Abstract
Purpose.
The pharmacokinetics of intrathecally administered antibody or small-molecule drugs in the human central nervous system (CNS) remains poorly understood. This study aimed to provide mechanistic and quantitative perspectives on the CNS pharmacokinetics of intrathecal chemotherapy, by using physiologically based pharmacokinetic (PBPK) modeling approach.
Experimental Design.
A novel CNS PBPK model platform was developed and verified, which accounted for the human CNS general anatomy and physiological processes governing drug distribution and disposition. The model was used to predict CNS pharmacokinetics of antibody (trastuzumab) and small-molecule drugs (methotrexate, abemaciclib, tucatinib) following intraventricular injection or intraventricular 24-h infusion, and to assess the key determinants of drug penetration into the deep brain parenchyma.
Results.
Intraventricularly administered antibody and small-molecule drugs exhibited distinct temporal and spatial distribution and disposition in human CNS. Both antibody and small-molecule drugs achieved supratherapeutic or therapeutic concentrations in the cerebrospinal fluid (CSF) compartments and adjacent brain tissue. While intrathecal small-molecule drugs penetrated the deep brain parenchyma to a negligible extent, intrathecal antibodies may achieve therapeutic concentrations in the deep brain parenchyma. Intraventricular 24-h infusion enabled prolonged CNS exposure to therapeutically relevant concentrations while avoiding excessively high and potentially neurotoxic drug concentrations.
Conclusions.
CNS PBPK modeling, in line with available clinical efficacy data, confirms the therapeutic value of intrathecal chemotherapy with antibody or small molecule drugs for treating neoplastic meningitis and warrants further clinical investigation of intrathecal antibody drugs to treat brain parenchyma tumors. Compared to intraventricular injection, intraventricular 24-h infusion may mitigate neurotoxicity while retaining potential efficacy.
INTRODUCTION
A key prerequisite for drug efficacy is achieving adequate exposure of pharmacologically active drug (i.e., unbound drug) at the site of action. Insufficient penetration of potentially effective chemotherapeutic agents into the human brain parenchyma or brain tumors is one of the major obstacles to efficacious treatment of primary or metastatic brain tumors (1–3). Restricted drug delivery into the brain following systemic administration is largely attributed to the blood-brain barrier (BBB), a physical and biochemical barrier separating the brain parenchyma from circulatory system (3). To bypass the BBB with the aim of improving therapeutic drug exposure in the brain, intrathecal administration of small-molecule chemotherapeutic agents (e.g., methotrexate) and antibody drugs (e.g., trastuzumab) has been clinically tested or used to patients with primary or metastatic brain cancer (4–7). However, controversial clinical efficacy data have been reported for different intrathecal drugs and in different brain tumor types. Notably, intrathecal chemotherapy appears to be clinically efficacious for the prevention or treatment of neoplastic meningitis (e.g. leptomeningeal metastases of breast cancer), while showing limited effectiveness in parenchyma brain tumors (e.g., glioblastoma) (4,8,9).
Following intrathecal drug administration either through the Ommaya intraventricular reservoir or lumbar puncture, the transport and spatial distribution of a drug in the human central nervous system (CNS) is determined by the CNS anatomical structure and physiological processes, as well as drug properties. Specifically, intrathecally administered drug would move along with cerebrospinal fluid (CSF) through the CSF circulation, whereby CSF flows through the ventricular system to the cranial and spinal subarachnoid spaces and is drained into the systemic circulation through the arachnoid villi or along the olfactory mucosa, cranial and spinal nerve sheaths (10). Depending on the drug properties, the drug may re-enter the brain parenchyma via the BBB after it is absorbed into the blood circulation via CSF circulation (11). In addition, intrathecal drug may distribute to the brain parenchyma, driven by a combination of simple diffusion and convective bulk flow. Simple diffusion is characterized by the dependence of diffusion rate on molecular size; and by contrary, convective bulk flow rate is independent of molecular size as all solutes are carried along with the moving medium at the same rate of fluid flow (12). The relative contribution of each process is dependent on the anatomical region and the size of the molecule. While simple diffusion plays a key role in facilitating drug distribution from the ventricular or subarachnoid CSF across the ependymal lining into the adjacent brain tissue, it is inefficient for drug distribution to the deep brain parenchyma at the distance > 2 mm away from the CSF tract (13). Studies using different molecular weight tracers indicate that convective bulk flow along paravascular spaces (also known as Virchow-Robin spaces) is the preferential pathway for CSF-interstitial fluid (ISF) exchange and interstitial solute clearance (14). The paravascular bulk flow pathway is also termed the glymphatic system given its role in interstitial solute clearance and dependence on the glial water channel aquaporin-4 (AQP4) (14–16).
The proper use of intrathecal chemotherapy for efficacious brain cancer treatment has been hindered by the lack of quantitative understanding of the pharmacokinetics of intrathecally administered small-molecule or antibody drugs in the human CNS. In particular, the temporal and spatial biodistribution of intrathecally administered drugs in the human brain parenchyma remains poorly understood, as it is difficult to be measured in patients due to the challenge of sampling and limitation of currently available imaging or analytical technologies. It is thus imperative to develop and apply innovative approaches to resolve the gap of our knowledge. Physiologically based pharmacokinetic (PBPK) modeling offers a unique mechanism-based computational modeling approach for quantitative prediction of CNS pharmacokinetics in humans, given its capability of incorporating biological system data and drug-specific data into a pharmacokinetic model and predicting in vivo kinetic processes based on mechanistic scaling of in vitro data (e.g., in vitro enzyme or transporter kinetic data) (17,18). While PBPK modeling of CNS pharmacokinetics has been explored by us and others, most studies were focused on systemic drug administration (19–24). The development and application of PBPK models for prediction of the human CNS pharmacokinetics of intrathecally administered small-molecule or antibody drugs remain largely understudied and underreported.
The purpose of this study was to provide mechanistic and quantitative perspectives on the CNS pharmacokinetics of intrathecal chemotherapy by using the PBPK modeling approach. A novel 6-compartment CNS PBPK model platform was developed to account for drug properties as well as the human CNS general anatomy and physiological processes governing drug distribution and disposition. The model was used to predict the CNS pharmacokinetics of intraventricularly administered antibody and small-molecule drugs with diverse drug properties (including trastuzumab, methotrexate, abemaciclib, and tucatinib), and to quantitatively assess the key determinants of drug penetration into the deep brain parenchyma.
METHODS
CNS PBPK model development
Model structure and assumptions.
A 6-compartment CNS (6-CNS) PBPK model structure (Figure 1) was designed to represent the general anatomy and physiology of the human CNS while maintaining reasonable simplicity needed for computational feasibility. The model has 6 compartments that represent brain blood compartment, 3 CSF compartments (including ventricular CSF, cranial subarachnoid space, and spinal subarachnoid space), and 2 brain parenchyma compartments (representing brain tissue adjacent to CSF compartments and deep brain parenchymal region with > 2 mm distance from CSF compartments).
Figure 1.
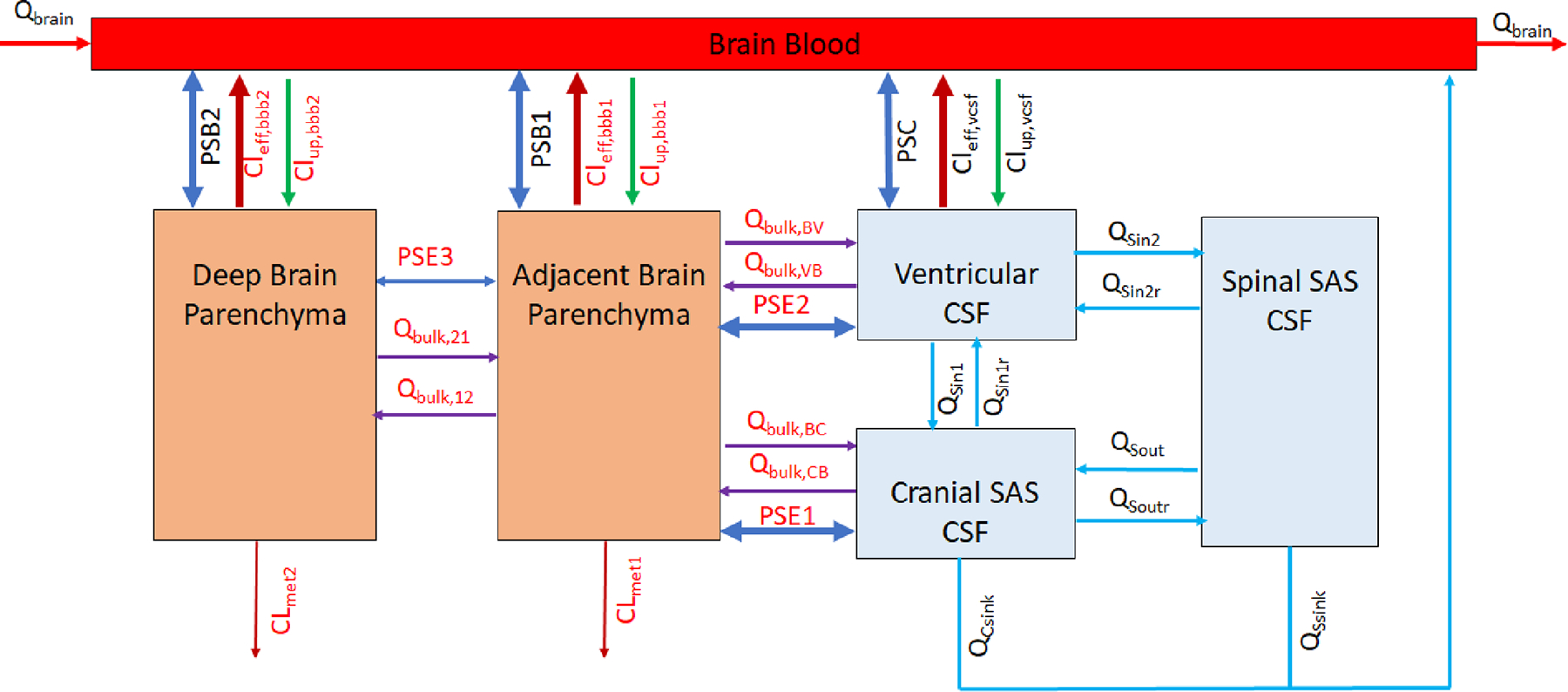
Model structure of the 6-compartment CNS (6-CNS) PBPK model. The model has 6 compartments that represent cerebral blood compartment, 3 CSF compartments (i.e., ventricular CSF, cranial and spinal subarachnoid CSF), and 2 brain parenchyma compartments (parenchyma region adjacent to CSF compartments and deep brain parenchymal region with > 2 mm distance from CSF compartments). Details on the fluid flow and drug transport in the 6-CNS model are described in the Method.
