Abstract
Introduction
Gastric cancer (GC) is the third leading cause of global cancer-related mortality. Despite the shifting burden of GC to low-and middle-income countries, the data regarding incidence, treatment, and outcomes in these settings are sparse. The primary aim of this systematic review was to aggregate all available data on GC in sub-Saharan Africa (SSA) to describe the variability in incidence across the region.
Methods
Studies reporting population-based primary data on GC in SSA were considered. The inclusion was limited to primary studies published between January 1995 and March 2022 which comprised of adult patients in SSA with GC. Studies without accessible full text in either French or English language were excluded. Unadjusted GC incidence rates with their standard errors for each study were recalculated from the crude numerators and denominators provided in individual studies.
Results
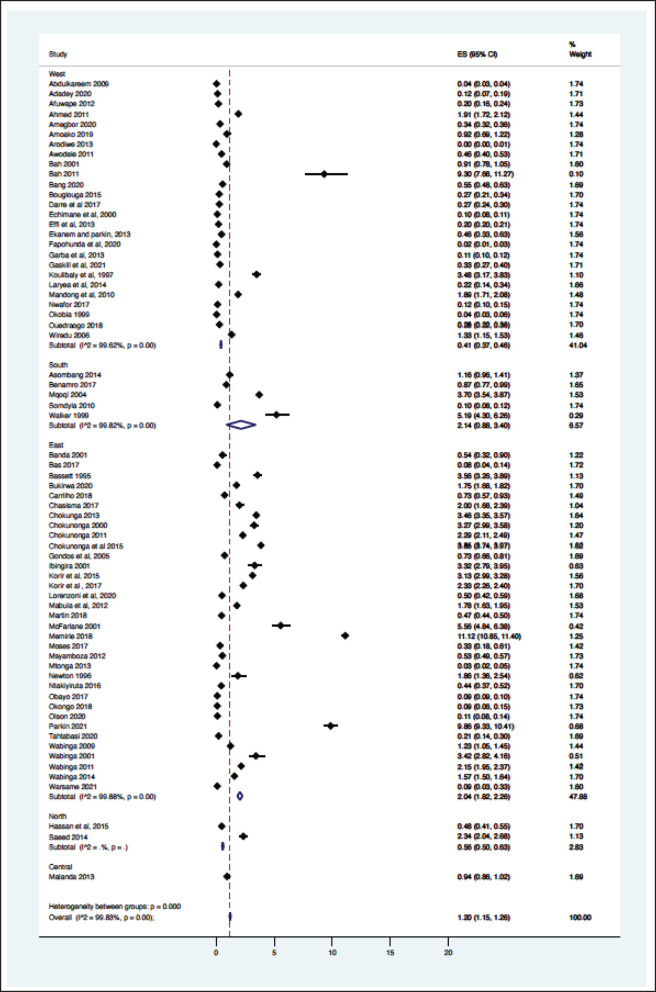
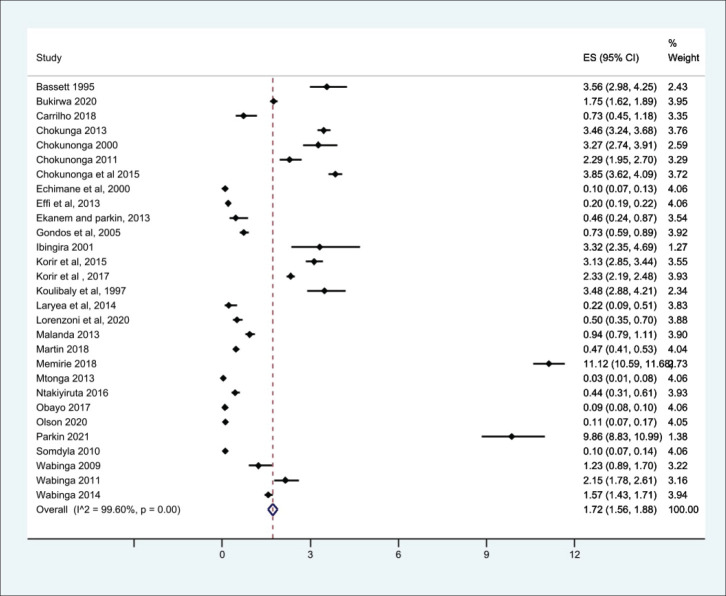
A total of 5,626 articles were identified in the initial search, of which, 69 studies were retained. Reported incidence rates ranged from a high of 5.56 GC cases per 100,000 in Greater Meru Kenya to a low of 0.04 GC cases per 100,000 people in Benin City Nigeria. The overall crude pooled incidence was 1.20 GC cases per 100, 000 (95%CI 1.15–1.26) with a variability of 99.83% (I2 p < 0.001). From the 29 high-quality population-based registry studies the crude pooled incidence was 1.71 GC cases per 100,000 people (95%CI 1.56–21.88) with a variability of 99.60%.
Conclusion
This systemic review demonstrates that GC incidence is highly variable across SSA. The limited data on GC treatment, mortality, and survival presents a significant challenge to providing a complete epidemiologic description of the burden of GC in SSA. There is a need for further robust data collection, exploration, and research studies on cancer care in SSA, with continued assessment of primary data availability.
Keywords: gastric cancer, adenocarcinoma, Sub-Saharan Africa, systematic review, incidence, epidemiology
Strengths and limitations of this study
Limitations
The employed search strategy is open to the biases of the search engines available to authors, potentially missing non-indexed reports published in regional journals.
Calculations in the study were limited by the availability of regional and population- based estimates that were truly reflective of a given study’s sample population.
Sparse GC data are available for the SSA countries.
Histological confirmation was reported in less than 50% of the studies which may have positively skewed the GC incidence. Histological confirmation is needed for accurate data reporting and scarcity of histological information could affect the epidemiology findings for GC incidence.
Strengths
This systematic review (SR) included studies which comprised of only primary data.
This study is the only SR on GC in SSA.
This study provides the most stringent review of high-quality data available to date to better inform efforts to bolster regional data collection.
Introduction
Globally gastric cancer (GC) is the fifth most common malignancy with over 1,250,000 new cases diagnosed annually. GC causes over 950,000 deaths each year and ranks as the third leading contributor to global cancer-related mortality [2]. While historically, the burden of GC has been attributed to higher-income countries, the shifting burden of non-communicable disease now attributes over 80% of GC-related deaths to low- and middle-income countries (LMICs) [2, 3]. The World Health Organisation 2020 report on cancer estimated 22,992 incident GC cases within Sub-Saharan Africa (SSA), amounting to over 20,000 deaths per year [4].
Current epidemiologic data on the burden of GC in LMICs is sparse [5]. GC is a multifactorial disease, impacted by genetic and environmental factors that result in wide epidemiologic variation. Up to 20-fold differences in incidence rates have been reported between different geographic regions [8]. Accurate epidemiologic data has historically been dependent on population-based registries. In the absence of high-quality data, cancer incidence, and mortality have been reported by relying on mathematical estimates, which may not appreciate the regional heterogenicity of GC rates or may under-represent GC incidence [9]. This is especially true for SSA, where only 10% of the population is included in population-based cancer registry data collection [10]. Further knowledge of GC incidence, treatment, and outcome is necessary for adequate treatment and public health planning.
The primary aim of this systematic review (SR) is to aggregate all available data on GC in SSA to describe the incidence rates and rate variability of GC in adults in SSA countries. Secondary aims include describing the treatment and mortality of GC in adults from SSA. The hypothesis is that GC is highly variable across the SSA region and this analysis has used primary studies to demonstrate this. The importance of GC variability indicates that the flow of risk factors to disease outcome differs by location. This is critical for public health planning which indicates that treatment and management approaches need to be customised to the variable GC situation at each location. This analysis will allow for a better understanding of the GC disease burden in SSA.
Methods
Eligibility criteria
Studies reporting population-based primary data on in SSA were considered. Country inclusion was in accordance with The World Bank definition of SSA. Primary data published between January 1995 and March 2022 which included adult patients in SSA with GC were included. Studies without accessible full text in either French or English language were excluded. All attempts to access texts and data were made, including contacting corresponding authors of publications. There was no involvement from any patients or the public in this SR.
Information sources, search strategy and study selection
The terms ‘GC’ OR ‘stomach cancer’ OR ‘gastric carcinoma’ OR ‘cancer of the stomach’ OR ‘stomach adenocarcinoma’ were queried when they appeared in the title, abstract or keyword of studies. The names of the SSA countries were applied without language restrictions. A full search strategy is included in Appendix 1. Published studies were identified through a comprehensive search of the following electronic databases; Web of Science, Embase, PubMed and Google Scholar. Duplicate studies were identified and removed. Abstracts were then screened by three authors (AR, PM, CG) to assess eligibility using the predetermined inclusion criteria. Full-text articles were then accessed and reviewed in detail to confirm appropriate inclusion and to extract relevant data. Additional citations were culled and included from the references of articles identified in the initial search. Citations were tracked using Zotero (6.0.8).
