Abstract
Heart failure with preserved ejection fraction (HFpEF) accounts for over 50% of all heart failure cases nationwide and continues to rise in its prevalence. The complex, multi‐organ involvement of the HFpEF clinical syndrome requires clinicians and investigators to adopt an integrative approach that considers the contribution of both cardiac and non‐cardiac function to HFpEF pathophysiology. Thus, this symposium review outlines the key points from presentations covering the contributions of disease‐related changes in cardiac function, arterial stiffness, peripheral vascular function, and oxygen delivery and utilization to exercise tolerance in patients with HFpEF. While many aspects of HFpEF pathophysiology remain poorly understood, there is accumulating evidence for a decline in vascular health in this patient group that may be remediable through pharmacological and lifestyle interventions and could improve outcomes and clinical status in this ever‐growing patient population.
Keywords: blood flow, exercise training, heart failure, vascular physiology
-
What is the topic of this review?
This symposium review provides an integrative view of how disease‐related changes in cardiac, vascular and skeletal muscle function contribute to exercise intolerance in patients with heart failure with preserved ejection fraction (HFpEF).
-
What advances does it highlight?
Emerging evidence continues to highlight the importance of considering both cardiac and non‐cardiac abnormalities in patients with HFpEF, with accumulating evidence for ‘plasticity’ in vascular function that emphasizes the need for a greater focus on strategies targeting the peripheral circulation as a means to improve exercise intolerance.
1. INTRODUCTION
Heart failure with preserved ejection fraction (HFpEF) accounts for over 50% of all heart failure cases in the United States and continues to rise (Tsao et al., 2018). This clinical syndrome is associated with poor quality of life and a dismal prognosis, with a 5‐year mortality rate that ranges from 50% to 75% (Dunlay et al., 2017). Dyspnoea upon exertion and severe exercise intolerance are defining features of HFpEF, symptoms that derive from disease‐related changes in multiple organ systems (Nayor et al., 2020). Indeed, beyond disease‐related changes in cardiac function such as elevated atrial filling pressure (Borlaug et al., 2010; Pfeffer et al., 2019; Reddy et al., 2018; Samuel et al., 2021) and chronotropic incompetence (Borlaug et al., 2010, Borlaug, Kane et al., 2016; Haykowsky et al., 2011), there is emerging evidence for abnormalities in vascular, skeletal muscle and pulmonary function that each appear to contribute to exercise intolerance in this patient group (Nayor et al., 2020) (Figure 1). The heterogeneous involvement of organ systems likely stems from the ‘constellation of comorbidities’ that characterize the HFpEF phenotype, including hypertension, pulmonary disease, type‐2 diabetes mellitus, morbid obesity and physical inactivity (Deichl et al., 2022). Facing the combined effect of multi‐organ involvement and polymorbidity, the search for effective treatment of HFpEF has yielded largely negative results. Indeed, while a small number of studies have identified the efficacy of pharmacological and lifestyle interventions on select physiological parameters, the majority of randomized clinical trials have been largely ineffective in identifying strategies for improving hard clinical outcomes in patients with HFpEF (Redfield & Borlaug, 2023). Thus, the complexity of this unique patient group continues to pose significant challenges in determining both underlying pathophysiology and in optimizing clinical care of patients with HFpEF.
FIGURE 1.
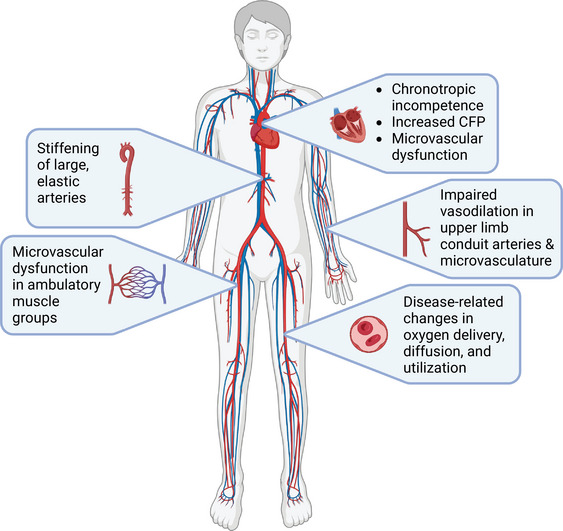
Overview of disease‐related changes in cardiac, vascular and skeletal muscle function that contribute to exercise intolerance in patients with HFpEF. CFP, cardiac filling pressure. Created with Biorender.com.
With recognition of the need to adopt an integrative approach when investigating HFpEF, the symposium ‘Exercise Intolerance in Heart Failure with a Preserved Ejection Fraction (HFpEF): Causes, Consequences, and the Journey Towards a Cure’, which took place at the ACSM 2022 World Congress on the Basic Science of Exercise and Vascular Health, brought together experts in several areas of physiology to explore how disease‐related changes in cardiac, vascular, and skeletal muscle function contribute to exercise intolerance in HFpEF. Select studies utilizing unique therapeutic approaches for mitigating vascular dysregulation in this clinical population were also presented, with an overall focus on new strategies for improving physical function and exercise capacity. Highlighted below are the salient points from each presentation, which together provided a comprehensive and contemporary view of ongoing efforts in further establishing the causes and consequences of exercise intolerance in patients with HFpEF, as well as an update on the journey towards improving outcomes in this ever‐growing patient group.
2. CARDIAC FUNCTION IN HFpEF
Abnormalities in cardiac structure, function and reserve each play a pivotal role in the pathophysiology of exercise intolerance in HFpEF (Pfeffer et al., 2019). Chronotropic incompetence and impairments in stroke volume reserve both limit the rise in cardiac output (CO) during exercise in HFpEF (Borlaug et al., 2010, Borlaug, Kane et al., 2016; Haykowsky et al., 2011), the latter of which is largely explained by abnormalities in systolic function (Borlaug et al., 2010; Haykowsky et al., 2011; Pandey et al., 2018) related to both contractile dysfunction and cardiac afterload (Borlaug et al., 2010; Reddy et al., 2017). Emerging evidence also implicates left atrial (LA) dysfunction as an important pathophysiological mechanism driving exercise intolerance in HFpEF, with impaired LA function and increased LA stiffness associated with abnormal exercise haemodynamics and peak oxygen uptake (Freed et al., 2016; Kusunose et al., 2012; Singleton et al., 2022). Similar observations have also been made with right atrial function and reserve (Kagami et al., 2022).
In addition to limitations in cardiac reserve, impairments in left ventricular diastolic compliance, relaxation reserve, and pericardial restraint lead to a marked rise in pulmonary capillary wedge pressure (PCWP) during exercise in HFpEF (Borlaug et al., 2010; Pfeffer et al., 2019; Reddy et al., 2018; Samuel et al., 2021). However, whether such steep elevations in cardiac filling pressures independently limits exercise remains incompletely understood, with at least one recent report dissociating the rise in PCWP from exercise tolerance (Sarma et al., 2022). Increases in PCWP during exercise, however, may cause lung congestion that could acutely promote dyspnoea (Obokata et al., 2018; Reddy et al., 2019). For example, using semi‐quantitative lung ultrasound, Simonovic et al. (2021) found that submaximal exercise increased the extent of B‐line formation (a marker of extravascular lung water) in HFpEF, coincident with significant variations in natriuretic peptides, greater tricuspid regurgitation, and diastolic dysfunction (i.e., E/e′). Likewise, Burrage et al. (2021) found a significant increase in lung water content in HFpEF following submaximal exercise (20 W), measured using a novel pulmonary proton density magnetic resonance imaging sequence. Moreover, using computed tomography, an increase in lung congestion immediately following submaximal exercise in HFpEF has been reported that appears to coincide with measurable impairments in lung diffusion capacity (Fermoyle et al., 2020, 2021). Thus, with recognition that the mechanisms responsible for exercise intolerance in HFpEF extend beyond the derangements in cardiac function that characterize this patient group, the contribution of abnormalities in cardiac structure, function and reserve should not be overlooked.
3. CORONARY MICROVASCULAR FUNCTION IN HFpEF
In patients with HFpEF, the presence of endothelium‐dependent and ‐independent coronary microvascular dysfunction is documented by reduced coronary blood flow response to intracoronary infusion of acetylcholine using Doppler flow wire with quantitative angiography (Yang et al., 2020) and an attenuated hyperaemic increase in coronary flow reserve in response to adenosine administration (Arnold et al., 2022; Yang et al., 2020). Similar to the peripheral vasculature, coronary microvascular endothelial dysfunction is thought to be mediated, in part, by oxidative stress and reduced nitric oxide (NO) bioavailability (Franssen et al., 2016). Interestingly, while there is a lack of relationship between endothelium‐dependent microvascular function and cardiac haemodynamics (Yang et al., 2020), endothelium‐independent coronary microvascular function has been associated with long‐term clinical outcomes (Arnold et al., 2022), mortality risks (Yang et al., 2020), and exertional haemodynamic abnormalities (i.e., higher filling pressure at peak exercise) and exercise intolerance in patients with HFpEF (Ahmad et al., 2021; Mahfouz et al., 2020). Therefore, given that patients with HFpEF may present with endothelial dysfunction at both the conduit artery and microvascular levels (discussed below), identification of strategies to improve this aspect of HFpEF‐related pathophysiology is of clinical significance.
