Abstract
INTRODUCTION
Clinical research in Alzheimer's disease (AD) lacks cohort diversity despite being a global health crisis. The Asian Cohort for Alzheimer's Disease (ACAD) was formed to address underrepresentation of Asians in research, and limited understanding of how genetics and non‐genetic/lifestyle factors impact this multi‐ethnic population.
METHODS
The ACAD started fully recruiting in October 2021 with one central coordination site, eight recruitment sites, and two analysis sites. We developed a comprehensive study protocol for outreach and recruitment, an extensive data collection packet, and a centralized data management system, in English, Chinese, Korean, and Vietnamese.
RESULTS
ACAD has recruited 606 participants with an additional 900 expressing interest in enrollment since program inception.
DISCUSSION
ACAD's traction indicates the feasibility of recruiting Asians for clinical research to enhance understanding of AD risk factors. ACAD will recruit > 5000 participants to identify genetic and non‐genetic/lifestyle AD risk factors, establish blood biomarker levels for AD diagnosis, and facilitate clinical trial readiness.
HIGHLIGHTS
The Asian Cohort for Alzheimer's Disease (ACAD) promotes awareness of under‐investment in clinical research for Asians.
We are recruiting Asian Americans and Canadians for novel insights into Alzheimer's disease.
We describe culturally appropriate recruitment strategies and data collection protocol.
ACAD addresses challenges of recruitment from heterogeneous Asian subcommunities.
We aim to implement a successful recruitment program that enrolls across three Asian subcommunities.
Keywords: Alzheimer's disease, Asian, biosamples, community‐based participatory research, dementia, environmental, genetics
1. INTRODUCTION
Underrepresentation of Asian American and Canadian (ASAC), African American, and Hispanic cohorts in Alzheimer's disease (AD) research is limiting our understanding of AD risk factors. AD, a global public health crisis with only symptomatic treatment options widely available (though recently disease‐modifying therapies have garnered US Food and Drug Administration approval), affects > 6 million people in the United States annually 1 , 2 , 3 and 32 million worldwide. 4
Racial minority populations in the United States and Canada are growing at a faster rate than non‐Hispanic Whites (NHW). According to the 2020 census, there were 23.7 million people of Asian descent in the United States, 5 the largest subgroups being Chinese, Indian, Filipino, Vietnamese, and Korean. Asians have increased from 0.5% of the population in 1970 to 5.6% in 2010 and will constitute the largest non‐European ancestry group by 2055, 6 a status already achieved in Canada. Similarly, the 2021 Canadian Census noted Asians represent 19.3% of Canadians, 7 with 62% of all immigrants entering from Asia between 2016 and 2021. 8 The number of ASACs ≥ 65 years old is projected to increase by 352% (21% of total ASAC population) by 2060, 9 underscoring the health and economic impacts of AD on these large sectors of aging populations in these two countries.
Despite their growing numbers, ASAC populations are markedly underrepresented in clinical research. Between 1992 and 2018, only 0.17% of the National Institutes of Health budget was dedicated to studying Asian Americans, Native Hawaiians, and Pacific Islanders. 10 Although the National Plan to Address Alzheimer's Disease introduced in 2012 set goals to diversify ethnic populations in research, 11 , 12 investment and resources for AD research in Asian Americans remain insufficient. For example, 2.6% of participants in the National Alzheimer's Coordinating Center (NACC) Uniform Data Set (UDS) have Asian ancestry, significantly less than African Americans (12.7%) and Hispanics (8%) and undersampled by half relative to the > 65 years old population. Similarly, in the Alzheimer's Disease Genetics Consortium (ADGC), the largest collaborative genetic study in the United States, only 385 are Asian American, representing 0.72% of all ADGC US samples, an undersampling of 1 in 7 (Table 1). Furthermore, ASAC participants in AD and related dementias (ADRD) interventional trials is minimal (< 2%). 13 , 14
TABLE 1.
Metrics to illustrate under‐sampling of Asian Americans > 65 years old in major Alzheimer's disease studies (NACC and US participants in ADGC) in the United States relative to others in the population.
| Population |
% US subpopulation |
% NACC | NACC relative difference to subpopulation * | % ADGC (US) | ADGC (US) relative difference to subpopulation * |
|---|---|---|---|---|---|
| Non‐Hispanic Whites | 75.80% | 76.62% | 1.01 | 70.01% | 0.93 |
| African Americans | 9.00% | 12.74% | 1.42 | 12.60% | 1.40 |
| Hispanic Americans | 9.00% | 8.05% | 0.89 | 16.67% | 1.85 |
| Asian Americans | 5.00% | 2.60% | 0.52 | 0.72% | 0.14 |
Abbreviations:ADGC, Alzheimer's Disease Genetic Consortium; NACC, National Alzheimer's Coordinating Center.
Fold difference in representation is noted below, where < 1 represents under‐representation in cohorts.
In addition to differences in AD risk, genetic loci effects and linkages vary across populations. 15 For example, the effect and incidence of the well‐known APOE ɛ4 allele 16 , 17 , 18 vary across racial/ethnic groups, with lifetime odds ratios (ORs) for ɛ4 homozygotes being 25, 14, and 8 for East Asians, NHW, and African Americans, respectively. 19 The increased effect size in East Asians may be due to the presence of a genetic modifier near the APOE gene. 19 Deeper sequencing of the genomic background upon which APOE occurs is warranted. Another example is SORL1, previously identified as a risk gene in NHW and confirmed in East Asians. 16 , 20 Greater inclusion of African American and Hispanic participants in genetic studies has improved understanding of locus heterogeneity in AD, but inclusion of East Asians (especially in North America) continues to lag.
Meanwhile, strong evidence suggests that sociocultural, lifestyle, and other environmental factors are associated with AD. 21 , 22 , 23 There is emerging evidence that dementia risk factor profiles vary across populations with distinct sociocultural features. 24 With differences in lifestyle and environmental exposures, ASACs may have unique AD risk profiles compared to Asians in Asia.
To address this major shortcoming, the Asian Cohort for Alzheimer's Disease (ACAD) was established to investigate genetic and non‐genetic AD risk factors among selected ASACs and to address the underrepresentation in AD research (Figure 1). ACAD initially focused on Chinese, Korean, and Vietnamese populations because of the co‐investigators’ existing connections and familiarities with these communities, and we hope to extend to the entire ASAC community in the future leveraging our experience in this pilot phase. ACAD will conduct a comprehensive survey of genetic and environmental risk factors in a longitudinal observational study to aid our understanding of the complexity and heterogeneity of AD, contributing insights into factors impacting AD, clinical trial readiness, diagnosis, and care.
FIGURE 1.
