Abstract
Substantial advances in the treatment of early onset scoliosis (EOS) over the past two to three decades have resulted in significant improvements in health-related quality of life of affected children. In addition to classifications that address the marked heterogeneity of this patient population, increasing understanding of the natural history of the disease, and new implants and treatment techniques have resulted in innovations unlike any other area of pediatric orthopedics. The growing understanding of the interaction between spinal and thoracic growth, as well as dependent lung maturation, has had a lasting impact on the treatment strategy of this potentially life-threatening disease. The previous treatment approach with early corrective fusion gave way to a growth-friendly concept. Despite the steady development of new growth-friendly surgical treatment options, whose efficacy still needs to be validated, as well as a revival of conservative growth control with serial casts and/or braces, the psychosocial burden of the long lasting and complication-prone treatments remains high. As a consequence, EOS still represents one of the greatest pediatric orthopedic challenges.
Keywords: Early onset scoliosis, growth guidance, growth-friendly surgery, health-related quality of life
Introduction
Early onset scoliosis (EOS) refers to a deformity of the spine (scoliosis and/or kyphosis), with or without involvement of the thorax, diagnosed before the age of 10. 1 In 2014, the Classification of Early Onset Scoliosis (C-EOS) has been introduced to encounter the heterogeneity of the affected patient population. 2 The C-EOS uses a continuous age prefix and four deformity characteristics: etiology, major curve magnitude, annual progression rate (APR), and kyphosis. In terms of etiology, a distinction is made between neuromuscular, congenital, syndromic, and idiopathic EOS. Validation of the C-EOS showed substantial to excellent interobserver and intraobserver reliability. 3 The natural history varies with the underlying disease and may be associated with increased mortality.4,5 At the same time, the principle of early definitive spinal fusion, which was still practiced until the beginning of the new millennium, has taught us not only revision rates of up to 39% but also the disastrous influence of a short (thoracic) spine on pulmonary function. 6 Children who underwent spondylodesis before the age of 9 showed a forced vital capacity <50% with associated restrictive ventilatory dysfunction when the length of the thoracic spine was significantly shortened (T1-T12 distance < 18 cm). 7 The interdependence of spinal and thoracic growth and lung maturation has been substantiated by the achievements of Dr Robert Campbell Jr. and his description of Thoracic Insufficiency Syndrome (TIS) as the inability of the thorax to provide normal respiration and lung development. 8 As a consequence, the conservative and surgical treatment of EOS must, in addition to controlling the deformity, primarily focus on maintaining, or ideally stimulating, the growth of the spine and thorax in order to achieve the targeted minimum T1-T12 distance of 18–22 cm, as a prerequisite for normal lung function. The development of new and the optimisation of existing treatment methods have recently been influenced by the consideration of aspects such as health-related quality of life (HrQoL) and psycho-social burdens for the affected children and their relatives. Additional comorbidities increase the already high-risk profile and require, in addition to early multidisciplinary management, treatment approaches with as few complications as possible and a minimum number of necessary (surgical and anesthetic) interventions.
In addition to a consequent use of the C-EOS, the validated Early Onset Scoliosis Questionnaire (EOSQ-24) should consistently be used to provide optimal and reproducible configuration of treatment management, outcome analysis, communication, and research. The EOSQ-24 consists of 24 items pertaining to the patient’s Health-related Quality of Life (HrQoL) during the past 4 weeks. 9 The 24 questions cover the following 11 areas: General Health, Pain/Discomfort, Pulmonary Function, Transfer, Physical Function, Daily Living, Fatigue/Energy Level, Emotion, Parental Impact, Financial Impact, and Satisfaction. The EOSQ-24 has been proven as a valid, reliable, and responsive instrument that is able to serve as a patient-reported outcome measure for EOS patients. 9 The questionnaire has meanwhile been validated in nine languages and can be found, for example, via the Pediatric Spine Foundation homepage (https://pediatricspinefoundation.org/). The efforts achieved to increase quality in treatment and research can be further improved by national and international multicenter studies with a relevant increase in patient numbers. 10
However, despite the aforementioned comprehensive and systematic approach to classification, treatment options, and outcome evaluation, there is still little consistent expert consensus on the optimal choice and design of adequate treatment for EOS. 11 In 2020, Hughes et al. 12 constructed a six-case survey that was sent to 20 EOS world thought-leaders with an average clinical experience of 24 years. The response rate was 100% with no consensus on any case.
Diagnosis
Medical history and physical examination
A comprehensive past and current medical history represents the fundament in the evaluation of a patient with EOS.13,14 The personal history begins with pregnancy and birth and includes further health, motor and neurological development, as well as previous surgical procedures or hospitalizations, neuromuscular or syndromic conditions, and respiratory infections. Family and social history can provide additional information about possible hereditary problems or environmental influences related to EOS. The disease-specific history should include age of onset, history of progression, and previous nonoperative and operative treatment. Current medications and known allergies should be documented. Additional neurological, urological, cardiological, neurosurgical, and endocrinological conditions should also be inquired. In the clinical examination, the overall health and nutritional status of the child must be considered, always including documentation of body height and weight, and dietry regimen. In ambulatory patients, a primarily dynamic assessment with observation of gait pattern and assessment of motor skills, such as toe-walking or bipedal and unipedal hopping, is very informative and allows the child to build confidence. In addition to a thorough general clinical examination, spine-specific assessment includes the evaluation of spine, chestwall, and rib deformities, as well as inspection for dimpling, sinus tracts, or other cutaneous signs of spinal dysraphism.
Radiographic evaluation
A posteroanterior and lateral full spine radiograph, preferably with a low-dose EOS imaging system and whenever possible in standing position, is the first line of diagnostic imaging. In addition to objectifying the spinal deformity and potential failure of segmentation and/or formation, abnormalities of the rib cage and ribs can be assessed and statements about skeletal maturity can be made based on the radiographic aspect of the different growth zones.
