Abstract
Background:
Image-guided thermal ablation is a minimally invasive local therapy for lung malignancies. NAVABLATE characterized the safety and performance of transbronchial microwave ablation (MWA) in the lung.
Methods:
The prospective, single-arm, 2-center NAVABLATE study (NCT03569111) evaluated transbronchial MWA in patients with histologically confirmed lung malignancies ≤30 mm in maximum diameter who were not candidates for, or who declined, both surgery and stereotactic body radiation therapy. Ablation of 1 nodule was allowed per subject. The nodule was reached with electromagnetic navigation bronchoscopy. Cone-beam computed tomography was used to verify the ablation catheter position and to evaluate the ablation zone postprocedure. The primary end point was composite adverse events related to the transbronchial MWA device through 1-month follow-up. Secondary end points included technical success (nodule reached and ablated according to the study protocol) and technique efficacy (satisfactory ablation based on 1-month follow-up imaging).
Results:
Thirty subjects (30 nodules; 66.7% primary lung, 33.3% oligometastatic) were enrolled from February 2019 to September 2020. The pre-procedure median nodule size was 12.5 mm (range 5 to 27 mm). Procedure-day technical success was 100% (30/30), with a mean ablative margin of 9.9±2.7 mm. One-month imaging showed 100% (30/30) technique efficacy. The composite adverse event rate related to the transbronchial MWA device through 1-month follow-up was 3.3% (1 subject, mild hemoptysis). No deaths or pneumothoraces occurred. Four subjects (13.3%) experienced grade 3 complications; none had grade 4 or 5.
Conclusion:
Transbronchial microwave ablation is an alternative treatment modality for malignant lung nodules ≤30 mm. There were no deaths or pneumothorax. In all, 13.3% of patients developed grade 3 or above complications.
Key Words: ablation techniques, bronchoscopy, lung cancer treatment, lung nodules, microwave
The increased adoption of lung cancer screening and incidental nodule management programs has improved the detection of tumors in the lung.1 While surgical resection remains the gold standard approach to treat small, localized malignant nodules, many patients are ineligible for surgical resection due to comorbidities. Local therapies, such as stereotactic body radiation therapy (SBRT) or thermal ablation, are recognized alternatives, especially for medically inoperable patients.2,3
Thermal ablation is a minimally invasive treatment option that is available for solitary lung tumors or selected metastatic tumors.4 Energy sources include radiofrequency ablation (RFA), microwave ablation (MWA), and cryoablation.5 In a meta-analysis of 34 studies of primary and metastatic lung cancer, the weighted average local recurrence rates following percutaneous ablation were 19.8% for RFA and 10.9% for MWA.6 In a meta-analysis of 53 studies, the median local tumor progression-free survival was 22 months (95% confidence interval [CI] 11.8–32.2) for RFA and 31.5 months (95% CI 19.0–44.0) for MWA.7 However, percutaneous thermal ablation carries a pooled pneumothorax risk of 34.3% (95% CI 25.9%–43.1%) following RFA and 33.9% (95% CI 23.8%–44.8%) following MWA, with intervention required in 12.3% (95% CI 6.8%–19.1%) and 11.0% (95% CI 4.5%–19.7%), respectively.7
Transbronchial thermal ablation8–11 may provide effective local control with a lower complication risk than percutaneous approaches. Transbronchial ablation may be associated with less pain than a percutaneous approach, more preserved lung function than surgery, and unlike SBRT, allows for repeated ablations in the event of local recurrence.12 The transbronchial approach also provides the ability to diagnose, stage with endobronchial ultrasound, and treat all in the same minimally invasive procedure based on current data. Several single-center retrospective studies and case reports have been published describing the use of transbronchial MWA for the local treatment of primary and metastatic tumors in the lung.9,11,13,14 The current paper is the largest prospective, multicenter evaluation of transbronchial microwave ablation in the lung.
MATERIAL AND METHODS
Trial Design and Enrollment Criteria
The prospective, single-arm, 2-center NAVABLATE study (NCT03569111) evaluated transbronchial microwave ablation using the Emprint ablation catheter kit with Thermosphere technology (Medtronic, Minneapolis, MN). The ablation device uses a saline-cooled ablation antenna with wavelength-controlled, field-based technology with the intent to deliver microwave energy in a spherical ablation zone.
Patients ≥18 years of age with a histologically confirmed malignant pulmonary nodule, ≤30 mm in maximum diameter, and over 5 mm from the pleura or fissure were eligible for enrollment. The study eligibility criteria required that subjects were not candidates for either lung surgery or SBRT or declined both of those treatments. All cases were reviewed by each institution’s multi-disciplinary board to determine whether or not they were candidates for surgery or SBRT. The specific reasons for subjects declining or not being candidates for surgery and SBRT were not collected in this study, but examples include pulmonary fibrosis, previous lung radiotherapy, patients unable to lie still and flat for the duration of treatment, patients not fulfilling the inclusion criteria for funded SBRT in the UK National Health Service, and patient preference of undergoing a single procedure instead of attending multiple sessions of SBRT. Subjects had not consented for the collection of racial or ethnic origin data; therefore, this data was not collected. In addition, nodules abutting the main bronchus, main pulmonary vasculature, esophagus, or trachea, and patients currently diagnosed with GOLD (Global Initiative for Chronic Obstructive Lung Disease) Stage IV chronic obstructive pulmonary disease (COPD) were excluded. If a patient had multiple nodules, only 1 was ablated in the study. Tissue diagnosis must have been obtained before the procedure or concurrently through ENB-guided biopsy during the index episode; however, malignancy must be confirmed before ablation. Both primary lung cancer and oligometastatic malignant nodules were included.
