To the Editor,
Obesity, one of the most serious health concerns worldwide, exacerbates periodontitis pathogenesis and reduces the effectiveness of treatments1. Conversely, periodontitis-associated inflammation disrupts systemic lipid and glucose homeostasis, the major risk factors for developing obesity-associated pathologies1. Accumulated studies have demonstrated that certain supplemental dietary lipids such as dietary polyunsaturated fatty acids, have beneficial outcomes for diabetic and obese patients. In addition to dietary lipid supply, the oral and gut microbiota produce fatty acids (FAs) which provide the host with essential nutrients and aid in defense against pathogens. Hydroxyhexanoic medium chain FAs (MCFAs; 6–12 carbons) are bacterial-derived lipids shown to have anti-inflammatory and insulin sensitization effects in diverse cell types such as hepatocytes2. However, whether and to what extent oral microbiota-produced MCFAs may ameliorate obesity-associated metabolic dysfunction are largely unknown.
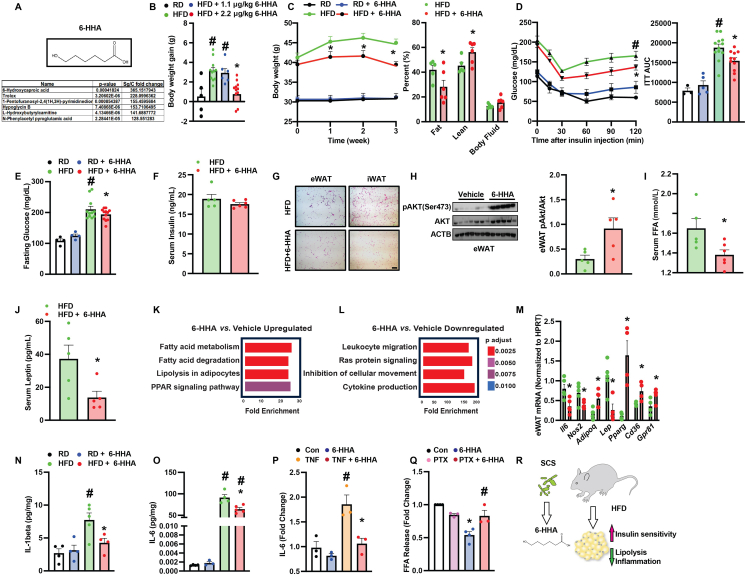
We recently demonstrated that spent culture supernatants (SCS) from the commensal oral bacterial species Streptococcus gordonii (Sg) mitigated periodontitis-associated inflammation3. To identify the anti-inflammatory mediator of Sg SCS, we performed an ultra performance liquid chromatography (UPLC) analysis and identified a total of 324 components significantly enriched as compared to controls (DMEM culture medium)3. Notably, 6-hydroxyhexanoic acid (6-HHA) was the only MCFA identified among the top enriched 12 components (Fig. 1A).
Figure 1.
6-Hydroxyhexanoic acid (6-HHA) ameliorates obesity-associated metabolic dysfunction. (A) Identification of 6-HHA. Top panel: structure of 6-HHA. Bottom panel: top enriched components of Sg SCS. (B) Body weight of mice fed on regular (RD) or high-fat diet (HFD; on diet for 16 weeks) followed by intraperitoneal (i.p.) injection with vehicle (water) or 6-HHA (1.1 or 2.2 μg/kg) every other day for additional three weeks. (C) Body weight and body composition, (D) insulin tolerance test (ITT) and AUC analysis, (E) 6-h fasting glucose and (F) serum insulin levels in 6-HHA (2.2 μg/kg) or vehicle-treated HFD mice (on diet for 16 weeks followed by 3 weeks of treatment). (G) Representative H&E images (20 ×) of epidydimal and inguinal adipose tissues (eWAT and iWAT, respectively; scale bar: 10 μm), and (H) Western blot analysis of tested proteins from eWAT of HFD mice in (C). Densitometric analysis is shown on the right panel, and data were normalized to ACTB. (I) Serum-free fatty acid (FFA) and (J) leptin levels in HFD mice 16-h post-fasting, and (K, L) RNA-seq analysis of eWAT of HFD mice in (C). KEGG categories were determined by Enrichr analysis. (M) Levels of mRNAs encoding the indicated genes in eWAT (data were normalized to Gapdh), and (N, O) serum levels of measured cytokines from mice in (C). (P) IL-6 levels in medium from differentiated 3T3-L1 cells treated with 0.1 μmol/L 6-HHA (24-h) in the presence or absence of 10 ng/mL TNFα. Data were first normalized to cellular protein content and then compared to control groups (con). (Q) FFA release from differentiated 3T3-L1 cells pretreated with 50 ng/mL PTX (24-h) in the presence or absence of 0.1 μmol/L 6-HHA. Cells were treated with 1 μmol/L isoproterenol (1-h) before measurement. Data were normalized to cellular protein content and represented as fold change compared to con. (R) Working model of the effects of 6-HHA on obesity-associated metabolic dysfunction. All data are presented as mean ± SEM. In B–O, n = 4–10 mice/group and each data point indicates one individual mouse. In P and Q, n = 3 (experimental replicates). ∗indicates statistical significance of 6-HHA treatment effect between HFD groups, TNF effect in the 6-HHA groups in (P), and 6-HHA effect in (Q); #indicates statistical significance of dietary effects (B, D, E, N, O), TNF effect (P), or PTX effects in cells with 6-HHA treatment (Q). Statistical significance was determined by Student's t-test (H–J, M) or one-way ANOVA followed by post hoc test (B, D, E, N–Q); P < 0.05.
