Abstract
Arthrospira/Limnospira is a popular botanical dietary supplement throughout the world and has been consumed as a food product for hundreds of years. Ongoing efforts from our research group are focused on evaluating the utility of a Limnospira-derived oral supplement (Immulina) in promoting resilience against influenza viral infection. Like other botanical extracts, Immulina is inherently a complex matrix with variation in the levels of its chemical constituents. Therefore, to ensure therapeutic consistency for future scientific research and clinical studies, we are developing standardization technology using a bioassay and chemical markers. Braun-type lipoproteins, a class of macromolecules that activate the Toll-like receptor (TLR)2/TLR1 signaling pathway, have been identified as a major active component within Immulina. Based on the mechanism of action of the Braun-type lipoproteins, an in vitro bioassay was established using the HEK-Blue hTLR2/TLR1 cell line to quantitate the immune-enhancing potency of Immulina. The objective of the current research was to validate that bioassay for Immulina activity quantification using the U.S. FDA guidance document for botanical drug development and U.S. Pharmacopeia recommendations. System suitability, reference standards and defining potency units were established. Validation of performance parameters included precision, specificity, accuracy, linearity, and range. Validating this bioassay for Immulina activity provides a tool for ensuring product consistency and quantifying the potency of this botanical for use in future research as well as material in the consumer market.
Keywords: Arthrospira, standardization, bioassay, immune, validation, spirulina
Introduction
It is well-accepted that botanicals are complex mixtures of hundreds of compounds and that the concentrations of these compounds often vary substantially. This inherent variation in the level of chemical constituents represents a quality control issue that has contributed to a lack of consistent product efficacy observed in clinical studies on some of these products [1]. To address this issue, chemical/analytical standardization methods are being used to verify that the levels of active compounds (when known) within these products are consistent. Although chemical standardization assays have been successful in many cases, they are not suitable for measuring the activity exhibited by families of high molecular weight compounds that are sometimes responsible for the health benefits of botanicals. Since the physiochemical properties of such macromolecular components do not cleanly predict or correlate strongly with their biological activity, the accurate assessment of the potency of these compounds cannot be fully achieved using only analytical techniques.
An analogous problem existed in the field of pharmaceutical biologics, an issue that was eventually solved by bioassay-based standardization to afford consistent quality of products. Biological standardization revolutionized the field of pharmaceutical biologics by providing a technology to ensure consistent product quality. Although analytical techniques are valuable, the majority of biotherapeutic products require bioassays to quantitate activity/potency. Consistent quality translates into products with reliable therapeutic effects for patient health. Hence, adapting the concept of biological standardization to botanicals would be expected to improve product quality (batch-to-batch consistency), like the success this approach has had with biologics. In fact, other researchers have already started using both biological assays and chemical methods as a complete standardization approach in the development and clinical evaluation of extracts derived from hop seed cones (Humulus lupulus L.) [2] and red clover aerial parts (Trifolium pratense L.)[3].
Our research group is focused on investigating the use of Immulina, an extract of Limnospira (formally Arthrospira) fusiformis, as an immune-based antiviral resilience promotor against influenza. Braun-type lipoproteins, a class of high molecular weight compounds, have been identified as the predominant macrophage-activating principal within this botanical [4]. Analysis of 12 Immulina samples extracted from biomass sourced from commercial growers in 10 different countries showed 12.5-fold activity variation between samples, highlighting the need for activity-based standardization of this product [5]. Efforts to design a bioassay-based standardization method for Immulina was driven based on the mechanism of action of the active lipoproteins component and resulted in selecting the HEK-Blue hTLR2/TLR1 cell line to quantitate its immune-enhancing activity [5]. The objective of the current research was to validate this bioassay so it can be employed as a tool for ensuring consistent quality product material for future use in research and clinical efficacy studies.
Materials and Methods
Chemicals and labware supplies.
Standards (Pam3CSK4, heat-killed E. coli 0111:B4 bacteria, Pam2CSK4 and ultra pure LPS from E. coli 0111:B4), QUANTI-Blue™ reagent and antibiotics (HEK-Blue™ Selection and Normocin™) were purchased from InvivoGen (San Diego, CA). Endotoxin-free water and 1X-phosphate buffered saline were purchased from MilliporeSigma (Burlington, MA). Cell culture medium Gibco™ Dulbecco’s Modified Eagle Medium (4.5 g/L D-glucose) and Pen-Strep were purchased from Fisher Scientific (Waltham, MA). HyClone characterized fetal bovine serum (US origin) was purchased from Cytiva (Marlborough, MA).
Regular labware was purchased from Fisher Scientific (Corning Costar 96-well plates #3595 and Fisherbrand 1.5 mL microfuge tube catalog #05-408-129), Eppendorf (200 μl multi-channel pipet tips catalog # 022491539, Hamburg, Germany) and Rainin Mettler Toledo (20 μl low retention multi-channel pipet tips, catalog #30389200, Oakland, CA). Low-binding labware was purchased from Eppendorf (200 μl multi-channel pipet tips catalog #022493022, LoBind 1.5 mL microfuge tubes catalog #30108442, Deepwell 96-well plate catalog #951032107) and Rainin Mettler Toledo (20 μl low retention multi-channel pipet tips, catalog #30389229). All endotoxin-free labware (plates and tips) were purchased from Associates of Cape Cod (Falmouth, MA).
