Abstract
INTRODUCTION
We investigated associations of Alzheimer's disease (AD) serum biomarkers with longitudinal changes in cognitive function from mid‐ to late life among women.
METHODS
The study population included 192 women with the median age of 53.3 years at baseline, from the Study of Women's Health Across the Nation Michigan Cohort, followed up over 14 years. Associations between baseline serum amyloid β (Aβ)42, the Aβ42/40 ratio, phosphorylated tau181 (p‐tau181), and total tau with longitudinal changes in cognition were evaluated using linear mixed effects models.
RESULTS
After adjusting for confounders, lower Aβ42/40 ratios were associated with faster declines in the Digit Span Backward Test. Higher p‐tau181 also showed a borderline statistically significant association with more rapid decline in the Symbol Digit Modalities Test.
DISCUSSION
Our findings suggest that mid‐life serum AD biomarkers could be associated with accelerated cognitive decline from mid‐ to late life in women. Future studies with larger samples are needed to validate and extend our findings.
Highlights
This study investigates midlife serum AD biomarkers on longitudinal cognitive function changes in women.
Mid‐life serum AD biomarkers are associated with accelerated cognitive decline.
A decrease in the Aβ42/40 ratio was associated with a faster decline in the DSB score.
A higher p‐tau181 concentration was associated with a faster decline in the SDMT score.
Keywords: Alzheimer's disease, amyloid β, biomarkers, cognitive function, midlife, tau, women
1. BACKGROUND
The growing prevalence of cognitive impairment and dementia in aging populations has led to heightened focus on delaying or preventing these aging‐related conditions. Midlife is increasingly recognized as a pivotal period, particularly for women, for early phases of processes producing cognitive decline and the subsequent development of dementias, including Alzheimer's disease (AD) and related dementias in later life. 1 The menopausal transition, characterized by sharp reduction in estrogen levels owing to irreversible ovarian alterations, is linked to the acceleration of cognitive decline in later life. 2 Higher prevalence of cardiometabolic risk factors in midlife women, such as hypertension and diabetes, is associated with elevated risk of cognitive decline and dementia in older age. 1 , 3 Given the vital role of midlife as an optimal window for early detection and prevention of cognitive decline and dementia, it is crucial to identify reliable predictors for cognitive decline.
AD biomarkers have potential to act as early predictors of cognitive decline in midlife, enabling timely interventions before the development of irreversible dementia in later life. Current assessment methods for pathological hallmarks of AD, such as amyloid β (Aβ) and tau proteins, involve positron emission tomography (PET) neuroimaging or cerebrospinal fluid (CSF) analysis. 4 Although these techniques yield valuable insights into AD‐associated brain pathology, their invasiveness, time‐consuming nature, and high costs limit widespread application in clinical practice, screening programs, and large epidemiologic studies. The development of highly sensitive immunoassays and advanced immunoprecipitation and liquid chromatography‐mass spectrometry assays now facilitate accurate detection of Aβ and tau proteins in blood samples. 4 , 5 These blood‐based biomarkers demonstrate strong correlations with measurements obtained through PET or CSF analysis, and can successfully predict cognitive decline, pathological alterations, and the clinical progression of dementia. 4 , 6 , 7 Blood‐based biomarkers hold significant promises for both clinical and research applications.
The majority of existing blood‐based biomarker research has focused on older populations or those at heightened risk for AD. 7 , 8 , 9 A few studies have investigated the relationship between AD biomarkers and cognitive decline from mid‐ to late life. 10 We examined associations of serum AD biomarkers, including Aβ42, the Aβ42/Aβ40 ratio, phosphorylated tau181 (p‐tau181), and total tau with longitudinal changes in cognitive function from mid‐ to late life among women in the Study of Women's Health Across the Nation (SWAN) Michigan Cohort. We hypothesized that a lower Aβ42/40 ratio and higher levels of p‐tau181 and total tau would be associated with worsening cognitive function and accelerated rates of cognitive decline.
RESEARCH IN CONTEXT
Systematic review: We searched PubMed for articles on the associations of blood‐based Alzheimer's disease (AD) biomarkers with cognitive decline and dementia. The majority of existing research focused on older populations or those at a heightened risk for AD. Few studies investigated the relationship between AD biomarkers and cognitive decline from mid‐ to late life.
Interpretation: Our study provides evidence that serum AD biomarkers, specifically lower Aβ42/40 ratios and higher p‐tau181 levels, are associated with faster cognitive declines from mid‐ to late life in women. These findings suggest that midlife blood AD biomarker assessments may serve as early predictors of cognitive decline, offering an opportunity for early detection and prevention before development of irreversible dementia.
