Abstract
Background and Aims
Haemodynamic instability is associated with peri-operative myocardial injury, particularly in patients receiving renin–angiotensin system (RAS) inhibitors (angiotensin-converting-enzyme inhibitors/angiotensin II receptor blockers). Whether stopping RAS inhibitors to minimise hypotension, or continuing RAS inhibitors to avoid hypertension, reduces peri-operative myocardial injury remains unclear.
Methods
From 31 July 2017 to 1 October 2021, patients aged ≥60 years undergoing elective non-cardiac surgery were randomly assigned to either discontinue or continue RAS inhibitors prescribed for existing medical conditions in six UK centres. Renin–angiotensin system inhibitors were withheld for different durations (2–3 days) before surgery, according to their pharmacokinetic profile. The primary outcome, masked to investigators, clinicians, and patients, was myocardial injury [plasma high-sensitivity troponin-T (hs-TnT) ≥ 15 ng/L within 48 h after surgery, or ≥5 ng/L increase when pre-operative hs-TnT ≥15 ng/L]. Pre-specified adverse haemodynamic events occurring within 48 h of surgery included acute hypertension (>180 mmHg) and hypotension requiring vasoactive therapy.
Results
Two hundred and sixty-two participants were randomized to continue (n = 132) or stop (n = 130) RAS inhibitors. Myocardial injury occurred in 58 (48.3%) patients randomized to discontinue, compared with 50 (41.3%) patients who continued, RAS inhibitors [odds ratio (for continuing): 0.77; 95% confidence interval (CI) 0.45–1.31]. Hypertensive adverse events were more frequent when RAS inhibitors were stopped [16 (12.4%)], compared with 7 (5.3%) who continued RAS inhibitors [odds ratio (for continuing): 0.4; 95% CI 0.16–1.00]. Hypotension rates were similar when RAS inhibitors were stopped [12 (9.3%)] or continued [11 (8.4%)].
Conclusions
Discontinuing RAS inhibitors before non-cardiac surgery did not reduce myocardial injury, and could increase the risk of clinically significant acute hypertension. These findings require confirmation in future studies.
Keywords: Myocardial injury, Non-cardiac surgery, Major cardiac events, Peri-operative care, Renin–angiotensin system inhibitors
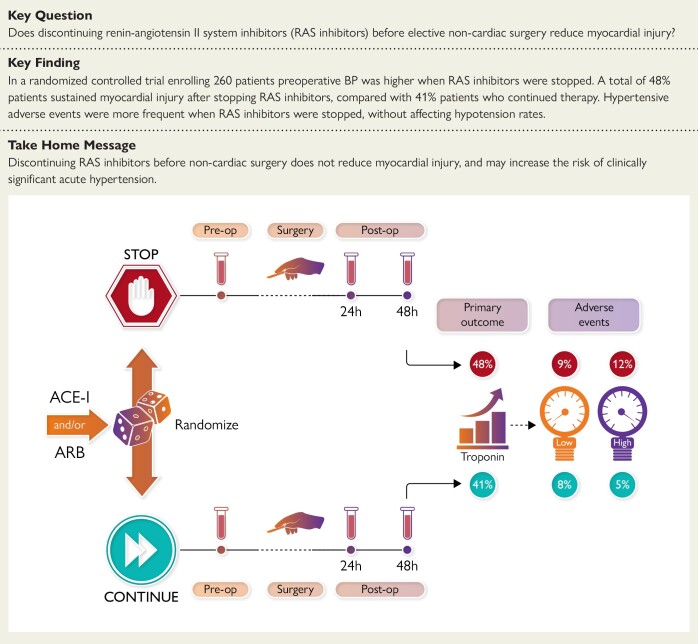
Structured Graphical Abstract
Structured Graphical Abstract.
In a randomized controlled trial enrolling 260 patients, did stopping renin–angiotensin system (RAS) inhibitors [angiotensin-converting-enzyme inhibitors (ACE-I)/angiotensin II receptor blockers (ARB)] before elective non-cardiac surgery reduce myocardial injury, taking into account the pharmacokinetic profile of individual RAS inhibitors? Pre-operative blood pressure was higher when RAS inhibitors were stopped. Forty-eight per cent of patients sustained myocardial injury after stopping RAS inhibitors, compared with 41% of patients who continued therapy. Hypertensive adverse events were more frequent when RAS inhibitors were stopped, without affecting hypotension rates. Discontinuing RAS inhibitors before non-cardiac surgery does not appear to reduce myocardial injury, and could increase the risk of clinically significant acute hypertension.
See the editorial comment for this article ‘Pre-operative withdrawal of renin-angiotensin inhibitors: time to re-visit current guidelines', by W.S. Beattie, https://doi.org/10.1093/eurheartj/ehad719.
Introduction
Myocardial injury after non-cardiac surgical procedures1,2 occurs frequently in patients with pre-existing cardio-metabolic pathology.3 Myocardial injury, independent of symptoms or ECG changes,2 increases the risk of death and further cardiovascular complications—even after discharge from hospital. Surgical patients at most risk of post-operative complications4,5 are commonly prescribed renin–angiotensin system (RAS) inhibitors [angiotensin-converting-enzyme inhibitors (ACE-I) and/or angiotensin II receptor blockers (ARB)] to treat hypertension, chronic kidney disease, and cardiac failure,6–8 which reduce organ injury, in part, by reducing systemic inflammation.9
The 2022 ESC guidelines reflect clinical uncertainty regarding RAS inhibitors during surgery.10–12 The dominance of observational studies,13 rather than randomized controlled trials with outcomes masked to investigators, have informed these divergent recommendations.10 RAS inhibitors are frequently stopped before surgery in the widely held belief that this prevents intra-operative hypotension,10,11 which may increase the risk of myocardial injury and death in older patients.14 Moreover, hypotension in combination with acute hypertension increases the risk even further of myocardial injury during the peri-operative period in non-cardiac surgery.15 The failure to restart RAS inhibitors after surgery, and hence likely increase the risk of poorly controlled blood pressure, is also associated with increased mortality.16,17 The markedly variable pharmacokinetic profiles of RAS inhibitors may also account for inconsistent study findings, since these reports assume that the drugs (particularly ARB) are no longer active during the early peri-operative period. Many clinicians simply stop ACE-I the day before surgery, but pharmacokinetic studies show that stopping these drugs for <24 h does not alter their biological activity.18
Accordingly, we conducted a multi-centre, randomized, open-label trial to assess whether the discontinuation of RAS inhibitors would reduce myocardial injury and post-operative complications, whilst assessing whether avoidance of haemodynamic instability would reduce post-operative morbidity.
