Abstract
The mechanism of DNA replication is conserved among papillomaviruses. The virus-encoded E1 and E2 proteins collaborate to target the origin and recruit host DNA replication proteins. Expression vectors of E1 and E2 proteins support homologous and heterologous papillomaviral origin replication in transiently transfected cells. Viral proteins from different genotypes can also collaborate, albeit with different efficiencies, indicating a certain degree of specificity in E1-E2 interactions. We report that, in the assays of our study, the human papillomavirus type 11 (HPV-11) E1 protein functioned with the HPV-16 E2 protein, whereas the HPV-16 E1 protein exhibited no detectable activity with the HPV-11 E2 protein. Taking advantage of this distinction, we used chimeric E1 proteins to delineate the E1 protein domains responsible for this specificity. Hybrids containing HPV-16 E1 amino-terminal residues up to residue 365 efficiently replicated either viral origin in the presence of either E2 protein. The reciprocal hybrids containing amino-terminal HPV-11 sequences exhibited a high activity with HPV-16 E2 but no activity with HPV-11 E2. Reciprocal hybrid proteins with the carboxyl-terminal 44 residues from either E1 had an intermediate property, but both collaborated more efficiently with HPV-16 E2 than with HPV-11 E2. In contrast, chimeras with a junction in the putative ATPase domain showed little or no activity with either E2 protein. We conclude that the E1 protein consists of distinct structural and functional domains, with the carboxyl-terminal 284 residues of the HPV-16 E1 protein being the primary determinant for E2 specificity during replication, and that chimeric exchanges in or bordering the ATPase domain inactivate the protein.
Human and animal papillomaviruses contain a double-stranded circular DNA genome. Replication of plasmids containing a papillomaviral origin sequence (ori) in cell-free systems is dependent on virus-encoded E1 and E2 proteins and the host DNA replication machinery (17, 43). Transfection of E1 and E2 expression plasmids into mammalian cells can support transient replication of an ori-containing plasmid (7, 42). The ori sequences are highly conserved among all papillomavirus types and consist of a known or putative E1 protein binding site and multiple copies of E2 protein binding sites in close proximity (for reviews, see references 8 and 37). The E1 protein binding sites are similar but not identical in sequence among different virus types, whereas all E2 proteins bind to the consensus sequence ACCN6GGT. Of all papillomavirus proteins, E1 is the most conserved. It is an ATPase and helicase (5, 15, 16, 25, 36, 44) and has sequence homology to the ATPase domain of the simian virus 40 (SV40) T antigen (9, 26), the initiator for SV40 ori replication (see Fig. 2A). As does the T antigen, the E1 protein binds to the ori and unwinds DNA in the presence of the host single-stranded DNA binding protein RPA and topoisomerase I. The human papillomavirus (HPV) and bovine papillomavirus type 1 (BPV-1) E1 proteins are thought to function as a helicase at the replication fork, since each is required during elongation (20). The BPV-1 E1 protein is known to interact with the 180-kDa catalytic subunit of the host DNA polymerase α, thereby bringing host replication proteins to the unwound ori (3, 30). The E2 protein is a transcription factor but also serves multiple functions in ori replication. It interacts with E1, stabilizing its binding to the ori (12, 24, 28, 34, 38, 43), and it also helps recruit host replication proteins into the initiation complex (20). In addition, E2 may prevent nucleosome formation around the ori in vivo (19). By virtue of its strong and specific affinity to the E2 protein binding site and its interaction with the E1 protein, the E2 protein is critical to the initiation of replication from ori (4, 6, 17, 22, 31, 33, 40), but it appears to be dispensable during elongation (20).
FIG. 2.