Future directions: Future studies with larger sample sizes are needed to validate and extend our findings.
2. METHODS
2.1. Study population
SWAN is an ongoing, multi‐racial/ethnic, community‐based, prospective study designed to characterize the natural history of menopause and physiological and psychosocial changes during the menopausal transition. 11 Initiated in 1996 to 1997, SWAN enrolled 3302 pre‐menopausal women from seven study sites across the United States. Eligibility criteria included age 42 to 52 years at enrollment, having an intact uterus, having at least one menstrual period in the prior 3 months, and not taking hormone medications in the previous 3 months. More details of the study design of SWAN were previously described. Institutional Review Board approval was obtained at each study site, and all participants provided signed informed consent at each study visit. 12
The current analyses encompassed 198 women from the SWAN Michigan Cohort, from whom stored serum samples were procured during the 7th SWAN follow‐up visit (2003 to 2004), denoted hereafter as the baseline. Each participant underwent at least two comprehensive cognitive function assessments between baseline and the 16th SWAN follow‐up visit in 2017. One participant was excluded from the analysis due to a lack of information on key covariates, and six incident stroke cases were censored during the follow‐up. We further excluded 13 participants with missing information of Aβ42 and Aβ40 serum levels, 12 with missing data of total tau serum levels, and 43 with missing data of p‐tau181 serum levels, all due to inadequate serum volume for assays. The final analytic sample comprised 184 participants for both Aβ42 and Aβ40 analyses (corresponding to 962 cognitive observations), 185 participants for total tau analysis (965 observations), and 154 participants for p‐tau181 analysis (799 observations). A flow chart of the analytic sample is shown in Figure 1.
FIGURE 1.
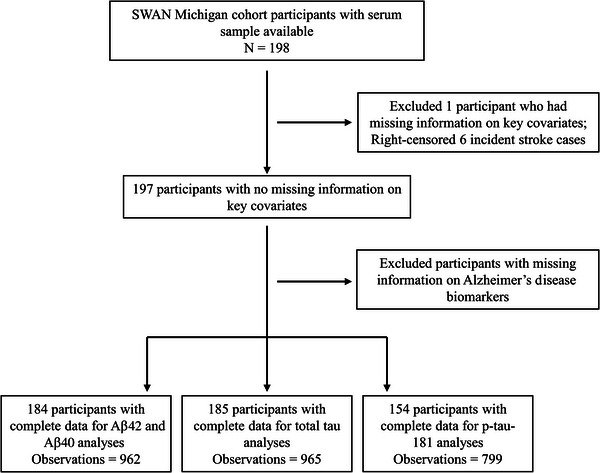
Flow chart of the study design. Aβ, amyloid β; SWAN, Study of Women's Health Across the Nation.
2.2. Cognitive function assessments
Cognitive functions were assessed with four standardized tests. The Symbol Digit Modalities Test (SDMT, range: 0 to 110) assessed processing speed, motor speed, visuospatial function, associative learning, and executive function. 13 The East Boston Memory Test (EBMT, range: 0 to 12) immediate recall component, and the EBMT delayed recall component, were used to measure verbal episodic memory. 14 The Digit Span Backward Test (DSB, range: 0 to 12) evaluated working memory and attention. 15 For each test, a higher score indicates better cognitive function. An adjudication panel resolved discrepancies. In SWAN, cognitive function was initially assessed during the 4th follow‐up visit (2000 to 2002). To minimize the “practice effect” whereby participants’ cognitive test scores improve over time due to learning from repeated testing, particularly during the first and second cognitive assessments, 2 the third cognitive assessment (7th follow‐up visit, 2003 to 2005) was chosen as the analytic baseline, at which the practice effect was no longer evident. 2
2.3. Serum AD biomarkers
Blood samples were collected prior to 10 am after an 8‐hour fasting period. The aliquoted specimens were stored at −80°C in ultra‐low freezers in the SWAN Repository without any instance of thawing. These samples were then shipped frozen to the Michigan Alzheimer's Disease Research Center Biomarker Core Lab at Michigan State University. Serum concentrations of Aβ42, Aβ40, total tau, and p‐tau181 were quantified using the Single Molecule Array (Simoa) assays (Quanterix, located in Billerica, MA, USA). Quality control was ensured by incorporating duplicate samples for each assay. The measured coefficient of variation remained below the 15% cutoff for all biomarkers. Pooled plasma bridge samples were also utilized across all assays as a part of quality control, and no inter‐assay discrepancies were detected.
