Abstract
The therapeutic landscape for patients with advanced or metastatic non–small cell lung cancer (NSCLC) is rapidly evolving due to advances in molecular testing and the development of new targeted therapies and immunotherapies. However, the efficacy of programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) inhibitors in advanced or metastatic patients with NSCLC whose tumors harbor BRAF V600E mutation, HER2/ERBB2 alteration, MET exon 14 skipping mutation, or RET rearrangement is not completely understood. A systematic literature review was performed to summarize evidence from clinical trials and observational studies on objective response rate, progression-free survival, and overall survival in patients whose tumors express these biomarkers and who were treated with PD-1/PD-L1 inhibitors. Searches of Embase, MEDLINE, conference abstracts, and a clinical trial registry identified a total of 12 unique studies: 4 studies included patients with BRAF V600E mutation, 6 studies included patients with HER2/ERBB2 alteration, 7 studies included patients with MET exon 14 skipping mutation, and 5 studies included patients with RET rearrangement. Across studies, there was heterogeneity in treatment and patient characteristics and a lack of reporting on many important predictive and prognostic factors, including treatment regimens, patients’ line of therapy, and tumor PD-L1 expression, which may explain the wide variation in objective response rate, progression-free survival, and overall survival across studies. Therefore, additional studies prospectively evaluating clinical outcomes of PD-1/PD-L1 inhibitors among patients with advanced or metastatic NSCLC whose tumors harbor emerging predictive or prognostic biomarkers are needed to determine whether this class of immunotherapy can provide additional survival benefits for these patients.
Key Words: non–small cell lung cancer, biomarkers, oncogenes, immune checkpoint inhibitors
Lung cancer is the leading cause of cancer-related mortality worldwide, with over 2.2 million new diagnoses and 1.8 million deaths estimated in 2020.1 Most (80%–90%) patients with lung cancer have non–small cell lung cancer (NSCLC), of whom ~70% are diagnosed in the advanced or metastatic stage.2–4 Promisingly, however, the therapeutic landscape for patients with advanced or metastatic NSCLC is rapidly evolving due to advances in molecular testing and the development of new therapies targeting specific molecular alterations.2,5–8 Recent studies show that a large proportion (28%–44%) of patients with NSCLC have tumors expressing biomarkers that are targetable by first-line (1L) or subsequent therapies approved for advanced or metastatic NSCLC.9–11 These biomarkers include genetic alterations in epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), ROS proto-oncogene 1 (ROS1), B-Raf proto-oncogene (BRAF), Erbb2 receptor tyrosine-protein kinase 2 (ERBB2)/human epidermal growth factor receptor 2 (HER2), Kirsten rat sarcoma virus (KRAS), MET proto-oncogene (MET), neurotrophic tyrosine receptor kinase (NTRK), and rearranged during transfection (RET).3,4 Thus, by considering tumor molecular profiles together with other patient characteristics, such as tumor histology, clinicians are increasingly able to select treatment regimens that are most likely to improve the clinical outcomes of individual patients with advanced or metastatic NSCLC.2–8
In addition to molecularly targeted therapies, immunotherapies including programmed death 1 (PD-1)/programmed death ligand 1 (PD-L1) inhibitors have also dramatically changed the way that NSCLC is treated and have led to survival benefits among patients with advanced or metastatic NSCLC with any PD-L1 expression level.12 Today, PD-1/PD-L1 inhibitors are predominately used in the 1L setting for patients with advanced or metastatic NSCLC whose tumors do not harbor EGFR, ALK, or ROS1 alterations. For patients whose tumors express an EGFR mutation or ALK rearrangement, treatment guidelines state that PD-1/PD-L1 inhibitors may be used for subsequent therapy after disease progression on a targeted EGFR or ALK inhibitor, respectively, although some evidence shows that PD-1/PD-L1 inhibitors may have less of a benefit for patients with certain EGFR or ALK alterations.3,4 In addition, randomized controlled trials show that PD-1/PD-L1 inhibitors delivered with or without chemotherapy in the 1L setting improve survival regardless of KRAS mutation status,13,14 and meta-analyses including studies evaluating PD-1/PD-L1 inhibitors delivered with or without chemotherapy in different treatment lines report greater improvements in survival in patients with KRAS mutant tumors than in patients with KRAS wild-type tumors.15,16 Emerging evidence also suggests that STK11 mutations may confer resistance to PD-1/PD-L1 inhibitors.17 Therefore, the clinical outcomes of PD-1/PD-L1 inhibitors may vary depending on the precise genetic alterations found in the tumors of patients with advanced or metastatic NSCLC.
Although there is accumulating evidence on the efficacy of PD-1/PD-L1 inhibitors among patients with advanced or metastatic NSCLC whose tumors express EGFR, ALK, ROS1, STK11, or KRAS alterations, there is a lack of synthesized evidence on the efficacy of these immunotherapies among patients who tumors express other molecular alterations that are predictive of response to specific therapies (ie, predictive biomarkers) or indicative of survival independent of the treatment received (ie, prognostic biomarkers). Therefore, to further understand the clinical outcomes of PD-1/PD-L1 inhibitors among patients with advanced or metastatic NSCLC whose tumors harbor emerging predictive or prognostic biomarkers, a systematic literature review (SLR) was performed to identify and summarize evidence from clinical trials and observational studies on objective response rate (ORR), progression-free survival (PFS), and overall survival (OS) among patients whose tumors express BRAF V600E mutation, HER2/ERBB2 alterations, MET exon 14 skipping mutation, or RET rearrangements and who were treated with PD-1/PD-L1 inhibitors.
