Abstract
Macrophage senescence, manifested by the special form of durable cell cycle arrest and chronic low-grade inflammation like senescence-associated secretory phenotype, has long been considered harmful. Persistent senescence of macrophages may lead to maladaptation, immune dysfunction, and finally the development of age-related diseases, infections, autoimmune diseases, and malignancies. However, it is a ubiquitous, multi-factorial, and dynamic complex phenomenon that also plays roles in remodeled processes, including wound repair and embryogenesis. In this review, we summarize some general molecular changes and several specific biomarkers during macrophage senescence, which may bring new sight to recognize senescent macrophages in different conditions. Also, we take an in-depth look at the functional changes in senescent macrophages, including metabolism, autophagy, polarization, phagocytosis, antigen presentation, and infiltration or recruitment. Furthermore, some degenerations and diseases associated with senescent macrophages as well as the mechanisms or relevant genetic regulations of senescent macrophages are integrated, not only emphasizing the possibility of regulating macrophage senescence to benefit age-associated diseases but also has an implication on the finding of potential targets or drugs clinically.
Key words: Macrophages, Senescence, Molecular changes, Functional alterations, Aging tissues, Age-associated diseases, Drug treatment, Genetic regulation
Graphical abstract
Senescent macrophages are characterized by specific molecular and functional changes, leading to tissue degenerations and diseases. Targeting macrophage senescence is expected to be a novel strategy for disease intervention.
1. Introduction
Cellular senescence, first observed in the cultured human fibroblasts by Hayflick and Moorhead in 19611, is featured with cell cycle arrest, DNA damage, oxidative damage, resistance to apoptosis, senescence-associated-β-galactosidase accumulation, and the senescence-associated heterochromatin foci2. Currently, more and more researches have generally considered that the whole organism aging is the consequence of senescent cell accumulation while cell senescence is also associated with a range of diseases, emphasizing the significance of cell senescence to organism1, 2, 3.
Due to the critical of immune cells to the body's defense, the concept of “immunosenescence” was also put forward to specify the stress-induced cell cycle inhibition of previously replication-competent immune cells by Walford in 19654,5. Similarly, immunosenescence is especially visible in the aging process and involved in the development of many diseases, such as neurodegenerative disorders and cancer6, 7, 8. Bone marrow, thymus, and peripheral lymphatic organs degrade to a certain extent, thus damaging immune cell recruitment and antigen presentation, and eventually leading to self-tissue damage9, 10, 11. It is found that there are lower naive cells, higher memory cells, and a significant telomere shortening of T and B cells in the elderly12,13. And senescent T and B cells participate in elevated proinflammatory cytokines, increased reactive oxygen species (ROS), and dysfunctional lysosomal deposits, which may have adverse effects on autoimmune diseases and increase the risk of infections such as COVID-1914, 15, 16. Although immunosenescence has been considered harmful for a long time, T cell senescence is found to facilitate the acceptance of allografts in transplant recipients, indicating the beneficial side of senescence in the absence of immune cell activation17.
Senescence-related secretion phenotype (SASP), the universal feature of cellular senescence, also promotes local inflammation and recruits immune cells aimed at eliminating damaged and senescent cells, thus contributing to inflammation relief, wound healing, and tissue remodeling18, 19, 20, 21. Due to the powerful phagocytes, macrophages play an extremely important role in this process. However, the significant decline of macrophage cytoplasmic spreading in old mice was later observed by Johnson for the first time in 1978, raising the subsequent studies on senescent macrophages22,23. Although there is still no exact trigger to macrophage senescence, several studies have revealed that gene mutation like Kras, and chemical exposure like lipopolysaccharide (LPS) may contribute to the senescence of macrophages24, 25, 26. In addition, it is also claimed that other senescent cells can secrete SASP in a paracrine manner and induce the senescence of tissues as well as macrophages, all of which together lead to the senescent environment and degenerations subsequently27.
Once senescent, the functions of macrophages get compromised, which may lead to the accumulation of abnormal cells and misfolded proteins in many diseases28. Although there is still less work on the senescent macrophages directly, aging may disrupt the physiological gene regulation and functions of macrophages, such as reducing its phagocytosis function, changing autophagy, metabolism, and other signals, while the immune balance of recombinant macrophages may reverse aging29, 30, 31, 32. Age-modified tissue-specific macrophages are associated with chronic low-grade inflammation, which mediates immunosuppressive effects and contributes to the development of diseases such as cancer28,33. In contrast, activated macrophages in some lesions resemble senescent cells and exhibit SASP, suggesting that senescent phenotypes in macrophages may be used for physiological stress and promote disease progression16,32. Therefore, macrophage senescence is related to numerous diseases but remains unsharp.
In this review, we summarized the common and specific molecular alterations and functional changes of senescent macrophages as well as their potential regulatory mechanisms. Particularly, we focused on the distinct states of senescent macrophages in different tissues or diseases, and laid out the participation of macrophage senescence in age-associated degenerations, thus exploring feasible strategies to benefit clinical therapy via targeting senescent macrophages.
2. Molecular changes in the senescent macrophages
It was raised that senescence occurred in embryos as featured with positive SA-β-galactosidase (SA-β-Gal) and negative Ki67 staining, and physiological senescence took place in adult organisms in a programmed manner34. The aging process of cells including macrophages could be analyzed with three parts, 1) the inducers like ROS and oncogenes cause the age-associated damage; 2) the responses to the damage such as retarded proliferation and SASP secretion; 3) integrative consequences of the responses and culprits of the aging phenotype like DNA damage and mitochondrial dysfunction35. Similar to other senescent cells, senescent macrophages share common changes like the increased level of SA-β-Gal, p16Ink4a, and SASP. Apart from these general molecular changes, senescent macrophages also show some specific signatures. Some researchers worked on the illustration of senescent macrophages and found several novel macrophage populations with newly identified markers through single-cell RNA-sequencing (scRNA-seq). More studies are needed to verify these specific markers with macrophage senescence. Also, we suggest two or more markers defining the senescent macrophages precisely.
2.1. General molecular changes in senescent macrophages
Senescent cells tend to produce a bioactive secretome, referred to as the SASP. Though SASP composition varies in different cell types and the nature of the initial stimulus, the core components are mainly proinflammatory cytokine interleukin-6 (IL6), CXC chemokine ligand 8 (CXCL8), extracellular matrix proteases such as matrix metalloproteinases (MMPs), and growth factor-β (TGF-β)2,36, 37, 38, 39. Generally, senescence has been considered a negative outcome of advanced cellular age. However, some studies revealed the benefits of senescent cells recently due to the long-term tumor suppression40. Interestingly, some researchers noted that in the diabetes wounds mice, macrophages tended to be senescent and could produce a CXC motif chemokine receptor 2 (CXCR2)-enriched SASP, which selectively induced chemokine and fibrotic markers like SERPINE1 in human dermal fibroblast and delayed wound healing41. Similarly, in the muscles from both young and old mice at 7 days post-injury, macrophages were senescent42. In the old mice at 14 days following injury, the CD11b+ macrophages displayed an SASP signature. The changed extracellular matrix in the injured muscle originating from old mice may promote cellular senescence and SASP in macrophages42. In the streptozotocin-induced hyperglycemia, elevated SASP genes were observed in both endothelial cells and macrophages in the diabetic mice kidney. Similarly, the hyperglycemic medium induced greater SA-β-gal positivity as well as SASP genes in cell line-derived macrophages43.
Another marker of senescent cells is the high expression of Cdkn2a and its protein product p16Ink4a. The accumulation of p16Ink4a positive macrophages was observed in young mice induced by senescent cells44. What's more, the treatment of systemic clodronate illustrated that p16Ink4a positive macrophages were the majority of senescent cells in old mice. Under the stimulation of ionizing radiation, the level of senescence-specific genes in pulmonary macrophages like p16Ink4a increased45,46, which drove the efferocytosis defects. Besides, in the mouse model of duchenne muscular dystrophy, the senescent cells were more in 8-week-old mice than in four-week-old mice, of which these senescent cells were mostly Cdkn2a-positive macrophages47. Pseudomonas aeruginosa can direct macrophage towards senescence with elevated levels of p16Ink4a 48. In streptozotocin-induced diabetic mice, young mice (18-week-old) presented the same inflammatory bone loss in the periodontal tissue and induction of p16/p21 as the aged mice (20-month-old), among which there were more p16Ink4a positive macrophages. It was supposed that these pro-inflammatory/senescent macrophages were in tight connection with periodontal damage49. Overall, in the aged mice or some diseases, senescent macrophages were p16Ink4a positive and they took great proportion in the p16Ink4a positive cells (regarded as senescent cells). Otherwise, the ionizing radiation-induced senescence or diseases like duchenne muscular dystrophy resulted in the p16Ink4a positive macrophages. While it raised concerns that the physiological stimuli rather than senescence can modulate the expression of p16Ink4a in macrophages, whether it's accurate to set p16Ink4a as a marker for senescent macrophages remains unknown50.
Cellular senescence is captured with enhanced SA-β-Gal activity because the lysosomal compartment expands in senescent cells. SA-β-Gal is regarded as an indicator of senescent cells. As mentioned above, ionizing radiation can increase the cellular senescence in the bone marrow-derived monocytes/macrophages (BMDMs), and up-regulated the expression of SA-β-Gal together with the SASP proinflammatory factors44. However, it is suggested that monocyte-to-macrophage differentiation was characterized by increased lysosome numbers51, the endo-lysosomal volume expansion after LPS stimulation as well and other essential organelles remodel to sustain the function52. The SA-β-gal activity was considered an unreliable marker of senescence in vivo, especially in macrophages50. Whether the SA-β-Gal is the hallmark of senescent macrophages is still under debate.
2.2. Specific markers in senescent macrophages
2.2.1. Lymphatic vessel endothelial hyaluronan receptor 1 (LYVE1)
The scRNA-seq analysis in old skeleton muscle illustrated altered numbers of LYVE1+ and LYVE1– macrophages, and suggested the abundance of LYVE1+ macrophages in old skeleton muscle is lower compared to the young skeleton muscle. LYVE1 is a major receptor for hyaluronan on lymphatic endothelial cells, which can act as a decoy receptor of low-molecular-weight hyaluronic acid fragments. Macrophage-derived LYVE1 can inhibit melanoma cell proliferation and promote lymphangiogenesis53,54.
Krasniewski's study defined four macrophage subpopulations (LYVE1+MHCIIhi, LYVE1+MHCIIlo, LYVE1–MHCIIhi, LYVE1−MHCIIlo) and currently LYVE1 represents M2-like macrophage. Their study on macrophages from old skeleton muscle also defined LYVE1 as an essential biomarker to distinguish old macrophages for the coherence between LYVE1– macrophages with elevated Gpnmb and Spp1 genes (markers of senescence and aging). Notably, the proinflammatory biomarkers, such as S100a8 and S100a9, increased in macrophages from aged skeletal muscle, which was opposed to the M2-like macrophage accumulation in old skeleton muscles though55. What's more, researchers also have found a high number of LYVE1+CD68+ macrophages around blood vessels in donors' episclera (whose mean age is 65.1 years old) since immunohistochemistry results showed the positive colocalization between CD68 and LYVE1 in the sclera54. Consistently, increased LYVE1+ CD11b+ cells were observed in aged mice, indicating the LYVE1+ macrophage population accumulated in the retinas of aged Kimba mice56.
Notably, some laboratories have established a sarcopenia (an age-related geriatric syndrome) animal model in the senescence-accelerated mouse prone-10, which represented the advanced senescence and diseases in age-associated pathologies57. The infiltration of senescent macrophages was captured in senescence-accelerated mouse prone-10 (SAMP10), but umbilical cord-derived mesenchymal stromal cells could retard these infiltrations and alleviate sarcopenia-related skeletal muscle atrophy and dysfunction58. It needs further investigation of the LYVE1+CD68+ macrophages to elucidate their functions as well as characteristics in age-related diseases as mentioned above.
2.2.2. Grancalcin (GCA)
Grancalcin is known as a Ca2+ binding protein with high expression in neutrophils. It's a member of the penta-EF-hand protein family and was found to regulate adherence and migration of immune cells59. Li et al.60 illustrated the underlying mechanism of skeleton aging for the abundant GCA secreted by macrophages and neutrophils. They discovered a significantly higher expression of GCA in the bone marrow supernatant of the older rats (24 months) compared with that from young ones (3 months) among 35 differentially expressed factors by mass spectrometry. Though neutrophils were with high expression of GCA, the number of changed pathways during aging is four, and only one is involved in inflammation. So, the researchers focus on other immune cells, macrophages. Of note, the number and proportion of GCA+ macrophages showed great differences between older and younger rats. Coherently, mice conditionally knockout of Gca in macrophages showed improved skeletal aging featured with more osteocalcin+osteoblasts than wide-type mice. What's more, young mice injected with recombinant GCA induced premature skeletal aging. Additionally, they also found GCA could bind to the plexin-B2 receptor and suppress the phosphorylation of FAK-SRC-YAP. To further elucidate this suppression, micro-CT was applied to capture the trabecular bone morphology in Gca-KO; skeleton stems cell conditionally knockout of Plxnb2 (Gca-KO; Prrx1 plxnb2−/+), which showed the abrogation of the rejuvenated bone phenotype of Gca-KO mice. To complete this study, they also investigated GCA-neutralizing antibodies to improve skeletal health. This whole research emphasized the prominent role of grancalcin+ macrophages in skeleton aging and enlightened us that grancalcin might be a marker for senescent macrophages. However, this still needs data to prove.