Transbronchial Microwave Ablation Procedure
All procedures were conducted under general anesthesia in a hybrid theater with both cone-beam computed tomography (CBCT; Artis zeego PURE platform, Siemens Healthineer, Germany or Allura Xper FD20, Koninklijke Philips N.V.) and fluoroscopy capabilities. A CBCT scan could be conducted to identify the target and reconfirm nodule eligibility criteria. Navigation to the target was conducted using the superDimension navigation system version 7 (Medtronic) as previously described.11 Once localized, the CrossCountry transbronchial access tool could be used at the investigator’s discretion to aid in creating a pathway through the nodule to obtain an optimal position for ablation. If necessary, a biopsy sample could also be obtained at this time. Following target access, the ablation catheter was placed inside the extended working channel and was unsheathed inside or alongside the target nodule. Correct positioning was verified using CBCT. The required power and time settings were determined from the product instruction for use, correlating power and time to the anticipated size of the ablation zone, to select an appropriately sized ablation zone that encompasses the nodule and a planned margin. The nodule was ablated. A CBCT scan at 10 minutes postablation was used to evaluate the ablation zone, and bracketed ablations were conducted as needed to achieve an adequate margin on all edges. Additional CBCT spins were utilized throughout the procedure as needed at the discretion of the operator. One-month follow-up imaging was obtained using conventional CT scans, along with clinical follow-up in all subjects. Example imaging at the described timepoints are presented in Fig. 1.
FIGURE 1.
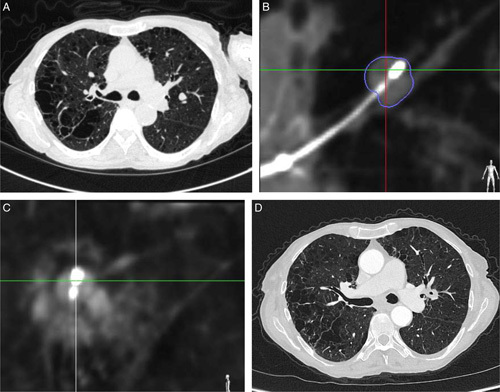
Seventy-five-year-old female, current smoker with emphysema and a 9 mm left upper lobe carcinoid tumor (A). The ablation catheter is placed at the nodule (B), and cone-beam CT shows a ground-glass opacity surrounding the lesion 10 minutes after ablation (C). Follow-up imaging shows consolidation of the ablated nodule (D). CT indicates computed tomography.
Outcomes Measures
The study primary end point was the composite rate of adverse events (both serious and nonserious) related to the transbronchial MWA device through a 1-month follow-up. The secondary safety end points were the composite rate of serious adverse events related to the transbronchial MWA device through 1-month follow-up and the composite rates of serious and all adverse events (both serious and nonserious) related to the study procedure or other study devices through 1-month follow-up. Secondary efficacy end points were technical success (target nodule reached and ablated according to the study protocol) and technique efficacy (satisfactory ablation as evidenced by the nodule being completely covered by the ablation zone on 1-month follow-up imaging).15 An independent medical monitor adjudicated all reported adverse events. Subject satisfaction, subject self-reported pain, and subject self-reported breathlessness were evaluated at 1 month postprocedure. Quality of life was also a secondary end point and was evaluated at baseline and at 1 month postprocedure using the EQ-5D-3L questionnaire.16
Statistics
Analyses were performed using SAS Version 9.4 (SAS Inc., Cary, NC) and data were summarized by descriptive statistics (for continuous variables) or frequencies and percentages (for categorical variables). A 1-way repeated measures analysis of variance test was used to evaluate whether the mean of the subject-reported pain scores differed over time.
RESULTS
Participants and Baseline Characteristics
Thirty subjects (30 nodules) were enrolled at 2 centers in the United Kingdom (16 subjects) and China (14 subjects) between February 2019 and September 2020. Subjects were a mean age of 68.4±10.3 years; 40% were female, and 66.7% had a history of current or former tobacco use. All lung nodules had a confirmed malignant diagnosis. Forty-seven percent (14/30) of subjects were not candidates for either surgery or SBRT, 33% (10/30) declined both surgery and SBRT, 17% (5/30) were not surgical candidates and declined SBRT, and 1 subject (3%) was not an SBRT candidate and declined surgery.