There is still no clear consensus regarding the routine performance of magnetic resonance imaging (MRI) in patients with EOS. In a global cohort of 836 patients, the overall prevalence of abnormal MRI findings was 24%. 15 In addition to the routine performance of an MRI prior to any planned spine intervention, we accordingly consider an MRI to be generally indicated also in case of an observational approach. However, in the absence of anamnestic and clinical neurological abnormalities, we try to postpone the MRI until it can be performed without the need for anesthesia.
An additional computed tomography (CT) scan is helpful to further investigate bony abnormalities and three-dimensional (3D) reconstructions or even CT-based 3D-printed models of the affected part of the spine can comprehensively illustrate the underlying bony pathology and can also be extremely valuable for planning a surgical intervention.
Conservative treatment
Although there have been no relevant innovations in the field of conservative treatment approaches for EOS in recent years, serial casting and/or the use of braces have revived increased attention due to the high complication rate and the associated need for unplanned re-operations (UPROR) in surgical growth-friendly techniques. This trend is also reflected in a consensus study on the evaluation and evolution of the preferred treatment options for EOS among a selection of renowned pediatric spine surgeons over the last 10 years.16,17 In 2020, 11 pediatric spinal surgeons with a mean number of 32 years in practice were invited to re-evaluate 315 idiopathic and neuromuscular EOS cases after a 10-year interval. Preferences for conservative management have increased, especially in younger children, and casting was preferred over bracing in infantile cases. 16
The methods used for bracing largely correspond to the principles of elongation, derotation, and flexion, based on the techniques of Mehta and Cotrel (traction and apical derotation) or Risser (traction and apical lateral translation), each described in the 1970s.18,19 While a sustainable correction or at least control of the deformity seems possible with noncongenital EOS in case of early treatment,19,20 conservative measures are often used to gain time until surgical intervention becomes necessary (“buy time strategy”) with some studies showing an average time gain of 2–3 years.21 –24
Despite the assumed non-invasive approach and the associated lower risk profile of conservative methods, possible effects on health-related quality of life (HrQoL) must be taken into account. 25 While patients with non-idiopathic EOS showed a decrease in the sub-domains “transfer” and “emotions” in the EOSQ-24, patients with idiopathic EOS experienced a deterioration in almost all sub-domains during and partly also after plaster or brace treatment. In addition, repetitive cast changes at 2- to 4-month intervals are traditionally associated with a high exposure to general anesthesia, which can lead to lasting negative effects on learning and behavioral functions, especially in children under 3 years of age. 26 However, a study investigating the radiological and clinical outcomes of serial body casting with and without general anesthesia in infantile idiopathic scoliosis (IIS) has shown that the hurdle of repetitive general anesthesia could be omitted in a selected population. 27 The study included 121 children who underwent serial casting for IIS. In 29 patients serial casting was performed awake, diverting the children’s attention with electronic devices. Although, these patients were older (median 3.4 vs 2.4 years), had a lower body mass index, and more severe curve magnitudes, they presented similar radiographic outcomes with regard to major curve correction, as well as gain in thoracic and total spine height, when compared to those who were casted under general anesthesia. This again is in contrast with the findings by Canavese et al. 28 who reported a better initial deformity correction in patients with juvenile scoliosis who were casted under general anesthesia, especially in combination with neuromuscular-blocking drugs. Further studies are necessary to address this relevant aspect more reliably.
Operative treatment