2.2.3. CD22
CD22 serves as an adhesion receptor for sialic acid-bearing ligands which is involved in the regulation of activation of B cells and antigen receptor signaling61. Tedder et al. revealed age-related genetic modifiers of microglial phagocytosis in the combination of CRISPR-Cas9 knockout screens with RNA-seq, and they identified CD22 as a negative regulator of phagocytosis in aged microglia. The expression of CD22 was remarkably upregulated in aged microglia. Particularly, the in situ hybridization on five brain regions especially the thalamus and cerebellum from aged mice showed the co-expression of CD22 in Tmem119+ microglia while the CD22+Tmem119+ microglia were almost completely absent in the young brain. CD22 bound sialic acid to negatively regulate BCR, the treatment of sialidase robustly promoted phagocytosis of BV2 but no effects in the simultaneous presence of sialic acid, suggesting the involvement of sialic acid in the CD22-induced inhibition of phagocytosis. Later experiments confirmed the inhibition of CD22 could restore microglial phagocytosis in aged mice (14–16 months) and promote the clearance of extracellular α-synuclein fibrils. What's more, the aged Cd22−/− mice exhibited improved spatial memory and associative memory compared to the aged wide-type mice. In all, CD22 could be the cause of compromised phagocytosis in aged microglia.
2.2.4. Glucose transporter 1 (GLUT1)
GLUT1 is a representative glucose transporter in macrophages and is correlated with the promotion of pro-inflammatory macrophages in the hyperglycemia condition, which is tightly linked to cellular senescence43,49. In the hyperglycemia-induced periodontitis, co-localization with GLUT1/F4-80/p16 showed widespread appearance indicating GLUT1 promoting macrophage senescence. What's more, the GLUT1 signaling contributed to the pre-establishment of the mature SASP response in macrophages as the coordinated elevated early SASP marker (interleukin-1β, IL-1β) in the augmented GLUT1 signaling. The inhibition of GLUT1 markedly reduced mTOR phosphorylation and the SASP response in the hyperglycemia-induced senescent macrophages49. That is to say, hyperglycemia could induce macrophage senescence with the presence of GLUT1. Different from hyperglycemia-induced macrophage senescence, the aged muscle macrophages (mainly aged M1 macrophages) showed impaired glycolysis and suppressed GLUT1 levels as well as undermined inflammatory transcriptome according to the single cell-RNA sequencing of macrophage metabolism and polarization in aged mice with disuse atrophy recovery62. In summary, GLUT1 showed alterations in disease-induced senescent macrophages and chronological aging macrophages, though the changes were discriminated, it still hinted to us that GLUT1 might be an indicator in the study of senescence in macrophages.
3. Functional changes in senescent macrophages
As mentioned above, senescent cells displayed mitochondrial dysfunction, and autophagy impairment after responding to the senescence inducers. Similarly, aging has a great impact on macrophages, including alterations in the metabolism and immune function63. In addition to the common functional features of senescent cells, like damaged metabolism and decreased autophagy level, we also summarized the specific characteristics in senescent macrophages, such as altered polarization, infiltration, phagocytosis, and antigen presentation.
3.1. Damaged metabolism
The initiation and maintenance of macrophage responses require high glycolytic and mitochondrial metabolism levels to meet the intense demand for energy and biosynthetic precursors64. Studies have shown notable oxygen consumption with decreased basal respiration, extracellular acidification rate, and reduced complex I and II activities in aged human monocyte-derived macrophages (MDMs)65. Besides, the de novo nicotinamide adenine dinucleotide (NAD) synthesis in aged human MDMs declined with the downregulated mitochondrial SIRT3 activity, which was also observed in mouse peritoneal macrophages. The disrupted NAD synthesis later undermined the phagocytosis, secretion of inflammatory cytokines, and resolution of inflammation in macrophages. Mitochondrial production of ROS was important in the skin's defense against pathogens65. What's more, the aged macrophages were observed as the dysfunction of mitochondria with reduced ATP production, the decrease of mitochondrial membrane potential, and increased oxidative stress, together with compromised antioxidant defense response66, 67, 68, 69.
Matrix metalloprotein-9 (MMP-9) which was involved in tissue repair and regulated the balance of the extracellular matrix was found to participate in cardiac aging70. In the model of SiO2-induced macrophage senescence, the level of MMP-9 was significantly elevated71. This is also by that in ionizing radiation-induced senescence in BMDMs45. Researchers have defined the chronological age (the actual age of an individual) and the biological age (phenotypic age of an individual by identifications of some biological signatures) by several inflammatory markers such as Bcl6, Ccl24, and Il4 in mice. MMP-9 was observed to be in great correlation with cardiac aging, since the MMP-9 null mice were biologically younger than their chronological age70.
Prostaglandin E2 (PGE2) is one of the lipids signaling molecules, as well as an essential regulator of immunity. Though it participates in the inflammation, it is observed an immunosuppressive effect on the inhibition of the activity of the “professional” antigen-presenting cell72. PGE2 tends to accumulate in macrophages from aged individuals and contributed to the immunosuppression. A study conducted by Hayek determined that PGE2 synthesis varied in peritoneal macrophage from 6-month-old mice and 24-month-old mice treated with LPS73. The PGE2 production induced by LPS was significantly larger in the aged macrophages compared to the young. The increased level of PGE2 was mainly the consequence of the upregulated cyclooxygenase-2 (COX-2) activity73. Similarly, IL-1β also promoted higher PGE2 production in peritoneal macrophage from aged mice than that from young mice74. Macrophages in aged mice also produced more PGE2 than those in young mice fed with a control diet (semipurified diets containing 30 parts per million vitamin E)75,76. These findings are in accordance that aged macrophages present the upregulated level of PGE2, which later undermine the adaptive immune response and help tumor escape.
As mentioned above, PGE2 plays a crucial role in the immune response. It's reported that PGE2 stimulated muscle stem cells in young mice and was essential to the regeneration of damaged muscles77. While, the PGE2 degrading enzyme, 15-hydroxyprostaglandin dehydrogenase (15-PGDH) was found to accumulate in aged skeletal muscle78. Surprisingly, the co-detection of 15-PGDH was macrophage markers CD11b and CD45 in the aged gastrocnemius muscles78. To further confirm this conclusion, the macrophages from young and aged mice hindlimb muscle were purified, a striking increase in 15-PGDH transcript level was observed. Hence, we suppose that in the aged muscle, 15-PGDH can be a characteristic of aged macrophages. While it still needs more research to verify.
3.2. Decreased autophagy level
Autophagy is a vital intracellular degradation mechanism that regulates cellular and organismal homeostasis79. Autophagy is thought to increase lifespan and reduce age-related degeneration including removal of accumulated toxic protein aggregates, degradation of damaged mitochondria, and reduced cell death80. Studies have revealed that autophagy level decreases significantly because of autophagy related gene 5 (ATG5) depression in aged mice. The number of LC3 puncta/macrophages was fewer in aged macrophages than that in young macrophages even under the stimulus of LPS/interferon-gamma (IFNγ)79. What's more, in the oxidized low-density lipoproteins (Ox-LDL)-induced RAW264.7 senescence which featured increased p21 and p16 levels, autophagy was decreased as the decreased formation of the autophagosome. In the treatment of 3-MA (autophagy inhibitor), the expression of Beclin1 was upregulated greatly while BCL-2 was reduced instead and 3-MA promoted senescent RAW264.731. Notably, the improvement of autophagy in aged BMDMs can restore polarization to that observed under young conditions81. Generally, the compromised autophagy in senescent macrophages can exacerbate the senescence process mutually.
3.3. Altered polarization tendency
As is known to all, macrophages are highly plastic cells and can respond to local environmental signals in different states82. They are classified as “classically activated” M1 and “alternatively activated” M2 macrophages83,84. The M1 phenotype is characterized by the expression of high levels of pro-inflammatory cytokines like tumor necrosis factor-alpha (TNFα), IFNγ, and IL6. M2 macrophages are involved in immunoregulation with anti-inflammatory cytokines such as IL4, and TGFβ. And researchers have found that the polarization condition in macrophages is different between aged and young individuals.
It was reported that M2-liked macrophages presented the pro-angiogenesis ability in the retina injury with an increased level of Interleukin 10 (IL10), and was observed with the reduction of Factor-related Apoptosis ligand (FasL), Interleukin 12, (IL12), and TNFα29. This suggests senescent macrophages may promote angiogenesis in other age-related diseases. What's more, M2-like macrophages were found to be the most abundant type of macrophages in human skeletal muscles and their frequencies increased during aging according to the investigation of M1 and M2 macrophages in skeletal muscles biopsies from a cohort of healthy human subjects (ranging between 27 and 89 years of age) systematically in the Genetic and Epigenetic Signatures of Translational Aging Laboratory Testing. That's the same in mice29,85. It is possible that the accumulation of anti-inflammatory macrophages in the old was attributed to the increased availability and sensitivity of IL-10 since researchers observed the increased percentage of CD206-positive macrophages under the IL-10 treatment. As mentioned above, MMP-9 was in relation to M1 macrophages. MMP-9 deletion reduced the cardiac M1 macrophages and increased M2 macrophages in aged mice70.
On the contrary, some studies have shown that aged macrophages tend to be M1-like. In the MDM collection from aged and young healthy donors, researchers detected the polarization markers (CD86, CD206) of macrophages in those donors’ peripheral blood. It turned out that the expression of CD86 was higher in the aged MDMs, which represented that the aged macrophages were in a proinflammatory state65. In the thioacetamide-induced acute liver failure, aging aggravated the liver injury, observed with the increased levels of proinflammatory mediators and SASP, which promoted the polarization of those aged macrophages towards the M1 phenotype81.
Conclusively, macrophages present different polarization state states in different tissues and organs in the aged mice. For example, in the elderly bone marrow, spleen, lymph nodes, lung, and muscle, the immunosuppressive M2 macrophages increase a lot86, 87, 88, while macrophages exhibit a more pro-inflammatory M1 phenotype compared to those from young mice in adipose tissue and liver89,90.
3.4. Downregulated phagocytosis
As the main cells of the immune system responsible for phagocytosis, it is found that the uptake of fluorescent myelin by several types of macrophages (peritoneal macrophages, BMDMs, and microglia) from aged mice (15–20 months old) significantly decreased compared to microglia isolated from neonate or young adult mice30. Besides, the primary peritoneal macrophages engulfing fluorescent E. coli particles vary in the aged and young mice91. Young macrophages showed a strong trend of phagocytic activity at the beginning of the dark phase during the circadian rhythmicity testing, while the aged macrophages completely lost this diurnal cariation91. In addition, age-related chronic exposure to TNFα could dampen the clearance of Streptococcus pneumoniae during lung infection by macrophages. However, the probable reason may be the lack of LC3-associated phagocytosis in senescent macrophages which resulted in the detriment in bacteria clearance and enhanced level of pro-inflammatory cytokines. This indicates the importance of LC3-associated phagocytosis in the innate immune defense against the S. pneumoniae infection92. In the senescence model of ionizing radiation, the macrophages showed diminished capacity to take up the fluorescent substrate but recovered with the treatment of ganiclovir93.
3.5. Reduced antigen presentation
As one of the vital antigen-presenting cells, macrophages show their antigen presentation with high expression of MHCII molecules, which can first recognize the pathogens or the damages and submit these harmful signals to T cells as soon as possible to respond to the invasions. Macrophages from aged donors showed decreased antigen-presenting capacity compared to those from young adult individuals94. This was due to the decreased expression of MHC class II molecules and the co-receptors, CD80 and CD8633,95, involved in the antigen presentation. While, in the latest study, researchers have found that lncRNA Aw112010 in old monocytes showed higher expression and induced the MHCII in aged macrophages, suggesting the elevated Aw112010 level can increase the MHCII expression among the aged subjects96.
Apart from that, significant downregulation of Toll-like receptor (TLR) expression on macrophages during aging has been described97,98. This downregulation to respond to bacteria combined with reduced antigen presentation capacity in elderly macrophages is likely to impact T cell function contributing to age-related immunosuppression33
3.6. Changed infiltration and recruitment
Functions of macrophages like migration, infiltration, and recruitment are compromised in aging. Aged macrophages tended to lose their ability to migrate into the wound, with consequent retention of the dermis and increased tissue damaging release of proinflammatory cytokines. The macrophages in aged mice also displayed delayed infiltration around the muscle in regenerating regions compared to the young99. To examine the age of skeletal muscle regeneration, a study worked on the acute time course of 3, 5, and 7 days following muscle damage discovered the poor infiltration in aged macrophages as well as the poor extracellular matrix remodeling. Data showed the same result in humans as the infiltration of CD206/CD68/DAPI macrophages in muscle was lower in old subjects rather than in young subjects, representing the compromised macrophage response during muscle regeneration in older individuals100. As impaired recruitment of macrophages was observed in aged (18-month-old) mice, Hachim et al.101 designed the sequential delivery of MCP-1/IL4 mesh, which was evaluated by subcutaneous implantation in both young mice and aged mice. Hopefully, their polypropylene meshes tended to restore the delayed recruitment and could be a potential biomaterial-based therapy to improve the outcomes in aging. However, in the aged mice, infiltration of macrophages in the crural muscles of aged mice was more than in young mice102. The recruitment of Mb macrophages may be mediated by the increased expression of CSF1 and CSF3 in aged myoepithelial cells and vascular endothelial cells.
