Abstract
Background
Ixora species are perennial shrubs and flowering plants belonging to the family Rubiaceae. The leaf and flower parts of Ixora coccinea (I. coccinea) andIxora alba (I. alba) were aimed at isolating their active fractions. The present study was to determine in vitro antitumor activity against malignant melanoma cell lines for phytosome formulation.
Materials and methods
Two species, I. coccinea (red flowers and leaves) and I. alba (white flowers and leaves), were selected, and this study focused on determining the active fraction by comparing the in vitro antimicrobial and antioxidant potentials of petroleum ether, chloroform, ethyl acetate, and hydroalcoholic (ethanol:water, 70:30 v/v) extracts. The identified potent extract was subjected to in vitro anticancer activity in malignant melanoma cell lines.
Results
A phytochemical study revealed phytosterols, flavonoids, proteins, amino acids, alkaloids, carbohydrates, phenols, tannins, and diterpenes. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay was used to evaluate the antioxidant effect of I. coccinea and I. alba leaf and flower extracts. In the DPPH assay, I. coccinea flower hydroalcoholic extract (ICFHA) had an IC50 value of 248.99 µg/mL, and I. coccinea leaf hydroalcoholic extract (ICLHA) had an IC50 value of 268.87 µg/mL. These two extracts had a lower value with a higher antioxidant effect. In the total antioxidant assay, I. coccinea leaf ethyl acetate extract (ICLEA) and I. coccinea leaf chloroform extract (ICLCE) have 77.4 ± 0.05 and 68.9 ± 0.03 mg of ascorbic acid equivalent per gm of extract, respectively. These two extracts exhibited a high antioxidant effect. The antimicrobial potential was evaluated using selected bacterial and fungal strains using the agar-well diffusion method. Petroleum ether and chloroform extracts of I. coccinea and I. alba leaves and flowers did not possess antimicrobial activity with any of the bacterial or fungal strains. An ethyl acetate extract and a hydroalcoholic extract of I. coccinea leaves and flowers showed antimicrobial activity against Enterococcus faecalis, Candida albicans, and Staphylococcus aureus. An ethyl acetate extract of I. coccinea flower and a hydroalcoholic extract of I. alba leaf showed a significant zone of inhibition when compared with standard chloramphenicol for all three selected strains, which may be due to the presence of active phytoconstituents. ICLHA showed a MIC of ≤300 µg/mL for Enterococcus faecalis and Staphylococcus aureus and ≤400 µg/mL for Candida albicans microbial strains. The high total flavonoid content was reported in ICLEA at 771.31 µg/mL and in I. coccinea flower ethyl acetate extract (ICFEA) at 694.69 µg/mL. High-performance thin layer chromatography (HPTLC) analysis showed a high quercetin (QCE) content in the ICLEA extract. To prove the in vitro skin anticancer activity, an MTT assay was performed for the ICLEA extract in a malignant melanoma cell line, and the IC50 value was reported as 7.96 µg/mL.
Conclusion
I. coccinea leaf ethyl acetate extract revealed a significant total flavonoid content in analysis through the aluminum chloride method, and the presence of a high QCE content was confirmed by HPTLC analysis. The in vitro skin anticancer activity of ICLEA was confirmed by the MTT assay; therefore, it was concluded that the ICLEA extract was a potent fraction and was selected to develop a phytosome.
Keywords: flower extract, leaf extract, in vitro skin anticancer study, phytosome formulation, quercetin, flavonoids, ixora alba, ixora coccinea
Introduction
Herbal medicine plays a significant role as an alternative to synthetic medication due to its reduced cost and lack of negative side effects. Plants have been used to separate various chemicals. Traditional medicine is practiced in many countries [1]. A family of flowering plants called Rubiaceae includes the genus Ixora. There were approximately 500 species of trees and shrubs. Some of the common names of Ixora are West Indian Jasmine, Rangan, Kheme, Ponna, Chann Tanea, Techi, Pan, Santan, Jarum-Jarum, Jungle Flame, and Jungle Geranium. Although more species were available, only a few Ixora species were cultivated, and their pharmacological effects were investigated [2]. The flower portions of plants that produce yellow, red, or blue coloration include flavonoids, which are well known for their antioxidant qualities and are responsible for protecting them from attack by microbes and insects. Compared with other active plant compounds, they have low toxicity [3]. Plant phenolics have several actions, including protection against pathogens, UV ray protection, and pigmentation [4].
There have been reports of quercetin (QCE), rutin, kaempferol-3-rutinoside, leucocyanidin glycoside, d-mannitol, and ursolic acid in the blooms of Ixora coccinea (I. coccinea). The blooms of I. coccinea also contain anthocyanins [5]. The heated decoction from shade-dried flowers and coconut oil is externally used to treat eczema. According to previous studies, the flower’s hydro-methanolic extract (70%) exhibits significant in vitro free radical scavenging action [6]. In mice, an aqueous leaf extract from I. coccinea showed antinociceptive activity [7]. Triterpenoid and ursolic acid are found in the flower extract of I. coccinea [8]. Researchers have discovered that the known alkaloid camptothecin is primarily responsible for the anticancer activity of the leaves of I. coccinea (Rubiaceae) [9]. Flowers from I. coccinea constitute a component of an ayurvedic formulation used to treat oral cancer [10]. Ixora alba (I. alba), a small- to medium-sized hardy shrub in the Rubiaceae family, is grown for ornamental purposes. Ixora species have been employed in traditional Indian medical systems for the treatment of ulcers, diarrhea, and, more recently, antitumor activity [11].
Materials and methods
Plant authentication
Selected Ixora species were authenticated by Prof. P. Jayaraman, PhD, Institute of Herbal Botany, Chennai: I. coccinea specimen certificate no.: PARC/2019/4016 and I. alba specimen certificate no.: PARC/2019/4017.
Extraction of plant material
The selected Ixora species, I. coccinea and I. alba, were collected in February and thoroughly washed with distilled water before being shadow-dried and used for further extraction. The plant materials were coarsely powdered and weighed to ensure complete extraction. Petroleum ether, chloroform, ethyl acetate, and hydroalcoholic (ethanol:water, 70:30, v/v) solvents were used for extraction. The maceration method was chosen to reduce the cost of evaporation and minimize heat damage to the thermolabile constituents. The plant materials were macerated for 72 hours, followed by 48 and 24 hours of maceration with occasional shaking. The extracts were filtered using Whatman filter paper, and the solvents were distilled, dried, stored in a container, and kept in a desiccator. The weight of the extracts was recorded. The yield was expressed as % w/w [12].
Elemental analysis
The instrument used for elemental analysis is inductively coupled plasma mass spectrometry (ICP-MS) [13]. The elements detected were arsenic (As), cadmium (Cd), lead (Pb), mercury (Hg), and iron (Fe).
Phytochemical analysis
Using various reagents, extracts of I. coccinea and I. alba were phytochemically screened for the presence of alkaloids, anthraquinone cardiac glycosides, coumarin flavonoid saponins, tannins, saponins, proteins, amino acids, and terpenoids [14].
Antioxidant studies
2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Assay
To conduct the radical scavenging assay, DPPH was purchased from Merck, Mumbai, India. It was prepared using methanol-based dissolution. Plant extract stock solutions (50 µg) from the stock series of 50 to 1000 µg were made. It was incubated for 30 minutes with 0.1 mL of extract and 1.9 mL of DPPH, and the absorbance of the reaction mixture was measured at 517 nm using a UV spectrophotometer. The formula was used to compute free radical scavenging [15].
% Inhibition = (OD of control- (OD of the test) / OD of control)) × 100
where the OD of the control is the absorbance of the negative control and the OD of the test is the absorbance of the sample.
Total Antioxidant Assay
A 1000 µg/mL stock solution (plant extract in dimethylsulfoxide (DMSO)) was prepared and diluted to concentrations ranging from 50 µg/mL to 1000 µg/mL. Ascorbic acid was used as a control. One milliliter of the reagent solution (0.6 M sulphuric sulfuric, 28 mM sodium phosphate, and 4 mM ammonium molybdate) was mixed with 0.1 mL of the sample solution, and the absorbance at 695 nm against the blank was measured using a UV-visible spectrophotometer. The antioxidant activity was measured in grams per milliliter of ascorbic acid equivalents (AAE) [16].
