Abstract
Objective
Geographic defect reconstruction in load‐bearing bones presents formidable challenges for orthopaedic surgeon. The use of 3D‐printed personalized implants presents a compelling opportunity to address this issue. This study aims to design, manufacture, and evaluate 3D‐printed personalized implants with irregular lattice porous structures for geographic defect reconstruction in load‐bearing bones, focusing on feasibility, osseointegration, and patient outcomes.
Methods
This retrospective study involved seven patients who received 3D‐printed personalized lattice implants for the reconstruction of geographic defects in load‐bearing bones. Personalized implants were customized for each patient. Randomized dodecahedron unit cells were incorporated within the implants to create the porous structure. The pore size and porosity were analyzed. Patient outcomes were assessed through a combination of clinical and radiological evaluations. Tomosynthesis‐Shimadzu metal artifact reduction technology (T‐SMART) was utilized to evaluate osseointegration. Functional outcomes were assessed according to the Musculoskeletal Tumor Society (MSTS) 93 score.
Results
Multiple pore sizes were observed in porous structures of the implant, with a wide distribution range (approximately 300–900 um). The porosity analysis results showed that the average porosity of irregular porous structures was around 75.03%. The average follow‐up time was 38.4 months, ranging from 25 to 50 months. Postoperative X‐rays showed that the implants matched the geographic bone defect well. Osseointegration assessments according to T‐SMART images indicated a high degree of bone‐to‐implant contact, along with favorable bone density around the implants. Patient outcomes assessments revealed significant improvements in functional outcomes, with the average MSTS score of 27.3 (range, 26–29). There was no implant‐related complication, such as aseptic loosening or structure failure.
Conclusion
3D‐printed personalized lattice implants offer an innovative and promising strategy for geographic defect reconstruction in load‐bearing bones. This approach has the potential to match the unique contours and geometry of the geographic bone defect and facilitate osteointegration.
Keywords: 3D‐Printed Personalized Implant, Geographic Defect, Lattice Structure, Load‐Bearing Bone, Osteointegration
3D‐printed personalized lattice implants for geographic defect reconstruction in load‐bearing bones. Randomized dodecahedron unit cells were incorporated within the implants. This approach has the potential to match the unique contours and geometry of the geographic bone defect and facilitate osteointegration.
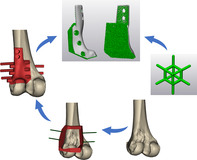
Introduction
Lower extremities are the most common anatomical site for primary malignant bone tumors. 1 Limb salvage surgery has become the preferred treatment method because of improvements in imaging technology, adjuvant chemotherapy, and surgical techniques. 2 , 3 In selected neoplastic patients, geographic tumor resections using multiplanar osteotomies around the lesion could preserve more bone stock for reconstruction, which is believed to enable better limb function. 4 , 5 , 6 , 7 , 8 However, geographic defect reconstruction in load‐bearing bones presents formidable challenges for orthopaedic surgeon. These defects often involve complex three‐dimensional (3D) geometries and compromise both the structural integrity and function of the affected bones. Traditional reconstruction approaches often involve the use of allografts, fibula autografts, or autogenous iliac crest, 5 , 6 , 7 which lack the precision and adaptability required for addressing the unique geometries of these defects.
The development of 3D printing technology has brought great changes to healthcare, especially in the field of orthopaedics. 9 , 10 , 11 , 12 , 13 3D printing technology provides outstanding advantages in customizing implants and devices. The personalized implants enable an accurate match of the patient's anatomy, ensuring optimal fit with bone defects. 14 , 15 , 16 Additionally, 3D printing allows the creation of porous structures that simulate the mechanical properties and trabecular structure of bones. 17 , 18 , 19 Therefore, personalized implants not only enhance surgical precision but also contribute to reduced surgery time and improved postoperative outcomes. The design and manufacture flexibility offered by 3D printing allows for the creation of porous structures within the implant, reducing stress shielding and promoting osteointegration. Hence, the use of 3D‐printed personalized implants presents a compelling opportunity to address the challenges associated with geographic defects in load‐bearing bones.