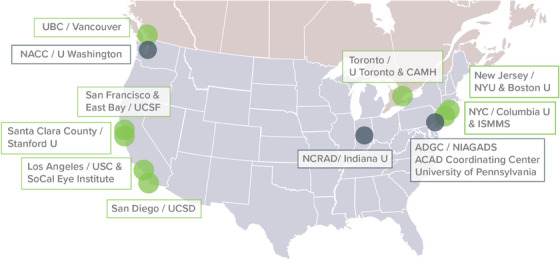
ACAD participating sites are listed on the map of the United States and the southern portion of Canada. Note: Recruiting sites in R56 phase (NYU, Columbia, UCSF, USC, SoCal Eye Institute, UCSD, UBC, UToronto/CAMH) and new recruiting sites (ISMMS, Stanford) are noted in green boxes, while coordinating sites (ACAD Coordinating Center, NCRAD, and NACC) are found in black boxes. NYU recruited Korean ancestry and UCSD recruited Vietnamese ancestry while the remaining sites recruited Chinese ancestry. The NYU lead investigator was previously affiliated with University of Massachusetts Boston when then study launched and then moved to NYU. ACAD, Asian Cohort for Alzheimer's Disease: ADGC, Alzheimer's Disease Genetic Consortium; CAMH, The Centre for Addiction and Mental Health; Indiana U, Indiana University; ISMMS, Icahn School of Medicine at Mount Sinai; NACC, National Alzheimer's Coordinating Center; NIAGADS, National Institute on Aging Genetics of Alzheimer's Disease Data Storage Site; NCRAD, National Centralized Repository for Alzheimer's Disease and Related Dementias; NYC, New York City; NYU, New York University; SoCal Eye Institute, Southern California Eye Institute; U, University; UBC, University of British Columbia; UCSD, University of California San Diego; UCSF, University of California San Francisco; USC, University of Southern California
2. METHODS
2.1. Overview
The long‐term goal of ACAD is to enroll 5081 participants (3893 Chinese, 795 Korean, and 393 Vietnamese Americans and Canadians), with one case for every two controls (≈1694 AD cases and 3387 controls) into an observational study. ACAD was initially designed to address the underrepresentation of Asians in AD research and to identify genetic risks for AD in this cohort. The study has since expanded to consider non‐genetic factors and biosample analysis, starting with recruitment at eight sites and two new sites being added (Figure 1). Given the lack of ASAC inclusion in AD research and the diverse spoken languages in the cohort (English, Mandarin, Cantonese, Korean, and Vietnamese), a comprehensive recruitment strategy was needed to meet our goals: each site shared common approaches but also used different approaches to reach their communities. For example, one site discussed using a community‐based geographical information system for recruitment of older Asian Americans. 25
RESEARCH IN CONTEXT
Systematic review: Asians have been underrepresented in Alzheimer's disease (AD) research. The genetic diversity, unique cultural backgrounds, and gene–environment interactions of Asians could provide novel insights on AD and promote clinical trial readiness.
Interpretation: To address the under‐investment of Asian populations in AD research, we launched the Asian Cohort for Alzheimer's Disease (ACAD), the first large‐scale genetic‐epidemiology AD study focusing on Asians in the United States and Canada. We have established culturally appropriate recruitment strategies and data collection protocol. We demonstrated feasibility to recruit and collect clinical data and biosamples, especially blood samples. This strategy may be of value for studies applying community recruitment, especially in minority populations.
Future directions: The success in ACAD's pilot phase lends strength to expand the research engagement, increasing recruitment numbers in the focused subpopulations and expanding the cohort to other Asian subpopulations. Data collected will support research projects on genetic and non‐genetic risk factors of AD for multi‐ethnic populations.
ACAD is composed of a multidisciplinary team of researchers, clinicians, and community partners with extensive collaborative history, experience, and leadership in AD research, including large collaborative genetics studies and Asian community outreach. Given that Asian American and Canadian inclusion in clinical research, including AD, is very limited, community‐based participatory research (CBPR) principles are being applied in outreach and recruitment with existing and new community partners. ACAD's approach addresses recruitment barriers, including language, lack of culturally and linguistically appropriate outreach, and identification of validated instruments to establish a harmonized data packet.
After a year of study design and protocol development, ACAD was awarded a National Institute on Aging (NIA) R56 High Priority Project award with a single institutional review board across US sites and corresponding research ethics board approval in Canada, and a centralized data entry portal for harmonized data collection by multilingual staff across all sites.
2.2. Outreach
CBPR principles were , applied with close coordination with sites, community partners, and the ACAD Community Advisory Board (CAB), who were bilingual and bicultural community leaders serving the ACAD study populations and recommended by ACAD, to facilitate recruitment. CBPR is crucial at all phases of research including recruitment, resource sharing, study design, and data interpretation. This is an ongoing, collaborative process between communities and researchers that recognizes respective strengths and assets and maintains sustainable collaborations. 25 , 26 , 27 , 28 CBPR approaches have been previously used in ADRD research recruitment 29 , 30 and ASAC research in other health conditions, 31 and have helped improve trial/research awareness and reduce language barriers in underrepresented populations—two substantial barriers for ASAC participation. 32
ACAD‐wide outreach materials (e.g., study flyer, PowerPoint slides, animated videos, and e‐cards) were developed by the Outreach and Education Workgroup. ACAD CAB members were either directly or indirectly involved in the development of the outreach materials that were made available to all sites to use “as‐is” and/or adapt for their local recruitment needs (e.g., site‐specific information). ACAD maintains a bilingual website (English and Chinese, with translations to Korean and Vietnamese in development). We developed multiple site‐specific recruitment strategies including partnering with community‐based groups/centers, health clinics/hospitals/providers, nursing homes, faith‐based institutions, Alzheimer's Association chapters, social/ethnic media, and participant referral. Support from the Collaborative Approach for Asian Americans and Pacific Islanders Research and Education (CARE) project 33 also helped recruit ACAD participants. ACAD was able to leverage these partnerships thanks to previous collaborations in existing research studies. ACAD sites and the Outreach and Education Workgroup monitor enrollment and proactively partner with community‐based organizations to conduct outreach and recruitment activities. Given that ACAD was launched during the COVID‐19 pandemic, early outreach activities were exclusively online. As restrictions were lifted, we are conducting more in‐person outreach and recruitment at health fairs and cultural events.
TABLE 2.
Items included in ACAD data collection packet.
| Sections | # of questions | Initial visit | Follow‐up |
|---|---|---|---|
| Demographics b | 15 | X | |
| Diet—Mediterranean diet adherence screener | 14 | X | X |
| Clinical Dementia Rating a | 6 | X | X |
| Early life enrichment | 53 | X | |
| Medical conditions/medications a | 21 | X | X |
| Geriatric Depression Scale a | 15 | X | X |
| Functional Assessment Scale a | 10 | X | X |
| Common objects memory test: 5 minute delayed recall and 5 minute delayed recognition | 5 | X | X |
| Cognitive Abilities Screening Instrument | 33 | X | X |
| Modified Mini‐Mental State Test | 15 | X | X |
| Additional cognitive tests (Clock Drawing, Category Fluency) | 3 | X | X |
| Neurological examination a | 4 | X | X |
| Family history of dementia b | 2 | X | X |
| Clinician's judgment of symptoms a | 16 | X | X |
| Imaging data | 3 | X | X |
| Neurological diagnosis a | 8 | X | X |
| Factors affecting testing | 2 | X | X |
| Consensus meeting worksheet | — | X | X |
Abbreviations: ACAD, Asian Cohort for Alzheimer's Disease; NACC, National Alzheimer's Coordinating Center; UDS, Uniform Data Set; WHICAP, Washington Heights/Inwood Columbia Aging Project.
Sections used in the NACC UDS initial visit packet.
Sections used in WHICAP. Korean participants responded to the Modified Mini‐Mental State Test instead of the Cognitive Abilities Screening Test.