Previously, we found 0.1 μmol/L 6-HHA suppressed proinflammatory cytokine production in human gingival fibroblasts3. Furthermore, 0.25 and 0.5 μmol/L 6-HHA downregulated inflammatory markers in epididymal white adipose fat pads isolated from mice on high-fat diet (HFD; eWAT; data not shown). To investigate the role of 6-HHA in the context of obesity in vivo, wildtype (WT; C57BL/6 J) HFD fed-mice were intraperitoneally (i.p.) injected with 6-HHA or vehicle every other day for 3 weeks at 1.1 or 2.2 μg/kg body weight. Compared to mice on regular chow (RD), mice on HFD for 16 weeks significantly gained more body weight (BW) (Fig. 1B). Dosage studies showed that 6-HHA (2.2 μg/kg) significantly decreased HFD-induced BW gain (Fig. 1B). We then focused on 6-HHA at the 2.2 μg/kg dose to investigate its functional role in the context of obesity.
To determine the effect of 6-HHA on adiposity in obesity, we first assessed the BW and body composition of 6-HHA-treated HFD mice and the vehicle controls. As shown in Fig. 1C, 6-HHA treatment significantly decreased obesity-associated weight gain and adiposity, and slightly increased lean mass compared to vehicle controls. Systemicaly, obesity disrupts systemic metabolic and energy balance. Therefore, we next measured whole-body metabolic profiles in HFD mice in the presence or absence of 6-HHA treatment by indirect mouse calorimetry analysis. As shown in Supporting Information Fig. S1A–S1C, treatment with 6-HHA did not alter activity, food intake or energy expenditure (EE) in HFD mice. However, the respiratory exchange ratio (RER) significantly shifted towards carbohydrate metabolism in the 6-HHA group compared to controls (Fig. S1D). Hyperglycemia and insulin resistance are major drivers for obesity-associated pathologies. Treatment with 6-HHA did not alter insulin tolerance in lean mice (Fig. 1D). However, 6-HHA significantly improved obesity-associated insulin intolerance and elevation of fasting glucose without altering serum insulin levels (Fig. 1D–F). Together, these data suggest that 6-HHA protects against obesity-associated systemic metabolic defects.
Obesity-disrupted systemic glucose and insulin homeostasis is attributed to metabolic dysfunction of multiple peripheral tissues. In HFD mice, 6-HHA significantly ameliorated hepatic steatosis and liver damage (serum alanine transaminase, ALT) (Fig. S11E–S1G) which was correlated with reduced hepatic expression of genes involved in lipogenesis and inflammation (Fig. S1H). Aberrant adipogenesis, chronic inflammation, insulin resistance and elevated lipolysis are common features of adipose dysfunction in obesity. Notably, 6-HHA treatment decreased crown-like structures in both inguinal (iWAT) and eWAT (Fig. 1G) and significantly improved adipose insulin sensitivity in eWAT of HFD mice (Fig. 1H). Systemically, 6-HHA lowered serum levels of free fatty acids (FFA) and leptin in HFD mice (Fig. 1I and J). At the cellular level, there were comparable levels of tested adipogenesis markers (Fig. S1I) and intracellular lipid accumulation (Fig. S1J) between 6-HHA and the control groups in differentiated 3T3-L1 cells. These observations were correlated with a comparable insulin action in differentiated 3T3-L1 cells between tested groups in vitro (Fig. S1K). Furthermore, 6-HHA had no effect on mature 3T3-L1-derived cytokine production under basal conditions but significantly suppressed tumor necrosis factor-α (TNF-α)-induced IL-6 secretion (Fig. 1P).