In recent years, the use of lattice cells has been advanced as a popular method for constructing porous structures of personalized implants, achieving lightweight and excellent interconnectivity. 18 Nevertheless, it's imperative to recognize the natural bone trabecular network is a complex and irregular porous structure. 20 Emerging research has underscored the advantages of irregular lattice porous designs that mirror the properties of natural bone, demonstrating their potential in promoting osteointegration. 21 , 22 Despite the apparent potential of these biomimetic designs, there remains a literature gap concerning their application in 3D‐printed personalized lattice implants, particularly for addressing geographic defects in weight‐bearing bones.
Therefore, this study aims to design, manufacture, and evaluate 3D‐printed personalized implants with irregular lattice porous structures for geographic defect reconstruction in load‐bearing bones, focusing on (i) feasibility of 3D‐printed personalized implants, (ii) osseointegration of irregular lattice porous structures.
Method
Patient Selection and Study Workflow
This is a retrospective study authorized by the Ethics Committee of our hospital. From June 2019 to July 2021, seven patients received 3D‐printed personalized lattice implants for the reconstruction of geographic defects in load‐bearing bones. All the patients meet the following inclusion criteria: (1) primary neoplasm at a load‐bearing bone; (2) geographic tumor resection; (3) follow‐up period of more than 2 years. Exclusion criteria were as follows: (1) life expectancy less than 6 months; (2) allergy to implant materials; (3) lack of complete follow‐up information. There were four females and three males, with a mean age of 29 years. The pathological diagnosis was osteosarcoma in six patients and sarcoma in one patient. Patient demographic and clinical characteristics, including gender, age, and pathological diagnosis were collected and shown in Table 1. Neoadjuvant chemotherapy was performed for all patients according to the guidelines for bone cancer. For each patient, written informed consent was obtained for the use of 3D‐printed personalized lattice implants.
TABLE 1.
Demographics, clinical characteristics, and implant porosity of seven patients.
| Patients | Sex | Age, year | Site | Diagnosis | Enneking zoning | Preoperative chemotherapy | Volume of the bone defects (cm3) | Implant porosity, % |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 44 | Femur | OSA | Metaphysis | 2 | 3.1 × 3.3 × 5.5 | 74.3 |
| 2 | F | 13 | Tibia | SA | Metaphysis | 3 | 2.8 × 3.7 × 9.0 | 75.5 |
| 3 | F | 31 | Femur | OSA | Diaphysis | 2 | 1.8 × 2.9 × 7.8 | 74.9 |
| 4 | F | 58 | Tibia | OSA | Metaphysis | 3 | 3.0 × 3.3 × 8.2 | 75.2 |
| 5 | M | 19 | Femur | OSA | Metaphysis | 3 | 4.5 × 5.1 × 7.5 | 75.1 |
| 6 | M | 25 | Femur | OSA | Metaphysis | 2 | 3.2 × 3.9 × 8.0 | 74.8 |
| 7 | F | 14 | Tibia | OSA | Diaphysis | 2 | 1.9 × 2.8 × 8.3 | 74.9 |
Abbreviations: OSA, osteosarcoma; SA, sarcoma.
This workflow included the following stages: (1) image acquisition and processing; (2) personalized implant and lattice structure design; (3) Ti‐6Al‐4V lattice implant manufacture; (4) tumor resection assisted with 3D‐printed bone‐cutting guides; (5) geographic defect reconstruction with personalized lattice implants; (6) follow‐up and outcomes assessment.
Image Acquisition and Processing
Preoperatively, patients underwent detailed radiographic examinations, including X‐ray, computed tomography (CT), magnetic resonance imaging (MRI), and single photon emission computed tomography (SPECT) for evaluation and preoperative planning (Figure 1). For each patient, CT and MRI data were collected in DICOM format.
FIGURE 1.
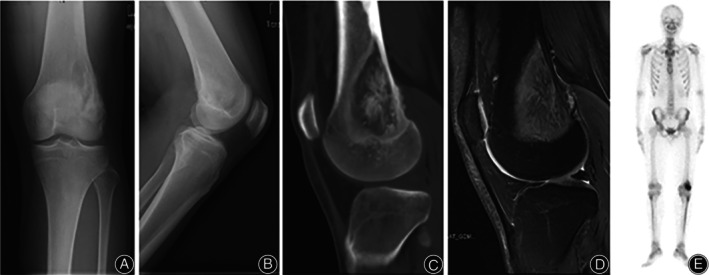
Preoperative anteroposterior (A) and lateral (B) X‐ray scans, computed tomography (CT) scan (C), magnetic resonance imaging (MRI) scan (D), and single photon emission computed tomography (SPECT) scan (E) of a 19‐year‐old patient with highly differentiated low‐grade intramedullary osteosarcoma involving the left femur.