Immulina sample preparation for TLR2/TLR1 dependent activity analysis.
Immulina™ lot 2290020 (ChromaDex, Los Angeles, CA) was used for all experiments and was manufactured from Limnospira fusiformis raw material (lot 20190820B, Dongtai Cibainian Biological Engineering Company, LTD, China). Complete taxonomic identification of the raw material (using sequence analysis and morphological examination) was previously performed [5]. This commercial extract was prepared by extraction of whole microalgae biomass with 50% aqueous ethanol at 80°C. Active components were precipitated by addition of 1 volume of cold 95% ethanol, collected by centrifugation, washed, and dried.
Immulina in water: samples were prepared fresh on the day of the assay by preparing a solution at 25.0 mg/mL. Samples were vortexed for 1 min every 5–10 minutes for 1 h at room temperature and then immediately diluted to the desired concentration and added to cells. All validation experiments used Immulina prepared using endotoxin-free water, except for the system suitability experiments on water quality which used endotoxin-free, Milli-Q and distilled waters.
Immulina in 2% sodium dodecyl sulfate (SDS): sample was prepared fresh on the day of the assay by preparing a solution at 25.0 mg/mL in 2% SDS followed by sonication for 45 minutes and then heating at 98 °C for 30 minutes. The sample was then cooled by transferring to 37 °C for 5 minutes and then left at room temperature for 10 minutes before performing dilutions for the assay.
TLR2/TLR1‐dependent activity assay.
HEK-Blue™ hTLR2/TLR1 cells (InvivoGen) were used to quantitate Immulina Braun-type lipoprotein dependent activity. This cell line is engineered to stably express genes of the TLR2/TLR1 signaling pathway and an NF‑kappa B-inducible secreted embryonic alkaline phosphatase (SEAP) reporter gene. Experiments were performed according to manufacturer’s instructions using a 96-well plate format. For the validation studies, cells were sub-cultured on average two days prior to each assay. On the assay day, samples and controls were added to cells and plates incubated for 24 h at 37 °C in 5% CO2. Activation was detected by incubating the cell culture supernatant with QUANTI-Blue™ reagent for 24 h and then SEAP levels evaluated using an optical density (OD) measurement at 635 nm (SpectraMax M5 plate reader, Molecular Devices, San Jose, CA).
Statistical analysis.
Analysis was done using GraphPad Prism 9.4.1. for one-way ANOVA followed by Dunnett’s multiple comparison test for comparison between more than two groups with significance defined by p<0.05. Unpaired Welch’s t-test was used for two group comparisons with significance defined by p<0.05 (two-tailed).
Results and Discussion
The U.S. FDA has provided a guidance for the industry document on botanical drug development [6]. Biological assays were recognized as one of the tools that can be used to ensure therapeutic consistency and quality. It was recommended that system suitability should be addressed to ensure reproducibility of the biological assay during routine use. Since biological assays are typically more variable than chemical methods [7], it is advisable to include a reference standard. Recommended validation parameters include precision, specificity, accuracy, linearity and range. In this study we followed the FDA guidance for establishing and validating a bioassay for quantitating the potency/activity of the Immulina extract using the commercially available HEK-Blue hTLR2-TLR1 cells [8]. For assessment of each validation parameter, we followed the recommended methods and number of replicates detailed in USP <1225> [9].
1). Establishing botanical reference material and reference standards:
Immulina™ lot 2290020 was selected as the botanical reference material. The active Immulina Braun-type lipoproteins have not been isolated and are therefore not available for use as a standard. Therefore, two positive controls/standards were evaluated: 1) Pam3CSK4 and 2) heat-killed E. coli 0111:B4 bacteria (HKEB). Pam3CSK4 is a synthetic molecule containing three fatty acids that mimics the lipid moiety of bacterial lipoproteins. This lipopeptide contains only 6 amino acids and is substantially smaller (molecular weight 1852.33 g/mol) than naturally occurring bacterial lipoproteins. HKEB contains wall components including naturally occurring bacterial lipoproteins that are likely to be structurally similar to those within Immulina. Both standards are commercially available with established storage and preparation protocols [10, 11].
An evaluation was performed to determine lot to lot consistency for each positive control. Two different lots for each positive control (purchased from InvivoGen) were tested in triplicate dose responses to determine EC50 values. Results indicate that there were no significant differences in potency between the lots for Pam3CSK4 (p=0.58) or for HKEB (p=0.37). This data suggests that there is good lot to lot consistency for these standards. However, since only two lots were analyzed, it is recommended that each new lot is tested to confirm equivalent potency.
