Abstract
Background.
Exchange transfusion (ET) has biologic plausibility as an adjunct to antimalarial drugs in treating severe malaria and has been used for decades despite limited evidence of its efficacy in improving survival. We examined the efficacy of ET as an adjunct treatment for severe malaria using US surveillance data and reviewed the literature to update recommendations.
Methods.
Patients with severe malaria reported to the US national malaria surveillance system during 1985–2010 were matched, and survival outcomes were compared between patients receiving and not receiving ET. The literature review used search terms “severe malaria” and “exchange transfusion.” Case reports and series, observational and case-control studies, and meta-analysis were included.
Results.
One hundred one patients receiving ET were matched to 314 patients not receiving ET. There was no statistically significant association between ET and survival outcome (odds ratio, 0.84; 95% confidence interval, .44–1.60). We found 87 articles, mostly case reports or series, showing successful use of ET, likely reporting bias. There were 12 comparative studies, most of which were retrospective cohort studies, underpowered with no significant differences in survival. A previously published meta-analysis of 8 comparative studies found no significant survival differences. Adverse events were rarely reported but included acute respiratory distress syndrome, ventricular fibrillation, and hypotension.
Conclusions.
Despite rapid parasite clearance times resulting from ET, there is no evidence for efficacy of ET as adjunctive therapy in severe malaria. Adjunct ET cannot be recommended. When rapidly acting antimalarials, specifically artemisinins, become more widely available, the biologic plausibility argument for ET will become less relevant.
Keywords: severe malaria, exchange transfusion, malaria treatment
Exchange transfusion (ET) has been used as an adjunct to antimalarial drugs for the treatment of severe malaria for decades since its first reported use in 1974 [1]. There is biologic plausibility to ET’s usefulness. In severe malaria, red blood cells infected with more mature stages of Plasmodium falciparum have decreased deformability and increased adhesiveness, causing sequestration of these cells in the microvasculature, local hypoxia, ischemia [2], and local release of proinflammatory cytokines [3, 4]. ET removes circulating infected red blood cells and toxic byproducts, and so should theoretically improve survival [5]. In fact, a goal of treating severe malaria is to clear parasitemia quickly [2], and ET has been observed to decrease parasitemia more rapidly than antimalarials alone. Mean parasite clearance times (PCTs) for oral quinine, intravenous quinine or quinidine, and intravenous artesunate average 75, 49, and 20 hours, respectively [6–8]. Adjunct ET given with intravenous quinidine halves the volume of parasites within 2–6 hours, and has a PCT of 24–46 hours. [9, 10]. In one study among patients receiving quinidine, the time it took for parasitemia to drop to <1% was the same in the ET and non-ET groups despite the ET group having a much higher baseline parasitemia, suggesting that ET dropped parasitemia more rapidly than quinidine alone [9]. However, the translation of rapidly dropping parasitemia with adjunct ET into improved survival has yet to be shown [11]. The widespread use of ET in many countries such as the United States as an adjunct to antimalarials for treatment of severe malaria continues despite an ongoing debate about its efficacy.
In the United States, the Centers for Disease Control and Prevention (CDC) has recommended that ET could be considered as an adjunct to intravenous quinidine (with doxycycline, tetracycline, or clindamycin) in patients with parasitemias >10% or if complications such as cerebral malaria, acute respiratory distress syndrome (ARDS), or renal compromise are present [9, 12]. Severe malaria is not commonly managed in the United States; of the 1691 cases of malaria reported in 2010 to the US National Malaria Surveillance System (NMSS), only 10% were classified as severe [13]. Cases of severe malaria reported to the NMSS from 1985 to 2010 were examined and a comprehensive literature review was done to evaluate the efficacy of ET as an adjunct to antimalarials for the treatment of severe malaria, and to update recommendations for its use.
METHODS
Patients with severe malaria and known survival outcome reported to the NMSS during 1985–2010 were identified. The NMSS is the oldest surveillance system at the CDC with well-established procedures for reporting cases. Reporting of confirmed cases of malaria to state health departments and to the CDC is required in the United States. Reporting to the CDC can also occur directly through a direct consultation with CDC malaria staff.
