Summary
Motile cilia have essential cellular functions in development, reproduction, and homeostasis. Genetic causes for motile ciliopathies have been identified, but the consequences on cellular functions beyond impaired motility remain unknown. Variants in CCDC39 and CCDC40 cause severe disease not explained by loss of motility. Using human cells with pathological variants in these genes, Chlamydomonas genetics, cryo-electron microscopy, single cell RNA transcriptomics, and proteomics, we identified perturbations in multiple cilia-independent pathways. Absence of the axonemal CCDC39/CCDC40 heterodimer results in loss of a connectome of over 90 proteins. The undocked connectome activates cell quality control pathways, switches multiciliated cell fate, impairs microtubule architecture, and creates a defective periciliary barrier. Both cilia-dependent and independent defects are likely responsible for the disease severity. Our findings provide a foundation for reconsidering the broad cellular impact of pathologic variants in ciliopathies and suggest new directions for therapies.
Keywords: cilia, primary ciliary dyskinesia, airway, dynein, proteomics, periciliary barrier, Notch-signaling
Introduction
Fluid propelled by motile cilia is an essential function during development at the embryonic node, and flow in the brain, reproductive, and respiratory tracts1–3. These tasks are accomplished by molecular motors with companion regulatory complexes docked along the length of the cilia’s microtubule skeleton, the axoneme4. Seminal studies of cilia from Chlamydomonas reinhardtii using freeze-fracture with transmission electronic microscopy (EM) uncovered a 96-nm repeating pattern of motors and other structures on the outer circumference of the nine doublet microtubules (DMT)5,6 (Figure 1A). Recent single particle cryo-EM of cilia provides exquisite detail that allows the identification of proteins and their position along the ciliary axoneme7–14,15. In parallel, advances in proteomics and genomics provide a catalog of motile ciliary components and pathogenic gene variants 16–21. These complementary discovery tools moved the understanding of cilia biology and human disease forward. However, a major gap exists in understanding how the ciliary proteins are placed in a precise, repeating pattern and how those processes are disrupted in disease variants.
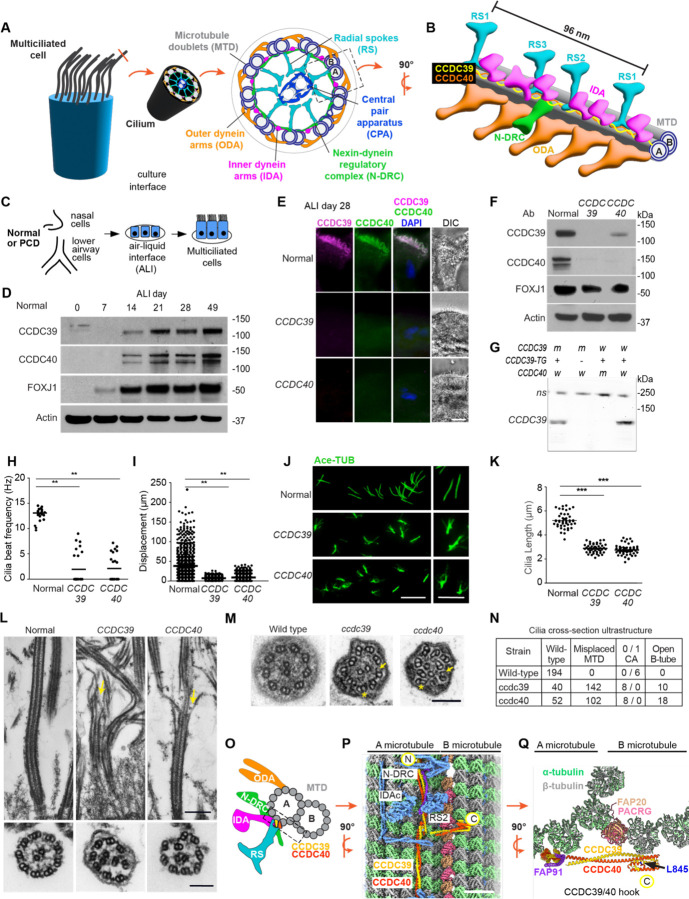
Figure 1. Loss of ciliary microtubular integrity of human multiciliated cells in CCDC39 and CCDC40 variants.
(A) Diagram of multiciliated cell, motile cilium cross-section with the nine microtubule doublets (DMT) linked to the major complexes. Dashed rectangle is expanded in Figure 1B.
(B) Diagram of a 96-nanometer unit showing the location of the CCDC39/CCDC40 heterodimer (yellow and orange ribbon) on the DMT. The major ciliary complexes are shown.
(C) Scheme for obtaining primary airway epithelial cells for culture at air-liquid interface (ALI). Cells are from normal individuals and those with CCDC39 and CCDC40 variants.
(D) Immunoblot (IB) detection of CCDC39, CCDC40, and FOXJ1 in normal ALI cultured cells during differentiation.
(E) Immunofluorescent (IF) detection of CCDC39 and CCDC40 in normal and variant cells cultured at ALI 28. Bar=10 μm (WU 182, WU146, respectively and in panels H-L).
(F) IB detection of CCDC39 and CCDC40 in normal and human variant cells.
(G) IB detection of CCDC39 and CCDC40 in Chlamydomonas wild-type and ccdc40 mutant.
(H) Cilia beat frequency in normal (n=2) and CCDC39 and CCDC40 variant cells.
(I) Cilia transport of microbeads on the surface of well-differentiated normal, CCDC39 and CCDC40 variant cells. Each point represents one bead.
(J) IF detection of acetylated α-tubulin (Ace-TUB) in cilia isolated from human normal and variant cells. Detail shows examples of cilia with splaying in variants. Bar=10 μm (left) and 5 μm (right)
(K) Quantification of cilia length from Panel J; n=47–50 cilia measured for each genotype from normal (n=3), CCDC39 and CCDC40.
(L) Transmission electron microscopy (TEM) of cilia from normal and variant cells showing the microtubules in longitudinal and cross section. Bar=500 nm (top) and 100 nm (bottom).
(M) TEM of cross-sections of cilia isolated from Chlamydomonas wild-type, ccdc39, and ccdc40. Asterisks indicate disorganized DMT. Arrows indicate an opening of the B-microtubule.
(N) Quantification of open B-microtubules from panel M; n=200 of each genotype. The total loss of the central apparatus in the ccdc39 and ccdc40 mutant strains is significantly different from wild-type (p<0.0001) by chi-squared and Fisher’s exact testing.
(O) Diagram of a cross-section of a DMT showing the ciliary complexes and the location of CCDC39/ CCDC40 on the DMT. Structures are as in panels A and B. The box outlined represents the region viewed at 900 in panel P.
(P) Three-dimensional structure of the Chlamydomonas axoneme resolved by cryo-EM showing CCDC39 and CCDC40 and indicated proteins on the surface of the DMT. The N terminus of CCDC39/40 is labeled (N). The C-terminus extends from the A- to the B-tubule (C). Bar=10 nm.
(Q) Detail of CCDC39/40 hook region from a predicted conformation of the C-terminal region of the CCDC39/40 heterodimer spanning from the A- to the B-microtubule. L845 is the location of the mutant residue in the temperature-sensitive ccdc39 Chlamydomonas mutant.
In H, I, K. The bar indicates the medium. Differences between groups were determined using Kruskal-Wallis with Dunn’s Multiple Comparison Test; *p<0.05, **p<0.01 are shown.
Insufficient cilia motor function in humans results in altered organ laterality, infertility, and respiratory tract infection, which are hallmarks of the genetic disease primary ciliary dyskinesia (PCD)22. Pathogenic variants in nearly 60 genes cause PCD23,24; the phenotypes provide powerful tools for uncovering cilia biology25,26. Variants of cilia genes code for dynein arms, the radial spokes (RS), nexin dynein regulatory complex (N-DRC) or central apparatus27–32 (Figure 1A, 1B). Paired with model organism genetics, these variants provide structure-function relationships, but do not inform the ciliary assembly process. A common cause of PCD includes pathologic variants in CCDC39 and CCDC4033–35. For unknown reasons, patients with CCDC39 or CCDC40 variants exhibit worse disease as indicated by decreased lung function and increased mucus plugging of airways, compared to individuals with variants in 20 other PCD genes36–39. Current dogma suggests that PCD is caused simply by ciliary dysmotility, however this concept does not explain the range of patient phenotypes. Thus, a second gap in understanding is how variants in different genes impact multiciliated cells.
CCDC39 and CCDC40 are alpha-helical proteins that form a heterodimer (CCDC39/40) repeating every 96-nm along the length of the DMT10 (Figure 1B). The dimer directly contacts most of the structures within the repeating unit7,9. These include proteins of the N-DRC and inner dynein arms (IDAs) that are missing in patients with CCDC39 and CCDC40 variants33,34. CCDC39/40 was suggested to be a molecular ruler for positioning of axonemal structures40. The ruler model proposed that CCDC39/40 measures a specific distance along the microtubule to direct the placement of IDAs and N-DRC40. An alternative model is that CCDC39/40 provides specific sites or “addresses” to dock ciliary complexes7,41.
We aim to identify how CCDC39/40 provides addresses and how its loss leads to severe lung disease. The variant cilia show reduced motility and are short. Proteomics of cilia from CCDC39 human variants show a devastating loss of 90 ciliary proteins, which defines a large “connectome” dependent on CCDC39/40 for anchoring. Loss of this connectome leads to the absence of major ciliary structures, impaired ciliary integrity, and an unexpected cytoplasmic burden of unanchored proteins, leading to cell stress. The findings provide insight into the cilia-independent impact of pathologic genetic variants on patients with motile ciliopathies.
Results
Loss of ciliary integrity in CCDC39 and CCDC40 variants
To understand the function of the human CCDC39/40 heterodimer, we obtained freshly isolated, primary airway epithelial cells from the respiratory tract of normal individuals and those with biallelic pathologic variants in CCDC39 or CCDC40. Most variants have frameshift mutations likely resulting in nonsense mediated decay of the RNA (Table S1). To avoid secondary insults on cilia that are caused by the inflamed airway environment in PCD, we isolated and expanded the airway basal progenitor cells in cultures with medium supplemented with antimicrobial drugs, then differentiated cells using the air-liquid interface condition (ALI) to generate multiciliated cells (Figure 1C). The onset of CCDC39 and CCDC40 expression during ALI differentiation coincides with the expression of the cilia transcription factor FOXJ1 (Figure 1D). The specificity of antibodies to CCDC39 and CCDC40 used for these studies was validated in cultured cells obtained from individuals with pathologic CCDC39 or CCDC40 variants by immunofluorescence and immunoblot analysis (Figure 1E, 1F). In some human variants we found that the unaffected partner protein is absent and in others, present by immunoblot analysis and is likely retained in the cytoplasm (Figure 1F, Figure S1A, S1D). CCDC39 protein is absent in the cytoplasm of the Chlamydomonas ccdc40 mutant (Figure 1G), which suggests that formation of the heterodimer enhances stability. Functional analysis of cilia in CCDC39 and CCDC40 variant human cultures show most are immotile or flicker (Figure 1H and Videos V1, V2). The surface transport of microbeads in variants is markedly decreased as expected (Figure 1I; Figure S1B, S1C; Videos V3-5).