4. ARTERIAL STIFFNESS AND AORTIC HAEMODYNAMICS IN HFpEF
Large elastic arteries, particularly the proximal aorta, play an important role in buffering oscillations in arterial blood pressure and blood flow that occur in response to LV contraction (Schultz et al., 2013). Age‐ and disease‐associated large artery stiffening and increased peripheral resistance decrease the buffering capacity of the proximal aorta, resulting in a greater mid‐late systolic afterload (augmentation pressure) and pulse pressure for any given stroke volume (Baksi et al., 2009). Patients with HFpEF have marked arterial stiffening beyond that observed with ageing (Hundley et al., 2001) and hypertension (Desai et al., 2009), or heart failure with a reduced ejection fraction (HFrEF) (Balmain et al., 2007). In patients with HFpEF, elevations in markers of arterial stiffness and associated wave reflections are associated with impaired systolic and diastolic function at rest and adverse cardiovascular outcomes (Namasivayam et al., 2022). Interestingly, while resting measures of arterial stiffness are poor independent predictors of functional capacity in HFpEF (Hundley et al., 2001; Kitzman, Herrington et al., 2013; Tartière‐Kesri et al., 2012; Zern et al., 2021), exercise unmasks a pathophysiological interaction between arterial stiffness, stroke volume and peripheral resistance (Reddy et al., 2017; Tartière‐Kesri et al., 2012) that contributes to greater wave reflection, augmentation pressure, pulse pressure and mid/late‐systolic LV afterload in HFpEF (Reddy et al., 2017; Tartière‐Kesri et al., 2012; Zern et al., 2021). The resulting increase in cardiac afterload is associated with diminished myocardial performance (Chirinos, Segers et al., 2019; Reddy et al., 2017), greater PCWP/CO response to exercise, and lower peak (Zern et al., 2021). However, the extent to which central arterial stiffness and pressure amplification contribute to exercise intolerance may be considerably heterogeneous due to the presence of specific comorbidities (Chirinos, Bhattacharya et al., 2019; Jain et al., 2021) and biological sex (Lau et al., 2022), and thus additional studies are needed to define the contribution of arterial stiffness to specific HFpEF phenotypes.
Several studies have investigated the effect of lifestyle and pharmacological interventions on central arterial stiffness and haemodynamics in patients with HFpEF. Moderate‐intensity aerobic exercise performed for 16–20 weeks showed no improvement in several indices of resting vascular stiffness/compliance or pulse wave velocity (PWV) in elderly patients with HFpEF (Kitzman, Brubaker et al., 2013, 2016). Similarly, neither longer duration (1 year) progressive endurance exercise training (Fujimoto et al., 2012) nor high intensity interval training (Gevaert et al., 2023) improved markers of vascular stiffness in patients with HFpEF. Interestingly, while caloric restriction leading to significant weight loss was insufficient to change arterial pulse wave velocity in older obese patients with HFpEF (Kitzman et al., 2016), the combination of aerobic exercise training and caloric restriction, with the addition of resistance exercise training, was effective in reducing arterial PWV in patients with HFpEF (Brubaker et al., 2022). While this combination of lifestyle interventions suggest that arterial stiffness is a modifiable feature of HFpEF pathophysiology, further investigations are needed to develop additional lifestyle interventions capable of reversing arterial stiffness in HFpEF, and to identify strategies capable of mitigating arterial stiffness earlier in the progression of HFpEF (Hearon, Dias et al., 2022).
The most commonly investigated pharmacological interventions to improve central aortic haemodynamics target the nitrate–nitrite–NO pathway (Chirinos et al., 2017). Inorganic nitrite administration reduces the magnitude of central aortic wave reflections in patients with HFpEF (Chirinos et al., 2017; Zamani et al., 2015), but has modest (Zamani et al., 2015) or no effect (Borlaug et al., 2018) on peak and exercise capacity. However, inorganic nitrite may have benefits during submaximal exercise (Borlaug, Melenovsky et al., 2016; Reddy et al., 2017, 2021), including improved oxygen uptake kinetics, cardiac output and lower pressure augmentation profile likely due to combined effects of nitrite on pulmonary, cardiac and peripheral vasodilatory function (Reddy et al., 2021). Future studies should consider the differential contribution of vascular stiffness within specific phenogroups of HFpEF which may dictate the responsiveness of interventions designed to target vascular stiffness.
5. PERIPHERAL CONDUIT VESSEL FUNCTION IN HFpEF
Conduit artery endothelial dysfunction, assessed via brachial artery flow‐mediated dilatation (FMD) testing, independently predicts cardiovascular disease‐related morbidity and mortality (Yeboah et al., 2009). The vasodilatory response to brachial artery FMD testing is partly mediated by NO (Green et al., 2014), suggesting that FMD may serve as a bioassay for NO bioavailability. Given the continued interest in pharmacotherapies targeting the NO pathway in patients with HFpEF, recent studies have sought to determine the magnitude of endothelial dysfunction, and whether it could be restored, in this patient group. While initial work failed to identify differences in FMD between patients and age‐matched controls in the upper (Gevaert et al., 2023; Haykowsky, Herrington et al., 2013) or lower (Hundley et al., 2007) limbs, more recent work has reported lower FMD values in HFpEF compared to healthy age‐matched (Kishimoto et al., 2017) and hypertensive (Farrero et al., 2014; Marechaux et al., 2016) control subjects. Interestingly, Lee, Barrett‐O'Keefe, Garten et al. (2016) observed that a decrease in %FMD in HFpEF compared to healthy age‐matched controls was no longer evident after the FMD response was normalized for the shear stimulus. While these disparate findings regarding endothelium‐dependent dilatation are most likely attributable to the marked heterogeneity within this patient population, collectively, the majority of studies to date indicate some decrement in conduit artery endothelial function in patients with HFpEF (Ambrosino et al., 2021).
Every 1% change in FMD has been reported to confer a reciprocal ∼13% change in risk for cardiovascular disease events (Inaba et al., 2010), suggesting that strategies seeking to improve endothelial function could provide a significant benefit in patients with HFpEF. Interestingly, neither moderate‐intensity endurance (Kitzman, Brubaker et al., 2013) nor high‐intensity interval (Angadi et al., 2015) exercise training improved FMD in patients with HFpEF, though some caution is warranted in interpretation, as training‐induced changes in vessel diameter and shear stimulus were not evaluated. In contrast, nutraceutical and pharmacological interventions have proven more effective. Both acute antioxidant supplementation (Ratchford et al., 2019) and 7 days of l‐citrulline administration (Ratchford et al., 2023) improved FMD in patients with HFpEF. While there is emerging evidence for favourable effects of newly developed heart failure pharmacotherapies such as sacubitril–valsartan on conduit artery endothelial function in HFrEF (Bunsawat, Ratchford, Alpenglow, Park et al., 2021), the impact of this drug class on vascular outcomes has not been determined in patients with HFpEF. However, expanded use of sacubitril–valsartan and other therapeutics (e.g., soluble guanylate cyclase stimulators and sodium–glucose cotransporter 2 inhibitors) in patients with EF > 40% presents an opportunity to determine the effect of these drugs on vascular health in HFpEF.
6. PERIPHERAL MICROVASCULAR FUNCTION IN HFpEF
Accumulating evidence has demonstrated a disease‐related attenuation in microvascular function in the peripheral circulation of patients with HFpEF, as evidenced by a marked reduction in the reactive hyperaemic response following lower arm cuff occlusion compared to age‐ and sex‐matched control participants (Lee, Barrett‐O'Keefe, Garten et al., 2016) and similarly aged hypertensive control participants (Marechaux et al., 2016). Locomotor muscle microvascular dysfunction, as determined by passive limb movement testing, has also been reported in patients with HFpEF (Francisco et al., 2021). Responses to passive limb movement are predominantly NO‐mediated, suggesting that disease‐related changes in NO signalling could be present, and may contribute to functional limitations (Trinity et al., 2012). Although a recent pilot study seeking to improve NO bioavailability through l‐Cit administration produced modest improvements in lower limb microvascular reactivity in patients with HFpEF, whether peripheral microvascular dysfunction can be mitigated through lifestyle or pharmacological interventions remains a largely unexplored area of investigation (Ratchford et al., 2023).