Antimicrobial activity using the agar-well diffusion method
To evaluate the antimicrobial activity of I. coccinea and I. alba, the concentrations of the samples used were 250 µg, 500 µg, and 1000 µg. DMSO was used as the control, and chloramphenicol (50 µg) was used as the standard. The medium used was Mueller-Hinton Media. Gram-positive strains of Staphylococcus aureus (ATCC-25932), Enterococcus faecalis (ATCC-29212), and Streptococcus pyogenes were used. Gram-negative strains of Escherichia coli (ATCC-25922), Klebsiella pneumoniae (ATCC-13883), and Pseudomonas aeruginosa (ATCC-27853) were used. Fungal strains of Candida albicans (ATCC-10231) and Cryptococcus neoformans (ATCC-14116) were used. Using a sterile cotton swab, bacterial solutions were evenly distributed over sterile agar Petri dishes. The plates were then held for five minutes. Then, 7-mm-diameter wells in each of the agar Petri dishes were punched, and each plate contained 30 μL of petroleum ether, chloroform, ethyl acetate, and hydroalcoholic extracts of the leaves and flowers of I. coccinea and I. alba. The plates were then left to diffuse at room temperature for one hour. Subsequently, the plates were incubated for 24 hours at 37°C. Following incubation, confluent bacterial growth was noticed, and the scale was used to quantify the length in millimeters of the bacterial growth inhibition zone. As a benchmark, chloramphenicol (50 μg/disc) was used. Every experiment was conducted in duplicate, and the averages were taken as the result [17].
Minimum inhibitory concentration (MIC)
The MIC was determined by the broth dilution method [18]. In a sterile test tube, 2 mL of brain heart infusion broth was added, and each 2 mL sample of 300 µg/mL, 400 µg/mL, and 500 µg/mL was mixed separately and diluted to 100 mL of organism suspension. The samples were incubated at 37°C for 24 hours. Staphylococcus aureus, Enterococcus faecalis, and Candida albicans were used in this study.
Total flavonoid content
The extracted sample in methanol was produced at 10 mg/mL. By diluting standard QCE (25-1000 µg/mL) in methanol, a calibration curve was created. Six hundred milliliters of methanol were used to dilute 200 µL of the QCE extract before adding 2.0 mL to 40 µL of a 10% (w/v) aluminum chloride solution and 40 µL of a 1 M potassium acetate solution. The combination was given 30 minutes to rest at room temperature. Subsequently, a UV-VIS spectrophotometer was used to quantify the mixture’s peak absorbance at 415 nm [19].
High-performance thin layer chromatography (HPTLC)
HPTLC was performed on 20-cm silica gel 60 HPTLC plates, which were dried at 110°C for 30 minutes before use. The silica gel-coated plate was used for all standards. Toluene, ethyl acetate, formic acid, and methanol (5.5:3:1:0.5 v/v/v/v) were used to develop plates in a horizontal or saturated twin-trough developing chamber. The retention factor was computed using the following formula:
Retention factor = distance moved by the sample/ distance moved by the solvent
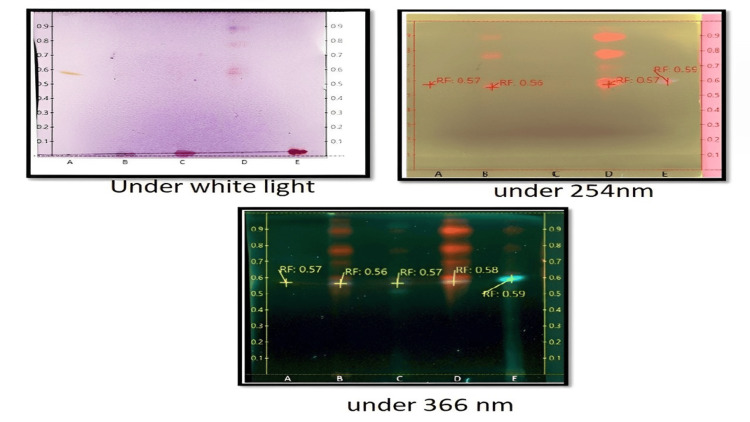
UV light is the most commonly used non-destructive visualization method for thin layer chromatography (TLC) plates. The TLC Visualizer was used to detect the plates containing standards and samples under white light illumination, long-wavelength UV light at 366 nm, and short-wavelength UV light at 254 nm.
The analyses were performed on 10 × 10 mm glass plates using a Linomat 5 (CAMAG, Muttenz, Switzerland) automated spray system. Based on the results obtained from the DPPH assay, total antioxidant, total flavonoid, and antimicrobial activity, the following I. coccinea leaf ethyl acetate (ICLEA), I. coccinea flower ethyl acetate (ICFEA), I. alba leaf chloroform (IALC), and I. alba flower ethyl acetate (IAFEA) extracts were selected for HPTLC analysis using QCE as the standard. All samples were applied with a 5 µl sample syringe. Five µL samples were used as 5-mm bands with a 10-mm distance between the band centers. Plates containing samples and the standard (QCE) were developed for 20 minutes in a saturated vertical glass chamber with a mobile phase. The migration distance of the mobile phase was calculated. The plates were dried and visualized in the TLC visualizer at various wavelengths after development.
QCE was quantified using an imaging processing method based on HPTLC. In brief, standard QCE concentrations of 10, 50, 100, 250, 500, and 1000 µg/mL were used. The sample was then taken at a concentration of 10 mg/ml and plotted alongside the standard in a silica-precoated TLC plate. The plate was then run through the same solvent system used in the previous standardized study (toluene:ethyl acetate:formic acid:methanol (5.5:3:1:0.5, v/v/v/v)) and sprayed with an ethanolic aluminum chloride solution to improve the visualization of the flavonoid-based sample spots. Finally, the derivatized (sprayed) plate was scanned using HPTLC image software for spot quantification [20].
In vitro anticancer evaluation using the MTT assay
The National Centre for Cell Science in Pune, India, donated human A375 cell lines (malignant melanoma cell lines), which were cultured in Eagle’s minimum essential medium. All cell cultures were maintained at 37°C with 100% relative humidity, 5% CO2, and 95% air. Twice a week, the dose was altered. Using trypsin and ethylene diamine tetraacetic acid, single suspensions were prepared. By diluting the suspension with 5% FBS, 1 × 105 cells/mL of cell suspension was obtained. In 96-well plates, 100 mL (10,000 cells per well) were planted. At 37°C, it was incubated. Different concentrations of 100, 50, 25, 12.5, 6.25, 3.125, and 1.5 µg/mL extract were used to treat the cells. After drug addition, the plates were incubated at 37 °C. As a control, a medium without samples was used.
Each well received 20 µL of MTT (5 mg/mL) in phosphate-buffered saline after 48 hours of incubation, followed by a four-hour incubation period at 37°C. The MTT-containing media was turned off, and formazan crystals were dissolved in 100 µL of DMSO. At 570 nm, the absorbance was measured using a microplate reader. The following formula was used to calculate the percentage of cell inhibition:
% cell inhibition was calculated as [100 − Abs (sample) / Abs (control)] × 100.
The same methodology was used to examine the impact of ICLEA on keratinocyte cell lines (HaCaT cell line) and human dermal fibroblasts under comparable circumstances [21].
Selection of the active fraction of Ixora species from the preliminary analysis for phytosomal formulation
I. coccinea and I. alba leaves and flower parts from the Ixora species were selected for the study. Sixteen extracts were prepared using various solvents, including petroleum ether, chloroform, ethyl acetate, and hydroalcoholics. The active fraction was identified by performing preliminary analyses such as phytochemical analysis, DPPH assay, total antioxidant assay, antimicrobial assay, total flavonoid content, and HPTLC analysis. Phytochemical analysis and antioxidant studies of the DPPH assay, the total antioxidant assay, and the antimicrobial assay were performed for all 16 extracts. Of the 16 extracts based on phytoconstituents, antibacterial effect, and free radical scavenging effect, six extracts (ICLEA, ICFEA, ICFHA, IALCE, IALHA, and IAFEA) were selected. The total flavonoid contents of these six selected extracts were calculated. From the flavonoid content comparison, only four extracts (ICLEA, ICFEA, IALCE, and IAFEA) were analyzed by HPTLC and quantified for QCE content. Finally, the ICLEA extract was selected and used for the phytosomal formulation.
Results
Extraction of plant material
The extractive value was calculated and reported as % w/w (Table 1).
Table 1. The extractive yield of Ixora coccinea and Ixora alba leaf and flower.
