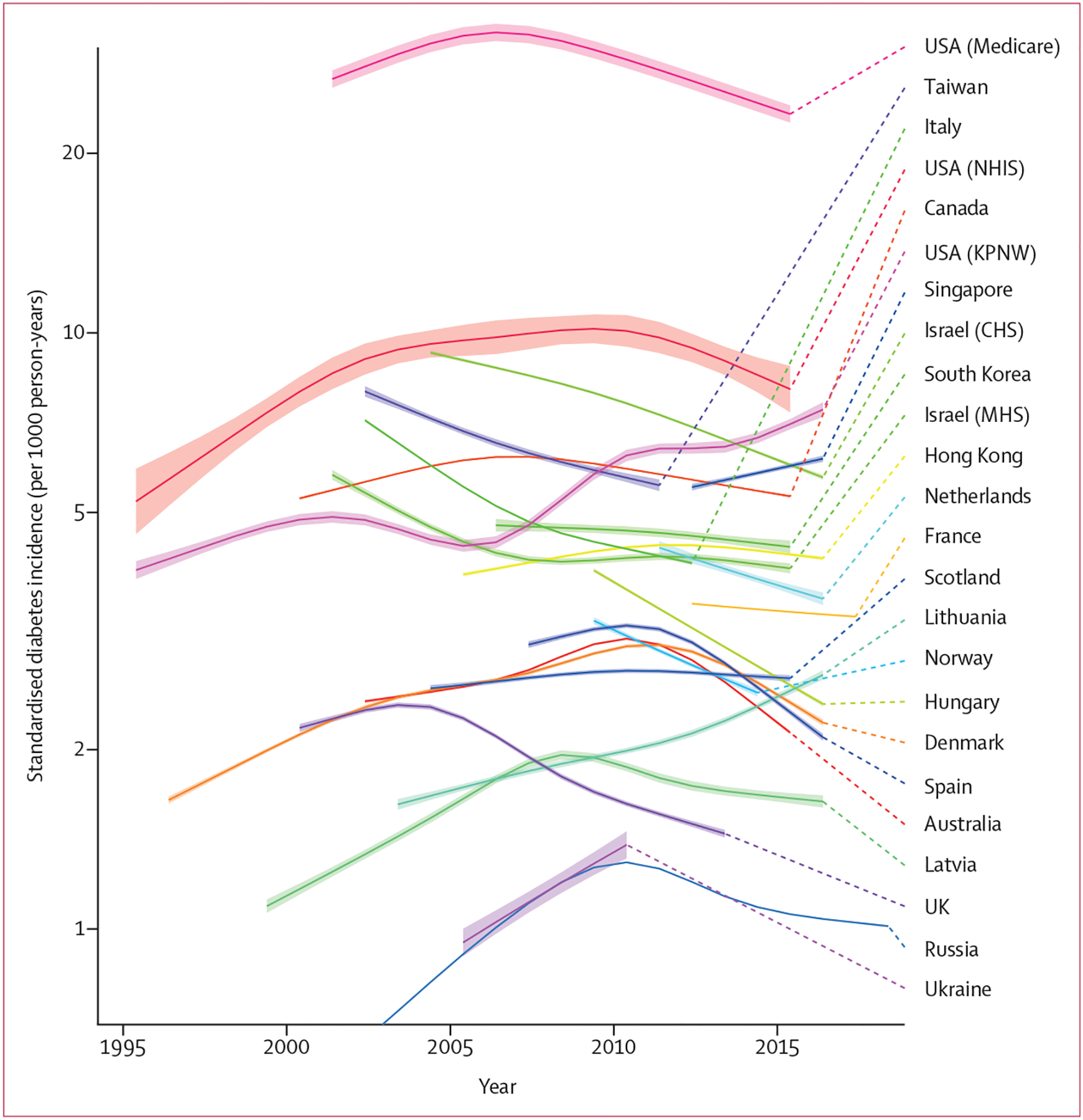
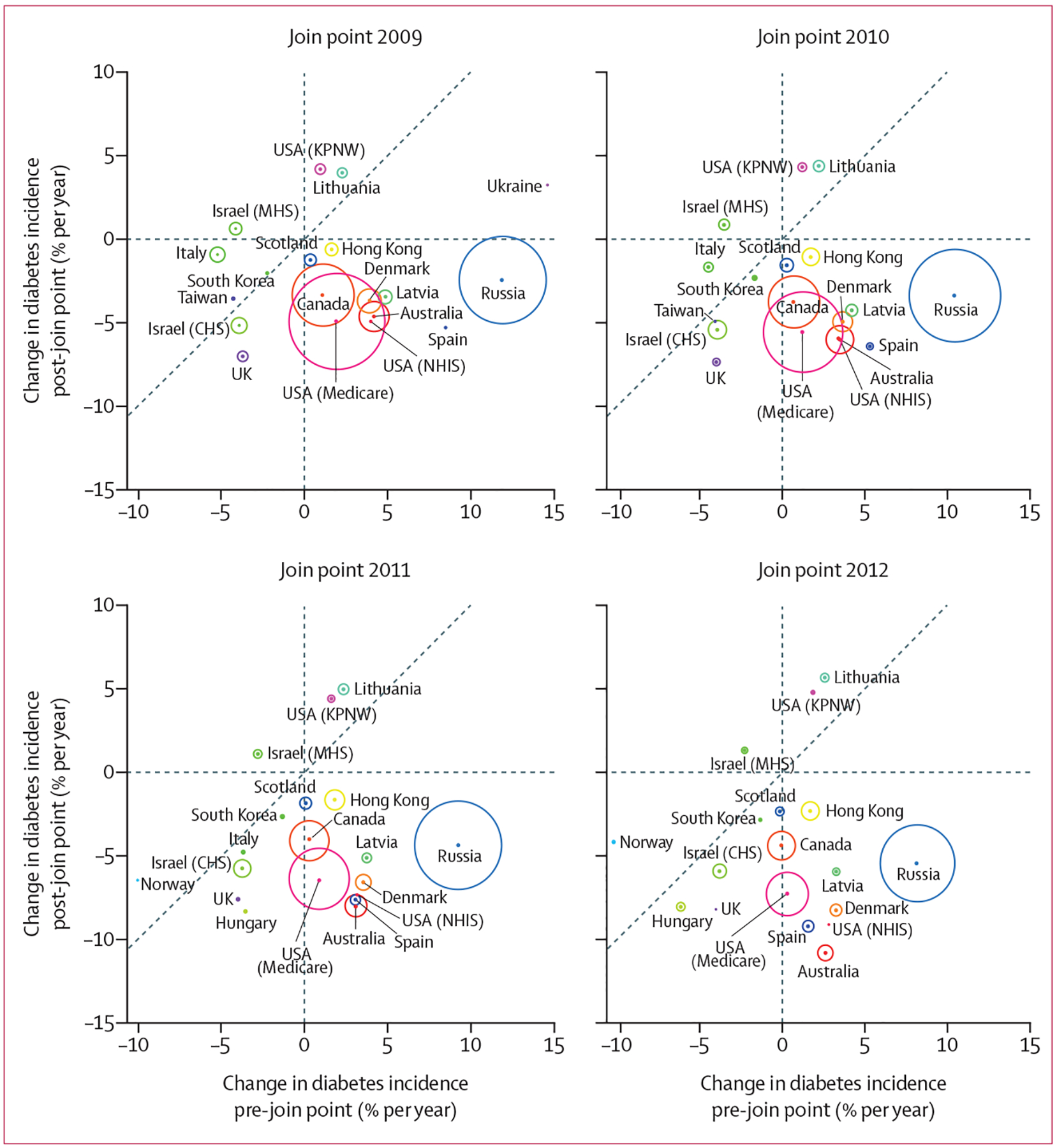
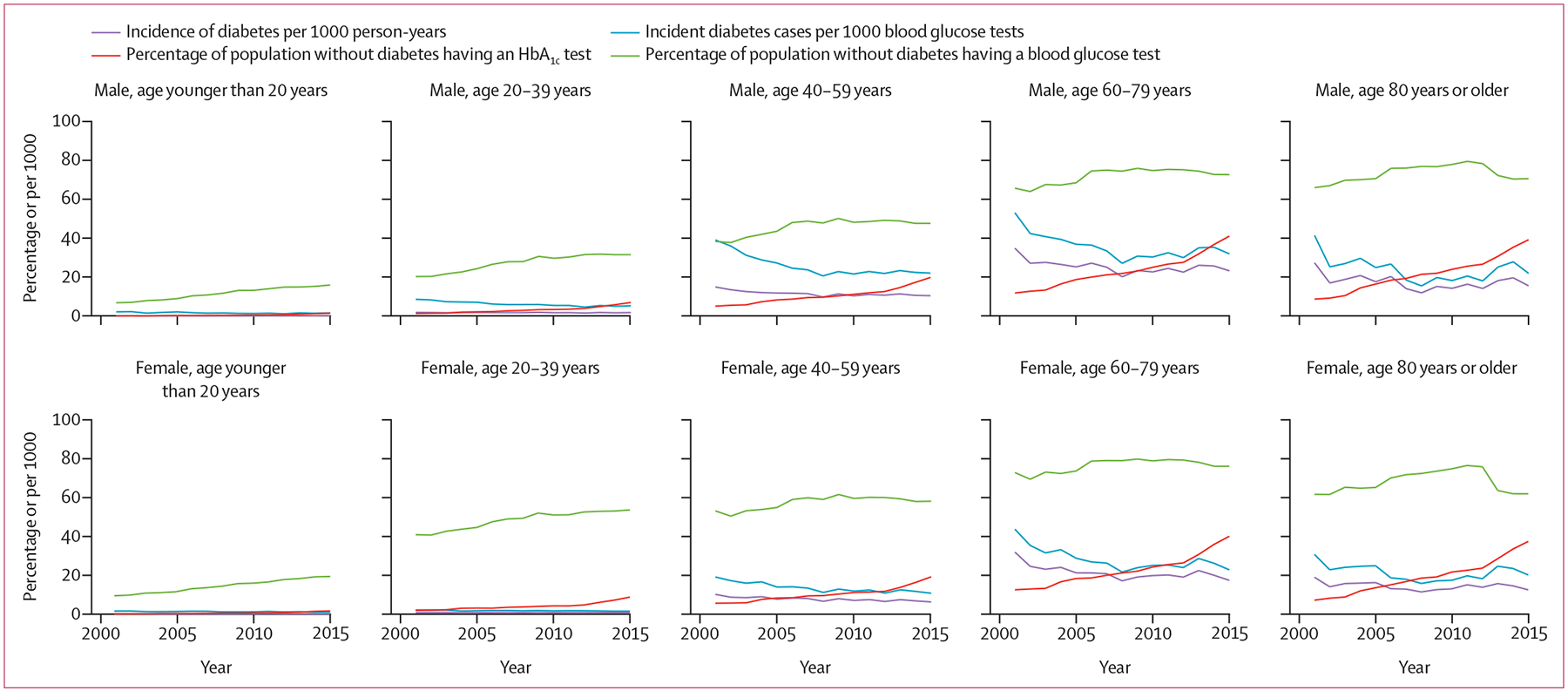
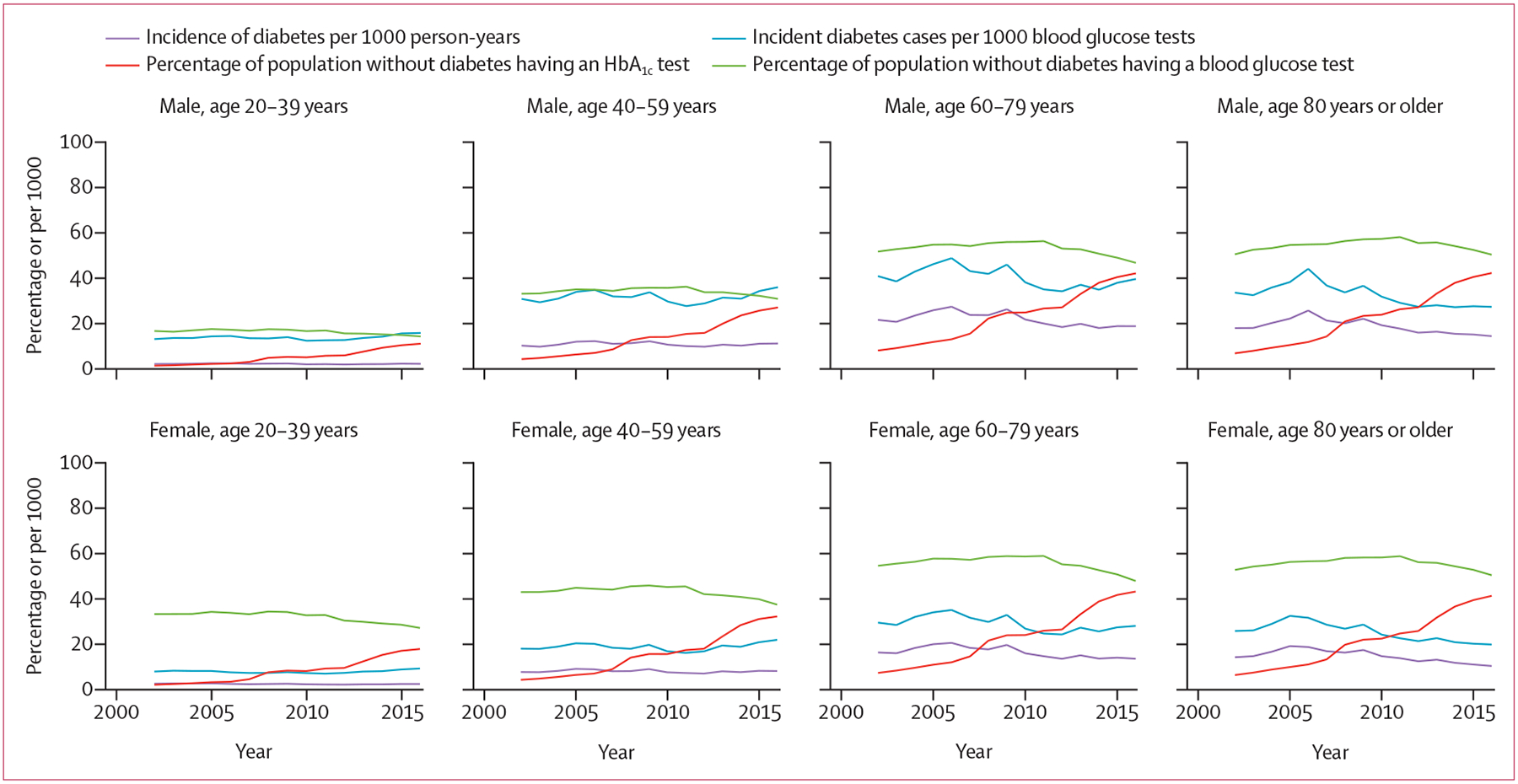
Dianna J Magliano
Dianna J Magliano
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Lei Chen
Lei Chen
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Rakibul M Islam
Rakibul M Islam
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Bendix Carstensen
Bendix Carstensen
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Edward W Gregg
Edward W Gregg
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Meda E Pavkov
Meda E Pavkov
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Linda J Andes
Linda J Andes
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Ran Balicer
Ran Balicer
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Marta Baviera
Marta Baviera
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Elise Boersma-van Dam
Elise Boersma-van Dam
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Gillian L Booth
Gillian L Booth
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Juliana C N Chan
Juliana C N Chan
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Yi Xian Chua
Yi Xian Chua
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Sandrine Fosse-Edorh
Sandrine Fosse-Edorh
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Sonsoles Fuentes
Sonsoles Fuentes
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Hanne L Gulseth
Hanne L Gulseth
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Romualdas Gurevicius
Romualdas Gurevicius
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Kyoung Hwa Ha
Kyoung Hwa Ha
