Abstract
Macrophages, as pivotal cells within the tumour microenvironment, significantly influence the impact of and reactions to treatments for solid tumours. The rapid evolution of bioengineering technology has revealed the vast potential of engineered macrophages in immunotherapy, disease diagnosis, and tissue engineering. Given this landscape, the goal of harnessing and innovating macrophages as a novel strategy for solid tumour immunotherapy cannot be overstated. The diverse strategies for engineered macrophages in the realm of cancer immunotherapy, encompassing macrophage drug delivery systems, chimeric antigen receptor macrophage therapy, and synergistic treatment approaches involving bacterial outer membrane vesicles and macrophages, are meticulously examined in this review. These methodologies are designed to enhance the therapeutic efficacy of macrophages against solid tumours, particularly those that are drug-resistant and metastatic. Collectively, these immunotherapies are poised to supplement and refine current solid tumour treatment paradigms, thus heralding a new frontier in the fight against malignant tumours.
Subject terms: Immunotherapy, Cancer therapy
Facts
The paradigm of cancer treatment has transitioned from a focus on the cancer itself to a treatment model centred on the tumour microenvironment.
Engineered macrophages can serve as carriers for anticancer or nanoparticle drugs.
CAR-Ms have broad application prospects in the context of immunotherapy for solid tumours.
A novel approach to cancer immunotherapy involves utilising tumour vaccines derived from bacterial outer membrane vesicles.
Open questions
What are the intrinsic mechanisms by which macrophages act as drug-delivery vehicles? What is their therapeutic efficacy?
CAR-M-cell therapy may be the main therapeutic approach for solid tumours in the future. Is this therapy feasible in clinical trials?
Can the interaction between OMVs and macrophages enhance their applicability and diversity in the design of tumour vaccines?
As a vital element within the solid tumour microenvironment, can macrophages serve as the focal point of interdisciplinary collaboration in the future for the advancement of related therapies?
Introduction
Cancer is a genomic disease in which a considerable number of somatic point mutations accumulate, leading to structural changes during its development and resulting in genomic instability [1, 2]. Cancer tissues, which are mainly composed of a substantial quantity of both neoplastic and nonneoplastic cells, modify the extracellular matrix and thus generate a unique tumour microenvironment (TME) [3, 4]. The TME is defined as an intricate and dense multicellular environment for tumorigenesis that consists of a substantial amount of different cells and cellular components, including multiple types of immune cells, endothelial cells, tumour-associated macrophages (TAMs), and a variety of other tissue-resident cell types [3, 5–7]. These cells act synergistically in tumour progression, invasion, metastasis, and response to immunotherapies [8]. Consequently, cancer treatment has transitioned from a model centred solely on the cancer itself to one focused on the tumour microenvironment, and cancer immunotherapy has consequently come onto the stage to revolutionise cancer treatment. However, its efficacy is still limited in most clinical settings [9, 10]. In recent years, cancer immunotherapy has been pursued through a myriad of approaches, including molecular targeted therapy, immune checkpoint inhibitors (such as PD-1/L1 and CTLA-4 inhibitors), adoptive cell immunotherapy (such as TIL, NK, CAR-T, CIK/DC-CIK), cytokine therapy, and tumour vaccines [2, 11–13].
Macrophages, as heterogeneous and multifunctional immune cells, play crucial roles in various biological processes, such as maintaining tissue homoeostasis, regulating cancer progression, and defending against pathogens. Their phenotype and functionality are intricately governed by the ambient microenvironment, and macrophages can demonstrate dual antitumour and tumour-promoting effects within the context of cancer [14, 15]. Polarised macrophages can be classified into two distinct subtypes, M1 macrophages and M2 macrophages, both of which are closely associated with tumour immunity [16]. Classically activated M1 macrophages are primarily involved in proinflammatory responses. Their activation is driven by factors such as lipopolysaccharide (LPS), interferon-γ (IFN-γ), granulocyte-macrophage colony-stimulating factor (GM-CSF), or other pathogen-associated molecular patterns [17–19]. Upon activation, proinflammatory factors, including IL-6, IL-12 and tumour necrosis factor (TNF), are produced. They possess the ability to identify and engulf tumour cells, thereby impeding tumour growth and metastasis. Furthermore, they can present tumour antigens to T cells, thus triggering specific immune responses and exerting antitumour effects [20–23]. M2 macrophages are macrophages that undergo alternative activation and primarily engage in anti-inflammatory responses; the activation of M2 macrophages is driven by macrophage colony-stimulating factor (M-CSF), IL-4, IL-10, IL-13, transforming growth factor-β (TGF-β), and glucocorticoids [16]. After polarization, cells can release anti-inflammatory cytokines (including IL-10, IL-13, IL-4, Arg-1, and CD206), thereby contributing to host defence, wound healing and tissue remodelling [24]. M2 macrophages can also promote tumour progression through various biological mechanisms, including the secretion of immunosuppressive molecules such as IL-10 and TGF-β, which hinder the function of other immune cells. Additionally, these cells secrete vascular endothelial growth factor (VEGF), which promotes tumour angiogenesis [25, 26].
TAMs are an important component of the TME, and they make up more than 50% of aggregate tumour cells [27]. The TME of solid tumours can recruit myeloid cells and lead to the extensive infiltration of immunosuppressive macrophages [28]. Macrophages in the TME are mainly derived from bone marrow-derived monocytes (BMDMs), which are recruited by tumour- or mechanism-derived chemokines [28–30]. Both M1 and M2 TAMs are present throughout all phases of tumour progression, with M1 macrophages prevailing during the initial stage and M2 macrophages dominating during the intermediate and advanced stages [31]. With tumour progression, M1 macrophages gradually polarise to M2 macrophages, and an increase in the quantity of M2 TAMs indicates a poor prognosis. Moreover, M2 macrophages promote tumour angiogenesis, leading to tumour progression; this role is the opposite of the role of M1 macrophages [32]. Therefore, decreasing the presence of M2 TAMs in the TME or fostering the conversion of M2 macrophages to M1 macrophages plays a significant role in the treatment of tumours [33].
Current research on macrophages in cancer immunotherapy is centred on unravelling their intricate functions within the tumour microenvironment and investigating strategies for translating this knowledge into clinical applications. A number of researchers have reported that engineered immune cells, which are an emerging form of immunotherapy, are immune cells that are engineered to recognise and respond to disease [34]. When these immune cells are introduced into patients, they can serve as a “living drug” that inhibits the growth of tumour cells [35]. Engineered macrophages, which are a subtype of modified immune cells, originate from macrophages and are primarily utilised for drug delivery, tissue repair, and antitumor applications through genetic engineering techniques [36–38]. Considering the abundance of macrophages in the TME, altering macrophages for solid tumour therapy has great potential. The techniques used to engineer macrophages and their immunotherapeutic impacts on the TME are the focus of this study.
Common techniques for engineering macrophages
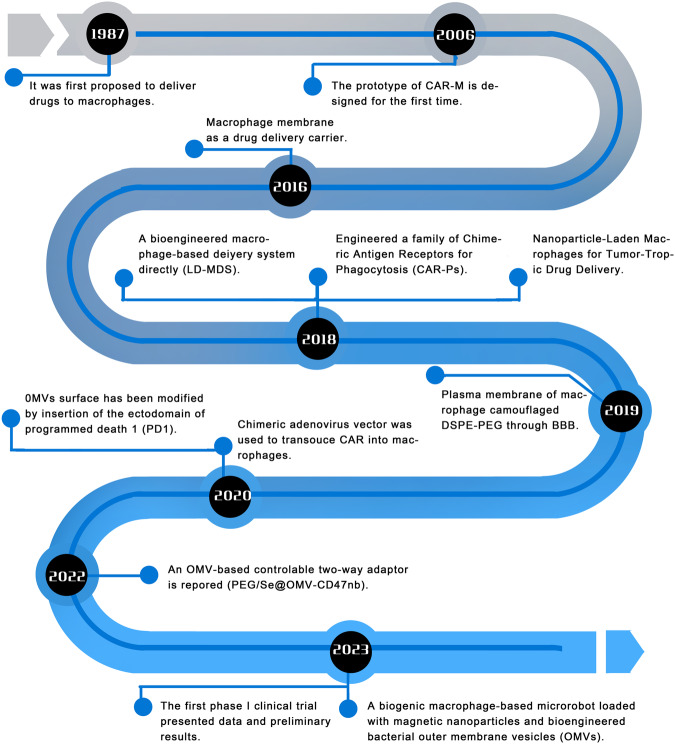
To date, there are three main methods for transforming macrophages into engineered macrophages. The process of developing engineered macrophages is shown in Fig. 1. First, macrophages were engineered for advanced drug delivery systems. However, the therapeutic effect is not optimal for solid tumours, mainly because the tumour has an immune barrier that reduces drug accessibility, which alters the therapeutic effect [39]. Macrophages, as an important part of the TME, possess the functions of natural immune cells and antigen-presenting cells and boast an extended half-life in blood; macrophages are essentially able to phagocytose foreign particles and specifically bind to tumour tissues [27, 40]. Therefore, macrophages can act as drug carriers and deliver drugs to tumour tissues. The first serious discussions and analyses of engineered macrophages for drug delivery emerged during the 1980s when micro particulates were proposed to function as a convenient platform for the targeted delivery of encapsulated drugs to cells within the mononuclear phagocyte series in vivo [41]. As research has progressed, macrophages were found to serve not only as direct or indirect carriers for drug delivery but also as encapsulate nanoparticles to facilitate drug delivery. Through these advancements, engineered macrophages have arisen as promising platforms for precision-targeted tumour therapy that are capable of overcoming immune barriers and drug delivery challenges encountered with traditional treatments [42, 43].
