Abstract
Objectives
To investigate the genomic diversity and β-lactam susceptibilities of Enterococcus faecalis collected from patients with infective endocarditis (IE).
Methods
We collected 60 contemporary E. faecalis isolates from definite or probable IE cases identified between 2018 and 2021 at the University of Pittsburgh Medical Center. We used whole-genome sequencing to study bacterial genomic diversity and employed antibiotic checkerboard assays and a one-compartment pharmacokinetic–pharmacodynamic (PK/PD) model to investigate bacterial susceptibility to ampicillin and ceftriaxone both alone and in combination.
Results
Genetically diverse E. faecalis were collected, however, isolates belonging to two STs, ST6 and ST179, were collected from 21/60 (35%) IE patients. All ST6 isolates encoded a previously described mutation upstream of penicillin-binding protein 4 (pbp4) that is associated with pbp4 overexpression. ST6 isolates had higher ceftriaxone MICs and higher fractional inhibitory concentration index values for ampicillin and ceftriaxone (AC) compared to other isolates, suggesting diminished in vitro AC synergy against this lineage. Introduction of the pbp4 upstream mutation found among ST6 isolates caused increased ceftriaxone resistance in a laboratory E. faecalis isolate. PK/PD testing showed that a representative ST6 isolate exhibited attenuated efficacy of AC combination therapy at humanized antibiotic exposures.
Conclusions
We find evidence for diminished in vitro AC activity among a subset of E. faecalis IE isolates with increased pbp4 expression. These findings suggest that alternate antibiotic combinations against diverse contemporary E. faecalis IE isolates should be evaluated.
Introduction
Enterococci are commensal inhabitants of the human gastrointestinal tract.1 In the antibiotic era, Enterococcus faecalis has emerged as a leading cause of infection, especially in immunocompromised patients.2 E. faecalis are a common cause of infective endocarditis (IE),3 and the prevalence of E. faecalis IE (EFIE) is steadily increasing.4 Despite the use of in vitro active antibiotic combination therapy for at least 6 weeks, mortality from EFIE remains as high as 30%.5,6 Treatment guidelines for EFIE include using the combination of ampicillin and ceftriaxone (AC) or ampicillin and gentamicin (AG).7–9 Prospective observational clinical studies in Europe have suggested that treatment of EFIE with AC is as efficacious as treatment with AG, and results in lower rates of renal toxicity.10,11 Nevertheless, EFIE mortality rates have not improved in decades.4
Previous studies documenting AC synergy have used a limited number of laboratory strains of E. faecalis, which may not represent the large genetic diversity of this species.12–14 We previously reviewed 190 EFIE cases from 2010 to 2017 at the University of Pittsburgh Medical Center (UPMC), and found higher overall 90-day mortality rates among patients treated with AC versus AG (21% versus 8%, P = 0.02).15 Whereas baseline differences between patients influenced these findings, it is possible that for some patients, AC may not be optimal. In this study, we investigated the genomic diversity and AC susceptibility of 60 contemporary E. faecalis isolates collected from patients with IE.
Materials and methods
Collection of isolates
E. faecalis isolates were collected from patients with definite or probable IE at UPMC between 2018 and 2021. Demographics and outcomes were collected through a retrospective search of patients’ electronic medical records. Bacterial isolates were collected as part of routine clinical care, and were stored as deidentified, pure bacterial cultures. This study was approved with a waiver of informed consent by the Institutional Review Board at the University of Pittsburgh (protocol no. STUDY22050046).
Whole-genome sequencing
Genomic DNA was extracted using a DNeasy Blood and Tissue Kit (Qiagen, Germantown, MD, USA) from 1 mL of bacterial cultures grown overnight in Brain Heart Infusion broth. Next-generation sequencing libraries were prepared with a Nextera XT kit (Illumina, San Diego, CA, USA). Libraries were sequenced on an Illumina MiSeq or NextSeq using 150-bp (NextSeq) or 300-bp (MiSeq) paired-end reads. Genomes were assembled with SPAdes v.3.13.0,16 annotated with prokka17 and compared to one another with Roary.18 A core genome phylogenetic tree was generated using RAxML with the GTRCAT substitution model and 100 iterations.19 STs, antimicrobial resistance genes and plasmid replicons were identified using the Center for Genomic Epidemiology (https://www.genomicepidemiology.org/) with default sequence coverage and identity search parameters. Illumina data for isolates newly sequenced in this study were submitted to NCBI under BioProject PRJNA729754 [accession numbers listed in Table S1 (available as Supplementary data at JAC Online)].
Antimicrobial susceptibility and synergy testing
AC susceptibilities and AC synergy were determined by a 96-well plate checkerboard assay.20 Briefly, 96-well plates were prepared containing 2-fold serial dilutions of each antibiotic, either alone or in combination, in 100 μL Mueller–Hinton Broth (MHB). Overnight E. faecalis cultures grown in MHB were diluted to OD600 = 0.1, were then diluted 1:1000 into fresh MHB and 100 μL of this dilution was transferred into each well of the 96-well plate (∼104 colony-forming units (cfu) per well). Plates were incubated for 24 hours at 37°C and growth in each well was analysed by both visual inspection and OD600 measurement using a Synergy H1 microplate reader (Biotek, Winooski, VT, USA). Assays were conducted on two replicate cultures grown on different days. MICs were identified as the lowest antibiotic concentration that inhibited bacterial growth. Fractional inhibitory concentration index (FICI) values were calculated with the following equation: FICI = (CA/MICA) + (CC/MICC), where MICA and MICC are the MICs of AC alone, and CA and CC are the concentrations of each drug in the well with the strongest inhibition. Concentration ranges tested were: ampicillin, 0.0039–4 mg/L and ceftriaxone, 16–1024 mg/L. MIC and FICI values were averaged across two biological replicates.
