Abstract
INTRODUCTION:
Whether 10-day short-course vonoprazan-amoxicillin dual therapy (VA-dual) is noninferior to the standard 14-day bismuth-based quadruple therapy (B-quadruple) against Helicobacter pylori eradication has not been determined. This trial aimed to compare the eradication rate, adverse events, and compliance of 10-day VA-dual regimen with standard 14-day B-quadruple regimen as first-line H. pylori treatment.
METHODS:
This prospective randomized clinical trial was performed at 3 institutions in eastern China. A total of 314 treatment-naive, H. pylori–infected patients were randomly assigned in a 1:1 ratio to either 10-day VA-dual group or 14-day B-quadruple group. Eradication success was determined by 13C-urea breath test at least 4 weeks after treatment. Eradication rates, adverse events, and compliance were compared between groups.
RESULTS:
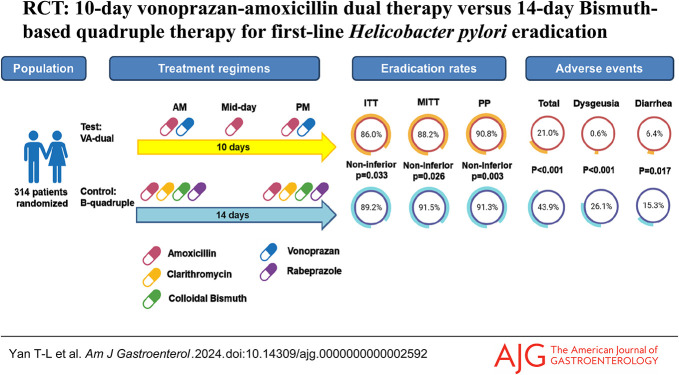
Eradication rates of VA-dual and B-quadruple groups were 86.0% and 89.2% (P = 0.389), respectively, by intention-to-treat (ITT) analysis; 88.2% and 91.5% (P = 0.338), respectively, by modified ITT analysis; and 90.8% and 91.3% (P = 0.884), respectively, by per-protocol (PP) analysis. The efficacy of the VA-dual remained noninferior to B-quadruple therapy in all ITT, modified ITT, and PP analyses. The incidence of adverse events in the VA-dual group was significantly lower compared with that in the B-quadruple group (P < 0.001). Poor compliance contributed to eradication failure in the VA-dual group (P < 0.001), while not in the B-quadruple group (P = 0.110).
DISCUSSION:
The 10-day VA-dual therapy provided satisfactory eradication rates of >90% (PP analysis) and lower rates of adverse events compared with standard 14-day B-quadruple therapy as first-line H. pylori therapy.
TRAIL REGISTRATION NUMBER:
ChiCTR2300070100.
KEYWORDS: Helicobacter pylori, dual therapy, vonoprazan, quadruple therapy, randomized clinical trial
INTRODUCTION
Helicobacter pylori is a widespread bacterium that typically infects the human gastric mucosa. H. pylori infection is universally acknowledged as a main risk factor of numerous gastrointestinal diseases, including gastritis, peptic ulcers, gastric carcinoma, and gastric lymphoma (1,2). Affecting approximately 50% of the global population, H. pylori infection has been a worldwide threat to human health (3,4).
Strategy of H. pylori screening and eradication in asymptomatic individuals is a cost-effective way to prevent gastric cancer (5). And nowadays, H. pylori eradication therapies are facing challenges with decreasing eradication rates, which mainly attribute to antimicrobial resistance such as clarithromycin, and are partially influenced by the efficacy of acid-suppressive drugs (6). Till now, international guidelines and consensus conferences still recommend bismuth-based quadruple (B-quadruple) therapies as first-line therapy for H. pylori infection in high clarithromycin resistance (resistance rate > 15%) areas (7). First-line B-quadruple therapies contain 2 types of antibiotics, a proton pump inhibitor (PPI) and bismuth, to overcome antimicrobial resistance and have reached an eradication rate approximately 90% (7,8). Despite improvement in the eradication rate, long-lasting B-quadruple regimens are restricted in clinical generalization owing to availability of conventional antibiotics (such as tetracycline and furazolidone) and bismuth and increasing side effects, poor compliance, and heavy cost. In addition, multiple antibiotics application could induce perturbation of fecal microbiota diversity and increase secondary antibiotic resistance (for instance, clarithromycin, levofloxacin, and metronidazole), which would limit the choice of rescue regimen in patients with a failure of first-line eradication (9). Therefore, there is an urgent need to explore novel regimens applying minimal antibiotics with shorter treatment duration while achieving satisfied H. pylori eradication rates.
