Abstract
Two chimeric proviruses comprising the U3 promoter and the nef gene of simian immunodeficiency virus (SIV) smmPBj1.9 in addition to other genomic regions of SIVagm3mc from African green monkeys (Cercopithecus aethiops) were constructed. The derived chimeric viruses (SIVagm3mc/SIVsmmPBj1.9) were both able to replicate in nonstimulated peripheral blood leukocytes from pig-tailed macaques (Macaca nemestrina), a biological property often correlated with acute pathogenicity. However, only one of the chimeric viruses was acutely pathogenic, inducing a rapid depletion of the peripheral CD4+ T cells in two infected pig-tailed macaques within 10 days after infection in a manner similar to infection with SIVsmmPBj1.9 itself. The other chimeric virus actively replicated during the first 8 weeks after experimental infection of two pig-tailed macaques but induced neither acute disease nor CD4+ T-cell depletion for 113 weeks after infection. Thus, the U3 promoter and the nef gene of SIVsmmPBj1.9 alone appear to be insufficient to confer acute pathogenicity to SIVagm3mc.
Experimental infections of African green monkeys (Cercopithecus aethiops) and pig-tailed macaques (Macaca nemestrina) with the molecular virus clone simian immunodeficiency virus (SIV) agm3mc follow the inapparent course seen during natural infections of African green monkeys with SIVagm (2, 3, 12, 16, 17, 19, 20). A single isolate (SIVagm9063) has been shown to induce lethal, AIDS-like disease in most experimentally infected pig-tailed macaques within 4 to 24 months, paralleled by a rapid decrease in the number of circulating CD4+ T cells (14). A severe CD4+ T-cell depletion and, in addition, a severe enteropathicity are the hallmarks of the acute viral disease induced by infection of pig-tailed macaques with isolate SIVsmmPBj14 from sooty mangabey monkeys (Cercocebus atys) (9) and its corresponding molecular virus clone SIVsmmPBj1.9 (4, 15). Infected macaques succumb within 2 weeks after infection. The critical role of the PBj14 nef gene in this disease was demonstrated by changing a single amino acid (R to Y [R→Y] at position 17) in SIVmac239 nef to match the nef gene of PBj14. The mutated viruses were also able to induce an acute and lethal disease in pig-tailed macaques (6, 7).
The acute pathogenicity of SIVsmmPBj14 and related chimeric viruses has been shown to correlate with their ability to replicate in nonstimulated peripheral blood mononuclear cells (PBMCs) (8, 10). A number of replication-competent SIVagm3mc/SIVsmmPBj1.9 chimeras were found to replicate in nonstimulated PBMCs from pig-tailed macaques (5). As this phenotype was characteristic for those chimeras retaining the U3 promoter region of SIVsmmPBj1.9, it seemed possible that infection of pig-tailed macaques with chimeras carrying both the nef gene and the U3 promoter region of SIVsmmPBj1.9 would also result in acute disease.
Two representative chimeric viruses (SIVagm3mc/SIVsmmPBj1.9) were chosen (Fig. 1), both containing the nef gene of SIVsmmPBj1.9 with the critical amino acid 17 substitution (R→Y) shown previously to confer the pathogenic SIVsmmPBj14 phenotype to SIVmac239 (6, 7). In addition, both chimeric genomes retained the U3 promoter region of SIVsmmPBj1.9. Briefly, the genome of chimeric virus HY-gag/pol/CR/nef/U3 comprised the region of SIVagm3mc env encoding the surface (SU) envelope glycoprotein and the N-terminal domain of the transmembrane (TM) protein (including the overlapping second exons of tat and rev) in the background of SIVsmmPBj1.9, including nef, both long terminal repeats and the gag-pol region (details of the construction are available from the authors upon request). In contrast, the genome of chimeric virus HY-TM/nef/U3 constructed as described previously (5) contained only the nef gene, the U3 promoter, and the region of env encoding the C-terminal part of TM from SIVsmmPBj1.9. The remaining genomic regions were derived from SIVagm3mc. The chimeric viruses HY-TM/nef/U3 and HY-gag/pol/CR/nef/U3, which were generated as described previously (5), replicated in C8166 cells as well as in phytohemagglutinin- and interleukin-2-stimulated macaque PBMCs with kinetics similar to those of the parental viruses SIVagm3mc and SIVsmmPBj1.9. However, although SIVagm3mc was unable to replicate in nonstimulated primary cells as expected, both chimeric viruses replicated similarly to SIVsmmPBj1.9 in these cells (data not shown).