ICLPE: Ixora coccinea leaf petroleum ether; ICLCE: Ixora coccinea leaf chloroform extract; ICLEA: Ixora coccinea leaf ethyl acetate; ICLHA: Ixora coccinea leaf hydroalcoholic; ICFPE: Ixora coccinea flower petroleum ether; ICFCE: Ixora coccinea flower chloroform extract; ICFEA: Ixora coccinea flower ethyl acetate; ICFHA: Ixora coccinea flower hydroalcoholic; IALPE: Ixora alba leaf petroleum ether; IALCE: Ixora alba leaf chloroform extract; IALEA: Ixora alba leaf ethyl acetate; IALHA: Ixora alba leaf hydroalcoholic; IAFPE: Ixora alba flower petroleum ether; IAFCE: Ixora alba flower chloroform extract; IAFEA: Ixora alba flower ethyl acetate; IAFHA: Ixora alba flower hydroalcoholic
| Plant and parts used | Solvent used | Extract | Extractive value in % | Plant and parts used | % weight of the extract | Extract | Extractive value in % |
| Ixora coccinea leaf | Petroleum ether | ICLPE | 2.65 | Ixora alba leaf | Petroleum ether | IALPE | 0.99 |
| Ixora coccinea leaf | Chloroform | ICLCE | 1.9 | Ixora alba leaf | Chloroform | IALCE | 3.39 |
| Ixora coccinea leaf | Ethyl acetate | ICLEA | 0.939 | Ixora alba leaf | Ethyl acetate | IALEA | 0.968 |
| Ixora coccinea leaf | Hydroalcoholic | ICLHA | 5.1 | Ixora alba leaf | Hydroalcoholic | IALHA | 2.8 |
| Ixora coccinea flower | Petroleum ether | ICFPE | 9.96 | Ixora alba flower | Petroleum ether | IAFPE | 1.16 |
| Ixora coccinea flower | Chloroform | ICFCE | 1.911 | Ixora alba flower | Chloroform | IAFCE | 1.143 |
| Ixora coccinea flower | Ethyl acetate | ICFEA | 0.9385 | Ixora alba flower | Ethyl acetate | IAFEA | 1.778 |
| Ixora coccinea flower | Hydroalcoholic | ICFHA | 3.7 | Ixora alba flower | Hydroalcoholic | IAFHA | 8.1 |
Elemental analysis
The contents of the elements detected in dried leaves and flowers are shown in Table 2.
Table 2. Elements detected and their content by elemental analysis.
BLQ: below limit of quantification; LOQ: limit of quantification
| Plant used for the analysis | Arsenic (As) mg/kg | Cadmium (Cd) mg/kg | Lead (Pb) mg/kg | Mercury (Hg) mg/kg | Iron (Fe) mg/kg |
| Ixora coccinea leaf (ICL) | BLQ (LOQ-0.1) | BLQ (LOQ-0.1) | 1.29 | BLQ (LOQ-0.1) | 42 |
| Ixora coccinea flower (ICF) | BLQ (LOQ-0.1) | BLQ (LOQ-0.1) | 1 | BLQ (LOQ-0.1) | 38 |
| Ixora alba leaf (IAL) | BLQ (LOQ-0.1) | BLQ (LOQ-0.1) | 0.40 | BLQ (LOQ-0.1) | 78 |
| Ixora alba flower (IAF) | BLQ (LOQ-0.1) | BLQ (LOQ-0.1) | 0.21 | BLQ (LOQ-0.1) | 104 |
Phytochemical analysis
The positive and negative results of phytochemical constituents present in the extract were shown as (+) and (-), respectively (Table 3).
Table 3. Photochemical analysis of petroleum ether, chloroform, ethyl acetate, and hydroalcoholic extracts of Ixora coccinea and Ixora alba leaves and flowers.
ICLPE: Ixora coccinea leaf petroleum ether; ICFPE: Ixora coccinea flower petroleum ether; IALPE: Ixora alba leaf petroleum ether; IAFPE: Ixora alba flower petroleum ether; ICLCE: Ixora coccinea leaf chloroform extract; ICFCE: Ixora coccinea flower chloroform extract; IALCE: Ixora alba leaf chloroform extract; IAFCE: Ixora alba flower chloroform extract; ICLEA: Ixora coccinea leaf ethyl acetate; ICFEA: Ixora coccinea flower ethyl acetate; IALEA: Ixora alba leaf ethyl acetate; IAFEA: Ixora alba flower ethyl acetate; ICLHA: Ixora coccinea leaf hydroalcoholic; ICFHA: Ixora coccinea flower hydroalcoholic; IALHA: Ixora alba leaf hydroalcoholic; IAFHA: Ixora alba flower hydroalcoholic; + indicates positive; − depicts negative
| Phytoconstituents | Petroleum ether extract | Chloroform extract | Ethyl acetate extract | Hydroalcoholic extract | |||||||||||||
| ICLPE | ICFPE | IALPE | IAFPE | ICLCE | ICFCE | IALCE | IAFCE | ICLEA | ICFEA | IALEA | IAFEA | ICLHA | ICFHA | IALHA | IAFHA | ||
| Alkaloid | − | − | − | − | + | + | + | + | + | + | + | + | − | − | − | − | |
| Carbohydrate | − | − | − | − | + | + | + | + | − | − | − | − | − | − | − | − | |
| Saponin | − | − | − | − | − | − | − | − | − | − | − | − | + | − | − | − | |
| Glycosides | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Phytosterols | + | + | + | + | − | + | − | + | + | + | − | + | + | + | + | + | |
| Phenols | − | − | − | − | + | − | + | + | − | − | − | − | − | − | − | − | |
| Tannins | − | − | − | − | − | + | − | + | − | − | − | − | − | − | − | − | |
| Flavonoids | + | + | + | + | − | + | − | + | + | + | + | + | + | + | + | + | |
| Proteins and amino acids | + | + | + | + | + | − | + | − | − | − | − | − | − | − | − | − | |
| Diterpene | − | − | − | − | − | + | − | + | + | + | + | + | + | + | − | − | |
DPPH assay
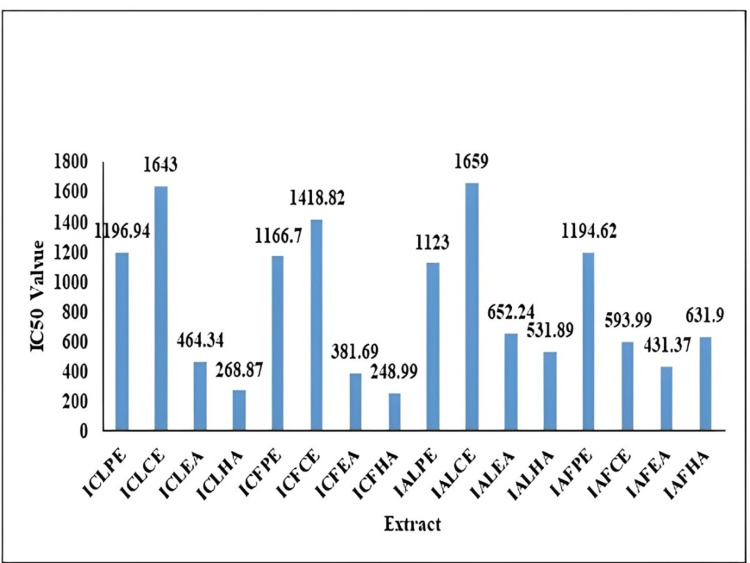
The mean IC50 values of all extracts are reported (Table 4). A graph representation of the IC50 values of various extracts of I. coccinea and I. alba was shown (Figure 1).
Table 4. IC50 value of Ixora coccinea and Ixora alba leaf and flower.