Dual therapy consisting of double-dose PPI and high-dose amoxicillin (≥3 g, administered 3–4 times daily) is the simplest regimen recommended by current guidelines and is highly promising with a real-world eradication rate of up to 90% in Asian treatment-naive patients (10–12). Success of dual therapy was, first, based on the low resistance rate of amoxicillin and, second, due to increasing intragastric pH induced by double-dose PPI, which synchronizes and enhances the effects of amoxicillin (13) in turn.
Vonoprazan, a novel potassium-competitive acid blocker providing a stronger and more sustained acid inhibitory effect than PPI, has shown immense potential to improve the H. pylori eradication rate. A pilot single-center study in China showed unsatisfactory eradication rate of 10-day vonoprazan-amoxicillin dual therapy (VA-dual) therapy (81.1% by PP analysis) (14). Subsequent trials demonstrated different results of comparable eradication rate of 14-day VA-dual regimen with standard 14-day B-quadruplet regimen (15) and 10-day VA-dual regimen vs 10-day B-quadruplet regimen (16). High-quality evidence has not emerged regarding efficacy of 10-day VA-dual therapy compared with currently recommended first-line 14-day B-quadruple regimen (7,8,10).
In this study, we aimed to evaluate the eradication rate, adverse events, and compliance of 10-day VA-dual regimen compared with standard 14-day B-quadruple therapy as first-line H. pylori treatment and to explore the factors associated with eradication failure.
METHODS
Study design and ethical issues
This study was designed as a multicenter, prospective, open-label, noninferiority, and randomized controlled trial and registered at the Chinese Clinical Trial Registry (www.chictr.org.cn) with the trial registration number ChiCTR2300070100. This study was conducted following the Declaration of Helsinki, and the study proposals were approved by the Clinical Research Ethics Committee of the First Affiliated Hospital, Zhejiang University School of Medicine. Written informed consents were obtained from all participants before enrollment. All authors had access to the study data and reviewed and approved the final manuscript.
Study population
Patients aged 18–70 years and diagnosed with H. pylori infection visited outpatient clinic of 3 medical centers in eastern China between April 2023 and June 2023 and were initially screened for eligibility. The detailed inclusion criteria were as follows: (i) participants aged 18–70 years; (ii) absent history of receiving H. pylori eradication therapy; and (iii) confirmed H. pylori infection by at least 1 of the following tests: urea breath test (UBT), histology examination, and positive bacterial culture.
The main exclusion criteria were as follows: (i) administration of antibiotics, bismuth, or acid inhibitor, including H2 receptor antagonist, PPI, or P-CAB 4 weeks before inclusion; (ii) pregnancy or lactation; (iii) allergy to any of the study drugs; (iv) history of gastrectomy; (v) gastric malignancy; (vi) gastroduodenal ulcer with recent hemorrhage or signs of hemorrhage within 4 weeks; (vii) preexisting serious diseases or clinical conditions that might interfere with the evaluation of study outcomes, including hepatic or renal dysfunction, heart disorders, etc; and (viii) uninterruptible administration of astemizole, cisapride, pimozide, terfenadine, ergotamine/dihydroergotamine, oral midazolam, colchicine, ticagrelor/ranolazine, statins, azanavir, or lipivirin.
Randomization and treatment
Participants were randomly assigned to receive 10-day vonoprazan-amoxicillin dual therapy or 14-day bismuth-based quadruple therapy in a 1:1 ratio using a computer-generated random allocation sequence. The sequence was concealed for all investigators. Patients and investigators were not blinded to allocation of treatment group.
The vonoprazan-amoxicillin dual regimen consisted of 20 mg of vonoprazan (Takeda Pharmaceutical, Tianjin, China) twice daily and 1 g of amoxicillin 3 times daily for 10 days. Vonoprazan was administered 30 minutes before breakfast and supper, and amoxicillin was administered 30 minutes after 3 meals. The bismuth-based quadruple regimen consisted of 10 mg of rabeprazole, 1 g of amoxicillin, 500 mg of clarithromycin, and 200 mg of colloidal bismuth twice daily for 14 days. Rabeprazole and colloidal bismuth were administered 30 minutes before breakfast and supper, and amoxicillin and clarithromycin were administered 30 minutes after breakfast and supper.
Procedures and study outcomes
Baseline demographics and characteristics of the patients were recorded. After enrollment, both written and oral detailed medical instructions were delivered to each patient. Possible adverse events and detailed matters that need attention were also informed.
