Abstract
Purpose:
This study aims to develop an accessible stepwise management algorithm for pediatric presentations of occipital condyle fractures (OCFs) based on a systematic review of the published literature regarding diagnostic evaluation, treatment, and outcomes.
Methods:
A systematic review of the literature was conducted on PubMed to locate English language studies reporting on the management of pediatric OCFs. Data extraction of clinical presentation, management strategies, imaging, and treatment outcome was performed.
Results:
A total of 15 studies reporting on 38 patients aged 18 years and younger presenting with OCFs were identified. Loss of consciousness (LOC), depressed level of consciousness, neck pain, decreased neck range of motion (ROM), and cranial nerve injury were the most common presenting symptoms. Diagnostic imaging included radiographs, computed tomography (CT) scans, magnetic resonance imaging (MRI), and functional radiographs to assess cervical stability. Treatment options varied and included soft collar, hard collar, and halo vest. All studies resulted in a complete healing of the OCF, with resolution of associated pain.
Conclusion:
The proposed treatment algorithm suggests a framework for the management of pediatric OCFs based on the available evidence (levels of evidence: 3, 4). This review of the literature indicated that a stepwise approach should be utilized in the management of isolated pediatric OCFs.
Keywords: Occipital condyle fracture, craniocervical junction, management algorithm, children, pediatrics
Introduction
Occipital condyle fractures (OCFs) are injuries at the craniocervical junction (CCJ), where the condyles, crucially linked with the first cervical vertebra, endure trauma. 1 OCFs in the pediatric population are not extensively described in the literature; despite often being considered rare,1–3 they manifest in 1%–16% of cervical spine injuries.1,2–5 OCFs are most associated with high-energy traumas, such as motor vehicle accidents (MVA) or falls from heights.1,2,4,6 OCFs in the pediatric population can occur even without indicative symptoms associated with other cervical spine injuries. 5 OCF symptoms can lead to severe neck pain, neurological deficits, headaches, altered consciousness, and difficulties in swallowing or speaking, and may also result in dangerous complications such as spinal cord compression and instability at the CCJ which may be life-threatening.2,4,7 Given their limited presence in existing literature concerning epidemiology and presentation, OCFs can be challenging to both identify and manage.
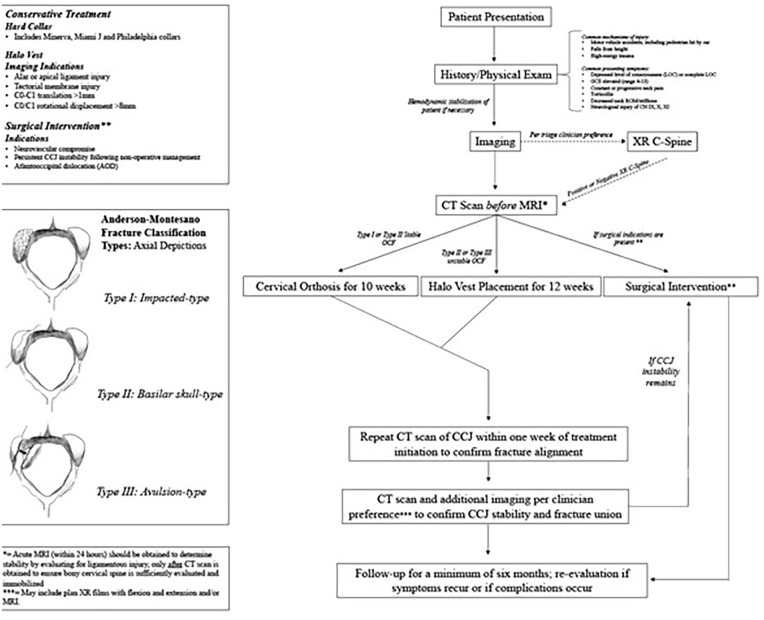
Management options for OCFs include observation, conservative strategies (soft collars, hard collars, and halo vests), and in severe cases, surgical occipital-cervical fusion.4,8,9 Best treatment for a patient is dependent on many factors including the severity of the OCF. OCF classification, often guiding treatment, is best described by Anderson and Montesano 10 in 1988, which presents three types of fractures (Figure 2). Type I is an OCF without displacement into the foramen magnum; Type II is a linear basioccipital fracture, that extends from the skull base to the foramen magnum; and Type III is a condylar avulsion near the alar ligament, which is described as an unstable fracture due to possible alar ligament and tectorial membrane stress and injury. Type I and II are typically considered stable; however, Type II may be classified as unstable if it shows features of severe dislocation, or tectorial membrane or ligamental tears. Type III is always considered unstable. OCFs were more recently further categorized by Tuli et al. in 1997, featuring magnetic resonance imaging (MRI) to evaluate stability of the condylar fracture. In the Tuli classification, Type I OCFs are non-displaced stable fractures; Type IIA OCFs are stable displaced fractures; and Type IIB OCFs are unstable displaced fractures. While the Tuli classification system is more contemporary, the studies highlighted in this review primarily employed the Anderson and Montesano criteria. We therefore utilized the Anderson and Montesano classification system, as well as patient age, size, symptom severity, and co-existing injuries (including ligamentous and other occipital–cervical injuries), as guidelines for optimal treatment.
Figure 2.
Management Algorithm of Pediatric Occipital Condyle Fracture.
Although OCF classification systems exist, and literature reporting OCFs in adults has increased as imaging techniques have improved, there remains no definitive consensus on optimal management for pediatric OCFs. This may create management variability and ambiguity and potentially lead to suboptimal treatment. This systematic review aims to evaluate pediatric OCF presentation, evaluation, treatment, and outcomes, with the goal of developing a well-informed management algorithm to guide clinical decision-making and improve patient care.
Methods
Search strategy
A systematic review was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) updated guidelines and checklist in 2020. 11 A digital search of MEDLINE (PubMed) was done between 2 August 2023 and 7 August 2023. The following terms were included in the search: “pediatric” or “child” or “children” or “adolescent” and “occipital condyle fracture” or “atlanto-occipital fracture.” All resulting studies were retrieved and stored to Covidence (app.covidence.org), a web-based software program used for streamlining the process of study selections and systematic review.