We previously observed that cilia of Chlamydomonas with null alleles in ccdc39 and ccdc40 were short with ciliary splaying42. Cilia in human CCDC39 and CCDC40 variants are approximately half the length of normal, when measured intact on the surface of cultured cells or following treatment of cells with a detergent-containing buffer that removes cilia from cells (Figure 1J, 1K). Immunostaining using an anti-tubulin antibody shows splaying in some cilia isolated from variants, likely caused by the loss of the nexin-dynein regulatory complex that links the DMT pairs. TEM of human variant axonemes in cross-section show the loss of organization of the nine DMTs as reported previously33,34,43. Notably, imaging the length of the cilia showed splayed microtubules (Figure 1L), which was not previously described in PCD patients. Analysis of TEM images of Chlamydomonas ccdc39 and ccdc40 identify other ultrastructural defects including misplaced DMTs and open B-tubules at the inner junction (Figure 1M, 1N), indicating the extensive loss of DMT integrity.
To determine how loss of CCDC39 or CCDC40 in the variants may lead to ciliary splaying and therefore instability, we used single particle cryo-EM of cilia from wild-type Chlamydomonas cells. We previously reported that CCDC39/40 is restricted to the groove between protofilaments A02 and A03, adjacent to IDAs, RS, and the N-DRC (Figure 1O, 1P)10. Previously, we were unable to resolve the C-terminus. With improved cryo-EM data processing and 3D classification, the last ~30 residues of the C-termini of CCDC39/40 are now resolved near the inner junction (Figure 1P). They extend as a hook from the A- to the B-tubule, forming a 4-helices bundle that agrees well with the AlphaFold prediction (Figure 1Q). The importance of the A-to-B tubule extension was illustrated by analysis of a CCDC39 variant (WU152), which carries a frameshift that is predicted to truncate the last 32 amino acids and shows persistence of CCDC39 and CCDC40 protein (Figure S1D). This individual has clinically severe PCD; cilia show lowered expression of CCD39 and CCDC40 and beat frequency (Figure S1E, S1F), suggesting an essential role for the CCDC39 C-terminal structure. A temperature-sensitive mutation in ccdc39 (L845P) is also within this hook region (Figure 1Q) and causes loss of the heterodimer at the restrictive temperature but reduced function at intermediate temperatures42. Taken together, the loss of CCDC39/40 function accounts for a unique defect in ciliary structure.
CCDC39/40 is required for assembly of a connectome of ciliary proteins via CARPs
Prior analysis of Chlamydomonas cilia in ccdc39 and ccdc40 mutants identified deficiencies in IDAs, N-DRC and tektin40,42. Other proteins were preserved, although studies were limited by antibody availability42. Proteomics of isolated cilia would provide a comprehensive assessment of the role of CCDC39/40. Cilia obtained from ALI preparations of cells from four normal donors identified 440 proteins for comparison to cilia from a CCDC39 variant individual (Figure 2A, 2B).
Figure 2. CCDC39/40 is required for assembly of a connectome of ciliary proteins.
(A) Scheme of cilia isolation from cultured human cells from normal (n=3) and CCDC39 variant (WU182) analyzed by mass spectrometry.
(B) Immunofluorescent (IF) detection of Ace-TUB and basal body protein centrin in cells after cilia isolation. Bar=10 μm.
(C) Heat map of mean levels of proteins normalized for tubulin in the CCDC39 variant compared to normal cilia. Proteins with direct CCDC39/40 contact noted by blue box.
(D) CCDC39/40 ciliary address recognition protein (CARP) with associated structure and references to experimental validation.
(E) Diagram of the relationship of CCDC39/40 with CARPs and ciliary structures (see Figure 1A for abbreviations).
Proteomics of cilia from the CCDC39 variant shows extensive loss of at least 90 proteins (Figure 2C, Table S2). Outer dynein arms are retained, but many of the known structural proteins are lost or reduced. All the IDA proteins are missing, as are 9 of the 12 N-DRC proteins; DRC5, DRC6, DRC12 were not detected in the normal cilia. CFAP73 and CFAP100, which regulate the normal waveform via the two-headed IDA44, are missing. Many radial spoke (RS) proteins are missing or reduced, as are several proteins within the central apparatus (CA), consistent with the loss of this structure in the cross-sections of the ccdc39 or 40 mutants (Figure 1L–1N). Many proteins with unknown roles and locations are also depleted or reduced (Figure 2C). Inspection of single particle cryo-EM reconstructions shows that at least 13 of the missing proteins are in direct contact with the CCDC39/40 heterodimer (Figure 2C)7–12,14,45–55. Each of these 13 missing proteins, plus MORN3, which is not present in our proteomics, is linked to a major cilia complex (Figure 2D). Due to this special arrangement, we refer to these 14 proteins as ciliary address recognition proteins (CARPs). The CARPs are diagrammed to indicate their position relative to ciliary structures (Figure 2E).
Ciliary radial spokes are differentially depleted in CCDC39/40 variants
RS act as a mechanotransducer to control ciliary waveform9,56. Chlamydomonas has two full radial spokes (RS1, RS2) and a shorter third RS (RS3) structure while humans have three full radial spokes within each 96-nm repeat unit (Figure 3A)7,9,15,57,58. Each RS contains unique proteins, as well as shared head and stalk proteins.
Figure 3. Ciliary radial spokes are differentially depleted in CCDC39/40 variants.
(A) Diagram of the radial spokes (RS) in Chlamydomonas and human in a 96-nm repeat along the DMT. Chlamydomonas has two complete and one partial RS. The human has three complete RS proteins at the bases. RSPH3 is shared by all RS.
(B) Immunofluorescent (IF) detection of Ace-TUB, CCDC39, and RS proteins in normal and CCDC39 variant airways. Arrow shows indicated protein. Bar=25 μm.
(C) IF detection of Ace-TUB, CCDC39, and radial spoke (RS) proteins in normal cells transduced with non-targeted or CCDC96-specific shRNA. Arrow shows indicated protein. Bar=25 μm.
(D) TEM of isolated Chlamydomonas wild-type and ccdc39 axonemes. Repeating distances of radial spokes within a 96-nm repeat (32 nm) and between repeats (96 nm) are indicated.
(E) Quantification of distance from panel D.
(F) Immunoblot detection of RS head protein RSP16 shared by all RS in Chlamydomonas strains.
We found that radial spokes are differentially depleted in the CCDC39/40 variants. Cryo-EM analysis shows that each of the three radial spokes is anchored to CCDC39/40 by different CARPs7,9. The loss of RS2 and RS3 proteins were investigated in CCDC39 and CCDC40 variants based on the availability of antibodies (Figure 3B, Figure S2A). By proteomic analysis the RS2 and RS3 CARP, CFAP917,52 is absent. Likewise, RS3-specific CARP, CCDC96, is absent and confirmed by immunostaining (Figure 2B, Figure S2A). Immunostaining confirms the reduction of RS2 stalk protein LRRC23, RS3-specific proteins CFAP61 and LRCC34. RSPH3, a shared RS protein, is present in the cilia of normal and variant cells by immunostaining, consistent with the assembly of some radial spokes. We used depletion of the RS3 CARP, CCDC96 in human cells to ask about its role for RS3. CCDC96 shRNA resulted in loss of CCDC96 and decrease in the surface transport of beads (Figure S2B-S2D). CCDC39 and RSPH3 were preserved (Figure 3C). There was loss of both LRRC34 and LRRC23, suggesting that CCDC96 plays a role in both RS2 and RS3 (Figure 3C).
Additionally, analysis shows that RS1 CARP (IQUB) is diminished. Chlamydomonas was used to confirm the retention of RS1 and loss of RS2 in the ccdc39 mutant. Analysis of longitudinal TEM images of ccdc39 show loss of the periodicity and rigidity of RS (Figure 3D and 3E). RSP16, a shared radial spoke head protein, is present in cilia isolated from both ccdc39 and fap253 (CARP and IQUB ortholog) by immunoblot analysis, confirming the presence of RS (Figure 3F). However, immunoblots of cilia from the ccdc39; fap253 double mutant lacks RSP16, indicating the complete loss of RS. The finding suggests that in the absence of CCDC39/40, RS1 still binds the microtubule lattice without address information but with variable positioning. Together, our observations suggest that CCDC39/40 provide unique addresses for the attachment of all RS via designated ciliary address recognition proteins (CARP).
Altered microtubule inner proteins in CCDC39/40 variants
Microtubule inner proteins (MIPs) are a network of proteins within the lumen of the A and B tubules that show 8-, 16-, and 48-nm periodicities10,12,13,59. Some MIPs directly contact outer surface proteins. In our proteomics of CCDC39 variant cilia, three of the four tektin proteins (TEKT2,3,4) are decreased compared to normal cilia (Figure 4A). TEK3 does not contact CCDC39/40 but was absent in variant cilia by proteomics and verified by immunolocalization (Figure 4A, 4B). Decreased TEK2 and TEK4 may result from an inability to form the TEK3-TEK2 and TEK3-TEK4 heterodimers7. Additionally, CFAP90 (inner junction) and CFAP141 (outer junction) are absent in our proteomics (Figure 4A). CFAP90 is localized at the inner junction10, near the hook of the CCDC39/40 heterodimer (Figure 1Q). There was no difference in normal and CCDC39 in the total number of peptides of other MIPS (Figure 4A). The 48-nm MIPS are present in the cilia by immunostaining (Figure 4C).
Figure 4. Disorganization of microtubule inner proteins (MIPs) in CCDC39/40 variants.
(A) Proteomic analysis of MIPs in normal and CCDC39 variant human cilia
(B) Immunofluorescent (IF) detection of TEKT3 in human cells (CCDC39, WU182). Airway, Bar=25 μm; cilia, Bar=2 μm.
(C) IF detection of MIPs in cilia cultured cells (CCDC40, WU146). Cells, Bar=5 μm; cilia, Bar=2 μm.
(D) MIPs in wild-type and ccdc39–2 Chlamydomonas identified by cryo-EM. DMT structure from wild-type has 48-nm periodicity applied and ccdc39–2 has 16-nm periodicity applied.