Publication bias and heterogeneity
Risk of bias was limited by maintaining a wide search criterion across multiple high quality electronic databases with a diversity of indexed articles.
Data extraction
Data collection included the following variables: surname of primary author, publication year, the country where the study was conducted, the study design, diagnostic method, the age group (age range including >18 years old), sample size, the number of GC reported, the overall cancer case number for the study population, the overall population size, regional or national population, differences by age or gender, treatment type, and the case fatality rate of GC in the study. Unadjusted GC incidence rates with their standard errors for each study were recalculated from the crude numerators and denominators provided in individual studies. Where denominators were not available regional and national population sizes were identified using a variety of sources such as macrotends.net, worldbank.org and countryeconomy.com [22–24]. The lowest population size value was used after verifying the values between various sources. Sub-group break downs (age, gender, race/ethnicity) were included. Age standardised rates were recorded without attempts to calculate backwards. Overall mortality and treatment strategies were recorded in crude numbers and percentages. To achieve a high level of reliability, three reviewers (AR, PM, CG) assessed the same articles and reconciled differences before adopting a final and complete data collection document.
Quality criteria
The strengthening reporting of observational studies in epidemiology (STROBE) checklist was used to assess the quality of the included studies. The final studies included in this SR were assessed using the STROBE checklist and ranked on the following criteria: (1) Inclusion of study in Cancer in Five Continents according to the International Agency for Research on Cancer (IARC) or the data was from a population-based registry [25] (2) 100% histologic confirmation of cancer from a regional registry or national registry (3) >80% histologic confirmation of cancer from a hospital registry (4) histologic confirmation of cancer from any type of registry. The highest rank went to papers included in ‘Cancer in Five Continents’ and regional registries. The second highest rank included regionally or nationally representative studies that were >80% histologically confirmed. The third-ranking category included hospital or pathology registries that showed histological confirmation. The final ranking category included registries that were not histologically confirmed. The quality of studies was given a rank from one to four.
Data synthesis and analysis
Unadjusted incidence with their standard errors for each study was recalculated based on the information of crude numerators and either denominators provided by individual studies or the regional population sizes. Regional and national population sizes were identified within the paper and when not reported, they were identified using online sources such as macrotends.net, worldbank.org and countryeconomy.com. Incidence rate patterns of GC across the various nations in SSA after recalculation were summarised. Descriptive analysis was done for the GC treatment and mortality studies. All data was stored in excel (Microsoft Inc, Redmond, WA, USA) analyses were conducted using StataSE (version 15.1, College Station, Texas, USA).
Results
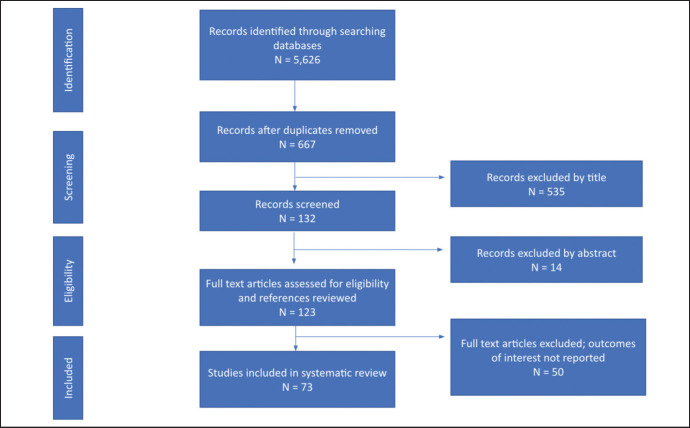
The database search returned a total of 5,626 articles including primary studies, case reports and reviews. Papers were collected from PubMed (694), Web of Science (4,887), and Google Scholar (45). After duplicates were removed there were 667 unique article titles left. Once the initial screening for titles including incidence of cancer in SSA was complete, 132 studies remained. From the 132 remaining studies, 14 papers were excluded by abstract alone, and the remaining 123 full-text articles were assessed for eligibility. References were searched but did not reveal additional relevant titles. After applying the selection criteria, 69 studies were finally retained in this review (Supplementary Figure S1).
From the 69 retained studies, we identified that the data collection was conducted across 50 unique study sites (regions or cities) and 23 countries. Most studies were conducted in East Africa [32] with Uganda appearing nine times across six unique locations, Zimbabwe appearing six times in both Harare and Bulawayo and Malawi appearing five times in Blantyre and Lilongwe (Supplementary Figure S2). West Africa had the second most studies [25]; Nigeria produced a total of nine studies across seven separate locations. East Africa and West Africa contributed 43% and 34% of the included studies respectively. North Africa provided studies from Sudan and South Africa provided four studies from Durban and the Eastern Cape (Table 1). Namibia, Botswana, South Sudan, Central African Republic, Chad, Niger and Mauritania are the SSA countries with missing primary GC data (Supplementary Figure S2).
Table 1. Characteristics of included studies describing GC incidence in SSA.
| Reference number | Author | Country | Region/City | Study period | Regional population | Study cancer population | GC cases | % Histologically confirmed GC cases | Registry type | Age group | Quality rank |
|---|---|---|---|---|---|---|---|---|---|---|---|
| [41] | Abdulkareem et al 2009 | Nigeria | 1995–2006 | 220,000,000 | 713 | 78 | 100 | Regional | All | 2 | |
| [42] | Adadey et al 2020 | Ghana | Volta | 2012–2014 | 6,900,000 | 567 | 8 | 0 | Regional | All | 3 |
| [43] | Amégbor et al 2020 | Togo | 1984–2008 | 112,000,000 | 5,251 | 379 | 100 | Pathology | All | 3 | |
| [44] | Afuwape et al 2012 | Nigeria | 2004–2009 | 25,000,000 | 49 | 89 | Hospital | Adults | 3 | ||
| [45] | Ahmed et al 2011 | Nigeria | Zaria | 1995–2009 | 9,375,000 | 179 | 100 | Hospital | Adults | 2 | |
| [46] | Amoako et al 2019 | Ghana | 2015 | 2,616,000 | 736 | 24 | 0 | Regional | All | 3 | |
| [48] | Arodiwe et al 2013 | Nigeria | 1995–2010 | 158,000,000 | 335 | 4 | 0 | Hospital | Adults | 4 | |
| [49] | Asombang et al 2014 | Zambia | Lusaka | 2010–2012 | 4,384,000 | 51 | 100 | Hospital | All | 3 | |
| [50] | Awodele et al 2011 | Nigeria | Lagos, Oyo | 2005–2009 | 48,000,000 | 5,094 | 221 | 0 | Hospital | All | 4 |
| [51] | Bah et al 2001 | Gambia | 1988–1997 | 10,152,000 | 2,957 | 92 | 21 | National | Al | 3 | |
| [52] | Bah et al 2011 | Gambia | 1993–1997 | 559,000 | 52 | 19 | National | All | 3 | ||
| [53] | Banda et al 2001 | Malawi | Blantyre | 1994–1998 | 2,600,000 | 2,259 | 14 | 39 | Regional | All | 4 |
| [54] | Bang et al 2020 | Cameroon | 2013–2018 | 19,000,000 | 1,105 | 105 | 100 | Hospital | All | 3 | |
| [55] | Baş et al 2017 | Somalia | 2016–2017 | 14,190,000 | 403 | 11 | 0 | Regional | Adults | 4 | |
| [57] | Bassett et al 1995 | Zimbabwe | Harare | 1990–1992 | 3,400,000 | 2,716 | 121 | 50 | Regional | All | 1 |
| [58] | Benamro et al 2017 | South Africa | Durban | 2009–2014 | 15,000,000 | 131 | 100 | Hospital | All | 3 | |
| [60] | Bouglouga et al 2015 | Togo | Lome | 2005–2012 | 11,200,000 | 250 | 30 | 100 | Hospital | All | 3 |
| [13] | Bukirwa et al 2021 | Uganda, | Kampala | 1991–2015 | 38,007,000 | 31,357 | 665 | 0 | Regional | All | 1 |