4. Senescent macrophages in degenerations of senile individuals
4.1. Aging skeleton and muscle
Bone homeostasis is strikingly disrupted with age, featuring a low degree of bone remodeling and increased marrow fat accumulation, resulting in skeletal fragility103, 104, 105, and diseases like osteoarthritis and osteoporosis106,107. Several studies have reported that senescent cells accumulated in the bone marrow and secreted SASP promoting skeletal aging108. Researchers have found that aged macrophages in the bone marrow were critical cell types during skeletal aging and released grancalcin60. It is reported that grancalcin secreted by aged macrophages induced an imbalance in osteogenesis versus adipogenesis of bone marrow stroma cells, accelerating skeletal aging. This offers a potential target for the treatment of age-related osteoporosis. It also hinders that grancalcin may be a marker for the aged macrophages in age-related bone diseases. Besides, glutamate dehydrogenase-1 (GLUD1) participated in the repression of muscle regeneration and functional recovery in response to aging102. Muscle weight reduced in mice with aging (18 months), GLUD1 repressed muscle regeneration and functional recovery in response to aging102. Knockout of Glud1 in aged macrophages altered infiltration and it only occurred in muscle, other organs like the brain, and liver remained unchanged. The inhibitor of GLUD1, R162 shew improved physical performance in aged mice muscle. Aged macrophages with elevated intracellular lipids, they alternatively activated phenotype promoting pathologic vascular proliferation109. What's more, in the model of the BaCl2-induced muscle injury in aged mice as mentioned before42. Recent studies found that elimination of senescent cells can reduce chronic systemic inflammation and repair tissues110,111. A broad method to overcome senescence was senolytics, one of the most popular senolytics is the combination of dasastinib (D) and quercetin (Q), which was first issued on the EbioMedicine and pioneered the anti-senescence therapy. The administration of D + Q shew effective clearance of senescent myonuclei as well as SA-β-gal positive macrophages secreting SASP42. D + Q can improve muscle regeneration and down-regulate genes associated with macrophages, such as Arg1.
4.2. Aging ovarian
As expected, the proportion phenotype of macrophages varies in young and aged ovaries. Xiao et al.112 had shown that M1 (CD45+F4/80+CD11c+CD206–) increased greatly in aged ovaries while no significant difference was found in M2 (CD45+F4/80+CD11c–CD206+) phenotype and the level of iNOS and CD11c was also increased in Western blot and immunostaining. Coherently, one of the highly scored hub genes Lcp2 was found to be positively correlated with M1 macrophages through the correlation analysis of genes and immune cells in ovarian aging113, suggesting a pro-inflammatory environment during ovarian aging. The method to rescue inflammation-related infertility in aged mice is M2-extracellular vesicles which can ameliorate the inflammatory microenvironment and improve ovarian function113. There is also a contradiction, Umehara et al.114 preferred to use antifibrosis drugs (pirfenidone and BGP-15) to eliminate the fibrotic collagen and dampen M2 macrophage polarization in reproductively old female mice (12 months) to maintain ovarian function and extend fertility. But both studies have reached a consensus that macrophages accumulate in aged ovarian, and the strategy to improve aged ovarian differs in the perspectives towards M2 macrophages. More studies should be carried out to make it clear.
4.3. Degeneration of enteric nervous system
The neurodegeneration of the Enteric nervous system featured neuronal loss and degenerative changes with age, macrophages also played an important role in maintaining gastrointestinal neuromuscular function115, 116, 117, 118, 119. And recent studies have shown that aging induces macrophages polarizing from anti-inflammatory ‘M2’ to proinflammatory ‘M1’ which was associated with a rise in cytokines and immune cells in the enteric nervous system120. The number of muscularis macrophages didn't differ significantly with age. However, the expression of M2 markers like CD206, FIZZ1, and TGFβ reduced with age. This phenomenon remained consistent in the transplantation of aged macrophages into lethally irradiated young mice with the results of reduced expression of CD206, FIZZ1, and TGFβ. In addition, the aged macrophages showed reduced suppression of lymphocyte proliferation compared to the young macrophages when macrophages were co-cultured with CD4+ T cells. This suggested cell-intrinsic factors and the aged microenvironment contribute to the age-dependent loss of anti-inflammatory phenotype120. And the decreased expression of the longevity gene FoxO3 was observed in aged macrophages which lost anti-inflammatory behavior compared to young mice. Aged macrophages might be in the senescence state. Whether FoxO3 is a potential target remains to be discussed.
4.4. Degeneration of nervous system
The energy-deficient state drives maladaptive pro-inflammatory responses in macrophages. While in the aged macrophages with high expression EP2 receptors, these macrophages are too fragile to support mitochondrial respiration and were finally in energy depletion and pro-inflammatory state32. The energy deficiency finally leads to the proinflammatory polarization state as well as the decreased phagocytosis, which dampens the scavenging of abnormal unfolded proteins by microglia in the central nervous system (CNS)32. The increased macrophages were observed in the 24-month mice and characterized by the round shape with lipid droplets originating from myelin indicating the activation of macrophages, named foamy macrophages121,122. Also, these aged foamy macrophages mainly express TREM2. Accumulation of these foamy macrophages in aged mice led to peripheral nerve degeneration. It was the first to prove the age associated with peripheral neuropathy is driven by Trem2+ foamy macrophages121. Additionally, microglia from the aged mice (24 months) increased a lot in corpus callosum and cerebellum123. Microglia in the neocortex exhibited age-related soma volume increase and loss of homogeneous tissue distribution as well as significantly decreased process speed124. Besides, the proportion of M1/M2 macrophages in CNS was related to remyelination. M2 macrophages could promote oligodendrocytes. The transfer of young macrophages into aged individuals has been regarded as a new routine to modify the aberrance in aged macrophages, regarded as a substitution of the senescent one, to some extent. Such as the treatment of parabiotic coupling young mice M2 to old mice which increased M2 densities during efficient remyelination125.
4.5. Other aged tissues
There was little research focusing on the senescent macrophages. Much more preferred the influences of aging on macrophages. Some of these studies also pointed out the relationship between senescent macrophages and aging. The study in Harvard Medical School performed a differential gene expression analysis and revealed the origins of Ma (fetal-derived, enriched for Mrc1, Adgre1) and Mb (adult-derived, enriched for H2-Aa, H2-Ab1) macrophages from mammary glands126. Aging-induced gene expression changes in Mb macrophages as well as the proportions of Ma and Mb macrophages indicating the replacement of fetal yolk sac with age and liver-derived macrophages by mature bone-marrow-derived cells took the dominant place126. In the aged Mb, cytokines like C–C chemokine ligand 5 (CCL5) increased, recruiting T cells and ligands like programmed death 1 (PD1) and immunoglobulin-like transcript 3 (ILT3) improved, promoting an immunosuppressive microenvironment.
As previously discussed, chemotherapy brought deleterious effects and caused abnormal senescence in individuals, the elimination of those senescent cells could be an effective method. In the ionizing radiation mice model, splenic cells presented SASP and increased levels of p16INK4a 93. Ganciclovir also shew the potentential of synovitis in p16-3MR mice could eliminate p16INK4a-positive cells. It could completely abrogate senescent macrophages (with an approximately 20-fold increase in p16INK4a expression) in the model of ionizing irradiation93. The mechanism of synolytics eliminating senescent cells remained further exploration, but it lighted the way to anti-senescence and treatment of age-associated diseases.
Apart from the degeneration of organs or systems in aged individuals, the loss of diurnally rhythmic innate immune responses was also found in association with aging. A study had shown that homeostatic immune responses strikingly declined and the circadian gene transcription downregulated in the aged macrophages. The loss of Kruppel-like factor 4 (Klf4), a transcription factor in regulating cell differentiation and reprogramming transcription factors in aged macrophages contributed to the disruption of circadian innate immune homeostasis, which might illustrate the age-associated loss of protective immune responses91.
5. Senescent macrophages in age-associated diseases
5.1. Age-related macular degeneration (AMD)
AMD is an inflammatory disease that develops with age and is the leading cause of blindness in people over 50 years old. The aged macrophages were with impaired cholesterol efflux which promoted macular degeneration109 and they also presented proangiogenic phenotype in AMD29. Abdoulaye discovered that macrophages from old mice (18 months) had higher levels of intracellular Ox-LDL and lower extruded Ox-LDL content in the supernatants compared to the young macrophages. Using LXR agonists T0-901317 or miR-33 inhibitors could restore cholesterol efflux in aged macrophages and repress macrophage regulation of vascular proliferation109.
5.2. Atherosclerosis
Similar to AMD, atherosclerosis is in close relationship with Ox-LDL and cholesterol crystals127. What's more, Ox-LDL induced senescence in macrophages, as seen by more SA-β-Gal positive in RAW264.7, and inhibited the recruitment of macrophages128. Recently, it has been reported that foamy macrophages with senescence signatures (X-galactosidase crystals) accumulated in the sub-endothelial space, inducing the atherosclerotic process with increased levels of inflammatory cytokines, chemokines, and metalloproteinases129. These senescent macrophages together with other p16Ink4a + senescent cells drove the formation of atherosclerotic plaques and finally created an environment to for further lesion growth.
5.3. Diseases in the central nervous system
There are several CNS diseases that are aging-related and non-resolving inflammation130. Usually, non-senescent macrophages are suggested to stimulate remyelination131. Remyelination defects occur with age characterized by low signal propagation and less protection for axons. In the aged mice (>15 months), the aged microglia and MDMs are unable to reach the lesions which contributes to the decline in remyelination and compromised uptake of myelin, resulting in the neurodegeneration diseases like multiple sclerosis30,132 and Alzheimer's disease32,133,134. Besides, the inflammatory infiltration of MAC1+IB4+ macrophages in aged mice was higher than that in young mice135.
CD36 (a receptor for the phagocytosis of oxidized phospholipids in myelin debris136) showed an overlap of 39.28% between scavenger receptor gene and RNA sequencing dataset for genes differentially expressed with age and activation state genes, which also represented reduced phagocytic activity in aging mice microglia132. The treatment of niacin upregulated CD36 expression, promoted myelin phagocytosis in 9–12-month mice as well as enhanced recruitment of oligodendrocyte progenitor cells and rejuvenated remyelination. Apart from the downregulation of CD36, genes in the retinoid X receptor were decreased with aging in both myelin-phagocytosing human monocytes and mouse macrophages30. Bexarotene as a retinoid X receptor agonist could partially restore myelin debris phagocytosis in aged macrophages and reverse the gene expression profile of monocytes to a more youthful profile in multiple sclerosis patients30.
5.4. Liver sterile inflammation
Aging is regarded as a contributor to promoting sterile inflammation, a form of pathogen-free inflammation mainly caused by ischemia, mechanical trauma, or drugs, and has been implicated in the pathogenesis of several liver diseases, such as liver cancer, nonalcoholic fatty liver disease, alcoholic liver disease137,138. Aged macrophages were observed with impaired mitophagy activation which led to the cytosolic release of mitochondrial DNA and promoted stimulator of interferon genes (STING) activation subsequently as well as the induction of proinflammatory responses in the liver. Besides, the deficiency of PINK1/Parkin-mediated polyubiquitination of mitochondria and mTOR/TFEB-mediated lysosomal biogenesis and function were also implicated in the aged macrophages characterized by damaged mitophagic flux138. The relationship between mitophagy dysfunction and STING activation in senescent macrophages could be a probable point cut to study sterile inflammatory liver injury. Suppression of STING could block the over-activation of NOD-like receptor protein 3 (NLRP3) signaling and excessive secretion of proinflammatory cytokines in the mitochondrial DNA-stimulated BMDMs from aged mice139.
5.5. Cancer
Tumor-associated macrophages are among the most abundant and essential immune cells in the tumor microenvironment140. They function in the orchestration of angiogenesis, extracellular matrix remodeling, cancer cell proliferation, metastasis, and immunosuppression141. However, macrophages are highly plastic cells that serve a multitude of functions in response to different environmental cues, thus may be double-edged swords in cancers142. In the early stage of tumor onset, macrophages may be antitumoral, they can kill tumor cells through phagocytosis, M1 polarization, and activating innate or adaptive immune responses. On the contrary, most macrophages are “educated” to enhance tumor progression and metastasis along with the change of tumor microenvironment by various mechanisms, including promoting the survival and proliferation of cancer cells as well as suppressing innate and adaptive immune responses140,141. Therefore, more and more strategies are utilized to eliminate cancer cells by targeting or reprogramming macrophages143.
Given the contribution of macrophages to the tumor microenvironment, some studies found that macrophage senescence also affects the progression of cancers. Old people are more susceptible to cancers like colorectal cancer, prostate cancer, and lung cancer144. It has been published that the infiltration of CD68+CD206+ cells in tumor tissues is higher in old patients (>60 years old) than in young patients145. The mix of CT26 cells and these tumor-associated macrophages showed that macrophages promoted CT26 proliferation, especially the aged tumor-associated macrophages presented a higher pro-tumorigenic effect. The p-NF-κB translocation to the nucleus was more significant in tumor-associated macrophages from aged mice compared with young. What's more, macrophages tended to polarize to M2 phenotype in aged mice, as seen by the higher Arg1 and less iNOS after the treatment with tumor condition medium, thus promoting tumor cell proliferation in aged mice. The upregulation of CD163 and VSIG4 (pro-tumor macrophage markers) in old patients with prostate cancer (>60 years) was remarkable, and associating with aggressive prostate cancer and poor outcomes146. Similarly, the alveolar macrophages (AMs, tissue-resident macrophages in the lung) were identified to be the most susceptible to senescence in Kras-mutant mice. Through scRNA-seq, the new cluster SIGLEC-F+ and CXCR1High AMs, which expressed several putative SASP factors (Spp1, Igf1, Ctsd, and Ctsb) and enriched negative regulators to proliferation (Ctnnb1, Ctsl), were identified as senescent AMs in Kras-tumorous lung. The senescent CXCR1High AMs accumulated with age and presented a counteraction against cytotoxic T cell accumulation during lung tumorigesis24. The study conducted by Haston et al.25 demonstrated the detrimental roles of senescent macrophages in lung cancer consistently. Additionally, the senescent macrophages took up the majority of senescent cells in early (premalignant) lesions, which used to be regarded as the origin of tumor cells or epithelial cells25. Generally, the infiltration of macrophages increases with age in cancers and most of them are pro-tumorigenic147.