Personalized Implant and Lattice Structure Design
The collected CT data was imported into Mimics V20.0 software (Materialize Corp., Leuven, Belgium) to segment anatomical 3D models of the bone. Following the determination of the precise tumor borders, osteotomy plains were determined. The distance between the tumor border and the osteotomy border was set as 2.0 and 0.5 cm at the diaphysis side and the metaphysis side, respectively. Then, osteotomies were simulated based on the anatomical 3D models. The patient‐specific bone‐cutting guide was designed consistent with the multiple osteotomy plains, with its internal surface fitting the host bone (Figure 2). Several K‐wire holes were set on the osteotomy guide for intraoperative fixation. The preliminary shape of the implant was designed by performing Boolean budgeting on mirrored 3D models from the corresponding normal part and post‐osteotomy anatomical 3D model. After that, specific features, including a side plate, screw holes, and suture holes were added to the implant model (Figures 2 and 3).
FIGURE 2.

Profile of the distal femur personalized implant and lattice structure design. (A) Segmenting anatomical 3D model of the distal femur; (B) simulating geographical tumor resection with a patient‐specific bone‐cutting guide; (C, D) designing the personalized implant; (E, F) incorporating dodecahedron unit cells within the implant; (G. H) porous analysis showed multiple pore sizes in irregular porous structures with a wide distribution.
FIGURE 3.

Profile of the proximal tibia personalized implant and lattice structure design. (A) segmenting anatomical 3D model of the proximal tibia; (B) simulating geographical reconstruction with a personalized implant; (C) details of the personalized implant; (D, E) incorporating dodecahedron unit cells within the implant; (F, G) porous analysis showed multiple pore sizes in irregular porous structures with a wide distribution.
3‐Matic V15.0 software (Materialize Corp., Leuven, Belgium) was employed to create the lattice structure. The dodecahedron unit cells were strategically incorporated to optimize biomechanical performance and osseointegration within the implant design. Unit cell parameters were adjusted, including unit cell size (2.6 mm) and strut thickness (400 μm). Then, the filling parameter was set as randomization. The pore size and porosity were analyzed (Figure S1). The finalized lattice structure design files were exported in the STL format.
Ti‐6Al‐4V Lattice Implant Manufacture
The lattice implants were fabricated using the electron beam melting (EBM) machine (ARCAM Q10plus, Mölndal, Sweden), utilizing the Ti‐6Al‐4V alloy powder (Chunli Corp., Beijing, China). Patient‐specific bone‐cutting guides were fabricated by selective laser sintering with nylon powder. In addition, resin anatomical 3D models were prepared. Preoperatively, re‐simulation was performed to ensure that the manufactured bone‐cutting guide and lattice implant matched resin anatomical 3D models with lesion and simulated resection, respectively.
Surgical Procedure
All the surgeries were performed by the same senior surgeon (CQT). An incision was made over the affected area to provide adequate access to the lesion in the femur or tibia. After visualization of the surgical site, multiplane osteotomies were performed assisted with the patient‐specific bone‐cutting guide. The bone bed was checked and shaped to ensure a precise fit for the 3D‐printed personalized implant (Figure 4). The implant was carefully inserted into the prepared bone defect. Following the confirmation of the implant position, screws were inserted to secure the implant in place. After successful implant placement, soft tissues, including muscles and tendons, were meticulously repositioned and sutured.
FIGURE 4.

Intraoperative photos of the reconstruction of the geographical defect with 3D‐printed Ti‐6Al‐4V lattice implant.