Fig. 1.
The evolution of engineered macrophages.
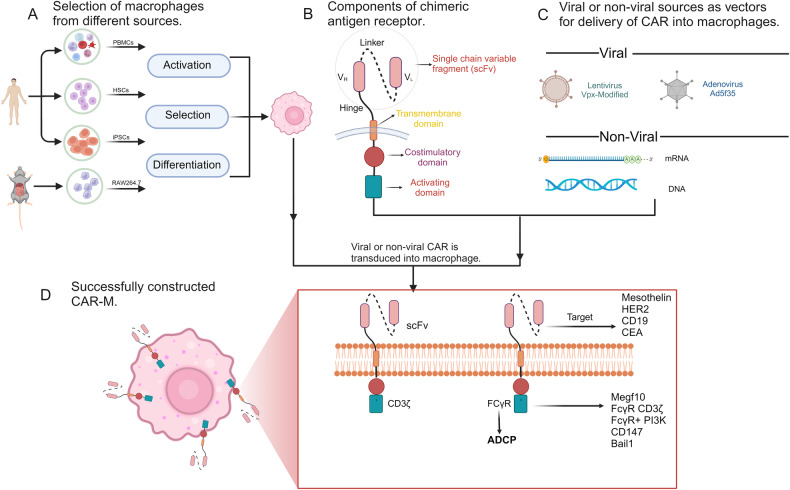
Chimeric antigen receptor-modified macrophages (CAR-Ms) have also been engineered. There has been tremendous progress in adoptive cell immunotherapy using CAR-T cells [44]. A challenging problem is the TME barrier formed by the extracellular matrix (ECM), which prevents CAR-T cells from infiltrating and persisting within the tumour immune microenvironment (TIME) [45–48]. A solution to this problem is the application of CAR-M therapy. The architecture of CARs in CAR-M therapy encompasses extracellular signalling domains that are designed to identify particular tumour antigens, hinge regions, transmembrane structural domains, and intracellular signalling structural domains (Fig. 2). Currently, scFv has been studied for use against common tumour targets, such as CD19 and HER2 [49–51]. Although the CAR-M intracellular domain does not contain immunoreceptor tyrosine-based activation motifs (ITAMs), it has CD3ζ chain-related functions [52, 53]. Macrophages can express the SH2 domain-containing kinase Syk, which binds to CD3ζ and transduces phagocytosis. Using chimeric antigen receptor technology, engineered macrophages (CAR-Ms) offer a promising approach for targeted therapy against specific tumour antigens. This innovative strategy provides not only a more precise and efficient treatment option for cancer patients but also the ability to surmount the constraints that have hindered the use of conventional CAR-T-cell therapy [54, 55].
Fig. 2. The structure of CAR-Ms.
A Macrophages from different sources, such as BMDMs, monocytes derived from human peripheral blood mononuclear cells (PBMCs), and mouse-derived Raw264.7 cells, were selected. B CARs were composed of an intracellular domain, an extracellular domain and a transmembrane domain. C CARs were successfully transfected into viral or nonviral vectors of macrophages. D CAR-Ms were successfully constructed, and the diversity of the extracellular antigen and intracellular domain was determined. (created with biorender.com).
The third is the application of bacterial outer membrane vesicles (OMVs) in engineered macrophages. OMVs, which were first thought to be the product of the disordered growth of gram-negative bacteria, are naturally occurring nanoparticles that are secreted by gram-negative bacteria with a particle size of 30-100 nm [56]. OMVs consist of antigens that originate from bacteria and various intrinsic pathogen-associated molecular patterns (PAMPs), which are highly immunogenic and, therefore can be used as bacterial vaccines and adjuvants to activate both humoral and cellular immune responses [57–59]. As vaccine carriers, OMV has more reliable safety performance than bacteria. Currently, there are various means for the synthetic modification of OMVs [60]. The first is by constructing different tumour antigen types immobilised on the surface of OMVs, such as Spy C and L7Ae [61, 62]. The second is to carry a PD-L1/CD47 nanoantibody that can be delivered to the TME to promote tumour-associated macrophage remodelling to an antitumor state [63, 64]. The third is to modify OMVs by synthetic methods to help maintain their stability in the human body and artificially control the release of antigens or antibodies, which plays a role in precise treatment [62, 65].
The aforementioned three methods for the engineered modification of macrophages represent the primary research areas in the current landscape. In the subsequent sections, I shall furnish a comprehensive exposition and delve into meticulous discussions regarding the advancement and utilisation of these engineering techniques.
Progress in the application of engineered macrophages in tumour immunotherapy
Over the past two decades, a multitude of scholars have endeavoured to ascertain whether engineered macrophages can play a role in boosting immunotherapy in cancer. Indeed, they demonstrated the role of engineered macrophages in enhancing the efficacy of immunotherapy. There is no doubt that engineered macrophages have broad prospects in future tumour immunotherapy [17, 34, 35, 39]. As a burgeoning form of immune cell therapy, engineered macrophages not only mitigate the immune system’s exposure to drug-related injury but also augment the immune response and fortify the efficacy of immunotherapy.
Engineered macrophages for advanced drug delivery systems
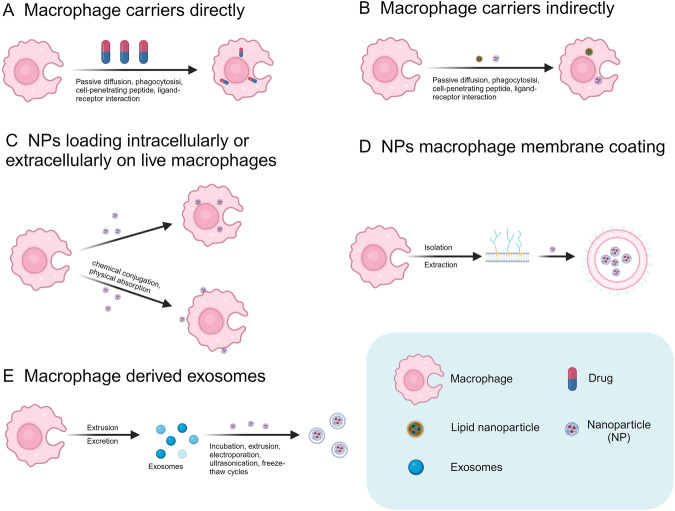
In this segment, three methodologies for employing engineered macrophages in drug delivery shall be elucidated (Fig. 3): 1) the use of engineered macrophages as direct drug carriers; 2) the use of engineered macrophages as indirect drug carriers; and 3) the use of engineered macrophage-encapsulated nanoparticles as drug carriers.
Fig. 3. The various ways engineered macrophages deliver drugs.
A Macrophages can be direct carriers for the transportation of anticancer drugs, and B macrophages can be indirect carriers for the transportation of anticancer drugs. C Nanomedicines can be loaded intracellularly or extracellularly on live macrophages, and D nanomedicines can be encapsulated by macrophage membranes. E Nanomedicine loading in macrophage exosomes. (created with biorender.com).
Engineered macrophages as direct drug carriers
There exist two primary reservoirs of M1 macrophage drug carriers, bone marrow-derived macrophages and RAW264.7 macrophages, which are capable of binding to cancer cells through their own α4 and β1 integrins and interacting with VCAM-1 [66]. Studies conducted earlier have demonstrated the loading of doxorubicin (DOX) into a mouse macrophage-like cell line (RAW264.7), which culminated in the establishment of a biomimetic drug delivery system (BDS). The generated BDS successfully targeted tumour cells and did not diminish their tumorigenic potential when treating metastatic 4T1 mouse mammary carcinoma cells. Furthermore, the BDS substantially extended the lifespan of mice [64, 67]. In a study by Huang et al., engineered macrophages (Oxa(IV)@ZnPc@M) was developed to carry nanodrugs consisting of oxaliplatin prodrugs and photosensitizers. These macrophages functioned as near-infra-red light-triggered drug carriers that are capable of inducing M1-type polarisation in vivo [68]. Oxa(IV)@ZnPc@M exhibited adept penetration into both primary and bone-metastatic tumours to achieve light-controlled drug release and chemo-photodynamic combination therapy, which not only provides precise drug release and improves the efficiency of drug administration but also induces immunogenic cellular death and plays an antitumour role. Within a specific dosage range, anticancer medications, including 5-fluorouracil, irinotecan, cisplatin, and gemcitabine, which can be combined with chemotherapy, radiotherapy, and immunotherapy, did not markedly impact the viability of macrophages [69].
In conclusion, this strategy not only enhances the local drug concentration and reduces systemic side effects but also assists in surmounting the immune barrier within the tumour microenvironment, thus revealing a novel strategy for addressing ailments such as cancer [70].