Study selection
All resulting studies retrieved were initially screened by title and abstract. Relevant studies progressed to full-text reviews, and eligibility criteria were applied to manuscripts describing case studies and systematic reviews of pediatric OCFs. Each study was independently screened and reviewed by first and third authors. Any inconsistencies were discussed and determined by the entirety of the authorship group. Exclusion criteria included (1) studies involving adult-only patients; (2) studies describing pediatric and adult cases, but with no distinction of pediatric from adult data; (3) case series describing CCJ injuries including OCFs, with no distinction of OCFs from other CCJ injuries; (4) studies with less than 1-month follow-up; (5) non-human studies; and (6) non-English studies.
Data extraction
Data collected from each study included first author; year of publication; number of pediatric cases; gender; mean age; age range; mechanism of injury (MOI); time from injury to treatment; co-occurring injuries; presenting symptoms; diagnostic imaging and results; Anderson and Montesano fracture type; treatment and treatment duration; imaging during treatment; post-treatment imaging; follow-up time; post-treatment recovery; and complications. For studies that did not clarify these categories, associated data were recorded as “not stated,” and these studies were excluded from our analysis.
Results
Search results
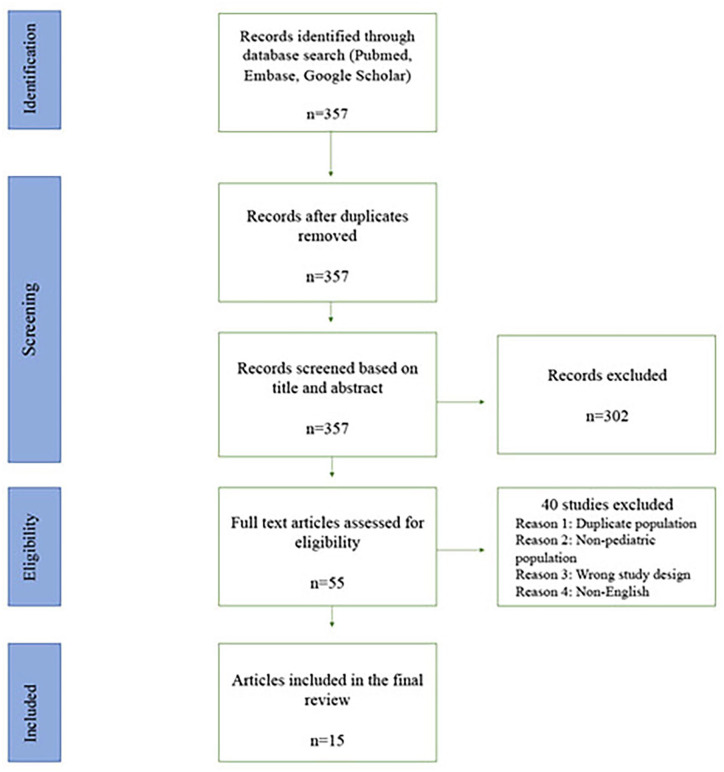
Our search resulted in 357 studies. After title and abstract screening, 55 articles underwent full-text review. A total of 15 studies qualified after applying exclusion criteria (Figure 1).
Figure 1.
PRISMA selection of studies.
Study characteristics
Among the 15 studies, six were large-cohort case reviews and systematic reviews (level of evidence 3) while nine were single case studies (level of evidence 4). These studies included a total of 38 patients 18 years and younger with OCFs. The data are presented below in order of presentation to treatment to best capture the evaluation process of OCFs.
Presentation and workup
Clinical presentation
All data describing presentation, including number of patients, mean age, MOI, and presenting symptomology, are listed in Table 1. Mean age among included studies ranged from 6 to 18 years. The most described presenting symptoms included loss of consciousness (LOC)/depressed level of consciousness (19/29 patients reported; 65.6%), with a Glasgow Coma Scale (GCS) ranging from 0 to 13 (average 10.75); neck pain (10/29; 34.5%); cranial nerve (CN) injury (8/28; 28.7%); torticollis (7/29; 24.1%); and limb paresis (5/29; 17.2%). Other studies described anterograde amnesia (1/29; 3.4%) and nausea/vomiting (1/29; 3.4%).
Table 1.
Summary of presenting characteristics, including average patient age, mechanism of injury, presenting symptoms, and associated injuries as described in included studies.