By cryo-EM data of Chlamydomonas cilia, the 48-nm MIPs are disrupted in the ccdc39 mutant compared to wild-type (Figure 4D). However, the MIPs with 8-nm and 16-nm periodicity are largely preserved (Figure 4D). The disorganization of Chlamydomonas MIPs contrasts with the structural analysis that only found the loss of tektin in human CCDC39/40 variants7. Like in our human proteomics, tektin was diminished in ccdc3942 and in N-DRC mutants (DRC1 and DRC2)60 (Figure 4D). We predict that missing and misplaced MIPs in the variants contribute to abnormal ciliary function.
CCDC39/40 connectome localization during cilia assembly
Our data suggest that the CCDC39/40 heterodimer creates a set of addresses for anchoring the CARPs on the ciliary axoneme. By this hypothesis, we predict a temporal pattern of trafficking, whereby the heterodimer would enter the cilia early, followed by the CARPs and finally those complexes that preassemble in the cytoplasm61. Guided by stages of ciliogenesis (Figure 5A), we examined trafficking of CCDC39/40 in normal cells. During early stages of multiciliated cell differentiation marked by centriole amplification, CCDC39 and 40 are expressed present in the cytoplasm, but do not directly colocalize with centrioles (Figure 5B). Just prior to cilia assembly, CCDC39 and CCDC40 are enriched at the apical domain but infrequently colocalize (Figure 5C). Subsequently, CCDC39/40 colocalize in short cilia as they begin to grow (Figure 5C).
Figure 5. CCDC39/40 traffics independently of connectome proteins.
(A) Scheme of motile ciliogenesis in air-liquid interface (ALI) cultures.
(B) Immunofluorescence (IF) detection of basal body protein centrin, CCDC39, and CDH1 during basal body amplification.
(C) IF detection of CCDC39 and CCDC40 at pre-cilia stage (arrow indicates co-localization).
(D) IF detection of Ace-TUB and CCDC40 with proteins that contact CCDC39/40 in cells with emerging cilia (arrow indicates co-localization).
(E) IF detection of Ace-TUB and CCDC40 with proteins that do not directly contact CCDC39/40 in cells with emerging cilia.
(F) IF detection of connectome member CFAP100 and basal body protein Centrin during early cilia growth.
(G) IF detection of Ace-TUB, CFAP73, and CFAP100 in mature normal and CCDC39 cells.
(H) Immunoprecipitation of CCDC40 in cells from early (ALI 14) and late (ALI 40) stages.
(I) IB of CCDC39, CCDC73, and CCDC100 in normal, and variant mature cells. Short (S) and long (L) exposure.
(J) Proposed schema of cilia assembly in normal and CCDC39 variant.
Bar=5 μm in B-G
We then tested the temporal pattern of trafficking of CCDC39/40, the CARPs, and the connectome during normal cilia assembly. The RS3 CARP (CCDC96) and IDA CARP (CFAP57) are localized with CCDC40 in very short cilia (Figure 5D). In contrast, non-CARPs (CFAP100, CFAP61), remain within the cytoplasm, near the basal bodies (Figure 5E, 5F). Localization of these connectome proteins in normal multiciliated cells in early cilia assembly may identify a staging site for later transport into the cilia. In later stages of ciliogenesis, connectome proteins are in the cilia (Figure 5G). In normal cells, immunoprecipitation with CCDC40 pulls down connectome proteins CFAP100 and CFAP73 to a greater degree at late compared to early stages of cilia growth (Figure 5H).
In CCDC39/40 variants, connectome proteins remain within the apical domain (Figure 5G), a location similar to the assembly staging site in normal early cilia (Figures 5E). Ultimately, the unbound connectome members are degraded (Figure 5I). We suggest cilia assembly proceeds with CCDC39/40 binding the DMTs, followed by CARP binding to the heterodimer, and subsequently, the remaining connectome. We cannot rule out that CARPs are co-transported with CCDC39/40. In sum, there is an orderly assembly sequence, dependent on CCDC39/40 and in the absence of the heterodimer, the proteins remain at the staging site or degrade (Figure 5J).
Single cell RNA transcriptomics reveals increased proteostasis in CCDC39/40 variants
To interrogate multiciliated cell dysfunction from CCDC39/40 loss, we used single cell RNA sequencing (scRNAseq). Transcriptomes were compared from normal (n=6) and variant (n=3) cell cultures. Cell types were identified using unsupervised clustering and annotated with known airway epithelial markers (Figure 6A)17. CCDC39 and CCDC40 are primarily expressed in the cluster of mature multiciliated cells (Cil3) (Figure 3SA). In this cluster, over 1800 genes are differentially expressed in the variants compared to normal (Extended Data Table). Enrichment analysis of differential gene expression in multiciliated cells (Cil3), identified cilia structure and function, proteostasis, cell stress, oxidative phosphorylation, and Notch signaling pathways (Figure 6B, Figure S3B-S3F). Genes upregulated have roles in: (1) proteostasis, including components of the E3 ubiquitin ligase complex62, multiple proteasome subunits, heat shock chaperone proteins that handle unfolded proteins and aggregates63 (Figure S3B, S3E); (2) cell stress response genes that include SAA1, SAA2, and SAA4, which are secreted acute phase reactants in sterile inflammation64,65 (Figure 6B), (3) mitochondrial complex I subunits of NADH ubiquinone oxidoreductase and complex V subunits of ATP synthase indicating upregulation of ATP production (Figure S3C); and (4) HES1, a target of Notch signaling that increases secretory cell differentiation and airway secretory cell proteins66–68 (Figure 6B). Together, these data indicate the presence of cellular stress in the variant cells, possibly due to the need to degrade the large burden of undocked connectome proteins. We examined the proteasome in variant cells using an antibody against PSMB6, a component of the 20S core subunit (Figure 6C). PSMB6 is located throughout in the cytoplasm of normal cells. In contrast, PSMB6 is localized just beneath the cilia in the variant cells. The pattern is coordinate with the location of undocked connectome members in variant cells (Figure 5H), suggesting a site-specific requirement for proteostasis. Consistent with our finding, increased proteostasis leads to increased ATP demand and oxidant production. These factors likely cause cell stress that may drive Notch-signaling69–72.
Figure 6. CCDC39/40 variant affect differentiation via Notch signaling.
(A) UMAP reductions of normal (n=6) and CCDC39/40 variant (CCDC39, WU182 and WU 157; CCDC40 WU146) differentiated airway epithelial cells showing clusters for basal (bas); secretory (sec); multiciliated (cil) cells.
(B) Dotplot shows manually annotated, differentially expressed genes in each cluster that show significantly increased expression in the variant compared to multiciliated cells. Ciliated cells are in purple.
(C) Immunofluorescent (IF) detection of Ace-TUB and PSMB6 in normal and CCDC39 (WU182) variant cells. Box identifies an example of PSMB6 in multiciliated cells. Arrow indicates the location of PSMB6 in the cytoplasm.
(D) Quantitation of cell numbers in each cluster from A. Comparison of Sec2 normal vs. CCDC39/CCDC40 (39/40) variant, p=0.0025.
(E) Immunohistochemistry for detection of PAS-positive mucous cells in the airway from a lung of a normal donor and a CCDC39 variant.
(F) IF detection of α-tubulin (α-TUB) and MUC5AC in cell cultures from unique normal donor (n=4), unique CCDC39/40 variants (n=4), and PCD variants DNAAF5, DNAH5, DNAI1 (n=1 per genotype).
(G) Quantitation of normal cells with genotypes CCDC39, CCDC40 and other PCD variants from Panels F and L. CCDC39 variant cells expressing control (Cont) and the CCDC39 Transgene (TG) are compared.
(H) IF detection of α-TUB and MUC5AC in CCDC39 variant cells. Cil, multiciliated cell; Muc, MUC5AC cell; MC, Multiciliated-MUC5AC cell.
(I) IF detection of MUC5AC and Ace-TUB in normal cells transduced with non-targeted CCDC39 or CCDC40 shRNA.
(J) Quantitation of panel I. Normal cells transduced with non-targeted, CCDC39, or CCDC40 shRNA are compared.
(K) IF detection of α-TUB and MUC5AC in iPSC cells that are cells derived from the control original iPSC from a normal donor, or CRISPR-Cas9 mediated deficiency of DNAH5 or CCDC40 in the normal iPSC. Bar=25 μm
(L) Quantitation of panel K. Each point represents a microscope field of cells from the control original iPSC line, DNAH5 CRISPR knockout, or CCDC40 CRISPR knockout. The control cells are compared to the knockout lines.
(M) IF detection of α-TUB and MUC5AC in transgene (TG) rescued variant cells transduced with a control (Cont) or FOXJ1-CCDC39 transgene. Quantitation of M is in panel G.
(N) IF detection of α-TUB and MUC5AC in unique normal and CCDC39 and CCDC40 variant cultures (ALI >28) day then treated with vehicle or NOTCH inhibitor DAPT for two weeks.
(O) Quantitation of panel N. Vehicle cells are compared to DAPT-treated for each group. Each point is the mean of fields of each condition in at least three independent experiments. Normal (n=3); CCDC39 (WU182, n=2); CCDC40 (WU146, n=2).
The bar indicates the medium. Differences between groups were determined using Mann-Whitney in D, G, O or Kruskal Wallis with Dunn’s Multiple comparison test in J and L; ns=non-significant, *p<0.05, **p<0.01, ***p<0.001.
Bar=5 m in C, H; Bar=25 μm in E, F, K, I, M, N.
CCDC39/40 variants switch fates in airway epithelial cells
Comparing variant and normal cells, scRNAseq analysis shows an increased number of secretory cells (Sec1 and Sec2) (Figure 3D), suggesting a shift in differentiation. The balance of airway multiciliated versus secretory cell differentiation is controlled by Notch66,73. In vivo, chronic airway inflammation is found in patients with asthma, cystic fibrosis, chronic obstructive pulmonary disease (COPD). These airways have increased mucous cells (also called goblet cells), marked by increased MUC5AC, a gel-forming, secreted mucin74. There is also an abundance of mucous cells in tissue samples obtained from an airway of a patient with a CCDC39 variant who underwent lung transplantation (Figure 6D). We examined the production of MUC5AC in ALI cultured cells. Control cultures show a high percentage of multiciliated cells, with few MUC5AC-expressing cells. In contrast, CCDC39/40 variant cultures have significantly more MUC5AC-expressing cells at the expense of multiciliated cells (Figure 6F, 6G). Cultured cells from PCD patients with variants in DNAAF5, DNAI1, and DNAH5 show significantly fewer MUC5AC-staining cells than the CCDC39/40 cultures (Figure 6F, 6G). A small number of cells expressing both MUC5AC and cilia proteins are present in the CCDC39/40 cultures and represent a transition state in cell differentiation75 (Figure 6H).
