Abstract
Introduction:
Methamphetamine use is highly prevalent among men who have sex with men (MSM), but knowledge of the long-term dynamics, and how they are affected by substance use treatment, is limited. This study aimed to describe trajectories of methamphetamine use among MSM, and to evaluate the impact of treatment for any kind of substance use on frequency of methamphetamine use.
Methods:
This analysis used data from a cohort of MSM in Los Angeles, CA who participated in semi-annual study visits from 2014–2022. Trajectories of methamphetamine use were characterized using a continuous time multistate Markov model with three states. States were defined using self-reported frequency of methamphetamine use in the past six months: frequent (daily), occasional (weekly or less), and never. The model estimated the association between receiving treatment for any kind of substance use and changes in state of frequency of methamphetamine use.
Results:
This analysis included 2348 study visits among 285 individuals who were followed up for an average of 4.4 years. Among participants who were in the frequent use state, 65% (n=26) of those who were receiving any kind of substance use treatment at a study visit had reduced their methamphetamine use at their next visit, compared to 33% (n=95) of those who were not receiving treatment. Controlling for age, race/ethnicity, and HIV-status, those who reported receiving current treatment for substance use were more likely to transition from occasional to no use (HR: 1.63, 95% CI: 1.10 – 2.42) and frequent to occasional use (HR: 4.25, 95% CI: 2.11 – 8.59) in comparison to those who did not report receiving current treatment for substance use.
Conclusions:
Findings from this dynamic modeling study provide a new method for assessing longitudinal methamphetamine use outcomes and add important evidence outside of clinical trials that substance use treatment may reduce methamphetamine use.
Keywords: methamphetamine, substance use treatment, multistate models, men who have sex with men
1. Introduction
Methamphetamine use is a significant public health problem that affects both mental and physical health. Between 2015 and 2019, the proportion of American adults who reported more than 100 days of methamphetamine use in the past year increased by 66%, and methamphetamine-involved overdose deaths increased by 180% (Han et al., 2021). Further, methamphetamine use disproportionately affects men who have sex with men (MSM), people living with HIV (PLWH), and especially MSM living with HIV (Rivera et al., 2021; Wohl et al., 2008). Methamphetamine may be used to enable sexual behaviors not common for MSM when not using the drug; its inhibition of judgement increases the likelihood of engaging in risky sexual behaviors such as condomless anal intercourse, which in turn increases the risk of HIV acquisition (Buchacz et al., 2005; Halkitis et al., 2009; Molitor et al., 1998; Reback et al., 2004). Methamphetamine use has also been linked to poor treatment outcomes and accelerated disease progression for MSM living with HIV; this may be explained by increased inflammation and viral replication caused by methamphetamine as well as the association between methamphetamine use and interference with and poor adherence to antiretroviral therapy (Brown et al., 2018; Carrico et al., 2019; Carrico, Shoptaw, et al., 2014; Lai et al., 2020; Passaro et al., 2015).
For many who use methamphetamine regularly, methamphetamine use is a highly dynamic behavior that involves changing patterns of use as well as periods of non-use, or methamphetamine abstinence. Among MSM, those who use methamphetamine can often be split into two groups, those who use frequently and those who use occasionally (weekend warriors) (Shoptaw et al., 2022). While frequency of methamphetamine use can range from daily to less than weekly among people who use the drug regularly, there appears to be some benefit in reducing the frequency of use, both in terms of negative social and physical health consequences including reductions in mental health conditions, cardiovascular issues, risk of overdose, and HIV/STI incidence (Aguilar & Sen, 2013; Halkitis et al., 2005; Javanbakht et al., 2020; Jones et al., 2022; Shoptaw et al., 2022). In order to better understand methamphetamine use, its determinants, and potential targets for intervention, it is advantageous to conceptualize its use as chronic and measure it longitudinally.
Trajectories of methamphetamine use over time, and of substance use in general, are understudied (Fuller et al., 2002; Hadland et al., 2017; Lloyd-Smith et al., 2009; Reddon et al., 2018). Latent class analyses have been used to describe stages of the substance use trajectory, but fail to consider the dynamic nature of this behavior (Genberg et al., 2011; McCarty-Caplan et al., 2014; Tobin et al., 2016). In fact, analyses that determine factors associated with group membership assume that group membership does not change over time. A study of the stability of latent classes in a population of MSM found that 26% of participants transitioned between classes over a period of 2.5 years (Card et al., 2018). While the authors of this study concluded that this suggests relative stability of latent classes for substance use trajectories, the limited follow-up period and substantial fraction of participants that transitioned suggest that further study is needed. In addition, the authors note that classes may be less stable in populations where substance use patterns are determined by fluctuating extrinsic factors such as income, housing, drug supply, and access to substance use treatment (Card et al., 2018).
The extent to which substance use treatment may change trajectories of methamphetamine use is poorly understood and is rarely considered in analyses that explore trajectories of substance use. There are few treatment options for methamphetamine use, and there is currently no FDA-approved medication (Brown & DeFulio, 2020; Cook et al., 2017; Courtney & Ray, 2014; Paulus & Stewart, 2020; Siefried et al., 2020). Recent clinical trials reported that Mirtazapine and combined Bupropion and extended-release Naltrexone reduced methamphetamine use over placebo, though access to these treatments remains limited (Coffin et al., 2020; Trivedi et al., 2021). Contingency management, which uses monetary and other incentives to encourage reductions in use, is currently the most effective treatment for methamphetamine use disorder; when patients complete a course of contingency management, return to use of methamphetamine can occur, which underscores the chronic nature of this disorder and underscores need for continued involvement in non-drug behaviors after the end of the treatment to maintain these reductions (Brown & DeFulio, 2020). Residential rehabilitation has been shown to reduce methamphetamine use in the first three months following detoxification, but the association was not sustained long term (McKetin et al., 2012). In addition, the availability and efficacy of 12-step programs and sponsorship is limited for stimulant use disorders (Donovan & Wells, 2007; Hatch-Maillette et al., 2016; Wendt et al., 2017).
