Abstract
Background
Exercise training as a component of pulmonary rehabilitation improves health‐related quality of life (HRQL) and exercise capacity in people with chronic obstructive pulmonary disease (COPD). However, some individuals may have difficulty performing exercise at an adequate intensity. Non‐invasive ventilation (NIV) during exercise improves exercise capacity and dyspnoea during a single exercise session. Consequently, NIV during exercise training may allow individuals to exercise at a higher intensity, which could lead to greater improvement in exercise capacity, HRQL and physical activity.
Objectives
To determine whether NIV during exercise training (as part of pulmonary rehabilitation) affects exercise capacity, HRQL and physical activity in people with COPD compared with exercise training alone or exercise training with sham NIV.
Search methods
We searched the following databases between January 1987 and November 2013 inclusive: The Cochrane Airways Group specialised register of trials, AMED, CENTRAL, CINAHL, EMBASE, LILACS, MEDLINE, PEDro, PsycINFO and PubMed.
Selection criteria
Randomised controlled trials that compared NIV during exercise training versus exercise training alone or exercise training with sham NIV in people with COPD were considered for inclusion in this review.
Data collection and analysis
Two review authors independently selected trials for inclusion in the review, extracted data and assessed risk of bias. Primary outcomes were exercise capacity, HRQL and physical activity; secondary outcomes were training intensity, physiological changes related to exercise training, dyspnoea, dropouts, adverse events and cost.
Main results
Six studies involving 126 participants who completed the study protocols were included. Most studies recruited participants with severe to very severe COPD (mean forced expiratory volume in one second (FEV1) ranged from 26% to 48% predicted). There was an increase in percentage change peak and endurance exercise capacity with NIV during training (mean difference in peak exercise capacity 17%, 95% confidence interval (CI) 7% to 27%, 60 participants, low‐quality evidence; mean difference in endurance exercise capacity 59%, 95% CI 4% to 114%, 48 participants, low‐quality evidence). However, there was no clear evidence of a difference between interventions for all other measures of exercise capacity. The results for HRQL assessed using the St George's Respiratory Questionnaire do not rule out an effect of NIV (total score mean 2.5 points, 95% CI ‐2.3 to 7.2, 48 participants, moderate‐quality evidence). Physical activity was not assessed in any study. There was an increase in training intensity with NIV during training of 13% (95% CI 1% to 27%, 67 participants, moderate‐quality evidence), and isoload lactate was lower with NIV (mean difference ‐0.97 mmol/L, 95% CI ‐1.58mmol/L to ‐0.36 mmol/L, 37 participants, moderate‐quality evidence). The effect of NIV on dyspnoea or the number of dropouts between interventions was uncertain, although again results were imprecise. No adverse events and no information regarding cost were reported. Only one study blinded participants, whereas three studies used blinded assessors. Adequate allocation concealment was reported in four studies.
Authors' conclusions
The small number of included studies with small numbers of participants, as well as the high risk of bias within some of the included studies, limited our ability to draw strong evidence‐based conclusions. Although NIV during lower limb exercise training may allow people with COPD to exercise at a higher training intensity and to achieve a greater physiological training effect compared with exercise training alone or exercise training with sham NIV, the effect on exercise capacity is unclear. Some evidence suggests that NIV during exercise training improves the percentage change in peak and endurance exercise capacity; however, these findings are not consistent across other measures of exercise capacity. There is no clear evidence that HRQL is better or worse with NIV during training. It is currently unknown whether the demonstrated benefits of NIV during exercise training are clinically worthwhile or cost‐effective.
Keywords: Humans; Noninvasive Ventilation; Exercise Tolerance; Exercise Tolerance/physiology; Forced Expiratory Volume; Forced Expiratory Volume/physiology; Physical Conditioning, Human; Physical Conditioning, Human/methods; Physical Conditioning, Human/physiology; Pulmonary Disease, Chronic Obstructive; Pulmonary Disease, Chronic Obstructive/physiopathology; Pulmonary Disease, Chronic Obstructive/rehabilitation; Randomized Controlled Trials as Topic
Plain language summary
Breathing support via a mask during exercise training for people with chronic obstructive pulmonary disease
Background: Quality of life and exercise tolerance are commonly reduced in people with chronic obstructive pulmonary disease (COPD). In addition, physical activity levels are lower compared with those of healthy people of a similar age. Exercise training as a part of a formal rehabilitation programme is an important component of management for people with COPD and has been shown to improve both quality of life and exercise tolerance. However, some individuals may have difficulty performing exercise at an adequate training intensity. Non‐invasive ventilation (NIV) is a method of providing breathing support using a machine called a ventilator. Breathing support is delivered via a mask that is worn over the nose, mouth or both, or via a mouthpiece. During a single exercise session, NIV has been shown to improve exercise tolerance and reduce breathlessness. Consequently, NIV used over multiple exercise sessions (during exercise training) may allow people with COPD to exercise at a higher intensity and potentially to achieve greater improvement in exercise tolerance, quality of life and physical activity.
Review question: We conducted a review to determine whether NIV during exercise training affects exercise tolerance, quality of life and physical activity compared with exercise training alone or exercise training with sham NIV (placebo) in people with COPD.
Study characteristics: The evidence is current to November 2013. We included six studies involving 126 participants who completed the study protocols. Most studies recruited participants with severe to very severe COPD. The average age of participants ranged from 63 to 71 years. Cycling or treadmill exercise training was performed in the studies. The duration of exercise training programmes ranged from six to twelve weeks.
Key results: The percentage change in peak exercise capacity increased by an average of 17% in three studies, and the percentage change in endurance exercise capacity by an average of 59% in two studies that provided NIV during training compared with training without NIV or training with sham NIV. However, these improvements in exercise capacity were not consistent findings as there was no clear evidence that NIV improved all other measures of exercise capacity. The results for quality of life were uncertain and our analysis did not exclude there being an effect with NIV during exercise training in two studies. Physical activity was not assessed in any of the studies. Non‐invasive ventilation allowed participants to exercise at a higher training intensity (average of 13% higher) in three studies, and evidence of a greater training effect on the muscles was found in two studies, as a marker in the blood (isoload blood lactate) was significantly lower by an average of 0.97 mmol/L. No information regarding adverse events or cost was reported. It is currently unknown whether demonstrated benefits of NIV during exercise training are clinically worthwhile or cost‐effective.
Quality of the evidence: This review was generally limited by the small number of included studies and the small numbers of participants within the included studies. The quality of the evidence was low for exercise capacity outcomes, largely because of issues with study design. Consequently, the effect of NIV during exercise training on exercise capacity is uncertain. The quality of the evidence for quality of life, training intensity and isoload blood lactate was moderate, and these findings can be interpreted with a greater degree of confidence.
Summary of findings
Summary of findings for the main comparison. Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation for people with chronic obstructive pulmonary disease.
| Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation for people with chronic obstructive pulmonary disease | ||||||
| Patient or population: people with chronic obstructive pulmonary disease Settings: outpatient Intervention: non‐invasive ventilation during exercise training Comparison: exercise training alone or exercise training with sham non‐invasive ventilation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Exercise training alone or exercise training with sham non‐invasive ventilation | Non‐invasive ventilation during exercise training | |||||
| Exercise capacity: percentage change in peak work rate Incremental cycle or incremental treadmill test Follow‐up: 6 to 8 weeks | Exercise capacity: percentage change in peak work rate in the control groups ranged from a mean of 9% to 38% | Mean exercise capacity: percentage change in peak work rate in the intervention groups was 17% higher (7% to 27% higher) | 17% (7% to 27%) | 60 (3 studies) | ⊕⊕⊝⊝ lowa | |
| Exercise capacity: percentage change constant work rate endurance time Constant work rate cycle endurance test Follow‐up: 6 to 8 weeks | Exercise capacity: percentage change constant work rate endurance time in the control groups ranged from a mean of 74% to 88% | Mean exercise capacity: percentage change constant work rate endurance time in the intervention groups was 59% higher (4% to 114% higher) | 59% (4% to 114%) | 48 (2 studies) | ⊕⊕⊝⊝ lowb,c | Mean change exceeds minimal important difference of 34% |
| Exercise capacity: endurance time (minutes) Constant work rate cycle endurance test Follow‐up: 6 to 8 weeks | Exercise capacity: endurance time (minutes) in the control groups ranged from a mean of 3.9 to 13.0 minutes | Mean exercise capacity: endurance time (minutes) in the intervention groups was 3.62 minutes higher (0.17 lower to 7.41 higher) | 3.62 minutes (‐0.17 to 7.41 minutes) | 48 (2 studies) | ⊕⊕⊝⊝ lowb,d | CI crosses zero but does not rule out an effect |
| Health‐related quality of life Change in total score of St George's Respiratory Questionnaire. Scale from 0 to 100 Follow‐up: 6 to 8 weeks | Mean health‐related quality of life in the intervention groups was 2.45 points higher (2.3 lower to 7.2 higher) | 2.45 points (‐2.3 to 7.2 points) | 48 (2 studies) | ⊕⊕⊕⊝ moderatee | CI crosses zero but does not rule out an effect | |
| Physical activity: not measured | See comment | See comment | Not estimable | – | See comment | This outcome was not reported in any of the included studies |
| Training intensity: fInal training session (% baseline peak work capacity) Follow‐up: 6 to 8 weeks | Training intensity: change from baseline (%) in the control groups ranged from a mean of 75% to 93% | Mean training intensity: change from baseline (%) in the intervention groups was 13% higher (1% to 27% higher) | 13% (1% to 27%) | 67 (3 studies) | ⊕⊕⊕⊝ moderatef | Heterogeneity between studies was explained by one study that recruited participants with milder disease compared with other studies in the analysis |
| Physiological outcomes: isoload blood lactate (mmol/L) Follow‐up: 6 to 12 weeks | Physiological outcomes: isoload blood lactate (mmol/L) in the control groups ranged from a mean of 2.50 to 2.61 mmol/L | Mean physiological outcomes: isoload blood lactate (mmol/L) in the intervention groups was 0.97 mmol/L lower (1.58 to 0.36 lower) | ‐0.97 mmol/L (‐1.58 to ‐0.36 mmol/L) | 37 (2 studies) | ⊕⊕⊕⊝ moderateg | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a‐2 for risk of bias: None of the studies blinded participants or trainers, and only one study used a blinded assessor. It was unclear whether allocation concealment was adequate in two of the studies. Also, one study reported significant between‐group differences in baseline peak exercise capacity. b‐1 for risk of bias: One study did not blind participants or use a blinded assessor. c‐1 for imprecision: wide 95% confidence interval. d‐1 for imprecision: 95% confidence interval includes no effect, and upper confidence limit crosses the minimal important difference for benefit. e‐1 for risk of bias: Participants were not blinded in one study. f‐1 for risk of bias: Participants were not blinded in two of the studies, and trainers were not blinded in any of the studies. g‐1 for risk of bias: None of the studies blinded participants or trainers, which may have resulted in performance bias and could have indirectly affected this outcome.
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a preventable but not curable disease that is generally progressive in nature (Viegi 2007). In 2010, COPD was one of the leading causes of mortality worldwide (Lozano 2012). Although variability between countries has been noted, it is estimated that the prevalence of COPD at GOLD (Global Initiative for Chronic Obstructive Lung Disease) stage II or higher (GOLD 2013) is 10.1% globally (Buist 2007). The economic and social costs of COPD are substantial (Pauwels 2004), and acute exacerbations of COPD that require admission to hospital are some of the largest contributors to direct healthcare costs (Viegi 2007). The number of years that people are living with disability due to COPD is also rising (Vos 2012).
Chronic obstructive pulmonary disease is characterised by expiratory flow limitation that is not fully reversible (O'Donnell 2006). In addition to pulmonary disease and dysfunction, COPD has a number of associated systemic manifestations including skeletal muscle dysfunction, weight loss and systemic inflammation (Agusti 2003). Dyspnoea is the hallmark symptom of COPD (Viegi 2007) and is more common in severe disease (Killian 1992). Dyspnoea can lead to a cycle of activity avoidance, deconditioning and reduced participation in society. Exercise capacity and health‐related quality of life (HRQL) are commonly reduced in people with COPD (Garrod 2006), and physical activity levels are lower than those of age‐matched healthy individuals (Pitta 2005).
Description of the intervention
Exercise training as a component of pulmonary rehabilitation is supported by high‐level evidence as one of the few effective interventions in the management of COPD (Rabe 2007; Ries 2007). Pulmonary rehabilitation has been shown to improve exercise capacity (Cambach 1999; Troosters 2000), HRQL and symptoms (Lacasse 2006), and to reduce the frequency of hospital admissions in those with a recent exacerbation (Puhan 2011). However, the effect of pulmonary rehabilitation on physical activity appears to be small (Ng 2012). High‐intensity exercise training may produce greater physiological improvement compared with lower‐intensity exercise training in people with COPD (Casaburi 1991; Gimenez 2000). However, some individuals may have difficulty performing exercise at an adequate intensity for the required duration (Maltais 1997) and may not achieve the same benefit from exercise training as those without a significant ventilatory limitation to exercise, particularly if peripheral muscle strength is relatively preserved (Garrod 2006; Plankeel 2005; Troosters 2001). Consequently, a number of adjuncts to exercise have been proposed, including non‐invasive ventilation (NIV), a type of breathing support delivered via a mask or mouthpiece.
How the intervention might work
In people with COPD, the use of NIV during a single session of lower limb exercise was shown in a systematic review (van't Hul 2002) to increase exercise endurance and reduce dyspnoea compared with exercise without NIV or exercise with sham NIV. Unloading of both inspiratory and expiratory components of the respiratory muscle pump has been observed with NIV during exercise (Kyroussis 2000), with the reduction in dyspnoea being proportional to respiratory muscle unloading (Maltais 1995). Improvement in pattern of breathing (Maltais 1995; van't Hul 2004) and in gas exchange (Dreher 2007; Hernandez 2001) was also noted. In addition, several extrapulmonary effects have been reported with NIV during exercise, including improved locomotor muscle perfusion (Borghi‐Silva 2008), decreased exercise‐induced lactic acidosis (Borghi‐Silva 2008; Polkey 2000) and associated reduction in symptoms of muscle fatigue (Bianchi 1998; Borghi‐Silva 2008).
Why it is important to do this review
Given the benefit of NIV during a single session of exercise, application of NIV over multiple sessions of exercise, that is, during exercise training, may allow people with COPD to exercise at a higher intensity for a greater duration. Therefore, exercise training with NIV could potentially lead to greater improvement in exercise capacity compared with exercise training alone. Such improvement in exercise capacity may also improve HRQL and increase physical activity levels in people with COPD.
Objectives
To determine whether NIV during exercise training (as part of pulmonary rehabilitation) affects exercise capacity, HRQL and physical activity in people with COPD compared with exercise training alone or exercise training with sham NIV.
Methods
Criteria for considering studies for this review
Types of studies
We included in this review randomised controlled trials (RCTs) comparing NIV during exercise training versus exercise training alone, or exercise training with sham NIV (control group). Randomised cross‐over trials were also considered for inclusion. Quasi‐RCTs, for example, those with alternate randomisation, were excluded.
Types of participants
Inclusion
We considered studies with participants with stable COPD for inclusion. Participants were considered to be stable if no history of an exacerbation was reported over the past month (Rabe 2007). The definition of COPD was based on:
a clinical diagnosis of COPD; and
a best recorded ratio of forced expiratory volume during one second (FEV1) over forced vital capacity (FVC) < 70% and a best recorded FEV1 < 80% predicted for individual study participants (equivalent to GOLD stage II to IV) (GOLD 2013).
Exclusion
We excluded studies that included participants with non‐COPD respiratory disease or participants with concomitant neuromuscular disease, a restrictive thoracic disorder, significant cardiac failure or cardiac disease if data from participants with COPD could not be analysed separately.
Types of interventions
Inclusion
The intervention for the active group consisted of the application of NIV (including bilevel, inspiratory pressure support and proportional assist ventilation) delivered via a mask or mouthpiece during all supervised exercise training sessions. The intervention for the control group was exercise training with or without sham NIV during all supervised exercise training sessions. Studies that involved the delivery of supplemental oxygen during exercise training in one group (e.g. exercise training with NIV and supplemental oxygen) were included provided that supplemental oxygen was also delivered to the alternative group (e.g. exercise training with supplemental oxygen). Similarly, studies that involved the use of nocturnal NIV were included only if both the actively treated group and the control group received nocturnal NIV. Training had to include lower limb and/or upper limb endurance exercise and had to comprise four or more weeks with a minimum of two supervised sessions per week.
Exclusion
Studies that used continuous positive airway pressure as the active treatment during exercise training were excluded.
Types of outcome measures
Primary outcomes
Exercise capacity (defined as peak exercise capacity, constant work rate (endurance) exercise capacity or functional exercise capacity measured post exercise training, without NIV).
Health‐related quality of life (measured using disease‐specific or generic HRQL instruments).
Physical activity: direct measurement (e.g. metabolic equivalents (METS), step count).
Secondary outcomes
Training intensity (e.g. peak training intensity, final session training intensity).
Physiological changes related to exercise training (e.g. blood lactate levels, minute ventilation).
Dyspnoea (e.g. Borg score, visual analogue scale score).
Dropouts.
Adverse events.
Cost.
Search methods for identification of studies
Electronic searches
We identified trials with assistance provided by the Cochrane Airways Group Trials Search Co‐ordinator using the Cochrane Airways Group Specialised Register of trials. This Register was derived from systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Cumulative Index to Nursing and Allied Health Literature (CINAHL), Allied and Complementary Medicine Database (AMED) and PsycINFO, and from handsearching of respiratory journals and meeting abstracts, including annual meetings of the American Thoracic Society, the European Respiratory Society and the British Thoracic Society. All records in the Specialised Register coded as 'COPD' between 1 January 1987 and 24 November 2013 were searched using the following terms: (exercis* or physical* or train* or rehabilitat* or conditioning or ergometry or treadmill or endurance or "upper limb") AND (non‐invasive* or noninvasive* or "non invasive*" or NIV or "positive pressure" or NIPPV or NPPV or "pressure support" or IPS or "assist* ventilation" or PAV or "ventilatory support" or bilevel or BVS or "mechanical ventilation" or "artificial ventilation" or "artificial respiration" or mask* or BiPAP or IPAP or EPAP or nasal* or "positive airway*"). The search commenced from 1 January 1987, as the first reports in the literature of NIV delivered via a mask were dated 1987 (Ellis 1987; Kerby 1987).
To reduce the risk of missing eligible studies, separate searches were conducted on the following databases across the same time period: AMED, CENTRAL, CINAHL, EMBASE, Latin American and Caribbean Health Science Information Database (LILACS), MEDLINE, Physiotherapy Evidence Database (PEDro), PsycINFO and PubMed. See Appendix 1 for a list of search strategies for each database. Several clinical trials registers and search engines were also screened: Australian New Zealand Clinical Trials Register (www.anzctr.org.au); ClinicalTrials.gov (www.ClinicalTrials.gov); International Standard Randomised Controlled Trial Number Register (www.controlled‐trials.com/isrctn/); Netherlands Trial Register (www.trialregister.nl/trialreg/index.asp); University hospital Medical Information Network (UMIN) (www.umin.ac.jp/ctr/index/); Google Scholar (http://scholar.google.com.au/); and Web of Science (http://thomsonreuters.com/web‐of‐science/).
Searching other resources
We screened reference lists of included studies and of review articles obtained from the initial search for additional studies that potentially met the inclusion criteria. Authors of the included trials and international experts in the field of NIV were contacted and were asked to identify any other published or unpublished studies involving NIV during exercise training in COPD. Four of the six authors of included trials responded (Bianchi 2002; Hawkins 2002; Toledo 2007; van 't Hul 2006), and 11 of the 18 experts responded. No additional trials were identified. We also screened conference abstracts from the following meetings: American College of Chest Physicians, Asia Pacific Society of Respirology, German Society for Pneumology and Respiratory Medicine and the Thoracic Society of Australia and New Zealand. Abstracts were included in this review, and no language restrictions were applied.
Data collection and analysis
Selection of studies
Two review authors (CM and AJP) independently selected studies for inclusion in the review. Initially, titles and abstracts were reviewed, and studies that obviously did not fit the inclusion criteria were discarded. Full papers of the remaining studies were obtained for closer evaluation. Studies that met the inclusion criteria were selected. A list of excluded trials compiled from the group of full papers included the primary reason for exclusion (see Characteristics of excluded studies for details). Disagreements in study selection were resolved by consensus. We calculated a kappa coefficient to determine agreement between the two review authors on study inclusion from the initial selection of full papers (from titles and abstracts) and from the second selection of included studies (from full papers).