Methods
Trial design and oversight
Before the trial started, SPACE was registered as a Clinical Trial of an Investigational Medicinal Product with Eudract on 2016-004141-90. The trial was also registered before the first patient was recruited with the International Traditional Medicine Clinical Trial Registry on 29 May 2017 as ISRCTN17251494 (doi.org/10.1186/ISRCTN17251494).
Patients underwent screening at six centres in the UK. The trial protocol (Supplementary data online) was approved by the London (City and East) Research Ethics Committee (16/LO/1495), the Health Research Authority (UK), and the Medicines and Healthcare products Regulatory Agency (UK). The trial was funded by the British Oxygen Company research chair grant from the Royal College of Anaesthetists, administered by the National Institute for Academic Anaesthesia and the National Institute for Health Research (NIHR); no industry support was provided. An independent steering committee whose members were unaware of trial-group assignments oversaw the conduct of the trial (Supplementary data online). A data and safety monitoring committee monitored patient safety in an unblinded manner (Supplementary data online).
Patients
Adults prescribed RAS inhibitors aged ≥60 years, with American Society of Anesthesiologists physical status grade 3 or above undergoing elective major surgery requiring general anaesthesia lasting longer than 120 min were eligible. Exclusion criteria included current participation in any other interventional clinical trials and myocardial infarction within the 3 months preceding surgery. We did not exclude patients taking ACE-I/ARB for left ventricular dysfunction (ejection fraction <50%). All patients provided written informed consent.
Randomization
Patients were randomly assigned in a 1:1 ratio either to discontinue or to continue RAS inhibitors. Randomization was performed by a centralized internet-based system. Minimization was used to ensure balance between the two groups for the following variables: centre, planned surgical procedure category, and ACE-I/ARB (ACE-I, ARB, or both). Each participant was allocated with 80% probability to the treatment group that minimizes between group differences in these factors among all participants recruited to the trial to date, and to the alternative group with 20% probability.
Treatment
For patients randomized to discontinue RAS inhibitors, the duration of cessation before surgery was defined by the pharmacokinetics of each drug (see Supplementary data online, Appendix for full protocol). For RAS inhibitors with a duration of action ≥24 h, the drug was discontinued 48 h prior to surgery. All other ACE-I and/or ARB were stopped on the morning of the day (i.e. 24–32 h) before surgery.
After randomization, participants received confirmation of which treatment group they have been allocated to and reminded of their randomized allocation by daily telephone call and/or text message, or in person if they were in hospital. Renin–angiotensin system inhibitors were restarted after surgery on the morning of post-operative day 2, in accord with recommendations by the ESC guidelines.12 Resumption or continuation of ACE-I and/or ARB did not involve dose modifications. Recommencing RAS inhibitors was delayed on post-operative day 2 if systolic blood pressure was <90 mmHg in the preceding 12 h, vasoactive therapy was required to maintain blood pressure and/or if acute kidney injury [as defined by Kidney Disease Improving Global Outcomes (KDIGO) criteria] had been sustained.19 The decision to restart ACE-I and/or ARB was confirmed by the principal investigator at each site. All other treatments (concomitant care) were administered according to usual peri-operative practice and were not influenced by trial-group allocation, in accord with recent ESC peri-operative guidelines.12
Follow-up
Blood samples were collected before induction of anaesthesia and on the morning 24 and 48 h after surgery. Investigators reviewed the medical record of participants (paper or electronic) at 24, 48, and 72 h after surgery. To minimise bias, follow-up data were collected by a study team member who was masked to the treatment group allocation. The schedule of assessments is detailed in the protocol.
Primary endpoint
The primary endpoint was the occurrence of myocardial injury, a binary variable based on plasma high-sensitivity troponin-T (hs-TnT) levels (fifth generation assay, Roche) measured in blood samples collected immediately before the induction of anaesthesia, and then at 24 and 48 h after surgery. Peri-operative myocardial injury was defined solely by the (hs)-cTn criterion, independent of symptoms or ECG changes,2 using the same threshold criteria as the VISION study.1 The primary endpoint was achieved provided either troponin-T ≥ 15 ng/L within 48 h after surgery with a pre-operative value <15 ng/L or troponin-T increase ≥5 ng/L within 48 h after surgery when pre-operative value ≥15 ng/L. Troponin-T was measured at a common central laboratory (The Doctor’s Laboratory, London, UK) by laboratory personnel masked to treatment allocation after the last randomized patient completed follow-up. We did not adjudicate whether raised troponin-T levels were primarily due to myocardial ischaemia by electrocardiographic criteria, since acute rises are associated with poorer outcomes.20 The primary outcome could not be met if the pre-operative sample was not collected or the day 1 and day 2 samples were not collected even though the pre-operative sample had been.
In accord with the Standardized Endpoints Consensus for peri-operative cardiovascular outcomes,21 we report myocardial injury and the rates of heart failure and myocardial infarction since they have significantly different outcome trajectories.2,22,23 The definitions for myocardial infarction, acute heart failure, and stroke are included in the Supplementary data online (as adopted by the Standardized Endpoints Consensus for peri-operative cardiovascular outcomes).21
Secondary endpoints
The secondary endpoints were: the highest absolute level of troponin-T measured within 48 h of surgery, clinically defined myocardial infarction within 30 days after randomization, clinically defined acute heart failure within 30 days after randomization, clinically defined stroke within 30 days after randomization, infection within 30 days after randomization, and mortality within 30 days of randomization.
Safety outcomes
Safety outcomes were hypotension deemed by the attending clinician to require pressor vasoactive medication delivered via central venous access (from induction of anaesthesia until 48 h after surgery), acute hypertension (systolic blood pressure >180 mmHg and/or diastolic blood pressure >100 mmHg, from the time of randomization until 48 h after surgery), and acute kidney injury (KDIGO grades 1–4) within 30 days after randomization. Secondary mechanistic outcomes included intra-operative hypotension and use of vasoactive therapy.
Statistical analysis
We determined that the enrolment of 260 patients (130 per trial group) would provide a power of 90% to determine a 20% absolute risk reduction in incidence of myocardial injury at an alpha level of 0.05, assuming a loss to follow-up of 5%. Sample size calculations were performed using STATA 14 (StataCorp, 2015, Stata Statistical Software: Release 14, College Station, TX).
All analyses were conducted according to the intention-to-treat principle, meaning that all randomly assigned patients with a recorded outcome for primary or secondary outcomes were included in the analysis, and analysed according to the group they were allocated. For analysis of the primary and each secondary outcome, we present: number of patients in each analysis, by treatment group; a summary statistic of the outcome [e.g. number (%)], by treatment group; estimated treatment effect and corresponding 95% confidence interval (CI); and two-sided P-value. The treatment effects calculated were for continuing vs. discontinuing RAS inhibitors. For all analyses, significance level of 5% was used. Analysis was conducted with Stata (version 14). Full details are provided in the statistical analysis plan, which was published online on 13 October 2021 (https://www.qmul.ac.uk/ccpmg/media/critical-care-and-pmg/documents/SPACE-SAP-v2.0.pdf).