A standardized follow-up phone call was performed at the end of treatment by an independent investigator. Patients' adverse events were recorded through standard questionnaire. Patients were asked to count and report the number of pills remaining at the end of the course. An independent investigator calculated the missing dosage. Good compliance was defined as taking at least 80% of the pills, thus missing ≤2 days in 10-day dual therapy or <3 days in 14-day quadruple therapy for each drugs. Poor compliance was defined as taking <80% of the prescribed drugs and excluded in per-protocol (PP) analyses. Investigator inquired and assessed the adverse events severity using the 1–4 grading system based on the Common Terminology Criteria for Adverse Events (CTCAE) V.5.0 (17).
The confirmation of H. pylori status was evaluated by a posttreatment 13C-UBT at least 4 weeks after the last dose. At the fourth week after the last dose, all patients were reminded to schedule reexamination and inquired drugs they used during the period waiting for posttreatment UBT. Due to COVID-19 outbreak in China, participants were allowed to complete UBT between 4 and 12 weeks after the last dose with cease of 2-week PPI/P-CAB and 4-week antimicrobials; otherwise, the UBT would need to be delayed. H. pylori status was considered as negative when the result was below 4.0% (delta over baseline).
The primary outcome of the study was the eradication rate of different regimens according to the intention-to-treat (ITT), modified intention-to-treat (mITT), and PP analyses. The ITT analysis was defined to include all randomized patients. The patients who were lost during follow-up or did not undergo posttreatment UBT were regarded as treatment failures in the ITT analysis. All randomized patients who received at least 1 dose of medication and reexamined UBT were included in the mITT analyses. The PP analysis included patients who took ≥80% of drug prescribed and underwent posttreatment UBT. The secondary outcomes included the frequency and severity of adverse events and compliance in both groups.
Sample size calculation and statistical analysis
The sample size was estimated as follows: According to previous studies, we assumed that eradication rates were 90% for both VA-dual and B-quadruple regimens (12,16,18); assuming a power of 80%, a 1-sided alpha of 0.025, and a noninferiority margin of −0.1 (−10%), at least 141 subjects in each group would be needed for the noninferiority analysis; assuming a 10% loss of follow-up, a total of 314 subjects (157 patients in each group) were planned for enrollment in this study.
Statistical analyses were performed using SPSS software version 26.0 (SPSS). Categorical variables were displayed as frequencies and proportions (%), and continuous variables were presented as the mean and SD. Continuous variables were compared by the Student t test or 1-way analysis of variance. Categorical variables were compared using the χ2 test. Comparative noninferiority of the 2 groups was assessed through hypothesis testing (1-sided test) and derivation of a 2-sided 95% confidence interval (CI). A P value of <0.05 was considered to be statistically significant.
RESULTS
Patient enrollment and baseline characteristics
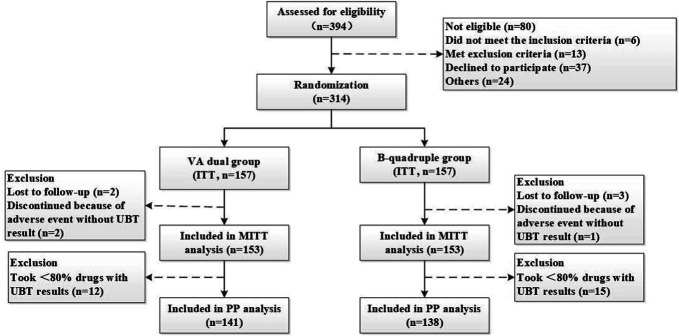
In total, 394 patients with H. pylori infection were assessed for eligibility. Three hundred fourteen patients were finally enrolled and randomly assigned to either the VA-dual or the B-quadruple treatment group, further included in the ITT analysis. Among the enrolled subjects, 2 subjects in the VA-dual group and 3 subjects in the B-quadruple group were lost to follow-up and 2 subjects in the VA-dual group and 1 subject in the B-quadruple group discontinued therapy because of adverse events without posttreatment UBT results. These 8 subjects were regarded as treatment failures in the ITT analysis and excluded in the mITT and PP analyses because of absence of posttreatment UBT results. Twelve subjects in the VA-dual group and 15 in B-quadruple were further excluded from PP analysis because of poor compliance (taken less than 80% of prescribed drugs) though with posttreatment UBT. The flow chart for patient enrollment is shown in Figure 1.
