Abstract
IMPORTANCE
Pulmonary fibrosis (PF) is characterized by progressive scarring of lung tissue and poor survival. Racial and ethnic minority populations face the greatest risk of morbidity and mortality from disparities impacting respiratory health, but the pattern of age at clinically relevant outcomes across diverse racial and ethnic populations with PF is unknown.
OBJECTIVE
To compare the age at PF-related outcomes and the heterogeneity in survival patterns among Hispanic, non-Hispanic Black, and non-Hispanic White participants.
DESIGN, SETTING, AND PARTICIPANTS
This cohort study included adult patients with a PF diagnosis and used data from prospective clinical registries: the Pulmonary Fibrosis Foundation Registry (PFFR) for the primary cohort and registries from 4 geographically distinct tertiary hospitals in the US for the external multicenter validation (EMV) cohort. Patients were followed between January 2003 and April 2021.
EXPOSURES
Race and ethnicity comparisons between Black, Hispanic, and White participants with PF.
MAIN OUTCOMES AND MEASURES
Age and sex distribution of participants were measured at the time of study enrollment. All-cause mortality and age at PF diagnosis, hospitalization, lung transplant, and death were assessed in participants over 14 389 person-years. Differences between racial and ethnic groups were compared using Wilcoxon rank sum tests, Bartlett 1-way analysis of variance, and χ2 tests, and crude mortality rates and rate ratios were assessed across racial and ethnic categories using Cox proportional hazards regression models.
RESULTS
In total, 4792 participants with PF were assessed (mean [SD] age, 66.1 [11.2] years; 2779 [58.0%] male; 488 [10.2%] Black, 319 [6.7%] Hispanic, and 3985 [83.2%] White); 1904 were in the PFFR and 2888 in the EMV cohort. Black patients with PF were consistently younger than White patients (mean [SD] age at baseline, 57.9 [12.0] vs 68.6 [9.6] years; P < .001). Hispanic and White patients were predominantly male (Hispanic: PFFR, 73 of 124 [58.9%] and EMV, 109 of 195 [55.9%]; and White: PFFR, 1090 of 1675 [65.1%] and EMV, 1373 of 2310 [59.4%]), while Black patients were less likely to be male (PFFR, 32 of 105 [30.5%] and EMV, 102 of 383 [26.6%]). Compared with White patients, Black patients had a lower crude mortality rate ratio (0.57 [95% CI, 0.31–0.97), but for Hispanic patients, the mortality rate ratio was similar to that of White patients (0.89; 95% CI, 0.57–1.35). Mean (SD) hospitalization events per person were highest among Black patients compared with Hispanic and White patients (Black: 3.6 [5.0]; Hispanic, 1.8 [1.4]; and White, 1.7 [1.3]; P < .001). Black patients were consistently younger than Hispanic and White patients at first hospitalization (mean [SD] age: Black, 59.4 [11.7] years; Hispanic, 67.5 [9.8] years; and White, 70.0 [9.3] years; P < .001), lung transplant (Black, 58.6 [8.6] years; Hispanic, 60.5 [6.1] years; and White, 66.9 [6.7] years; P < .001), and death (Black, 68.7 [8.4] years; Hispanic, 72.9 [7.6] years; and White, 73.5 [8.7] years; P < .001). These findings remained consistent in the replication cohort and in sensitivity analyses within prespecified deciles of age groups.
CONCLUSIONS AND RELEVANCE
In this cohort study of participants with PF, racial and ethnic disparities, especially among Black patients, were found in PF-related outcomes, including earlier onset of death. Further research is essential to identify and mitigate the underlying responsible factors.
Introduction
Death rates from chronic respiratory diseases have recently increased, largely driven by the rising burden of interstitial lung diseases (ILDs) doubling mortality rates over the past 4 decades.1,2 Pulmonary fibrosis (PF), a form of ILD, is characterized by destruction of lung tissue and accounts for the highest increase in mortality rates.3,4 The disproportionate impact exerted by ILD on PF-related outcomes such as respiratory-related deaths is a function of its epidemiological burden, greater disease severity, and an increasingly aging population, culminating in widespread recognition of ILD as the foremost indication for lung transplant in the US.5,6
Racial and ethnic minority populations face the greatest risk of morbidity and mortality from health disparities and preexisting socioeconomic inequities.7–9 Black patients have high rates of respiratory impairment and more frequent pulmonary involvement with autoimmune disease, are 3 times as likely to die of obstructive lung diseases like asthma, and may have differential survival in ILD when compared with White individuals.10–14 While these disparities impact factors that span the spectrum from diagnosis to the time of death or lung transplant, poor enrollment of racial and ethnic minority individuals in ILD registries and clinical trials has limited our understanding of the interrelationship between health disparities and racial and ethnic differences in outcomes among patients with PF.
As PF is deemed more prevalent in White individuals,15 the age at which clinically relevant outcomes occur in racial and ethnic minority populations is less well understood. We hypothesized that the age at clinically relevant outcomes among patients with PF differs by racial or ethnic category. Therefore, our study sought to evaluate the age at PF-related outcomes (diagnosis, hospitalization, lung transplant, and death) and the heterogeneity in survival patterns among White, Black, and Hispanic participants with PF in a nationally acquired US registry and to validate these findings with data from 4 geographically disparate tertiary care centers with PF expertise.
Methods
Study Setting
This cohort study was conducted using the Pulmonary Fibrosis Foundation (PFF) patient registry as the primary cohort. Independent replication of findings was performed within an external multicenter validation (EMV) cohort of prospective registries from 4 geographically distinct tertiary hospitals. The PFF Registry (PFFR), acquired from the PFF Care Center Network (PFF-CCN), the largest nationwide consortium network of PF centers in the US, is a multicenter clinical registry of patients with ILD that contains data on more than 2000 patients across the US collected since 2016.16 All registry resources used from the primary and replication cohorts contain details on ILD diagnosis, lung function indices, radiographic data, and clinical course for enrolled patients. All patients provided written informed consent at the time of center registry enrollment. This study was approved by the respective institutional review board for each participating center and followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.17
Study Population and Design
We performed a retrospective analysis of prospectively enrolled consenting patients with a multidisciplinary PF diagnosis including idiopathic PF (IPF), connective tissue disease–related ILD (CTD-ILD), fibrotic hypersensitivity pneumonitis (fHP), and unclassifiable or other subtypes such as pulmonary sarcoidosis, pneumoconiosis, and pleuroparenchymal fibroelastosis within the PFFR (March 2016 through February 2020), which was the primary cohort, and 4 tertiary hospital registries, which composed the EMV replication cohort: The University of Chicago, The University of Texas Southwestern Medical Center, University of California San Francisco, and University of California, Davis (January 2003 through April 2021). Given the heterogeneity of PF, our study was designed to assess findings in a replication cohort using currently available clinical data from geographically disparate sources without the constraints of more restrictive criteria within the national PFFR, where patient enrollment was enriched for specific PF subtypes such as IPF. The design of the PFF-CCN and PFFR has been previously published.16,18 Patients in the EMV replication cohort were enrolled in their hospital registries at ILD diagnosis; therefore, all those concurrently enrolled in the PFFR were excluded from the EMV replication cohort to avoid duplications. Data on PF-specific therapy for the University of California, Davis, cohort was unavailable at the time of institutional review board study approval. To compare clinically relevant outcomes among patients with PF, categorization of self-reported race and ethnicity was implemented per federally defined US Census Bureau standards on race (American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or Pacific Islander, and White) and ethnicity (Hispanic or not Hispanic).19 Patients in 1 of 3 predefined racial and ethnic categories—Black (not Hispanic), Hispanic, or White (not Hispanic)—were included in the analysis.