2). Establishing system suitability:
Selecting a relevant biological assay is driven by the intended use of the botanical and, if known, the product’s mechanism of action. Previous research from our lab identified lipoproteins of the Braun-type as a major monocyte/macrophage activating component of Immulina that activate the TLR2/TLR1 signaling pathway [5]. Therefore, we selected to use the HEK-Blue hTLR2/TLR1 cell line that is engineered for specific detection of ligands for the hTLR2/TLR1 heterodimer. Optimized protocols for cell maintenance, culture conditions and screening assay are provided by the manufacture [8]. All recommended procedures and storage conditions were followed. For the purpose of validating this cell line for Immulina activity standardization, we evaluated system suitability based on three common parameters/testing conditions to determine their impact on the quantitation of activity.
Type of labware: macromolecules that contain both hydrophilic and lipophilic components, such as LPS, have been reported to exhibit a tendency to adsorb to container surfaces [12]. Braun-type lipoproteins have similar amphipathic properties and may therefore also non-specifically adsorb to surfaces. To address this possibility, we investigated whether the use of different types of common labware (regular, low-binding and endotoxin-free) affected activity in the hTLR2-TLR1 bioassay. For each type of labware, all needed laboratory consumables were obtained (pipet tips, tubes, and 96-well plates) and used to test the Immulina reference standard, Pam3CSK4 and HKEB in triplicate at 5 concentrations. Dose responses were then used to calculate EC50 values. Our results showed no significant effect between the different types of labware on the EC50 activity for any of the three agents in this assay system (Table 1). Since regular labware is more affordable and widely available, it was used for all validation experiments and routine assays.
Matrix effect of detergent: in aqueous solvents, macromolecules containing lipid moieties can form spherical vesicles. Disruption of such aggregates using detergents could potentially expose more molecules for detection (for example, see research on LPS by Piluso and Martinez [13]). In our bioassay system, we evaluated SDS and determined that the final concentration of detergent in cell cultures needs to be less than 0.001% to prevent solvent interference. To determine the matrix effect of detergent, Immulina extract dissolved in 2% SDS was compared to material dissolved in endotoxin-free water. Comparison of EC50 values (calculated from dose responses of 5 concentrations, performed in triplicate) indicated that there was no significant difference between the two samples (data not shown). For routine assay use and the validation studies, dissolving Immulina in water was selected as the solvent of choice to avoid the limitation of substantially diluting SDS to avoid solvent interference.
Water quality for preparation of stock solutions: bacteria (and their derived components such as endotoxin) are found everywhere in the environment, including water used as lab solvents. This can potentially be an added source of variation/contamination, especially for biological assays designed for detection of immune activating agents. To avoid this potential issue, endotoxin-free water is supplied by the manufacture to prepare stock solutions for the positive controls (Pam3CSK4 and HKEB). To determine whether grade/quality of water is an important factor in activity determination of Immulina, extract material was dissolved in different grades of water and activity of the samples compared. Our results showed no significant variation in the EC50 values (calculated from dose responses of 5 concentrations, performed in triplicate) for Immulina dissolved in distilled water, Milli-Q water or endotoxin-free water (Table 2). For all our validation experiments, endotoxin-free water was used for the preparation of Immulina stock solutions.
Table 1.
Effect of different types of labware on Pam3CSK4, HKEB and Immulina EC50 values. EC50 represent the concentration (ng/mL for Pam3CSK4 and Immulina, or bacteria cells × 103 for HKEB) of sample required to induce activation for the TLR2/TLR1 signaling pathway to levels 50% of those achieved by Pam3CSK4 (100 ng/mL). No statistically significant differences in activity were observed. Values represent mean EC50 values calculated from triplicate dose response determinations.
| Labware | EC50 value ± SD | |
|---|---|---|
| Pam3CSK4 | Regular | 5.12 ± 2.11 |
| Low-binding | 4.09 ± 0.44 | |
| Endotoxin-free | 3.62 ± 0.44 | |
| HKEB | Regular | 670 ± 479 |
| Low-binding | 316 ± 73 | |
| Endotoxin-free | 210 ± 8 | |
| Immulina | Regular | 1270 ± 760 |
| Low-binding | 665 ± 27 | |
| Endotoxin-free | 764 ± 132 |
Table 2.
Effect of different grades of waters on Immulina EC50. EC50 is the concentration (ng/mL) of Immulina material required to induce activation for the TLR2/TLR1 signaling pathway to levels 50% of those achieved by Pam3CSK4 (100 ng/mL). No statistically significant differences in activity were observed. Values represent mean EC50 values calculated from triplicate dose response determinations.
| Type of water | EC50 value ± SD |
|---|---|
| Endotoxin-free | 1280 ± 110 |
| Deionized | 1340 ± 470 |
| Milli-Q | 1090 ± 110 |
3). Definition of potency units:
Assignment of potency units to reference standards used in biological standardization assays is typically an arbitrary decision [14]. For the current bioassay we define 1 unit of activity to equal the amount of extract added to the test well that is required to induce activation to 50% of maximal inducible levels.
The following method can be used to assign units of potency to Immulina extract material:
Estimate an EC50 value (the concentration of sample required to induce activation to 50% of maximal level inducible by Pam3CSK4 tested at 100 ng/mL).