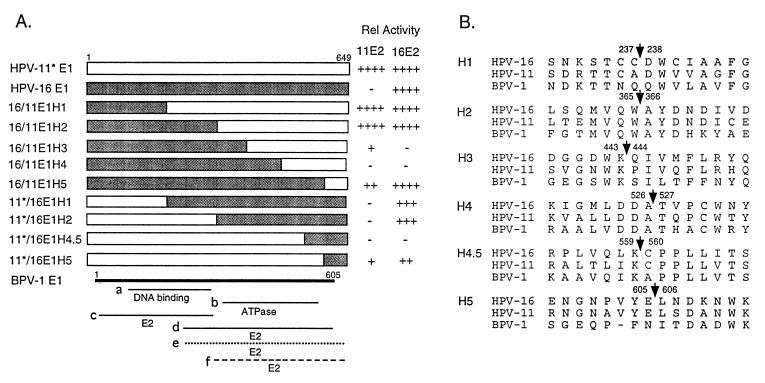
Structures, functional domains, and relative replication activities of wild-type and hybrid HPV E1 proteins. (A) Schematic representations of wild-type and hybrid E1 proteins and relative replication activities. The shaded boxes represent regions derived from HPV-16 E1, and the open boxes represent those derived from HPV-11 E1. To minimize replication differences that might be attributable to mRNA translation efficiencies, the 5′ untranslated sequence and first 5 codons of HPV-11 and of 11/16 hybrid proteins were all replaced with those from HPV-16 E1 and are denoted with an asterisk in the designation. This modification resulted in a net change of two amino acids. The BPV-1 E1 protein is shown as a thick line. Numbers refer to amino acid residues. Thin solid lines represent the BPV-1 E1 protein domains defined to be involved in E2 interactions (lines c and d) or DNA binding (line a), based on protein interaction assays in vitro or in the yeast two-hybrid system (1, 18, 23, 32, 41), or the ATPase domain (line b) based on the homology to the SV40 T antigen and mutagenic analysis (8, 26, 28). The dotted line (e) approximates the E1 domain of HPV-16 and HPV-33 involved in interaction with the E2 protein (29, 45). The dashed line (f) signifies the domain of HPV-16 E1 responsible for E2 specificity during replication (this study). After subtraction of background signals, the relative replication activities (Rel Activity) of each E1 protein in the presence of HPV-11 or HPV-16 E2 protein were determined by PhosphorImager quantification of the results shown in Fig. 3 and 4A. The results are shown to the right of each clone. The activities achieved with HPV-16 E1 and E2 were taken as 100% and are assigned a score of ++++. Scores: −, relative activity of less than 5%, including signals detectable only after a long exposure; +, activity between 5 and 10%; ++, activity between 10 and 50%; +++, activity between 50 and 90%; ++++, activity over 90%. (B) Peptide sequences spanning the junctions of hybrid E1 proteins. The numbers flanking the arrows are amino acid residues where protein coding switches from one virus type to the other.
Because of the homologous nature of the ori and of E1 and E2 proteins, proteins from one virus type can efficiently replicate either a homologous or heterologous viral ori. In contrast, mixed pairs of proteins support ori replication with various levels of efficiency (2, 7, 10, 13, 39). These observations indicate that a degree of specificity in E1-E2 protein interactions must exist. Binding studies in vitro and the yeast two-hybrid system have yielded conflicting results with regard to the E1 domain which interacts with the E2 protein. Some investigators have reported that the amino-terminal portion of the E1 protein of BPV-1 and HPV type 31 (HPV-31) contains the major DNA binding and E2 interaction domains (1, 11, 18, 41). However, others have demonstrated that the C-terminal portion of the BPV-1 E1 protein (residues 162 to 605), the HPV-16 E1 protein (residues 144 to 649), or the HPV-33 E1 protein (residues 312 to 644) was necessary for E2 binding (23, 29, 32, 45) (see Fig. 2A). A study to examine the functional interactions between E1 and E2 proteins during replication would be of significant interest. Here we report such an investigation.
To examine the interactions between the E1 and E2 proteins, we tested combinations of HPV-11 and HPV-16 E1 and E2 proteins for their ability to support ori replication in transfected human 293 cells. The HPV-11 proteins were each expressed from the eukaryotic vector pMT2 as described previously (7). The interrupted HPV-16 E1 open reading frame (ORF) in the prototype genomic HPV-16 clone (35) was repaired by inserting a G residue between nucleotide positions (nt) 1138 and 1139 (27) by site-directed mutagenesis. The PvuII-HincII and AvaII-MstII restriction fragments spanning the HPV-16 E1 ORF (nt 685 to 3211) and the E2 ORF (nt 2714 to 4337), respectively, were cloned into pMTX, a derivative of the pMT2 vector containing a multiple cloning site. The ori plasmid contained either the HPV-11 ori (nt 7730 to 7933/1 to 99) or the HPV-16 ori (nt 7455 to 7906/1 to 111) in pUC19.
ori replication by matched or mixed pairs of HPV-11 and HPV-16 E1 and E2 proteins.
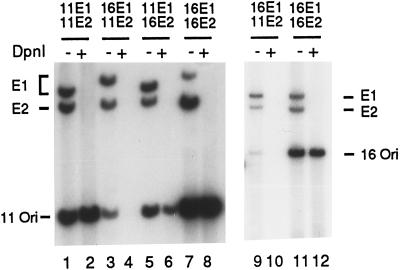
Transient replication assays were conducted as previously described (7). Briefly, 48 h after electroporation of 5 μg each of the expression vectors and 0.5 μg of the ori plasmid, low-molecular-weight DNA was harvested by alkaline Hirt lysis and digested with HindIII alone, which linearized all three plasmids, or with both HindIII and DpnI, which eliminated all unreplicated input DNA and linearized the replicated DNA. The digestion products were separated electrophoretically in a 0.8% agarose gel and then revealed by Southern blot hybridization with [α-32P]dCTP-labeled ori plasmid probes generated by random-priming reactions. These results showed that the matched protein pairs were able to replicate both HPV-11 and HPV-16 ori plasmids efficiently (Fig. 1, lanes 1 and 2, 7 and 8, and 11 and 12; see also Fig. 4B, lane 5). Cotransfection of the HPV-11 E1 expression vector with the HPV-16 E2 expression plasmid supported ori replication with a slightly reduced efficiency relative to that achieved with either homologous protein pair (Fig. 1, compare lanes 5 and 6 to 1 and 2 and to 7 and 8). In contrast, the HPV-16 E1 protein consistently failed to replicate either HPV ori plasmid in the presence of HPV-11 E2 (Fig. 1, lanes 3 and 4 and lanes 9 and 10; see also Fig. 3, lane 7, and Fig. 4A, lane 1). Furthermore, these results were reproducible when the cells were transfected with E1 and E2 expression plasmids in a wide range of relative quantities (data not shown). The stringent discrimination between the two E2 proteins exhibited by HPV-16 E1 and the lack of specificity by the HPV-11 E1 provided us with an opportunity to investigate the domains involved in functional interactions during replication by using chimeric E1 proteins.