Construction of pbp4 isogenic mutants
Isogenic pbp4 mutants were constructed in the OG1RF laboratory strain21 using the pMAD vector.22 Briefly, a ∼1.8 kb region centred on the locus of interest was amplified by PCR from genomic DNA extracted from the DVT676 (ST179, pbp4 P520S) or DVT680 (ST6, pbp4 −82delT) isolate. PCR products were ligated to the pMAD vector using Gibson assembly and vectors were transformed into NEB 5-alpha E. coli. Vectors were then transformed into electrocompetent E. faecalis OG1RF,23 and transformants were selected on Brain Heart Infusion agar containing 25 mg/L erythromycin and grown at 30°C. Allelic replacement was performed as previously described22 and resulting mutants were confirmed by whole-genome sequencing to confirm presence of desired mutations and absence of off-target mutations.
In vitro one-compartment pharmacokinetic/pharmacodynamic (PK/PD) model
A one-compartment PK/PD model was used to simulate free serum concentrations of ampicillin dosed at 2 g every 4 hours (fCmax 120 µg/mL, half-life 1 h, protein binding 20%) and ceftriaxone dosed at 2 g every 12 hours (fCmax 25.7 µg/mL, half-life 6 h, protein binding 90%).7 A central reservoir of cation-adjusted MHB in a glass model with four ports (inflow, outflow, sampling and ventilation) was used and maintained at 37°C. Peristaltic pumps controlled the flow of media to achieve desired antibiotic concentrations. In combination studies, flow rates were set to achieve ampicillin exposures given a shorter half-life and supplemental ceftriaxone was added using methods described by Blaser et al.24 AC were purchased from the UPMC pharmacy and were prepared according to the manufacturer’s instructions. All agents were infused over 30 minutes by programmable syringe pumps maintained at 4°C. Samples of 1 mL volume were removed at 0, 2, 6, 10, 24, 27, 30, 48, 51, 54 and 72 hours, centrifuged at 5000 rpm for 5 minutes, washed in saline three times and resuspended to the original volume to prevent antibiotic carryover.25 Each sample was then serially 10-fold diluted and plated onto cation-adjusted MHB agar to enumerate surviving bacteria down to a limit of detection of 0.7 log10 cfu/mL, which was achieved by plating 200 µL per sample, allowing detection as low as 5 cfu/mL. A standard inoculum of 1 × 106 cfu/mL was used for all studies; bactericidal activity was defined as a ≥3-log10 cfu/mL decrease. All experiments were performed in duplicate and a representative curve for each isolate was used for analysis.
Statistics
Differences in rep gene abundance between different STs were assessed with unpaired two-tailed t-tests. Differences in antibiotic MIC and FICI values were assessed with ordinary one-way analysis of variance (ANOVA) with multiple comparisons.
Results
Genomic diversity of EFIE isolates
A total of 60 isolates were collected between 2018 and 2021 from patients with probable or definite E. faecalis IE based on modified Duke’s criteria26,27 (Table S1). The median age of the patients was 64.5 years and 72% were male (Table 1). AC combination therapy was the first-line treatment for 50 patients (83.3%), and all-cause 30- and 90-day mortality rates were 21.7% and 30.0%, respectively (Table 1).
Table 1.
Demographics and clinical features of EFIE patients
| Variable | N = 60 |
|---|---|
| Age at admissiona, years | 64.5 (49.5, 74) |
| Male sex | 43 (71.7) |
| White race | 48 (80.0) |
| Definite IEb | 38 (63.3) |
| Length of bacteraemiaa, hoursc | 63.9 (41.7, 107.0) |
| Native valve IE | 27 (45.0) |
| Prosthetic valve IE | 14 (23.3) |
| Definitive treatment with AC | 50 (83.3) |
| Length of hospitalizationa, days | 13.5 (10, 34.5) |
| Positive E. faecalis bacteraemia within 3 months of treatment | 3 (5.0) |
| Death during hospital admission | 15 (25.0) |
| 30-day mortality | 13 (21.7) |
| 90-day mortality | 20 (30.0) |
| Readmission within 3 monthsd, any cause | 28 (46.7) |
IE, infective endocarditis; AC, ampicillin/ceftriaxone.
aMedian values (interquartile ranges) are reported. For all other variables, the number of patients (% of patients) is reported.
bDefinite IE based on the modified Duke Criteria for endocarditis.
cEstimated duration of bacteraemia from first positive blood culture collection to either last positive culture or first negative culture.
dPercentage readmission based on patients who survived initial hospitalization.
The genomes of all isolates were sequenced on the Illumina platform and a core genome phylogeny was constructed (Table S1, Figure 1). Isolates were found to belong to 27 multi-locus sequence types (with single locus variants, SLVs, counted along with their closest assigned ST). ST6 (n = 11) and ST179 (n = 10) were the most prevalent STs observed, together accounting for 35% of collected isolates. Isolates belonging to ST40 (n = 6), ST16 (n = 5), ST34 (n = 3), ST59 (n = 2), ST64 (n = 2) and ST310 (n = 2) were also identified in more than one patient. E. faecalis isolates from all other patients belonged to unique STs (Figure 1).