5.6. Lung fibrosis
Alveolar macrophages are the most abundant innate immune cells in the lung. The compromised phagocytosis of apoptotic cells by alveolar macrophages contributes to severe lung damage in elderly individuals. It was shown that alveolar macrophages tended to decrease with aging and exhibited both M1 and M2 features with enhanced expression levels of Nos2, Ccl8 for M1, and Arg1, Cxcl13 for M2148. Sigle-cell RNA sequencing discovered the majority of alveolar macrophages was different in aged and young mice, because 81.73% of alveolar macrophages from young mice are in cluster 2, which was later substituted by cluster 1 in alveolar macrophages from aged mice. The top 15 marker genes in cluster 1 were Gstm1, Serpine1, Cybb, Pdk4, and Cd63. The MARCO+alveolar macrophages increased among the aged alveolar macrophages and they produced more CCL6148. It has been confirmed that MARCO+ alveolar macrophages promoted pulmonary fibrosis in aged mice while the removal of MARCO+ alveolar macrophages in aged mice showed attenuated pulmonary fibrosis.
5.7. Diabetes and wound
Wound-induced senescence remains transient and macrophages can clear senescent cells, facilitating tissue repair in the M2 polarization state. In models of delayed healing like diabetes, macrophages are in heightened proinflammatory response and secrete high levels of SASP149. Holly found the SA-β-gal+cells increased in diabetes and aged mice with wounds on Day 7 after injury149. Other senescence genes like Cdkn2a (p16) and Trp53 (p53) also showed higher expression. Macrophages took a large proportion of these senescent cells in diabetes wounds. Undoubtedly, these macrophages were SA-β-gal positive in diabetes mice and presented reduced polarization with less expression of iNOS and ARG1. What's more, CXC motif chemokine ligand 2 (CXCL2) secretion was increased in diabetes macrophages and induced senescence in human dermal fibroblasts, which was linked to diabetes wound healing41. By the use of SB265610 (blockade of CXCR2), the wound healing was accelerated and senescent macrophages were also reduced in the diabetes wound mice model. Recently, some studies worked on the cell–cell interaction to modify the dysfunction in senescent macrophages. Mesenchymal stem cells (MSC) co-cultured with aged macrophages showed increased phagocytic capabilities, which was necessary in initiating reparative responses in the wound. Additionally, MSC appeared to promote the conversation of M1 to M2, and increased the secretion of IL10, as well as inhibit the level of IL1β, IL1729,150,151.
6. Genetic regulations and mechanisms of senescent macrophages
6.1. Regulation of SASP in senescent macrophages
There were several mediators inducing SASP, like RIG-1, inflammasome, and damage-associated molecular patterns. Among them, NLRP3 inflammasome is an innate immune sensor that induce the production of inflammatory cytokines IL-1β and IL-18, thus regulating the SASP importantly. Based on this, sirtuin 2 (a cytosolic deacetylase) was found to acetylate NLRP3, quenching the activation of NLRP3 inflammasome in aged macrophages and improving the insulin signaling in old white adipose tissues152. Besides, the epigenetic reader bromodomain and extra-terminal domain protein 4 (BRD4) could bind to acetylated histones and then recruit to superenchancers adjacent to SASP genes153. Hence, BRD4 executes cellular senescence directly and acts as a tumor suppressor2,153. In macrophages, LPS can promote the senescence of macrophages, the function of which may be also related to the increased BRD4 expression154. While, chemical and genetic inhibition of BRD4 (or I-BET762, siBRD4) reduces SASP and also limits the circuit of paracrine enlarged senescence circumstances in senescent macrophages154. Additionally, post-transcriptional control of SASP in senescent macrophages could be a potential perspective to treat neurodegenerative diseases155. mTOR- and translation-related genes were upregulated in aged microglia. The ablation of Rheb1 (an upstream activator of mTORC1) could diminish the binding of elF4E to elF4G, and finally mild neural sickness behavior with decreased cytokine protein levels155. In brief, there are many regulators of the SASP of senescent macrophages, including transcription factors, epigenetic regulatory signals, and post-transcriptional regulators, although some have yet to be discovered. Interfering with these factors may also alter the aging state of macrophages as well as related diseases.
6.2. Regulators of universal hallmarks in senescent macrophages
Like SASP, there are also some regulators to the universal hallmarks in senescent macrophages, such as DNA damage response, p16, and so on. As mentioned above, the presence of DNA double-strand breaks will activate the DNA damage response, which then induces the senescence of macrophages2,156. Knockdown of heme oxygenase-1 (HO-1, an enzyme that removes heme) resulted in an impaired DNA damage response with the observation of lower Ki67 levels as well as higher H2AXγ and SA-β-Gal expression157. Inversely, heme arginate treatment increased HO-1 expression in kidney-resident macrophages significantly and then ameliorated renal failure in aged mice158. Since p16 is a cyclin-dependent kinase inhibitor and often accumulated in senescent cells, the high-level activation of p16INK4a promoter in macrophages was observed with increased expression of lysosomal mRNAs and β-galactosidase activity159. That's the same in p16 deletion mice, which ameliorated the proinflammatory level of macrophages160. What's more, Bmi-1 was found to bind to PRC2 and then occupy the promoter of p16, resulting in the methylation and transcription inhibition of p16161. However, the function of Bmi-1 on senescent macrophages remains unknown.
6.3. Relevant mechanisms involved in senescent macrophages-associated diseases
The senescent macrophage-associated diseases include various abnormalities of organs and systems in the individual. Several common mechanisms have been summarized across these diseases. Firstly, the imbalance of polarization between M1 and M2 plays a central role in senescent macrophage-associated diseases. The wrong domination of the macrophage polarization state could lead to disorders. Senescent macrophages polarized toward M1 in several diseases like liver sterile inflammation, diabetes wounds, and atherosclerosis. They induced excessive STING activation instead of producing anti-inflammatory cytokines (IL-10, TGF-β) or lipid mediators (resolvins, protectins)162, 163, 164, which contributed to proinflammatory responses in the liver and exacerbated liver sterile inflammation138. That is the same in diabetes wounds, senescent macrophages presented altered retention of polarization and secreted CXCR2 at a high level41. The delayed transition from M1 to M2 and less pro-healing mediators release led to more chemoattractant cytokines and drove the negative loop with the influx of other pro-inflammatory macrophages165. It was induced that senescent macrophages recruited monocytes by enhancing Vcam1, and Mcp1 expression and promoted inflammatory cytokines with elevated SASP components (Mmp3, Mmp13, Tnfα) in atherosclerosis mice129. Mutually, aggregated LDL induced macrophage senescence with increased ROS production and secretion of pro-inflammatory cytokines, which could also be inhibited by lysosomotropic antioxidant, cysteamine166,167. While, in prostate cancer and macular degeneration, senescent macrophages exhibited M2 polarization. Senescent macrophages in macular degeneration increased M2 relevant markers IL10, and CD163 significantly and exhibited an anti-inflammatory profile that was too incapable of regulating aberrant angiogenesis109.
Damaged phagocytosis of senescent macrophages also incurs diseases like macular degeneration, CNS diseases, and pulmonary fibrosis. Studies discovered that the low response to exogenous lipid and weak cholesterol efflux capability in senescent macrophages contribute to the defeat of the inhibition of vascular proliferation29,109. Reduced phagocytic activity could cause deserted myelin accumulation in the central nervous system, promoting the development of multiple sclerosis132 and accumulating apoptotic cells in lung fibrosis148.
Macrophages dominate the innate immune in cancers by surveillance of tumors and presentation of MHC I to cytotoxic T cells as well as phagocytosis of cellular debris. Senescent macrophages display a pro-tumorigenesis function to the contrary. It has been revealed the p-NF-κB translocation in senescent macrophages and these macrophages undermine the cytotoxicity of T cells146. In an oncogenic Kras-driven lung cancer mouse model, senescent AMs inhibited the accumulation of cytotoxic T cells (PRF+CD8+ T cells, PRF+CD4+ T cells and GZMB+PRF+CD4+ cells), aggravating lesion formation24.
As mentioned above, many functions of macrophages are involved in senescent macrophage-related diseases. Thus, we speculate that mechanisms related to macrophage function may also participate in these diseases. For example, there are several signaling pathways mediating the polarization of macrophages, like IRF/STAT1 signaling in M1 polarization andIL4, and IL13 signaling in M2 polarization168, 169, 170. It was also reported that the STING signaling could increase phagocytosis of macrophages. The inhibition of adhesion and degranulation-protein adaptor protein potentiated STAT1 nuclear entry, which finally promote macrophages to phagocyte171,172. However, more research is needed to explain whether these conventional mechanisms apply to senescent macrophages in diseases.
7. Concluding remarks
As the first-responder to the pathogens and damages in the immune response, macrophages play a vital role in the function of phagocytosis and polarization towards different situations to mediate the inflammation inside individuals. Senescent macrophages are usually featured with an unbalanced polarization state, compromised phagocytosis, impaired migration, and damaged autophagy. Due to the abnormal accumulation and the aberrant functions of senescent macrophages, aged people tend to be unhealthy or with acerbated diseases. Despite the increasing understanding of the role of senescent macrophages in the development of individuals and diseases, many fundamental questions are still unanswered and follow-up studies in more detail are still required.
Whether the macrophages in aged individuals represent the senescent macrophages, and what's the difference between macrophages in aged individuals and macrophages featured with SASP like the common senescent cells? There's a proposal that when the tissue (composited with functional cells) gets damaged, the cells initiate senescence in situ and induce accumulation of immune cells. The macrophages and T cells that are recruited can clear those senescent cells while progenitor cells repopulate and regenerate the damaged tissue. However, the persistent damage and less clearance of senescent cells can result in organ aging. This senescence-clearance-regeneration model is applied to occasional adult somatic damage, tumors are excluded173. Li et al.60 revealed that GCA + senescent macrophages promote skeletal aging, and the conditioned medium from old macrophages reduced myoblast proliferation, impacting myogenic repair processes174, which stands in line with Muñoz's unified model that senescent macrophages contribute to degenerations and diseases. On the other hand, whether aging should have influenced on macrophage senescence process is still in the air. Abundant studies have regarded macrophages from aged individuals as senescent macrophages and Lin et al.175 revealed that macrophages from old mice exhibited numerous signs of aging like dysregulated cholesterol homeostasis and oxidative respiration. Age-associated diseases also affect macrophage functions. Aged wounds and diabetic wounds had more senescent cells and aging causes a shift in macrophage polarization, impairing its suppressive properties on lymphocytes. Besides, macrophages, which can be recruited by other senescent cells, are found to be susceptible to senescence and are particularly subject to more cell-extrinsic factors in the aged microenvironment91,120,176. Thus, despite the awareness of diseases/degenerations and senescent macrophages in aged individuals/organs, their causal or precedence relationship remains complex and fuzzy.
Notably, we have found that senescent macrophages play various functions in different diseases or organs which indicates that treatments should be specialized for the distinctive characteristics of senescent macrophages. In the cognitive decline, senescent macrophages turn to behave abnormally in phagocytosis for the dampened scavenging of abnormal unfolded proteins in the CNS32,177. The imbalanced polarization state in senescent macrophages also contributes to the development of malignant cancers. More senescent macrophages tend to M2 phenotype promoting tumor cells proliferating and counteract against cytotoxic T lymphocytes24,25,146. Contrarily, in the ovarian, senescent macrophages turn to the M1 phenotype with a higher level of iNOS which causes ovarian aging. Secretions of senescent macrophages are also in close relationship with some organ disorders. Grancalcin produced by senescent macrophages would exacerbate skeletal aging for the impaired balance between osteogenesis and adipogenesis of bone marrow stroma cells60. Additionally, metabolic disturbances like chaotic cholesterol levels in senescent macrophages cause age-related macular degeneration for their proangiogenic function109.
As we have summed before, senescent macrophages serve different functions in distinct contexts, so it's necessary to characterize senescent macrophages more precisely. Grancalcin in bone marrow macrophages can be regarded as the hallmarks of senescent macrophages. However, there is still less known about other specific signs that indicate the senescent process in macrophages. To date, the major limitation in senescent cells is the lack of single, universal, or model-specific biomarkers. Therefore, the combination of multiple markers (SA-β-Gal, p21, SASP) simultaneously is suggested to differentiate true senescent cells from other quiescent or differentiated counterparts2. Similarly, we propose that can also be applied to the identification of senescent macrophages: the combination of multiple biomarkers like SASP (a common senescent mark), limited proliferation (shortened telomerase), altered function of macrophages (polarization, phagocytosis, and so on), as well as specific hallmarks like grancalcin in particular diseases or organs (Fig. 1). In summary, it is supposed to work more on the clear definition of senescent macrophages and the particular markers of senescent macrophages.