ICLPE: Ixora coccinea leaf petroleum ether; ICLCE: Ixora coccinea leaf chloroform extract; ICLEA: Ixora coccinea leaf ethyl acetate; ICLHA: Ixora coccinea leaf hydroalcoholic; ICFPE: Ixora coccinea flower petroleum ether; ICFCE: Ixora coccinea flower chloroform extract; ICFEA: Ixora coccinea flower ethyl acetate; ICFHA: Ixora coccinea flower hydroalcoholic; IALPE: Ixora alba leaf petroleum ether; IALCE: Ixora alba leaf chloroform extract; IALEA: Ixora alba leaf ethyl acetate; IALHA: Ixora alba leaf hydroalcoholic; IAFPE: Ixora alba flower petroleum ether; IAFCE: Ixora alba flower chloroform extract; IAFEA: Ixora alba flower ethyl acetate); IAFHA: Ixora alba flower hydroalcoholic; SD: standard deviation); IC50: half-maximal inhibitory concentration
Values are means of triplicate experiments
| Sample ID | IC50 value mean ± SD | Extract | IC50 value mean ± SD |
| Ixora coccinea | Ixora alba | ||
| ICLPE | 1196.94 ± 0.04 | IALPE | 1123 ± 0.02 |
| ICLCE | 1643 ± 0.02 | IALCE | 1659 ± 0.01 |
| ICLEA | 464.34 ± 0.05 | IALEA | 652.24 ± 0.00 |
| ICLHA | 268.87 ± 0.01 | IALHA | 531.89 ± 0.01 |
| ICFPE | 1166.7 ± 0.01 | IAFPE | 1194.62 ± 0.01 |
| ICFCE | 1418.82 ± 0.02 | IAFCE | 593.99 ± 0.03 |
| ICFEA | 381.69 ± 0.04 | IAFEA | 431.37 ± 0.02 |
| ICFHA | 248.99 ± 0.05 | IAFHA | 631.9 ± 0.01 |
Figure 1. IC50 value of Ixora coccinea and Ixora alba leaf and flower.
ICLPE: Ixora coccinea leaf petroleum ether; ICLCE: Ixora coccinea leaf chloroform extract; ICLEA: Ixora coccinea leaf ethyl acetate; ICLHA: Ixora coccinea leaf hydroalcoholic; ICFPE: Ixora coccinea flower petroleum ether; ICFCE: Ixora coccinea flower chloroform extract; ICFEA: Ixora coccinea flower ethyl acetate; ICFHA: Ixora coccinea flower hydroalcoholic; IALPE: Ixora alba leaf petroleum ether; IALCE: Ixora alba leaf chloroform extract; IALEA: Ixora alba leaf ethyl acetate; IALHA: Ixora alba leaf hydroalcoholic; IAFPE: Ixora alba flower petroleum ether; IAFCE: Ixora alba flower chloroform extract; IAFEA: Ixora alba flower ethyl acetate; IAFHA: Ixora alba flower hydroalcoholic; IC50: half-maximal inhibitory concentration
Total antioxidant assay
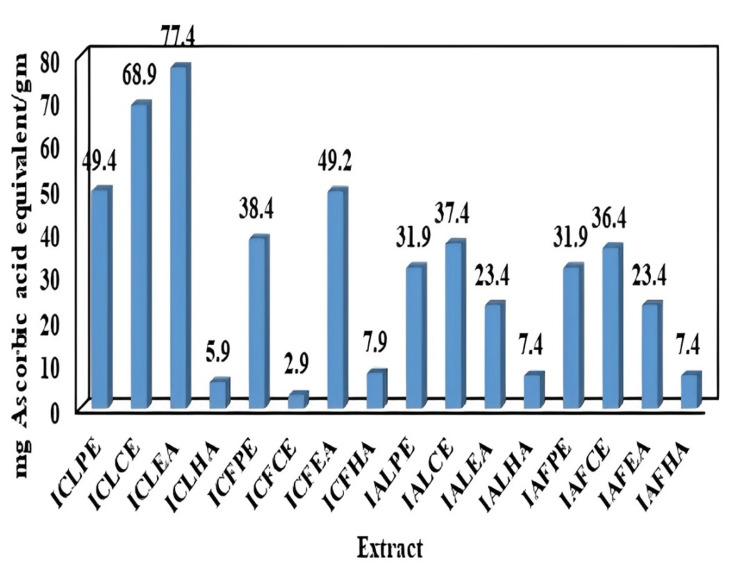
The milligrams of AAE per gram of extract are reported (Table 5) and the graph represents the comparison of all the extracts (Figure 2).
Table 5. Milligrams of AAE per gram of Ixora coccinea and Ixora alba leaf and flower.
ICLPE: Ixora coccinea leaf petroleum ether; ICLCE: Ixora coccinea leaf chloroform extract; ICLEA: Ixora coccinea leaf ethyl acetate; ICLHA: Ixora coccinea leaf hydroalcoholic; ICFPE: Ixora coccinea flower petroleum ether; ICFCE: Ixora coccinea flower chloroform extract; ICFEA: Ixora coccinea flower ethyl acetate; ICFHA: Ixora coccinea flower hydroalcoholic; IALPE: Ixora alba leaf petroleum ether; IALCE: Ixora alba leaf chloroform extract; IALEA: Ixora alba leaf ethyl acetate; IALHA: Ixora alba leaf hydroalcoholic; IAFPE: Ixora alba flower petroleum ether; IAFCE: Ixora alba flower chloroform extract; IAFEA: Ixora alba flower ethyl acetate; IAFHA: Ixora alba flower hydroalcoholic; SD: standard deviation; AAE: ascorbic acid equivalents
Values are means of triplicate experiments
| Extract | mg AAE/g mean ± SD | Extract | mg AAE/g mean ± SD |
| Ixora coccinea | Ixora alba | ||
| ICLPE | 49.4 ± 0.01 | IALPE | 31.9 ± 0.03 |
| ICLCE | 68.9 ± 0.03 | IALCE | 37.4 ± 0.02 |
| ICLEA | 77.4 ± 0.05 | IALEA | 23.4 ± 0.01 |
| ICLHA | 5.9 ± 0.02 | IALHA | 7.4 ± 0.02 |
| ICFPE | 38.4 ± 0.01 | IAFPE | 31.9 ± 0.02 |
| ICFCE | 2.9 ± 0.04 | IAFCE | 36.4 ± 0.03 |
| ICFEA | 49.2 ± 0.03 | IAFEA | 23.4 ± 0.04 |
| ICFHA | 7.9 ± 0.05 | IAFHA | 7.4 ± 0.01 |
Figure 2. Milligram of AAE per gram of Ixora coccinea and Ixora alba leaf and flower.
ICLPE: Ixora coccinea leaf petroleum ether; ICLCE: Ixora coccinea leaf chloroform extract; ICLEA: Ixora coccinea leaf ethyl acetate; ICLHA: Ixora coccinea leaf hydroalcoholic; ICFPE: Ixora coccinea flower petroleum ether; ICFCE: Ixora coccinea flower chloroform extract; ICFEA: Ixora coccinea flower ethyl acetate; ICFHA: Ixora coccinea flower hydroalcoholic; IALPE: Ixora alba leaf petroleum ether; IALCE: Ixora alba leaf chloroform extract; IALEA: Ixora alba leaf ethyl acetate; IALHA: Ixora alba leaf hydroalcoholic; IAFPE: Ixora alba flower petroleum ether; IAFCE: Ixora alba flower chloroform extract; IAFEA: Ixora alba flower ethyl acetate; IAFHA: Ixora alba flower hydroalcoholic; AAE: ascorbic acid equivalent
Antimicrobial studies
Extracts of I. coccinea and I. alba leaf and flower parts showed significant antimicrobial activity (Tables 6-7).
Table 6. Antimicrobial activity of petroleum ether, chloroform, ethyl acetate, and hydroalcoholic extracts of Ixora coccinea and Ixora alba leaf and flower.
ICLPE: Ixora coccinea leaf petroleum ether; ICLCE: Ixora coccinea leaf chloroform extract; ICLEA: Ixora coccinea leaf ethyl acetate; ICLHA: Ixora coccinea leaf hydroalcoholic; ICFPE: Ixora coccinea flower petroleum ether; ICFCE: Ixora coccinea flower chloroform extract; ICFEA: Ixora coccinea flower ethyl acetate; ICFHA: Ixora coccinea flower hydroalcoholic; IALPE: Ixora alba leaf petroleum ether; IALCE: Ixora alba leaf chloroform extract; IALEA: Ixora alba leaf ethyl acetate; IALHA: Ixora alba leaf hydroalcoholic; IAFPE: Ixora alba flower petroleum ether; IAFCE: Ixora alba flower chloroform extract; IAFEA: Ixora alba flower ethyl acetate; IAFHA: Ixora alba flower hydroalcoholic; STD: standard chloramphenicol; + depicts positive; - depicts negative
| Microbial strains | Petroleum ether extract | Chloroform extract | Ethyl acetate extract | Hydroalcoholic extract | ||||||||||||||
| STD | ICLPE | ICFPE | IALPE | IAFPE | ICLCE | ICFCE | IALCE | IAFCE | ICLEA | ICFEA | IALEA | IAFEA | ICLHA | ICFHA | IALHA | IAFHA | ||
| Staphylococcus aureus | + | − | − | − | − | − | − | − | − | + | + | + | + | + | + | + | + | |
| Streptococcus pyogenes | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Enterococcus faecalis | + | − | − | − | − | − | − | − | − | + | + | + | + | + | + | + | + | |
| Escherichia coli | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Klebsiella pneumoniae | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Pseudomonas aeruginosa | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| Candida albicans | + | − | − | − | − | − | − | − | − | + | + | + | + | + | + | + | + | |
| Cryptococcus neoformans | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
Table 7. Zone of inhibition of Ixora coccinea and Ixora alba.