MATERIALS AND METHODS
This SLR was performed and reported in accordance with “PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analyses)” 2020 guidelines.18
Database searches were conducted in Embase, MEDLINE, and Cochrane Central Register of Controlled Trials (CENTRAL) through the OVID platform on March 15, 2022. To identify relevant studies that were not yet published in full-text format, searches for conference abstracts from American Society of Clinical Oncology, European Society for Medical Oncology, European Society for Medical Oncology Asia, International Association for the Study of Lung Cancer’s World Conference on Lung Cancer, American Association for Cancer Research, Society for Immunotherapy of Cancer, and International Association for the Study of Lung Cancer’s Targeted Therapies of Lung Cancer from 2020 to 2021 were performed. Finally, the US National Institutes of Health’s Clinical Trial Registry (http://www.clinicaltrials.gov) was manually searched to identify completed studies not yet published with results available. Search strategies are provided in Supplemental Digital Content 1, Tables S1–S3 (Supplemental Digital Content 1, http://links.lww.com/JIT/A802, Supplemental Digital Content 2, http://links.lww.com/JIT/A803, Supplemental Digital Content 3, http://links.lww.com/JIT/A804).
Title/abstract screening, full-text screening, data extraction, and quality assessment were performed by 2 independent reviewers, with discrepancies between reviewers resolved by discussion and the involvement of a third reviewer, if needed. Studies were eligible for inclusion in the SLR if they were randomized controlled trials, nonrandomized clinical trials, or observational studies evaluating the efficacy or effectiveness of PD-1/PD-L1 inhibitors (atezolizumab, cemiplimab, durvalumab, nivolumab, or pembrolizumab) in adult patients with recurrent or de novo stage III or IV NSCLC who were not candidates for surgical resection or definitive chemoradiation and for whom there was positive detection of one or more of the following biomarkers: BRAF V600E mutation, HER2/ERBB2 alteration, MET exon 14 skipping mutation, or RET rearrangement. For studies enrolling a mixed population of patients with and without detectable biomarkers, only studies reporting outcomes by biomarker status were eligible for inclusion. Studies evaluating pooled treatments (ie, different PD-1/PD-L1-based regimens pooled together in a single treatment group) were eligible for inclusion if at least 80% of patients19 in the study were treated with a PD-1/PD-L1 inhibitor. Publications were excluded from the SLR if they were studies conducted exclusively in biomarker-negative or unknown populations, studies reporting biomarker subgroups with <5 patients, case reports or case series describing <5 patients, editorials, commentaries, or review articles. Only English-language reports were eligible for inclusion.
Data were extracted on study characteristics, baseline patient characteristics, treatment characteristics, and reported outcomes. Quality assessment of included studies was performed with the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies,20 which was used for both observational studies and clinical trials that were not randomized based on patients’ biomarker status, making the biomarker subgroup analysis more akin to a cohort study design.
RESULTS
Out of a total of 5862 records identified through searches of Embase, MEDLINE, CENTRAL, conference proceedings, and ClinicalTrials.gov, 13 reports describing 12 unique studies were selected for inclusion in the SLR (Fig. 1). Eleven studies were reported as full-text journal articles and 1 study was reported as a conference abstract and accompanying ClinicalTrials.gov record. The quality of these 12 studies as evaluated using the Newcastle-Ottawa Quality Assessment Scale for Cohort Studies is reported in Supplemental Digital Content 1, Table S4 (Supplemental Digital Content 4, http://links.lww.com/JIT/A805).
FIGURE 1.
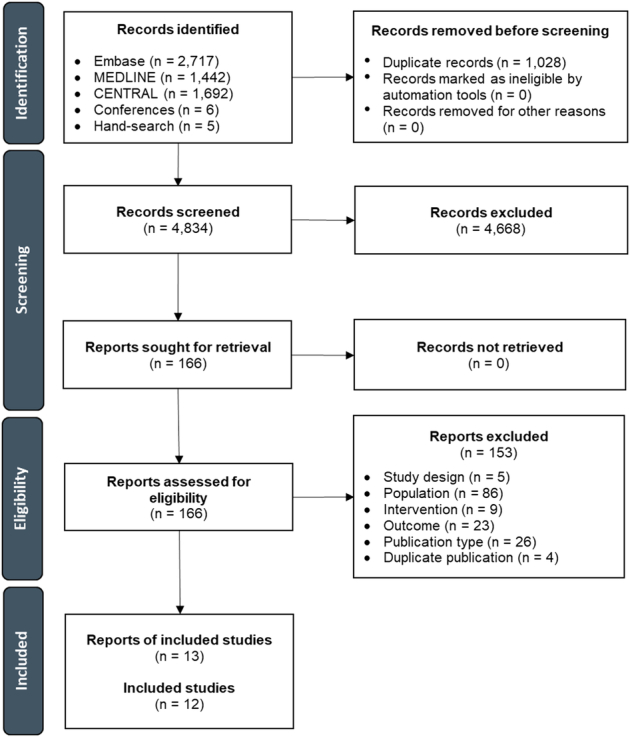
PRISMA flow diagram depicting the study selection process. PRISMA indicates Preferred Reporting Items for Systematic Reviews and Meta-analyses.
BRAF V600E Mutation
Between 1% and 5% of patients with NSCLC harbor BRAF mutations, among whom over half exhibit point mutation in BRAF V600E, which leads to persistent activation of downstream signaling in the mitogen-activated protein kinase pathway, resulting in uncontrolled cell proliferation.21
This SLR identified 4 observational studies22–25 that included patients with BRAF V600E mutation who were treated with PD-1/PD-L1 inhibitors (Table 1). All 4 studies evaluated pooled PD-1/PD-L1 inhibitors (ie, different PD-1/PD-L1-based regimens pooled together in a single treatment group) delivered as monotherapy. Three studies included patients in a 1L+ treatment setting, whereas one study did not specify patients’ line of therapy. Other patient characteristics, including age, sex, and performance status, are shown in Supplemental Digital Content 1, Table S5 (Supplemental Digital Content 5, http://links.lww.com/JIT/A806).
TABLE 1.