There has been minimal research on longitudinal trajectories of substance use, and no specific studies of methamphetamine use trajectories to our knowledge. Analyses that account for the long-term, dynamic nature of methamphetamine use among MSM over time have potential to characterize methamphetamine use trajectories and the ways in which substance use treatment may change them. This study aims to describe trajectories of methamphetamine use in a cohort of MSM in Los Angeles, and to determine the association between reporting treatment for substance use and changes in these trajectories.
2. Materials and Methods
2.1. Study Population
The present analysis used data from mSTUDY, a National Institute on Drug Abuse (NIDA)-funded cohort in Los Angeles, CA. mSTUDY began enrolling participants in 2014. Participants were recruited using direct outreach including flyers and advertising on social media; study visits took place at two sites including a community-based organization providing services to the lesbian, gay, bisexual, and transgender community, and a university-based research clinic. Follow-up is ongoing. Inclusion criteria for mSTUDY include being between age 18 and 45 at baseline, assigned male sex at birth, ability to provide informed consent, and willingness to return for follow-up visits. Participants include those people living with HIV and those who reported high risk for HIV acquisition (condomless anal intercourse with a male partner in the past six months). The present analysis was restricted to the 285 participants (51% of the parent cohort) who reported methamphetamine use at one or more study visits and includes follow-up data collected through February 2022.
2.2. Data Collection and Measures
At each of the semi-annual visits, participants completed a self-administered, computer-based questionnaire which was available in both English and Spanish. As part of the questionnaire, participants reported on recent (past six months) substance use. Frequency of methamphetamine use was measured using the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST) that was adapted to capture substance use in the past six months (Humeniuk et al., 2010). Participants reported frequency of use as daily, weekly, monthly, less than monthly, once, or never; weekly use was defined as at least one time per week, monthly use was defined as at least one time per month and less than monthly use was defined as less than once per month but more than once in the past six months. For this analysis, frequent use was defined as daily use and occasional use was defined as weekly or less. Substance use treatment was based on an affirmative response to the questions that asked, “are you currently receiving treatment for substance use, including alcohol?” HIV status was based on laboratory testing and every six months, blood samples were collected for HIV testing among those not living with HIV. Due to the COVID-19 pandemic, between March 2020 and June 2021, in-person research activities were curtailed and precluded the collection of blood for HIV testing.
All participants were scheduled to return every six months and the study procedures were repeated at each visit. Visits typically lasted 60–90 minutes and participants were compensated between $70 and $90 depending on the visit. The University of California, Los Angeles Institutional Review Board approved the study, and all participants provided written informed consent.
2.3. Model Description
Trajectories of methamphetamine use were characterized using a continuous time Markov Chain, a probabilistic mathematical model that assumes that the future state of the system depends only on the current state of the system (Grimmett & Stirzaker, 2001). The model tracks the state S of an individual at time t. The ways an individual may move through the states of the model are defined by transition intensities, which represent the instantaneous risk of transition between two states.
Trajectories of methamphetamine use were defined using three states of frequency: none, occasional, and frequent. The model did not include an absorbing state for loss to follow-up or death; since mSTUDY began, 93% of participants remain enrolled and there has been less than 1% mortality.
The model was designed with seven possible transitions, as represented by the arrows in Figure 1. Participants could increase frequency of methamphetamine use from none to occasional or occasional to frequent, decrease frequency from frequent to occasional or occasional to none, or they could remain in each of the three respective states. It was assumed that model parameters do not change over time, and that there were no transitions between non-adjacent states in the model. Participants who reported non-adjacent transitions at consecutive study visits were assumed to have traveled through the adjacent state during the intervening 6 months.
Figure 1.
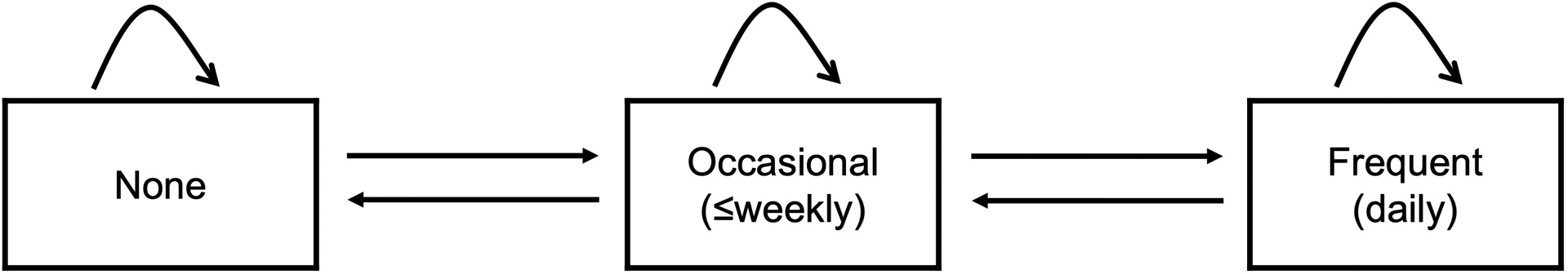
Trajectories of methamphetamine use model diagram.
Parameter estimation was performed using the msm package in R (Jackson, 2011). Using the observed state of each participant at each study visit, maximum likelihood estimation was used to estimate the transition intensities. The estimation method assumed that the observed data were panel data from a time-homogeneous process. Panel data are data collected on a continuous time process that is observed periodically; the time of observation does not influence the observed value or the transition intensities. This is a reasonable assumption, as mSTUDY participants are observed biannually, and the timing of study visits do not influence frequency of methamphetamine use.