Engineered macrophages as indirect drug carriers
In contrast, when macrophages serve as indirect conveyors of anticancer medications, they not only bypass the effects of drugs on the macrophages but also increase the drug-loading ratios [71]. Engineered macrophages mainly transport liposomes or nanoparticles loaded with anticancer drugs. The present findings confirmed that drugs loaded via nanoparticles are more efficient than those carried directly [56, 72]. Recently, investigators have examined the effects of M1 macrophages instead of macrophage-loaded drugs in the treatment of glioma. First, poly (lactide-coglycolide) nanoparticles were combined with primary M1 macrophages to create M1 macrophage-loaded nanoparticles [73]. Second, in vitro cellular assays indicated that M1 macrophages not only exhibited formidable tumour-suppressing effects but also facilitated the transport of antineoplastic agents across the blood-brain barrier into tumour tissues [74, 75]. Finally, M1 macrophage-loaded nanoparticles seemed to improve the targeting efficiency and efficacy of chemotherapeutic drugs in glioma treatment. Similarly, the development of hybrid exosomes through the fusion of macrophage extracellular vesicles with synthetic liposomes represents a novel approach for drug delivery in cancer therapy. These hybrid exosomes have the capability to encapsulate chemotherapeutic agents, thereby increasing their cytotoxicity towards cancer cells. Moreover, these exosomes exhibit pH-sensitive drug release, particularly within acidic tumour environments, thus facilitating the precise delivery of therapeutic agents to cancer cells. This innovative approach has immense potential for augmenting the efficacy and precision of cancer therapeutics [76].
The findings suggested that macrophages, as indirect carriers of drugs, possess the capability to traverse the blood-brain barrier not only in gliomas but also in some other solid tumours through the immunosuppressive microenvironment. This innovation not only enhances the precise targeting and therapeutic efficacy of drugs against tumours but also optimises drug potency, thereby significantly enhancing the overall response to cancer immunotherapy [73, 77–81].
Engineered macrophage-encapsulated nanoparticles as drug carriers
Currently, there are three main methods of engineering macrophage-encapsulated nanoparticles (Fig. 3): nanomedicines are loaded inside or on the surface of living macrophages; nanomedicines are encapsulated by macrophage membranes; and nanomedicines are loaded in macrophage exosomes. The macrophage membrane surface abounds with lipids and proteins, thus presenting an opportunity for loading nanodrugs through chemical conjugation or physical adsorption [72]. The drug loading method can effectively prolong the drug circulation time, specifically by targeting deeper regions of the tumour and augmenting the effectiveness of the anticancer medication [72, 82]. In a recent study, macrophage-encapsulated mesoporous silica cocoon materials were developed to deliver DOX to 4T1 tumours [83]. Macrophage membrane-encapsulated mesoporous silica cocoon materials were able to effectively prolong the survival of nanoparticles in the blood circulatory system and increase their accumulation in tumours, suggesting that macrophage membranes can act as attachment sites for nanomedicines to target tumours and improve therapeutic efficacy.
Macrophage membrane drug delivery is principally segmented into four steps: macrophage isolation, purification, the preparation of nanocarrier cores and fusion of the “core-shell” structure. This drug delivery method disrupts the structure of macrophages and uses cell membranes to encapsulate nanomedicines [84]. A more recent study using a drug delivery system composed of nanogemcitabine encapsulated by a macrophage membrane showed that the drug delivery system promoted lymphocyte infiltration into the tumour with effective intratumor infiltration and drug release ability, thereby significantly removing multifunctional immunosuppressive cells [85]. Furthermore, this system enhances lymphocyte infiltration into tumour regions and, in combination with anti-PD-L1 drugs, effectively decreases the number of nonfunctional cells; thus, this system showed encouraging outcomes in various tumour models.
Compared with synthetic nanoparticles, the use of exosomes as nanocarriers for targeted drug delivery has many advantages [86]. Macrophage-derived exosome vesicles can be used as carriers of anticancer drugs; these vesicles can maintain the same favourable surface properties as macrophage membranes, enter the body and fuse with the membrane for cellular uptake [87]. To address the impact of the blood-brain barrier, as well as the hypoxic microenvironment, on the treatment of glioblastoma, Wu et al. generated a silica nanoparticle encapsulating catalase (CAT@SiO2) that adheres to exosomes derived from macrophages, thus forming CSI@Ex-A, which exhibits remarkable blood-brain barrier penetration and adept targeting of cancer cells [88]. CAT released by tumour cells after the endocytosis of CSI@Ex-A catalyses the decomposition of hydrogen peroxide, thereby generating oxygen to mitigate tumour hypoxia. More generally, these basic findings are consistent with research showing that the drugs carried by exosomes have good biocompatibility and long cycle times while improving the drug loading rate and safety [76, 88].
Macrophages can serve as drug carriers to target diseased tissues by leveraging their inherent migratory properties to deliver therapeutic payloads precisely to target sites. This approach capitalises on the extended half-life of macrophages in blood and their responsiveness to the pathological microenvironment, thereby minimising drug distribution in healthy tissues and associated side effects. Although this method has yet to be implemented clinically, future research endeavours will delve into optimising the design of macrophages as drug delivery vehicles and overcoming the challenges associated with clinical translation. The goal of these efforts is the clinical application of this promising strategy in treating various diseases [39, 89, 90].
Chimeric antigen receptor-modified engineered macrophages
Numerous endeavours have sought to employ CAR-Ms for cancer treatment (Table 1). Figure 4 illustrates the mechanism by which chimeric antigen receptor macrophages target solid tumours. During 2006, the initial in-depth discussions and analyses of CAR-Ms began with the fusion of a single-chain Fv molecule targeting human carcinoembryonic antigen (CEA) with the transmembrane and cell membrane structural domains of human CD64. This construct was transferred into monocytes using adenoviral vectors and chimerized within the cell membrane [91]. These results clearly show that CAR-M effectively delays the growth of CEA-positive tumour cells in vitro.
Table 1.
All studies of CAR-M-based therapies to date, as well as the composition, macrophage source, and mode of CAR-M delivery.
| Macrophage source | Gene delivery | Target antigens | Extracellular/ Intracellular domains | Application strategy | Clinical trials | Refs. |
|---|---|---|---|---|---|---|
| Monocytes derived from human PBMCs | Adenoviral vector | CEA |
Extra:scFv Intra: CD64 |
In vitro | / | [91] |
| PBMCs | MaxCyte GT System | Mesothelin |
Extra: scFv Intra:CD3ζ |
In vitro | NCT03608618 | [50] |
| J774A.I Macrophages BMDMs | Lentiviral vector |
CD19 CD22 |
Extra: scFv Intra: Megf10, FcγR CD3ζ, FcγR + PI3K |
In vitro | / | [117] |
| Raw264.7 | Lentiviral vector | HER2 |
Extra: scFv Intra: CD147 |
In vitro | / | [95] |
| THP-1 monocyte-derived macrophages | Adenoviral vector(Ad5f35) |
CD19 HER2 |
Extra: scFv Intra: CD3ζ |
In vitro | NCT04660929 | [28] |
| iPSCs from PBMCs | Lentiviral vector |
CD19 Mesothelin |
Extra: scFv Intra: CD86-FcγR I |
In vitro | / | [118] |
| PBMC | Ad5f35 | HER2 |
Extra: scFv Intra: CD3ζ |
In vitro | / | [119] |
|
RAW264.7, M2 BMDMs, M2 TAMs |
Non-viral vector: jetPEI-macrophage(MPEI) | CD19 ALK |
Extra: scFv Intra: CD28-CD3 |
In vivo | / | [120] |
| Raw264.7 monocyte | Lentiviral vector | CCR7 |
Extra:CCL19 Intra:TLR2,TLR4,TLR6,MerTK,4-1BB-CD3ζ |
In vitro | / | [121] |
| THP-1 BMDMs RAW 264.7 | nanoporter(NP)-hydrogel | CD133 |
Extra: scFv Intra: CD3ζ |
In vivo | / | [51] |
| THP-1 hematopoietic stem and progenitor cells (HSPCs) | Lentiviral vector | CEA |
Extra: scFv Intra: CD28-CD3ζ |
In vitro | / | [122] |
| RAW264.7 BMDMs | Lipid nanoparticle (LNP) | CD19 |
Extra: scFv Intra: CD3ζ |
In vitro | / | [123] |
| Human primary peritoneal macrophages | Lentiviral vector | HER2 |
Extra: scFv Intra: FcεR1γ |
In vitro, In vivo |
/ | [124] |
| BMDMs | Plasmids | ErbB2 |
Extra: scFv Intra: CD3ζ |
In vitro, In vivo |
/ | [125] |
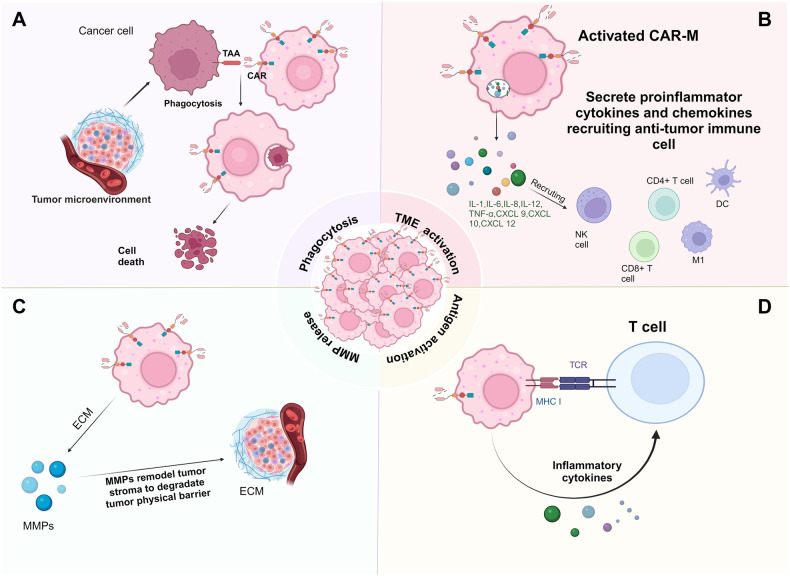
Fig. 4. Major mechanisms of solid tumour clearance by CAR-Ms.