FIG. 1.
Transient replication by combinations of HPV-11 and HPV-16 E1 and E2 proteins. Replication assays of an HPV-11 ori plasmid (nt 7730 to 7933/1 to 99) (lanes 1 to 8) or an HPV-16 ori plasmid (nt 7455 to 7906/1 to 111) (lanes 9 to 12) were conducted with human 293 cells as described in the text. The E1 and E2 expression vector plasmids used are indicated above each pair of lanes. Low-molecular-weight DNA was harvested 48 h posttransfection. Half of the recovered DNA from one 100-mm plate was subjected to HindIII and DpnI double digestion (DpnI +). The other half was subjected to HindIII digestion, which linearized the three plasmids (DpnI −). The products were separated electrophoretically in a 0.8% agarose gel and transferred to a nitrocellulose membrane. The membrane was probed with the [α-32P]dCTP-labeled, origin-containing plasmid and exposed to X-ray film overnight. Only replicated DNA was resistant to DpnI digestion (marked Ori), whereas the unreplicated DNA was digested to small fragments and migrated ahead of the linearized, newly replicated ori plasmid (see Fig. 3 and 4).
FIG. 4.
Transient replication by HPV-11*/16 E1 hybrid proteins in combination with either HPV-16 or HPV-11 E2 protein. (A) Replication of an HPV-11 ori plasmid; (B) replication of an HPV-16 ori plasmid.
FIG. 3.
Transient replication of an HPV-11 ori plasmid by HPV-16/11 E1 hybrid proteins in combination with either HPV-16 or HPV-11 E2 protein. Replication assays were conducted as described in the legend to Fig. 1. The low-molecular-weight DNA was digested overnight with both DpnI and HindIII. Fragments that migrated faster than the linearized ori plasmid were derived from DpnI-sensitive input DNA. Similar results were obtained when an HPV-16 ori plasmid was used (data not shown).
Reciprocal HPV-16/11 and HPV-11*/16 hybrid proteins.
Nine hybrid E1 protein genes were constructed (Fig. 2). Each hybrid E1 gene encodes a protein identical in length (649 amino acids) to that encoded by the wild-type HPV-11 or HPV-16 gene or a modified HPV-11 E1 gene (designated 11*; described below). The junctions of the hybrid proteins were selected based on one of two criteria or both. Either they were located near a boundary of functional domains inferred from the homologous BPV-1 E1 protein or they were within a highly conserved region to minimize the probability of generating a misfolded, nonfunctional protein (Fig. 2B). Five HPV-16/11 (16/11) hybrid E1 genes contained HPV-16 E1 sequences at the 5′ portion and the balance from HPV-11 E1 sequences at the 3′ portion. We prepared four HPV-11/16 (11/16) hybrid E1 protein genes in which the sequences encoding the amino-terminal portion were derived from HPV-11, with the balance from HPV-16. To facilitate a quantitative comparison among various wild-type and hybrid E1 genes, we modified the 5′ untranslated sequence and the first 5 amino acids of the 11/16 hybrid gene to those of HPV-16 E1 (Fig. 2A, designated HPV-11*/16 E1H1, E1H2, E1H4.5, and E1H5). This modification resulted in a net change of 2 amino acids from MADDS of HPV-11 to MADPA of HPV-16. These two altered residues are not conserved among different HPVs. The reason for making the HPV-11*/16 hybrid genes is the following. We note that HPV-16 proteins always supported a higher level of replication than HPV-11 proteins (Fig. 1, compare lanes 7 and 8 to lanes 1 and 2). This distinction could in part be due to differences intrinsic to the viral proteins, to differences in levels of protein expression, or both. By constructing clones that had identical sequences in the 5′ untranslated region and around the translation initiation codon, we could at least reduce differences in replication that may have originated from variances in protein translation efficiency among the different E1 genes. To provide a reference protein, we also prepared an HPV-11 E1 gene with the same modification and designated it HPV-11*E1. In transient replication assays, HPV-11*E1 supported the replication of either the HPV-11 or HPV-16 ori plasmid in the presence of either E2 protein as efficiently as that promoted by HPV-16 E1 and E2 proteins (Fig. 3, compare lanes 1 and 8 to lane 14; Fig. 4A, compare lanes 6 and 12 to lane 7; and data not shown). These results demonstrate that HPV-11*E1 is fully functional and that the difference previously observed between the wild-type HPV-11 and HPV-16 E1 proteins in the presence of their respective homologous E2 proteins is due at least in part to the levels of protein expression. This modification minimized the difference and made it feasible to quantify the levels of replication by the panel of E1 proteins.