2.4. Analytic Strategy
Before modeling trajectories of methamphetamine use, univariate and bivariate analyses were conducted to describe the study sample. Baseline methamphetamine use, age, race/ethnicity, and HIV-status were compared for those who did and did not report receiving current treatment for substance use. In addition, the number of observed transitions among those who did and did not report receiving current treatment for substance use was computed by tabulating participants’ frequency of methamphetamine use at visit t-1 and visit t.
Three increasingly complex multistate models were analyzed. The first model estimated trajectories of methamphetamine use without covariates. The second model compared trajectories of methamphetamine use for those who did and did not report receiving current treatment for substance use. The third model built upon the second model by controlling for age, race/ethnicity, and HIV-status. All covariates except race/ethnicity were time-varying, and it was assumed that they were constant between study visits, and that the estimated transition intensity was dependent upon the value of the covariate at visit t-1 (Jackson, 2011).
The estimated transition intensities from each model were used to present more intuitive parameterizations: the mean sojourn time and the probability that the next transition is to state S. Mean sojourn time was defined as the amount of time in years that individuals spend in each state before transitioning to another state.31
To compare transition rates for those who did and did not report receiving current treatment for substance use, hazard ratios for the association of reporting current treatment for substance use and transition between states of methamphetamine frequency were computed. Model fit was assessed by using likelihood ratio tests to compare each of the three models.
3. Results
This analysis included 285 unique individuals who reported methamphetamine use on at least one visit, of whom 15.8% (n=45) reported receiving current treatment for substance use at baseline. At baseline, 23.5% (n=67) of participants reported no methamphetamine use in the past six months, 57.5% (n=164) reported occasional use, and 18.9% (n=54) reported frequent use. The mean baseline age was 32.5 years old, and 40.8% (n=116) identified as Black/African American, 36.6% (n=104) identified as Hispanic/Latinx/Spanish, 15.5% (n=44) identified as white, and 7.0% (n=20) identified as another race or ethnicity. The proportion of participants who identified as Black/African American was higher among those who reported not receiving current treatment for substance use, and the proportion of participants who identified as Hispanic/Latinx/Spanish and who were living with HIV was higher among those who reported receiving current treatment for substance use. (Table 1). Among 104 participants who reported receiving treatment for substance use at some point over the course of the study, 53.8% (n=56) reported receiving treatment for substance use at two or more consecutive study visits.
Table 1.
Baseline characteristics of mSTUDY participants
| Receiving Treatment for Substance Use | |||
|---|---|---|---|
| Totala | Yes | No | |
| (n=285) | (n=45) | (n=240) | |
| n (%) | n (%) | n (%) | |
| Methamphetamine Use b | |||
| None | 67 (23.5) | 12 (26.7) | 55 (22.9) |
| Occasional | 164 (57.5) | 23 (51.1) | 141 (58.8) |
| Frequent | 54 (18.9) | 10 (22.2) | 44 (18.3) |
| Age (Mean, SD) | 32.5 (6.7) | 33.2 (6.0) | 32.4 (6.9) |
| Race/Ethnicity | |||
| Black/African American, NH | 116 (40.8) | 10 (22.2) | 106 (44.4) |
| Hispanic/Latinx/Spanish | 104 (36.6) | 25 (55.6) | 79 (33.1) |
| White, NH | 44 (15.5) | 8 (17.8) | 36 (15.1) |
| Other, NH | 20 (7.0) | 2 (4.4) | 18 (7.5) |
| HIV-Positive | 181 (63.5) | 35 (77.8) | 146 (60.8) |
| Number of Visits (Mean, SD) | 8.2 (3.7) | 8.3 (3.7) | 8.2 (3.7) |
| Years of Follow-Up (Mean, SD) | 4.4 (1.9) | 4.4 (1.9) | 4.4 (1.9) |
Participants reported methamphetamine use during one or more visits
Self-reported frequency of use in the past six months (occasional defined as weekly or less, frequent defined as daily).
Abbreviations: NH = Non-Hispanic
Between August 2014 and February 2022 these individuals had a combined 2,348 study visits and 2,063 observed transitions between states of methamphetamine frequency. On average, participants had 8.2 study visits over the course of 4.4 years. Average follow-up time was comparable for those who reported receiving current treatment for substance use and those who did not.
Comparisons of observed transitions between states of methamphetamine use for those who did and did not report receiving current treatment for substance use at visit t-1 are shown in Figure 2. Of 2,063 observed transitions, 12.0% (n=247) were among participants who reported receiving current treatment for substance use at visit t-1, and 88.0% (n=1816) were among those who did not report receiving current treatment for substance use at visit t-1. Among participants who reported frequent use at visit t-1, 35.0% (n=14) of those who were receiving any kind of substance use treatment remained in the frequent use state at their next visit, compared to 67.5% (n=197) of those who were not receiving treatment. It was most common to remain in the same state between visit t-1 and t, and non-adjacent transitions were relatively rare.
Figure 2.

Observed transitions between states of methamphetamine use comparing those who reported receiving substance use treatment at visit t-1 compared to those who did not.
When modeling trajectories of methamphetamine use without covariates, an average of 1.6 years (95% CI: 1.4–1.8) of nonuse elapsed before participants transitioned to occasional use. An average of 1 year (95% CI: 0.9–1.1) of occasional use elapsed before participants transitioned to no use with a probability of 0.64 (95% CI: 0.6–0.7) or frequent use with a probability of 0.36 (95% CI: 0.3–0.4). An average of 1.2 years (95% CI: 1.0–1.4) of frequent use elapsed before participants transitioned to occasional use (Table 2).