| Author (reference) | Number of patients (n) | Mean age (range, if applicable) (years) | Mode of injury (number of patients affected, if stated) | Presenting symptoms (number of patients affected, if stated) | Associated injuries |
|---|---|---|---|---|---|
| Abat et al. 12 | 1 | 17 | MVA | • GCS 13 • Temporal • Disorientation • Incoherent speech |
• Ulnar, radial, clavicle fractures • Pseudo-meningocele • Brachial plexus injury |
| Anderson and Montesano 10 | 2 | 10.5 (3–18) | MVA (2) | • LOC (2) • Neck pain once patient regained consciousness (1) |
Not stated |
| Bakhshi et al. 13 | 2 | 12.61 (9–16) | Not stated | Not stated | Not stated |
| Capuano et al. 14 | 2 | 15.5 (14–17) | High energy trauma (2) | • GCS 7 (2) • Cranial nerve 9, 10, 11 injury (2) |
• Diffuse brain edema • Epidural hematoma • C1-C2 fracture |
| Curri et al. 15 | 1 | 16 | Pedestrian struck by vehicle | • Deep Coma • Decerebration at four limbs • Abnormal position of head/torticollis • Stiff neck when awake |
• Thin subdural clot drained by use of a craniotomy |
| Harding-Smith et al. 16 | 1 | 18 | MVA | • LOC • “Tilted head”/torticollis |
• Right squamous temporal bone fracture with possible involvement of petrous portion |
| Kapapa et al. 17 | 1 | 15 | Falling off a carousel in a school yard | • Progressive neck pain • Head in a fixed position/torticollis |
None |
| Kelly and Parrish 18 | 1 | 16 | MVA | • GCS 9 | • Bleeding from occipital scalp wound • Frontal lobe hematoma |
| Leventhal et al. 8 | 2 | 17 | MVA | • GCS 10 • GCS 4 |
• Mild subluxation C4–5 • Mandibular fracture • Left pneumothorax |
| Momjian et al. 2 | 15 | 10 (7 months–15 years) | • MVA (4) • Pedestrian struck by vehicle (5) • Head injury (6) including 1. Fell off a donkey and was dragged 2. Skiing accident 3. Fell from a height 4. Trampoline accident 5. Father fell on head of child |
• Depressed consciousness or LOC (10) • GCS 13, 11, 8, 6, 5, 3 • Neck pain (2) • Torticollis/rigid neck (3) • Cranial nerve injury (3) • CN 5, 6, 7, 9, 10, 12 • Limb paresis (3) |
• Brain stem hemorrhage • C1 fracture • Brainstem hematoma • Hydrocephalus • Subarachnoid hemorrhage with hematoma • Cerebral edema • Frontal intracranial hemorrhage • Right lower limb fracture • Spinal cord contusion • “Multiple organ injury” • Mandible, forearm, rib, arm fractures • Pulmonary contusion • Diffuse axonal injury • Hepatic laceration • Aspiration pneumonia |
| Strehle and Tolinov 5 | 1 | 6 | Pedestrian struck by vehicle | • Anterograde amnesia • Nausea/vomiting • Neck pain |
• Occipital bone fracture |
| Tomaszewski et al. 4 | 6 | 15.83 (14.7–18) | • MVA (3) • Pedestrian struck by vehicle (1) • Fall from height (1) • Bike accident (1) |
Not stated | • Frontal bone fracture • Frontal sinus fracture • Frontal bone contusion • Lung contusion • Brain concussion pneumothorax • Neurogenic vocal cord injury • Aphasia • Subdural hematoma • Nasal bone fracture • Subarachnoid hemorrhage • Th3–5 transverse process fracture • Radial fracture • Orbit fracture • Maxillary sinus fracture |
| Ucler and Yucetas 19 | 1 | 16 | MVA | • Neck pain • Hypoglossal nerve injury (day 3 post-injury) |
None |
| Wasserberg and Bartlett 20 | 1 | 16 | MVA | Not stated | “Multiple injuries” with traumatic brain injury |
| Peeters and Verbeeten 21 | 1 | 17 | MVA | • Neck pain • Stiff neck/torticollis |
Mandibular fracture |
GCS: Glasgow Coma Scale; MVA: motor vehicle accident; LOC: loss of consciousness; CN: cranial nerve.
MOI included MVAs (16/35; 45.7% patient-reported), pedestrian–car collisions (6/35; 17.14%), or other high-energy traumas (13/35, 37.14%), including falling from a playground carousel and being dragged by a donkey. Associated injuries varied, and most commonly featured traumatic brain injury (18/33; 54.5%), including hemorrhages/hematomas, other cervical spine injury (8/28; 28.5%), facial fractures (7/34; 20.6%), and other corporeal injuries such as radial, ulnar, pelvic and clavicular fractures, pneumothoraxes, and other organ injury (6/34; 17.6%). A complete list of injuries can be found in Table 1.
Evaluation
All data describing evaluation of patients with OCF are listed in Table 2. The studies included initially evaluated patients using radiographs (XR), computed tomography (CT), and MRI. Method used varied by the year which the article was written, with more recent articles preferring CT scans. Of the 15 studies that utilized XR, all report that OCFs were not seen initially. Momjian et al. 2 noted that XR will nearly always fail to demonstrate OCFs, but that prevertebral swelling with asymmetry of the dens may indicate OCF presence and should trigger further evaluation. Two of our studies described this finding.2,16 In the 13 studies that utilized CT, 100.0% reported detection of OCF, in addition to associated subluxation or additional fracture fragments used to classify fracture type. Specifically, CTs and MRIs in Tomaszewski et al. 4 revealed a translation between 3 and 4 mm between C0 and C1, and a rotational displacement of C0/C1 by 10 and 12 degrees.4,9 Three studies (20%) commented on use of MRI in this setting,2,14 reporting associated injury to ligaments of atlantooccipital joints, joint capsules, and nearby vertebral disks as far caudal as C2–3.
Table 2.
Summary of diagnostic evaluation, including type of imaging utilized, OCF laterality, and OCF type.
| Author (reference) | Number of patients (n) | Diagnostic imaging utilized | OCF laterality (side; number of patients) | OCF type (number of patients) |
|---|---|---|---|---|
| Abat et al. 12 | 1 | • Cervical XR • CT head and neck • MRI |
Unilateral (R) | Type I (1) |
| Anderson and Montesano 10 | 2 | • CT | Unilateral (side not stated) (2) | Type I (1) Type III (1) |
| Bakhshi et al. 13 | 2 | Not stated | Not Stated | Not stated |
| Capuano et al. 14 | 2 | • XR • CT • MRI |
Unilateral (L; 2) | Type III (2) |
| Curri et al. 15 | 1 | • XR • CT scan |
Unilateral (R) | Type II (1) |
| Harding-Smith et al. 16 | 1 | • XR • hypocycloidal tomography |
Unilateral (R) | Type I (1) |
| Kapapa et al. 17 | 1 | • CT | Unilateral (L) | Type I (1) |
| Kelly and Parrish 18 | 1 | • XR • CT |
Unilateral (R) | Type III (1) |
| Leventhal et al. 8 | 2 | • CT • XR |
Unilateral (R; 2) | Type I (1) Type II (1) |
| Momjian et al. 2 | 15 | • XR • MRI • CT |
Unilateral (13) Bilateral (2) |
Type I (5) Type II (4) Type III (6) |
| Strehle and Tolinov 5 | 1 | • CT • XR |
Unilateral (L) | Type II (1) |
| Tomaszewski et al. 4 | 6 | • CT • MRI |
Side not stated—all unilateral | Type I (3) Type III (3) |
| Ucler and Yucetas 19 | 1 | • CT | Unilateral (L) | Type II (1) |
| Wasserberg and Bartlett 20 | 1 | • CT • Coronal reconstructions |
Unilateral (R) | Type III (1) |
| Peeters and Verbeeten 21 | 1 | • Cervical XR • CT neck • Open mouth XR |
Unilateral (R) | Type III (1) |
OCF: occipital condyle fracture; XR: radiographs; CT: computed tomography; MRI: magnetic resonance imaging.