Figure 1.
Core genome phylogeny of EFIE isolates. A single-copy core genome phylogeny was constructed for 60 E. faecalis isolates from patients with probable or definite IE. The midpoint-rooted RAxML tree was built from single nucleotide polymorphisms in 1918 single copy core genes identified with Roary. Tips are annotated with isolate name, ST, pbp4 mutations, acquired antibiotic resistance-associated genes, plasmid rep genes and presence of CRISPR–Cas loci. SLV, single locus variant; aac(6′)-aph(2″), aph(2′)-Ic, aph(3′)-III and ant(6)-Ia, aminoglycoside resistance; erm(B), erythromycin resistance; tet(M) and tet(L), tetracycline resistance; dfrG, trimethoprim resistance.
Mutations in the low-affinity penicillin-binding protein 4 (pbp4) have been previously described in E. faecalis and have been shown to affect susceptibility to β-lactam agents.28–30 We observed pbp4 mutations in isolates belonging to ST6 and ST179 (Table S1, Figure 1). All ST6 isolates carried a single base deletion 82 nucleotides upstream of pbp4 (−82delT), while all ST179 isolates as well as three additional isolates (two ST64 isolates and one ST1271 isolate) harboured a non-synonymous P520S mutation in pbp4. We also screened all genomes for acquired antimicrobial resistance genes using ResFinder31 (Figure 1). Vancomycin resistance genes were absent, and no acquired β-lactamase genes nor β-lactamase production were identified in any isolate. Genes predicted to confer high-level aminoglycoside resistance were found in every ST6 isolate, while outside of ST6 they were rare except for ST16. Macrolide, tetracycline and trimethoprim resistance genes were also frequently found in ST6, ST16 and ST179 isolates (Figure 1). All genomes were also screened for plasmid rep genes using PlasmidFinder,32 which identified eight different rep genes that together were detected in 46 (76.7%) isolate genomes (Figure 1). ST6 and ST179 isolates carried more rep genes than isolates belonging to other STs (ST6, mean 2.46 versus 1.13 rep genes, P = 0.0002; ST179, mean 2.20 versus 1.13 rep genes, P = 0.0015). Finally, we examined all isolates for the presence of intact clustered regularly interspaced short palindromic repeats (CRISPR)–Cas loci and found that intact loci were present in 39 (65%) isolate genomes distributed across the phylogenetic tree (Figure 1). CRISPR–Cas presence was not associated with resistance gene nor plasmid rep gene abundance, by contrast with previous studies.33,34
To test whether the E. faecalis isolates that we sampled were specific to our location or were more broadly reflective of EFIE in the USA, we assessed the genomic diversity of EFIE isolates collected in Houston, Texas and Detroit, Michigan as part of the VENOUS I study35 (Table S2, Figure S1). Among the 18 EFIE isolate genomes included in that study, ST6 and ST179 were identified in seven (38.9%) isolates, although ST6 isolates were only sampled from Detroit and ST179 isolates were only sampled from Houston (Table S2). All four ST6 isolates and one additional ST778 isolate had the same pbp4 −82delT mutation as was detected among ST6 isolates from Pittsburgh. Additionally, all three ST179 isolates plus two additional ST64 and ST777 isolates had the pbp4 P520S mutation that was seen in the ST179 isolates from Pittsburgh (Figure S1). Taken together, these data indicate that ST6 and ST179 are frequently isolated from patients with EFIE in the USA, and that both lineages carry pbp4 mutations predicted to affect β-lactam susceptibility.
ST6 isolates are more resistant to ceftriaxone and show diminished AC synergy
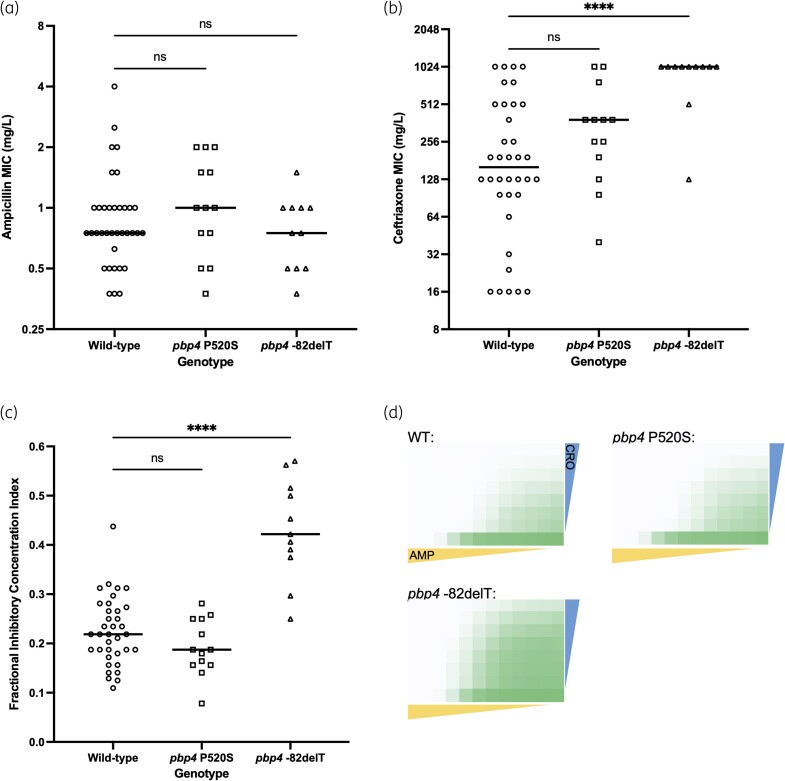
To investigate β-lactam susceptibility among EFIE isolates, we measured AC MICs against all 60 isolates we collected (Figure 2a and b). To understand how mutations in pbp4 impact antibiotic susceptibility, we analysed isolates based on their pbp4 genotypes. Ampicillin MICs were ≤4 mg/L for all isolates and did not vary significantly between pbp4 genotypes (Table S1, Figure 2a). Ceftriaxone MICs were more variable and ranged from 16 mg/L to 1024 mg/L. Median ceftriaxone MICs were higher for isolates encoding the pbp4 P520S mutation compared to pbp4 wild-type isolates; however, the difference was not statistically significant. ST6 isolates, all of which encoded the pbp4 −82delT mutation, had higher ceftriaxone MIC values compared to wild-type isolates (mean ceftriaxone MIC = 896 mg/L versus 303 mg/L, P < 0.0001) (Figure 2b).