It is possible that the chronic inflammatory environment in the airways of CCDC39 and CCDC40 patients account for the increased mucous cells in vitro, as observed in cultures of cells from COPD subjects76,77. Accordingly, we depleted CCDC39 or CCDC40 from normal donor airway cells using shRNA (Figure 6I, 6J, Figure S4A-D). CCDC39 or CCDC40 depleted cultures show increased numbers of MUC5AC-expressing cells and fewer multiciliated cells than controls. Thus, the shift in the differentiation phenotype is unlikely to be attributable to effects of a chronically infected, inflamed PCD airway. To exclude the possibility that the MUC5AC phenotype was the result of exposure of cells to an airway environment, we assessed cultures of inducible pluripotent stem cells (iPSC) that underwent CCDC40 or DNAH5 deletion by CRISPR-CAS9 editing78. Cells were differentiated to airway basal cells, then cultured using ALI conditions. CCDC40 deficient cells show more MUC5AC-expressing cells compared to those with a DNAH5 deletion or the control iPS cells (Figure 6K, 6L; Figure S4E). These findings suggest that the CCDC39 or CCDC40 patient cells or their airway basal cells are not altered by the environment. Finally, lentivirus-mediated delivery of a transgene expressing CCDC39 under control of the FOXJ1 promotor (Transgene, TG) in the CCDC39 variant cells showed rescue compared to a control lentivirus (Cont) (Figures 6M, 6G, Figure S5A-S5E). We observed a decrease in the percentage of MUC5AC cells and an increased percentage of ciliated cells (Figure 6G, 6L). Thus, the shift in cell fate likely occurs by the transition of multiciliated to secretory cells via a genetics loss of CCDC39/40 and is consistent with increased mucus plugging of airways observed in computed tomography imaging of patients with CCDC39/40 variants39.
To ask if the shift in differentiation from multiciliated to mucous cells in the CCDC39/40 cultures was mediated by Notch, we inhibited Notch signaling with the γ-secretase inhibitor DAPT79. We delayed treatment of the variant cultures with vehicle or DAPT until after ALI day 28, when MUC5AC cells were increased. In cells treated with DAPT, the number of MUC5AC-expressing cells was markedly decreased and acetylated α-tubulin expressing cells was increased compared to a persistence of MUC5AC-expressing cells in vehicle-treated variant cells (Figure 6N, 6O). We conclude that Notch signaling is required to shift the differentiation of the multiciliated variant cells to mucous cells and that continuous inhibition of Notch signaling blocks the switch from multiciliated to mucous cells. As expected, the CCDC39 and CCDC40 variants remain immotile after treatment (Figure S4F). Taken together, CCDC39/40 deficiency results in loss of ciliary proteins, increased intracellular stress, activated Notch activity, and altered maintenance of multiciliated cell differentiation.
CCDC39/40 variants disrupt the periciliary barrier
We defined the intracellular impact of a failed connectome, but the effect of the altered cilia integrity remains unaddressed. In addition to ciliary motility, the cilia are an intrinsic component of the periciliary barrier. Seminal studies defined the periciliary barrier as containing a brush-like polymer of tethered mucins (MUC1, MUC4, MUC16) that surround the cilia and act as a selective filter to particle entry74,80. One striking feature of the CCDC39/40 variants is ciliary splaying (Figure 1L-N). To better visualize the DMT splaying, we used scanning EM. Cells were untreated or treated with detergent-containing buffer that disrupts the ciliary membrane (Figure 7A). Normal cilia exhibit intact DMTs in both conditions. CCDC39/40 variants show DMT splaying, particularly at the distal ends in the untreated condition and further unraveling of the DMT with detergent treatment (Figure 7A-C). Normal isolated cilia show little splaying regardless of treatment. In contrast, at least 15% of variant isolated cilia show splaying with no treatment, while about 50% splay when the membrane is removed. Splaying, as well as cilia length and cilia beat frequency were rescued using a CCDC39 transgene (Figures 7F-J, Supplement S5A-F; Video V6).
Figure 7. CCDC39/40 variants have disrupted periciliary barrier.
(A) Scanning EM of normal and variant (CCDC39, WU152; CCDC40, WU151) cilia from cells untreated and treated to disrupt the ciliary membrane. Bar=1 μm.
(B) Immunofluorescent (IF) detection of Ace-TUB to identify splaying in cilia untreated and treated with buffer to remove cilia membranes from cultured cells. Bar=10 μm.
(C) Quantitation of splaying in cilia from cultured cells from normal (n=2) and variant cells (CCDC39, WU152, WU182; CCDC40, WU146, WU151).
(D) Scheme for isolation of fresh cilia by nasal brush biopsy and IF staining.
(E) Quantitation of IF detection of splaying by Ace-TUB in cilia from normal (n=3) and variant cells (CCDC39, WU120, WU152; CCDC40, WU149, WU151). There was no difference between CCDC39 and CCDC40.
(F) Scheme of normal CCDC39 transgene delivery to CCDC39 variant cells (WU182) using control and FOXJ1-CCDC39 rescue transgene lentiviruses (Lenti).
(G) IF detection of Ace-TUB to identify cilia length in cells from CCDC39 variant transduced with control (top) and rescue (bottom) lentiviruses in panel F. Bar=10 μm.
(H) Quantification of cilia length in CCDC39 variant cells from panel G, transduced with control or transgene (TG) lentivirus; n=150–200 cilia per condition in over 20 fields.
(I) Quantitation of cilia beat frequency (CBF) in CCDC39 variant cells and cells transduced with control lentivirus, low or high concentration of the CCDC39 TG.
(J) Quantification of cilia splaying in cells transduced with control and TG. n=300–500 cilia per condition from over 20 fields.
(K) IF detection of MUC4 in the periciliary region of cultures from normal, DNAH5 (WU165) variant, and two CCDC39 variant cells non-transduced and following transgene rescue. Bar=5 μm.
(L) Scheme of periciliary barrier assessment performed by addition of microparticles to the apical surface for 15 min prior to fixation and imaging.
(M) Fluorescence particles relative to cilia in cultures from normal and variant (CCDC39, WU182; CCDC40, WU146; DNAAF5, WU108; DNAI1, WU103). Bar=5 μm.
(N) Detection of fluorescent particles in CCDC39 variant (WU182) transduced with the rescue CCDC39 transgene as in panel F, Bar=5 μm.
(O) Quantitation of fluorescent particles in the periciliary space from panels and L and M. Differences in the indicated conditions were determined compared to normal cells.
In C, E, I, N the bar indicates the medium. Difference between groups determined by Kruskal-Wallis and with a Dunn’s Multiple Comparison Test. In H the unpaired two-tailed t-test. In J, the Mann-Whitney test ns=non-significant; ***p<0.001.
To determine the effect of the respiratory tract environment, we examined the extent of cilia splaying from freshly obtained samples (Figure 7D). Using non-detergent conditions, the median number of splayed cilia in freshly obtained and cultured samples were similar (Figure 7C, 7E). Surprisingly, untreated, fresh samples were significantly more variable in their splaying phenotype (F=0.03) (Figure 7D, 7E), suggesting that the respiratory tract environment destabilizes the ciliary membrane.
The periciliary barrier relies on both intact cilia and inter-ciliary tethered mucins74,80. MUC4 is present within the ciliary region of normal and DNAH5 variant cells but absent in the periciliary layer of cells with CCDC39 variants (Figure 7K). Its presence in the periciliary layer of the CCDC39 variant can be rescued by the transgene (Figure 7K), suggesting that the periciliary barrier is abnormal due to the variant. While it is difficult to visualize the polymer-like structure of this barrier, it can be functionally defined as being impervious to particles that are greater or equal to 40 nm in diameter80. To test the barrier function of variant multiciliated cells, we labeled the cilia of live cells using a fluorescent reporter, SPY-tubulin. Fluorescent-coated spherical particles (100 nm or 40 nm) were added to the apical surface (Figures 7L). The particles penetrate the periciliary space of the CCDC39 and CCDC40 variant cells, but not those of control cells (Figures 7M, 7O). Likewise, there is no detectable entry of microparticles in cells from individuals with PCD due to DNAI1 or DNAAF5 variants, which also have non-beating cilia. The periciliary space function in CCDC39 cells was restored with the transgene (Figures 7N, 7O). The findings suggest that there is an incompetent periciliary barrier in CCDC39 and CCDC40 variant cells, increasing susceptibility to injury and infection that is not related to ciliary beating, contributing to severe lung disease in affected patients.
Discussion
The CCDC39/40 heterodimer appears by cryo-EM as a ribbon-like structure that repeats every 96-nm along the length of the outer doublet microtubules7,10. We propose that the heterodimer guides multiple structural complexes to their correct positions by providing addresses for their docking. CCDC39/40 anchors an extensive connectome of over 90 proteins that is much greater than suggested by earlier studies33,34,40,42. The severe lung disease in CCDC39/40 patients may be explained by the large burden of the undocked connectome. This proteotoxic load is postulated to cause alterations in the multiciliated cell transcriptional and differentiation programs. In addition to the intracellular effects, we suggest that the microtubular disorganization results in a change in the periciliary barrier from signals in the extracellular environment.
Ciliary address recognition proteins (CARP) identify addresses on CCDC39/40 for docking major ciliary complexes.
Cilia of the CCDC39 variant were missing 50 proteins while 40 other proteins were significantly decreased. Notably, 14 of the missing proteins directly contact CCD39/40 and are likely to be the ciliary address recognition proteins (CARPs) for IDA, the N-DRC, and the RSs. CARPs attach to specific amino acid sequences of CCDC39/40 (the addresses) (Figure 2D, 2E)7–12,14,45–55. During assembly, CARPs enter cilia coincident with CCDC39/40. The IDA and RS are prefabricated in the cytoplasm into megadalton complexes but do not include the CARPs61,81–83. The entry and docking of these structures follow the CARPs (Figure 5C-E). Therefore, the loss of CCDC39/40, and thus the addresses for CARPs in the variants result in the failure of docking of entire complexes (Figure 3B, Figure 5H). Our findings are relevant as variants in several CARPs are diseases-causing. CFAP57, DRC1, DRC2 and DRC4 variants result in PCD associated with the loss of the respective IDA or N-DRC complex47,84–86. Variants in CCDC146 and ZYMND12 result in human male sterility. We do not know the extent of protein loss in these variants, or their impact on multiciliated cell function.