Table 2.
Full parameterization of continuous-time multistate Markov model
| Mean Sojourn Time | Probability State S is Next | ||||
|---|---|---|---|---|---|
| None | Occasional | Frequent | Occasional to None | Occasional to Frequent | |
| Years (95% CI) | Years (95% CI) | Years (95% CI) | Probability (95% CI) | Probability (95% CI) | |
| No Covariates | 1.57 (1.36, 1.83) | 0.97 (0.87, 1.09) | 1.16 (0.96, 1.41) | 0.64 (0.59, 0.70) | 0.36 (0.30, 0.41) |
| Receiving Treatment for Substance Use (Bivariate) | |||||
| Yes | 1.82 (1.13, 2.93) | 0.61 (0.43, 0.87) | 0.38 (0.22, 0.66) | 0.62 (0.43, 0.79) | 0.38 (0.21, 0.57) |
| No | 1.55 (1.33, 1.81) | 1.04 (0.92, 1.17) | 1.37 (1.11, 1.70) | 0.64 (0.57, 0.69) | 0.36 (0.31, 0.43) |
| Receiving Treatment for Substance Use (Adjusteda) | |||||
| Yes | 2.10 (0.82, 5.39) | 0.38 (0.19, 0.76) | 0.20 (0.06, 0.70) | 0.74 (0.39, 0.92) | 0.26 (0.08, 0.61) |
| No | 1.88 (0.85, 4.17) | 0.65 (0.34, 1.23) | 0.87 (0.27, 2.84) | 0.78 (0.50, 0.93) | 0.22 (0.07, 0.50) |
Adjusted for age, race/ethnicity, and HIV-status
Trajectories of methamphetamine use are compared for those who did and did not report receiving current treatment for substance use in Table 2. Controlling for age, race/ethnicity, and HIV-status, participants who reported receiving current treatment for substance use did not use methamphetamine for an average of 2.1 years (95% CI: 0.8 – 5.4) before transitioning to occasional use. They used methamphetamine occasionally for an average of 0.4 years (95% CI: 0.2 – 0.8) and then had a 0.7 (95% CI: 0.4 – 0.9) probability of transitioning to non-use and a 0.3 (95% CI: 0.1 – 0.6) probability of transitioning to frequent use. They used methamphetamine frequently for an average of 0.2 years (95% CI: 0.1 – 0.7) before transitioning to occasional use.
In comparison, those who did not report receiving current treatment for substance use did not use methamphetamine for an average of 1.9 years (95% CI: 0.9 – 4.2) before transitioning to occasional use. They used methamphetamine occasionally for an average of 0.7 years (95% CI: 0.3 – 1.2) and then had a 0.8 (95% CI: 0.5 – 0.9) probability of transitioning to non-use and a 0.2 (95% CI: 0.1– 0.5) probability of transitioning to frequent use. They used methamphetamine frequently for an average of 0.9 years (95% CI: 0.3 – 2.8) before transitioning to occasional use.
Controlling for age, race/ethnicity, and HIV-status, those who reported receiving current treatment for substance use were more likely to transition from occasional to no use (HR: 1.6, 95% CI: 1.1 – 2.4) and from frequent to occasional use (HR: 4.3, 95% CI: 2.1 – 8.6) in comparison to those who did not report receiving current treatment for substance use (Table 3). Receiving current treatment for substance use was not associated with transition from no to occasional use or occasional to frequent use.
Table 3.
Association of receiving treatment for substance use and transitions between states of frequency of methamphetamine use
| Transition | ||||
|---|---|---|---|---|
| None to Occasional | Occasional to None | Occasional to Frequent | Frequent to Occasional | |
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |
| Bivariate Model | ||||
| Receiving Treatment for Substance Use | 0.85 (0.52, 1.41) | 1.65 (1.13, 2.41) | 1.77 (0.85, 3.71) | 3.58 (2.00, 6.39) |
| Adjusted Model | ||||
| Receiving Treatment for Substance Use | 0.90 (0.54, 1.49) | 1.63 (1.10, 2.42) | 2.01 (0.84, 4.81) | 4.25 (2.11, 8.59) |
| Age at visit | 1.01 (0.98, 1.03) | 0.99 (0.97, 1.01) | 1.00 (0.97, 1.03) | 0.99 (0.96, 1.02) |
| Race/Ethnicity | ||||
| Black/African American | 1.43 (1.02, 2.00) | 0.98 (0.71, 1.36) | 1.18 (0.76, 1.83) | 1.01 (0.63, 1.60) |
| Hispanic/Latinx/Spanish | 1.02 (0.62, 1.66) | 1.15 (0.74, 1.78) | 0.86 (0.46, 1.60) | 0.67 (0.34, 1.31) |
| Other | 1.24 (0.70, 2.19) | 1.14 (0.63, 2.09) | 1.97 (0.93, 4.14) | 0.73 (0.33, 1.57) |
| White | 1.00 | 1.00 | 1.00 | 1.00 |
| HIV-Positive | 0.67 (0.47, 0.95) | 0.55 (0.41, 0.75) | 0.93 (0.61, 1.41) | 1.09 (0.68, 1.73) |
Abbreviations: HR=Hazard Ratio
Likelihood ratio tests revealed that the bivariate model improved model fit over the model without covariates (p<0.001), and that the fully adjusted model also improved model fit over the bivariate model (p<0.001).
4. Discussion
In this study, a continuous-time multistate Markov model provided insight into trajectories of methamphetamine use in a population of MSM living with or at high risk of acquiring HIV. Receiving current treatment for substance use was associated with both transition from frequent to occasional use states and transition from occasional to no use states, suggesting that treatment for substance use may lead to reduced frequency of methamphetamine use.