Follow‐Up and Outcomes Assessment
The patient was followed monthly during the first 3 months, and every 3 months thereafter. Patient outcomes were assessed through a combination of clinical and radiological evaluations. In detail, X‐rays and CT scans were used to monitor implant place, stability, and alignment. Tomosynthesis‐Shimadzu metal artifact reduction technology (T‐SMART) was utilized to evaluate osseointegration. Functional outcomes were assessed according to the Musculoskeletal Tumor Society (MSTS) 93 score, which includes pain, function, emotional acceptance, use of supports, walking ability, and gait. 23
Results
Ti‐6Al‐4V Lattice Implants Design
The personalized lattice implants matched the unique contours and geometry of the geographic bone defect. The implant‐plate connection was a solid part to ensure mechanical strength. Particularly, the popliteal surface of the distal femur implants was a solid part to protect the posterior popliteal artery, which is adjacent to the popliteal surface of the distal femur and the posterior part of the knee joint capsule.
Through porous analysis in 3‐Matic, multiple pore sizes in irregular porous structures were observed (Figures 2G,H and 3F,G), with a wide distribution range (approximately 300–900 um). The porosity analysis results showed that the average porosity of irregular porous structures was around 75.03% in these seven samples, ranging from 74.8% to 75.5%.
Clinical and Radiologic Outcomes
The average follow‐up time was 38.4 months, ranging from 25 to 50 months (Table 2). No intraoperative complication was observed, such as periprosthetic fracture, implant breakage, or vascular and nerve damage. Negative surgical margins were achieved in all patients according to postoperative pathological examination of specimens. During the follow‐up period, no local recurrence or tumor metastasis was detected. Postoperative X‐rays showed that 3D‐printed personalized lattice implants matched the geographic bone defect well. In addition, osseointegration assessments according to T‐SMART images indicated a high degree of bone‐to‐implant contact, along with favorable bone density around the implants (Figure 5). Patient outcomes assessments revealed significant improvements in functional outcomes, with the average MSTS score of 27.3 (range, 26–29). Patients reported enhanced mobility and reduced discomfort. There was no implant‐related complication, such as aseptic loosening or structure failure.
TABLE 2.
Surgery details and follow‐up outcomes of seven patients.
| Patients | Operative time, minutes | Intraoperative bleeding, ml | Follow‐up, months | MSTS score | Complications | Oncological status |
|---|---|---|---|---|---|---|
| 1 | 130 | 100 | 50 | 28 | NA | NED |
| 2 | 170 | 150 | 47 | 27 | NA | NED |
| 3 | 180 | 250 | 45 | 26 | NA | NED |
| 4 | 150 | 150 | 39 | 29 | NA | NED |
| 5 | 160 | 150 | 34 | 28 | NA | NED |
| 6 | 120 | 100 | 29 | 26 | NA | NED |
| 7 | 120 | 100 | 25 | 27 | NA | NED |
Abbreviations: NA, none; NED, no evidence of disease.
FIGURE 5.

Osseointegration assessment over time using continuous Tomosynthesis‐Shimadzu metal artifact reduction technology (T‐SMART) images: (A) 3 months after surgery, early indications of osseointegration and favorable bone density surrounding the 3D‐printed lattice implant; (B) 6 months after surgery, the ongoing development of osseointegration; (C) 9 months after surgery: the ongoing development of osseointegration; (D) 1 year after surgery: enhanced bone‐to‐implant contact and continued osseointegration; (E) 1.5 years after surgery: continued osseointegration at the plate; (F) 2 years after surgery: continued osseointegration at the plate.
Discussion
3D‐printed patient‐specific implant as an innovative approach has the potential to revolutionize the field of bone defect reconstruction. In the present study, 3D‐printed personalized lattice implants were used to reconstruct geographic defects in load‐bearing bones. Our results revealed that the implants matched the unique contours and geometry of the geographic bone defect and facilitated osteointegration.