Figure 1.
Flowchart of patient enrollment. ITT, intention-to-treat; mITT, modified intention-to-treat; PP, per protocol; VA-dual, vonoprazan and amoxicillin dual therapy, B-quadruple, bismuth-based quadruple therapy, UBT, urea breath test.
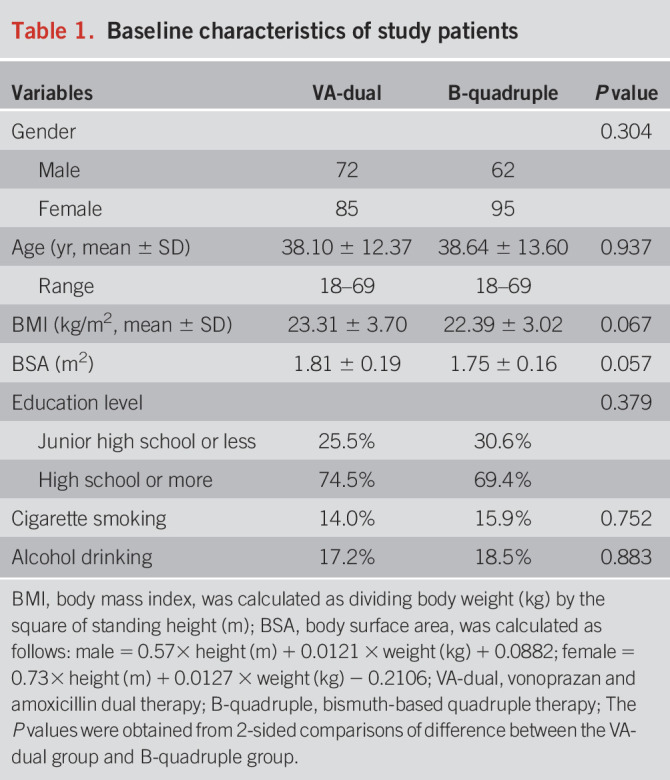
Baseline characteristics are summarized in Table 1. In total, 157 patients (72 men and 85 women) were enrolled in the VA-dual group, and 157 patients (62 men and 95 women) were enrolled in the B-quadruple group (P = 0.304). The mean age was 38.10 years (SD 12.37) in the VA-dual therapy group and 38.64 years (SD 13.60) in the B-quadruple therapy group (P = 0.937). There was no significant difference between baseline characteristics of the 2 groups in terms of gender, age, body mass index, body surface area, educational level, and history of tobacco or alcohol use.
Table 1.
Baseline characteristics of study patients
H. pylori eradication rates
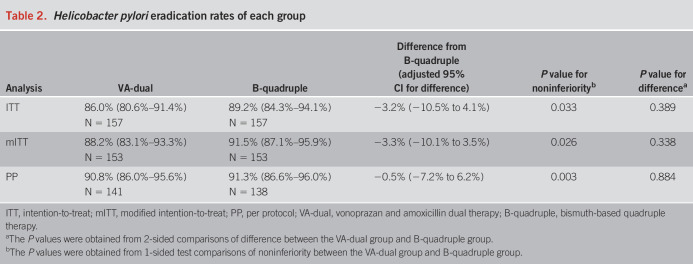
H. pylori eradication rates of each group in ITT, mITT, and PP analyses are listed in Table 2. In the ITT and mITT analyses, the H. pylori eradication rate was 86.0% (95% CI: 80.6%–91.4%, 135/157) and 88.2% (95% CI: 83.1%–93.3%, 135/153), respectively, in the VA-dual group, while 89.2% (95% CI: 84.3%–94.1%, 140/157) and 91.5% (95% CI: 87.1%–95.9%, 140/153), respectively, in the B-quadruple group. In the PP analysis, the H. pylori eradication rate was 90.8% (95% CI: 86.0%–95.6%, 128/141) and 91.3% (95% CI: 86.6%–96.0%, 126/138) in the VA-dual and B-quadruple groups, respectively. There was no significant difference in the overall eradication rates between 2 groups (P = 0.389, 0.338, and 0.884 in the ITT, mITT, and PP analyses, respectively).
Table 2.
Helicobacter pylori eradication rates of each group
In noninferiority test of VA-dual compared with B-quadruple therapy, the difference in eradication rates between the 2 groups was −3.2% (95% CI: −10.5% to 4.1%) in the ITT analysis, −3.3% (95% CI: −10.1% to 3.5%) in the mITT analysis, and −0.5% (95% CI: −7.2% to 6.2%) in the PP analysis. The lower bound of the 95% CI for the difference of eradication rates of the VA-dual group from the B-quadruple group was greater than the prespecified noninferiority margin in the ITT, mITT, and PP analyses, which indicated noninferiority efficacy of VA-dual therapy to standard B-quadruple therapy.