Data extraction and management
Two review authors (CM and AJP) independently extracted data from the included studies onto a predesigned form. We recorded the following information: study methods; participant characteristics; interventions; outcomes; and results. Although NIV was used during exercise training in the actively treated groups, post‐training primary and secondary outcome data were extracted only when study participants were evaluated while off NIV (e.g. unassisted test of exercise capacity). Discrepancies in the extracted data were resolved by consensus. If data were not presented numerically, a software programme (Engauge Digitizer, http://digitizer.sourceforge.net/) was used by one review author (KKW) to convert graphical images to numerical data. Two other review authors (CM and AJP) independently manually extracted numerical data from each graph using enlarged copies of the images. Discrepancies were resolved by consensus. Authors of included studies were contacted and were asked to provide missing information when applicable.
Assessment of risk of bias in included studies
Two review authors (CM and AJP) independently assessed the internal validity of the included studies. The strategy recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) was used and included assessment of randomisation sequence generation; allocation concealment; blinding; completeness of outcome assessment; selective outcome reporting; and other potential sources of bias. Unblinded studies were included in this review. Each item was graded as high, low or unclear risk of bias. Disagreements were resolved by consensus. Study authors were contacted to provide additional information when needed.
Studies with multiple treatment groups
One study (Johnson 2002) consisted of two intervention groups and one control group. Data were extracted only from the intervention group that used NIV during exercise training and from the control group, which performed exercise training alone.
Measures of treatment effect
We recorded mean postintervention values and mean changes from baseline values and standard deviations (SDs) for continuous variables from both groups within each study. The mean difference (MD) and the 95% confidence interval (CI) were used when continuous data measured on the same scale were combined. The standardised mean difference (SMD) was used when studies reported data measured on different scales that could not be calculated back to a common scale. When possible, estimates of treatment effect and confidence limits were related to the minimal important difference (MID) for each outcome. When dichotomous data were combined, the treatment effect was defined as the odds ratio (OR) with 95% CI.
Unit of analysis issues
The unit of analysis was the participant.
Dealing with missing data
If the number of dropouts was large (> 15%), and results from intention‐to‐treat analyses (ITT) and per‐protocol analyses were reported, data were extracted from ITT analyses. If ITT analyses were not reported, data from the per‐protocol analyses were extracted for use in the meta‐analysis. If incomplete statistical results were reported in an included study for a given outcome (e.g. point estimate but no measure of variability), we contacted the study author and asked for the missing data. If the missing data were not provided, data were not extracted from the study for that particular outcome.
Assessment of heterogeneity
The effect of heterogeneity was quantified using the I2 statistic. The I2 statistic indicates the percentage of the total variation in observed intervention effects across studies that is due to heterogeneity rather than to chance alone (Deeks 2011). The following thresholds have been suggested to guide the interpretation of I2: 0% to 40% might not be important; 30% to 60% may indicate moderate heterogeneity; 50% to 90% may indicate substantial heterogeneity; and 75% to 100% represents considerable heterogeneity (Deeks 2011).
Assessment of reporting biases
As a result of the small number of included trials, we were not able to produce meaningful funnel plots to assess the likelihood of publication bias (Sterne 2011).
Data synthesis
When the included studies were clinically homogeneous, data were combined using Review Manager 5 software (RevMan 2012), and forest plots were generated. We used a fixed‐effect model for all analyses unless a moderate or greater degree of heterogeneity was detected (I2 > 30%), in which case we used a random‐effects model.
Subgroup analysis and investigation of heterogeneity
The small number of studies included in this review precluded the investigation of heterogeneity between studies and the performance of subgroup analyses. However, if more studies are included in future updates of this review, the following subgroup analyses will be considered if I2 indicates a moderate or higher level of heterogeneity (I2 > 30%).
Study population (e.g. moderate vs severe to very severe disease (GOLD 2013)).
Blinding versus no blinding.
Type of exercise (e.g. treadmill vs cycling training, upper limb vs lower limb training).
Ventilatory settings (e.g. low‐ vs high‐level ventilatory assistance, mode of ventilation).
With versus without the use of supplemental oxygen during exercise training.
Duration of the training programme (e.g. standard vs long).
Primary limitation to peak exercise (e.g. ventilatory limited vs limited by leg fatigue).
Sensitivity analysis
We performed sensitivity analyses to determine the effects of the following on results: methodological design (blinding and allocation concealment), participant characteristics (disease severity), characteristics of the intervention (programme duration) and between‐group differences at baseline. Sensitivity analyses were limited to outcomes that included data from three or more studies in the initial analysis.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
The initial search of electronic databases identified 12,392 potentially relevant reports of studies. Of these, we excluded 12,299 by title and abstract. Full papers of the remaining 93 publications were retrieved for closer inspection. Substantial agreement was reported (Landis 1977) between the two review authors in selection of publications for retrieval of full papers and closer inspection (kappa = 0.78). After the full papers were examined, an additional article was identified from the study reference lists and was retrieved for detailed evaluation. Of the 94 full papers, six met the inclusion criteria of the present review. Perfect agreement was noted between the two review authors for final selection of included studies (kappa = 1.0). A flow chart of the study selection process is displayed in Figure 1. The latest search was run 24 November 2013.
1.
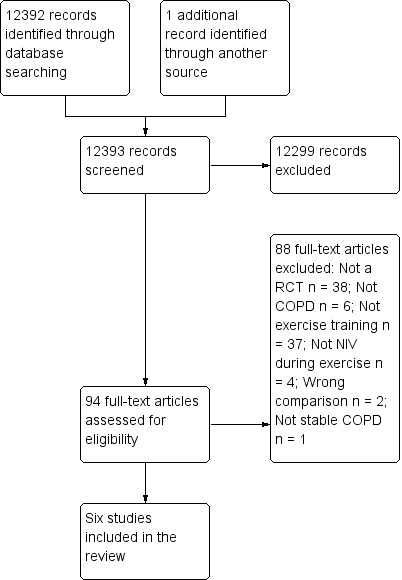
Study flow diagram.
Included studies
In total, six RCTs were included in the review (Bianchi 2002; Hawkins 2002; Johnson 2002; Reuveny 2005; Toledo 2007; van 't Hul 2006). Details of each included study are outlined in Characteristics of included studies, and a summary is provided in Appendix 2. All trials used a parallel‐group design and were published in English. When all studies were combined, data from a total of 126 participants who completed the study protocols (i.e. excluding dropouts) were analysed (control: N = 63; NIV during exercise training: N = 63). Individual study sample sizes ranged from 18 to 29 participants. Studies were conducted in Italy, the United Kingdom, the United States of America, Israel, Brazil and The Netherlands. The mean age of participants ranged from 63 to 71 years. Most participants were male (n = 93 of 108 participants from five studies; one study did not report the sex of participants). Most studies recruited participants with severe to very severe COPD (mean FEV1 26% to 41% predicted), and one study recruited participants with moderate to severe COPD (Bianchi 2002).
Exercise training programmes were conducted in the outpatient setting: two were hospital based (Bianchi 2002; Hawkins 2002); four were based in non‐hospital centres (Johnson 2002; Reuveny 2005; Toledo 2007; van 't Hul 2006). Exercise training programmes were similar between studies; most were conducted over six to eight weeks, with two to three sessions per week of 30 to 45 minutes of exercise training per session at a moderately high intensity. One study (Johnson 2002) encouraged participants to perform additional unsupervised exercise at home (without NIV). Based on log book records, this resulted in an average of two extra exercise sessions per week. All studies involved lower limb exercise training. None of the studies assessed upper limb training.
A variety of modes of NIV were used during exercise training, including bilevel, proportional assist ventilation (PAV) and inspiratory pressure support (IPS), with low to moderate levels of ventilatory support. Only one study compared exercise training with NIV versus exercise training with sham NIV (van 't Hul 2006). The remaining studies used exercise training without NIV as the control intervention. Three studies used supplemental oxygen during exercise training (Hawkins 2002; Johnson 2002; Reuveny 2005). Delivery of oxygen was reported as equivalent between groups. None of the studies included participants receiving domiciliary NIV.
All studies used exercise capacity to evaluate treatment effects, and two studies evaluated HRQL. None of the studies used physical activity as an outcome measure. We attempted to contact authors from all six trials to obtain additional information about study design, outcomes or funding support for the study. Three study authors provided the requested information, one gave a partial response and two did not respond.
Excluded studies
A list of studies excluded (N = 88) during the second round of selection (i.e. from the list of full papers that were evaluated in detail) and reasons for exclusion are presented in Characteristics of excluded studies. The primary reasons for exclusion included the following: not an RCT (N = 38); exercise training not evaluated (N = 37); no COPD (N = 6); NIV not used during exercise (N = 4); wrong comparison (N = 2); no stable COPD (N = 1). Of the excluded trials in which the wrong comparison was made, one study (Pires Di Lorenzo 2003) compared nocturnal NIV plus exercise training versus NIV during exercise training without nocturnal NIV. This study was excluded because nocturnal NIV has been shown to augment the benefits of pulmonary rehabilitation (Duiverman 2008; Garrod 2000; Kohnlein 2009), and this could have confounded the results. The second study (Borghi‐Silva 2010) compared supplemental oxygen during exercise training versus NIV during exercise training. This study was excluded because supplemental oxygen during exercise training has been shown to increase both training intensity and exercise capacity in people with COPD compared with exercise training alone (Emtner 2003), which also could have confounded the results. Four excluded studies were written in Portuguese, three in German, one in Russian, one in French and one in Norwegian. The abstract and method sections of these studies were translated before exclusion. The remaining studies were published in English.
Risk of bias in included studies
Details of the review authors' judgements on risk of bias for each included study can be seen in Figure 2, Figure 3 and Characteristics of included studies.
2.
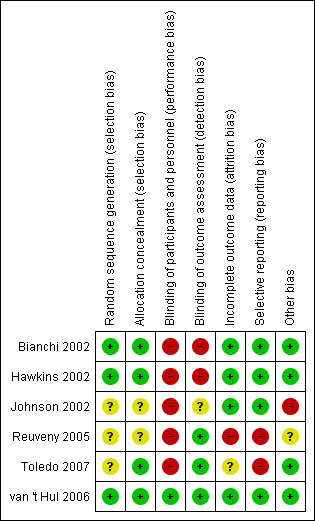
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
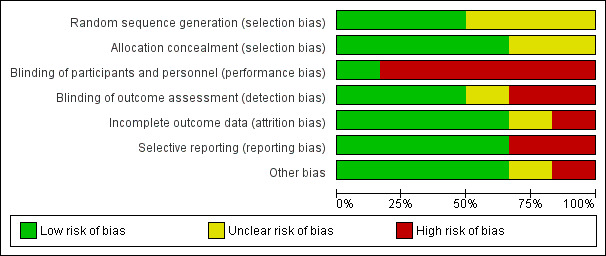
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
The method of randomisation sequence generation was described and was judged to be adequate in half of the studies (Bianchi 2002; Hawkins 2002; van 't Hul 2006). The remaining three studies did not report randomisation sequence generation (Johnson 2002; Reuveny 2005; Toledo 2007), and inability to contact study authors prevented a conclusive assessment of bias in two studies (Johnson 2002; Reuveny 2005). However, despite the use of sealed, opaque envelopes to conceal group allocation, it may have been possible to predict group allocation for a small number of participants (4/29) in the study by (Hawkins 2002), as randomisation blocks were of a fixed size, the study was performed at a single centre and investigators were not blinded to group allocation.
Blinding
Personnel who trained participants were not blinded to group allocation in any of the studies. Similarly, participants were not blinded in most of the studies; this may have introduced bias for outcomes such as exercise capacity and HRQL, whereas physiological outcomes were less likely to be affected. Consequently, high risk of performance bias was observed for five of the six studies. Lack of blinding of participants largely reflects the difficulty of providing an adequate sham intervention for NIV during exercise training. However, one study (van 't Hul 2006) did blind participants using sham NIV (IPS 5 cmH2O), which previously has been shown to have an equivalent effect on exercise performance as unassisted exercise in people with severe COPD (van't Hul 2004). Half of the studies (Reuveny 2005; Toledo 2007; van 't Hul 2006) reported using blinded assessors to evaluate clinical outcomes. Two studies (Bianchi 2002; Hawkins 2002) did not use blinded assessors and were judged as having high risk of detection bias. One study (Johnson 2002) did not report whether outcome assessors were blinded, and the study author could not be contacted to provide clarification.
Incomplete outcome data
Five studies reported the number of dropouts and the reasons for dropping out, and one study (Toledo 2007) did not report the number of dropouts. Intolerance of NIV was reported as a reason for dropping out in two studies: In one study (Reuveny 2005), all dropouts from the NIV during training group (n = 3/12 or 25%) were due to NIV intolerance; in the other study (Bianchi 2002), 28% of participants (n = 5/18) dropped out as the result of NIV intolerance. An ITT analysis was performed in two studies (Bianchi 2002; van 't Hul 2006). The study authors stated that the results did not differ from per‐protocol analyses, although data from ITT analyses were not reported.
Selective reporting
Although most studies were free from selective outcome reporting, two studies (Reuveny 2005; Toledo 2007) did not report results for between‐group comparisons for exercise capacity or for a number of physiological variables despite reporting post‐training within‐group differences.
Other potential sources of bias
In one study (Johnson 2002), the results may have been confounded by contamination, as the group randomly assigned to exercise training with NIV also performed unsupervised exercise training without NIV for an average of two sessions per week. In addition, the same study (Johnson 2002) reported significant between‐group differences in baseline exercise capacity, which may have affected the response to NIV during exercise training. The efficacy of the control intervention (unassisted exercise training) was questionable in one study (Reuveny 2005), as within‐group improvement in exercise capacity did not occur. The group that trained with NIV did improve. However, as trainers and participants were not blinded to the intervention, bias cannot be excluded. However, the progression of training intensity was standardised, which should have helped to ensure that participants were exposed to the same training programme.
Effects of interventions
See: Table 1
See Table 1.
Primary outcomes
Exercise capacity
Peak exercise capacity
All six trials included in the review reported the effects of NIV during exercise training on peak exercise capacity. Three studies (Bianchi 2002; Hawkins 2002; Reuveny 2005) assessed peak exercise capacity using an incremental cycle ergometer test in a combined total of 28 participants who trained with NIV and 29 participants who trained without NIV. No clear evidence of a difference was found between training with or without NIV (MD 6.34 watts; 95% CI ‐1.66 to 14.34; Analysis 1.1). Two studies evaluated peak exercise capacity using incremental treadmill tests. One study (Johnson 2002) used a protocol that increased walking speed and incline, with performance measured in METS, and the other study (Toledo 2007) used a protocol that increased walking speed only, while performance was measured in kilometres per hour. Although both studies used incremental treadmill tests to assess peak exercise capacity, results were not combined, as different constructs were measured (one protocol measured peak work, the other measured peak walking speed) (Table 2). Peak oxygen consumption during an incremental treadmill test was also reported in two studies (Reuveny 2005; Toledo 2007). No clear evidence of a difference was found between exercise training with or without NIV (MD 0.12 L/min; 95% CI ‐0.08 to 0.31; Analysis 1.2). The remaining study (van 't Hul 2006) measured peak exercise capacity using the incremental shuttle walk test (ISWT) (Singh 1992). The individual study effect size of 17.0 metres (95% CI ‐ 2.4 to 36.4) was lower than the reported MID of 47.5 metres (95% CI 38.6 to 56.8) for this test (Singh 2008) (Table 2). A significant difference in peak exercise capacity in favour of training with NIV was observed when the percentage change in peak work rate was assessed in three studies (Hawkins 2002; Johnson 2002; Reuveny 2005) in a combined total of 30 participants who received NIV during exercise training and 30 participants who received exercise training alone (MD 17%; 95% CI 7 to 27; Figure 4; Analysis 1.3).
1.1. Analysis.
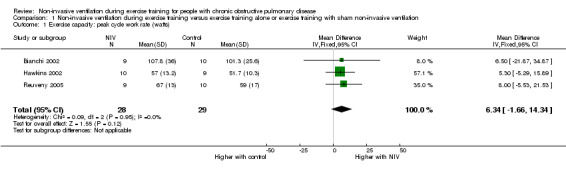
Comparison 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, Outcome 1 Exercise capacity: peak cycle work rate (watts).
1. Results for individual studies.
| Outcome or subgroup | Study | Participants |
Effect estimate (mean difference (95% confidence interval)) |
| Exercise capacity | |||
| Peak work rate (metabolic equivalents (METS)) | Johnson 2002 | 22 | 0.2 (‐0.8 to 1.2) |
| Peak treadmill walking speed (km/h) | Toledo 2007 | 18 | 0 (‐0.9 to 0.9) |
| Peak oxygen consumption (% change) | Reuveny 2005 | 19 | 21 (5 to 37) |
| Incremental shuttle walk test (m) | van 't Hul 2006 | 29 | 17.0 (‐2.4 to 36.4) |
| Incremental shuttle walk test (% change) | van 't Hul 2006 | 29 | 13 (2 to 24) |
| Six‐minute walk test (m) | Bianchi 2002 | 19 | 4.3 (‐64.1 to 72.7) |
| Incremental treadmill test distance (m) | Toledo 2007 | 18 | 30.9 (‐250.6 to 312.4) |
| Training intensity | |||
| Peak cycle training intensity (W) | Hawkins 2002 | 19 | 5.0 (‐4.0 to 14.0) |
| Peak treadmill training speed (km/h) | Reuveny 2005 | 19 | 0 (‐0.5 to 0.5) |
| Physiological outcomes | |||
| Peak exercise minute ventilation (L/min) | Reuveny 2005 | 19 | 10.7 (‐4.0 to 25.5) |
| Peak exercise aerobic threshold (% change) | Reuveny 2005 | 19 | 6 (‐8 to 21) |
| Peak exercise oxygen pulse (mL/beat) | Reuveny 2005 | 19 | 1.0 (‐0.8 to 2.8) |
1.2. Analysis.
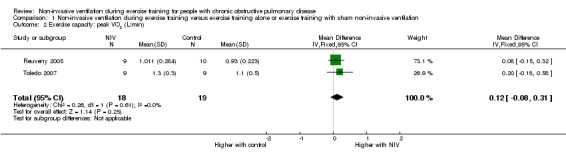
Comparison 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, Outcome 2 Exercise capacity: peak VO2 (L/min).
4.
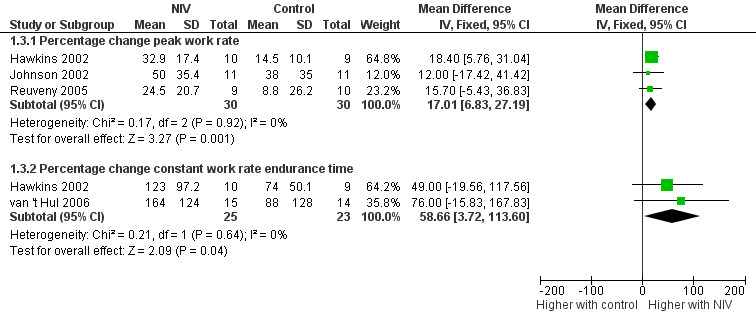
Forest plot of comparison: 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, outcome: 1.3 Exercise capacity: percentage change.
1.3. Analysis.
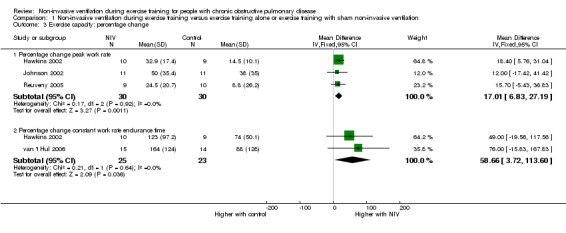
Comparison 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, Outcome 3 Exercise capacity: percentage change.
Three sensitivity analyses were also performed (Table 3). The analysis for peak work rate (watts) was re‐run first after exclusion of data from one study (Bianchi 2002) that recruited participants with milder disease severity, and second after exclusion of data from another study (Reuveny 2005) that did not report adequate allocation concealment. No change in effect size was observed in either case. Finally, the analysis for percentage change in peak work rate was rerun without data from one study (Johnson 2002) with significant between‐group differences in exercise capacity at baseline. Similarly, no differences in effect size were observed.