Follow-up and Study Outcomes
Patients entered the study cohorts on the date of registry enrollment, and all patients were followed up until occurrence of death, lung transplant, the end of the study period, or loss to follow-up. Patients were censored if alive without transplant at the end of the study period or when lost to follow-up. Person-time was averaged at 30 days per month from registry enrollment to study end point. We quantified clinically relevant milestones of interest in the natural history of PF, including ILD diagnosis, hospitalizations, lung transplant, and all-cause mortality. We compared age at occurrence of these outcomes across racial and ethnic categories.
Statistical Analysis
We summarized baseline characteristics using descriptive statistics and present these as means with SDs, medians with IQRs, or counts with proportions, as appropriate. The primary aim was to determine if age at PF-related outcomes and survival patterns differed across Black, Hispanic, and White patients with PF. We compared differences in clinical milestones, time to event, and outcomes between racial and ethnic groups using Wilcoxon rank sum tests, the Bartlett 1-way analysis of variance, and χ2 tests, as indicated. We assessed crude mortality rates and rate ratios across racial and ethnic categories. In assessment of age at PF-related outcomes substratified by age group in deciles (<50, 50–59, 60–69, and 70–80 years), the primary and replication cohorts were analyzed separately; then a meta-analysis across both cohorts was performed.
To assess whether racial and ethnic categories were associated with differential transplant-free survival from time of enrollment, Poisson generalized linear models with a logistic regression link were first used to assess mortality incidence rate ratios across Black, Hispanic, and White patients; then we constructed multivariable Cox proportional hazards regression models with robust variances for hazard ratio (HR) estimation. Survival curves were plotted using the Kaplan-Meier survival estimator, and the log-rank test was used for group comparisons. The HRs for death are reported with study participants of White race and ethnicity as the reference group. In all Cox proportional hazards regression analyses, mortality and lung transplant were considered a composite outcome. All multivariable Cox models were adjusted for potential confounders selected a priori. These were known prognostic determinants in PF, such as age, sex, PF subtype, and physiologic indices of disease severity such as forced vital capacity (FVC), diffusing capacity of the lung for carbon monoxide (DLCO), and Gender-Age-Physiology Index (GAP) score (possible range, −2 to 8, with higher scores indicating worse prognosis). Outcome modeling incorporated a random effect to account for heterogeneity across hospitals as well as clustering of racial and ethnic groups within hospitals. Given the study hypothesis that differences across racial and ethnic groups would exist in 4 main temporal domains (time to diagnosis, time to hospitalization, time to lung transplant, and time to death), 2-sided, Bonferroni-corrected P < .05 was considered statistically significant.
In sensitivity analyses, we performed patient stratification by center to assess consistency of study results across sites. Quintile stratification of age groups was performed in deciles and categorized by race and ethnicity, and the Jonckheere-Terpstra nonparametric test was used to assess the trend in age across ordered racial and ethnic groups within the pooled population. Analyses were performed without imputation, as missing covariates were infrequent in our cohort (<5%). Postestimation tests demonstrated goodness of fit for all models. We tested the proportional hazards assumption by examining covariates over time and by regressing Schoenfeld residuals over time in the Cox proportional hazards regression survival models, and all models evaluated passed this test. All analyses were conducted using Stata, version 17 (StataCorp LLC), and code is available upon request.
Results
Patient Characteristics
We identified 5275 patients with a diagnosis of PF from January 2003 through April 2021 within the study population, of whom 4792 patients (90.8%), assessed over 14 389 person-years, met eligibility criteria (mean [SD] age, 66.1 [11.2] years; 488 [10.2%] Black, 319 [6.7%] Hispanic, and 3985 [83.2%] White), including 1904 in the primary cohort and 2888 in the replication cohort (Table 1 and eFigure 1 and eTable 1 in Supplement 1). The mean (SD) age at enrollment of patients into the PFFR was 67.8 (10.1) years, while the mean (SD) age for the EMV replication cohort was 65.0 (11.9) years. Most enrolled study participants were men (overall: 2779 [58.0%] male, 2013 [42.0%] female; PFFR: 1195 [62.8%] male, 709 [37.2%] female; EMV: 1584 [54.8%] male, 1304 [45.2%] female), had previously smoked tobacco, and were overweight. Lung function was moderately impaired in the PFFR cohort, with a mean (SD) percent predicted FVC of 68.5 (18.1), percent predicted DLCO of 42.7 (17.3), and GAP score of 3.4 (2.0). These data were similar to those of the EMV cohort except for a slightly higher mean (SD) percent predicted DLCO of 51.4 (21.6). In both cohorts, the most frequent PF subcategory was IPF, and a substantial minority had received antifibrotic therapy.
Table 1.
Baseline Characteristics of the Study Population, Stratified by Race and Ethnicity
| Characteristic | Patientsa | P value | |||
|---|---|---|---|---|---|
| Black (n = 488) | Hispanic (n = 319) | White (n = 3985) | All (N = 4792) | ||
| PFFR cohort | |||||
| Patients, No. | 105 | 124 | 1675 | 1904 | |
| Age, mean (SD), y | 57.9 (12.0) | 65.4 (10.6) | 68.6 (9.6) | 67.8 (10.1) | <.001 |
| Sex | |||||
| Female | 73 (69.5) | 51 (41.1) | 585 (34.9) | 709 (37.2) | <.001 |
| Male | 32 (30.5) | 73 (58.9) | 1090 (65.1) | 1195 (62.8) | |
| Ever smoker | 47 (44.8) | 70 (56.5) | 1008 (60.2) | 1125 (59.1) | .006 |
| BMI, mean (SD) | 31.0 (7.8) | 29.9 (5.5) | 29.4 (5.7) | 29.5 (5.9) | .02 |
| Lung function measure, mean (SD) | |||||
| FVC, % predicted | 68.2 (21.7) | 60.9 (17.0) | 69.0 (17.8) | 68.5 (18.1) | <.001 |
| DLCO, % predicted | 40.0 (23.0) | 42.5 (14.9) | 42.9 (17.1) | 42.7 (17.3) | .25 |
| GAP scoreb | 1.9 (2.1) | 2.8 (1.9) | 3.6 (1.9) | 3.4 (2.0) | <.001 |
| PF subcategory | |||||