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Thomas R Hird
Thomas R Hird
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
György Jermendy
György Jermendy
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Mykola D Khalangot
Mykola D Khalangot
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Dae Jung Kim
Dae Jung Kim
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Zoltán Kiss
Zoltán Kiss
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Victor I Kravchenko
Victor I Kravchenko
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Maya Leventer-Roberts
Maya Leventer-Roberts
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Chun-Yi Lin
Chun-Yi Lin
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Andrea O Y Luk
Andrea O Y Luk
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Manel Mata-Cases
Manel Mata-Cases
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Didac Mauricio
Didac Mauricio
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Gregory A Nichols
Gregory A Nichols
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Mark M Nielen
Mark M Nielen
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Deanette Pang
Deanette Pang
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Sanjoy K Paul
Sanjoy K Paul
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Catherine Pelletier
Catherine Pelletier
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Santa Pildava
Santa Pildava
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Avi Porath
Avi Porath
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Stephanie H Read
Stephanie H Read
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Maria Carla Roncaglioni
Maria Carla Roncaglioni
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Paz Lopez-Doriga Ruiz
Paz Lopez-Doriga Ruiz
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Marina Shestakova
Marina Shestakova
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Olga Vikulova
Olga Vikulova
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Kang-Ling Wang
Kang-Ling Wang
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Sarah H Wild
Sarah H Wild
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Naama Yekutiel
Naama Yekutiel
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1,
Jonathan E Shaw
Jonathan E Shaw
1Department of Diabetes and Population Health, Baker Heart and Diabetes Institute, Melbourne, VIC, Australia (Prof D J Magliano PhD, L Chen PhD, T R Hird PhD, Prof J E Shaw MD); School of Public Health and Preventive Medicine, Monash University, Melbourne, VIC, Australia (Prof D J Magliano, R M Islam PhD, T R Hird, Prof J E Shaw); Clinical Epidemiology, Steno Diabetes Center Copenhagen, Gentofte, Denmark (B Carstensen MSc [Stat]); Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, UK (Prof E W Gregg PhD); Division of Diabetes Translation, Centers for Disease Control and Prevention, Atlanta, GA, USA (M E Pavkov MD, L J Andes PhD); Clalit Research Institute, Clalit Health Services, Tel Aviv, Israel (Prof R Balicer MD, M Leventer-Roberts MD); Laboratory of Cardiovascular Prevention, Mario Negri Institute for Pharmacological Research IRCCS, Milan, Italy (M Baviera PharmD, M C Roncaglioni MSc); Department of General Practice, Netherlands Institute for Health Services