Feasibility of 3D‐Printed Personalized Implants
One of the key strengths of 3D‐printed personalized implants is the ability to be tailored to the unique defect of each patient. 24 In the past with grafts (autologous or allogeneic) for geographic defect reconstruction, surgeons had to spend time meticulously cutting the grafts to adapt to the defects. 5 , 6 Meanwhile, the accuracy of the grafts was unsatisfactory through this free‐handed method. Furthermore, patients were at risk of complications such as graft absorption and non‐union. 8 Compared with bone grafting, 3D‐printed personalized implants used in this work ensured a precise fit with the geographic defect and minimized the need for intraoperative adjustments. In addition, the implant‐plate connection was designed as a sturdy solid component to maintain mechanical strength, and a solid popliteal surface was added to the distal femoral implant, specifically to protect the posterior popliteal artery. All these demonstrate the design flexibility of personalized implants. Nevertheless, it was worth noting that 3D‐printed personalized implants have higher requirements for the accuracy of tumor resection and must be consistent with surgical planning. Computer navigation and patient‐specific bone‐cutting guides are commonly used techniques. 4 , 8 In the present study, relatively simple bone‐cutting guides were used. Postoperative X‐rays showed that the implants matched the geographic bone defect well. In addition, postoperative pathology examination of the specimen showed negative margins. Therefore, the strategy of using 3D‐printed personalized implants for geographic defect reconstruction was feasible following tumor resection assisted with the bone‐cutting guides.
Osseointegration of Irregular Lattice Porous Structures
Stress shielding arises from discrepancies between the mechanical properties of the implant and the adjacent bone, which can lead to bone resorption and subsequent implant loosening. 25 Traditional solid implants, due to their often significantly stiffer properties compared to natural bone, can exacerbate this stress shielding. However, with the advent of 3D printing, there has been a transformative shift in implant design. 3D‐printed porous implants offer a unique solution to address the stress shielding dilemma. 26 By introducing controlled porosity into the implant, it is possible to adjust its mechanical properties, making them more closely match those of the adjacent bone. In the present study, the implants were designed with irregular lattice porous structure, possessing a reasonable porosity of 75% approximately. By doing so, the stiffness difference between the implant and the bone was reduced remarkably, thereby reducing the possibility of stress shielding. This would ensure a more uniform distribution of mechanical loads across the bone‐implant interface. In addition, irregular porous structures, closely resembling the intricate network of bone trabeculae, offer significant advantages in promoting effective bone integration when used in 3D‐printed porous implant design. 27 , 28 This biomimetic design enhances cell infiltration, nutrient diffusion, and osteogenic cell adhesion, creating an environment conducive to rapid osseointegration. 20 In the present study, irregular porous structures designed using randomly distributed dodecahedral unit cells exhibit pore size diversity and complexity. In our cases, T‐SMART images indicated a high degree of bone‐to‐implant contact, along with favorable bone density around the implants. The positive results indicated that this irregular lattice implant has the potential to reduce stress shielding and facilitate osseointegration.
Strengths and Limitations
This study presents an innovative approach by utilizing 3D‐printed personalized lattice implants for geographic defect reconstruction. This approach offers the advantage of tailoring implants to the unique defect of each patient. The incorporation of irregular lattice porous structures shows the advanced design of our implants. This biomimetic design, inspired by natural bone trabeculae, has the potential to promote osseointegration.
Nevertheless, this study has several limitations. Firstly, being a retrospective study, there is no comparison group included. Secondly, this study involved a relatively small cohort of seven patients, which could potentially affect the generalizability of our findings. While this study includes a follow‐up period ranging from 25 to 50 months, extended observations to thoroughly assess the long‐term stability and performance of the 3D‐printed implants are needed.
Prospects of Clinical Application
The use of 3D‐printed personalized lattice implants has shown promise in improving functional outcomes for patients. By restoring the natural biomechanics of the affected joint or bone, patients often experience good mobility and reduced pain. This can significantly improve their overall quality of life.
Conclusion
This study involved a retrospective analysis of seven patients who underwent 3D‐printed personalized lattice implants for the reconstruction of geographic defects in load‐bearing bones. The comprehensive evaluation included clinical and radiological assessments. The results demonstrated that the personalized implants successfully matched the unique contours of the geographic bone defects, promoting significant improvements in functional outcomes. The lattice structure design showed multiple pore sizes with a wide distribution range, emphasizing the biomimetic nature of the irregular lattice porous structures. T‐SMART images revealed a high degree of bone‐to‐implant contact and favorable bone density around the implants. Notably, the absence of implant‐related complications, such as aseptic loosening or structure failure, further strengthened the positive outcomes of this technique. In conclusion, 3D‐printed personalized lattice implants offer an innovative and promising strategy for reconstructing geographic defects in load‐bearing bones.