Adverse events and compliance
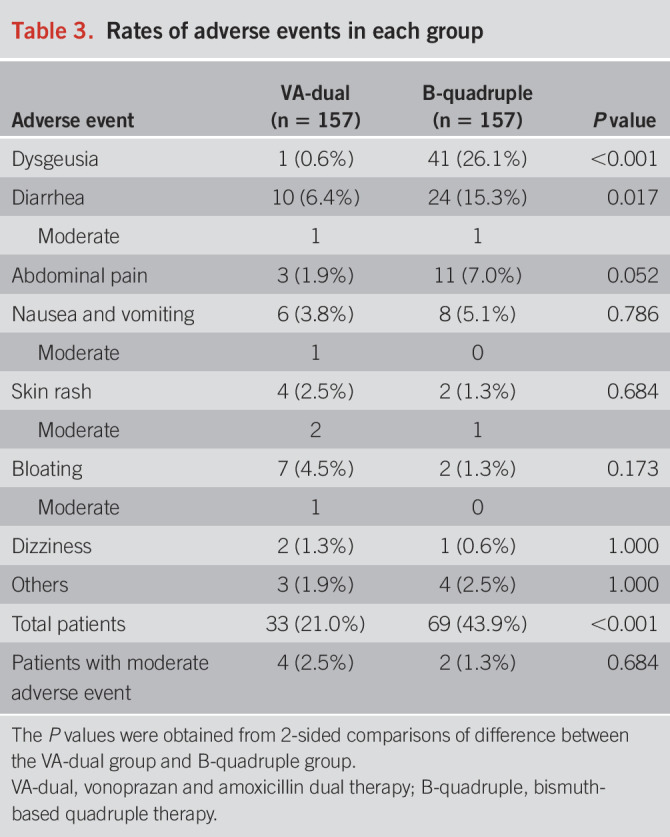
The adverse events of all patients are summarized in Table 3. The total rate of adverse events in the B-quadruple group was significantly higher than that in the VA-dual group (43.9% vs 21.0%, P < 0.001). Among all the adverse events, dysgeusia (26.1% vs 0.6%, P < 0.001) and diarrhea (15.3% vs 6.4%, P = 0.017) occurred more frequently in the B-quadruple group than in the VA-dual group, while the rates of abdominal pain, nausea and vomiting, skin rash, bloating, dizziness, and others were similar. Overall, 94.6% (122/129) of the adverse events were mild (grade 1 in CTCAE) and 5.4% (7/129) were moderate (grade 2 in CTCAE). There was no presence of severe adverse events (grades 3–4 in CTCAE).
Table 3.
Rates of adverse events in each group
A total of 15 patients in the VA-dual group and 18 patients in the B-quadruple group failed to take at least 80% of the prescribed drugs. The rates of good compliance were similar in the VA-dual group and B-quadruple group (90.4% vs 88.5%, P = 0.713). A total of 4 patients discontinued because of adverse events. Among these 4 patients, 1 patient in the B-quadruple group experienced rash on the ankles on the second day and 1 patient in the VA-dual group experienced rash on the arms on the second day; they discontinued the therapy on the next day (without follow-up UBT). One patient in the VA-dual group had rash all over the body on the sixth day and discontinued the therapy on the same day; then the rash disappeared spontaneously and his follow-up UBT showed successful eradication. One patient in the VA-dual group experienced moderate bloating and nausea, and then she discontinued the therapy from the third day because subsequent gastroscopy suggested gastric retention (without follow-up UBT).