Research, Utrecht, Netherlands (E Boersma-van Dam MSc, M M Nielen PhD); Department of Medicine, University of Toronto, Toronto, ON, Canada (Prof G L Booth MD); Department of Medicine and Therapeutics, Chinese University of Hong Kong, Hong Kong Special Administrative Region, China (Prof J C N Chan MD, A O Y Luk MD); Epidemiology and Disease Control Division, Public Health Group, Ministry of Health, Singapore (Y X Chua MSc, D Pang BSocSci [Hons]); Department of Non-Communicable Diseases and Trauma, Santé Publique France, Saint-Maurice, France (S Fosse-Edorh MS, S Fuentes MPH); Department for Chronic Diseases and Ageing, Norwegian Institute of Public Health, Oslo, Norway (H L Gulseth MD, P Lopez-Doriga Ruiz MD); Center of Health Information, Institute of Hygiene, Vilnius, Lithuania (Prof R Gurevicius MD); Faculty of Public Governance and Business, Mykolas Romeris University, Vilnius, Lithuania (Prof R Gurevicius); Department of Endocrinology and Metabolism, Ajou University School of Medicine, Suwon, South Korea (K H Ha PhD, Prof D J Kim MD); 3rd Medical Department, Bajcsy-Zsilinszky Hospital, Budapest, Hungary (G Jermendy DSc); Komisarenko Institute of Endocrinology and Metabolism, National Academy of Medical Sciences, Kyiv, Ukraine (Prof M D Khalangot ScD, Prof V I Kravchenko ScD); Endocrinology Department, Shupyk National Medical Academy of Postgraduate Education, Kyiv, Ukraine (Prof M D Khalangot); 2nd Department of Medicine and Nephrological Center, Medical Faculty, University of Pécs, Pécs, Hungary (Z Kiss MD); Department of Pediatrics and Department of Environmental Medicine and Public Health, Icahn School of Medicine