Abstract
Morbidity associated with autograft harvest has led to the need for alternative bone grafts during fusion surgical procedures. The purpose of this study is to evaluate the efficacy of a cellular bone allograft (CBA) in patients who underwent foot/ankle fusion surgery. Retrospective data of patients who underwent foot/ankle arthrodesis using a CBA between 2016 and 2021 were collected from a single site. Patients were at least 18 years of age at the time of surgery and had ankle/foot surgery with Trinity ELITE CBA as the primary or only bone graft. Patients’ radiographic union was assessed at three (3) months, six (6) months, nine (9) months, and twelve (12) months. Twenty-two (22) patients and 29 joints were evaluated. The mean age and BMI of the cohort were 54±9yrs and 30.5±6kg/m2, respectively. The surgical indications were degenerative joint diseases, trauma, and arthritis. All patients except one had at least one risk factor for non-union. At 12 months, 21 of the 22 patients (95%) attained successful fusion with an average time of 6 months. In addition, there was a 100% fusion among patients with prior failed fusion, nicotine use, diabetes, neuropathy, and osteoporosis. There was no significant difference in time to fusion between patients with non-union risk factor(s) ≤ 1 and ≥ 2 (p=0.71). No complication or adverse event was reported following the surgery. The use of CBA resulted in high fusion among patients with the risk of non-union. CBA is a viable bone graft substitute for autograft in foot/ankle arthrodesis procedures.
Keywords: Cellular Bone Allograft, Arthrodesis, Fusion, Ankle
Introduction
Foot and ankle fusion (arthrodesis) is often suggested to patients with ankle arthritis or degenerative joint diseases when conservative approaches fail.1,2 A successful arthrodesis is vital to improve mobility and reduce pain in this population.2 However, risk factors such as nicotine use, age, high adiposity, diabetes, etc., can cause an unsuccessful bone fusion (non-union), resulting in revision surgery.3 Surgical complications such as blood clots, pain, infection, cost, non-union, etc., are higher with a revision surgery than the original surgery; therefore it is essential to have a complete union at the first surgical procedure.4 Another critical factor that contributes to the success of an arthrodesis procedure is the choice of bone graft used during the procedure. Bone grafting is used as an adjunct to fusion, especially in patients with unhealthy bones or at risk for non-union.5,6
To have a successful bone fusion, the bone graft must possess the three key elements to support effective graft remodeling and incorporation, which are osteoconductive properties, osteoinductive potential and osteogenic capabilities.7 Autograft has been the gold standard for arthrodesis procedures. However, the complications of harvesting autograft are well documented and include vascular injuries, chronic donor-site pain, blood loss, deformity, bowel injury, hernia, prolonged surgical time, infections at the donor site, neurologic injuries, deep hematoma formation requiring surgical intervention, increased hospitalization time, significant donor site morbidity, etc.8–11 Also, autograft is not always viable for patients with poor bone quality due to their age or medical conditions.12 The limitations associated with autografts have resulted in the development of a number of alternative bone grafts, which include cortico-cancellous allografts, demineralized bone matrix (DBM), bone morphogenic proteins (BMP), synthetics, ceramics, peptide-enhanced grafts, and cellular bone allografts (CBA).
CBA is derived from cadaveric bone and is carefully processed and cryopreserved to maintain the native cells and proteins in its matrix. In addition to providing an osteoconductive scaffold to support bone growth and repopulation by the patient’s own cells, these grafts contain viable osteogenic cells and an osteoinductive demineralized cortical bone component.13–15 Collectively, these features provide all the grafting elements necessary for a successful bone/joint fusion.16
Studies have shown the clinical advantage of CBAs in lumbar and cervical surgical procedures while eliminating the risk associated with autograft harvesting.16,17 However, there is currently limited information on the efficacy and safety of CBA with foot/ankle arthrodesis, especially in patients with risk for non-union. This study aims to evaluate the rate of fusion and complications in patients who underwent foot/ankle arthrodesis procedures using a CBA. The result of this study may provide valuable insight into the efficacy of CBA as an alternative to autograft in foot and ankle arthrodesis.