FIG. 1.
Proviral structures of chimeric SIVagm3mc/SIVsmmPBj1.9. Viral genomic regions derived from SIVsmmPBj1.9 are shown in grey, and those derived from SIVagm3mc are shown in white. Whereas the genomic regions of chimeric virus HY-TM/nef/U3 are colinear with the respective regions of SIVagm3mc or SIVsmmPBj1.9, the chimeric TM protein of virus HY-gag/pol/CR/nef/U3 is extended by 35 amino acids of SIVsmmPBj1.9 preceding the nef gene. LTR, long terminal repeat.
In order to test the ability of the chimeric virus HY-gag/pol/CR/nef/U3 to induce an acute viral disease, two pig-tailed macaques (termed Nem 145 and Nem 151) were infected with 6 × 105 50% tissue culture infective doses (TCID50) of virus grown in C8166 cells. In parallel, two macaques (Nem 82 and Nem 149) were infected with 6 × 105 TCID50 of the parental virus SIVsmmPBj1.9. All animals developed acute disease characterized by fever, rash, lymphadenopathy, and diarrhea and had to be sacrificed 8 to 10 days after infection. On day 10, the cell-associated virus loads in the peripheral blood of the animals infected with the chimera were 500 (Nem 145) and 7,000 (Nem 151) infected cells per 106 PBMCs. Nem 149 and Nem 82 (infected by SIVsmmPBj1.9) had viral loads of 2,000 and 34,000 infected cells per 106 PBMCs, respectively, thus confirming previous reports for macaques infected by SIVsmmPBj14 and its molecular virus clones (23). In all animals the number of infected lymph node mononuclear cells was 2- to 18-fold higher than the number of infected PBMCs (data not shown). CD4+ T-cell numbers had decreased 8 to 10 days after infection from about 1,500 to <250 per μl in the animals infected with the chimera and from 1,000 to <400 per μl in the animals infected with SIVsmmPBj1.9. Concomitantly, the number of CD8+ T lymphocytes decreased from >1,250 per μl to <750 per μl in the two chimera-infected animals and in SIVsmmPBj1.9-infected Nem 82. No such depletion of the CD8+ T cells was observed in the second macaque (Nem 149) infected with the parental virus. Pathological examinations revealed similar lesions in the intestinal tract of the two macaques infected with chimeric virus HY-gag/pol/CR/nef/U3 and of the two macaques infected with parental virus SIVsmmPBj1.9. In conclusion, HY-gag/pol/CR/nef/U3 was able to induce an acute viral and enteropathic disease in infected pig-tailed macaques similar to that seen with the parental viruses SIVsmmPBj1.9 and SIVsmmPBj14 (4, 9, 11).
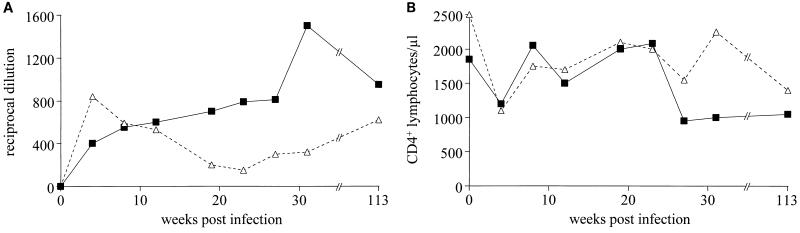
Two pig-tailed macaques (Nem 83 and Nem 144) were subsequently infected with 6 × 105 TCID50 of the chimeric virus HY-TM/nef/U3. Both animals remained healthy, showing no clinical signs of disease and a constant level of SIV-specific antibodies for 113 weeks after infection (Fig. 2A), consistent with the development of a chronic infection. The number of peripheral CD4+ (Fig. 2B) and CD8+ T lymphocytes (data not shown) initially decreased after 4 weeks of infection but returned to normal thereafter. At 27, 31, and 113 weeks after infection, around 1,000 CD4+ T cells were detected in the peripheral blood of Nem 83, whereas the number of CD4+ T cells fluctuated in the peripheral blood of Nem 144, until decreasing to 1,200 at 113 weeks after infection.