ICLEA: Ixora coccinea leaf ethyl acetate; ICFEA: Ixora coccinea flower ethyl acetate; IALEA: Ixora alba leaf ethyl acetate; IAFEA: Ixora alba flower ethyl acetate; ICLHA: Ixora coccinea leaf hydroalcoholic; ICFHA: Ixora coccinea flower hydroalcoholic; IALHA: Ixora alba leaf hydroalcoholic; IAFHA: Ixora alba flower hydroalcoholic; STD: chloramphenicol standard
| ICLEA | ICFEA | IALEA | IAFEA | |||||||||||||
| Sample | STD | 250 µg | 500 µg | 1000 µg | STD | 250 µg | 500 µg | 1000 µg | STD | 250 µg | 500 µg | 1000 µg | STD | 250 µg | 500 µg | 1000 µg |
| Organism | Zone of inhibition (mm) | Zone of inhibition (mm) | ||||||||||||||
| Staphylococcus aureus | 18 | 10 | 14 | 15 | 17 | 10 | 12 | 14 | 15 | 6 | 8 | 10 | 15 | 6 | 8 | 9 |
| Enterococcus faecalis | 15 | 8 | 10 | 12 | 16 | 10 | 12 | 13 | 16 | 8 | 10 | 11 | 17 | 12 | 14 | 15 |
| Candida albicans | 20 | 8 | 10 | 12 | 21 | 9 | 12 | 14 | 21 | 8 | 10 | 12 | 20 | 8 | 9 | 10 |
| ICLHA | ICFHA | IALHA | IAFHA | |||||||||||||
| Sample | STD | 250 µg | 500 µg | 1000 µg | STD | 250 µg | 500 µg | 1000 µg | STD | 250 µg | 500 µg | 1000 µg | STD | 250 µg | 500 µg | 1000 µg |
| Organism | Zone of inhibition (mm) | Zone of inhibition (mm) | ||||||||||||||
| Staphylococcus aureus | 15 | 8 | 10 | 12 | 13 | 10 | 12 | 13 | 18 | 10 | 12 | 14 | 20 | 10 | 12 | 14 |
| Enterococcus faecalis | 15 | 10 | 11 | 12 | 15 | 9 | 10 | 12 | 16 | 13 | 14 | 15 | 18 | 12 | 13 | 14 |
| Candida albicans | 20 | 10 | 12 | 13 | 20 | 9 | 10 | 11 | 21 | 13 | 15 | 17 | 18 | 11 | 12 | 14 |
MIC
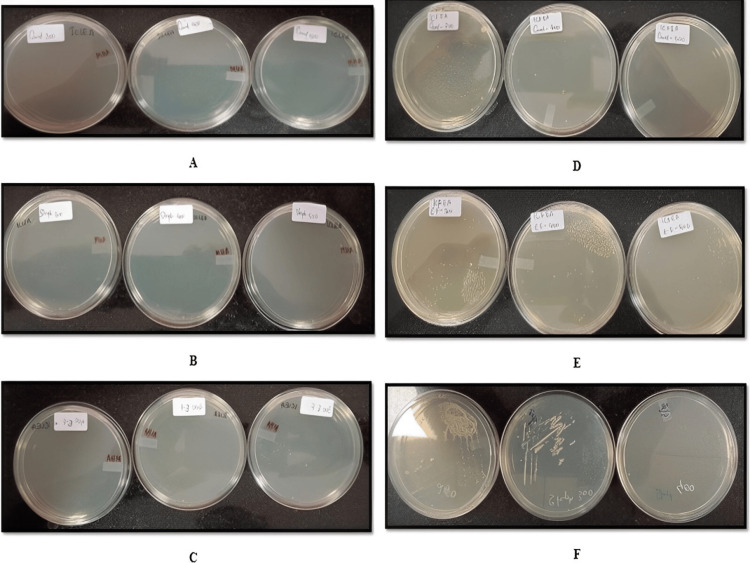
The MICs of the ethyl acetate extract of Ixora coccinea leaf and flower against Staphylococcus aureus, Enterococcus faecalis, and Candida albicans are reported (Figure 3). The MICs of the ethyl acetate extract and hydroalcoholic extracts of I. coccinea and I. alba against Staphylococcus aureus,Enterococcus faecalis, and Candida albicans are reported (Table 8).
Table 8. Minimal inhibitory concentrations of ethyl acetate and hydroalcoholic extracts of Ixora coccinea and Ixora alba leaf and flower.
ICLEA: Ixora coccinea leaf ethyl acetate; ICFEA: Ixora coccinea flower ethyl acetate; IALEA: Ixora alba leaf ethyl acetate; IAFEA: Ixora alba flower ethyl acetate; ICLHA: Ixora coccinea leaf hydroalcoholic; ICFHA: Ixora coccinea flower hydroalcoholic; IALHA: Ixora alba leaf hydroalcoholic; IAFHA: Ixora alba flower hydroalcoholic
| Microbial strain | MIC of the plant extract | |||||||
| Ixora coccinea | Ixora alba | |||||||
| ICLEA | ICFEA | ICLHA | ICFHA | IALEA | IAFEA | IALHA | IAFHA | |
| Candida albicans | 500 µg | 400 µg | 400 µg | 400 µg | 400 µg | 400 µg | 300 µg | 1000 µg |
| Enterococcus faecalis | 400 µg | 500 µg | 300 µg | 1000 µg | 1000 µg | 1000 µg | 400 µg | 1000 µg |
| Staphylococcus aureus | 500 µg | 500 µg | 300 µg | 1000 µg | 1000 µg | 1000 µg | 400 µg | 1000 µg |
Figure 3. MIC of ethyl acetate extract of Ixora coccinea leaf and flower.
A: MIC of Ixora coccinea leaf ethyl acetate extract in Candida albicans. B: MIC of Ixora coccinea leaf ethyl acetate extract in Staphylococcus aureus. C: MIC of Ixora coccinea leaf ethyl acetate extract in Enterococcus faecalis. D: MIC of Ixora coccinea flower ethyl acetate extract in Candida albicans. E: MIC of Ixora coccinea flower ethyl acetate extract in Enterococcus faecalis. F: MIC of Ixora coccinea flower ethyl acetate extract in Staphylococcus aureus
MIC: minimum inhibitory concentration
Total flavonoid content
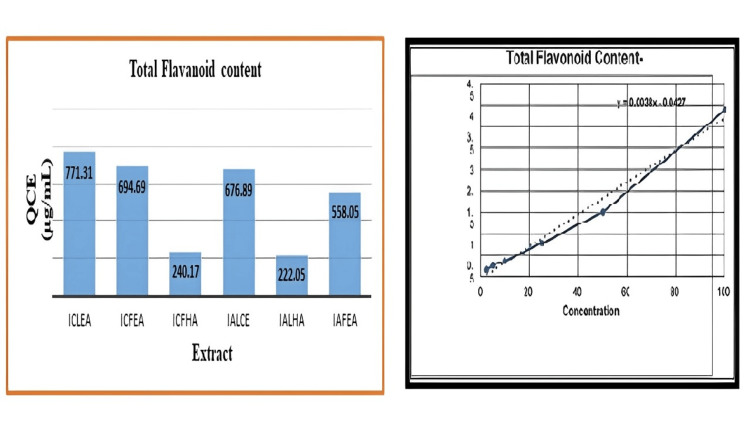
The results of the total flavonoid content of different extracts, I. coccinea and I. alba, are reported (Table 9, Figure 4).
Table 9. Total flavonoid content of QCE (µg/mL).