Method
The authors collected retrospective, non-randomized, standard-of-care follow-up data from a single site (Temple University Health System) on patients who underwent single or multi-joint fusion surgery in the foot/ankle using Trinity™ ELITE CBA (Orthofix Inc.). Subjects (n=22) with one-year follow-up data were included in this study. The Institutional Review Board (IRB) approved this study, and informed consent was waived. Clinical records were assessed to obtain de-identified data on patients’ demographics, health history, surgical data, fusion status, adverse events/complications, and risk factors for non-union. Data were collected following Good Clinical Practice Requirements.
Patients were included if they were 18+ years of age at the time of surgery, had undergone ankle, subtalar, tarsometatarsal, or mid-foot fusion surgery using a CBA. Patients were excluded if they had been diagnosed with Charcot Neuropathy.
Surgical procedure
A single surgeon performed an ankle arthrodesis procedure on all patients included in the study, with the procedure being performed at a single site. The surgeon employed the standard operative technique to access the operative site and to prepare opposing joint surfaces, using traditional instrumentation and standard postoperative care regimens. Joints involved in the procedure are tibiotalar, subtalar, calcaneocuboid, and talonavicular. Surgical procedures included single, double (i.e. calcaneocuboid and talonavicular), and triple joint arthrodesis (i.e. subtalar, calcaneocuboid, and talonavicular).
Endpoints
The investigator assessed the primary endpoint of patients’ fusion at three (3) months, six (6) months, nine (9) months, and twelve (12) months from three views of standard radiographs (AP, lateral, and oblique) or a CT scan. Fusion was considered successful when bones in the joints were permanently fused, via bridging bone assessment, following radiograph review and physical examination. The incidence of adverse events was assessed as the secondary endpoint of the clinical outcome, and safety information was collected following FDA regulations. It included the number of adverse events and the investigators’ evaluation of the severity and possible relationship of the adverse events to the treatment.
Statistical analysis
SAS analytical software (SAS Institute for data management, North Carolina) was used for the data analysis. The data was analyzed to calculate the percentage of successful fusion at three (3) months, six (6) months, nine (9) months, and twelve (12) months. Mean, standard deviation, and range were calculated for the patient’s demographic data and health information. Fusion rate was assessed in patients with risks of non-union, defined by age ≥ 60 years, BMI > 24.9kg/m2, exposure to nicotine, and patients with diabetes, neuropathy, and osteoporosis. Independent sample t-test was used to analyze the difference in time to fusion between patients with exposure to nicotine (previous and current users) and patients with no exposure to nicotine, patients with and without diabetes/neuropathy, non-obese patients (BMI < 30 kg/m2) and obese patients (BMI ≥ 30 kg/m2), and younger patients (age < 60 years) and older patients (age ≥ 60 years). Fisher’s Exact test was done to evaluate the difference in time to fusion between patients with one (1) or no risk factor and patients with more than one (1) risk factor of non-union. We accepted significance at 0.05 alpha.
Result
Out of the 22 patients included in the study, 12 (55%) were males, and 10 (45%) were females. Mean age of the patients was 55 years ± 8 years (range 35 years – 66 years), mean weight of patients was 188lbs ± 33lbs (range 101lbs - 261lbs), mean BMI 30.5kg/m2 ± 5.6kg/m2 (range 17.3kg/m2 - 41.6kg/m2). Table 1 shows patients’ individual data.
Table 1. Patients’ data.