Hybrid genes 16/11 E1H1 through E1H4 were generated by PCR amplification followed by exchanging a restriction fragment with the wild-type HPV-16 E1 gene in the expression vector. Hybrid gene 16/11 E1H5 was prepared similarly except that a HindIII site was introduced downstream of the hybrid E1 gene during PCR amplification. Using a similar strategy, we constructed hybrid genes containing the amino terminus of HPV-11 E1 and the balance from HPV-16 E1 except for the extra steps to replace their 5′ ends with that of the HPV-16 E1 gene as follows. We took advantage of a PstI site located in the fifth codon of the HPV-16 E1 gene. A PstI site was generated by PCR amplification at the comparable site in HPV-11 E1 or 11/16 hybrid E1, and an EcoRI site was introduced at the 3′ flanking sequence. After digestion with PstI and EcoRI, the fragment containing the bulk of the E1 gene was ligated to the vector fragment of similarly digested pMTX-16E1. Hence, all of the E1 sequences were located at the same site in the pMTX vector and had identical sequences at the 5′ ends of the mRNAs. All junction sequences or the entire sequences generated by PCR were confirmed by sequencing.
The domain of HPV-16 E1 protein which confers E2 protein specificity during replication.
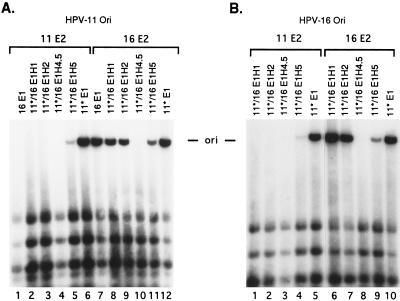
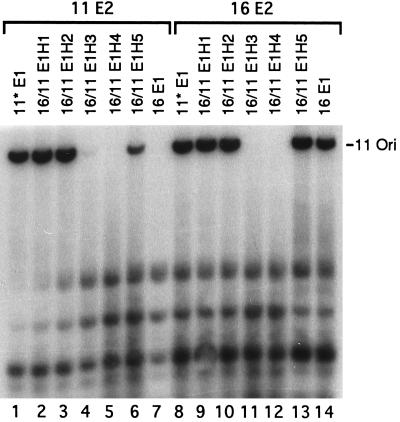
The 16/11 E1H1 and 16/11 E1H2 hybrid proteins contain amino acids 1 to 237 and 1 to 365, respectively, from HPV-16 E1 and the balance from HPV-11 E1. The junctions are located in the middle or at the carboxyl-terminal boundary of the putative DNA and E2 binding domains by analogy to BPV-1 (Fig. 2A). Figure 3 presents the results of transient replications of an HPV-11 ori with the expression vectors of 16/11 hybrid E1 proteins in combination with the HPV-11 or HPV-16 E2 expression vector. Unlike the wild-type HPV-16 E1 protein, chimeric proteins 16/11 E1H1 and E1H2 supported efficient replication in the presence of either E2 protein, a property shared with HPV-11 E1 (Fig. 3, compare lanes 1 to 3 and 7 to lanes 9 to 11 and 14). Similar results were observed when the HPV-16 ori plasmid was used in the test (data not shown). These results demonstrate that the amino-terminal region up to residue 365 of HPV-16 E1 is not responsible for functional discrimination of the two E2 proteins during replication. By inference, the carboxyl-terminal domain of the HPV-16 E1 protein is expected to constitute the determinant. We then tested the reciprocal 11*/16 hybrid E1 genes. Transient replication assays of an HPV-11 or HPV-16 ori showed that neither 11*/16 E1H1 nor 11*/16 E1H2 had detectable replication activity with HPV-11 E2 while both supported efficient replication of either viral ori in the presence of HPV-16 E2 (Fig. 4A, compare lanes 1, 2, and 3 to lanes 7, 8, and 9; Fig. 4B, compare lanes 1, 2, and 5 to lanes 6, 7, and 10). These properties are similar to those of the HPV-16 E1 protein and support the previous conclusion that the carboxyl-terminal region of the HPV-16 E1 is responsible for E2 specificity. This conclusion is supported by the replication properties of reciprocal H5 hybrid proteins described below.
Inactivation of the E1 protein by a chimeric junction in the ATPase domain.