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
Ethics Statement
This study was performed in accordance with the Declaration of Helsinki as revised in 2008 and was approved by the Ethics Committee of West China Hospital. The patients signed the informed consent form before surgery.
Author Contributions
ZZL, MXL, LM, and CQT were involved with the concept and design of this manuscript. YQZ, JW, and TJG were involved with the acquisition of the subject and data. ZZL, YQZ, JW, and CQT were involved in the design of the prosthesis. YTW, XHH, YL, YZ, LM, and CQT were involved in the postsurgical evaluation of the patient. All authors contributed to the article and approved the submitted version.
Funding Information
This work was supported by 1·3·5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC18036) and the Science and Technology Research Program of Sichuan Province (2022YFG0109).
Supporting information
Figure S1. The formula for calculating the porosity of the personalized Ti‐6Al‐4V lattice implant.
Zhuangzhuang Li and Minxun Lu contributed equally to this study and share first authorship.
Contributor Information
Li Min, Email: minli1204@scu.edu.cn.
Chongqi Tu, Email: tucq@scu.edu.cn.
References
- 1. Nomikos GC, Murphey MD, Kransdorf MJ, Bancroft LW, Peterson JJ. Primary bone tumors of the lower extremities. Radiol Clin. 2002;40(5):971–990. [DOI] [PubMed] [Google Scholar]
- 2. Liu Q, He H, Duan Z, Zeng H, Yuan Y, Wang Z, et al. Intercalary allograft to reconstruct large‐segment diaphysis defects after resection of lower extremity malignant bone tumor. Cancer Manag Res. 2020;12:4299–4308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanders P, Spierings J, Albergo J, Bus MPA, Fiocco M, Farfalli GL, et al. Long‐term clinical outcomes of intercalary allograft reconstruction for lower‐extremity bone tumors. J Bone Joint Surg Am. 2020;102(12):1042–1049. [DOI] [PubMed] [Google Scholar]
- 4. Wong KC, Sze LKY, Kumta SM. Complex joint‐preserving bone tumor resection and reconstruction using computer navigation and 3D‐printed patient‐specific guides: a technical note of three cases. J Orthopaedic Transl. 2021;29:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Agarwal M, Puri A, Anchan C, Shah M, Jambhekar N. Hemicortical excision for low‐grade selected surface sarcomas of bone. Clin Orthop Relat Res. 2007;459:161–166. [DOI] [PubMed] [Google Scholar]
- 6. Avedian RS, Haydon RC, Peabody TD. Multiplanar osteotomy with limited wide margins: a tissue preserving surgical technique for high‐grade bone sarcomas. Clin Orthop Relat Res. 2010;468:2754–2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Deijkers R, Bloem R, Hogendoorn P, Verlaan JJ, Kroon HM, Taminiau AHM. Hemicortical allograft reconstruction after resection of low‐grade malignant bone tumours. J Bone Joint Surg Br. 2002;84(7):1009–1014. [DOI] [PubMed] [Google Scholar]
- 8. Benady A, Meyer JS, Ran Y, Mor Y, Gurel R, Rumack N, et al. Intercalary and geographic lower limb tumor resections with the use of 3D printed patient specific instruments‐when less is more. J Orthop. 2022;32:36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alemayehu DG, Zhang Z, Tahir E, et al. Preoperative planning using 3D printing technology in orthopedic surgery. Biomed Res Int. 2021;2021:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malik HH, Darwood AR, Shaunak S, Kulatilake P, el‐Hilly AA, Mulki O, et al. Three‐dimensional printing in surgery: a review of current surgical applications. J Surg Res. 2015;199(2):512–522. [DOI] [PubMed] [Google Scholar]
- 11. Kumar Gupta D, Ali MH, Ali A, et al. 3D printing technology in healthcare: applications, regulatory understanding, IP repository and clinical trial status. J Drug Target. 2022;30(2):131–150. [DOI] [PubMed] [Google Scholar]
- 12. Bozkurt Y, Karayel E. 3D printing technology; methods, biomedical applications, future opportunities and trends. J Mater Res Technol. 2021;14:1430–1450. [Google Scholar]