ICLEA: Ixora coccinea leaf ethyl acetate; ICFEA: Ixora coccinea flower ethyl acetate; ICFHA: Ixora coccinea flower hydroalcoholic; IALCE: Ixora alba leaf chloroform extract; IALHA: Ixora alba leaf hydroalcoholic; IAFEA: Ixora alba flower ethyl acetate; QCE: quercetin; * mean of triplicate
| Concentration of QCE (µg/mL) | *Absorbance of the standard at 415 nm | Sample | Concentration of the extract (mg/mL) | *Absorbance of the extract at 415 nm | Total flavonoid content (QCE) (µg/mL) |
| 25 | 0.1587 | ICLEA | 10 | 2.8883 | 771.31 |
| 50 | 0.2620 | ICFEA | 10 | 2.5972 | 694.69 |
| 100 | 0.3721 | ICFHA | 10 | 0.8699 | 240.17 |
| 250 | 0.7797 | IALCE | 10 | 2.5295 | 676.89 |
| 500 | 1.5032 | IALHA | 10 | 0.8011 | 222.05 |
| 1000 | 3.8906 | IAFEA | 10 | 2.0779 | 558.05 |
Figure 4. QCE (µg/mL) in Ixora coccinea and Ixora alba (leaf and flower) and the calibration curve of QCE (25-1000 µg/mL).
ICLEA: Ixora coccinea leaf ethyl acetate; ICFEA: Ixora coccinea flower ethyl acetate; ICFHA: Ixora coccinea flower hydroalcoholic; IALCE: Ixora alba leaf chloroform extract; IALHA: Ixora alba leaf hydroalcoholic; IAFEA: Ixora alba flower ethyl acetate; QCE: quercetin
HPTLC analysis
The spots of the different extracts under different conditions are shown (Figure 5). The retention factor calculated for each extracted sample and QCE concentration were reported (Tables 10-11).
Table 10. Rf value.
A: QCE; B: ICLEA; C: ICFEA; D: IALCE; E: IAFEA
ICLEA: Ixora coccinea leaf ethyl acetate; ICFEA: Ixora coccinea flower ethyl acetate; IALCE: Ixora alba leaf chloroform extract; IAFEA: Ixora alba flower ethyl acetate; Rf: retention factor; QCE: quercetin
| Sample code | Sample name | Solvent system | Rf value under white light | Rf value under 254 nm | Rf value under 366 nm |
| A | QCE | Toluene:ethyl acetate:formic acid:methanol (5.5:3:1:0.5, v/v/v/v) | 0.57 | 0.57 | 0.57 |
| B | ICLEA | - | 0.56 | 0.56 | |
| C | ICFEA | - | - | 0.57 | |
| D | IALCE | 0.58 | 0.57 | 0.58 | |
| E | IAFEA | - | 0.59 | 0.59 |
Table 11. QCE concentration (µg/mL) in ICLEA and IALCE.
ICLEA: Ixora coccinea leaf ethyl acetate; IALCE: Ixora alba leaf chloroform extract; QCE: quercetin
| Sample | QCE concentration (µg/mL) |
| ICLEA | 24.44 |
| IALCE | 22.37 |
Figure 5. Visualization of a spot.
A: QCE; B: ICLEA; C: ICFEA; D: IALCE; E: IAFEA
ICLEA: Ixora coccinea leaf ethyl acetate; ICFEA: Ixora coccinea flower ethyl acetate; IALCE: Ixora alba leaf chloroform extract; IAFEA: Ixora alba flower ethyl acetate; QCE: quercetin
In vitro anticancer evaluation by MTT assay
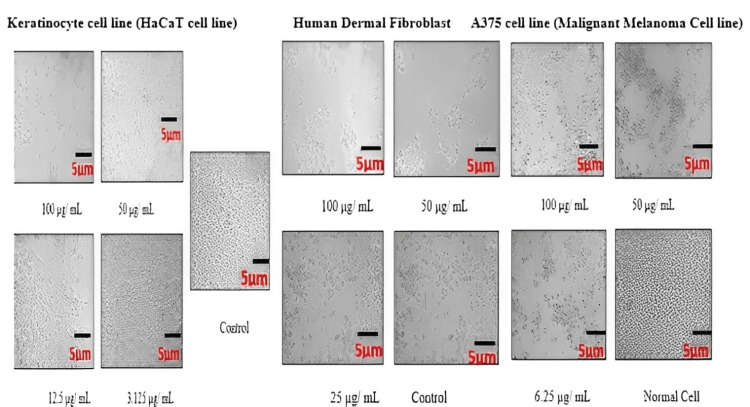
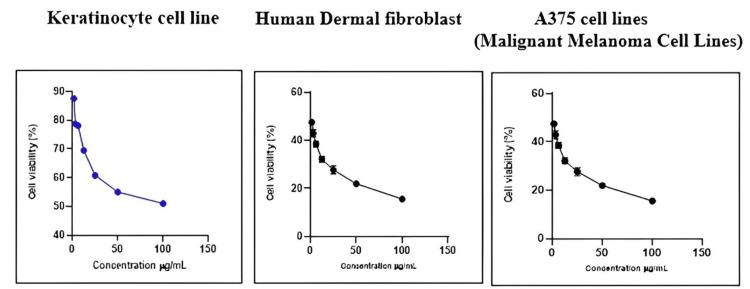
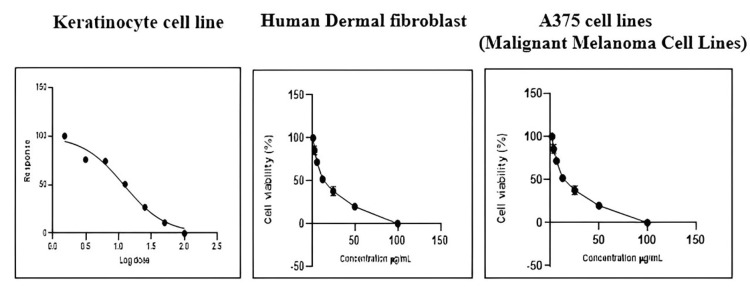
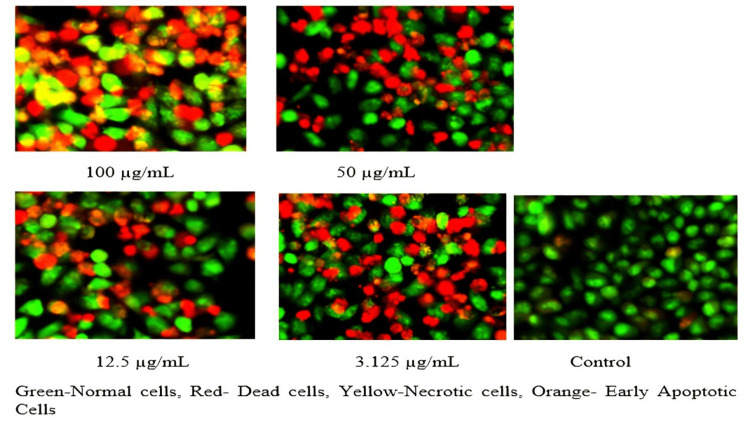
The percentage of live and dead cells of the HaCaT cell line, A375 cell lines (malignant melanoma cell lines), and human dermal fibroblasts treated with ICLEA extract are reported (Table 12). An increase in the concentration of the ICLEA extract inhibited cell growth (Figure 6). The results for cell viability and the nonlinear regression curve fit are shown in Figures 7-8. Live and dead cells were differentiated by staining with acridine orange and ethidium bromide (Figure 9).
Table 12. Percentage of live and dead cells from different cell lines treated with I. coccinea leaf ethyl acetate extract.
SD: standard deviation; IC50: half-maximal inhibitory concentration; SEM: standard error of mean; HaCaT cell line: keratinocyte cell line
Values are means of triplicate experiments
| Cell line used | HaCaT cell line | Human dermal fibroblasts | A375 cell lines (malignant melanoma cell lines) | |||||||||
| Concentration of extract (µg/mL) | % cell death mean | SD | SEM | % live cells | % cell death | SD | SEM | % live cells | % cell death | SD | SEM | % live cells |
| 100 | 48.98 | 1.39 | 0.80 | 51.02 | 63.98 | 0.56 | 0.32 | 36.02 | 86.30 | 7.95 | 4.59 | 13.70 |
| 50 | 44.97 | 0.98 | 0.56 | 55.03 | 49.77 | 2.17 | 1.25 | 50.23 | 84.24 | 1.27 | 0.73 | 15.76 |
| 25 | 39.18 | 1.74 | 1.00 | 60.82 | 41.19 | 0.66 | 0.38 | 58.81 | 66.34 | 7.29 | 4.21 | 33.66 |
| 12.5 | 30.48 | 1.42 | 0.82 | 69.52 | 32.58 | 0.58 | 0.34 | 67.42 | 59.98 | 5.94 | 3.43 | 40.02 |
| 6.25 | 21.84 | 1.27 | 0.74 | 78.16 | 29.97 | 0.56 | 0.32 | 70.03 | 53.55 | 4.69 | 2.71 | 46.45 |
| 3.125 | 21.22 | 1.01 | 0.58 | 78.78 | 27.63 | 0.65 | 0.38 | 72.37 | 37.54 | 2.40 | 1.39 | 62.46 |
| 1.5 | 12.38 | 1.58 | 0.91 | 87.62 | 15.42 | 4.12 | 2.38 | 84.58 | 24.67 | 3.57 | 2.06 | 75.33 |
| Control | 100 | 100.00 | 100 | |||||||||
| IC50 | 98.52 µg/mL | IC50 | 58.52 µg/mL | IC50 | 7.96 µg/mL | |||||||
Figure 6. Visual live cells from different cell lines after I. coccinea leaf ethyl acetate extract treatment.