To characterize the broad effect of 6-HHA on adipose tissues in the context of obesity, we next performed a bulk RNAseq analysis in the eWAT from 6-HHA treated HFD mice and controls. Treatment of 6-HHA suppressed adipose proinflammatory signatures (Fig. 1L and M) which were concomitant with reduced serum levels of IL-1β and IL-6 (Fig. 1N and O) as well as IL-6 production from mature adipocytes (Fig. 1P). Conversely, 6-HHA significantly induced signaling pathways involved in fatty acid metabolism (Fig. 1K. Indeed, we found that 6-HHA reduced serum FFA levels in HFD mice (Fig. 1I) suggesting a potential negative role of 6-HHA in regulating lipolysis, a process regulated by G protein-coupled receptors (GPCRs)4. To answer whether 6-HHA inhibits GPCR-mediated adipocyte lipolysis, we next treated differentiated 3T3-L1 cells with pertussis toxin (PTX), a well-known inhibitor of the Gαi family that inhibits lipolysis signaling cascades5, prior to 6-HHA exposure. As shown in Fig. 1Q, the isoproterenol (ISO)-induced FFA release from mature adipocytes was significantly reduced by 6-HHA. However, this 6-HHA-mediated anti-lipolytic effect was dampened in the presence of PTX indicating an inhibitory role for 6-HHA in Gαi-mediated adipocyte-FFA release (Fig. 1Q).
In summary, we have identified and characterized the effect of a novel bacterial commensal metabolite, 6-HHA, on improving adiposity, systemic inflammation, systemic energy and metabolic homeostasis in mice with diet-induced obesity (Fig. 1R). Together these findings suggest that supplementation with microbial-derived MCFAs may possess the dual therapeutic potential to improve periodontitis and obesity-associated metabolic disorders. We recognize that the protective role of 6-HHA on systemic metabolic homeostasis observed in rodents might not be phenocopied in humans. Furthermore, identifying the in vivo origins of 6-HHA (oral vs. gut microbiome) as well as the main tissue/cell type specific GPCR-targets of 6-HHA are areas of interest for future studies.
Author contributions
Sara C. Sebag, Liu Hong and Ling Yang designed the research. Sara C. Sebag, Meihua Hao, Qingwen Qian, Chawin Upara, Min Zhu, and Qiong Ding performed the research. Sara C. Sebag, Meihua Hao, Qingwen Qian, Liu Hong and Ling Yang analyzed the data. Sara C. Sebag, Liu Hong and Ling Yang wrote the article. All authors have read and approved the final version of this manuscript and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of interest
The authors declare no competing financial interests.
Acknowledgments
Ling Yang is supported by R01DK108835 and R01DK126817 (USA), and Sara C. Sebag and Liu Hong are supported by R01DE026433 (USA).
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Supporting data to this article can be found online at https://doi.org/10.1016/j.apsb.2024.01.002.
Contributor Information
Liu Hong, Email: liu-hong@uiowa.edu.
Ling Yang, Email: ling-yang@uiowa.edu.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Jepsen S., Suvan J., Deschner J. The association of periodontal diseases with metabolic syndrome and obesity. Periodontol 2000. 2020;83:125–153. doi: 10.1111/prd.12326. [DOI] [PubMed] [Google Scholar]
- 2.Wang B., Li L., Fu J., Yu P., Gong D., Zeng C., et al. Effects of long-chain and medium-chain fatty acids on apoptosis and oxidative stress in human liver cells with steatosis. J Food Sci. 2016;81:H794–H800. doi: 10.1111/1750-3841.13210. [DOI] [PubMed] [Google Scholar]
- 3.Shu Y., Upara C., Ding Q., Zhu M., Zeng E., Banas J.A., et al. Spent culture supernatant of Streptococcus gordonii mitigates inflammation of human periodontal cells and inhibits proliferation of pathogenic oral microbes. J Periodontol. 2023;94:575–585. doi: 10.1002/JPER.22-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Malfacini D., Pfeifer A. GPCR in adipose tissue function-focus on lipolysis. Biomedicines. 2023;11:588. doi: 10.3390/biomedicines11020588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Katada T., Ui M. ADP ribosylation of the specific membrane protein of C6 cells by islet-activating protein associated with modification of adenylate cyclase activity. J Biol Chem. 1982;257:7210–7216. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.