2.3. Translation
English‐language materials were translated into the major languages used in ACAD—Chinese (written–simplified and traditional; spoken—Mandarin and Cantonese), Korean, and Vietnamese, using a multi‐step translation process guided by the World Health Organization's process of forward and reverse translation, consensus verification, and reconciliation. 34 This translation process was implemented by a team of multilingual faculty, clinicians, staff, and community leaders (including CAB members) who have extensive practical translation experience. These translations are used to reflect both literal and sociocultural accuracy across languages.
2.4. Application of best practices and data collection packet harmonization
ACAD adopted relevant materials from the Washington Heights/Inwood Columbia Aging Project (WHICAP) 35 as WHICAP's community‐based research extended to multiple racial and ethnic groups, using multilingual infrastructure and multi‐site data collection. For further multi‐site harmonization and to make data accessible for collaborative studies, ACAD data collection is aligned with NACC Uniform Dataset (UDS), 36 which captures demographics, medical history, cognitive assessments, daily living activities, and depressive symptoms.
ACAD also surveys additional relevant demographics such as educational equivalents, immigration history, and Rush Early Life Enrichment Inventory 37 data to capture physical, cultural, and cognitive activity items at epochs of participants’ lives during childhood, adolescence, and mid‐life. We adapted the Mediterranean Diet Assessment Scale (MEDAS) 38 , 39 items to apply to Asian cultures. A complete listing of the ACAD data collection packet (DCP) is provided in Table 2. Biosamples (blood for DNA and plasma or saliva for DNA) were also collected for analysis against non‐genetic and lived experience data.
Data entry operations are coordinated by the Data Management Workgroup, which collects DCP information in multiple languages in a centralized database powered by Research Electronic Data Capture (REDCap), 40 , 41 which is expandable for future multi‐institute collaborations.
3. RESULTS
3.1. Current progress in recruitment
After materials development and regulatory approval, the first participant was enrolled in late July 2021. By October 2021 more than half of the eight sites had enrolled their first participant and by December 2022, 1580 people had expressed interest in ACAD, 606 had enrolled in the study, and 560 had completed the study (Figure 2). ACAD participant demographics are provided in Table 3, including information on ethnicity, sex, age, and education attainment and national average for comparison. 42 Notably, we demonstrated the ability to recruit across all groups, ages, and education attainments, with approximately two thirds of participants being women, aligned with relative proportions seen in AD. To address COVID‐19 pandemic challenges, a virtual strategy for recruitment and assessment was developed.
FIGURE 2.
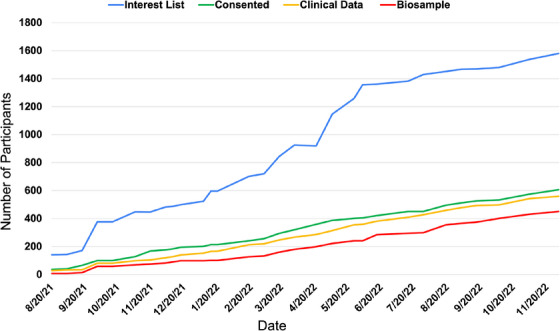
Recruitment statistics for ACAD. Note: After the development of ACAD data collection materials, recruitment began in August 2021, with all sites actively recruiting by October 2021. As of December 1, 2022, a total of 1580 individuals have indicated interest (blue line) and we have seen a steady incline of consented participants (green line) and participants completing the clinical package (Part C; yellow). Biosample (either blood or saliva; red) collections are usually completed at the end of their participation and lag Part C completion. ACAD, Asian Cohort for Alzheimer's Disease
TABLE 3.
Participant demographic characteristics as of December 2022.
| Chinese | Korean | Vietnamese | Total | 2021 ACS | |||||
|---|---|---|---|---|---|---|---|---|---|
| N | (%) | N | (%) | N | (%) | N | (%) | (%) | |
| Participants | 448 | 54 | 25 | 527 | |||||
| Sex | |||||||||
| Male | 149 | (33.3) | 18 | (33.3) | 11 | (44.0) | 178 | (33.8) | (44) |
| Female | 299 | (66.7) | 38 | (66.7) | 14 | (56.0) | 349 | (66.2) | (56) |
| Age range, year | |||||||||
| 60–69 | 182 | (40.6) | 8 | (14.8) | 11 | (44.0) | 201 | (38.1) | (52.9) |
| 70–79 | 173 | (38.6) | 22 | (40.7) | 7 | (28.0) | 202 | (38.3) | (32.1) |
| 80–89 | 80 | (17.9) | 19 | (35.2) | 5 | (20.0) | 104 | (19.7) | (12.2) |
| 90 and older | 13 | (2.9) | 5 | (9.3) | 2 | (8.0) | 20 | (3.8) | (2.8) |
| Education | |||||||||
| Grade school or less | 36 | (8.0) | 9 | (16.7) | 3 | (12.0) | 48 | (9.1) | (15.8) |
| Some high school | 35 | (7.8) | 2 | (3.7) | 3 | (12.0) | 40 | (7.6) | (3.6) |
| High school/GED | 81 | (18.1) | 9 | (16.7) | 9 | (36.0) | 99 | (18.8) | (19.4) |
| Some college or technical school | 80 | (17.9) | 9 | (16.7) | 2 | (8.0) | 91 | (17.3) | (19.2) |
| College degree | 150 | (33.5) | 24 | (44.4) | 6 | (24.0) | 180 | (34.2) | (24.2) |
| Graduate or professional degree | 66 | (14.7) | 1 | (1.9) | 2 | (8.0) | 48 | (13.1) | (15.6) |
Note: A total of 560 participants completed data collection while recruiting sites completed data submission to REDCap of 527 out of 560 (94.1%) participants. To provide the national average as the benchmark, we report the 2021 ACS 1‐year estimate, Asian American of age 60 to 99 (top‐coded) sex, age, and educational attainment. The Asian American in 2021 ACS was defined as “Asian alone or in combination with one or more other races,” which is more inclusive than of only our three intended populations.
Abbreviations: ACS, American Community Survey; GED, General Educational Development test; REDCap, Research Electronic Data Capture.
The cohort consists of women and men of Chinese/Korean/Vietnamese ancestry, ≥ 60 years with or without dementia based on Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐V) 43 criteria. (See Table 4 for inclusion/exclusion criteria.) Informants assisted cognitively impaired participants in data collection. Signed informed consents were obtained from participants or their legally authorized representatives. Figure 2 illustrates the process from outreach to consent and participation, and data quality assurance (QA).
TABLE 4.
Inclusion and exclusion criteria for ACAD pilot participants.
| Criteria | Inclusion | Exclusion |
|---|---|---|
| Ancestry | Chinese/Korean/Vietnamese ancestry | |
| Language | Fluent in English, Chinese (Mandarin or Cantonese), Korean, or Vietnamese | |
| Demographics | 60 years old or older | |
| Diagnosis | Cognitively healthy or diagnosis of AD‐type dementia | Neuropsychiatric diagnoses that would indicate non‐neurodegenerative causes of cognitive impairment |
| Other | If participant has significant cognitive impairment, study partner is available to participate | |
| Able to provide a biosample (saliva or blood) |
Abbreviations: ACAD, Asian Cohort for Alzheimer's Disease; AD, Alzheimer's disease.