Multiply the EC50 value (ng/mL) by the total volume (mL) per well used in the bioassay to assess the level of activity. This calculation will give the amount of extract material that exhibits 1 unit of activity.
For example, if an Immulina extract exhibits an EC50 value of 1000 ng/mL when tested in 0.200 mL, then it would be assigned a value of 1.00 unit per 200 ng of extract (1000 ng/mL × 0.200 mL).
4). Validation parameter 1 - assessment of precision:
Precision refers to the closeness of agreement between independent results of measurements obtained under defined conditions. It describes the random error of measurements and has no relation to the accuracy or trueness of a value [15]. Our bioassay repeatability (intra-assay precision) was measured by testing four independent samples of Immulina reference material (each sample tested at 5 concentrations in triplicate to determine EC50 values). The intra-assay coefficient of variation (CV) was 21.9%. Intermediate precision (inter-assay precision) was determined by testing three independent samples of Immulina reference material in experiments performed on different days (each sample tested at 5 concentrations in triplicate to determine EC50 values). The inter-assay CV was calculated at 40.8%. The reproducibility precision (interlaboratory) was not determined as it was beyond the scope of the current study.
5). Validation parameter 2 - assessment of specificity:
Specificity defines how well the HEK-Blue hTLR2/TLR1 assay is capable of quantitating activity due to the Immulina Braun-type lipoproteins. Since botanical extracts (such as Immulina) are complex mixtures, various matrix components could potentially interfere with quantitation of the targeted activity. Although the cell line used in our assay is engineered for selective detection of only TLR2/TLR1 agonists, we evaluated whether there was any interference resulting from the presence of structurally similar lipoproteins and LPS.
The TLR2/TLR1 heterodimer detects triacylated lipoproteins such as those present within Immulina [5] and the synthetic triacylated lipopeptide Pam3CSK4. Although structurally similar diacylated lipoproteins/lipopeptides do not activate the TLR2/TLR1 signaling pathway, we evaluated whether the presence of these molecules could cause assay interference. Pam2CSK4 (a synthetic diacylated lipopeptide) was added at two concentrations (50.0 and 100 ng/mL) in combination with three different doses of Immulina (370, 1130 and 3300 ng/mL). Only one combination showed significant interference – activation of cells by the highest concentration of Pam2CSK4 (100 ng/mL) plus the lowest concentration of Immulina (370 ng/mL) was significantly lower than Immulina alone (Fig. 1A, p = 0.02). However, the EC50 value for Immulina was not statistically different from the EC50 value of the combination of Immulina plus Pam2CSK4 (data not shown). In agreement with previous research [5], Pam2CSK4 did not increase SEAP levels above background at any concentration tested (Fig. 1A).
Figure 1.
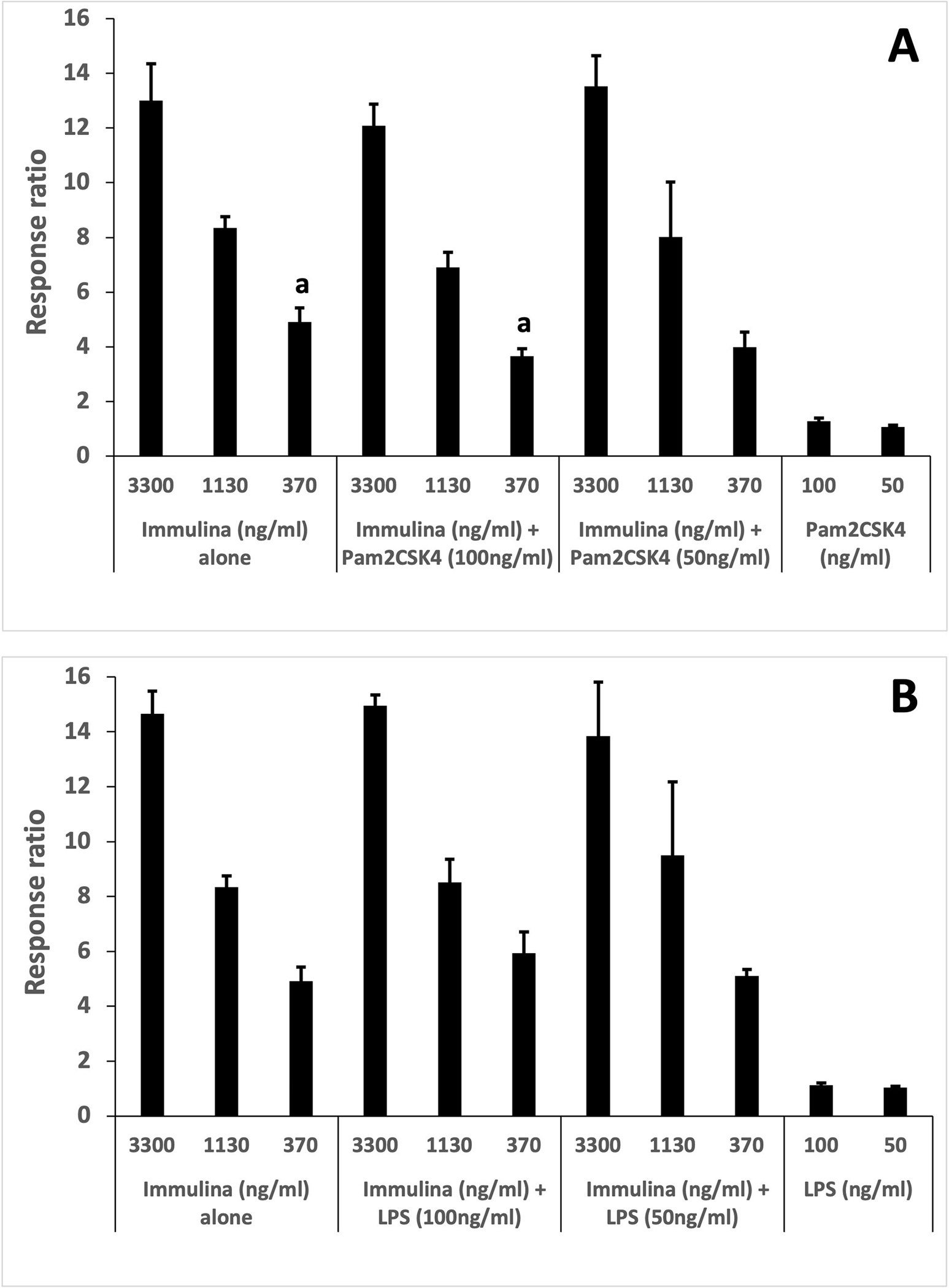
Determining specificity of the HEK-Blue hTLR2/TLR1 assay. Potential interference was evaluated for (A) Pam2CSK4 and (B) LPS. Both compounds were tested at two concentrations alone and in combination with three doses of Immulina (values represent the average of triplicate determinations). Response ratio ± SD is defined as OD of sample/OD of untreated cells. The control value for untreated cells was 1.00 ± 0.01. Statistical comparisons were performed between each concentration of “Immulina alone” and the combinations of “Immulina + Pam2CSK4 (at 100 and 50ng/ml)” (A). Similar statistical analyses were performed between “Immulina alone” and the combinations of “Immulina + LPS” (B). Treatments with “a” are significantly different from each other (p=0.02).