Figure 7. Percentage of cell viability of different cell lines after I. coccinea leaf ethyl acetate extract treatment.
Figure 8. Nonlinear regression curve fit of different cell lines after I. coccinea leaf ethyl acetate extract treatment.
Figure 9. Live and dead staining with acridine orange and ethidium bromide in I. coccinea leaf ethyl acetate extract at different concentrations.
Discussion
The selected Ixora species of I. coccinea and I. alba leaf and flowers were subjected to extraction by petroleum ether, chloroform, ethyl acetate, and hydroalcoholic (ethanol: water, 70:30, v/v) via the maceration method. The prepared extracts of I. coccinea were given the following IDs: ICLPE, ICLCE, ICLEAE, ICLHAE, ICFPE, ICFCE, ICFEAE, and ICFHAE. In the leaf part of the selected Ixora species, the highest percentage yield was obtained for the ICLHA extract. The prepared extracts of I. alba were given the following IDs: IALPE, IALCE, IALEAE, IALHAE, IAFPE, IACFCE, IAFEAE, and IAFHAE. In the flower of the selected Ixora species, the highest percentage yield was obtained for the ICFPE extract.
Elemental analysis performed by ICP-MS proved that arsenic (As), cadmium (Cd), and mercury (Hg) levels were below the limit of quantification. Lead (Pb) was detected to be very low, and iron (Fe) content was present in the dried leaves and flowers of I. coccinea and I. alba.
All extracts were tested for the presence of phytoconstituents using standard procedures. In phytochemical analysis, alkaloids, phytosterols, flavonoids, proteins, amino acids, and diterpenes were predominantly present in chloroform, ethyl acetate, and hydroalcoholic extracts of I. coccinea and I. alba.
In the DPPH assay, the percentage of the scavenging effect of the plant extract was estimated. The IC50 value was calculated. The hydroalcoholic extract of I. coccinea was shown to have a good scavenging effect compared with other extracts. Less inhibition was reported in the petroleum ether extract. The total antioxidant assay was performed using the phosphomolybdenum method. The highest total antioxidant capacity was proven in ICLEA at 77.4 ± 0.05 mg; in ICFEA it was 49.2 ± 0.03 mg; in IALCE it was 37.4 ± 0.02 mg; and in IAFCE it was 36.4 ± 0.03 mg.
The antimicrobial activity of I. coccinea, I. alba leaf, and flower extracts was evaluated against various bacterial and fungal strains such as Staphylococcus aureus, Streptococcus pyogenes, Enterococcus faecalis, Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Candida albicans, and Cryptococcus neoformans. Petroleum ether and chloroform extract did not possess any zones of inhibition for the above eight strains. Ethyl acetate and hydroalcoholic extract showed a significant zone of inhibition with Staphylococcus aureus, Enterococcus faecalis, and Candida albicans, which was comparable with standard chloramphenicol. Ethyl acetate and hydroalcoholic extract showed antimicrobial activity, which may be due to the presence of phytosterol and flavonoid phytoconstituents in I. coccinea and I. alba. Results concluded that all ethyl acetate and hydroalcoholic extract fractions showed ≤400 µg of antimicrobial activity with all three strains.
Using the aluminum chloride method, the total flavonoid content was determined. In four extracts (ICLEA, ICFEA, IALCE, and IAFEA), the QCE content was greater than 500 µg/mL, and two extracts (ICFHA and IALHA) had a lower total flavonoid content. From the results, it was evident that a predominant QCE appearance was seen for both ICLEA and IALCE under all three illumination sources and was concluded by the retention factor; in particular, IALCE has bright red/yellow spots under white light similar to QCE. Only at 366 nm was the fluorescence QCE appearance observed for ICFEA. At 366 nm, fluorescence was observed for sample IAFEA, hinting that a flavone-type flavonoid may be present. Among the four samples, IAFEA displayed a single band under different illumination sources, indicating that it is a highly pure compound compared with the other extracts. The ICLEA extract has a high flavonoid content; the band produced was equivalent to QCE; therefore, it is identified as a promising extract for phytosome formulation.
It was found that 100 µg of ICLEA extract showed 48.98% inhibition in the HaCaT cell line. The IC50 value was found to be 98.52 µg/mL. Treatment of the ICLEA extract sample in the HaCaT cell line and human dermal fibroblasts did not affect normal cell functioning. It was proven that ICLEA did not cause any damage to normal cells. The ICLEA extract treatment on A375 cell lines (malignant melanoma cell lines) at various concentrations proved that an increase in the concentration increases cell growth inhibition.
Pornima et al. reported that ethanol, water, and hydroethanolic extracts of I. coccinea leaves have antioxidant potential [22]. Haji et al., by HPLC analysis, identified that pyrocatechol, catechin, and chlorogenic acid were most abundant in the root of I. coccinea. They proved that methanol has antioxidant and antibacterial effects [23].
Panikkar et al. formulated an ayurvedic oil from the I. coccinea and Cortus sativum flowers. The formulation was subjected to animals to identify the prevention of Dalton's lymphoma, a solid tumor, and the oil was reported to arrest the further growth of the tumor cell [24].
Latha et al. reported that the extract of I. coccinea flower obtained from n-Hexane, which was administered to Swiss albino mice for skin carcinogenic studies, caused a significant decrease in the mean number of papillomas in these mice. By spectrometrically identifying the presence of triterpenoid ursolic acid, which has already been reported to significantly prevent skin tumors [25].
Prabhu et al. evaluated the in vitro cytotoxicity activity of a methanolic extract of fresh flowers from I. coccinea. GC-MS analysis of the methanol extract identified the presence of cyclopentaneundecanoic acid, methyl ester (40.36%), and dibutyl phthalate (26.83%) as the major compounds. The methanolic extract showed an IC50 value of 250 µg/mL and 300 µg/mL against DLA and EAC cell lines, respectively [26].
Limitations
The phytosome gel formulation from the identified active fraction of the I. coccinea leaf ethyl acetate extract has not been prepared. Thus, phytosome preparation and evaluation of gel properties shall be studied. In the current study, the in vitro cytotoxicity effect in a cell line was only proved; further phytosomal gel in vivo studies for acute dermal toxicity and cell-induced cytotoxicity effects can be conducted to support the skin cancer activity of the I. coccinea leaf active fraction.
Conclusions
From this preliminary analysis, it was concluded that I. coccinea leaf ethyl acetate has promising antioxidant and antimicrobial properties. This may be due to the presence of a high flavonoid content. The in vitro cytotoxicity by the MTT assay has proven that this extract does not affect normal cells; it inhibits only A375 cell lines (malignant melanoma cell lines) at a low concentration. Various studies have shown that plant extracts containing flavonoids can be expressed as phytosomes to reduce problems arising from pharmacokinetics and bioavailability. Hence, phytosomes can be formulated from I. coccinea leaf ethyl acetate extract, which may be a potent candidate for various ailments.
Acknowledgments
The authors express their gratitude to the principal and management of the Sri Ramachandra Faculty of Pharmacy, Sri Ramachandra Institute of Higher Education and Research, for their ongoing support and encouragement.
The authors have declared that no competing interests exist.