For further delineation of functional domains, we tested 16/11 E1H3, E1H4, and E1H5, which contain amino acid residues 1 to 443, 1 to 526, and 1 to 605 from HPV-16 E1, respectively, and the balance from HPV-11 E1. The junctions reside within or border on the putative ATPase domain (Fig. 2A). 16/11 E1H3 showed a very low replication activity with HPV-11 E2, and the activity with HPV-16 E2 was detectable only after prolonged exposure (Fig. 3, lanes 4 and 11). 16/11 E1H4 had no detectable activity with either E2 protein (Fig. 3, lanes 5 and 12). In contrast, 16/11 E1H5 had an activity comparable to that of HPV-16 E1 or HPV-11* E1 in the presence of HPV-16 E2 (Fig. 3, compare lane 13 to lanes 8 and 14). Interestingly, this hybrid now gained a moderate activity in collaboration with HPV-11 E2 (Fig. 3, lane 6).
Two 11*/16 hybrid genes with a junction near the carboxyl terminus were tested. The exchange in 11*/16 E1H4.5 occurs between residues 559 and 560, whereas the 11*/16 E1H5 is the reciprocal hybrid of 16/11 E1H5 (Fig. 2). Transient replication assays of the HPV-11 or HPV-16 ori by 11*/16 E1H4.5 revealed no detectable activity with either HPV-11 E2 or HPV-16 E2 (Fig. 4A, lanes 4 and 10; Fig. 4B, lanes 3 and 8). Only after extended exposure was a very weak replication signal observed, and only with HPV-16 E2 (data not shown). Transient replication assays of 11*/16 E1H5 showed a low activity with HPV-11 E2 and a higher activity with HPV-16 E2 (Fig. 4A, compare lanes 5 and 11; Fig. 4B, compare lanes 4 and 9). Therefore, our results showed that 16/11 E1H3, 16/11 E1H4, and 11*/16 E1H4.5 were severely crippled in their function in transient replication assays. Since the reciprocal H5 hybrids have a property intermediate between the HPV-11 E1, HPV-11*E1 (or 16/11 E1H1 or E1H2), and HPV-16 E1 (or 11*/16 E1H1 or E1H2), we suggest that the carboxyl-terminal 44 amino acids of HPV-16 contribute to but do not comprise the entire determinant for E2 specificity.
The 16/11 E1H3 and E1H4 hybrid proteins had little or no detectable replication activity, whereas most other hybrid proteins had a moderate to high activity in the presence of at least one of the E2 proteins. To ascertain that these hybrid genes indeed express full-length proteins, we tested for the presence of E1 proteins in transfected COS-7 cells. To eliminate variations in antibody reactivities with hybrid proteins, HPV-16 E1 and each of the 16/11 hybrid E1 genes were recloned into pMT2 with a glutamic acid-rich (EE) epitope tagged at the amino terminus. EE-epitope-tagged HPV-11 and HPV-16 E1 proteins function in transient and in cell-free replication systems (17, 21). After transfection of individual expression vectors into COS-7 cells, the E1 proteins were detected by immunoblot with anti-EE monoclonal antibody (14). Figure 5 shows that all had mobilities identical to that of the EE-epitope tagged HPV-16 protein purified from insect Sf9 cells infected with a recombinant baculovirus (rightmost lane) and that there were no significant differences in expression levels under these conditions. These results indicate that the inactivity of the H3 and H4 (and possibly that of H4.5) chimeras is likely mainly due to a loss of function, with at most a minor effect from variations in steady-state protein levels in the transfected cells.
FIG. 5.
Expression of wild-type and HPV-16/11 hybrid E1 proteins in COS-7 cells. The EE-epitope-tagged wild-type and hybrid E1 proteins were each expressed from the pMT2 vector. After 24 h of incubation, the transfected cells were disrupted in 200 μl of lysis buffer. Forty microliters of each lysate was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. The E1 proteins were detected by monoclonal antibody to the EE epitope (14). The rightmost lane contains EE-epitope-tagged HPV-16 E1 protein purified from insect Sf9 cells infected with a recombinant baculovirus (21).
Multiple structural and functional domains of the E1 protein.