Details of the NMSS have been described elsewhere [13]. Severe malaria was defined as malaria infection plus at least 1 of the following; cerebral malaria, renal failure, ARDS, severe anemia (hemoglobin level <7 g/dL), jaundice, parasitemia level >5%, acidosis, hypotension, or disseminated intravascular congestion [12]. The criterion “receipt of an antimalarial regimen” was defined as administration of any antimalarial regimen recommended by the CDC for the treatment of malaria [14] at any time during the hospitalization. Regimens specific for severe malaria were not targeted because before 2007 the NMSS did not distinguish between quinine and quinidine use, whereas after 2007, quinine and quinidine are listed distinctly. In the United States, intravenous quinine is not available, and therefore intravenous quinidine is the recommended antimalarial for severe malaria whereas oral quinine is recommended only for uncomplicated malaria. Parenteral artesunate is currently available only through the CDC under an investigational new drug (IND) protocol.
To evaluate survival outcomes, patients who received ET were matched by propensity score, without replacement, to at most 4 controls who had not received ET. Propensity scores were obtained for each patient via a multivariable logistic regression model with variables included noted in Table 1. Of note, factors that could influence survival outcome were chosen for this model and include age, completion of appropriate prophylaxis, antimalarial regimen received, ARDS, cerebral malaria, renal failure, P. falciparum species, type of hospital, possible immune status to malaria, and parasite density. “Possibly semi-immune” individuals were defined as those who at the time lived in a malaria-endemic area and were visiting the United States, or US residents who traveled to visit friends and relatives (for US residents, country of origin and date of immigration were not available). A maximum difference in propensity scores for each match was set at 0.1. Quality of the matches was evaluated by comparing proportions of the aforementioned variables between the matched and unmatched groups. Additional factors known to be associated with poor prognosis were also examined. Finally, the survival outcome of the matched patients was examined using multivariate logistic regression with additional predictors chosen by finding the model with the lowest Bayesian information criterion value. All analyses were performed using SAS software, version 9.3 (SAS Institute, Inc, Cary, North Carolina) and used the 5% level of significance. Retrospective power analyses were also performed in PASS [15] to determine the sample sizes or rates needed to find a significant difference.
Table 1.
Demographic and Clinical Characteristics of Unmatched and Matched Cohorts
| Summary Statistics | Mortality Rates | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||
| Unmatched Cohort (No.a) | Matched Cohort (No.a) | Unmatched Cohort (No.a) | Matched Cohort (No.a) | |||||||
|
|
|
|
|
|||||||
| Characteristic | ET | Non-ET | P Value | ET | Non-ET | P Value | ET | Non-ET | ET | Non-ET |
|
| ||||||||||
| Age, yb,c (mean) | 38.0 (105) | 37.9 (704) | .97 | 37.0 (101) | 37.3 (314) | .91 | ||||
| Female sexb | 54.8 (104) | 66.8 (683) | .02 | 54.5 (101) | 59.6 (314) | .37 | 12.3 (7) | 11.2 (51) | 12.7 (7) | 17.1 (32) |
| Pregnant | 2.9 (35) | 9.5 (168) | .20 | 2.9 (34) | 6.0 (83) | .49 | 0.0 (0) | 0.0 (0) | 0.0 (0) | 0.0 (0) |