Surgical treatment of spinal deformities dates back more than 100 years. After the original goal of preventing further worsening of scoliosis and lowering mortality by aiming for uninstrumented bony fusion, the possibility of—at least partial—correction of a deformity by instrumented spondylodesis emerged with spinal implants introduced by pioneers such as Harrington, Luque, Cotrel, and Dubousset from the 1960s onwards. 29 Although primarily designed for adolescent and adult patients, these techniques were increasingly used for EOS that could no longer be controlled conservatively. After the initial use of periodically lengthened unilateral Harrington rods, or Luque trolley systems for passive growth control, a “claw foundation” with anchorage of a growing rod proximal and distal to the deformity, sometimes combined with apical fusion, has been described in the early 1990s. 30 Based on this, the use of dual growing rods with spine-based fixation above and below the deformity became the gold standard in the surgical treatment of progressive EOS. 31 In 2014, the different techniques for surgical growth-friendly management were divided into 3 groups, distinguishing between distraction- and compression-based systems, and growth-guiding techniques, which is still valid in principle. 32 However, with the introduction of motorized magnetically controlled growing rods (MCGRs), the heterogeneity in the choice of growth-friendly implants for the index surgery has decreased massively with a marked predominance of MCGR. 33 Recently, efforts have also been made to systematically characterize an older, heterogeneous EOS patient population, the so-called “tweeners,” who qualify for either growth-friendly treatment options or definitive fusion due to their age and skeletal maturity. 34
Vertical expandable prosthetic titanium rib (VEPTR®)
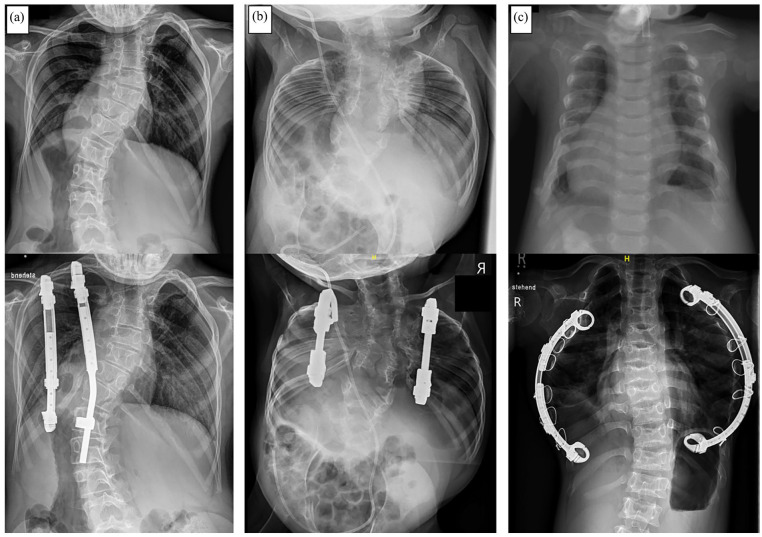
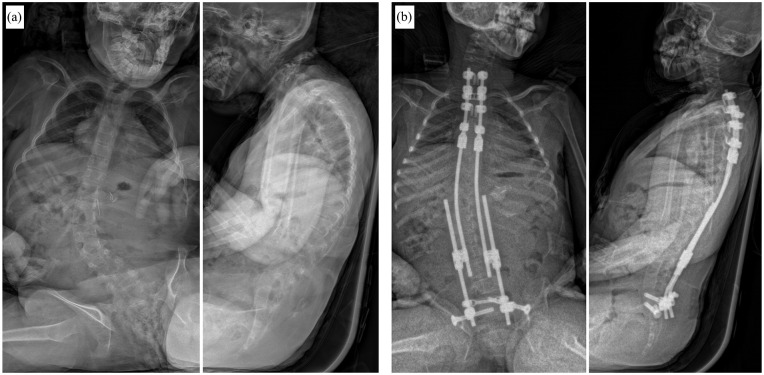
The main indication for VEPTR, originally provided by the inventor, Dr Campbell Jr, is the presence of TIS in skeletally immature patients. From an anatomical perspective, in addition to missing or fused ribs, a hypoplastic thorax, or EOS of congenital or neurogenic etiology without rib anomalies are also indications for VEPTR treatment (Figure 1). 35 Although often clinically evident, changes in lung function in VEPTR-treated patients are difficult to objectify with standard pulmonary function tests, and newer techniques based on dynamic magnetic resonance imaging may provide valuable evidence. 36
Figure 1.
Common indications for the use of Vertical Expandable Prosthetic Titanium Rib (VEPTR) as a distraction-based growth-friendly technique in case of thoracic insufficiency syndrome (TIS): (a) Congenital early onset scoliosis (EOS) in combination with fused ribs. (b) Volume-depletion deformity (VDD) type IIIa in a patient with Jarcho-Levin syndrome with a foreshortened thorax. (c) VDD type IIIb in a patient with Jeune syndrome and a transverse constricted thorax.
The potential to improve lung function while indirectly controlling the spinal deformity has continuously expanded the range of indications for VEPTR, not least due to frequent reports of complications with traditional growing rods (TGR). However, the initial enthusiasm for spine-sparing deformity correction has gradually faded with an increasing number of reported complications, including the evidence of extraspinal ossifications along the implants and across ribs. 37 The avoidance of repetitive surgical implant lengthening with the availability of motorized distraction-based implants has further reduced the use of VEPTR.
As with other distraction-based systems, VEPTR primarily follows a delaying fusion strategy, and the limited data and heterogeneity of the treated population limit the decision-making process for so-called “graduates” of growth-sparing surgery. In many cases, the decision on when to stop lengthening is less surgeon driven than more determined by curve progression, lack of further distraction, or complications. 38 Previous evidence of outcomes after final fusion with modest correction and high complication and reoperation rates, both in VEPTR patients and with other growth-friendly systems, prompted to rethink the original idea of controlling the deformity during growth and aiming for significant correction with conversion to final fusion (Figure 2).39 –41
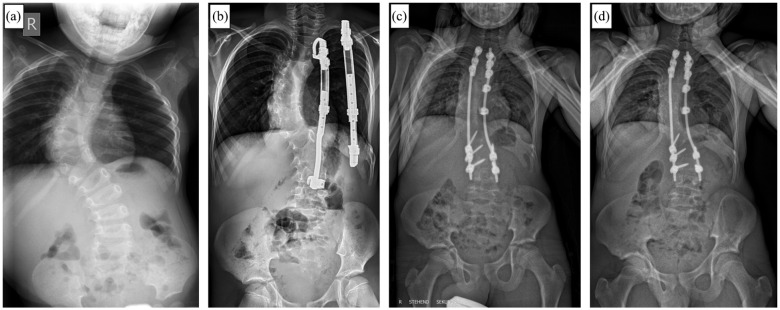
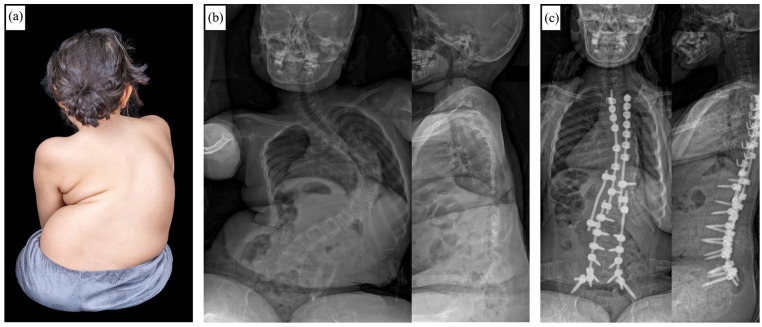
Figure 2.
Course of a patient with congenital scoliosis and concave fused ribs before (a) and during (b) Vertical Expandable Prosthetic Titanium Rib (VEPTR) treatment. After frustrating lengthening attempts due to spontaneous stiffening of the spine and thorax over time, final fusion surgery had to be performed before reaching skeletal maturity (c). (d) Follow-up x-ray 3 years after conversion to final fusion.