FIG. 2.
(A) Titers of anti-SIV antibodies in sera of pig-tailed macaques infected with chimeric virus HY-TM/nef/U3. Serial dilutions of sera from macaque Nem 83 (squares) and macaque Nem 144 (triangles) were tested against whole SIVagm antigen by enzyme-linked immunosorbent assay (2) prior to inoculation (day 0) and thereafter. (B) Numbers of CD4+ T cells in peripheral blood of pig-tailed macaque Nem 83 (squares) and macaque Nem 144 (triangles) prior to the experimental infection (day 0) with chimeric virus HY-TM/nef/U3 and thereafter. The numbers of CD4+ T cells per microliter of blood were measured as described previously (2).
At 1 and 4 weeks after infection, virus could be isolated from PBMCs of both infected pig-tailed macaques by previously published methods (2), whereas in the following weeks, attempts to isolate virus from PBMCs were unsuccessful, indicating a low level of virus replication in vivo. Detection of cells harboring proviruses was therefore attempted by PCR. By using 0.6 μg of purified DNA isolated from the PBMCs of both animals and the nef-specific oligonucleotide primers Pnef(+) (CCAAGGTCGACATGGGTGGCGTTACCTCCTCCAAG), Pnef(−) (GTCAGCCTCGAGTTAGCTTGTTTTCTTCTTGTCAGC), Pnef1(+) (AAGCAGTCGACAAGCAGCGCAGGCGTGGTGG), and Pnef1(−) (AATTTCTCGAGCCATTTTTAAAAGGCCTCTTG) in nested PCRs, proviral DNA was consistently detected at weeks 4 and 8 after infection in both infected macaques and in one of four attempts at 19 weeks after infection in macaque Nem 144.
In order to demonstrate the continuous presence in vivo of the functional nef gene derived from SIVsmmPBj1.9 we analyzed the genetic variability of nef in PBMC DNA from both pig-tailed macaques at 4 and 8 weeks after infection. Specific oligonucleotide primers were used in the nested PCR described above to specifically amplify the core nef gene region from nucleotide 43 to 762 comprising the critical codon 17 and the SH2 binding domain (6, 7). The amplified fragments were cloned into plasmid pGem11 ZF(+) or by TA cloning into pGem-T (Boehringer Ingelheim Bioproducts Partnership, Heidelberg, Germany) and entirely sequenced.
Four and 8 weeks after infection, six or eight cloned sequences obtained from PBMC DNA of both macaques (Nem 83 and Nem 144) infected with chimeric virus HY-TM/nef/U3 were analyzed (Table 1). Of the 28 sequences, 27 displayed neither a rearrangement nor a nucleotide deletion or insertion which would lead to a shift of the nef reading frame. Most importantly, no amino acid changes within the putative SH2 binding domain from codon 17 to 20 were observed. Interestingly, a number of sequences derived from Nem 83 and Nem 144 were found to contain premature stop codons due to a G→A substitution in the tryptophan codon (Table 1). Such stop codon insertions are often generated by mutations of tryptophan codons in lentiviruses due to the known G→A hypermutation (26). The amino acid divergence compared to PBj1.9 Nef was 0.5 to 1.8% in macaque Nem 83 and 0.9 to 3.7% in macaque Nem 144, a degree of variability comparable to that of SIVsmm-PBj14 at 6 to 12 weeks after clonal infection (24).
TABLE 1.
Genetic variability of the nef gene during the first 8 weeks of infection with chimeric virus HY-TM/nef/U3
| Clonea | Mutation or insertion at codon no.