Figure 2.
Variability in AC susceptibility among EFIE isolates. MICs of (a) ampicillin and (b) ceftriaxone among 60 EFIE isolates. MIC values were averaged between biological replicate experiments, and isolates are grouped based on their pbp4 genotype. (c) FICI values collected from AC in vitro synergy testing. In (a)–(c), horizontal lines show median values and P values are from ordinary one-way ANOVA comparing each pbp4 mutant group with the wild-type group. ns, not significant. ****P < 0.0001. (d) Average bacterial growth (OD600) in checkerboard assays separated by pbp4 genotype. Growth was averaged among all assays conducted for isolates possessing each genotype. WT, wild-type. Coloured triangles show decreasing ampicillin (yellow; range tested was 0.0039–4 mg/L) and ceftriaxone (blue; range tested was 16–1024 mg/L) concentrations across and down each plate, respectively. The same antibiotic concentrations were tested against all isolates. Green shading indicates bacterial growth after 24 hours in each assay well, with darker shading indicating higher OD600 values.
To investigate how effectively AC could inhibit the growth of EFIE isolates, we performed two-antibiotic checkerboard assays on the 60 isolates we collected.20 We found that FICI values were below 0.5 for all but four isolates, all of which belonged to ST6 (Figure 2c). FICI values among isolates carrying the pbp4 −82delT mutation (all ST6 isolates) were significantly higher than pbp4 wild-type isolates (P < 0.0001). We also examined average bacterial growth (OD600) across the checkerboard assay plates among isolates encoding different pbp4 mutations (Figure 2d). Bacterial growth was similar between pbp4 wild-type and P520S isolates, whereas isolates encoding the pbp4 −82delT mutation showed more growth in wells containing higher concentrations of both antibiotics. These data suggest that AC may be less active against ST6 isolates, perhaps due to the pbp4 −82delT mutation they encode.
To test the effect of the pbp4 mutations we identified on AC susceptibility in a clean genetic background, we performed allelic replacement experiments to introduce the pbp4 P520S and −82delT mutations into the E. faecalis OG1RF laboratory strain.21 Compared to the wild-type OG1RF strain, the OG1RF derivative strain carrying the pbp4 P520S mutation showed no difference in ampicillin MIC and a 2-fold decrease in ceftriaxone MIC (Table 2). The strain carrying the pbp4 −82delT mutation also showed no difference in ampicillin MIC, however, there was a 16-fold increase in its ceftriaxone MIC. Together, these data suggest that while the pbp4 P520S mutation does not affect ampicillin or ceftriaxone susceptibility, the pbp4 −82delT mutation contributes to the higher ceftriaxone MICs observed among ST6 EFIE isolates.
Table 2.
AC susceptibility of OG1RF wild-type and isogenic pbp4 mutant strains
| Strain background | pbp4 genotype | Ampicillin MIC (mg/L) | Ceftriaxone MIC (mg/L) |
|---|---|---|---|
| OG1RF | Wild-type | 1 | 16 |
| OG1RF | P520S | 1 | 8 |
| OG1RF | −82delT | 1 | 256 |
pbp4, penicillin-binding protein 4.
In vitro pharmacokinetic/pharmacodynamic responses to AC
Next, we tested whether clinical isolates with or without pbp4 mutations showed differences in AC susceptibility in the presence of dynamic drug exposures that mimic those achieved in patients. To do so, we employed a one-compartment PK/PD model to measure killing activity and suppression of bacterial regrowth following exposures to AC alone or together (Figure 3). Three representative EFIE isolates encoding either wild-type pbp4 (isolate DVT685, ST869), pbp4 P520S (isolate DVT684, ST179) or pbp4 −82delT (isolate DVT871, ST6) were selected. Simulated antibiotic exposures were selected based on those recommended by consensus guidelines (Ampicillin 2 g q 4 h, Ceftriaxone 2 g q 12 h).7 Exposure to ampicillin alone resulted in rapid killing of all three isolates, with log-kills ranging from 3.07 to 4.91 log10 cfu/mL at 10 hours. Notably, the DVT871 isolate belonging to ST6 and encoding the pbp4 −82delT mutation demonstrated bacterial regrowth after initial killing in the presence of ampicillin alone as well as AC together (Figure 3). These data suggest that ST6 isolates encoding the pbp4 −82delT mutation display decreased susceptibility to AC synergy under assay conditions and at drug concentrations that reflect what E. faecalis are likely to experience in vivo during infection.