2. Sensitivity analysis.
| Outcome | Initial analysis | Sensitivity analysis | |||
| Effect estimate |
Study removed |
Reason for removal |
Included studies |
Effect estimate | |
| Exercise capacity | |||||
| Peak cycle work rate (watts) | 6.34 (‐1.66 to 14.34) | a | Disease severity | b, d | 6.33 (‐2.02 to 14.67) |
| Peak cycle work rate (watts) | 6.34 (‐1.66 to 14.34) | d | Allocation concealment | a, b | 5.45 (‐4.48 to 15.37) |
| Peak work rate (% change) | 17 (7 to 27) | c | Baseline differences | b, d | 18 (7 to 29) |
| Training intensity | |||||
| Training intensity (% change) | 13 (1 to 27) | a | Disease severity | b, f | 20 (12 to 28) |
| Training intensity (% change) | 13 (1 to 27) | f | Blinding | a, b | 10 (‐9 to 28) |
| Physiological outcomes | |||||
| Isotime exercise VE (L/min) | ‐0.08 (‐2.82 to 2.67) | a | Disease severity | b, f | ‐0.68 (‐4.89 to 3.52) |
| Peak exercise VE (L/min) | 2.68 (‐2.02 to 7.37) | a | Disease severity | b, d | 2.60 (‐2.35 to 7.55) |
| Isotime exercise VE (L/min) | ‐0.08 (‐2.82 to 2.67) | f | Blinding | a, b | 0.30 (‐2.70 to 3.30) |
| Peak exercise La (mmol/L) | ‐0.35 (‐1.10 to 0.41) | d | Allocation concealment | b, e | ‐0.61 (‐1.59 to 0.37) |
| Peak exercise VE (L/min) | 2.68 (‐2.02 to 7.37) | d | Allocation concealment | a, b | 2.54 (‐3.09 to 8.16) |
| Peak exercise La (mmol/L) | ‐0.35 (‐1.10 to 0.41) | e | Programme duration | b, d | 0.04 (‐0.55 to 0.62) |
| Dyspnoea | |||||
| Isotime dyspnoea (Borg) | ‐0.33 (‐0.83 to 0.16) | a | Disease severity | b, e | ‐0.34 (‐0.86 to 0.17) |
| Isotime dyspnoea (Borg) | ‐0.33 (‐0.83 to 0.16) | e | Programme duration | a, b | ‐0.39 (‐0.92 to 0.14) |
| Dropouts | |||||
| Dropouts | OR 1.26 (0.61 to 2.59) | a | Disease severity | b, c, d, f | OR 1.07 (0.46 to 2.48) |
Effect estimate is presented as mean difference (95% confidence interval) unless otherwise indicated;aBianchi 2002; bHawkins 2002; cJohnson 2002; dReuveny 2005; eToledo 2007; fvan 't Hul 2006.
La: lactate; OR: odds ratio; VE: minute ventilation.
Endurance exercise capacity
Endurance exercise capacity was assessed with a constant work rate cycle ergometer test in two studies (Hawkins 2002; van 't Hul 2006) in a combined total of 25 participants who trained with NIV and 23 participants who performed exercise training alone or with sham NIV. The reported MID for the constant work rate cycle endurance test (performed at 75% peak work capacity) is 101 seconds (95% CI 86 to 116) (Puente‐Maestu 2009). A trend for increased exercise endurance was found to favour exercise training with NIV (MD 3.62 minutes; 95% CI ‐0.17 to 7.41; Analysis 1.4). However, the lower limit of the confidence interval crossed zero. When the summary effect for each study was expressed as the percentage change from baseline, rather than as post‐intervention values, a significant effect in favour of exercise training with NIV was observed when the results were combined (MD 59%; 95% CI 4 to 114; Figure 4; Analysis 1.3). Although the mean effect size for percentage change in endurance time was greater than the reported MID for percentage change in constant work rate cycle endurance of 34% (95% CI 29 to 39) (Puente‐Maestu 2009), the lower limit of the confidence interval was less than the MID.
1.4. Analysis.
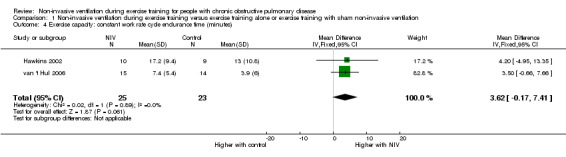
Comparison 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, Outcome 4 Exercise capacity: constant work rate cycle endurance time (minutes).
Functional exercise capacity
Functional exercise capacity was measured in one study (Bianchi 2002) by the six‐minute walk test (6MWT). The MID for the 6MWT in people with COPD is 25 metres (95% CI 20 to 61) (Holland 2010). Individual study results demonstrated no statistically or clinically significant difference between training with NIV and exercise training alone (MD 4.3 metres; 95% CI ‐64.1 to 72.7) (Table 2).
Health‐related quality of life
Health‐related quality of life was measured in two studies (Bianchi 2002; van 't Hul 2006) with the St George’s Respiratory Questionnaire (SGRQ) in a total of 24 participants who trained with NIV and 24 participants who trained without NIV or with sham NIV. A reduction of four points in the SGRQ total score represents a clinically worthwhile improvement in HRQL (Jones 2002). No clear evidence of an effect on HRQL was found for the SGRQ total score (MD 2.5 points; 95% CI ‐2.3 to 7.2). Similar results were found for the three subscales of the SGRQ: symptoms (MD 0.9 points; 95% CI ‐10.2 to 11.9); activity (MD 0.1 points; 95% CI ‐14.9 to 15.0); and impacts (MD 0.1 points; 95% CI ‐6.8 to 7.1) (Figure 5; Analysis 1.5). Heterogeneity between studies was considerable (I2 = 77%) for the activity subsection of the SGRQ.
5.
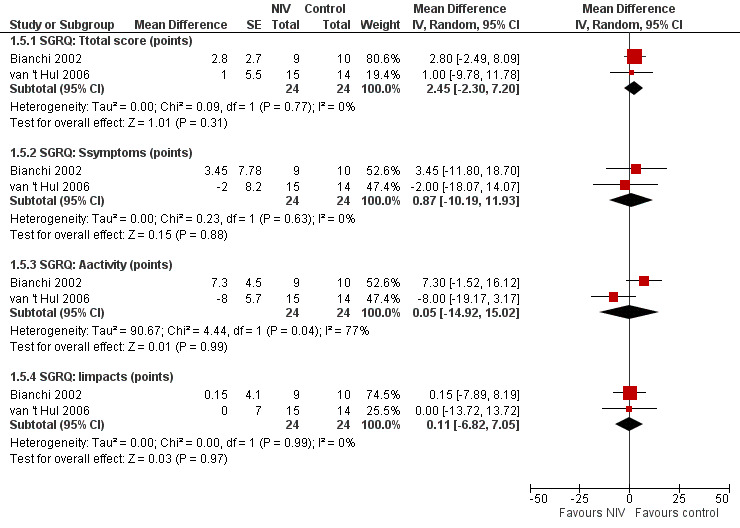
Forest plot of comparison: 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, outcome: 1.5 Health‐related quality of life: St George's Respiratory Questionnaire.
1.5. Analysis.
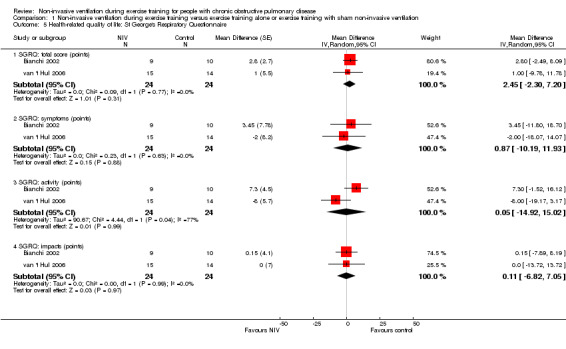
Comparison 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, Outcome 5 Health‐related quality of life: St George's Respiratory Questionnaire.
Physical activity
None of the included studies reported physical activity as an outcome.
Secondary outcomes
Training intensity
Three studies (Bianchi 2002; Hawkins 2002; van 't Hul 2006) reported the training intensity achieved during the final training session (expressed as a percentage of baseline peak work capacity) in a combined total of 34 participants who trained with NIV and 33 participants who performed exercise training alone or with sham NIV. A significant effect on training intensity was found to favour training with NIV during exercise (MD 13%; 95% CI 1 to 27; Figure 6; Analysis 1.6). However, heterogeneity between studies was substantial (I2 = 72%).
6.
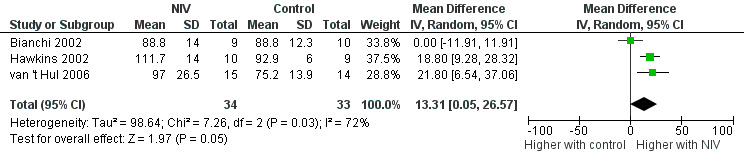
Forest plot of comparison: 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, outcome: 1.6 Training intensity: Final training session (% baseline peak work capacity).
1.6. Analysis.
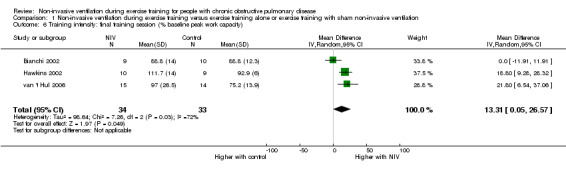
Comparison 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, Outcome 6 Training intensity: final training session (% baseline peak work capacity).
Two sensitivity analyses were conducted (Table 3). First, the analysis was rerun without data from one study (Bianchi 2002) that recruited participants with milder disease. The effect size increased to a mean of 20% (95% CI 12 to 28), and heterogeneity was reduced to 0%. Second, the analysis was rerun without data from one study (van 't Hul 2006) that blinded participants to determine whether the effect size was different (e.g. overestimated) if only studies with unblinded participants were included. The effect size was slightly reduced and the 95% CI widened, with the lower limit of the 95% CI crossing zero (MD 10%; 95% CI ‐9 to 28). Heterogeneity also increased to I2 = 83%.
Physiological outcomes
A significant decrease in isoload blood lactate was observed to favour training with NIV when data from two studies (Hawkins 2002; Toledo 2007) with 19 participants who trained with NIV and 18 participants who trained without NIV (MD ‐0.97 mmol/L; 95% CI ‐1.58 to ‐0.36; Figure 7; Analysis 1.7) were combined. There was no clear evidence of an effect between exercise training with NIV and exercise training alone or exercise training with sham NIV for peak exercise blood lactate, isotime exercise minute ventilation (VE), post‐training peak exercise VE, or change in oxygen consumption at the anaerobic threshold (Analysis 1.8; Analysis 1.9; Analysis 1.10; Analysis 1.11). A moderate level of heterogeneity between studies was found for the analysis of peak exercise blood lactate (I2 = 59%).
7.
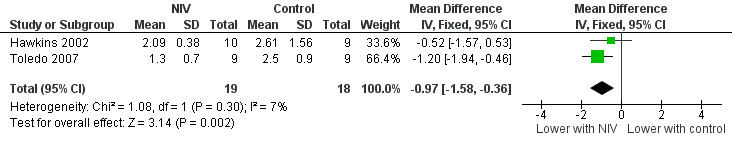
Forest plot of comparison: 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, outcome: 1.7 Physiological outcomes: Isoload lactate (mmol/L).
1.7. Analysis.
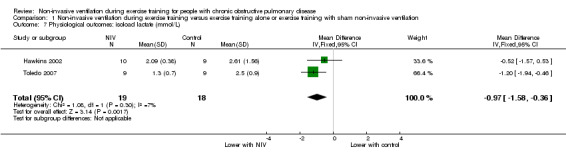
Comparison 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, Outcome 7 Physiological outcomes: isoload lactate (mmol/L).
1.8. Analysis.
Comparison 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, Outcome 8 Physiological outcomes: peak exercise lactate (mmol/L).
1.9. Analysis.
Comparison 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, Outcome 9 Physiological outcomes: isotime exercise minute ventilation (L/min).
1.10. Analysis.
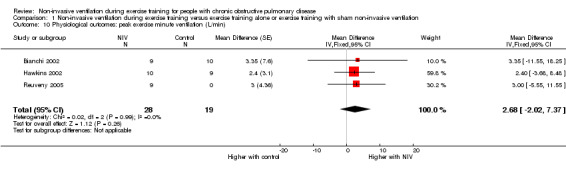
Comparison 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, Outcome 10 Physiological outcomes: peak exercise minute ventilation (L/min).
1.11. Analysis.
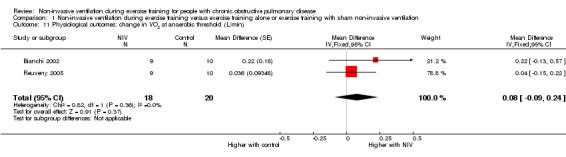
Comparison 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, Outcome 11 Physiological outcomes: change in VO2 at anaerobic threshold (L/min).
Several sensitivity analyses were conducted (Table 3). First, analyses for isotime exercise VE and for peak exercise VE were rerun without data from one study (Bianchi 2002) that recruited participants with milder disease. For each outcome, the effect size did not change. The analysis for isotime exercise VE was rerun without data from one study (van 't Hul 2006), which blinded participants to determine whether the effect size was different if only studies with unblinded participants were included. The effect size did not change substantially. Analyses for peak exercise blood lactate and peak exercise VE were also rerun with data excluded from one study (Reuveny 2005) that did not report adequate allocation concealment. A slight increase in effect size was noted for peak exercise blood lactate from ‐0.35 mmol/L (95% CI ‐1.10 to 0.41) in the initial analysis to ‐0.62 mmol/L (95% CI ‐1.22 to ‐0.01), and heterogeneity between studies did not change. The effect size for peak exercise VE was not altered. Finally, the effect size for peak exercise blood lactate was mildly reduced to 0.04 mmol/L (95% CI ‐0.55 to 0.62) when the analysis was repeated without data from one study (Toledo 2007) with a programme duration approximately twice as long as that of other studies included in the review, and heterogeneity between studies decreased to 0%.
Dyspnoea
Post‐training isotime exercise dyspnoea was measured in three studies (Bianchi 2002; Hawkins 2002; Toledo 2007) in a total of 28 participants who trained with NIV and 28 participants who performed exercise training alone. No significant effect on dyspnoea, as measured on the Borg scale, was noted between participants performing exercise training with and without NIV (MD ‐0.18; 95% CI ‐1.09 to 0.72; Analysis 1.12). A sensitivity analysis that excluded data from one study (Bianchi 2002), which recruited participants with milder disease, did not change the size of the effect. Similarly, a sensitivity analysis that excluded data from one study (Toledo 2007) with a longer programme duration did not alter the effect size (Table 3).
1.12. Analysis.
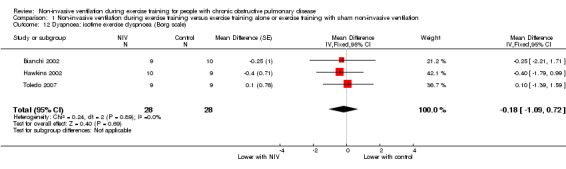
Comparison 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, Outcome 12 Dyspnoea: isotime exercise dyspnoea (Borg scale).
Dropouts
Dropouts were reported in five studies (Bianchi 2002; Hawkins 2002; Johnson 2002; Reuveny 2005; van 't Hul 2006) from a total of 151 participants (78 participants who were randomly assigned to exercise training with NIV and 73 participants who were randomly assigned to exercise training without NIV or with sham NIV). There was no evidence of a clear effect on dropouts with NIV during exercise training compared with exercise training alone, or exercise training with sham NIV (OR 1.26; 95% CI 0.61 to 2.59; Analysis 1.13). A sensitivity analysis that excluded data from one study (Bianchi 2002), which recruited participants with milder disease, did not change the magnitude of the effect (Table 3).
1.13. Analysis.
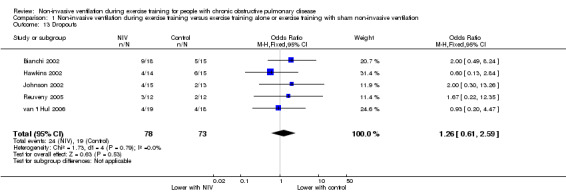
Comparison 1 Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation, Outcome 13 Dropouts.
Adverse events
Adverse events were not reported in any of the studies.
Cost
Cost was not reported in any of the studies.
Discussion
Summary of main results
Pulmonary rehabilitation, with exercise training as a key component, is well established as a standard of care for people with COPD, with demonstrated improvement in exercise capacity, HRQL and dyspnoea (Lacasse 2006). The aim of this systematic review was to determine whether NIV during exercise training could provide benefit for exercise capacity, HRQL and physical activity above that of exercise training alone in people with COPD. The current review showed that NIV during exercise training allowed participants to achieve a greater percentage improvement in lower limb peak and endurance exercise capacity, to exercise at a higher training intensity and to gain a greater physiological training effect compared with exercise training alone or exercise training with sham NIV. There was no clear evidence that HRQL was better or worse with NIV during exercise training, and the effect of NIV during exercise training on physical activity is unknown, as none of the included studies reported this outcome.
Results for the effect of NIV during exercise training on exercise capacity should be interpreted with caution, as differences were found only when percentage change from baseline values rather than post‐intervention values were used in analyses. One possible explanation for the difference in results is that if large interindividual or intergroup baseline differences were present, the use of change from baseline values rather than postintervention values would provide greater statistical power to detect treatment effects. In addition, the overall quality of the evidence for percentage change in peak and endurance exercise capacity was judged as low (see Table 1). The clinical significance of the treatment effect for percentage change in peak exercise capacity is unknown, and the effect size may have been exaggerated because of the high risk of bias of studies included in the analysis. Endurance exercise capacity may be more relevant to people with COPD than peak exercise capacity, given that most daily activities are performed at a submaximal level (Pitta 2005). However, interpretation of the clinical significance of the effect size for percentage change in endurance exercise capacity is also unclear. Although the mean effect of 59% was above the reported MID of 34% (Puente‐Maestu 2009), the 95% CI was very wide, with the lower limit of the CI (4%) considerably below the MID.
The finding of an improvement in some aspects of exercise capacity with NIV during exercise training compared with exercise training alone or exercise training with sham NIV may relate to the fact that NIV during exercise training permits higher‐intensity exercise training and results in a greater physiological training effect, as reflected by lower isoload blood lactate levels. It is interesting to note that although isoload lactate was reduced with NIV during exercise training, no evidence was found of a significant reduction in isotime VE or isotime dyspnoea. Although overall assessments of the quality of the evidence for training intensity and isoload lactate were moderate (see Table 1), it is unknown whether the size of the treatment effects is clinically meaningful.
As no cure for COPD is known, treatment aims to relieve symptoms, slow disease progression, optimise function and overall health and prevent and treat exacerbations (GOLD 2013). As such, HRQL is an important outcome for people living with COPD. Although the overall quality of the evidence for HRQL was judged as moderate (see Table 1), only two of the studies included in this review assessed HRQL. In addition, significant heterogeneity was found across studies for the activity subsection of the SGRQ and could not be investigated further because of the small number of studies included in the analysis. As a result, the effect of NIV during exercise training on this domain remains uncertain. The effect of NIV during exercise training on functional exercise capacity is also unclear, as this outcome was measured in only one study (Bianchi 2002) by the 6MWT. Changes in six‐minute walk distance are an important prognostic indicator for people with COPD and have been shown to relate to mortality (Polkey 2013) and risk of hospitalisation (Spruit 2012). Similarly, the effect of NIV during upper limb exercise training is unknown, as none of the included studies used upper limb exercise as a training modality. Upper limb training is recommended as part of a comprehensive pulmonary rehabilitation programme (Spruit 2013), and some evidence suggests that NIV during unsupported arm exercise improves endurance exercise capacity during a single exercise session (Menadue 2009a). Consequently, HRQL, functional exercise capacity and upper limb training should be considered as outcomes for future studies.
Among the combined total of 63 participants who trained with NIV, no adverse events were reported. However, as the total number of participants who trained with NIV was relatively small, the effect of NIV during exercise training on adverse events is unclear in people with moderate to very severe COPD.
Overall completeness and applicability of evidence
The studies included in the current review recruited participants with severe to very severe COPD (GOLD 2013), with the exception of one study (Bianchi 2002), which recruited participants with moderate to severe COPD. The impact of disease severity on the efficacy of training with NIV could not be formally assessed in the present review. However, based on outcomes from the individual included studies, it appears that disease severity could be an important factor in patient selection for this technique, with greater benefit reported in studies in which individuals with severe to very severe COPD were recruited, compared with those with moderate disease (Bianchi 2002). In addition to selecting people with severe COPD, two studies (Reuveny 2005; van 't Hul 2006) selected participants who demonstrated a very limited ventilatory reserve at peak unassisted exercise, suggesting a ventilatory limitation to exercise. In the latter study (van 't Hul 2006), participants were included only if they were tolerant of NIV, indicating that participants were highly selected. It is unclear whether a trial of NIV was undertaken to test acceptability before enrolment in the other included studies. Three of the included studies (Reuveny 2005; Toledo 2007; van 't Hul 2006) also did not report the number of patients screened during the recruitment process. Of those studies that did report the number of patients screened during the recruitment process (Bianchi 2002; Hawkins 2002; Johnson 2002), no information was provided regarding the number of patients who declined to take part because of the intervention (NIV). Subsequently, the potential for participants to have been highly selected cannot be excluded. In addition, two studies reported dropouts due to poor tolerance of NIV (Bianchi 2002; Reuveny 2005), which could have related to selection of participants with less severe COPD (Bianchi 2002) or the provision of lower levels of ventilatory support (Bianchi 2002; Reuveny 2005) compared with other studies (Hawkins 2002; van 't Hul 2006). Consequently, the findings of the present review may not be applicable to all people with moderate to very severe COPD.