The lung nodules were primary lung cancer in 20/30 subjects (66.7%) and oligometastatic in 10/30 (33.3%; 8 colorectal, 1 thymus, and 1 pancreatic). Primary lung cancer included 15 with adenocarcinoma, 2 squamous cell carcinoma, 2 carcinoid tumor, and 1 non-small-cell lung cancer not otherwise specified. Among subjects with primary lung cancer, 50.0% had COPD and 45% had prior lung surgery (Table 1).
TABLE 1.
Subject Characteristics
| All subjects (n=30) | Primary lung cancer (n=20) | Oligometastatic (n=10) | |
|---|---|---|---|
| Age at consent (y) | 68.4±10.3 (49–90) | 72.2±9.3 (56–90) | 60.9±8.2 (49–75) |
| Female | 12/30 (40) | 8/20 (40.0) | 4/10 (40.0) |
| Male | 18/30 (60.0) | 12/20 (60.0) | 6/10 (60.0) |
| History or presence of tobacco use | 20/30 (66.7) | 14/20 (70.0) | 6/10 (60.0) |
| COPD, % (n/N) | 10/30 (33) | 10/20 (50.0) | 0/10 (0.0) |
| FEV1 (% predicted) | 85±23 (33–127) | 78±20 (53–118) | 99±25 (33–127) |
| FVC (% predicted) | 100±17 (66–130) | 97±17 (66–127) | 106±16 (75–130) |
| DLCO (% predicted) | 72±19 (41–109) | 65±19 (41–109) | 85±12 (67–105) |
| Prior lobectomy | 9/30 (30.0) | 7/20 (35.0) | 2/10 (20.0) |
| Prior wedge resection | 5/30 (16.7) | 4/20 (20.0) | 1/10 (10.0) |
| Prior pneumonectomy | 2/30 (6.7) | 2/20 (10.0) | 0/10 (0.0) |
| Prior radiotherapy | 1/30 (3.3) | 1/20 (5.0) | 0/10 (0.0) |
Data are presented as n/N (%) or mean±SD (mix-max).
COPD indicates chronic obstructive pulmonary disease; DLCO, diffusing capacity of the lung; FVC, forced vital capacity (FVC); FEV1, forced expiratory volume in one second. Lung function data not available in one primary lung subject
The nodules ranged in size from 5 to 27 mm (mean 13.7 mm; Table 2), with a median of 12.5 mm in both primary lung cancer and oligometastatic cases. Nodules were in the periphery in 63.3% (19/30) and the mid-zone in 36.7% (11/30), with none located in the proximal zone. The distance from the nodule to the pleura ranged from 6 to 44 mm.
TABLE 2.
Nodule Characteristics (N=30 nodules)
| Maximum nodule size (mm) | 12.5 (10, 15) 13.7±5.3 (5–27) |
| Location | |
| Right upper lobe | 10/30 (33.3) |
| Right middle lobe | 2/30 (6.7) |
| Right lower lobe | 8/30 (26.7) |
| Left upper lobe | 6/30 (20.0) |
| Left lower lobe | 4/30 (13.3) |
| Peripheral third of lung | 19/30 (63.3) |
| Middle third of the lung | 11/30 (36.7) |
| Proximal third of lung | 0/30 (0.0) |
| Suzuki class* (N=15 nodules) | |
| Class 1–4 | 8/15 (53.3) |
| Class 5–6 | 7/15 (46.7) |
| Bronchus sign present | 14/30 (46.7) |
| Nodule distance to closest fissure (mm) | 35 (23, 52) 36.9±18.8 (6–75) |
| Nodule distance to pleura (mm) | 18 (13, 30) 21.6±11.1 (6–44) |
| Blood vessel ≥3 mm inside target nodule | 0/30 (0.0) |
| Blood vessel ≥3 mm within 5 mm of target nodule | 5/30 (16.7) |
Data are presented as n/N (%), mean±SD (mix-max), or median (Q1, Q3).
Suzuki classification17 for adenocarcinoma of the lung includes.
Type 1: Pure (simple) GGO.
Type 2: Semiconsolidation (an area of intermediate homogeneous increase in density).
Type 3: Halo (area consisting of solid part and surrounding GGO halo).
Type 4: Mixed (an area consisting of GGO and solid part having air-bronchogram).
Type 5: Solid pattern with GGO.
Type 6: Solid pattern.
GGO indicates ground-glass opacity.
Ablation Procedure Characteristics
Ablation procedure details are presented in Table 3. Concurrent biopsy with on-site histology was conducted during the study index procedure in 33.3% (10/30) of subjects, all at a single study site. Twenty-two subjects (73.3%) required only 1 ablation, 7 subjects (23.3%) required 2 overlapping ablation zones, and 1 subject (3.3%) required 3 ablation zones to fully cover the single target nodule with adequate margins. The mean number of CBCT spins was 11.1±5.4 (range 5 to 29). The median air kerma radiation dose for CBCT was 388.6 mGy (range 57.0 to 2425.8 mGy). The median bronchoscopy procedure time measured by the insertion and removal of the bronchoscope (inclusive of navigation, positioning, biopsy if applicable, and ablation) was 123 minutes, with a range of 54 to 247 minutes.