| Prophylaxis taken | 17.9 (106) | 14.1 (711) | .29 | 17.8 (101) | 17.2 (314) | .89 | 10.5 (2) | 14.0 (14) | 11.1 (2) | 25.9 (14) |
| Antimalarial regimen receivedb,d | 22.9 (105) | 43.3 (711) | <.0001 | 22.8 (101) | 23.9 (314) | .82 | 20.8 (5) | 6.8 (21) | 21.7 (5) | 12.0 (9) |
| Acute respiratory distress syndromeb | 22.6 (106) | 22.4 (711) | .95 | 23.8 (101) | 22.0 (314) | .71 | 16.7 (4) | 13.2 (21) | 16.7 (4) | 15.9 (11) |
| Cerebral malariab,c | 69.8 (106) | 37.3 (711) | <.0001 | 69.3 (101) | 63.7 (314) | .30 | 17.6 (13) | 17.7 (47) | 17.1 (12) | 19.5 (39) |
| Renal failureb,c | 43.4 (106) | 44.4 (711) | .84 | 41.6 (101) | 43.0 (314) | .80 | 30.4 (14) | 14.2 (45) | 31.0 (13) | 25.2 (34) |
| Plasmodium falciparum speciesb | 98.1 (104) | 84.8 (652) | .0002 | 98.0 (101) | 97.8 (314) | .88 | 17.6 (18) | 11.8 (65) | 17.2 (17) | 16.3 (50) |
| Hospital typeb | ||||||||||
| Teaching | 43.4 (106) | 30.0 (711) | .006 | 45.5 (101) | 35.0 (314) | .06 | 8.7 (4) | 5.6 (12) | 8.7 (4) | 6.4 (7) |
| >200-bed community | 21.7 (106) | 26.2 (711) | .33 | 19.8 (101) | 21.3 (314) | .74 | 21.7 (5) | 10.8 (20) | 25.0 (5) | 19.4 (13) |
| 100–200-bed community | 5.7 (106) | 8.3 (711) | .35 | 5.9 (101) | 4.1 (314) | .45 | 33.3 (2) | 3.4 (2) | 33.3 (2) | 20.0 (1) |
| <100-bed community | 1.9 (106) | 3.1 (711) | .49 | 2.0 (101) | 2.5 (314) | .75 | 0.0 (0) | 4.5 (1) | 0.0 (0) | 12.5 (1) |
| Unknown | 27.4 (106) | 32.5 (711) | .29 | 26.7 (101) | 36.9 (314) | .06 | 27.6 (8) | 16.5 (38) | 25.9 (7) | 24.1 (28) |
| Possible immune statusb,c | ||||||||||
| Possibly semi-immune | 31.1 (106) | 41.8 (711) | .04 | 30.7 (101) | 28.3 (314) | .65 | 12.1 (4) | 5.7 (17) | 12.9 (4) | 6.7 (6) |
| Possibly not immune | 36.8 (106) | 28.6 (711) | .08 | 37.6 (101) | 36.6 (314) | .86 | 17.9 (7) | 9.9 (20) | 18.4 (7) | 11.3 (13) |
| Unknown | 32.1 (106) | 29.7 (711) | .62 | 31.7 (101) | 35.0 (314) | .54 | 23.5 (8) | 17.1 (36) | 21.9 (7) | 28.2 (31) |
| Parasite densityb | ||||||||||
| <10% | 1.9 (106) | 8.0 (711) | .02 | 2.0 (101) | 2.9 (314) | .63 | 0.0 (0) | 7.0 (4) | 0.0 (0) | 22.2 (2) |
| ≥10% | 6.6 (106) | 4.8 (711) | .42 | 6.9 (101) | 4.8 (314) | .40 | 14.3 (1) | 8.8 (3) | 14.3 (1) | 20.0 (3) |
| Unknown | 91.5 (106) | 87.2 (711) | .21 | 91.1 (101) | 92.4 (314) | .68 | 18.6 (18) | 10.6 (66) | 18.5 (17) | 15.5 (45) |
| Propensity score (mean) | 0.25 (101) | 0.12 (711) | 0.25 (101) | 0.20 (314) | ||||||
Data are presented as % (No.) unless otherwise indicated.
Abbreviation: ET, exchange transfusion.
Total No. differs for some characteristics because of missing data.
Characteristics used in propensityscore model.
Included as covariate in final outcome model.
Documented to have received a drug regimen recommended by the Centers for Disease Control and Prevention for malaria.
The literature was reviewed for evidence of ET efficacy and for adverse events related to ET by searching the PubMed database using the keywords “exchange transfusion” and “malaria.” All articles with content relevant to ET as an adjunct treatment for malaria, with patient survival outcome, were included. Articles without data, such as malaria treatment guidelines and commentaries, were excluded. The included articles were classified according to type of study: case reports and series, comparative studies, and meta-analyses. Articles not in English were reviewed by persons who spoke that language, with the exception of Finnish, Italian, Norwegian, Swedish, Dutch, and Turkish, which were translated via an online translating service.