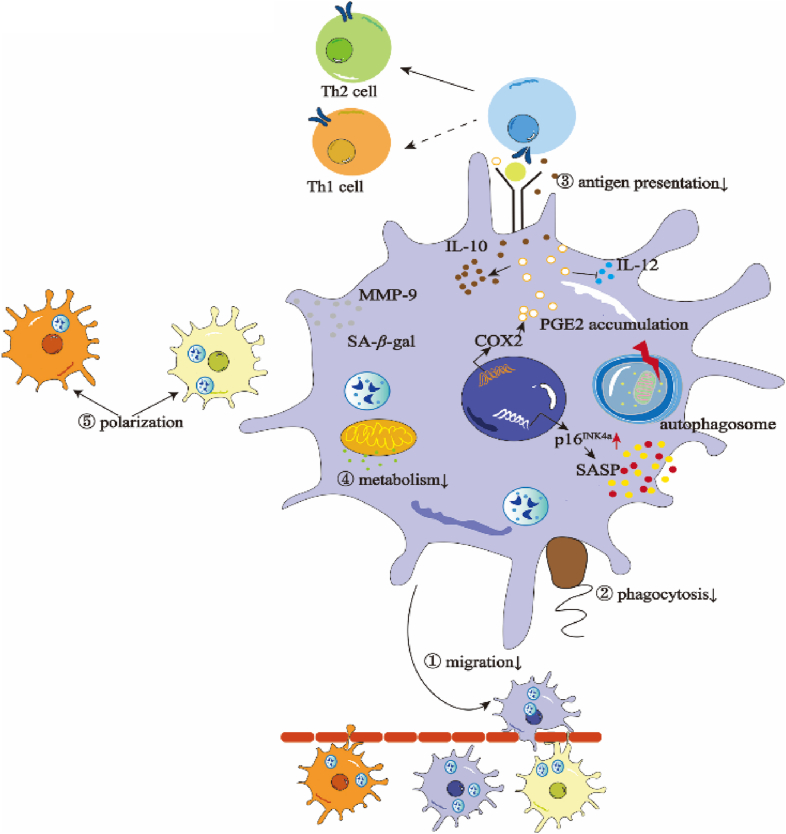
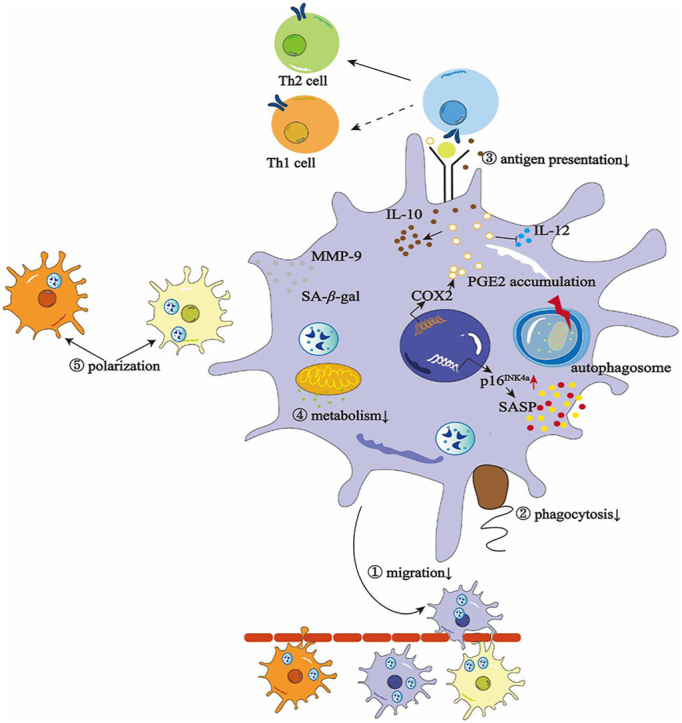
Figure 1.
Functional and molecular alterations in senescent macrophages. Senescent macrophages are characterized by upregulated p16INK4a and accumulation of SA-β-gal, SASP, MMP-9, and PGE2. These abnormal accumulation and expression levels finally undermine macrophage functions like compromised migration through barriers, poor phagocytosis level to defend against bacteria and other infections, less effective antigen presentation towards T cells, low metabolism with deficient glucose and other metabolism productions contributing to other dysfunctions, imbalanced polarization which lead to the diseases and degenerations.
Since the senescent macrophages play an important role both in the development of the individuals and the process of diseases149, drugs that target macrophage senescence could be an effective means of treating the diseases (Table 1). Generally, some therapeutic methods to avert the morbigenous effects of senescence are senolytics and senomorphics2,178,179. Senolytics works on the removal of the aberrant accumulation of senescent cells while xenomorphic emphasizes the elimination of the key attributes of senescence-like SASP. The depletion of senescent cells can be directed by quercetin and dasatinib which have been introduced previously. In addition, the cardiac glycoside ouabain and EF24 treatment targeting BCL-2 to promote apoptosis and kill senescent cells180,181. Some natural molecules like urolithin A attenuate d-galactose-induced brain aging182,183 and prolong the lifespan of C. elegans184. Rapamycin, the autophagy inducer improves immune function by reducing senescent markers in immune cells in the Vav-iCre+/−; Ercc1–/flox mice (a model of immunosenescence)182,185. The SASP can be modulated by NAD+/NADH metabolism, while the nicotinamide phosphoribosyl transferase activators have been shown to increase NAD+ levels which functions in a neuroprotective behavior186,187. Directedly targeting key components of the SASP or their receptor was also an effective gynomorphic. Alanine-serine-cysteine transporter type-2 (ASCT2), a high-affinity glutamine transporter can inhibit the senescence of hepatic stellate cells by promoting SASP, disturbing IL-1α/NF-κB feedback loop188. Apart from these strategies applied to senescent cells, there are several specific treatments targeting senescent macrophages such as R162, inhibiting GLUD1 and improving infiltration of aged macrophages as we mentioned before. Though there are few studies working on the treatment of senescent macrophages, we summed up several therapies as seen in Table 1. Besides, it's worth noting that crosstalk between other cells and macrophages can be a potential breakthrough point in many diseases.
Table 1.
Treatment and improvement towards senescent macrophages in degenerations and age-associated diseases.
| Treatment | Degenerations/diseases/models | Mechanism | Ref. |
|---|---|---|---|
| Dasastinib & quercetin | BaCl2-induced muscle injury in aged mice | General elimination of senescent cells including senescent macrophages | 42 |
| Ganciclovir | Ionizing irradiation-induced senescence in splenic cells | General elimination of senescent cells including senescent macrophages | 93 |
| R162 | Aged muscle | Inhibition of GLUD1 & promotion of glutamine synthetase activity and improve of infiltration in aged macrophages | 102 |
| Niacin | Aged demyelinated mice | Upregulation of CD36 & augment of phagocytosis of aged microglia and macrophages | 30 |
| T0-901317 | Senescent macrophages from aged mice (>18 months) & aged mice | Downregulation of ABCA1 & restoration of cholesterol efflux in aged macrophages & altered phenotype as M2 macrophages | 109 |
| SB265610 | Diabetes wound mice | Blockade of CXCR2 & reduction of senescent macrophages & acceleration of skin wound healing | 41 |
| Parabiotic coupling young mice M2 macrophages to old mice | CNS demyelination | Increase of M2 macrophages densities & restoration of remyelination efficiency | 125 |
| Mesenchymal stem cells | Skin wound mice | Promotion of the conversation of aged macrophage polarization & restoration of tensile strength in aged mice | 150 |
| JQ-1 | LPS-induced senescent macrophages | Inhibition of BRD4 & decrease of SASP in senescent macrophages | 26 |
| Cysteamine | Smase-LDL induced senescent macrophages | Antioxidation of LDL in lysosome & inhibition of secretion of TNF-α, IL-6 | 166 |
Acknowledgments
This study was supported by the Fundamental Research Funds for the Central Universities (No. 226-2023-00114, China), National Natural Science Foundation of China (Nos. 82222069 and 82104181), the Key R&D Program of Zhejiang (No. 2022C03143, China), and the Huadong Medicine Joint Funds of the Zhejiang Provincial Natural Science Foundation of China (No. LHDMD22H310004).
Author contributions
Qinjie Weng and Jiajia Wang conceived and designed the project. Longling Wang, and Wenxiang Hong drafted the manuscript. Longling Wang, Wenxiang Hong, and Hong Zhu revised the manuscript. Qiaojun He and Bo Yang gave some critical suggestions to the manuscript. All authors approved the final version of the manuscript.
Conflicts of interest
The authors declare no conflicts of interest.
Footnotes
Peer review under the responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
Contributor Information
Jiajia Wang, Email: wangjiajia3301@zju.edu.cn.
Qinjie Weng, Email: wengqinjie@zju.edu.cn.
References
- 1.Hayflick L., Moorhead P.S. The serial cultivation of human diploid cell strains. Exp Cell Res. 1961;25:585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 2.Di Micco R., Krizhanovsky V., Baker D., d'Adda di Fagagna F. Cellular senescence in ageing: from mechanisms to therapeutic opportunities. Nat Rev Mol Cell Biol. 2021;22:75–95. doi: 10.1038/s41580-020-00314-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim H.N., Chang J., Shao L., Han L., Iyer S., Manolagas S.C., et al. DNA damage and senescence in osteoprogenitors expressing Osx1 may cause their decrease with age. Aging Cell. 2017;16:693–703. doi: 10.1111/acel.12597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walford R.L. The immunologic theory of aging. Gerontol. 1964;4:195–197. doi: 10.1093/geront/4.4.195. [DOI] [PubMed] [Google Scholar]
- 5.Liu Z., Liang Q., Ren Y., Guo C., Ge X., Wang L., et al. Immunosenescence: molecular mechanisms and diseases. Signal Transduct Targeted Ther. 2023;8:200. doi: 10.1038/s41392-023-01451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y., Dong C., Han Y., Gu Z., Sun C. Immunosenescence, aging and successful aging. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.942796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.He S., Sharpless N.E. Senescence in health and disease. Cell. 2017;169:1000–1011. doi: 10.1016/j.cell.2017.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sui D., Li C., Tang X., Meng X., Ding J., Yang Q., et al. Sialic acid-mediated photochemotherapy enhances infiltration of CD8+ T cells from tumor-draining lymph nodes into tumors of immunosenescent mice. Acta Pharm Sin B. 2023;13:425–439. doi: 10.1016/j.apsb.2022.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner V.M., Mabbott N.A. Structural and functional changes to lymph nodes in ageing mice. Immunology. 2017;151:239–247. doi: 10.1111/imm.12727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirakawa K., Sano M.T. Cell immunosenescence in aging, obesity, and cardiovascular disease. Cells. 2021;10:2435. doi: 10.3390/cells10092435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Snoeck H.W. Aging of the hematopoietic system. Curr Opin Hematol. 2013;20:355–361. doi: 10.1097/MOH.0b013e3283623c77. [DOI] [PubMed] [Google Scholar]
- 12.Listì F., Candore G., Modica M.A., Russo M., Di Lorenzo G., Esposito-Pellitteri M., et al. A study of serum immunoglobulin levels in elderly persons that provides new insights into B cell immunosenescence. Ann N Y Acad Sci. 2006;1089:487–495. doi: 10.1196/annals.1386.013. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez I.J., Lalinde Ruiz N., Llano León M., Martínez Enríquez L., Montilla Velásquez M.D.P., Ortiz Aguirre J.P., et al. Immunosenescence study of t cells: a systematic review. Front Immunol. 2020;11 doi: 10.3389/fimmu.2020.604591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ritzel R.M., Crapser J., Patel A.R., Verma R., Grenier J.M., Chauhan A., et al. Age-associated resident memory CD8 T cells in the central nervous system are primed to potentiate inflammation after ischemic brain injury. J Immunol. 2016;196:3318–3330. doi: 10.4049/jimmunol.1502021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y., Klein S.L., Garibaldi B.T., Li H., Wu C., Osevala N.M., et al. Aging in COVID-19: vulnerability, immunity and intervention. Ageing Res Rev. 2021;65 doi: 10.1016/j.arr.2020.101205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang W., Hickson L.J., Eirin A., Kirkland J.L., Lerman L.O. Cellular senescence: the good, the bad and the unknown. Nat Rev Nephrol. 2022;18:611–627. doi: 10.1038/s41581-022-00601-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trzonkowski P., Debska-Slizień A., Jankowska M., Wardowska A., Carvalho-Gaspar M., Hak Ł., et al. Immunosenescence increases the rate of acceptance of kidney allotransplants in elderly recipients through exhaustion of CD4+ T-cells. Mech Ageing Dev. 2010;131:96–104. doi: 10.1016/j.mad.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Lunin S.M., Novoselova E.G., Glushkova O.V., Parfenyuk S.B., Novoselova T.V., Khrenov M.O. Cell senescence and central regulators of immune response. Int J Mol Sci. 2022;23:4109. doi: 10.3390/ijms23084109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ritschka B., Storer M., Mas A., Heinzmann F., Ortells M.C., Morton J.P., et al. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017;31:172–183. doi: 10.1101/gad.290635.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosteiro L., Pantoja C., Alcazar N., Marión R.M., Chondronasiou D., Rovira M., et al. Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science. 2016;354:aaf4445. doi: 10.1126/science.aaf4445. [DOI] [PubMed] [Google Scholar]
- 21.Chen R., Zhang S., Liu F., Xia L., Wang C., Sandoghchian Shotorbani S., et al. Renewal of embryonic and neonatal-derived cardiac-resident macrophages in response to environmental cues abrogated their potential to promote cardiomyocyte proliferation via Jagged-1–Notch1. Acta Pharm Sin B. 2023;13:128–141. doi: 10.1016/j.apsb.2022.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.De La Fuente M. Changes in the macrophage function with aging. Comp Biochem Physiol A Comp Physiol. 1985;81:935–938. doi: 10.1016/0300-9629(85)90933-8. [DOI] [PubMed] [Google Scholar]
- 23.Johnson D.R., Fernandes G., Douglas S.D. Age related decline in cytoplasmic spreading of mouse peritoneal macrophages. Dev Comp Immunol. 1978;2:347–354. doi: 10.1016/s0145-305x(78)80077-9. [DOI] [PubMed] [Google Scholar]
- 24.Prieto L.I., Sturmlechner I., Graves S.I., Zhang C., Goplen N.P., Yi E.S., et al. Senescent alveolar macrophages promote early-stage lung tumorigenesis. Cancer Cell. 2023;41:1261–1275.e6. doi: 10.1016/j.ccell.2023.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haston S., Gonzalez-Gualda E., Morsli S., Ge J., Reen V., Calderwood A., et al. Clearance of senescent macrophages ameliorates tumorigenesis in KRAS-driven lung cancer. Cancer Cell. 2023;41:1242–1260.e6. doi: 10.1016/j.ccell.2023.05.004. [DOI] [PubMed] [Google Scholar]
- 26.Wang H., Fu H., Zhu R., Wu X., Ji X., Li X., et al. BRD4 contributes to LPS-induced macrophage senescence and promotes progression of atherosclerosis-associated lipid uptake. Aging. 2020;12:9240–9259. doi: 10.18632/aging.103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Calcinotto A., Kohli J., Zagato E., Pellegrini L., Demaria M., Alimonti A. Cellular senescence: aging, cancer, and injury. Physiol Rev. 2019;99:1047–1078. doi: 10.1152/physrev.00020.2018. [DOI] [PubMed] [Google Scholar]