| IPF | 17 (16.2) | 60 (48.4) | 1102 (65.8) | 1179 (61.9) | <.001 |
| CTD-ILD | 57 (54.3) | 27 (21.8) | 227 (13.6) | 311 (16.3) | <.001 |
| fHP | 6 (5.7) | 15 (12.1) | 126 (7.5) | 147 (7.7) | .16 |
| Unclassifiable or other | 25 (23.8) | 22 (17.7) | 220 (13.1) | 267 (14.0) | .004 |
| PF-specific therapy | |||||
| Antifibrotic | 9 (8.6) | 33 (26.6) | 646 (38.6) | 688 (36.1) | <.001 |
| Corticosteroids | 17 (16.2) | 6 (4.8) | 110 (6.6) | 133 (7.0) | .001 |
| None | 462 (94.7) | 280 (87.8) | 3229 (81.0) | 3971 (82.9) | <.001 |
| Supplemental oxygen | 42 (40.0) | 67 (54.0) | 741 (44.2) | 850 (44.6) | .07 |
| EMV cohort | |||||
| Patients, No. | 383 | 195 | 2310 | 2888 | |
| Age, mean (SD), y | 57.3 (13.6) | 61.4 (12.3) | 66.6 (11.0) | 65.0 (11.9) | <.001 |
| Sex | |||||
| Female | 281 (73.4) | 86 (44.1) | 937 (40.6) | 1304 (45.2) | <.001 |
| Male | 102 (26.6) | 109 (55.9) | 1373 (59.4) | 1584 (54.8) | |
| Ever smoker | 168 (44.6) | 82 (42.3) | 1370 (60.1)c | 1620 (56.9) | <.001 |
| BMI, mean (SD) | 30.1 (7.1) | 27.9 (6.1) | 29.1 (6.0) | 29.1 (6.1) | .005 |
| Lung function measure, mean (SD) | |||||
| FVC, % predicted | 59.5 (17.2) | 64.8 (18.7) | 69.0 (19.0) | 67.5 (19.1) | <.001 |
| DLCO, % predicted | 48.9 (22.1) | 49.2 (22.2) | 51.9 (21.5) | 51.4 (21.6) | .03 |
| GAP scoreb | 3.0 (1.5) | 3.0 (1.6) | 3.1 (1.6) | 3.1 (1.6) | .17 |
| PF subcategory | |||||
| IPF | 29 (7.8) | 80 (41.5) | 1034 (45.5) | 1143 (39.6) | <.001 |
| CTD-ILD | 148 (38.6) | 53 (27.2) | 290 (12.6) | 491 (17.0) | <.001 |
| fHP | 23 (6.0) | 28 (14.4) | 321 (13.9) | 372 (12.9) | <.001 |
| Unclassifiable or other | 173 (45.2) | 32 (16.4) | 630 (27.3) | 835 (28.9) | <.001 |
| PF-specific therapyd | |||||
| Antifibrotic | 14 (3.9) | 32 (20.7) | 411 (20.9) | 457 (18.4) | <.001 |
| Corticosteroids | 267 (74.6) | 70 (45.2) | 916 (46.8) | 1253 (50.7) | <.001 |
| None | 102 (26.7) | 93 (47.7) | 983 (42.6) | 1178 (40.8) | <.001 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CTD-ILD, connective tissue disease– associated interstitial lung disease; DLCO, diffusing capacity of the lungs for carbon monoxide; EMV, external multicenter validation; fHP, fibrotic hypersensitivity pneumonitis; FVC, forced vital capacity; GAP, Gender-Age-Physiology Index; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; PF, pulmonary fibrosis; PFFR, Pulmonary Fibrosis Foundation Registry.
Categorical variables are presented as the number (percentage) of patients; continuous variables are presented as the mean (SD).
Score range, −2 to 8, with higher scores indicating worse prognosis.
Exception for participants in the EMV cohort: ever smoker, n = 2849.
Data on the PF-specific therapy were unavailable for the University of California, Davis, subgroup of the EMV cohort.
Differences Across Racial and Ethnic Groups at Study Enrollment
Black patients diagnosed with PF were consistently younger than Hispanic and White patients (mean [SD] age at baseline in the PFFR: Black, 57.9 [12.0] years; Hispanic, 65.4 [10.6] years; White, 68.6 [9.6] years; P < .001; EMV: Black, 57.3 [13.6] years; Hispanic, 61.4 [12.3] years; White, 66.6 [11.0] years; P < .001). Black patients with PF were least likely to be male (PFFR, 32 of 105 [30.5%]; EMV, 102 of 383 [26.6%]) and had the highest mean (SD) body mass index (calculated as weight in kilograms divided by height in meters squared) (PFFR, 31.0 [7.8]; EMV, 30.1 [7.1]). In contrast, Hispanic and White patients were predominantly male (PFFR: Hispanic, 73 of 124 [58.9%]; White, 1090 of 1675 [65.1%]; EMV: Hispanic, 109 of 195 [55.9%]; White, 1373 of 2310 [59.4%]), and White patients had the highest prevalence of tobacco smokers (PFFR, 1008 of 1675 [60.2%]; EMV, 1370 of 2278 [60.1%]). When assessing lung function impairment across racial and ethnic groups, Black patients had the lowest mean (SD) percent predicted DLCO (PFFR: 40.0 [23.0]; EMV, 48.9 [22.1]) and GAP scores (PFFR, 1.9 [2.1]; EMV, 3.0 [1.5]), while the mean (SD) percent predicted FVC was highest in White patients (PFFR, 69.0 [17.8]; EMV, 69.0 [19.0]) and lower in Hispanic patients (PFFR, 60.9 [17.0]; EMV, 64.8 [18.7]).
The PF subcategories and specific therapies varied by racial category in both cohorts. For example, IPF was most prevalent in White patients, whereas fHP was most prevalent in Hispanic patients. Black patients had the highest prevalence of CTD-ILD in the PFFR (Black, 57 of 105 [54.3%]; Hispanic, 27 of 124 [21.8%]; White, 227 of 1675 [13.6%]; P < .001) and the EMV cohort (Black, 148 of 383 [38.6%]; Hispanic, 53 of 195 [27.2%]; and White, 290 of 2310 [12.6%]; P < .001). Further, Black patients had the highest prevalence of unclassifiable or other PF subcategories. The proportion of patients treated with antifibrotic therapy was higher among Hispanic and White patients, while Black patients had the highest proportion of corticosteroid therapy.
Association of Race and Ethnicity With PF Outcomes
The median follow-up time for the PFFR cohort was 2.4 years (IQR, 1.5–3.1 years), during which 397 deaths and 185 lung transplants occurred, while the EMV cohort had a longer median follow-up time at 3.4 years (IQR, 1.4–7.5 years), during which 1042 deaths and 250 lung transplants occurred. A minority of patients in both cohorts were censored at loss to follow-up (PFFR: 223 of 1904 [11.7%] [Black, 8 of 105 (7.6%); Hispanic, 16 of 124 (12.9%); White, 199 of 1675 (11.9%)]; EMV: 452 of 2888 [15.7%] [Black, 55 of 383 (14.4%); Hispanic, 61 of 195 (31.3%); and White, 336 of 2310 (14.5%)]). Black patients had the longest mean survival time and the lowest number of deaths per 100 person-years. When compared with White patients, Black patients consistently had a lower crude mortality rate ratio (PFFR: 0.57; 95% CI, 0.31–0.97; P = .03; EMV: 0.56; 95% CI, 0.46–0.68; P < .001). Conversely, the mortality rate ratio among Hispanic patients (PFFR: 0.89; 95% CI, 0.57–1.35; P = .61; EMV: 0.91; 95% CI, 0.74–1.11; P = .36) was similar to that of White patients (Table 2). Similarly, the unadjusted HR for death was lower in Black patients, but similar in Hispanic patients, when compared with White patients (eTable 2 in Supplement 1). In adjusted Cox proportional hazards regression models, the mortality for all 3 racial and ethnic categories was similar within the PFFR; however, in the EMV cohort, the adjusted mortality HR remained lower in Black patients when compared with White patients.
Table 2.