The fluid flow and drug transport in the 6-CNS model are driven by physiological processes of CSF circulation, size-dependent simple diffusion and paravascular convective bulk flow, as well as passive permeability and/or transporter-mediated active transport at the BBB and blood-CSF barriers. CSF is produced primarily by the choroid plexuses of the brain ventricles (with the average production rate if 0.021 L/h in humans) (25,26). CSF along with intraventricularly administered drug flows through the ventricular system to the cranial and spinal subarachnoid spaces (at the flow rate Qsin1 and Qsin2 respectively) and is subsequently drained into the bloodstream through the arachnoid villi and along the olfactory mucosa, cranial and spinal nerve sheaths (at the total absorption rates QCsink and QSsink respectively) (10,26). Drug distribution from the subarachnoid CSF spaces across the ependymal lining into the adjacent brain tissue mainly relies on molecular size-dependent diffusion (at the diffusion rates PSE1 and PSE2 respectively); direct diffusion (PSE3) between the adjacent and deep brain parenchyma is assumed to be negligible. Intrathecally administered drug may penetrate to the deep brain parenchyma via the glymphatic system (14). Specifically, ventricular or cranial subarachnoid CSF along with solutes and drug molecules influx through paraarterial spaces into the brain interstitial (at the convective bulk flow rates Qbulk,VB, Qbulk,CB, and Qbulk,12 respectively), and subsequently the mixture of brain ISF and CSF flows along paravenous spaces back to the ventricle CSF and cranial subarachnoid CSF compartments (at the convective bulk flow rates Qbulk,BV, Qbulk,BC, and Qbulk,21 respectively). Metabolism in the brain parenchyma is allowed in the model, but it is assumed to be negligible for the model drugs in the present study. Drug transport across the BBB between the brain blood and the adjacent and deep brain parenchyma compartments and drug transporter across the blood-CSF barrier between the brain blood and ventricular CSF compartments is governed by passive transcellular permeability and transporter-mediated active transport (efflux or uptake clearance) (19,20). It is assumed that transcellular permeability allows only unbound and unionized drug to pass, and transporters act upon unbound drug (including both unionized and ionized species) (19,20).
System- and drug-specific parameters.
Table 1 summarizes the system-specific parameters, the population mean values, and assumptions or references (26–30). Supplementary Table S1 summarizes the drug-specific parameters for model drugs. Briefly, physicochemical properties (i.e., molecular weight, PKa and LogP) were obtained from PubChem. In vitro parameters for passive permeability, in vitro ABCB1- and ABCG2-mediated intrinsic clearance, as well as drug binding to plasma proteins and brain tissues were determined experimentally by us previously or obtained from published studies (19,20,31–33). In vivo parameters for passive clearance at the BBB (parameterized as the passive permeability-BBB surface area product, PSB) and blood-CSF barrier (parameterized as the permeability-blood-CSF barrier surface area product, PSC) as well as ABCB1- and ABCG2-mediated active efflux clearance at the BBB were estimated using in vitro-in vivo extrapolation approach), as described by us previously (19,20). Of note, the adjacent brain and deep brain parenchyma compartments may have different interstitial pH or tissue composition, which may result in different drug ionization or tissue binding. While the current model simulations were performed assuming the drug ionization or tissue binding remained the same in the adjacent and deep brain parenchyma compartments, the developed models could incorporate different parameters for the two brain parenchyma compartments if needed.
Table 1.
System-specific parameters for the 6-compartment CNS PBPK model
| Parameters | Descriptions | Values | References or Assumptions |
|---|---|---|---|
|
| |||
| Vbb (L) | Volume of brain blood | 0.0630 | 5% of brain volume (1260 cm3 in men and 1130 cm3 in women) (ref 26, 27) |
| Vbm1 (L) | Volume of adjacent brain tissue | 0.1200 | 10% of brain volume (average brain volume, 1.2 L) |
| Vbm2 (L) | Volume of deep brain parenchyma | 1.0800 | 90% of brain volume (average brain volume, 1.2 L) |
| Vvcsf (L) | Volume of ventricular CSF | 0.0251 | 16.7% of total CSF (0.15 L) (ref 26) |
| Vccsf (L) | Volume of cranial subarachnoid CSF | 0.0450 | 30% of total CSF (0.15 L) (ref 26) |
| Vscsf (L) | Volume of spinal subarachnoid CSF | 0.0800 | SCSF is 80 mL (ref 28) |
| Qbrain (L/h) | Cerebral blood flow | 39.0000 | 600–700 mL per min or 15% of the cardiac output (ref 28) |
| Qbulk,BC (L/h) | Convective bulk flow rate from the adjacent brain tissue to cranial subarachnoid CSF | 0.0101 | 80% of Qbulk,21 |
| Qbulk,CB (L/h) | Convective bulk flow rate from the cranial subarachnoid CSF to adjacent brain tissue | 0.0101 | Qbulk,CB equals Qbulk,BC to maintain fluid balance |
| Qbulk,BV (L/h) | Convective bulk flow rate from the adjacent brain tissue to ventricular CSF | 0.0025 | 20% of Qbulk,BC |
| Qbulk,VB (L/h) | Convective bulk flow rate from the ventricular CSF to adjacent brain tissue | 0.0025 | Qbulk,VB equals Qbulk,BV to maintain fluid balance |
| Qbulk,12 (L/h) | Convective bulk flow rate from the adjacent brain tissue to deep brain parenchyma | 0.0126 | Qbulk,12 equals Qbulk,21 to maintain fluid balance |
| Qbulk,21 (L/h) | Convective bulk flow rate from the deep brain parenchyma to adjacent brain tissue | 0.0126 | Average convective bulk flow is 0.15 uL/min/g in human brain (ref 29) |
| Qcsink (L/h) | Absorption rate of cranial subarachnoid CSF into blood circulation | 0.0130 | 62% of CSF production rate (0.021 L/h) (ref 30) |
| Qssink (L/h) | Absorption rate of spinal subarachnoid CSF into blood circulation | 0.0080 | 38% of CSF production rate (0.021 L/h) (ref 30) |
| Qsin1 (L/h) | CSF flow rate from the ventricle to cranial subarachnoid space | 0.0126 | 60% of CSF production rate (0.021 L/h) (ref 30) |
| Qsin1r (L/h) | CSF back flow rate from the cranial subarachnoid space to ventricle | 0.0013 | 10% of Qsin1 |
| Qsin2 (L/h) | CSF flow rate from the ventricle to spinal subarachnoid space | 0.0084 | 40% of CSF production rate (0.021 L/h) (ref 30) |
| Qsin2r (L/h) | CSF back flow rate from the spinal subarachnoid space to ventricle | 0.0008 | 10% of Qsin2 |
| Qsout (L/h) | CSF flow rate from the spinal subarachnoid space to cranial subarachnoid space | 0.0004 | Qsout = Qcsink - Qsin1 to maintain fluid balance |
| Qsoutr (L/h) | CSF flow rate from the cranial subarachnoid space to spinal subarachnoid space | 0.0000 | 10% of Qsout |
Model simulations
Differential equations (presented in Supplementary Methods) were written to describe fluid flow and drug transport in the 6-CNS model. R programming was used for model simulations to predict the drug concentration – time profiles of intrathecally administered model drugs in the CNS compartments.
Intrathecal chemotherapy is often achieved through the Ommaya intraventricular reservoir or lumbar puncture. Intraventricular injection via Ommaya reservoir is more commonly used clinically because it is easier to apply in clinic and better tolerated by patients and moreover, produces higher and more consistent intraventricular drug concentrations (34). Thus, in this study, model simulations were performed following Ommaya reservoir intraventricular injection or 24-h infusion of a single dose or multiple doses using the dosing regimens matched to those reported in clinical studies (for trastuzumab and methotrexate) or hypothesized if no intrathecal clinical studies have been reported (for tucatinib and abemaciclib).
For model validation, the observed clinical CSF data (available for trastuzumab and methotrexate) (34,35) were used to verify the model-predicted CSF pharmacokinetic profiles. As observed clinical CSF data were published for intraventricularly injected cytarabine (Ara C) and etoposide (36,37), additional simulations were performed to compare the predicted and observed CSF data for these two drugs to further validate the model. In addition, since observed drug concentration data in the human brain parenchyma were not available, the published data from tracer studies and clinical efficacy data of intrathecal chemotherapy (14,35,38) were used to support or indirectly verify the model predictions of drug brain exposure.