We planned to analyse the primary outcome using a mixed effects logistic regression model with patients clustered by centre. However, owing to a near-zero intra-cluster correlation, the model did not converge, so instead we fitted a single-level model ignoring clustering. The model was adjusted for minimization variables [planned surgical procedure category and ACE-I/ARB (ACE-I, ARB, or both)] and the following pre-specified baseline covariates: age, sex. All covariates were entered into the model as fixed factors, with age being included as a continuous variable, assuming a linear association with the primary outcome. The analysis of the primary outcome was conducted using a logistic regression model which gives an estimated adjusted odds ratio (OR) and 95% CI.
The continuously distributed secondary outcomes (absolute peak troponin levels) were analysed using a mixed effects linear regression model adjusted for the same covariates as the primary analysis and taking into account clustering by centre. Secondary outcome for infection within 30 days of surgery was analysed with the use of a logistic regression model adjusted for minimization variables [planned surgical procedure category and ACE-I/ARB (ACE-I, ARB, or both)]. For all other secondary outcomes, no statistical analysis was performed (pre-peer review) due to low event rates. Categorical safety outcome measures were summarized as the percentage of patients with these events. Pre-specified sensitivity analyses for the primary outcome were conducted under the missing not at random assumption over a range of plausible scenarios to assess how robust the results were to departures from missing at random on the treatment estimates.
Results
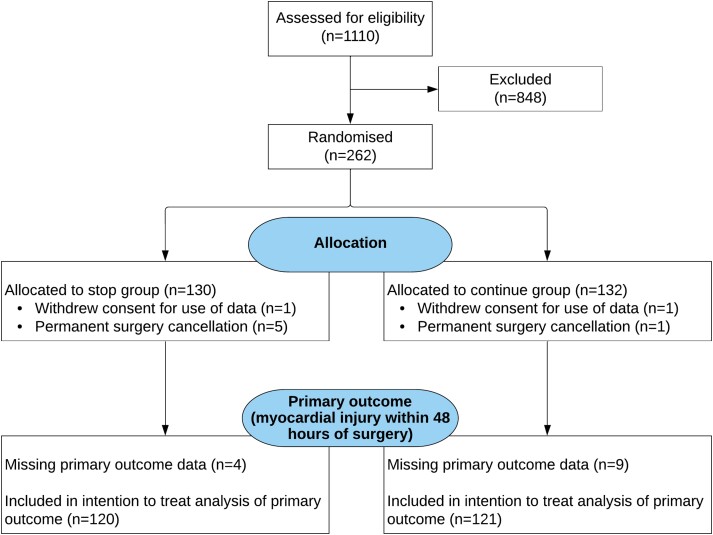
From 31 July 2017 to 1 October 2021, a total of 1110 patients were screened at six participating centres. Two hundred and sixty-two participants underwent randomization; 130 were assigned to the discontinuation group and 132 were assigned to the continuation group. Two patients withdrew their data from the study after group allocation, with 260 retained in the trial (Figure 1). Recruitment was temporarily suspended in accord with the sponsor requirements during the two UK peaks of the COVID-19 pandemic (Supplementary data online, Figure S1). The median age was 71 years, with 126 (48%) female patients (Table 1). The majority of patients were predominantly prescribed RAS inhibitors for hypertension (97%), with diabetes mellitus having been diagnosed in 74 patients (29%). Most patients were taking statins (70%) and other antihypertensive medicines.
Figure 1.
CONSORT diagram. Details are shown regarding the screening, potential eligibility, randomized assignments, and disposition of the trial patients. Details regarding the methods that were used for screening and the determining of eligibility are provided in the Supplementary data online, Appendix. Before surgery, there were 6 patients for whom surgery was cancelled after randomization had occurred (for surgical/oncologic reasons). Two additional patients withdrew their consent after randomization but before attending for surgery. Once admitted for surgery, primary outcome data was missing for 4 patients allocated to stop their RAS inhibitor, and in 9 patients randomized to continue their RAS therapy. The primary outcome could not be met if the pre-operative sample was not collected or the day 1 and day 2 samples were not collected even though the pre-operative sample had been. The reasons for this were either that 11 patients refused to give all the required additional blood sample (but consented to continue in the trial) or samples were not obtained by research staff (n = 2).
Table 1.
Baseline characteristics
| Stopa | Continueb | |
|---|---|---|
| Sex—no. (%) | ||
| Male | 66 (51.2) | 68 (51.9) |
| Female | 63 (48.8) | 63 (48.1) |
| Ethnicity (n; % black/Afro-Carribean) | 6 (5) | 4 (3) |
| Age (years) | 72 (67–78) | 71 (66–76) |
| Current smoker—no. (%) | 12 (9.4) | 10 (7.6) |
| American Society of Anaesthesiology grade—no. (%) | ||
| III | 125 (98.4) | 128 (100.0) |
| IV | 2 (1.6) | 0 (0.0) |
| Chronic comorbid disease—no. (%)c | ||
| COPD | 14 (11.0) | 18 (13.7) |
| Asthma | 13 (10.2) | 18 (13.7) |
| Interstitial lung disease or pulmonary fibrosis | 0 (0.0) | 1 (<1) |
| Ischaemic heart disease | 28 (22.0) | 26 (19.8) |
| Diabetes mellitus | 33 (26.0) | 41 (31.3) |
| Heart failure | 10 (7.9) | 6 (4.6) |
| Liver cirrhosis | 1 (<1) | 2 (1.5) |
| Active cancer | 31 (24.4) | 32 (24.6) |
| Stroke or transient ischaemic attack | 9 (7.1) | 10 (7.6) |
| Peripheral vascular disease | 8 (6.3) | 7 (5.3) |
| Hypertension | 124 (97.6) | 127 (96.9) |
| Any treated infections within the previous month | 2 (1.6) | 4 (3.1) |
| Planned surgical procedure—no. (%) | ||
| Surgery involving the gut | 24 (18.6) | 25 (19.1) |
| All other surgery | 105 (81.4) | 106 (80.9) |
| Class of drug routinely taken—no. (%) | ||
| ACE-I | 83 (64.3) | 79 (60.3) |
| ARB | 46 (35.7) | 52 (39.7) |
| Site—no. (%) | ||
| County Durham and Darlington NHS Foundation Trust | 2 (1.6) | 5 (3.8) |
| Plymouth Hospitals NHS Trust | 15 (11.6) | 16 (12.2) |
| Barts Health NHS Trustd | 78 (60.5) | 76 (58.0) |
| University College London Hospitals | 28 (21.7) | 27 (20.6) |
| University Hospitals Bristol NHS Foundation Trust | 6 (4.7) | 7 (5.3) |
| Surgical procedure performed—no. (%) | ||
| Surgery involving the gut | 22 (17.9) | 23 (18.4) |
| All other surgery | 101 (82.1) | 102 (81.6) |
| Pre-operative blood test results | ||
| Haemoglobin (g/L) | 134 (125–143) | 133 (123–144) |
| Creatinine (µmol/L) | 83 (69–101) | 82 (66–98) |
| Cardiovascular medication—no. (%) | ||
| Beta blocker | 38 (29.9) | 38 (29.0) |
| Calcium channel antagonist | 46 (36.2) | 47 (35.9) |
| Doxazosin | 13 (10.2) | 11 (8.4) |
| Diuretic | 39 (30.7) | 39 (29.8) |
| Statin | 94 (74.0) | 86 (65.6) |
| Nitrate | 14 (11.0) | 12 (9.2) |
| Anti-platelet agents | 43 (33.9) | 51 (38.9) |
Data are given as median (interquartile range) unless otherwise specified.