2.4. Covariates
Data on participants' age, self‐identified race (Black or White), and level of education (high school or less, some college, or college degree or higher) were collected using self‐administered questionnaires. Smoking status (never smoker, former smoker, or current smoker), frequency of alcohol consumption (less than 1 drink/month, 1 drink/month to 1 drink/week, or more than 1 drink/week), and menopausal status (pre‐menopausal, early peri‐menopausal, late peri‐menopausal, post‐menopausal, or unknown due to hormone therapy use) were assessed via standardized interviews. Depressive symptoms were evaluated using the Center for Epidemiologic Studies Depression (CES‐D) Scale.
2.5. Statistical analysis
Distributions of participants’ characteristics and AD biomarker concentrations were examined at baseline. Correlations between AD biomarker concentrations were also calculated.
We employed linear mixed effects models to examine the associations of baseline AD biomarker measurements, including Aβ42, the Aβ42/40 ratio, total tau, and p‐tau181, with repeatedly measured SDMT, DSB, and EBMT scores. To account for within‐participant correlations of recurring cognitive assessments, random intercepts were included in all models. To determine if baseline AD biomarkers were associated with the rate of change in cognitive functions over time, we incorporated multiplicative interaction terms between the elapsed time from baseline and AD biomarker concentrations into each model. Time was modeled using a linear term of year. To better compare the associations of different biomarkers with cognitive scores, we further standardized the biomarkers by subtracting the mean of the corresponding concentrations divided by their standard deviation (SD). Effect estimates were then interpreted as changes in cognitive outcomes associated with one SD increase in AD biomarkers for more comparable results. The covariates adjusted in the models were selected based on a priori knowledge and included the non‐time‐varying variables age (baseline), race, and education; and time‐varying variables comprising follow‐up time, smoking status, alcohol drinking, menopausal status, and CES‐D score. We adjusted p values for multiple comparisons for associations of AD biomarkers with each cognitive outcome at a false discovery rate (FDR) of 0.05 using the Benjamini‐Hochberg method. 16
In the sensitivity analysis, we adjusted for the multiplicative interaction between age at baseline and follow‐up time in all regression models to further control for potential confounding due to age. All analyses were conducted using R, version 4.3.1 (www.R‐project.org).
3. RESULTS
The study population of 192 women with at least one AD biomarker available had a median (interquartile range [IQR]) age at baseline of 53.3 (51.0, 55.6) years, and median (IQR) age at the last follow‐up visit of 64.0 (61.4, 66.3) years. The median (IQR) follow‐up time was 8.5 (11,7, 12.1) years. At baseline, the majority were non‐smokers, consumed alcohol fewer than once per month, and were post‐menopausal (Table 1). Over half of the participants identified as Black (60.9%), and the remaining were White (39.1%).
TABLE 1.
Characteristics of the study population with at least one Alzheimer's disease biomarker available at baseline (N = 192).
| Characteristics | Distribution |
|---|---|
| Age, years, median (IQR) | 53.3 (51.0, 55.6) |
| Follow‐up time, years, median (IQR) | 8.5 (11,7, 12.1) |
| Race, n (%) | |
| White | 75 (39.1) |
| Black | 117 (60.9) |
| Education, n (%) | |
| High school or less | 134 (69.8) |
| Some college | 29 (15.1) |
| College and above | 29 (15.1) |
| Smoking status, n (%) | |
| Never smoked | 105 (54,7) |
| Former smoker | 52 (27.1) |
| Current smoker | 35 (18.2) |
| Alcohol drinking, n (%) | |
| <1 drink/month | 122 (63.5) |
| 1 drink/month to 1 drink/week | 39 (20.3) |
| >1 drink/week | 31 (16.2) |
| Menopausal status, n (%) | |
| Pre‐menopausal | 2 (1.0) |
| Early peri‐menopausal | 52 (27.1) |
| Late peri‐menopausal | 23 (12.0) |
| Post‐menopausal | 101 (59.9) |
| Unknown * | 14 (7.3) |
| Center for epidemiologic studies depression scale, mean (SD) | 9.3 (9.6) |
| Symbol digit modalities test (SDMT), mean (SD) | 53.5 (11.5) |
| Digit span backwards test (DSB), mean (SD) | 6.2 (2.3) |
| East Boston Memory Test (EBMT) immediate recall, mean (SD) | 10.0 (1.8) |
| EBMT delayed recall, mean (SD) | 9.7 (2.0) |
| Aβ42, pg/mL, mean (SD) | 7.9 (4.4) |
| Aβ40, pg/mL, mean (SD) | 157.0 (87.2) |
| Aβ42/40 ratio, mean (SD) | 0.06 (0.04) |
| Total tau, pg/mL, mean (SD) | 3.4 (2.8) |
| Phosphorylated tau181, pg/mL, mean (SD) | 15.9 (10.9) |
Abbreviations: Aβ, amyloid β; IQR, interquartile range; SD, standard deviation.