Although the studies included in the present review were reasonably homogeneous and representative of current clinical practice with respect to the exercise training programmes, substantial diversity was reported regarding the delivery of NIV. Three different NIV modes were used (bilevel, PAV and IPS), and ventilatory support ranged from a low to a moderate level. None of the included studies assessed high‐level pressure support, which has shown promising results during ground walking in people with very severe COPD (Dreher 2007). During pressure preset ventilation, the amount of tidal volume assistance delivered will vary, depending on factors such as respiratory system compliance, airways resistance and inspiratory time (Mehta 2001). As a result, a given level of pressure support can have a different effect on tidal volume between participants and even within an individual, for example, if dynamic hyperinflation occurs during exercise and respiratory system compliance is reduced. However, as subgroup analyses could not be performed, the influence of these factors on the treatment effects associated with NIV during exercise training is unclear. As yet, the optimal mode and settings for NIV during exercise training are unknown. FInally, although NIV during exercise training could potentially benefit select individuals with COPD, implementation of this technique does have resource implications and would require experienced staff and access to appropriate equipment and may involve extra costs, which could limit the feasibility of this technique in some settings.
Quality of the evidence
Six studies with a combined total of 126 participants who completed the study protocols (63 with NIV during exercise training; 63 with exercise training alone or exercise training with sham NIV) were included in the current review. Limitations in the literature were noted in terms of the small number of studies included in the analyses, the small numbers of participants within the included studies and issues related to methodological quality such as lack of blinding or inadequate reporting of allocation concealment. These limitations are reflected in assessments of the quality of the evidence, which ranged from low for exercise capacity outcomes to moderate for HRQL, training intensity and post training isoload blood lactate levels (see Table 1).
The key methodological limitation of the studies was lack of blinding. Only one study blinded participants, three used blinded assessors and none of the included studies blinded trainers, which may have introduced performance or detection bias. Consequently, important outcomes such as exercise capacity and HRQL could be influenced by bias, as all analyses included data from unblinded studies. Unblinded studies have been shown to overestimate treatment effect size by 9% compared with blinded studies (Pildal 2007). Blinding an intervention such as NIV is difficult but may be achieved with the use of sham NIV. However, if an inappropriate sham NIV is used, the treatment effect size could be altered by sham NIV either impeding exercise performance or assisting exercise performance compared with what would have occurred during unassisted exercise. Therefore a sham NIV would have to be shown to be appropriate for a given patient population before commencement of a training study. For example, the sham NIV used by one study (van 't Hul 2006) was previously demonstrated to have an equivalent effect on exercise performance as unassisted exercise (van't Hul 2004). Sensitivity analyses were performed to determine whether lack of blinding exaggerated effect sizes in the present review. Because of the small number of included studies, this could be performed only for isotime exercise VE and training intensity. No substantial changes in effect size were observed, suggesting that these results are robust.
Allocation concealment was adequately performed and reported in most of the trials. However, two studies (Johnson 2002; Reuveny 2005) did not provide an adequate description of allocation concealment in the paper, and the study authors could not be contacted to provide additional information. In addition, although allocation concealment was adequately described in another study (Hawkins 2002), it may have been compromised for a small number of participants as the result of block randomisation. If the size of the blocks used during block randomisation is fixed and known, it may be possible to predict future group allocation for some participants in an unblinded trial (Berger 2005). Inclusion of studies without adequate allocation concealment has been reported to overestimate effect size by 18% to 37% (Moher 1998, Pildal 2007). In the present review, only a limited number of sensitivity analyses could be conducted to assess the impact of including trials without adequate description of allocation concealment. The change in effect size was small to negligible for peak work rate (watts), training intensity, peak exercise blood lactate and peak exercise VE, indicating that these results were also robust.
Another factor that may have impacted the results of the current review is lack of statistical power. Only six studies were eligible for inclusion in the review, and within each study, sample sizes were generally small, with dropout rates in five of the studies ranging from 21% to 42%. In addition, a variety of measurement tools were used to assess outcomes of interest. Consequently, only a small number of meta‐analyses could be performed, often with results from only two to four studies combined, occasionally with data from as few as 37 participants. Statistical power also was probably compromised in individual studies. Two studies (Reuveny 2005; Toledo 2007) failed to present post‐training results for between‐group comparisons of expected outcomes such as exercise capacity, despite conducting parallel RCTs to assess the effects of training with NIV versus exercise training alone. This reporting bias may have occurred because significantly different results between groups were not detected. Another study (Hawkins 2002) was powered to assess post‐training isoload blood lactate. However, the combination of dropouts and difficulty gaining vascular access in some participants reduced the power of the study to detect differences between groups. When isoload blood lactate data from this study were combined with data from Toledo 2007 in the present review, statistical power was improved, and a difference between interventions was found to favour training with NIV. A larger number of RCTs with greater numbers of participants are needed to achieve sufficient statistical power to confidently assess the effects of NIV during exercise training on key outcomes.
Significant heterogeneity across studies was detected in only three analyses: HRQL (activity subsection of the SGRQ); peak exercise blood lactate; and training intensity. The most likely reason for heterogeneity for the activity subsection of the SGRQ was a difference in disease severity between the two studies. The condition of participants from one study (van 't Hul 2006) was more severe (based on FEV1 and ventilatory reserve at peak exercise) than that of participants recruited by the second study (Bianchi 2002), and a trend was found to favour NIV during training improving this outcome. In contrast, the second study (Bianchi 2002) reported a trend for improvement in this outcome in favour of the control group. It is unlikely that the treatment effect was overestimated by the first study (van 't Hul 2006), as allocation concealment was adequate and both participants and assessors were blinded. However subgroup and sensitivity analyses could not be performed to investigate the cause of heterogeneity, as only two studies reported this outcome. Three studies were included in the analysis of peak exercise blood lactate. The training programme in one study (Toledo 2007) was substantially longer (12 weeks) than the programmes in the other two studies (six to eight weeks) (Hawkins 2002; Reuveny 2005). Greater training effects can be achieved with a training programme duration of 12 weeks or longer in comparison with programmes with a duration of six to eight weeks (Ries 2007). This factor appeared to account for the difference in effect sizes between studies, as demonstrated in a sensitivity analysis for which data from the study with the longer programme duration (Toledo 2007) were removed, with the summary effect size reduced and I2 decreased to zero. Finally, differences in disease severity appeared to explain heterogeneity across studies in the analysis of training intensity. Two studies (Hawkins 2002; van 't Hul 2006) recruited participants with severe to very severe COPD and reported an increase in training intensity with NIV during exercise training compared with control, whereas another study (Bianchi 2002) recruited a 'milder' group of participants and found no difference in training intensity between those who trained with NIV and the control group, suggesting that individuals with less severe disease may not derive benefit from NIV during exercise. A sensitivity analysis that excluded data from Bianchi 2002 increased the summary effect size and reduced I2 to zero.
Potential biases in the review process
Strengths of the review process include adherence to a predefined protocol (Menadue 2009b), with the exception of several small alterations (see Differences between protocol and review), and the performance of a comprehensive literature search (including non‐English trials and grey literature). A potential weakness of the review process was the inability to assess for the likelihood of publication bias because of the small number of included trials. To reduce the risk of publication bias, a number of clinical trial registers were searched, conference abstracts were reviewed and international experts in the field of NIV were asked to identify further published or unpublished trials. However, no additional potential studies were found. Not all of the studies included in the present review reported results in favour of NIV during exercise training. Nevertheless, publication bias cannot be excluded. Finally, not all of the authors of included studies could be contacted to provide additional information regarding study design or data. This may have affected the judgement of some categories of risk of bias and limited the data included in meta‐analyses for some outcomes.
Agreements and disagreements with other studies or reviews
Two non‐Cochrane systematic reviews and meta‐analyses have previously investigated the effects of NIV during exercise. Ricci 2013 evaluated the physiological effects of NIV during exercise training in people with stable COPD compared with control (exercise training alone or exercise training with sham NIV or supplemental oxygen). In addition to the six RCTs included in the present review, Ricci 2013 included one quasi‐randomised study (Costes 2003) and one study that compared supplemental oxygen during exercise training with NIV during exercise training (Borghi‐Silva 2010). In contrast to the present review, Ricci 2013 found no difference between NIV during exercise training and control with respect to lactate. Also no difference between NIV during exercise training and control was found for heart rate, oxygen consumption (VO2) and workload. However, very limited data were provided, as the study authors did not state which studies were included in each meta‐analysis and did not report the summary effect for outcomes other than VO2. The second systematic review (van't Hul 2002) reported significant benefit for exercise endurance time and dyspnoea during a single application of NIV during exercise in people with COPD compared with control (exercise without NIV). The present review also found some evidence that endurance exercise capacity may be improved with NIV during exercise, although, in contrast to van't Hul 2002, for which included studies tested endurance exercise capacity while participants breathed on NIV, studies included in the present review tested exercise capacity post training and without NIV. Also, the present review did not find a reduction in dyspnoea associated with NIV during training. However, as dyspnoea was measured after training and without NIV during exercise in the studies included in the present review, the difference in results may simply reflect the fact that the reduction in dyspnoea is a temporary phenomenon related to respiratory muscle unloading (Maltais 1995) as a direct result of the application of NIV.
To date, three literature reviews have specifically addressed the role of NIV during exercise training as part of a pulmonary rehabilitation programme (Araujo 2005; Corner 2010; De Backer 2010). Meta‐analyses were not performed in any of the reviews. Araujo 2005 did not discuss any of the studies included in the present review, and Corner 2010 included five RCTs from the current review (Bianchi 2002; Hawkins 2002; Johnson 2002; Reuveny 2005; van 't Hul 2006) and one quasi‐randomised study (Costes 2003) that was excluded from the present review, and excluded one RCT (Toledo 2007) that was included in the present review. De Backer 2010 included all six RCTs from the present review, as well as one quasi‐randomised study (Costes 2003) and one randomised cross‐over study (Barakat 2007), all of which were excluded from the present review. Conclusions were similar between the three reviews, namely, that NIV may permit patients with moderate to very severe COPD to train at a higher intensity and gain greater improvement in exercise capacity compared with exercise training alone. The current review largely supports these findings. However, it is unclear whether the observed improvement in exercise capacity is clinically worthwhile.
Several pulmonary rehabilitation practice guidelines have included recommendations regarding the role of NIV as an adjunct to exercise training during pulmonary rehabilitation. Most recently, the American Thoracic Society/European Respiratory Society Statement 'Key Concepts and Advances in Pulmonary Rehabilitation' (Spruit 2013) referred to the findings of the literature review by Corner 2010, which concluded that NIV appears to enhance the effects of exercise training, with greatest benefit observed in individuals with severe disease. It was also stated that as NIV is a difficult and labour‐intensive intervention, its use may be feasible only in centres with significant expertise with NIV, and for individuals with demonstrated benefit from using NIV during exercise (Spruit 2013). Also in 2013, the British Thoracic Society Guideline on Pulmonary Rehabilitation in Adults (Bolton 2013) stated that NIV should not be used routinely during pulmonary rehabilitation in patients with chronic hypercapnic respiratory failure who do not already receive domiciliary NIV, and that patients with chronic hypercapnic respiratory failure who use domiciliary NIV should be offered the opportunity to exercise with NIV during pulmonary rehabilitation, provided that this is tolerable and accepted by the patient. In 2007, The Joint American College of Chest Physicians/American Association of Cardiovascular and Pulmonary Rehabilitation Evidence‐Based Clinical Practice Guidelines stated that NIV during exercise training may be of benefit for select patients with severe COPD and may permit modest improvements in exercise performance above that of exercise training alone (Ries 2007). The current review provides evidence to support some of these recommendations, for example that NIV during exercise may lead to improvement in endurance exercise capacity above that of exercise training alone. However, regarding patient selection for this technique, although the results of individual studies suggest that individuals with severe to very severe COPD may respond better to NIV during exercise training than those with milder disease, an insufficient number of included studies in the present review precluded the performance of subgroup analyses to determine the effect of disease severity on outcomes. The present review does not provide evidence for the use of NIV during exercise training in people with chronic hypercapnic respiratory failure secondary to COPD, as none of the studies included in the present review recruited participants with chronic hypercapnia. However, as benefit for exercise capacity and dyspnoea has been observed in this population during an acute application of NIV during exercise (Bianchi 1998), investigation of the role of NIV during exercise training is warranted. Some guidelines (Spruit 2013) recommend selecting individuals for NIV during training who have previously demonstrated an acute benefit from exercise with NIV. However, currently no evidence is available to suggest that selecting individuals on these grounds will result in greater training effects with NIV, as the predictive validity of the acute response is low (van 't Hul 2006).
Although a number of pulmonary rehabilitation review articles (Spruit 2013; Troosters 2005; Troosters 2010) allude to NIV during exercise training as a difficult, costly and time‐consuming intervention, none of the studies included in the present review reported the additional costs associated with using NIV during exercise training when compared with exercise training alone. One of the included studies (Bianchi 2002) did report that staff spent an average of 11 ± 3 minutes setting up the ventilator. However, it is unclear whether this time was spent during the initial session or during each session of the rehabilitation programme, or how this compared with the amount of time spent with the group who performed exercise training without NIV. The issue of cost, in terms of staff time and additional resources, requires further investigation before conclusions can be drawn as to whether NIV could or could not be a cost‐effective adjunct to exercise training for people with COPD.
Authors' conclusions
Implications for practice.
This review provides evidence that NIV during exercise training may allow people with COPD to exercise at a higher training intensity and to achieve a greater physiological training effect compared with exercise training alone or exercise training with sham NIV. Although some evidence suggests that NIV during exercise training improves the percentage change in peak and endurance exercise capacity, these findings are not consistent across other measures of peak and endurance exercise capacity. The results for quality of life were uncertain and our analysis did not exclude there being an effect with NIV during exercise. It is currently unknown whether the demonstrated benefit of NIV during exercise training is clinically worthwhile or cost‐effective.
Implications for research.
To conclusively determine the effects of NIV during exercise training, additional RCTs with larger numbers of participants are needed. It is essential that studies have a strong methodological design to minimise the risk of bias, as well as high‐quality reporting to enable accurate assessment of the risk of bias. In particular, blinding of participants, trainers and assessors is required, although arguably difficult, with an intervention such as NIV. Important outcomes that should be evaluated include endurance exercise capacity, HRQL and physical activity. Assessment of exercise capacity using tests for which the MID is known may help to clarify whether clinically relevant improvements in exercise capacity can be obtained. Longer‐term follow‐up of study participants (e.g. 12 months) should also be performed. In addition, future studies need to quantify the extra time and costs associated with NIV during exercise training, so that this potential barrier can be weighed against any potential benefits.
The optimal mode and settings for NIV during exercise training are not well defined and may directly alter the efficacy of NIV during exercise training. However, a larger number of studies are required before subgroup analyses can be performed to determine the effects of ventilator mode and settings on important outcomes. Studies included in the present review used only low to moderate levels of ventilatory support during exercise training. Assessment of high‐level ventilatory support during exercise training is warranted. Evaluation of NIV during exercise training in subgroups, such as those with or without a limited ventilatory reserve at peak (unassisted) exercise and individuals with or without significant dynamic hyperinflation during exercise, may help to better define a target population for this intervention. People with chronic hypercapnic respiratory failure who are considered appropriate candidates for nocturnal NIV could potentially benefit from NIV during exercise training and should be assessed in future studies.
Acknowledgements
Thank you to Elizabeth Stovold and Susan Hansen for assistance with the search strategy and for conducting searches of the Cochrane Airways Group Register of Trials, and to Dr Emma Welsh, Toby Lasserson and Emma Jackson for support provided throughout the review process. Also, thank you to Professor Jennifer Alison for advice and comments on the review.
Phillippa Poole was the Editor for this review and commented critically on the review.
Appendices
Appendix 1. Search strategies
A randomised controlled trial (RCT) filter was applied to all database searches except for CENTRAL and PEDro, as these databases contain only controlled trials and systematic reviews. The filter was developed by grouping database‐specific terms for ‘RCT’ using ‘or.’ Then, database‐specific search terms for ‘NIV' were grouped using ‘or,’ database‐specific search terms for ‘Exercise’ were grouped using ‘or’ and database‐specific search terms for ‘COPD’ were grouped using ‘or.’ Afterwards, the following four search strategies were used for each database: (I) ‘RCT’ and ‘COPD’ and ‘NIV’ and ‘Exercise’; (ii) ‘RCT’ and ‘COPD’ and ‘NIV’; (iii) ‘RCT’ and ‘COPD’ and ‘Exercise’; and (iv) ‘NIV’ and ‘Exercise.’ We searched the following databases via Ovid: EMBASE; MEDLINE; Allied and Complementary Medicine Database (AMED); Cumulative Index to Nursing and Allied Health Literature (CINAHL); PsycINFO. See below for detailed search strategies for each database.
Allied and Complementary Medicine Database (AMED)
| RCT | NIV | Exercise | COPD | |
|
Search terms |
#1 Randomized controlled trial.pt. #2 Controlled clinical trial.pt. #3 Randomized.ab. #4 Placebo.ab. #5 Randomly.ab. #6 Trial.ab. #7 Groups.ab. #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 (animals not (humans and animals)).sh. #10 #8 not #9 |
#11 Respiration, Artificial/ or Positive‐Pressure Respiration/ #12 Intermittent Positive‐Pressure Ventilation/ #13 Ventilators mechanical/ #14 #11 or #12 or #13 |
#15 Exercise/ or Exercise Therapy/ or Exercise Test/ or Exercise Tolerance/ #16 "Physical Education and Training"/ #17 Rehabilitation/ #18 Physical Fitness/ or Physical Endurance/ #19 Ergometry/ #20 Walking/ #21 Bicycling/ #22 Upper Extremity/ #23 #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 |
#24 Pulmonary Disease, Chronic Obstructive/ #25 Lung diseases obstructive/ #26 #24 or #25 |
The following combinations of groups of search terms using ‘and’ was performed: RCT (#10) and COPD (#26) and NIV (#14) and Exercise (#23); RCT (#10) and COPD (#26) and NIV (#14); RCT (#10) and COPD (#26) and Exercise (#23); NIV (#14) and Exercise (#23).
Cochrane Central Register of Controlled Trials (CENTRAL)
This search was performed in addition to the search conducted by the Cochrane Airways Group Trials Search Co‐ordinator. CENTRAL is a database of randomised trials and systematic reviews; therefore a randomised controlled trial filter was not required.
| NIV | Exercise | COPD | |
|
Search terms |
#1 Non‐invasive ventilation #2 NIV #3 Proportional assist ventilation #4 PAV #5 Inspiratory pressure support #6 IPS #7 #1 or #2 or #3 or #4 or #5 or #6 |
#8 Exercise | #9 COPD |
The following combinations of groups of search terms using ‘and’ was performed: COPD (#9) and NIV (#7) and Exercise (#8); COPD (#9) and NIV (#7); COPD (#9) and Exercise (#8); NIV (#7) and Exercise (#8).
Cumulative Index to Nursing and Allied Health Literature (CINAHL)
| RCT | NIV | Exercise | COPD | |
|
Search terms |
#1 Clinical trials or placebo |
#2 Positive pressure ventilation #3 Ventilators, mechanical #4 #2 or #3 |
#5 Exercise #6 Physical endurance #7 Endurance #8 Rehabilitation #9 Rehabilitation exercise #10 Conditioning #11 Cardiopulmonary #12 Ergometry #13 Treadmills #14 Exercise test #15 Upper extremity #16 Upper extremity exercises #17 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 |
#18 Lung diseases, obstructive |
The following combinations of groups of search terms using ‘and’ was performed: RCT (#1) and COPD (#18) and NIV (#4) and Exercise (#17); RCT (#1) and COPD (#18) and NIV (#4); RCT (#1) and COPD (#18) and Exercise (#17); NIV (#4) and Exercise (#17).
EMBASE
| RCT | NIV | Exercise | COPD | |
|
Search terms |
#1 Random$ #2 Factorial$ #3 Crossover$ #4 Cross over$ #5 Cross‐over$ #6 Placebo$ #7 Doubl$ adj blind$ #8 Singl$ adj blind$ #9 Assign$ #10 Allocate$ #11 Volunteer$ #12 Crossover‐procedure #13 Double‐blind procedure #14 Single‐blind procedure #15 Randomized controlled trial #16 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 |
#17 Artificial ventilation #18 Assisted ventilation #19 Intermittent positive pressure ventilation #20 Pressure support ventilation #21 #17 or #18 or #19 or #20 |
#22 Exercise #23 Arm exercise #24 Exercise test #25 Exercise tolerance #26 Treadmill exercise #27 Leg exercise #28 Pulmonary rehabilitation #29 Rehabilitation #30 Training #31 Ergometry #32 #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 |
#33 Chronic obstructive pulmonary disease |
The following combinations of groups of search terms using ‘and’ was performed: RCT (#16) and COPD (#33) and NIV (#21) and Exercise (#32); RCT (#16) and COPD (#33) and NIV (#21); RCT (#16) and COPD (#33) and Exercise (#32); NIV (#21) and Exercise (#32).