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 19 | 26 | 32 | 48 | 70 | 71 | 72 | 81 | 87 | 94 | 105 | 107 | 109 | 130 | 147 | 148 | 149 | 194 | 211 | 224 | 230 | |
| 83/4/1 | W→⊗b | E→K | E→K | G→E | W→⊗ | E→K | |||||||||||||||
| 83/4/2 | P→S | E→K | H→P | ||||||||||||||||||
| 83/4/3 | P→S | E→K | |||||||||||||||||||
| 83/4/4 | E→K | ||||||||||||||||||||
| 83/4/5 | E→D | P→S | M→V | E→K | |||||||||||||||||
| 83/4/6 | R→K | ||||||||||||||||||||
| 83/8/1 | P→S | ||||||||||||||||||||
| 83/8/2 | P→S | ||||||||||||||||||||
| 83/8/3 | P→S | ||||||||||||||||||||
| 83/8/4 | W→⊗ | D→N | M→I | P→S | R→K | E→K | G→E | ||||||||||||||
| 83/8/5 | W→⊗ | P→S | |||||||||||||||||||
| 83/8/6 | F→S | ||||||||||||||||||||
| 83/8/7 | F→S | ||||||||||||||||||||
| 83/8/8 | (G+c) ⊗ | F→S | |||||||||||||||||||
| 144/4/1 | W→⊗ | D→N | M→V | P→S | R→K | W→⊗ | |||||||||||||||
| 144/4/2 | W→⊗ | D→N | M→I | D→N | P→S | R→K | E→K | G→E | W→⊗ | F→S | E→K | ||||||||||
| 144/4/3 | W→⊗ | M→I | D→N | P→S | R→K | E→K | G→E | W→⊗ | F→S | E→K | |||||||||||
| 144/4/4 | P→S | F→S | |||||||||||||||||||
| 144/4/5 | W→⊗ | D→N | M→I | D→N | P→S | R→K | E→K | G→E | W→⊗ | F→S | E→K | ||||||||||
| 144/4/6 | P→S | F→S | |||||||||||||||||||
| 144/4/7 | P→S | I→F | |||||||||||||||||||
| 144/8/1 | W→⊗ | D→N | M→I | D→N | P→S | R→K | E→K | G→E | F→S | E→K | |||||||||||
| 144/8/2 | P→S | F→S | |||||||||||||||||||
| 144/8/3 | P→S | F→S | |||||||||||||||||||
| 144/8/4 | P→S | F→S | |||||||||||||||||||
| 144/8/5 | K→N | P→S | F→Y | ||||||||||||||||||
| 144/8/6 | M→I | P→S | |||||||||||||||||||
| 144/8/7 | R→K | P→S | E→K | ||||||||||||||||||
| C1 | M→L | P→S | E→K | F→S | |||||||||||||||||
| C2 | P→S | M→I | E→K | F→S | |||||||||||||||||
| C3 | R→K | M→L | P→S | E→K | F→S | ||||||||||||||||
| C4 | P→S | ||||||||||||||||||||
Cloned sequences are named according to animal, week after infection, and number of sequence (e.g., for the first clone listed, 83/4/1 refers to macaque Nem 83 at 4 weeks after infection [sequence 1]). Clones C1 to C4 are sequences cloned from C8166 cells after a second round of infection with the virus stock.
⊗, stop codon TGA.
(G +), G nucleotide insertion; frameshift extending the Nef reading frame by an unrelated 11 amino acids following codon 71.
The sequences of the nef genes and other critical regions in the genome of chimeric virus HY-TM/nef/U3 were verified in the virus stock as previously described (5) before infecting animals Nem 83 and Nem 144. No sequence changes, particularly in the nef gene and in the U3 promoter, were found. However, as observed in vivo, amino acid changes in positions 105 (P→S), 148 (E→K), and 224 (F→S) were found in viral DNA detected in C8166 cells after a second round of infection with the virus stock (Table 1, clones C1 to C4), indicating a certain bias towards these codon mutations during replication of chimera HY-TM/nef/U3. However the critical tyrosine codon in position 17 was unchanged in all sequences detected in vitro and in vivo.