TABLE 3.
Ablation Procedure Characteristics (N=39 procedures in 30 subjects)
| Final catheter position before ablation | |
| Distal end adjacent to the nodule | 20/30 (66.7) |
| Nodule max. diameter ≥20 mm | 2/30 (6.7) |
| Nodule max. diameter <20 mm | 18/30 (63.3) |
| Distal end within the nodule | 10/30 (33.3) |
| Nodule max. diameter ≥20 mm | 3/30 (10) |
| Nodule max. diameter <20 mm | 7/30 (23.3) |
| Subjects with 1 ablation | 22/30 (73.3) |
| Subjects with 2 ablations | 7/30 (23.3) |
| Subjects with 3 ablations | 1/30 (3.3) |
| Total number of CBCT spins per subject | 10 (6, 14) 11.1±5.4 (5–29) |
| Total radiation dose, Cumulative Air Kerma (mGy)* | 407.6 (207, 1036) 697.8±724.3 (68.4–3358) |
| Average ablation time per ablation (min) | 10 (7, 10) 8.1±2.3 (3.5–10) |
| Procedure time (min) | |
| Entire case (bronchoscope in/out) | 123 (89, 155) 126.8±45.4 (54–247) |
| Navigation time, including positioning (locatable guide in/ablation catheter in) | 52 (41, 88) 62.2±30.3 (15–127) |
| Ablation procedure only (ablation catheter in/out) | 39 (34, 58) 54.8±34.6 (22–159) |
| Approximate average ablative margin (mm) | 10 (8, 12) 9.9±2.7 (5–15) |
| Nodules with any edge lacking 5 mm margin | 3/30 (10.0) |
| Technical success† | 30/30 (100.0) |
Data are presented as n/N (%), mean±SD (mix-max), or median (Q1, Q3).
Radiation dose was not available in mGy units for 1 subject and was excluded.
Nodule treatment according to the study protocol.15
CBCT indicates cone-beam computed tomography.
In post hoc analyses, we did not observe any consistent effects of learning curve, inclusion of concurrent biopsy, nodule size, lobe location, or the use of a positioning tool on the overall procedure time. The total procedure time was lower in cases with a single ablation (median 105.5 min) versus bracketed ablations (169.5 min).
The ablation power was set to 100 Watts (W) for most procedures (37/39), while 2 procedures used 75 W power. The ablation times were most commonly 10 minutes (21/39; 53.8%) or 7 minutes (8/39; 20.5%); the average ablation time was 8.1±2.3 minutes (range 3.5 to 10 min).
Needle puncture was used to access the lesion in 19 cases, including 18 using the transbronchial access tool and 1 using both the CrossCountry tool and a needle (Arcpoint pulmonary needle, Medtronic). Following positioning, the distal end of the ablation catheter was within the nodule or adjacent to the nodule in all cases. The mean postprocedure average ablation margin was 9.9±2.7 mm, with 90% of nodules (27/30) achieving at least a 5 mm margin on all edges.
Safety
Adverse events are reported in Table 4. The primary end point rate (composite adverse events related to the transbronchial MWA device through 1-month follow-up) was 3.3%, which was a single case of mild [nonserious, Common Terminology Criteria for Adverse Events (CTCAE) grade 1] hemoptysis reported by the subject in a phone discussion on day 5 postablation. The patient returned for a chest CT scan on day 6 postablation, which showed a satisfactory ablation zone smaller than the postprocedure CT, and reported that the symptom had resolved without treatment. There were no serious adverse events related to the ablation device.
TABLE 4.