Summary of Studies Evaluating PD-1/PD-L1 Inhibitor Treatment Among Patients With BRAF V600E Mutation
| Study | Treatment | Line of therapy | Alteration type | No. patients | PD-L1 1%–49% (%) | PD-L1 ≥50% (%) | ORR (%) | Median PFS (mo) (95% CI) | Median OS (mo) (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Dudnik 201822 | Nivolumab, pembrolizumab, or atezolizumab | 1L+ | V600E mutation | 11* | 32† | 42† | — | — | 33.9 (4.6–NR) |
| Guisier 202023 | Single-agent nivolumab, pembrolizumab, or other immunotherapy agent | 1L+ | V600E mutation | 26 | — | 4 | 26.1 | 5.3 (2.1–NR) | 22.5 (8.3–NR) |
| Mazieres 201924 | Single-agent nivolumab, pembrolizumab, atezolizumab, durvalumab, or other PD-1/PD-L1 inhibitor | Not specified | V600E mutation | 17 | — | 55.6‡ | — | 1.8 (1–4.6) | 8.2 (1.1–NR) |
| Negrao 202125 | Single-agent atezolizumab, durvalumab, nivolumab, or pembrolizumab | 1L+ | V600E mutation | 30§ | ∥ | ∥ | — | 9.79 (7.59–NE) | 20.83 (7.49–NE) |
Advanced disease only subgroup.
Overall PD-L1 expression level reported for patients with any disease stage.
Overall PD-L1 expression level reported for patients with any BRAF exon 15 mutation (V600E and non-V600E mutations).
Clinico-Genomic Database immunotherapy cohort.
22% of patients had PD-L1 expression ≥1%.
1L indicates first-line; NE, not estimable; NR, not reached; ORR, objective response rate; OS, overall survival; PD-1, programmed death protein 1; PD-L1, programmed death ligand 1; PFS, progression-free survival.
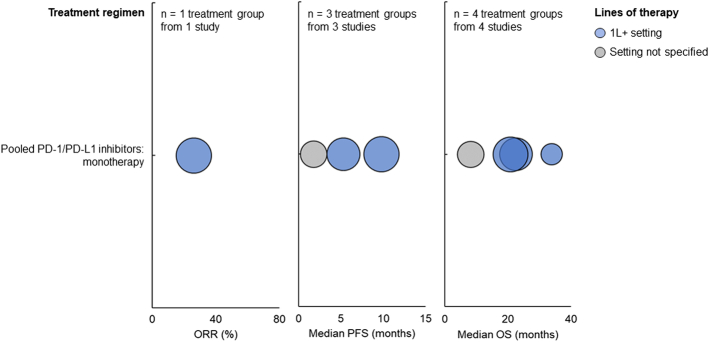
For patients with BRAF V600E mutation treated with pooled PD-1/PD-L1 monotherapy in the 1L+ setting, ORR was 26.1%, median PFS ranged from 5.3 to 9.79 months, and median OS ranged from 20.83 to 33.9 months (Fig. 2). For patients treated with pooled PD-1/PD-L1 monotherapy whose line of therapy was not specified, the median PFS was 1.8 months, and the median OS was 8.2 months.
FIGURE 2.
ORR (left), median PFS (middle), and median OS (right) among patients with BRAF V600E mutation treated with PD-1/PD-L1 inhibitors. Each bubble corresponds to one treatment group, with bubble size reflecting the number of patients within each group. The y-axis categories correspond to different treatment regimens, and the bubble color indicates the patients’ line of therapy. ORR indicates objective response rate; OS, overall survival; PD-1, programmed death protein 1; PD-L1, programmed death ligand 1; PFS, progression-free survival.
ERBB2/HER2 Alteration
ERBB2/HER alterations, including mutations, amplification, and overexpression, are observed in 1%–4%, 2%–5%, and 2%–30% of patients with NSCLC, respectively.26 All HER2 alterations lead to persistent activation of HER2 and multiple downstream signaling pathways, resulting in cell growth, metastasis, and angiogenesis.
This SLR identified 6 observational studies23–25,27–29 that included patients with HER2 alteration who were treated with PD-1/PD-L1 inhibitors (Table 2). These studies describe patient subgroups with a variety of HER2 alterations, including exon 20 insertions, transmembrane or juxtamembrane domain point mutations between exons 17 and 19, and mutations in codons 755 or 770–785. Some studies evaluated pooled PD-1/PD-L1 inhibitors delivered as monotherapy (n = 3), other studies evaluated pooled PD-1/PD-L1 inhibitors delivered as monotherapy or combination therapy (n = 2; with or without a CTLA4 inhibitor or another unspecified immune checkpoint inhibitor), and 1 study evaluated 2 treatment groups of pooled PD-1/PD-L1 inhibitors delivered as monotherapy. Most studies (n = 5) included patients in a 1L+ setting, whereas one study did not specify patients’ line of therapy. Other patient characteristics are shown in Supplemental Digital Content 1, Table S6 (Supplemental Digital Content 6, http://links.lww.com/JIT/A807).
TABLE 2.
Summary of Studies Evaluating PD-1/PD-L1 Inhibitor Treatment Among Patients With ERBB2/HER2 Alteration
| Study | Treatment | Line of therapy | Alteration type | No.patients | PD-L1 1%–49% (%) | PD-L1 ≥50% (%) | ORR (%) | Median PFS (mo) (95% CI) | Median OS (mo) (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Cinaursero 201927 | Nivolumab or pembrolizumab | 1L+ | Unspecified mutation | 6 | — | — | — | * | * |
| Guisier 202023 | Single-agent nivolumab, pembrolizumab, or other immunotherapy agent | 1L+ | Exon 20 insertion | 23 | 6 | 37 | 27.3 | 2.2 (1.7–15.2) | 20.4 (9.3–NR) |
| Lau 202128 | Pembrolizumab, nivolumab, or other PD-1 inhibitor±CTLA4 inhibitor | 1L+ | Exon 20 insertion or transmembrane or juxtamembrane domain point mutation between exons 17 and 19 | 14 | 38 | 23 | 29 | 3.6 (1.6–NR) | — |
| Mazieres 201924 | Single-agent nivolumab, pembrolizumab, atezolizumab, durvalumab, or other PD-1/PD-L1 inhibitor | Not specified | Exon 20 activating mutation | 29 | † | † | 7.4 | 2.5 (1.8–3.5) | 20.3 (7.8–NR) |
| Negrao 202125 | Single-agent atezolizumab, durvalumab, nivolumab, or pembrolizumab | 1L+ | Mutation in codons 755 or 770–785 | 15‡ | δ | δ | 8 | 1.88 (1.6–2.1) | 16.8 (3.1–30.6) |
| Single-agent atezolizumab, durvalumab, nivolumab, or pembrolizumab | 1L+ | Mutation in codons 755 or 770–785 | 28∥ | ¶ | ¶ | — | 3.02 (1.81–NE) | 10.81 (5.62–NE) | |
| Yoh 202129 | Pembrolizumab, nivolumab, atezolizumab, or PD-L1 inhibitor±another ICI received in clinical trial# | 1L+ | Exon 20 insertion | 9 | — | — | 33 | — | — |
Study does not report median value but instead reports hazard ratio for mutation versus wild-type comparison.