Twelve studies (36 patients) described OCF injury type. Twelve patients (33.3%) were reported to have Type I OCF, with a mean age of 12.9 years (range = 3–17). Eight patients (22.2%) were reported to have Type II OCF, with a mean age of 6.2 years (range = 7 months–17 years). Sixteen patients (44.4%) were reported to have Type III OCF, with mean age 14.6 years (range = 2–18). Among the 36 patients for whom laterality was reported, 34 (94.4%) patients had unilateral OCFs, while only two patients (5.6%) had Type I bilateral OCFs.
Treatment options
Eight studies4,5,12,14,17–20 described time from injury to initial management. Seven (77.8%) of these studies described immediate or in-hospital treatment within 24 h.4,5,12,17–20 One study initiated treatment 1 day after the patient presented with progressive neck pain. 5 One study, featuring two patients, described treatment at 10 and 15 days, respectively. 14 A summary of all treatment options by fracture type can be found in Table 3.
Table 3.
Summary of treatments, treatment length, and imaging during treatment.
| Author (reference) | Number of patients (n) | Treatment by fracture type | Length of treatment | Imaging during treatment |
|---|---|---|---|---|
| Abat et al. 12 | 1 | Type I: rigid cervical collar | Type I: 2 months | Not stated |
| Anderson and Montesano 10 | 2 | Type I: soft collar Type III: rigid collar (Minerva) |
Type I: 8 weeks Type II: 12 weeks |
Not stated |
| Bakhshi et al. 13 | 2 | Type III: pinless halo vest | Type III: 42, 60 days | Not stated |
| Capuano et al. 14 | 2 | Type II: – Rigid (Philadelphia) collar – Halo vest (complex patient) |
Type II: 12 weeks | Not stated |
| Curri et al. 15 | 1 | Type II: neck collar | Type II: 6 months | Not stated |
| Harding-Smith et al. 16 | 1 | Type III: neck collar (5 months) | Type III: 5 months | Not stated |
| Kapapa et al. 17 | 1 | Type I: rigid (Miami J) neck collar and light pain medication for 2 days | Type I: 5 weeks | Not stated |
| Kelly and Parrish 18 | 1 | Type III: halo vest (3 months) | Type III: 3 months | Not stated |
| Leventhal et al. 8 | 2 | Type I: rigid (Philadelphia) collar Type II: rigid (Philadelphia) collar |
Type I: 10 weeks Type II: 10 weeks |
Not stated |
| Momjian et al. 2 | 15 | Type I: – 4 rigid collar – 1 not stated Type II: – 1 “cervical brace” – 1 rigid collar – 1 not stated – 1 no treatment Type III: – 1 ventriculoperitoneal shunt followed by halo vest – 1 applied traction for 7 days then rigid collar – 1 soft collar – 2 halo vest |
Type I: – Rigid collar; 8, 4, 7, and 8–12 weeks Type II: – Rigid collar 8–12 weeks; otherwise not stated Type III: – Rigid collar: 2 months – Halo vest (3 months, 6 weeks) |
Not stated |
| Strehle and Tolinov 5 | 1 | Type II: soft collar (8 weeks) | Type II: 8 weeks | • CT scan 1 week into treatment revealed signs of healing, no bone fragment displacement |
| Tomaszewski et al. 4 | 6 | Type I: rigid (Minerva) collar (not stated) Type III: halo vest |
• Type I: not stated • Type III: 12.5, 13, 14 weeks |
• CT at median of 1.3 days, range 1–2 days, to confirm fracture alignment • CT scan before halo vest removal to confirm fracture consolidation |
| Ucler and Yucetas 19 | 1 | Type II: rigid cervical collar and methylprednisolone for hypoglossal nerve palsy | Type II: 8 weeks | Not stated |
| Wasserberg and Bartlett 20 | 1 | Type III: skeletal traction to reduce subluxation followed by collar immobilization | Not stated | Not stated |
| Peeters and Verbeeten 21 | 1 | Type III: “immobilization of head and neck” | Not stated | Not stated |
CT: computed tomography.
Non-operative
Management options included no treatment, soft cervical collars, rigid cervical collars (including Philadelphia. Miami J, and Minerva braces), and halo vest treatment with or without traction. Patients with Type I OCF were predominantly treated by rigid cervical collars (10/11 with treatment described), although one study did utilize soft cervical collars. Treatment duration following rigid cervical collar application for Type I OCFs ranged from 5 to 10 weeks (average of 7.6 weeks).
Patients with Type II OCF were also commonly treated by rigid cervical collar (3/7), with one study utilizing soft collar. Two studies utilized “cervical collar” treatment with no further clarification. One patient was not immobilized. One patient was given methylprednisolone for hypoglossal nerve palsy. 19 Type II OCF treatment duration following rigid collar application ranged from 8 to 12 weeks. Another study, which did not clarify the type of collar used, treated for 6 months.
Patients with Type III OCF were treated with a halo vest (8/12), skeletal traction followed by rigid cervical collar (2/12), soft collar due to reported apparent stability (1/12), and rigid cervical collar (1/12). Two patients received a non-specified neck collar. Duration of treatment following halo vest placement or rigid collar immobilization was typically 3 months.