COPD, chronic obstructive pulmonary disease.
aOne patient withdrew consent for use of data in the stop group.
bOne patient withdrew consent for use of data in the continue group.
cPatient may have more than one chronic comorbid disease.
dIncorporates two separate hospitals, Royal London Hospital and Barts Health Orthopaedic Centre at Newham Hospital.
Treatment adherence after randomization
Adherence to treatment after randomization was confirmed in 110 patients (85%) in the discontinuation group and in 104 patients (79%) in the continuation group (Table 2). There were no protocol deviations before admission to hospital. Sixteen (12%) patients in the continuation group had their RAS inhibitor omitted, rather than restart 48 h as per protocol, after surgery. These deviations were triggered by the local clinical team for clinical concerns not stipulated by our protocol.
Table 2.
Adherence to treatment after randomization
| Protocol deviations—no. (%) | Summary measure | |
|---|---|---|
| Stopa | Continueb | |
| (n = 129/130) | (n = 131/132) | |
| Patients with ≥1 treatment deviation | 19 (14.7) | 27 (20.6) |
| Total number of deviations | 19 | 34 |
| Number of treatment deviations per patient | ||
| 0 | 110 (85.3) | 104 (79.4) |
| 1 | 19 (14.7) | 22 (16.8) |
| ≥2 | 0 (0.0) | 5 (3.8) |
| Type of deviationc | ||
| Participant in the stop group did receive ACE-I and/or ARB | 2 (1.6) | N/A |
| Participant in the continuation group did not receive ACE-I and/or ARB | N/A | 16 (12.2) |
| Other | 17 (13.2) | 11 (8.4) |
| Blood sample not collected | 3 | 4 |
| Ward drug omission | 10 | N/A |
| Randomized < 72 h before surgery | 1 | 6 |
| Drug given against protocol | 1 | 1 |
| Change in planned surgical procedure | 1 | 0 |
| Staff limitations | 1 | 0 |
aOne patient withdrew consent for use of data in the stop group.
bOne patient withdrew consent for use of data in the continue group.
cPatient may have more than one deviation.
Intervention
Before randomization, systolic and mean arterial blood pressure (MAP) readings in the pre-assessment clinic were similar between patients randomly allocated to either continue (systolic: 138 ± 20 mmHg; MAP: 95 ± 11 mmHg) or stop (systolic: 140 ± 21 mmHg; MAP: 97 ± 12 mmHg) their RAS inhibitor. On the day of surgery, the first arterial blood pressure reading did not change from pre-assessment clinic readings in patients allocated to continue RAS inhibitors (+3 mmHg, 95% CI −3 to 8 mmHg). Both systolic and MAP readings were higher in patients who had been randomized to stop RAS inhibitors, compared to their pre-operative readings. Systolic blood pressure increased by 16 mmHg, 95% CI 10–22 mmHg; MAP increased by 10 mmHg, 95% CI 6–13 mmHg. The clinical management was similar between patients randomized to either stop or continue their RAS inhibitor (Table 3).
Table 3.
Peri-operative management
| Clinical management | Stopa | Continueb |
|---|---|---|
| Surgical technique—no. (%) | ||
| Open surgical technique used during surgery | 102 (82.9) | 102 (82.3) |
| Laparoscopic or laparoscopic-assisted technique | 18 (14.6) | 19 (15.3) |
| Laparoscopic converted to open | 3 (2.4) | 3 (2.4) |
| Anaesthetic technique—no. (%) | ||
| General anaesthesia alone | 36 (31.3) | 38 (33.9) |
| General + epidural anaesthesia | 18 (15.7) | 16 (14.3) |
| General + spinal anaesthesia | 29 (25.2) | 31 (27.7) |
| General + other regional anaesthesia | 20 (17.4) | 17 (15.2) |
| Regional anaesthesia with sedation | 12 (10.4) | 10 (8.9) |
| Planned level of care on the first night after surgery—no. (%) | ||
| Surgical ward | 87 (70.7) | 90 (70.3) |
| Critical care | 36 (29.3) | 38 (29.7) |
| Blood pressure during surgery | ||
| Systolic blood pressure <90 mmHg during surgery | 40 (32.5) | 55 (44.0) |
| Total dose of metaraminol during surgery (mg) | ||
| Median (IQR) | 0.5 (0.0–4.5) | 2.3 (0.0–7.3) |
| Other pressor support (norepinephrine) | 12 (9.8) | 12 (9.6) |
| Arrhythmias | 3 (2.5) | 1 (<1) |
| Intravenous fluids during surgery | ||
| Total volume of intravenous fluid administered excluding blood products (mL) | ||
| Median (IQR) | 1600 (1000–2000) | 2000 (1000–2000) |
| Total volume of blood products administered (mL) | ||
| Median (IQR) | 0 (0–0) | 0 (0–0) |
| Lactate measurement (mmol/L) | ||
| Median (IQR) | 1.3 (0.9–1.9) | 1.5 (0.8–2.0) |
SD, standard deviation; IQR, interquartile range.
aOne patient withdrew consent for use of data and five patients did not have surgery in the stop group.
bOne patient withdrew consent for use of data and one patient did not have surgery in the continue group.
Primary outcome
From the 241/260 patients who had blood samples collected (Table 4), myocardial injury occurred in 50 (41%) patients who continued RAS inhibitor therapy, compared to 58 (48%) patients who discontinued RAS inhibitors (OR 0.77; 95% CI 0.45–1.31; P = .33) by 48 h after surgery. Sixty-two patients had hs-TnT >15 ng/L before surgery (31 in each group); for patients without a pre-operative troponin elevation, myocardial injury was sustained by 19/90 (21%) patients who continued RAS inhibitor therapy, compared to 27/89 (30%) patients who discontinued RAS inhibitors. The results from the sensitivity analysis indicate that the analysis of the primary outcome is robust to departures from the missing-at-random assumption, as detailed in the Supplementary data online. When we excluded patients who had regional anaesthesia with sedation, the primary outcome was observed in 46/111 (41.4%) patients who continued their RAS inhibitor, compared with 55/108 (50.9%) patients allocated to stop (OR 0.68; 95% CI 0.40–1.16).