Bacteria are present essentially everywhere in our environment and as a result so are immunostimulatory bacterial-derived components such as LPS. Botanicals are not sterile products and will contain various levels of LPS originating from endophytes, epiphytes and/or bacterial associated with growing/harvesting/processing conditions. To assess whether LPS could interfere with activity quantitation in our bioassay, two different concentrations of the LPS (50.0 and 100 ng/mL) were added to three different concentrations of Immulina (370, 1130 and 3300 ng/mL). None of the combinations exhibited any significant inhibition or enhancement of activity (Fig. 1B). Not surprisingly, the EC50 value for Immulina was not statistically different from the EC50 value of the combination of Immulina plus LPS (data not shown). Also, as expected there was no TLR2/TLR1-dependent activation for any of the LPS concentrations tested alone (Fig. 1B).
6). Validation parameter 3 - assessment of accuracy:
Accuracy describes how closely experimental values obtained from the HEK-Blue hTLR2/TLR1 assay agree with the accepted known or predicted value [9]. One limitation of using an in vitro bioassay for standardization of a botanical extract is that non-active components (nuisance compounds) within the sample may either inhibit or enhance the detected level of activity. Although there is no perfect system for detection of all mechanisms by which nuisance compounds could interfere with detection of activity, a useful approach to determine accuracy is by “spiking” the sample with a known quantity of a reference standard. This approach is valuable when the active component is not fully characterized and/or multiple compounds are responsible for the activity (such as a class of macromolecules). The data obtained from “spiking” experiments is used to develop an acceptance criterion for determining whether matrix components are significantly interfering with quantitation of potency. In the current study, the two positive controls were used for spiking and the dose selected for each standard was based on their approximate EC50 value (5 ng/mL for Pam3CSK4 and 1.1 × 106 cells/mL for HKEB).
Determination of accuracy was performed using two approaches (Table 3). In the first approach (Method “A”) Immulina extract was spiked with each of the two positive controls and for the second approach (Method “B”), NaOH-treated Immulina was spiked with each of the two positive controls. In Method B, the Braun-type lipoproteins in Immulina were inactivated by treating the extract with 1M NaOH (37 °C, 1 hr) followed by neutralization with HCl. The purpose of this approach was to evaluate potential interference from the extract components, in the absence of the active molecules. NaOH-treated Immulina exhibited no detectable TLR2/TLR1-dependent activity above values obtained for untreated cells at concentrations between 10.0 μg/mL and 123 ng/mL, confirming that the Braun-type lipoproteins were inactivated by NaOH. Inactivated Immulina (at 4 concentrations) was spiked with each positive control and percent interference determined (Table 3).
Table 3.