Figure 3.
One-compartment pharmacodynamic modelling of ampicillin, ceftriaxone and their combination against three different EFIE isolates belonging to different sequence types. Bacterial counts over time for cultures treated with no drug (black), ampicillin alone (yellow), ceftriaxone alone (blue) or both ampicillin and ceftriaxone (green). Isolate ID, ST, pbp4 genotype and ampicillin (AMP) and ceftriaxone (CRO) MICs are shown above each graph. Dots indicate measured time points, and values plotted on the x-axis are below the limit of detection of 0.7 log10 cfu/mL.
Discussion
In this study, we characterized the genomic diversity and antibiotic susceptibility of 60 E. faecalis isolates from patients with probable or definite IE treated at a single medical centre between 2018 and 2021. Because AC is the current front-line therapy regimen for EFIE,9 we focused on the ability of AC to inhibit E. faecalis in vitro, using MIC and checkerboard assays to measure growth inhibition as well as a dynamic one-compartment PK/PD model of bacterial killing. While we found that AC was active in vitro against most EFIE isolates, susceptibility to ceftriaxone and AC were variable among the different isolates tested. In particular, ST6 isolates displayed elevated ceftriaxone MICs and a representative ST6 isolate showed attenuated AC synergy at humanized antibiotic exposures. These findings have potential implications for clinical management of EFIE and suggest that AC may not be equally effective against all E. faecalis isolates.
Among the EFIE isolates we collected, we observed abundant genetic diversity as well as enrichment of a small number of genetic lineages. ST6 was the most frequently observed lineage at our centre, accounting for 18% of all EFIE cases. All ST6 isolates carried a mutation 82 nucleotides upstream of pbp4 (−82delT) that was previously shown to cause overexpression of pbp4 and increased β-lactam resistance.29,30 This phenomenon correlated with our observation that ST6 isolates were less susceptible to ceftriaxone compared to isolates with wild-type pbp4 sequences, and that ST6 isolates showed diminished AC synergy compared to the other tested isolates. We also observed a P520S mutation in pbp4 among 13 different isolates belonging to three different STs. In E. faecalis, PBP4 is a 680-amino acid membrane protein with three penicillin-binding motifs (STFK, SDN and KTG), which are located in a penicillin-binding module between amino acids 350–680.35 The P520S substitution falls within the region between the active site-defining SDN and KTG motifs, and this mutation has been previously found to cause increased β-lactam resistance in E. faecalis.28,29,36 In contrast to these previous reports, we did not find significant differences in ampicillin or ceftriaxone MICs, nor in AC FICIs, between wild-type isolates versus those with the pbp4 P520S mutation. The single ST179 isolate we tested in the one-compartment PK/PD experiment, however, retained low levels of viable bacteria under AC pressure throughout the assay. While these data suggest that the pbp4 P520S mutation might provide increased tolerance of AC without affecting overt resistance, additional testing is necessary to determine whether this is indeed the case.
Previous clinical studies have shown that combination therapy with AC is non-inferior to AG.10,11 Because the use of ceftriaxone avoids the nephrotoxicity and therapeutic drug monitoring associated with gentamicin use,12 AC is now used almost universally to treat EFIE.9,37 Nearly 80% of the patients in this study were treated with AC, and no patients received AG. Our findings of variable in vitro susceptibility to ceftriaxone and diminished AC synergy among a subset of isolates suggest that AC may not be the optimal treatment for all patients with EFIE. The ST6 isolates we collected were all predicted to display high-level gentamicin resistance, meaning that gentamicin would be ineffective for the same patients who may be at higher risk for poor outcomes due to decreased AC susceptibility. Therapeutic alternatives to AC that were given to patients in this study included ceftaroline, daptomycin and vancomycin, however, these agents (and combinations of them) have not been rigorously assessed for the treatment of EFIE. Nonetheless, our findings suggest that alternative therapies to AC should be explored and tested, particularly against ST6 isolates.
Most available data on AC synergy against enterococci are from in vitro testing,38,39 a rabbit model of IE12,40 and previous clinical studies.41–44 In addition to in vitro susceptibility testing, we used a one-compartment PK/PD model to monitor the effect of AC exposure on E. faecalis bacterial viability over time. We simulated in vivo exposures during AC treatment of patients with IE by modelling human dosing regimens and serum free drug concentrations. The PK/PD experiments we conducted showed that AC was rapidly bactericidal within the first 24 hours across isolates with varying pbp4 genotypes. After 24 hours, however, bacterial regrowth was evident against a ST6 clinical isolate harbouring the −82delT mutation upstream of pbp4 when exposed to either ampicillin alone or to AC. These data suggest that this mutation not only impacts ceftriaxone MICs, but over time it also attenuates ampicillin-mediated killing activity. Future studies are needed to validate these findings against additional clinical isolates, and against isogenic strains with varying pbp4 mutations.