- 28.Lian J., Yue Y., Yu W., Zhang Y. Immunosenescence: a key player in cancer development. J Hematol Oncol. 2020;13:151. doi: 10.1186/s13045-020-00986-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly J., Ali Khan A., Yin J., Ferguson T.A., Apte R.S. Senescence regulates macrophage activation and angiogenic fate at sites of tissue injury in mice. J Clin Invest. 2007;117:3421–3426. doi: 10.1172/JCI32430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Natrajan M.S., de la Fuente A.G., Crawford A.H., Linehan E., Nuñez V., Johnson K.R., et al. Retinoid X receptor activation reverses age-related deficiencies in myelin debris phagocytosis and remyelination. Brain. 2015;138:3581–3597. doi: 10.1093/brain/awv289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cao H., Jia Q., Yan L., Chen C., Xing S., Shen D. Quercetin suppresses the progression of atherosclerosis by regulating MST1-mediated autophagy in ox-LDL-induced RAW264.7 macrophage foam cells. Int J Mol Sci. 2019;20:6093. doi: 10.3390/ijms20236093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minhas P.S., Latif-Hernandez A., McReynolds M.R., Durairaj A.S., Wang Q., Rubin A., et al. Restoring metabolism of myeloid cells reverses cognitive decline in ageing. Nature. 2021;590:122–128. doi: 10.1038/s41586-020-03160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jackaman C., Tomay F., Duong L., Abdol Razak N.B., Pixley F.J., Metharom P., et al. Aging and cancer: the role of macrophages and neutrophils. Ageing Res Rev. 2017;36:105–116. doi: 10.1016/j.arr.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Muñoz-Espín D., Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 35.McHugh D., Gil J. Senescence and aging: causes, consequences, and therapeutic avenues. J Cell Biol. 2018;217:65–77. doi: 10.1083/jcb.201708092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coppé J.P., Desprez P.Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coppé J.P., Patil C.K., Rodier F., Krtolica A., Beauséjour C.M., Parrinello S., et al. A human-like senescence-associated secretory phenotype is conserved in mouse cells dependent on physiological oxygen. PLoS One. 2010;5 doi: 10.1371/journal.pone.0009188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Coppé J.P., Patil C.K., Rodier F., Sun Y., Muñoz D.P., Goldstein J., et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferreira-Gonzalez S., Lu W.Y., Raven A., Dwyer B., Man T.Y., O'Duibhir E., et al. Paracrine cellular senescence exacerbates biliary injury and impairs regeneration. Nat Commun. 2018;9:1020. doi: 10.1038/s41467-018-03299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coppé J.-P., Desprez P.-Y., Krtolica A., Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilkinson H.N., Clowes C., Banyard K.L., Matteuci P., Mace K.A., Hardman M.J. Elevated local senescence in diabetic wound healing is linked to pathological repair via CXCR2. J Invest Dermatol. 2019;139:1171–1181.e6. doi: 10.1016/j.jid.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Dungan C.M., Murach K.A., Zdunek C.J., Tang Z.J., VonLehmden G.L., Brightwell C.R., et al. Deletion of SA β-Gal+ cells using senolytics improves muscle regeneration in old mice. Aging Cell. 2022;21 doi: 10.1111/acel.13528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prattichizzo F., De Nigris V., Mancuso E., Spiga R., Giuliani A., Matacchione G., et al. Short-term sustained hyperglycaemia fosters an archetypal senescence-associated secretory phenotype in endothelial cells and macrophages. Redox Biol. 2018;15:170–181. doi: 10.1016/j.redox.2017.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall B.M., Balan V., Gleiberman A.S., Strom E., Krasnov P., Virtuoso L.P., et al. Aging of mice is associated with p16(Ink4a)- and β-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging (Albany NY) 2016;8:1294–1315. doi: 10.18632/aging.100991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su L., Dong Y., Wang Y., Wang Y., Guan B., Lu Y., et al. Potential role of senescent macrophages in radiation-induced pulmonary fibrosis. Cell Death Dis. 2021;12:527. doi: 10.1038/s41419-021-03811-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sadhu S., Decker C., Sansbury B.E., Marinello M., Seyfried A., Howard J., et al. Radiation-induced macrophage senescence impairs resolution programs and drives cardiovascular inflammation. J Immunol. 2021;207:1812–1823. doi: 10.4049/jimmunol.2100284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Young L.V., Morrison W., Campbell C., Moore E.C., Arsenault M.G., Dial A.G., et al. Loss of dystrophin expression in skeletal muscle is associated with senescence of macrophages and endothelial cells. Am J Physiol Cell Physiol. 2021;321:C94–C103. doi: 10.1152/ajpcell.00397.2020. [DOI] [PubMed] [Google Scholar]
- 48.Li H., Luo Y.F., Wang Y.S., Yang Q., Xiao Y.L., Cai H.R., et al. Using ROS as a second messenger, NADPH oxidase 2 mediates macrophage senescence via interaction with NF-κB during Pseudomonas aeruginosa infection. Oxid Med Cell Longev. 2018;2018 doi: 10.1155/2018/9741838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang Q., Nie L., Zhao P., Zhou X., Ding Y., Chen Q., et al. Diabetes fuels periodontal lesions via GLUT1-driven macrophage inflammaging. Int J Oral Sci. 2021;13:11. doi: 10.1038/s41368-021-00116-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall B.M., Balan V., Gleiberman A.S., Strom E., Krasnov P., Virtuoso L.P., et al. p16(Ink4a) and senescence-associated β-galactosidase can be induced in macrophages as part of a reversible response to physiological stimuli. Aging (Albany NY) 2017;9:1867–1884. doi: 10.18632/aging.101268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Daigneault M., Preston J.A., Marriott H.M., Whyte M.K.B., Dockrell D.H. The identification of markers of macrophage differentiation in PMA-stimulated THP-1 cells and monocyte-derived macrophages. PLoS One. 2010;5 doi: 10.1371/journal.pone.0008668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hipolito V.E.B., Diaz J.A., Tandoc K.V., Oertlin C., Ristau J., Chauhan N., et al. Enhanced translation expands the endo-lysosome size and promotes antigen presentation during phagocyte activation. PLoS Biol. 2019;17 doi: 10.1371/journal.pbio.3000535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dollt C., Becker K., Michel J., Melchers S., Weis C.A., Schledzewski K., et al. The shedded ectodomain of Lyve-1 expressed on M2-like tumor-associated macrophages inhibits melanoma cell proliferation. Oncotarget. 2017;8:103682–103692. doi: 10.18632/oncotarget.21771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Carvalho A.M., Reis R.L., Pashkuleva I. Hyaluronan receptors as mediators and modulators of the tumor microenvironment. Adv Healthcare Mater. 2023;12 doi: 10.1002/adhm.202202118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Krasniewski L.K., Chakraborty P., Cui C.Y., Mazan-Mamczarz K., Dunn C., Piao Y., et al. Single-cell analysis of skeletal muscle macrophages reveals age-associated functional subpopulations. Elife. 2022;11 doi: 10.7554/eLife.77974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wada I., Nakao S., Yamaguchi M., Kaizu Y., Arima M., Sawa S., et al. Retinal VEGF-A overexpression is not sufficient to induce lymphangiogenesis regardless of VEGF-C upregulation and Lyve1+ macrophage infiltration. Invest Ophthalmol Vis Sci. 2021;62:17. doi: 10.1167/iovs.62.13.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Takeda T., Matsushita T., Kurozumi M., Takemura K., Higuchi K., Hosokawa M. Pathobiology of the senescence-accelerated mouse (SAM) Exp Gerontol. 1997;32:117–127. doi: 10.1016/s0531-5565(96)00068-x. [DOI] [PubMed] [Google Scholar]
- 58.Piao L., Huang Z., Inoue A., Kuzuya M., Cheng X.W. Human umbilical cord-derived mesenchymal stromal cells ameliorate aging-associated skeletal muscle atrophy and dysfunction by modulating apoptosis and mitochondrial damage in SAMP10 mice. Stem Cell Res Ther. 2022;13:226. doi: 10.1186/s13287-022-02895-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jia J., Han Q., Borregaard N., Lollike K., Cygler M. Crystal structure of human grancalcin, a member of the penta-EF-hand protein family. J Mol Biol. 2000;300:1271–1281. doi: 10.1006/jmbi.2000.3925. [DOI] [PubMed] [Google Scholar]
- 60.Li C.J., Xiao Y., Sun Y.C., He W.Z., Liu L., Huang M., et al. Senescent immune cells release grancalcin to promote skeletal aging. Cell Metabol. 2021;33:1957–1973.e6. doi: 10.1016/j.cmet.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 61.Tedder T.F., Tuscano J., Sato S., Kehrl J.H. CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annu Rev Immunol. 1997;15:481–504. doi: 10.1146/annurev.immunol.15.1.481. [DOI] [PubMed] [Google Scholar]
- 62.Fix D.K., Ekiz H.A., Petrocelli J.J., McKenzie A.M., Mahmassani Z.S., O'Connell R.M., et al. Disrupted macrophage metabolic reprogramming in aged soleus muscle during early recovery following disuse atrophy. Aging Cell. 2021;20 doi: 10.1111/acel.13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Beek A.A., Van den Bossche J., Mastroberardino P.G., de Winther M.P.J., Leenen P.J.M. Metabolic alterations in aging macrophages: ingredients for inflammaging?. Trends Immunol. 2019;40:113–127. doi: 10.1016/j.it.2018.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Pearce E.L., Pearce E.J. Metabolic pathways in immune cell activation and quiescence. Immunity. 2013;38:633–643. doi: 10.1016/j.immuni.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Minhas P.S., Liu L., Moon P.K., Joshi A.U., Dove C., Mhatre S., et al. Macrophage de novo NAD+ synthesis specifies immune function in aging and inflammation. Nat Immunol. 2019;20:50–63. doi: 10.1038/s41590-018-0255-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yarbro J.R., Emmons R.S., Pence B.D. Macrophage immunometabolism and inflammaging: roles of mitochondrial dysfunction, cellular senescence, CD38, and NAD. Immunometabolism. 2020;2 doi: 10.20900/immunometab20200026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Plataki M., Cho S.J., Harris R.M., Huang H.R., Yun H.S., Schiffer K.T., et al. Mitochondrial dysfunction in aged macrophages and lung during primary Streptococcus pneumoniae infection is improved with pirfenidone. Sci Rep. 2019;9:971. doi: 10.1038/s41598-018-37438-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Guimarães G.R., Almeida P.P., de Oliveira Santos L., Rodrigues L.P., de Carvalho J.L., Boroni M. Hallmarks of aging in macrophages: consequences to skin inflammaging. Cells. 2021;10:1323. doi: 10.3390/cells10061323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Linehan E., Dombrowski Y., Snoddy R., Fallon P.G., Kissenpfennig A., Fitzgerald D.C. Aging impairs peritoneal but not bone marrow-derived macrophage phagocytosis. Aging Cell. 2014;13:699–708. doi: 10.1111/acel.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ma Y., Chiao Y.A., Clark R., Flynn E.R., Yabluchanskiy A., Ghasemi O., et al. Deriving a cardiac ageing signature to reveal MMP-9-dependent inflammatory signalling in senescence. Cardiovasc Res. 2015;106:421–431. doi: 10.1093/cvr/cvv128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Geng F., Xu M., Zhao L., Zhang H., Li J., Jin F., et al. Quercetin alleviates pulmonary fibrosis in mice exposed to silica by inhibiting macrophage senescence. Front Pharmacol. 2022;13 doi: 10.3389/fphar.2022.912029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu D., Meydani S.N. Mechanism of age-associated up-regulation in macrophage PGE2 synthesis. Brain Behav Immun. 2004;18:487–494. doi: 10.1016/j.bbi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 73.Hayek M.G., Mura C., Wu D., Beharka A.A., Han S.N., Paulson K.E., et al. Enhanced expression of inducible cyclooxygenase with age in murine macrophages. J Immunol. 1997;159:2445–2451. [PubMed] [Google Scholar]
- 74.Wu D., Marko M., Claycombe K., Paulson K.E., Meydani S.N. Ceramide-induced and age-associated increase in macrophage COX-2 expression is mediated through up-regulation of NF-κB activity. J Biol Chem. 2003;278:10983–10992. doi: 10.1074/jbc.M207470200. [DOI] [PubMed] [Google Scholar]
- 75.Han S.N., Wu D., Ha W.K., Beharka A., Smith D.E., Bender B.S., et al. Vitamin E supplementation increases T helper 1 cytokine production in old mice infected with influenza virus. Immunology. 2000;100:487–493. doi: 10.1046/j.1365-2567.2000.00070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Beharka A.A., Wu D., Serafini M., Meydani S.N. Mechanism of vitamin E inhibition of cyclooxygenase activity in macrophages from old mice: role of peroxynitrite. Free Radic Biol Med. 2002;32:503–511. doi: 10.1016/s0891-5849(01)00817-6. [DOI] [PubMed] [Google Scholar]
- 77.Ho A.T.V., Palla A.R., Blake M.R., Yucel N.D., Wang Y.X., Magnusson K.E.G., et al. Prostaglandin E2 is essential for efficacious skeletal muscle stem-cell function, augmenting regeneration and strength. Proc Natl Acad Sci U S A. 2017;114:6675–6684. doi: 10.1073/pnas.1705420114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Palla A.R., Ravichandran M., Wang Y.X., Alexandrova L., Yang A.V., Kraft P., et al. Inhibition of prostaglandin-degrading enzyme 15-PGDH rejuvenates aged muscle mass and strength. Science. 2021;371 doi: 10.1126/science.abc8059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stranks A.J., Hansen A.L., Panse I., Mortensen M., Ferguson D.J., Puleston D.J., et al. Autophagy controls acquisition of aging features in macrophages. J Innate Immun. 2015;7:375–391. doi: 10.1159/000370112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubinsztein D.C., Mariño G., Kroemer G. Autophagy and aging. Cell. 2011;146:682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 81.Liu R., Cui J., Sun Y., Xu W., Wang Z., Wu M., et al. Autophagy deficiency promotes M1 macrophage polarization to exacerbate acute liver injury via ATG5 repression during aging. Cell Death Dis. 2021;7:397. doi: 10.1038/s41420-021-00797-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Butcher M.J., Galkina E.V. Phenotypic and functional heterogeneity of macrophages and dendritic cell subsets in the healthy and atherosclerosis-prone aorta. Front Physiol. 2012;3:44. doi: 10.3389/fphys.2012.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mantovani A., Sica A., Sozzani S., Allavena P., Vecchi A., Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004;25:677–686. doi: 10.1016/j.it.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 84.Mills C.D., Kincaid K., Alt J.M., Heilman M.J., Hill A.M. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.164.12.6166. [DOI] [PubMed] [Google Scholar]