Imaging was occasionally obtained during treatment and following its completion. Two studies utilized CT scan during the treatment period at a median of 1.3 days to confirm fracture alignment, 4 and at 1 week 5 to observe preliminary signs of fracture healing. Post-management imaging was described by five studies. One study obtained CT prior to final halo removal, 4 two obtained CT to confirm fracture healing after completion of treatment course,17,18 one obtained XR for this same purpose, 15 and one obtained flexion/extension cervical XR. 8 For patients who received halo vests, all underwent MRI within 9 days (median = 5.6 days, range = 3–9 days) of halo removal.4,8
Operative
Two patients required operative intervention in the form of ventriculoperitoneal shunt 4 and craniotomy. 15 These patients presented with Type III and Type II OCFs, respectively. In addition, one patient had persistent C1-C2 instability after a Type III OCF and received fixation-fusion. 2 No occipital-cervical fusions were performed in our review for isolated OCFs.
Outcomes
All data describing outcomes, mean follow-up period, post-treatment CCJ functionality, and complications are included in Table 4. Mean follow-up was 12.1 months (range = 1–72 months). Twelve studies described post-treatment CCJ functionality. All confirmed complete return of neck range of motion (ROM) without pain, except for one patient who had neck pain with head movement 1 month after the injury. 17 CT and XR imaging at follow-up confirmed fracture consolidation.2,8,17,15 One patient demonstrated continued hypoglossal nerve palsy. 20 Two patients had persistent CN deficits due to injuries impacting the brainstem (brainstem hemorrhage and medullar hematoma). Three patients who presented with limb paresis had persistent limb movement deficits following treatment; paresis in all cases was due to non-OCF causes. 2 Three studies described complications related to the OCF itself. These included superficial infection at site of halo vest pins (1/32; 3.1%), 4 chin irritation due to collar placement (1/32; 3.1%), 13 and persistent hypoglossal nerve palsy (1/32; 3.1%). 20 No other patients described complications related to OCF injury or treatment (29/32; 90.6%).
Table 4.
Summary of post-treatment outcomes, imaging after treatment, stated follow-up, and complications.
| Author (reference) | Number of patients (n) | Post-treatment outcomes | Imaging after treatment | Stated follow-up (mean; range) | Complications |
|---|---|---|---|---|---|
| Abat et al. 12 | 1 | • Not stated | • Not stated | • 2 months | • None |
| Anderson and Montesano 10 | 2 | • Pain-free, full ROM | • Not stated | • 30 months; 14–36 months | • None |
| Bakhshi et al. 13 | 2 | • Pain-free, full ROM | • Not stated | • 51 days; 42–60 days | • Skin irritation on chin from immobilization |
| Capuano et al. 14 | 2 | • Pain-free, full ROM | • Not stated | • 2 years | • None |
| Curri et al. 15 | 1 | • Pain-free, full ROM | • XR showing fracture healing | • 6 months | • None |
| Harding-Smith et al. 16 | 1 | • Symptom free | • Not stated | • 16 months | • None |
| Kapapa et al. 17 | 1 | • “Pain intermittently after mindless movements” | • CT scan showing reorganization of fracture fragments | • 4 weeks | • None |
| Kelly and Parrish 18 | 1 | • Pain-free, full ROM | • CT scan showing healing of fracture | • 4 months | • None |
| Leventhal et al. 8 | 2 | • Pain-free, full ROM | • Flexion and extension cervical films showing OCF resolution | • 3 months | • None |
| Momjian et al. 2 | 15 | • Remaining deficits related to other injuries: (a) Cranial nerve deficits due to brainstem hemorrhage (3) (b) Persistent quadriparesis (3) |
• Fracture consolidation (2) | • Not stated | • Persistent C1-C2 instability treated by a secondary fixation-fusion |
| Strehle and Tolinov 5 | 1 | • Pain-free, full ROM | • None stated | • 8 weeks | • None |
| Tomaszewski et al. 4 | 6 | • Confirmed fracture consolidation in all patients | • None stated | • Median 49 months; 6–72 months | • One superficial infection caused by halo vest pins |
| Ucler and Yucetas 19 | 1 | • Neck pain treated with physiotherapy | • None stated | • 3 months | • None |
| Wasserberg and Bartlett 20 | 1 | • Pain-free, full ROM; none | • None stated | • 3 months | • Hypoglossal palsy |
| Peeters and Verbeeten 21 | 1 | • Not stated | • None stated | • Not stated | • None |
ROM: range of motion; XR: radiographs; CT: computed tomography; OCF: occipital condyle fracture.
Management algorithm
The below stepwise management algorithm is proposed based on existing literature describing presentation, evaluation, treatment, and outcomes of pediatric OCFs (Figure 2). A high index of suspicion is warranted to begin OCF evaluation; however, clinicians should have a low threshold to begin this pathway among patients with high-risk presentation. Initial characteristics that may suggest elevated risk include high-energy MOI or the common presenting symptoms identified. Hemodynamic stabilization should always precede further evaluation of the cervical spine, with careful consideration for cervical protection.
Once stabilized, the patient should undergo imaging. XR has routinely been shown to be insufficient regarding OCF; however, some clinicians may elect to begin with XR in the setting of other suspected injuries. Negative XR, particularly in the setting of the above presentation, should not be considered sufficient to rule out OCF. CT scan followed by MRI should be obtained to detect OCFs and concurrent CCJ instability including atlantooccipital dislocation (AOD), soft-tissue injury, ligamentous tears, and tectorial membrane involvement.
Following imaging, OCF classification and C-spine stability should guide immediate treatment. For stable Type I or II OCFs, rigid cervical collar for 10 weeks is recommended, to err on the side of caution in more extensive Type II fractures. For unstable Type II or III OCF, halo vest placement for 12 weeks is the most appropriate treatment method. We recommend CT scan within 1 week of treatment initiation to confirm fracture alignment. Following treatment termination, we recommend a CT scan within 1 week to confirm OCF healing, with additional imaging obtained to confirm CCJ stability. These may include dynamic XR of the lateral cervical spine with flexion and extension and/or MRI, based on the patient’s clinical condition and evidence of remaining symptoms.