Due to the more restrained use of VEPTR in recent years, there are only few valid data regarding patient-reported outcome. The complication-prone course and the necessity for repetitive surgical lengthening with encumbering cosmetic consequences explain the poor evaluations in regard to “pain”, “self-image”, and “function” (Figure 3). 42
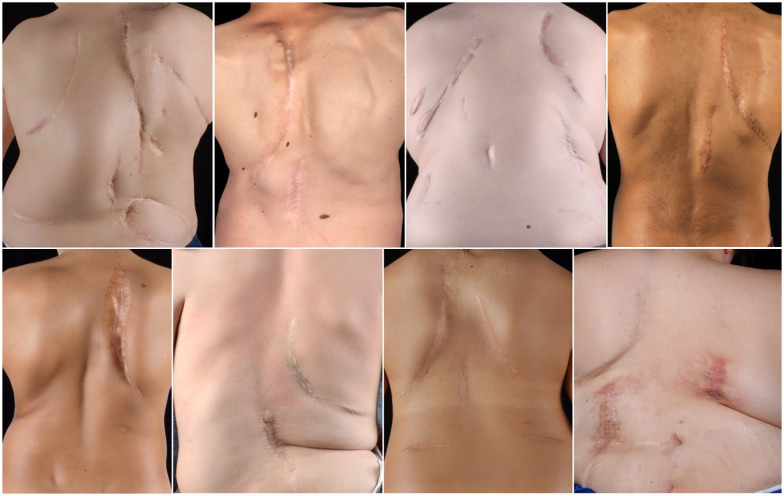
Figure 3.
Clinical pictures of different patients treated with Vertical Expandable Prosthetic Titanium Rib (VEPTR) System. Tipically, a parascapular approach is used for the proximal rib anchors. Depending on the location of the distal foundation, the incision is either performed in the midline (for rib-to-spine constructs) or above the iliac crest (for rib-to-pelvis constructs). Due to the need for repetitive surgical implant lengthening, the scars tend to become broad and hypertrophic.
Growing rods
Progressive EOS in patients without thoracic or rib abnormalities and a normally segmented spine are the classic indications for Growing Rods (Figure 4). In the era of MCGR, the use of TGR has been significantly reduced. However, TGRs can still play a relevant role in patients of short stature with stiff, hyperkyphotic curves. 43 Dual rods are superior to single rods in terms of complications. 31 The extent to which this is true in regard of correction and control of deformity, as well as gain in length is controversial.31,44
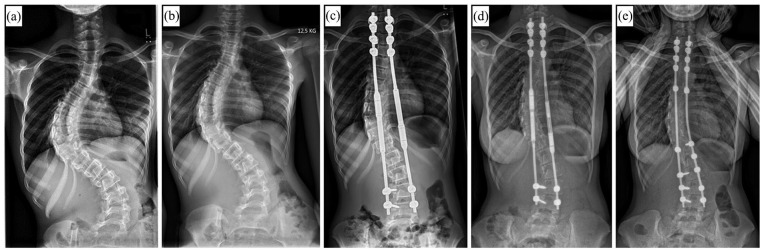
Figure 4.
X-rays of a 9-year-old female patient with syndromic early onset scoliosis (EOS) before treatement (a), after 2 weeks of preoperative halo gravity traction (b), and after index surgery with magnetically controlled growing rods (MCGR) (c). (d) Radiograph 4 years after index surgery showing convex-sided failure to lengthen. (e) 2 years after conversion to definitive instrumented spinal fusion.
MCGRs do not require open lengthening, which, in addition to a reduction in peri- and intraoperative complications, has a particular effect on the psychosocial burden of patients and their relatives. 45 Accordingly, MCGRs are now used in >80% of index operations in EOS. 46 However, the initial enthusiasm gave way to the humiliating results of progressive complication rates with increasing follow-up,47,48 and it can be assumed that this number will soon decline. Based on an expert consensus, inadequate skin and soft tissue cover, a stiff spinal curve, a sagittal curve apex above T3, hyperkyphosis, or patients requiring repetitive MRI for their care were recently defined as contraindications for the use of MCGR. 49 Whereas in parallel, a syndromic EOS patient without prior surgical treatment and the possibility of using a 90-mm actuator was declared an ideal candidate for MCGR. 50 The supposed cost savings of MCGR over TGR has also recently been questioned due to frequent reports of early implant failure 51 and by the latest Field Safety Notice (FSN) in December 2021 in Europe, urgently recommending to change or remove the implant(s) after implantation time of no more than 2 years (MAGEC and Precice systems CE Marks FAQs_7December2021_Final (nuvasive.com)).
The predominance of a motorized implants in the treatment of EOS has clearly underlined the claim for non-invasive surgical growth management. Hopefully, further optimisation of existing, or development of new motorized implants can address this need and broaden the options for the treating physicians.
Guided growth
The high costs and the associated limited global availability of motorized implants, as well as the high complication and reoperation rates of all classic distraction-based techniques, have again paved the way for « guided growth » implants. Traditional systems, such as the Shilla technique or Luque Trolley, have not been able to sustainably be established. In addition to the lack of growth stimulation and the presumably lower correction rate, reports of premature autofusion with the Luque trolley or a significant risk of crankshaft phenomenon with the Shilla system have led to a hesitant use of these techniques.52,53 New techniques are currently being validated. Like growing rods, these systems are based on a bipolar spine-based anchorage proximal and distal to the deformity. While the Spring Distraction System (SDS) shall be validated for all EOS aetiologies, the One-Way Self-Expanding Rod (OWSER) system is now mainly recommended for the treatment of neuromuscular EOS (Figure 5).54,55 The available short-term results are encouraging but must stand the test of time.56,57
Figure 5.
One-Way Self-Expanding Rod (OWSER) technique as a growth guiding concept in neuromuscular Early Onset Scoliosis (EOS): (a) Preoperative anteroposterior (ap) an lateral x-ray of a 9-year-old female patient with progressive neuromuscular EOS. (b) Postoperative ap and lateral x-ray after instrumentation with the OWSER system.