3.2. ACAD recruitment
In the R56 pilot phase, ACAD focused on recruitment of Chinese Americans and Canadians to demonstrate feasibility of the overall study design. Additionally, a smaller scale effort to recruit Korean and Vietnamese participants helped validate the recruitment protocol and data collection instruments. To show appreciation for ACAD participants’ time and effort, the honorarium provided was up to $100, with variation due to site‐specific standard operating procedures. An additional $25 honorarium was given when a study partner assisted with data collection. As capacity and success in recruitment of Chinese, Korean, and Vietnamese participants builds, we will use a similar strategy of local sociocultural programs to recruit other Asian subpopulations (e.g., Filipino, Japanese, Asian Indian) to ACAD. Eight recruitment sites launched at the commencement of ACAD. Two additional sites will join ACAD in the future. Figure 1 shows coordinating sites and all institutional collaborators.
3.3. Establishing workgroups for assuring rigor
Outreach and Education, Clinical, Data Management (DM), Biosample, and Training QA (TQA) workgroups were created to help streamline study management and ensure rigor. The DM Workgroup implemented the DCP and other outreach forms for entry into REDCap in four languages (English/Chinese/Korean/Vietnamese) and developed the Data Management Plan and the Data Entry Guidance. A controlled access to REDCap was established and allowed site personnel to only view or edit site‐specific data. The TQA Workgroup developed a training curriculum for pre‐screening, human subject protection and consenting, administration of clinical tests to ensure cross‐site compliance, and procedures for sample collection/data entry. Recruiting site staff onboarded by the TQA were trained through orientation sessions, instructive documents, and mock videos of neuropsychological testing and neurological examinations to ensure inter‐site reliability. To protect data integrity and improve data quality, DM and TQA workgroups collaborated to design and implement protocols of data QA with continued monitoring by the Clinical, DM, and TQA workgroups to maintain best practices and remedy inconsistencies. This QA is critical for high‐fidelity data collection across sites and future collaborative research.
3.4. Community outreach development and activities
Due to the COVID‐19 pandemic, 36 of the 46 activities (October 2020–December 2022) were conducted virtually. As some areas reopened, in‐person events began at community centers, clinics, senior residences, and outdoors (e.g., health fairs, Walk to End Alzheimer's). Overall, the outreach/recruitment attendance was > 8800, with 75% 60+ years of age. Events were conducted by bi(tri)lingual/bicultural ACAD team members or healthcare professionals in one or multiple languages (Table 5).
TABLE 5.
Number of outreach activities between October 2020 and December 2022 by language.
| Single language | |||
|---|---|---|---|
| English | Cantonese | Korean | Mandarin |
| 23 | 4 | 1 | 9 |
| Multi‐language | |
|---|---|
| Cantonese and Mandarin | English and at least one Asian language |
| 1 | 8 |
To support CBPR principles, community outreach, and engagement, a CAB was formed to support the Outreach and Education Workgroup. The CAB has met quarterly to provide valuable feedback on study procedures and material development (including the DCP) and has been promoting ACAD to their respective communities and networks. The CAB consists of members representing all ethnic groups in ACAD and professionals with vast experience in community engagement and participation.
3.5. Data collection and consensus‐based diagnoses
The DCP was designed to be administered in up to three sessions and contains a total of 17 sections (Table 1 and Figure 3). There are > 200 questions in the DCP to cover: participant demographics; , family, medical, and lifestyle factors across the individual's lifespan; cognitive assessments including validated instruments—Cognitive Abilities Screening Instrument (CASI) for English, Mandarin, 44 Cantonese, or Vietnamese 45 speakers, or the Modified Mental Status Examination (3MS) for Korean speakers; 46 additional cognitive assessments for list learning, figure drawing, and verbal fluency; the Functional Assessment Scale (FAS) 47 and a brief neurological examination from NACC; 48 all of which were used for an initial diagnosis discussed locally at each site's consensus conference. Assessments were conducted in person or by videoconferencing including pre‐screening, consenting, instructions for biosample collection, and cognitive and neurological assessments typically taking a total of 2.5 to 4 hours. Each component was administered by ACAD‐certified professionals to ensure inter‐site rigor. Follow‐up assessment is planned on a subset of the DCP sections (Table 2).
FIGURE 3.
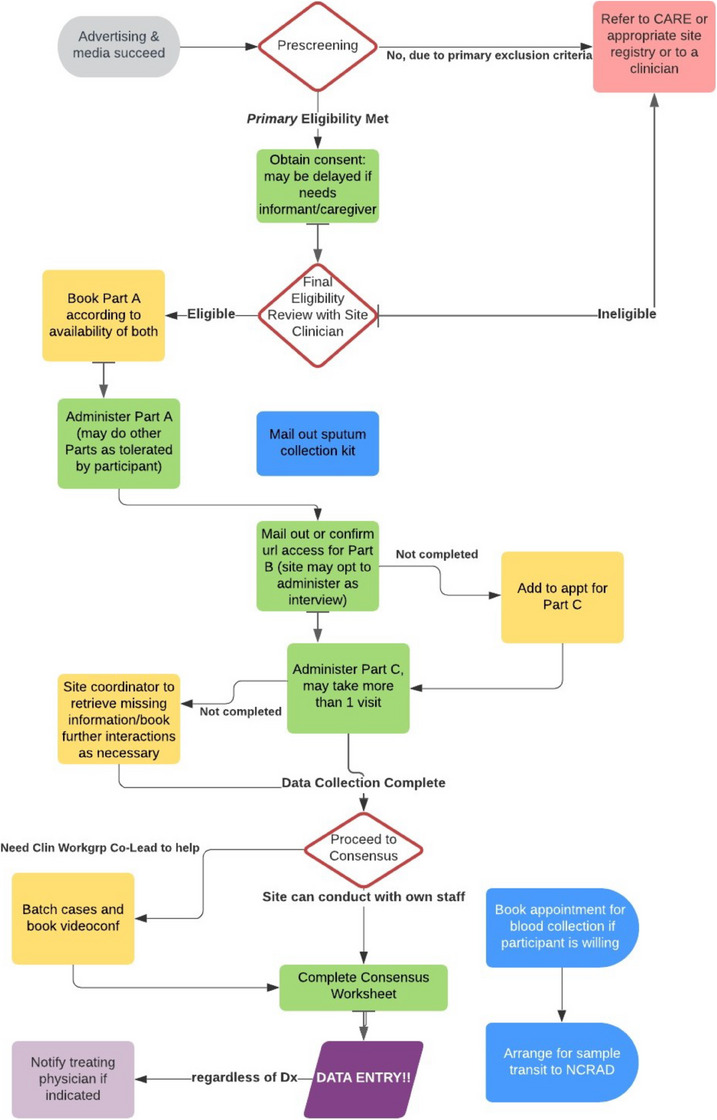
ACAD recruitment and assessment workflow. Note: ACAD recruitment and assessment workflow, including Recruitment/Prescreening; Assessment and Biosample Collection, and Data Collection; and Diagnosis consensus, Data Entry and Quality Assurance (QA), and Biosample Shipment to NCRAD. Participant assessment can range from a single to several visits, while consensus meetings, QA, and biosample transport were done in batches. ACAD, Asian Cohort for Alzheimer's Disease; NCRAD, National Centralized Repository for Alzheimer's Disease and Related Dementias
We were able to achieve high tractability in completing the study, with 96% of consented participants completing the first section (Part A), and 92% of participants completing all parts of this study. As ACAD is ongoing, this number also includes recently added participants who have not had time to complete the entire study, likely underestimating participant completion rates. Of the two sites (San Diego/University of California San Diego and New Jersey/University of Massachusetts Boston) that had concluded recruitment, we achieved a 96% and 100% completion rate.