To better understand the key determinants of intrathecal drug penetration into the deep brain parenchyma, simulations were performed to illustrate the impacts of paravascular convective bulk flow rates, BBB passive permeability, and BBB active efflux clearance on the concentration – time profiles of the model drugs in the deep brain parenchyma following intraventricular injection of a single dose.
Data Availability
The data generated in this study are available within the paper and its Supplementary Materials. The raw simulation data used to create graphs are available from the corresponding author upon reasonable request.
RESULTS
Figure 2 and Figure 3 show the model-predicted unbound (i.e., pharmacologically relevant) drug concentration – time profiles of trastuzumab, methotrexate, abemaciclib, and tucatinib in the 3 CSF compartments and 2 brain parenchyma compartments following intraventricular injection or intraventricular 24-h infusion of a single dose or multiple doses (twice weekly for 2 weeks). The model predictions of CSF pharmacokinetics were validated by comparisons of the predicted and observed clinical CSF data for trastuzumab (Figure 2A), methotrexate (Figure 2C), cytarabine (Supplementary Figure S1A), and etoposide (Supplementary Figure S1B) following intraventricular injection. The CNS pharmacokinetic parameters for trastuzumab, methotrexate, abemaciclib, and tucatinib are summarized in Table 2. Detailed descriptions of the CNS pharmacokinetics for individual drugs are presented as follows.
Figure 2.
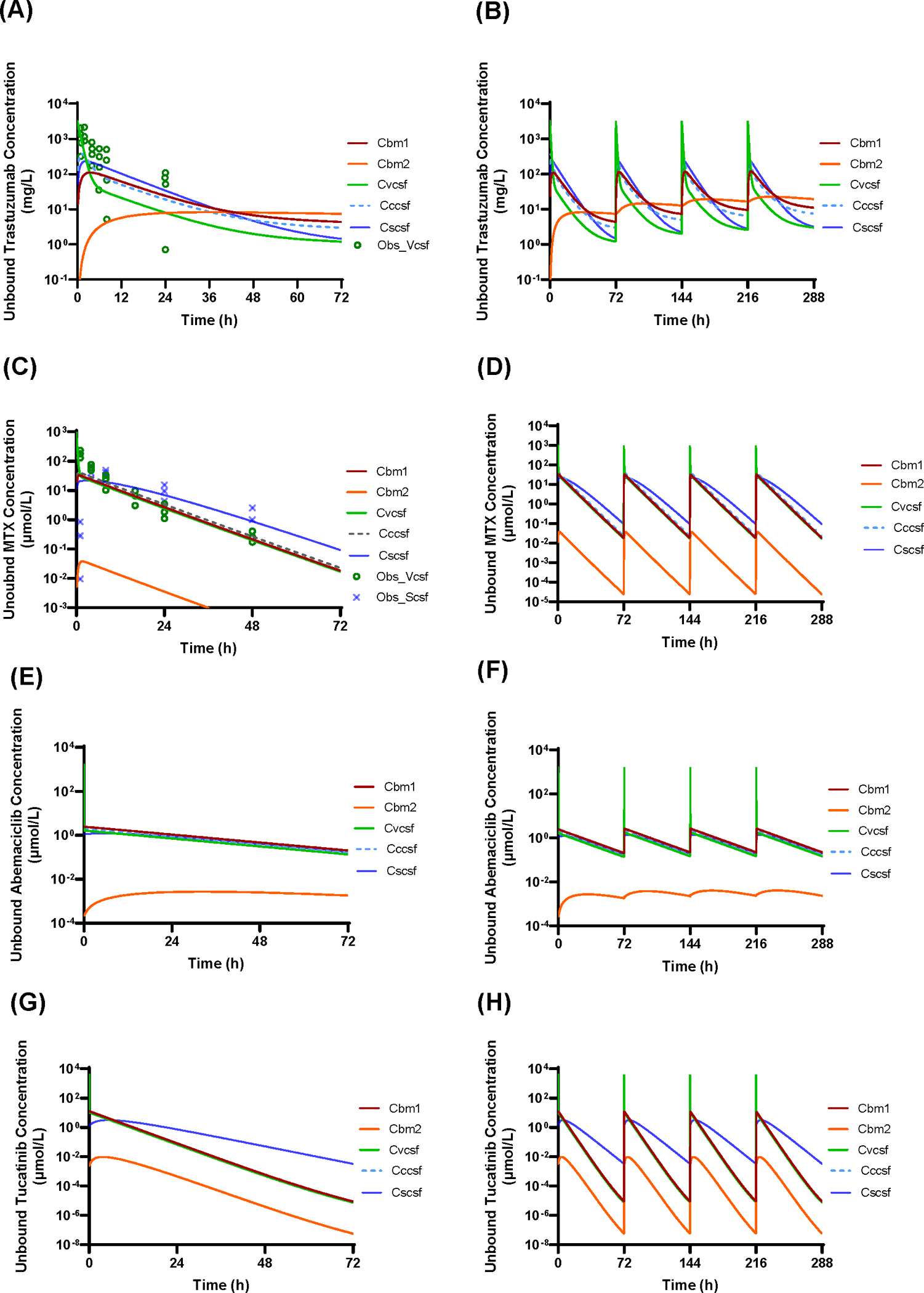
Model-predicted unbound drug – concentration time profiles in the ventricular CSF (Cvcsf), cranial subarachnoid CSF (Cccsf), spinal subarachnoid (Cscsf), adjacent brain tissue (Cbm1), and deep brain parenchyma (Cbm2) following intraventricular injection. (A and B) Trastuzumab given at a single dose of 80 mg or twice weekly for 2 weeks. (C and D) Methotrexate given at a single dose of 24.76 μmol (11.25 mg) or twice weekly for 2 weeks. (E and F) Abemaciclib given at a single dose of 59.2 μmol (30 mg) or twice weekly for 2 weeks. (G and H) Tucatinib given at a single dose of 125 μmol (60 mg) or twice weekly for 2 weeks. Lines represent model-predicted concentration – time profiles. ○ and x symbols represent the observed concentrations of trastuzumab or methotrexate in ventricular CSF and spinal CSF in patients (as reported in reference 34 and 35).
Figure 3.
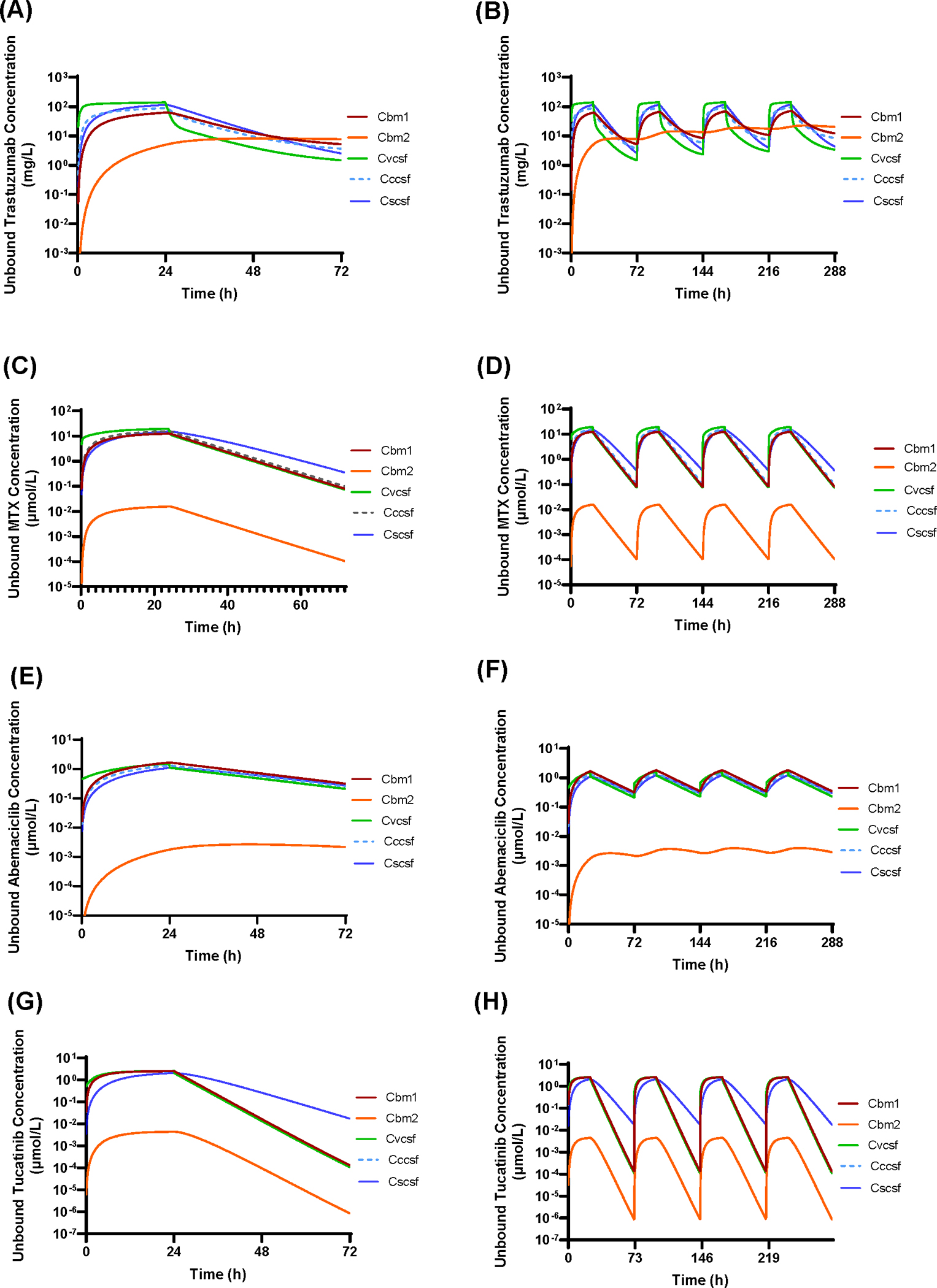
Model-predicted unbound drug – concentration time profiles in the ventricular CSF (Cvcsf), cranial subarachnoid CSF (Cccsf), spinal subarachnoid (Cscsf), adjacent brain tissue (Cbm1), and deep brain parenchyma (Cbm2), following intraventricular 24-h infusion. (A and B) Trastuzumab given at a single dose of 80 mg or twice weekly for 2 weeks. (C and D) Methotrexate given at a single dose of 24.76 μmol (11.25 mg) or twice weekly for 2 weeks. (E and F) Abemaciclib given at a single dose of 59.2 μmol (30 mg) or twice weekly for 2 weeks. (G and H) Tucatinib given at a single dose of 125 μmol (60 mg) or twice weekly for 2 weeks.