Additionally, those who reported receiving current treatment for substance use did not report significant increases in frequency of methamphetamine use. While we have no data to detail whether substance use treatment was specific to methamphetamine, these findings suggest that MSM who report involvement with substance use treatment behave in ways that reduce the cumulative reported frequency of their methamphetamine use. This finding is supported by other data which suggest that people in substance use treatment engage in behavioral self-monitoring independent of treatment type (Foxx & Brown, 1979). It is possible that being in substance use treatment sensitizes the individuals to pay attention to the frequency of one’s use of methamphetamine. Whatever the mechanism, the signal that substance use treatment produces downward influences on trajectories of methamphetamine use in this group provides evidence for harm reduction strategies that incorporate these ideas.
Consistent with these findings, those who report receiving current treatment for substance use have longer periods of abstinence and shorter periods of occasional and frequent use of methamphetamine than their peers who do not report receiving current treatment for substance use. This finding, taken together with the strong association between receiving treatment for substance use and transition from frequent down to occasional use, highlights the importance of treatment programs that focus on reducing use, rather than requiring commitment to and achievement of complete abstinence. In fact, prior work has shown that reductions in frequency of methamphetamine use, independent of involvement in substance use treatment, may decrease the likelihood of many of the harmful physical effects of methamphetamine such as psychosis, cardiovascular complications, infectious disease acquisition, overdose, and death as well as improve social relationship status, economic status, and mental health status (Carrico, Flentje, et al., 2014; Corsi et al., 2019; Prakash et al., 2017; Shoptaw et al., 2022).
By considering multiple transitions, rather than just cessation, this model highlights substance use treatment outcomes related to both reduction in use and abstinence. It is well-established that treatment options for methamphetamine use are limited, especially for those who report frequent use (Coffin et al., 2020; Cook et al., 2017; Siefried et al., 2020; Trivedi et al., 2021). While our study made the important observation that substance use treatment may reduce frequency of methamphetamine use in an observational cohort of MSM, further research is needed to better understand how methamphetamine use trajectories may differ depending on the type of substance use treatment (e.g., contingency management, medication, residential rehabilitation) that a patient is receiving as well as whether they sought treatment on their own or if it was court-ordered. Such studies could employ the novel conceptualization of longitudinal methamphetamine use presented in this analysis to provide important insight into the long-term efficacy of specific modalities used to treat methamphetamine use and how self-motivation to enter treatment may influence efficacy. Additionally, future models should explore trajectories of methamphetamine use following discontinuation or completion of substance use treatment in order to determine if reductions in use persist beyond the treatment period.
Further work must also consider interventions that could increase not only the rate of transition from occasional use to abstinence, but also the rate of transition from frequent to occasional use. Future studies would benefit from more extensive data sources with larger sample sizes to consider how additional time-varying background factors as well as different types of treatment work together to influence changes in frequency of methamphetamine use over long periods of time.
The multistate Markov model used in this study provides important insight into the rates at which MSM transition between states of frequency of methamphetamine use, and reveals key observations that would not have been visible under a conventional static risk factor analysis. Multistate Markov models are widely used in public health research to study chronic disease progression, but they have rarely been applied to studies of substance use. One previous modeling study focused on transition from cannabis use to illicit substance use, and another considered transitions from onset to regular use of alcohol and tobacco among adolescents (Mayet et al., 2011; Mayet et al., 2012). However, multistate Markov models have not been previously used to study multiple transitions within a methamphetamine use trajectory, and they have not been used to study substance use treatment.
4.1. Limitations
It is important to keep in mind the limitations of this study. First, nearly all of the data for this analysis was collected by self-report. Social desirability bias is particularly likely when collecting data about sensitive behaviors such as substance use, where participants are likely to underreport these behaviors. However, the use of computer-assisted self-interviewing (CASI) rather than face-to-face interviews, helps to minimize this bias.
The model used in this analysis makes strong assumptions that must be considered. First, the model assumes that all parameter values are constant over time and that all observed non-adjacent transitions occurred via travel through adjacent states. In addition, Markov models assume that the future state of the system depends only on the current state of the system; it is likely that the system is not entirely memory-less and that previous methamphetamine use has some amount of influence on transition between states. Lastly, participants were observed biannually, so if the true sojourn time for a particular state is less than six months, that could be missed by the data collection process and hence not captured by the model.
This study is also limited by its sample size. Sparse data limited the number of covariates that could be included in the fully adjusted model; additional factors, including use of other substances, mental health, and housing likely confound this association as well as mask additional heterogeneity in the estimated transition intensities. Of particular note, most mSTUDY participants use multiple substances; the decreases in methamphetamine use observed in this study may be associated with changes in use of other substances, which the model does not capture.
Lastly, the results of this study should be generalized with caution. mSTUDY is a convenience sample that may not be representative of all MSM in Los Angeles or people who use methamphetamine in other settings or locations. mSTUDY provides connections to healthcare and other services for participants, so those who choose to remain enrolled may be more motivated to change substance use behaviors or have better access to treatment. In particular, people living in Los Angeles who seek treatment for methamphetamine use have access to an unusually extensive array of options including in-person- and agency-based harm reduction programs, outpatient treatment programs that feature contingency management and culturally tailored cognitive behavioral therapy, and clinical trials of new/emerging behavioral and medical treatments for methamphetamine use disorder. Additionally, the mSTUDY cohort is by purpose designed to over-recruit MSM who hold minority racial and ethnic identities in order to enrich the sample with participants who have disproportionate risks for HIV incidence in Los Angeles; thus the high prevalence of engagement with substance use treatment reported by participants who identify as Hispanic or Latinx could be spurious and/or relate to the lack of similar sized White and Asian MSM that might provide a fair comparator to the observed correlation.