With the persistent challenges of surgical growth guidance associated with current techniques, discussion has recently been raised regarding the optimal treatment of an older, biologically more advanced population of patients with EOS, the so-called “tweeners,” in whom in addition to growth-friendly approaches primary final fusion may be considered. Based on a three-round survey, consensus (≥70% agreement) was reached among experts in regard of chronological age (8–10 years in girls; 9–11 years in boys), as well as inclusion of Sanders stage (<4). Patients with Sanders stage ≥4, closed triradiate cartilage, and postmenarchal should no longer be included in the tweener group and should preferably undergo corrective spondylodesis (Figure 6). 34
Figure 6.
(a) Clinical photograph of a 9-year-old non-ambulant female patient with cerebral palsy with rapidly progressive neuromuscular early onset scoliosis. Posteroanterior and lateral radiographs preoperative (b) and after posterior instrumented spinal fusion surgery without previous growth-friendly surgery (c).
Similar criteria are found in the anterior convex growth-guiding system approaches that have been highly controversial in recent years. Modern anterior vertebral body tethering techniques are primarily dedicated to be used in skeletally immature patients with adolescent idiopathic scoliosis. 58 Recently, however, there has been an expansion of the range of indications in non-idiopathic and even EOS, particularly in the previously defined tweener category.59,60 The limited available results with short follow-up periods do not yet allow an adequate assessment, and the patients treated with this technique did not really comply with the EOS definition in terms of age.
The extensive enthusiasm for growth-friendly surgery leads to certain approaches receiving little or no attention in many publications. Congenital EOS, for example, can often be sustainably treated by a single surgical procedure at an early age to prevent long-term and stressful treatments, as well as functional restrictions and growth deficit. This is especially true for congenital EOS in which no additional rib fusions are present or in which only single or few segments of the spine are affected. Surgical options range from convex-side growth inhibition using pedicle screw epiphyseodesis, to partial or total (hemi-)vertebra resection (Figure 7). 61
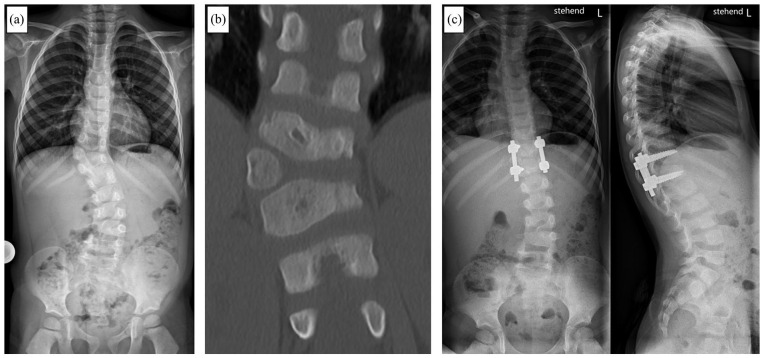
Figure 7.
A 4-year-old male patient with congenital early onset scoliosis (EOS). Anteroposterior (ap) x-ray (a) and computed tomography (b) showing a fully segmented accessory hemi-vertebra T11/T12. (c) ap and lateral xray after hemi-vertebra resection an instrumented monosegmental spinal fusion of the adjacent segments.
Current challenges and future perspectives
Recent years have taught us that there is no cookbook approach in the treatment of EOS. A multidisciplinary team represents the basis for an optimized individual strategy, in which the principles of indication of the available growth-friendly options, as well as recent pharmacological and medical advances must be considered. With the fading enthusiasm for motorized implants as a comprehensive problem solver, a more differentiated approach will be necessary again in the future. Fundamental problems, such as progressive stiffening of the spine, diminished gains in length, and a kyphosing effect remain unavoidable with rod-based systems.37,51,62 –64 In addition, there are implant-specific problems, such as metallosis in MCGR, whose long-term consequences we do not yet know. 65 The different growth-friendly strategies can be used selectively based on the EOS etiology, for example modern growth guiding techniques such as the one-way self-expanding rod system (OWSER) in neuromuscular EOS, or they can be predetermined by anatomical features, for example as hybrid constructs (Figure 8). In addition, there is a distinct trend back to a time-buying non-operative initial treatment approach, opposed to early definitive fusion in older EOS patients. 16
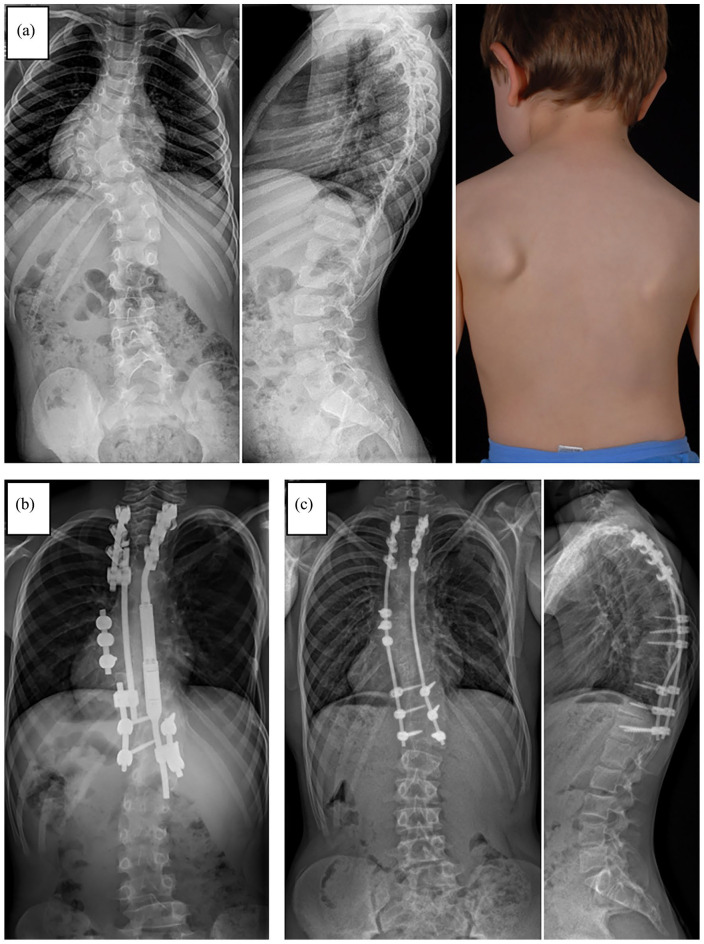
Figure 8.