This study had several limitations. Despite nearly 3 years of isolate collection from a tertiary medical centre, we collected a relatively small number of E. faecalis isolates. Additionally, while we hypothesize that patients with IE due to E. faecalis ST6 may experience worse outcomes due to diminished AC synergy mediated by mutations in pbp4, our sample size was too small to test this hypothesis. We plan to continue to collect EFIE isolates from our centre and other locations, and to ultimately build a cohort that is large enough to identify associations between bacterial genotypes, in vitro antibiotic susceptibilities and clinical outcomes. Another potential limitation is that the checkerboard and one-compartment PK/PD assays employed may not recapitulate treatment outcomes of patients. We used a starting inoculum of 1 × 106 cfu/mL for these studies, which may be considered low for modelling high-inoculum infections like endocarditis. Our results, however, are in line with prior PK/PD studies that tested E. faecalis inoculums ranging from 1 × 107 to 1 × 108 cfu/mL.13,37 Additionally, we did not directly measure antibiotic exposures during the one-compartment PK/PD assays, and assays were only performed on representative isolates due to the high costs associated with these assays. Finally, biofilm formation is likely to play a role in modulating antibiotic susceptibility during endocarditis; however, in this study we did not measure how antibiotic susceptibility and biofilm formation might be linked to one another. This will be a focus of our future work, in which we also plan to use relevant animal models of infection to assess the effectiveness of AC (as well as other alternative combinations) against genetically diverse EFIE isolates.
Overall, this study provides an initial description of the genomic diversity and variability in in vitro AC susceptibility among contemporary EFIE isolates at a single medical centre. Our analysis shows that bacteria belonging to two different STs dominate the EFIE population at our centre. Both STs carry mutations that alter the E. faecalis pbp4, and our in vitro data suggest that ST6 isolates in particular may be difficult to eradicate with AC. Further studies are needed to understand whether and how these findings impact clinical outcomes in patients with EFIE. Such studies could lead to improved treatment strategies for EFIE, which would ideally help address the high morbidity and mortality associated with these infections.
Supplementary Material
Acknowledgements
We gratefully acknowledge all members of the Van Tyne laboratory for helpful discussions throughout the course of this study.
Contributor Information
Kevin J Westbrook, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Gayatri Shankar Chilambi, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Madison E Stellfox, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Hayley R Nordstrom, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Yanhong Li, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; Tsinghua University School of Medicine, Beijing, China.
Alina Iovleva, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Niyati H Shah, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Chelsea E Jones, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Ellen G Kline, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Kevin M Squires, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
William R Miller, Division of Infectious Diseases, Houston Methodist Hospital, Houston, TX, USA; Center for Infectious Diseases, Houston Methodist Research Institute, Houston, TX, USA.
Truc T Tran, Division of Infectious Diseases, Houston Methodist Hospital, Houston, TX, USA; Center for Infectious Diseases, Houston Methodist Research Institute, Houston, TX, USA.
Cesar A Arias, Division of Infectious Diseases, Houston Methodist Hospital, Houston, TX, USA; Center for Infectious Diseases, Houston Methodist Research Institute, Houston, TX, USA; Department of Medicine, Weill Cornell Medical College, New York, NewYork, USA.
Yohei Doi, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Ryan K Shields, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Daria Van Tyne, Division of Infectious Diseases, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; Center for Evolutionary Biology and Medicine, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Funding
This work was supported by National Institutes of Health grant R21AI164018 to D.V.T., and by the Department of Medicine at the University of Pittsburgh School of Medicine. M.E.S. was supported by grant T32AI138954 from the National Institutes of Health. A.I. was supported by the Physician Scientist Incubator Program at the University of Pittsburgh, which is sponsored by the Burrows Wellcome Fund. W.R.M was supported by grant K08AI135093 from the National Institutes of Health. C.A.A. was supported by NIH grants K24AI121296, R01AI134637, R01AI48342 and P01AI152999. Y.D. was supported by grants R01AI104895 and R21AI151362 from the National Institutes of Health. R.K.S. was supported by grants R03AI144636 and R21AI151363 from the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Transparency declarations
W.R.M. has received grants and/or honoraria from Merck and Entasis Therapeutics. C.A.A. has received grant funding from Merck, MeMed Diagnostics and Entasis pharmaceuticals. Y.D. has served as a consultant for GSK, Meiji Seika Pharma, Shionogi, Gilead, MSD, AbbVie, Pfizer, Moderna and bioMérieux, has received honoraria from MSD, Shinogi, Gilead and FujiFilm, and has received investigator-initiated funding from Entasis and Shionogi. R.K.S. has served as a consultant for Allergan, Cidara, Entasis, GlaxoSmithKline, Melinta, Menarini, Merck, Pifzer, Shionogi, Utility and Venatorx, and has received investigator-initiated funding from Merck, Melinta, Roche, Shionogi and Venatorx. All other authors have nothing to declare.
Supplementary data
Figure S1 and Tables S1 and S2 are available as Supplementary data at JAC Online.