Table 4.
Primary and secondary outcomes
| Outcomes | Number of patients with available data—no. (%) | Summary measure | Treatment effect (95% CI) | P-value | ||
|---|---|---|---|---|---|---|
| Stopa (n = 124/130) | Continueb (n = 130/132) | Stop | Continue | |||
| Primary outcome | ||||||
| Myocardial injuryc,g | 120 (96.8) | 121 (93.1) | 58 (48.3) | 50 (41.3) | 0.77 (0.45, 1.31)e | .33 |
| Secondary outcomes | ||||||
| Peak level Troponin-Tc | 120 (96.8) | 121 (93.1) | 18.4 (19.5) | 16.8 (12.7) | −0.16 (−3.13, 2.82)f | .92 |
| Infectiond,g | 124 (100.0) | 123 (94.6) | 26 (21.0) | 24 (19.5) | 0.93 (0.50, 1.76)e | .83 |
| Myocardial infarctiond | 124 (100.0) | 123 (94.6) | 3 (2.4) | 0 (0.0) | ||
| Acute heart failured | 124 (100.0) | 123 (94.6) | 2 (1.6) | 0 (0.0) | ||
| Stroked | 124 (100.0) | 123 (94.6) | 1 (0.8) | 0 (0.0) | ||
| Deathd | 124 (100.0) | 123 (94.6) | 1 (0.8) | 2 (1.6) | ||
aOne patient withdrew consent for use of data and five patients did not have surgery in the stop group.
bOne patient withdrew consent for use of data and one patient did not have surgery in the continue group.
cWithin 48 h of surgery.
dWithin 30 days of surgery.
eOdds ratio.
fMean difference.
gModel taking into account clustering by site did not converge and hence a logistic regression model was fitted ignoring clustering.
Secondary outcomes
Peak troponin-T levels were similar between groups (mean difference, −0.16 ng/L; 95% CI −3.13–2.82). To account for the skewed distribution, a post hoc analysis of peak troponin using the Wilcoxon–Mann–Whitney test also found no difference in peak troponin levels between stop and continue groups (z = 0.117, P = .91). Post-operative infections occurred in 26 (21.0%) in the stop group, compared to 24 (19.5%) patients who continued RAS inhibitors (OR 0.93; 95% CI 0.50–1.76; P = .83). Clinically diagnosed myocardial infarction, stroke, and death were recorded in fewer than 10 participants.
Adverse events
A similar number of pre-specified adverse events (n = 115) were recorded in patients randomized to discontinue RAS inhibitors [43 (33%)], compared with 43 (33%) in the continuation group (Table 5). Only hypertensive adverse events were more frequent from the time of randomization to 48 h after surgery when RAS inhibitors were discontinued [16 (12.4%) vs. 7 (5.3%) (OR 0.40, 95% CI 0.16–1.00; numbers needed to harm (NNH): 14, 95% CI: 7 (NNH) to 517 (numbers needed to benefit)]. Hypotension treated by attending clinicians with vasoactive infusions requiring via central venous catheterization was similar when RAS inhibitors were discontinued [12 (9.3%)] or continued [11 (8.4%)]. The combination of adverse hypotension and hypertension was more common when RAS inhibitors were discontinued (28/129, 21.7%), compared to continuation group (17/131, 13.0%), and associated with delayed hospital discharge (Supplementary data online).
Table 5.
Pre-specified adverse events
| Adverse events—no. (%) | Summary measure | ||
|---|---|---|---|
| Stopa (n = 124/130) | Continueb (n = 130/132) | P-values | |
| Patients with ≥1 adverse events | 43 (34.7) | 43 (33.1) | .93 |
| Hypotensionc | 12 (9.7) | 11 (8.5) | .80 |
| Hypertensiond | 16 (12.9) | 7 (5.4) | .05 |
| Acute kidney injurye | 14 (11.3) | 12 (9.2) | .65 |
| Other | 15 (12.1) | 19 (14.6) | .49 |
Other adverse events failing to meet the pre-specified definitions that were reported by research staff are listed in the Supplementary data online.
aOne patient withdrew consent for use of data and five patients did not have surgery in the stop group.
bOne patient withdrew consent for use of data and one patient did not have surgery in the continue group.
cSafety outcomes were hypotension deemed by the attending clinician to require pressor vasoactive medication delivered via central venous access (from induction of anaesthesia until 48 h after surgery).
dAcute hypertension (systolic blood pressure >180 mmHg and/or diastolic blood pressure >100 mmHg, from the time of randomization until 48 h after surgery).
eAcute kidney injury (KDIGO grades 1–4) within 30 days after randomization.
Intra-operatively, continuation of RAS inhibitors resulted in 55 (44%) patients recording systolic blood pressure <90 mmHg (Table 3), compared with 40 (33%) patients in whom RAS inhibitors were discontinued (OR 1.63; 95% CI 0.97–2.73; P = .06). The median intra-operative dose required to maintain blood pressure using the alpha-1 vasoconstrictor agent metaraminol in patients randomized to continue RAS inhibitors was 2.3 mg (0.0–7.3), compared to patients in whom RAS inhibitors were discontinued [0.5 mg (0.0–4.5)].
Discussion
The main finding of this trial was that the incidence of myocardial injury was similar between patients who stopped and continued RAS inhibitors during the peri-operative period. However, patients who stopped RAS inhibitors did experience more hypertensive events. By contrast, there was no difference between groups in the need for vasoactive therapy to treat hypotension within 48 h of surgery (Structured Graphical Abstract). We also noted that the relative risk of myocardial injury between groups, if replicated in a larger clinical effectiveness trial, may indicate significant benefits to continuing RAS inhibitors during the peri-operative period. It is also important to emphasise that we used a drug-specific protocol for stopping different RAS inhibitors before surgery, to ensure the duration reflected the pharmacokinetic profile of each drug.