5. Conclusions
Findings from this study are important in that they provide a new model for measuring outcomes from methamphetamine use longitudinally. Consequences to methamphetamine use for most users accrue gradually, so viewing methamphetamine use in terms of periods of use frequency (“sojourns”), which can increase, decrease, or remain static, offers a novel way of understanding the cumulative impacts of those consequences. Findings from these kinds of analyses may point to events or to opportunities that might be pivotal in shifting the duration of “sojourns” of methamphetamine use to avoid negative consequences that are less intensive than formal drug treatment episodes.
This study highlights the utility of multistate Markov models in substance abuse research, and future studies should consider using this method to further delve into trajectories of methamphetamine use. The present analysis focused on frequency, an important characteristic of one’s methamphetamine use. However, there are other relevant markers of use including craving, interference with responsibilities, and mode of administration (Hasin et al., 2013; Koob & Le Moal, 2001). In the future, a questionnaire that assesses multiple dimensions of methamphetamine use (such as the ASSIST) could be used to characterize low, moderate, and high severity. These categories could define the states of a multistate Markov model that provides new insight into the dynamic nature of methamphetamine use (Humeniuk et al., 2010).
Acknowledgements
The authors wish to thank the mSTUDY staff and participants who make this research possible.
Funding Source
mSTUDY is funded by the National Institute on Drug Abuse (U01DA036267) and Drs Shoptaw and Gorbach are the MPIs.
Abbreviations:
- MSM
men who have sex with men
- PLWH
people living with HIV
- ASSIST
Alcohol, Smoking and Substance Involvement Screening Test
- HR
hazard ratio
Footnotes
Declaration of Competing Interest
The authors have no competing interests to declare.
CRediT Authorship Statement
Allison D. Rosen: Conceptualization, Methodology, Formal analysis, Writing - Original Draft, Visualization. Marjan Javanbakht: Methodology, Formal analysis, Data Curation, Writing - Review & Editing. Steven J. Shoptaw: Conceptualization, Resources, Writing - Review & Editing, Supervision, Funding acquisition. Marissa J. Seamans: Methodology, Formal analysis, Writing - Review & Editing. James O. Lloyd-Smith: Conceptualization, Methodology, Formal analysis, Writing - Review & Editing, Supervision. Pamina M. Gorbach: Conceptualization, Resources, Writing - Review & Editing, Supervision, Funding acquisition
References
- Aguilar JP, & Sen S (2013). The Culture of Methamphetamine: Reframing Gay Men’s Methamphetamine Use. Journal of Human Behavior in the Social Environment, 23(3), 370–382. 10.1080/10911359.2013.764204 [DOI] [Google Scholar]
- Brown HD, & DeFulio A (2020, Nov 1). Contingency management for the treatment of methamphetamine use disorder: A systematic review. Drug Alcohol Depend, 216, 108307. 10.1016/j.drugalcdep.2020.108307 [DOI] [PubMed] [Google Scholar]
- Brown MJ, Serovich JM, Laschober TC, & Kimberly JA (2018, Oct). Age and racial disparities in substance use and self-reported viral suppression among men who have sex with men with HIV. Int J STD AIDS, 29(12), 1174–1182. 10.1177/0956462418779663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchacz K, McFarland W, Kellogg TA, Loeb L, Holmberg SD, Dilley J, & Klausner JD (2005, Sep 2). Amphetamine use is associated with increased HIV incidence among men who have sex with men in San Francisco. AIDS, 19(13), 1423–1424. https://www.ncbi.nlm.nih.gov/pubmed/16103774 [DOI] [PubMed] [Google Scholar]
- Card KG, Armstrong HL, Carter A, Cui Z, Wang L, Zhu J, Lachowsky NJ, Moore DM, Hogg RS, & Roth EA (2018, Jul 1). Assessing the longitudinal stability of latent classes of substance use among gay, bisexual, and other men who have sex with men. Drug Alcohol Depend, 188, 348–355. 10.1016/j.drugalcdep.2018.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Flentje A, Gruber VA, Woods WJ, Discepola MV, Dilworth SE, Neilands TB, Jain J, & Siever MD (2014, Jun). Community-based harm reduction substance abuse treatment with methamphetamine-using men who have sex with men. J Urban Health, 91(3), 555–567. 10.1007/s11524-014-9870-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Hunt PW, Neilands TB, Dilworth SE, Martin JN, Deeks SG, & Riley ED (2019, Jan 1). Stimulant Use and Viral Suppression in the Era of Universal Antiretroviral Therapy. J Acquir Immune Defic Syndr, 80(1), 89–93. 10.1097/qai.0000000000001867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Shoptaw S, Cox C, Stall R, Li X, Ostrow DG, Vlahov D, & Plankey MW (2014, Dec 15). Stimulant use and progression to AIDS or mortality after the initiation of highly active antiretroviral therapy. J Acquir Immune Defic Syndr, 67(5), 508–513. 10.1097/QAI.0000000000000364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin PO, Santos GM, Hern J, Vittinghoff E, Walker JE, Matheson T, Santos D, Colfax G, & Batki SL (2020, Mar 1). Effects of Mirtazapine for Methamphetamine Use Disorder Among Cisgender Men and Transgender Women Who Have Sex With Men: A Placebo-Controlled Randomized Clinical Trial. JAMA Psychiatry, 77(3), 246–255. 