Table 2.
Pharmacokinetic parameters following intraventricular injection or intraventricular 24-h infusion a
| Intraventricular injection | Intraventricular 24-h infusion | ||||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Trastuzumabb | T1/2 (h) | Tmax (h) | Cmax (mg/L) | C72h (mg/L) | AUC0–72h (mg/L*h) | Tmax (h) | Cmax (mg/L) | C72h (mg/L) | AUC0–72h (mg/L*h) |
| Cbm1 | 71.54 | 3.53 | 110.51 | 4.36 | 1922.31 | 24.22 | 62.41 | 5.23 | 1864.22 |
| Cbm2 | 165.68 | 36.49 | 8.25 | 7.39 | 501.90 | 50.77 | 8.18 | 7.76 | 410.26 |
| Cccsf | 65.00 | 1.18 | 374.23 | 2.98 | 2447.93 | 24.05 | 88.81 | 3.65 | 2409.46 |
| Cscsf | 24.39 | 2.35 | 232.61 | 1.45 | 3144.97 | 24.13 | 114.37 | 2.52 | 3121.30 |
| Cvcsf | 63.67 | 0.00 | 3200.00 | 1.20 | 3459.37 | 23.88 | 138.61 | 1.48 | 3442.08 |
|
|
|
||||||||
| Methotrexatec | T1/2 (h) | Tmax (h) | Cmax (μmol/L) | C72h (μmol/L) | AUC0–72h (μmol/L*h) | Tmax (h) | Cmax (μmol/L) | C72h (μmol/L) | AUC0–72h (μmol/L*h) |
| Cbm1 | 6.75 | 0.84 | 32.61 | 0.02 | 326.33 | 24.05 | 12.55 | 0.08 | 326.11 |
| Cbm2 | 6.76 | 1.68 | 0.04 | 2.41E-05 | 0.41 | 24.13 | 0.02 | 1.05E-04 | 0.41 |
| Cccsf | 6.76 | 0.67 | 41.64 | 41.64 | 406.14 | 24.05 | 15.63 | 0.10 | 406.00 |
| Cscsf | 7.36 | 3.03 | 21.65 | 21.65 | 446.42 | 24.05 | 15.25 | 0.35 | 443.86 |
| Cvcsf | 6.77 | 0.00 | 990.40 | 990.40 | 491.05 | 23.88 | 19.44 | 0.07 | 489.34 |
|
|
|
||||||||
| Abemaciclibd | T1/2 (h) | Tmax (h) | Cmax (μmol/L) | C72h (μmol/L) | AUC0–72h (μmol/L*h) | Tmax (h) | Cmax (μmol/L) | C72h (μmol/L) | AUC0–72h (μmol/L*h) |
| Cbm1 | 20.02 | 0.07 | 2.45 | 0.20 | 64.96 | 24.05 | 1.66 | 0.32 | 61.75 |
| Cbm2 | 44.59 | 32.29 | 0.00 | 1.81E-03 | 0.16 | 45.00 | 0.00 | 2.16E-03 | 0.13 |
| Cccsf | 20.02 | 0.07 | 1.95 | 0.16 | 51.79 | 24.05 | 1.33 | 0.25 | 49.24 |
| Cscsf | 20.41 | 5.77 | 1.22 | 0.18 | 47.37 | 24.05 | 1.10 | 0.27 | 44.58 |
| Cvcsf | 20.02 | 0.00 | 1657.60 | 0.13 | 60.17 | 23.88 | 1.54 | 0.21 | 51.36 |
|
|
|
||||||||
| Tucatinibe | T1/2 (h) | Tmax (h) | Cmax (μmol/L) | C72h (μmol/L) | AUC0–72h (μmol/L*h) | Tmax (h) | Cmax (μmol/L) | C72h (μmol/L) | AUC0–72h (μmol/L*h) |
| Cbm1 | 4.45 | 0.07 | 12.71 | 8.87E-06 | 60.71 | 24.00 | 2.53 | 1.30E-04 | 61.13 |
| Cbm2 | 4.42 | 3.75 | 0.01 | 5.69E-08 | 0.11 | 24.00 | 0.00 | 8.47E-07 | 0.11 |
| Cccsf | 5.54 | 0.07 | 12.49 | 8.80E-06 | 59.69 | 24.00 | 2.49 | 1.29E-04 | 60.08 |
| Cscsf | 6.05 | 5.26 | 3.16 | 3.26E-03 | 56.20 | 24.00 | 2.07 | 1.73E-02 | 56.12 |
| Cvcsf | 4.45 | 0.00 | 4000.00 | 7.55E-06 | 97.59 | 24.00 | 2.55 | 1.08E-04 | 61.63 |
Pharmacokinetic parameters in the CNS compartments, including the ventricular CSF (Cvcsf), cranial subarachnoid CSF (Cccsf), spinal subarachnoid (Cscsf), adjacent brain tissue (Cbm1), and deep brain parenchyma (Cbm2), were estimated using non-compartmental analysis.
Trastuzumab was given at a single dose of 80 mg.
Methotrexate was given at a single dose of 24.76 μmol (11.25 mg).
Abemaciclib was given at a single dose of 59.2 μmol (30 mg, 10% of the standard daily oral dose).
Tucatinib was given at a single dose of 125 μmol (60 mg, 10% of the standard daily oral dose).
Abbreviations: AUC0–72h, area under unbound drug concentration – time curve during one dosing interval (72 h); C72h, trough drug concentration; Cmax, peak drug concentration; Tmax, time to reach the peak concentration; T1/2, terminal half-life.
CNS pharmacokinetics of intrathecal trastuzumab
Trastuzumab, a monoclonal antibody targeting the HER2 receptor, is approved as the first line treatment for patients with HER2-positive breast cancer. Intravenous administered trastuzumab demonstrates extremely poor CNS penetration due to its large molecule weight (145 KDa); specifically, trastuzumab CSF concentrations are ~ 0.3% of its plasma concentrations following weekly intravenous regimen (39). Here, trastuzumab is a model drug representing antibody drugs with extremely low passive permeability and no interaction with efflux or uptake transporters at the BBB.
In a recently reported phase I/II study of intrathecal trastuzumab in HER2-positive cancer patients with leptomeningeal metastases, ventricular CSF concentrations of trastuzumab were determined following intraventricular injection of 80 mg via Ommaya reservoir (35). The model-predicted mean ventricular CSF concentration – time profile was in agreement with the clinically observed data (despite a large inter-individual variability) following the same dosing regimen (Figure 2A), suggesting that the system-specific and trastuzumab drug-specific parameters were reasonably well defined in the 6-CNS PBPK model. Following intraventricular injection (80 mg), trastuzumab reached an excessively high peak concentration (Cmax) of 3200 mg/L in the ventricular CSF, from which it distributed into the cranial subarachnoid CSF (achieving the Cmax of 374 mg/L at ~ 1.2 h), spinal subarachnoid CSF (achieving the Cmax of 233 mg/L at ~ 2.4 h), and adjacent brain parenchyma (achieving the unbound drug Cmax of 110 mg/L at ~ 3.5 h); and simultaneously it slowly penetrated into the deep brain parenchyma (achieving Cmax of 8.2 mg/L at ~ 36 h) (Figure 2A and Table 2). The elimination half-life (T1/2) in the ventricular CSF, cranial and spinal subarachnoid CSF was estimated to be 64, 65, and 24 h, respectively (Table 2), which was in accordance with the observed T1/2 in the spinal CSF in the patients following intraventricular injection (40). The elimination of trastuzumab from the deep brain parenchyma was slow (T1/2, 166 h), leading to drug accumulation in the deep brain parenchyma following twice weekly dosing regimen (Figure 2B). Notably, the steady-state unbound trastuzumab concentration in the deep brain parenchyma (assuming unbound fraction of 0.5 in the brain) was maintained at ~ 10 mg/L (Figure 2B), which was considered as therapeutically effective concentration (40,41). The overall pharmacologically relevant drug exposure, as measured by the area under unbound drug concentration – time curve during one dosing interval (AUC0–72h), in the deep brain parenchyma was about 15% of the AUC0–72h in the ventricular CSF (Table 2), suggesting that considerably large amount of trastuzumab penetrated from CSF to the deep parenchyma.
Following intraventricular 24-h infusion of a same dose (80 mg) as injection, trastuzumab achieved considerably lower Cmax in all 3 CSF compartments and adjacent brain tissue (e.g., 23-fold lower Cmax in the ventricular CSF), while remaining similar Cmax and steady-state drug concentration (~ 10 mg/L) in the deep brain parenchyma (Figure 3A and 3B and Table 2). Notably, intraventricular 24-h infusion and injection resulted in the same overall pharmacologically relevant drug exposure (AUC0–72h) in each CNS compartments (Table 2).