Determining accuracy of the HEK-Blue hTLR2-TLR1 assay for quantitation of Immulina potency. Immulina (Method A) and NaOH-treated Immulina (Method B) samples were evaluated in triplicate at 4 concentrations, alone and spiked with Pam3CSK4 (5.00 ng/mL) or HKEB (1.10 × 106 cells/mL). Percent interference ± SD was calculated using the following formula: [(activity of spiked Immulina – activity of unspiked Immulina) / (activity exhibited by the concentration of added Pam3CSK4 spike or HKEB spike)] × 100. 100% is defined as no interference of sample matrix components.
| Immulina Concentration (μg/mL) | Method A - Immulina | Method B - NaOH-treated Immulina | ||
|---|---|---|---|---|
| Percent Interference | Percent Interference | |||
| Spiked with Pam3CSK4 | Spiked with HKEB | Spiked with Pam3CSK4 | Spiked with HKEB | |
| 3.3 | 86.1 ± 47.9 | 75.3 ± 14.3 | 57.7 ± 22.3 | 69.5 ± 34.8 |
| 1.1 | 70.2 ± 6.9 | 77.8 ± 10.8 | 51.7 ± 11.7 | 67.2 ± 15.6 |
| 0.37 | 70.8 ± 15.1 | 46.8 ± 5.7 | 52.2 ± 8.8 | 62.7 ± 22.9 |
| 0.12 | 50.8 ± 14.9 | 66.7 ± 29.4 | 58.6 ± 12.8 | 76.5 ± 28.1 |
| Mean = 69.5 | Mean = 66.7 | Mean = 55.1 | Mean = 68.9 | |
The criterion selected for acceptable assay accuracy was that the detected sample activity due to the spike was within 2-fold (50–200%) of the activity of the positive control tested alone (at the “spike” concentration). This acceptance criterion has been used in other in vitro potency assays. For example, quantitation of endotoxin units using the commercial Limulus Amebocyte Lysate assay kit (Associates of Cape Cod Inc.) includes a routine step in the protocol that detects interference by “spiking” the sample with a known amount of endotoxin standard. The mean percent interference for samples analyzed with both Method A and Method B, spiked with each positive control, were all within the acceptable range (Table 3).
7). Validation parameter 4 - assessment of linearity and range:
Linearity refers to the ability of the bioassay system to yield a dose-response that is directly proportional to the concentration of sample/extract tested. Range is interval between the lower and higher levels of activity that is suitable for linearity [9]. Both linearity and range are defined by the assay and hence have no acceptance criteria. In our in vitro bioassay, activity is expressed between 0% to 100% activation. Unknown samples showing 100% activation will have to be diluted and re-run since activity beyond 100% is not quantifiable.
A dose response curve for Immulina using log transformation of the concentration (x-axis) and percent activation (y-axis) exhibits a sigmoidal curve (Fig. 2A). A representative example of the linear range is depicted Fig. 2B (R2 value of 0.9965) and is typically the interval between about 10% and 90% activation.
Figure 2.
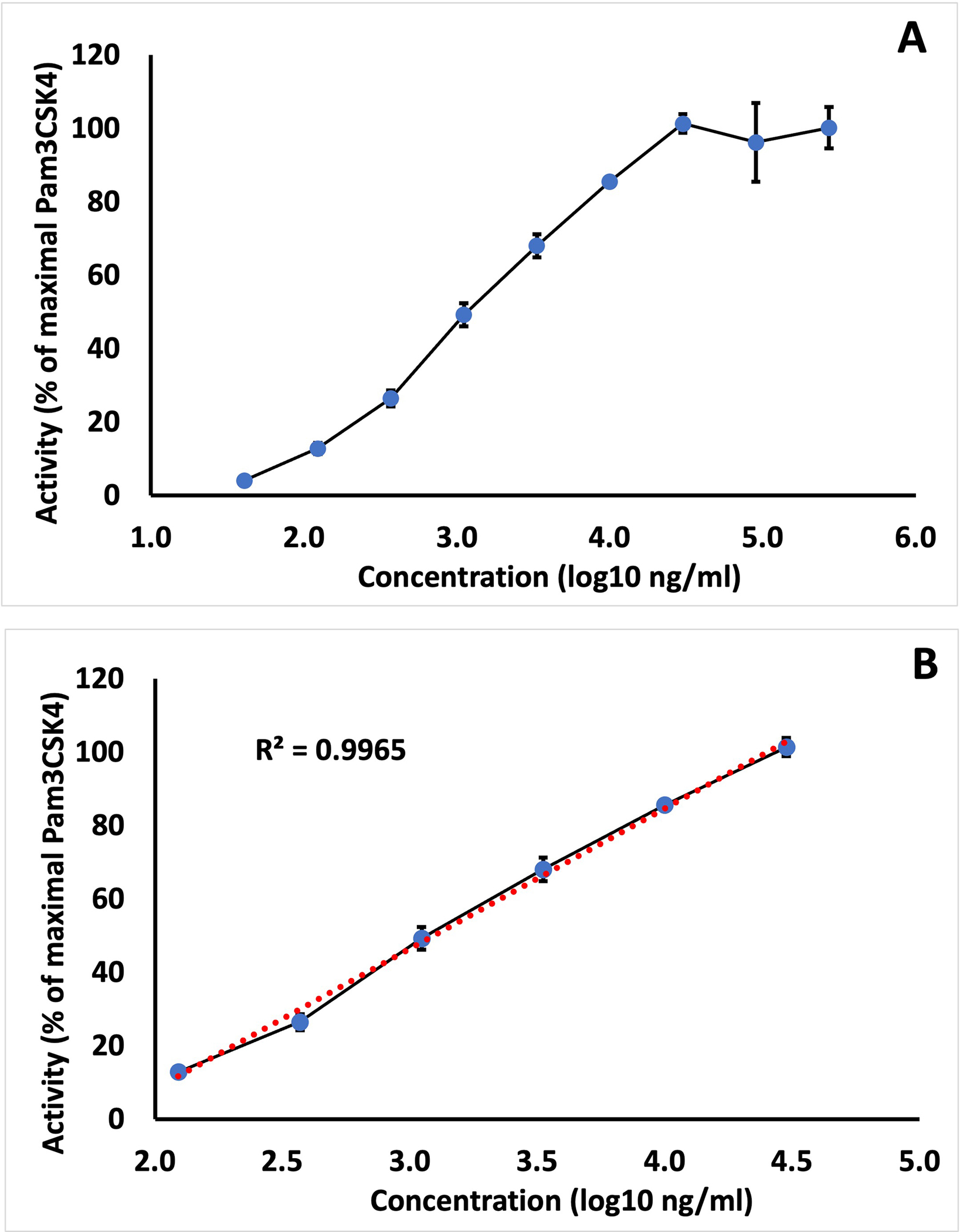
Determining assay linearity using the HEK-Blue hTLR2/TLR1 cells. (A) Representative dose response of Immulina evaluated for concentrations between 0.04 to 270 μg/mL. (B) Linear range for Immulina represents interval between 0.120 to 30.0 μg/mL. Values represent mean ± SD from triplicate determinations. Activity expressed as a percentage relative to maximal activation exhibited by Pam3CSK4 (tested at 100 ng/mL).