Menopausal status unknown due to hormone therapy or hysterectomy.
The mean (SD) of cognitive test scores at baseline was 53.5 (11.5) for SDMT, 6.2 (2.3) for DSB, 10.0 (1.8) for EBMT immediate recall, and 9.7 (2.0) for EBMT delayed recall (Table 1). The mean (SD) of AD biomarker concentrations was 7.9 (4.4) pg/mL for Aβ42, 157.0 (87.2) pg/mL for Aβ40, 0.06 (0.04) for the Aβ42/40 ratio, 3.4 (2.8) pg/mL for total tau, and 15.9 (10.9) pg/mL for p‐tau181. Aβ42, Aβ40, total tau, and p‐tau181 were positively correlated, while negatively correlated with the Aβ42/40 ratio (Figure S1).
After adjustment for age at baseline, race, education, follow‐up time, smoking status, alcohol drinking, menopausal status, CES‐D, and interaction terms between AD biomarker concentrations and follow‐up in the linear mixed effects model, a lower Aβ42/40 ratio was associated with a more rapid decline in the DSB score (Table 2). On average, the DSB score decreased −0.06 (95% confidence interval [CI]: −0.11, 0) annually, while an SD decrease in the Aβ42/40 ratio was associated with a further decline of 0.03 (95% CI: 0.01, 0.06) in the DSB score annually (p for interaction = 0.03). However, the interaction was no longer significant (FDR = 0.12) after adjustment for multiple comparisons using FDR. Additionally, participants with higher p‐tau181 concentrations exhibited a faster decline in the SBMT score (−0.08, 95% CI: −0.18, 0.01), though the interaction was borderline statistically significant (p = 0.08). No associations of AD biomarkers with other cognitive function scores were observed.
TABLE 2.
Changes in cognitive scores and their rates of changes per standard deviation increase in Alzheimer's disease biomarker concentrations from linear mixed effects models.
| AD biomarkers | SD | Time effect (95% CI) | Main effect (95% CI) | Interaction with time (95% CI) | p for interaction |
|---|---|---|---|---|---|
| Symbol Digit Modalities Test | |||||
| Aβ42, pg/mL | 4.4 | −0.38 (−0.58, −0.18) | −1.19 (−2.66, 0.28) | 0.03 (−0.06, 0.12) | 0.57 |
| Aβ42/40 ratio | 0.04 | −0.31 (−0.51, −0.12) | −0.14 (−1.59, 1.31) | −0.01 (−0.12, 0.09) | 0.82 |
| Total tau, pg/mL | 2.8 | −0.33 (−0.49, −0.18) | 1.06 (−0.41, 2.53) | 0.01 (−0.09, 0.10) | 0.92 |
| P‐tau181, pg/mL | 10.9 | −0.26 (−0.44, −0.07) | 0.57 (−1.00, 2.15) | −0.08 (−0.18, 0.01) | 0.08 |
| Digit Span Backwards Test | |||||
| Aβ42, pg/mL | 4.4 | 0.01 (−0.05, 0.06) | 0.03 (−0.26, 0.31) | −0.01 (−0.03, 0.02) | 0.64 |
| Aβ42/40 ratio | 0.04 | −0.06 (−0.11, 0) | −0.04 (−0.32, 0.23) | 0.03 (0.01, 0.06) | 0.03 |
| Total tau, pg/mL | 2.8 | 0.01 (−0.04, 0.05) | 0.07 (−0.22, 0.35) | −0.01 (−0.03, 0.02) | 0.54 |
| P‐tau181, pg/mL | 10.9 | −0.02 (−0.07, 0.03) | −0.09 (−0.42, 0.24) | 0.02 (−0.01, 0.05) | 0.17 |
| East Boston Memory Test immediate recall | |||||
| Aβ42, pg/mL | 4.4 | −0.03 (−0.08, 0.02) | −0.04 (−0.25, 0.17) | 0.01 (−0.01, 0.04) | 0.31 |
| Aβ42/40 ratio | 0.04 | −0.03 (−0.08, 0.02) | −0.15 (−0.36, 0.05) | 0.02 (−0.01, 0.05) | 0.22 |
| Total tau, pg/mL | 2.8 | −0.02 (−0.07, 0.01) | 0.03 (−0.18, 0.23) | 0.02 (−0.01, 0.04) | 0.11 |
| P‐tau181, pg/mL | 10.9 | −0.03 (−0.08, 0.02) | 0.03 (−0.20, 0.26) | 0.02 (−0.01, 0.05) | 0.11 |
| East Boston Memory Test–Delayed recall | |||||
| Aβ42, pg/mL | 4.4 | −0.03 (−0.08, 0.03) | −0.06 (−0.28, 0.16) | 0.01 (−0.01, 0.03) | 0.42 |
| Aβ42/40 ratio | 0.04 | −0.02 (−0.07, 0.03) | −0.13 (−0.35, 0.08) | 0.01 (−0.02, 0.03) | 0.73 |
| Total tau, pg/mL | 2.8 | −0.02 (−0.06, 0.02) | 0.11 (−0.11, 0.33) | 0.01 (−0.01, 0.04) | 0.28 |
| P‐tau181, pg/mL | 10.9 | −0.04 (−0.09, 0.01) | 0.05 (−0.19, 0.30) | 0.02 (−0.01, 0.05) | 0.12 |
Note: All models were adjusted for age at baseline, race, education, follow‐up time (year), smoking status, alcohol drinking, menopausal status, and Center for Epidemiologic Studies Depression Scale score. Multiplicative interaction terms between AD biomarkers and follow‐up time were also included in the models to estimate the associations between AD biomarkers and rate of changes in cognitive scores.