PD-L1 <10%: 84.6%, PD-L1 ≥10%: 15.4%.
MD Anderson Cancer Center cohort.
PD-L1 ≥1%: 13%.
Clinico-Genomic Database immunotherapy cohort.
PD-L1 ≥1%: 4%.
1% of patients in the total study cohort (n = 260) were treated in a clinical trial.
CTLA4 indicates cytotoxic T-lymphocyte–associated protein 4; ICI, immune checkpoint inhibitor; 1L, first-line; NE, not estimable; NR, not reached; ORR, objective response rate; OS, overall survival; PD-1, programmed death protein 1; PD-L1, programmed death ligand 1; PFS, progression-free survival.
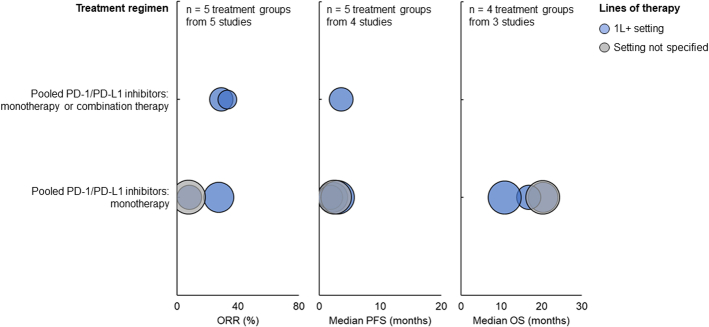
For patients with HER2 alterations treated with pooled PD-1/PD-L1 monotherapy in the 1L+ setting, ORR ranged from 8% to 27.3%, median PFS ranged from 1.88 to 3.02 months, and median OS ranged from 10.81 to 20.4 months (Fig. 3). For patients treated with pooled PD-1/PD-L1 monotherapy or combination therapy in the 1L+ setting, ORR ranged from 29% to 33%, and median PFS was 3.6 months. For patients treated with pooled PD-1/PD-L1 monotherapy whose line of therapy was not specified, ORR was 7.4%, median PFS was 2.5 months, and median OS was 20.3 months.
FIGURE 3.
ORR (left), median PFS (middle), and median OS (right) among patients with ERBB2/HER2 alteration treated with PD-1/PD-L1 inhibitors. Each bubble corresponds to one treatment group, with bubble size reflecting the number of patients within each group. The y-axis categories correspond to different treatment regimens, and the bubble color indicates the patients’ line of therapy. ORR indicates objective response rate; OS, overall survival; PD-1, programmed death protein 1; PD-L1, programmed death ligand 1; PFS, progression-free survival.
MET Exon 14 Skipping Mutation
Up to 3% of patients with NSCLC exhibit MET exon 14 skipping mutation, which leads to impaired MET protein degradation and excessive activation of MET signaling, thereby driving tumor cell proliferation, survival, invasion, and metastasis.30
This SLR identified 1 randomized controlled trial31,32 and 6 observational studies23–25,29,33,34 that included patients with MET exon 14 skipping mutation who were treated with PD-1/PD-L1 inhibitors (Table 3). Most studies exclusively described patients with exon 14 skipping mutation (n = 5), whereas one study described patients with either exon 14 or amplification mutations. Some studies evaluated pooled PD-1/PD-L1 inhibitors delivered as monotherapy (n = 4), other studies evaluated pooled PD-1/PD-L1 inhibitors delivered as monotherapy or combination therapy (n = 2; with ipilimumab or another unspecified immune checkpoint inhibitor), and 1 study evaluated nivolumab-based combination therapy (with capmatinib). Most studies (n = 6) included patients in a 1L+ setting, whereas 1 study did not specify patients’ line of therapy. Other patient characteristics are shown in Supplemental Digital Content 1, Table S7 (Supplemental Digital Content 7, http://links.lww.com/JIT/A808).
TABLE 3.
Summary of Studies Evaluating PD-1/PD-L1 Inhibitor Treatment Among Patients With MET exon 14 Skipping Mutation
| Study | Treatment | Line of therapy | Alteration type | No. patients | PD-L1 1%–49% (%) | PD-L1 ≥50% (%) | ORR (%) | Median PFS (mo) (95% CI) | Median OS (mo) (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Felip 202131 | Nivolumab + capmatinib | 1L+ | MET amplification or exon 14 mutation* | 16 | — | — | — | 13.8 (3.5–19.2) | — |
| Guisier 202023 | Single-agent nivolumab, pembrolizumab, or other immunotherapy agent | 1L+ | Exon 14 skipping | 30 | 11 | 22 | 35.7 | 4.9 (2–11.4) | 13.4 (9.4–NR) |
| Kauffmann-Guerrero 202033 | Nivolumab, pembrolizumab, or atezolizumab monotherapy | 1L+ | Exon 14 skipping | 8 | 37.5 | 50 | 37.5 | 13.25 (—) | — |
| Mazieres 201924 | Single-agent nivolumab, pembrolizumab, atezolizumab, durvalumab, or other PD-1/PD-L1 inhibitor | Not specified | Exon 14 skipping | 23 | † | † | — | 4.7 (1.8–7.8) | 25 (18.4–NR) |
| Negrao 202125 | Single-agent atezolizumab, durvalumab, nivolumab, or pembrolizumab | 1L+ | Exon 14 skipping | 28‡ | δ | δ | — | 2.69 (1.97–NE) | 12.25 (5.85–NE) |
| Sabari 201834 | Pembrolizumab, nivolumab, atezolizumab, durvalumab, or nivolumab + ipilimumab | 1L+ | Exon 14 skipping | 21 | 22 | 41 | 17 | 1.9 (1.7–2.7) | 18.2 (12.9–NR) |
| Yoh 202129 | Pembrolizumab, nivolumab, atezolizumab, or PD-1/PD-L1 inhibitor±another ICI received in a clinical trial | 1L+ | Exon 14 skipping | 9∥ | — | — | 25 | — | — |
Immunohistochemistry = 3+ in ≥50% tumor cells or immunohistochemistry = 2+ in ≥50% tumor cells and gene copy number ≥5 or MET exon 14+.