10.1001/jamapsychiatry.2019.3655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook R, Quinn B, Heinzerling K, & Shoptaw S (2017, Jun). Dropout in clinical trials of pharmacological treatment for methamphetamine dependence: the role of initial abstinence. Addiction, 112(6), 1077–1085. 10.1111/add.13765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corsi KF, Shoptaw S, Alishahi M, & Booth RE (2019, Feb). Interventions to Reduce Drug Use Among Methamphetamine Users at Risk for HIV. Curr HIV/AIDS Rep, 16(1), 29–36. 10.1007/s11904-019-00423-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney KE, & Ray LA (2014, Oct 1). Methamphetamine: an update on epidemiology, pharmacology, clinical phenomenology, and treatment literature. Drug Alcohol Depend, 143, 11–21. 10.1016/j.drugalcdep.2014.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan DM, & Wells EA (2007, Apr). ‘Tweaking 12-Step’: the potential role of 12-Step self-help group involvement in methamphetamine recovery. Addiction, 102 Suppl 1, 121–129. 10.1111/j.1360-0443.2007.01773.x [DOI] [PubMed] [Google Scholar]
- Foxx RM, & Brown RA (1979, Spring). Nicotine fading and self-monitoring for cigarette abstinence or controlled smoking. J Appl Behav Anal, 12(1), 111–125. 10.1901/jaba.1979.12-111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller CM, Vlahov D, Ompad DC, Shah N, Arria A, & Strathdee SA (2002, Apr 1). High-risk behaviors associated with transition from illicit non-injection to injection drug use among adolescent and young adult drug users: a case-control study. Drug Alcohol Depend, 66(2), 189–198. [DOI] [PubMed] [Google Scholar]
- Genberg BL, Gange SJ, Go VF, Celentano DD, Kirk GD, & Mehta SH (2011, Apr 1). Trajectories of injection drug use over 20 years (1988–2008) in Baltimore, Maryland. Am J Epidemiol, 173(7), 829–836. 10.1093/aje/kwq441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmett G, & Stirzaker D (2001). Probability and random processes (3rd ed.). Oxford University Press. [Google Scholar]
- Hadland SE, Wood E, Nosova E, Kerr T, & DeBeck K (2017, Nov). Cessation of Injecting and Preceding Drug Use Patterns Among a Prospective Cohort of Street-Involved Youth. J Adolesc Health, 61(5), 612–618. 10.1016/j.jadohealth.2017.05.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halkitis PN, Fischgrund BN, & Parsons JT (2005). Explanations for methamphetamine use among gay and bisexual men in New York City. Subst Use Misuse, 40(9–10), 1331–1345. 10.1081/JA-200066900 [DOI] [PubMed] [Google Scholar]
- Halkitis PN, Mukherjee PP, & Palamar JJ (2009, Aug). Longitudinal modeling of methamphetamine use and sexual risk behaviors in gay and bisexual men. AIDS Behav, 13(4), 783–791. 10.1007/s10461-008-9432-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han B, Compton WM, Jones CM, Einstein EB, & Volkow ND (2021). Methamphetamine Use, Methamphetamine Use Disorder, and Associated Overdose Deaths Among US Adults. JAMA Psychiatry, 78(12), 1329–1342. 10.1001/jamapsychiatry.2021.2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, O’Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M, & Grant BF (2013, Aug). DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry, 170(8), 834–851. 10.1176/appi.ajp.2013.12060782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch-Maillette M, Wells EA, Doyle SR, Brigham GS, Daley D, DiCenzo J, Donovan D, Garrett S, Horigian VE, Jenkins L, Killeen T, Owens M, & Perl HI (2016, Sep). Predictors of 12-Step Attendance and Participation for Individuals With Stimulant Use Disorders. J Subst Abuse Treat, 68, 74–82. 10.1016/j.jsat.2016.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humeniuk R, Henry-Edwards S, Ali R, Poznyak V, Monteiro MG, & World Health, O. (2010, 2010). The Alcohol, Smoking and Substance involvement Screening Test (ASSIST): manual for use in primary care / prepared by R. HumeniukƯ [et al]. https://apps.who.int/iris/handle/10665/44320
- Jackson C (2011). Multi-State Models for Panel Data: The msm Package for R. Journal of Statistical Software, 38(8), 1–28. [Google Scholar]
- Javanbakht M, Shoptaw S, Ragsdale A, Brookmeyer R, Bolan R, & Gorbach PM (2020, Feb 1). Depressive symptoms and substance use: Changes overtime among a cohort of HIV-positive and HIV-negative MSM. Drug Alcohol Depend, 207, 107770. 10.1016/j.drugalcdep.2019.107770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Houry D, Han B, Baldwin G, Vivolo-Kantor A, & Compton WM (2022, Feb). Methamphetamine use in the United States: epidemiological update and implications for prevention, treatment, and harm reduction. Ann N Y Acad Sci, 1508(1), 3–22. 10.1111/nyas.14688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, & Le Moal M (2001, Feb). Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology, 24(2), 97–129. 10.1016/S0893-133X(00)00195-0 [DOI] [PubMed] [Google Scholar]