References
- 1. Van Tyne D, Gilmore MS. Friend turned foe: evolution of enterococcal virulence and antibiotic resistance. Annu Rev Microbiol 2014; 68: 337–56. 10.1146/annurev-micro-091213-113003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Arias CA, Murray BE. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 2012; 10: 266–78. 10.1038/nrmicro2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Murdoch DR, Corey GR, Hoen B et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-prospective cohort study. Arch Intern Med 2009; 169: 463–73. 10.1001/archinternmed.2008.603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chirouze C, Athan E, Alla F et al. Enterococcal endocarditis in the beginning of the 21st century: analysis from the International Collaboration on Endocarditis-prospective cohort study. Clin Microbiol Infect 2013; 19: 1140–7. 10.1111/1469-0691.12166 [DOI] [PubMed] [Google Scholar]
- 5. Pericas JM, Cervera C, Moreno A et al. Outcome of Enterococcus faecalis infective endocarditis according to the length of antibiotic therapy: preliminary data from a cohort of 78 patients. PLoS ONE 2018; 13: e0192387. 10.1371/journal.pone.0192387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pericas JM, Llopis J, Munoz P et al. A contemporary picture of Enterococcal endocarditis. J Am Coll Cardiol 2020; 75: 482–94. 10.1016/j.jacc.2019.11.047 [DOI] [PubMed] [Google Scholar]
- 7. Baddour LM, Wilson WR, Bayer AS et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation 2005; 111: e394–434. 10.1161/CIRCULATIONAHA.105.165564 [DOI] [PubMed] [Google Scholar]
- 8. Dressel DC, Tornatore-Reuscher MA, Boschman CR et al. Synergistic effect of gentamicin plus ampicillin on enterococci with differing sensitivity to gentamicin: a phenotypic assessment of NCCLS guidelines. Diagn Microbiol Infect Dis 1999; 35: 219–25. 10.1016/S0732-8893(99)00088-7 [DOI] [PubMed] [Google Scholar]
- 9. Delgado V, Ajmone Marsan N, de Waha S et al. 2023 ESC guidelines for the management of endocarditis. Eur Heart J 2023; 44: 3948–4042. 10.1093/eurheartj/ehad193 [DOI] [PubMed] [Google Scholar]
- 10. Fernandez-Hidalgo N, Almirante B, Gavalda J et al. Ampicillin plus ceftriaxone is as effective as ampicillin plus gentamicin for treating Enterococcus faecalis infective endocarditis. Clin Infect Dis 2013; 56: 1261–8. 10.1093/cid/cit052 [DOI] [PubMed] [Google Scholar]
- 11. Pericas JM, Cervera C, del Rio A et al. Changes in the treatment of Enterococcus faecalis infective endocarditis in Spain in the last 15 years: from ampicillin plus gentamicin to ampicillin plus ceftriaxone. Clin Microbiol Infect 2014; 20: O1075–83. 10.1111/1469-0691.12756 [DOI] [PubMed] [Google Scholar]
- 12. Gavalda J, Torres C, Tenorio C et al. Efficacy of ampicillin plus ceftriaxone in treatment of experimental endocarditis due to Enterococcus faecalis strains highly resistant to aminoglycosides. Antimicrob Agents Chemother 1999; 43: 639–46. 10.1128/AAC.43.3.639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Luther MK, Rice LB, LaPlante KL. Ampicillin in combination with ceftaroline, cefepime, or ceftriaxone demonstrates equivalent activities in a high-inoculum Enterococcus faecalis infection model. Antimicrob Agents Chemother 2016; 60: 3178–82. 10.1128/AAC.03126-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thieme L, Klinger-Strobel M, Hartung A et al. In vitro synergism and anti-biofilm activity of ampicillin, gentamicin, ceftaroline and ceftriaxone against Enterococcus faecalis. J Antimicrob Chemother 2018; 73: 1553–61. 10.1093/jac/dky051 [DOI] [PubMed] [Google Scholar]
- 15. Shah NH, Shutt KA, Doi Y. Ampicillin-ceftriaxone vs ampicillin-gentamicin for definitive therapy of Enterococcus faecalis infective endocarditis: a propensity score–matched, retrospective cohort analysis. Open Forum Infect Dis 2021; 8: ofab102. 10.1093/ofid/ofab102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bankevich A, Nurk S, Antipov D et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 2012; 19: 455–77. 10.1089/cmb.2012.0021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seemann T. Prokka: rapid prokaryotic genome annotation. Bioinformatics 2014; 30: 2068–9. 10.1093/bioinformatics/btu153 [DOI] [PubMed] [Google Scholar]
- 18. Page AJ, Cummins CA, Hunt M et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics 2015; 31: 3691–3. 10.1093/bioinformatics/btv421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stamatakis A. RAxML-VI-HPC: maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics 2006; 22: 2688–90. 10.1093/bioinformatics/btl446 [DOI] [PubMed] [Google Scholar]
- 20. Kim SH, Park C, Chun HS et al. Pilot screening to determine antimicrobial synergies in a multidrug-resistant bacterial strain library. Microb Drug Resist 2016; 22: 372–8. 10.1089/mdr.2015.0251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bourgogne A, Garsin DA, Qin X et al. Large scale variation in Enterococcus faecalis illustrated by the genome analysis of strain OG1RF. Genome Biol 2008; 9: R110. 10.1186/gb-2008-9-7-r110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hebert L, Courtin P, Torelli R et al. Enterococcus faecalis constitutes an unusual bacterial model in lysozyme resistance. Infect Immun 2007; 75: 5390–8. 10.1128/IAI.00571-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dunny GM, Lee LN, LeBlanc DJ. Improved electroporation and cloning vector system for Gram-positive bacteria. Appl Environ Microbiol 1991; 57: 1194–201. 10.1128/aem.57.4.1194-1201.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blaser J. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J Antimicrob Chemother 1985; 15 Suppl A:125–30. 10.1093/jac/15.suppl_A.125 [DOI] [PubMed] [Google Scholar]