Adverse Events* through One-Month Follow-up (Primary and Secondary End Points)
| Outcome | n/N subjects (%) [n events] | |
|---|---|---|
| Composite rate of all adverse events related to the ablation device (primary endpoint)† | 1/30 (3.3) [1] (Hemoptysis) |
|
| Composite rate of all serious adverse events related to the ablation device† | 0/30 (0.0) [0] | |
| Composite rate of all adverse events related to the study procedure or study devices (including events related to anesthesia and the entire bronchoscopic procedure) | 21/30 (70.0) [51] | |
| CTCAE Grade <3 (detailed below)‡ | 20/30 (66.7) [42] | |
| Bradycardia§ | 1/30 (3.3) [1] | |
| Chest discomfort | 2/30 (6.7) [2] | |
| Cough | 2/30 (6.7) [2] | |
| Dyspnea | 7/30 (23.3) [7] | |
| Hemoptysis | 5/30 (16.7) [5] | |
| Lower respiratory tract infection | 2/30(6.7) [2] | |
| Oropharyngeal pain | 3/30 (10) [3] | |
| Pleural effusion | 2/30 (6.7) [2] | |
| Pleural thickening | 1/30 (3.3) [1] | |
| Postablation syndrome | 1/30 (3.3) [1] | |
| Procedural pain | 13/30 (43.3) [15] | |
| Unwanted awareness during anesthesia | 1/30 (3.3) [1] | |
| CTCAE Grade 3 | 4/30 (13.3) [9] | |
| Composite rate of all serious adverse events related to the study procedure or other study devices (detailed below)‖ | 4/30 (13.3) [9]¶ | |
| Subject | Event(s) | Description |
| 1 | Postablation syndrome | The patient developed a fever of 38°C while still in the hospital the day after ablation and was kept in the hospital overnight for observation. |
| Postprocedure pleuritic chest pain | Right pleuritic chest pain on the day after ablation. Treated with a standard dose of paracetamol, tramadol, and Voltaren SR for pain control during hospitalization. | |
| Ablation site infection | The patient was admitted to the emergency room with fever on day 6 after ablation. Sputum culture was positive for Acinetobacter the following day. The patient was admitted and treated with intravenous ampicillin and sulbactam. | |
| 2 | Pleural effusion | The ablation zone involves the visceral pleura. The patient developed right pleural effusion on day 2 while still in the hospital after the study procedure. The patient was treated with oxygen a nasal cannula and drainage with a 12 French catheter. |
| 3 | Postprocedure pleuritic chest pain | The patient complained of chest pain on day 4 postprocedure, thought to be the result of inflammation (below) and worsened by cough induced by postnasal drip from allergic rhinitis. |
| Pleuritis | Postablation pleural inflammation (day 16 postprocedure) with no evidence of infection. | |
| Hemoptysis | Blood-stained sputum treated with oral transamin | |
| 4 | Breathlessness | The patient experienced 2 weeks of breathlessness postprocedure and was admitted for ultrasound-guided effusion drainage and COPD treatment. |
| Pleural effusion | One-month follow-up CT showed small pleural effusion. |
Data are presented as n/N (%) [n events]
Adverse events are defined according to ISO 14155:2011 as any untoward medical occurrence, unintended disease or injury, or untoward clinical signs (including abnormal laboratory findings) whether or not related to the investigational medical device.
The Emprint Ablation Catheter Kit with Thermosphere Technology
Patients may have had more than one event. CTCAE Grades are as follows:18
Grade 1: Mild; asymptomatic or mild symptoms; clinical or diagnostic observations only; intervention not indicated.
Grade 2: Moderate; minimal, local, or noninvasive intervention indicated; limiting age-appropriate instrumental activities of daily living.
Grade 3: Severe or medically significant but not immediately life-threatening; hospitalization or prolongation of hospitalization indicated; disabling; limiting self-care activities of daily living.
The adverse event was self-limiting and occurred during the inspiratory breath hold for CBCT.
Other study devices may include, but are not limited to, the navigation system, the locatable guide, the extended working channel, transbronchial access tools and other endoscopic tools. There were no serious adverse events related to the Emprint Ablation Catheter Kit with Thermosphere Technology.
A total of 9 serious adverse events (all rated as CTCAE Grade 3) related to the study procedure or other study devices occurred in 4 subjects.
COPD indicates chronic obstructive pulmonary disease; CT, computed tomography; CTCAE, Common Terminology Criteria for Adverse Events.
Nine serious adverse events in 4 subjects (13.3%), all rated as CTCAE grade 3, were considered by the medical monitor to be related to the study procedure or other study devices (Table 4). A total of 51 adverse events were reported in 21 subjects (29 grade 1 events, 13 grade 2 events, and 9 grade 3 events); these included all events related to any aspect of the study transbronchial procedure (including anesthesia, navigation, biopsy, and ablation). There were no CTCAE grade 4 or 5 events, no pneumothoraces, and no deaths.
Performance
Technical success (target nodule reached and ablated according to the study protocol)15 was 100% (30/30), Table 5. One-month follow-up imaging was performed by conventional CT in all 30 subjects. Technique efficacy was 100% (30/30), defined as a satisfactory ablation as evidenced by the follow-up CT scan.15 There was no new metastatic disease or lymphadenopathy. No repeat treatments of any ablated nodules had occurred or were planned as of the 1-month follow-up.
TABLE 5.
Nodule Follow-Up
| One-month follow-up imaging was performed | 30/30 (100.0) |
|---|---|
| Days from index procedure to follow-up imaging | 32 (28, 34) 31.6±4.8 (22–46) |
| Ablation zone size at follow-up (mm) | 32 (28.5, 40) 34.2±10.5 (10–59) |
| Technique efficacy* | 30/30 (100.0) |
| Repeat treatment of study (ablated) nodule | 0/30 (0.0) |
| Planned retreatments of study (ablated) nodule | 0/30 (0.0) |
Data are presented as n/N (%), mean±SD (min-max), or median (Q1, Q3).
Complete ablation as evidenced by 1-month imaging.
Subject Satisfaction and Quality of Life
On a scale of 0 to 10 (0 being no pain), subjects’ self-reported pain and discomfort was 1.5±2.2 immediately after the ablation procedure, 1.4±1.8 1 week after the procedure, and 0.5±1.1 one month after the procedure (P=0.016). On a scale of 1 to 5 (1 being the least), subjects rated their breathlessness at 1.8±1.0, 1.6±1.0, and 1.5±0.9 immediately after, at 1 week, and at 1-month postablation, respectively. On a scale of 1 to 5 (1 being “extremely unlikely” and 5 “extremely likely”), subjects rated their willingness to have another transbronchial ablation procedure, if necessary, at 4.8±0.8 (range 2 to 5).