at Mount Sinai, New York, NY, USA (M Leventer-Roberts); General Clinical Research Center, Taipei Veterans General Hospital, Taipei, Taiwan (C-Y Lin MSc, K-L Wang MD); CIBER of Diabetes and Associated Metabolic Diseases, Instituto de Salud Carlos III, Barcelona, Spain (M Mata-Cases PhD, D Mauricio MD); DAP-Cat Group, Institut Català de la Salut, Unitat de Suport a la Recerca Barcelona Ciutat, Institut Universitari d’Investigació en Atenció Primària Jordi Gol, Barcelona, Spain (M Mata-Cases, D Mauricio); Department of Endocrinology and Nutrition, Hospital de la Santa Creu i Sant Pau, Autonomous University of Barcelona, Barcelona, Spain (D Mauricio); Science Programs Department, Kaiser Permanente Center for Health Research, Portland, OR, USA (G A Nichols PhD); Melbourne EpiCentre, University of Melbourne, Melbourne, VIC, Australia (Prof S K Paul PhD); Centre for Surveillance and Applied Research, Public Health Agency of Canada, Ottawa, ON, Canada (C Pelletier MSc); Research and Health Statistics Department, Centre for Disease Prevention and Control, Riga, Latvia (S Pildava Mg Sc Sal); Research Institute, Maccabi Healthcare Services, Tel Aviv, Israel (Prof A Porath MD, N Yekutiel MSc); Faculty of Health, Ben Gurion University, Beer-Sheva, Israel (Prof A Porath); Usher Institute, University of Edinburgh, Edinburgh, UK (S H Read PhD, Prof S H Wild PhD); Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway (P Lopez-Doriga Ruiz); Diabetes Institute, Endocrinology Research Center, Moscow, Russia (Prof M Shestakova PhD, O Vikulova PhD); School of Life Sciences, Latrobe University, Bundoora, VIC, Australia (Prof J E Shaw)
1