Peri-operative trials examining whether RAS inhibitors are organ protective or detrimental have been inconsistent.24 A major factor in the interpretation of preceding studies is when RAS inhibitors were stopped and when they were restarted. Until SPACE, no preceding trials have adopted a pharmacokinetically based rationale for stopping RAS inhibitors. In contrast to the recently published pragmatic hypotension-avoidance trial embedded in POISE-3,25 our trial specifically targeted RAS inhibitors. Moreover, we stopped ACE-I/ARB in an individualized manner, based on their distinct pharmacokinetic properties. Given that this class of drugs was stopped <24 h before surgery in POISE-3, it seems very unlikely that their withdrawal would meaningfully impact on blood pressure on the day of surgery. Indeed, our individualized protocol resulted in substantially higher pre-operative arterial blood pressure when RAS inhibitors were stopped. The pre-operative omission of other cardiovascular drugs in POISE-3, as well as their reintroduction after surgery, was a highly complex intervention with lower compliance than we achieved with SPACE (e.g. 57%–68% on the day of surgery).
For most ACE-I alone, at least 24 h cessation is required. For example, the duration of action for lisinopril and ramipril, the commonest ACE-I used by patients recruited into SPACE, is 24 h, with half-life elimination times of 12–17 h, respectively. The lack of consistency in when RAS inhibitors have been stopped before—and restarted after—non-cardiac surgery explains, in part, the considerable variability in international recommendations for the management of RAS inhibitors in surgical patients. The current evidence base relies mostly on retrospective studies with a strong risk of bias and small, single-centre randomized trials that had non-blinded primary outcomes. A similar sized trial to ours examined haemodynamic changes after 275 patients were randomized to either continuing ACE-I or omitting a single ACE-I dose. Intra-operative hypotension occurred less frequently after ACE-I omission, accompanied by lower vasopressor use.26 Similar to our study, post-operative hypertension was more frequent when ACE-I were omitted, although fluid requirements did not differ between groups. In the absence of markers for organ injury, the relevance of these haemodynamic alterations remained unclear. Moreover, recent randomized controlled trial data do not support universally targeting higher intra-operative blood pressures to reduce post-operative complications.27
In our study, we specifically focussed on older patients aged >60 years, who are at most risk of myocardial injury, rather than younger patients (age ≥ 18 years) with markedly less risk of morbidity. Three ongoing, pragmatic multi-centre trials are also examining the management of RAS inhibitors in patients aged ≥ 18 years undergoing non-cardiac surgery.28–30 In contrast to our study, discontinuation of RAS inhibitors has not been designed in these studies according to individual pharmacokinetic profiles of these drugs. Moreover, these trials do not protocolise when RAS inhibitor treatment should be resumed. Two large database studies in non-cardiac16 and cardiac surgery17 have identified that failure to restart RAS inhibitor treatment is strongly associated with excess morbidity and mortality, suggesting that protocolization of resuming therapy is likely to be an important confounder in interpreting unblinded outcomes beyond the early post-operative stage. Indeed, our data suggest that clinically significant acute hypertension is a notable feature of stopping RAS inhibitors peri-operatively. However, caution is warranted in interpreting these results, given the event rates. Furthermore, our post hoc analyses show that blood pressure lability is more common after discontinuation of RAS inhibitors, as evidenced by the combination of acute hypertension and intra-operative hypotension.
Most attention on peri-operative ACE-I/ARB use has focussed on hypotension. However, the VISION investigators also reported that acute hypertension was independently associated with myocardial injury.15 Mechanistically, even a brief elevation in pre-load produced by transient increases in arterial blood pressure produces mechanical stretch-induced myocyte injury resulting in raised plasma troponin levels.31 Pre-load-induced myocyte injury may therefore explain many cardiac troponin I elevations seen in the absence of clinical signs or symptoms of myocardial ischaemia. Despite myocardial injury under these experimental conditions, left ventricular systolic function was preserved. Similarly, acute increases in blood pressure as a result of ACE-I/ARB may contribute to myocardial injury.
Although there is no universally accepted definition of peri-operative myocardial injury, the definition we used is consistent with the findings and/or recommendations of the VISION study,1 American Heart Association,32 and the recent ESC guidelines.12 Whilst our trial did not have sufficient power to investigate the effect of the discontinuation of RAS inhibitors on clinically detected cardiovascular events or mortality, the use of an objective-blinded primary outcome—troponin-T—serves as a robust surrogate for both early and delayed poorer outcomes after non-cardiac surgery. The similar incidence of acute kidney injury and all-cause complications is consistent with a lack of difference in early objective evidence for myocardial injury. Our trial has several additional limitations. Non-White ethnic backgrounds were under-represented, which limits the generalizability of our results to other racial or ethnic groups. Failure to adhere to the randomly assigned management strategy may have influenced the results. The open-label design of the trial may have influenced clinical care, although the blinded primary outcome is likely to reduce the impact of subjective changes in clinical management. We also acknowledge that despite careful research team management of RAS inhibitor prescriptions after surgery, deviations were still triggered by the local clinical team for clinical concerns not stipulated by our protocol. The findings may not be generalizable to patients receiving RAS inhibitors for indications other than mainly hypertension, although around 30% had diabetes mellitus in our study. However, patients with heart failure comprised <10% of our trial population. Establishing whether raised troponin-T levels were primarily due to myocardial ischaemia by electrocardiographic criteria may be instructive, although acute rises are associated with poorer outcomes regardless of aetiology.
In conclusion, discontinuation of RAS inhibitors in patients undergoing non-cardiac surgery did not lead to a clinically relevant reduction in myocardial injury and/or other complications, but did increase the risk of clinically significant hypertensive events. These findings require confirmation in future studies.
Supplementary Material
Acknowledgements
The trial was designed by the first and last authors. The first author wrote the first draft of the manuscript, which was then edited by all the co-authors; no other medical writing assistance was provided. The authors had access to the results and take responsibility for the accuracy and completeness of the data, for the fidelity of the trial to the protocol, and for the decision to submit the manuscript for publication.
Contributor Information
Gareth L Ackland, Translational Medicine and Therapeutics, William Harvey Research Institute, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK.
Akshaykumar Patel, Translational Medicine and Therapeutics, William Harvey Research Institute, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK.
Tom E F Abbott, Translational Medicine and Therapeutics, William Harvey Research Institute, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK.
Salma Begum, Translational Medicine and Therapeutics, William Harvey Research Institute, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK.
Priyanthi Dias, Translational Medicine and Therapeutics, William Harvey Research Institute, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK.
David R Crane, Translational Medicine and Therapeutics, William Harvey Research Institute, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK.
Sameer Somanath, County Durham and Darlington NHS Foundation Trust, Darlington, UK.
Alexander Middleditch, University Hospitals Bristol NHS Foundation Trust, UK.
Stuart Cleland, University Hospitals Plymouth NHS Trust, UK.
Ana Gutierrez del Arroyo, Translational Medicine and Therapeutics, William Harvey Research Institute, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK.
David Brealey, Bloomsbury Institute of Intensive Care Medicine, University College London, London, UK; UCL Hospitals NHS Foundation Trust, London, UK; NIHR University College London Hospitals Biomedical Research Centre, London, UK.