CNS pharmacokinetics of intrathecal methotrexate
Methotrexate is the most commonly used chemotherapeutic agent for intrathecal administration. The most common intrathecal dose used in adults is 10 – 15 mg (22 – 33 μmol) per injection, given twice weekly for 4 weeks and then once weekly for an additional 4 weeks followed by once monthly maintenance dosing. Since methotrexate is a weak acid drug with PKa1 of 4.8 and PKa2 of 5.5, > 99% of the drug is ionized at the physiological pH 7.4. Thus, it exhibits low transcellular passive permeability with an apparent permeability (Papp) of 0.09 × 10−6 cm/s across the MDCKII cell monolayer (31), based on which the apparent passive clearance at the human BBB was estimated to be 0.06 L/h at pH 7.4 given the average brain blood vasculature surface area of 20 m2. Methotrexate is a substrate of ABCG2, with the estimated efflux clearance of 10 L/h at the human BBB. Here, methotrexate represents a small-molecule drug with low passive permeability but high/moderate active efflux clearance at the human BBB.
Following intraventricular injection of 11.25 mg (or 24.76 μmol), the model-predicted mean methotrexate concentration – time profiles in the ventricular and spinal CSF compartments (Figure 2C) were in good agreement with the clinically observed data following the same dosing regimen (34), suggesting that the system-specific and methotrexate drug-specific parameters were reasonably well defined in the 6-CNS PBPK model. Following intraventricular injection (24.76 μmol), methotrexate immediately reached the Cmax of 990 μmol/L in the ventricular CSF, followed by bio-exponential decline with the T1/2 of 0.14 and 6.8 h, respectively (Figure 2C). From the ventricular CSF, the drug rapidly distributed into the cranial subarachnoid CSF (Cmax, 41.6 μmol/L; Tmax, 0.67 h) and adjacent brain tissue (unbound Cmax, 32.6 μmol/L; Tmax, 0.84 h), while relatively slowly distributing into the spinal subarachnoid CSF (Cmax, 21.6 μmol/L; Tmax, 3.0 h) and deep brain parenchyma (unbound Cmax, 0.04 μmol/L; Tmax, 1.7 h) (Table 2). Methotrexate shared a similar elimination T1/2 (~ 6.8–7.4 h) from all CNS compartments (Table 2). Given the long dosing interval (72 h) and relatively short elimination T1/2, methotrexate was not accumulated in the CNS following the twice weekly dosing regimen (Figure 2D).
In comparison with intraventricular injection, intraventricular 24-h infusion of a same dose (24.76 μmol) resulted in considerably lower Cmax in all CNS compartments (e.g., ~ 50-fold lower Cmax in the ventricular CSF), while maintaining the same overall pharmacologically relevant drug exposure (AUC0–72h) in all CNS compartments (Figure 3C and 3D and Table 2).
Although the clinically therapeutic concentration of methotrexate has not been well established yet, 2 μmol/L unbound methotrexate was considered as the minimum effective concentration based on ex vivo studies (42). Following intraventricular injection or 24-h infusion (24.76 μmol), methotrexate remained unbound concentrations above 2 μmol/L for approximately 30 – 48 h or 44 – 58 h, respectively, in the 3 CSF compartments and adjacent brain tissue (Figure 2C and 3C). On the contrary, a negligible amount of the drug reached the deep brain parenchyma, where the unbound drug Cmax and AUC0–72h were about 3 orders of magnitude lower than those in other CNS compartments (Table 2). These data suggested that intrathecal methotrexate treatment would likely achieve and maintain therapeutic concentrations in the CSF and adjacent brain tissue, but not in the deep brain parenchyma (with > 2 mm distance from the CSF tract).
CNS pharmacokinetics of intrathecal abemaciclib
Abemaciclib, an orally bioavailable, selective, small molecule CDK4/6 inhibitor, is approved as monotherapy or combination with hormone therapy for treating hormone receptor-positive, HER2-negative metastatic breast cancers (43). Abemaciclib exhibits high transcellular passive permeability, with the Papp of 9.1 × 10−6 cm/s across the MDCKII cell monolayer (20). It is a weak substrate of ABCB1 and ABCG2 with the estimated total active efflux clearance of 0.97 L/h at the human BBB (20). Here, abemaciclib represents a model drug with high passive permeability but low active efflux clearance at the BBB.
Since no clinical studies have been reported to administer abemaciclib intrathecally, simulations were performed following a hypothesized dosing regimen, i.e., intraventricular injection of 59.2 μmol (or 30 mg, 10% of the standard daily oral dose) as a single dose or twice weekly for two weeks (Figure 2E and 2F). Following intraventricular injection, abemaciclib immediately reached an excessively high unbound drug Cmax (1658 μmol/L) in the ventricular CSF, from which it rapidly distributed to the cranial and spinal subarachnoid CSF as well as adjacent brain parenchyma, achieving the unbound drug Cmax of 1.95, 1.22, and 2.45 μmol/L, respectively (Table 2). The overall drug exposure (i.e. unbound drug AUC0–72h) in the 3 CSF compartments and adjacent brain parenchyma was similar, indicating a rapid drug distribution and equilibrium in these CNS compartments. By contrary, the drug distribution to the deep brain parenchyma was slow (Tmax, 32.3 h) and to a negligible extent (with the unbound drug AUC0–72h being < 0.3% of that achieved in the other CNS compartments) (Table 2). Abemaciclib appeared to accumulate and maintain a steady-state drug level despite low (~ 0.003 μmol/L) in the deep brain parenchyma following twice weekly intraventricular dosing regimen (Figure 2F).
In comparison with intraventricular injection, intraventricular 24-h infusion resulted in ~ 1000-fold lower Cmax in the ventricular CSF, and slightly lower Cmax (1.1 – 1.7 μmol/L vs. 1.2 – 2.5 μmol/L) in the cranial and spinal subarachnoid CSF and adjacent brain parenchyma (Figure 3E and 3F and Table 2). Notably, intraventricular 24-h infusion and injection resulted in the same overall pharmacologically relevant drug exposure (AUC0–72h) in all CNS compartments (Table 2).
CNS pharmacokinetics of intrathecal tucatinib
Tucatinib, a reversible, highly specific tyrosine kinase inhibitor of HER2, is approved for use in combination with trastuzumab and capecitabine for the treatment of patients with advanced or metastatic HER2-positive breast cancer, including patients with brain metastases. Tucatinib exhibits a high transcellular passive permeability with the Papp of 12.6 × 10−6 cm/s across the MDCKII cell monolayer (19). It is a substrate of ABCB1 and ABCG2, with the estimated active efflux clearance of 11.5 L/h at the human BBB (19). Here, tucatinib serves as a model drug with high passive permeability and high active efflux clearance at the BBB.
Since no clinical studies of intrathecal tucatinib have been reported, simulations were performed following a hypothesized dosing regimen, i.e., intraventricular injection or 24-h infusion of 125 μmol (or 60 mg, 10% of the standard daily oral dose) as a single dose or twice weekly for 2 weeks (Figure 2G, 2H, 3G, and 3H). Immediately following intraventricular injection, tucatinib reached the unbound drug Cmax of 4000 μmol/L in the ventricular CSF, from which the drug rapidly distributed to the cranial subarachnoid CSF and adjacent brain parenchyma, achieving the unbound Cmax of 12.5 and 12.7 μmol/L, respectively; simultaneously, it relatively slowly distributed into the spinal subarachnoid CSF (unbound Cmax, 3.2 μmol/L) and the deep brain parenchyma (unbound Cmax, 0.01 μmol/L) (Table 2). Tucatinib achieved similar overall drug exposure (i.e. AUC0–72h) in the 3 CSF compartments and adjacent brain parenchyma, indicating a rapid drug distribution and equilibrium in these CNS compartments; whereas, a negligible amount of the drug reached the deep parenchyma compartment (Table 2). The drug shared similar elimination T1/2 (~ 4.4 h) from the ventricular CSF and two brain parenchyma compartments, while it was relatively slowly eliminated from the cranial and spinal subarachnoid CSF (T1/2, 5.5 – 6.0 h) (Figure 2G and Table 2).
As compared to intraventricular injection, intraventricular 24-h infusion (125 μmol) resulted in considerably lower unbound drug Cmax in all CNS compartments (e.g., ~ 1500-fold lower Cmax in the ventricular CSF), while maintaining similar overall pharmacologically relevant drug exposure (AUC0–72h) in all CNS compartments (Figure 3G and 3H and Table 2). The drug did not accumulate in the CNS following twice weekly intraventricular injection or infusion (Figure 2H and 3H).
Key determinants of intrathecal drug penetration into the deep brain parenchyma
The penetration and disposition of intraventricularly administered drug in the deep brain parenchyma is driven by the collective contributions of multiple simultaneous physiological processes, specifically, paravascular convective bulk flows through the glymphatic system and drug transport at the BBB via bidirectional passive permeability and/or transporter-mediated active efflux clearance. Simulations were performed to quantitatively assess the impacts of convective bulk flow rates, BBB passive permeability, and BBB active efflux clearance on the unbound drug concentration – time profiles of 4 model drugs in the deep brain parenchyma following a single-dose intraventricular injection (Figure 4). In general, as paravascular convective bulk flow rates increased, the rate and extent of intrathecal drug penetration into the deep parenchyma would increase (Figure 4A, 4B, 4C, 4D). Higher BBB passive permeability would lead to faster drug deep brain penetration but to a less extent (Figure 4E, 4F, 4G, 4H). Notably, the magnitude of impact of each process was dependent on the drug properties. Specifically, convective bulk flow rates and BBB passive permeability had profound impacts on the deep brain drug penetration of trastuzumab and abemaciclib, both drugs with insignificant active efflux clearance at the BBB (Figure 4A, 4B, 4E, 4F). By contrast, the impacts were less apparent for methotrexate and tucatinib, both drugs showing high active efflux clearance at the BBB (Figure 4C, 4D, 4G, 4H). For these drugs, the change of BBB active efflux clearance had a predominant impact on the drug deep brain penetration; specifically, the decrease of active efflux clearance at the BBB would enhance the extent of penetration while decreasing the rate of penetration (Figure 4I and 4J).