A CAR-Ms engulf and kill tumour cells by recognising tumour cell-associated antigens. B Activated CAR-Ms can secrete proinflammatory factors and chemokines to recruit antitumour immune cells to tumour tissues. C CAR-Ms can induce the secretion of matrix metalloproteinases to degrade dense tumour ECM. D CAR-Ms can coordinate an antitumour T-cell response by recruiting T cells and cross-presenting antigens from phagocytosed cells. (created with biorender.com).
CAR-Ms were first demonstrated to exert effective antitumour effects in vivo in a recent study by Klichinsky et al., who used the targeting of the solid tumour antigen mesothelin or HER2 as the antigenic structural recognition domain and CD3ζ as the intracellular structural domain [28]. Concurrently, they engineered a minimally replicating chimeric adenoviral vector (Ad5f35) for macrophage-mediated CAR delivery [92]. The findings indicated that Ad5f35-transformed human macrophages exhibited robust CAR expression, leading to dose- and time-dependent elimination of the HER2+ ovarian cancer cell line SKOV3 by CAR-Ms. The extent of tumour phagocytosis and cytotoxicity exhibited a direct correlation with both the magnitude of CAR expression and the level of target antigen expression. Moreover, HER2-CAR-Ms demonstrated the ability to polarise M2 macrophages into M1 macrophages, incite the formation of an inflammatory TME, and increase T cell cytotoxicity against tumours.
One pivotal factor contributing to the inefficacy of CAR-T therapy in solid tumours lies in the challenge of T-cell infiltration into tumour tissues, which are enriched with dense and distinctive ECM [93]. The compact tissue structure of tumour tissues constitutes a physical impediment that constrains the migration of T cells. Matrix metalloproteinases (MMPs) primarily regulate the synthesis and degradation of the ECM [94]. In response to this constraint, efforts to formulate CAR-147-M, which is composed of a monoclonal antibody in a single-chain format that specifically targets human HER2 and encompasses the hinge region of IghG1, were made. Additionally, CAR-147-M incorporates the transmembrane and intracellular domains derived from mouse CD147 molecules [95]. These findings indicated that CAR-147 macrophages attenuated tumour collagen deposition and facilitated T-cell infiltration into tumours; however, in vitro, CAR-147 macrophages did not impede tumour cell proliferation.
The FDA has granted approval to nine CAR-T-cell therapies for haematologic malignancies (Table 2) [44]. Two clinical trials based on CAR-M therapeutic interventions are already available (NCT05007379 and NCT04660929). The initial one is the drug candidate CT-0508 from Carisma Therapeutics, which is tailored to patients with relapsed/refractory HER2 overexpressing tumours (Phase I trial). This study enrolled 18 patients with HER2-positive solid tumours for an inaugural investigation into the efficacy of CAR-M transduction via adenovirus. Carisma Therapeutics unveiled data and preliminary findings from a phase I clinical trial of CT-0508 catering to individuals afflicted with HER2-positive solid tumours, as highlighted at the American Society for Gene and Cell Therapy conference in 2023, with a particular emphasis on its safety, tolerability, efficacy in cell production, transportation, and modulation of the tumour microenvironment [48]. Another notable finding is that Maxyte’s MCY-M11 leverages mRNA-transfected PBMCs to express mesothelin-targeting CARs, including CAR-Ms, as a therapeutic intervention for patients with recurrent or resistant cases of ovarian cancer and peritoneal mesothelioma. Nevertheless, there is an ongoing recruitment of volunteers for the phase I clinical trials [50]. In addition, a great quantity of other clinical trials related to CAR-M therapy are underway (NCT06224738, NCT04405778 and NCT05164666). The further development of CAR-M therapy will require strong evidence from clinical trials.
Table 2.
Nine marketed CAR-T therapeutic drugs.
| Drug name | Target | Indications | Complete remission rates | Approval time | Listed country |
|---|---|---|---|---|---|
| Kymriah | CD19 | Relapsed or refractory diffuse large B-cell lymphoma; B cell precursor acute lymphoblastic leukaemia | >90% | 2017.8 | America |
| Yescarta | CD19 | Relapsed or refractory diffuse large B-cell lymphoma; Recurrent or refractory follicular cell lymphoma | 51% | 2017.10 | America |
| Tescarta | CD19 | Recurrent or refractory mantle cell lymphoma | 67% | 2020.7 | America |
| Breyanz | CD19 | Relapsed or refractory diffuse large B-cell lymphoma | 54% | 2021.2 | America |
| Abecma | BCMA | Relapsed or refractory multiple myeloma | 28% | 2021.3 | America |
| Axicabtagene Ciloleucel | CD19 | Relapsed or refractory diffuse large B-cell lymphoma | / | 2021.6 | China |
| Relmacabtagene Autoleucel | CD19 | Relapsed or refractory diffuse large B-cell lymphoma | / | 2021.9 | China |
| Carvykti | BCMA | Relapsed or refractory multiple myeloma | 78% | 2022.2 | America |
| Relmacabtagene Autoleucel | BCMA | Relapsed or refractory multiple myeloma | / | 2023.6 | China |
Application of bacterial outer membrane vesicles in engineered macrophages
Regulating and reprogramming macrophages by constructing OMVs that target the TME is crucial for immunotherapy [33]. In this section, two strategies by which OMVs synergistically regulate macrophages in the TME are discussed: (1) OMVs directly regulate engineered macrophages, and (2) OMVs indirectly regulate engineered macrophages (Fig. 5).
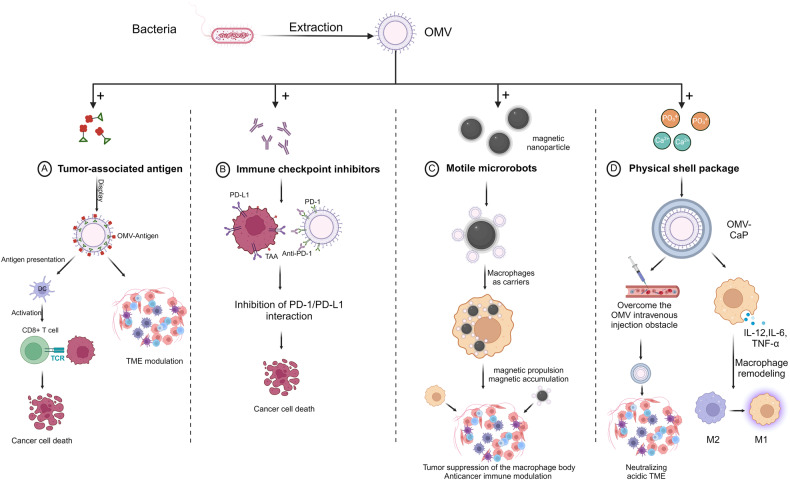
Fig. 5. The main mechanism by which bacterial outer membrane vesicles are modified to treat solid tumours.
A Tumour-associated antigens carried by OMVs activate T cells to induce the immune system to kill cancer cells in vivo. B Immune checkpoint inhibitors carried by OMVs can block immunosuppression. C Macrophages carrying magnetically driven OMVs can accurately target tumour tissues. D Physically encapsulated OMVs can overcome intravenous obstruction and induce the conversion of M2 macrophages to M1 macrophages. (created with biorender.com).
OMV directly regulate engineered macrophages
Past investigations have documented the utilisation of a calcium phosphate shell to encase OMVs [96]. CaP not only overcomes the obstacles of antibody-dependent clearance and high toxicity induced by OMVs when administered intravenously, but CaP is also sensitive to an acidic TME and can promote neutralisation of the acidic TME. In vivo, injection of the modified OMV-CaPs precipitated the transition of tumour-associated macrophages from the M2 to the M1 state, which improved the therapeutic efficacy of tumour treatment. OMV-CaP administration also increased the level of secreted cytokines (IL-12, IL-6, TNF-α, and IFN-γ) in tumour tissues (e.g., breast cancer) and promoted an antitumour immune response. This finding suggested that OMVs have the powerful ability to remodel the TME.
Owing to the diminished oxygen levels within the TME, tumour cells under hypoxic circumstances can facilitate metastasis by suppressing glycolysis in macrophages, thus reducing their competition for glucose with neovascularized epithelial cells [97]. Therefore, in a previous study, researchers modified OMVs for macrophages by synthesising REDD1-siRNA with the pH-sensitive linkers cis aconitum anhydride and paclitaxel [98]. The findings revealed a decrease in the mRNA expression of Redd1 during in vitro experiments, and the phagocytic capacity and number of macrophages polarised from the M2 to M1 phenotype increased. The results of the study clearly demonstrated that OMVs can reprogram macrophages to enhance the efficacy of tumour immunotherapy [99].
The interplay between CD47 and signal-regulated protein α (SIRPα) initiates an inhibitory signalling pathway, which leads to malignant cell evasion of macrophage phagocytosis [100]. OMV-CD47nb, a bidirectional adapter, can simultaneously interact with CD47 on tumour cells and Toll-like receptors (TLRs) on TAMs and block “do not eat me” signalling on tumour cells while polarising TAMs to the M1 phenotype. The outermost PEG/Se layer additionally mitigated the immunogenicity and toxicity of intravenously injected OMVs. Conversely, localised irradiation at the tumour site disrupts the PEG/Se layer, resulting in a more potent and targeted release of OMVs into the TME [101, 102]. This finding suggests that OMVs can improve treatment efficacy when combined with immune checkpoint inhibitor therapy [62].