Association of Racial and Ethnic Categories With Pulmonary Fibrosis Outcomes in the PFFR and EMV Cohorts
| Outcome | Patients | P value | ||
|---|---|---|---|---|
| Black | Hispanic | White | ||
| PFFR cohort | ||||
| Patients, No. | 105 | 124 | 1675 | NA |
| Survival time, mean (95% CI), moa | 33 (32–35) | 32 (30–34) | 31 (31–32) | .09 |
| Deaths, No. (%) | 14 (13.3) | 24 (19.4) | 359 (21.4) | .13 |
| Crude mortality rate, events/100 person-years | 5.4 | 8.4 | 9.4 | <.001 |
| Mortality rate ratio (95% CI) | 0.57 (0.31–0.97) | NA | 1 [Reference] | .03 |
| NA | 0.89 (0.57–1.35) | 1 [Reference] | .61 | |
| Unadjusted HR (95% CI) | 0.65 (0.43–0.98) | NA | 1 [Reference] | .04 |
| NA | 0.98 (0.70–1.36) | 1 [Reference] | .88 | |
| Adjusted HR (95% CI)b | 1.03 (0.67–1.57) | NA | 1 [Reference] | .91 |
| NA | 1.12 (0.80–1.56) | 1 [Reference] | .52 | |
| Participants hospitalized, No. (%) | 36 (34.3) | 50 (40.3) | 575 (34.3) | .40 |
| Hospitalizations per person, mean (SD), No. | 3.6 (5.0) | 1.8 (1.4) | 1.7 (1.3) | <.001 |
| Lung transplant, No. (%) | 9 (8.6) | 14 (11.3) | 162 (9.7) | .77 |
| EMV cohort | ||||
| Patients, No. | 383 | 195 | 2310 | NA |
| Survival time, mean (95% CI), moa | 182 (169–195) | 98 (84–112) | 115 (110–121) | <.001 |
| Deaths, No. (%)c | 89/374 (23.8) | 64/164 (39.0) | 889/2117 (42.0) | <.001 |
| Crude mortality rate, events/100 person-years | 3.5 | 8.5 | 8.6 | <.001 |
| Mortality rate ratio (95% CI) | 0.56 (0.46–0.68) | NA | 1 [Reference] | <.001 |
| NA | 0.91 (0.74–1.11) | 1 [Reference] | .36 | |
| Unadjusted HR (95% CI) | 0.45 (0.37–0.55) | NA | 1 [Reference] | <.001 |
| NA | 1.01 (0.81–1.27) | 1 [Reference] | .91 | |
| Adjusted HR (95% CI)b | 0.68 (0.55–0.84) | NA | 1 [Reference] | <.001 |
| NA | 0.82 (0.65–1.03) | 1 [Reference] | .09 | |
| Participants hospitalized, No. (%)d | 93/183 (50.8) | 27/70 (38.6) | 195/807 (24.2) | <.001 |
| Hospitalizations per person, mean (SD) | 2.6 (2.2) | 1.9 (1.7) | 1.8 (1.4) | <.001 |
| Lung transplant, No. (%)c | 21/374 (5.6) | 18/164 (11.0) | 219/2117 (10.3) | .02 |
Abbreviations: EMV, external multicenter validation; HR, hazard ratio; NA, not applicable; PFFR, Pulmonary Fibrosis Foundation Registry.
Computed from the point of registry enrollment.
Cox proportional hazards regression models adjusted for age, sex, forced vital capacity, diffusing capacity of the lungs for carbon monoxide, interstitial lung disease subtype, and hospital center.
Exception for data availability on lung transplantation and decedents for the EMV cohort; n = 2655.
Exception for data availability on hospitalization for EMV cohort; n = 1094.
Temporal Patterns and Rates of Outcomes Across Racial and Ethnic Groups
Among decedents in the PFFR, the mean (SD) time to death did not differ significantly among Black (18.0 [9.2] months), Hispanic (17.4 [9.2] months), and White participants (15.6 [10.0] months) (P = .48). This was in contrast to the EMV cohort, where the mean (SD) time to death was longest in Black decedents (45.8 [41.2] months; P = .02) but similar between Hispanic and White decedents (37.2 [30.5] months and 35.2 [32.9] months, respectively) (Figure 1A and B). Kaplan-Meier analyses suggested improved survival patterns in Black patients in both the EMV and PFFR cohorts (Figure 1C and D). The proportion of patients who received a lung transplant was lower in Black patients than Hispanic or White patients (PFFR: Black, 9 of 105 [8.6%]; Hispanic, 14 of 124 [11.3%]; White, 162 of 1675 [9.7%]; P = .77; EMV: Black, 21 of 374 [5.6%]; Hispanic, 18 of 164 [11.0%]; and White, 219 of 2117 [10.3%]; P = .02). Although hospitalizations were frequent across patients from all racial and ethnic categories, during the study period Black patients had the highest mean (SD) number of hospitalization events per person, while mean (SD) hospitalizations per person were less frequent in Hispanic and White patients (PFFR: Black, 3.6 [5.0]; Hispanic, 1.8 [1.4]; White, 1.7 [1.3]; P < .001; EMV: Black, 2.6 [2.2]; Hispanic, 1.9 [1.7]; White, 1.8 [1.4]; P < .001).
Figure 1. Survival Patterns Stratified by Racial and Ethnic Category.
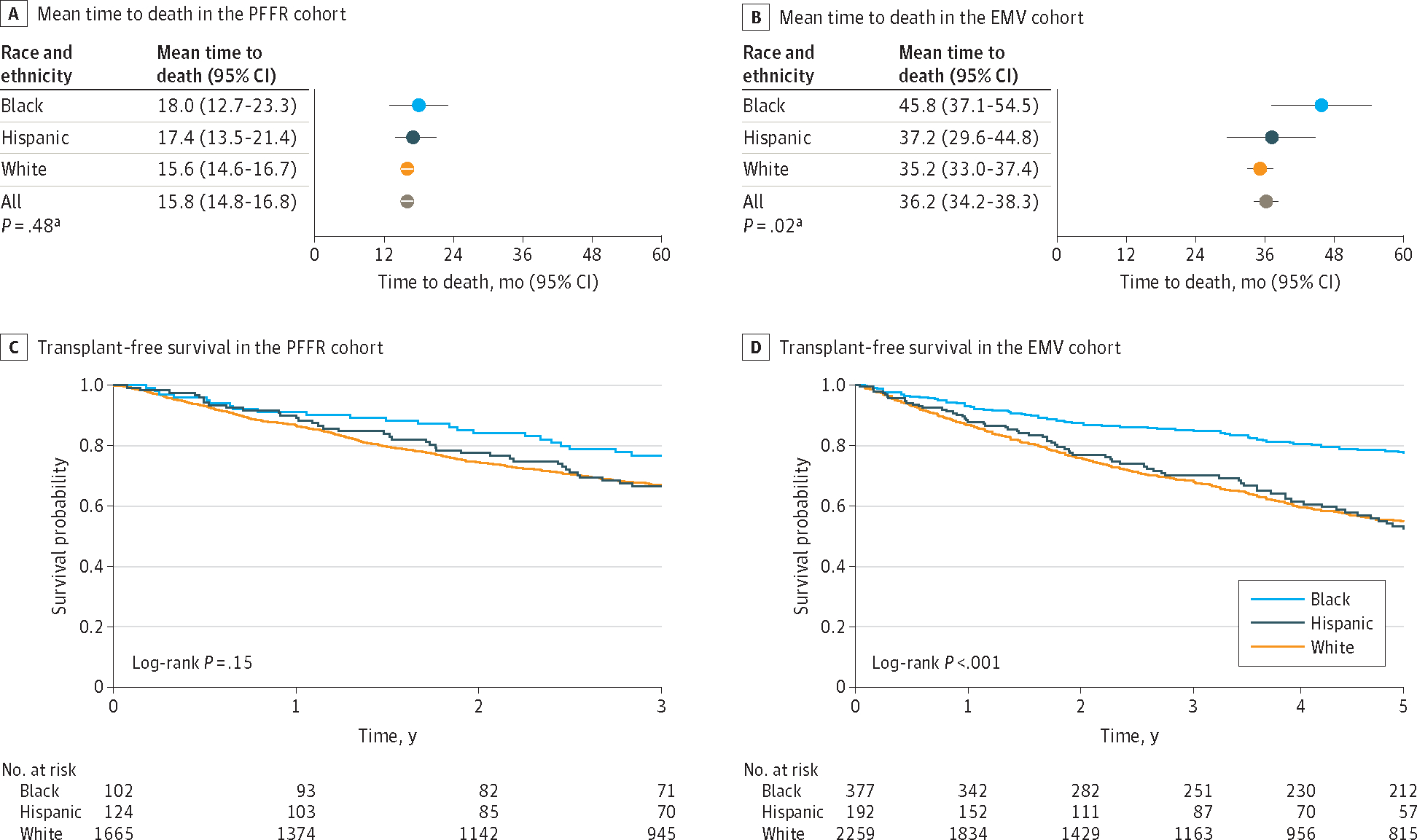
PFFR indicates Pulmonary Fibrosis Foundation Registry; EMV, external multicenter validation.
a P values are for the Bartlett 1-way analysis of variance test for equal variances comparing all 3 racial groups.