Figure 4.
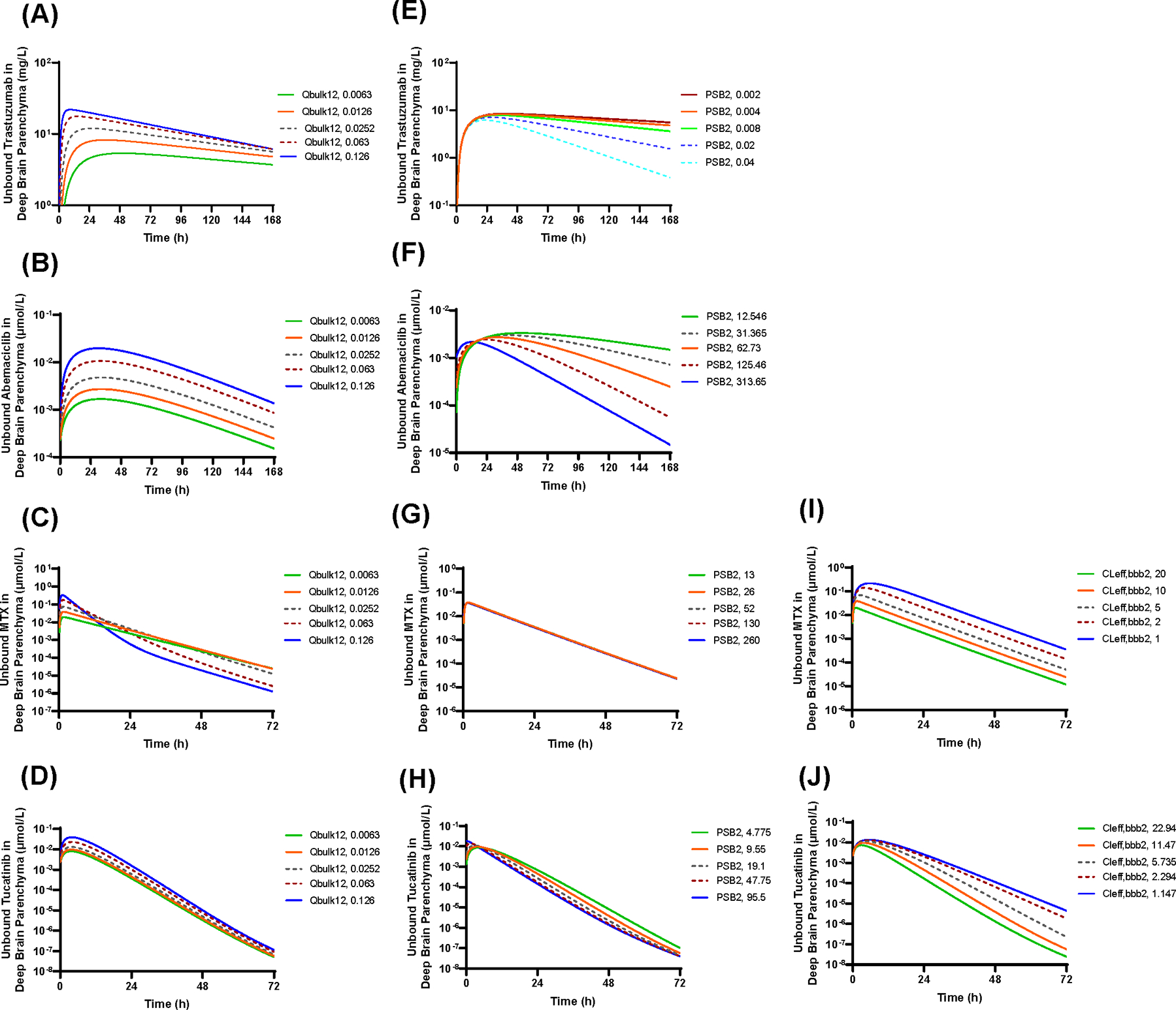
Key determinants of the intrathecal drug penetration into the deep brain parenchyma. (A-D) Impact of the paravascular convective bulk flow rates (Qbulk,12) on the unbound drug concentration – time profiles of trastuzumab (A), methotrexate (B), abemaciclib (C), and tucatinib (D) in the deep brain parenchyma following intraventricular injection. (E-H) Impact of BBB passive permeability (PSB2) on the unbound drug concentration – time profiles of trastuzumab (E), methotrexate (F), abemaciclib (G), and tucatinib (H) in the deep brain parenchyma following intraventricular injection. (I and J) Impact of BBB active efflux clearance (CLeff,bbb2) on the unbound drug concentration – time profiles of methotrexate (I) and tucatinib (J) in the deep brain parenchyma following intraventricular injection.
DISCUSSION
Using a novel CNS PBPK modeling approach, we demonstrate that intraventricularly administered antibody and small-molecule drugs exhibit distinct temporal and spatial distribution in the human CNS. Generally, intrathecal administration of both antibody and small-molecule drugs achieves supratherapeutic or therapeutic drug concentrations in the CSF compartments and adjacent brain tissue (< 2 mm away from the CSF tract). While negligible amounts of intrathecal small-molecule drugs penetrate the deep brain parenchyma, intrathecal antibody drugs (such as trastuzumab) penetrate to a larger extent and may achieve therapeutically effective drug exposure in the deep brain parenchyma.
The mechanistic 6-CNS PBPK model considers both simple diffusion and paravascular convective bulk flow as the mechanisms for drug distribution from CSF to the brain parenchyma (12). While drug distribution to the brain tissue adjacent to the CSF tract is mainly driven by simple diffusion, drug penetration from CSF into the deep brain parenchyma predominantly relies on the paravascular convective bulk flow. The directionality of CSF flow along paravascular spaces is driven by arterial pulsation, which allows the influx of cranial subarachnoid CSF along with solutes/drug molecules from periarterial spaces into the brain parenchyma interstitial space and subsequent clearance of the CSF/ISF mixture through perivenous spaces back to the cranial subarachnoid space (14,16,44). Convective bulk flow along paravascular spaces has been confirmed by the clearance of intra-parenchymally injected radiolabeled tracers of different molecular weight (spanning a five-fold range in diffusion coefficient) from the rat brain at similar rates (29). The calculated CSF/ISF outflow rates range from 0.10 to 0.29 μl·min−1·g brain−1 in the different regions of rat or rabbit brain (29). Based on these values, the average paravascular convective bulk flow rate is estimated to be 0.0126 L/h in a typical person (with brain weight of 1400 g), which is used in the PBPK modeling.
The drug would interact with the BBB during its passage through the paravascular space and brain interstitial space. As illustrated by the PBPK model simulations, the passive permeability and active efflux clearance at the BBB have profound impacts on intrathecal drug penetration into the deep brain parenchyma (Figure 4). If the drug readily moves across the BBB due to either high bi-directional transcellular passive permeability (e.g., abemaciclib) or high transporter-mediated active efflux clearance at the BBB (e.g., methotrexate) or both mechanisms (e.g, tucatinib), it would be rapidly cleared to the cerebral or systemic circulation during its passage through the paravascular space; therefore, a negligible amount of the drug would be expected to reach the deep brain parenchyma from CSF. For such drugs, intrathecal administration would not provide an advantage to drug delivery to the deep brain parenchyma over the systemic intravenous or oral administration. On the contrary, antibody drugs (e.g., trastuzumab) exhibit poor BBB permeability in either direction (from the blood to brain or the brain to blood) because of the extremely low transcellular passive permeability (due to large molecular size) and no affinity to BBB uptake or efflux transporters. The 6-CNS PBPK model suggests that the ratio of BBB permeability (the sum of apparent passive permeability clearance and active efflux clearance at the BBB) to paravascular convective bulk flow rate is 0.33 for trastuzumab, as compared to 802, 370, and 1606 for methotrexate, abemaciclib, and tucatinib, respectively (Table 1 and Supplementary Table S1). Therefore, it is not surprising that intrathecal antibody drug trastuzumab penetrates the deep brain parenchyma to a larger extent and maintains there much longer, as compared to small-molecule drugs (Table 2). The PBPK model simulations are in line with published experimental data demonstrating that the paravascular convective fluid flow effectively delivers high-molecular weight tracers (with low BBB permeability and no interaction with BBB transporters) from CSF to the whole mouse brain including the deep brain parenchyma (14). Collectively, the PBPK modeling, along with a growing body of experimental evidence (14,45,46) support further clinical evaluation of intrathecal administration as a potential effective route to enhance delivery of antibody drugs into the deep brain parenchyma by harnessing the paravascular convective bulk flow (or glymphatic pathway).
Notably, the PBPK model simulations indicate that as paravascular convective bulk flow rate increases, the antibody drug trastuzumab penetrates the deep brain parenchyma quicker and to a larger extent (Figure 4A). Interestingly, animal studies demonstrate that paravascular convective bulk flows increase under conditions where the brain interstitial space is expanded as can occur during sleep or in the cerebral edema area surrounding brain tumors (10,47). This has important therapeutic implications suggesting that intrathecal chemotherapy with antibody drugs may be more efficacious to treat parenchyma brain tumors when they are administered during sleep. Further studies are needed to test this hypothesis.