RESULTS
We identified 1317 patients with severe malaria, 817 of whom had known survival outcome. Of these 817 patients, 106 received ET. A total of 101 ET patients were matched to 314 non-ET patients. Five patients with severe malaria who received ET and later survived were omitted due to missing data on matching variables. Characteristics of the ET vs non-ET patients are presented in Table 1, showing pre- and postmatching proportions and means and appropriate tests for association (Pearson χ2, Fisher exact, or t tests). Overall, matching successfully reduced any significant differences in characteristics between ET and non-ET groups. The differences in proportions increased for a minority of characteristics, but these differences were small and were not significant. The proportions of other factors that might have influenced survival such as presence of ARDS, cerebral malaria, renal failure, parasitemia, and malaria immune status were similar in both groups. Mortality rates for subgroups of patients within ET and non-ET groups are presented in Table 1. The overall mortality rates of the ET and non-ET groups were 17.8% and 15.9%, respectively. There was no statistically significant association between ET and survival outcome (odds ratio [OR], 0.84; 95% confidence interval [CI], .44–1.60), after adjusting for the associations between survival and age, cerebral malaria, and renal failure. Given the observed rates and a 1:4 case-to-control ratio, 5071 ET patients and 20 284 non-ET patients would have been needed to achieve a 90% power with a 2-sided test. In other words, with an observed mortality rate of 15.9% in the non-ET group, the ET group would have needed a mortality rate of 4.6% (to show improved survival) or 31.3% (to show worsened survival) to obtain 90% power to find a significant difference from the non-ET group.
Literature Review
A total of 193 articles were found (Figure 1). Of these, 171 had content related to ET as adjunct therapy for treatment of severe malaria. Eighty-five of these articles were excluded because they were commentaries, guidelines, or general reviews of severe malaria with no data. Therefore, a total of 86 articles were included: 62 in English, 9 in German, 7 in French, 5 in Spanish, 1 in Danish, 1 in Italian, and 1 in Norwegian.
Figure 1.
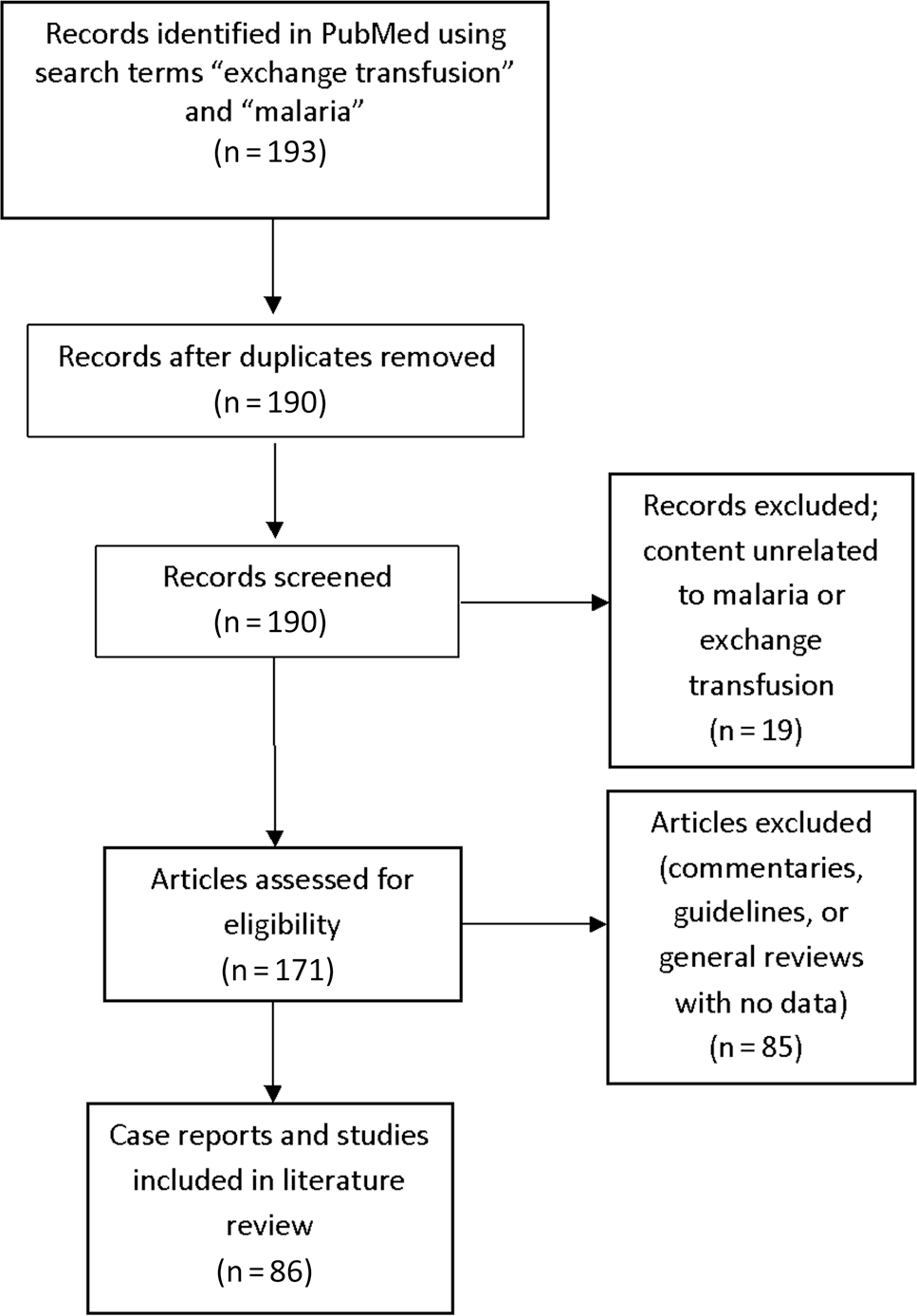
Flow diagram of articles selected for the literature review.