Factors affecting eradication rates
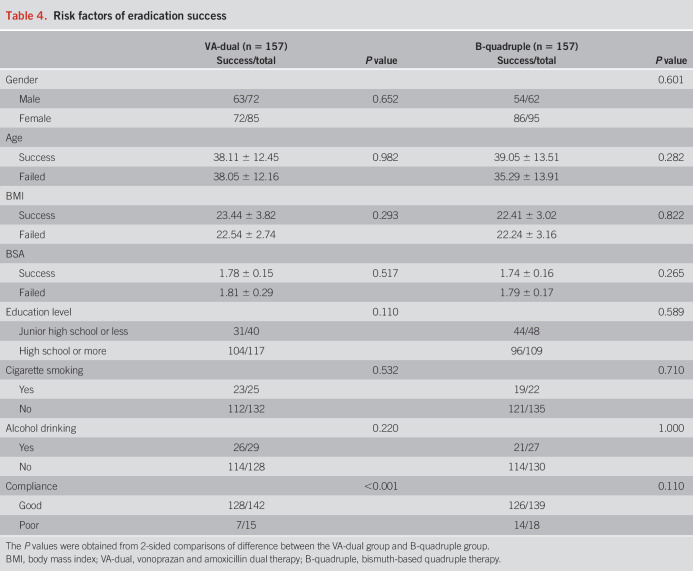
We performed stepwise logistic regression analyses to explore factors associated with eradication failure. As summarized in Table 4, the eradication rates in the VA-dual group were remarkably higher among good compliant patients compared with poor compliance (90.1% vs 46.7%, P < 0.001). While in the B-quadruple group, good compliance did not result in significant higher eradication rate (90.6% vs 77.8%, P = 0.110). Gender, age, body mass index, body surface area, education level, and history of cigarette or alcohol use were not associated with eradication failure.
Table 4.
Risk factors of eradication success
DISCUSSION
In this multicenter, prospective, open-label, noninferiority, and randomized controlled trial, we evaluated the efficiency and safety of 10-day VA-dual regimen compared with standard 14-day B-quadruple regimen as first-line H. pylori treatment. Results showed that VA-dual therapy achieved a delectable eradication rate of 90.8% in PP analysis and was comparable with B-quadruple therapy in ITT, mITT, and PP analyses. Besides, the adverse event occurred less common in the VA-dual group than in the B-quadruple group, particularly regarding abnormal taste and diarrhea. Eradication failure attributed to poor compliance in the VA-dual group.
The 10-day VA-dual therapy as first-line treatment has shown remarkable benefits, compared with 14-day B-quadruple therapy. First, the cost-effectiveness of H. pylori regimens is a crucial consideration for promoting population-based H. pylori screening and eradication strategy in China. The 10-day VA-dual therapy applied minimal variety of the drugs with shorter treatment duration and therefore occupies less health insurance cost. Take head clinical center of our study, for example, the cost of 10-day VA-dual regimen was 216.54 RMB/patient (29.7 USD), while the cost of 14-day B-quadruple regimen was 389.42 RMB/patient (53.5 USD), thus a 44% expense reduction can be obtained in each patient. Second, amoxicillin has well-known advantages such as being highly available, rare resistance and almost no development of secondary resistance, having few side effects other than allergies, and having a low impact on the intestinal flora (19). Therefore, antimicrobial susceptibility testing is not necessary before VA-dual therapy, and even if the eradication fails, future antibiotic would still face wide selection. Third, vonoprazan twice daily administration could archive similar intragastric pH compared with high dose PPI. The stronger and more sustained acid inhibitory effect enhances antibiotic function by decreasing the minimal inhibitory concentration, increasing the chemical stability, and increasing the concentration of antibiotics in succus gastricus (20,21).
Treatment duration and antibiotics selection are of fierce debate among H. pylori experts. Recent studies have provided evidence of high-dose amoxicillin (3 g, administered 3 or 4 times/d)–based VA dual therapy in treatment optimization from traditional triple and quadruple regimen (15,18). A multicenter randomized clinical trial in China showed a satisfied eradication rate of 95.6% for 14-day VA dual therapy, confirming treatment potential for high-dose amoxicillin-based VA dual therapy as the first-line H. pylori treatment (15). However, according to current evidence, whether the duration could be shortened to 10 days was controversial (14,16). To provide higher level of evidence in standardizing and optimizing the regimen, we designed this randomized controlled trial to compare 10-day VA (with amoxicillin of 3 g, administered 3 times/d) with current first-line standard 14-day B-quadruple regimen. Delightfully, we proved an eradication rate of 10-day VA treatment up to 90% and less adverse events compared with standard 14-day B-quadruple regimen, providing strong evidence of shorter-course therapy as an alternative in Chinese population.
It is noteworthy that poor compliance (treatment duration <8 days) was significantly associated with eradication failure in the VA-dual group, while not in the B-quadruple group, indicating a possible just right course for VA-dual of 10 days, but a redundant course for B-quadruple for 14 days. It is a reasonable speculation that eradication failure of B-quadruple owes to other reasons such as clarithromycin resistance, rather than poor compliance. Regardless, sticking to the prescription was essential to eradication success in VA-dual therapy.