7. PERIPHERAL DETERMINANTS OF OXYGEN UTILIZATION IN HFpEF
During graded cardiopulmonary exercise testing, functional capacity (peak ) can be split into its components according to the Fick principle to quantify the relative contribution of central () and peripheral (a–vO2 difference) components of exercise intolerance. While lower peak observed during exercise in HFpEF can be interpreted as a central limitation, cardiac output responses during exercise should always be interpreted in the context of metabolic demand (Bhella et al., 2011). When the response to exercise is normalized to (/ slope) in patients with HFpEF, most studies report a preserved or exaggerated / relationship (Bhella et al., 2011; Haykowsky et al., 2011; Namasivayam et al., 2022; Obokata et al., 2017, 2018; Zamani et al., 2020). While these findings are not universal (Abudiab et al., 2013) and are likely to be influenced by body position (supine vs. upright) and comorbidities (Obokata et al., 2017), they do support several investigations indicating that impaired oxygen extraction (a–vO2 difference) is a key determinant of exercise intolerance in HFpEF (Bhella et al., 2011; Dhakal et al., 2015; Haykowsky et al., 2011). Broadly, a–vO2 difference is determined by convective transport of oxygen via blood flow, the diffusive transport of oxygen from red blood cells to the mitochondria, and the utilization of oxygen within the myocyte. HFpEF patients appear to exhibit abnormalities in each of these steps along the oxygen transport cascade, as discussed below.
8. EXERCISING SKELETAL MUSCLE BLOOD FLOW IN HFpEF
Patients with HFpEF consistently demonstrate a blunted fall in systemic vascular resistance during exercise, especially in the upright position, which is a primary contributor to exercise intolerance (Pandey et al., 2018). Investigations utilizing smaller muscle mass exercise that isolate peripheral mechanisms of oxygen transport and utilization independent of central cardiopulmonary limitations have identified striking impairments in the regulation of skeletal muscle blood flow in patients with HFpEF. Indeed, studies utilizing isolated knee‐extensor exercise (up to 15 W, ∼75–90% of peak work rate) indicate that patients with HFpEF have 15–25% lower leg blood flow compared to age‐matched control participants due primarily to a lower vasodilatory response to exercise (Hearon, Samels et al., 2022; Lee, Barrett‐O'Keefe, Nelson et al., 2016). Similarly, investigations employing handgrip exercise (30–45% of maximal voluntary contraction, MVC) documented a 20–40% lower forearm blood flow in patients with HFpEF compared to age‐matched hypertensives that was apparent primarily at higher exercise intensities (Ratchford et al., 2020). However, findings in the forearm are not universal, as another investigation indicates preserved forearm blood flow when compared to age matched and hypertensive controls at ∼70% of MVC (Zamani et al., 2020).
The mechanisms responsible for the decrement in blood flow during exercise are unclear, and may differ according to HFpEF phenotype. For example, patients with HFpEF who are obese have 30–40% lower forearm blood flow compared to those without obesity during progressive, rhythmic handgrip exercise that is accompanied by a marked elevation in proinflammatory cytokines (Ratchford et al., 2022). The degree of adiposity is also associated with poor peripheral oxygen utilization (a–vO2 difference) during handgrip exercise (Zamani et al., 2020). The direct mechanism for obesity‐associated impairments in peripheral oxygen utilization are likely multifactorial and could include the combined effects of adipose tissue on blood flow distribution and skeletal muscle mitochondrial function (Molina et al., 2016). Disease‐related changes in circulating vasoactive substances may also play a prominent role. Indeed, there is now accumulating evidence for sympathetic nervous system (SNS) overactivity at rest (Seravalle et al., 2019) and during exercise (Badrov et al., 2022; Bunsawat, Ratchford, Alpenglow, Ryan et al., 2021) in patients with HFpEF, which may promote excess vasoconstriction and limit the hyperaemic response during exercise. A recent study from Alpenglow et al. (2023) supports this interesting possibility, providing initial evidence for a diminished ability to attenuate SNS‐mediated vasoconstriction in the exercising limb of patients with HFpEF compared to healthy aged‐matched controls. Further, prior work has also identified an important role of non‐adrenergic vasoconstrictor signalling pathways including endothelin‐A (Barrett‐O'Keefe et al., 2015) and the renin–angiotensin–aldosterone system (Wray et al., 2008) in the regulation of exercising muscle blood flow in older, healthy adults. Remarkably, almost nothing is currently known regarding the role of non‐adrenergic signalling in the regulation of muscle blood flow during exercise in patients with HFpEF, and thus further studies are certainly warranted to explore these potential mechanisms of vascular dysregulation, and how it may relate to exercise intolerance, in this patient group.
9. SKELETAL MUSCLE OXYGEN UTILIZATION IN HFpEF
Patients with HFpEF exhibit increased intermuscular fat deposition and poor skeletal muscle functional performance compared to healthy age‐matched controls (Haykowsky, Brubaker et al., 2013), which are associated with reduced peak (Haykowsky et al., 2014, 2018). Further, capillary‐to‐fibre ratio is reduced in HFpEF, which may impair diffusive capacity, and a shift towards greater type II muscle fibres is associated with reduced oxidative capacity and efficiency (Kitzman et al., 2014; Zamani et al., 2021) and slower onset kinetics during submaximal exercise (Hearon et al., 2019; Krustrup et al., 2008). Severe structural, biochemical and bioenergetic abnormalities have been identified in HFpEF, including impaired mitochondrial content, abnormal mitochondrial fusion and reduced activity of citrate synthase and other proteins integral to proper mitochondrial function (Molina et al., 2016; Zamani et al., 2021). The net result of these structural and biochemical alterations observed in HFpEF is a primary impairment in O2 diffusion (Houstis et al., 2018) and/or utilization resulting in depletion of high energy phosphates and early onset of skeletal muscle fatigue (Weiss et al., 2017).
10. EFFECTS OF EXERCISE TRAINING ON DETERMINANTS OF IN HFpEF
Several investigations have been undertaken to examine the effect of supervised exercise training in patients with HFpEF. While the modality, frequency and duration of exercise interventions among these trials varies, supervised exercise training programmes are generally effective at improving functional capacity and quality of life. Indeed, meta‐analyses of randomized supervised exercise training in HFpEF show an increase relative peak of ∼12% (2 ml/kg/min), a clinically meaningful improvement (Keteyian et al., 2010; Nayor et al., 2020; Sachdev et al., 2023). Regarding the mechanism of improvement in functional capacity, exercise training has been strikingly ineffective at improving left ventricular volume, pressure, compliance or function (stroke volume, cardiac output) during exercise (Fu et al., 2016; Fujimoto et al., 2012; Haykowsky et al., 2012; Mueller et al., 2021; Smart et al., 2012; Tucker et al., 2018). Therefore, it appears that non‐cardiac, peripheral adaptations are the primary mechanisms of improved peak after exercise training in patients with HFpEF. Improvements in peak arterial‐venous oxygen content (a–vO2) difference are consistently improved by exercise training and can account for as much as 85% of the improvement in peak (Borlaug et al., 2010; Fu et al., 2016; Fujimoto et al., 2012; Haykowsky et al., 2012; Tucker et al., 2018). However, the mechanisms responsible for improved peripheral oxygen utilization remain poorly understood. Exercise hyperaemia is determined primarily by the resistance vasculature of the skeletal muscle as opposed to the large elastic arteries typically assessed by flow mediated dilatation. Further, the mechanisms that govern exercise vasodilatation are likely more diverse that the mechanisms that govern traditional assessments of conduit vascular function (Hearon & Dinenno, 2016). Therefore, despite the limited ability of exercise training to improve these traditional markers of vascular function (Gevaert et al., 2023; Tucker et al., 2018), habitual exercise has been shown to improve vascular responses during acute bouts of exercise. A small investigation in patients with HFpEF who completed 8 weeks of isolated knee extensor exercise training showed improvements in leg vascular conductance (vasodilatation) during exercise compared to pre‐training values that was associated with an improvement in peak a–vO2 difference and functional capacity (Hearon, Samels et al., 2022). Therefore, some level of vascular plasticity may exist in patients with HFpEF. Less is known regarding the biochemical adaptation of skeletal muscle to exercise training. Findings from a large (n = 100) 20‐week intervention comparing caloric restriction to aerobic exercise training demonstrated that although neither intervention independently reduced intramuscular fat deposition, the change in the ratio of skeletal muscle to intermuscular fat was associated with improvements in peak in patients with HFpEF (Kitzman et al., 2016). However, the combination of aerobic exercise and/or resistance exercise with caloric restriction was effective in lowering intramuscular fat deposition (Brubaker et al., 2022). Whether these markers of skeletal muscle metabolic health are accompanied by improvements in diffusive or oxidative capacity of skeletal muscle remains to be determined.