The extent of replication shown in Fig. 3 and 4A was quantified by PhosphorImager and compared to the activities achieved by HPV-16*E1 and HPV-11 E2. The results are shown in Fig. 2A. Several conclusions can be drawn. (i) The HPV-11 E1 protein is more flexible in its collaboration with a heterologous E2 protein than is the HPV-16 E1 protein, which does not function in the presence of HPV-11 E2 (Fig. 1, 3, and 4). (ii) The E1 protein can be divided into at least three structural and functional domains on the basis of the ability of hybrid proteins to function in transient replication assays. The amino-terminal residues 1 to 237 or 1 to 365 (E1H1 and E1H2) and the carboxyl-terminal 44 amino acids (E1H5) can be exchanged between the two viral proteins and result in hybrid proteins that exhibit low (5 to 10%), moderate (10 to 50%), or high (50 to 90%) replication activity in the presence of one or both E2 proteins. However, residues 366 to 605, which comprise the putative ATPase domain (H3, H4, and H4.5), are very sensitive to changes within the domain or in the flanking regions, since the hybrid proteins are largely or entirely nonfunctional in the presence of either E2 protein (Fig. 3 and 4). Whether this loss of function is due to the inactivation of the ATPase and helicase activities or to other defects is not known. (iii) The amino-terminal 56% of the HPV-16 E1 protein (residues 1 to 365) does not play a role in E2 protein specificity, as 16/11 E1H1 and E1H2 exhibited high replication activities with both E2 proteins (Fig. 3). That the carboxyl-terminal 44% of the HPV-16 protein (residues 366 to 649) is responsible for this discrimination is substantiated by the reciprocal 11*/16 E1H1 and E1H2 proteins. These latter hybrid proteins exhibited functional properties similar to those of the wild-type HPV-16 E1 and replicated either HPV-16 or HPV-11 ori only in the presence of HPV-16 E2 (Fig. 4). (iv) The reciprocal E1H5 proteins functioned with either E2 protein. Since the discrimination between the two E2 proteins by the hybrid protein containing residues 606 to 649 of HPV-16 is not as stringent as that by the HPV-16 or 11*/16 E1H2 protein that contains HPV-16 E1 residues 366 to 649 (Fig. 3 and 4), we suggest that the domains spanning residues 366 to 605 and residues 606 to 649 of HPV-16 E1 both contribute to the selectivity for the HPV-16 E2 protein. Conversely, in 16/11 H5 and H2, the corresponding domains of HPV-11 E1 confer increasing activities with the HPV-11 E2 protein. Considered together, these two domains spanning residues 366 to 649 of HPV-16 E1 comprise the primary determinant for E2 protein specificity during replication. This conclusion, however, does not necessarily rule out the possibility that the amino-terminal domain of the E1 protein also interacts with E2. But such an interaction does not lead to an E2 selectivity. Taking into account previous reports that the carboxyl domain of HPV E1 binds to the E2 protein, a mechanistic interpretation of our observations is the following. The noncooperativity of the HPV-16 E1 protein with the HPV-11 E2 protein is due to steric interference involving subregions within the carboxyl-terminal 44% of the protein, thereby negating or preventing positive interactions between other subregions within the same domain. (v) The E2 specificity is not related to the ori plasmid used, since similar results were also obtained with an HPV-16 ori plasmid (Fig. 1 and 4B and data not shown). In summary, we have constructed reciprocal chimeric E1 proteins between HPV-11 and HPV-16. The results of transient replication assays demonstrate that the E1 protein consists of at least three structural and functional domains and that the region of HPV-16 E1 responsible for discriminating E2 proteins resides within the carboxyl-terminal 44% of the protein.
Acknowledgments
This work was supported by USPHS grant CA36200.
We thank Harald zur Hausen and Lutz Gissmann for providing cloned HPV-11 and HPV-16 DNAs and Biing-Yuan Lin for technical assistance.
REFERENCES
- 1.Benson J D, Howley P M. Amino-terminal domains of bovine papillomavirus type 1 E1 and E2 proteins participate in complex formation. J Virol. 1995;69:4364–4372. doi: 10.1128/jvi.69.7.4364-4372.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg M, Stenlund A. Functional interactions between papillomavirus E1 and E2 proteins. J Virol. 1997;71:3853–3863. doi: 10.1128/jvi.71.5.3853-3863.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonne-Andrea C, Santucci S, Clertant P, Tillier F. Bovine papillomavirus E1 protein binds specifically DNA polymerase α but not replication protein A. J Virol. 1995;69:2341–2350. doi: 10.1128/jvi.69.4.2341-2350.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonne-Andrea C, Tillier F, McShan G D, van Wilson G, Clertant P. Bovine papillomavirus type 1 DNA replication: the transcriptional activator E2 acts in vitro as a specificity factor. J Virol. 1997;71:6805–6815. doi: 10.1128/jvi.71.9.6805-6815.