| Gender | Race | Age | BMI | Smoking Status | Time to fusion | Number of joints | Surgical indication | Prior health condition |
Prior
failed Fusion |
|---|---|---|---|---|---|---|---|---|---|
| F | black | 42.0 | 28.0 | current | 3 | 1 | Degenerative Joint Diseases | Yes | |
| M | Caucasian | 57.0 | 31.3 | current | 3 | 1 | Degenerative Joint Diseases | ||
| F | Caucasian | 57.0 | 36.4 | former | 6 | 1 | Degenerative Joint Diseases | ||
| M | Black | 50.0 | 32.6 | Never | 6 | 1 | trauma | Diabetes, neuropathy | Yes |
| F | Caucasian | 64.0 | 35.5 | Never | 9 | 3 | arthritis | osteoporosis, neuropathy | |
| F | Caucasian | 64.0 | 17.3 | Never | 12 | 3 | Degenerative Joint Diseases | osteoporosis, neuropathy | |
| M | Caucasian | 54.0 | 26.3 | current | 3 | 1 | arthritis | Yes | |
| M | Black | 55.0 | 29.6 | Never | Not fused | 3 | trauma | ||
| M | Caucasian | 48.0 | 34.4 | Never | 6 | 1 | Degenerative Joint Diseases | ||
| M | Caucasian | 46.0 | 41.6 | Never | 3 | 1 | Degenerative Joint Diseases | ||
| M | Caucasian | 40.0 | 29.5 | Never | 3 | 1 | Degenerative Joint Diseases | ||
| M | Black | 35.0 | 25.8 | Never | 6 | 2 | trauma | ||
| F | Caucasian | 57.0 | 28.0 | Never | 6 | 1 | Degenerative Joint Diseases | diabetes | |
| F | Black | 60.0 | 28.5 | Never | 12 | 1 | Degenerative Joint Diseases | osteoporosis | |
| F | Caucasian | 66.0 | 35.7 | current | 3 | 1 | Degenerative Joint Diseases | ||
| M | Caucasian | 59.0 | 28.8 | former | 12 | 1 | Degenerative Joint Diseases | ||
| M | Caucasian | 58.0 | 25.4 | current | 6 | 1 | Degenerative Joint Diseases | diabetes | |
| F | Caucasian | 57.0 | 31.2 | Never | 6 | 1 | Degenerative Joint Diseases | diabetes | |
| F | Black | 60.0 | 36.9 | Never | 12 | 1 | arthritis | ||
| F | Caucasian | 65.0 | 25.2 | Never | 6 | 1 | trauma | ||
| M | Caucasian | 59.0 | 23.8 | Never | 6 | 1 | Degenerative Joint Diseases | ||
| M | Caucasian | 54.0 | 39.1 | Never | 6 | 1 | arthritis | ||
| Mean | 54.9 ± 8 | 30.5 ± 5.5 | 6±3 | 29 |
Table 2 shows the surgical site information. There were 29 Arthrodesis, which included 18 patients with one (1) joint treated, one (1) patient with two (2) joints treated, and three (3) patients with three (3) joints treated. The primary surgical indications for the patients were degenerative joint diseases (14), arthritis (4), and trauma (4).
Table 2. Surgical data.
| Operative site treated | n (%) |
| Ankle | 5 |
| Subtalar | 12 |
| Tarsometatarsal | 4 |
| Mid-foot | 1 |
| # of Joints treated | n (%) |
| One | 18 |
| Two | 1 |
| Three | 3 |
| Surgical approach | |
| Anterior | 15 |
| Lateral | 7 |
| Operative Leg | |
| Right | 10 |
| Left | 12 |
There were five (5) current nicotine users, two (2) former nicotine users, and fifteen (15) of the patients who had never been exposed to nicotine use. At the time of surgery, three (3) patients were diagnosed with diabetes, one (1) patient had diabetes and neuropathy, two (2) patients had osteoporosis and neuropathy, and one (1) patient had osteoporosis alone. Three (3) patients had revision surgery due to a prior incidence of non-union. Table 1 includes information on the patient’s clinical risk factors.
Fusion
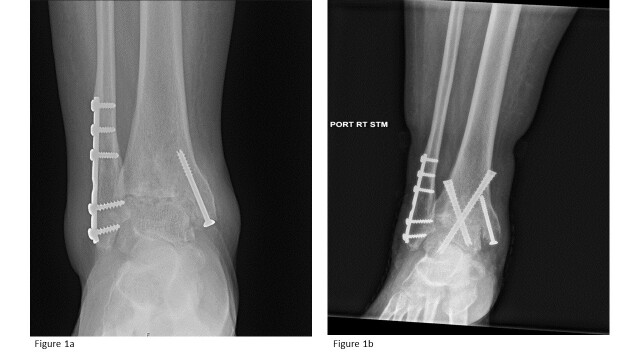
21/22 (95%) of the patients had a successful radiographic fusion by 12 months (Table 3). Figure 1 shows a sample of radiograph pre-operation and post-operation.