CCDC39/40-dependent ciliary proteins with unknown localization are missing or reduced
There are approximately 32 proteins with unknown roles that are missing or reduced in cilia from the CCDC39 variant; most of these are transcribed primarily in multiciliated tissues or testes (Figure 2C). VCP and UBXN11 are reduced; UBXN11 is reported to interact with the proteosome quality control AAA-ATPase, VCP87. A related protein, UBXN10 along with VCP, controls primary cilia length. The reduction of UBXN11 may contribute to the short cilia of the CCDC39/40 variants87. Other proteins that are absent may belong to RS3, as current structural maps are incomplete. In future studies it will be important to understand the interaction of these uncharacterized proteins with CCDC39/40 using high resolution cryo-EM and proteomics.
CCDC39/40 variants have an impaired periciliary barrier
Two striking features of the CCDC39/40 variant cilia is their short length and ciliary splaying via the loss of N-DRC (Figure 2C)8,41,88. Loss of N-DRC proteins also causes microtubule misalignment in PCD patients with DRC4 variants, but splaying was not investigated. Our SEM of the CCDC39/40 variants in cultured cells shows that removal the ciliary membranes increased splaying substantially (Figure 7A-C). Interestingly, the degree of cilia splaying observed in fresh nasal biopsies of individuals with CCDC39/40 variants was more variable (Figure 7E). We suggest that this significant variability in splaying occurs from inflammatory environmental insults on the ciliary membrane, which normally functions to support ciliary integrity.
The tethered mucin MUC4 was decreased in the periciliary layer (Figure 7K), suggesting a role of the ciliary membrane to establish or maintain the interconnected polymer structure. The relationship between ciliary integrity and a functional periciliary barrier has not been explored beforehand. The periciliary space plays an important role in separating the mucus layer and its contents from the airway epithelia74,80. We observed that fluorescent-labeled microbeads enter the periciliary space of variant cells but not in normal cells, which suggests that pathogens and foreign particles may more easily perturb multiciliary cells in the variants. Short cilia could also impact the function of the periciliary barrier. Disruption of the periciliary layer would further impair airway host defense and contribute to severe lung disease in these patients.
CCDC39/40 variants have mucous cell metaplasia in vitro
We were surprised to find that in vitro differentiation of CCDC39/40 variant cells led to mucous cell metaplasia (Figure 6F, 6G). These findings are consistent with increased airway mucus plugging observed by x-ray imaging lungs of patients with CCDC39 and CCDC40 variants. Cultures of airway cells from individuals with COPD and cystic fibrosis show a similar phenotype that is proposed to arise from altered basal cell differentiation in response to a chronically inflamed environment76,77,79,89. Four lines of evidence suggest that the altered cell fate switching observed in CCDC39/40 variants is associated with the genotype and not the environment. First, an increase in mucous cells was not observed in cultures of cells with other PCD variants that have immotile cilia and were isolated from an inflamed environment. Second, mucous cell metaplasia was observed in cells from normal individuals after depleting CCDC39 and CCDC40 by shRNA (Figure 6I, 6J). Third, an increased number of mucous cells was present in cultures of CCDC40 knockout iPS cells derived from peripheral blood cells of a healthy donor. Fourth, transgene rescue with a normal CCDC39 gene in the CCDC39 variant cells reversed the mucous cell phenotype (Figure 6K, 6L). These lines of evidence strongly suggest that the loss of CCDC39/40 affects the shift in the cell fate of airway cells.
Single cell transcriptomics shows alterations in multiple pathways that may contribute to the mucous cell phenotype. Oxidant stress is reported to increase the number of mucous cells and expression of MUC5AC in airway epithelial cells and associated with intracellular ER stress or oxidants from neutrophilic inflammation, and cigarette smoke71,72,74. SAA1, SAA2 and SAA4 are upregulated in the variant multiciliated cells (Figure 6B). These secreted proteins are known to respond to injury, and activate inflammatory pathways in neighboring cells that may provide Notch signaling to multiciliated cells65. The observation that DAPT treatment decreased mucous cell number in the variant suggests inhibition of Notch signaling as an adjunctive therapy for patients with CCDC39/40 variants66.
Increased transcription of proteosome subunits, heat shock chaperones, and E3 ligases is consistent with increased proteostatic stress63. We estimate that proteins from the 6 inner dynein arms, on each ~6 μm long cilium, from an average of 200 cilia per cell will result in one billion kilodaltons of protein that must be continually degraded by each cell. We propose that the unbound connectome proteins are responsible for generating the proteotoxic stress. The dramatic re-localization of the proteasome protein PMSB6 to the apical region of the multiciliated cell is concurrent with accumulation of unbound connectome proteins at what we term the “assembly staging zone” (Figures 5H, 6C). This similar localization of the proteosome and unbound connectome may indicate a demand for local quality control. Proteostasis requires significant ATP, which is consistent with the increased transcription of mitochondrial genes (Figure S3C). CCDC39/40 variants share transcriptional pathways with neurodegenerative diseases (Alzheimer’s, Parkinson’s, and Huntington Disease), which have increased protein burden resulting in proteotoxicity and ATP demand63,90 (Figure S3E). Of note, neurodegenerative disease and a growing number of other diseases are being treated using inhibitors of ubiquitin and proteosome pathway modulators91.
Limitations of the study
We report an extensive disruption the ciliary proteome from the CCDCC39 variant, but we have not examined the proteome from the CCDC40 variant. However, there is interdependence between CCDC39 and CCDC40 proteins as described in Chlamydomonas22, and we validated a similar loss of selected proteins in both CCDC39 and CCDC40 variant cells (e.g., Figure 3, Figure S3). Second, our proposal is that CCDC39/40 provides amino acid sequences as addresses for attachment of CARPs to CCDC39/40 as supported by multiple cryo-EM models and experimental data (Figure 2D). The mutagenesis of these addresses will be the focus of future studies. Finally, we do not know if other PCD variants will lead to similar proteostatic stress or ciliary barrier impairment by the disruption of ciliary membranes due to airway inflammation.
Supplementary Material
Acknowledgements.
Transmission EM was performed with the assistance of Dr. Wandy Beatty in the Department of Molecular Microbiology at Washington University and scanning EM by Dr. Sanja Sviben in the Washington University Center for Cellular Imaging (WUCCI), which is supported by Washington University School of Medicine, The Children’s Discovery Institute of Washington University and St. Louis Children’s Hospital (CDI-CORE-2015-505 and CDI-CORE-2019-813), and the Foundation for Barnes-Jewish Hospital (3770). Proteomics was performed with the assistance of Dr. Shin-Cheng Tzeng in the Donald Danforth Plant Science Center Proteomic and Mass Spectrometry Core. We thank Greg Longmore (Washington University) for the UbiC-YFP lentivirus transfer plasmid and Mehmet Kesimer (University of North Carolina, Chapel Hill) for the anti-MUC4 antibody.
Funding:
National Institutes of Health: R01 HL146601 (SLB), R01 HL128370 (SLB, SKD, MRM); R35 GM131909 (SKD); K08 HL150223 (AH); R01 GM138854 (RZ); R01 HL139799 (F.J.H.). Cystic Fibrosis Foundation Harry Shwachman Award BERICA22Q0 (AB). Barnes Jewish Hospital Foundation (SLB)
Funding Statement
National Institutes of Health: R01 HL146601 (SLB), R01 HL128370 (SLB, SKD, MRM); R35 GM131909 (SKD); K08 HL150223 (AH); R01 GM138854 (RZ); R01 HL139799 (F.J.H.). Cystic Fibrosis Foundation Harry Shwachman Award BERICA22Q0 (AB). Barnes Jewish Hospital Foundation (SLB)
Footnotes
Declaration of Interest
The authors declare no competing interests
References
- 1.Derderian C., Canales G.I., and Reiter J.F. (2023). Seriously cilia: A tiny organelle illuminates evolution, disease, and intercellular communication. Dev Cell 58, 1333–1349. 10.1016/j.devcel.2023.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Katoh T.A., Omori T., Mizuno K., Sai X., Minegishi K., Ikawa Y., Nishimura H., Itabashi T., Kajikawa E., Hiver S., et al. (2023). Immotile cilia mechanically sense the direction of fluid flow for left-right determination. Science 379, 66–71. 10.1126/science.abq8148. [DOI] [PubMed] [Google Scholar]
- 3.Olstad E.W., Ringers C., Hansen J.N., Wens A., Brandt C., Wachten D., Yaksi E., and Jurisch-Yaksi N. (2019). Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development. Curr Biol 29, 229–241 e226. 10.1016/j.cub.2018.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klena N., and Pigino G. (2022). Structural Biology of Cilia and Intraflagellar Transport. Annu Rev Cell Dev Biol 38, 103–123. 10.1146/annurev-cellbio-120219-034238. [DOI] [PubMed] [Google Scholar]