Surgical intervention via occipital–cervical decompression and fusion may be indicated for patients with AOD, evidence of neurovascular compromise, or failed non-operative management as defined by persistent instability.2,4,10,7 Ultimately, conservative management should be first-line in all isolated, non-complex cases.
Follow-up for these patients should be a minimum of 6 months.12,14,19,20 Repeat imaging at that time may be obtained per clinician preference. Re-evaluation should be performed if any symptoms persist or there is a recurrence of any symptoms.
Discussion
This review aimed to evaluate the existing literature for OCFs in the pediatric population to formulate a systematic and well-informed management algorithm. Although OCFs have historically been considered rare, recent literature review shows increasing evidence that they are often underdiagnosed and occur more than previously thought.2,4,5,7,12,16 Given the absence of consensus regarding pediatric OCF management, the development of a comprehensive management algorithm is imperative to guide clinical decision-making and ensure standardized patient care, particularly due to the comparatively small amount of existing literature on this subject. This review serves as a comprehensive collection of the most common MOIs, presenting symptoms, diagnostic imaging techniques, and treatment options available for pediatric OCFs.
Presentation and workup
OCFs frequently manifest with a range of symptoms after high-energy traumas. The most common MOI identified in our population included MVAs, pedestrian–car collisions, and other high-energy traumas such as fall from a height. Of these, MVA was most common, accounting for nearly half of the patients in this review (45.7%). It is essential to emphasize that any trauma to the cervical spine or base of the skull should raise clinical suspicion of OCF and warrant further workup.
Most commonly, patients presented with depressed level of consciousness or complete LOC (65.6%), neck pain (34.5%), and CN injury (28.7%). The most commonly injured nerves included CN IX, X, XI, and particularly XII, due to their location relative to this region. Other commonly presenting symptoms included torticollis and limb paresis. The range of symptoms emphasizes the often varied and complex presentations associated with these fractures, particularly given their propensity to co-occur with a wide range of other injuries. It should be acknowledged that some of our cases reported little to no symptoms; we therefore emphasize that any child in a high-energy trauma should be evaluated for OCFs.
Once OCF is suspected, CCJ imaging is necessary to confirm the OCF. Although XRs have been historically used since OCFs were first described by Sir Charles Bell in 1817, 22 our data strongly underscore their limited ability to detect OCFs, as none of our studies were able to identify OCF through plain XRs alone.2,5,8,12,14,18,21 In our algorithm, we suggest XR use only if there is suspicion of other cervical injuries, facial fractures, or corporeal injuries that may otherwise impact patient treatment. All 13 studies that used CT indicated its high sensitivity in detecting fractures. CT visualization of OCFs is necessary for the Anderson and Montesano classification and stability determination and will direct treatment. Therefore, CT performed in 1–2 mm12,14,19 slices should remain the predominant initial imaging modality of OCFs. Similarly, MRI, used in three of our studies, was utilized to assess atlantooccipital joint, joint capsule, ligamentous, and soft-tissue injury as an indication of fracture stability.
For unstable fractures, CT and MRI revealed a translation ranging between 3 and 4 mm between C0 and C1 and a rotational displacement of C0/C1 by 10 and 12 degrees.4,9,20 This range of findings is similar to the Tuli criteria definitions, 9 which describe instability as >1 mm C0 and C1 translation or >8 degrees of C0/C1 axial rotation, among other criteria such as >7 mm C1 overhang onto C2, >45 degrees of C1-C2 to one side, >4 mm C1-C2 translation, and ligament injury. Notably, while these findings were described in a majority adult population, these established measurements can largely be applied to the population included in this review as the majority sustained their injury during or after puberty. 23
Treatment options
Timely intervention for OCFs is imperative. Patients who are not stabilized at the scene should receive some form of immobilization as soon as OCF is suspected. Due to the potential long-lasting impact of OCF, treatment should be initiated within hours after injury.
We propose that fracture type and stability guide treatment. Treatment types include non-surgical versus surgical management, with a strong preference for non-surgical management. For Type I OCFs, rigid cervical collars were the primary choice, with treatment ranging from 5 to 10 weeks. Furthermore, two Type I patients in our review had bilateral OCFs. These patients were treated similarly to unilateral Type I patients. Although higher mortality has been associated with bilateral fractures in the adult population, 7 our review demonstrated similar outcomes to unilateral fracture patients. 4 Type II OCFs were managed similarly to Type I OCFs in our review. Similar to stable Type I OCFs, we recommend 10 weeks of rigid collar immobilization, due to the possibility of more extensive Type II fracture. Type II OCF can also be identified as unstable through CT and MRI imaging (Tuli Type IIb). In this case, treatment would be similar to Type III OCF below.
Type III OCFs are considered inherently unstable. Most Type III OCFs were treated by halo vests in our review. In addition, two Type III OCF cases utilized skeletal traction followed by halo vest or rigid cervical collar. Halo vest treatment duration was 3 months, and 8 to 12 weeks for collar placement with or without temporary traction. Among the described treatment options, we recommend a halo vest for a 12-week period. Halo vests were first described in 1968 for cervical instability in Polio patients, using pins anchored to the cranium, connected to a body cast, for superior immobilization. 24 Since its development, halo vests have become in many ways a gold standard of rigid cervical immobilization treatment in the pediatric population.13,25,26 Studies have shown its superiority and recommended it as a primary treatment modality to provide axial traction, 27 and pre- or post-operative CCJ reduction. 28 Thus, we recommend halo vest above other treatment modalities for all uncomplicated unstable OCFs.