Table 3. Patients’ follow-up fusion rate.
| Fusion status / Months | 3months | 6months | 9months | 12months |
|---|---|---|---|---|
| Fused | 6(27%) | 16(72%) | 17(77%) | 21(95%) |
| Not fused | 16(72%) | 6(27%) | 5(23%) | 1(5%) |
Figure 1.
All the patients with diabetes had a successful fusion by six (6) months. All the patients who were current nicotine users had a successful fusion by six (6) months, all three (3) patients who had revision surgery showed successful fusion by six (6) months., and all the patients with osteoporosis had a successful fusion by twelve (12) months. The average time to fusion for all the patients was six (6) months.
There was no significant difference in the time to fusion between patients exposed to nicotine (n = 7) and patients who had never used nicotine (n = 14), (p = 0.25). There was no significant difference in the time to fusion between patients with diabetes/neuropathy (n = 6) and those without diabetes/neuropathy (n = 15), (p = 0.15). Obese patients (n = 11) had no difference in time to fusion compared to non-obese (n = 10), (p = 0.29). There was no difference in time-to-fusion between younger (n = 15) and older patients (n = 6), (p=0.45). Overall, there was no significant difference in the time to fusion between patients with ≤ 1 risk factor and patients with ≥ 2 risk factor for non-union.
Adverse events
There were no bone graft related adverse events reported.
Discussion
The rate of successful fusion following ankle/foot arthrodesis varies depending on factors such as bone graft material, risk of non-union, etc. The choice of bone graft material is a major contributory factor modifiable by the surgeon. To avoid the complications associated with autografts, surgeons are beginning to evaluate the efficacy of alternative bone grafts on the market. In this retrospective study, we evaluated the safety and efficacy of CBA among patients with high risk(s) of non-union. The result of this study indicates that using CBA may be an effective and safe alternative for improving radiological outcomes of ankle/foot arthrodesis in high-risk patients.
The main finding of our study is that 95% of the patients in this study had a successful fusion after an average follow-up period of 6 months. Other studies have reported a fusion rate between 52% and 97% following a foot/ankle arthrodesis procedure. The fusion rate reported in our study is similar to those reported in studies with patients that underwent foot/ankle arthrodesis using autografts and other CBA. A retrospective study reported a 97% fusion success among the patients who underwent arthrodesis surgery using the distal tibia bone graft (autograft) after an average follow-up period of 23.3 months.18 Similarly, a logistic regression analysis of data from 159 research articles compared fusion success following ankle/foot arthrodesis surgeries using autograft and cancellous CBA. The study reported a fusion rate of 93.7% for cancellous autograft, 94.2% for structural autograft, and 93.3% for cancellous allograft.19 A clinical comparison between two CBAs showed a 93.6% fusion success at 12 months in the patients following the arthrodesis procedure.20
Ensuring a successful fusion following an ankle/foot arthrodesis is always the goal of the patient and surgeon to avoid the complications associated with revision surgery. Also, it is essential to have a successful fusion after ankle/foot arthrodesis because there are limited alternatives to treat the associated condition. Therefore, choosing bone graft material is an important decision, especially for patients with the risk of nonunion or prior failed fusion. All three patients in our study who were treated because of prior failed fusion experienced successful fusion by 12 months following the use the CBA.
Risk factors such as age, BMI, diabetes, neuropathy, osteoporosis, nicotine use, etc., have been identified to predominantly cause nonunion of the joint/bone.21–26 Our study shows successful fusion in patients with these risk factors, which is higher than those reported in the literature with the use of autograft. A retrospective analysis among younger and older patients treated with autograft shows that the joints of younger adults (< 60 years) had >2 times the odds of successful fusion compared with the older adults (≥ 60 years). In addition, the study revealed a 72% fusion success in younger adults and a 52% fusion success in older adults following a surgical procedure using autografts.27 A prospective study that used autograft for foot/ankle arthrodesis shows a fusion success rate of 66.4% and 75.2% at 6 months and 12 months respectively. The high fusion experienced with the patients in our study may suggest that CBA possess an appropriate level of endogenous osteogenic cells and osteoinductive factors resulting in faster and more complete bone healing. This finding is supported by previous studies that have shown the efficacy of CBA following foot/ankle procedures. Another published retrospective study evaluated the efficacy of a CBA following foot/ankle arthrodesis in patients at risk of non-union. 83% of the patients experienced a successful fusion after a mean follow-up period of 13 months.12 Although our study has a smaller sample size, the high fusion of 95% in 1year experienced by the patients with risk(s) for non-union might suggest CBA has a better option for successful fusion in people with risk(s) for non-union when compared to autograft.