Latin American and Caribbean Health Science Information Database (LILACS)
| RCT | NIV | Exercise | COPD | |
|
Search terms |
#1 Random$ #2 Placebo$ #3 Trial$ #4 #1 or #2 or #3 |
#5 Respiration, artificial |
#6 Exercise$ #7 Physical$ #8 Train$ #9 Rehabilitat$ #10 Conditioning #11 Ergometry #12 Treadmill #13 Endurance #14 #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 |
#15 COPD #16 COAD #17 DPOC #18 Emphysema$ #19 Bronchit #20 #15 or #16 or #17 or #18 or #19 |
The following combinations of groups of search terms using ‘and’ was performed: RCT (#4) and COPD (#20) and NIV (#5) and Exercise (#14); RCT (#4) and COPD (#20) and NIV (#5); RCT (#4) and COPD (#20) and Exercise (#14); NIV (#5) and Exercise (#14).
MEDLINE
| RCT | NIV | Exercise | COPD | |
|
Search terms |
#1 Randomized controlled trial.pt. #2 Controlled clinical trial.pt. #3 Randomized.ab. #4 Placebo.ab. #5 Randomly.ab. #6 Trial.ab. #7 Groups.ab. #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 (animals not (humans and animals)).sh. #10 #8 not #9 |
#11 Respiration, Artificial/ or Positive‐Pressure Respiration #12 Intermittent Positive‐Pressure Ventilation #13 #11 or #12 |
#14 Exercise/ or Exercise Therapy/or Exercise Test/ or Exercise Tolerance
#15 "Physical Education and Training"
#16 Rehabilitation
#17 Physical Fitness/or Physical Endurance
#18 Ergometry
#19 Walking #20 Bicycling #21 Upper Extremity #22 #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 |
#23 Pulmonary Disease, Chronic Obstructive |
The following combinations of groups of search terms using ‘and’ was performed: RCT (#10) and COPD (#23) and NIV (#13) and Exercise (#22); RCT (#10) and COPD (#23) and NIV (#13); RCT (#10) and COPD (#23) and Exercise (#22); NIV (#13) and Exercise (#22).
PEDro
PEDro is a database of randomised trials and systematic reviews; therefore a randomised controlled trial filter was not required. PEDro was searched with the following terms: COPD and NIV or pressure support or ventilation and exercise or training; COPD and NIV or pressure support or ventilation; NIV or pressure support or ventilation and exercise or training; NIV and exercise.
PsycINFO
| RCT | NIV | Exercise | COPD | |
|
Search terms |
#1 Randomized controlled trial.pt. #2 Controlled clinical trial.pt. #3 Randomized.ab. #4 Placebo.ab. #5 Randomly.ab. #6 Trial.ab. #7 Groups.ab. #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 (animals not (humans and animals)).sh. #10 #8 not #9 |
#11 Respiration, Artificial/ or Positive‐Pressure Respiration #12 Intermittent Positive‐Pressure Ventilation #13 Ventilators mechanical #14 Exp Artificial Respiration #15 #11 or #12 or #13 or #14 |
#16 Exercise/ or Exercise Therapy/ or Exercise Test/ or Exercise Tolerance #17 "Physical Education and Training" #18 Rehabilitation #19 Physical Fitness/ or Physical Endurance #20 Ergometry #21 Walking #22 Bicycling #23 Upper Extremity #24 #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 |
#25 Pulmonary Disease, Chronic Obstructive #26 Lung diseases obstructive #27 Exp Lung Disorders #28 #25 or #26 or #27 |
The following combinations of groups of search terms using ‘and’ was performed: RCT (#10) and COPD (#28) and NIV (#15) and Exercise (#24); RCT (#10) and COPD (#28) and NIV (#15); RCT (#10) and COPD (#28) and Exercise (#24); NIV (#15) and Exercise (#24).
PubMed
| RCT | NIV | Exercise | COPD | |
|
Search terms |
#1 Randomized controlled trial #2 Controlled clinical trial #3 Randomized #4 Placebo #5 Randomly #6 Trial #7 Groups #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 |
#9 Respiration, artificial #10 Positive pressure respiration #11 Intermittent positive‐pressure ventilation #12 #9 or #10 or #11 |
#13 Exercise #14 Exercise test #15 Exercise therapy #16 Exercise tolerance #17 Physical education and training #18 Rehabilitation #19 Physical endurance #20 Physical fitness #21 Ergometry #22 Walking #23 Bicycling #24 Upper extremity #25 #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 |
#26 Pulmonary disease, chronic obstructive |
The following combinations of groups of search terms using ‘and’ was performed: RCT (#8) and COPD (#26) and NIV (#12) and Exercise (#25); RCT (#8) and COPD (#26) and NIV (#12); RCT (#8) and COPD (#26) and Exercise (#25); NIV (#12) and Exercise (#25).
Appendix 2. Summary of characteristics of included studies
| Bianchi 2002 | Hawkins 2002 | Johnson 2002 | Reuveny 2005 | Toledo 2007 | van 't Hul 2006 | |
| Sample size | 19 | 19 | 22 | 19 | 18 | 29 |
| Disease severity | Moderate to severe | Very severe | Severe to very severe | Severe to very severe | Severe to very severe | Severe |
| Setting | Outpatient, hospital‐based | Outpatient, hospital‐based | Outpatient, centre‐based | Outpatient, centre‐based | Outpatient, centre‐based | Outpatient, centre‐based |
| Programme length | 6 weeks | 6 weeks | 6 weeks | 8 weeks | 12 weeks | 8 weeks |
| Supervised sessions/wk | 3 | 3 | 2 | 2 | 3 | 3 |
| Session duration | 30 minutes | 30 minutes | 20 minutes | 45 minutes | 30 minutes | 45 minutes |
| Type of exercise | Cycling | Cycling | Treadmill | Treadmill | Treadmill | Cycling |
| Training intensity | 50% to 70% peak work capacity | 70% peak work capacity | 50% to 60% maximum METs | 65% to 70% initial maximum walking speed | 70% baseline walk speed | 65% peak work capacity |
| Comparison | Unassisted versus NIV | Unassisted versus NIV | Unassisted versus NIV | Unassisted versus NIV | Unassisted versus NIV | Sham NIV versus NIV |
| NIV mode | PAV | PAV | Bilevel | Bilevel | Bilevel | IPS |
| NIV settings | FA: 3.5 (1.6) cmH2O/L/s VA: 6.6 (2.2) cmH2O/L CPAP: 2 cmH2O |
FA: 3.6 (0.7) cmH2O/L/s VA: 12.7 (1.5) cmH2O/L CPAP: 0 |
IPAP: 8 to 12 cmH2O EPAP: 2 cmH2O |
IPAP: 7 to 10 cmH2O EPAP: 2 cmH2O |
IPAP: 10 to 15 cmH2O EPAP: 4 to 6 cmH2O |
IPS: 5 versus IPS: 10 cmH2O EPAP: 0 cmH2O |
CPAP: continuous positive airway pressure; EPAP: expiratory positive airway pressure; FA: flow assist; IPAP: inspiratory positive airway pressure; IPS: inspiratory pressure support; METs: metabolic equivalents; NIV: non‐invasive ventilation; PAV: proportional assist ventilation; VA: volume assist.
Data and analyses
Comparison 1. Non‐invasive ventilation during exercise training versus exercise training alone or exercise training with sham non‐invasive ventilation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Exercise capacity: peak cycle work rate (watts) | 3 | 57 | Mean Difference (IV, Fixed, 95% CI) | 6.34 [‐1.66, 14.34] |
| 2 Exercise capacity: peak VO2 (L/min) | 2 | 37 | Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.08, 0.31] |
| 3 Exercise capacity: percentage change | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Percentage change peak work rate | 3 | 60 | Mean Difference (IV, Fixed, 95% CI) | 17.01 [6.83, 27.19] |
| 3.2 Percentage change constant work rate endurance time | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | 58.66 [3.72, 113.60] |
| 4 Exercise capacity: constant work rate cycle endurance time (minutes) | 2 | 48 | Mean Difference (IV, Fixed, 95% CI) | 3.62 [‐0.17, 7.41] |
| 5 Health‐related quality of life: St George's Respiratory Questionnaire | 2 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 5.1 SGRQ: total score (points) | 2 | 48 | Mean Difference (Random, 95% CI) | 2.45 [‐2.30, 7.20] |
| 5.2 SGRQ: symptoms (points) | 2 | 48 | Mean Difference (Random, 95% CI) | 0.87 [‐10.19, 11.93] |
| 5.3 SGRQ: activity (points) | 2 | 48 | Mean Difference (Random, 95% CI) | 0.05 [‐14.92, 15.02] |
| 5.4 SGRQ: impacts (points) | 2 | 48 | Mean Difference (Random, 95% CI) | 0.11 [‐6.82, 7.05] |
| 6 Training intensity: final training session (% baseline peak work capacity) | 3 | 67 | Mean Difference (IV, Random, 95% CI) | 13.31 [0.05, 26.57] |
| 7 Physiological outcomes: isoload lactate (mmol/L) | 2 | 37 | Mean Difference (IV, Fixed, 95% CI) | ‐0.97 [‐1.58, ‐0.36] |
| 8 Physiological outcomes: peak exercise lactate (mmol/L) | 3 | 56 | Mean Difference (IV, Random, 95% CI) | ‐0.35 [‐1.10, 0.41] |
| 9 Physiological outcomes: isotime exercise minute ventilation (L/min) | 3 | 53 | Mean Difference (Fixed, 95% CI) | ‐0.08 [‐2.82, 2.67] |
| 10 Physiological outcomes: peak exercise minute ventilation (L/min) | 3 | 47 | Mean Difference (Fixed, 95% CI) | 2.68 [‐2.02, 7.37] |
| 11 Physiological outcomes: change in VO2 at anaerobic threshold (L/min) | 2 | 38 | Mean Difference (Fixed, 95% CI) | 0.08 [‐0.09, 0.24] |
| 12 Dyspnoea: isotime exercise dyspnoea (Borg scale) | 3 | 56 | Mean Difference (Fixed, 95% CI) | ‐0.18 [‐1.09, 0.72] |
| 13 Dropouts | 5 | 151 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.61, 2.59] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bianchi 2002.
| Methods |
Study design: parallel randomised controlled trial Inclusion criteria: male participants with COPD diagnosed by American Thoracic Society criteria; referred to outpatient pulmonary rehabilitation; stable clinical condition Exclusion criteria: chronic respiratory failure; other organ failure; cancer; inability to co‐operate; arterial exercise–induced hypertension (systolic blood pressure > 200 mmHg or diastolic blood pressure > 130 mmHg) Statistical analysis: between groups: repeated measures analysis of variance; differences between dropouts and programme completers: Fisher’s exact test; P value < 0.05 significance |
|
| Participants |
Participants recruited from: referred to outpatient multi‐disciplinary pulmonary rehabilitation programme Number screened: 83 Sample size: 9 NIV group; 10 control group Age mean (range), years: 64 (61 to 67) NIV group; 65 (61 to 69) control group Gender: 0 female participants NIV group; 0 female participants control group FEV1 mean (SD) % predicted: 48 (19) NIV group; 40 (12) control group FVC mean (SD) % predicted: 77 (15) NIV group; 74 (19) control group RV mean (SD) % predicted: 161 (69) NIV group; 181 (49) control group PaO2 mean (SD) kPa: 10.0 (1.1) NIV group; 10.0 (1.1) control group PaCO2 mean (SD) kPa: 5.2 (0.6) NIV group; 5.2 (0.5) control group PImax mean (SD) cmH2O: 82.9 (25.8) NIV group; 72.4 (23.4) control group PEmax mean (SD) cmH2O: 147.9 (26.8) NIV group, 139.2 (25.1) control group Domiciliary NIV: nil Long‐term oxygen therapy: nil |
|
| Interventions |
NIV: proportional assist ventilation: flow assist 3.5 (1.6) cmH2O/L/s, volume assist 6.6 (2.2) cmH2O/L with EPAP 2 cmH2O during exercise training Control: unassisted exercise training Supplemental oxygen during exercise training: no Type of exercise training: cycle ergometry Intensity of training: 50% to 70% of peak work capacity Number of sessions per week: 3 Duration of each session: 30 minutes, supervised Total duration of training: 6 weeks Number of training sessions attended (/18): NIV group 17.4 ± 1.3 sessions; control group 17.0 ± 1.8 sessions Programme setting: outpatient, hospital‐based |
|
| Outcomes | Maximal cycle ergometry: unassisted, load increased 10 watts/min; six‐minute walk test; dyspnoea; leg discomfort; St George's Respiratory Questionnaire Evaluation: assessment performed at baseline and immediately following the training programme |
|
| Dropouts | A total of 33 participants were recruited. Of the 18 randomly assigned to NIV, 9 completed the protocol (50% dropout rate). Reasons for dropout: intolerance of NIV (N = 5); acute exacerbation of COPD (N = 2); exercise‐induced hypertension (N = 1); and unexpected coronary disease (N = 1). Of the 15 randomly assigned to the control group, 10 completed the protocol (33% dropout rate). Reasons for dropout: acute exacerbation of COPD (N = 2); exercise‐induced hypertension (N = 2); unexpected coronary disease (N = 1) | |
| Funding | No information provided | |
| Notes |
Country: Italy Study author contacted: full response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "The sequence of allocation was computer generated" |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "...concealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (from report): "Patients and investigators were unblinded during the study" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (from report): "Patients and investigators were unblinded during the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Total number of participants recruited, number of dropouts from each group and reasons for dropping out are reported. Dropout rate was higher in the group that trained with NIV (44%) compared with the group that trained without NIV (33%). Five of the eight dropouts in the NIV group were due to NIV intolerance. Both intention‐to‐treat analyses and per‐protocol analyses were performed for key outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: Published report contains all expected outcomes, including those that were prespecified |
| Other bias | Low risk | None evident |
Hawkins 2002.
| Methods |
Study design: parallel randomised controlled trial Inclusion criteria: severe COPD; current smoker or had a history of smoking Exclusion criteria: orthopaedic or cardiovascular contraindications to exercise Statistical analysis: within groups: paired t‐tests; between groups: unpaired t‐tests; strength of relationship between physiological variables: Pearson’s correlation coefficient; P value < 0.05 significance |
|
| Participants |
Participants recruited from: respiratory medicine clinic Number screened: 34 Sample size: 10 NIV group; 9 control group Age mean (SD), years: 66 (7) NIV group; 68 (9) control group Gender: 0 female participants NIV group; 2 female participants control group FEV1 mean (SD) % predicted: 28 (7) NIV group; 26 (7) control group RV/TLC mean (SD) %: 62 (9) NIV group; 59 (15) control group PaO2 mean (SD) kPa: 8.6 (0.9) NIV group; 8.1 (1.2) control group PaCO2 mean (SD) kPa: 5.6 (0.7) NIV group; 5.8 (0.9) control group Domiciliary NIV: no information provided Long‐term oxygen therapy: no information provided |
|
| Interventions |
NIV: proportional assist ventilation: flow assist 3.6 (0.7) cmH2O/L/s, volume assist 12.7 (1.5) cmH2O/L during exercise training Control: unassisted exercise training Supplemental oxygen during exercise training: yes (two participants with unassisted training; one participant with non‐invasive ventilation during exercise training) Type of exercise training: cycle ergometry Intensity of training: initially 70% of peak work capacity, then increased progressively by 5 watts when able to maintain the existing work rate for cycle of 30 minutes Number of sessions per week: 3 Duration of each session: 30 minutes, supervised Total duration of training: 6 weeks Number of training sessions attended (/18): not reported Programme setting: outpatient, hospital‐based |
|
| Outcomes | Isoload arterialised venous blood lactate concentration; maximal cycle ergometry: unassisted, workload increased 5 to 10 watts/min; constant power cycle ergometry: unassisted, at 70% of baseline peak work capacity Evaluation: Baseline testing was performed on two occasions before the training programme was begun, and on two occasions within one week of completion of the training programme |
|
| Dropouts | A total of 29 participants were recruited. Of the 14 randomly assigned to NIV, 10 completed the protocol (29% dropout rate). Of the 15 randomly assigned to the control group, 9 completed the protocol (40% dropout rate). Reasons for dropout: acute exacerbation of COPD (N = 4); non‐compliance with the exercise programme (N = 4); non‐pulmonary hospitalisation (N = 2) | |
| Funding | Peter Hawkins was funded by grant (F97/1) from the British Lung Foundation. Dimitra Nikoletou was funded by Respironics Inc, which also provided the ventilators used in the study | |
| Notes |
Country: United Kingdom Study author contacted: full response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "The envelopes were shuffled and then numbered in sequence" |
| Allocation concealment (selection bias) | Low risk | Quote (from report): "Patients were randomised using sealed envelopes" Quote (from correspondence): "The envelopes were opaque" Quote (from correspondence): "Subjects were randomised in groups of 6" Comment: It may have been possible to predict group allocation for 4/29 participants because the randomisation blocks were of a fixed size at a single centre and investigators were not blinded to group allocation. However, as the proportion of participants potentially affected was very low, the overall judgement of risk of bias was low |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Participants and trainers would not have been blinded, as sham non‐invasive ventilation was not used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote (from correspondence): "The investigators performing the post exercise test were aware which group the subject was in" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Total number of participants recruited, number of dropouts from each group and reasons for dropping out are reported. Numbers of dropouts were reasonably balanced between groups. Per‐protocol analyses were performed and data reported for key outcomes. An intention‐to‐treat analysis was not reported |
| Selective reporting (reporting bias) | Low risk | Comment: Published report contains all expected outcomes, including those that were prespecified |
| Other bias | Low risk | None evident |
Johnson 2002.
| Methods |
Study design: parallel randomised controlled trial Inclusion criteria: diagnosis of COPD; FEV1 < 50% predicted; ability to walk on a treadmill; referred to outpatient pulmonary rehabilitation Exclusion criteria: exertional angina; congestive cardiac failure; valvular heart disease; uncontrolled cardiac dysrhythmias; other conditions limiting ability to exercise or use a nasal mask Statistical analysis: within groups: paired t‐tests; between groups: unpaired t‐tests; P value < 0.05 significance |
|
| Participants |
Participants recruited from: referred to outpatient pulmonary rehabilitation programme Number screened: 39 Sample size: 11 NIV group; 11 control group; 10 heliox group Age mean (SD), years: 69 (9) NIV group; 67 (8) control group; 72 (9) heliox group Gender: 3 female participants NIV group; 4 female participants control group; 6 female participants heliox group FEV1 mean (SD) % predicted: 32 (9) NIV group; 31 (11) control group; 34 (13) heliox group FVC mean (SD) % predicted: 57 (15) NIV group; 53 (10) control group; 57 (16) heliox group RV mean (SD) % predicted: 203 (63) NIV group; 199 (73) control group; 191 (66) heliox group PaO2 mean (SD) mmHg: 72 (10) NIV group; 69.2 (9) control group; 70.3 (6.0) heliox group PaCO2 mean (SD) mmHg: 42.4 (4.3) NIV group; 43.3 (4.5) control group; 43.5 (3.7) heliox group Domiciliary NIV: no information provided Long‐term oxygen therapy: no information provided |
|
| Interventions |
NIV: bilevel support: IPAP 8 to 12 cmH2O, EPAP 2 cmH2O during exercise training Control: humidified air at 10 L/min via a non‐rebreather mask during exercise training Heliox: 10 L/min of humidified heliox (79% helium, 21% oxygen) via a non‐rebreather mask during exercise training Supplemental oxygen during exercise training: yes. Oxygen was titrated to maintain oxygen saturation of at least 90% throughout the entire testing and training periods. Maximum oxygen delivery flow rate during unassisted training: 2.7 ± 1.6 L/min; during training with NIV: 2.6 ± 1.8 L/min. Information was not provided regarding the number of participants in each group who used supplemental oxygen Type of exercise training: treadmill Intensity of training: 50% to 60% of maximum METS based on initial incremental treadmill test, then increased (speed and grade) once 20 minutes of continuous exercise was achieved at this level Number of sessions per week: 2 Duration of each session: 20 minutes, supervised Total duration of training: 6 weeks Number of training sessions attended (/12): Quote: "Nearly all of the patients were present for 10 sessions" Programme setting: outpatient, centre‐based |
|
| Outcomes | Incremental maximal treadmill test: unassisted, workload increased 0.5 mph every 4 minutes, when predetermined maximum speed achieved, incline was increased at 3% every 4 minutes Evaluation: Testing was performed the week before and within the week following completion of training |
|
| Dropouts | A total of 39 participants were recruited. Of the 15 randomly assigned to NIV, 11 completed the protocol (27% dropout rate). Of the 13 randomly assigned to the control group, 11 completed the protocol (15% dropout rate). Of the 11 randomly assigned to Heliox, 10 completed the protocol (9% dropout rate). Reasons for dropout: exertional angina (N = 1); congestive heart failure (N = 1); flare of chronic liver disease (N = 1); acute exacerbation of COPD (N = 1); tibial fracture (N = 1); scheduling conflict (N = 1); non‐compliance (N = 1) | |
| Funding | Funded through Brooke Army Medical Center local research funds | |
| Notes |
Country: United States of America Study author contacted: no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: No information provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (from report): "No attempt was made to blind the Heliox group and unassisted exercise training group patients to their training modality" Comment: It would not have been possible to blind participants or trainers in the NIV training group either |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided regarding blinding of outcome assessors. This probably was not done |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Total number of participants recruited, number of dropouts from each group and reasons for dropping out are reported. Numbers of dropouts were reasonably well balanced between groups. Per‐protocol analyses (not intention‐to‐treat analyses) were performed for key outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: Published report contains all expected outcomes, including those that were prespecified |
| Other bias | High risk | Quote (from report): "Review of the patient's exercise logs showed that they uniformly exercised more than the supervised sessions, with an average total of approximately four times per week. Hence, our comparison of training modalities represented only a portion of the patients exercise sessions since the others were done at home" Comment: Participants performed two supervised sessions per week according to group allocation (unassisted, with heliox or with non‐invasive ventilation). The unsupervised, unassisted training at home (without non‐invasive ventilation or without Heliox) may have confounded the results (contamination) Comment: baseline difference between groups for key outcomes (non‐invasive ventilation group significantly lower compared with unassisted group for unassisted exercise time and unassisted maximum workload) |
Reuveny 2005.