With the aim of understanding the mechanisms underlying the apathogenicity of SIVagm in its natural or in heterologous hosts, we had previously analyzed the biological activities in vitro of a variety of chimeric SIVagm3mc/SIVsmmPBj1.9 viruses (5). Four of five chimeric viruses were able to replicate in the permissive CD4+ T-cell line C8166 and in stimulated PBMCs from pig-tailed macaques. Moreover, those variants harboring the U3 promoter of SIVsmmPBj1.9 within their genomes also replicated in nonstimulated PBMCs, an ability known to be associated for SIVsmmPBj14 and related chimeric viruses with the acutely pathogenic phenotype in vivo (8, 11). Here, the chimeric virus HY-gag/pol/CR/nef/U3, containing within its genome the U3 promoter, the nef gene, and other regions of the SIVsmmPBj1.9 genome, was also shown to replicate in nonstimulated pig-tailed macaque PBMCs and to induce acute disease. Comparable studies with chimeras derived from acutely pathogenic SIVsmmPBj6.6 and minimally pathogenic SIVsmmH4 (21, 22) suggest that multiple viral genetic determinants are necessary for the development of acute disease. It was shown by Du et al. (6) and by Tao et al. (25) that multiple NFκB-binding sites in the U3 region of SIVsmmPBj14 were not required for acute disease. However, the gag gene of SIVsmmPBj and the 5′ region of its nef gene (including the critical tyrosine at position 17) were suggested to be a requirement for disease induction. The results described here are consistent with this, because the gag and nef genes of SIVsmmPBj1.9 were present in the genome of the acutely pathogenic HY-gag/pol/CR/nef/U3.
In contrast, the chimeric virus HY-TM/nef/U3, although able to replicate in nonstimulated PBMCs, was unable to induce acute disease or CD4+ cell depletion in pig-tailed macaques. During the first 8 weeks, the infected macaques were viremic, as shown by repeated isolation of replication-competent chimeric virus from the peripheral blood and consistent detection of proviral DNA in PBMCs using nef specific PCR. In accordance with an active replication in vivo the nef genes acquired a degree of variation similar to that described for SIVsmmPBj14 nef following clonal infection (24). In addition, with one exception, none of the nef sequences obtained during the first 8 weeks after infection showed deletions or insertions, indicating that the gene was indeed functional. Therefore, during the first 8 weeks after infection, active replication of chimeric virus HY-TM/nef/U3 containing an intact PBj-derived nef gene could have resulted in the induction of an acute viral disease but did not.
The lack of disease induction by chimeric virus HY-TM/nef/U3 contrasts with the results of Du et al. (6, 7), showing that a single amino acid substitution at position 17 (R→Y) in nef converted the pathogenic SIVmac239 into an acutely lethal and enteropathic virus similar to SIVsmmPBj14. However, SIVmac239 and SIVagm3mc, from which the chimeras with PBj14 nef genes were derived, are themselves very different with respect to their pathogenicity (1). Whereas SIVmac239 is already a moderately pathogenic virus able to induce AIDS-like disease in macaques (18), SIVagm3mc is completely apathogenic in these animals. Therefore, the nef gene of SIVsmmPBj14 and related virus clones can convert moderately pathogenic SIV into acutely lethal and enteropathic viruses but is unable to confer the same phenotype to apathogenic SIVagm3mc. This is also in agreement with the studies performed with chimeric SIVsmmH4/SIVsmmPBj mentioned above (21, 22). SIVsmmH4 is classified as a minimally pathogenic lentivirus (13) and is therefore more related (in terms of in vivo phenotype) to the apathogenic SIVagm3mc used here. At least two of the chimeric SIVsmmH4/SIVsmmPBj carrying PBj14 nef including the critical tyrosine at position 17 were also unable to induce acute disease in macaques (21, 22). In conclusion, the SIVsmmPBj nef gene is a critical and single determinant of acute pathogenicity when introduced into the genomes of SIV strains which by themselves are able to induce AIDS in macaques. It is, however, not sufficient to convert apathogenic or minimally pathogenic SIV strains into acutely pathogenic viruses.