On the EQ-5D-3L scale,16 subjects’ overall assessment of their current health state was 74.6±12.8 (range 50 to 90) at baseline versus 77.4±14.2 (range 47 to 100) at 1-month postablation (on a scale of 0 to 100, with 100 being the best imaginable health state), representing a change from baseline of 2.8±15.9. Fifty percent of subjects noted an improvement in their overall health state, 26.7% reported a decrease, and 23.3% reported no change.
DISCUSSION
To date, NAVABLATE is the largest prospective, multicenter evaluation of transbronchial MWA in histologically confirmed malignant lung nodules. The study aimed to characterize the safety and short-term performance of the transbronchial ablation device in patients with limited alternative treatment options. There was a low (3.3%) rate of device-related adverse events, no serious adverse events related to the transbronchial MWA device, no pneumothoraces, and no deaths. The overall CTCAE grade 3 or above rate was 13.3%, with no grade 4 or 5 events. In addition, the procedure-day technical success and 1-month technique efficacy were both 100% (30/30).
Transbronchial thermal ablation may be associated with less risk than percutaneous thermal ablation. Pritchett et al reported no pneumothoraces occurred in all 10 subjects where image-guided transbronchial MWA was performed.19 Yuan et al reported pneumothorax in 34.3% following percutaneous RFA (12.3% requiring intervention) and 33.9% following percutaneous MWA (11.0% requiring intervention).7 Lyons et al, 2015, reported on 8 studies of percutaneous RFA to treat pulmonary metastases from colorectal cancer. Pneumothorax rates ranged from 32% to 70%, with chest tube drainage required at 23%. Nelson et al, 2019, reported pneumothorax requiring a chest tube in 1% to 15% of patients across 10 percutaneous ablation studies, with 8 studies reporting rates between 5% and 15%.20 Among these meta-analyses, a total of 4 procedure-related deaths were reported in 3 studies.21–23 In contrast, there were no pneumothoraces or deaths following transbronchial MWA in NAVABLATE.
Pleural effusion is another common complication of percutaneous thermal ablation. Pleural effusion occurred in 5.2% of patients undergoing RFA and 9.6% of patients undergoing MWA patients in the Yuan 2019 meta-analysis, of which 0.6% and 0.3%, respectively, were considered severe events that required intervention.7 In NAVABLATE, there were a total of 4 subjects with pleural effusions: 2 requiring intervention (CTCAE grade 3) and 2 not requiring intervention (CTCAE grade 1). A comprehensive comparison of pleural effusion rates between percutaneous and bronchoscopic thermal ablation will require a larger data set, as there were 2979 at-risk patients in the Yuan et al analysis versus only 30 in NAVABLATE.
In NAVABLATE, minor complications (CTCAE grade <3) occurred in 20/30 subjects while major complications (CTCAE grade ≥3) occurred in 4/30 subjects. However, as a safety study, NAVABLATE captured all adverse events related to the ablation device, other study devices, or the procedure (including anesthesia) regardless of severity, as defined by international safety reporting guidelines.24 In contrast, single-center papers often report only clinically significant events without standardized definitions. The composite rate of major complications in NAVABLATE (13.3%) is similar to the pooled major complication rates reported in the meta-analyses of percutaneous thermal ablation by Jiang et al (11.5%),6 Li et al (6%),25 and Lyons et al (range 7% to 33%)26 and those reported for SBRT by Devpura et al (5.5%),27 Timmerman et al (16.4%),28 and Nyman et al (14.6%).29 Based on the data in NAVABLATE, transbronchial MWA has a lower pneumothorax rate than percutaneous thermal ablation and a similar adverse event rate to SBRT.
The median total bronchoscopy procedure time in NAVABLATE was 123 minutes, ranging from 54 to 247 minutes. Of note, this total procedure time was inclusive of navigation, positioning, biopsy frozen section histologic confirmation, and ablation. Time navigating and positioning was a median of 52 minutes, and time actively ablating was a median of 10 minutes per ablation. There were 3 cases with a procedure time greater than 200 minutes, all of which required 2 ablations and an increased number of CBCT spins due to difficulty in navigation and positioning. We found no effects of the learning curve, inclusion of concurrent biopsy, nodule size, lobe location, or the use of a positioning tool on the overall procedure time. However, the NAVABLATE sample size was too small to support meaningful multivariate analyses.