11. CLINICAL PHENOTYPES OF HFpEF
There is an increasing recognition that HFpEF is not a well‐defined clinical entity, such that patients with HFpEF may present with a combination of cardiovascular, metabolic, renal and/or geriatric conditions (Samson et al., 2016). The heterogeneity in clinical phenotypes of HFpEF contributes to the complex pathophysiology and manifestations that add great challenges to clinical care and warrants increased efforts to incorporate ‘phenomapping’ of this clinical syndrome (Cohen et al., 2020; Kao et al., 2015; Shah et al., 2015). This is important, because patients with HFpEF have a wide range of clinical profiles, including older age, female sex, history of hypertension, diabetes, obesity, atrial fibrillation, chronic kidney disease and coronary artery disease, and present with variable underlying cardiac and non‐cardiac abnormalities in structure and function (Lee et al., 2009; Owan et al., 2006; Shah & Pfeffer, 2012). Therefore, understanding how various clinical phenotypes of HFpEF differentially affect underlying pathophysiological processes will aid in identification of, and responsiveness to, targeted therapeutic interventions in this ever‐growing patient population (Peters et al., 2023).
12. SUMMARY
Exercise intolerance is a hallmark characteristic of patients with HFpEF that is likely the consequence of dysregulation across a spectrum of disease‐related changes in cardiovascular health, including deficits in cardiac function, increased arterial stiffness, peripheral vascular dysfunction, and impairments in oxygen delivery and utilization (Figure 1). Fortunately, there is now promising evidence for ‘plasticity’, particularly in the peripheral circulation, providing a renewed sense of optimism for the potential benefit of lifestyle and pharmacological therapies in this patient group.
AUTHOR CONTRIBUTIONS
All authors conceived and discussed the content of the manuscript, approved the final version of the manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All persons designated as authors qualify for authorship, and all those who qualify for authorship are listed.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Bunsawat, K. , Nelson, M. D. , Hearon Jr., C. M. , & Wray, D. W. (2024). Exercise intolerance in heart failure with preserved ejection fraction: Causes, consequences and the journey towards a cure. Experimental Physiology, 109, 502–512. 10.1113/EP090674
Handling Editor: Toby Mundel
This review was presented at the ‘Exercise Intolerance in Heart Failure with a Preserved Ejection Fraction (HFpEF): Causes, Consequences, and the Journey Towards a Cure’ symposium, which took place at the ACSM 2022 World Congress on the Basic Science of Exercise and Vascular Health, held in San Diego, CA.
REFERENCES
- Abudiab, M. M. , Redfield, M. M. , Melenovsky, V. , Olson, T. P. , Kass, D. A. , Johnson, B. D. , & Borlaug, B. A. (2013). Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. European Journal of Heart Failure, 15(7), 776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad, A. , Corban, M. T. , Toya, T. , Verbrugge, F. H. , Sara, J. D. , Lerman, L. O. , Borlaug, B. A. , & Lerman, A. (2021). Coronary microvascular dysfunction is associated with exertional haemodynamic abnormalities in patients with heart failure with preserved ejection fraction. European Journal of Heart Failure, 23(5), 765–772. [DOI] [PubMed] [Google Scholar]
- Alpenglow, J. K. , Bunsawat, K. , Francisco, M. A. , Craig, J. C. , Iacovelli, J. J. , Ryan, J. J. , & Wray, D. W. (2023). Evidence of impaired functional sympatholysis in patients with heart failure with preserved ejection fraction. American Journal of Physiology‐Heart and Circulatory Physiology, 325(4), H806–H813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosino, P. , Papa, A. , Buonauro, A. , Mosella, M. , Calcaterra, I. , Spedicato, G. A. , Maniscalco, M. , & Di Minno, M. N. D. (2021). Clinical assessment of endothelial function in heart failure with preserved ejection fraction: A meta‐analysis with meta‐regressions. European Journal of Clinical Investigation, 51(8), e13552. [DOI] [PubMed] [Google Scholar]
- Angadi, S. S. , Mookadam, F. , Lee, C. D. , Tucker, W. J. , Haykowsky, M. J. , & Gaesser, G. A. (2015). High‐intensity interval training vs. moderate‐intensity continuous exercise training in heart failure with preserved ejection fraction: A pilot study. Journal of Applied Physiology, 119(6), 753–758. [DOI] [PubMed] [Google Scholar]
- Arnold, J. R. , Kanagala, P. , Budgeon, C. A. , Jerosch‐Herold, M. , Gulsin, G. S. , Singh, A. , Khan, J. N. , Chan, D. C. S. , Squire, I. B. , Ng, L. L. , & McCann, G. P. (2022). Prevalence and prognostic significance of microvascular dysfunction in heart failure with preserved ejection fraction. JACC: Cardiovascular Imaging, 15(6), 1001–1011. [DOI] [PubMed] [Google Scholar]
- Badrov, M. B. , Notarius, C. F. , Keys, E. , & Floras, J. S. (2022). Muscle sympathetic excitatory response to dynamic 1‐leg cycling in heart failure with preserved ejection fraction. JACC: Case Reports, 4(22), 1501–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baksi, A. J. , Treibel, T. A. , Davies, J. E. , Hadjiloizou, N. , Foale, R. A. , Parker, K. H. , Francis, D. P. , Mayet, J. , & Hughes, A. D. (2009). A meta‐analysis of the mechanism of blood pressure change with aging. Journal of the American College of Cardiology, 54(22), 2087–2092. [DOI] [PubMed] [Google Scholar]
- Balmain, S. , Padmanabhan, N. , Ferrell, W. R. , Morton, J. J. , & McMurray, J. J. (2007). Differences in arterial compliance, microvascular function and venous capacitance between patients with heart failure and either preserved or reduced left ventricular systolic function. European Journal of Heart Failure, 9(9), 865–871. [DOI] [PubMed] [Google Scholar]
- Barrett‐O'Keefe, Z. , Ives, S. J. , Trinity, J. D. , Morgan, G. , Rossman, M. J. , Donato, A. J. , Runnels, S. , Morgan, D. E. , Gmelch, B. S. , Bledsoe, A. D. , Richardson, R. S. , & Wray, D. W. (2015). Endothelin‐A‐mediated vasoconstriction during exercise with advancing age. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 70(5), 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhella, P. S. , Prasad, A. , Heinicke, K. , Hastings, J. L. , Arbab‐Zadeh, A. , Adams‐Huet, B. , Pacini, E. L. , Shibata, S. , Palmer, M. D. , Newcomer, B. R. , & Levine, B. D. (2011). Abnormal haemodynamic response to exercise in heart failure with preserved ejection fraction. European Journal of Heart Failure, 13(12), 1296–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlaug, B. A. , Anstrom, K. J. , Lewis, G. D. , Shah, S. J. , Levine, J. A. , Koepp, G. A. , Givertz, M. M. , Felker, G. M. , LeWinter, M. M. , Mann, D. L. , Margulies, K. B. , Smith, A. L. , Tang, W. H. W. , Whellan, D. J. , Chen, H. H. , Davila‐Roman, V. G. , McNulty, S. , Desvigne‐Nickens, P. , Hernandez, A. F. , …, Redfield, M. M. (2018). Effect of inorganic nitrite vs placebo on exercise capacity among patients with heart failure with preserved ejection fraction: The INDIE‐HFpEF randomized clinical trial. Journal of the American Medical Association, 320(17), 1764–1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlaug, B. A. , Kane, G. C. , Melenovsky, V. , & Olson, T. P. (2016). Abnormal right ventricular‐pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. European Heart Journal, 37(43), 3293–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlaug, B. A. , Melenovsky, V. , & Koepp, K. E. (2016). Inhaled sodium nitrite improves rest and exercise hemodynamics in heart failure with preserved ejection fraction. Circulation Research, 119(7), 880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlaug, B. A. , Olson, T. P. , Lam, C. S. , Flood, K. S. , Lerman, A. , Johnson, B. D. , & Redfield, M. M. (2010). Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. Journal of the American College of Cardiology, 56(11), 845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker, P. H. , Nicklas, B. J. , Houston, D. K. , Hundley, W. G. , Chen, H. , Molina, M. , Lyles, W. M. , Nelson, B. , Upadhya, B. , Newland, R. , & Kitzman, D. W. (2022). A randomized, controlled trial of resistance training added to caloric restriction plus aerobic exercise training in obese heart failure with preserved ejection fraction. Circulation: Heart Failure, 16(2), e010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunsawat, K. , Ratchford, S. M. , Alpenglow, J. K. , Park, S. H. , Jarrett, C. L. , Stehlik, J. , Smith, A. S. , Richardson, R. S. , & Wray, D. W. (2021). Sacubitril‐valsartan improves conduit vessel function and functional capacity and reduces inflammation in heart failure with reduced ejection fraction. Journal of Applied Physiology, 130(1), 256–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunsawat, K. , Ratchford, S. M. , Alpenglow, J. K. , Ryan, J. J. , Richardson, R. S. , & Wray, D. W. (2021). Direct assessment of muscle sympathetic nerve activity during exercise in heart failure with preserved ejection fraction: A case report. Journal of Cardiac Failure, 27(1), 114–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burrage, M. K. , Hundertmark, M. , Valkovič, L. , Watson, W. D. , Rayner, J. , Sabharwal, N. , Ferreira, V. M. , Neubauer, S. , Miller, J. J. , Rider, O. J. , & Lewis, A. J. M. (2021). Energetic basis for exercise‐induced pulmonary congestion in heart failure with preserved ejection fraction. Circulation, 144(21), 1664–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirinos, J. A. , Bhattacharya, P. , Kumar, A. , Proto, E. , Konda, P. , Segers, P. , Akers, S. R. , Townsend, R. R. , & Zamani, P. (2019). Impact of diabetes mellitus on ventricular structure, arterial stiffness, and pulsatile hemodynamics in heart failure with preserved ejection fraction. Journal of the American Heart Association, 8(4), e011457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirinos, J. A. , Londono‐Hoyos, F. , Zamani, P. , Beraun, M. , Haines, P. , Vasim, I. , Varakantam, S. , Phan, T. S. , Cappola, T. P. , Margulies, K. B. , Townsend, R. R. , & Segers, P. (2017). Effects of organic and inorganic nitrate on aortic and carotid haemodynamics in heart failure with preserved ejection fraction. European Journal of Heart Failure, 19(11), 1507–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirinos, J. A. , Segers, P. , Hughes, T. , & Townsend, R. (2019). Large‐artery stiffness in health and disease: JACC state‐of‐the‐art review. Journal of the American College of Cardiology, 74(9), 1237–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen, J. B. , Schrauben, S. J. , Zhao, L. , Basso, M. D. , Cvijic, M. E. , Li, Z. , Yarde, M. , Wang, Z. , Bhattacharya, P. T. , Chirinos, D. A. , Prenner, S. , Zamani, P. , Seiffert, D. A. , Car, B. D. , Gordon, D. A. , Margulies, K. , Cappola, T. , & Chirinos, J. A. (2020). Clinical phenogroups in heart failure with preserved ejection fraction: detailed phenotypes, prognosis, and response to spironolactone. JACC: Heart Failure, 8(3), 172–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichl, A. , Wachter, R. , & Edelmann, F. (2022). Comorbidities in heart failure with preserved ejection fraction. Herz, 47(4), 301–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai, A. S. , Mitchell, G. F. , Fang, J. C. , & Creager, M. A. (2009). Central aortic stiffness is increased in patients with heart failure and preserved ejection fraction. Journal of Cardiac Failure, 15(8), 658–664. [DOI] [PubMed] [Google Scholar]
- Dhakal, B. P. , Malhotra, R. , Murphy, R. M. , Pappagianopoulos, P. P. , Baggish, A. L. , Weiner, R. B. , Houstis, N. E. , Eisman, A. S. , Hough, S. S. , & Lewis, G. D. (2015). Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: The role of abnormal peripheral oxygen extraction. Circulation: Heart Failure, 8(2), 286–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlay, S. M. , Roger, V. L. , & Redfield, M. M. (2017). Epidemiology of heart failure with preserved ejection fraction. Nature Reviews Cardiology, 14(10), 591–602. [DOI] [PubMed] [Google Scholar]
- Farrero, M. , Blanco, I. , Batlle, M. , Santiago, E. , Cardona, M. , Vidal, B. , Castel, M. A. , Sitges, M. , Barbera, J. A. , & Perez‐Villa, F. (2014). Pulmonary hypertension is related to peripheral endothelial dysfunction in heart failure with preserved ejection fraction. Circulation: Heart Failure, 7(5), 791–798. [DOI] [PubMed] [Google Scholar]
- Fermoyle, C. C. , Stewart, G. M. , Borlaug, B. A. , & Johnson, B. D. (2020). Effects of exercise on thoracic blood volumes, lung fluid accumulation, and pulmonary diffusing capacity in heart failure with preserved ejection fraction. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 319(5), R602–R609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermoyle, C. C. , Stewart, G. M. , Borlaug, B. A. , & Johnson, B. D. (2021). Simultaneous measurement of lung diffusing capacity and pulmonary hemodynamics reveals exertional alveolar‐capillary dysfunction in heart failure with preserved ejection fraction. Journal of the American Heart Association, 10(16), e019950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco, M. A. , Lee, J. F. , Barrett‐O'Keefe, Z. , Groot, H. J. , Ratchford, S. M. , Bunsawat, K. , Alpenglow, J. K. , Ryan, J. J. , Nativi, J. N. , Richardson, R. S. , & Wray, D. W. (2021). Locomotor muscle microvascular dysfunction in heart failure with preserved ejection fraction. Hypertension, 78(6), 1750–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen, C. , Chen, S. , Unger, A. , Korkmaz, H. I. , De Keulenaer, G. W. , Tschope, C. , Leite‐Moreira, A. F. , Musters, R. , Niessen, H. W. , Linke, W. A. , Paulus, W. J. , & Hamdani, N. (2016). Myocardial microvascular inflammatory endothelial activation in heart failure with preserved ejection fraction. JACC: Heart Failure, 4(4), 312–324. [DOI] [PubMed] [Google Scholar]
- Freed, B. H. , Daruwalla, V. , Cheng, J. Y. , Aguilar, F. G. , Beussink, L. , Choi, A. , Klein, D. A. , Dixon, D. , Baldridge, A. , Rasmussen‐Torvik, L. J. , Maganti, K. , & Shah, S. J. (2016). Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: Importance of left atrial strain. Circulation: Cardiovascular Imaging, 9(3), e003754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu, T. C. , Yang, N. I. , Wang, C. H. , Cherng, W. J. , Chou, S. L. , Pan, T. L. , & Wang, J. S. (2016). Aerobic interval training elicits different hemodynamic adaptations between heart failure patients with preserved and reduced ejection fraction. American Journal of Physical Medicine & Rehabilitation, 95(1), 15–27. [DOI] [PubMed] [Google Scholar]
- Fujimoto, N. , Prasad, A. , Hastings, J. L. , Bhella, P. S. , Shibata, S. , Palmer, D. , & Levine, B. D. (2012). Cardiovascular effects of 1 year of progressive endurance exercise training in patients with heart failure with preserved ejection fraction. American Heart Journal, 164(6), 869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gevaert, A. B. , Böhm, B. , Hartmann, H. , Goovaerts, I. , Stoop, T. , Van De Heyning, C. M. , Beckers, P. J. , Baldassarri, F. , Mueller, S. , Oberhoffer, R. , Duvinage, A. , Haykowsky, M. J. , Wisløff, U. , Adams, V. , Pieske, B. , Halle, M. , & Van Craenenbroeck, E. M. (2023). Effect of training on vascular function and repair in heart failure with preserved ejection fraction. JACC: Heart Failure, 11(4), 454–464. [DOI] [PubMed] [Google Scholar]
- Green, D. J. , Dawson, E. A. , Groenewoud, H. M. , Jones, H. , & Thijssen, D. H. (2014). Is flow‐mediated dilation nitric oxide mediated?: A meta‐analysis. Hypertension, 63(2), 376–382. [DOI] [PubMed] [Google Scholar]