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bream G L, Ohmstede C A, Phelps W C. Characterization of human papillomavirus type 11 E1 and E2 proteins expressed in insect cells. J Virol. 1993;67:2655–2663. doi: 10.1128/jvi.67.5.2655-2663.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiang C-M, Dong G, Broker T R, Chow L T. Control of human papillomavirus type 11 origin of replication by the E2 family of transcription regulatory proteins. J Virol. 1992;66:5224–5231. doi: 10.1128/jvi.66.9.5224-5231.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiang C-M, Ustav M, Stenlund A, Ho T F, Broker T R, Chow L T. Viral E1 and E2 proteins support replication of homologous and heterologous papillomaviral origins. Proc Natl Acad Sci USA. 1992;89:5799–5803. doi: 10.1073/pnas.89.13.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chow L T, Broker T R. Papillomavirus DNA replication. Intervirology. 1994;37:150–158. doi: 10.1159/000150373. [DOI] [PubMed] [Google Scholar]
- 9.Clertant P, Seif I. A common function for polyoma virus large-T and papillomavirus E1 proteins. Nature. 1984;311:276–279. doi: 10.1038/311276a0. [DOI] [PubMed] [Google Scholar]
- 10.Del Vecchio A, Romanczuk H, Howley P M, Baker C C. Transient replication of human papillomavirus DNAs. J Virol. 1992;66:5949–5958. doi: 10.1128/jvi.66.10.5949-5958.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frattini M G, Laimins L A. The role of the E1 and E2 proteins in the replication of human papillomavirus type 31b. Virology. 1994;204:799–804. doi: 10.1006/viro.1994.1596. [DOI] [PubMed] [Google Scholar]
- 12.Frattini M G, Laimins L A. Binding of the human papillomavirus E1 origin-recognition protein is regulated through complex formation with the E2 enhancer-binding protein. Proc Natl Acad Sci USA. 1994;91:12398–12402. doi: 10.1073/pnas.91.26.12398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gopalakrishnan V, Khan S A. E1 protein of human papillomavirus type 1a is sufficient for initiation of viral DNA replication. Proc Natl Acad Sci USA. 1994;91:9597–9601. doi: 10.1073/pnas.91.20.9597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grussenmeyer T, Scheidtmann K H, Hutchinson M A, Eckhart W, Walter G. Complexes of polyoma virus medium T antigen and cellular proteins. Proc Natl Acad Sci USA. 1985;82:7952–7954. doi: 10.1073/pnas.82.23.7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hughes F J, Romanos M A. E1 protein of human papillomavirus is a DNA helicase/ATPase. Nucleic Acids Res. 1993;21:5817–5823. doi: 10.1093/nar/21.25.5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jenkins O, Earnshaw D, Sarginson G, Del Vecchio A, Tsai J, Kallender H, Amergadzie B, Browne M. Characterization of the helicase and ATPase activity of human papillomavirus type 6b E1 protein. J Gen Virol. 1996;77:1805–1809. doi: 10.1099/0022-1317-77-8-1805. [DOI] [PubMed] [Google Scholar]
- 17.Kuo S-R, Liu J-S, Broker T R, Chow L T. Cell-free replication of the human papillomavirus DNA with homologous viral E1 and E2 proteins and human cell extracts. J Biol Chem. 1994;269:24058–24065. [PubMed] [Google Scholar]
- 18.Leng X, Ludes-Meyers J H, Wilson V G. Isolation of an amino-terminal region of bovine papillomavirus type 1 E1 protein that retains origin binding and E2 interaction capability. J Virol. 1997;71:848–852. doi: 10.1128/jvi.71.1.848-852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li R, Botchan M R. Acidic transcription factors alleviate nucleosome-mediated repression of DNA replication of bovine papillomavirus type 1. Proc Natl Acad Sci USA. 1994;91:7051–7055. doi: 10.1073/pnas.91.15.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu J-S, Kuo S-R, Broker T R, Chow L T. The functions of human papillomavirus type 11 E1, E2 and E2C proteins in cell-free DNA replication. J Biol Chem. 1995;270:27283–27291. doi: 10.1074/jbc.270.45.27283. [DOI] [PubMed] [Google Scholar]
- 21.Liu, J.-S., S.-R. Kuo, T. R. Broker, and L. T. Chow. Unpublished results.
- 22.Lu J Z, Sun Y N, Rose R C, Bonnez W, McCance D J. Two E2 binding sites (E2-BS) alone or one E2-BS plus an A/T-rich region are minimal requirements for the replication of the human papillomavirus type 11 origin. J Virol. 1993;67:7131–7139. doi: 10.1128/jvi.67.12.7131-7139.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lusky M, Fontane E. Formation of the complex of bovine papillomavirus E1 and E2 proteins is modulated by E2 phosphorylation and depends upon sequences within the carboxyl terminus of E1. Proc Natl Acad Sci USA. 1991;88:6363–6367. doi: 10.1073/pnas.88.14.6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lusky M, Hurwitz J, Seo Y S. Cooperative assembly of the bovine papilloma virus E1 and E2 proteins on the replication origin requires an intact E2 binding site. J Biol Chem. 1993;268:15795–15803. [PubMed] [Google Scholar]
- 25.MacPherson P, Thorner L, Parker J M, Botchan M. The bovine papilloma virus E1 protein has ATPase activity essential to viral DNA replication and efficient transformation in cells. Virology. 1994;204:403–408. doi: 10.1006/viro.1994.1544. [DOI] [PubMed] [Google Scholar]