Differences in Age at Outcomes Across Racial and Ethnic Groups With PF
Paralleling the disparities in age at study enrollment, Black patients had the youngest mean (SD) age at first hospitalization with PF when compared with Hispanic and White patients in the PFFR (Black, 59.4 [11.7] years; Hispanic, 67.5 [9.8] years; White, 70.0 [9.3] years; P < .001) and the EMV cohort (Black, 59.7 [12.3] years; Hispanic, 64.9 [8.2] years; White, 64.6 [9.3] years; P = .008) (Figure 2A and B and eFigure 2 in Supplement 1). These age differences between Black patients compared with Hispanic or White patients were mostly preserved through the clinical course of disease such that at the time of lung transplant, Black patients who received lung transplants remained youngest in contrast to Hispanic and White patients in the PFFR (mean [SD] age: Black, 58.6 [8.6] years; Hispanic, 60.5 [6.1] years; and White, 66.9 [6.7] years; P < .001) and the EMV cohort (Black, 52.0 [8.5] years; Hispanic, 61.8 [9.6] years; and White, 62.8 [7.1] years; P < .001) (Figure 2C and eFigure 2 in Supplement 1). With disease progression, these age disparities persisted until the time of death such that among the subpopulation of decedents, Black patients were consistently younger compared with Hispanic and White patients in the PFFR (mean [SD] age: Black, 68.7 [8.4] years; Hispanic, 72.9 [7.6] years; and White, 73.5 [8.7] years; P < .001) and the EMV cohort (Black, 65.0 [13.1] years; Hispanic, 68.2 [10.9] years; and White, 71.9 [9.2] years; P < .001) (Figure 2D and eFigure 2 in Supplement 1).
Figure 2. Mean Age at Outcomes Among Study Participants With Pulmonary Fibrosis, Stratified by Race and Ethnicity.
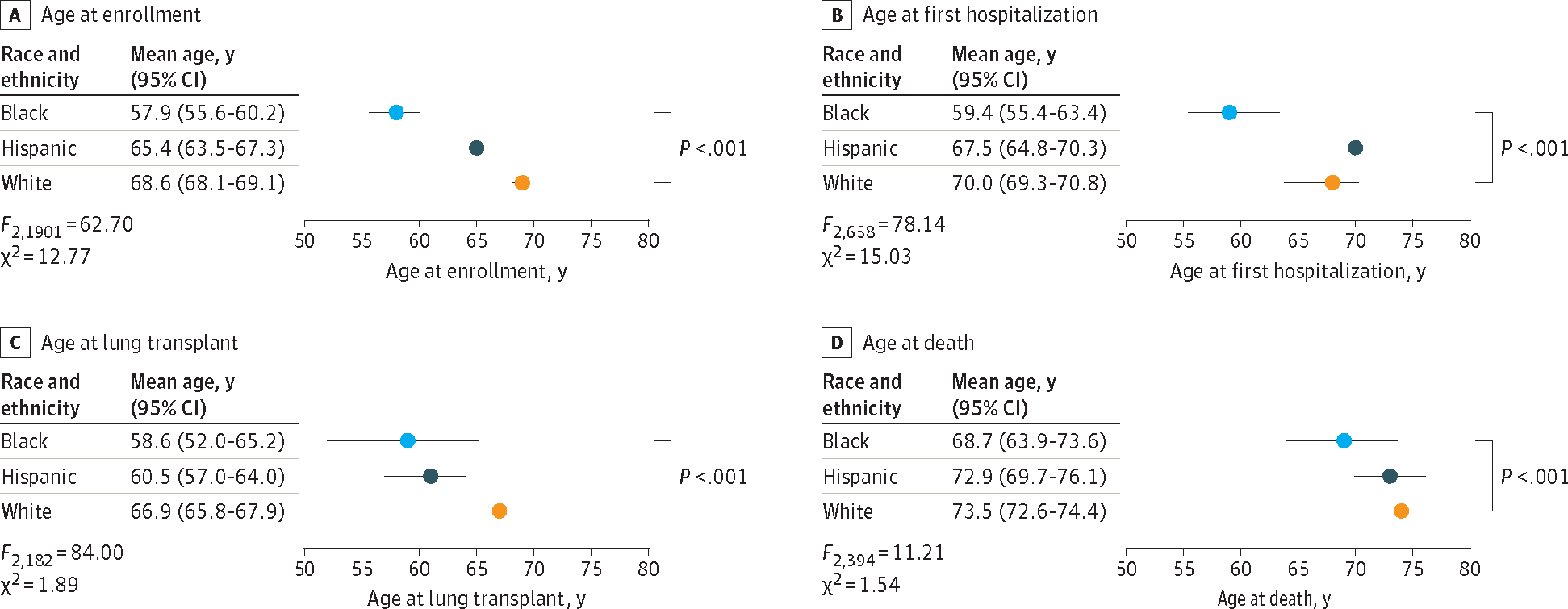
P values are for analysis of variance comparing all 3 racial groups.
In sensitivity analyses, assessment for effect modification by quintile stratification of age groups within racial and ethnic categories showed consistency with aggregated results for all PF outcomes analyzed (Figure 3 and eFigure 3 in Supplement 1). Black patients were consistently younger than White patients within the majority of age group deciles, while Hispanic patients were intermediate in age rankings. These findings remained consistent when the median age at PF outcomes across Black, Hispanic, and White patients was analyzed. Given the greater prevalence of CTD-ILD and lower prevalence of IPF among Black patients in both cohorts, we performed additional sensitivity analyses in which we stratified by IPF and non-IPF and by CTD-ILD and non–CTD-ILD subtypes of PF. In both cohorts, the age at outcomes consistently trended with the aggregated results for all PF outcomes analyzed (eFigures 4 through 8 in Supplement 1).
Figure 3.