| [61] | Carrilho et al 2019 | Mozambique | Maputo | 2015–2016 | 2,200,000 | 1,705 | 16 | 76 | Regional | All | 1 |
| [62] | Chasimpha et al 2017 | Malawi | Blantyre | 2008–2010 | 2,046,000 | 3,711 | 41 | 100 | Regional | All | 2 |
| [63] | Chokunonga et al 2013 | Zimbabwe | Harare | 1991–2010 | 27,000,000 | 28,319 | 933 | 0 | Regional | All | 1 |
| [63] | Chokunonga et al 2000 | Zimbabwe | Harare | 1993–1995 | 3,696,000 | 3,571 | 121 | 59 | Regional | All | 1 |
| [64] | Chokunonga et al 2011 | Zimbabwe, | Harare | 1993–1997 | 6,278,000 | 2,300 | 144 | 67,4 | Regional | All | 1 |
| [65] | Chokunonga et al 2015 | Zimbabwe | Harare | 1991–2010 | 27,000,000 | 37,787 | 1,040 | 0 | Regional | All | 1 |
| [65] | Darre et al 2017 | Togo | Lome | 2009–2016 | 54,968,097 | 1,738 | 147 | 100 | Hospital | All | 3 |
| [66] | Echimane et al 2000 | Ivory Coast | Abidjan | 1995–1997 | 38,007,000 | 2,815 | 37 | 0 | Regional | All | 1 |
| [67] | Effi et al 2013 | Ivory Coast | Abidjan | 1984–2009 | 384,000,000 | 12,841 | 782 | 100 | Regional | All | 1 |
| [68] | Ekanem and Parkin 2016 | Nigeria | Calabar | 2009–2013 | 1,967,000 | 719 | 9 | 100 | Regional | All | 1 |
| [70] | Fapohunda et al 2020 | Nigeria | Lagos | 2015 | 50,800,000 | 548 | 9 | 0 | Hospital | All | 4 |
| [72] | Garba et al 2013 | Niger | 1992–2009 | 212,000,000 | 7,031 | 236 | 42 | National | All | 3 | |
| [73] | Gaskill et al 2021 | Ghana | 2014–2015 | 30,000,000 | 2,562 | 98 | 0 | Hospital | Adults | 4 | |
| [74] | Gondos et al 2005 | Uganda | Kampala | 1993–1997 (2002 follow-up) | 12,500,000 | 1,831 | 91 | 48 | Regional | Adults | 1 |
| [76] | El Hassan et al 2015 | Sudan | Khartoum | 2000–2004 | 18,450,000 | 1,958 | 88 | 100 | Hospital | Adults | 3 |
| [77] | Ibingira 2001 | Uganda | Kampala | 1995 | 964,000 | 32 | 100 | Hospital | Adults | 1 | |
| [80] | Korir et al 2015 | Kenya | Nairobi | 2004–2008 | 13,922,000 | 8,982 | 436 | 83 | Regional | All | 1 |
| [81] | Korir et al 2017 | Kenya | Nairobi | 2000–2014 | 43,700,000 | 1,019 | 0 | Regional | All | 1 | |
| [82] | Koulibaly et al 1997 | Guinea | Conakry | 1992–1995 | 3,043,300 | 2,064 | 106 | 0 | National | All | 1 |
| [83] | Laryea et al 2014 | Ghana | Kumasi, | 2012 | 2,300,000 | 253 | 5 | 74 | Regional | All | 1 |
| [84] | Lorenzoni et al 2020 | Mozambique | Beira and Maputo | 201–4–2017 | 6,629,000 | 4,373 | 33 | 70 | Regional | All | 1 |
| [6] | Mabula et al 2012 | Tanzania | Mabula | 2006–2011 | 13,000,000 | 5,134 | 232 | 100 | Hospital | Adults | 3 |
| [93] | Nsondé Malanda 2013 | Congo | Brazaville | 1998–2009 | 14,428,000 | 135 | 0 | Regional | All | 1 | |
| [85] | Mandong et al 2010 | Nigeria | Plataeu State | 1985–2004 | 10,862,000 | 5,705 | 205 | 100 | Hospital | All | 3 |
| [85] | Martin et al 2018 | Rwanda | 2012–2016 | 51,000,000 | 229 | 100 | Hospital | All | 1 | ||
| [86] | McFarlane et al 2001 | Kenya | Greater Meru | 1991–1993 | 3,600,000 | 200 | 0 | Hospital | Adults | 4 | |
| [87] | Memirie et al 2018 | Ethiopia | Addis Ababa | 2012–2015 | 14,539,000 | 64,285 | 1,617 | 89 | Regional | All | 1 |
| [89] | Moses et al 2017 | Malawi | Lilongwe | 2009–2012 | 3,000,000 | 1,453 | 10 | 0 | Hospital | Adults | 4 |
| [90] | Msyamboza et al 2012 | Malawi | 2007–2010 | 56,400,000 | 18,946 | 299 | 0 | National | All | 4 | |
| [91] | Mtonga et al 2013 | Malawi | Blantyre | 2010–2010 | 15,000,000 | 244 | 5 | 100 | Hospital | All | 3 |
| [93] | Mqoqi et al 2004 | South Africa | 1998–1999 | 60,908 | 1,999 | 0 | National | All | 1 | ||
| [92] | Newton et al 1996 | Rwanda | Butare | 1991–1993 | 2,100,000 | 455 | 39 | 0 | Regional | All | 4 |
| [94] | Ntakiyiruta 2009 | Rwanda | 2006–2007 | 8,000,000 | 35 | 57 | Hospital | All | 1 | ||
| [95] | Nwafor and Nwafor 2018 | Nigeria | Akwa Ibom | 2007–2015 | 36,391,420 | 1,186 | 45 | 100 | Hospital | All | 3 |
| [96] | Obayo et al 2017 | Uganda | 5 regional hospitals | 2002–2011 | 292,000,000 | 270 | 100 | Hospital | All | 1 | |
| [98] | Okobia et al 1999 | Nigeria | Benin City | 1989–1998 | 104,500,000 | 816 | 44 | 36 | Hospital | all | 4 |
| [97] | Okongo et al 2018 | Uganda | Acholi | 2013–2016 | 18,000,000 | 1,627 | 17 | 0 | Regional | All | 4 |
| [98] | Olson et al 2020 | Tanzania | Lake Zone | 2008–2016 | 15,000,000 | 2,772 | 16 | 0 | Hospital | All | 1 |
| [99] | Ouedraogo et al 2018 | Burkina Faso | North-east | 2013–2017 | 22,000,000 | 352 | 61 | 0 | Hospital | All | 4 |
| [16] | Parkin et al 2021 | Zimbabwe | Bulawayo | 2011–2015 | 3,257,000 | 4,105 | 321 | 100 | Regional | All | 1 |
| [101] | Saeed et al 2014 | Sudan | Khartoum | 2009–2010 | 8,900,000 | 6,771 | 208 | 0 | Regional | All | 4 |
| [105] | Somdyla et al 2010 | South Africa | Eastern Cape | 1998–2002 | 33,000,000 | 2,501 | 33 | 0 | Regional | All | 1 |
| [102] | Tahtabasi et al 2020 | Somalia | Mogadishu | 2017–2019 | 6,300,000 | 1,306 | 13 | 100 | Hospital | All | 3 |
| [107] | Wabinga et al 2009 | Uganda | Kyadondo | 1995–1997 | 3,000,000 | 1,290 | 37 | 0 | Regional | All | 1 |
| [108] | Wabinga et al 2001 | Uganda | Mbarara | 1995–1999 | 1,460,000 | 585 | 50 | 0 | Hospital | All | 3 |
| [14] | Wabinga et al 2011 | Uganda | Kampala | 1993–1997 | 4,833,000 | 2,523 | 104 | 0 | Regional | All | 1 |
| [110] | Wabinga et al 2014 | Uganda | Kyadondo | 1991–2010 | 31,529,670 | 22,494 | 494 | 0 | Regional | All | 1 |
| [105] | Walker et al 1999 | South Africa | Durban | 1995 | 2,100,000 | 3,823 | 109 | 0 | Hospital | All | 4 |
| [106] | Warsame et al 2021 | Somalia | Mogadishu | 2019 | 2,180,000 | 126 | 2 | 0 | Hospital | Adults | 4 |
| [107] | Wiredu et al 2006 | Ghana | Accra | 1991–2000 | 14,000,000 | 3,659 | 186 | 0 | Hospital | All | 4 |
! = stratified by age and sex
Data was primarily extracted from regional registries, which accounted for 42% (N = 31) of the study sites, followed by retrospective reviews of hospital records (40%, N = 29). The remaining data were recorded from national registries (9%, N = 7) or retrospective reviews of pathology department data (8%, N = 6). Of the studies reviewed, 32% (N = 24) contained >90% histologically confirmed GC cases. A grading system was implemented to assess the quality of included studies: only 8% (N = 6) were from national registries and therefore ranked as the highest quality studies. Most studies (56%, N = 42) were from regional or national registries with <80% of the GC cases being histologically confirmed (Table 1).
From the 69 registry-based primary data sets, the overall crude pooled incidence was 1.20 GC cases per 100,000 people (CI 1.15–1.26). The variability between incidence calculation was 99.83% (I2 p < 0.001) (Figure 1). From the 29 high-quality population-based registry studies the crude pooled incidence was 1.71 GC cases per 100,000 people (95%CI 1.56–21.88) and the variability between studies was 99.60% (Figure 2). The GC incidence variability for West Africa I2 = 99.62 (p = 0.00), Southern Africa I2 = 99.82 (p = 0.00) and East Africa I2 = 99.88 (p = 0.00) indicates significant inter- and intra-regional variability within SSA (Figure 1). The study site with the highest incidence was Greater Meru in Kenya with an incidence of 5.56 GC cases per 100,000 people. The study site with the lowest incidence was 0.04 GC cases per 100,000 people in Benin City of Nigeria (Figure 1).