Abbreviations: Aβ, amyloid β; AD, Alzheimer's disease; CI, confidence interval; p‐tau181: phosphorylated tau181; SD, standard deviation.
In the sensitivity analysis of further adjusting for interaction terms between age at baseline and follow‐up time, we observed no differences compared to our primary analysis (Table S1).
4. DISCUSSION
In this longitudinal study of mid‐ to late‐life women following over 14 years from the SWAN Michigan cohort, we found that a lower serum Aβ42/40 ratio at baseline was significantly associated with subsequent faster decline in DSB scores, reflecting accelerated decline in attention and working memory. However, this interaction was no longer statistically significant after adjusting for multiple comparisons. We found that higher serum p‐tau181 concentrations were associated with faster declines in SDMT scores, though this association was marginally significant. Both associations were independent of age, race, education, smoking status, alcohol drinking, menopausal status, and depressive symptoms.
Our finding that lower Aβ42/40 ratios were associated with more rapid declines in DSB scores is in line with findings from other studies, though investigations specifically targeting midlife populations remain scarce. Specifically, a study from the Wisconsin Registry for Alzheimer's Prevention (WRAP) with 184 cognitively healthy participants in late middle age (mean age = 60) reported that higher Aβ levels, as measured by PET scans, were associated with greater rates of decline in delayed memory and executive functioning. Notably, similar to our results, the WRAP study did not observe significant associations in their cross‐sectional analysis at baseline, strengthening the value of longitudinal studies in this domain. 17 , 18 Additionally, our results also align with several studies that have examined the associations of blood‐based Aβ with cognitive functions, cognitive impairment, and dementia, although primarily in older populations. For instance, lower plasma Aβ42/40 ratios were associated with more pronounced declines of Mini‐Mental State Examination scores and composite cognitive scores among 483 older adults aged 70 or older, followed over a median of 3.9 years in the Multidomain Alzheimer Preventive Trial. 7 Another longitudinal study of 563 elderly cognitively normal volunteers, with a median age of 78 years, showed that participants with lower plasma Aβ42/40 ratios had significantly greater risk of mild cognitive impairment (MCI) or AD over a median follow‐up period of 3.7 years. 8 The Health ABC Study of 997 older adults (mean age 74.0 years) found that lower plasma Aβ42/40 ratios were associated with greater 9‐year cognitive declines, particularly among those with low cognitive reserve. 9 Most recently, the Atherosclerosis Risk in Communities cohort study found that higher midlife plasma Aβ42/40 ratios were associated with a 37% lower risk of MCI/dementia among 2284 participants aged around 59.2 years at baseline over a 9‐year follow‐up. 10 These findings collectively suggest that the blood Aβ42/40 ratio might reflect underlying brain pathologies occurring prior to manifestation of cognitive impairment features. Our study is one of the first to demonstrate a possibly prospective association between the blood Aβ42/40 ratio and cognitive decline among midlife women. Our findings from this 14‐year longitudinal study involving a cohort of midlife women with a median age of 53.3 years at baseline highlight the potential utility of blood Aβ42/40 as an early predictor of cognitive impairments. It is also important to note that our finding was not significant after adjustment for multiple comparisons. This highlights the need for further research with large samples to confirm these findings.