Rupert M Pearse, Translational Medicine and Therapeutics, William Harvey Research Institute, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK.
the Stopping Perioperative ACE-inhibitors or angiotensin-II receptor blockers (SPACE) trial investigators:
Gareth Ackland, Tim Martin, Maria Fernandez, Fatima Seidu, Mari-Liis Pakats, Otto Mahr, Neil MacDonald, Filipa Dos Santos, Amaia Arrieta Garcia, Ruzena Uddin, Salma Begum, Rupert Pearse, Emily Subhedar, Yize Wan, Akshaykumar Patel, Tasnin Shahid, Mevan Gooneratne, Charlotte Trainer, Bethan Griffiths, Steven Dunkley, Shaun May, Sophie Walker, Alexander Fowler, Timothy Stephens, Monica Oliveira, Marta Januszewska, Edyta Niebrzegowska, Vanessa Amaral, Jamila Kassam, Sophie Young, Shanaz Ahmad, Jan Whalley, Ryan Haines, Sara Hui, Rob Hammond, David Crane, David Brealey, Sohail Bampoe, Robert Stephens, Anna Reyes, Gladys Martir, Chimverly Diaz, Stuart Cleland, Gary Minto, Natasha Wilmshurst, Debbie-Claire Affleck, Tracy Ward, Gavin Werrett, Susan Cummins, Alan Amber, Andrew Biffen, Stephen Boumphrey, Elizabeth Cann, Charlotte Eglinton, Elaine Jones, Memory Mwadeyi, Sam Piesley, Richard Cowan, Julie Alderton, Fiona Reed, Joanne Smith, Amy Turner, Lorraine Madziva, Abigail Patrick, Penny Harris, Harry Lang, Alexander Middleditch, Anthony Pickering, Catherine O'Donovan, Rebecca Houlihan, Rosina Jarvis, Andrew Shrimpton, Toni Farmery, Katy Tucker, Danielle Davis, Sameer Somanth, Louise Duncan, Helen Melsom, Sarah Clark, Melanie Kent, Michelle Wood, Ami Laidlaw, Tracy Matheson-Smith, Kathryn Potts, Andrea Kay, Stefanie Hobson, John Sear, Vikas Kapil, Andrew Archbold, Matt Wilson, Drilona Dndrejaj, Dennis Ly, Akshaykumar Patel, Toby Richards, Simon Finney, and Steve Harris
Supplementary data
Supplementary data are available at European Heart Journal online.
Declarations
Disclosure of Interest
All authors declare no disclosure of interest for this contribution.
Data Availability
The study-related documents can be made available by contacting the corresponding author.
Funding
G.L.A. was supported by the National Institute for Academic Anaesthesia (British Oxygen Company research chair grant); NIHR Advanced Fellowship [NIHR300097], and a British Heart Foundation Programme grants (RG/14/4/30736; RG/19/5/34463). T.E.F.A. and R.M.P. were supported by NIHR.
Ethical Approval
This study was approved by the London (City and East) Research Ethics Committee (16/LO/1495), the Health Research Authority (UK), and the Medicines and Healthcare products Regulatory Agency (UK).
Pre-registered Clinical Trial Number
SPACE was registered as a Clinical Trial of an Investigational Medicinal Product with Eudract on 2016-004141-90. The trial was also registered before the first patient was recruited with the International Traditional Medicine Clinical Trial Registry on 29 May 2017 as ISRCTN17251494 (doi.org/10.1186/ISRCTN17251494).
References
- 1. Devereaux PJ, Biccard BM, Sigamani A, Xavier D, Chan MTV, Srinathan SK, et al. Association of postoperative high-sensitivity troponin levels with myocardial injury and 30-day mortality among patients undergoing noncardiac surgery. JAMA 2017;317:1642–1642. 10.1001/jama.2017.4360 [DOI] [PubMed] [Google Scholar]
- 2. Puelacher C, Gualandro DM, Glarner N, Lurati Buse G, Lampart A, Bolliger D, et al. Long-term outcomes of perioperative myocardial infarction/injury after non-cardiac surgery. Eur Heart J 2023;44:1690–701. 10.1093/eurheartj/ehac798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fowler AJ, Wan YI, Prowle JR, Chew M, Campbell D, Cuthbertson B, et al. Long-term mortality following complications after elective surgery: a secondary analysis of pooled data from two prospective cohort studies. Br J Anaesth 2022;129:588–97. 10.1016/j.bja.2022.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pearse RM, Harrison DA, MacDonald N, Gillies MA, Blunt M, Ackland GL, et al. Effect of a perioperative, cardiac output-guided hemodynamic therapy algorithm on outcomes following major gastrointestinal surgery: a randomized clinical trial and systematic review. JAMA 2014;311:2181–90. 10.1001/jama.2014.5305 [DOI] [PubMed] [Google Scholar]
- 5. Ackland GL, Iqbal S, Paredes LG, Toner A, Lyness C, Jenkins N, et al. Individualised oxygen delivery targeted haemodynamic therapy in high-risk surgical patients: a multicentre, randomised, double-blind, controlled, mechanistic trial. Lancet Respir Med 2015;3:33–41. 10.1016/S2213-2600(14)70205-X [DOI] [PubMed] [Google Scholar]
- 6. Fox KM; EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease Investigators . Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised, double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet 2003;362:782–8. 10.1016/S0140-6736(03)14286-9 [DOI] [PubMed] [Google Scholar]
- 7. Heart Outcomes Prevention Evaluation Study Investigators; Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med 2000;342:145–53. 10.1056/NEJM200001203420301 [DOI] [PubMed] [Google Scholar]
- 8. Visseren FLJ, Mach F, Smulders YM, Carballo D, Koskinas KC, Back M, et al. 2021 ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–337. 10.1093/eurheartj/ehab484 [DOI] [PubMed] [Google Scholar]
- 9. Benigni A, Cassis P, Remuzzi G. Angiotensin II revisited: new roles in inflammation, immunology and aging. EMBO Mol Med 2010;2:247–57. 10.1002/emmm.201000080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mets B. To stop or not? Anesth Analg 2015;120:1413–9. 10.1213/ANE.0000000000000758 [DOI] [PubMed] [Google Scholar]
- 11. Sear JW. Perioperative renin-angiotensin blockade: to continue or discontinue, that is the question!. Anesth Analg 2014;118:909–11. 10.1213/ANE.0000000000000204 [DOI] [PubMed] [Google Scholar]
- 12. Halvorsen S, Mehilli J, Cassese S, Hall TS, Abdelhamid M, Barbato E, et al. 2022 ESC guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur Heart J 2022;43:3826–924. 10.1093/eurheartj/ehac270 [DOI] [PubMed] [Google Scholar]
- 13. Roshanov PS, Rochwerg B, Patel A, Salehian O, Duceppe E, Belley-Cote EP, et al. Withholding versus continuing angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers before noncardiac surgery: an analysis of the vascular events in noncardiac surgery patIents cOhort evaluatioN prospective cohort. Anesthesiology 2017;126:16–27. 10.1097/ALN.0000000000001404 [DOI] [PubMed] [Google Scholar]