Conclusions
In the current research a biological standardization assay to quantify the potency of Immulina, using HEK-Blue hTLR2/TLR1 cells, has been validated based on recommendations from the United States FDA and Pharmacopeia (Table 4). The validated bioassay provides a valuable tool that can be used, together with existing analytical characterization methods, to ensure consistent and properly characterized Immulina botanical material for scientific investigations as well as the consumer market. Use of biologically standardized Immulina in future clinical studies will provide the information to define the units of biological potency needed to produce a therapeutic effect. Implementing biological standardization assays in the field of botanical dietary supplements will likely improve product quality (batch-to-batch consistency), similar to the success this approach has had in the fields of pharmaceuticals and vitamins.
Table 4.
Summary of HEK-Blue hTLR2/TLR1 in vitro assay validation for biological standardization of Immulina activity.
| Specifications | Parameters | ||
|---|---|---|---|
| Cell line | HEK-Blue hTLR2/TLR1 (InvivoGen) | Accuracy | Acceptance criterion: activity within 2-fold (50–200%) |
| Reference standards | 1) Pam3CSK4 synthetic lipopeptide | Precision | 21.9% intra-assay CV |
| 2) Heat-killed E. coli 0111:B4 | 40.8% inter-assay CV | ||
| Unit definition | 1 unit = amount of extract added to test well to obtain an EC50 value | Specificity | Activity (EC50 value) not affected by presence of other lipoproteins and LPS |
| System suitability | Suitable for use with different types of labware and different grades of water. Avoid detergents. | Linearity | Linearity attained by log10 transformation of concentration, R2=0.997 |
Assay recommendation for testing unknown samples: determine average EC50 value from running a triplicate dose response and then calculate units of potency using reference standard curves.
Acknowledgements
Research reported in this publication was supported by the Office of Dietary Supplements and National Center for Complementary and Integrative Health of the National Institutes of Health under Award Number U19AT010838. The content is solely the authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health. Additional funding was also provided by a grant from the USDA, Agricultural Research Service Specific Cooperative Agreement No. 58-6060-6-015.
Biographies
Mona Haron, PhD, is a Research Scientist, National Center for Natural Products Research, School of Pharmacy, University of Mississippi. Research focus; pharmacological properties of medicinal plants and their phytochemical constituents particularly immune enhancing and neuro-active agents. A special interest in studying the pharmacokinetic herb-drug interactions and safety of herbal supplements.
Jin Zhang, PhD, is a Research Scientist at the National Center for Natural Products Research, School of Pharmacy, University of Mississippi, with extensive research experience in natural products/biological chemistry. Jin Zhang’s interests include immune enhancing, anticancer, cell signaling, high throughput screening, isolation and structure elucidation of biologically active compounds in natural products.