For patients with any MET alteration, PD-L1 1%–49%: 28.3%, PD-L1 ≥50%: 46.7%.
Clinico-Genomic Database immunotherapy cohort.
PD-L1 ≥1%: 9%.
1% of patients in the overall study cohort (n = 260) were treated in a clinical trial.
ICI indicates immune checkpoint inhibitor; 1L, first-line; NE, not estimable; NR, not reached; ORR, objective response rate; OS, overall survival; PD-1, programmed death protein 1; PD-L1, programmed death ligand 1; PFS, progression-free survival.
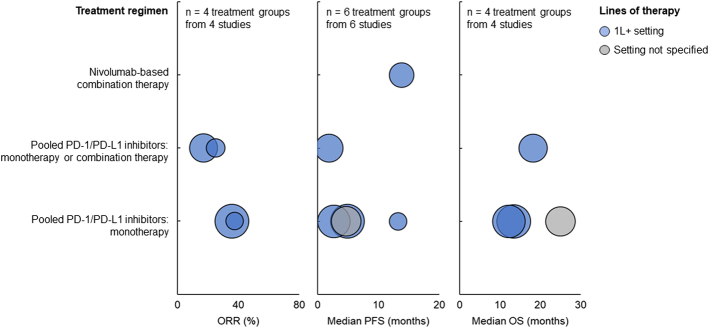
For patients with MET exon 14 skipping mutation who were treated with nivolumab-based combination therapy in the 1L+ setting, the median PFS was 13.8 months. For patients treated with pooled PD-1/PD-L1 monotherapy in the 1L+ setting, ORR ranged from 35.7% to 37.5%, median PFS ranged from 2.69 to 13.25 months, and median OS ranged from 12.25 to 13.4 months (Fig. 4). For patients treated with pooled PD-1/PD-L1 monotherapy or combination therapy in the 1L+ setting, ORR ranged from 17% to 25%, median PFS was 1.9 months, and median OS was 18.2 months. For patients treated with pooled PD-1/PD-L1 monotherapy whose line of therapy was not specified, the median PFS was 4.7 months, and the median OS was 25 months.
FIGURE 4.
ORR (left), median PFS (middle), and median OS (right) among patients with MET exon 14 skipping mutation treated with PD-1/PD-L1 inhibitors. Each bubble corresponds to one treatment group, with bubble size reflecting the number of patients within each group. The y-axis categories correspond to different treatment regimens, and the bubble color indicates the patients’ line of therapy. ORR indicates objective response rate; OS, overall survival; PD-1, programmed death protein 1; PD-L1, programmed death ligand 1; PFS, progression-free survival.
RET Rearrangement
RET rearrangements, which are found in 1%–2% of patients with NSCLC, can lead to constitutive mitogen-activated protein kinase or phosphoinositide 3-kinase pathway activation and hence uncontrolled cell proliferation, growth, differentiation, and survival.35
This SLR identified 5 observational studies23,24,29,36,37 that included patients with RET rearrangement who were treated with PD-1/PD-L1 inhibitors (Table 4). Most studies described patients with RET fusion (n = 3), whereas other studies described patients with RET translocation (n = 1) or did not specify the type of RET rearrangement (n = 1). Some studies evaluated pooled PD-1/PD-L1 inhibitors delivered as monotherapy (n = 2), 1 study evaluated pooled PD-1/PD-L1 inhibitors delivered as monotherapy or combination therapy (with or without another unspecified immune checkpoint inhibitor), 1 study evaluated pembrolizumab-based combination therapy (with pemetrexed + platinum or other systemic therapy), and 1 study evaluated 2 treatment groups: pooled PD-1/PD-L1 inhibitors delivered as monotherapy or combination therapy (with or without unspecified other agents) and pembrolizumab-based combination therapy (with pemetrexed + carboplatin). Two studies included patients in a 1L+ setting, 1 study included patients in a 1L setting, 1 study did not specify patients’ line of therapy, and 1 study included multiple cohorts of patients in 1L or second-line settings. Other patient characteristics are shown in Supplemental Digital Content 1, Table S8 (Supplemental Digital Content 8, http://links.lww.com/JIT/A809).
TABLE 4.