The subjects enrolled in NAVABLATE included 2/3 with primary lung cancer and 1/3 with oligometastatic disease (primarily colorectal). This is similar to the proportions observed in a meta-analysis of 34 studies evaluating percutaneous thermal ablation.6 While surgical resection is the preferred treatment modality for localized lung nodules, patients with primary lung cancer in NAVABLATE had poorer lung function than those with metastatic lesions, including 50% with COPD, and prior lung surgery in 45%. While over half (53%, 16/30) of NAVABLATE subjects were candidates for surgery and/or SBRT, 10/30 declined both of those procedures, and 6/30 declined the one procedure for which they were eligible. Thus, thermal ablation was an acceptable alternative treatment for these patients. Furthermore, a biopsy was conducted in the same setting (just before the ablation procedure) in one-third of subjects, suggesting that a single stage procedure approach of concurrent biopsy and treatment is feasible. Staging by endobronchial ultrasound is also possible in the same procedure.
Limitations
This is a single-arm, 2-center study of 30 subjects with 1-month follow-up. The inclusion of selected patients with nodules ≤30 mm in maximum diameter and ≥5 mm from the pleura may not represent the entire population of patients undergoing transbronchial MWA. The short-term measures of technical success and technique efficacy were based on standardized terminology and reporting criteria for image-guided tumor ablation15; however, longer follow-up is required to provide a full evaluation of local control and recurrence. These results will need to be replicated in a larger study among users with varying experience levels, a broader subject population, and employing longer follow-ups.
CONCLUSIONS
In conclusion, transbronchial MWA is a treatment option for patients with primary or metastatic malignant lung nodules ≤30 mm who have limited alternative treatment options. Future studies will be needed to evaluate longer-term outcomes in a larger data set.
ACKNOWLEDGMENTS
Th authors thank Haiying Lin of Medtronic for providing biostatistical analysis. The medical writing support was provided by Kristin L. Hood, PhD, of Medtronic in accordance with Good Publication Practice (GPP3) guidelines. The authors had full access to all study data and final responsibility for the decision to submit for publication. The Emprint ablation catheter with Thermosphere technology is not approved or cleared in all geographies.
Footnotes
Presented at the European Respiratory Society 2021 International Congress on September 5, 2021.
The corresponding author attests that all listed authors qualify for authorship under the ICMJE Recommendations (www.icmje.org). K.K.W.L. contributed to the study concept and design; K.K.W.L., R.W.H.L., R.B., and C.S.H.N. enrolled subjects and collected data; all authors contributed to the data interpretation, reviewed and critically revised all drafts of the manuscript for important intellectual content, and approved the final version to be published.
All patients provided written informed consent to participate in the study. No direct patient identifiers have been included in the paper.
This study was conducted in accordance with the Declaration of Helsinki and all local regulatory requirements. The protocol was approved by the Joint Chinese University of Hong Kong—New Territories East Cluster Clinical Research Ethics Committee and the Health Research Authority London—City & East Research Ethics Committee.
The NAVABLATE aggregate data set has been made publicly available on ClinicalTrials.gov (NCT03569111). Aggregate data are also available from the corresponding author upon reasonable request.
The study is sponsored and funded by Medtronic (Minneapolis, MN).
Disclosure: K.K.W.L., R.W.H.L., R.B., and C.S.H.N. are investigators in the Medtronic-sponsored NAVABLATE study, and their institutions have received research funds from Medtronic for participation. K.K.W.L. is a consultant for Medtronic, Philips, and Johnson and Johnson. R.W.H.L. is a consultant for Medtronic and Siemens Healthineer. J.K. is a full-time employee of Medtronic. C.S.H.N. is a consultant for Johnson and Johnson, Medtronic, and Siemens Healthineer.
Contributor Information
Kelvin K.W. Lau, Email: kelvin.lau@nhs.net.
Rainbow W.H. Lau, Email: rainbowlau@surgery.cuhk.edu.hk.
Ralitsa Baranowski, Email: ralitsa@baranowski.info.
Julie Krzykowski, Email: julie.krzykowski@medtronic.com.
Calvin S.H. Ng, Email: calvinng@surgery.cuhk.edu.hk.
REFERENCES
- 1.Gould MK, Tang T, Liu IL, et al. Recent Trends in the Identification of Incidental Pulmonary Nodules. Am J Respir Crit Care Med. 2015;192:1208–1214. [DOI] [PubMed] [Google Scholar]
- 2.Callister ME, Baldwin DR, Akram AR, et al. British Thoracic Society guidelines for the investigation and management of pulmonary nodules. Thorax. 2015;70(suppl 2):ii1–ii54. [DOI] [PubMed] [Google Scholar]
- 3.Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J. 2009;34:17–41. [DOI] [PubMed] [Google Scholar]
- 4.Non-small cell lung cancer, Version 2. 2021, March 3, 2021, NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network. 2021; Accessed June 1, 2021. Current guidelines are available at NCCN.org.