Figure 1. Crude incidence for all data sets describing GC in SSA (N = 69).
Figure 2. Crude incidence from the high-quality population-based registry studies describing GC in SSA.
Twelve (16%) of the studies included treatment information and were primarily from Nigeria (N = 3) and Rwanda (N = 2). The majority describe either palliation or curative resection (N = 11) and a few described adjuncts like chemotherapy (N = 4), in Rwanda, South Africa, Tanzania and Cameroon. In Nigeria, between 47% and 86% of patients had surgery with at least half described as palliative.
Twelve (16%) of studies included survival data, with the described time periods ranging from 1991 to 2018 across nine different countries. Median survivals were provided by approximately 33.3% of the studies (N = 3), and five studies described absolute survival in 5 years. The median survival periods reported ranged from 4.7 to 13.6 (Table 3).
Table 3. Mortality data reported in available studies on GC from SSA.
| Reference number | Author | Country | Region/City | Study period | GC cases | Outcomes | ||
|---|---|---|---|---|---|---|---|---|
| Post-operative complications | Mortality rate (%) | Survival | ||||||
| [42] | Adadey 2020 | Ghana | Volta | 2012–2014 | 8 | 90 All ages = 0.09 60–69 = 1.21 >70 = 0.96 |
||
| [45] | Ahmed 2011 | Nigeria | Zaria | 1995–2009 | 179 | 47% (post resection: 1 during index hospitalization) | Median 13.6 months | |
| [49] | Asombang et al 2014 | Zambia | Lusaka | 2010–2012 | 51 | Median 4.6 months | ||
| [52] | Bah et al 2011 | Gambia | 1993–1997 | 52 | 4.5% 1 year: 17.8% 3 years: 17.8% 5 years: 4.5% |
|||
| [54] | Bang 2020 | Cameroon | 2013–2018 | 105 | Mean =5.91 months 85 dead |
5 years: 19.0% | ||
| [64] | Chokunonga et al 2011 | Zimbabwe | Harare | 1993–1997 | 144 | 82 1 year: 37.5% 3-year: 19.0% 5-year: 18.1% |
||
| [74] | Gondos et al 2005 | Uganda | Kampala | 1993–1997 | 91 | 0% | ||
| [6] | Mabula et al 2012 | Tanzania | Mabula | 2006–2011 | 232 | 86 | 82 | 5 years survival rate: 6.9% 76 patients followed up 156 lost to follow up |
| [85] | Martin et al 2018 | Rwanda | 2012–2016 | 229 | 31 | |||
| [98] | Okobia 1999 | Nigeria | Benin City | 1989–1998 | 44 | 50 (N = 26) 7 died within 30 days of hospitalisation |
||
| [109] | Wabinga 2011 | Uganda | Kampala | 1993–1997 | 104 | 100 1 year: 39.1 3 years: 7.5 5 years: 0 |
Median survival 4.2 months | |
| [107] | Wiredu et al 2006 | Ghana | Accra | 1991–2000 | 186 | 100 | ||
Discussion
This SR is the first pooled analysis for GC incidence using primary data from SSA countries. While the pooled analysis shows an average incidence rate of 1.20 GC cases per 100,000 people (CI 1.15–1.26) in SSA, we demonstrate a significant incident variability between individual SSA countries and regions. Most data included in these studies were obtained from hospital registries or hospital data, with <10% included in national cancer registries and <50% containing histological confirmation. Analysis of pooled data limited to high-quality studies reported a similarly low incidence rate (2.12 cases/100,000 people) but retained significant variability. Less than 20% of studies reported treatment or mortality data.
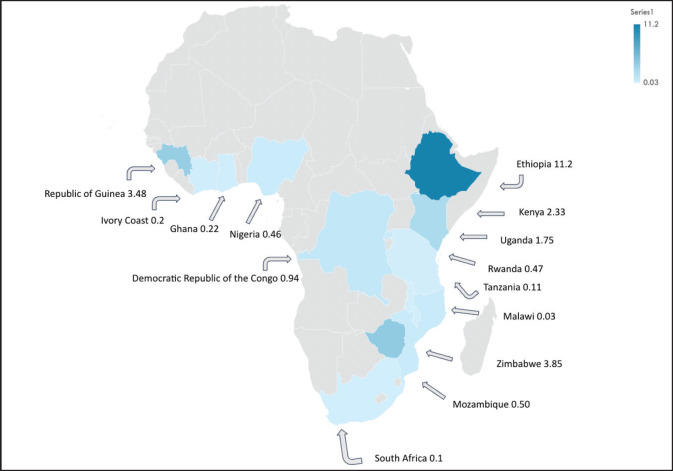
The methodology used in this analysis is similar to other SRs and meta-analyses to estimate cancer incidence in SSA [10, 11], where reliance on population and hospital-based registries leads to similar incidence rate heterogeneity [10, 11]. We found significant heterogenicity (I2 >99%, p < 0.001) among the entire cohort and within geographic regional groups. This finding was maintained when the analysis was limited to only the highest quality data, as identified with the incidence of two East African nations, Ethiopia, and Rwanda with a GC incidence of 11.2 and 0.47 cases per 100,000 people respectively. The GC incidence rates in SSA are comparable to North America and Europe but far lower than Asia. The GLOBOCAN 2020 data shows the GC incidence rates were 5.7 in Northern America, 5.9 in Northern Europe, 4.6 in Western Europe, 8.5 in Western Europe and 22.4 in Eastern Asia where exposure to Helicobacter pylori is high and extensive genomic analysis have demonstrated genetic predisposition [31 ,39]. The regional variation of GC is well established, attributed to different risk factors, genetic predisposition, dietary practices, environmental exposures and access to healthcare [3, 4]. Helicobacter pylori is a key risk factor for GC and some SSA countries have a known high prevalence of this dependent on multiple factors including geographic elevation, water sources, and sanitation infrastructure [27]. Mozambique and Zimbabwe have a high consumption of smoked, salted, and pickled foods, which are known risk factors for GC [28]. Tobacco consumption is widespread in Ethiopia and Tanzania which may contribute to increased GC among involved populations [29]. Our findings of high incidence variability highlight the importance of local data to better understand the true incidence rate and specific risk factor for any given community.
Namibia, Botswana, South Sudan, Central African Republic, Chad, Niger and Mauritania has missing GC data, the North African and Southern African region provided limited GC data, whilst West and East Africa provided the bulk of the data used in this analysis. Nigeria, Malawi, Zimbabwe, and Uganda provided most of the data. This speaks to the longstanding, high quality successful cancer registries in SSA, including the Harare, Kampala and Eastern Cape population-based registries [13, 16, 17]. The large gap from missing data contributes to the true incidence of GC in SSA remaining unknown. A dire need for these registries to be replicated in other African countries remains, highlighted by the geographic variability in cancer epidemiology and existing gaps in GC diagnosis and care in SSA. The data quality is vastly different among SSA countries, closing the data gap and standardising the quality of data collection will minimise the incidence bias that is currently present for GC in SSA [38].
This SR incident results are similar to rates previously reported by modelling estimates [1]. The 2020 IARC (GLOBOCAN) estimated a SSA GC incidence rate of 2.1 cases per 100,000 people [7, 8]. Likewise, Institute for Health Metrics and Evaluation’s (IHME) Global Burden of Disease estimates a crude incidence rate of 3.21 cases per 100,000 [21]. The overall reported incidence rates are similar to these modelled, validating the accuracy of the average over the studies included. GLOBOCAN and IHME used different models to produce estimates of GC rates as a means to overcome the known limitations in primary data availability and quality on GC in SSA. However, these modelling methodologies are limited by the validity of assumptions regarding disease incidence and may not reflect the true disease burden in the population [26]. The GLOBOCAN methodology derived incidence rates from available registry information or from the average of rates from neighbouring countries, extrapolated over the known population of an entire country [8]. Only 12% of the countries in SSA had national data for these estimates, with 36% of countries incident rates relying on neighbouring country data [9]. The IHME rate was produced by estimating mortality rates for GC through registry information and then dividing by modelled mortality-to-incidence ratios. While this methodology maximizes data coverage, it is based on reported case fatality rates, which may overestimate incidence in areas with worse than expected mortality. The methodology utilised in this SR was stringent as only primary data were included in the analysis and regional and national population sizes were verified with global population databases [22–24]. While our results are comparable to the modelled estimates over SSA as a whole, they have the added benefit of improved GC incidence characterisation within subregions as evident by the high heterogenicity of reported rates [26]. These metrics may provide a more precise understanding of localised disease trends upon which disease prevention and management approaches may be planned.