The distinct CNS pharmacokinetic characteristics of intrathecally administered antibody and small-molecule drugs provide a pharmacological explanation for controversial clinical outcomes of intrathecal chemotherapy. Given the supratherapeutic or therapeutic drug concentrations achieved in the CSF compartments and adjacent brain tissue following intrathecal administration, it is not surprising that intrathecal therapy with either small-molecule or antibody drugs shows clinically meaningful intracranial activity and overall survival benefits in patients with neoplastic meningitis where tumor cells spread to the CSF and adjacent tissue (4,8,9). As the proof-of-concept, the PBPK modeling demonstrates that intrathecally administered three small-molecule model drugs (including methotrexate, abemaciclib, and tucatinib) all achieve supratherapeutic or therapeutic drug concentrations in the CSF compartments and adjacent brain tissue. However, it should be noted that only water-soluble drugs (e.g., methotrexate and cytarabine) are suitable for clinical use as intrathecal therapy for prevention or treatment of neoplastic meningitis. This is mainly because appropriate drug formulations (i.e., preservative-free and organic solvent-free drug solution at a physiological pH and osmolarity) are required for intrathecal drug administration (48), while such formulations are not feasible for hydrophobic drugs such as abemaciclib and tucatinib.
In line with the PBPK model simulations showing that intrathecal antibody (but not small-molecular drugs) may achieve therapeutically effective concentrations in the deep brain parenchyma, clinically meaningful intracranial efficacy or overall survival benefit has been demonstrated for intrathecal therapy with antibody drugs, but not small-molecule drugs, in patients with parenchyma brain tumors (35,38). Notably, a recently published phase I/II study demonstrated that following intraventricular injection of trastuzumab at the recommended phase II dose 80 mg twice weeks, the median overall survival was 10.5 months (95% CI 5.2–20.9), as compared to the historical median overall survival of 3 – 4 months, in heavily pretreated HER2-positive breast cancer patients with leptomeningeal metastases and concurrent brain parenchyma metastases (35). In addition, intraventricular administration of another antibody drug, rituximab (an anti-CD20 monoclonal antibody), also demonstrated clinical efficacy in heavily pretreated patients with recurrent CNS non-Hodgkin’s lymphoma, in which among 10 patients, 6 exhibited meningeal response, 2 had intraocular response, and 1 exhibited resolution of parenchyma metastasis (38). Collectively, the CNS PBPK modeling, in accordance with the observed favorable clinical efficacy, supports further clinical investigation of intrathecal antibody drugs for the treatment of not only neoplastic meningitis but also brain parenchyma tumors.
Intrathecal chemotherapy, often given by intraventricular injection via the Ommaya intraventricular reservoir, is commonly used to treat leptomeningeal metastases, a devastating complication of advanced cancer with the most common primary cancer being breast cancer, lung cancer, melanoma, and gastrointestinal malignancies (49). However, intrathecal chemotherapy (such as methotrexate and cytarabine) frequently cause symptoms of meningeal irritation, and occasionally cases of weakness and paralysis and rare instances of severe encephalopathy may occur (50,51). The neurologic toxicities and complications are likely attributable to the supratherapeutic and potentially toxic drug concentrations achieved in the CSF compartments and adjacent brain tissue following intraventricular injection, as revealed by the CNS PBPK modeling (Figure 2 and Table 2). By contrary, intraventricular 24-h infusion produced lower, yet potential therapeutic effective, drug peak concentrations in the CSF and adjacent brain tissue, while maintaining prolonged and equivalent overall drug exposure in all CNS compartments (Figure 3 and Table 2). Hence, 24-h intraventricular infusion may mitigate neurotoxicity associated with post-injection supratherapeutic or toxic drug concentrations in the CNS, while retaining potential efficacy.
In conclusion, the CNS PBPK modeling, in line with available clinical efficacy data, confirms the therapeutic value of intrathecal chemotherapy with antibody or small molecule drugs for treating neoplastic meningitis and warrants further clinical investigation of intrathecal antibody drugs to treat brain parenchyma tumors. In addition, the PBPK modeling supports further randomized clinical studies to compare the safety and efficacy of intraventricular injection and 24-h infusion; the obtained results will provide critical information to guide clinical practice for efficacious and safer use of intrathecal chemotherapy.
Supplementary Material
Translational Relevance.
The optimal use of intrathecal chemotherapy for brain cancer treatment is hindered by the lack of quantitative understanding of the CNS pharmacokinetics of intrathecally administered antibody or small-molecule drugs. Particularly, the temporal and spatial drug distribution and disposition in the human brain parenchyma remains poorly understood, as it is difficult to be measured in patients due to the challenge of sampling and limitation of analytical technologies. To resolve this gap of knowledge, we developed a novel CNS PBPK model platform for mechanistic prediction of CNS pharmacokinetics. We demonstrated that intrathecally administered antibody and small-molecule drugs exhibited distinct temporal and spatial distribution and disposition in the human CNS. Our study shed key pharmacological insights into the controversial clinical efficacy, and moreover, provided critical information to guide selection of the right drugs and optimal dosing regiments for efficacious and safer use of intrathecal chemotherapy to treat neoplastic meningitis or brain parenchyma tumors.
Acknowledgement
This study is supported by the National Cancer Institute of the National Institute of Health (NIH) R01CA255124 (JL) and NIH Cancer Center Support Grant P30CA022453 (Karmanos Cancer Institute Pharmacology and Metabolomics Core, Biostatistics and Bioinformatics Core).
Footnotes
Conflict of Interest: The authors declare no potential conflict of interest.
References
- 1.Miller JJ, Wen PY. Emerging targeted therapies for glioma. Expert Opin Emerg Drugs 2016;21(4):441–52 doi 10.1080/14728214.2016.1257609. [DOI] [PubMed] [Google Scholar]
- 2.Henderson JT, Piquette-Miller M. Blood-brain barrier: an impediment to neuropharmaceuticals. Clin Pharmacol Ther 2015;97(4):308–13 doi 10.1002/cpt.77. [DOI] [PubMed] [Google Scholar]
- 3.Bhowmik A, Khan R, Ghosh MK. Blood brain barrier: a challenge for effectual therapy of brain tumors. Biomed Res Int 2015;2015:320941 doi 10.1155/2015/320941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arkenau HT, Chong G, Cunningham D, Watkins D, Agarwal R, Sirohi B, et al. The role of intrathecal chemotherapy prophylaxis in patients with diffuse large B-cell lymphoma. Ann Oncol 2007;18(3):541–5 doi 10.1093/annonc/mdl434. [DOI] [PubMed] [Google Scholar]
- 5.Lu NT, Raizer J, Gabor EP, Liu NM, Vu JQ, Slamon DJ, et al. Intrathecal trastuzumab: immunotherapy improves the prognosis of leptomeningeal metastases in HER-2+ breast cancer patient. J Immunother Cancer 2015;3:41 doi 10.1186/s40425-015-0084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zagouri F, Zoumpourlis P, Le Rhun E, Bartsch R, Zografos E, Apostolidou K, et al. Intrathecal administration of anti-HER2 treatment for the treatment of meningeal carcinomatosis in breast cancer: A metanalysis with meta-regression. Cancer Treat Rev 2020;88:102046 doi 10.1016/j.ctrv.2020.102046. [DOI] [PubMed] [Google Scholar]
- 7.Oberkampf F, Gutierrez M, Trabelsi Grati O, Le Rhun E, Tredan O, Turbiez I, et al. Phase II study of intrathecal administration of trastuzumab in patients with HER2-positive breast cancer with leptomeningeal metastasis. Neuro Oncol 2023;25(2):365–74 doi 10.1093/neuonc/noac180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bleyer WA, Poplack DG. Intraventricular versus intralumbar methotrexate for central-nervous-system leukemia: prolonged remission with the Ommaya reservoir. Med Pediatr Oncol 1979;6(3):207–13 doi 10.1002/mpo.2950060304. [DOI] [PubMed] [Google Scholar]
- 9.Sullivan MP, Humphrey GB, Vietti TJ, Haggard ME, Lee E. Superiority of conventional intrathecal methotrexate therapy with maintenance over intensive intrathecal methotrexate therapy, unmaintained, or radiotherapy (2000–2500 rads tumor dose) in treatment for meningeal leukemia. Cancer 1975;35(4):1066–73 doi . [DOI] [PubMed] [Google Scholar]
- 10.Hladky SB, Barrand MA. Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 2014;11(1):26 doi 10.1186/2045-8118-11-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pardridge WM. CSF, blood-brain barrier, and brain drug delivery. Expert Opin Drug Deliv 2016;13(7):963–75 doi 10.1517/17425247.2016.1171315. [DOI] [PubMed] [Google Scholar]
- 12.Groothuis DR, Vavra MW, Schlageter KE, Kang EW, Itskovich AC, Hertzler S, et al. Efflux of drugs and solutes from brain: the interactive roles of diffusional transcapillary transport, bulk flow and capillary transporters. J Cereb Blood Flow Metab 2007;27(1):43–56 doi 10.1038/sj.jcbfm.9600315. [DOI] [PubMed] [Google Scholar]