- 25. Eng RH, Smith SM, Cherubin CE et al. Evaluation of two methods for overcoming the antibiotic carry-over effect. Eur J Clin Microbiol Infect Dis 1991; 10: 34–8. 10.1007/BF01967095 [DOI] [PubMed] [Google Scholar]
- 26. Durack DT, Lukes AS, Bright DK. New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service. Am J Med 1994; 96: 200–9. 10.1016/0002-9343(94)90143-0 [DOI] [PubMed] [Google Scholar]
- 27. Li JS, Sexton DJ, Mick N et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clin Infect Dis 2000; 30: 633–8. 10.1086/313753 [DOI] [PubMed] [Google Scholar]
- 28. Ono S, Muratani T, Matsumoto T. Mechanisms of resistance to imipenem and ampicillin in Enterococcus faecalis. Antimicrob Agents Chemother 2005; 49: 2954–8. 10.1128/AAC.49.7.2954-2958.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rice LB, Desbonnet C, Tait-Kamradt A et al. Structural and regulatory changes in PBP4 trigger decreased beta-lactam susceptibility in Enterococcus faecalis. MBio 2018; 9: e00361-18. 10.1128/mBio.00361-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Van Tyne D, Manson AL, Huycke MM et al. Impact of antibiotic treatment and host innate immune pressure on enterococcal adaptation in the human bloodstream. Sci Transl Med 2019; 11: eaat8418. 10.1126/scitranslmed.aat8418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zankari E, Hasman H, Cosentino S et al. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 2012; 67: 2640–4. 10.1093/jac/dks261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Carattoli A, Zankari E, Garcia-Fernandez A et al. In silico detection and typing of plasmids using PlasmidFinder and plasmid multilocus sequence typing. Antimicrob Agents Chemother 2014; 58: 3895–903. 10.1128/AAC.02412-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Palmer KL, Gilmore MS. Multidrug-resistant enterococci lack CRISPR-cas. MBio 2010; 1: e00227-10. 10.1128/mBio.00227-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tao S, Chen H, Li N et al. Association of CRISPR-cas system with the antibiotic resistance and virulence genes in nosocomial isolates of Enterococcus. Infect Drug Resist 2022; 15: 6939–49. 10.2147/IDR.S388354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Contreras GA, Munita JM, Simar S et al. Contemporary clinical and molecular epidemiology of vancomycin-resistant Enterococcal bacteremia: a prospective multicenter cohort study (VENOUS I). Open Forum Infect Dis 2022; 9: ofab616. 10.1093/ofid/ofab616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hiraga N, Muratani T, Naito S et al. Genetic analysis of faropenem-resistant Enterococcus faecalis in urinary isolates. J Antibiot (Tokyo) 2008; 61: 213–21. 10.1038/ja.2008.31 [DOI] [PubMed] [Google Scholar]
- 37. Beganovic M, Luther MK, Rice LB et al. A review of combination antimicrobial therapy for Enterococcus faecalis bloodstream infections and infective endocarditis. Clin Infect Dis 2018; 67: 303–9. 10.1093/cid/ciy064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mainardi JL, Gutmann L, Acar JF et al. Synergistic effect of amoxicillin and cefotaxime against Enterococcus faecalis. Antimicrob Agents Chemother 1995; 39: 1984–7. 10.1128/AAC.39.9.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pasticci MB, Mencacci A, Moretti A et al. In vitro antimicrobial activity of ampicillin-ceftriaxone and ampicillin-ertapenem combinations against clinical isolates of Enterococcus faecalis with high levels of aminoglycoside resistance. Open Microbiol J 2008; 2: 79–84. 10.2174/1874285800802010079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gavalda J, Onrubia PL, Gomez MT et al. Efficacy of ampicillin combined with ceftriaxone and gentamicin in the treatment of experimental endocarditis due to Enterococcus faecalis with no high-level resistance to aminoglycosides. J Antimicrob Chemother 2003; 52: 514–7. 10.1093/jac/dkg360 [DOI] [PubMed] [Google Scholar]
- 41. El Rafei A, DeSimone DC, Narichania AD et al. Comparison of dual beta-lactam therapy to penicillin-aminoglycoside combination in treatment of Enterococcus faecalis infective endocarditis. J Infect 2018; 77: 398–404. 10.1016/j.jinf.2018.06.013 [DOI] [PubMed] [Google Scholar]
- 42. Gil-Navarro MV, Lopez-Cortes LE, Luque-Marquez R et al. Outpatient parenteral antimicrobial therapy in Enterococcus faecalis infective endocarditis. J Clin Pharm Ther 2018; 43: 220–3. 10.1111/jcpt.12635 [DOI] [PubMed] [Google Scholar]
- 43. Peterson SC, Lau TTY, Ensom MHH. Combination of ceftriaxone and ampicillin for the treatment of enterococcal endocarditis: a qualitative systematic review. Ann Pharmacother 2017; 51: 496–503. 10.1177/1060028017692357 [DOI] [PubMed] [Google Scholar]
- 44. Tascini C, Doria R, Leonildi A et al. Efficacy of the combination ampicillin plus ceftriaxone in the treatment of a case of Enterococcal endocarditis due to Enterococcus faecalis highly resistant to gentamicin: efficacy of the “ex vivo” synergism method. J Chemother 2004; 16: 400–3. 10.1179/joc.2004.16.4.400 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.