We evaluated the difference in time to fusion among the patients with the risk of non-union, and there was no significant difference in time to fusion between patients with low risk (≤ 1 risk) and patients with higher risk (≥ 2 risks). More specifically, there was no significant difference in the time to fusion between patients with a BMI ≤ 30kg/m2 and BMI > 30kg/m2. Also, there was no difference in time to fusion between patients who have/had exposure to nicotine and patients who have never been exposed to nicotine. The high fusion rate in these patients and the lack of difference in the fusion status between patients with high and low risk of non-union shows that CBA can mitigate the risk of non-union following foot/ankle arthrodesis in this population.
Another important finding of this study is that the use of CBA did not result in any adverse events or complications. The safety of using a bone graft material is an important consideration, as previous studies have shown that the use of autografts may be associated with a higher risk of infection or complication.9,10,
Although this study shows a success rate with the use of the CBA, the study has some limitations. The study is retrospective, with data from a single site and surgeon. However, the data were all collected before the planning of this study; hence the result is less likely to be subjected to bias. In addition, the sample size of patients in this study is relatively small; a multi-site prospective study with a larger sample size is needed to evaluate further the safety and efficacy of this CBA in the ankle/foot arthrodesis procedure.
Conclusion
Foot/ankle procedures using CBA resulted in a high fusion rate in patients with risk(s) of non-union. The fusion rate reported in this study is higher or similar to those reported in articles using autograft for bone fusion in foot/ankle arthrodesis procedures. There were no reports of graft rejection, postoperative adverse events, or complications associated with the use of the CBA. The result of this study suggests that CBAs can be used as a safe and effective substitute for autografts in patients with a risk of non-union during foot/ankle arthrodesis while avoiding complications associated with harvesting autografts. Further studies are needed to confirm these findings and explore the long-term outcomes of CBA following foot/ankle procedures.
Authors Contributions
EG: Study conception/design, data acquisition, data analysis, data interpretation, revised the manuscript for important intellectual content, and approved the final version.
DP: Data analysis, data interpretation, revised the manuscript for important intellectual content, and final approval of the version to be published.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Acknowledgment
We thank Adeola Sanni of Sanadex, Inc., who assisted in writing and implementing the authors’ revisions. Also, the authors appreciate Paul Rutti for helping with the statistical data analysis.
Funding Statement
Orthofix Inc funded this study.