| Methods |
Study design: parallel randomised controlled trial Inclusion criteria: diagnosis of severe COPD based on smoking history, clinical findings and pulmonary function testing; FEV1 < 40% predicted and < 12% reversibility; ventilatory limitation to exercise, defined as exercise breathing reserve < 5 L/min Exclusion criteria: cardiovascular, orthopaedic or neuromuscular disease that could limit exercise capacity Statistical analysis: within groups: paired t‐tests; between groups: ANOVA; linear regression for differences in breathing pattern; P value < 0.05 significance |
|
| Participants |
Participants recruited from: no information provided Number screened: no information provided Sample size: 9 NIV group; 10 control group Age mean (SD), years: 64 (9) NIV group; 63 (9) control group Gender: 1 female participant; 18 male participants FEV1 mean (SD) % predicted: 32 (4) NIV group; 33 (9) control group FEV1/FVC mean (SD) %: 59 (16) NIV group; 58 (16) control group RV mean (SD) % predicted: 194 (34) NIV group; 215 (56) control group RV/TLC mean (SD) % predicted: 164 (19) NIV group; 165 (17) control group Domiciliary NIV: no information provided Long‐term oxygen therapy: nocturnal oxygen used in four participants from the NIV group and two participants from the control group |
|
| Interventions |
NIV: bilevel support: IPAP 7 to 10 cmH2O, EPAP 2 cmH2O during exercise training Control: unassisted exercise training Supplemental oxygen during exercise training: yes. Supplemental oxygen was provided to 8/10 participants in the control group and 9/9 participants in the NIV group to maintain oxygen saturation of at least 92% during training Type of exercise training: treadmill Intensity of training: initially started at > 2 km/h based on what the participant could maintain for 15 minutes. Speed increased at 0.2 km/h each week after participants could maintain the target intensity for 45 minutes, with the aim of achieving 65% to 70% of the initial maximum walking speed. Zero slope Number of sessions per week: 2 Duration of each session: 45 minutes Total duration of training: 8 weeks Number of training sessions attended (/16): not reported Programme setting: outpatient, centre‐based |
|
| Outcomes | Maximal cycle ergometry: unassisted, load increased 15 watts/min Evaluation: Testing was performed within one week before and at the end of the programme |
|
| Dropouts | A total of 24 participants were recruited. Of the 12 randomly assigned to NIV, 9 completed the protocol (25% dropout rate). Reasons for dropout: failed to adjust to the mask (N = 3). Of the 12 randomly assigned to the control group, 10 completed the protocol (17% dropout rate). Reasons for dropout: lung transplant (N = 1); back pain (N = 1) | |
| Funding | Supported in part by a grant from the Israel Lung Association, Tel Aviv | |
| Notes |
Country: Israel Study author contacted: no response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Unclear risk | Quote (from report): "sealed envelope." Unsure if opaque etc |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote (from report): "Although it was not feasible to blind the patients or the trainers to the condition of their exercise training" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from report): "...the investigators conducting exercise testing and interpreting the exercise data did so without knowing to which group the patients were assigned" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote (from report): "Of the 24 patients 5 did not complete the study: 3 from the BiPAP group, who failed to adjust to the mask, and 2 from the control group—one due to lung transplant and one because of back pain" Comment: Total number of participants recruited, number of dropouts from each group and reasons for dropping out are reported. Numbers of dropouts were reasonably well balanced between groups. However, all dropouts in the non‐invasive ventilation group were due to mask intolerance, which may have introduced bias, as only participants who could tolerate non‐invasive ventilation were included in the analysis, as opposed to those who were allocated. An intention‐to‐treat analysis was not reported |
| Selective reporting (reporting bias) | High risk | Comment: Study report fails to include results for a key outcome that would be expected to have been reported for such a study Between‐group results were not reported for post‐training exercise capacity or for most physiological outcomes. Primary study outcome(s) were not stated. Instead, the study authors report that certain significant changes (when compared with baseline) were detected in the group that trained with NIV only (not the control group). The only between‐groups comparisons reported were for training intensity and change in peak exercise tidal volume |
| Other bias | Unclear risk | Quote (from report): "...no improvement was found in the control group. This may have been due to the relatively short twice‐weekly training, while effective protocols reported in the literature utilized between three and five training sessions per week" Comment: As the control group did not improve with pulmonary rehabilitation, the efficacy of the rehabilitation programme appears questionable. The group that trained with non‐invasive ventilation did improve. However, as both trainers and participants were not blinded to the intervention, bias cannot be ruled out, although the progression of training intensity was standardised; this should have helped to ensure that participants were exposed to the same type of training programme |
Toledo 2007.
| Methods |
Study design: parallel randomised controlled trial Inclusion criteria: clinical and spirometric diagnosis of COPD; FEV1 < 60% predicted and FEV1/FVC < 70%; clinically stable for a minimum of six months Exclusion criteria: no associated cardiovascular, orthopaedic or neuromuscular disorders or reactive hypertension related to effort that would impede involvement in the programme Statistical analysis: within groups: Wilcoxon test; between‐group comparisons: Mann‐Whitney U‐test; P value < 0.05 significance |
|
| Participants |
Participants recruited from: referred to respiratory physical therapy service at a university hospital Number screened: no information provided Sample size: 9 NIV group; 9 control group Age mean (SD), years: 67 (11) NIV group; 67 (9) control group Gender: numbers not stated FEV1 mean (SD) % predicted: 33 (10) NIV group; 34 (8) control group FVC mean (SD) % predicted: 63 (18) NIV group; 55 (11) control group Domiciliary NIV: no information provided Long‐term oxygen therapy: no information provided |
|
| Interventions |
NIV: bilevel support: IPAP 10 to 15 cmH2O, EPAP 4 to 6 cmH2O during exercise training Control: unassisted exercise training Supplemental oxygen during exercise training: no Type of exercise training: treadmill Intensity of training: 70% of maximum baseline speed Number of sessions per week: 3 Duration of each session: 30 minutes Total duration of training: 12 weeks Number of training sessions attended (/36): not reported Programme setting: outpatient, centre‐based |
|
| Outcomes | Incremental treadmill test, increasing 0.5 km/h every 2 minutes; blood lactate; respiratory muscle strength Evaluation: no information provided |
|
| Dropouts | A total of 18 participants completed the protocol (NIV group N = 9, control group N = 9). No information was given regarding dropouts | |
| Funding | No information provided | |
| Notes |
Country: Brazil Study author contacted: partial response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no information provided |
| Allocation concealment (selection bias) | Low risk | Quote (from correspondence): "...randomised into 2 groups by opaque and sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: It would not have been possible to blind participants or trainers, as sham non‐invasive ventilation was not used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from correspondence): "Tests were made with 3 assessors and they did not know the group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: Number of dropouts from each group (if any) and reasons for dropping out are not reported. Total number of participants recruited was stated (N = 18), and all of the 18 participants were included in the analysis |
| Selective reporting (reporting bias) | High risk | Comment: Study report fails to include results for a key outcome that would be expected to have been reported for such a study Between‐group results were not reported for post‐training exercise capacity or for most of the physiological outcomes. Primary study outcome(s) were not stated. It is not clear whether the study was powered to detect a change between groups. Instead, the study authors report that certain significant changes (when compared with baseline) were detected in the group that trained with non‐invasive ventilation only (not the control group). The only between‐group comparison reported was isoload lactate |
| Other bias | Low risk | None evident |
van 't Hul 2006.
| Methods |
Study design: parallel randomised controlled trial Inclusion criteria: diagnosis of COPD based on GOLD criteria; FEV1 < 60% predicted; breathing reserve at maximal exercise < 20% of maximum voluntary ventilation; peak minute ventilation < 50 L/min; resting PaO2 > 60 mmHg; SpO2 at maximal exercise > 85%; 40 to 75 years of age Statistical analysis: within groups: paired t‐tests or Wilcoxon test; between groups: independent samples t‐tests or Mann‐Whitney U‐test; strength of associations: Pearson’s correlation coefficient; P < 0.05 significance |
|
| Participants |
Participants recruited from: information not available from trial report Number screened: information not available from trial report Sample size: 15 NIV group; 14 control group Age mean (SD), years: 70 (5) NIV group; 71 (4) control group Gender: 4 female participants NIV group; 1 female participant control group FEV1 mean (SD) % predicted: 41 (10) NIV group; 38 (9) control group FVC mean (SD) % predicted: 87 (14) NIV group; 76 (14) control group FEV1/FVC mean (SD) %: 34 (6) NIV group; 36 (8) control group RV mean (SD) % predicted: 161 (18) NIV group; 164 (50) control group PImax cmH2O: 58 (20) NIV group; 59 (21) control group PEmax cmH2O: 100 (44) NIV group; 111 (28) control group Domiciliary NIV: nil Long‐term oxygen therapy: no information provided |
|
| Interventions |
NIV: inspiratory pressure support 10 cmH2O during exercise training Control: sham IPS 5 cmH2O during exercise training Supplemental oxygen during exercise training: no Type of exercise training: cycle ergometry Intensity of training: initially 65% of peak work capacity, then increased progressively by 5% of peak work rate when able to cycle for > 15 minutes Number of sessions per week: 3 Duration of each session: 45 minutes, supervised Total duration of training: 8 weeks Number of training sessions attended (/24): Participants were allowed to miss up to 4 training sessions, provided that the training period was extended (from 8 weeks to a maximum of 9 to 10 weeks) to ensure that each participant completed all 24 training sessions Program setting: outpatient, centre‐based |
|
| Outcomes | Incremental shuttle walk test; constant work rate cycle endurance time (75% of baseline peak work rate); St. George's Resiratory Questionnaire Evaluation: baseline measurements performed within 2 weeks before the start of the training period. Post‐training measurements took place within 2 weeks following the final training session |
|
| Dropouts | A total of 37 participants were recruited. Of the 19 randomly assigned to NIV, 15 completed the protocol (21% dropout rate). Reasons for dropout: acute exacerbation of COPD (N = 3); worsening general fatigue (N = 1). Of the 18 randomly assigned to the control group, 14 completed the protocol (22% dropout rate). Reasons for dropout: exacerbation of COPD (N = 3); cerebrovascular accident (N = 1) | |
| Funding | The study was supported by a grant from the Dutch Lung Foundation | |
| Notes |
Country: The Netherlands Study author contacted: full response |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (from correspondence): "...concealed envelopes and block randomisation. Each block (envelope) contained four cards. Two cards with 'experimental condition' and two cards with 'control condition' written on it. When a patient got to be randomised an independent observer drew one of the cards out of this envelope. After four procedures the envelope was empty and the next envelope was used, and so on" |
| Allocation concealment (selection bias) | Low risk | Quote (from report): "Patients were randomly allocated (concealed envelopes)" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote (from report): "...not possible to blind the three physiotherapists to the inspiratory pressure support (IPS) intensity patients were training with, but patients were kept naive with respect to randomisation outcome" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote (from report): "All measurements were performed by one independent investigator (A. van't Hul), who was not involved in the training and who was kept blinded to randomisation outcome" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Total number of participants recruited, number of dropouts from each group and reasons for dropping out are reported. Both intention‐to‐treat analyses and per‐protocol analyses were performed for key outcomes |
| Selective reporting (reporting bias) | Low risk | Comment: Published report contains all expected outcomes, including those that were prespecified |
| Other bias | Low risk | None evident |
ANOVA: analysis of variance; COPD: chronic obstructive pulmonary disease; EPAP: expiratory positive airway pressure; FEV1: forced expiratory volume in one second; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; IPAP: inspiratory positive airway pressure; METS: metabolic equivalents; NIV: non‐invasive ventilation; PaO2: arterial partial pressure of oxygen; PaCO2: arterial partial pressure of carbon dioxide; PEmax: maximal expiratory pressure; PImax: maximal inspiratory pressure; RCT: randomised controlled trial; RV: residual volume; SD: standard deviation; SpO2: oxygen saturation; TLC: total lung capacity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Allan 2009 | Not exercise training |
| Amann 2010 | Not exercise training |
| Ambrosino 2000 | Not an RCT |
| Ambrosino 2004 | Not an RCT |
| Ambrosino 2006 | Not an RCT |
| Ambrosino 2011 | Not an RCT |
| Anonymous 2007 | Not an RCT |
| Arad 1992 | Not an RCT |
| Araujo 2005 | Not an RCT |
| Bach 1992 | Not an RCT |
| Bach 1993 | Not an RCT |
| Baer 1989 | Not an RCT |
| Barakat 2007 | Not an RCT |
| Bianchi 1998 | Not exercise training |
| Borghi‐Silva 2005 | Not exercise training |
| Borghi‐Silva 2008 | Not exercise training |
| Borghi‐Silva 2009 | Not exercise training |
| Borghi‐Silva 2010 | Wrong comparison |
| Boye 1994 | Not NIV during exercise |
| Bullemer 1999 | Not an RCT |
| Carrascossa 2010 | Not exercise training |
| Chaturvedi 2011 | Not exercise training |
| Chen 2012 | Not COPD |
| Chiang 2006a | Not COPD |
| Chiang 2006b | Not exercise training |
| Corner 2010 | Not an RCT |
| Costa 2006 | Not an RCT |
| Costes 2003 | Not an RCT |
| De Backer 2010 | Not an RCT |
| den Hartog 2003 | Not an RCT |
| Dieperink 2006 | Not an RCT |
| Dolmage 1997 | Not exercise training |
| Dreher 2007 | Not exercise training |
| Dreher 2008 | Not an RCT |
| Dreher 2009 | Not exercise training |
| Dreher 2010 | Not COPD |
| Duiverman 2008 | Not NIV during exercise |
| Duiverman 2011 | Not NIV during exercise |
| Dyer 2011 | Not stable COPD |
| Gallagher 1989 | Not COPD |
| Garrod 2000 | Not NIV during exercise |
| Hernandes 2012 | Not an RCT |
| Hernandez 2001 | Not exercise training |
| Highcock 2003 | Not exercise training |
| Hussain 2011 | Not exercise training |
| Jackson 1991 | Not an RCT |
| Keilty 1994 | Not exercise training |
| Kleinsasser 2004 | Not COPD |
| Kohnlein 2009 | Not NIV during exercise |
| Kyroussis 2000 | Not exercise training |
| Maltais 1995 | Not exercise training |
| Martin 2005 | Not an RCT |
| Medvedev 2007 | Not COPD |
| Menadue 2009a | Not exercise training |
| Moga 2012 | Not an RCT |
| Monteiro 2012 | Not an RCT |
| Nicolini 2013 | Not exercise training |
| O'Donnell 1988a | Not exercise training |
| O'Donnell 1988b | Not exercise training |
| Oliveira 2010 | Not exercise training |
| Padkao 2010 | Not exercise training |
| Pepin 2010 | Not an RCT |
| Pessoa 2012 | Not exercise training |
| Petrof 1990 | Not exercise training |
| Pires Di Lorenzo 2003 | Wrong comparison |
| Poggi 2006 | Not exercise training |
| Polkey 1996 | Not exercise training |
| Polkey 2000 | Not exercise training |
| Poon 1987 | Not an RCT |
| Porszasz 2013 | Not exercise training |
| Puhan 2004 | Not an RCT |
| Revill 2000 | Not exercise training |
| Ricci 2013 | Not an RCT |
| Rochester 2013 | Not an RCT |
| Rodrigues 2013 | Not exercise training |
| Schmidt 1999 | Not an RCT |
| Schonhofer 2003 | Not an RCT |
| Schonhofer 2008 | Not an RCT |
| Skobel 2011 | Not an RCT |
| Soo Hoo 2003 | Not an RCT |
| Spruit 2007 | Not an RCT |
| van't Hul 2002 | Not an RCT |
| van't Hul 2004 | Not exercise training |
| Vitacca 2006 | Not exercise training |
| Walterspacher 2013 | Not exercise training |
| Wibmer 2013 | Not NIV during exercise |
| Wijkstra 2011 | Not an RCT |
| ZuWallack 2008 | Not an RCT |
Differences between protocol and review
The following changes were made to the published protocol (Menadue 2009b) during the review process: an addition was made to the criteria for the types of interventions that were eligible for inclusion in the review whereby studies that involved the use of nocturnal NIV were included only if both the actively treated group and the control group received nocturnal NIV; rather than data extraction being performed by one review author and verified by another review author, data from the included studies were extracted independently by two review authors; post training outcome data were only extracted if study participants were evaluated off NIV (e.g. unassisted test of exercise capacity); when dichotomous data were combined, the treatment effect was defined as the OR with 95% CI; if ITT analyses were not reported, data from the per‐protocol analyses were extracted for use in the meta‐analysis; a fixed effect model was used for analyses with I2 less than 30%, otherwise a random effects model was used; ventilator settings (including level of ventilatory assistance and mode of ventilation) were added as a factor to be considered for subgroup analyses; sensitivity analyses were performed for outcomes that included data from studies for which baseline differences between groups were accounted for by using change from baseline data rather than post intervention data. In addition, a summary of findings table was presented. Both the summary of findings table and the outcomes presented in the table were not planned a priori.
Contributions of authors
Collette Menadue: initiation and writing of protocol and manuscript, data extraction and analysis.
Amanda Piper: protocol development, data extraction, manuscript review.
Alex van't Hul: protocol development, manuscript review.
Keith Wong: protocol development, data extraction, manuscript review.
Sources of support
Internal sources
Royal Prince Alfred Hospital, Australia.
External sources
No sources of support supplied
Declarations of interest
Amanda Piper has received honoraria for educational presentations conducted on behalf of Respironics, Australia; ResMed, Australia; and Weinmann, Germany. She has also received a grant from the ResMed Foundation. The sleep laboratory of Collette Menadue, Amanda Piper and Keith Wong has previously received industry‐sponsored project grants from ResMed, Australia, and positive airway pressure equipment for other research projects from Philips Respironics, Australia; Air Liquide, Australia; and MayoHealthcare, Australia. Alex van't Hul is an author of one of the studies included in the present review.