Summary of Studies Evaluating PD-1/PD-L1 Inhibitor Treatment Among Patients With RET Rearrangements
| Study | Treatment | Line of therapy | Alteration type | No. patients | PD-L1 1% to 49% (%) | PD-L1 ≥50% (%) | ORR (%) | Median PFS (mo) (95% CI) | Median OS (mo) (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Guisier 202023 | Single-agent nivolumab, pembrolizumab, or unspecified other immunotherapy agent | 1L+ | Translocation | 9 | — | 38 | 37.5 | 7.6 (2.3–NR) | NR (26.8–NR) |
| Mazieres 201924 | Single-agent nivolumab, pembrolizumab, atezolizumab, durvalumab, or other PD-1/PD-L1 inhibitor | Not specified | Unspecified rearrangement | 16 | — | 50 | 6.3 | 2.1 (1.3–4.7) | 21.3 (3.8–28) |
| Yoh 202129 | Pembrolizumab, nivolumab, atezolizumab, or PD-1/PD-L1 inhibitor + another ICI in a clinical trial‡ | 1L+ | Fusion | 13 | — | — | 33 | — | — |
| Bhandari 202136 | Atezolizumab, ipilimumab, nivolumab, or pembrolizumab±other agents | 1L | Fusion | 17* | — | — | 53.9 | 4.2 (1.4–8.4) | 19.1 (6.9–NR) |
| 2L | Fusion | 11* | — | — | 33.3 | 4.4 (1.5–NR) | 16 (3.7–NR) | ||
| Pembrolizumab + pemetrexed + carboplatin | 1L | Fusion | 12* | — | — | 70 | 5.4 (1.4–14.2) | 19 (6.9–NR) | |
| 1L | Fusion | 7† | — | — | — | — | NR (17.3–NR) | ||
| Hess 202137 | Pembrolizumab + pemetrexed + platinum | 1L | Fusion | 9 | 57.1 | 28.6 | 75 | 6.6 (0.4–NR) | NR (NE–NE) |
Clinico-Genomic Database cohort.
Guardant Health Database cohort.
1% of patients in the overall study cohort (n = 260) were treated in a clinical trial.
ICI indicates immune checkpoint inhibitor; 1L, first-line; 2L, second-line; NE, not estimable; NR, not reached; ORR, objective response rate; OS, overall survival; PD-1, programmed death protein 1; PD-L1, programmed death ligand 1; PFS, progression-free survival.
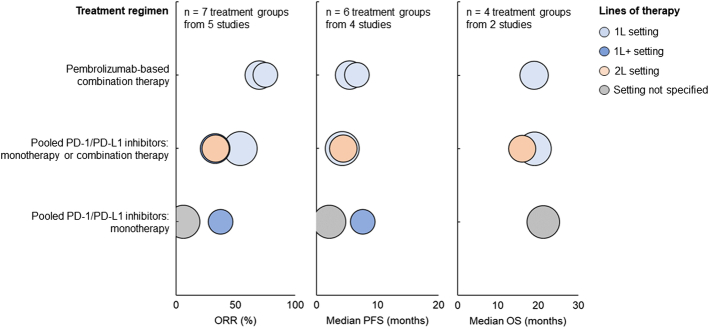
For patients treated with pembrolizumab-based combination therapy in the 1L setting, all of whom had RET fusion, ORR was 70%, median PFS was 5.4 months, and median OS was 19 months (Fig. 5). For patients treated with pooled PD-1/PD-L1 monotherapy or combination therapy in the 1L setting, all of whom had RET fusion, ORR ranged from 53.9% to 75%, median PFS ranged from 4.2 to 6.6 months, and median OS was 19.1 months. For patients treated with pooled PD-1/PD-L1 monotherapy in the 1L+ setting, all of whom had RET translocation, ORR was 37.5%, and median PFS was 7.6 months. For patients treated with pooled PD-1/PD-L1 monotherapy or combination therapy in the 1L+ setting, all of whom had RET fusion, ORR was 33%. For patients treated with pooled PD-1/PD-L1 monotherapy or combination therapy in the second-line setting, all of whom had RET fusion, ORR was 33.3%, median PFS was 4.4 months, and median OS was 16 months. For patients treated with pooled PD-1/PD-L1 monotherapy whose line of therapy was not specified, all of whom had unspecified RET rearrangements, ORR was 6.3%, median PFS was 2.1 months, and median OS was 21.3 months.
FIGURE 5.
ORR (left), median PFS (middle), and median OS (right) among patients with RET rearrangement treated with PD-1/PD-L1 inhibitors. Each bubble corresponds to one treatment group, with bubble size reflecting the number of patients within each group. The y-axis categories correspond to different treatment regimens, and the bubble color indicates the patients’ line of therapy. ORR indicates objective response rate; OS, overall survival; PD-1, programmed death protein 1; PD-L1, programmed death ligand 1; PFS, progression-free survival.
DISCUSSION
The objective of this study was to identify and summarize evidence from clinical trials and observational studies reporting clinical outcomes of patients with advanced or metastatic NSCLC harboring BRAF V600E mutation, ERBB2/HER2 alteration, MET exon 14 skipping mutation, or RET rearrangement and treated with PD-1/PD-L1 inhibitors. The overall evidence base of all included studies consisted primarily of observational studies, and the one interventional study identified (which included patients with MET exon 14 skipping mutation) analyzed outcomes by biomarker status in a post hoc manner. Most studies evaluated different PD-1/PD-L1 inhibitors pooled together in a single treatment group without separating the agents when reporting outcomes. Among these studies, there was substantial heterogeneity in whether the PD-1/PD-L1 inhibitors were delivered as monotherapy or in combination with other immunotherapy or chemotherapy agents. Across studies, evaluated PD-1/PD-L1 inhibitors included pembrolizumab, nivolumab, atezolizumab, and durvalumab, with no studies evaluating cemiplimab. Although the line of therapy is a known predictor of survival outcomes among patients with cancer,38 most studies included patient populations with mixed lines of therapy (eg, 1L+) and did not report outcomes by the line of therapy, and some studies did not report information on line of therapy. Furthermore, many studies did not report tumor PD-L1 expression level, which is a known predictor of patient response to PD-1/PD-L1 inhibitors.12 Thus, the wide variation in reported ORR, PFS, and OS across studies suggests that the efficacy of PD-1/PD-L1 inhibitors within subgroups of patients with advanced or metastatic NSCLC expressing certain biomarkers depends on key treatment and patient characteristics, including treatment regimen, patients’ line of therapy, and tumor PD-L1 expression level.