- 85.Cui C.Y., Driscoll R.K., Piao Y., Chia C.W., Gorospe M., Ferrucci L. Skewed macrophage polarization in aging skeletal muscle. Aging Cell. 2019;18 doi: 10.1111/acel.13032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gomez C.R., Hirano S., Cutro B.T., Birjandi S., Baila H., Nomellini V., et al. Advanced age exacerbates the pulmonary inflammatory response after lipopolysaccharide exposure. Crit Care Med. 2007;35:246–251. doi: 10.1097/01.CCM.0000251639.05135.E0. [DOI] [PubMed] [Google Scholar]
- 87.Jackaman C., Dye D.E., Nelson D.J. IL-2/CD40-activated macrophages rescue age and tumor-induced T cell dysfunction in elderly mice. Age (Dordr) 2014;36:9655. doi: 10.1007/s11357-014-9655-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y., Wehling-Henricks M., Samengo G., Tidball J.G. Increases of M2a macrophages and fibrosis in aging muscle are influenced by bone marrow aging and negatively regulated by muscle-derived nitric oxide. Aging Cell. 2015;14:678–688. doi: 10.1111/acel.12350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fontana L., Zhao E., Amir M., Dong H., Tanaka K., Czaja M.J. Aging promotes the development of diet-induced murine steatohepatitis but not steatosis. Hepatology. 2013;57:995–1004. doi: 10.1002/hep.26099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lumeng C.N., Liu J., Geletka L., Delaney C., Delproposto J., Desai A., et al. Aging is associated with an increase in T cells and inflammatory macrophages in visceral adipose tissue. J Immunol. 2011;187:6208–6216. doi: 10.4049/jimmunol.1102188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Blacher E., Tsai C., Litichevskiy L., Shipony Z., Iweka C.A., Schneider K.M., et al. Aging disrupts circadian gene regulation and function in macrophages. Nat Immunol. 2022;23:229–236. doi: 10.1038/s41590-021-01083-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Inomata M., Xu S., Chandra P., Meydani S.N., Takemura G., Philips J.A., et al. Macrophage LC3-associated phagocytosis is an immune defense against Streptococcus pneumoniae that diminishes with host aging. Proc Natl Acad Sci U S A. 2020;117:33561–33569. doi: 10.1073/pnas.2015368117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Palacio L., Goyer M.L., Maggiorani D., Espinosa A., Villeneuve N., Bourbonnais S., et al. Restored immune cell functions upon clearance of senescence in the irradiated splenic environment. Aging Cell. 2019;18 doi: 10.1111/acel.12971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gon Y., Hashimoto S., Hayashi S., Koura T., Matsumoto K., Horie T. Lower serum concentrations of cytokines in elderly patients with pneumonia and the impaired production of cytokines by peripheral blood monocytes in the elderly. Clin Exp Immunol. 1996;106:120–126. [PubMed] [Google Scholar]
- 95.van Duin D., Allore H.G., Mohanty S., Ginter S., Newman F.K., Belshe R.B., et al. Prevaccine determination of the expression of costimulatory B7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J Infect Dis. 2007;195:1590–1597. doi: 10.1086/516788. [DOI] [PubMed] [Google Scholar]
- 96.Barman P.K., Shin J.E., Lewis S.A., Kang S., Wu D., Wang Y., et al. Production of MHCII-expressing classical monocytes increases during aging in mice and humans. Aging Cell. 2022;21 doi: 10.1111/acel.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liang S., Domon H., Hosur K.B., Wang M., Hajishengallis G. Age-related alterations in innate immune receptor expression and ability of macrophages to respond to pathogen challenge in vitro. Mech Ageing Dev. 2009;130:538–546. doi: 10.1016/j.mad.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Renshaw M., Rockwell J., Engleman C., Gewirtz A., Katz J., Sambhara S. Cutting edge: impaired Toll-like receptor expression and function in aging. J Immunol. 2002;169:4697–4701. doi: 10.4049/jimmunol.169.9.4697. [DOI] [PubMed] [Google Scholar]
- 99.Rahman F.A., Angus S.A., Stokes K., Karpowicz P., Krause M.P. Impaired ECM remodeling and macrophage activity define necrosis and regeneration following damage in aged skeletal muscle. Int J Mol Sci. 2020;21:4575. doi: 10.3390/ijms21134575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ahmadi M., Karlsen A., Mehling J., Soendenbroe C., Mackey A.L., Hyldahl R.D. Aging is associated with an altered macrophage response during human skeletal muscle regeneration. Exp Gerontol. 2022;169 doi: 10.1016/j.exger.2022.111974. [DOI] [PubMed] [Google Scholar]
- 101.Hachim D., Iftikhar A., LoPresti S.T., Nolfi A.L., Ravichandar S., Skillen C.D., et al. Distinct release strategies are required to modulate macrophage phenotype in young versus aged animals. J Control Release. 2019;305:65–74. doi: 10.1016/j.jconrel.2019.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shang M., Cappellesso F., Amorim R., Serneels J., Virga F., Eelen G., et al. Macrophage-derived glutamine boosts satellite cells and muscle regeneration. Nature. 2020;587:626–631. doi: 10.1038/s41586-020-2857-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang G., Liu J. Targeting senescent immune cells to rejuvenate the aging skeleton. Cell Metabol. 2021;33:1903–1905. doi: 10.1016/j.cmet.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 104.Ambrosi T.H., Scialdone A., Graja A., Gohlke S., Jank A.M., Bocian C., et al. Adipocyte accumulation in the bone marrow during obesity and aging impairs stem cell-based hematopoietic and bone regeneration. Cell Stem Cell. 2017;20:771–784.e6. doi: 10.1016/j.stem.2017.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Shim K.S., Hwang Y.H., Jang S.A., Kim T., Ha H. Water extract of Lysimachia christinae inhibits trabecular bone loss and fat accumulation in ovariectomized mice. Nutrients. 2020;12:1927. doi: 10.3390/nu12071927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boer C.G., Radjabzadeh D., Medina-Gomez C., Garmaeva S., Schiphof D., Arp P., et al. Intestinal microbiome composition and its relation to joint pain and inflammation. Nat Commun. 2019;10:4881. doi: 10.1038/s41467-019-12873-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rowe M.A., Harper L.R., McNulty M.A., Lau A.G., Carlson C.S., Leng L., et al. Reduced osteoarthritis severity in aged mice with deletion of macrophage migration inhibitory factor. Arthritis Rheumatol. 2017;69:352–361. doi: 10.1002/art.39844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Farr J.N., Xu M., Weivoda M.M., Monroe D.G., Fraser D.G., Onken J.L., et al. Targeting cellular senescence prevents age-related bone loss in mice. Nat Med. 2017;23:1072–1079. doi: 10.1038/nm.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sene A., Khan Aslam A., Cox D., Nakamura Rei E.I., Santeford A., Kim Bryan M., et al. Impaired cholesterol efflux in senescent macrophages promotes age-related macular degeneration. Cell Metabol. 2013;17:549–561. doi: 10.1016/j.cmet.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Baker D.J., Wijshake T., Tchkonia T., LeBrasseur N.K., Childs B.G., van de Sluis B., et al. Clearance of p16Ink4a-positive senescent cells delays ageing-associated disorders. Nature. 2011;479:232–236. doi: 10.1038/nature10600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lee J.S., Mo Y., Gan H., Burgess R.J., Baker D.J., van Deursen J.M., et al. Pak2 kinase promotes cellular senescence and organismal aging. Proc Natl Acad Sci U S A. 2019;116:13311–13319. doi: 10.1073/pnas.1903847116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Xiao Y., Peng X., Peng Y., Zhang C., Liu W., Yang W., et al. Macrophage-derived extracellular vesicles regulate follicular activation and improve ovarian function in old mice by modulating local environment. Clin Transl Med. 2022;12 doi: 10.1002/ctm2.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ma L., Lu H., Chen R., Wu M., Jin Y., Zhang J., et al. Identification of key genes and potential new biomarkers for ovarian aging: a study based on rna-sequencing data. Front Genet. 2020;11 doi: 10.3389/fgene.2020.590660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Umehara T., Winstanley Y.E., Andreas E., Morimoto A., Williams E.J., Smith K.M., et al. Female reproductive life span is extended by targeted removal of fibrotic collagen from the mouse ovary. Sci Adv. 2022;8:eabn4564. doi: 10.1126/sciadv.abn4564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gabanyi I., Muller P.A., Feighery L., Oliveira T.Y., Costa-Pinto F.A., Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell. 2016;164:378–391. doi: 10.1016/j.cell.2015.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Muller P.A., Koscsó B., Rajani G.M., Stevanovic K., Berres M.L., Hashimoto D., et al. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell. 2014;158:300–313. doi: 10.1016/j.cell.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cipriani G., Gibbons S.J., Verhulst P.J., Choi K.M., Eisenman S.T., Hein S.S., et al. Diabetic Csf1op/op mice lacking macrophages are protected against the development of delayed gastric emptying. Cell Mol Gastroenterol Hepatol. 2016;2:40–47. doi: 10.1016/j.jcmgh.2015.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Matteoli G., Gomez-Pinilla P.J., Nemethova A., Di Giovangiulio M., Cailotto C., van Bree S.H., et al. A distinct vagal anti-inflammatory pathway modulates intestinal muscularis resident macrophages independent of the spleen. Gut. 2014;63:938–948. doi: 10.1136/gutjnl-2013-304676. [DOI] [PubMed] [Google Scholar]
- 119.Wehner S., Behrendt F.F., Lyutenski B.N., Lysson M., Bauer A.J., Hirner A., et al. Inhibition of macrophage function prevents intestinal inflammation and postoperative ileus in rodents. Gut. 2007;56:176–185. doi: 10.1136/gut.2005.089615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Becker L., Nguyen L., Gill J., Kulkarni S., Pasricha P.J., Habtezion A. Age-dependent shift in macrophage polarisation causes inflammation-mediated degeneration of enteric nervous system. Gut. 2018;67:827–836. doi: 10.1136/gutjnl-2016-312940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yuan X., Klein D., Kerscher S., West B.L., Weis J., Katona I., et al. Macrophage depletion ameliorates peripheral neuropathy in aging mice. J Neurosci. 2018;38:4610–4620. doi: 10.1523/JNEUROSCI.3030-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ryan C.B., Choi J.S., Al-Ali H., Lee J.K. Myelin and non-myelin debris contribute to foamy macrophage formation after spinal cord injury. Neurobiol Dis. 2022;163 doi: 10.1016/j.nbd.2021.105608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Poliani P.L., Wang Y., Fontana E., Robinette M.L., Yamanishi Y., Gilfillan S., et al. TREM2 sustains microglial expansion during aging and response to demyelination. J Clin Invest. 2015;125:2161–2170. doi: 10.1172/JCI77983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hefendehl J.K., Neher J.J., Sühs R.B., Kohsaka S., Skodras A., Jucker M. Homeostatic and injury-induced microglia behavior in the aging brain. Aging Cell. 2014;13:60–69. doi: 10.1111/acel.12149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miron V.E., Boyd A., Zhao J.W., Yuen T.J., Ruckh J.M., Shadrach J.L., et al. M2 microglia and macrophages drive oligodendrocyte differentiation during CNS remyelination. Nat Neurosci. 2013;16:1211–1218. doi: 10.1038/nn.3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li C.M., Shapiro H., Tsiobikas C., Selfors L.M., Chen H., Rosenbluth J., et al. Aging-associated alterations in mammary epithelia and stroma revealed by single-cell RNA sequencing. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.He X., Fan X., Bai B., Lu N., Zhang S., Zhang L. Pyroptosis is a critical immune-inflammatory response involved in atherosclerosis. Pharmacol Res. 2021;165 doi: 10.1016/j.phrs.2021.105447. [DOI] [PubMed] [Google Scholar]
- 128.Ning Y., Zhang M., Du Y.H., Zhang H.N., Li L.Y., Qin Y.W., et al. Effects of thyroid hormone on macrophage dysfunction induced by oxidized low-density lipoprotein. Acta Physiol Sin. 2018;70:141–148. [PubMed] [Google Scholar]
- 129.Childs B.G., Baker D.J., Wijshake T., Conover C.A., Campisi J., van Deursen J.M. Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Science. 2016;354:472–477. doi: 10.1126/science.aaf6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Han X., Sun S., Sun Y., Song Q., Zhu J., Song N., et al. Small molecule-driven NLRP3 inflammation inhibition via interplay between ubiquitination and autophagy: implications for Parkinson disease. Autophagy. 2019;15:1860–1881. doi: 10.1080/15548627.2019.1596481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kotter M.R., Li W.W., Zhao C., Franklin R.J. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26:328–332. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Rawji K.S., Young A.M.H., Ghosh T., Michaels N.J., Mirzaei R., Kappen J., et al. Niacin-mediated rejuvenation of macrophage/microglia enhances remyelination of the aging central nervous system. Acta Neuropathol. 2020;139:893–909. doi: 10.1007/s00401-020-02129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Griciuc A., Serrano-Pozo A., Parrado A.R., Lesinski A.N., Asselin C.N., Mullin K., et al. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bowman G.L., Dayon L., Kirkland R., Wojcik J., Peyratout G., Severin I.C., et al. Blood–brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement. 2018;14:1640–1650. doi: 10.1016/j.jalz.2018.06.2857. [DOI] [PubMed] [Google Scholar]