References
- Mann Roger A., Rongstad Kurt M. Foot & Ankle International. 1. Vol. 19. SAGE Publications; Arthrodesis of the ankle: a critical analysis; pp. 3–9. [DOI] [PubMed] [Google Scholar]
- Abidi Nicholas A., Gruen Gary S., Conti Stephen F. Journal of the American Academy of Orthopaedic Surgeons. 3. Vol. 8. Ovid Technologies (Wolters Kluwer Health); Ankle arthrodesis: indications and techniques; pp. 200–209. [DOI] [PubMed] [Google Scholar]
- Perlman Mark H., Thordarson David B. Foot & Ankle International. 8. Vol. 20. SAGE Publications; Ankle fusion in a high risk population: an assessment of nonunion risk factors; pp. 491–496. [DOI] [PubMed] [Google Scholar]
- Bigsby Ewan, Cowie Simon, Middleton Rory G., Kemp Mark, Hepple Steve. The Journal of Foot and Ankle Surgery. 4. Vol. 53. Elsevier BV; Complications after revision surgery of malreduced ankle fractures; pp. 426–428. [DOI] [PubMed] [Google Scholar]
- Grabowski G., Cornett C. A. Journal of the American Academy of Orthopaedic Surgeons. 1. Vol. 21. Ovid Technologies (Wolters Kluwer Health); Bone graft and bone graft substitutes in spine surgery: current concepts and controversies; pp. 51–60. [DOI] [PubMed] [Google Scholar]
- Contemporary alternatives to synthetic bone grafts for spine surgery. Brandoff J. F., Silber J. S., Vaccaro A. R. 2008Am J Orthop (Belle Mead NJ) 37(8):410–4. [PubMed] [Google Scholar]
- Devescovi Valentina, Leonardi Elisa, Ciapetti Gabriela, Cenni Elisabetta. La Chirurgia degli Organi di Movimento. 3. Vol. 92. Springer Science and Business Media LLC; Growth factors in bone repair; pp. 161–168. [DOI] [PubMed] [Google Scholar]
- Arrington Edward D., Smith William J., Chambers Henry G., Bucknell Allan L., Davino Nelson A. Clinical Orthopaedics & Related Research. 329. Ovid Technologies (Wolters Kluwer Health); Complications of iliac crest bone graft harvesting; pp. 300–309. [DOI] [PubMed] [Google Scholar]
- Dimitriou Rozalia, Mataliotakis George I., Angoules Antonios G., Kanakaris Nikolaos K., Giannoudis Peter V. Injury. Suppl 2. Vol. 42. Elsevier BV; Complications following autologous bone graft harvesting from the iliac crest and using the RIA: a systematic review; pp. S3–S15. [DOI] [PubMed] [Google Scholar]
- Calori G.M., Colombo M., Mazza E.L., Mazzola S., Malagoli E., Mineo G.V. Injury. Suppl 6. Vol. 45. Elsevier BV; Incidence of donor site morbidity following harvesting from iliac crest or RIA graft; pp. S116–S120. [DOI] [PubMed] [Google Scholar]
- Baumhauer Judith, Pinzur Michael S., Donahue Rafe, Beasley William, DiGiovanni Christopher. Foot & Ankle International. 2. Vol. 35. SAGE Publications; Site Selection and Pain Outcome After Autologous Bone Graft Harvest; pp. 104–107. [DOI] [PubMed] [Google Scholar]
- Dekker Travis J., White Peter, Adams Samuel B. Foot & Ankle International. 3. Vol. 38. SAGE Publications; Efficacy of a Cellular Bone Allograft for Foot and Ankle Arthrodesis and Revision Nonunion Procedures; pp. 277–282. [DOI] [PubMed] [Google Scholar]
- Roberts Timothy T., Rosenbaum Andrew J. Organogenesis. 4. Vol. 8. Informa UK Limited; Bone grafts, bone substitutes and orthobiologics: the bridge between basic science and clinical advancements in fracture healing; pp. 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolvien Tim, Barbeck Mike, Wenisch Sabine, Amling Michael, Krause Matthias. International Journal of Molecular Sciences. 10. Vol. 19. MDPI AG; Cellular Mechanisms Responsible for Success and Failure of Bone Substitute Materials; p. 2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgafy Hossein, Wetzell Bradley, Gillette Marshall, Semaan Hassan, Rowland Andrea, Balboa Christopher A., Mierzwa Thomas A., McLean Julie B., Dorsch Kimberly, Moore Mark A. BMC Musculoskeletal Disorders. 