- 5.Goodenough U.W., and Heuser J.E. (1985). Substructure of inner dynein arms, radial spokes, and the central pair/projection complex of cilia and flagella. J Cell Biol 100, 2008–2018. 10.1083/jcb.100.6.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodenough U.W., and Heuser J.E. (1982). Substructure of the outer dynein arm. J Cell Biol 95, 798–815. 10.1083/jcb.95.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walton T., Gui M., Velkova S., Fassad M.R., Hirst R.A., Haarman E., O'Callaghan C., Bottier M., Burgoyne T., Mitchison H.M., and Brown A. (2023). Axonemal structures reveal mechanoregulatory and disease mechanisms. Nature 618, 625–633. 10.1038/s41586-023-06140-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghanaeian A., Majhi S., McCafferty C.L., Nami B., Black C.S., Yang S.K., Legal T., Papoulas O., Janowska M., Valente-Paterno M., et al. (2023). Integrated modeling of the Nexin-dynein regulatory complex reveals its regulatory mechanism. Nat Commun 14, 5741. 10.1038/s41467-023-41480-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gui M., Ma M., Sze-Tu E., Wang X., Koh F., Zhong E.D., Berger B., Davis J.H., Dutcher S.K., Zhang R., and Brown A. (2021). Structures of radial spokes and associated complexes important for ciliary motility. Nat Struct Mol Biol 28, 29–37. 10.1038/s41594-020-00530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma M., Stoyanova M., Rademacher G., Dutcher S.K., Brown A., and Zhang R. (2019). Structure of the Decorated Ciliary Doublet Microtubule. Cell 179, 909–922.e912. 10.1016/j.cell.2019.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gui M., Farley H., Anujan P., Anderson J.R., Maxwell D.W., Whitchurch J.B., Botsch J.J., Qiu T., Meleppattu S., Singh S.K., et al. (2021). De novo identification of mammalian ciliary motility proteins using cryo-EM. Cell 184, 5791–5806 e5719. 10.1016/j.cell.2021.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kubo S., Black C.S., Joachimiak E., Yang S.K., Legal T., Peri K., Khalifa A.A.Z., Ghanaeian A., McCafferty C.L., Valente-Paterno M., et al. (2023). Native doublet microtubules from Tetrahymena thermophila reveal the importance of outer junction proteins. Nat Commun 14, 2168. 10.1038/s41467-023-37868-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung M.R., Zeng J., Wang X., Roelofs M.C., Huang W., Zenezini Chiozzi R., Hevler J.F., Heck A.J.R., Dutcher S.K., Brown A., et al. (2023). Structural specializations of the sperm tail. Cell 186, 2880–2896 e2817. 10.1016/j.cell.2023.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gui M., Wang X., Dutcher S.K., Brown A., and Zhang R. (2022). Ciliary central apparatus structure reveals mechanisms of microtubule patterning. Nat Struct Mol Biol 29, 483–492. 10.1038/s41594-022-00770-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meng X., Xu C., Li J., Qiu B., Luo J., Hong Q., Tong Y., Fang C., Feng Y., Ma R., et al. (2024). Multi-scale structures of the mammalian radial spoke and divergence of axonemal complexes in ependymal cilia. Nat Commun 15, 362. 10.1038/s41467-023-44577-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blackburn K., Bustamante-Marin X., Yin W., Goshe M.B., and Ostrowski L.E. (2017). Quantitative Proteomic Analysis of Human Airway Cilia Identifies Previously Uncharacterized Proteins of High Abundance. J Proteome Res 16, 1579–1592. 10.1021/acs.jproteome.6b00972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Plasschaert L.W., Žilionis R., Choo-Wing R., Savova V., Knehr J., Roma G., Klein A.M., and Jaffe A.B. (2018). A single-cell atlas of the airway epithelium reveals the CFTR-rich pulmonary ionocyte. Nature 560, 377–381. 10.1038/s41586-018-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao H., Zhu L., Zhu Y., Cao J., Li S., Huang Q., Xu T., Huang X., Yan X., and Zhu X. (2013). The Cep63 paralogue Deup1 enables massive de novo centriole biogenesis for vertebrate multiciliogenesis. Nat Cell Biol 15, 1434–1444. 10.1038/ncb2880. [DOI] [PubMed] [Google Scholar]
- 19.Yu X., Ng C.P., Habacher H., and Roy S. (2008). Foxj1 transcription factors are master regulators of the motile ciliogenic program. Nat Genet 40, 1445–1453. 10.1038/ng.263. [DOI] [PubMed] [Google Scholar]
- 20.Inglis P.N., Boroevich K.A., and Leroux M.R. (2006). Piecing together a ciliome. Trends Genet 22, 491–500. 10.1016/j.tig.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Li J.B., Gerdes J.M., Haycraft C.J., Fan Y., Teslovich T.M., May-Simera H., Li H., Blacque O.E., Li L., Leitch C.C., et al. (2004). Comparative genomics identifies a flagellar and basal body proteome that includes the BBS5 human disease gene. Cell 117, 541–552. 10.1016/s0092-8674(04)00450-7. [DOI] [PubMed] [Google Scholar]
- 22.Wallmeier J., Nielsen K.G., Kuehni C.E., Lucas J.S., Leigh M.W., Zariwala M.A., and Omran H. (2020). Motile ciliopathies. Nat Rev Dis Primers 6, 77. 10.1038/s41572-020-0209-6. [DOI] [PubMed] [Google Scholar]
- 23.Raidt J., Loges N.T., Olbrich H., Wallmeier J., Pennekamp P., and Omran H. (2023). Primary ciliary dyskinesia. Presse Med 52, 104171. 10.1016/j.lpm.2023.104171. [DOI] [PubMed] [Google Scholar]
- 24.Legendre M., Zaragosi L.E., and Mitchison H.M. (2021). Motile cilia and airway disease. Semin Cell Dev Biol 110, 19–33. 10.1016/j.semcdb.2020.11.007. [DOI] [PubMed] [Google Scholar]
- 25.Afzelius B.A. (1976). A human syndrome caused by immotile cilia. Science 193, 317–319. 10.1126/science.1084576. [DOI] [PubMed] [Google Scholar]
- 26.Omran H., Kobayashi D., Olbrich H., Tsukahara T., Loges N.T., Hagiwara H., Zhang Q., Leblond G., O'Toole E., Hara C., et al. (2008). Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature 456, 611–616. 10.1038/nature07471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olbrich H., Schmidts M., Werner C., Onoufriadis A., Loges N.T., Raidt J., Banki N.F., Shoemark A., Burgoyne T., Al Turki S., et al. (2012). Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left-right body asymmetry. Am J Hum Genet 91, 672–684. 10.1016/j.ajhg.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loges N.T., Antony D., Maver A., Deardorff M.A., Güleç E.Y., Gezdirici A., Nöthe-Menchen T., Höben I.M., Jelten L., Frank D., et al. (2018). Recessive DNAH9 Loss-of-Function Mutations Cause Laterality Defects and Subtle Respiratory Ciliary-Beating Defects. Am J Hum Genet 103, 995–1008. 10.1016/j.ajhg.2018.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olbrich H., Häffner K., Kispert A., Völkel A., Volz A., Sasmaz G., Reinhardt R., Hennig S., Lehrach H., Konietzko N., et al. (2002). Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left-right asymmetry. Nat Genet 30, 143–144. 10.1038/ng817. [DOI] [PubMed] [Google Scholar]
- 30.Schwabe G.C., Hoffmann K., Loges N.T., Birker D., Rossier C., de Santi M.M., Olbrich H., Fliegauf M., Failly M., Liebers U., et al. (2008). Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum Mutat 29, 289–298. 10.1002/humu.20656. [DOI] [PubMed] [Google Scholar]
- 31.Castleman V.H., Romio L., Chodhari R., Hirst R.A., de Castro S.C., Parker K.A., Ybot-Gonzalez P., Emes R.D., Wilson S.W., Wallis C., et al. (2009). Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. Am J Hum Genet 84, 197–209. 10.1016/j.ajhg.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pennarun G., Escudier E., Chapelin C., Bridoux A.M., Cacheux V., Roger G., Clément A., Goossens M., Amselem S., and Duriez B. (1999). Loss-of-function mutations in a human gene related to Chlamydomonas reinhardtii dynein IC78 result in primary ciliary dyskinesia. Am J Hum Genet 65, 1508–1519. 10.1086/302683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Merveille A.C., Davis E.E., Becker-Heck A., Legendre M., Amirav I., Bataille G., Belmont J., Beydon N., Billen F., Clément A., et al. (2011). CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat Genet 43, 72–78. 10.1038/ng.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Becker-Heck A., Zohn I.E., Okabe N., Pollock A., Lenhart K.B., Sullivan-Brown J., McSheene J., Loges N.T., Olbrich H., Haeffner K., et al. (2011). The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left-right axis formation. Nat Genet 43, 79–84. 10.1038/ng.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Antony D., Becker-Heck A., Zariwala M.A., Schmidts M., Onoufriadis A., Forouhan M., Wilson R., Taylor-Cox T., Dewar A., Jackson C., et al. (2013). Mutations in CCDC39 and CCDC40 are the major cause of primary ciliary dyskinesia with axonemal disorganization and absent inner dynein arms. Hum Mutat 34, 462–472. 10.1002/humu.22261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis S.D., Rosenfeld M., Lee H.S., Ferkol T.W., Sagel S.D., Dell S.D., Milla C., Pittman J.E., Shapiro A.J., Sullivan K.M., et al. (2019). Primary Ciliary Dyskinesia: Longitudinal Study of Lung Disease by Ultrastructure Defect and Genotype. Am J Respir Crit Care Med 199, 190–198. 10.1164/rccm.201803-0548OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pifferi M., Bush A., Mariani F., Piras M., Michelucci A., Cangiotti A., Di Cicco M., Caligo M.A., Miccoli M., Boner A.L., and Peroni D. (2020). Lung Function Longitudinal Study by Phenotype and Genotype in Primary Ciliary Dyskinesia. Chest 158, 117–120. 10.1016/j.chest.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 38.Shoemark A., Rubbo B., Legendre M., Fassad M.R., Haarman E.G., Best S., Bon I.C.M., Brandsma J., Burgel P.R., Carlsson G., et al. (2021). Topological data analysis reveals genotype-phenotype relationships in primary ciliary dyskinesia. Eur Respir J 58. 10.1183/13993003.02359-2020. [DOI] [PubMed] [Google Scholar]
- 39.Kinghorn B., Rosenfeld M., Sullivan E., Onchiri F., Ferkol T.W., Sagel S.D., Dell S.D., Milla C., Shapiro A.J., Sullivan K.M., et al. (2023). Airway Disease in Children with Primary Ciliary Dyskinesia: Impact of Ciliary Ultrastructure Defect and Genotype. Ann Am Thorac Soc 20, 539–547. 10.1513/AnnalsATS.202206-524OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oda T., Yanagisawa H., Kamiya R., and Kikkawa M. (2014). A molecular ruler determines the repeat length in eukaryotic cilia and flagella. Science 346, 857–860. 10.1126/science.1260214. [DOI] [PubMed] [Google Scholar]
- 41.Bower R., Tritschler D., Mills K.V., Heuser T., Nicastro D., and Porter M.E. (2018). DRC2/CCDC65 is a central hub for assembly of the nexin-dynein regulatory complex and other regulators of ciliary and flagellar motility. Mol Biol Cell 29, 137–153. 10.1091/mbc.E17-08-0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin H., Zhang Z., Guo S., Chen F., Kessler J.M., Wang Y.M., and Dutcher S.K. (2015). A NIMA-Related Kinase Suppresses the Flagellar Instability Associated with the Loss of Multiple Axonemal Structures. PLoS Genet 11, e1005508. 10.1371/journal.pgen.1005508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blanchon S., Legendre M., Copin B., Duquesnoy P., Montantin G., Kott E., Dastot F., Jeanson L., Cachanado M., Rousseau A., et al. (2012). Delineation of CCDC39/CCDC40 mutation spectrum and associated phenotypes in primary ciliary dyskinesia. J Med Genet 49, 410–416. 10.1136/jmedgenet-2012-100867. [DOI] [PubMed] [Google Scholar]