- 13. Bhushan J, Grover V. Additive manufacturing: current concepts, methods, and applications in oral health care. Biomanufacturing. Cham: Springer; 2019. p. 103–122. [Google Scholar]
- 14. Wong K, Kumta S, Geel N, Demol J. One‐step reconstruction with a 3D‐printed, biomechanically evaluated custom implant after complex pelvic tumor resection. Comput Aided Surg. 2015;20(1):14–23. [DOI] [PubMed] [Google Scholar]
- 15. Zhao D, Tang F, Min L, Lu M, Wang J, Zhang Y, et al. Intercalary reconstruction of the “ultra‐critical sized bone defect” by 3D‐printed porous prosthesis after resection of tibial malignant tumor. Cancer Manag Res. 2020;12:2503–2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu L, Qin H, Tan J, Cheng Z, Luo X, Tan H, et al. Clinical study of 3D printed personalized prosthesis in the treatment of bone defect after pelvic tumor resection. J Orthopaedic Transl. 2021;29:163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang B, Pei X, Zhou C, Fan Y, Jiang Q, Ronca A, et al. The biomimetic design and 3D printing of customized mechanical properties porous Ti6Al4V scaffold for load‐bearing bone reconstruction. Mater Des. 2018;152:30–39. [Google Scholar]
- 18. Pei X, Wu L, Zhou C, Fan H, Gou M, Li Z, et al. 3D printed titanium scaffolds with homogeneous diamond‐like structures mimicking that of the osteocyte microenvironment and its bone regeneration study. Biofabrication. 2020;13(1):015008. [DOI] [PubMed] [Google Scholar]
- 19. Kumawat S, Deshmukh SR, Ghorpade R. Fabrication of Ti‐6Al‐4v cellular lattice structure using selective laser melting for orthopedic use: a review. Mater Today Proc. 2023;8:53‐70. [Google Scholar]
- 20. Chao L, Jiao C, Liang H, Xie D, Shen L, Liu Z. Analysis of mechanical properties and permeability of trabecular‐like porous scaffold by additive manufacturing. Front Bioeng Biotechnol. 2021;9:779854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pei X, Wang L, Zhou C, Wu L, Lei H, Fan S, et al. Ti6Al4V orthopedic implant with biomimetic heterogeneous structure via 3D printing for improving osteogenesis. Mater Des. 2022;221:110964. [Google Scholar]
- 22. Pei X, Wu L, Lei H, Zhou C, Fan H, Li Z, et al. Fabrication of customized Ti6AI4V heterogeneous scaffolds with selective laser melting: optimization of the architecture for orthopedic implant applications. Acta Biomater. 2021;126:485–495. [DOI] [PubMed] [Google Scholar]
- 23. Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res (1976–2007). 1993;286:241–246. [PubMed] [Google Scholar]
- 24. Mobbs RJ, Coughlan M, Thompson R, Sutterlin CE, Phan K. The utility of 3D printing for surgical planning and patient‐specific implant design for complex spinal pathologies: case report. J Neurosurg Spine. 2017;26(4):513–518. [DOI] [PubMed] [Google Scholar]
- 25. Arabnejad S, Johnston B, Tanzer M, Pasini D. Fully porous 3D printed titanium femoral stem to reduce stress‐shielding following total hip arthroplasty. J Orthop Res. 2017;35(8):1774–1783. [DOI] [PubMed] [Google Scholar]
- 26. Abar B, Alonso‐Calleja A, Kelly A, Kelly C, Gall K, West JL. 3D printing of high‐strength, porous, elastomeric structures to promote tissue integration of implants. J Biomed Mater Res A. 2021;109(1):54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Z, Zhang M, Liu Z, Wang Y, Dong W, Zhao S, et al. Biomimetic design strategy of complex porous structure based on 3D printing Ti‐6Al‐4V scaffolds for enhanced osseointegration. Mater Des. 2022;218:110721. [Google Scholar]
- 28. Ren B, Wan Y, Liu C, Wang H, Yu M, Zhang X, et al. Improved osseointegration of 3D printed Ti‐6Al‐4V implant with a hierarchical micro/nano surface topography: an in vitro and in vivo study. Mater Sci Eng C. 2021;118:111505. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. The formula for calculating the porosity of the personalized Ti‐6Al‐4V lattice implant.