We established a consensus‐based diagnosis process for study participants to determine diagnostic categories: probable/possible AD, mild cognitive impairment, and normal control (including Subjective cognitive complaint). The Clinical Dementia Rating (CDR) scale and FAS were preferably administered to an informant, but normal controls or those participants who declined to involve an informant responded to both themselves. An informant was defined as someone who regularly interacts with the participant and has known the participant for many years. ACAD investigators reviewed the entire DCP for prior exclusionary neurological disorders, expression of cognitive complaints, and, when rarely available, pre‐existing neuroimaging reports. Cases are diagnosed through consensus conferences at each site, with randomized external review by two neurologists (T.W.C., V.H.), co‐chairs of the Clinical Workgroup, to assure harmonization of diagnoses among all ACAD sites. This process echoes diagnostic procedures in Chinese cross‐sectional studies of community‐based dementia prevalence. 49
3.6. Biosample collection and processing
To identify genetic risk factors, biosamples (saliva/blood) were collected from every participant. For saliva, samples were collected on site if participants were present in person, at homes or clinics if assessments were performed off site but in person, or via mail if the study was conducted remotely. Recognizing the importance of using AD plasma biomarkers, blood collection was preferred. Blood was collected either on site, or using a remote laboratory service (at a lab or a traveling phlebotomist) for those who consented. We piloted blood collection feasibility at two sites to identify the likelihood our populations would provide blood over saliva if given the opportunity. Despite sociological factors resisting blood donations, feasibility at the Toronto (Chinese) and New Jersey (Korean) sites was high at 62% and 72%, respectively, suggesting similar rates of participation for providing blood. In addition, two other sites (Vancouver/University of British Columbia and New York/Columbia University) offered the opportunity to participants who came on site to provide blood. When we examined these four sites, the blood collection rate was 177 of the first 383 (54%) participants, overriding concerns we had of cultural fear of giving blood and demonstrating feasibility in older Asian adults. The ACAD Outreach and Education Workgroup generated materials to encourage blood sample donation by addressing sociocultural concerns, mitigating fear of pain or blood, and focusing on the impact of participation in research including support for future generations. In addition to ACAD investigations, additional plasma and DNA samples are banked to support future collaborative research. Sample collection procedures are detailed in a procedural manual developed by the National Centralized Repository for Alzheimer's Disease and Related Dementias (NCRAD) for ACAD where samples are banked. DNA has been successfully isolated from collected samples and yielded comparable results with prior AD studies conducted at NCRAD, 50 , 51 suggesting consistent sample quality.
4. DISCUSSION
4.1. Key findings and significance
The lifetime experiences and communities of Asians in the United States and Canada are legacies of their languages, cultures, and immigration histories, thus requiring a tailored strategy to effectively recruit these populations into research. Older ASACs have sociocultural characteristics notably different from other groups, including language, culture, health beliefs, health attitudes, health behaviors, living environment, and social networks that can be facilitators or barriers to ADRD screening and research. 52 , 53 Many ASACs still have stigma toward dementia 54 , 55 , 56 , 57 , 58 that further disincentivizes research participation. However, these unique non‐genetic factors, including lived experiences (e.g., diet, language, climate, and lifetime exposure to pathogens) and cultural and social determinants of health, may be the strength in facilitating novel AD research but are largely unaccounted for in the limited number of ASAC AD studies. 59 Through ACAD, we showed there is willingness to participate in clinical research on AD and this supports further enrollment in other studies, which has been lacking. This bodes well for future research with a lens for inclusion of Asian background, providing comprehensive lessons learned that will enable more research.
The genetic architecture contributing to AD risk is complex, including notable allelic heterogeneity (different alleles at a single gene locus causing the same outcome) and pleiotropy (one gene affecting multiple traits) across diverse ancestral populations. In the past decade, improved genotyping and sequencing technologies have identified > 75 AD susceptibility loci in large genome‐wide association studies. 60 These studies have been conducted largely in NHW populations, despite AD also affecting individuals of diverse ancestries. 60 , 61 Though ASACs’ immigration history is too brief to play a role in their AD genetic compositions, there will be distinctions between ASACs and populations from where they or their ancestors emigrated, due to admixture/marriage across subpopulations and genetic drift. Furthermore, the non‐genetic factors may impact genetic risk of AD through key gene interactions, thus highlighting the value‐added of ASAC AD studies in areas other than Asia.
4.2. Considerations, challenges, and lessons learned
ACAD is the first study of ASACs in the United States and Canada designed to comprehensively investigate genetic contributions to AD and their interactions with non‐genetic risk factors and to establish resources that meaningfully expand knowledge about ASACs and AD while supporting community needs. As such, ACAD has had to make some important decisions on study design from the beginning.
Four main characteristics of ASAC communities have significantly influenced the ACAD study design. First, ASAC communities are highly concentrated in major metropolitan areas. Second, many older ASACs have limited English proficiency. Third, there is a culture of shame about dementia and fourth, in‐person visits are less feasible due to mobility issues. Consequently, building trust through active engagement of older ASACs in their communities is imperative for effective recruitment. Chinese, Korean, and Vietnamese subpopulations were prioritized in part due to their population sizes in the United States and Canada, as well as the backgrounds of ACAD investigators and existing community connections. Only recruiting from Chinese populations misses the opportunity to test the robustness of the ACAD data collection protocol across subpopulations and risks the impression of equating Asians to Chinese alone. The current study is the first step for researchers to engage a broader section of ASACs and demonstrates the feasibility and commitment to leading collaborations and building trust in ASAC communities. Future ACAD cohorts will include other ASAC subpopulations.
Most genome‐wide association studies rely on the clinical diagnosis of AD, with plasma biomarkers, particularly analysis of amyloid, tau, and neurodegeneration (ATN). However, other genetic epidemiology studies such as the NIA Genetics Initiative for Late‐Onset Alzheimer's Disease (NIA‐LOAD) and WHICAP have used ATN criteria for establishing diagnosis and to exclude non‐AD etiologies (e.g., people with histories of significant stroke and cardiovascular events are excluded to reduce vascular dementia cases) and increase the likelihood of a probable/possible AD diagnosis. The NIA‐LOAD study has found the same variants with only slightly weaker effect sizes using diagnoses established without neuroimaging compared to ADRC clinical and autopsy‐confirmed cohorts. 62 , 63 In the latest International Genomics of Alzheimer's Project (IGAP) study, 60 association signals from the UK Biobank AD cases (using medical record diagnosis listings or even self‐reported diagnoses) replicated results from biomarker‐ or autopsy‐confirmed cohorts.
Due to increasing traction for biofluid analysis as potential diagnostic tools, and paucity of biosamples from Asian populations, it is imperative to increase blood collection for ATN biomarkers and establish ASAC‐specific cutoffs, which can be critical to enhance clinical diagnosis of AD but not required to generate reliable genetic risk signals. This resource is also needed to identify new biomarkers of disease. To this end, we will continue to increase the likelihood of blood collection, demonstrating the importance of giving blood over saliva and destigmatizing the fear and cultural reluctance to provide blood through greater awareness through information literature and programs.