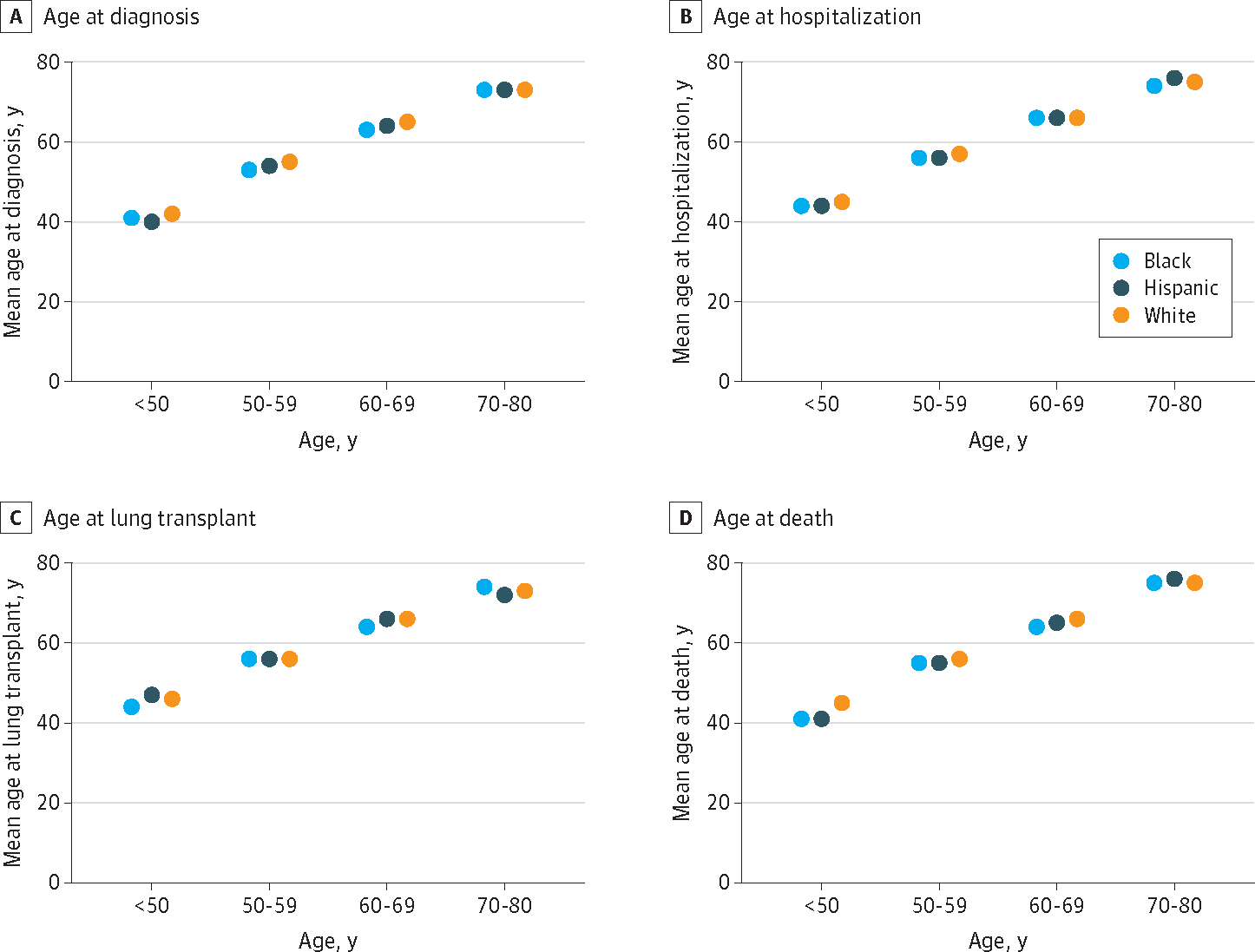
Distribution of Age at Outcomes Among Participants With Pulmonary Fibrosis, Stratified by Race and Ethnicity
Discussion
This study demonstrated substantial racial and ethnic disparities in PF outcomes over the life span of affected racial and ethnic minority individuals. Most notably, Black patients with PF were diagnosed and hospitalized, underwent lung transplant, and died at a younger age than Hispanic and White patients. Mortality rates also appeared to be lower in Black individuals than Hispanic and White individuals. However, any modest gain in life expectancy presumably accrued by Black patients fell short of the age differences, considering that PF diagnosis occurred a decade earlier in Black compared with White patients. The onset of debility attributable to PF generally occurs around middle age, and the median age at PF diagnosis typically occurs between the ages of 60 and 70 years.20 Thus, earlier onset of disease likely exerts considerable consequences on quality of life, hospitalization frequency, and functional capacity of affected persons. Black individuals reportedly often experience a delay in time to disease diagnosis,21,22 raising concern that even the apparent earlier PF diagnosis in Black patients might be delayed. Consistent with current trends in national epidemiological data in which racial and ethnic minority populations have lower waiting list access to lung transplants23 and are more frequently hospitalized,24 our study showed lower lung transplant rates and disproportionately higher hospitalization rates among Black and Hispanic patients with PF compared with White patients, further underscoring the impact of health care disparities on these racial and ethnic minority populations.
Our findings also reveal intriguing demographic patterns across racial and ethnic groups. In lockstep with the male predominance of the overall study population, most White patients with PF were men, contrasting with the female predominance in the Black subpopulation. In tandem with this divergent sex distribution, PF diagnostic subcategories differed in their patterns of distribution across racial and ethnic groups. The predominant diagnosis in White patients was IPF, while fHP was most commonly diagnosed among Hispanic patients and CTD-ILD was 3 to 4 times more common in Black patients compared with White patients. As autoimmune disease is more frequently reported in Black individuals25,26 and pet birds are often present in Hispanic households,27 this raises the possibility that confirmation bias in the medical diagnosis–making process results in the observed diagnostic differences across racial and ethnic groups.28–31 For example, in a study that examined US decedents with IPF within the National Center for Health Statistics database, Black decedents were less likely to be coded with IPF than were White decedents.15 Conversely, Hispanic decedents were more likely to be coded with IPF at the time of death.15 This idiosyncrasy necessitates the use of clinical data from geographically disparate centers with ILD expertise and carefully curated data sets that prioritize diagnostic ascertainment for assessing outcome disparities across racial and ethnic subgroups.
Black race, in particular, has been associated with increased symptomatic burden, frequent hospitalizations, and reduced life expectancy in numerous respiratory diseases.10–13 Unlike Hispanic and White patients, Black patients with PF in the current study had a notable increase in their survival time after diagnosis. These findings were similar to those previously described in a multicenter cohort of patients with various forms of ILD, in which race was demonstrated as an independent factor associated with survival,14 raising the possibility of lead-time bias, as earlier recognition of symptomatic disease could conceivably prompt the institution of medically necessary interventions. However, this potential benefit may have been constrained by previously well-described socioeconomic factors that disproportionately impact racial and ethnic minority populations,32,33 such that any improvement in survival time is insufficient to offset the earlier age at death. Unsurprisingly, the intermediate survival time observed in Hispanic patients may have resulted from their ethnic categorization as distinct from race, since these patients may have been Black Hispanic or White Hispanic patients.