- Haykowsky, M. J. , Brubaker, P. H. , John, J. M. , Stewart, K. P. , Morgan, T. M. , & Kitzman, D. W. (2011). Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. Journal of the American College of Cardiology, 58(3), 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haykowsky, M. J. , Brubaker, P. H. , Morgan, T. M. , Kritchevsky, S. , Eggebeen, J. , & Kitzman, D. W. (2013). Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: Role of lean body mass. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 68(8), 968–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haykowsky, M. J. , Brubaker, P. H. , Stewart, K. P. , Morgan, T. M. , Eggebeen, J. , & Kitzman, D. W. (2012). Effect of endurance training on the determinants of peak exercise oxygen consumption in elderly patients with stable compensated heart failure and preserved ejection fraction. Journal of the American College of Cardiology, 60(2), 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haykowsky, M. J. , Herrington, D. M. , Brubaker, P. H. , Morgan, T. M. , Hundley, W. G. , & Kitzman, D. W. (2013). Relationship of flow‐mediated arterial dilation and exercise capacity in older patients with heart failure and preserved ejection fraction. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 68(2), 161–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haykowsky, M. J. , Kouba, E. J. , Brubaker, P. H. , Nicklas, B. J. , Eggebeen, J. , & Kitzman, D. W. (2014). Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. American Journal of Cardiology, 113(7), 1211–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haykowsky, M. J. , Nicklas, B. J. , Brubaker, P. H. , Hundley, W. G. , Brinkley, T. E. , Upadhya, B. , Becton, J. T. , Nelson, M. D. , Chen, H. , & Kitzman, D. W. (2018). Regional Adipose Distribution and its Relationship to Exercise Intolerance in Older Obese Patients Who Have Heart Failure With Preserved Ejection Fraction. JACC: Heart Failure, 6(8), 640–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearon, C. M., Jr. , Dias, K. A. , MacNamara, J. P. , Hieda, M. , Mantha, Y. , Harada, R. , Samels, M. , Morris, M. , Szczepaniak, L. S. , Levine, B. D. , & Sarma, S. (2022). 1 Year HIIT and Omega‐3 fatty acids to improve cardiometabolic risk in Stage‐A heart failure. JACC: Heart Failure, 10(4), 238–249. [DOI] [PubMed] [Google Scholar]
- Hearon, C. M., Jr. , & Dinenno, F. A. (2016). Regulation of skeletal muscle blood flow during exercise in ageing humans. The Journal of Physiology, 594(8), 2261–2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearon, C. M., Jr. , Samels, M. , Dias, K. A. , MacNamara, J. P. , Levine, B. D. , & Sarma, S. (2022). Isolated knee extensor exercise training improves skeletal muscle vasodilation, blood flow, and functional capacity in patients with HFpEF. Physiological Reports, 10(15), e15419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearon, C. M., Jr. , Sarma, S. , Dias, K. A. , Hieda, M. , & Levine, B. D. (2019). Impaired oxygen uptake kinetics in heart failure with preserved ejection fraction. Heart, 105(20), 1552–1558. [DOI] [PubMed] [Google Scholar]
- Houstis, N. E. , Eisman, A. S. , Pappagianopoulos, P. P. , Wooster, L. , Bailey, C. S. , Wagner, P. D. , & Lewis, G. D. (2018). Exercise intolerance in heart failure with preserved ejection fraction: Diagnosing and ranking its causes using personalized O(2) pathway analysis. Circulation, 137(2), 148–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hundley, W. G. , Bayram, E. , Hamilton, C. A. , Hamilton, E. A. , Morgan, T. M. , Darty, S. N. , Stewart, K. P. , Link, K. M. , Herrington, D. M. , & Kitzman, D. W. (2007). Leg flow‐mediated arterial dilation in elderly patients with heart failure and normal left ventricular ejection fraction. American Journal of Physiology‐Heart and Circulatory Physiology, 292(3), H1427–H1434. [DOI] [PubMed] [Google Scholar]
- Hundley, W. G. , Kitzman, D. W. , Morgan, T. M. , Hamilton, C. A. , Darty, S. N. , Stewart, K. P. , Herrington, D. M. , Link, K. M. , & Little, W. C. (2001). Cardiac cycle‐dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. Journal of the American College of Cardiology, 38(3), 796–802. [DOI] [PubMed] [Google Scholar]
- Inaba, Y. , Chen, J. A. , & Bergmann, S. R. (2010). Prediction of future cardiovascular outcomes by flow‐mediated vasodilatation of brachial artery: A meta‐analysis. The International Journal of Cardiovascular Imaging, 26(6), 631–640. [DOI] [PubMed] [Google Scholar]
- Jain, S. , Obeid, M. J. , Yenigalla, S. , Paravathaneni, M. , Gadela, N. V. , Singh, G. , Kulkarni, V. , Kondaveety, S. , Gade, K. C. , Lee, J. , Kulick‐Soper, C. M. , Sanchez, N. , Satija, V. , Hashmath, Z. , Zamani, P. , Akers, S. , & Chirinos, J. A. (2021). Impact of chronic obstructive pulmonary disease in heart failure with preserved ejection fraction. American Journal of Cardiology, 149, 47–56. [DOI] [PubMed] [Google Scholar]
- Kagami, K. , Harada, T. , Yoshida, K. , Amanai, S. , Kato, T. , Wada, N. , Adachi, T. , & Obokata, M. (2022). Impaired right atrial reserve function in heart failure with preserved ejection fraction. Journal of the American Society of Echocardiography, 35(8), 836–845. [DOI] [PubMed] [Google Scholar]
- Kao, D. P. , Lewsey, J. D. , Anand, I. S. , Massie, B. M. , Zile, M. R. , Carson, P. E. , McKelvie, R. S. , Komajda, M. , McMurray, J. J. , & Lindenfeld, J. (2015). Characterization of subgroups of heart failure patients with preserved ejection fraction with possible implications for prognosis and treatment response. European Journal of Heart Failure, 17(9), 925–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keteyian, S. J. , Brawner, C. A. , Ehrman, J. K. , Ivanhoe, R. , Boehmer, J. P. , & Abraham, W. T. (2010). Reproducibility of peak oxygen uptake and other cardiopulmonary exercise parameters: Implications for clinical trials and clinical practice. Chest, 138(4), 950–955. [DOI] [PubMed] [Google Scholar]
- Kishimoto, S. , Kajikawa, M. , Maruhashi, T. , Iwamoto, Y. , Matsumoto, T. , Iwamoto, A. , Oda, N. , Matsui, S. , Hidaka, T. , Kihara, Y. , Chayama, K. , Goto, C. , Aibara, Y. , Nakashima, A. , Noma, K. , & Higashi, Y. (2017). Endothelial dysfunction and abnormal vascular structure are simultaneously present in patients with heart failure with preserved ejection fraction. International Journal of Cardiology, 231, 181–187. [DOI] [PubMed] [Google Scholar]
- Kitzman, D. W. , Brubaker, P. , Morgan, T. , Haykowsky, M. , Hundley, G. , Kraus, W. E. , Eggebeen, J. , & Nicklas, B. J. (2016). Effect of caloric restriction or aerobic exercise training on peak oxygen consumption and quality of life in obese older patients with heart failure with preserved ejection fraction: A randomized clinical trial. Journal of the American Medical Association, 315(1), 36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzman, D. W. , Brubaker, P. H. , Herrington, D. M. , Morgan, T. M. , Stewart, K. P. , Hundley, W. G. , Abdelhamed, A. , & Haykowsky, M. J. (2013). Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: A randomized, controlled, single‐blind trial. Journal of the American College of Cardiology, 62(7), 584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzman, D. W. , Herrington, D. M. , Brubaker, P. H. , Moore, J. B. , Eggebeen, J. , & Haykowsky, M. J. (2013). Carotid arterial stiffness and its relationship to exercise intolerance in older patients with heart failure and preserved ejection fraction. Hypertension, 61(1), 112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzman, D. W. , Nicklas, B. , Kraus, W. E. , Lyles, M. F. , Eggebeen, J. , Morgan, T. M. , & Haykowsky, M. (2014). Skeletal muscle abnormalities and exercise intolerance in older patients with heart failure and preserved ejection fraction. American Journal of Physiology‐Heart and Circulatory Physiology, 306(9), H1364–H1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup, P. , Secher, N. H. , Relu, M. U. , Hellsten, Y. , Söderlund, K. , & Bangsbo, J. (2008). Neuromuscular blockade of slow twitch muscle fibres elevates muscle oxygen uptake and energy turnover during submaximal exercise in humans. The Journal of Physiology, 586(24), 6037–6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusunose, K. , Motoki, H. , Popovic, Z. B. , Thomas, J. D. , Klein, A. L. , & Marwick, T. H. (2012). Independent association of left atrial function with exercise capacity in patients with preserved ejection fraction. Heart, 98(17), 1311–1317. [DOI] [PubMed] [Google Scholar]