OMVs indirectly regulate engineered macrophages
Cancer immunotherapy can block immunosuppressive TME through immune checkpoint inhibitors, such as anti-PD 1/PD-L1 therapy [103]. OMVs that express PD1 bind specifically to tumour cell membranes, resulting in PD-L1 internalisation and disruption of PD-L1-mediated immunosuppression, thereby promoting T-cell proliferation [104]. Recent research suggested that an OMV-based mRNA delivery platform (OMV-LL) was constructed by combining an RNA-binding protein (L7Ae) and a lysosomal escape protein (Listeria monocytogenes lysin O); this strategy emulates the innate generation of tumour antigens within malignant cells [105]. These findings demonstrated the ability to provoke a vigorous, antigen-specific immune response and enduring immunity. This study provides a “plug-and-play” strategy to implement mRNA antigens in an OMV-based platform, which could be widely applied for personalised tumour vaccines [106].
Recent studies using magnetically driven immune macrophage microrobot (MΦ-OMV) analysis have suggested that controlling MΦ-OMV by magnetic operation can target tumour site aggregation and play a role in precise guidance [107]. Importantly, MΦ-OMV contains two antitumour peptides (hirudin and mastoparan 1), which inhibit tumour angiogenesis and promote cancer cell apoptosis, respectively. Moreover, tumours can be eliminated through a variety of mechanisms [108, 109]. This design methodology holds immense promise for fabricating dynamic medical vectors that meet the rigorous criteria of clinical trials, including commendable biocompatibility with minimal adverse effects, controlled propulsion, tumour targeting, and multimodal therapies, which are promising for the future of medicine. Furthermore, gold nanoparticles combined with OMVs to generate Au-OMVs, when combined with radiotherapy, were able to induce macrophage chemotaxis towards glioma cells [106, 110, 111]. In a recent study, a multifunctional nanosystem (mU@OMVs) was constructed based on OMVs. This system blocks efferocytosis, which acts on M2 macrophages to reduce the phagocytosis of apoptotic cells [112]. Recent evidence suggests that bacterial-derived OMVs possess the dual functions of carriers and adjuvants, so they are more suitable as carriers for tumour vaccines than synthetic nanoparticles [106].
In conclusion, OMVs play a pivotal role in engineered macrophages by activating their immune response through the transport of microbial-related molecular patterns, thereby enhancing their phagocytic capabilities, facilitating antigen presentation, and potentially expanding macrophages to target cancer cells by presenting specific antigens or expressing tumour-related peptides. Furthermore, OMVs, which serve as nanoscale carriers, can be utilized for drug delivery by transporting therapeutic agents directly into the tumour microenvironment, thereby contributing to cancer treatment. Currently, this strategy remains in the research phase but holds significant promise for future clinical applications.
Conclusions and future outlook
As mentioned above, significant advancements have been achieved in the engineering of macrophages for cancer immunotherapy. The ability of these “living drugs” to precisely target the TME, as well as solid tumours, has ameliorated the shortcomings of cancer immunotherapy in solid tumours, but there are still many shortcomings and challenges with this novel therapy.
First, in terms of modifying macrophage drug delivery, the mechanism governing the recruitment and polarisation of macrophages in the TME remains elusive [40, 113, 114]. Similarly, due to the complex nature of the TME, macrophage-mediated drug delivery may not be sufficient to eliminate tumours. In terms of safety, engineered macrophages may elicit unpredictable immune responses, potentially resulting in immune system overactivation and leading to inflammation, autoimmune diseases, and other unforeseen risks. Regarding efficacy, the behaviour of engineered macrophages in vivo can vary among individuals, leading to significant differences in their antitumor effects across different patients. Addressing the challenge of controlling variations in efficacy among individuals poses a significant hurdle. In the future, if this therapy can be combined with immune checkpoint blockade (ICD) therapy, phototherapy and other therapies, such developments could further augment the efficacy of tumour treatment. Second, in terms of CAR-M therapy, the technology is still immature. Although clinical trials of this immunotherapy are available, the results have not been announced. An additional groundbreaking discovery revealed that the TME has the capacity to divert tumour-resident CAR-Ms towards a phenotype that supports tumour growth, in contrast to preclinical modelling results (CAR-Ms reprogramme the TME) [115]. Because of this potential limitation, it is imperative for us to delve into the mechanism underlying this phenomenon. Third, OMVs synergise with macrophages to alter the TME. The utilisation of the OMV-based adapter strategy extends to therapeutic approaches involving other immune checkpoints, such as the CD24-Siglec-10 axis [62]. The current limitations are that this strategy is technically difficult to perform, and quantification and timeliness are difficult to ensure. Future studies designed to answer these questions will certainly help increase OMV applicability and design diversity in the field of tumour vaccines. Fourth, in the realm of clinical translation, the development and production costs associated with engineered macrophage therapies are typically substantial, thus necessitating stringent patient requirements. Addressing cost considerations and managing patient relationships in clinical applications are of paramount importance. In conclusion, engineered macrophages, as an emerging cancer treatment modality, present both potential and challenges [116].
In the face of these obstacles, macrophages have the potential to emerge as a potent tool for the treatment of solid tumours. Future progress in cancer immunotherapy will require collaborative interdisciplinary endeavours, with combination therapies harnessing the collective knowledge of proteins, chemistry, materials, and bioengineering to revolutionise immune cell therapies for individuals battling cancer.
Acknowledgements
We are grateful for the financial support from the Medical Scientific and Technological Research Project of Henan Province (Grant No. SBGJ202102121) to J.H. and the National Natural Science Foundation of China (Grant No. U2004112) to W.Y.
Author contributions
J.H. was involved in the conception of the study. S.Y. and Y.W. were involved in the study design. Y.Y., J.J., and Y.F. were involved in the data collection and preparing the first draft of the paper. J.H. and W.Y. reviewed the paper and provided critical comments. All authors approved the final version of the paper.
Data availability
All the data supporting the findings of this study are available from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This review did not require ethical approval.
Footnotes
Edited by Professor Thomas Kaufmann
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Shuaixi Yang, Yuhang Wang.
Contributor Information
Weitang Yuan, Email: 13673384555@163.com.
Junhong Hu, Email: hujunhong@zzu.edu.cn.
References
- 1.Martínez-Jiménez F, Muiños F, Sentís I, Deu-Pons J, Reyes-Salazar I, Arnedo-Pac C, et al. A compendium of mutational cancer driver genes. Nat Rev Cancer. 2020;20:555–72. doi: 10.1038/s41568-020-0290-x. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807–21. doi: 10.1038/s41423-020-0488-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Visser KE, Joyce JA. The evolving tumor microenvironment: from cancer initiation to metastatic outgrowth. Cancer Cell. 2023;41:374–403. doi: 10.1016/j.ccell.2023.02.016. [DOI] [PubMed] [Google Scholar]
- 4.Barkley D, Moncada R, Pour M, Liberman DA, Dryg I, Werba G, et al. Cancer cell states recur across tumor types and form specific interactions with the tumor microenvironment. Nat Genet. 2022;54:1192–201. doi: 10.1038/s41588-022-01141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bejarano L, Jordāo MJC, Joyce JA. Therapeutic targeting of the tumor microenvironment. Cancer Discov. 2021;11:933–59. doi: 10.1158/2159-8290.CD-20-1808. [DOI] [PubMed] [Google Scholar]
- 6.Casey SC, Amedei A, Aquilano K, Azmi AS, Benencia F, Bhakta D, et al. Cancer prevention and therapy through the modulation of the tumor microenvironment. Semin Cancer Biol. 2015;35:S199–S223. doi: 10.1016/j.semcancer.2015.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elhanani O, Ben-Uri R, Keren L. Spatial profiling technologies illuminate the tumor microenvironment. Cancer Cell. 2023;41:404–20. doi: 10.1016/j.ccell.2023.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Jin MZ, Jin WL. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct Target Ther. 2020;5:166. doi: 10.1038/s41392-020-00280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiam-Galvez KJ, Allen BM, Spitzer MH. Systemic immunity in cancer. Nat Rev Cancer. 2021;21:345–59. doi: 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity. 2020;52:17–35. doi: 10.1016/j.immuni.2019.12.011. [DOI] [PubMed] [Google Scholar]
