Ekpini E, Coulibaly R, Porter A, Braun M M, Zabban M L K, Andou J, Timite A, Assi-Adou J, DeCock K M. HIV-1 and HIV-2 infection in children in Abidjan, Côte d’Ivoire. J Acquired Immune Defic Syndr. 1992;5:513–517. [PubMed] [Google Scholar]
- 12.Higgins D G, Sharp P M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988;73:237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman A D, Banapour B, Levy J A. Characterization of the AIDS-associated retrovirus reverse transcriptase and optimal conditions for its detection in virions. Virology. 1985;147:326–335. doi: 10.1016/0042-6822(85)90135-7. [DOI] [PubMed] [Google Scholar]
- 14.Kanki, P. J., and K. M. De Cock. 1994. Epidemiology and natural history of HIV-2. AIDS 8(Suppl. 1):s85–s93.
- 15.Kliks S C, Wara D W, Landers D V, Levy J A. Features of HIV-1 that could influence maternal-child transmission. JAMA. 1994;272:467–474. [PubMed] [Google Scholar]
- 16.Learn G H, Korber B T M, Foley B, Hahn B H, Wolinsky S M, Mullins J M. Maintaining the integrity of human immunodeficiency virus sequence databases. J Virol. 1996;70:5720–5730. doi: 10.1128/jvi.70.8.5720-5730.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matheron S, Courpotin C, Simon F, DiMaria H, Balloul H, Bartzack S, Dormont D, Brun-Vézinet F, Saimot A G, Coulaud J P. Vertical transmission of HIV-2. Lancet. 1990;335:1103–1104. doi: 10.1016/0140-6736(90)92682-8. [DOI] [PubMed] [Google Scholar]
- 18.McGann K A, Collman R, Kolson D L, Gonzalez-Scarano F, Coukos G, Coutifaris C, Strauss J F, Nathanson N. Human immunodeficiency virus type 1 causes productive infection of macrophages in primary placental cell cultures. J Infect Dis. 1994;169:746–753. doi: 10.1093/infdis/169.4.746. [DOI] [PubMed] [Google Scholar]
- 19.Ministério da Saúde, Portugal. SIDA: situação em Portugal. Boletim Epidemiológico da C. N. L. C. S. 97, Março, 31. Lisbon, Portugal: Ministério da Saúde; 1997. [Google Scholar]
- 20.Morgan G, Wilkins H A, Pepin J, Jobe O, Brewster D, Whittle H. AIDS following mother-to-child transmission of HIV-2. AIDS. 1990;4:879–882. doi: 10.1097/00002030-199009000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Morikawa Y, Moore J P, Wilkinson A J, Jones I M. Reduction in CD4 binding affinity associated with removal of a single glycosylation site in the external glycoprotein of HIV-2. Virology. 1991;180:853–856. doi: 10.1016/0042-6822(91)90106-l. [DOI] [PubMed] [Google Scholar]
- 22.Myers G, Korber B, Smith R F, Berzosfsky J A, Pavlakis G N. Human retroviruses and AIDS 1994: a compilation and analysis of nucleic acid and amino acid sequences. Los Alamos, N.Mex: Theoretical Biology and Biophysics Group, Los Alamos National Laboratory; 1994. [Google Scholar]
- 23.Olsen G J, Matsuda H, Hagstrom R, Overbeek R. fastDNAml, a tool for construction of phylogenetic trees of DNA sequences using maximum likelihood. Comput Appl Biosci. 1994;10:41–48. doi: 10.1093/bioinformatics/10.1.41. [DOI] [PubMed] [Google Scholar]
- 24.Ometto L, Zanotto C, Maccabruni A, Caselli D, Truscia D, Giaquinto C, Ruga E, Chieco-Bianchi L, De Rossi A. Viral phenotype and host-cell susceptibility to HIV-1 infection as risk factors for mother-to-child HIV-1 transmission. AIDS. 1995;9:427–434. [PubMed] [Google Scholar]
- 25.Peckam C, Gibb D. Mother-to-child transmission of the human immunodeficiency virus. N Engl J Med. 1995;333:298–302. doi: 10.1056/NEJM199508033330507. [DOI] [PubMed] [Google Scholar]
- 26.Poulsen A G, Kvinesdal B B, Aaby P, Lisse I M, Gottschau A, Molbak K, Dias F, Lauritzen E. Lack of evidence of vertical transmission of human immunodeficiency virus type 2 in a sample of the general population in Bissau. J Acquired Immune Defic Syndr. 1992;5:25–30. [PubMed] [Google Scholar]
- 27.Scarlatti G, Hodara V, Rossi P, Muggiasca L, Bucceri A, Albert J, Fenyö E M. Transmission of human immunodeficiency virus type 1 (HIV-1) from mother to child correlates with viral phenotype. Virology. 1993;197:624–629. doi: 10.1006/viro.1993.1637. [DOI] [PubMed] [Google Scholar]
- 28.Schuitmaker H, Koostra N A, de Goede R E Y, De Wolf F, Miedema F, Tersmette M. Monocytotropic human immunodeficiency virus type 1 (HIV-1) variants detectable in all stages of HIV-1 infection lack T-cell line tropism and syncytium-inducing ability in primary T-cell culture. J Virol. 1991;65:356–363. doi: 10.1128/jvi.65.1.356-363.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith S W, Overbeek R, Woese C R, Gilbert W, Gillevet P M. The genetic data environment an expandable GUI for multiple sequence analysis. Comput Appl Biosci. 1994;10:671–675. doi: 10.1093/bioinformatics/10.6.671. [DOI] [PubMed] [Google Scholar]
- 30.Taveira N C, Bex F, Burny A, Sharp P M, Santos-Ferreira M O, Moniz-Pereira J. Molecular characterization of the env gene from a non-syncytium-inducing HIV-2 isolate (HIV-2 ALI) AIDS Res Hum Retroviruses. 1994;10:223–224. doi: 10.1089/aid.1994.10.223. [DOI] [PubMed] [Google Scholar]
- 31.Tersmette M, De Goede R E Y, Al B J, Winkel I, Gruters B, Cuypers H T, Huisman H G, Miedema F. Differential syncytium-inducing capacity of human immunodeficiency virus isolates: frequent detection of syncytium-inducing isolates in patients with acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. J Virol. 1988;62:2026–2032. doi: 10.1128/jvi.62.6.2026-2032.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolinsky S M, Wike C M, Korber B T M, Hutto C, Parks W P, Rosenblum L L, Kunstman K J, Furtado M R, Muñoz J L. Selective transmission of human immunodeficiency virus type-1 variants from mothers to infants. Science. 1992;255:1134–1137. doi: 10.1126/science.1546316. [DOI] [PubMed] [Google Scholar]
- 33.Working Group on Mother-to-Child Transmission of HIV. Rates of mother-to-child transmission of HIV-1 in Africa, America, and Europe: results from 13 perinatal studies. J Acquired Immune Defic Syndr. 1995;8:506–510. doi: 10.1097/00042560-199504120-00011. [DOI] [PubMed] [Google Scholar]