We found 73 case reports and case series summarizing the clinical course of 157 patients (Supplementary Data). Almost all described successful use of ET for the management of severe malaria; there were only 9 deaths reported. It is possible that successful use of ET is more likely to be reported, which would bias the information available in case reports, and better evidence would be provided by comparative studies.
Twelve comparative studies were found. To consolidate the evidence from 8 of these nonrandomized comparative studies that took place in various countries (n = 279), Riddle et al performed a thorough meta-analysis [11]. Their summary OR of 1.2 (95% CI, .7–2.1) suggested that ET had no effect on survival. A secondary analysis using patient-specific data from 4 of the studies (n = 73 patients, 28 received ET), although underpowered, found that ET was not associated with increased survival (OR, 0.3; 95% CI, .1–2.4) even after adjusting for malaria-immunity status, initial parasitemia, and number of World Health Organization (WHO) criteria met for severe malaria.
The 4 remaining comparative studies not included in the meta-analysis are 1 randomized controlled trial, and 3 others published after the Riddle meta-analysis article [11]. The one randomized controlled trial, described briefly in a letter and not peer reviewed [16], found no difference in survival. However, the sample size was small (N = 8), and some patients received chloroquine in an area with known chloroquine resistance. The other 3 studies were retrospective cohort studies. The first study examined patients receiving intravenous quinine for severe malaria and compared 25 patients receiving adjunct ET to 31 patients not receiving ET [10], with mean initial parasitemias of 10% and 6%, respectively. Despite observing that the time to reduce parasitemia by 90% was significantly less among those with ET vs those without (24.2 hours vs 38.7 hours, respectively), there were no differences in survival. The second study found 1 death in 5 patients receiving adjunct ET, and no deaths among 8 patients on intravenous quinine and clindamycin alone [17]. The third study showed no difference in survival between patients receiving adjunct ET and those receiving quinine or artesunate alone [18]. All of these studies were underpowered to detect statistically significant differences in survival.
Risks attributed to the ET procedure include fluid overload, complications from blood transfusion, infection at the transfusion site, cerebral hemorrhage, and electrolyte abnormalities [19]. There is very limited reporting of adverse events of ET used for malaria. In the 86 articles included in this review, there were only 6 articles that mention adverse events possibly related to the ET procedure. The most commonly described event was ARDS within 1 day following ET. This was described in 5 patients, of whom 2 died [20–22]. In all of these cases, ARDS was not attributed directly to ET, as ARDS is a known complication of severe malaria. One article on severe malaria in children noted 2 cases of congestive heart failure–like symptoms directly following ET requiring medical intervention; both children survived [23]. Two other events have been described occurring during ET and were more strongly attributed to ET. In 2 patients, while receiving ET, hypotension developed in 1, and ventricular fibrillation in the other, resulting in death in both patients [24, 25].
Although reports of adverse events of ET for malaria are scarce, complications of ET or other apheresis procedures to manage other diseases have been well documented [19, 26–28]. One multicenter study of adverse events associated with therapeutic apheresis procedures found the highest adverse event rate for red blood cell exchange (10.28%) compared to other apheresis procedures; no deaths attributed to apheresis procedures were observed [28]. This study reported a cumulative list of adverse events for all apheresis procedures including citrate-related nausea or vomiting, drops in systolic blood pressure <80 mm Hg, and vasovagal nausea or vomiting; no adverse events specific to red blood cell exchange were mentioned. Almost 90% of the transfusion reactions observed in this study were associated with plasma exchange, and not red blood cell exchange. Complications associated with blood transfusion would be expected to apply to ET, but in practice seem to be rarely reported.