- Lau, E. S. , Panah, L. G. , Zern, E. K. , Liu, E. E. , Farrell, R. , Schoenike, M. W. , Namasivayam, M. , Churchill, T. W. , Curreri, L. , Malhotra, R. , Nayor, M. , Lewis, G. D. , & Ho, J. E. (2022). Arterial Stiffness and Vascular Load in HFpEF: Differences Among Women and Men. Journal of Cardiac Failure, 28(2), 202–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, D. S. , Gona, P. , Vasan, R. S. , Larson, M. G. , Benjamin, E. J. , Wang, T. J. , Tu, J. V. , & Levy, D. (2009). Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: Insights from the framingham heart study of the national heart, lung, and blood institute. Circulation, 119(24), 3070–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. F. , Barrett‐O'Keefe, Z. , Garten, R. S. , Nelson, A. D. , Ryan, J. J. , Nativi, J. N. , Richardson, R. S. , & Wray, D. W. (2016). Evidence of microvascular dysfunction in heart failure with preserved ejection fraction. Heart, 102(4), 278–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, J. F. , Barrett‐O'Keefe, Z. , Nelson, A. D. , Garten, R. S. , Ryan, J. J. , Nativi‐Nicolau, J. N. , Richardson, R. S. , & Wray, D. W. (2016). Impaired skeletal muscle vasodilation during exercise in heart failure with preserved ejection fraction. International Journal of Cardiology, 211, 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahfouz, R. A. , Gouda, M. , & Abdelhamid, M. (2020). Relation of microvascular dysfunction and exercise tolerance in patients with heart failure with preserved ejection fraction. Echocardiography, 37(8), 1192–1198. [DOI] [PubMed] [Google Scholar]
- Marechaux, S. , Samson, R. , van Belle, E. , Breyne, J. , de Monte, J. , Dedrie, C. , Chebai, N. , Menet, A. , Banfi, C. , Bouabdallaoui, N. , Le Jemtel, T. H. , & Ennezat, P. V. (2016). Vascular and microvascular endothelial function in heart failure with preserved ejection fraction. Journal of Cardiac Failure, 22(1), 3–11. [DOI] [PubMed] [Google Scholar]
- Molina, A. J. , Bharadwaj, M. S. , Van Horn, C. , Nicklas, B. J. , Lyles, M. F. , Eggebeen, J. , Haykowsky, M. J. , Brubaker, P. H. , & Kitzman, D. W. (2016). Skeletal muscle mitochondrial content, oxidative capacity, and Mfn2 expression are reduced in older patients with heart failure and preserved ejection fraction and are related to exercise intolerance. JACC: Heart Failure, 4(8), 636–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller, S. , Winzer, E. B. , Duvinage, A. , Gevaert, A. B. , Edelmann, F. , Haller, B. , Pieske‐Kraigher, E. , Beckers, P. , Bobenko, A. , Hommel, J. , Van de Heyning, C. M. , Esefeld, K. , von Korn, P. , Christle, J. W. , Haykowsky, M. J. , Linke, A. , Wisløff, U. , Adams, V. , Pieske, B. , … Group, O.‐C. S. (2021). Effect of high‐intensity interval training, moderate continuous training, or guideline‐based physical activity advice on peak oxygen consumption in patients with heart failure with preserved ejection fraction: A randomized clinical trial. Journal of the American Medical Association, 325(6), 542–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namasivayam, M. , Lau, E. S. , Zern, E. K. , Schoenike, M. W. , Hardin, K. M. , Sbarbaro, J. A. , Cunningham, T. F. , Farrell, R. M. , Rouvina, J. , Kowal, A. , Bhat, R. R. , Brooks, L. C. , Nayor, M. , Shah, R. V. , Ho, J. E. , Malhotra, R. , & Lewis, G. D. (2022). Exercise blood pressure in heart failure with preserved and reduced ejection fraction. JACC: Heart Failure, 10(4), 278–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayor, M. , Houstis, N. E. , Namasivayam, M. , Rouvina, J. , Hardin, C. , Shah, R. V. , Ho, J. E. , Malhotra, R. , & Lewis, G. D. (2020). Impaired exercise tolerance in heart failure with preserved ejection fraction: Quantification of multiorgan system reserve capacity. JACC: Heart Failure, 8(8), 605–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obokata, M. , Olson, T. P. , Reddy, Y. N. V. , Melenovsky, V. , Kane, G. C. , & Borlaug, B. A. (2018). Haemodynamics, dyspnoea, and pulmonary reserve in heart failure with preserved ejection fraction. European Heart Journal, 39(30), 2810–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obokata, M. , Reddy, Y. N. V. , Pislaru, S. V. , Melenovsky, V. , & Borlaug, B. A. (2017). Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation, 136(1), 6–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owan, T. E. , Hodge, D. O. , Herges, R. M. , Jacobsen, S. J. , Roger, V. L. , & Redfield, M. M. (2006). Trends in prevalence and outcome of heart failure with preserved ejection fraction. New England Journal of Medicine, 355(3), 251–259. [DOI] [PubMed] [Google Scholar]
- Pandey, A. , Khera, R. , Park, B. , Haykowsky, M. , Borlaug, B. A. , Lewis, G. D. , Kitzman, D. W. , Butler, J. , & Berry, J. D. (2018). Relative impairments in hemodynamic exercise reserve parameters in heart failure with preserved ejection fraction: A study‐level pooled analysis. JACC: Heart Failure, 6(2), 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, A. E. , Tromp, J. , Shah, S. J. , Lam, C. S. P. , Lewis, G. D. , Borlaug, B. A. , Sharma, K. , Pandey, A. , Sweitzer, N. K. , Kitzman, D. W. , & Mentz, R. J. (2023). Phenomapping in heart failure with preserved ejection fraction: Insights, limitations, and future directions. Cardiovascular Research, 118(18), 3403–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer, M. A. , Shah, A. M. , & Borlaug, B. A. (2019). Heart failure with preserved ejection fraction in perspective. Circulation Research, 124(11), 1598–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratchford, S. M. , Bunsawat, K. , Alpenglow, J. K. , Zhao, J. , Wright, J. B. , Ryan, J. J. , & Wray, D. W. (2023). Improved vascular function and functional capacity following l‐citrulline administration in patients with heart failure with preserved ejection fraction: A single‐arm, open‐label, prospective pilot study. Journal of Applied Physiology, 134(2), 328–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratchford, S. M. , Clifton, H. L. , Gifford, J. R. , LaSalle, D. T. , Thurston, T. S. , Bunsawat, K. , Alpenglow, J. K. , Richardson, R. S. , Wright, J. B. , Ryan, J. J. , & Wray, D. W. (2019). Impact of acute antioxidant administration on inflammation and vascular function in heart failure with preserved ejection fraction. American Journal of Physiology‐Regulatory, Integrative and Comparative Physiology, 317(5), R607–R614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratchford, S. M. , Clifton, H. L. , La Salle, D. T. , Broxterman, R. M. , Lee, J. F. , Ryan, J. J. , Hopkins, P. N. , Wright, J. B. , Trinity, J. D. , Richardson, R. S. , & Wray, D. W. (2020). Cardiovascular responses to rhythmic handgrip exercise in heart failure with preserved ejection fraction. Journal of Applied Physiology, 129(6), 1267–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratchford, S. M. , Lee, J. F. , Bunsawat, K. , Alpenglow, J. K. , Zhao, J. , Ma, C. L. , Ryan, J. J. , Khor, L. L. , & Wray, D. W. (2022). The impact of obesity on the regulation of muscle blood flow during exercise in patients with heart failure with a preserved ejection fraction. Journal of Applied Physiology, 132(5), 1240–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, Y. N. V. , Andersen, M. J. , Obokata, M. , Koepp, K. E. , Kane, G. C. , Melenovsky, V. , Olson, T. P. , & Borlaug, B. A. (2017). Arterial stiffening with exercise in patients with heart failure and preserved ejection fraction. Journal of the American College of Cardiology, 70(2), 136–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, Y. N. V. , Obokata, M. , Wiley, B. , Koepp, K. E. , Jorgenson, C. C. , Egbe, A. , Melenovsky, V. , Carter, R. E. , & Borlaug, B. A. (2019). The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. European Heart Journal, 40(45), 3721–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, Y. N. V. , Olson, T. P. , Obokata, M. , Melenovsky, V. , & Borlaug, B. A. (2018). Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC: Heart Failure, 6(8), 665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy, Y. N. V. , Stewart, G. M. , Obokata, M. , Koepp, K. E. , & Borlaug, B. A. (2021). Peripheral and pulmonary effects of inorganic nitrite during exercise in heart failure with preserved ejection fraction. European Journal of Heart Failure, 23(5), 814–823. [DOI] [PubMed] [Google Scholar]
- Redfield, M. M. , & Borlaug, B. A. (2023). Heart failure with preserved ejection fraction: A review. Journal of the American Medical Association, 329(10), 827–838. [DOI] [PubMed] [Google Scholar]