Based on our data, we recommend CT within 1 week of treatment initiation to confirm fracture alignment.2,4 While we recognize that this is an increased amount of radiation exposure for the pediatric patients, we contend that the invaluable insights into the injury and treatment process warrant the two to three CT scans the child undergoes, considering the substantial anatomic importance of OCFs and the associated risks of destabilization. Serial imaging is also supported by the literature, with Ucler and Yucetas 19 and other works highlighting that CT scanning at set time intervals is essential for thorough neurological examination.4,15
Operative intervention was not utilized within our patient population, apart from two operations for associated injuries. In addition, one patient received fixation-fusion due to C1-C2 instability after initial ventriculoperitoneal shunt placement and halo vest treatment. 2 Surgical management for OCFs is uncommon, with occipital–cervical fusion typically serving as the primary procedure to stabilize the CCJ. To our knowledge, there is no literature on occipital–cervical fusions on isolated pediatric OCFs without AOD. Nonetheless, our exhaustive review in conjunction with existing literature for adult patients prompts surgical consideration for the following conditions: (1) neurovascular compromise; (2) persistent CCJ instability following non-operative intervention; or (3) AOD.2,9 Notably, some studies indicate that the risk of AOD in adult cases of OCFs is upward of 9.7%7,19 and poses the highest morbidity and mortality of any OCF. 7 No comparable studies in the pediatric population exist. Given our small population of OCFs, none of which were treated by surgical management, and all of which resulted in excellent outcomes, we recommend that physicians use prudent clinical expertise to determine the necessity of operative intervention. Our findings and literature review support that our conservative management algorithm above should be first-line treatment for non-complicated OCFs and is nearly always sufficient for treatment. Surgery should be used only in special and comparatively extreme circumstances in the skeletally mature patient.2,4,5,9,19
Outcomes and follow-up
Key outcomes assessed included post-treatment CCJ functionality, neck ROM restoration, pain resolution, and alleviation of CN deficits. All but one patient reported complete painless return of neck ROM. A single case had persistent neck pain with head movement at 4 weeks from time-of-injury. 17 There was a lack of clarity regarding symptom resolution at follow-up. Our review also noted one case of hypoglossal nerve palsy emerging 3 months after treatment. 20 This was the only case that had continued CN deficits. The only two other complications were one case of superficial infection at the site of halo vest pins, 4 which resolved with treatment, and chin irritation following collar placement. 13 Overall, most patients (90.6%) experienced no complications related to either their injury or treatment.
Although our review had inconsistent reporting of post-treatment imaging, CT was most often utilized. We recommend cervical CT scans be conducted within 1 week of treatment completion to confirm OCF healing, with additional imaging to confirm CCJ stability obtained, if there is evidence of persisting symptoms or deficits, including plain XR films with flexion and extension, and/or MRI. It is important for physicians to consider that obtaining dynamic XR may prove challenging and uncomfortable for patients after extended periods of immobilization of the cervical spine. All patients should be closely monitored and undergo a thorough physical examination to confirm lack of CN impingement or threat. Despite minimal recurrence of symptoms in our review, we recommend follow-up for symptom evaluation for a minimum of 6 months, given reported reappearance of delayed CN palsies, with one study citing a 38% incidence rate in adult patients. 9 This may be attributed to osseous and fibrous tissue proliferation related to the healing process or to inadequate stabilization. Beyond the 6-month recommendation, due to a paucity of evidence in our systematic review, we cannot advise a standardized follow-up plan and recommend that re-evaluation be performed if there is symptom recurrence mirroring presenting symptomology, if complications occur, or as deemed appropriate at the physician’s discretion.
Limitations
While this systematic review captures a comprehensive portrayal of OCFs in the pediatric population, there are limitations worth acknowledging. The first is the dearth of literature dedicated to pediatric OCFs. This necessitated the inclusion of single case reviews, which, although cumulatively contribute to a substantial amount of data, may not fully encapsulate broader trends as comprehensively as larger cohorts of case reviews, and introduces the potential for selection bias. Relatedly, it should be noted that there are many papers that describe AODs or other cervical injuries, which often have concomitant OCFs. These papers were ultimately excluded due to lack of data-defining OCF presence and therefore limit the algorithm’s applicability to OCFs without more serious cervical injuries. This is reflected in our lack of surgical patients, as these co-existing injuries are often indications for operative intervention. Second, there was heterogeneity present in our data collection. In instances where classification type was not present, authors defined type based on provided images. Some studies did not clarify imaging timing, follow-up, or specific orthosis type. For this reason, treatment options are contingent upon clinical context and surgeon expertise and depend on individual case presentations. Further research with larger-scale studies is necessary to validate and refine the algorithm and to specifically define instability as seen by imaging in the pediatric population. The addition of longer follow-up periods, standardized reporting, and prospective data incorporation will enhance the external validity of this management algorithm. Nonetheless, despite the lack of extensive literature on this topic, we believe this is a comprehensive and necessary review of the existing literature given the potential for underdiagnosed OCFs to cause long-lasting, permanent harm.
Conclusion
Pediatric OCFs are a rare but potentially life-altering or life-threatening injury, for which there currently exists no standardized guidelines for clinicians to follow. This allows increased potential for errors in management, which may have long-term consequences for patients. This systematic review fills this critical knowledge gap by offering a framework for clinical decision-making, providing a valuable resource for guiding physicians in streamlining and optimizing care for pediatric OCFs.
Supplemental Material
Supplemental material, sj-pdf-1-cho-10.1177_18632521241229301 for Occipital condyle fracture in the pediatric population: A management algorithm and systematic review by Mary M Morcos, David S Liu, Alexander R Farid, Pokmeng See and Grant D Hogue in Journal of Children’s Orthopaedics
Footnotes
Author contributions: M.M.M. contributed to writing—original draft; writing—review and editing; methodology; analysis; and visualization. D.S.L. contributed to conceptualization; writing—original draft; writing—review and editing; and methodology. A.R.F. contributed to writing—original draft; writing—review and editing; methodology; analysis; and visualization. P.S. contributed to conceptualization; methodology; and writing—reviewing and editing. GDH contributed to conceptualization; methodology; writing—reviewing and editing; and supervision.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: There are no human participants in this article and informed consent is not applicable.