DISCUSSION
The efficacy of ET on improving survival for patients with severe malaria has not been supported by this study and is not well supported in the literature despite its biologic plausibility. There are potential serious, though rarely observed or reported, adverse events associated with ET. Complications of severe malaria such as ARDS and hypotension from sepsis are often difficult to separate from the possible adverse events of ET, and perhaps this is why there is little mention of adverse events in the literature. Therefore, taking into account the lack of data for ET efficacy and the possibility of adverse events associated with ET, antimalarials should remain the mainstay of treatment of severe malaria.
WHO recommends artesunate as the first-line treatment for severe malaria [29]. Artemisinin-based antimalarials have a PCT of <24 hours [6]. Unlike ET, which removes only circulating infected red blood cells, artemisinins are able to kill sequestered parasites and prevent trophozoites from maturing, allowing for splenic clearance and prevention of further sequestration. Artemisinins were also found to reduce mortality among inpatients with severe P. falciparum malaria by 34.7% compared to quinine [30]. There is limited evidence describing the use of ET combined with artemisinins. One recent retrospective observational study of patients with severe P. falciparum malaria found that the parasite elimination curves of either artemisinin or quinine did not change significantly when manual ET was added [18]. Another study described 3 patients who received parenteral artesunate alone (n = 1) or with ET (n = 2) [31]. A 50% reduction of parasitemia was achieved in 16.7 hours among the patient receiving artesunate alone, and 13.2 hours and 10.7 hours among the 2 patients receiving both ET and artesunate. All patients had parasitemias <1% within 16–24 hours and all survived. The reduction in parasitemia between artesunate vs artesunate plus ET differed by at most 6 hours; in this small group, there was no survival difference. On a cautionary note, the development of artesunate resistance, observed as prolonged PCT in very limited locations [32], could portend a decrease in artesunate’s efficacy on improving survival. The issue of using artesunate plus ET in those with artemisinin-resistant strains of plasmodium may need examination in the future.
This comprehensive review of ET examines 26 years of US experience with adjunct ET in the context of the entirety of the published literature. Ideally a randomized case-control study would provide the evidence needed to determine ET’s efficacy for severe malaria; unfortunately, there are no such published studies. It is unlikely that such a study would become available as sample size requirements, estimated at about 400 patients [11], would be difficult to achieve in areas where ET might be available. The availability of comparative studies have also been limited as in the >10 years since the meta-analysis by Riddle et al, only 2 other studies have been published. Previous comparison studies in the literature have been limited by the disparity in the comparison groups; patients receiving ET are usually a sicker group than those not receiving ET. We attempted to address this limitation by matching to achieve a more equitable clinical status between the 2 groups.
However, there are still several limitations to our study, largely due to inherent limitations with the NMSS data. NMSS data is passively collected, so cases may be underreported. However, severe malaria cases are more likely than uncomplicated cases to be captured in the system because these cases result in hospitalization and CDC clinical consultation. NMSS also has built-in redundancies to increase case finding. Therefore, it is unlikely that there are a large number of unreported cases of severe malaria. Another limitation to the NMSS is incomplete data for some of the variables of interest. This affected which patients could be included in the analysis. Of patients with severe malaria, 35% were excluded because of missing outcome information. Also, 5 patients with severe malaria received ET and survived, but did not have enough clinical information to be included in the matching. Furthermore, although we intended to match on as many characteristics for severe malaria as were available, parasite density (exact parasite density per microliter or as a percentage of red blood cells), jaundice, acidosis, and hypotension were ultimately not used for matching because these data were rarely captured. One characteristic used for matching, receipt of an antimalarial regimen, depended on the availability of the treatment information. This information was available for only 22.8% and 23.9% of ET and non-ET groups, respectively. Patients who had treatment information available were matched to each other. Those without treatment information were similarly matched. There were also missing data for time between onset of symptoms and presentation for medical care, so we could not include delays in seeking care as a factor that might have influenced outcome. Finally, the NMSS does not routinely collect certain variables of interest to this study, such as a detailed clinical course and timing of when antimalarials were received. Because of this, we could not examine adverse events due to ET, factors that could influence survival such as critical care survival scores, outcomes such as fever duration and resolution of complications, and receipt of an appropriate antimalarial within 24 hours of presentation.