4.3. Future directions
ACAD's mission will continue to achieve the target sample size of 5081 participants. The design will include a 2‐year follow‐up to capture conversions from cognitively normal or mild cognitive impairment to AD or other dementias, allowing further explorations of genetic and non‐genetic risk factors for AD. Hypothesis‐driven projects supported by ACAD will address questions on factors impacting AD in ASAC populations.
Through our preparations and findings, ACAD can grow in many important directions including: (1) enrolling multiple subpopulations at existing sites, (2) collaborating with ADRCs and other cohorts, (3) reporting recruitment strategies and outcomes to foster recruitment of additional Asian subpopulations (e.g., Asian Indians, Japanese, Filipinos) in major ASACs in ACAD and other communities (e.g., Houston, Texas), (4) sequencing and analyzing complete genomes (Alzheimer's Disease Sequencing Project) to identify rare genetic/structural risk variants, (5) identifying novel plasma biomarkers relevant to ASACs, (6) generating magnetic resonance imaging/positron emission tomography imaging data for analyses, and (7) expanding the ACAD data collection protocol to include sleep and other variables. As the largest ASAC study of its kind, ACAD will be an important research and community resource with implications for AD diagnosis and recruitment of ASACs, a group under‐represented in research and medicine. Ultimately, this inclusion will enhance clinical trial readiness by identifying Asian‐specific biomarker criteria and promoting AD education to raise awareness and reduce stigma, allowing for greater potential to participate in other studies, provide more comprehensive understanding to AD pathophysiology, and offer additional perspectives in dementia prevention strategies.
CONFLICT OF INTEREST STATEMENT
Dr. Jennifer S. Yokoyama is supported by NIH‐NIA R01 AG062588, R01 AG057234, P30 AG062422; NIH‐NINDS U54 NS123985; the Rainwater Charitable Foundation; the Alzheimer's Association; the Global Brain Health Institute; and the Mary Oakley Foundation. She serves on the scientific advisory board for the Epstein Family Alzheimer's Research Collaboration. Dr. Howard Feldman reports grant funding from the NIH (1R56AG069130‐01) and University of Pennsylvania Department of Laboratory Medicine & Pathology (Dr. Li‐San Wang) to UCSD for the work under consideration for publication. Outside of the submitted work, Dr. Feldman reports: grant funding from Annovis (QR Pharma), Vivoryon (Probiodrug), AC Immune, and LuMind; service agreements for consulting activities with LuMind, Axon Neuroscience, Arrowhead Pharmaceuticals, Genentech (DSMB), Roche/Banner (DMC), Tau Consortium (SAB), Samus Therapeutics, Biosplice Therapeutics, Novo Nordisk Inc. (evoke SC), and Janssen Research & Development LLC with no personal funds received and all payments to UCSD. He also reports a philanthropic donation to UCSD from the Epstein Family Alzheimer's Disease Collaboration for therapeutic research in AD. Outside of the submitted work, Dr. Mingyao Li reports research funds from Biogen. Outside of the submitted work, Dr. Rhoda Au reports research funds from Signant Health, Biogen, and Davos Alzheimer's Collaborative. Dr. Jeffrey Dage is an inventor on patents or patent applications of Eli Lilly and Company relating to the assays, methods, reagents, and/or compositions of matter related to measurement of P‐tau217. Dr. Jeffrey Dage has served as a consultant for Eisai, Abbvie, Genotix Biotechnologies Inc,, Gates Ventures, Karuna Therapeutics, AlzPath Inc,, Cognito Therapeutics, Inc., and received research support from ADx Neurosciences, Fujirebio, AlzPath Inc,, Roche Diagnostics. and Eli Lilly and Company in the past 2 years. Dr. Jeffrey Dage has received speaker fees from Eli Lilly and Company. Dr. Dage has stock or stock options in Eli Lilly and Company, Genotix Biotechnologies, AlzPath Inc., and Monument Biosciences. Other authors do not have any relevant conflicts of interest to disclose. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All participants have provided informed consent to participate in the present study.
Supporting information
Supporting information
ACKNOWLEDGMENTS
We are grateful to the participants and families whose help and participation made this work possible. We sincerely thank the entire ACAD team and the ACAD Community Advisory Board members for their assistance with study design, recruitment, and data collection. We also thank Ms. Brittany Caramanna and Mr. Carlos Thomas Jr. for their efforts. This study was supported by the National Institute on Aging Grant R56 AG069130 and U19 AG079774. Samples collected in this study are deposited at the National Centralized Repository for Alzheimer's Diseases and Related Dementias (NCRAD), which receives government support under a cooperative agreement grant U24 AG021886. The study was also supported in part by the National Institute on Aging Grant R24 AG063718, R01 AG083926, R01 AG080469, P30 AG062422, R21 AG068757, U19 AG068753, R21 AG077649, K23 AG062750, UH2 AG083258, R61 AG083582, R01 AG083840, Centre for Addiction and Mental Health Discovery Fund 3000028, the National Eye Institute Grant U10 EY017337, Korea Brain Research Institute grant 23‐BR‐03‐05, as well as Alzheimer's Association grant AACSFD‐22‐972143 and ALZ‐NAN‐22‐928181.
Ho P‐C, Yu WH, Tee BL, et al. Asian Cohort for Alzheimer's Disease (ACAD) pilot study on genetic and non‐genetic risk factors for Alzheimer's disease among Asian Americans and Canadians. Alzheimer's Dement. 2024;20:2058–2071. 10.1002/alz.13611
Pei‐Chuan Ho, Wai Haung Yu, Gyungah R. Jun, Van M. Ta Park, Tiffany W. Chow, and Li‐San Wang contributed equally to this study.
DATA AVAILABILITY STATEMENT
The recruitment resources, including the data collection packet, are currently accessible to ACAD members. We will share the resources with research communities in the future by requests as well as via our website, https://acadstudy.org.