- 26.Mansky K C, Batiza A, Lambert P F. Bovine papillomavirus type 1 E1 and simian virus 40 large T antigen share regions of sequence similarity required for multiple functions. J Virol. 1997;71:7600–7608. doi: 10.1128/jvi.71.10.7600-7608.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsukura T, Kanda T, Furuno A, Yoshikawa H, Kawana T, Yoshiike K. Cloning of monomeric human papillomavirus type 16 DNA integrated within cell DNA from a cervical carcinoma. J Virol. 1986;58:979–982. doi: 10.1128/jvi.58.3.979-982.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohr I J, Clark R, Sun S, Androphy E J, MacPherson P, Botchan M R. Targeting the E1 replication protein to the papillomavirus origin of replication by complex formation with the E2 transactivator. Science. 1990;250:1694–1699. doi: 10.1126/science.2176744. [DOI] [PubMed] [Google Scholar]
- 29.Muller F, Sapp M. Domains of the E1 protein of human papillomavirus type 33 involved in binding to the E2 protein. Virology. 1996;219:247–256. doi: 10.1006/viro.1996.0242. [DOI] [PubMed] [Google Scholar]
- 30.Park P, Copeland W, Yang L, Wang T, Botchan M R, Mohr I J. The cellular DNA polymerase α-primase is required for papillomavirus DNA replication and associates with the viral E1 helicase. Proc Natl Acad Sci USA. 1994;91:8700–8704. doi: 10.1073/pnas.91.18.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Remm M, Brain R, Jenkins J R. The E2 binding sites determine the efficiency of replication for the origin of human papillomavirus type 18. Nucleic Acids Res. 1992;20:6015–6021. doi: 10.1093/nar/20.22.6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sarafi T R, McBride A A. Domains of the BPV-1 E1 replication protein required for origin-specific DNA binding and interaction with the E2 transactivator. Virology. 1995;211:385–396. doi: 10.1006/viro.1995.1421. [DOI] [PubMed] [Google Scholar]
- 33.Sedman J, Stenlund A. Co-operative interaction between the initiator E1 and the transcriptional activator E2 is required for replicator specific DNA replication of bovine papillomavirus in vivo and in vitro. EMBO J. 1995;14:6218–6228. doi: 10.1002/j.1460-2075.1995.tb00312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sedman T, Sedman J, Stenlund A. Binding of E1 and E2 proteins to the origin of replication of bovine papillomavirus. J Virol. 1997;71:2887–2896. doi: 10.1128/jvi.71.4.2887-2896.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seedorf K, Krämmer G, Dürst M, Suhai S, Röwekamp W G. Human papillomavirus type 16 DNA sequence. Virology. 1985;145:181–185. doi: 10.1016/0042-6822(85)90214-4. [DOI] [PubMed] [Google Scholar]
- 36.Seo Y S, Müller F, Lusky M, Hurwitz J. Bovine papillomavirus (BPV)-encoded E1 protein contains multiple activities required for BPV DNA replication. Proc Natl Acad Sci USA. 1993;90:702–706. doi: 10.1073/pnas.90.2.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stenlund A. Papillomavirus DNA replication. In: De Pamphlis M L, editor. DNA replication in eukaryotic cells. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. pp. 679–697. [Google Scholar]
- 38.Sun Y N, Lu J Z, McCance D J. Mapping of HPV-11 E1 binding site and determination of other important cis elements for replication of the origin. Virology. 1996;216:219–222. doi: 10.1006/viro.1996.0050. [DOI] [PubMed] [Google Scholar]
- 39.Sverdrup F, Khan S A. Replication of human papillomavirus (HPV) DNAs supported by the HPV type 18 E1 and E2 proteins. J Virol. 1994;68:505–509. doi: 10.1128/jvi.68.1.505-509.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sverdrup F, Khan S A. Two E2 binding sites alone are sufficient to function as the minimal origin of replication of human papillomavirus type 18 DNA. J Virol. 1995;69:1319–1323. doi: 10.1128/jvi.69.2.1319-1323.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thorner L K, Lim D A, Botchan M R. DNA-binding domain of bovine papillomavirus type 1 E1 helicase: structural and functional aspects. J Virol. 1993;67:6000–6014. doi: 10.1128/jvi.67.10.6000-6014.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ustav M, Stenlund A. Transient replication of BPV-1 requires two viral polypeptides encoded by the E1 and E2 open reading frames. EMBO J. 1991;10:449–457. doi: 10.1002/j.1460-2075.1991.tb07967.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang L, Li R, Mohr I J, Clark R, Botchan M R. Activation of BPV-1 replication in vitro by the transcription factor E2. Nature. 1991;353:628–632. doi: 10.1038/353628a0. [DOI] [PubMed] [Google Scholar]
- 44.Yang L, Mohr I, Fouts E, Lim D A, Nohaile M, Botchan M. The E1 protein of bovine papillomavirus 1 is an ATP-dependent DNA helicase. Proc Natl Acad Sci USA. 1993;90:5086–5090. doi: 10.1073/pnas.90.11.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yasugi T, Benson J D, Sakai H, Vidal M, Howley P M. Mapping and characterization of the interaction domains of human papillomavirus type 16 E1 and E2 proteins. J Virol. 1997;71:891–899. doi: 10.1128/jvi.71.2.891-899.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]