In most SSA nations, access to healthcare is limited with decreased treatment capacity leading to increased mortality from GC [30]. Less than 20% of all the studies provide information for treatment or survival. From the available data, most surgeries reported were palliative. This is possibly a result of poor surveillance, late-stage presentation, and lack of treatment options upon presentation [5]. Late-stage GC diagnosis, healthcare costs, inadequate diagnostic tools and limited healthcare workers all contribute to restricted GC treatment in SSA [5, 6]. The African enigma which hypothesizes the disassociation between GC and H. pylori infection is important to consider in further research [108]. The scarcity of robust data for GC in SSA, and the association between H. pylori and GC in SSA not being widely researched may indicate the African enigma is not as convincing as prior belief [108, 109]. Despite these limitations, treatment data are essential to establishing context-relevant treatment guidelines [33]. Future data collection could focus on the current standard of GC care in SSA and available palliative services.
Population-based GC disease registries such as the successful Harare, Eastern Cape and Kampala registries identifies demographic and geographic discrepancies in incidence and mortality rates which is valuable for improving cancer care and developing population specific control measures [34, 37]. Granular data collected from hospitals, clinics and laboratories will give better clarity of the epidemiology and risk factors that shape the local epidemiology of GC. This information on GC in SSA is vital to achieve a better understanding of the local and regional disease landscape and demonstrate the impact of local public health improvements accounting for the high regional variability of GC in SSA [35]. Establishing and maintaining population-based registries at local levels pose a challenge to most SSA regions due to limited healthcare infrastructure, shortage of trained data collectors, high costs, time involvement, and accuracy needed for proper data collection [36]. Disease registry success may be possible by focussing on local-level data collection that is aligned with and monitored by national data collection guidelines to ensure adherence to high-quality standards. Regional and national health systems will benefit from investing in GC surveillance programmes, national control programmes and training of health workers for accurate data collection [34, 40].
This review was limited by several factors. The employed search strategy is open to the biases of the search engines available to authors, potentially missing non-indexed reports published in regional journals. Additionally, our calculations were limited by the availability of regional and population-based estimates that were truly reflective of a given study’s sample population. Despite these limitations, we provide the most stringent review of high-quality data available to date to better inform efforts to bolster regional data collection. Moreover, we likely underestimate the true burden of GC disease in SSA as the studies that do exist are limited by selection bias for those who receive care for GC.
Conclusion
This study demonstrates the high variability of GC incidence across SSA, independent of data quality. We calculated an overall rate of 1.20 cases per 100,000 people, similar to modelled estimated but highlighting the large regions devoid of primary data. The variable GC incidence rates from the contributing studies of this SR highlight the need for further development of population-based cancer registries [19, 20].
Conflicts of interest
Our authors have no competing interests and the contents of this manuscript have not been published elsewhere. There are no conflicts of interest to disclose.
Funding
PROSPERO number CRD42022341498.
No external funding or funding by any author has been received for this study
Table 2. Treatment data reported in available studies on GC from SSA.
| Study reference number | Author | Country | Region/City | Study period | Regional population | Study cancer population | GC cases | Treatment | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Chemotherapy | Surgery | Radiation | ||||||||
| [44] | Afuwape et al 2012 | Nigeria | 2004–2009 | 25,000,000 | 49 | Curative = 10 Palliative = 13 |
||||
| [45] | Ahmed et al 2011 | Nigeria | Zaria | 1995–2009 | 9,375,000 | 179 | 57 | Total = 155 (D1 = 37 and D2 = 50) Curative = 38 Palliative = 68 |
||
| [49] | Asombang et al 2014 | Zambia | Lusaka | 2010–2012 | 4,384,000 | 51 | 6 | Palliative = 12 | 6 | |
| [54] | Bang et al 2020 | Cameroon | 2013–2018 | 19,000,000 | 1,105 | 105 | Curative = 30 Palliative = 77 |
Curative =32 Palliative = 16 |
||
| [58] | Benamro 2017 | South Africa | Durban | 2009–2014 | 15,000,000 | 131 | Curative = 21 Palliative = 40 |
Curative = 36 Curative = 1 Palliative = 12 |
17 | |
| [73] | Gaskill et al 2021 | Ghana | 2014–2015 | 30,000,000 | 2,562 | 98 | ||||
| [77] | Ibingira 2001 | Uganda | Kampala | 1995 | 964,000 | 32 | Curative = 2 Palliative = 15 Diagnostic/Biopsy = 18 |
|||
| [6] | Mabula et al 2012 | Tanzania | Mabula | 2006–2011 | 13,000,000 | 5,134 | 232 | Curative = 53 | Curative = 53 (Total =1 and Partial =52) Palliative =120 Biopsy = 50 |
|
| [85] | Martin et al 2018 | Rwanda | 2012–2016 | 51,000,000 | 229 | Curative = 15 Palliative = 13 |
Curative = 30 Palliative = 87 |
|||
| [94] | Ntakiyiruta 2009 | Rwanda | 2006–2007 | 8,000,000 | 35 | Curative = 1 Palliative = 34 |
||||
| [98] | Okobia 1999 | Nigeria | Benin City | 1989–1998 | 104,500,000 | 816 | 44 | |||
Appendix: GC SR
Methods – search strategy
Articles applicable to this GC SR were identified by a PubMed, Google Scholar and Web of Science search between 1st January 1995 and 1st June 2022. GC related search terms comprising ‘GC’, ‘stomach neoplasm’, ‘gastric neoplasm’, ‘cancer of the stomach’, ‘GC’ and ‘stomach cancer’ were applied to the search. The SSA country name and the SSA regional was applied with no language restriction.
The final search terms used was ‘(Cancer OR mass OR malignancy OR adenocarcinoma OR carcinoma OR neoplasm OR tumour) AND (Gastric OR stomach) AND (Botswana OR Burkina Faso OR Burundi OR Cabo Verde OR Cameroon OR central African republic OR Chad OR Comoros OR Democratic Republic of the Congo OR Congo OR Cote D’Ivoire OR Djibouti OR Equatorial Guinea OR Eritrea OR Swaziland OR Ethiopia OR Gabon OR Gambia OR Ghana OR Guinea OR Guinea Bissau OR Kenya OR Lesotho OR Liberia OR Madagascar OR Malawi OR Mali OR Mauritania OR Mauritius OR Mozambique OR Namibia OR Niger OR Nigeria OR Rwanda OR Sao Tome OR Senegal OR Seychelles OR Sierra Leone OR Somalia OR South Africa OR Sudan OR Tanzania OR Togo OR Uganda OR Zambia OR Zimbabwe OR SSA OR Africa)’
The participants included will be from primary GC studies from 1st January1995 to 1st June 2022 comprising of adult patients above the age of 18 years in SSA who have or had GC. Randomised control trials, cohort studies, case-control studies, and cross-sectional primary data studies of adult GC patients in SSA were included in this analysis. The primary exposure is GC. The primary outcomes of interest are incidence, treatment, and mortality. Studies that describe treatment interventions for GC including both surgery and chemoradiation were included. GC studies conducted outside of SSA or inclusive of non-African countries, paediatric cancer studies, benign gastric tumours, and gastric lymphoma were excluded. Letters to the editor, case reports, case series, narrative reviews, commentaries, perspectives, literature reviews, meta-analyses, medical reports, and non-peer reviewed publications were excluded from the analysis.
All references identified after deployment of the search strategy were exported using Zotero software. All records obtained from the various databases were combined in a single Zotero folder and all duplicates removed. The final search results underwent a title review to assess if the abstracts may contain relevant information for the study. After elimination of non-relevant titles, an abstract review was done prior to a full text review of pertinent studies. A data extraction form was employed on Microsoft Excel to record information on; the surname of primary author, year of publication, country of study, diagnostic method, registry type, study design, age group (age rages including >18 years old), sample size, GC case numbers, overall cancer case number for study population, overall population size, age or gender stratification, treatment type and numbers, and the case fatality rate of GC in the study. References of all relevant articles for additional data sources missed during our search was scanned and included where full texts were retrieved. References of pertinent reviews were also scanned.
5,558 records were identified with the online database search. An initial record screening and removal of duplicated records resulted in 667 studies remaining. Five hundred and thirty-five records were excluded after title screening and 132 study abstracts were screened. After the abstract screening 14 studies were excluded and 123 studies were assessed for eligibility by full text reading and reference reviewal. A total of 85 studies were included in this SR after 38 full text articles were excluded due to outcomes of interest not being reported.
Two reviewers (CG and PM) independently evaluated the titles of studies obtained from the searches. All three reviewers (CG, PM and AR) reviewed abstracts of papers obtained, after which the full texts of potentially eligible papers were retrieved. The three reviewers independently reviewed the full text of each potentially eligible study, compared their results, and resolved any discrepancies by discussion. For duplicate studies published in more than one report, the study reporting the largest sample size was considered. Studies with inaccessible full text either online or from the corresponding author was excluded. Methodological quality and risk of bias of included studies were assessed using the STROBE checklist. https://www.strobe-statement.org/
A SR with descriptive analysis was performed after the final data collection. To determine the incidence of GC, the crude GC case numbers (numerators), the regional population and the population of people with diagnosis of cancer (denominators) were considered. Overall mortality and treatment strategies are reported in crude numbers and percentages. Incidence patterns of GC across the various nations in SSA are summarised after the recalculation of incidence was done.