This SLR involved highly sensitive searches in the peer-reviewed literature, as well as searches of recent conferences and a clinical trial registry to identify unpublished trials with results available. Thus, the relatively small number of studies identified that evaluated patients with advanced or metastatic NSCLC with certain targetable biomarkers is likely due to a lack of relevant studies in the literature. Also, the review process was guided by predefined eligibility criteria, and data quality was ensured through the involvement of 2 independent reviewers in the study selection and data extraction processes. Despite the strengths of this SLR methodology, however, some limitations should be acknowledged. First, some studies did not report patient characteristics for a specific biomarker subgroup (eg, ERBB2/HER2 mutation) but rather for a larger biomarker-evaluable subgroup (eg, all patients evaluated for ERBB2/HER2 status, including both mutation and wild-type). As patients expressing a specific biomarker may systematically differ in certain baseline characteristics from the overall patient population, this lack of reported patient characteristics for specific biomarker subgroups makes it difficult to determine their similarity across studies. Second, there could be considerable differences among studies in the biomarker test assay used and the timing of testing, resulting in between-study variations in definitions of biomarker status. Third, as with any SLR, the evidence base continues to evolve quickly; as such, there is a risk that recent clinical trials that have been published since March 2022 may not have been captured. Fourth, SLRs are limited by the use of published data. Although there is a risk of publication bias, as some clinical trials fail to be published, whereas others are published only as abstracts, this risk may be mitigated by our extensive search of conference abstracts.
CONCLUSIONS
Considering the evidence base for each biomarker separately, the presence of large heterogeneity in key study characteristics (eg, study design, treatment regimen, and patients’ line of therapy) as well as the fragmentary availability of efficacy outcomes precludes meaningful conclusions regarding the efficacy of PD-1/PD-L1 inhibitors depending on biomarker status. Moreover, the small number of studies identified for each biomarker indicates that gaps exist in the literature on the efficacy of PD-1/PD-L1 inhibitors for treating patients with advanced or metastatic NSCLC with a known molecular biomarker. Despite the accumulation of studies of patients with EGFR, ALK, ROS1, STK11, or KRAS alterations,15–17,39,40 studies of patients with BRAF V600E mutation, ERBB2/HER2 alteration, MET exon 14 skipping mutation, or RET rearrangement are still lacking. Also, as most identified studies evaluated pooled PD-1/PD-L1 inhibitors among patients receiving mixed lines of therapy, there is limited data on the efficacy of specific PD-1/PD-L1 inhibitors among patients receiving a certain line of therapy. Therefore, in light of the availability of new targeted therapies for BRAF, ERBB2/HER2, MET, or RET alterations and the widespread use of immunotherapy among patients with advanced or metastatic NSCLC, it is important for prospective studies to evaluate the outcomes of PD-1/PD-L1 inhibitors among patients whose tumors harbor these biomarkers to determine whether this class of immunotherapy can provide additional survival benefits.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Akanksha Jaiswal (PRECISIONheor) and Dweeti Nayak (PRECISIONheor) for their contributions to this study.
Conflicts of Interest/Financial Disclosures
S.O., B.Z., and A.A. report full-time employment with Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and stock ownership of Merck & Co., Inc., Rahway, NJ, USA. K.G.A. and A.M.F. are employees of PRECISIONheor, a health care research consultancy that received funding from Merck Sharp & Dohme LLC to conduct the research described in this manuscript.
Footnotes
Supplemental Digital Content is available for this article. Direct URL citations are provided in the HTML and PDF versions of this article on the journal's website, www.immunotherapy-journal.com.
Contributor Information
Katherine G. Akers, Email: katherine.akers@precisionvh.com.
Sabine Oskar, Email: sabine.oskar@merck.com.
Andrew M. Frederickson, Email: andrew.frederickson@precisionvh.com.
Ashwini Arunachalam, Email: ashwini.arunachalam@merck.com.
REFERENCES
- 1. Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. [DOI] [PubMed] [Google Scholar]
- 2. Melosky B, Wheatley-Price P, Juergens RA, et al. The rapidly evolving landscape of novel targeted therapies in advanced non-small cell lung cancer. Lung Cancer. 2021;160:136–151. [DOI] [PubMed] [Google Scholar]
- 3. Hendriks LE, Kerr KM, Menis J, et al. Oncogene-addicted metastatic non-small-cell lung cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:339–357. [DOI] [PubMed] [Google Scholar]
- 4. NCCN. NCCN Clinical Practice Guidelines in Oncology: Non-Small Cell Lung Cancer, Version 3.2023. Updated April 13, 2023. Accessed May 2, 2023. https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- 5. Kerr KM, Bibeau F, Thunnissen E, et al. The evolving landscape of biomarker testing for non-small cell lung cancer in Europe. Lung Cancer. 2021;154:161–175. [DOI] [PubMed] [Google Scholar]
- 6. Gregg JP, Li T, Yoneda KY. Molecular testing strategies in non-small cell lung cancer: optimizing the diagnostic journey. Transl Lung Cancer Res. 2019;8:286–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non–small cell lung cancer: a review. JAMA. 2019;322:764–774. [DOI] [PubMed] [Google Scholar]
- 8. Guo H, Zhang J, Qin C, et al. Biomarker-targeted therapies in non–small cell lung cancer: current status and perspectives. Cells. 2022;11:3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Adib E, Nassar AH, Abou Alaiwi S, et al. Variation in targetable genomic alterations in non-small cell lung cancer by genetic ancestry, sex, smoking history, and histology. Genome Med. 2022;14:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hou H, Yang X, Zhang J, et al. Discovery of targetable genetic alterations in advanced non-small cell lung cancer using a next-generation sequencing-based circulating tumor DNA assay. Sci Rep. 2017;7:14605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stephan-Falkenau S, Streubel A, Mairinger T, et al. Landscape of genomic alterations and PD-L1 expression in early-stage non-small-cell lung cancer (NSCLC)—a single center, retrospective observational study. Int J Mol Sci. 2022;23:12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bodor JN, Boumber Y, Borghaei H. Biomarkers for immune checkpoint inhibition in non–small cell lung cancer (NSCLC). Cancer. 2020;126:260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mok TSK, Lopes G, Cho BC, et al. Associations of tissue tumor mutational burden and mutational status with clinical outcomes in KEYNOTE-042: pembrolizumab versus chemotherapy for advanced PD-L1-positive NSCLC. Ann Oncol. 2023;34:377–388. [DOI] [PubMed] [Google Scholar]