Although our study was underpowered to detect a difference in mortality <10%, and due to the limitations of the NMSS, this dataset represents >25 years of treating severe malaria in the United States and is the largest study so far of adjunct ET in a uniform population. Despite having good biological plausibility for its usefulness in the treatment of severe malaria, adjunct ET does not appear to improve survival and so cannot be recommended. Antimalarials continue to be the cornerstone for treatment of severe malaria. As rapidly acting antimalarials, specifically artemisinins that can reduce parasitemia as fast as ET, become more widely available, the biologic plausibility argument for ET will become less relevant.
Supplementary Material
Acknowledgments.
Parenteral artesunate is available through the Centers for Disease Control and Prevention (CDC) under an investigational new drug (IND) protocol. To enroll a patient with severe malaria in the IND protocol, contact the CDC Malaria Hotline: +1 (770) 488–7788 (Monday through Friday, 8 am–4:30 pm EST) or after office hours call the Emergency Operations Center +1 (770) 488–7100.
Footnotes
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
References
- 1.Gyr K, Speck B, Ritz R, Cornu P, Buckner CD. Cerebral tropical malaria with blackwater fever. A current diagnostic and therapeutic problem [in German]. Schweiz Med Wochenschr 1974; 104:1628–30. [PubMed] [Google Scholar]
- 2.World Health Organization, Communicable Diseases Cluster. Severe falciparum malaria. Trans R Soc Trop Med Hyg 2000; 94(suppl 1):S1–90. [PubMed] [Google Scholar]
- 3.Clark IA, Budd AC, Alleva LM, Cowden WB. Human malarial disease: a consequence of inflammatory cytokine release. Malar J 2006; 5:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chuncharunee S, Jootar JS, Leelasiri A, et al. Levels of serum tumor necrosis factor alpha in relation to clinical involvement and treatment among Thai adults with Plasmodium falciparum malaria. J Med Assoc Thai 1997; 80(suppl 1):S72–5. [PubMed] [Google Scholar]
- 5.Valbonesi M, Bruni R. Clinical application of therapeutic erythrocytapheresis (TEA). Transfus Sci 2000; 22:183–94. [DOI] [PubMed] [Google Scholar]
- 6.Hien TT, White NJ. Qinghaosu. Lancet 1993; 341:603–8. [DOI] [PubMed] [Google Scholar]
- 7.Phillips P, Nantel S, Benny WB. Exchange transfusion as an adjunct to the treatment of severe falciparum malaria: case report and review. Rev Infect Dis 1990; 12:1100–8. [DOI] [PubMed] [Google Scholar]
- 8.Karbwang J, Na-Bangchang K, Thanavibul A, Bunnag D, Chongsuphajaisiddhi T, Harinasuta T. Comparison of oral artesunate and quinine plus tetracycline in acute uncomplicated falciparum malaria. Bull World Health Organ 1994; 72:233–8. [PMC free article] [PubMed] [Google Scholar]
- 9.Miller KD, Greenberg AE, Campbell CC. Treatment of severe malaria in the United States with a continuous infusion of quinidine gluconate and exchange transfusion. N Engl J Med 1989; 321:65–70. [DOI] [PubMed] [Google Scholar]
- 10.van Genderen PJ, Hesselink DA, Bezemer JM, Wismans PJ, Overbosch D. Efficacy and safety of exchange transfusion as an adjunct therapy for severe Plasmodium falciparum malaria in nonimmune travelers: a 10-year single-center experience with a standardized treatment protocol. Transfusion 2010; 50:787–94. [DOI] [PubMed] [Google Scholar]
- 11.Riddle MS, Jackson JL, Sanders JW, Blazes DL. Exchange transfusion as an adjunct therapy in severe Plasmodium falciparum malaria: a meta-analysis. Clin Infect Dis 2002; 34:1192–8. [DOI] [PubMed] [Google Scholar]
- 12.Griffith KS, Lewis LS, Mali S, Parise ME. Treatment of malaria in the United States: a systematic review. JAMA 2007; 297:2264–77. [DOI] [PubMed] [Google Scholar]
- 13.Mali S, Kachur SP, Arguin PM, et al. Malaria surveillance—United States, 2010. MMWR Surveill Summ 2012; 61:1–17. [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Guidelines for treatment of malaria in the United States. Available at: http://www.cdc.gov/malaria/resources/pdf/treatmenttable.pdf. Accessed 13 May 2013.