- 44.Yamamoto R., Song K., Yanagisawa H.A., Fox L., Yagi T., Wirschell M., Hirono M., Kamiya R., Nicastro D., and Sale W.S. (2013). The MIA complex is a conserved and novel dynein regulator essential for normal ciliary motility. J Cell Biol 201, 263–278. 10.1083/jcb.201211048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fu G., Augspurger K., Sakizadeh J., Reck J., Bower R., Tritschler D., Gui L., Nicastro D., and Porter M.E. (2023). The ciliary MBO2 complex targets assembly of inner arm dynein b and reveals additional doublet microtubule asymmetries. bioRxiv. 10.1101/2023.07.31.551375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin J., Le T.V., Augspurger K., Tritschler D., Bower R., Fu G., Perrone C., O'Toole E.T., Mills K.V., Dymek E., et al. (2019). FAP57/WDR65 targets assembly of a subset of inner arm dyneins and connects to regulatory hubs in cilia. Mol Biol Cell 30, 2659–2680. 10.1091/mbc.E19-07-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bustamante-Marin X.M., Horani A., Stoyanova M., Charng W.L., Bottier M., Sears P.R., Yin W.N., Daniels L.A., Bowen H., Conrad D.F., et al. (2020). Mutation of CFAP57, a protein required for the asymmetric targeting of a subset of inner dynein arms in Chlamydomonas, causes primary ciliary dyskinesia. PLoS Genet 16, e1008691. 10.1371/journal.pgen.1008691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dacheux D., Martinez G., Broster Reix C.E., Beurois J., Lores P., Tounkara M., Dupuy J.W., Robinson D.R., Loeuillet C., Lambert E., et al. (2023). Novel axonemal protein ZMYND12 interacts with TTC29 and DNAH1, and is required for male fertility and flagellum function. Elife 12. 10.7554/eLife.87698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gui L., Song K., Tritschler D., Bower R., Yan S., Dai A., Augspurger K., Sakizadeh J., Grzemska M., Ni T., et al. (2019). Scaffold subunits support associated subunit assembly in the Chlamydomonas ciliary nexin-dynein regulatory complex. Proc Natl Acad Sci U S A 116, 23152–23162. 10.1073/pnas.1910960116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dymek E.E., Heuser T., Nicastro D., and Smith E.F. (2011). The CSC is required for complete radial spoke assembly and wild-type ciliary motility. Mol Biol Cell 22, 2520–2531. 10.1091/mbc.E11-03-0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heuser T., Dymek E.E., Lin J., Smith E.F., and Nicastro D. (2012). The CSC connects three major axonemal complexes involved in dynein regulation. Mol Biol Cell 23, 3143–3155. 10.1091/mbc.E12-05-0357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bicka M., Joachimiak E., Urbanska P., Osinka A., Konopka A., Bulska E., and Wloga D. (2022). Cfap91-Dependent Stability of the RS2 and RS3 Base Proteins and Adjacent Inner Dynein Arms in Tetrahymena Cilia. Cells 11. 10.3390/cells11244048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bazan R., Schrofel A., Joachimiak E., Poprzeczko M., Pigino G., and Wloga D. (2021). Ccdc113/Ccdc96 complex, a novel regulator of ciliary beating that connects radial spoke 3 to dynein g and the nexin link. PLoS Genet 17, e1009388. 10.1371/journal.pgen.1009388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urbanska P., Joachimiak E., Bazan R., Fu G., Poprzeczko M., Fabczak H., Nicastro D., and Wloga D. (2018). Ciliary proteins Fap43 and Fap44 interact with each other and are essential for proper cilia and flagella beating. Cell Mol Life Sci 75, 4479–4493. 10.1007/s00018-018-2819-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang X., Xiao Z., Zhang J., Xu C., Liu S., Cheng L., Zhou S., Zhao S., Zhang Y., Wu J., et al. (2022). Differential requirements of IQUB for the assembly of radial spoke 1 and the motility of mouse cilia and flagella. Cell Rep 41, 111683. 10.1016/j.celrep.2022.111683. [DOI] [PubMed] [Google Scholar]
- 56.Brokaw C.J., and Luck D.J. (1985). Bending patterns of chlamydomonas flagella: III. A radial spoke head deficient mutant and a central pair deficient mutant. Cell Motil 5, 195–208. 10.1002/cm.970050303. [DOI] [PubMed] [Google Scholar]
- 57.Chen Z., Greenan G.A., Shiozaki M., Liu Y., Skinner W.M., Zhao X., Zhao S., Yan R., Yu Z., Lishko P.V., et al. (2023). In situ cryo-electron tomography reveals the asymmetric architecture of mammalian sperm axonemes. Nat Struct Mol Biol 30, 360–369. 10.1038/s41594-022-00861-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Y., Pinskey J., Lin J., Yin W., Sears P.R., Daniels L.A., Zariwala M.A., Knowles M.R., Ostrowski L.E., and Nicastro D. (2021). Structural insights into the cause of human RSPH4A primary ciliary dyskinesia. Mol Biol Cell 32, 1202–1209. 10.1091/mbc.E20-12-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fabritius A.S., Bayless B.A., Li S., Stoddard D., Heydeck W., Ebmeier C.C., Anderson L., Gunnels T., Nachiappan C., Whittall J.B., et al. (2021). Proteomic analysis of microtubule inner proteins (MIPs) in Rib72 null Tetrahymena cells reveals functional MIPs. Mol Biol Cell 32, br8. 10.1091/mbc.E20-12-0786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yanagisawa H.A., and Kamiya R. (2004). A tektin homologue is decreased in chlamydomonas mutants lacking an axonemal inner-arm dynein. Mol Biol Cell 15, 2105–2115. 10.1091/mbc.e03-11-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang P., Diener D.R., Yang C., Kohno T., Pazour G.J., Dienes J.M., Agrin N.S., King S.M., Sale W.S., Kamiya R., et al. (2006). Radial spoke proteins of Chlamydomonas flagella. J Cell Sci 119, 1165–1174. 10.1242/jcs.02811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramachandran S., Osterhaus S.R., Parekh K.R., Jacobi A.M., Behlke M.A., and McCray P.B. Jr. (2016). SYVN1, NEDD8, and FBXO2 Proteins Regulate ΔF508 Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Ubiquitin-mediated Proteasomal Degradation. J Biol Chem 291, 25489–25504. 10.1074/jbc.M116.754283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Powers E.T., Morimoto R.I., Dillin A., Kelly J.W., and Balch W.E. (2009). Biological and chemical approaches to diseases of proteostasis deficiency. Annu Rev Biochem 78, 959–991. 10.1146/annurev.biochem.052308.114844. [DOI] [PubMed] [Google Scholar]
- 64.Meek R.L., and Benditt E.P. (1986). Amyloid A gene family expression in different mouse tissues. J Exp Med 164, 2006–2017. 10.1084/jem.164.6.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee J.Y., Hall J.A., Kroehling L., Wu L., Najar T., Nguyen H.H., Lin W.Y., Yeung S.T., Silva H.M., Li D., et al. (2020). Serum Amyloid A Proteins Induce Pathogenic Th17 Cells and Promote Inflammatory Disease. Cell 183, 2036–2039. 10.1016/j.cell.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lafkas D., Shelton A., Chiu C., de Leon Boenig G., Chen Y., Stawicki S.S., Siltanen C., Reichelt M., Zhou M., Wu X., et al. (2015). Therapeutic antibodies reveal Notch control of transdifferentiation in the adult lung. Nature 528, 127–131. 10.1038/nature15715. [DOI] [PubMed] [Google Scholar]
- 67.Reynolds S.D., Hill C.L., Alsudayri A., Lallier S.W., Wijeratne S., Tan Z.H., Chiang T., and Cormet-Boyaka E. (2022). Assemblies of JAG1 and JAG2 determine tracheobronchial cell fate in mucosecretory lung disease. JCI Insight 7. 10.1172/jci.insight.157380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bingle C.D., Wilson K., Lunn H., Barnes F.A., High A.S., Wallace W.A., Rassl D., Campos M.A., Ribeiro M., and Bingle L. (2010). Human LPLUNC1 is a secreted product of goblet cells and minor glands of the respiratory and upper aerodigestive tracts. Histochem Cell Biol 133, 505–515. 10.1007/s00418-010-0683-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schneider J.L., Rowe J.H., Garcia-de-Alba C., Kim C.F., Sharpe A.H., and Haigis M.C. (2021). The aging lung: Physiology, disease, and immunity. Cell 184, 1990–2019. 10.1016/j.cell.2021.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sbodio J.I., Snyder S.H., and Paul B.D. (2019). Redox Mechanisms in Neurodegeneration: From Disease Outcomes to Therapeutic Opportunities. Antioxid Redox Signal 30, 1450–1499. 10.1089/ars.2017.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Casalino-Matsuda S.M., Monzón M.E., and Forteza R.M. (2006). Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium. Am J Respir Cell Mol Biol 34, 581–591. 10.1165/rcmb.2005-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nishida Y., Yagi H., Ota M., Tanaka A., Sato K., Inoue T., Yamada S., Arakawa N., Ishige T., Kobayashi Y., et al. (2023). Oxidative stress induces MUC5AC expression through mitochondrial damage-dependent STING signaling in human bronchial epithelial cells. FASEB Bioadv 5, 171–181. 10.1096/fba.2022-00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tsao P.N., Vasconcelos M., Izvolsky K.I., Qian J., Lu J., and Cardoso W.V. (2009). Notch signaling controls the balance of ciliated and secretory cell fates in developing airways. Development 136, 2297–2307. 10.1242/dev.034884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hill D.B., Button B., Rubinstein M., and Boucher R.C. (2022). Physiology and pathophysiology of human airway mucus. Physiol Rev 102, 1757–1836. 10.1152/physrev.00004.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tyner J.W., Kim E.Y., Ide K., Pelletier M.R., Roswit W.T., Morton J.D., Battaile J.T., Patel A.C., Patterson G.A., Castro M., et al. (2006). Blocking airway mucous cell metaplasia by inhibiting EGFR antiapoptosis and IL-13 transdifferentiation signals. J Clin Invest 116, 309–321. 10.1172/jci25167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rao W., Wang S., Duleba M., Niroula S., Goller K., Xie J., Mahalingam R., Neupane R., Liew A.A., Vincent M., et al. (2020). Regenerative Metaplastic Clones in COPD Lung Drive Inflammation and Fibrosis. Cell 181, 848–864.e818. 10.1016/j.cell.2020.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gohy S., Carlier F.M., Fregimilicka C., Detry B., Lecocq M., Ladjemi M.Z., Verleden S., Hoton D., Weynand B., Bouzin C., and Pilette C. (2019). Altered generation of ciliated cells in chronic obstructive pulmonary disease. Sci Rep 9, 17963. 