Our results suggest a potential link between elevated serum p‐tau181 concentrations and faster declines in SDMT scores, though this association is only marginally statistically significant. A few studies have examined this association in midlife. For example, a study of 209 cognitively normal middle‐aged to older participants (mean age = 60.5) from the Adult Children Study reported that CSF p‐tau181 was associated with a faster rate of decline in global cognition. 19 Another investigation of 167 initially cognitively unimpaired participants from the WRAP study in late middle age found that those with tau pathophysiology, as determined by PET scans, exhibited accelerated decline in retrospective cognition compared to those without elevated biomarkers. 20 In populations with older ages or elevated risks of dementia, associations between p‐tau181 concentrations and cognitive decline have been more extensively documented. In a cross‐sectional study of 451 participants at various stages of cognitive decline, higher serum p‐tau181 concentrations were negatively correlated with global cognition. 21 This correlation was strongest among participants with Clinical Dementia Rating scores of 1 or more. A retrospective study involving 404 participants from three independent cohorts, and a cross‐sectional analysis of 243 participants, found that elevated plasma p‐tau181 concentrations were associated with poorer performance across multiple cognitive assessments in AD/MCI patients, particularly those who were Aβ positive. 22 , 23 Blood p‐tau181 concentrations may begin to increase decades before the deposition of tau aggregates and onset of AD clinical features. 24 A recent longitudinal study of 185 participants (mean age = 73.3) from the Alzheimer's Disease Neuroimaging Initiative reported that higher plasma p‐tau181 concentrations were associated with decreased global cognition, executive function, memory, language, and visuospatial functioning in prodromal AD. 6 Future studies focusing on mid‐ to late‐life populations are warranted to verify our results and assess the potential of blood p‐tau181 as a predictive marker for early‐stage cognitive decline, even among individuals with normal cognition.
The main strength of our study lies in its utilization of a longitudinal cohort of midlife women, followed for up to 14 years. To the best of our knowledge, this is the first investigation into the relationships between midlife blood‐based AD biomarkers and longitudinal changes in cognitive function with follow‐up over a decade. Repeated assessments of cognitive function also permitted some assessments of the temporal relations of AD biomarkers and cognitive changes. Our study's sample size, drawn from the SWAN Michigan Cohort, constrained our statistical power to detect more potential associations. The SWAN Michigan Cohort, by design, did not include men and was limited to Black and White participants, restricting the generalizability of our findings. Future research involving larger and more diverse populations in midlife is necessary to extend and validate our findings. Additionally, AD biomarkers were only measured at baseline in the current study. It is possible that changes in AD biomarkers, in addition to baseline values, are associated with accelerated cognitive declines. 25 We encourage future studies to include repeated measurements of AD biomarkers to investigate this hypothesis. Lastly, despite adjusting for numerous known confounders, we were unable to eliminate potential residual confounding, particularly related to APOE4 genotypes, 26 due to the lack of available data. In future studies, we will extract DNA from plasma for reliable APOE genotyping, which would help account for potential residual confounding and offer additional insights into gene–environment interactions.
In conclusion, our study suggests that serum AD biomarkers, specifically lower Aβ42/40 ratios and higher p‐tau181 levels, could be potentially associated with faster cognitive declines from mid‐ to late life in women. These findings suggest that midlife blood AD biomarker assessments may serve as early predictors of cognitive decline, offering an opportunity for early detection and prevention before development of irreversible dementia. Future studies with larger sample sizes and more diverse populations are needed to validate and extend our findings, and further work is needed to translate these biomarkers into practical clinical and public health applications for prediction of cognitive decline and dementia prevention.
CONFLICT OF INTEREST STATEMENT
The authors declare they have no actual or potential competing interests. Author disclosures are available in the supporting information
CONSENT STATEMENT
Institutional Review Board approval was obtained at each study site of SWAN, and all participants provided signed informed consent at each study visit.