- 15.Hintze J PASS 11 Available at: http://ncss.wpengine.netdna-cdn.com/wp-content/uploads/2012/09/PASS11QuickStart.pdf. Accessed 1 October 2012. [Google Scholar]
- 16.Saddler M Treatment of severe malaria by exchange transfusion. N Engl J Med 1990; 322:58–9. [DOI] [PubMed] [Google Scholar]
- 17.Auer-Hackenberg L, Staudinger T, Bojic A, et al. Automated red blood cell exchange as an adjunctive treatment for severe Plasmodium falciparum malaria at the Vienna General Hospital in Austria: a retrospective cohort study. Malar J 2012; 11:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kreeftmeijer-Vegter AR, Melo Mde M, de Vries PJ, Koelewijn R, van Hellemond JJ, van Genderen PJ. Manual blood exchange transfusion does not significantly contribute to parasite clearance in artesunate-treated individuals with imported severe Plasmodium falciparum malaria. Malar J 2013; 12:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szczepiorkowski ZM, Bandarenko N, Kim HC, et al. Guidelines on the use of therapeutic apheresis in clinical practice: evidence-based approach from the Apheresis Applications Committee of the American Society for Apheresis. J Clin Apher 2007; 22:106–75. [DOI] [PubMed] [Google Scholar]
- 20.Bellmann R, Sturm W, Pechlaner C, et al. Imported malaria: six cases of severe Plasmodium falciparum infection in Innsbruck, Austria, within a period of five weeks (February/March 1999). Wien Klin Wochenschr 2000; 112:453–8. [PubMed] [Google Scholar]
- 21.Molla S, de La Rubia J, Arriaga F, Fernandez MJ, Carpio N, Marty ML. Role of exchange transfusion in patients with severe falciparum malaria: report of six cases. Haematologica 2001; 86:208–9. [PubMed] [Google Scholar]
- 22.Moorkens GH, Zachée PB, Coolen LJ, et al. Exchange transfusion for fulminant Plasmodium falciparum infection. Int J Artif Organs 1991; 14:122–3. [PubMed] [Google Scholar]
- 23.McCaslin RI, Pikis A, Rodriguez WJ. Pediatric Plasmodium falciparium malaria: a ten-year experience from Washington, DC. Pediatr Infect Dis J 1994; 13:709–15. [PubMed] [Google Scholar]
- 24.Mirete Ferrer C, Masia Canuto M, Navarro Ruiz A, Gutierrez Rodero F. Fatal cerebral malaria caused by Plasmodium falciparum after treatment with exchange transfusion [in Spanish]. Med Clin (Barc) 2000; 115:38. [DOI] [PubMed] [Google Scholar]
- 25.Looareesuwan S, Phillips RE, Karbwang J, White NJ, Flegg PJ, Warrell DA. Plasmodium falciparum hyperparasitaemia: use of exchange transfusion in seven patients and a review of the literature. Q J Med 1990; 75:471–81. [PubMed] [Google Scholar]
- 26.Norda R, Stegmayr BG Swedish Apheresis Group. Therapeutic apheresis in Sweden: update of epidemiology and adverse events. Transfus Apher Sci 2003; 29:159–66. [DOI] [PubMed] [Google Scholar]
- 27.Boga C, Kozanoglu I, Ozdogu H, Ozyurek E. Plasma exchange in critically ill patients with sickle cell disease. Transfus Apher Sci 2007; 37:17–22. [DOI] [PubMed] [Google Scholar]
- 28.McLeod BC, Sniecinski I, Ciavarella D, et al. Frequency of immediate adverse effects associated with therapeutic apheresis. Transfusion 1999; 39:282–8. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization. Guidelines for the treatment of malaria. Geneva, Switzerland: WHO, 2011. [Google Scholar]
- 30.Dondorp A, Nosten F, Stepniewska K, Day N, White N; South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) Group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 2005; 366:717–25. [DOI] [PubMed] [Google Scholar]
- 31.Harris P, Price S, Senthuran S, Cochupanachimootil J, Norton R. Automated erythrocytapheresis for severe falciparum malaria. Intern Med J 2011; 41(1a):60–3. [DOI] [PubMed] [Google Scholar]
- 32.Dondorp AM, Nosten F, Yi P, et al. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 2009; 361:455–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