The disparities in age at diagnosis of PF raise several questions that demand answers: What underlying pathophysiologic processes drive the earlier onset of PF in Black patients? Is the greater prevalence of autoimmunity in Black individuals an epiphenomenon, or does it truly drive PF onset? Why is it that earlier disease recognition and intervention do not completely abrogate the age disparities and lead to similar age at terminal end points? Ultimately, there is unquestionably a need for greater enrollment of racial and ethnic minority populations into registries to help us begin to find meaningful answers to these questions. Concerted efforts toward this might comprise broadening enrollment sites to include institutions that provide care to underserved and racial and ethnic minority populations, improving access to high-quality primary and subspecialty pulmonology care, and mitigating factors that perpetuate these racial differences in PF-related outcomes.33
Limitations
Our study has several limitations. First, the PF subcategory of CTD-ILD within the Black cohort was 3 to 4 times larger than that of the White cohort, and idiopathic PF was comparatively less frequent among Black patients compared with White patients. The disproportionately greater incidence of CTD-ILD among Black patients may have influenced the prevalence of hospitalizations and lung transplant. Although our statistical survival models were adjusted for diagnostic subtypes of PF, it is possible that differences in the underlying PF subcategories likely led to the younger age at presentation, at hospitalization, and at death among Black patients. Similarly, varying densities of specific ethnic and racial groups within registries and the greater female prevalence among Black patients may have influenced the crude mean age at outcomes. However, the retrospective nature of this investigation limits our ability to infer causality in any of the relationships assessed. Further, even after substratifying the cohorts by PF subtype, the trend of lower age at outcomes among Black compared with White patients remained consistent. Second, as we were unable to conduct ascertainment of genetic ancestry, we used patients’ self-identified race and ethnicity as documented within the registries. Because race is a complex social construct that frequently reflects an individual’s perception of their familial origin and sociocultural environment, “race and ethnicity” as defined in the context of this study may be a surrogate for several unmeasured confounders. Third, conceivably, there may have been differences between dates of diagnosis and enrollment at a few PFF-CCN centers. However, because PF diagnosis and registry enrollment typically occur contemporaneously for most PFFR sites and diagnosis reascertainment was systematically performed at enrollment, the time of enrollment was uniformly accepted as the time of diagnosis across all study cohorts. Fourth, available follow-up time for the PFFR was constrained to 3 years, potentially limiting our ability to fully assess long-term survival. However, the estimates of our mortality outcomes were highly consistent across the primary and replication cohorts, reinforcing confidence in our results.
Conclusions
The findings of this cohort study suggest that racial disparities in PF may be associated with earlier onset of terminal outcomes among racial and ethnic minority populations, especially Black patients. The disparities in age were pervasive and ran through the natural history of PF from diagnosis through disease progression, culminating in early occurrence of hospitalization, lung transplant, and death among racial and ethnic minority populations. Further research is essential to identify and mitigate the underlying responsible factors.
Supplementary Material
Key Points.
Question
Does the age at onset of pulmonary fibrosis–related outcomes differ among Black, Hispanic, and White adults?
Findings
In this cohort study of 4792 participants with pulmonary fibrosis across the US between January 2003 and April 2021, Black patients were significantly younger than Hispanic and White patients at the time of first hospitalization, lung transplant, and death. The number of hospitalizations was also higher among Black patients vs Hispanic and White patients.
Meaning
These findings suggest that racial and ethnic minority populations may experience disparities in pulmonary fibrosis–related outcomes; policy initiatives and interventions for pulmonary fibrosis should consider factors underlying these disparities.
Funding/Support:
This study was funded by grants K23HL146942, K23HL138190, K23HL148498, and R01HL093096 from the NHBLI, NIH. The PFF Registry is supported by Genentech, Boehringer Ingelheim, United Therapeutics, InterMune, the Gerry and Bill Cowlin Foundation, Mr and Mrs Chuck and Monica McQuaid, the Peter L. O’Neill Memorial Fund, Three Lakes Foundation, and others.
Role of the Funder/Sponsor:
The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: Dr Adegunsoye reported receiving grants from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH) during the conduct of the study; personal fees from Boehringer Ingelheim, Genentech, and Roche Pharmaceuticals outside the submitted work; and grants from the Pulmonary Fibrosis Foundation (PFF) and the American College of Chest Physicians outside the submitted work. Ms White reported receiving payments from the PFF to the University of Michigan to be the data coordinating center for the PFF Registry during the conduct of the study. Dr Kaul reported receiving a career development award from the NHBLI, NIH and grants from the PFF and the US Department of Defense (DOD). Dr Newton reported receiving grants from the NHLBI, NIH during the conduct of the study and personal fees from Boehringer Ingelheim outside the submitted work. Dr Oldham reported receiving grants from the NHLBI, NIH during the conduct of the study; grants from Boehringer Ingelheim outside the submitted work; and personal fees from Boehringer Ingelheim, Genentech, AmMax Bio, Lupin, Novartis, Endeavor Biomedicines, and Gatehouse Bio outside the submitted work. Dr Lee reported receiving grants from the NHLBI, NIH and the PFF during the conduct of the study. Prof Flaherty reported receiving personal fees from Boehringer Ingelheim, Roche/Genentech, Bellerophon, Shionogi, DevPro, AstraZeneca, Pure Health, Horizon, FibroGen, Sun Pharmaceuticals, Pliant, United Therapeutics, Arrowhead, Lupin, Polarean, PureTech, Trevi Therapeutics, CSL Behring, Daewoong, DisperSol, Immumet, NeRRe Therapeutics, Insilco, and Vicore outside the submitted work. Dr Wolters reported receiving grants from Boehringer Ingelheim, Genentech, Roche, and Pliant and personal fees from Blade Therapeutics, Roche, and Boehringer Ingelheim outside the submitted work. Dr C. M. Garcia reported receiving grants from the NIH during the conduct of the study, receiving grants from the DOD and Boehringer Ingelheim to her institution outside the submitted work, and consulting for Rejuvenation Technologies. Dr Strek reported receiving institutional funding for interstitial lung disease research from Boehringer Ingelheim, Galapagos, the NIH, and Novartis; honoraria from Genentech for serving on an adjudication committee; grants from Boehringer Ingelheim and Galapagos; nonfinancial support (medical writing assistance) from Boehringer Ingelheim; and personal fees from Fibrinogen outside the submitted work. No other disclosures were reported.
Data Sharing Statement: See Supplement 2.
Contributor Information
Ayodeji Adegunsoye, Section of Pulmonary and Critical Care Medicine, Department of Medicine, The University of Chicago, Chicago, Illinois.
Elizabeth Freiheit, Department of Biostatistics, School of Public Health, University of Michigan, Ann Arbor.
Emily N. White, Department of Biostatistics, School of Public Health, University of Michigan, Ann Arbor.
Bhavika Kaul, Section of Pulmonary, Critical Care, Allergy and Sleep Medicine, Department of Medicine, University of California San Francisco.
Chad A. Newton, Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, University of Texas Southwestern, Dallas.
Justin M. Oldham, Division of Pulmonary & Critical Care Medicine, Department of Medicine, University of Michigan, Ann Arbor.
Cathryn T. Lee, Section of Pulmonary and Critical Care Medicine, Department of Medicine, The University of Chicago, Chicago, Illinois.
Jonathan Chung, Department of Radiology, The University of Chicago, Chicago, Illinois.
Nicole Garcia, Section of Pulmonary and Critical Care Medicine, Department of Medicine, The University of Chicago, Chicago, Illinois.
Sahand Ghodrati, Division of Pulmonary, Critical Care, and Sleep Medicine, University of California, Davis, Sacramento.
Rekha Vij, Section of Pulmonary and Critical Care Medicine, Department of Medicine, The University of Chicago, Chicago, Illinois.
Renea Jablonski, Section of Pulmonary and Critical Care Medicine, Department of Medicine, The University of Chicago, Chicago, Illinois.
Kevin R. Flaherty, Division of Pulmonary & Critical Care Medicine, Department of Medicine, University of Michigan, Ann Arbor.
Paul J. Wolters, Section of Pulmonary, Critical Care, Allergy and Sleep Medicine, Department of Medicine, University of California San Francisco.
Christine Kim Garcia, Division of Pulmonary, Allergy and Critical Care Medicine, Columbia University, New York, New York.
Mary E. Strek, Section of Pulmonary and Critical Care Medicine, Department of Medicine, The University of Chicago, Chicago, Illinois.