- 11.Lin MJ, Svensson-Arvelund J, Lubitz GS, Marabelle A, Melero I, Brown BD, et al. Cancer vaccines: the next immunotherapy frontier. Nat Cancer. 2022;3:911–26. doi: 10.1038/s43018-022-00418-6. [DOI] [PubMed] [Google Scholar]
- 12.Laskowski TJ, Biederstädt A, Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer. 2022;22:557–75. doi: 10.1038/s41568-022-00491-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng M, Mo Y, Wang Y, Wu P, Zhang Y, Xiong F, et al. Neoantigen vaccine: an emerging tumor immunotherapy. Mol Cancer. 2019;18:128. doi: 10.1186/s12943-019-1055-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Locati M, Curtale G, Mantovani A. Diversity, mechanisms, and significance of macrophage plasticity. Annu Rev Pathol. 2020;15:123–47. doi: 10.1146/annurev-pathmechdis-012418-012718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233:6425–40. doi: 10.1002/jcp.26429. [DOI] [PubMed] [Google Scholar]
- 16.Yunna C, Mengru H, Lei W, Weidong C. Macrophage M1/M2 polarization. Eur J Pharmacol. 2020;877:173090. doi: 10.1016/j.ejphar.2020.173090. [DOI] [PubMed] [Google Scholar]
- 17.Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-based approaches for cancer immunotherapy. Cancer Res. 2021;81:1201–8. doi: 10.1158/0008-5472.CAN-20-2990. [DOI] [PubMed] [Google Scholar]
- 18.Jaguin M, Houlbert N, Fardel O, Lecureur V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol. 2013;281:51–61. doi: 10.1016/j.cellimm.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 19.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–11. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 20.Gunassekaran GR, Poongkavithai Vadevoo SM, Baek MC, Lee B. M1 macrophage exosomes engineered to foster M1 polarization and target the IL-4 receptor inhibit tumor growth by reprogramming tumor-associated macrophages into M1-like macrophages. Biomaterials. 2021;278:121137. doi: 10.1016/j.biomaterials.2021.121137. [DOI] [PubMed] [Google Scholar]
- 21.Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, et al. The immune landscape of cancer. Immunity. 2019;51:411–2. doi: 10.1016/j.immuni.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000prime Rep. 2014;6:13. doi: 10.12703/P6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015:816460. doi: 10.1155/2015/816460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. 2018;19:1801. [DOI] [PMC free article] [PubMed]
- 26.Chen S, Saeed A, Liu Q, Jiang Q, Xu H, Xiao GG, et al. Macrophages in immunoregulation and therapeutics. Signal Transduct Target Ther. 2023;8:207. doi: 10.1038/s41392-023-01452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baslan T, Hicks J. Unravelling biology and shifting paradigms in cancer with single-cell sequencing. Nat Rev Cancer. 2017;17:557–69. doi: 10.1038/nrc.2017.58. [DOI] [PubMed] [Google Scholar]
- 28.Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38:947–53. doi: 10.1038/s41587-020-0462-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61. doi: 10.1016/j.immuni.2014.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee HW, Choi HJ, Ha SJ, Lee KT, Kwon YG. Recruitment of monocytes/macrophages in different tumor microenvironments. Biochim Biophys Acta. 2013;1835:170–9. doi: 10.1016/j.bbcan.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 31.Boutilier AJ, Elsawa SF. Macrophage polarization states in the tumor microenvironment. Int J Mol Sci. 2021;22:6995. [DOI] [PMC free article] [PubMed]
- 32.Pan Y, Yu Y, Wang X, Zhang T. Tumor-associated macrophages in tumor immunity. Front Immunol. 2020;11:583084. doi: 10.3389/fimmu.2020.583084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang H, Yung MMH, Ngan HYS, Chan KKL, Chan DW. The impact of the tumor microenvironment on macrophage polarization in cancer metastatic progression. Int J Mol Sci. 2021;22:6560. [DOI] [PMC free article] [PubMed]
- 34.Weber EW, Maus MV, Mackall CL. The emerging landscape of immune cell therapies. Cell. 2020;181:46–62. doi: 10.1016/j.cell.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Irvine DJ, Maus MV, Mooney DJ, Wong WW. The future of engineered immune cell therapies. Science. 2022;378:853–8. doi: 10.1126/science.abq6990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gu C, Geng X, Wu Y, Dai Y, Zeng J, Wang Z, et al. Engineered macrophage membrane-coated nanoparticles with enhanced CCR2 expression promote spinal cord injury repair by suppressing neuroinflammation and neuronal death. Small (Weinheim an der Bergstrasse, Germany). 2023;20:e2305659. [DOI] [PubMed]
- 37.Yin T, Fan Q, Hu F, Ma X, Yin Y, Wang B, et al. Engineered macrophage-membrane-coated nanoparticles with enhanced PD-1 expression induce immunomodulation for a synergistic and targeted antiglioblastoma activity. Nano Lett. 2022;22:6606–14. doi: 10.1021/acs.nanolett.2c01863. [DOI] [PubMed] [Google Scholar]
- 38.Moyes KW, Lieberman NA, Kreuser SA, Chinn H, Winter C, Deutsch G, et al. Genetically engineered macrophages: a potential platform for cancer immunotherapy. Hum Gene Ther. 2017;28:200–15. doi: 10.1089/hum.2016.060. [DOI] [PubMed] [Google Scholar]
- 39.Xia Y, Rao L, Yao H, Wang Z, Ning P, Chen X. Engineering macrophages for cancer immunotherapy and drug delivery. Adv Mater (Deerfield Beach, Fla) 2020;32:e2002054. doi: 10.1002/adma.202002054. [DOI] [PubMed] [Google Scholar]
- 40.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol. 2011;11:723–37. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirsh R, Bugelski PJ, Poste G. Drug delivery to macrophages for the therapy of cancer and infectious diseases. Ann N Y Acad Sci. 1987;507:141–54. doi: 10.1111/j.1749-6632.1987.tb45797.x. [DOI] [PubMed] [Google Scholar]
- 42.Lu S, Zhang C, Wang J, Zhao L, Li G. Research progress in nano-drug delivery systems based on the characteristics of the liver cancer microenvironment. Biomed Pharmacother. 2024;170:116059. doi: 10.1016/j.biopha.2023.116059. [DOI] [PubMed] [Google Scholar]
- 43.Wang X, Li Y, Pu X, Liu G, Qin H, Wan W, et al. Macrophage-related therapeutic strategies: regulation of phenotypic switching and construction of drug delivery systems. Pharmacol Res. 2024;199:107022. doi: 10.1016/j.phrs.2023.107022. [DOI] [PubMed] [Google Scholar]
- 44.Abbasi S, Totmaj MA, Abbasi M, Hajazimian S, Goleij P, Behroozi J, et al. Chimeric antigen receptor T (CAR-T) cells: Novel cell therapy for hematological malignancies. Cancer Med. 2023;12:7844–58. doi: 10.1002/cam4.5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PloS ONE. 2012;7:e50946. doi: 10.1371/journal.pone.0050946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Aloia MM, Zizzari IG, Sacchetti B, Pierelli L, Alimandi M. CAR-T cells: the long and winding road to solid tumors. Cell Death Dis. 2018;9:282. doi: 10.1038/s41419-018-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shah NN, Fry TJ. Mechanisms of resistance to CAR T cell therapy. Nat Rev Clin Oncol. 2019;16:372–85. doi: 10.1038/s41571-019-0184-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Y, Yang J, Zhang T, Gu H. Emerging advances in nanobiomaterials-assisted chimeric antigen receptor (CAR)-macrophages for tumor immunotherapy. Front Bioeng Biotechnol. 2023;11:1211687. doi: 10.3389/fbioe.2023.1211687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen Y, Yu Z, Tan X, Jiang H, Xu Z, Fang Y, et al. CAR-macrophage: a new immunotherapy candidate against solid tumors. Biomed Pharmacother. 2021;139:111605. doi: 10.1016/j.biopha.2021.111605. [DOI] [PubMed] [Google Scholar]
- 50.Hung CF, Xu X, Li L, Ma Y, Jin Q, Viley A, et al. Development of anti-human mesothelin-targeted chimeric antigen receptor messenger RNA-transfected peripheral blood lymphocytes for ovarian cancer therapy. Hum Gene Ther. 2018;29:614–25. doi: 10.1089/hum.2017.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen C, Jing W, Chen Y, Wang G, Abdalla M, Gao L, et al. Intracavity generation of glioma stem cell-specific CAR macrophages primes locoregional immunity for postoperative glioblastoma therapy. Sci Transl Med. 2022;14:eabn1128. doi: 10.1126/scitranslmed.abn1128. [DOI] [PubMed] [Google Scholar]
- 52.Pan K, Farrukh H, Chittepu V, Xu H, Pan CX, Zhu Z. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res. 2022;41:119. doi: 10.1186/s13046-022-02327-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu M, Liu J, Liang Z, Dai K, Gan J, Wang Q, et al. CAR-macrophages and CAR-T cells synergistically kill tumor cells in vitro. Cells. 2022;11:3692. [DOI] [PMC free article] [PubMed]