- 5.Palussiere J, Catena V, Lagarde P, et al. Primary tumors of the lung: should we consider thermal ablation as a valid therapeutic option? Int J Hyperthermia. 2019;36:46–52. [DOI] [PubMed] [Google Scholar]
- 6.Jiang B, McClure MA, Chen T, et al. Efficacy and safety of thermal ablation of lung malignancies: A Network meta-analysis. Ann Thorac Med. 2018;13:243–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yuan Z, Wang Y, Zhang J, et al. A meta-analysis of clinical outcomes after radiofrequency ablation and microwave ablation for lung cancer and pulmonary metastases. J Am Coll Radiol. 2019;16:302–314. [DOI] [PubMed] [Google Scholar]
- 8.Koizumi T, Tsushima K, Tanabe T, et al. Bronchoscopy-guided cooled radiofrequency ablation as a novel intervention therapy for peripheral lung cancer. Respiration. 2015;90:47–55. [DOI] [PubMed] [Google Scholar]
- 9.Yu PSY, Chu CM, Lau RWH, et al. Hybrid theater facilitates lung-preserving multimodal treatment for multiple pulmonary metastases. Ann Thorac Surg. 2021;111:e89–e92. [DOI] [PubMed] [Google Scholar]
- 10.Qu R, Tu D, Hu S, et al. Electromagnetic navigation bronchoscopy-guided microwave ablation combined with uniportal video-assisted thoracoscopic surgery for multiple ground glass opacities. Ann Thorac Surg. 2022;113:1307–1315. [DOI] [PubMed] [Google Scholar]
- 11.Chan JWY, Lau RWH, Ngai JCL, et al. Transbronchial microwave ablation of lung nodules with electromagnetic navigation bronchoscopy guidance-a novel technique and initial experience with 30 cases. Transl Lung Cancer Res. 2021;10:1608–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao H, Okano S, Pelecanos A, et al. Repeat thermal ablation for local progression of lung tumours: how safe and efficacious is it? Mini-invasive Surg. 2018;2:26. [Google Scholar]
- 13.Lau K, Spiers A, Pritchett M, et al. P1.05-06 bronchoscopic image-guided microwave ablation of peripheral lung tumours – early results. J Thorac Oncol. 2018;13:S542. [Google Scholar]
- 14.Mak KL, Chan JWY, Lau RWH, et al. Management of bronchopleural fistula with endobronchial valve in hybrid operating room following transbronchial microwave ablation. Interact Cardiovasc Thorac Surg. 2021;33:992–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed M, Solbiati L, Brace CL, et al. Image-guided tumor ablation: standardization of terminology and reporting criteria--a 10-year update. J Vasc Interv Radiol. 2014;25:1691–1705 e1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.EuroQol G. EuroQol--a new facility for the measurement of health-related quality of life. Health Policy. 1990;16:199–208. [DOI] [PubMed] [Google Scholar]
- 17.Suzuki K, Kusumoto M, Watanabe S, et al. Radiologic classification of small adenocarcinoma of the lung: radiologic-pathologic correlation and its prognostic impact. Ann Thorac Surg. 2006;81:413–419. [DOI] [PubMed] [Google Scholar]
- 18.National Cancer Institute, Cancer Therapy Evaluation Program, United States Department of Health and Human Services, National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Accessed July 12, 2021. https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_50.
- 19.Pritchett M, Reisenauer J, Kern R, et al. Novel image-guided flexible-probe transbronchial microwave ablation for stage 1 lung cancer. Respiration. 2023;102:182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson DB, Tam AL, Mitchell KG, et al. Local recurrence after microwave ablation of lung malignancies: a systematic review. Ann Thorac Surg. 2019;107:1876–1883. [DOI] [PubMed] [Google Scholar]
- 21.Healey TT, March BT, Baird G, et al. Microwave ablation for lung neoplasms: a retrospective analysis of long-term results. J Vasc Interv Radiol. 2017;28:206–211. [DOI] [PubMed] [Google Scholar]
- 22.Gillams A, Khan Z, Osborn P, et al. Survival after radiofrequency ablation in 122 patients with inoperable colorectal lung metastases. Cardiovasc Intervent Radiol. 2013;36:724–730. [DOI] [PubMed] [Google Scholar]
- 23.Zheng A, Wang X, Yang X, et al. Major complications after lung microwave ablation: a single-center experience on 204 sessions. Ann Thorac Surg. 2014;98:243–248. [DOI] [PubMed] [Google Scholar]
- 24.ISO 14155:2011: Clinical investigation of medical devices for human subjects — Good clinical practice. Accessed August 6, 2021. https://www.iso.org/standard/45557.html.
- 25.Li G, Xue M, Chen W, et al. Efficacy and safety of radiofrequency ablation for lung cancers: A systematic review and meta-analysis. Eur J Radiol. 2018;100:92–98. [DOI] [PubMed] [Google Scholar]
- 26.Lyons NJ, Pathak S, Daniels IR, et al. Percutaneous management of pulmonary metastases arising from colorectal cancer; a systematic review. Eur J Surg Oncol. 2015;41:1447–1455. [DOI] [PubMed] [Google Scholar]
- 27.Devpura S, Feldman AM, Rusu SD, et al. An analysis of clinical toxic effects and quality of life as a function of radiation dose and volume after lung stereotactic body radiation therapy. Adv Radiat Oncol. 2021;6:100815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303:1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nyman J, Hallqvist A, Lund JA, et al. SPACE - A randomized study of SBRT versus conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121:1–8. [DOI] [PubMed] [Google Scholar]