- 13.Blasberg RG, Patlak C, Fenstermacher JD. Intrathecal chemotherapy: brain tissue profiles after ventriculocisternal perfusion. J Pharmacol Exp Ther 1975;195(1):73–83. [PubMed] [Google Scholar]
- 14.Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med 2012;4(147):147ra11 doi 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuroscience Nedergaard M.. Garbage truck of the brain. Science 2013;340(6140):1529–30 doi 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mestre H, Hablitz LM, Xavier AL, Feng W, Zou W, Pu T, et al. Aquaporin-4-dependent glymphatic solute transport in the rodent brain. Elife 2018;7 doi 10.7554/eLife.40070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao P, Zhang L, Grillo JA, Liu Q, Bullock JM, Moon YJ, et al. Applications of physiologically based pharmacokinetic (PBPK) modeling and simulation during regulatory review. Clin Pharmacol Ther 2011;89(2):259–67 doi 10.1038/clpt.2010.298. [DOI] [PubMed] [Google Scholar]
- 18.Poulin P, Theil FP. Prediction of pharmacokinetics prior to in vivo studies. II. Generic physiologically based pharmacokinetic models of drug disposition. J Pharm Sci 2002;91(5):1358–70 doi 10.1002/jps.10128. [DOI] [PubMed] [Google Scholar]
- 19.Li J, Jiang J, Bao X, Kumar V, Alley SC, Peterson S, et al. Mechanistic Modeling of Central Nervous System Pharmacokinetics and Target Engagement of HER2 Tyrosine Kinase Inhibitors to Inform Treatment of Breast Cancer Brain Metastases. Clin Cancer Res 2022;28(15):3329–41 doi 10.1158/1078-0432.CCR-22-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li J, Jiang J, Wu J, Bao X, Sanai N. Physiologically Based Pharmacokinetic Modeling of Central Nervous System Pharmacokinetics of CDK4/6 Inhibitors to Guide Selection of Drug and Dosing Regimen for Brain Cancer Treatment. Clin Pharmacol Ther 2021;109(2):494–506 doi 10.1002/cpt.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Wu J, Bao X, Honea N, Xie Y, Kim S, et al. Quantitative and Mechanistic Understanding of AZD1775 Penetration across Human Blood-Brain Barrier in Glioblastoma Patients Using an IVIVE-PBPK Modeling Approach. Clin Cancer Res 2017;23(24):7454–66 doi 10.1158/1078-0432.CCR-17-0983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamoto Y, Valitalo PA, Wong YC, Huntjens DR, Proost JH, Vermeulen A, et al. Prediction of human CNS pharmacokinetics using a physiologically-based pharmacokinetic modeling approach. Eur J Pharm Sci 2018;112:168–79 doi 10.1016/j.ejps.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Chang HY, Wu S, Meno-Tetang G, Shah DK. A translational platform PBPK model for antibody disposition in the brain. J Pharmacokinet Pharmacodyn 2019;46(4):319–38 doi 10.1007/s10928-019-09641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Badhan RK, Chenel M, Penny JI. Development of a physiologically-based pharmacokinetic model of the rat central nervous system. Pharmaceutics 2014;6(1):97–136 doi 10.3390/pharmaceutics6010097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kimelberg HK. Water homeostasis in the brain: basic concepts. Neuroscience 2004;129(4):851–60 doi 10.1016/j.neuroscience.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 26.Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis 2011;128(6):309–16 doi 10.1016/j.anorl.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Cosgrove KP, Mazure CM, Staley JK. Evolving knowledge of sex differences in brain structure, function, and chemistry. Biol Psychiatry 2007;62(8):847–55 doi 10.1016/j.biopsych.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinnatamby CS. Last’s Anatomy. Churchill Livingstone/Elsevier; 2011. [Google Scholar]
- 29.Cserr HF, Cooper DN, Suri PK, Patlak CS. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol 1981;240(4):F319–28 doi 10.1152/ajprenal.1981.240.4.F319. [DOI] [PubMed] [Google Scholar]
- 30.Edsbagge M, Tisell M, Jacobsson L, Wikkelso C. Spinal CSF absorption in healthy individuals. Am J Physiol Regul Integr Comp Physiol 2004;287(6):R1450–5 doi 10.1152/ajpregu.00215.2004. [DOI] [PubMed] [Google Scholar]
- 31.Furubayashi T, Inoue D, Nishiyama N, Tanaka A, Yutani R, Kimura S, et al. Comparison of Various Cell Lines and Three-Dimensional Mucociliary Tissue Model Systems to Estimate Drug Permeability Using an In Vitro Transport Study to Predict Nasal Drug Absorption in Rats. Pharmaceutics 2020;12(1) doi 10.3390/pharmaceutics12010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. IV. A microscopic model of the perivascular distribution. Microvasc Res 1991;41(2):252–72 doi 10.1016/0026-2862(91)90026-8. [DOI] [PubMed] [Google Scholar]
- 33.Wang Q, Rager JD, Weinstein K, Kardos PS, Dobson GL, Li J, et al. Evaluation of the MDR-MDCK cell line as a permeability screen for the blood-brain barrier. Int J Pharm 2005;288(2):349–59 doi 10.1016/j.ijpharm.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Shapiro WR, Young DF, Mehta BM. Methotrexate: distribution in cerebrospinal fluid after intravenous, ventricular and lumbar injections. N Engl J Med 1975;293(4):161–6 doi 10.1056/NEJM197507242930402. [DOI] [PubMed] [Google Scholar]
- 35.Kumthekar PU, Avram MJ, Lassman AB, Lin NU, Lee E, Grimm SA, et al. A phase I/II study of intrathecal trastuzumab in human epidermal growth factor receptor 2-positive (HER2-positive) cancer with leptomeningeal metastases: Safety, efficacy, and cerebrospinal fluid pharmacokinetics. Neuro Oncol 2023;25(3):557–65 doi 10.1093/neuonc/noac195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fleischhack G, Reif S, Hasan C, Jaehde U, Hettmer S, Bode U. Feasibility of intraventricular administration of etoposide in patients with metastatic brain tumours. Br J Cancer 2001;84(11):1453–9 doi 10.1054/bjoc.2001.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zimm S, Collins JM, Miser J, Chatterji D, Poplack DG. Cytosine arabinoside cerebrospinal fluid kinetics. Clin Pharmacol Ther 1984;35(6):826–30 doi 10.1038/clpt.1984.120. [DOI] [PubMed] [Google Scholar]
- 38.Rubenstein JL, Fridlyand J, Abrey L, Shen A, Karch J, Wang E, et al. Phase I study of intraventricular administration of rituximab in patients with recurrent CNS and intraocular lymphoma. J Clin Oncol 2007;25(11):1350–6 doi 10.1200/JCO.2006.09.7311. [DOI] [PubMed] [Google Scholar]
- 39.Pestalozzi BC, Brignoli S. Trastuzumab in CSF. J Clin Oncol 2000;18(11):2349–51 doi 10.1200/JCO.2000.18.11.2349. [DOI] [PubMed] [Google Scholar]
- 40.Bousquet G, Darrouzain F, de Bazelaire C, Ternant D, Barranger E, Winterman S, et al. Intrathecal Trastuzumab Halts Progression of CNS Metastases in Breast Cancer. J Clin Oncol 2016;34(16):e151–5 doi 10.1200/JCO.2012.44.8894. [DOI] [PubMed] [Google Scholar]
- 41.Tokuda Y, Watanabe T, Omuro Y, Ando M, Katsumata N, Okumura A, et al. Dose escalation and pharmacokinetic study of a humanized anti-HER2 monoclonal antibody in patients with HER2/neu-overexpressing metastatic breast cancer. Br J Cancer 1999;81(8):1419–25 doi 10.1038/sj.bjc.6690343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hryniuk WM, Bertino JR. Treatment of leukemia with large doses of methotrexate and folinic acid: clinical-biochemical correlates. J Clin Invest 1969;48(11):2140–55 doi 10.1172/JCI106181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shah M, Nunes MR, Stearns V. CDK4/6 Inhibitors: Game Changers in the Management of Hormone Receptor-Positive Advanced Breast Cancer? Oncology (Williston Park) 2018;32(5):216–22. [PMC free article] [PubMed] [Google Scholar]
- 44.Hadaczek P, Yamashita Y, Mirek H, Tamas L, Bohn MC, Noble C, et al. The “perivascular pump” driven by arterial pulsation is a powerful mechanism for the distribution of therapeutic molecules within the brain. Mol Ther 2006;14(1):69–78 doi 10.1016/j.ymthe.2006.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Calias P, Banks WA, Begley D, Scarpa M, Dickson P. Intrathecal delivery of protein therapeutics to the brain: a critical reassessment. Pharmacol Ther 2014;144(2):114–22 doi 10.1016/j.pharmthera.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Naseri Kouzehgarani G, Feldsien T, Engelhard HH, Mirakhur KK, Phipps C, Nimmrich V, et al. Harnessing cerebrospinal fluid circulation for drug delivery to brain tissues. Adv Drug Deliv Rev 2021;173:20–59 doi 10.1016/j.addr.2021.03.002. [DOI] [PubMed] [Google Scholar]
- 47.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science 2013;342(6156):373–7 doi 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cook AM, Mieure KD, Owen RD, Pesaturo AB, Hatton J. Intracerebroventricular administration of drugs. Pharmacotherapy 2009;29(7):832–45 doi 10.1592/phco.29.7.832. [DOI] [PubMed] [Google Scholar]
- 49.Kaplan JG, DeSouza TG, Farkash A, Shafran B, Pack D, Rehman F, et al. Leptomeningeal metastases: comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol 1990;9(3):225–9 doi 10.1007/BF02341153. [DOI] [PubMed] [Google Scholar]
- 50.Fujimoto T [Pharmacokinetics of intrathecal chemotherapy and clinical problems]. Gan To Kagaku Ryoho 1984;11(8):1536–42. [PubMed] [Google Scholar]
- 51.Weigel R, Senn P, Weis J, Krauss JK. Severe complications after intrathecal methotrexate (MTX) for treatment of primary central nervous system lymphoma (PCNSL). Clin Neurol Neurosurg 2004;106(2):82–7 doi 10.1016/j.clineuro.2003.09.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in this study are available within the paper and its Supplementary Materials. The raw simulation data used to create graphs are available from the corresponding author upon reasonable request.