(a) X-ray and clinical photograph of a 6-year-old male patient with congenital early onset scoliosis (EOS). (b) X-ray after growth-friendly surgery with a hybrid construct including a magnetically controlled growing rod (MAGEC®) on the concave side in combination with apical fusion and a passively sliding construct on the convex side. (c) Posteroanterior and lateral x-ray after conversion to final instrumented spinal fusion. Due to spontaneous bony fusion between the proximal and distal foundation no additional anchors were used (apart from the initial convex-sided apical fusion screws).
The heterogeneity of patients included under the umbrella of EOS and the overall low incidence mandate a systematic approach with multicenter research projects and a global exchange among experts. In addition, healthcare and industry partners must prioritize their ethical obligations to fund research and empower the development of improved treatment options.
Supplemental Material
Supplemental material, sj-pdf-1-cho-10.1177_18632521241228141 for Diagnostic and therapeutic strategies in early onset scoliosis: A current concept review by Daniel Studer and Carol Claudius Hasler in Journal of Children’s Orthopaedics
Footnotes
Author contributions: D.S.: Conceptualization and drafting of the manuscript.
C.C.H.: Critical revision of the manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical statement: Approval not needed because of the article type. This article does not contain any studies with human or animal participants. Written informed consent was obtained from the individuals for the publication of any potentially identifiable images in this article.
Supplemental material: Supplemental material for this article is available online.
References
- 1. El-Hawary R, Akbarnia BA. Early onset scoliosis: time for consensus. Spine Deform 2015; 3(2): 105–106. [DOI] [PubMed] [Google Scholar]
- 2. Williams BA, Matsumoto H, McCalla DJ, et al. Development and initial validation of the classification of early-onset scoliosis (C-EOS). J Bone Joint Surg Am 2014; 96(16): 1359–1367. [DOI] [PubMed] [Google Scholar]
- 3. Dragsted C, Ohrt-Nissen S, Hallager DW, et al. Reproducibility of the classification of early onset scoliosis (C-EOS). Spine Deform 2020; 8(2): 285–293. [DOI] [PubMed] [Google Scholar]
- 4. Karol LA. The natural history of early-onset scoliosis. J Pediatr Orthop 2019; 39(6 Suppl. 1): S38–S43. [DOI] [PubMed] [Google Scholar]
- 5. Pehrsson K, Larsson S, Oden A, et al. Long-term follow-up of patients with untreated scoliosis: a study of mortality, causes of death, and symptoms. Spine 1992; 17(9): 1091–1096. [DOI] [PubMed] [Google Scholar]
- 6. Karol LA. Early definitive spinal fusion in young children: what we have learned. Clin Orthop Relat Res 2011; 469(5): 1323–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Karol LA, Johnston C, Mladenov K, et al. Pulmonary function following early thoracic fusion in non-neuromuscular scoliosis. J Bone Joint Surg Am 2008; 90(6): 1272–1281. [DOI] [PubMed] [Google Scholar]
- 8. Campbell RM, Jr, Smith MD, Mayes TC, et al. The characteristics of thoracic insufficiency syndrome associated with fused ribs and congenital scoliosis. J Bone Joint Surg Am 2003; 85(3): 399–408. [DOI] [PubMed] [Google Scholar]
- 9. Matsumoto H, Williams B, Park HY, et al. The final 24-Item Early Onset Scoliosis Questionnaires (EOSQ-24): validity, reliability and responsiveness. J Pediatr Orthop 2018; 38(3): 144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Johnson MA, Lott C, Clark AJ, et al. Changes in research quality and surgical trends at the international congress on early-onset scoliosis. Spine Deform 2023; 11(3): 707–713. [DOI] [PubMed] [Google Scholar]
- 11. Hasler CC. Early-onset scoliosis: contemporary decision-making and treatment options. J Pediatr Orthop 2018; 38(Suppl. 1): S13–S20. [DOI] [PubMed] [Google Scholar]
- 12. Hughes MS, Swarup I, Makarewich CA, et al. Expert consensus for early onset scoliosis surgery. J Pediatr Orthop 2020; 40(7): e621–e628. [DOI] [PubMed] [Google Scholar]
- 13. Akbarnia B, Yazici M, Thompson G. The growing spine. 2nd ed. Berlin; Heidelberg: Springer, 2016. [Google Scholar]
- 14. Ashebo L, Anari JB, Cahill PJ. Update on the diagnosis and management of early-onset scoliosis. Curr Rev Musculoskelet Med 2023; 16: 447–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams BA, McClung A, Blakemore LC, et al. MRI utilization and rates of abnormal pretreatment MRI findings in early-onset scoliosis: review of a global cohort. Spine Deform 2020; 8(5): 1099–1107. [DOI] [PubMed] [Google Scholar]
- 16. Matsumoto H, Fano AN, Quan T, et al. Re-evaluating consensus and uncertainty among treatment options for early onset scoliosis: a 10-year update. Spine Deform 2023; 11(1): 11–25. [DOI] [PubMed] [Google Scholar]
- 17. Corona J, Miller DJ, Downs J, et al. Evaluating the extent of clinical uncertainty among treatment options for patients with early-onset scoliosis. J Bone Joint Surg Am 2013; 95(10): e67. [DOI] [PubMed] [Google Scholar]
- 18. Risser JC. Scoliosis treated by cast correction and spine fusion. Clin Orthop Relat Res 1976(116): 86–94. [PubMed] [Google Scholar]
- 19. Mehta MH. Growth as a corrective force in the early treatment of progressive infantile scoliosis. J Bone Joint Surg Br 2005; 87(9): 1237–1247. [DOI] [PubMed] [Google Scholar]