New
References
References to studies included in this review
Bianchi 2002 {published data only}
- Bianchi L, Foglio K, Porta R, Baiardi R, Vitacca M, Ambrosino N. Lack of additional effect of adjunct of assisted ventilation to pulmonary rehabilitation in mild COPD patients. Respiratory Medicine 2002;96(5):359‐67. [DOI] [PubMed] [Google Scholar]
Hawkins 2002 {published and unpublished data}
- Hawkins P, Johnson LC, Nikoletou D, Hamnegard CH, Sherwood R, Polkey MI, et al. Proportional assist ventilation as an aid to exercise training in severe chronic obstructive pulmonary disease. Thorax 2002;57(10):853‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P, Johnson LC, Nikoletou D, Moxham J. Physiological training in severe chronic obstructive pulmonary disease (COPD) is possible using proportional assist ventilation (PAV). Thorax 1999;54(Suppl 3):A61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins P, Johnson LC, Nikoletou D, Moxham J. Physiological training in severe chronic obstructive pulmonary disease (COPD) using proportional assist ventilation (PAV). American Journal of Respiratory and Critical Care Medicine 2000;161(3 Suppl):A255. [Google Scholar]
Johnson 2002 {published data only (unpublished sought but not used)}
- Johnson JE, Gavin DJ, Adams‐Dramiga S. Effects of training with heliox and noninvasive positive pressure ventilation on exercise ability in patients with severe COPD. Chest 2002;122(2):464‐72. [DOI] [PubMed] [Google Scholar]
Reuveny 2005 {published data only (unpublished sought but not used)}
- Reuveny R, Ben‐Dov I, Gaides M, Reichert N. Ventilatory support during training improves training benefit in severe chronic airway obstruction. Israel Medical Association Journal 2005;7(3):151‐5. [PubMed] [Google Scholar]
- Reuveny R, Ben‐Dov I, Reichert N. Positive pressure ventilation training effect in patients with COPD. American Journal of Respiratory and Critical Care Medicine 2001;163(5 Suppl):A20. [Google Scholar]
Toledo 2007 {published and unpublished data}
- Toledo A, Borghi‐Silva A, Sampaio LM, Ribeiro KP, Baldissera V, Costa D. The impact of noninvasive ventilation during the physical training in patients with moderate‐to‐severe chronic obstructive pulmonary disease (COPD). Clinics (Sao Paulo) 2007;62(2):113‐20. [DOI] [PubMed] [Google Scholar]
van 't Hul 2006 {published data only}
- 't Hul A, Gosselink R, Hollander P, Postmus P, Kwakkel G. Training with inspiratory pressure support in patients with severe COPD. European Respiratory Journal 2006;27(1):65‐72. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Allan 2009 {published data only}
- Allan PF, Thomas KV, Ward MR, Harris AD, Naworol GA, Ward JA. Feasibility study of noninvasive ventilation with helium‐oxygen gas flow for chronic obstructive pulmonary disease during exercise. Respiratory Care 2009;54(9):1175‐82. [PubMed] [Google Scholar]
Amann 2010 {published data only}
- Amann M, Regan MS, Kobitary M, Eldridge MW, Boutellier U, Pegelow DF, et al. Impact of pulmonary system limitations on locomotor muscle fatigue in patients with COPD. American Journal of Physiology ‐ Regulatory Integrative and Comparative Physiology 2010;299(1):R314‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ambrosino 2000 {published data only}
- Ambrosino N. Exercise and noninvasive ventilatory support. Monaldi Archives for Chest Disease 2000;55(3):242‐6. [PubMed] [Google Scholar]
Ambrosino 2004 {published data only}
- Ambrosino N, Strambi S. New strategies to improve exercise tolerance in chronic obstructive pulmonary disease. European Respiratory Journal 2004;24(2):313‐22. [DOI] [PubMed] [Google Scholar]
Ambrosino 2006 {published data only}
- Ambrosino N. Assisted ventilation as an aid to exercise training: a mechanical doping?. European Respiratory Journal 2006;27(1):3‐5. [DOI] [PubMed] [Google Scholar]
Ambrosino 2011 {published data only}
- Ambrosino N, Guarracino F. Unusual applications of noninvasive ventilation. European Respiratory Journal 2011;38(2):440‐9. [DOI] [PubMed] [Google Scholar]
Anonymous 2007 {published data only}
- Anonymous. COPD ‐ Non‐invasive ventilation makes training effective [COPD ‐ Nichtinvasive Beatmung macht Training effektiver]. Pneumologie 2007;61(10):623. [DOI] [PubMed] [Google Scholar]
Arad 1992 {published data only}
- Arad M, Heruti R, Shaham E, Atsmon J, Epstein Y. The effects of powered air supply to the respiratory protective device on respiration parameters during rest and exercise. Chest 1992;102(6):1800‐4. [DOI] [PubMed] [Google Scholar]
Araujo 2005 {published data only}
- Araujo RB, Camisasca MT, Britto RR, Parreira VF. The noninvasive ventilation use in pulmonary rehabilitation of chronic obstructive pulmonary disease patients: a literature review [O uso da ventilação não‐invasiva na reabilitação pulmonar em pacientes portadores da doença pulmonar obstrutiva crônica: uma revisão de literatura]. Fisioterapia em Movimento 2005;18(1):49‐57. [Google Scholar]
Bach 1992 {published data only}
- Bach JR. Mechanical exsufflation, noninvasive ventilation, and new strategies for pulmonary rehabilitation and sleep disordered breathing. Bulletin of the New York Academy of Medicine 1992;68(2):321‐40. [PMC free article] [PubMed] [Google Scholar]
Bach 1993 {published data only}
- Bach JR, Lee HJ. New therapeutic techniques and strategies in pulmonary rehabilitation. Yonsei Medical Journal 1993;34(3):201‐11. [DOI] [PubMed] [Google Scholar]
Baer 1989 {published data only}
- Baer R, Eiken O. Effects of continuous positive‐ and negative‐pressure breathing on the pattern of breathing in man during exercise. Acta Physiologica Scandinavica 1989;137(2):301‐7. [DOI] [PubMed] [Google Scholar]
Barakat 2007 {published data only}
- Barakat S, Michele G, Nesme P, Nicole V, Guy A. Effect of a noninvasive ventilatory support during exercise of a program in pulmonary rehabilitation in patients with COPD. International Journal of Chronic Obstructive Pulmonary Disease 2007;2(4):585‐91. [PMC free article] [PubMed] [Google Scholar]
Bianchi 1998 {published data only}
- Bianchi L, Foglio K, Pagani M, Vitacca M, Rossi A, Ambrosino N. Effects of proportional assist ventilation on exercise tolerance in COPD patients with chronic hypercapnia. European Respiratory Journal 1998;11(2):422‐7. [DOI] [PubMed] [Google Scholar]
Borghi‐Silva 2005 {published data only}
- Borghi‐Silva A, Sampaio LMM, Toledo A, Pincelli MP, Costa D. Acute effects of BiPAP application on physical exercise tolerance among chronic obstructive pulmonary disease patients [Efeitos agudos da aplicacao do BiPAP sobre a tolerancia ao exercicio fisico em pacientes com doenca pulmonar obstrutiva cronica (DPOC)]. Revista Brasileira de Fisioterapia 2005;9(3):273‐80. [Google Scholar]
Borghi‐Silva 2008 {published data only}
- Borghi‐Silva A, Oliveira CC, Carrascosa C, Maia J, Berton D, Queiroga F, et al. Respiratory muscle unloading improves leg muscle oxygenation during exercise in patients with COPD. Thorax 2008;63:910‐5. [DOI] [PubMed] [Google Scholar]
Borghi‐Silva 2009 {published data only}
- Borghi‐Silva A, Thommazo L, Pantoni CBF, Mendes RG, Fatima Salvini T, Costa D. Non‐invasive ventilation improves peripheral oxygen saturation and reduces fatigability of quadriceps in patients with COPD. Respirology 2009;14:537‐44. [DOI] [PubMed] [Google Scholar]
Borghi‐Silva 2010 {published data only}
- Borghi‐Silva A, Mendes RG, Sampaio LMM, Souza HCD, Tania Salvini T, Costa D. Noninvasive ventilation is better than oxygen supplementation to improve performance during a physical training program in COPD patients ‐ A randomised study. European Respiratory Journal 2010;36(Suppl 54):3651. [Google Scholar]
- Borghi‐Silva A, Mendes RG, Toledo AC, Malosa Sampaio LM, Silva TP, Kunikushita LN, et al. Adjuncts to physical training of patients with severe COPD: oxygen or noninvasive ventilation?. Respiratory Care 2010;55(7):885‐94. [PubMed] [Google Scholar]
- Borghi‐Silva A, Mendes RG, Toledo AC, Malosa Sampaio LM, Silva TP, Kunikushita LN, et al. Different adjuncts during physical training in severe chronic obstructive pulmonary disease patients: oxygen or non‐invasive ventilation?. American Journal of Respiratory and Critical Care Medicine 2010;181:A6502. [Google Scholar]
Boye 1994 {published data only}
- Boye NP, Udjus E, Ottersen I, Refsum HE. Physiotherapy in chronic obstructive pulmonary disease with and without mask training [Fysioterapi vedkronisk obstruktiv lungesykdom med og uten masketrening]. Tidsskr Nor Laegeforen 1994;114(22):2606‐8. [PubMed] [Google Scholar]
Bullemer 1999 {published data only}
- Bullemer F, Kroworsch P, Heindl S, Winkler‐Wehgartner S, Karg O. Exercise tolerance of patients under nasal intermittent positive pressure ventilation (NiPPV) [Korperliche belastbarkeit von patienten mit atempumpenschwache unter intermittierender selbstbeatmung ]. Medizinische Klinik 1999;94(1 Spec No):29‐31. [PubMed] [Google Scholar]
Carrascossa 2010 {published data only}
- Carrascossa CR, Oliveira CC, Borghi‐Silva A, Ferreira EM, Maya J, Queiroga F Jr, et al. Haemodynamic effects of proportional assist ventilation during high‐intensity exercise in patients with chronic obstructive pulmonary disease. Respirology 2010;15(8):1185‐91. [DOI] [PubMed] [Google Scholar]
Chaturvedi 2011 {published data only}
- Chaturvedi RK, Zidulka A. Use of continuous negative pressure around the chest increases exercise performance in chronic obstructive pulmonary disease patients: a pilot study. Canadian Respiratory Journal 2011;18(1):6‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2012 {published data only}
- Chen YH, Lin HL, Hsiao HF, Chou LT, Kao KC, Huang CC, et al. Effects of exercise training on pulmonary mechanics and functional status in patients with prolonged mechanical ventilation. Respiratory Care 2012;57(5):727‐34. [DOI] [PubMed] [Google Scholar]
Chiang 2006a {published data only}
- Chiang LL, Wang LY, Wu CP, Wu HD, Wu YT. Effects of physical training on functional status in patients with prolonged mechanical ventilation. Physical Therapy 2006;86(9):1271‐81. [DOI] [PubMed] [Google Scholar]
Chiang 2006b {published data only}
- Chiang LL, Yu CT, Liu CY, Lo YL, Kuo HP, Lin HC. Six‐month nocturnal nasal positive pressure ventilation improves respiratory muscle capacity and exercise endurance in patients with chronic hypercapnic respiratory failure. Journal of the Formosan Medical Association 2006;105(6):459‐67. [DOI] [PubMed] [Google Scholar]
Corner 2010 {published data only}
- Corner E, Garrod R. Does the addition of non‐invasive ventilation during pulmonary rehabilitation in patients with chronic obstructive pulmonary disease augment patient outcome in exercise tolerance? A literature review. Physiotherapy Research International 2010;15(1):5‐15. [DOI] [PubMed] [Google Scholar]
Costa 2006 {published data only}
- Costa D, Toledo A, Borghi‐Silva A, Sampaio LM. Influence of noninvasive ventilation by BiPAP on exercise tolerance and respiratory muscle strength in chronic obstructive pulmonary disease patients (COPD) [INFLUÊNCIA DA VENTILAÇÃO NÃO INVASIVA POR MEIO DO BIPAP® SOBRE A TOLERÂNCIA AO EXERCÍCIO FÍSICO E FORÇA MUSCULAR RESPIRATÓRIA EM PACIENTES COM DOENÇA PULMONAR OBSTRUTIVA CRÔNICA (DPOC)]. Revista Latino‐Americano Enfermagem 2006;14(3):378‐82. [DOI] [PubMed] [Google Scholar]
Costes 2003 {published data only}
- Costes F, Agresti A, Court‐Fortune I, Roche F, Vergnon JM, Barthelemy JC. Noninvasive ventilation during exercise training improves exercise tolerance in patients with chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation 2003;23(4):307‐13. [DOI] [PubMed] [Google Scholar]
De Backer 2010 {published data only}
- Backer LA, Ides K, Daems D, Dieriks B, Backer WA, Germonpre P. Pulmonary rehabilitation and non‐invasive ventilation in COPD. Acta Clinica Belgica 2010;65(5):330‐5. [DOI] [PubMed] [Google Scholar]
den Hartog 2003 {published data only}
- Hartog EA, Heus R. Positive pressure breathing during rest and exercise. Applied Ergonomics 2003;34(2):185‐94. [DOI] [PubMed] [Google Scholar]
Dieperink 2006 {published data only}
- Dieperink W, Goorhuis JF, Weerd W, Hazenberg A, Zijlstra JG, Nijsten MW. Walking with continuous positive airway pressure. European Respiratory Journal 2006;27(4):853‐5. [DOI] [PubMed] [Google Scholar]
Dolmage 1997 {published data only}
- Dolmage TE, Goldstein RS. Proportional assist ventilation and exercise tolerance in subjects with COPD. Chest 1997;111(4):948‐54. [DOI] [PubMed] [Google Scholar]
Dreher 2007 {published data only}
- Dreher M, Storre JH, Windisch W. Noninvasive ventilation during walking in patients with severe COPD: a randomised cross‐over trial. European Respiratory Journal 2007;29(5):930‐6. [DOI] [PubMed] [Google Scholar]
Dreher 2008 {published data only}
- Dreher M, Kenn K, Windisch W. Non‐invasive ventilation and physical exercise in patients with COPD [Nichtinvasive Beatmung und körperliche Belastung bei Patienten mit COPD]. Pneumologie 2008;62(3):162‐8. [DOI] [PubMed] [Google Scholar]
Dreher 2009 {published data only}
- Dreher M, Doncheva E, Schwoerer A, Walterspacher S, Sonntag F, Kabitz HJ, et al. Preserving oxygenation during walking in severe chronic obstructive pulmonary disease: noninvasive ventilation versus oxygen therapy. Respiration 2009;78(2):154‐60. [DOI] [PubMed] [Google Scholar]
Dreher 2010 {published data only}
- Dreher M, Kabitz H‐J, Burgardt V, Walterspacher S, Windisch W. Proportional assist ventilation improves exercise capacity in patients with obesity. Respiration 2010;80(2):106‐11. [DOI] [PubMed] [Google Scholar]
Duiverman 2008 {published data only}
- Duiverman ML, Wempe JB, Bladder G, Jansen DF, Kerstjens HA, Zijlstra JG, et al. Nocturnal non‐invasive ventilation in addition to rehabilitation in hypercapnic patients with COPD. Thorax 2008;63(12):1052‐7. [DOI] [PubMed] [Google Scholar]
Duiverman 2011 {published data only}
- Duiverman ML, Wempe JB, Bladder G, Vonk J, Kerstjens HAM, Wijkstra PJ. Two‐year nocturnal noninvasive ventilation added to rehabilitation in hypercapnic COPD patients. American Journal of Respiratory and Critical Care Medicine 2010;181:A3056. [Google Scholar]
- Duiverman ML, Wempe JB, Bladder G, Vonk JM, Zijlstra JG, Kerstjens HA, et al. Two‐year home‐based nocturnal noninvasive ventilation added to rehabilitation in chronic obstructive pulmonary disease patients: a randomised controlled trial. Respiratory Research 2011;12:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dyer 2011 {published data only}
- Dyer F, Flude L, Bazari F, Jolley C, Polkey MI, Hopkinson NS, et al. Non‐invasive ventilation (NIV) as an aid to rehabilitation in acute respiratory disease. BMC Pulmonary Medicine 2011;11:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallagher 1989 {published data only}
- Gallagher CG, Younes M. Effect of pressure assist on ventilation and respiratory mechanics in heavy exercise. Journal of Applied Physiology 1989;66(4):1824‐37. [DOI] [PubMed] [Google Scholar]
Garrod 2000 {published data only}
- Garrod R, Mikelsons C, Paul E, Wedzicha JA. Randomised controlled trial of domiciliary non‐invasive positive pressure ventilation and physical training in severe COPD. American Journal of Respiratory and Critical Care Medicine 2000;61(Suppl 3):A255. [DOI] [PubMed] [Google Scholar]
- Garrod R, Mikelsons C, Paul EA, Wedzicha JA. Effects on exercise tolerance of the addition of domiciliary non‐invasive positive pressure ventilation to physical training in severe COPD. Thorax 1999;54(Suppl 3):A61. [Google Scholar]
- Garrod R, Mikelsons C, Paul EA, Wedzicha JA. Randomized controlled trial of domiciliary noninvasive positive pressure ventilation and physical training in severe chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;162(4 Pt 1):1335‐41. [DOI] [PubMed] [Google Scholar]
Hernandes 2012 {published data only}
- Hernandes NA, Pitta F. Use of expiratory positive airway pressure delivered by a spring load resistor during exercise: a new tool to optimise exercise training in patients with COPD?. Respiratory Care 2012;57(9):1530‐1. [DOI] [PubMed] [Google Scholar]
Hernandez 2001 {published data only}
- Hernandez P, Maltais F, Gursahaney A, LeBlanc P, Navalesi P, Gottfried SB. Proportional assist ventilation (PAV) improves exercise performance in severe COPD. European Respiratory Journal 1995;8(Suppl 19):502s. [DOI] [PubMed] [Google Scholar]
- Hernandez P, Maltais F, Gursahaney A, Leblanc P, Gottfried SB. Proportional assist ventilation may improve exercise performance in severe chronic obstructive pulmonary disease. Journal of Cardiopulmonary Rehabilitation 2001;21(3):135‐42. [DOI] [PubMed] [Google Scholar]
Highcock 2003 {published data only}
- Highcock MP, Shneerson JM, Smith IE. Increased ventilation with NiIPPV does not necessarily improve exercise capacity in COPD. European Respiratory Journal 2003;22(1):100‐5. [DOI] [PubMed] [Google Scholar]
Hussain 2011 {published data only}
- Hussain O, Collins EG, Adiguzel N, Langbein WE, Tobin MJ, Laghi F. Contrasting pressure‐support ventilation and helium‐oxygen during exercise in severe COPD. Respiratory Medicine 2011;105(3):494‐505. [DOI] [PubMed] [Google Scholar]
Jackson 1991 {published data only}
- Jackson NC. Pulmonary rehabilitation for mechanically ventilated patients. Critical Care Nursing Clinics of North America 1991;3(4):591‐600. [PubMed] [Google Scholar]
Keilty 1994 {published data only}
- Keilty SE, Ponte J, Fleming TA, Moxham J. Effect of inspiratory pressure support on exercise tolerance and breathlessness in patients with severe stable chronic obstructive pulmonary disease. Thorax 1994;49(10):990‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kleinsasser 2004 {published data only}
- Kleinsasser A, Goedecke A, Hoermann C, Maier S, Schaefer A, Keller C, et al. Proportional assist ventilation reduces the work of breathing during exercise at moderate altitude. High Altitude Medicine and Biology 2004;5(4):420‐8. [DOI] [PubMed] [Google Scholar]
Kohnlein 2009 {published data only}
- Kohnlein T, Schonheit‐Kenn U, Winterkamp S, Welte T, Kenn K. Noninvasive ventilation in pulmonary rehabilitation of COPD patients. Respiratory Medicine 2009;103(9):1329‐36. [DOI] [PubMed] [Google Scholar]
Kyroussis 2000 {published data only}
- Kyroussis D, Polkey MI, Hamnegard CH, Mills GH, Green M, Moxham J. Respiratory muscle activity in patients with COPD walking to exhaustion with and without pressure support. European Respiratory Journal 2000;15(4):649‐55. [DOI] [PubMed] [Google Scholar]
Maltais 1995 {published data only}
- Maltais F, Reissmann H, Gottfried SB. Pressure support reduces inspiratory effort and dyspnoea during exercise in chronic airflow obstruction. American Journal of Respiratory and Critical Care Medicine 1995;151(4):1027‐33. [DOI] [PubMed] [Google Scholar]
Martin 2005 {published data only}
- Martin UJ, Hincapie L, Nimchuk M, Gaughan J, Criner GJ. Impact of whole‐body rehabilitation in patients receiving chronic mechanical ventilation. Critical Care Medicine 2005;33(10):2259‐65. [DOI] [PubMed] [Google Scholar]
Medvedev 2007 {published data only}
- Medvedev DV, Gorbaneva EP, Iumatova SN, Kuznetsova TIu, Solopov IN, Katuntsev VP. Evaluation of benefits of the course of positive pressure breathing training on exercise performance. Aviakosmicheskaia i Ekologicheskaia Meditsina 2007;41(3):14‐8. [PubMed] [Google Scholar]
Menadue 2009a {published data only}
- Menadue C, Alison JA, Piper AJ, Flunt D, Ellis ER. Non‐invasive ventilation during arm exercise and ground walking in patients with chronic hypercapnic respiratory failure. Respirology 2009;14(2):251‐9. [DOI] [PubMed] [Google Scholar]
- Menadue C, Ellis ER, Piper AJ, Flunt D, Alison JA. Non‐invasive ventilation during unsupported arm exercise and walking in patients with chronic hypercapnic respiratory failure. Respirology 2007;12(Suppl 4):A129. [DOI] [PubMed] [Google Scholar]