- 54.Moradinasab S, Pourbagheri-Sigaroodi A, Ghaffari SH, Bashash D. Targeting macrophage-mediated tumor cell phagocytosis: An overview of phagocytosis checkpoints blockade, nanomedicine intervention, and engineered CAR-macrophage therapy. Int Immunopharmacol. 2022;103:108499. doi: 10.1016/j.intimp.2021.108499. [DOI] [PubMed] [Google Scholar]
- 55.Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69. doi: 10.1038/s41408-021-00459-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang J, Cheng KM, Li Y, Xu JQ, Chen YW, Ma NN, et al. Personalized cancer vaccines from bacteria-derived outer membrane vesicles with antibody-mediated persistent uptake by dendritic cells. Fundam Res. 2022;2:23–36. doi: 10.1016/j.fmre.2021.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15:375–87. doi: 10.1038/nri3837. [DOI] [PubMed] [Google Scholar]
- 58.Kuzmich NN, Sivak KV, Chubarev VN, Porozov YB, Savateeva-Lyubimova TN, Peri F. TLR4 signaling pathway modulators as potential therapeutics in inflammation and sepsis. Vaccines. 2017;5:34. [DOI] [PMC free article] [PubMed]
- 59.Sartorio MG, Pardue EJ, Feldman MF, Haurat MF. Bacterial outer membrane vesicles: from discovery to applications. Annu Rev Microbiol. 2021;75:609–30. doi: 10.1146/annurev-micro-052821-031444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Micoli F, MacLennan CA. Outer membrane vesicle vaccines. Semin Immunol. 2020;50:101433. doi: 10.1016/j.smim.2020.101433. [DOI] [PubMed] [Google Scholar]
- 61.Cheng K, Zhao R, Li Y, Qi Y, Wang Y, Zhang Y, et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology. Nat Commun. 2021;12:2041. doi: 10.1038/s41467-021-22308-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Feng Q, Ma X, Cheng K, Liu G, Li Y, Yue Y, et al. Engineered bacterial outer membrane vesicles as controllable two-way adaptors to activate macrophage phagocytosis for improved tumor immunotherapy. Adv Mater (Deerfield Beach, Fla) 2022;34:e2206200. doi: 10.1002/adma.202206200. [DOI] [PubMed] [Google Scholar]
- 63.Su Z, Dong S, Chen Y, Huang T, Qin B, Yang Q, et al. Microfluidics-enabled nanovesicle delivers CD47/PD-L1 antibodies to enhance antitumor immunity and reduce immunotoxicity in lung adenocarcinoma. Adv Sci (Weinh, Baden-Wurtt, Ger) 2023;10:e2206213. doi: 10.1002/advs.202206213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuerban K, Gao X, Zhang H, Liu J, Dong M, Wu L, et al. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm Sin B. 2020;10:1534–48. doi: 10.1016/j.apsb.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang S, Huang W, Li K, Yao Y, Yang X, Bai H, et al. Engineered outer membrane vesicle is potent to elicit HPV16E7-specific cellular immunity in a mouse model of TC-1 graft tumor. Int J Nanomed. 2017;12:6813–25. doi: 10.2147/IJN.S143264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cao H, Dan Z, He X, Zhang Z, Yu H, Yin Q, et al. Liposomes Coated with Isolated Macrophage Membrane Can Target Lung Metastasis of Breast Cancer. ACS Nano. 2016;10:7738–48. doi: 10.1021/acsnano.6b03148. [DOI] [PubMed] [Google Scholar]
- 67.Fu J, Wang D, Mei D, Zhang H, Wang Z, He B, et al. Macrophage mediated biomimetic delivery system for the treatment of lung metastasis of breast cancer. J Controlled Release. 2015;204:11–19. doi: 10.1016/j.jconrel.2015.01.039. [DOI] [PubMed] [Google Scholar]
- 68.Huang Y, Guan Z, Dai X, Shen Y, Wei Q, Ren L, et al. Engineered macrophages as near-infrared light activated drug vectors for chemo-photodynamic therapy of primary and bone metastatic breast cancer. Nat Commun. 2021;12:4310. doi: 10.1038/s41467-021-24564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sakai H, Kokura S, Ishikawa T, Tsuchiya R, Okajima M, Matsuyama T, et al. Effects of anticancer agents on cell viability, proliferative activity and cytokine production of peripheral blood mononuclear cells. J Clin Biochem Nutr. 2013;52:64–71. doi: 10.3164/jcbn.12-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xiao Q, Li X, Li Y, Wu Z, Xu C, Chen Z, et al. Biological drug and drug delivery-mediated immunotherapy. Acta Pharm Sin B. 2021;11:941–60. doi: 10.1016/j.apsb.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li W, Su Z, Hao M, Ju C, Zhang C. Cytopharmaceuticals: an emerging paradigm for drug delivery. J Controlled Release. 2020;328:313–24. doi: 10.1016/j.jconrel.2020.08.063. [DOI] [PubMed] [Google Scholar]
- 72.Facklam AL, Volpatti LR, Anderson DG. Biomaterials for personalized cell therapy. Adv Mater (Deerfield Beach, Fla) 2020;32:e1902005. doi: 10.1002/adma.201902005. [DOI] [PubMed] [Google Scholar]
- 73.Pang L, Zhu Y, Qin J, Zhao W, Wang J. Primary M1 macrophages as multifunctional carrier combined with PLGA nanoparticle delivering anticancer drug for efficient glioma therapy. Drug Deliv. 2018;25:1922–31. doi: 10.1080/10717544.2018.1502839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang X, Ding H, Li Z, Peng Y, Tan H, Wang C, et al. Exploration and functionalization of M1-macrophage extracellular vesicles for effective accumulation in glioblastoma and strong synergistic therapeutic effects. Signal Transduct Target Ther. 2022;7:74. doi: 10.1038/s41392-022-00894-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kim J, Zhu Y, Chen S, Wang D, Zhang S, Xia J, et al. Anti-glioma effect of ginseng-derived exosomes-like nanoparticles by active blood-brain-barrier penetration and tumor microenvironment modulation. J Nanobiotechnol. 2023;21:253. doi: 10.1186/s12951-023-02006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rayamajhi S, Nguyen TDT, Marasini R, Aryal S. Macrophage-derived exosome-mimetic hybrid vesicles for tumor targeted drug delivery. Acta Biomater. 2019;94:482–94. doi: 10.1016/j.actbio.2019.05.054. [DOI] [PubMed] [Google Scholar]
- 77.Ikehara Y, Niwa T, Biao L, Ikehara SK, Ohashi N, Kobayashi T, et al. A carbohydrate recognition-based drug delivery and controlled release system using intraperitoneal macrophages as a cellular vehicle. Cancer Res. 2006;66:8740–8. doi: 10.1158/0008-5472.CAN-06-0470. [DOI] [PubMed] [Google Scholar]
- 78.Dalzon B, Guidetti M, Testemale D, Reymond S, Proux O, Vollaire J, et al. Utility of macrophages in an antitumor strategy based on the vectorization of iron oxide nanoparticles. Nanoscale. 2019;11:9341–52. doi: 10.1039/C8NR03364A. [DOI] [PubMed] [Google Scholar]
- 79.Choi MR, Stanton-Maxey KJ, Stanley JK, Levin CS, Bardhan R, Akin D, et al. A cellular Trojan Horse for delivery of therapeutic nanoparticles into tumors. Nano Lett. 2007;7:3759–65. doi: 10.1021/nl072209h. [DOI] [PubMed] [Google Scholar]
- 80.Muthana M, Giannoudis A, Scott SD, Fang HY, Coffelt SB, Morrow FJ, et al. Use of macrophages to target therapeutic adenovirus to human prostate tumors. Cancer Res. 2011;71:1805–15. doi: 10.1158/0008-5472.CAN-10-2349. [DOI] [PubMed] [Google Scholar]
- 81.Choi J, Kim HY, Ju EJ, Jung J, Park J, Chung HK, et al. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33:4195–203. doi: 10.1016/j.biomaterials.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 82.Song S, Xia H, Guo M, Wang S, Zhang S, Ma P, et al. Role of macrophage in nanomedicine-based disease treatment. Drug Deliv. 2021;28:752–66. doi: 10.1080/10717544.2021.1909175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xuan M, Shao J, Dai L, He Q, Li J. Macrophage cell membrane camouflaged mesoporous silica nanocapsules for in vivo cancer therapy. Adv Healthc Mater. 2015;4:1645–52. doi: 10.1002/adhm.201500129. [DOI] [PubMed] [Google Scholar]
- 84.Liu Y, Luo J, Chen X, Liu W, Chen T. Cell membrane coating technology: a promising strategy for biomedical applications. Nano-Micro Lett. 2019;11:100. doi: 10.1007/s40820-019-0330-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li J, Wu Y, Wang J, Xu X, Zhang A, Li Y, et al. Macrophage membrane-coated nano-gemcitabine promotes lymphocyte infiltration and synergizes AntiPD-L1 to restore the tumoricidal function. ACS Nano. 2023;17:322–36. doi: 10.1021/acsnano.2c07861. [DOI] [PubMed] [Google Scholar]
- 86.Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11:3183–95. doi: 10.7150/thno.52570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science (New York, NY). 2020;367:eaau6977. [DOI] [PMC free article] [PubMed]
- 88.Wu T, Liu Y, Cao Y, Liu Z. Engineering macrophage exosome disguised biodegradable nanoplatform for enhanced sonodynamic therapy of glioblastoma. Adv Mater (Deerfield Beach, Fla) 2022;34:e2110364. doi: 10.1002/adma.202110364. [DOI] [PubMed] [Google Scholar]
- 89.Liu P, Gao C, Chen H, Vong CT, Wu X, Tang X, et al. Receptor-mediated targeted drug delivery systems for treatment of inflammatory bowel disease: Opportunities and emerging strategies. Acta Pharm Sin B. 2021;11:2798–818. doi: 10.1016/j.apsb.2020.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y, Cai K, Li C, Guo Q, Chen Q, He X, et al. Macrophage-membrane-coated nanoparticles for tumor-targeted chemotherapy. Nano Lett. 2018;18:1908–15. doi: 10.1021/acs.nanolett.7b05263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Biglari A, Southgate TD, Fairbairn LJ, Gilham DE. Human monocytes expressing a CEA-specific chimeric CD64 receptor specifically target CEA-expressing tumour cells in vitro and in vivo. Gene Ther. 2006;13:602–10. doi: 10.1038/sj.gt.3302706. [DOI] [PubMed] [Google Scholar]