- 20. Fedorak GT, MacWilliams BA, Stasikelis P, et al. Age-stratified outcomes of mehta casting in idiopathic early-onset scoliosis: a multicenter review. J Bone Joint Surg Am 2022; 104(22): 1977–1983. [DOI] [PubMed] [Google Scholar]
- 21. Fletcher ND, McClung A, Rathjen KE, et al. Serial casting as a delay tactic in the treatment of moderate-to-severe early-onset scoliosis. J Pediatr Orthop 2012; 32(7): 664–671. [DOI] [PubMed] [Google Scholar]
- 22. Baulesh DM, Huh J, Judkins T, et al. The role of serial casting in early-onset scoliosis (EOS). J Pediatr Orthop 2012; 32(7): 658–663. [DOI] [PubMed] [Google Scholar]
- 23. LaValva S, Adams A, MacAlpine E, et al. Serial casting in neuromuscular and syndromic early-onset scoliosis (EOS) can delay surgery over 2 years. J Pediatr Orthop 2020; 40(8): e772–e779. [DOI] [PubMed] [Google Scholar]
- 24. Demirkiran HG, Bekmez S, Celilov R, et al. Serial derotational casting in congenital scoliosis as a time-buying strategy. J Pediatr Orthop 2015; 35(1): 43–49. [DOI] [PubMed] [Google Scholar]
- 25. Matsumoto H, Auran E, Fields MW, et al. Serial casting for early onset scoliosis and its effects on health-related quality of life during and after discontinuation of treatment. Spine Deform 2020; 8(6): 1361–1367. [DOI] [PubMed] [Google Scholar]
- 26. Hu D, Flick RP, Zaccariello MJ, et al. Association between exposure of young children to procedures requiring general anesthesia and learning and behavioral outcomes in a population-based birth cohort. Anesthesiology 2017; 127(2): 227–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. LaValva SM, MacAlpine EM, Kawakami N, et al. Awake serial body casting for the management of infantile idiopathic scoliosis: is general anesthesia necessary. Spine Deform 2020; 8(5): 1109–1115. [DOI] [PubMed] [Google Scholar]
- 28. Canavese F, Dimeglio A. Serial elongation derotation flexion casting in children with infantile and juvenile scoliosis. Ann Transl Med 2020; 8(2): 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hasler CC. A brief overview of 100 years of history of surgical treatment for adolescent idiopathic scoliosis. J Child Orthop 2013; 7(1): 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Morin C. Pediatric cotrel-dubousset instrumentation system. In: Bridwell KH, Dewald RL. (eds) The textbook of spinal deformity. Philadelphia, PA: J. B. Lipincott, 1991, pp. 212–217. [Google Scholar]
- 31. Thompson GH, Akbarnia BA, Kostial P, et al. Comparison of single and dual growing rod techniques followed through definitive surgery: a preliminary study. Spine 2005; 30(18): 2039–2044. [DOI] [PubMed] [Google Scholar]
- 32. Skaggs DL, Akbarnia BA, Flynn JM, et al. A classification of growth friendly spine implants. J Pediatr Orthop 2014; 34(3): 260–274. [DOI] [PubMed] [Google Scholar]
- 33. Murphy RF, Neel GB, Barfield WR, et al. Trends in the utilization of implants in index procedures for early onset scoliosis from the Pediatric Spine Study Group. J Pediatr Orthop 2022; 42(9): e912–e916. [DOI] [PubMed] [Google Scholar]
- 34. Quan T, Matsumoto H, Bonsignore-Opp L, et al. Definition of tweener: consensus among experts in treating early-onset scoliosis. J Pediatr Orthop 2023; 43(3): e215–e222. [DOI] [PubMed] [Google Scholar]
- 35. Studer D, Hasler CC. Long term outcome of vertical expandable prosthetic titanium rib treatment in children with early onset scoliosis. Ann Transl Med 2020; 8(2): 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tong Y, Udupa JK, McDonough JM, et al. Assessment of regional functional effects of surgical treatment in thoracic insufficiency syndrome via dynamic magnetic resonance imaging. J Bone Joint Surg Am 2023; 105(1): 53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zivkovic V, Büchler P, Ovadia D, et al. Extraspinal ossifications after implantation of vertical expandable prosthetic titanium ribs (VEPTRs). J Child Orthop 2014; 8(3): 237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pizones J, Martín-Buitrago MP, Sánchez Márquez JM, et al. Decision making of graduation in patients with early-onset scoliosis at the end of distraction-based programs: risks and benefits of definitive fusion. Spine Deform 2018; 6(3): 308–313. [DOI] [PubMed] [Google Scholar]
- 39. Studer D, Büchler P, Hasler CC. Radiographic outcome and complication rate of 34 graduates after treatment with vertical expandable prosthetic titanium rib (VEPTR): a single center report. J Pediatr Orthop 2019; 39(10): e731–e736. [DOI] [PubMed] [Google Scholar]
- 40. Sawyer JR, de Mendonça RG, Flynn TS, et al. Complications and radiographic outcomes of posterior spinal fusion and observation in patients who have undergone distraction-based treatment for early onset scoliosis. Spine Deform 2016; 4(6): 407–412. [DOI] [PubMed] [Google Scholar]
- 41. Flynn JM, Tomlinson LA, Pawelek J, et al. Growing-rod graduates: lessons learned from ninety-nine patients who completed lengthening. J Bone Joint Surg Am 2013; 95(19): 1745–1750. [DOI] [PubMed] [Google Scholar]
- 42. Haapala H, Heiskanen S, Syvänen J, et al. Surgical and health-related quality of life outcomes in children with congenital scoliosis during 5-year follow-up: comparison to age and sex-matched healthy controls. J Pediatr Orthop 2023; 43(6): e451–e457. [DOI] [PubMed] [Google Scholar]