REFERENCES
- 1. Brookmeyer R, Gray S, Kawas C. Projections of Alzheimer's disease in the United States and the public health impact of delaying disease onset. Am J Public Health. 1998;88(9):1337‐1342. doi: 10.2105/ajph.88.9.1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sloane PD, Zimmerman S, Suchindran C, et al. The public health impact of Alzheimer's disease, 2000‐2050: potential implication of treatment advances. Annu Rev Public Health. 2002;23:213‐231. doi: 10.1146/annurev.publhealth.23.100901.140525 [DOI] [PubMed] [Google Scholar]
- 3. Alzheimer's Association . 2014 Alzheimer's disease facts and figures. Alzheimers Dement. 2014;10(2):e47‐92. doi: 10.1016/j.jalz.2014.02.001 [DOI] [PubMed] [Google Scholar]
- 4. Gustavsson A, Norton N, Fast T, et al. Global estimates on the number of persons across the Alzheimer's disease continuum. Alzheimers Dement. 2023;2023;19:658‐670.doi: 10.1002/alz.12694 [DOI] [PubMed] [Google Scholar]
- 5. United States Census Bureau . Annual Estimates of the Resident Population by Single Year of Age and Sex for the United States: April 1, 2020 to July 1, 2021 (NC‐EST2021‐SR11H) . 2022. https://www.census.gov/data/tables/time‐series/demo/popest/2020s‐national‐detail.html
- 6. Pew Research Center . Key findings about U.S. immigrants. Accessed January 27, 2023, https://www.pewresearch.org/fact‐tank/2020/08/20/key‐findings‐about‐u‐s‐immigrants/
- 7. Statistics Canada . Citizenship by visible minority and immigrant status and period of immigration: Canada, provinces and territories and federal electoral districts (2013 Representation Order). Accessed October 26, 2022, https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=9810030301
- 8. Statistics Canada . Immigrants make up the largest share of the population in over 150 years and continue to shape who we are as Canadians. https://www150.statcan.gc.ca/n1/daily‐quotidien/221026/dq221026a‐eng.htm
- 9. National Asian Pacific Center on Aging . Asian Americans and Pacific Islanders in the United States Aged 65 Years and Older: Population, Nativity, and Language. Accessed July 11, 2018, https://napca.org/wp‐content/uploads/2017/10/65‐population‐repo
- 10. Doan LN, Takata Y, Sakuma KK, Irvin VL. Trends in clinical research including Asian American, Native Hawaiian, and Pacific Islander participants funded by the US National Institutes of Health, 1992 to 2018. JAMA Netw Open. 2019;2(7):e197432. doi: 10.1001/jamanetworkopen.2019.7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alzheimer's Association International Conference . New National Strategy For Recruitment And Participation In Alzheimer's Disease Clinical Trials Takes Shape. Accessed September 5, 2018, https://www.alz.org/aaic/downloads2018/Mon‐pm‐briefing‐clinical‐research‐recruitment‐strategy.pdf
- 12. National Institute on Aging . NIA and the National Plan to Address Alzheimer's Disease. Accessed September 5, 2018, https://www.nia.nih.gov/about/nia‐and‐national‐plan‐address‐alzheimers‐disease
- 13. Faison WE, Schultz SK, Aerssens J, et al. Potential ethnic modifiers in the assessment and treatment of Alzheimer's disease: challenges for the future. Int Psychogeriatr. 2007;19(3):539‐558. doi: 10.1017/S104161020700511X [DOI] [PubMed] [Google Scholar]
- 14. Raman R, Quiroz YT, Langford O, et al. Disparities by Race and ethnicity among adults recruited for a preclinical Alzheimer disease trial. JAMA Network Open. 2021;4(7):e2114364‐e2114364. doi: 10.1001/jamanetworkopen.2021.14364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lake J, Solsberg CW, Kim JJ, et al. Multi‐ancestry meta‐analysis and fine‐mapping in Alzheimer's disease. medRxiv. 2022:2022.08.04.22278442. doi: 10.1101/2022.08.04.22278442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Miyashita A, Koike A, Jun G, et al. SORL1 is genetically associated with late‐onset Alzheimer's disease in Japanese, Koreans and Caucasians. PLoS One. 2013;8(4):e58618. doi: 10.1371/journal.pone.0058618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reitz C, Jun G, Naj A, et al. Variants in the ATP‐binding cassette transporter (ABCA7), apolipoprotein E ε4,and the risk of late‐onset Alzheimer disease in African Americans. JAMA. 2013;309(14):1483‐1492. doi: 10.1001/jama.2013.2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Jun GR, Chung J, Mez J, et al. Transethnic genome‐wide scan identifies novel Alzheimer's disease loci. Alzheimers Dement. 2017;13(7):727‐738. doi: 10.1016/j.jalz.2016.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Choi KY, Lee JJ, Gunasekaran TI, et al. APOE promoter polymorphism‐219T/G is an effect modifier of the influence of APOE ε4 on Alzheimer's disease risk in a multiracial sample. J. Clin. Med. 2019;8(8). doi: 10.3390/jcm8081236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shigemizu D, Mitsumori R, Akiyama S, et al. Ethnic and trans‐ethnic genome‐wide association studies identify new loci influencing Japanese Alzheimer's disease risk. Transl Psychiatry. 2021;11(1):151. doi: 10.1038/s41398-021-01272-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H, Summary of the evidence on modifiable risk factors for cognitive decline and dementia: a population‐based perspective. Alzheimers Dement. 2015;11(6):718‐726. doi: 10.1016/j.jalz.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 22. Sabbagh MN, Perez A, Holland TM, et al. Primary prevention recommendations to reduce the risk of cognitive decline. Alzheimers Dement. 2022;18(8):1569‐1579. doi: 10.1002/alz.12535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Xu W, Tan L, Wang HF, et al. Meta‐analysis of modifiable risk factors for Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2015;86(12):1299‐1306. doi: 10.1136/jnnp-2015-310548 [DOI] [PubMed] [Google Scholar]
- 24. Mukadam N, Sommerlad A, Huntley J, Livingston G. Population attributable fractions for risk factors for dementia in low‐income and middle‐income countries: an analysis using cross‐sectional survey data. Lancet Glob Health. 2019;7(5):e596‐e603. doi: 10.1016/S2214-109X(19)30074-9 [DOI] [PubMed] [Google Scholar]
- 25. Lee H, Ha H, Yim S, et al. Using community‐based geographical information system (GIS) to recruit older Asian Americans in an Alzheimer's disease study. BMJ Open. 2023;13(8):e072761. doi: 10.1136/bmjopen-2023-072761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Israel BAEE, Schulz AJ, Parker EA, Introduction to methods in community‐based participatory research for health. In: Israel BA EE, Schulz AJ, Parker E, ed. Methods in Community‐Based Participatory Research For Health. Jossey‐Bass; 2005:3‐26. [Google Scholar]
- 27. Faridi Z, Grunbaum JA, Gray BS, Franks A, Simoes E. Community‐based participatory research: necessary next steps. Prev Chronic Dis. 2007;4(3):A70. [PMC free article] [PubMed] [Google Scholar]
- 28. Minkler M, Blackwell AG, Thompson M, Tamir H. Community‐based participatory research: implications for public health funding. Am J Public Health. 2003;93(8):1210‐1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Green‐Harris G, Koscik RL, Houston S, et al. Using asset‐based community involvement to address health disparities and increase African American participation in AD research: experiences from the Wisconsin Alzheimer's institute. Alzheimer's & Dementia. 2017;13(7):P897‐P898. doi: 10.1016/j.jalz.2017.07.308 [DOI] [Google Scholar]
- 30. Austrom MG, Bachman J, Altmeyer L, Gao S, Farlow M. A collaborative Alzheimer disease research exchange using a community‐based Helpline as a recruitment tool. Alzheimer Dis Assoc Disord. 2010;24(Suppl):S49‐53. [PubMed] [Google Scholar]
- 31. Katigbak C, Foley M, Robert L, Hutchinson MK, Experiences and Lessons Learned in Using Community‐Based Participatory Research to Recruit Asian American Immigrant Research Participants. J Nurs Scholarsh. 2016;48(2):210‐218. doi: 10.1111/jnu.12194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grill JD, Karlawish J. Addressing the challenges to successful recruitment and retention in Alzheimer's disease clinical trials. Alzheimers Res Ther. 2010;2(6):34. doi: 10.1186/alzrt58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ta Park VM, Meyer OL, Tsoh JY, et al. The collaborative approach for Asian Americans and Pacific Islanders Research and Education (CARE): a recruitment registry for Alzheimer's disease and related dementias, aging, and caregiver‐related research. Alzheimers Dement. 2022. doi: 10.1002/alz.12667. Apr 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. World Health Organization . Process of translation and adaptation of instruments.