A narrative summary of findings regarding overall mortality and treatment strategies are reported due to the heterogeneity and paucity of data. All data analyses were conducted using StataSE (version 15.1, College Station, Texas, USA).
GC in SSA – an SR of primary data
Supplementary figures
Figure S1. PRISMA flowchart diagram of the study selection.
Figure S2. Gastric incidence map with case incidence per 100,000 people1.
Footnotes
Estimated size incidence cases per 100,000 described here. Data selected from the most recently published high-quality population-based registry reports
References
- 1.International agency for Research on Cancer, The Global Cancer Observatory. Sub-Saharan African Hub Fact Sheet, Globocan 2020. Lyon: International agency for Research on Cancer, The Global Cancer Observatory; 2021. [Google Scholar]
- 2.Asombang AW, Rahman R, Ibdah JA. Gastric cancer in Africa: current management and outcomes. World J Gastroenterol. 2014;20(14):3875–3879. doi: 10.3748/wjg.v20.i14.3875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morhason-Bello IO, Odedina F, Rebbeck TR, et al. Challenges and opportunities in cancer control in Africa: a perspective from the African Organisation for Research and Training in Cancer. Lancet Oncol. 2013;14:e142–e151. doi: 10.1016/S1470-2045(12)70482-5. [DOI] [PubMed] [Google Scholar]
- 4.Hamdi Y, Abdeljaoued-Tej I, Zatchi AA, et al. Cancer in Africa: the untold story. Front Oncol. 2021;11:650117. doi: 10.3389/fonc.2021.650117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kingham TP, Alatise OI, Vanderpuye V, et al. Treatment of cancer in sub-Saharan Africa. Lancet Oncol. 2014;14:e158–e167. doi: 10.1016/S1470-2045(12)70472-2. [DOI] [PubMed] [Google Scholar]
- 6.Mabula JB, Mchembe MD, Koy M, et al. Gastric cancer at a university teaching hospital in northwestern Tanzania: a retrospective review of 232 cases. World J Surg Oncol. 2012;10:257. doi: 10.1186/1477-7819-10-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 8. Globocan 2020 WHO report.
- 9.Ferlay J, Ervik M, Lam F, et al. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer; [09/10/18]. [ https://GCo.iarc.fr/today] [Google Scholar]
- 10.Cumberbatch MG, Jubber I, Black PC, et al. Epidemiology of bladder cancer: a systematic review and contemporary update of risk factors in 2018. Eur Urol. 2018;74:784–795. doi: 10.1016/j.eururo.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Adeloye D, Sowunmi OY, Jacobs W, et al. Estimating the incidence of breast cancer in Africa: a systematic review and meta-analysis. J Glob Health. 2018;8(1):010419. doi: 10.7189/jogh.08.010419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ajani JA, D'Amico TA, Bentrem DJ, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Nat Compr Cancer Netw. 2022;20(2):167–192. doi: 10.6004/jnccn.2022.0008. [DOI] [PubMed] [Google Scholar]
- 13.Bukirwa P, Wabinga H, Nambooze S, et al. Trends in the incidence of cancer in Kampala, Uganda, 1991 to 2015. Int J Cancer. 2021;148:2129–2138. doi: 10.1002/ijc.33373. [DOI] [PubMed] [Google Scholar]
- 14.Wabinga H, Parkin DM, Nambooze S, et al. Cancer survival in Kampala, Uganda, 1993−1997. IARC Sci Publ. 2011;162:243–247. [PubMed] [Google Scholar]
- 15.Chokunonga E, Windridge P, Sasieni P, et al. Black–white differences in cancer risk in Harare, Zimbabwe, during 1991–2010. Int J Cancer. 2016;138:1416–1421. doi: 10.1002/ijc.29883. [DOI] [PubMed] [Google Scholar]
- 16.Parkin DM, Chingonzoh T, Vuma S, et al. Changes in the incidence of cancer in Bulawayo, Zimbabwe over a 50-year period. Cancer Epidemiol Biomarkers Prev. 2021;30(5):867–873. doi: 10.1158/1055-9965.EPI-20-0669. [DOI] [PubMed] [Google Scholar]
- 17.Somdyala NI, Bradshaw D, Gelderblom WC, et al. Cancer incidence in a rural population of South Africa, 1998–2002. Int J Cancer. 2010;127:2420–2429. doi: 10.1002/ijc.25246. [DOI] [PubMed] [Google Scholar]
- 18.Somdyala NI, Parkin DM, Sithole N, et al. Trends in cancer incidence in rural Eastern Cape Province; South Africa, 1998–2012. Int J Cancer. 2015;136:E470–E474. doi: 10.1002/ijc.29224. [DOI] [PubMed] [Google Scholar]
- 19.Moodley J, Constant D, Mwaka AD, et al. Anticipated help seeking behaviour and barriers to seeking care for possible breast and cervical cancer symptoms in Uganda and South Africa. ecancermedicalscience. 2021;15:1171. doi: 10.3332/ecancer.2021.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lubuzo B, Ginindza T, Hlongwana K. Exploring barriers to lung cancer patient access, diagnosis, referral and treatment in Kwazulu-Natal, South Africa: the health providers’ perspectives. Transl Lung Cancer Res. 2019;8(4):380–391. doi: 10.21037/tlcr.2019.08.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Institute for Health Metrics and Evaluation (IHME) report.
- 22. https://www.macrotrends.net/countries .
- 23. https://data.worldbank.org/
- 24. https://countryeconomy.com/demography/population .
- 25. https://afcrn.org/
- 26.Ioannidis JPA. Coronavirus disease 2019: the harms of exaggerated information and non-evidence-based measures. Eur J Clin Invest. 2020;50(4):e13222. doi: 10.1111/eci.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fock KM, Ang TL. Epidemiology of Helicobacter pylori infection and gastric cancer in Asia. J Gastroenterol Hepatol. 2010;25(4):479–486. doi: 10.1111/j.1440-1746.2009.06188.x. [DOI] [PubMed] [Google Scholar]
- 28.Marques-Vidal P, Ravasco P, Ermelinda Camilo M. Gastric cancer: epidemiology, prevention, and therapy. Nutr Cancer. 2004;48(2):149–156. [Google Scholar]
- 29.Akinyemiju T, Abera S, Ahmed M, et al. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the Global, Regional, and National level: results from the global burden of disease study 2015. JAMA Oncol. 2017;3(10):1683–1691. doi: 10.1001/jamaoncol.2017.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferlay J, Soerjomataram I, Ervik M, et al. Lyon: International Agency for Research on Cancer; 2013. [ http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx] [Google Scholar]
- 31.Ferlay J, Ervik M, Lam F, et al. Lyon: International Agency for Research on Cancer; 2023. [26/04/23]. [ https://gco.iarc.fr/today/home] [Google Scholar]
- 32.Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14(1):26–38. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sexton RE, Al Hallak MN, Diab M, et al. Gastric cancer: a comprehensive review of current and future treatment strategies. Cancer Metastasis Rev. 2020;39(4):1179–1203. doi: 10.1007/s10555-020-09925-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gliklich RE, Dreyer NA, Leavy MB. Registries for Evaluating Patient Outcomes: A User's Guide. 3rd. Rockville: Agency for Healthcare Research and Quality; 2014. [ https://www.ncbi.nlm.nih.gov/books/NBK208643/] [PubMed] [Google Scholar]
- 35.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023–1075. doi: 10.1016/S0140-6736(17)33326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Selvin E, Parrinello CM, Sacks DB, et al. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160(8):517–525. doi: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cruz-Flores S, Rabinstein A, Biller J, et al. Racial-ethnic disparities in stroke care: the American experience: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(7):2091–2116. doi: 10.1161/STR.0b013e3182213e24. [DOI] [PubMed] [Google Scholar]
- 38.Gini A, Jansen EEL, Zielonke N, et al. Impact of colorectal cancer screening on cancer-specific mortality in Europe: a systematic review. Eur J Cancer. 2020;127:224–235. doi: 10.1016/j.ejca.2019.12.014. [DOI] [PubMed] [Google Scholar]