Several limitations are inevitable when explaining the results of this study. First, antimicrobial susceptibility testing was not performed in this study. However, the resistance rate of amoxicillin is still rare in China, and absence of antimicrobial susceptibility testing is not unendurable for VA-dual regimen (15). On the contrary, even in regions with high clarithromycin resistance, clarithromycin-based B-quadruple therapy would also achieve high eradication rate of more than 90%, which weakens the influence of missing antimicrobial susceptibility testing in this study (22). Second, patients allergic to penicillin or regions with high amoxicillin resistance are not suitable for VA-dual and appropriate regimen without amoxicillin needs further exploration. Last, open label without blinding may have introduced treatment bias and may have affected the reporting of adverse events.
In conclusion, this study revealed a satisfactory H. pylori eradication rate of 90.8% for 10-day VA-dual therapy in Eastern China with low adverse event rate compared with standard 14-day B-quadruple treatment. VA-dual is a promising cost-effective regimen and worth generalization in population-based H. pylori screening and eradication strategy.
CONFLICTS OF INTEREST
Guarantors of the article: Yi Chen, MD.
Specific author contributions: T.Y., L.L. and Y.C.: designed the trial. T.Y., J.W., X.-J.H., Y.-B.Z., L.-J.L., Y.-J.W., Z.-W.W., J.-G.G., C.-F.X., H.M., and S.-M.L.: contributed to patient enrollment. J.W.: collected and analyzed data. T.Y.: drafted the manuscript. L.L. and Y.C.: supervised the whole process.
Financial support: This work was supported by the National Natural Science Foundation of China (81600447), National Natural Science Foundation of China (81970498), and Natural Science Foundation of Zhejiang Province (LZ22H030002).
Potential competing interests: Nothing to report.
Data availability: Analytic methods will be available within publication other researchers. Individual participant data will not be shared.
Study Highlights.
WHAT IS KNOWN
✓ Helicobacter pylori eradication is of great attention in clinical care.
✓ Treatment options diverse, which boost heavy economy burden.
✓ It is in urgent need to be simplified and standardized.
WHAT IS NEW HERE
✓ Ten-day vonoprazan-amoxicillin dual therapy provided satisfactory eradication rates of >90% (PP analysis).
✓ Ten-day vonoprazan-amoxicillin dual therapy was noninferior to standard 14-day B-quadruple therapy as first-line H. pylori treatment.
Footnotes
Tian-Lian Yan, Jing-Hua Wang contributed equally to this work.
Contributor Information
Tian-Lian Yan, Email: ytl1201@zju.edu.cn.
Jing-Hua Wang, Email: wjh614@zju.edu.cn.
Xin-Jue He, Email: hexinjue@zju.edu.cn.
Ya-Bi Zhu, Email: 18957091362@163.com.
Lin-Jie Lu, Email: lulin_jie@126.com.
Yan-Jiao Wang, Email: wyajiao@163.com.
Zi-Wei Wang, Email: wangzwmimi0033@sina.com.
Jian-Guo Gao, Email: gjg0633706@163.com.
Cheng-Fu Xu, Email: xiaofu@zju.edu.cn.
Han Ma, Email: mahan@zju.edu.cn.
Shuang-Mei Luan, Email: lslsm2016@163.com.