10.1038/s41598-019-54292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hawkins F.J., Suzuki S., Beermann M.L., Barillà C., Wang R., Villacorta-Martin C., Berical A., Jean J.C., Le Suer J., Matte T., et al. (2021). Derivation of Airway Basal Stem Cells from Human Pluripotent Stem Cells. Cell Stem Cell 28, 79–95.e78. 10.1016/j.stem.2020.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vladar E.K., Kunimoto K., Rojas-Hernandez L.S., Spano J.M., Sellers Z.M., Joo N.S., Cooney R.A., Axelrod J.D., and Milla C.E. (2023). Notch signaling inactivation by small molecule γ-secretase inhibitors restores the multiciliated cell population in the airway epithelium. Am J Physiol Lung Cell Mol Physiol 324, L771–l782. 10.1152/ajplung.00382.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Button B., Cai L.H., Ehre C., Kesimer M., Hill D.B., Sheehan J.K., Boucher R.C., and Rubinstein M. (2012). A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337, 937–941. 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Qin H., Diener D.R., Geimer S., Cole D.G., and Rosenbaum J.L. (2004). Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body. J Cell Biol 164, 255–266. 10.1083/jcb.200308132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Viswanadha R., Hunter E.L., Yamamoto R., Wirschell M., Alford L.M., Dutcher S.K., and Sale W.S. (2014). The ciliary inner dynein arm, I1 dynein, is assembled in the cytoplasm and transported by IFT before axonemal docking. Cytoskeleton (Hoboken) 71, 573–586. 10.1002/cm.21192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamamoto R., Obbineni J.M., Alford L.M., Ide T., Owa M., Hwang J., Kon T., Inaba K., James N., King S.M., et al. (2017). Chlamydomonas DYX1C1/PF23 is essential for axonemal assembly and proper morphology of inner dynein arms. PLoS Genet 13, e1006996. 10.1371/journal.pgen.1006996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wirschell M., Olbrich H., Werner C., Tritschler D., Bower R., Sale W.S., Loges N.T., Pennekamp P., Lindberg S., Stenram U., et al. (2013). The nexin-dynein regulatory complex subunit DRC1 is essential for motile cilia function in algae and humans. Nat Genet 45, 262–268. 10.1038/ng.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Horani A., Brody S.L., Ferkol T.W., Shoseyov D., Wasserman M.G., Ta-shma A., Wilson K.S., Bayly P.V., Amirav I., Cohen-Cymberknoh M., et al. (2013). CCDC65 mutation causes primary ciliary dyskinesia with normal ultrastructure and hyperkinetic cilia. PLoS One 8, e72299. 10.1371/journal.pone.0072299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Olbrich H., Cremers C., Loges N.T., Werner C., Nielsen K.G., Marthin J.K., Philipsen M., Wallmeier J., Pennekamp P., Menchen T., et al. (2015). Loss-of-Function GAS8 Mutations Cause Primary Ciliary Dyskinesia and Disrupt the Nexin-Dynein Regulatory Complex. Am J Hum Genet 97, 546–554. 10.1016/j.ajhg.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Raman M., Sergeev M., Garnaas M., Lydeard J.R., Huttlin E.L., Goessling W., Shah J.V., and Harper J.W. (2015). Systematic proteomics of the VCP-UBXD adaptor network identifies a role for UBXN10 in regulating ciliogenesis. Nat Cell Biol 17, 1356–1369. 10.1038/ncb3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Alford L.M., Stoddard D., Li J.H., Hunter E.L., Tritschler D., Bower R., Nicastro D., Porter M.E., and Sale W.S. (2016). The nexin link and B-tubule glutamylation maintain the alignment of outer doublets in the ciliary axoneme. Cytoskeleton (Hoboken) 73, 331–340. 10.1002/cm.21301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Byers D.E., Alexander-Brett J., Patel A.C., Agapov E., Dang-Vu G., Jin X., Wu K., You Y., Alevy Y., Girard J.P., et al. (2013). Long-term IL-33-producing epithelial progenitor cells in chronic obstructive lung disease. J Clin Invest 123, 3967–3982. 10.1172/jci65570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Labbadia J., and Morimoto R.I. (2015). The biology of proteostasis in aging and disease. Annu Rev Biochem 84, 435–464. 10.1146/annurev-biochem-060614-033955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ciechanover A., and Brundin P. (2003). The ubiquitin proteasome system in neurodegenerative diseases: sometimes the chicken, sometimes the egg. Neuron 40, 427–446. 10.1016/s0896-6273(03)00606-8. [DOI] [PubMed] [Google Scholar]
- 92.Horani A., Ustione A., Huang T., Firth A.L., Pan J., Gunsten S.P., Haspel J.A., Piston D.W., and Brody S.L. (2018). Establishment of the early cilia preassembly protein complex during motile ciliogenesis. Proc Natl Acad Sci U S A 115, E1221–e1228. 10.1073/pnas.1715915115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Shapiro A.J., Davis S.D., Polineni D., Manion M., Rosenfeld M., Dell S.D., Chilvers M.A., Ferkol T.W., Zariwala M.A., Sagel S.D., et al. (2018). Diagnosis of Primary Ciliary Dyskinesia. An Official American Thoracic Society Clinical Practice Guideline. Am J Respir Crit Care Med 197, e24–e39. 10.1164/rccm.201805-0819ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.You Y., and Brody S.L. (2013). Culture and differentiation of mouse tracheal epithelial cells. Methods Mol Biol 945, 123–143. 10.1007/978-1-62703-125-7_9. [DOI] [PubMed] [Google Scholar]
- 95.Horani A., Nath A., Wasserman M.G., Huang T., and Brody S.L. (2013). Rho-associated protein kinase inhibition enhances airway epithelial Basal-cell proliferation and lentivirus transduction. Am J Respir Cell Mol Biol 49, 341–347. 10.1165/rcmb.2013-0046TE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mou H., Vinarsky V., Tata P.R., Brazauskas K., Choi S.H., Crooke A.K., Zhang B., Solomon G.M., Turner B., Bihler H., et al. (2016). Dual SMAD Signaling Inhibition Enables Long-Term Expansion of Diverse Epithelial Basal Cells. Cell Stem Cell 19, 217–231. 10.1016/j.stem.2016.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lin H., Guo S., and Dutcher S.K. (2018). RPGRIP1L helps to establish the ciliary gate for entry of proteins. J Cell Sci 131. 10.1242/jcs.220905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Awata J., Takada S., Standley C., Lechtreck K.F., Bellve K.D., Pazour G.J., Fogarty K.E., and Witman G.B. (2014). NPHP4 controls ciliary trafficking of membrane proteins and large soluble proteins at the transition zone. J Cell Sci 127, 4714–4727. 10.1242/jcs.155275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li X., Zhang R., Patena W., Gang S.S., Blum S.R., Ivanova N., Yue R., Robertson J.M., Lefebvre P.A., Fitz-Gibbon S.T., et al. (2016). An Indexed, Mapped Mutant Library Enables Reverse Genetics Studies of Biological Processes in Chlamydomonas reinhardtii. Plant Cell 28, 367–387. 10.1105/tpc.15.00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dutcher S.K. (2023). Practical aspects of mating and tetrad analysis. In The Chlamydomonas Sourcebook, (Elsevier; ), pp. 315–323. [Google Scholar]
- 101.Patel D.A., Patel A.C., Nolan W.C., Zhang Y., and Holtzman M.J. (2012). High throughput screening for small molecule enhancers of the interferon signaling pathway to drive next-generation antiviral drug discovery. PLoS One 7, e36594. 10.1371/journal.pone.0036594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pan J., You Y., Huang T., and Brody S.L. (2007). RhoA-mediated apical actin enrichment is required for ciliogenesis and promoted by Foxj1. J Cell Sci 120, 1868–1876. 10.1242/jcs.005306. [DOI] [PubMed] [Google Scholar]
- 103.You Y., Richer E.J., Huang T., and Brody S.L. (2002). Growth and differentiation of mouse tracheal epithelial cells: selection of a proliferative population. Am J Physiol Lung Cell Mol Physiol 283, L1315–1321. 10.1152/ajplung.00169.2002. [DOI] [PubMed] [Google Scholar]
- 104.Horani A., Gupta D.K., Xu J., Xu H., Carmen Puga-Molina L.D., Santi C.M., Ramagiri S., Brennan S.K., Pan J., Koenitzer J.R., et al. (2023). The effect of Dnaaf5 gene dosage on primary ciliary dyskinesia phenotypes. JCI Insight 8. 10.1172/jci.insight.168836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang C., Compton M.M., and Yang P. (2005). Dimeric novel HSP40 is incorporated into the radial spoke complex during the assembly process in flagella. Mol Biol Cell 16, 637–648. 10.1091/mbc.e04-09-0787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Feng Y., Nie L., Thakur M.D., Su Q., Chi Z., Zhao Y., and Longmore G.D. (2010). A multifunctional lentiviral-based gene knockdown with concurrent rescue that controls for off-target effects of RNAi. Genomics Proteomics Bioinformatics 8, 238–245. 10.1016/S1672-0229(10)60025-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang Y., Huang G., Shornick L.P., Roswit W.T., Shipley J.M., Brody S.L., and Holtzman M.J. (2007). A transgenic FOXJ1-Cre system for gene inactivation in ciliated epithelial cells. Am J Respir Cell Mol Biol 36, 515–519. 10.1165/rcmb.2006-0475RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Preble A.M., Giddings T.H. Jr., and Dutcher S.K. (2001). Extragenic bypass suppressors of mutations in the essential gene BLD2 promote assembly of basal bodies with abnormal microtubules in Chlamydomonas reinhardtii. Genetics 157, 163–181. 10.1093/genetics/157.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.O'Toole E.T., Giddings T.H., McIntosh J.R., and Dutcher S.K. (2003). Three-dimensional organization of basal bodies from wild-type and delta-tubulin deletion strains of Chlamydomonas reinhardtii. Mol Biol Cell 14, 2999–3012. 10.1091/mbc.e02-11-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dutcher S.K., Li L., Lin H., Meyer L., Giddings T.H. Jr., Kwan A.L., and Lewis B.L. (2012). Whole-Genome Sequencing to Identify Mutants and Polymorphisms in Chlamydomonas reinhardtii. G3 (Bethesda) 2, 15–22. 10.1534/g3.111.000919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schorb M., Haberbosch I., Hagen W.J.H., Schwab Y., and Mastronarde D.N. (2019). Software tools for automated transmission electron microscopy. Nat Methods 16, 471–477. 10.1038/s41592-019-0396-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Punjani A., Rubinstein J.L., Fleet D.J., and Brubaker M.A. (2017). cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat Methods 14, 290–296. 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 113.Grigorieff N. (2007). FREALIGN: high-resolution refinement of single particle structures. J Struct Biol 157, 117–125. 10.1016/j.jsb.2006.05.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.