Acknowledgments
This work was supported by grant Ci 10/9-1 of the Deutsche Forschungsgemeinschaft to Klaus Cichutek.
S. Dewhurst (University of Rochester Medical Center, Rochester, N.Y.) is gratefully acknowledged for the kind donation of plasmid pSIVsmmPBj1.9. We thank D. Kahlenberg for excellent technical assistance, M. Selbert for expert automatic DNA sequencing, and S. Wagener for editorial assistance.
REFERENCES
- 1.Baier M, Werner A, Cichutek K, Garber C, Müller C, Kraus G, Ferdinand F J, Hartung S, Papas T S, Kurth R. Molecularly cloned simian immunodeficiency virus SIVagm3 is highly divergent from other SIVagm isolates and is biologically active in vitro and in vivo. J Virol. 1989;63:5119–5123. doi: 10.1128/jvi.63.12.5119-5123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beer B, Scherer J, zur Megede J, Norley S, Baier M, Kurth R. Lack of dichotomy between virus load of peripheral blood and lymph nodes during long-term simian immunodeficiency virus infection of African green monkeys. Virology. 1996;219:367–375. doi: 10.1006/viro.1996.0262. [DOI] [PubMed] [Google Scholar]
- 3.Cichutek, K., and S. Norley. 1993. Lack of immune suppression in SIV-infected natural hosts. AIDS 7(Suppl. 1):25–35. [PubMed]
- 4.Dewhurst S, Embretson J E, Anderson D C, Mullins J I, Fultz P N. Sequence analysis and acute pathogenicity of molecularly cloned SIVsmm-PBj14. Nature. 1990;345:636–640. doi: 10.1038/345636a0. [DOI] [PubMed] [Google Scholar]
- 5.Dittmar M T, Cichutek K, Fultz P N, Kurth R. The U3 promoter region of the acutely lethal simian immunodeficiency virus clone smmPBj1.9 confers related biological activity on the apathogenic clone agm3mc. Proc Natl Acad Sci USA. 1995;92:1362–1366. doi: 10.1073/pnas.92.5.1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du Z, Ilyinskii P O, Sasseville V G, Newstein M, Lackner A A, Desrosiers R C. Requirements for lymphocyte activation by unusual strains of simian immunodeficiency virus. J Virol. 1996;70:4157–4161. doi: 10.1128/jvi.70.6.4157-4161.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Du Z, Lang S M, Sasseville V G, Lackner A A, Ilyinskii P O, Daniel M D, Jung J U, Desrosiers R C. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell. 1995;82:665–674. doi: 10.1016/0092-8674(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 8.Fultz P. Replication of an acutely lethal simian immunodeficiency virus activates and induces proliferation of lymphocytes. J Virol. 1991;65:4902–4909. doi: 10.1128/jvi.65.9.4902-4909.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fultz P F, McClure H M, Anderson D C, Switzer W M. Identification and biologic characterization of an acutely lethal variant of simian immunodeficiency virus from sooty mangabeys (SIV/SMM) AIDS Res Hum Retroviruses. 1989;5:397–409. doi: 10.1089/aid.1989.5.397. [DOI] [PubMed] [Google Scholar]
- 10.Fultz P N, Anderson D C, McClure H M, Dewhurst S, Mullins J I. SIVsmm infection of macaque and mangabey monkeys: correlation between in vivo and in vitro properties of different isolates. Dev Biol Stand. 1990;72:253–258. [PubMed] [Google Scholar]
- 11.Fultz P N, Zack P M. Unique lentivirus-host interactions: SIVsmmPBj14 infection in macaques. Virus Res. 1994;32:205–225. doi: 10.1016/0168-1702(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 12.Hartung S, Boller K, Cichutek K, Norley S G, Kurth R. Quantitation of a lentivirus in its natural host: simian immunodeficiency virus in African green monkeys. J Virol. 1992;66:2143–2149. doi: 10.1128/jvi.66.4.2143-2149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hirsch V M, Olmsted R A, Murphey-Corb M, Purcell R H, Johnson P R. An African primate lentivirus (SIVsm) closely related to HIV-2. Nature. 1989;339:389–391. doi: 10.1038/339389a0. [DOI] [PubMed] [Google Scholar]