1. Vol. 22. Springer Science and Business Media LLC; Lumbar spine fusion outcomes using a cellular bone allograft with lineage-committed bone-forming cells in 96 patients; p. 699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wind Joshua, Park Daniel, Lansford Todd, Nunley Pierce, Peppers Timothy, Russo Anthony, Hassanzadeh Hamid, Sembrano Jonathan N., Yoo Jung, Sales Jonathan. Neurology International. 4. Vol. 14. MDPI AG; Twelve-Month Results from a Prospective Clinical Study Evaluating the Efficacy and Safety of Cellular Bone Allograft in Subjects Undergoing Lumbar Spinal Fusion; pp. 875–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musante David B., Firtha Michael E., Atkinson Brent L., Hahn Rebekah, Ryaby James T., Linovitz Raymond J. Journal of Orthopaedic Surgery and Research. 1. Vol. 11. Springer Science and Business Media LLC; Clinical evaluation of an allogeneic bone matrix containing viable osteogenic cells in patients undergoing one- and two-level posterolateral lumbar arthrodesis with decompressive laminectomy; p. 63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danziger Marc B., Abdo Richard V., Decker J. Elliot. Foot & Ankle International. 4. Vol. 16. SAGE Publications; Distal tibia bone graft for arthrodesis of the foot and ankle; pp. 187–190. [DOI] [PubMed] [Google Scholar]
- Lareau Craig R., Deren Matthew E., Fantry Amanda, Donahue Rafe M.J., DiGiovanni Christopher W. Foot and Ankle Surgery. 3. Vol. 21. Elsevier BV; Does autogenous bone graft work? A logistic regression analysis of data from 159 papers in the foot and ankle literature; pp. 150–159. [DOI] [PubMed] [Google Scholar]
- Loveland Jeff D, Waldorff Erik I, He David Y, Atkinson Brent L. Journal of Stem Cell Research & Therapy. 10. Vol. 7. OMICS Publishing Group; A Retrospective Clinical Comparison of Two Allogeneic Bone Matrices Containing Viable Osteogenic Cells in Patients Undergoing Foot and/or Ankle Arthrodesis; pp. 1–7. [DOI] [Google Scholar]
- Jones Carroll P., Loveland Jeffrey, Atkinson Brent L., Ryaby James T., Linovitz Raymond J., Nunley James A. Foot & Ankle International. 10. Vol. 36. SAGE Publications; Prospective, Multicenter Evaluation of Allogeneic Bone Matrix Containing Viable Osteogenic Cells in Foot and/or Ankle Arthrodesis; pp. 1129–1137. [DOI] [PubMed] [Google Scholar]
- Fink B., Niggemeyer O., Schneider T., Strauss J.M., Rüther W. Foot and Ankle Surgery. 3. Vol. 2. Elsevier BV; Reasons for non-unions after arthrodeses of the ankle; pp. 145–154. [DOI] [Google Scholar]
- Zura Robert, Mehta Samir, Della Rocca Gregory J., Steen R. Grant. JBJS Reviews. 1. Vol. 4. Ovid Technologies (Wolters Kluwer Health); Biological Risk Factors for Nonunion of Bone Fracture. [DOI] [PubMed] [Google Scholar]
- Thevendran Gowreeson, Shah Kalpesh, Pinney Stephen J, Younger Alastair SE. Journal of Orthopaedic Surgery. 1. Vol. 25. SAGE Publications; Perceived risk factors for nonunion following foot and ankle arthrodesis; p. 2309499017692703. [DOI] [PubMed] [Google Scholar]
- Liska Franz, Haller Bernhard, Voss Andreas, Mehl Julian, Imhoff Florian B., Willinger Lukas, Imhoff Andreas B. Knee Surgery, Sports Traumatology, Arthroscopy. 9. Vol. 26. Wiley; Smoking and obesity influence the risk of nonunion in lateral opening wedge, closing wedge and torsional distal femoral osteotomies; pp. 2551–2557. [DOI] [PubMed] [Google Scholar]
- O’Connor Kathryn M., Johnson Jeffrey E., McCormick Jeremy James, Klein Sandra E. Foot & Ankle International. 8. Vol. 37. SAGE Publications; Clinical and Operative Factors Related to Successful Revision Arthrodesis in the Foot and Ankle; pp. 809–815. [DOI] [PubMed] [Google Scholar]
- Berlet Gregory C., Baumhauer Judith F., Glazebrook Mark, Haddad Steven L., Younger Alastair, Quiton Jovelyn D., Fitch David A., Daniels Timothy R., DiGiovanni Christopher W. JBJS Open Access. 4. Vol. 5. Ovid Technologies (Wolters Kluwer Health); The Impact of Patient Age on Foot and Ankle Arthrodesis Supplemented with Autograft or an Autograft Alternative (rhPDGF-BB/β-TCP) p. e20.00056. [DOI] [PMC free article] [PubMed] [Google Scholar]