- 43. Varley ES, Pawelek JB, Mundis GM, Jr, et al. The role of traditional growing rods in the era of magnetically controlled growing rods for the treatment of early-onset scoliosis. Spine Deform 2021; 9(5): 1465–1472. [DOI] [PubMed] [Google Scholar]
- 44. Wang T, Fan N, Zang L, et al. Comparative efficacy and complications of single and dual growing rods for early-onset scoliosis: an updated meta-analysis. Eur Spine J 2023; 32(1): 167–180. [DOI] [PubMed] [Google Scholar]
- 45. Matsumoto H, Skaggs DL, Akbarnia BA, et al. Comparing health-related quality of life and burden of care between early-onset scoliosis patients treated with magnetically controlled growing rods and traditional growing rods: a multicenter study. Spine Deform 2021; 9(1): 239–245. [DOI] [PubMed] [Google Scholar]
- 46. Klyce W, Mitchell SL, Pawelek J, et al. Characterizing use of growth-friendly implants for early-onset scoliosis: a 10-year update. J Pediatr Orthop 2020; 40(8): e740–e746. [DOI] [PubMed] [Google Scholar]
- 47. Lebel DE, Rocos B, Helenius I, et al. Magnetically controlled growing rods graduation: deformity control with high complication rate. Spine 2021; 46(20): E1105–E1112. [DOI] [PubMed] [Google Scholar]
- 48. Choi E, Yaszay B, Mundis G, et al. Implant complications after magnetically controlled growing rods for early onset scoliosis: a multicenter retrospective review. J Pediatr Orthop 2017; 37(8): e588–e592. [DOI] [PubMed] [Google Scholar]
- 49. Matsumoto H, Sinha R, Roye BD, et al. Contraindications to magnetically controlled growing rods: consensus among experts in treating early onset scoliosis. Spine Deform 2022; 10(6): 1289–1297. [DOI] [PubMed] [Google Scholar]
- 50. Heyer JH, Anari JB, Baldwin KD, et al. Lengthening behavior of magnetically controlled growing rods in early-onset scoliosis: a multicenter study. J Bone Joint Surg Am 2022; 104(24): 2186–2194. [DOI] [PubMed] [Google Scholar]
- 51. Shaw KA, Bassett P, Ramo BA, et al. The evolving stall rate of magnetically controlled growing rods beyond 2 years follow-up. Spine Deform 2023; 11(2): 487–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mardjetko SM, Hammerberg KW, Lubicky JP, et al. The Luque trolley revisited. Spine 1992; 17(5): 582–589. [DOI] [PubMed] [Google Scholar]
- 53. Wilkinson JT, Songy CE, Bumpass DB, et al. Curve modulation and apex migration using shilla growth guidance rods for early-onset scoliosis at 5-year follow-up. J Pediatr Orthop 2019; 39(8): 400–405. [DOI] [PubMed] [Google Scholar]
- 54. Miladi L, Khouri N, Pradon J, et al. One-way self-expanding rod for early-onset scoliosis: early results of a clinical trial of 20 patients. Eur Spine J 2021; 30(3): 749–758. [DOI] [PubMed] [Google Scholar]
- 55. Lemans JVC, Wijdicks SPJ, Castelein RM, et al. Spring distraction system for dynamic growth guidance of early onset scoliosis: two-year prospective follow-up of 24 patients. Spine J 2021; 21(4): 671–681. [DOI] [PubMed] [Google Scholar]
- 56. Tabeling CS, Lemans JVC, Topet A, et al. The spring distraction system for growth-friendly surgical treatment of early onset scoliosis: a preliminary report on clinical results and safety after design iterations in a prospective clinical trial. J Clin Med 2022; 11(13): 3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gaume M, Hajj R, Khouri N, et al. One-way self-expanding rod in neuromuscular scoliosis: preliminary results of a prospective series of 21 patients. JB JS Open Access 2021; 6(4): e2100089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Parent S, Shen J. Anterior vertebral body growth-modulation tethering in idiopathic scoliosis: surgical technique. J Am Acad Orthop Surg 2020; 28(17): 693–699. [DOI] [PubMed] [Google Scholar]
- 59. Baroncini A, Courvoisier A. The different applications of vertebral body tethering: narrative review and clinical experience. J Orthop 2023; 37: 86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Mackey C, Hanstein R, Lo Y, et al. Magnetically controlled growing rods (MCGR) versus single posterior spinal fusion (PSF) versus vertebral body tether (VBT) in older early onset scoliosis (EOS) patients: how do early outcomes compare? Spine 2022; 47(4): 295–302. [DOI] [PubMed] [Google Scholar]
- 61. Ruf M. [Surgical treatment of congenital scoliosis]. Oper Orthop Traumatol. Epub ahead of print 19 September 2023. DOI: 10.1007/s00064-023-00827-5. [DOI] [PubMed] [Google Scholar]
- 62. Cahill PJ, Marvil S, Cuddihy L, et al. Autofusion in the immature spine treated with growing rods. Spine 2010; 35(22): E1199–E1203. [DOI] [PubMed] [Google Scholar]
- 63. Sankar WN, Skaggs DL, Yazici M, et al. Lengthening of dual growing rods and the law of diminishing returns. Spine 2011; 36(10): 806–809. [DOI] [PubMed] [Google Scholar]
- 64. El-Hawary R, Sturm P, Cahill P, et al. What is the risk of developing proximal junctional kyphosis during growth friendly treatments for early-onset scoliosis. J Pediatr Orthop 2017; 37(2): 86–91. [DOI] [PubMed] [Google Scholar]
- 65. Zhang T, Sze KY, Peng ZW, et al. Systematic investigation of metallosis associated with magnetically controlled growing rod implantation for early-onset scoliosis. Bone Joint J 2020; 102-B(10): 1375–1383. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-cho-10.1177_18632521241228141 for Diagnostic and therapeutic strategies in early onset scoliosis: A current concept review by Daniel Studer and Carol Claudius Hasler in Journal of Children’s Orthopaedics