- 35. Brickman AM, Manly JJ, Honig LS, et al. Plasma p‐tau181, p‐tau217, and other blood‐based Alzheimer's disease biomarkers in a multi‐ethnic, community study. Alzheimers Dement. 2021;17(8):1353‐1364. doi: 10.1002/alz.12301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Beekly DL, Ramos EM, Lee WW, et al. The National Alzheimer's Coordinating Center (NACC) database: the Uniform Data Set. Alzheimer Dis Assoc Disord. 2007;21(3):249‐258. doi: 10.1097/WAD.0b013e318142774e [DOI] [PubMed] [Google Scholar]
- 37. Landau SM, Marks SM, Mormino EC, et al. Association of lifetime cognitive engagement and low β‐amyloid deposition. Arch Neurol. 2012;69(5):623‐629. doi: 10.1001/archneurol.2011.2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schröder H, Fitó M, Estruch R, et al. A short screener is valid for assessing Mediterranean diet adherence among older Spanish men and women. J Nutr. 2011;141(6):1140‐1145. doi: 10.3945/jn.110.135566 [DOI] [PubMed] [Google Scholar]
- 39. Martínez‐González MA, García‐Arellano A, Toledo E, et al. A 14‐item Mediterranean diet assessment tool and obesity indexes among high‐risk subjects: the PREDIMED trial. PLoS One. 2012;7(8):e43134. doi: 10.1371/journal.pone.0043134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377‐381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. U.S .Census Bureau . 2021 ACS 1‐Year Estimates Public Use Microdata Sample. Accessed August 11, 2023. https://data.census.gov/mdat/#/
- 43. American Psychiatric Association , American Psychiatric Association . DSM‐5 Task Force. Diagnostic and Statistical Manual of Mental Disorders: DSM‐5. 5th ed. American Psychiatric Association; 2013:xliv, 947 p. [Google Scholar]
- 44. Liu HC, Chou P, Lin KN, et al. Assessing cognitive abilities and dementia in a predominantly illiterate population of older individuals in Kinmen. Psychol Med. 1994;24(3):763‐770. doi: 10.1017/s0033291700027914 [DOI] [PubMed] [Google Scholar]
- 45. Dick MB, Teng EL, Kempler D, Davis DS, Taussig IM. The cross‐cultural neuropsychological test battery (CCNB): effects of age, education, ethnicity, and cognitive status on performance. In: Ferraro FR, ed. Minority and Cross‐Cultural Aspects of Neuropsychological Assessment. Swets & Zeitlinger; 2002:17‐41. [Google Scholar]
- 46. Jeong S‐K. Population‐based norms for the Korean Mini‐Mental State Examination and Korean version of modified Mini‐Mental State Examination. J Korean Neurol Assoc. 2007;25(1):1‐9. [Google Scholar]
- 47. Loewenstein DA, Amigo E, Duara R, et al. A new scale for the assessment of functional status in Alzheimer's disease and related disorders. J Gerontol. 1989;44(4):P114‐121. doi: 10.1093/geronj/44.4.p114 [DOI] [PubMed] [Google Scholar]
- 48. Pfeffer RI, Kurosaki TT, Harrah CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323‐329. doi: 10.1093/geronj/37.3.323 [DOI] [PubMed] [Google Scholar]
- 49. Zhang ZX, Zahner GE, Roman GC, et al. Socio‐demographic variation of dementia subtypes in China: methodology and results of a prevalence study in Beijing, Chengdu, Shanghai, and Xian. Neuroepidemiology. 2006;27(4):177‐187. doi: 10.1159/000096131 [DOI] [PubMed] [Google Scholar]
- 50. Wong R, Michaels‐Obregon A, Palloni A. Cohort profile: the Mexican Health and Aging Study (MHAS). Int J Epidemiol. 2017;46(2):e2. doi: 10.1093/ije/dyu263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Melikyan ZA, Greenia DE, Corrada MM, Hester MM, Kawas CH, Grill JD. Recruiting the oldest‐old for clinical research. Alzheimer Dis Assoc Disord. 2019;33(2):160‐162. doi: 10.1097/WAD.0000000000000260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lee S, Martinez G, Ma GX, et al. Barriers to health care access in 13 Asian American communities. Am J Health Behav. 2010;34(1):21‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dong X, Chang ES, Wong E, Wong B, Skarupski KA, Simon MA. Assessing the health needs of Chinese Older Adults: findings from a Community‐based participatory research study in Chicago's Chinatown. J Aging Res. 2011;2010:124246. doi: 10.4061/2010/124246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Liu D, Hinton L, Tran C, Hinton D, Barker JC. Reexamining the relationships among dementia, stigma, and aging in immigrant Chinese and Vietnamese family caregivers. J Cross Cult Gerontol. 2008;23(3):283‐299. doi: 10.1007/s10823-008-9075-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Eng KJ, Woo BK. Knowledge of dementia community resources and stigma among Chinese American immigrants. Gen Hosp Psychiatry. 2015;37(1):e3‐4. doi: 10.1016/j.genhosppsych.2014.11.003 [DOI] [PubMed] [Google Scholar]
- 56. Woo BK, Chung JO. Public stigma associated with dementia in a Chinese‐American immigrant population. J Am Geriatr Soc. 2013;61(10):1832‐1833. doi: 10.1111/jgs.12472 [DOI] [PubMed] [Google Scholar]
- 57. Chao SZ, Lai NB, Tse MM, et al. Recruitment of Chinese American elders into dementia research: the UCSF ADRC experience. Gerontologist. 2011;51(Suppl 1):S125‐133. doi: 10.1093/geront/gnr033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yuri J, Kim G, Chiriboga D. Knowledge of Alzheimer's Disease, feelings of shame, and awareness of services among Korean American elders. Journal of Aging and Health. 2010;22(4):419‐433. doi: 10.1177/0898264309360672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tom SE. Understanding diversity in life course social determinants of Dementia risk and resilience in the Asian American older adult population. JAMA Network Open. 2023;6(3):e231668‐e231668. doi: 10.1001/jamanetworkopen.2023.1668 [DOI] [PubMed] [Google Scholar]
- 60. Bellenguez C, Kucukali F, Jansen IE, et al. New insights into the genetic etiology of Alzheimer's disease and related dementias. Nat Genet. 2022;54(4):412‐436. doi: 10.1038/s41588-022-01024-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kunkle BWG‐BB, Sims R, et al. Meta‐analysis of genetic association with diagnosed Alzheimer's disease identifies novel risk loci and implicates Abeta, Tau, immunity and lipid processing. bioRxiv. 2018; [Google Scholar]
- 62. Chung J, Zhang X, Allen M, et al. Genome‐wide pleiotropy analysis of neuropathological traits related to Alzheimer's disease. Alzheimers Res Ther. 2018;10(1):22. doi: 10.1186/s13195-018-0349-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Beecham GW, Hamilton K, Naj AC, et al. Genome‐wide association meta‐analysis of neuropathologic features of Alzheimer's disease and related dementias. PLoS Genet. 2014;10(9):e1004606. doi: 10.1371/journal.pgen.1004606 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information
Data Availability Statement
The recruitment resources, including the data collection packet, are currently accessible to ACAD members. We will share the resources with research communities in the future by requests as well as via our website, https://acadstudy.org.