- 39.Katoh H, Ishikawa S. Lifestyles, genetics, and future perspectives on gastric cancer in east Asian populations. J Hum Genet. 2021;66:887–899. doi: 10.1038/s10038-021-00960-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Maione L, Chanson P. National acromegaly registries. Best Pract Res Clin Endocrinol Metabol. 2019;33(2):101264. doi: 10.1016/j.beem.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 41.Abdulkareem FB, Faduyile FA, Daramola AO. Malignant gastrointestinal tumours in south western Nigeria: a histopathologic analysis of 713 cases West Afr J Med. 2009;28(3):173–176. doi: 10.4314/wajm.v28i3.48478. [DOI] [PubMed] [Google Scholar]
- 42.Adadey SM, Languon S, Ayee R, et al. Incidence and mortality of cancer in the Volta Region of Ghana. Exp Biol Med. 2020;245(12):1058–1065. doi: 10.1177/1535370220931514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Amégbor K, Darre T, Ayéna KD, et al. Cancers in Togo from 1984 to 2008: epidemiological and pathological aspects of 5251 cases. J Cancer Epidemiol. 2011;2011:319872. doi: 10.1155/2011/319872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Afuwape OO, Irabor DO, Ladipo JK, et al. A review of the current profile of gastric cancer presentation in the university college hospital Ibadan, a tertiary health care institution in the tropics. J Gastrointest Cancer. 2012;43(2):177–180. doi: 10.1007/s12029-011-9259-z. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed A, Ukwenya AY, Makama JG, et al. Management and outcome of gastric carcinoma in Zaria, Nigeria. Afr Health Sci. 2011;11(3):353–361. [PMC free article] [PubMed] [Google Scholar]
- 46.Amoako YA, Awuah B, Larsen-Reindorf R, et al. Malignant tumours in urban Ghana: evidence from the city of Kumasi. BMC Cancer. 2019;19(1):267. doi: 10.1186/s12885-019-5480-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Joutei HA, Mahfoud W, Sadaoui I, et al. Étude des caractéristiques épidémiologiques cliniques et anatomopathologiques de l’adénocarcinome gastrique chez une population Marocaine. Ann Pathol. 2020;40(6):442–446. doi: 10.1016/j.annpat.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 48.Arodiwe EB, Ike SO, Nwokediuko SC, et al. Pattern of cancer deaths in the medical wards of a teaching hospital in South East Nigeria. Niger J Clin Pract. 2013;16(4):505–510. doi: 10.4103/1119-3077.116901. [DOI] [PubMed] [Google Scholar]
- 49.Asombang AW, Kayamba V, Turner-Moss E, et al. Gastric malignancy survival in Zambia, Southern Africa: a two year follow up study. Med J Zamb. 2014;41:13–18. [PMC free article] [PubMed] [Google Scholar]
- 50.Awodele O, Adeyomoye AA, Awodele DF, et al. Cancer distribution pattern in south-western Nigeria. Tanzan J Health Res. 2011;13(2):125–131. doi: 10.4314/thrb.v13i2.55226. [DOI] [PubMed] [Google Scholar]
- 51.Bah E, Parkin DM, Hall AJ, et al. Cancer in the Gambia: 1988–97. Br J Cancer. 2001;84(9):1207–1214. doi: 10.1054/bjoc.2001.1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bah E, Sam O, Whittle H, et al. Cancer survival in the Gambia, 1993–1997. IARC Sci Publ. 2011;162:97–100. [PubMed] [Google Scholar]
- 53.Banda LT, Parkin DM, Dzamalala CP, et al. Cancer incidence in Blantyre, Malawi 1994–1998. Trop Med Int Health. 2001;6(4):296–304. doi: 10.1046/j.1365-3156.2001.00707.x. [DOI] [PubMed] [Google Scholar]
- 54.Bang GA, Savom EP, Oumarou BN, et al. Clinical epidemiology and mortality risk factors of gastric cancer in a sub-Saharan African setting: a retrospective analysis of 120 cases in Yaoundé (Cameroon) Pan Afr Med J. 2020;37:104. doi: 10.11604/pamj.2020.37.104.25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Baş Y, Hassan HA, Adıgüzel C, et al. The distribution of cancer cases in Somalia. Semin Oncol. 2017;44(3):178–186. doi: 10.1053/j.seminoncol.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 56.Bassène ML, Sy D, Dia D, et al. Le cancer gastrique : étude descriptive de 101 cas dans le centre d'endoscopie digestive du CHU Aristide Le Dantec [Stomach cancer: a descriptive study of 101 cases at the gastrointestinal endoscopy center at Aristide Le Dantec University Hospital] Med Sante Trop. 2015;25(4):377–380. doi: 10.1684/mst.2014.0384. [DOI] [PubMed] [Google Scholar]
- 57.Bassett MT, Chokunonga E, Mauchaza B, et al. Cancer in the African population of Harare, Zimbabwe, 1990–1992. Int J Cancer. 1995;63(1):29–36. doi: 10.1002/ijc.2910630107. [DOI] [PubMed] [Google Scholar]
- 58.Benamro F, Sartorius B, Clarke DL, et al. The spectrum of gastric cancer as seen in a large quaternary hospital in KwaZulu-Natal, South Africa. South Afr Med J. 2017;107(2):130–133. doi: 10.7196/SAMJ.2017.v107i2.11383. [DOI] [PubMed] [Google Scholar]
- 59.Bodalal Z, Azzuz R, Bendardaf R. Cancers in Eastern Libya: first results from Benghazi Medical Center. World J Gastroenterol. 2014;20(20):6293–6301. doi: 10.3748/wjg.v20.i20.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bouglouga O, Lawson-Ananissoh LM, Bagny A, et al. Cancer de l'estomac: aspects épidémiologiques, cliniques et histologiques au CHU Campus de Lomé (Togo) [Stomach cancer: epidemiological, clinical and histological aspects at the Lome Campus teaching hospital (Togo)] Med Sante Trop. 2015;25(1):65–68. doi: 10.1684/mst.2014.0415. [DOI] [PubMed] [Google Scholar]
- 61.Carrilho C, Fontes F, Tulsidás S, et al. Cancer incidence in Mozambique in 2015–2016: data from the Maputo Central Hospital Cancer Registry. Eur J Cancer Prev. 2019;28(4):373–376. doi: 10.1097/CEJ.0000000000000457. [DOI] [PubMed] [Google Scholar]
- 62.Chasimpha SJD, Parkin DM, Masamba L, et al. Three-year cancer incidence in Blantyre, Malawi (2008–2010) Int J Cancer. 2017;141(4):694–700. doi: 10.1002/ijc.30777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chokunonga E, Borok MZ, Chirenje ZM, et al. Trends in the incidence of cancer in the black population of Harare, Zimbabwe 1991–2010. Int J Cancer. 2013;133(3):721–729. doi: 10.1002/ijc.28063. [DOI] [PubMed] [Google Scholar]
- 64.Chokunonga E, Borok MZ, Chirenje ZM, et al. Cancer survival in Harare, Zimbabwe, 1993–1997. IARC Sci Publ. 2011;162:249–255. [PubMed] [Google Scholar]
- 65.Darre T, Kpatcha TM, Bagny A, et al. Descriptive epidemiology of cancers in Togo from 2009 to 2016. Asian Pac J Cancer Prev APJCP. 2017;18(12):3407–3411. doi: 10.22034/APJCP.2017.18.12.3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Echimane AK, Ahnoux AA, Adoubi I, et al. Cancer incidence in Abidjan, Ivory Coast: first results from the cancer registry, 1995–1997. Cancer. 2000;89(3):653–663. doi: 10.1002/1097-0142(20000801)89:3&lt;653::AID-CNCR22&gt;3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 67.Effi AB, Koffi KE, Doukouré B, et al. Épidémiologie descriptive des cancers en Côte d’Ivoire. Bull Cancer. 2013;100(2):119–125. doi: 10.1684/bdc.2013.1695. [DOI] [PubMed] [Google Scholar]
- 68.Ekanem IO, Parkin DM. Five year cancer incidence in Calabar, Nigeria (2009–2013) Cancer Epidemiol. 2016;42:167–172. doi: 10.1016/j.canep.2016.04.014. [DOI] [PubMed] [Google Scholar]
- 69.Elghali MA, Gouader A, Bouriga R, et al. Gastric cancers in Central Tunisia: evolution specificities through two decades and relation with Helicobacter pylori. Oncology. 2018;95(2):121–128. doi: 10.1159/000488488. [DOI] [PubMed] [Google Scholar]
- 70.Fapohunda A, Fakolade A, Omiye J, et al. Cancer presentation patterns in Lagos, Nigeria: experience from a private cancer center. J Public Health Afr. 2020;11(2):1138. doi: 10.4081/jphia.2020.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Feuchtner J, Mathewos A, Solomon A, et al. Addis Ababa population-based pattern of cancer therapy, Ethiopia. PLoS One. 2019;14(9):e0219519. doi: 10.1371/journal.pone.0219519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Garba SM, Zaki HM, Arfaoui A, et al. Épidémiologie des cancers au Niger, 1992 à 2009. Bull Cancer. 2013;100(2):127–133. doi: 10.1684/bdc.2013.1699. [DOI] [PubMed] [Google Scholar]
- 73.Gaskill CE, Gyedu A, Stewart B, et al. Improving global surgical oncology benchmarks: defining the unmet need for cancer surgery in Ghana. World J Surg. 2021;45(9):2661–2669. doi: 10.1007/s00268-021-06197-y. [DOI] [PubMed] [Google Scholar]