Supporting information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The Study of Women's Health Across the Nation (SWAN) has grant support from NIH, DHHS, NIA, the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women's Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, and U19AG063720). This study was supported by grants from the National Institute on Aging (NIA) R01‐AG070897, NIA Michigan Alzheimer's Disease Research Center grant P30AG072931, the University of Michigan Alzheimer's Disease Center (Berger Endowment), and the National Institute of Environmental Health Sciences (NIEHS) Michigan Lifestage Environmental Exposures and Disease (M‐LEEaD) Center grant P30ES017885. The study was also supported by the SWAN Repository (U01AG017719). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH. Clinical Centers: University of Michigan, Ann Arbor—Carrie Karvonen‐Gutierrez, PI 2021–present, Siobán Harlow, PI 2011–2021, MaryFran Sowers, PI 1994–2011; Massachusetts General Hospital, Boston, MA—Sherri‐Ann Burnett‐Bowie, PI 2020–present, Joel Finkelstein, PI 1999–2020, Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Imke Janssen, PI 2020–present, Howard Kravitz, PI 2009–2020, Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser—Elaine Waetjen and Monique Hedderson, PIs 2020–present, Ellen Gold, PI 1994–2020; University of California, Los Angeles—Arun Karlamangla, PI 2020–present, Gail Greendale, PI 1994–2020; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011, Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry–New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA—Rebecca Thurston, PI 2020–present, Karen Matthews, PI 1994–2020. NIH Program Office: National Institute on Aging, Bethesda, MD—Rosaly Correa‐de‐Araujo, 2020–present, Chhanda Dutta, 2016–present, Winifred Rossi, 2012–2016, Sherry Sherman, 1994–2012, Marcia Ory, 1994–2001. National Institute of Nursing Research, Bethesda, MD, Program Officers: Central Laboratory, University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services); NIA Biorepository—Rosaly Correa‐de‐Araujo, 2019–present; SWAN Repository, University of Michigan, Ann Arbor—Siobán Harlow, 2013–2018, Dan McConnell, 2011–2013, MaryFran Sowers, 2000–2011; Coordinating Center, University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012–present, Kim Sutton‐Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001. Steering Committee: Susan Johnson, Current Chair; Chris Gallagher, Former Chair. The authors thank the study staff at each site and all the women who participated in SWAN.
Wang X, Bakulski KM, Karvonen‐Gutierrez CA, et al. Blood‐based biomarkers for Alzheimer's disease and cognitive function from mid‐ to late life. Alzheimer's Dement. 2024;20:1807–1814. 10.1002/alz.13583
REFERENCES
- 1. Deckers K, Van Boxtel MPJ, Schiepers OJG, et al. Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observational studies. Int J Geriatr Psychiatry. 2015;30(3):234‐246. doi: 10.1002/GPS.4245 [DOI] [PubMed] [Google Scholar]
- 2. Karlamangla AS, Lachman ME, Han WJ, Huang MH, Greendale GA. Evidence for cognitive aging in midlife women: study of Women's health across the nation. PLOS ONE. 2017;12(1). doi: 10.1371/JOURNAL.PONE.0169008. e0169008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Derby CA, Hutchins F, Greendale GA, et al. Cardiovascular risk and midlife cognitive decline in the Study of Women's Health Across the Nation. Alzheimers Dement. 2021;17(8):1342‐1352. doi: 10.1002/alz.12300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teunissen CE, Verberk IMW, Thijssen EH, et al. Blood‐based biomarkers for Alzheimer's disease: towards clinical implementation. Lancet Neurol. 2022;21(1):66‐77. doi: 10.1016/S1474-4422(21)00361-6 [DOI] [PubMed] [Google Scholar]
- 5. Palmqvist S, Insel PS, Stomrud E, et al. Cerebrospinal fluid and plasma biomarker trajectories with increasing amyloid deposition in Alzheimer's disease. EMBO Molecular Medicine. 2019;11(12):e11170. doi: 10.15252/emmm.201911170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang YL, Chen J, Du ZL, et al. Plasma p‐tau181 level predicts neurodegeneration and progression to Alzheimer's dementia: a longitudinal study. Front Neurol. 2021;12:1337. doi: 10.3389/FNEUR.2021.695696/BIBTEX [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Giudici KV, de Souto, Barreto P, Guyonnet S, et al. Assessment of Plasma amyloid‐β42/40 and cognitive decline among community‐dwelling older adults. JAMA Network Open. 2020;3(12). doi: 10.1001/jamanetworkopen.2020.28634. e2028634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graff‐Radford NR, Crook JE, Lucas J, et al. Association of low plasma Aβ42/Aβ40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64(3):354‐362. doi: 10.1001/archneur.64.3.354 [DOI] [PubMed] [Google Scholar]