- Sachdev, V. , Sharma, K. , Keteyian, S. J. , Alcain, C. F. , Desvigne‐Nickens, P. , Fleg, J. L. , Florea, V. G. , Franklin, B. A. , Guglin, M. , Halle, M. , Leifer, E. S. , Panjrath, G. , Tinsley, E. A. , Wong, R. P. , & Kitzman, D. W. (2023). Supervised exercise training for chronic heart failure with preserved ejection fraction: A scientific statement from the American Heart Association and American College of Cardiology. Journal of the American College of Cardiology, 147(16), e699–e715. [DOI] [PubMed] [Google Scholar]
- Samson, R. , Jaiswal, A. , Ennezat, P. V. , Cassidy, M. , & Le Jemtel, T. H. (2016). Clinical phenotypes in heart failure with preserved ejection fraction. Journal of the American Heart Association, 5(1), e002477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel, T. J. , Kitzman, D. W. , Haykowsky, M. J. , Upadhya, B. , Brubaker, P. , Nelson, M. B. , Hundley, W. G. , & Nelson, M. D. (2021). Left ventricular diastolic dysfunction and exercise intolerance in obese heart failure with preserved ejection fraction. American Journal of Physiology‐Heart and Circulatory Physiology, 320(4), H1535–H1542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma, S. , MacNamara, J. P. , Balmain, B. N. , Hearo, C. M., Jr. , Wakeham, D. J. , Tomlinson, A. R. , Hynan, L. S. , Babb, T. G. , & Levine, B. D. (2022). Challenging the hemodynamic hypothesis in heart failure with preserved ejection fraction: Is exercise capacity limited by elevated pulmonary capillary wedge pressure? Circulation, 147(5), 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz, M. G. , Davies, J. E. , Roberts‐Thomson, P. , Black, J. A. , Hughes, A. D. , & Sharman, J. E. (2013). Exercise central (aortic) blood pressure is predominantly driven by forward traveling waves, not wave reflection. Hypertension, 62(1), 175–182. [DOI] [PubMed] [Google Scholar]
- Seravalle, G. , Quarti‐Trevano, F. , Dell'Oro, R. , Gronda, E. , Spaziani, D. , Facchetti, R. , Cuspidi, C. , Mancia, G. , & Grassi, G. (2019). Sympathetic and baroreflex alterations in congestive heart failure with preserved, midrange and reduced ejection fraction. Journal of Hypertension, 37(2), 443–448. [DOI] [PubMed] [Google Scholar]
- Shah, A. M. , & Pfeffer, M. A. (2012). The many faces of heart failure with preserved ejection fraction. Nature Reviews Cardiology, 9(10), 555–556. [DOI] [PubMed] [Google Scholar]
- Shah, S. J. , Katz, D. H. , Selvaraj, S. , Burke, M. A. , Yancy, C. W. , Gheorghiade, M. , Bonow, R. O. , Huang, C. C. , & Deo, R. C. (2015). Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation, 131(3), 269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonovic, D. , Coiro, S. , Deljanin‐Ilic, M. , Kobayashi, M. , Carluccio, E. , Girerd, N. , & Ambrosio, G. (2021). Exercise‐induced B‐lines in heart failure with preserved ejection fraction occur along with diastolic function worsening. ESC Heart Failure, 8(6), 5068–5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton, M. J. , Nelson, M. B. , Samuel, T. J. , Kitzman, D. W. , Brubaker, P. , Haykowsky, M. J. , Upadhya, B. , Chen, H. , & Nelson, M. D. (2022). Left atrial stiffness index independently predicts exercise intolerance and quality of life in older, obese patients with heart failure with preserved ejection fraction. Journal of Cardiac Failure, 28(4), 567–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart, N. A. , Haluska, B. , Jeffriess, L. , & Leung, D. (2012). Exercise training in heart failure with preserved systolic function: A randomized controlled trial of the effects on cardiac function and functional capacity. Congestive Heart Failure, 18(6), 295–301. [DOI] [PubMed] [Google Scholar]
- Tartière‐Kesri, L. , Tartière, J. M. , Logeart, D. , Beauvais, F. , & Cohen Solal, A. (2012). Increased proximal arterial stiffness and cardiac response with moderate exercise in patients with heart failure and preserved ejection fraction. Journal of the American College of Cardiology, 59(5), 455–461. [DOI] [PubMed] [Google Scholar]
- Trinity, J. D. , Groot, H. J. , Layec, G. , Rossman, M. J. , Ives, S. J. , Runnels, S. , Gmelch, B. , Bledsoe, A. , & Richardson, R. S. (2012). Nitric oxide and passive limb movement: A new approach to assess vascular function. The Journal of Physiology, 590(6), 1413–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao, C. W. , Lyass, A. , Enserro, D. , Larson, M. G. , Ho, J. E. , Kizer, J. R. , Gottdiener, J. S. , Psaty, B. M. , & Vasan, R. S. (2018). Temporal trends in the incidence of and mortality associated with heart failure with preserved and reduced ejection fraction. JACC: Heart Failure, 6, 678–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker, W. J. , Lijauco, C. C. , Hearon, C. M., Jr. , Angadi, S. S. , Nelson, M. D. , Sarma, S. , Nanayakkara, S. , La Gerche, A. , & Haykowsky, M. J. (2018). Mechanisms of the improvement in peak VO(2) with exercise training in heart failure with reduced or preserved ejection fraction. Heart Lung Circulation, 27(1), 9–21. [DOI] [PubMed] [Google Scholar]
- Weiss, K. , Schär, M. , Panjrath, G. S. , Zhang, Y. , Sharma, K. , Bottomley, P. A. , Golozar, A. , Steinberg, A. , Gerstenblith, G. , Russell, S. D. , & Weiss, R. G. (2017). Fatigability, exercise intolerance, and abnormal skeletal muscle energetics in heart failure. Circulation: Heart Failure, 10(7), e004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray, D. W. , Nishiyama, S. K. , Harris, R. A. , & Richardson, R. S. (2008). Angiotensin II in the elderly: Impact of angiotensin II type 1 receptor sensitivity on peripheral hemodynamics. Hypertension, 51(6), 1611–1616. [DOI] [PubMed] [Google Scholar]
- Yang, J. H. , Obokata, M. , Reddy, Y. N. V. , Redfield, M. M. , Lerman, A. , & Borlaug, B. A. (2020). Endothelium‐dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. European Journal of Heart Failure, 22(3), 432–441. [DOI] [PubMed] [Google Scholar]
- Yeboah, J. , Folsom, A. R. , Burke, G. L. , Johnson, C. , Polak, J. F. , Post, W. , Lima, J. A. , Crouse, J. R. , & Herrington, D. M. (2009). Predictive value of brachial flow‐mediated dilation for incident cardiovascular events in a population‐based study: The multi‐ethnic study of atherosclerosis. Circulation, 120(6), 502–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani, P. , Proto, E. A. , Mazurek, J. A. , Prenner, S. B. , Margulies, K. B. , Townsend, R. R. , Kelly, D. P. , Arany, Z. , Poole, D. C. , Wagner, P. D. , & Chirinos, J. A. (2020). Peripheral determinants of oxygen utilization in heart failure with preserved ejection fraction: Central role of adiposity. JACC: Basic to Translational Science, 5(3), 211–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani, P. , Proto, E. A. , Wilson, N. , Fazelinia, H. , Ding, H. , Spruce, L. A. , Davila, A., Jr. , Hanff, T. C. , Mazurek, J. A. , Prenner, S. B. , Desjardins, B. , Margulies, K. B. , Kelly, D. P. , Arany, Z. , Doulias, P. T. , Elrod, J. W. , Allen, M. E. , McCormack, S. E. , Schur, G. M. , … Chirinos, J. A. (2021). Multimodality assessment of heart failure with preserved ejection fraction skeletal muscle reveals differences in the machinery of energy fuel metabolism. ESC Heart Failure, 8(4), 2698–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamani, P. , Rawat, D. , Shiva‐Kumar, P. , Geraci, S. , Bhuva, R. , Konda, P. , Doulias, P. T. , Ischiropoulos, H. , Townsend, R. R. , Margulies, K. B. , Cappola, T. P. , Poole, D. C. , & Chirinos, J. A. (2015). Effect of inorganic nitrate on exercise capacity in heart failure with preserved ejection fraction. Circulation, 131(4), 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zern, E. K. , Ho, J. E. , Panah, L. G. , Lau, E. S. , Liu, E. , Farrell, R. , Sbarbaro, J. A. , Schoenike, M. W. , Pappagianopoulos, P. P. , Namasivayam, M. , Malhotra, R. , Nayor, M. , & Lewis, G. D. (2021). Exercise intolerance in heart failure with preserved ejection fraction: Arterial stiffness and aabnormal left ventricular hemodynamic responses during exercise. Journal of Cardiac Failure, 27(6), 625–634. [DOI] [PMC free article] [PubMed] [Google Scholar]