- 74.Gondos A, Brenner H, Wabinga H, et al. Cancer survival in Kampala, Uganda. Br J Cancer. 2005;92(9):1808–1812. doi: 10.1038/sj.bjc.6602540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gyorki DE, Muyco A, Kushner AL, et al. Cancer surgery in low-income countries: an unmet need. Arch Surg. 2012;147(12):1135–1140. doi: 10.1001/archsurg.2012.1265. [DOI] [PubMed] [Google Scholar]
- 76.El Hassan A, El Hassan L, Mudawi H, et al. Malignant gastric tumors in Sudan: a report from a single pathology center. Hematol/Oncol Stem Cell Ther. 2008;1(2):130–132. doi: 10.1016/S1658-3876(08)50044-6. [DOI] [PubMed] [Google Scholar]
- 77.Ibingira CB. Management of cancer of the stomach in Mulago Hospital Kampala, Uganda. East Afr Med J. 2001;78(5):233–237. doi: 10.4314/eamj.v78i5.9044. [DOI] [PubMed] [Google Scholar]
- 78.Jedy-Agba E, Curado MP, Ogunbiyi O, et al. Cancer incidence in Nigeria: a report from population-based cancer registries. Cancer Epidemiol. 2012;36(5):e271–e278. doi: 10.1016/j.canep.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson O, Ersumo T, Ali A. Gastric carcinoma at Tikur Anbessa Hospital, Addis Ababa. East Afr Med J. 2000;77(1):27–30. [PubMed] [Google Scholar]
- 80.Korir A, Okerosi N, Ronoh V, et al. Incidence of cancer in Nairobi, Kenya (2004–2008) Int J Cancer. 2015;137(9):2053–2059. doi: 10.1002/ijc.29674. [DOI] [PubMed] [Google Scholar]
- 81.Korir A, Yu Wang E, Sasieni P, et al. Cancer risks in Nairobi (2000–2014) by ethnic group. Int J Cancer. 2017;140(4):788–797. doi: 10.1002/ijc.30502. [DOI] [PubMed] [Google Scholar]
- 82.Koulibaly M, Kabba IS, Cissé A, et al. Cancer incidence in Conakry, Guinea: first results from the cancer registry 1992–1995. Int J Cancer. 1997;70(1):39–45. doi: 10.1002/(SICI)1097-0215(19970106)70:1<39::AID-IJC6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 83.Laryea DO, Awuah B, Amoako YA, et al. Cancer incidence in Ghana, 2012: evidence from a population-based cancer registry. BMC Cancer. 2014;14:362. doi: 10.1186/1471-2407-14-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lorenzoni CF, Ferro J, Carrilho C, et al. Cancer in Mozambique: results from two population-based cancer registries. Int J Cancer. 2020;147(6):1629–1637. doi: 10.1002/ijc.32953. [DOI] [PubMed] [Google Scholar]
- 85.Martin AN, Silverstein A, Ssebuufu R, et al. Impact of delayed care on surgical management of patients with gastric cancer in a low-resource setting. J Surg Oncol. 2018;118(8):1237–1242. doi: 10.1002/jso.25286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McFarlane G, Forman D, Sitas F. A minimum estimate for the incidence of gastric cancer in Eastern Kenya. Br J Cancer. 2001;85(9):1322–1325. doi: 10.1054/bjoc.2001.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Memirie ST, Habtemariam MK, Asefa M, et al. Estimates of cancer incidence in Ethiopia in 2015 using population-based registry data. J Glob Oncol. 2018;4:1–11. doi: 10.1200/JGO.17.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Missaoui N, Trabelsi A, Parkin DM, et al. Trends in the incidence of cancer in the Sousse region, Tunisia, 1993–2006. Int J Cancer. 2010;127(11):2669–2677. doi: 10.1002/ijc.25490. [DOI] [PubMed] [Google Scholar]
- 89.Moses A, Mwafongo A, Chikasema M, et al. Risk factors for common cancers among patients at Kamuzu Central Hospital in Lilongwe, Malawi: a retrospective cohort study. Malawi Med J. 2017;29(2):136–141. doi: 10.4314/mmj.v29i2.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Msyamboza KP, Dzamalala C, Mdokwe C, et al. Burden of cancer in Malawi; common types, incidence and trends: national population-based cancer registry. BMC Res Notes. 2012;5:149. doi: 10.1186/1756-0500-5-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mtonga P, Masamba L, Milner D, et al. Biopsy case mix and diagnostic yield at a Malawian central hospital. Malawi Med J. 2013;25(3):62–64. [PMC free article] [PubMed] [Google Scholar]
- 92.Newton R, Ngilimana PJ, Grulich A, et al. Cancer in Rwanda. Int J Cancer. 1996;66(1):75–81. doi: 10.1002/(SICI)1097-0215(19960328)66:1<75::AID-IJC14>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 93.Nsondé Malanda J, Nkoua Mbon JB, Bambara AT, et al. Douze années de fonctionnement du registre des cancers de Brazzaville [Twelve years of working of Brazzaville cancer registry] Bull Cancer. 2013;100(2):135–139. doi: 10.1684/bdc.2013.1701. [DOI] [PubMed] [Google Scholar]
- 94.Ntakiyiruta G. Gastric cancers at Kibogora Hospital. East Cent Afr J Surg (Online) 2009;14(1):130–134. [Google Scholar]
- 95.Nwafor CC, Nwafor NN. The pattern and distribution of cancers in Akwa Ibom State, Nigeria. Niger J Clin Pract. 2018;21(5):603–608. doi: 10.4103/njcp.njcp_316_17. [DOI] [PubMed] [Google Scholar]
- 96.Obayo S, Lukwago L, Orem J, et al. Gastrointestinal malignancies at five regional referral hospitals in Uganda. Afr Health Sci. 2017;17(4):1051–1058. doi: 10.4314/ahs.v17i4.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Okongo F, Ogwang DM, Liu B, et al. Cancer incidence in Northern Uganda (2013–2016) Int J Cancer. 2019;144:2985–2991. doi: 10.1002/ijc.32053. [DOI] [PubMed] [Google Scholar]
- 98.Olson AC, Afyusisye F, Egger J, et al. Cancer incidence and treatment utilization patterns at a regional cancer center in Tanzania from 2008–2016: initial report of 2,772 cases. Cancer Epidemiol. 2020;67:101772. doi: 10.1016/j.canep.2020.101772. [DOI] [PubMed] [Google Scholar]
- 99.Ouedraogo S, Ouedraogo S, Kambire JL, et al. Profil épidémiologique, clinique, histologique et thérapeutique des cancers digestifs primitifs dans les régions nord et est du Burkina Faso. Bull Cancer. 2018;105(12):1119–1125. doi: 10.1016/j.bulcan.2018.09.001. [DOI] [PubMed] [Google Scholar]
- 100.Parkin DM, Nambooze S, Wabwire-Mangen F, et al. Changing cancer incidence in Kampala, Uganda, 1991–2006. Int J Cancer. 2010;126(5):1187–1195. doi: 10.1002/ijc.24838. [DOI] [PubMed] [Google Scholar]
- 101.Saeed IE, Weng HY, Mohamed KH, et al. Cancer incidence in Khartoum, Sudan: first results from the cancer registry, 2009–2010. Cancer Med. 2014;3(4):1075–1084. doi: 10.1002/cam4.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tahtabasi M, Mohamud Abdullahi I, Kalayci M, et al. Cancer incidence and distribution at a tertiary care hospital in Somalia from 2017 to 2020: an initial report of 1306 cases. Cancer Manag Res. 2020;12:8599–8611. doi: 10.2147/CMAR.S277202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wabinga HR, Parkin DM, Wabwire-Mangen F, et al. Trends in cancer incidence in Kyadondo County, Uganda, 1960–1997. Br J Cancer. 2000;82(9):1585–1592. doi: 10.1054/bjoc.1999.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wabinga HR. Pattern of cancer in Mbarara, Uganda. East Afr Med J. 2002;79(4):193–197. doi: 10.4314/eamj.v79i4.8877. [DOI] [PubMed] [Google Scholar]
- 105.Walker AR, Halse J. Pattern of cancer in Indian patients hospitalized in Durban, South Africa. Eur J Cancer Prev. 1999;8(3):247–254. doi: 10.1097/00008469-199906000-00013. [DOI] [PubMed] [Google Scholar]
- 106.Warsame AA, Sönmez RE, Muse MM, et al. Prevalence of cancer related to sociodemographic characteristics and prevention strategies in Mogadishu, Somalia. Bangladesh J Med Sci. 2021;20(4):756–761. doi: 10.3329/bjms.v20i4.54130. [DOI] [Google Scholar]
- 107.Wiredu EK, Armah HB. Cancer mortality patterns in Ghana: a 10-year review of autopsies and hospital mortality. BMC Public Health. 2006;6:159. doi: 10.1186/1471-2458-6-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dong SXM. “Peptic ulcers are an infectious disease caused by Helicobacter pylori” is an illusion in medical research. 2023. Preprints 2023020297.
- 109.Cavadas B, Leite M, Pedro N, et al. Shedding light on the African enigma: in vitro testing ofHomo sapiens-Helicobacter pylori coevolution. Microorganisms. 2021;9:240. doi: 10.3390/microorganisms9020240. [DOI] [PMC free article] [PubMed] [Google Scholar]