Amar Chittiboyina, PhD, Assistant Director, National Center for Natural Products Research, Research Institute of Pharmaceutical Sciences, School of Pharmacy, University of Mississippi. Broad background in organic chemistry, with specific training and expertise in organic and medicinal chemistry, natural products, and analytical chemistry. Holder of several national, international patents, has authored over 120 peer-reviewed research articles in various scientific journals. His research interests include biogenesis of secondary metabolites; development of synthetic methods for commercial viability; safer and greener ways to produce natural products in gram quantities; synthesis and structure-activity relationships (SAR); implementation of orthogonal methods for the quality assessment of phytochemicals in various matrices; development of non-animal alternative methods in dermatotoxicology; application of computational tools to address the adverse effects such as potential herb-herb and herb-drug interactions associated with phytochemicals. Dr. Chittiboyina received his Ph.D. degree from the National Chemical Laboratory, Pune, India, followed by postdoctoral training at the Department of Medicinal Chemistry, University of Mississippi. Dr. Chittiboyina serves as a consultant for several small biotech companies and well versed in grantsmanship and technology transfer aspects.
Ikhlas Khan, PhD, is Director and Research Professor of Pharmacognosy at the National Center for Natural Products Research. Main areas of research include phytomedicine, drug discovery, medicinal plants research, analytical fingerprinting for standardization of herbal products, bio-analytical approaches to improvement of product quality and safety, development of analytical methods for phytochemical characterization of biologically active natural products.
Nirmal Pugh, PhD, is a Principal Scientist at the National Center for Natural Products Research with a background in pharmacognosy and performs research on botanicals.
Footnotes
Declaration of Interest Statement
NDP and IAK acknowledge financial interest in Immulina. All other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability
All data and materials are available upon request by contacting Dr. Mona Haron (email: mhharon@olemiss.edu).
References:
- 1.Shipkowski KA, et al. , Naturally complex: Perspectives and challenges associated with Botanical Dietary Supplement Safety assessment. Food and Chemical Toxicology, 2018. 118: p. 963–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krause E, et al. , Biological and chemical standardization of a hop (Humulus lupulus) botanical dietary supplement. Biomedical Chromatography, 2014. 28(6): p. 729–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piersen C, et al. , Chemical and biological characterization and clinical evaluation of botanical dietary supplements: a phase I red clover extract as a model. Current medicinal chemistry, 2004. 11(11): p. 1361–1374. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen CH, et al. , Enhancement of natural killer cell activity in healthy subjects by Immulina®, a Spirulina extract enriched for Braun-type lipoproteins. Planta medica, 2010. 76(16): p. 1802–1808. [DOI] [PubMed] [Google Scholar]
- 5.Huh J, et al. , Utility of fatty acid profile and in vitro immune cell activation for chemical and biological standardization of Arthrospira/Limnospira. Scientific Reports, 2022. 12(1): 15657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.FDA Revised Botanical Drug Development, Guidance for Industry: https://www.fda.gov/media/93113/download (accessed April 4, 2023)
- 7.Wu C, et al. , Scientific and regulatory approach to botanical drug development: a US FDA perspective. Journal of natural products, 2020. 83(2): p. 552–562. [DOI] [PubMed] [Google Scholar]
- 8.HEK-Blue™ hTLR2-TLR1 Cells. SEAP reporter 293 cells expressing the human TLR2 and TLR1 genes. Catalog code: hkb-htlr21. https://www.invivogen.com/sites/default/files/invivogen/products/files/hek-blue_htlr2-tlr1_tds.pdf. Date accessed: May 16, 2023.
- 9.United States Pharmacopeia Method #1225 - Validation of compendial procedures, https://online.uspnf.com/uspnf/document/1_GUID-E2C6F9E8-EA71-4B72-A7BA-76ABD5E72964_4_en-US. Date accessed: March 26, 2021.
- 10.Pam3CSK4. Synthetic triacylated lipoprotein; TLR2/TLR1 Ligand. Catalog code: tlrl-pms. https://www.invivogen.com/sites/default/files/invivogen/products/files/pam3csk4_tds.pdf. Date accessed: May 16,2023.
- 11.HKEB, Heat Killed E.coli 0111:B4 - TLR2 & TLR4 agonist. Catalog # tlrl-hkeb2. https://www.invivogen.com/sites/default/files/invivogen/products/files/hkeb_tds.pdf. Date accessed: May 16, 2023.
- 12.Novitsky TJ, Schmidt-Gengenbach J, and Remillard JF, Factors affecting recovery of endotoxin adsorbed to container surfaces. PDA Journal of Pharmaceutical Science and Technology, 1986. 40(6): p. 284–286. [PubMed] [Google Scholar]
- 13.Piluso L and Martinez M, Resolving liposomal inhibition of quantitative LAL methods. PDA journal of pharmaceutical science and technology, 1999. 53(5): p. 260–263. [PubMed] [Google Scholar]
- 14.Mire-Sluis AR, Progress in the use of biological assays during the development of biotechnology products. Pharmaceutical research, 2001. 18(9): p. 1239–1246. [DOI] [PubMed] [Google Scholar]
- 15.Chesher D, Evaluating assay precision. The Clinical Biochemist. Reviews, 2008. 29(Suppl 1): p. S23–6. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data and materials are available upon request by contacting Dr. Mona Haron (email: mhharon@olemiss.edu).