- 92.Nilsson M, Ljungberg J, Richter J, Kiefer T, Magnusson M, Lieber A, et al. Development of an adenoviral vector system with adenovirus serotype 35 tropism; efficient transient gene transfer into primary malignant hematopoietic cells. J Gene Med. 2004;6:631–41. doi: 10.1002/jgm.543. [DOI] [PubMed] [Google Scholar]
- 93.Talts JF, Wirl G, Dictor M, Muller WJ, Fässler R. Tenascin-C modulates tumor stroma and monocyte/macrophage recruitment but not tumor growth or metastasis in a mouse strain with spontaneous mammary cancer. J Cell Sci. 1999;112:1855–64. doi: 10.1242/jcs.112.12.1855. [DOI] [PubMed] [Google Scholar]
- 94.Hynes RO, Naba A. Overview of the matrisome-an inventory of extracellular matrix constituents and functions. Cold Spring Harb Perspect Biol. 2012;4:a004903. doi: 10.1101/cshperspect.a004903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang W, Liu L, Su H, Liu Q, Shen J, Dai H, et al. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br J Cancer. 2019;121:837–45. doi: 10.1038/s41416-019-0578-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qing S, Lyu C, Zhu L, Pan C, Wang S, Li F, et al. Biomineralized bacterial outer membrane vesicles potentiate safe and efficient tumor microenvironment reprogramming for anticancer therapy. Adv Mater (Deerfield Beach, Fla) 2020;32:e2002085. doi: 10.1002/adma.202002085. [DOI] [PubMed] [Google Scholar]
- 97.Wenes M, Shang M, Di Matteo M, Goveia J, Martín-Pérez R, Serneels J, et al. Macrophage metabolism controls tumor blood vessel morphogenesis and metastasis. Cell Metab. 2016;24:701–15. doi: 10.1016/j.cmet.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 98.Shoshani T, Faerman A, Mett I, Zelin E, Tenne T, Gorodin S, et al. Identification of a novel hypoxia-inducible factor 1-responsive gene, RTP801, involved in apoptosis. Mol Cell Biol. 2002;22:2283–93. doi: 10.1128/MCB.22.7.2283-2293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Guo Q, Li X, Zhou W, Chu Y, Chen Q, Zhang Y, et al. Sequentially triggered bacterial outer membrane vesicles for macrophage metabolism modulation and tumor metastasis suppression. ACS Nano. 2021;15:13826–38. doi: 10.1021/acsnano.1c05613. [DOI] [PubMed] [Google Scholar]
- 100.Zhang W, Huang Q, Xiao W, Zhao Y, Pi J, Xu H, et al. Advances in anti-tumor treatments targeting the CD47/SIRPα Axis. Front Immunol. 2020;11:18. doi: 10.3389/fimmu.2020.00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Logtenberg MEW, Scheeren FA, Schumacher TN. The CD47-SIRPα immune checkpoint. Immunity. 2020;52:742–52. doi: 10.1016/j.immuni.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Dacek MM, Kurtz KG, Wallisch P, Pierre SA, Khayat S, Bourne CM, et al. Potentiating antibody-dependent killing of cancers with CAR T cells secreting CD47-SIRPα checkpoint blocker. Blood. 2023;141:2003–15. doi: 10.1182/blood.2022016101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Li Y, Zhao R, Cheng K, Zhang K, Wang Y, Zhang Y, et al. Bacterial outer membrane vesicles presenting programmed death 1 for improved cancer immunotherapy via immune activation and checkpoint inhibition. ACS Nano. 2020;14:16698–711. doi: 10.1021/acsnano.0c03776. [DOI] [PubMed] [Google Scholar]
- 105.Li Y, Ma X, Yue Y, Zhang K, Cheng K, Feng Q, et al. Rapid surface display of mRNA antigens by bacteria-derived outer membrane vesicles for a personalized tumor vaccine. Adv Mater (Deerfield Beach, Fla) 2022;34:e2109984. doi: 10.1002/adma.202109984. [DOI] [PubMed] [Google Scholar]
- 106.Zhao X, Zhao R, Nie G. Nanocarriers based on bacterial membrane materials for cancer vaccine delivery. Nat Protoc. 2022;17:2240–74. doi: 10.1038/s41596-022-00713-7. [DOI] [PubMed] [Google Scholar]
- 107.Li Y, Cong Z, Xie L, Tang S, Ren C, Peng X, et al. Magnetically powered immunogenic macrophage microrobots for targeted multimodal cancer therapy. Small (Weinheim an der Bergstrasse, Germany). 2023;19:e2301489. [DOI] [PubMed]
- 108.Li G, Lei Q, Wang F, Deng D, Wang S, Tian L, et al. Fluorinated polymer mediated transmucosal peptide delivery for intravesical instillation therapy of bladder cancer. Small (Weinh der Bergstr, Ger) 2019;15:e1900936. doi: 10.1002/smll.201900936. [DOI] [PubMed] [Google Scholar]
- 109.Jiang T, Shen S, Wang T, Li M, He B, Mo R. A substrate-selective enzyme-catalysis assembly strategy for oligopeptide hydrogel-assisted combinatorial protein delivery. Nano Lett. 2017;17:7447–54. doi: 10.1021/acs.nanolett.7b03371. [DOI] [PubMed] [Google Scholar]
- 110.Zhuang WR, Wang Y, Nie W, Lei Y, Liang C, He J, et al. Bacterial outer membrane vesicle based versatile nanosystem boosts the efferocytosis blockade triggered tumor-specific immunity. Nat Commun. 2023;14:1675. doi: 10.1038/s41467-023-37369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen MH, Liu TY, Chen YC, Chen MH. Combining augmented radiotherapy and immunotherapy through a nano-gold and bacterial outer-membrane vesicle complex for the treatment of glioblastoma. Nanomaterials (Basel, Switzerland). 2021;11:1661. [DOI] [PMC free article] [PubMed]
- 112.Tajbakhsh A, Gheibi Hayat SM, Movahedpour A, Savardashtaki A, Loveless R, Barreto GE, et al. The complex roles of efferocytosis in cancer development, metastasis, and treatment. Biomed Pharmacother. 2021;140:111776. doi: 10.1016/j.biopha.2021.111776. [DOI] [PubMed] [Google Scholar]
- 113.Li C, Xu X, Wei S, Jiang P, Xue L, Wang J. Tumor-associated macrophages: potential therapeutic strategies and future prospects in cancer. J Immunother Cancer. 2021;9:e001341. [DOI] [PMC free article] [PubMed]
- 114.Kumari N, Choi SH. Tumor-associated macrophages in cancer: recent advancements in cancer nanoimmunotherapies. J Exp Clin Cancer Res. 2022;41:68. doi: 10.1186/s13046-022-02272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Netea-Maier RT, Smit JWA, Netea MG. Metabolic changes in tumor cells and tumor-associated macrophages: a mutual relationship. Cancer Lett. 2018;413:102–9. doi: 10.1016/j.canlet.2017.10.037. [DOI] [PubMed] [Google Scholar]
- 116.Sloas C, Gill S, Klichinsky M. Engineered CAR-macrophages as adoptive immunotherapies for solid tumors. Front Immunol. 2021;12:783305. doi: 10.3389/fimmu.2021.783305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Morrissey MA, Williamson AP, Steinbach AM, Roberts EW, Kern N, Headley MB, et al. Chimeric antigen receptors that trigger phagocytosis. eLife. 2018;7:e36688. [DOI] [PMC free article] [PubMed]
- 118.Zhang L, Tian L, Dai X, Yu H, Wang J, Lei A, et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J Hematol Oncol. 2020;13:153. doi: 10.1186/s13045-020-00983-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gabitova L, Menchel B, Gabbasov R, Pierini S, Best A, Ross K, et al. Anti-HER2 CAR monocytes demonstrate targeted anti-tumor activity and enable a single day cell manufacturing process. Cancer Res. 2021;81:1530.
- 120.Kang M, Lee SH, Kwon M, Byun J, Kim D, Kim C, et al. Nanocomplex-mediated in vivo programming to chimeric antigen receptor-M1 macrophages for cancer therapy. Adv Mater (Deerfield Beach, Fla) 2021;33:e2103258. doi: 10.1002/adma.202103258. [DOI] [PubMed] [Google Scholar]
- 121.Niu Z, Chen G, Chang W, Sun P, Luo Z, Zhang H, et al. Chimeric antigen receptor-modified macrophages trigger systemic anti-tumour immunity. J Pathol. 2021;253:247–57. doi: 10.1002/path.5585. [DOI] [PubMed] [Google Scholar]
- 122.Paasch D, Meyer J, Stamopoulou A, Lenz D, Kuehle J, Kloos D, et al. Ex vivo generation of CAR macrophages from hematopoietic stem and progenitor cells for use in cancer therapy. Cells. 2022;11:994. [DOI] [PMC free article] [PubMed]
- 123.Ye Z, Chen J, Zhao X, Li Y, Harmon J, Huang C, et al. In vitro engineering chimeric antigen receptor macrophages and T cells by lipid nanoparticle-mediated mRNA delivery. ACS Biomater Sci Eng. 2022;8:722–33. doi: 10.1021/acsbiomaterials.1c01532. [DOI] [PubMed] [Google Scholar]
- 124.Dong X, Fan J, Xie W, Wu X, Wei J, He Z, et al. Efficacy evaluation of chimeric antigen receptor-modified human peritoneal macrophages in the treatment of gastric cancer. Br J Cancer. 2023;129:551–62. [DOI] [PMC free article] [PubMed]
- 125.Gao L, Shi C, Yang Z, Jing W, Han M, Zhang J, et al. Convection-enhanced delivery of nanoencapsulated gene locoregionally yielding ErbB2/Her2-specific CAR-macrophages for brainstem glioma immunotherapy. J Nanobiotechnol. 2023;21:56. doi: 10.1186/s12951-023-01810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data supporting the findings of this study are available from the corresponding author upon reasonable request.