- 135.Ruckh J.M., Zhao J.W., Shadrach J.L., van Wijngaarden P., Rao T.N., Wagers A.J., et al. Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Podrez E.A., Poliakov E., Shen Z., Zhang R., Deng Y., Sun M., et al. A novel family of atherogenic oxidized phospholipids promotes macrophage foam cell formation via the scavenger receptor CD36 and is enriched in atherosclerotic lesions. J Biol Chem. 2002;277:38517–38523. doi: 10.1074/jbc.M205924200. [DOI] [PubMed] [Google Scholar]
- 137.Xu D., Tian Y., Xia Q., Ke B. The cGAS–STING pathway: novel perspectives in liver diseases. Front Immunol. 2021;12 doi: 10.3389/fimmu.2021.682736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhong W., Rao Z., Xu J., Sun Y., Hu H., Wang P., et al. Defective mitophagy in aged macrophages promotes mitochondrial DNA cytosolic leakage to activate STING signaling during liver sterile inflammation. Aging Cell. 2022;21 doi: 10.1111/acel.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhong W., Rao Z., Rao J., Han G., Wang P., Jiang T., et al. Aging aggravated liver ischemia and reperfusion injury by promoting STING-mediated NLRP3 activation in macrophages. Aging Cell. 2020;19 doi: 10.1111/acel.13186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lopez-Yrigoyen M., Cassetta L., Pollard J.W. Macrophage targeting in cancer. Ann N Y Acad Sci. 2021;1499:18–41. doi: 10.1111/nyas.14377. [DOI] [PubMed] [Google Scholar]
- 141.Mantovani A., Allavena P., Marchesi F., Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21:799–820. doi: 10.1038/s41573-022-00520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Anderson N.R., Minutolo N.G., Gill S., Klichinsky M. Macrophage-based approaches for cancer immunotherapy. Cancer Res. 2021;81:1201–1208. doi: 10.1158/0008-5472.CAN-20-2990. [DOI] [PubMed] [Google Scholar]
- 143.Zhang L., Tian L., Dai X., Yu H., Wang J., Lei A., et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J Hematol Oncol. 2020;13:153. doi: 10.1186/s13045-020-00983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Magalhães J.P. How ageing processes influence cancer. Nat Rev Cancer. 2013;13:357–365. doi: 10.1038/nrc3497. [DOI] [PubMed] [Google Scholar]
- 145.Li Y., Zhao Y., Gao Y., Li Y., Liu M., Xu N., et al. Age-related macrophage alterations are associated with carcinogenesis of colorectal cancer. Carcinogenesis. 2022;43:1039–1049. doi: 10.1093/carcin/bgac088. [DOI] [PubMed] [Google Scholar]
- 146.Bianchi-Frias D., Damodarasamy M., Hernandez S.A., Gil da Costa R.M., Vakar-Lopez F., Coleman I.M., et al. The aged microenvironment influences the tumorigenic potential of malignant prostate epithelial cells. Mol Cancer Res. 2019;17:321–331. doi: 10.1158/1541-7786.MCR-18-0522. [DOI] [PubMed] [Google Scholar]
- 147.Walters H. Senescent macrophages drive lung cancer and accumulate in aging. Nat Aging. 2023;3:757. doi: 10.1038/s43587-023-00459-1. [DOI] [PubMed] [Google Scholar]
- 148.Chen Y., Hao X., Li M., Tian Z., Cheng M. UGRP1-modulated MARCO+ alveolar macrophages contribute to age-related lung fibrosis. Immun Ageing. 2023;20:14. doi: 10.1186/s12979-023-00338-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bannon P., Wood S., Restivo T., Campbell L., Hardman M.J., Mace K.A. Diabetes induces stable intrinsic changes to myeloid cells that contribute to chronic inflammation during wound healing in mice. Dis Model Mech. 2013;6:1434–1447. doi: 10.1242/dmm.012237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lee S., Szilagyi E., Chen L., Premanand K., DiPietro L.A., Ennis W., et al. Activated mesenchymal stem cells increase wound tensile strength in aged mouse model via macrophages. J Surg Res. 2013;181:20–24. doi: 10.1016/j.jss.2012.05.040. [DOI] [PubMed] [Google Scholar]
- 151.Pajarinen J., Lin T., Gibon E., Kohno Y., Maruyama M., Nathan K., et al. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials. 2019;196:80–89. doi: 10.1016/j.biomaterials.2017.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.He M., Chiang H.H., Luo H., Zheng Z., Qiao Q., Wang L., et al. An acetylation switch of the NLRP3 inflammasome regulates aging-associated chronic inflammation and insulin resistance. Cell Metabol. 2020;31:580–591.e5. doi: 10.1016/j.cmet.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Birch J., Gil J. Senescence and the SASP: many therapeutic avenues. Genes Dev. 2020;34:1565–1576. doi: 10.1101/gad.343129.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wang H., Fu H., Zhu R., Wu X., Ji X., Li X., et al. BRD4 contributes to LPS-induced macrophage senescence and promotes progression of atherosclerosis-associated lipid uptake. Aging (Albany NY) 2020;12:9240–9259. doi: 10.18632/aging.103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Keane L., Antignano I., Riechers S.P., Zollinger R., Dumas A.A., Offermann N., et al. mTOR-dependent translation amplifies microglia priming in aging mice. J Clin Invest. 2021;131 doi: 10.1172/JCI132727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Muñoz-Espín D., Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 157.Hedblom A., Hejazi S.M., Canesin G., Choudhury R., Hanafy K.A., Csizmadia E., et al. Heme detoxification by heme oxygenase-1 reinstates proliferative and immune balances upon genotoxic tissue injury. Cell Death Dis. 2019;10:72. doi: 10.1038/s41419-019-1342-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ferenbach D.A., Nkejabega N.C., McKay J., Choudhary A.K., Vernon M.A., Beesley M.F., et al. The induction of macrophage hemeoxygenase-1 is protective during acute kidney injury in aging mice. Kidney Int. 2011;79:966–976. doi: 10.1038/ki.2010.535. [DOI] [PubMed] [Google Scholar]
- 159.Liu J.Y., Souroullas G.P., Diekman B.O., Krishnamurthy J., Hall B.M., Sorrentino J.A., et al. Cells exhibiting strong p16(INK4a) promoter activation in vivo display features of senescence. Proc Natl Acad Sci U S A. 2019;116:2603–2611. doi: 10.1073/pnas.1818313116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Zhou J., Hou C., Chen H., Qin Z., Miao Z., Zhao J., et al. P16INK4a deletion ameliorates damage of intestinal epithelial barrier and microbial dysbiosis in a stress-induced premature senescence model of Bmi-1 deficiency. Front Cell Dev Biol. 2021;9 doi: 10.3389/fcell.2021.671564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Rayess H., Wang M.B., Srivatsan E.S. Cellular senescence and tumor suppressor gene p16. Int J Cancer. 2012;130:1715–1725. doi: 10.1002/ijc.27316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Freire-de-Lima C.G., Xiao Y.Q., Gardai S.J., Bratton D.L., Schiemann W.P., Henson P.M. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem. 2006;281:38376–38384. doi: 10.1074/jbc.M605146200. [DOI] [PubMed] [Google Scholar]
- 163.Serhan C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510:92–101. doi: 10.1038/nature13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.McDonald B., Kubes P. Innate immune cell trafficking and function during sterile inflammation of the liver. Gastroenterology. 2016;151:1087–1095. doi: 10.1053/j.gastro.2016.09.048. [DOI] [PubMed] [Google Scholar]
- 165.Sharifiaghdam M., Shaabani E., Faridi-Majidi R., De Smedt S.C., Braeckmans K., Fraire J.C. Macrophages as a therapeutic target to promote diabetic wound healing. Mol Ther. 2022;30:2891–2908. doi: 10.1016/j.ymthe.2022.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ahmad F., Leake D.S. Lysosomal oxidation of LDL alters lysosomal pH, induces senescence, and increases secretion of pro-inflammatory cytokines in human macrophages. J Lipid Res. 2019;60:98–110. doi: 10.1194/jlr.M088245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Xiang Q., Tian F., Xu J., Du X., Zhang S., Liu L. New insight into dyslipidemia-induced cellular senescence in atherosclerosis. Biol Rev Camb Phil Soc. 2022;97:1844–1867. doi: 10.1111/brv.12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ni Y., Zhuge F., Nagashimada M., Ota T. Novel action of carotenoids on non-alcoholic fatty liver disease: macrophage polarization and liver homeostasis. Nutrients. 2016;8 8:391. doi: 10.3390/nu8070391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Galván-Peña S., O'Neill L.A. Metabolic reprograming in macrophage polarization. Front Immunol. 2014;5:420. doi: 10.3389/fimmu.2014.00420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yang T., Hu Y., Miao J., Chen J., Liu J., Cheng Y., et al. A BRD4 PROTAC nanodrug for glioma therapy via the intervention of tumor cells proliferation, apoptosis and M2 macrophages polarization. Acta Pharm Sin B. 2022;12:2658–2671. doi: 10.1016/j.apsb.2022.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Moore J.A., Mistry J.J., Hellmich C., Horton R.H., Wojtowicz E.E., Jibril A., et al. LC3-associated phagocytosis in bone marrow macrophages suppresses acute myeloid leukemia progression through STING activation. J Clin Invest. 2022;132 doi: 10.1172/JCI153157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xiong Y., Li Y., Cui X., Zhang L., Yang X., Liu H. ADAP restraint of STAT1 signaling regulates macrophage phagocytosis in immune thrombocytopenia. Cell Mol Immunol. 2022;19:898–912. doi: 10.1038/s41423-022-00881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Muñoz-Espín D., Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol. 2014;15:482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 174.Tobin S.W., Alibhai F.J., Wlodarek L., Yeganeh A., Millar S., Wu J., et al. Delineating the relationship between immune system aging and myogenesis in muscle repair. Aging Cell. 2021;20 doi: 10.1111/acel.13312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lin J.B., Sene A., Santeford A., Fujiwara H., Sidhu R., Ligon M.M., et al. Oxysterol signatures distinguish age-related macular degeneration from physiologic aging. EBioMedicine. 2018;32:9–20. doi: 10.1016/j.ebiom.2018.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Martini H., Iacovoni J.S., Maggiorani D., Dutaur M., Marsal D.J., Roncalli J., et al. Aging induces cardiac mesenchymal stromal cell senescence and promotes endothelial cell fate of the CD90+ subset. Aging Cell. 2019;18 doi: 10.1111/acel.13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Wang D., Gu X., Ma X., Chen J., Zhang Q., Yu Z., et al. Nanopolyphenol rejuvenates microglial surveillance of multiple misfolded proteins through metabolic reprogramming. Acta Pharm Sin B. 2023;13:834–851. doi: 10.1016/j.apsb.2022.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Zhang L., Pitcher L.E., Yousefzadeh M.J., Niedernhofer L.J., Robbins P.D., Zhu Y. Cellular senescence: a key therapeutic target in aging and diseases. J Clin Invest. 2022;132 doi: 10.1172/JCI158450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gasek N.S., Kuchel G.A., Kirkland J.L., Xu M. Strategies for targeting senescent cells in human disease. Nat Aging. 2021;1:870–879. doi: 10.1038/s43587-021-00121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Triana-Martínez F., Picallos-Rabina P., Da Silva-Álvarez S., Pietrocola F., Llanos S., Rodilla V., et al. Identification and characterization of cardiac glycosides as senolytic compounds. Nat Commun. 2019;10:4731. doi: 10.1038/s41467-019-12888-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li W., He Y., Zhang R., Zheng G., Zhou D. The curcumin analog EF24 is a novel senolytic agent. Aging (Albany NY) 2019;11:771–782. doi: 10.18632/aging.101787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Upadhyay A. Natural compounds in the regulation of proteostatic pathways: an invincible artillery against stress, ageing, and diseases. Acta Pharm Sin B. 2021;11:2995–3014. doi: 10.1016/j.apsb.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Chen P., Chen F., Lei J., Li Q., Zhou B. Activation of the miR-34a-mediated SIRT1/mTOR signaling pathway by urolithin A attenuates d-galactose-induced brain aging in mice. Neurotherapeutics. 2019;16:1269–1282. doi: 10.1007/s13311-019-00753-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ryu D., Mouchiroud L., Andreux P.A., Katsyuba E., Moullan N., Nicolet-Dit-Félix A.A., et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat Med. 2016;22:879–888. doi: 10.1038/nm.4132. [DOI] [PubMed] [Google Scholar]
- 185.Yousefzadeh M.J., Flores R.R., Zhu Y., Schmiechen Z.C., Brooks R.W., Trussoni C.E., et al. An aged immune system drives senescence and ageing of solid organs. Nature. 2021;594:100–105. doi: 10.1038/s41586-021-03547-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tang S., Garzon Sanz M., Smith O., Krämer A., Egbase D., Caton P.W., et al. Chemistry-led investigations into the mode of action of NAMPT activators, resulting in the discovery of non-pyridyl class NAMPT activators. Acta Pharm Sin B. 2023;13:709–721. doi: 10.1016/j.apsb.2022.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yao H., Liu M., Wang L., Zu Y., Wu C., Li C., et al. Discovery of small-molecule activators of nicotinamide phosphoribosyltransferase (NAMPT) and their preclinical neuroprotective activity. Cell Res. 2022;32:570–584. doi: 10.1038/s41422-022-00651-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Wang F., Li Z., Chen L., Yang T., Liang B., Zhang Z., et al. Inhibition of ASCT2 induces hepatic stellate cell senescence with modified proinflammatory secretome through an IL-1α/NF-κB feedback pathway to inhibit liver fibrosis. Acta Pharm Sin B. 2022;12:3618–3638. doi: 10.1016/j.apsb.2022.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]