Author Contributions
Concept and design: Jasmin Sajini Rajayan, Karthik Rajendran, Vinodhini Chandrasekar, Chamundeeswari Duraipandian
Acquisition, analysis, or interpretation of data: Jasmin Sajini Rajayan, Karthik Rajendran, Vinodhini Chandrasekar, Chamundeeswari Duraipandian
Drafting of the manuscript: Jasmin Sajini Rajayan, Karthik Rajendran, Vinodhini Chandrasekar, Chamundeeswari Duraipandian
Critical review of the manuscript for important intellectual content: Jasmin Sajini Rajayan, Karthik Rajendran, Vinodhini Chandrasekar, Chamundeeswari Duraipandian
Supervision: Karthik Rajendran, Vinodhini Chandrasekar, Chamundeeswari Duraipandian
Human Ethics
Consent was obtained or waived by all participants in this study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.Extraction and isolation of active constituents from Ixora chinensis Lam leaves. Sunitha D, Hemalatha K, Manthripragada BR, Chary N. https://www.derpharmachemica.com/pharma-chemica/extraction-and-isolation-of-active-constituents-from-ixora-chinensis-lam-leaves.pdf Der Pharma Chemica. 2015;7:434–441. [Google Scholar]
- 2.A review on phytochemical and pharmacological activity of genus Ixora. Kharat AR, Nambiar VV, Tarkasband YS, Pujari RR. https://www.ijrpc.com/files/20-3158.pdf Int J Res Pharm Chem. 2013;3:628–635. [Google Scholar]
- 3.Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Rauha JP, Remes S, Heinonen M, et al. Int J Food Microbiol. 2000;25:3–12. doi: 10.1016/s0168-1605(00)00218-x. [DOI] [PubMed] [Google Scholar]
- 4.Phytochemical studies of Ixora coccinea Linn-an ethnobotanical plant from Karwar District. Damle S, Sharon K. https://www.researchgate.net/publication/318153195_PHYTOCHEMICAL_STUDIES_OF_Ixora_coccinea_Linn-AN_ETHNOBOTANICAL_PLANT_FROM_KARWAR_DISTRICT#fullTextFileContent Int J Bio Pharm Allied Sci . 2017;6:1403–1415. [Google Scholar]
- 5.Srinivasan GV. Phytochemical investigation and biological studies of secondary metabolites from some folk medicinal plants. Malappuram, India: University of Calicut; 2015. [Google Scholar]
- 6.Ixora coccinea Linn.: traditional uses, phytochemistry and pharmacology. Manjeshwar SB, Poruthukaran JK. https://link.springer.com/article/10.1007/s11655-011-0881-3. Chin J Integr Med. 2012;18:72–79. doi: 10.1007/s11655-011-0881-3. [DOI] [PubMed] [Google Scholar]
- 7.Anti-inflammatory activity of the aqueous leaf extract of Ixora coccinea. Ratnasooriya WD, Deraniyagala SA, Galhena G, Liyanage SS, Bathige SD, Jayakody JR. Pharm Bio. 2005;43:147–152. [Google Scholar]
- 8.Modulatory effects of ixora coccinea flower on cyclophosphamide-induced toxicity in mice. Latha PG, Panikkar KR. https://pubmed.ncbi.nlm.nih.gov/10479765/ Phytother Res. 1999;13:517–520. doi: 10.1002/(sici)1099-1573(199909)13:6<517::aid-ptr524>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Occurrence of camptothecin an anticancer drug from Ixora coccinea Linn. Saravanan P, Boopalan E. https://www.researchgate.net/publication/236143530_Occurrence_of_Camptothecin_an_Anticancer_Drug_From_Ixora_coccinea_Linn Int J Appl Biol. 2011;2:30–34. [Google Scholar]
- 10.Cytotoxic and antitumour principles from Ixora coccinea flowers. Latha PG, Panikkar KR. Cancer Lett. 1998;14:197–202. doi: 10.1016/s0304-3835(98)00140-2. [DOI] [PubMed] [Google Scholar]
- 11.Pharmacognostic & phytochemical characterization of Ixora alba L. Talpade MB, Chachad D. https://www.academia.edu/23957441/PHARMACOGNOSTIC_and_PHYTOCHEMICAL_CHARACTERIZATION_OF_IXORA_ALBA_L World J Pharm Res. 2016;5:1015–1022. [Google Scholar]
- 12.In-vitro free radical scavenging activity of Ixora coccinea L. Saha MR, Alam MA, Akter R, Jahangir R. https://www.researchgate.net/publication/26521940_In-vitro_free_radical_scavenging_activity_of_Ixora_coccinea_L#fullTextFileContent Bangladesh J Pharmacol. 2008;15:90–96. [Google Scholar]
- 13.Inductively coupled plasma-mass spectrometry (ICP-MS) and -optical emission spectroscopy (ICP-OES) for determination of essential minerals in closed acid digestates of peanuts (Arachis hypogaea L.) Phan-Thien KY, Wright GC, Lee NA. Food Chem. 2012;1:453–460. [Google Scholar]
- 14.Ixora coccinea Linn.: traditional uses, phytochemistry and pharmacology. Baliga MS, Kurian PJ. Chin J Integr Med. 2012;18:72–79. doi: 10.1007/s11655-011-0881-3. [DOI] [PubMed] [Google Scholar]
- 15.Total antioxidant capacity. Bartosz G. Adv Clin Chem. 2003;37:219–292. doi: 10.1016/s0065-2423(03)37010-6. [DOI] [PubMed] [Google Scholar]
- 16.Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. Saeed N, Khan MR, Shabbir M. BMC Complement Altern Med. 2012;12:221. doi: 10.1186/1472-6882-12-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Study of antimicrobial properties of Catharanthus roseus by Agar well diffusion method. Chaman S, Sharma G, Reshi AK, Shalini Shalini. https://scienztech.org/index.php/irjpas/article/view/498 Int J Pharm Sci Res. 2013;31:65–68. [Google Scholar]
- 18.Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Wiegand I, Hilpert K, Hancock RE. https://www.nature.com/articles/nprot.2007.521. Nat Protoc. 2008;3:163–175. doi: 10.1038/nprot.2007.521. [DOI] [PubMed] [Google Scholar]
- 19.Antioxidant activity and total phenolic content of methanol extracts of Ixora coccinea. Torey A, Sasidharan S, Latha LY, Sudhakaran S, Ramanathan S. Pharm Biol. 2010;48:1119–1123. doi: 10.3109/13880200903490505. [DOI] [PubMed] [Google Scholar]
- 20.A rapid RP-HPTLC densitometry method for simultaneous determination of major flavonoids in important medicinal plants. Bhandari P, Kumar N, Gupta AP, Singh B, Kaul VK. J Sep Sci. 2007;30:2092–2096. doi: 10.1002/jssc.200700066. [DOI] [PubMed] [Google Scholar]
- 21.In-vitro cytotoxicity activity of Solanum nigrum extract against HeLa cell line and Vero cell line. Patel S, Gheewala N, Suthar A, Shah A, Patel SK. https://www.researchgate.net/publication/228492173_In-Vitro_cytotoxicity_activity_of_Solanum_Nigrum_extract_against_Hela_cell_line_and_Vero_cell_line#fullTextFileContent Int J Pharm Pharm Sci. 2009;1:38–46. [Google Scholar]
- 22.Comparative analysis of three leaf extracts of Ixora Coccinea linn. for their protective and anti-oxidant potentials and correlation with analytical data. Shyam P, Suresh PK. https://www.researchgate.net/publication/286465640_Comparative_analysis_of_three_leaf_extracts_of_Ixora_Coccinea_linn_for_their_protective_and_anti-oxidant_potentials_and_correlation_with_analytical_data Int J Pharma Bio Sci. 2013;4:937–949. [Google Scholar]
- 23.Antioxidant and antimicrobial activities of Ixora coccinea root and quantification of phenolic compounds using HPLC. Muhammad H, Qasim M, Ikram A, et al. S Afr J Bot. 2020;135:71–79. [Google Scholar]
- 24.Anti - tumour activity of an ayurvedic oil preparation. Panikar KR, Bhanumathy P, Raghunath PN. https://pubmed.ncbi.nlm.nih.gov/22557556/ Anc Sci Life. 1986;6:107–108. [PMC free article] [PubMed] [Google Scholar]
- 25.Inhibition of chemical carcinogenesis in mice by ixora coccinea flowers. Latha PG, Panikkar KR. Pharm Biol. 2000;38:152–156. doi: 10.1076/1388-0209(200004)3821-1FT152. [DOI] [PubMed] [Google Scholar]
- 26.GC-MS analysis of bioactive compounds present in the petroleum ether, chloroform and methanol extract of Ixora coccinea’s flower and in-vitro cytotoxic activity of methanolic extract against DLA and EAC cancer cell lines. Prabhu V, Poonkodi K, Mini R, Vimala Devi K, Karthigai Priya M. http://ijrar.com/upload_issue/ijrar_issue_20542652.pdf Int J Res Anal Rev. 2018;5:801–807. [Google Scholar]