REFERENCES
- 1.Dwyer-Lindgren L, Bertozzi-Villa A, Stubbs RW, et al. Trends and patterns of differences in chronic respiratory disease mortality among US counties, 1980–2014. JAMA. 2017;318(12):1136–1149. doi: 10.1001/jama.2017.11747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wijsenbeek M, Suzuki A, Maher TM. Interstitial lung diseases. Lancet. 2022;400(10354):769–786. doi: 10.1016/S0140-6736(22)01052-2 [DOI] [PubMed] [Google Scholar]
- 3.Wijsenbeek M, Cottin V. Spectrum of fibrotic lung diseases. N Engl J Med. 2020;383(10):958–968. doi: 10.1056/NEJMra2005230 [DOI] [PubMed] [Google Scholar]
- 4.Johannson KA, Chaudhuri N, Adegunsoye A, Wolters PJ. Treatment of fibrotic interstitial lung disease: current approaches and future directions. Lancet. 2021;398(10309):1450–1460. doi: 10.1016/S0140-6736(21)01826–2 [DOI] [PubMed] [Google Scholar]
- 5.Chambers DC, Perch M, Zuckermann A, et al. ; International Society for Heart and Lung Transplantation. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-eighth adult lung transplantation report—2021: focus on recipient characteristics. J Heart Lung Transplant. 2021;40(10):1060–1072. doi: 10.1016/j.healun.2021.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kapnadak SG, Raghu G. Lung transplantation for interstitial lung disease. Eur Respir Rev. 2021;30(161):210017. doi: 10.1183/16000617.0017-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiao D, Lange C, Beaty TH, et al. ; NHLBI GO Exome Sequencing Project; COPDGene Investigators. Exome sequencing analysis in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;193(12):1353–1363. doi: 10.1164/rccm.201506-1223OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yancy CW. COVID-19 and African Americans. JAMA. 2020;323(19):1891–1892. doi: 10.1001/jama.2020.6548 [DOI] [PubMed] [Google Scholar]
- 9.Foreman MG, Zhang L, Murphy J, et al. ; COPDGene Investigators. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene Study. Am J Respir Crit Care Med. 2011;184(4):414–420. doi: 10.1164/rccm.201011-1928OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adegunsoye A, Strek ME. Therapeutic approach to adult fibrotic lung diseases. Chest. 2016;150(6):1371–1386. doi: 10.1016/j.chest.2016.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaz Fragoso CA, McAvay G, Gill TM, Concato J, Quanjer PH, Van Ness PH. Ethnic differences in respiratory impairment. Thorax. 2014;69(1):55–62. doi: 10.1136/thoraxjnl-2013-203631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke CR, Erickson SE, Eisner MD, Martin GS. Trends in the incidence of noncardiogenic acute respiratory failure: the role of race. Crit Care Med. 2012;40(5):1532–1538. doi: 10.1097/CCM.0b013e31824518f2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heron M, Hoyert DL, Murphy SL, Xu J, Kochanek KD, Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57(14):1–134. [PubMed] [Google Scholar]
- 14.Adegunsoye A, Oldham JM, Bellam SK, et al. African-American race and mortality in interstitial lung disease: a multicentre propensity-matched analysis. Eur Respir J. 2018;51(6):1800255. doi: 10.1183/13993003.00255-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swigris JJ, Olson AL, Huie TJ, et al. Ethnic and racial differences in the presence of idiopathic pulmonary fibrosis at death. Respir Med. 2012;106(4):588–593. doi: 10.1016/j.rmed.2012.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang BR, Edwards R, Freiheit EA, et al. The Pulmonary Fibrosis Foundation Patient Registry: rationale, design, and methods. Ann Am Thorac Soc. 2020;17(12):1620–1628. doi: 10.1513/AnnalsATS.202001-035SD [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–1457. doi: 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 18.Adegunsoye AO, Moore J, Green E, Strek ME; Pulmonary Fibrosis Foundation. Racial differences in age at diagnosis and mortality in the Pulmonary Fibrosis Foundation Registry. Am J Respir Crit Care Med. 2019;199:A5841. doi: 10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A5841 [DOI] [Google Scholar]
- 19.Arias E, Heron M, Xu J. United States life tables, 2014. Natl Vital Stat Rep. 2017;66(4):1–64. [PubMed] [Google Scholar]
- 20.Adegunsoye A, Oldham JM, Bellam SK, et al. Computed tomography honeycombing identifies a progressive fibrotic phenotype with increased mortality across diverse interstitial lung diseases. Ann Am Thorac Soc. 2019;16(5):580–588. doi: 10.1513/AnnalsATS.201807-443OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cai C, Gaffney A, McGregor A, et al. Racial and ethnic disparities in outpatient visit rates across 29 specialties. JAMA Intern Med. 2021;181(11):1525–1527. doi: 10.1001/jamainternmed.2021.3771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pritchard D, Adegunsoye A, Lafond E, et al. Diagnostic test interpretation and referral delay in patients with interstitial lung disease. Respir Res. 2019;20(1):253. doi: 10.1186/s12931-019-1228-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mooney JJ, Hedlin H, Mohabir P, Bhattacharya J, Dhillon GS. Racial and ethnic disparities in lung transplant listing and waitlist outcomes. J Heart Lung Transplant. 2018;37(3):394–400. doi: 10.1016/j.healun.2017.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bime C, Poongkunran C, Borgstrom M, et al. Racial differences in mortality from severe acute respiratory failure in the United States, 2008–2012. Ann Am Thorac Soc. 2016;13(12):2184–2189. doi: 10.1513/AnnalsATS.201605-359OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts MH, Erdei E. Comparative United States autoimmune disease rates for 2010–2016 by sex, geographic region, and race. Autoimmun Rev. 2020;19(1):102423. doi: 10.1016/j.autrev.2019.102423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lewis MJ, Jawad AS. The effect of ethnicity and genetic ancestry on the epidemiology, clinical features and outcome of systemic lupus erythematosus. Rheumatology (Oxford). 2017;56(suppl 1):i67–i77. [DOI] [PubMed] [Google Scholar]
- 27.Roldán-Clarà B, Toledo VM, Espejel I. The use of birds as pets in Mexico. J Ethnobiol Ethnomed. 2017;13(1):35. doi: 10.1186/s13002-017-0161-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elston DM. Confirmation bias in medical decision-making. J Am Acad Dermatol. 2020;82(3):572. doi: 10.1016/j.jaad.2019.06.1286 [DOI] [PubMed] [Google Scholar]
- 29.Pines JM. Profiles in patient safety: confirmation bias in emergency medicine. Acad Emerg Med. 2006;13(1):90–94. doi: 10.1111/j.1553-2712.2006.tb00990.x [DOI] [PubMed] [Google Scholar]
- 30.Marinescu DC, Raghu G, Remy-Jardin M, et al. Integration and application of clinical practice guidelines for the diagnosis of idiopathic pulmonary fibrosis and fibrotic hypersensitivity pneumonitis. Chest. 2022;162(3):614–629. doi: 10.1016/j.chest.2022.06.013 [DOI] [PubMed] [Google Scholar]
- 31.Jalbert AC, Siafa L, Ramanakumar AV, Assayag D. Gender and racial equity in clinical research for idiopathic pulmonary fibrosis: a systematic review and meta-analysis. Eur Respir J. 2022;59(3):2102969. doi: 10.1183/13993003.02969-2021 [DOI] [PubMed] [Google Scholar]
- 32.Wallace J, Jiang K, Goldsmith-Pinkham P, Song Z. Changes in racial and ethnic disparities in access to care and health among US adults at age 65 years. JAMA Intern Med. 2021;181(9):1207–1215. doi: 10.1001/jamainternmed.2021.3922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adegunsoye A, Vela M, Saunders M. Racial disparities in pulmonary fibrosis and the impact on the Black population. Arch Bronconeumol. 2022;58(8):590–592. doi: 10.1016/j.arbres.2021.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