ORCID iD: Mary M Morcos  https://orcid.org/0000-0001-9269-0042
https://orcid.org/0000-0001-9269-0042
Supplemental material: Supplemental material for this article is available online.
References
- 1. Caroli E, Rocchi G, Orlando ER, et al. Occipital condyle fractures: report of five cases and literature review. Eur Spine J 2005; 14(5): 487–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Momjian S, Dehdashti AR, Kehrli P, et al. Occipital condyle fractures in children. Pediatr Neurosurg 2003; 38(5): 265–270. [DOI] [PubMed] [Google Scholar]
- 3. Neeman Z, Bloom AI. Occipital condyle fractures in the pediatric population. Radiographics 2003; 23(6): 1699–1701. [DOI] [PubMed] [Google Scholar]
- 4. Tomaszewski R, Gap A, Lucyga M, et al. Treatment of unstable occipital condylar fractures in children—a STROBE-compliant investigation. Medicina (B Aires) 2021; 57: 530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Strehle EM, Tolinov V. Occipital condylar fractures in children: rare or underdiagnosed? Dentomaxillofac Radiol 2014; 41: 175–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Malham GM, Ackland HM, Jones R, et al. Occipital condyle fractures: incidence and clinical follow-up at a level 1 trauma centre. Emerg Radiol 2009; 16(4): 291–297. [DOI] [PubMed] [Google Scholar]
- 7. Mueller FJ, Fuechtmeier B, Kinner B, et al. Occipital condyle fractures. Prospective follow-up of 31 cases within 5 years at a level 1 trauma centre. Eur Spine J 2012; 21: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leventhal MR, Boydston WR, Sebes JI, et al. The diagnosis and treatment of fractures of the occipital condyle. Orthopedics 1992; 15(8): 944–947. [DOI] [PubMed] [Google Scholar]
- 9. Tuli S, Tator CH, Fehlings MG, et al. Occipital condyle fractures. Neurosurgery 1997; 41: 368–377. [DOI] [PubMed] [Google Scholar]
- 10. Anderson PA, Montesano PX. Morphology and treatment of occipital condyle fractures. Spine (Phila Pa 1976) 1988; 13(7): 731–736. [DOI] [PubMed] [Google Scholar]
- 11. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harding-Smith J, MacIntosh PK, Sherbon KJ. Fracture of the occipital condyle. A case report and review of the literature. J Bone Joint Surg Am 1981; 63: 1170–1171. [PubMed] [Google Scholar]
- 13. Capuano C, Costagliola C, Shamsaldin M, et al. Occipital condyle fractures: a hidden nosologic entity. Acta Neurochir (Wien) 2004; 146(8): 779–784. [DOI] [PubMed] [Google Scholar]
- 14. Abat F, Soria L, García-Casas O, et al. Occipital condyle fracture: clinical case and a review of the literature. Rev Esp Cir Ortop Traumatol 2012; 56(1): 67–71. [DOI] [PubMed] [Google Scholar]
- 15. Kapapa T, Tschan CA, König K, et al. Fracture of the occipital condyle caused by minor trauma in child. J Pediatr Surg 2006; 41(10): 1774–1776. [DOI] [PubMed] [Google Scholar]
- 16. Kelly A, Parrish R. Fracture of the occipital condyle: the forgotten part of the neck. J Accid Emerg Med 2000; 17(3): 220–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ucler N, Yucetas SC. Occipital condyle fracture extending to the inferior part of the clivus. Pediatr Neurosurg 2018; 53(4): 282–285. [DOI] [PubMed] [Google Scholar]
- 18. Wasserberg J, Bartlett RJ. Occipital condyle fractures diagnosed by high-definition CT and coronal reconstructions. Neuroradiology 1995; 37(5): 370–373. [DOI] [PubMed] [Google Scholar]
- 19. Curri D, Cervellini P, Zanusso M, et al. Isolated fracture of occipital condyle. Case report. J Neurosurg Sci 1988; 32: 157–159. [PubMed] [Google Scholar]
- 20. Bakhshi H, Banskota B, Kushare I, et al. Pinless halo in the pediatric population: indications and complications. J Pediatr Orthop 2015; 35: 374–378. [DOI] [PubMed] [Google Scholar]
- 21. Bell C. Surgical observations. Middlesex Hosp J 1817; 4: 469–470. [Google Scholar]
- 22. Peeters F, Verbeeten B. Evaluation of occipital condyle fracture and atlantic fracture, two uncommon complications of cranio-vertebral trauma. Rofo 1983; 138(5): 631–633. [DOI] [PubMed] [Google Scholar]
- 23. Tomaszewski R, Kler J, Pethe K, et al. Evaluation of using the Anderson-Montesano and the Tuli classifications in pediatric patients with occipital condyle fractures. J Orthop Surg Res 2021; 16: 449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nickel VL, Perry J, Garrett A, et al. The halo. A spinal skeletal traction fixation device. By Vernon L. Nickel, Jacquelin Perry, Alice Garrett, and Malcolm Heppenstall, 1968. Clin Orthop Relat Res 1989; 239: 4–11. [PubMed] [Google Scholar]
- 25. Arkader A, Hosalkar HS, Drummond DS, et al. Analysis of halo-orthoses application in children less than three years old. J Child Orthop 2007; 1(6): 337–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mueller DG, Mueller K. Three case studies involving the use of a noninvasive halo for cervical stabilization. J Prosthet Orthot 2005; 17: 40–46. [Google Scholar]
- 27. Kostuik JP. Indications for the use of the halo immobilization. Clin Orthop Relat Res 1981; 154: 46–50. [PubMed] [Google Scholar]
- 28. Botte MJ, Byrne TP, Abrams RA, et al. Halo skeletal fixation: techniques of application and prevention of complications. J Am Acad Orthop Surg 1996; 4(1): 44–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-cho-10.1177_18632521241229301 for Occipital condyle fracture in the pediatric population: A management algorithm and systematic review by Mary M Morcos, David S Liu, Alexander R Farid, Pokmeng See and Grant D Hogue in Journal of Children’s Orthopaedics