REFERENCES
- 1.Boltin D, Niv Y, Schutte K, et al. Review: Helicobacter pylori and non-malignant upper gastrointestinal diseases. Helicobacter 2019;24(Suppl 1):e12637. [DOI] [PubMed] [Google Scholar]
- 2.Sugano K. Effect of Helicobacter pylori eradication on the incidence of gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2019;22:435–45. [DOI] [PubMed] [Google Scholar]
- 3.Li M, Sun Y, Yang J, et al. Time trends and other sources of variation in Helicobacter pylori infection in mainland China: A systematic review and meta-analysis. Helicobacter 2020;25:e12729. [DOI] [PubMed] [Google Scholar]
- 4.Yuan C, Adeloye D, Luk TT, et al. The global prevalence of and factors associated with Helicobacter pylori infection in children: A systematic review and meta-analysis. Lancet Child Adolesc Health 2022;6:185–94. [DOI] [PubMed] [Google Scholar]
- 5.Han Y, Yan T, Ma H, et al. Cost-effectiveness analysis of Helicobacter pylori eradication therapy for prevention of gastric cancer: A Markov model. Dig Dis Sci 2020;65:1679–88. [DOI] [PubMed] [Google Scholar]
- 6.Yan TL, Gao JG, Wang JH, et al. Current status of Helicobacter pylori eradication and risk factors for eradication failure. World J Gastroenterol 2020;26:4846–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu WZ, Xie Y, Lu H, et al. Fifth Chinese national consensus report on the management of Helicobacter pylori infection. Helicobacter 2018;23:e12475. [DOI] [PubMed] [Google Scholar]
- 8.Malfertheiner P, Megraud F, Rokkas T, et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022:1–39. doi: 10.1136/gutjnl-2022-327745 [DOI] [PubMed] [Google Scholar]
- 9.Liou JM, Jiang XT, Chen CC, et al. Second-line levofloxacin-based quadruple therapy versus bismuth-based quadruple therapy for Helicobacter pylori eradication and long-term changes to the gut microbiota and antibiotic resistome: A multicentre, open-label, randomised controlled trial. Lancet Gastroenterol Hepatol 2023;8:228–41. [DOI] [PubMed] [Google Scholar]
- 10.Zhou L, Lu H, Song Z, et al. 2022 Chinese national clinical practice guideline on Helicobacter pylori eradication treatment. Chin Med J (Engl) 2022;135:2899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tai WC, Liang CM, Kuo CM, et al. A 14 day esomeprazole- and amoxicillin-containing high-dose dual therapy regimen achieves a high eradication rate as first-line anti-Helicobacter pylori treatment in Taiwan: A prospective randomized trial. J Antimicrob Chemother 2019;74:1718–24. [DOI] [PubMed] [Google Scholar]
- 12.Yu L, Luo L, Long X, et al. High-dose PPI-amoxicillin dual therapy with or without bismuth for first-line Helicobacter pylori therapy: A randomized trial. Helicobacter 2019;24:e12596. [DOI] [PubMed] [Google Scholar]
- 13.Yang J, Zhang Y, Fan L, et al. Eradication efficacy of modified dual therapy compared with bismuth-containing quadruple therapy as a first-line treatment of Helicobacter pylori. Am J Gastroenterol 2019;114:437–45. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y, Xu X, Ouyang YB, et al. Optimization of vonoprazan-amoxicillin dual therapy for eradicating Helicobacter pyloriinfection in China: A prospective, randomized clinical pilot study. Helicobacter 2022;27:e12896. [DOI] [PubMed] [Google Scholar]
- 15.Hu J, Mei H, Su NY, et al. Eradication rates of Helicobacter pylori in treatment-naive patients following 14-day vonoprazan-amoxicillin dual therapy: A multicenter randomized controlled trial in China. Helicobacter 2023;28:e12970. [DOI] [PubMed] [Google Scholar]
- 16.Qian HS, Li WJ, Dang YN, et al. Ten-day vonoprazan-amoxicillin dual therapy as a first-line treatment of Helicobacter pylori infection compared with bismuth-containing quadruple therapy. Am J Gastroenterol 2022;118:627–34. [DOI] [PubMed] [Google Scholar]
- 17.U.S. Department of Health and Human Services NIoH, National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. The United States of America. National Institutes of Health, 2017. (https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm#ctc_50). Accessed March 18, 2023. [Google Scholar]
- 18.Chey WD, Megraud F, Laine L, et al. Vonoprazan triple and dual therapy for Helicobacter pylori infection in the United States and Europe: Randomized clinical trial. Gastroenterology 2022;163:608–19. [DOI] [PubMed] [Google Scholar]
- 19.Hu Y, Xu X, Ouyang YB, et al. Altered gut microbiota and short-Chain fatty acids after vonoprazan-amoxicillin dual therapy for Helicobacter pylori eradication. Front Cell Infect Microbiol 2022;12:881968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scarpignato C, Howden CW, Leifke E, et al. A translational pharmacokinetic/pharmacodynamic approach supports optimal vonoprazan dosing for erosive oesophagitis and Helicobacter pylori infection. Aliment Pharmacol Ther 2023;58:16–25. [DOI] [PubMed] [Google Scholar]
- 21.Kiyotoki S, Nishikawa J, Sakaida I. Efficacy of vonoprazan for Helicobacter pylori eradication. Intern Med 2020;59:153–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long X, Chen Q, Yu L, et al. Bismuth improves efficacy of proton-pump inhibitor clarithromycin, metronidazole triple Helicobacter pylori therapy despite a high prevalence of antimicrobial resistance. Helicobacter 2018;23:e12485. [DOI] [PubMed] [Google Scholar]