- 14. Gadgeel S, Rodriguez-Abreu D, Felip E, et al. LBA5-KRAS mutational status and efficacy in KEYNOTE-189: pembrolizumab (pembro) plus chemotherapy (chemo) vs placebo plus chemo as first-line therapy for metastatic non-squamous NSCLC. Ann Oncol. 2019;30:xi64–xi65. [Google Scholar]
- 15. Landre T, Justeau G, Assié J-B, et al. Anti-PD-(L) 1 for KRAS-mutant advanced non-small–cell lung cancers: a meta-analysis of randomized–controlled trials. Cancer Immunol Immunother. 2022;71:719–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kim JH, Kim HS, Kim BJ. Prognostic value of KRAS mutation in advanced non-small-cell lung cancer treated with immune checkpoint inhibitors: a meta-analysis and review. Oncotarget. 2017;8:48248–48252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sumbly V, Landry I. Unraveling the role of STK11/LKB1 in non-small cell lung cancer. Cureus. 2022;14:e21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. IQWiG . General Methods. 2022. Accessed Nov 20, 2023. https://www.iqwig.de/methoden/general-methods_version-6-1.pdf [Google Scholar]
- 20. Wells G, Shea B, O’Connell D, et al. Newcastle-Ottawa quality assessment scale cohort studies. University of Ottawa. 2014. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Google Scholar]
- 21. O’Leary CG, Andelkovic V, Ladwa R, et al. Targeting BRAF mutations in non-small cell lung cancer. Transl Lung Cancer Res. 2019;8:1119–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dudnik E, Peled N, Nechushtan H, et al. BRAF mutant lung cancer: programmed death ligand 1 expression, tumor mutational burden, microsatellite instability status, and response to immune check-point inhibitors. J Thorac Oncol. 2018;13:1128–1137. [DOI] [PubMed] [Google Scholar]
- 23. Guisier F, Dubos-Arvis C, Viñas F, et al. Efficacy and safety of anti–PD-1 immunotherapy in patients with advanced NSCLC with BRAF, HER2, or MET mutations or RET translocation: GFPC 01-2018. J Thorac Oncol. 2020;15:628–636. [DOI] [PubMed] [Google Scholar]
- 24. Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Ann Oncol. 2019;30:1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Negrao MV, Skoulidis F, Montesion M, et al. Oncogene-specific differences in tumor mutational burden, PD-L1 expression, and outcomes from immunotherapy in non-small cell lung cancer. J Immunother Cancer. 2021;9:e002891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Riudavets M, Sullivan I, Abdayem P, et al. Targeting HER2 in non-small-cell lung cancer (NSCLC): a glimpse of hope? An updated review on therapeutic strategies in NSCLC harbouring HER2 alterations. ESMO Open. 2021;6:100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cinausero M, Laprovitera N, Maglio GD, et al. KRAS and ERBB-family genetic alterations affect response to PD-1 inhibitors in metastatic nonsquamous NSCLC. Ther Adv Med Oncol. 2019;11:1758835919885540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lau SC, Fares AF, Le LW, et al. Subtypes of EGFR-and HER2-mutant metastatic NSCLC influence response to immune checkpoint inhibitors. Clin Lung Cancer. 2021;22:253–259. [DOI] [PubMed] [Google Scholar]
- 29. Yoh K, Matsumoto S, Furuya N, et al. Comprehensive assessment of PD-L1 expression, tumor mutational burden, and oncogenic driver alterations in non-small cell lung cancer patients treated with immune checkpoint inhibitors. Lung Cancer. 2021;159:128–134. [DOI] [PubMed] [Google Scholar]
- 30. Fujino T, Suda K, Mitsudomi T. Lung cancer with MET exon 14 skipping mutation: genetic feature, current treatments, and future challenges. Lung Cancer. 2021;12:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Felip E, Minotti V, Tan D, et al. P76.03 efficacy and safety of capmatinib plus nivolumab in pretreated patients with EGFR wild-type non–small cell lung cancer. J Thorac Oncol. 2021;16:S585–S586. [Google Scholar]
- 32. CT.gov . Study of efficacy and safety of nivolumab in combination with EGF816 and of nivolumab in combination with INC280 in patients with previously treated non-small cell lung cancer. Accessed: March 15, 2022. https://clinicaltrials.gov/ct2/show/NCT02323126
- 33. Kauffmann-Guerrero D, Tufman A, Kahnert K, et al. Response to checkpoint inhibition in non-small cell lung cancer with molecular driver alterations. Oncol Res Treat. 2020;43:289–298. [DOI] [PubMed] [Google Scholar]
- 34. Sabari J, Leonardi G, Shu C, et al. PD-L1 expression, tumor mutational burden, and response to immunotherapy in patients with MET exon 14 altered lung cancers. Ann Oncol. 2018;29:2085–2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cascetta P, Sforza V, Manzo A, et al. RET inhibitors in non-small-cell lung cancer. Cancers. 2021;13:4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bhandari NR, Hess LM, Han Y, et al. Efficacy of immune checkpoint inhibitor therapy in patients with RET fusion-positive non-small-cell lung cancer. Immunotherapy. 2021;13:893–904. [DOI] [PubMed] [Google Scholar]
- 37. Hess LM, Han Y, Zhu YE, et al. Characteristics and outcomes of patients with RET-fusion positive non-small lung cancer in real-world practice in the United States. BMC Cancer. 2021;21:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bailey CH, Jameson G, Sima C, et al. Progression-free survival decreases with each subsequent therapy in patients presenting for phase I clinical trials. J Cancer. 2012;3:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu T, Ding S, Dang J, et al. First-line immune checkpoint inhibitors for advanced non-small cell lung cancer with wild-type epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK): a systematic review and network meta-analysis. J Thorac Dis. 2019;11:2899–2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Guaitoli G, Tiseo M, Di Maio M, et al. Immune checkpoint inhibitors in oncogene-addicted non-small cell lung cancer: a systematic review and meta-analysis. Transl Lung Cancer Res. 2021;10:2890–2916. [DOI] [PMC free article] [PubMed] [Google Scholar]