- 14.Hirsch V M, Dapolito G, Johnson P R, Elkins W E, London W T, Montali R, Goldstein S, Brown C. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J Virol. 1995;69:955–967. doi: 10.1128/jvi.69.2.955-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Israel Z R, Dean G A, Maul D H, O’Neil S P, Dreitz M J, Mullins J I, Fultz P N, Hoover E A. Early pathogenesis of disease caused by SIVsmmPBj14 molecular clone 1.9 in macaques. AIDS Res Hum Retroviruses. 1993;9:277–286. doi: 10.1089/aid.1993.9.277. [DOI] [PubMed] [Google Scholar]
- 16.Johnson P R, Goldstein S, London W T, Fomsgaard A, Hirsch V M. Molecular clones of SIVsm and SIVagm: experimental infection of macaques and African green monkeys. J Med Primatol. 1990;19:279–286. [PubMed] [Google Scholar]
- 17.Kanki P J, Alroy J, Essex M. Isolation of T-lymphotropic retrovirus related to HTLV III/LAV from wild-caught African green monkeys. Science. 1985;230:951–954. doi: 10.1126/science.2997923. [DOI] [PubMed] [Google Scholar]
- 18.Kestler H, Kodama T, Ringler D, Marthas M, Pedersen N, Lackner A, Regier D, Sehgal P, Daniel M, King N, Desrosiers R. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990;248:1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- 19.Kraus G, Werner A, Baier M, Binninger D, Ferdinand F-J, Norley S, Kurth R. Isolation of human immunodeficiency virus-related simian immunodeficiency viruses from African green monkeys. Proc Natl Acad Sci USA. 1989;86:2892–2896. doi: 10.1073/pnas.86.8.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norley S G, Kraus G, Ennen J, Bonilla J, König H, Kurth R. Immunological studies of the basis for the apathogenicity of simian immunodeficiency virus from African green monkey. Proc Natl Acad Sci USA. 1990;87:9067–9071. doi: 10.1073/pnas.87.22.9067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Novembre F J, Johnson P R, Lewis M G, Anderson D C, Klumpp S, McClure H M, Hirsch V M. Multiple viral determinants contribute to pathogenicity of the acutely lethal simian immunodeficiency virus SIVsmmPBj variant. J Virol. 1993;67:2466–2474. doi: 10.1128/jvi.67.5.2466-2474.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Novembre F J, Saucier M M, Hirsch V M, Johnson P R, McClure H M. Viral determinants in SIVsmmPBj pathogenesis. J Med Primatol. 1994;23:136–145. doi: 10.1111/j.1600-0684.1994.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 23.Schwiebert R, Fultz P N. Immune activation and viral burden in acute disease induced by simian immunodeficiency virus SIVsmmPBj14: correlation between in vivo and in vitro events. J Virol. 1994;68:5538–5547. doi: 10.1128/jvi.68.9.5538-5547.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwiebert R S, Tao B, Fultz P N. Loss of the SIVsmmPBj14 phenotype and nef genotype during long-term survival of macaques infected by mucosal routes. Virology. 1997;230:82–92. doi: 10.1006/viro.1997.8469. [DOI] [PubMed] [Google Scholar]
- 25.Tao B, Fultz P N. Molecular and biological analysis of quasispecies during evolution of a virulent simian immunodeficiency virus, SIVsmmPBj14. J Virol. 1995;69:2031–2037. doi: 10.1128/jvi.69.4.2031-2037.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vartanian J-P, Meyerhans A, Åsjö B, Wain-Hobson S. Selection, recombination, and G→A hypermutation of human immunodeficiency virus type 1 genomes. J Virol. 1991;65:1779–1788. doi: 10.1128/jvi.65.4.1779-1788.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]