- 14. Sessler DI, Bloomstone JA, Aronson S, Berry C, Gan TJ, Kellum JA, et al. Perioperative quality initiative consensus statement on intraoperative blood pressure, risk and outcomes for elective surgery. Br J Anaesth 2019;122:563–74. 10.1016/j.bja.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 15. Abbott TEF, Pearse RM, Archbold RA, Ahmad T, Niebrzegowska E, Wragg A, et al. A prospective international multicentre cohort study of intraoperative heart rate and systolic blood pressure and myocardial injury after noncardiac surgery: results of the VISION study. Anesth Analg 2018;126:1936–45. 10.1213/ANE.0000000000002560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee SM, Takemoto S, Wallace AW. Association between withholding angiotensin receptor blockers in the early postoperative period and 30-day mortality: a cohort study of the veterans affairs healthcare system. Anesthesiology 2015;123:288–306. 10.1097/ALN.0000000000000739 [DOI] [PubMed] [Google Scholar]
- 17. Mudumbai SC, Takemoto S, Cason BA, Au S, Upadhyay A, Wallace AW. Thirty-day mortality risk associated with the postoperative nonresumption of angiotensin-converting enzyme inhibitors: a retrospective study of the Veterans Affairs Healthcare System. J Hosp Med 2014;9:289–96. 10.1002/jhm.2182 [DOI] [PubMed] [Google Scholar]
- 18. Song JC, White CM. Clinical pharmacokinetics and selective pharmacodynamics of new angiotensin converting enzyme inhibitors: an update. Clin Pharmacokinet 2002;41:207–24. 10.2165/00003088-200241030-00005 [DOI] [PubMed] [Google Scholar]
- 19. O’Connor ME, Kirwan CJ, Pearse RM, Prowle JR. Incidence and associations of acute kidney injury after major abdominal surgery. Intensive Care Med 2016;42:521–30. 10.1007/s00134-015-4157-7 [DOI] [PubMed] [Google Scholar]
- 20. Ackland GL, Abbott TEF, Jones TF, Leuwer M, Pearse RM, Investigators V-U, et al. Early elevation in plasma high-sensitivity troponin T and morbidity after elective noncardiac surgery: prospective multicentre observational cohort study. Br J Anaesth 2020;124:535–43. 10.1016/j.bja.2020.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beattie WS, Lalu M, Bocock M, Feng S, Wijeysundera DN, Nagele P, et al. Systematic review and consensus definitions for the standardized endpoints in perioperative medicine (StEP) initiative: cardiovascular outcomes. Br J Anaesth 2021;126:56–66. 10.1016/j.bja.2020.09.023 [DOI] [PubMed] [Google Scholar]
- 22. Vascular Events in Noncardiac Surgery Patients Cohort Evaluation Study Investigators; Spence J, LeManach Y, Chan MTV, Wang CY, Sigamani A, et al. Association between complications and death within 30 days after noncardiac surgery. CMAJ 2019;191:E830–7. 10.1503/cmaj.190221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Beattie WS, Wijeysundera DN, Chan MTV, Peyton PJ, Leslie K, Paech MJ, et al. Implication of major adverse postoperative events and myocardial injury on disability and survival: a planned subanalysis of the ENIGMA-II trial. Anesth Analg 2018;127:1118–26. 10.1213/ANE.0000000000003310 [DOI] [PubMed] [Google Scholar]
- 24. Hollmann C, Fernandes NL, Biccard BM. A systematic review of outcomes associated with withholding or continuing angiotensin-converting enzyme inhibitors and angiotensin receptor blockers before noncardiac surgery. Anesth Analg 2018;127:678–87. 10.1213/ANE.0000000000002837 [DOI] [PubMed] [Google Scholar]
- 25. Marcucci M, Painter TW, Conen D, Lomivorotov V, Sessler DI, Chan MTV, et al. Hypotension-avoidance versus hypertension-avoidance strategies in noncardiac surgery: an international randomized controlled trial. Ann Intern Med 2023;176:605–14. 10.7326/M22-3157 [DOI] [PubMed] [Google Scholar]
- 26. Shiffermiller JF, Monson BJ, Vokoun CW, Beachy MW, Smith MP, Sullivan JN, et al. Prospective randomized evaluation of preoperative angiotensin-converting enzyme inhibition (PREOP-ACEI). J Hosp Med 2018;13:661–7. 10.12788/jhm.3036 [DOI] [PubMed] [Google Scholar]
- 27. Wanner PM, Wulff DU, Djurdjevic M, Korte W, Schnider TW, Filipovic M. Targeting higher intraoperative blood pressures does not reduce adverse cardiovascular events following noncardiac surgery. J Am Coll Cardiol 2021;78:1753–64. 10.1016/j.jacc.2021.08.048 [DOI] [PubMed] [Google Scholar]
- 28. Legrand M, Futier E, Leone M, Deniau B, Mebazaa A, Plaud B, et al. Impact of renin-angiotensin system inhibitors continuation versus discontinuation on outcome after major surgery: protocol of a multicenter randomized, controlled trial (STOP-or-NOT trial). Trials 2019;20:160. 10.1186/s13063-019-3247-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Misra S, Parida S, Sahajanandan R, Behera BK, Senthilnathan M, Mariappan R, et al. The effect of continuing versus withholding angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers on mortality and major adverse cardiovascular events in hypertensive patients undergoing elective non-cardiac surgery: study protocol for a multi-centric open-label randomised controlled trial. Trials 2022;23:670. 10.1186/s13063-022-06616-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. UMC Utrecht . AIPOP: Management of angiotensin inhibitors during the perioperative period. https://www.umcutrecht.nl/en/wetenschappelijk-onderzoek/aipop-management-of-angiotensin-inhibitors-during-the-perioperative-period (2 March 2023, date last accessed).
- 31. Weil BR, Suzuki G, Young RF, Iyer V, Canty JMJR. Troponin release and reversible left ventricular dysfunction after transient pressure overload. J Am Coll Cardiol 2018;71:2906–16. 10.1016/j.jacc.2018.04.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ruetzler K, Smilowitz NR, Berger JS, Devereaux PJ, Maron BA, Newby LK, et al. Diagnosis and management of patients with myocardial injury after noncardiac surgery: a scientific statement from the American heart association. Circulation 2021;144:e287–305. 10.1161/CIR.0000000000001024 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The study-related documents can be made available by contacting the corresponding author.