- 9. Yaffe K, Weston A, Graff‐Radford NR, et al. Association of plasma β‐Amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011;305(3):261‐266. doi: 10.1001/jama.2010.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sullivan KJ, Blackshear C, Simino J, et al. Association of midlife plasma amyloid‐β levels with cognitive impairment in late life: the ARIC neurocognitive study. Neurol. 2021;97(11):e1123‐e1131. doi: 10.1212/WNL.0000000000012482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Santoro N, Sutton‐Tyrrell K. The SWAN song: study of Women's health across the Nation's recurring themes. Obstet Gynecol Clin North Am. 2011;38(3):417‐423. doi: 10.1016/j.ogc.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang X, Ding N, Harlow SD, et al. Exposure to heavy metals and hormone levels in midlife women: the Study of Women's Health Across the Nation (SWAN). Environ Pollut. 2022;317:120740. doi: 10.1016/J.ENVPOL.2022.120740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Salthouse TA. The processing‐speed theory of adult age differences in cognition. Psychol Rev. 1996;103(3):403‐428. doi: 10.1037/0033-295X.103.3.403 [DOI] [PubMed] [Google Scholar]
- 14. Albert M, Smith LA, Scherr PA, Taylor JO, Evans DA, Funkenstein HH. Use of brief cognitive tests to identify individuals in the community with clinically diagnosed Alzheimer's disease. Int J Neurosci. 1991;57(3‐4):167‐178. doi: 10.3109/00207459109150691 [DOI] [PubMed] [Google Scholar]
- 15. Tulsky DS, Price LR. The joint WAIS‐III and WMS‐III factor structure: development and cross‐validation of a six‐factor model of cognitive functioning. Psychological Assessment. 2003;15(2):149‐162. doi: 10.1037/1040-3590.15.2.149 [DOI] [PubMed] [Google Scholar]
- 16. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statistical Society Series B (Methodological). 1995;57(1):289‐300. [Google Scholar]
- 17. Clark LR, Racine AM, Koscik RL, et al. Beta‐amyloid and cognitive decline in late middle age: findings from the Wisconsin Registry for Alzheimer's prevention study. Alzheimer Dement. 2016;12(7):805‐814. doi: 10.1016/j.jalz.2015.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Okonkwo OC, Oh JM, Koscik R, et al. Amyloid burden, neuronal function, and cognitive decline in middle‐aged adults at risk for Alzheimer's disease. J Int Neuropsychol Society. 2014;20(4):422‐433. doi: 10.1017/S1355617714000113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiong C, Jasielec MS, Weng H, et al. Longitudinal relationships among biomarkers for Alzheimer disease in the Adult Children Study. Neurol. 2016;86(16):1499‐1506. doi: 10.1212/WNL.0000000000002593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Betthauser TJ, Koscik RL, Jonaitis EM, et al. Amyloid and tau imaging biomarkers explain cognitive decline from late middle‐age. Brain. 2020;143(1):320‐335. doi: 10.1093/brain/awz378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiao Z, Wu X, Wu W, et al. Plasma biomarker profiles and the correlation with cognitive function across the clinical spectrum of Alzheimer's disease. Alzheimers Res Ther. 2021;13:123. doi: 10.1186/s13195-021-00864-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thijssen EH, La Joie R, Wolf A, et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer's disease and frontotemporal lobar degeneration. Nat Med. 2020;26(3):387‐397. doi: 10.1038/s41591-020-0762-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huang Y, Li Y, Xie F, Guo Q. Associations of plasma phosphorylated tau181 and neurofilament light chain with brain amyloid burden and cognition in objectively defined subtle cognitive decline patients. CNS Neurosci Ther. 2022;28(12):2195‐2205. doi: 10.1111/cns.13962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barthélemy NR, Li Y, Joseph‐Mathurin N, et al. A soluble phosphorylated tau signature links tau, amyloid and the evolution of stages of dominantly inherited Alzheimer's disease. Nat Med. 2020;26(3):398‐407. doi: 10.1038/s41591-020-0781-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luo J, Agboola F, Grant E, et al. Accelerated longitudinal changes and ordering of Alzheimer disease biomarkers across the adult lifespan. Brain. 2022;145(12):4459‐4473. doi: 10.1093/brain/awac238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Serrano‐Pozo A, Das S, Hyman BT. APOE and Alzheimer's disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021;20(1):68‐80. doi: 10.1016/S1474-4422(20)30412-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information