Moga 2012 {published data only}
- Moga AM, Marchie M, Saey D, Spahija J. Mechanisms of non‐pharmacologic adjunct therapies used during exercise in COPD. Respiratory Medicine 2012;106(5):614‐26. [DOI] [PubMed] [Google Scholar]
Monteiro 2012 {published data only}
- Monteiro MB, Berton DC, Moreira MA, Menna‐Barreto SS, Teixeira PJ. Effects of expiratory positive airway pressure on dynamic hyperinflation during exercise in patients with COPD. Respiratory Care 2012;57(9):1405‐12. [DOI] [PubMed] [Google Scholar]
Nicolini 2013 {published data only}
- Nicolini A, Merliak F, Barlascini C. Use of positive expiratory pressure during six minute walk test: results in patients with moderate to severe chronic obstructive pulmonary disease. Multidisciplinary Respiratory Medicine 2013;8(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Donnell 1988a {published data only}
- O'Donnell DE, Sanii R, Giesbrecht G, Younes M. Effect of continuous positive airway pressure on respiratory sensation in patients with chronic obstructive pulmonary disease during submaximal exercise. American Review of Respiratory Disease 1988;138(5):1185‐91. [DOI] [PubMed] [Google Scholar]
O'Donnell 1988b {published data only}
- O'Donnell DE, Sanii R, Younes M. Improvement in exercise endurance in patients with chronic airflow limitation using continuous positive airway pressure. American Review of Respiratory Disease 1988;138(6):1510‐4. [DOI] [PubMed] [Google Scholar]
Oliveira 2010 {published data only}
- Oliveira CC, Carrascosa CR, Borghi‐Silva A, Berton DC, Queiroga F Jr, Ferreira EMV, et al. Influence of respiratory pressure support on haemodynamics and exercise tolerance in patients with COPD. European Journal of Applied Physiology 2010;109(4):681‐9. [DOI] [PubMed] [Google Scholar]
Padkao 2010 {published data only}
- Padkao T, Boonsawat W, Jones CU. Conical‐PEP is safe, reduces lung hyperinflation and contributes to improved exercise endurance in patients with COPD: a randomised cross‐over trial. Journal of Physiotherapy 2010;56(1):33‐9. [DOI] [PubMed] [Google Scholar]
Pepin 2010 {published data only}
- Pepin J‐L, Labeix P, Costes F. Rehabilitation of long‐term ventilated patients: can noninvasive ventilation be of help?. Revue des Maladies Respiratoires Actualites 2010;2(6):624‐7. [Google Scholar]
Pessoa 2012 {published data only}
- Pessoa IM, Costa D, Velloso M, Mancuzo E, Reis MA, Parreira VF. Effects of noninvasive ventilation on dynamic hyperinflation of patients with COPD during activities of daily living with upper limbs. Revista Brasileira de Fisioterapia 2012;16(1):61‐7. [PubMed] [Google Scholar]
Petrof 1990 {published data only}
- Petrof BJ, Calderini E, Gottfried SB. Effect of CPAP on respiratory effort and dyspnoea during exercise in severe COPD. Journal of Applied Physiology 1990;69(1):179‐88. [DOI] [PubMed] [Google Scholar]
Pires Di Lorenzo 2003 {published data only}
- Pires Di Lorenzo VA, Borghi‐Silva A, Sampaio LMM, Jamami M, Oishi J, Costa D. Effect of physical and respiratory muscle training in patients with chronic obstructive pulmonary disease (COPD) undergoing BiPAP [Efeitos do treinamento fisico e muscular respiratório em pacientes com COPD grave submetidos ao BiPAP]. Revista Brasileira Fisioterapia 2003;7(1):69‐76. [Google Scholar]
Poggi 2006 {published data only}
- Poggi R, Appendini L, Polese G, Colombo R, Donner CF, Rossi A. Noninvasive proportional assist ventilation and pressure support ventilation during arm elevation in patients with chronic respiratory failure. A preliminary, physiologic study. Respiratory Medicine 2006;100(6):972‐9. [DOI] [PubMed] [Google Scholar]
Polkey 1996 {published data only}
- Polkey MI, Kyroussis D, Keilty SEJ, Hamnegard CH, Mills GH, Moxham J. Inspiratory pressure support reduces inspiratory muscle loading caused by exhaustive treadmill walking in patients with severe COPD. European Respiratory Journal 1995;8(Suppl 19):346s. [Google Scholar]
- Polkey MI, Kyroussis D, Mills GH, Hamnegard CH, Keilty SE, Green M, et al. Inspiratory pressure support reduces slowing of inspiratory muscle relaxation rate during exhaustive treadmill walking in severe COPD. American Journal of Respiratory & Critical Care Medicine 1996;154(4 Pt 1):1146‐50. [DOI] [PubMed] [Google Scholar]
Polkey 2000 {published data only}
- Polkey MI, Hawkins P, Kyroussis D, Ellum SG, Sherwood R, Moxham J. Inspiratory pressure support prolongs exercise induced lactataemia in severe COPD. Thorax 2000;55(7):547‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polkey MI, Kyroussis D, Sherwood R, Flemming TA, Wood CN, Peters TJ, et al. Inspiratory pressure support (IPS) prolongs exercise‐induced lactataemia in COPD. European Respiratory Journal 1996;9(Suppl 22):379s. [Google Scholar]
Poon 1987 {published data only}
- Poon C‐S, Ward SA, Whipp BJ. Influence of inspiratory assistance on ventilatory control during moderate exercise. Journal of Applied Physiology 1987;62(2):551‐60. [DOI] [PubMed] [Google Scholar]
Porszasz 2013 {published data only}
- Porszasz J, Cao R, Morishige R, Eykern LA, Stenzler A, Casaburi R. Physiologic effects of an ambulatory ventilation system in chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 2013;188(3):334‐42. [DOI] [PubMed] [Google Scholar]
Puhan 2004 {published data only}
- Puhan MA, Schunemann HJ, Frey M, Bachmann LM. Value of supplemental interventions to enhance the effectiveness of physical exercise during respiratory rehabilitation in COPD patients. A systematic review. Respiratory Research 2004;5(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Revill 2000 {published data only}
- Revill SM, Singh SJ, Morgan MD. Randomized controlled trial of ambulatory oxygen and an ambulatory ventilator on endurance exercise in COPD. Respiratory Medicine 2000;94(8):778‐83. [DOI] [PubMed] [Google Scholar]
- Revill SM, Singh SJ, Morgan MDL. Effects of ambulatory inspiratory pressure support (IPS) and ambulatory oxygen on walking endurance capacity in severe COPD. European Respiratory Journal 1998;12(Suppl 28):262s. [Google Scholar]
Ricci 2013 {published data only}
Rochester 2013 {published data only}
- Rochester CL, Maltais F. Innovate to ambulate: creating opportunities for patients with chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 2013;188(3):265‐7. [DOI] [PubMed] [Google Scholar]
Rodrigues 2013 {published data only}
- Rodrigues MK, Oliveira MF, Soares A, Trepton E, Neder JA. Additive effects of non‐invasive ventilation to hyperoxia on cerebral oxygenation in COPD patients with exercise‐related O2 desaturation. Clinical Physiology & Functional Imaging 2013;33(4):274‐81. [DOI] [PubMed] [Google Scholar]
Schmidt 1999 {published data only}
- Schmidt MJ, Laier‐Groeneveld G, Criee CP. Fitness training during nasal ventilation in diseases limited by dyspnoea [Fitnesstraining unter nasaler beatmung bei dyspnoelimitierter belastbarkeit ]. Pneumologie 1999;53( Suppl 2) :S109‐12. [PubMed] [Google Scholar]
Schonhofer 2003 {published data only}
- Schonhofer B, Zimmermann C, Abramek P, Suchi S, Kohler D, Polkey MI. Non‐invasive mechanical ventilation improves walking distance but not quadriceps strength in chronic respiratory failure. Respiratory Medicine 2003;97(7):818‐24. [DOI] [PubMed] [Google Scholar]
Schonhofer 2008 {published data only}
- Schonhofer B, Dellweg D, Suchi S, Kohler D. Exercise endurance before and after long‐term noninvasive ventilation in patients with chronic respiratory failure. Respiration 2008;75(3):296‐303. [DOI] [PubMed] [Google Scholar]
Skobel 2011 {published data only}
- Skobel E, Jendralski A, Skobel E, Randerath W, Norra C. Influence of pulmonary rehabilitation on quality of life and anxiety in patients with severe COPD and long‐term ventilation. Atemwegs und Lungenkrankheiten 2011;37(7):257‐66. [Google Scholar]
Soo Hoo 2003 {published data only}
- Soo Hoo GW. Nonpharmacologic adjuncts to training during pulmonary rehabilitation: the role of supplemental oxygen and noninvasive ventilation. Journal of Rehabilitation Research & Development 2003;40(5(Suppl 2)):81‐97. [DOI] [PubMed] [Google Scholar]
Spruit 2007 {published data only}
- Spruit MA, Wouters EF. New modalities of pulmonary rehabilitation in patients with chronic obstructive pulmonary disease. Sports Medicine 2007;37(6):501‐18. [DOI] [PubMed] [Google Scholar]
van't Hul 2002 {published data only}
- van't Hul A, Kwakkel G, Gosselink R. The acute effects of noninvasive ventilatory support during exercise on exercise endurance and dyspnoea in patients with chronic obstructive pulmonary disease: a systematic review. Journal of Cardiopulmonary Rehabilitation 2002;22(4):290‐7. [DOI] [PubMed] [Google Scholar]
van't Hul 2004 {published data only}
- van't Hul A, Gosselink R, Hollander P, Postmus P, Kwakkel G. Acute effects of inspiratory pressure support during exercise in patients with COPD. European Respiratory Journal 2004;23(1):34‐40. [DOI] [PubMed] [Google Scholar]
Vitacca 2006 {published data only}
- Vitacca M, Bianchi L, Sarva M, Paneroni M, Balbi B. Physiological responses to arm exercise in difficult to wean patients with chronic obstructive pulmonary disease. Intensive Care Medicine 2006;32(8):1159‐66. [DOI] [PubMed] [Google Scholar]
Walterspacher 2013 {published data only}
- Walterspacher S, Walker DJ, Kabitz HJ, Windisch W, Dreher M. The effect of continuous positive airway pressure on stair‐climbing performance in severe COPD patients. Journal of Chronic Obstructive Pulmonary Disease 2013;10(2):193‐9. [DOI] [PubMed] [Google Scholar]
Wibmer 2013 {published data only}
Wijkstra 2011 {published data only}
- Wijkstra PJ, Wempe JB. New tools in pulmonary rehabilitation. European Respiratory Journal 2011;38(6):1468‐74. [DOI] [PubMed] [Google Scholar]
ZuWallack 2008 {published data only}
- ZuWallack RL. The roles of bronchodilators, supplemental oxygen, and ventilatory assistance in the pulmonary rehabilitation of patients with chronic obstructive pulmonary disease. Respiratory Care 2008;53(9):1190‐5. [PubMed] [Google Scholar]
Additional references
Agusti 2003
- Agusti AG, Noguera A, Sauleda J, Sala E, Pons J. Systemic effects of chronic obstructive pulmonary disease. European Respiratory Journal 2003;21(2):347‐60. [DOI] [PubMed] [Google Scholar]
Berger 2005
- Berger VW. Quantifying the magnitude of baseline covariate imbalances resulting from selection bias in randomised clinical trials. Biometrical Journal 2005;47(2):119‐27. [DOI] [PubMed] [Google Scholar]
Bolton 2013
- Bolton CE, Bevan‐Smith EF, Blakey JD, Crowe P, Elkin SL, Garrod R, et al. British Thoracic Society guideline on pulmonary rehabilitation in adults. Thorax 2013;68:ii1‐ii30. [DOI] [PubMed] [Google Scholar]
Buist 2007
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD study): a population‐based prevalence study. Lancet 2007;370(9589):741‐50. [DOI] [PubMed] [Google Scholar]
Cambach 1999
- Cambach W, Wagenaar RC, Koelman TW, Keimpema AR, Kemper HC. The long‐term effects of pulmonary rehabilitation in patients with asthma and chronic obstructive pulmonary disease: a research synthesis. Archives of Physical Medicine and Rehabilitation 1999;80(1):103‐11. [DOI] [PubMed] [Google Scholar]
Casaburi 1991
- Casaburi R, Patessio A, Ioli F, Zanaboni S, Donner CF, Wasserman K. Reductions in exercise lactic acidosis and ventilation as a result of exercise training in patients with obstructive lung disease. American Review of Respiratory Disease 1991;143(1):9‐18. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. New York: Wiley‐Blackwell, 2008. [Google Scholar]
Ellis 1987
- Ellis ER, Bye PT, Bruderer JW, Sullivan CE. Treatment of respiratory failure during sleep in patients with neuromuscular disease. Positive‐pressure ventilation via a nose mask . American Review of Respiratory Disease 1987;135(1):148‐52. [DOI] [PubMed] [Google Scholar]
Emtner 2003
- Emtner M, Porszasz J, Burns M, Somfay A, Casaburi R. Benefits of supplemental oxygen in exercise training in non‐hypoxaemic chronic obstructive pulmonary disease patients. American Journal of Respiratory and Critical Care Medicine 2003;168(9):1034‐42. [DOI] [PubMed] [Google Scholar]
Garrod 2006
- Garrod R, Marshall J, Barley E, Jones PW. Predictors of success and failure in pulmonary rehabilitation. European Respiratory Journal 2006;27(4):788‐94. [DOI] [PubMed] [Google Scholar]
Gimenez 2000
- Gimenez M, Servera E, Vergara P, Bach JR, Polu JM. Endurance training in patients with chronic obstructive pulmonary disease: a comparison of high versus moderate intensity. Archives of Physical Medicine and Rehabilitation 2000;81(1):102‐9. [DOI] [PubMed] [Google Scholar]
GOLD 2013
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease [updated 2013]. http://www.goldcopd.org/ (accessed 9 March 2013).
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March 2011]. UK: The Cochrane Collaboration, 2011. [Google Scholar]
Holland 2010
- Holland AE, Hill CJ, Rasekaba T, Lee A, Naughton MT, McDonald CF. Updating the minimal important difference for six‐minute walk distance in patients with chronic obstructive pulmonary disease. Archives of Physical Medicine and Rehabilitation 2010;91(2):221‐5. [DOI] [PubMed] [Google Scholar]
Jones 2002
- Jones PW. Interpreting thresholds for a clinically significant change in health status in asthma and COPD. European Respiratory Journal 2002;19(3):398‐404. [DOI] [PubMed] [Google Scholar]
Kerby 1987
- Kerby GR, Mayer LS, Pingleton SK. Nocturnal positive pressure ventilation via nasal mask. American Review of Respiratory Disease 1987;135(3):738‐40. [DOI] [PubMed] [Google Scholar]
Killian 1992
- Killian KJ, Leblanc P, Martin DH, Summers E, Jones NL, Campbell EJ. Exercise capacity and ventilatory, circulatory, and symptom limitation in patients with chronic airflow limitation. American Review of Respiratory Disease 1992;146(4):935‐40. [DOI] [PubMed] [Google Scholar]
Lacasse 2006
- Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003793.pub2] [DOI] [PubMed] [Google Scholar]
Landis 1977
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33(1):159‐74. [PubMed] [Google Scholar]
Lozano 2012
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2095‐128. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maltais 1997
- Maltais F, LeBlanc P, Jobin J, Berube C, Bruneau J, Carrier L, et al. Intensity of training and physiologic adaptation in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1997;155(2):555‐61. [DOI] [PubMed] [Google Scholar]
Mehta 2001
- Mehta S, Hill NS. Noninvasive ventilation. American Journal of Respiratory and Critical Care Medicine 2001;163(2):540‐77. [DOI] [PubMed] [Google Scholar]
Menadue 2009b
- Menadue C, Piper AJ, van't Hul AJ, Wong KK. Non‐invasive ventilation during exercise training for people with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007714] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Ng 2012
- Ng CLW, Mackney J, Jenkins S, Hill K. Does exercise training change physical activity in people with COPD? A systematic review and meta‐analysis. Chronic Respiratory Disease 2012;9:17‐26. [DOI] [PubMed] [Google Scholar]
O'Donnell 2006
- O’Donnell DE, Laveneziana P. Physiology and consequences of lung hyperinflation in COPD. European Respiratory Reveiw 2006;15(100):61‐7. [Google Scholar]
Pauwels 2004
- Pauwels RA, Rabe KF. Burden and clinical features of chronic obstructive pulmonary disease (COPD). Lancet 2004;364(9434):613‐20. [DOI] [PubMed] [Google Scholar]
Pildal 2007
- Pildal J, Hrobjartsson A, Jorgensen KJ, Hilden J, Altman DG, Gotzsche PC. Impact of allocation concealment on conclusions drawn from meta‐analyses of randomised trials. International Journal of Epidemiology 2007;36(4):847‐57. [DOI] [PubMed] [Google Scholar]
Pitta 2005
- Pitta F, Troosters T, Spruit MA, Probst VS, Decramer M, Gosselink R. Characteristics of physical activities in daily life in chronic obstructive pulmonary disease. American Journal of Respiratory and Criticial Care Medicine 2005;171(9):972‐7. [DOI] [PubMed] [Google Scholar]
Plankeel 2005
- Plankeel JF, McMullen B, MacIntyre NR. Exercise outcomes after pulmonary rehabilitation depend on the initial mechanism of exercise limitation among non‐oxygen‐dependent COPD patients. Chest 2005;127(1):110‐6. [DOI] [PubMed] [Google Scholar]
Polkey 2013
- Polkey MI, Spruit MA, Edwards LD, Watkins ML, Pinto‐Plata V, Vestbo J, et al. Six‐minute‐walk test in chronic obstructive pulmonary disease: minimal clinically important difference for death or hospitalisation. American Journal of Respiratory & Critical Care Medicine 2013;187(4):382‐6. [DOI] [PubMed] [Google Scholar]
Puente‐Maestu 2009
- Puente‐Maestu L, Villar F, Miguel J, Stringer WW, Sanz P, Sanz ML, et al. Clinical relevance of constant power exercise duration changes in COPD. European Respiratory Journal 2009;34:340‐5. [DOI] [PubMed] [Google Scholar]
Puhan 2011
- Puhan MA, Gimeno‐Santos E, Scharplatz M, Troosters T, Walters EH, Steurer J. Pulmonary rehabilitation following exacerbations of chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD005305.pub3] [DOI] [PubMed] [Google Scholar]
Rabe 2007
- Rabe KF, Hurd S, Anzueto A, Barnes PJ, Buist SA, Calverley P, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. American Journal of Respiratory and Critical Care Medicine 2007;176(6):532‐55. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Ries 2007
- Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence‐based clinical practice guidelines. Chest 2007;131(Suppl 5):4s‐42s. [DOI] [PubMed] [Google Scholar]
Singh 1992
- Singh SJ, Morgan MD, Scott S, Walters D, Hardman AE. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992;47(12):1019‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Singh 2008
- Singh SJ, Jones PW, Evans R, Morgan MD. Minimum clinically important improvement for the incremental shuttle walking test. Thorax 63;9:775‐7. [DOI] [PubMed] [Google Scholar]
Spruit 2012
- Spruit MA, Polkey MI, Celli B, Edwards LD, Watkins ML, Pinto‐Plata V, et al. Predicting outcomes from 6‐minute walk distance in chronic obstructive pulmonary disease. Journal of the American Medical Directors Association 2012;13:291‐7. [DOI] [PubMed] [Google Scholar]
Spruit 2013
- Spruit M, Singh SJ, Garvey C, ZuWallack R, Nici L, Rochester C, et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory & Critical Care Medicine 2013;188(8):e13‐e64. [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Intervention. New York: Wiley‐Blackwell, 2011. [Google Scholar]
Troosters 2000
- Troosters T, Gosselink R, Decramer M. Short‐ and long‐term effects of outpatient rehabilitation in patients with chronic obstructive pulmonary disease: a randomised trial. American Journal of Medicine 2000;109(3):207‐12. [DOI] [PubMed] [Google Scholar]
Troosters 2001
- Troosters T, Gosselink R, Decramer M. Exercise training in COPD: how to distinguish responders from non‐responders. Journal of Cardiopulmonary Rehabilitation 2001;21(1):10‐7. [DOI] [PubMed] [Google Scholar]
Troosters 2005
- Troosters T, Casaburi R, Gosselink R, Decramer M. Pulmonary rehabilitation in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2005;172(1):19‐38. [DOI] [PubMed] [Google Scholar]
Troosters 2010
- Troosters T, Gosselink R, Janssens W, Decramer M. Exercise training and pulmonary rehabilitation: new insights and remaining challenges. European Respiratory Review 2010;19(115):24‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Viegi 2007
- Viegi G, Pistelli F, Sherrill DL, Maio S, Baldacci S, Carrozzi L. Definition, epidemiology and natural history of COPD. European Respiratory Journal 2007;30(5):993‐1013. [DOI] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380:2163‐96. [DOI] [PMC free article] [PubMed] [Google Scholar]