- Lai HH, Kuo YC, Kuo CJ, Lai YJ, Chen M, Chen YT, Chen CC, Yen MY, Hu BS, Wang TH, Wang CC, Kuo LL, Yen TF, Chuang PH, & Yen YF (2020, Apr 28). Methamphetamine Use Associated with Non-adherence to Antiretroviral Treatment in Men Who Have Sex with Men. Sci Rep, 10(1), 7131. 10.1038/s41598-020-64069-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Smith E, Wood E, Li K, Montaner JS, & Kerr T (2009, Jan 1). Incidence and determinants of initiation into cocaine injection and correlates of frequent cocaine injectors. Drug Alcohol Depend, 99(1–3), 176–182. 10.1016/j.drugalcdep.2008.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayet A, Legleye S, Chau N, & Falissard B (2011, Nov). Transitions between tobacco and cannabis use among adolescents: a multi-state modeling of progression from onset to daily use. Addict Behav, 36(11), 1101–1105. 10.1016/j.addbeh.2011.06.009 [DOI] [PubMed] [Google Scholar]
- Mayet A, Legleye S, Falissard B, & Chau N (2012, Feb). Cannabis use stages as predictors of subsequent initiation with other illicit drugs among French adolescents: use of a multi-state model. Addict Behav, 37(2), 160–166. 10.1016/j.addbeh.2011.09.012 [DOI] [PubMed] [Google Scholar]
- McCarty-Caplan D, Jantz I, & Swartz J (2014, Jul). MSM and drug use: A latent class analysis of drug use and related sexual risk behaviors. AIDS Behav, 18(7), 1339–1351. 10.1007/s10461-013-0622-x [DOI] [PubMed] [Google Scholar]
- McKetin R, Najman JM, Baker AL, Lubman DI, Dawe S, Ali R, Lee NK, Mattick RP, & Mamun A (2012, Nov). Evaluating the impact of community-based treatment options on methamphetamine use: findings from the Methamphetamine Treatment Evaluation Study (MATES). Addiction, 107(11), 1998–2008. 10.1111/j.1360-0443.2012.03933.x [DOI] [PubMed] [Google Scholar]
- Molitor F, Truax SR, Ruiz JD, & Sun RK (1998, Feb). Association of methamphetamine use during sex with risky sexual behaviors and HIV infection among non-injection drug users. West J Med, 168(2), 93–97. https://www.ncbi.nlm.nih.gov/pubmed/9499742 [PMC free article] [PubMed] [Google Scholar]
- Passaro RC, Pandhare J, Qian HZ, & Dash C (2015, Sep). The Complex Interaction Between Methamphetamine Abuse and HIV-1 Pathogenesis. J Neuroimmune Pharmacol, 10(3), 477–486. 10.1007/s11481-015-9604-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulus MP, & Stewart JL (2020, Sep 1). Neurobiology, Clinical Presentation, and Treatment of Methamphetamine Use Disorder: A Review. JAMA Psychiatry, 77(9), 959–966. 10.1001/jamapsychiatry.2020.0246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash MD, Tangalakis K, Antonipillai J, Stojanovska L, Nurgali K, & Apostolopoulos V (2017, Jun). Methamphetamine: Effects on the brain, gut and immune system. Pharmacol Res, 120, 60–67. 10.1016/j.phrs.2017.03.009 [DOI] [PubMed] [Google Scholar]
- Reback CJ, Larkins S, & Shoptaw S (2004, Mar). Changes in the meaning of sexual risk behaviors among gay and bisexual male methamphetamine abusers before and after drug treatment. AIDS Behav, 8(1), 87–98. 10.1023/b:aibe.0000017528.39338.75 [DOI] [PubMed] [Google Scholar]
- Reddon H, DeBeck K, Socias ME, Dong H, Wood E, Montaner J, Kerr T, & Milloy MJ (2018, Mar). Cannabis use is associated with lower rates of initiation of injection drug use among street-involved youth: A longitudinal analysis. Drug Alcohol Rev, 37(3), 421–428. 10.1111/dar.12667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera AV, Harriman G, Carrillo SA, & Braunstein SL (2021, Apr). Trends in Methamphetamine Use Among Men Who Have Sex with Men in New York City, 2004–2017. AIDS Behav, 25(4), 1210–1218. 10.1007/s10461-020-03097-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoptaw S, Li MJ, Javanbakht M, Ragsdale A, Goodman-Meza D, & Gorbach PM (2022, Mar 1). Frequency of reported methamphetamine use linked to prevalence of clinical conditions, sexual risk behaviors, and social adversity in diverse men who have sex with men in Los Angeles. Drug Alcohol Depend, 232, 109320. 10.1016/j.drugalcdep.2022.109320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siefried KJ, Acheson LS, Lintzeris N, & Ezard N (2020, Apr). Pharmacological Treatment of Methamphetamine/Amphetamine Dependence: A Systematic Review. CNS Drugs, 34(4), 337–365. 10.1007/s40263-020-00711-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin KE, Yang C, King K, Latkin CA, & Curriero FC (2016, Mar). Associations Between Drug and Alcohol Use Patterns and Sexual Risk in a Sample of African American Men Who Have Sex with Men. AIDS Behav, 20(3), 590–599. 10.1007/s10461-015-1214-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, Walker R, Ling W, Dela Cruz A, Sharma G, Carmody T, Ghitza UE, Wahle A, Kim M, Shores-Wilson K, Sparenborg S, Coffin P, Schmitz J, Wiest K, Bart G, Sonne SC, Wakhlu S, Rush AJ, Nunes EV, & Shoptaw S (2021, Jan 14). Bupropion and Naltrexone in Methamphetamine Use Disorder. The New England journal of medicine, 384(2), 140–153. 10.1056/NEJMoa2020214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt DC, Hallgren KA, Daley DC, & Donovan DM (2017, Mar). Predictors and Outcomes of Twelve-Step Sponsorship of Stimulant Users: Secondary Analyses of a Multisite Randomized Clinical Trial. J Stud Alcohol Drugs, 78(2), 287–295. 10.15288/jsad.2017.78.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohl AR, Frye DM, & Johnson DF (2008, Sep). Demographic characteristics and sexual behaviors associated with methamphetamine use among MSM and non-MSM diagnosed with AIDS in Los Angeles County. AIDS Behav, 12(5), 705–712. 10.1007/s10461-007-9315-7 [DOI] [PubMed] [Google Scholar]


