Abstract
The introduction of antiretroviral therapy (ART) has significantly prolonged the lifespan of people living with human immunodeficiency virus (PLWH). However, the sustained use of this drug regimen has also been associated with a cluster of metabolic anomalies, including renal toxicity, which can lead to the development of kidney diseases. In this study, we reviewed studies examining kidney disease in PLWH sourced from electronic databases such as PubMed/MEDLINE, Scopus, and Google Scholar, as well as gray literature. The narrative synthesis of data from these clinical studies demonstrated that the serum levels of cystatin C remained unchanged or were not affected in PLWH on ART, while the creatinine-based glomerular filtration rate (GFR) fluctuated. In fact, some of the included studies showed that the creatinine-based GFR was increased in PLWH taking tenofovir disoproxil fumarate-containing ART, perhaps indicating that the use of both cystatin C- and creatinine-based GFRs is vital to monitor the development of kidney disease in PLWH. Clinical data summarized within this study indicate the potential detrimental effects of tenofovir-based ART regimens in causing renal tubular injury, while highlighting the possible beneficial effects of dolutegravir-based ART on improving the kidney function in PLWH. However, the summarized literature remains limited, while further clinical studies are required to provide insights into the potential use of cystatin C as a biomarker for kidney disease in PLWH.
Keywords: HIV, highly active antiretroviral therapy, biomarker, cystatin C, kidney function, nephropathy
1. Introduction
Chronic kidney disease is a progressive medical condition that affects approximately 10% of the global population, with over 800 million reported cases worldwide (1). The International Society of Nephrology Global Health Atlas survey for Africa has estimated the prevalence of this condition to be similarly high in South Africa, especially aggravated/exacerbated by socioeconomic inequalities (2). Older individuals, including people with other disease conditions such as diabetes mellitus, hypertension, and human immunodeficiency virus (HIV), present with the greatest burden of kidney disease (3). Prominent features of chronic kidney disease include accelerated fibrosis and a decline in the rate of glomerular filtration, which can progress to end-stage kidney diseases and is associated with new-onset kidney injury (4). End-stage kidney failure occurs when the kidneys are no longer able to meet day-to-day requirements, while acute kidney injury is considered an abrupt reduction in the kidney function, which incorporates both structural damage and functional loss (5). Chronic kidney disease has become even more prevalent among people living with HIV (PLWH) compared to the general population and is correlated with an increased risk of adverse outcomes, such as heart failure, diabetes mellitus, and cardiovascular disease (6–8). Because of the many developing consequences associated with the sustained use of antiretroviral therapy (ART) (9, 10), there has been an enhanced need to understand the status of kidney disease in PLWH (11–13).
There is no argument that the effective use of ART has certainly prolonged the lifespan of PLWH (14). However, disparities linked with treatment adherence, dose selection, and the presence of other chronic conditions could contribute to disruptions in the organ function (15), including kidney toxicity (6, 7). The common methods of assessing the renal function in PLWH include the serum creatinine-based estimated glomerular filtration rate (eGFR) or creatinine clearance and/or urinalysis of proteinuria, glucose, or phosphate excretion (16, 17). However, evidence points to the limitations of these methods, as they may not be sensitive enough to detect kidney toxicity in PLWH (18–20).
Currently, growing evidence underscores the importance of making use of other alternative markers, such as serum cystatin C, to estimate GFR or renal tubular injury in PLWH (21, 22). Human cystatin C is a low-molecular-weight cysteine-rich protein that is synthesized by almost all tissues of the body (23). Despite the substantiated evidence that efficient kidneys can regulate serum cystatin C concentration to meet body’s homeostatic requirements, there is growing evidence to suggest an inverse correlation between GFR and serum cystatin C (20, 22, 24). Most recent evidence also shows that cystatin C could effectively identify high-risk chronic kidney disease individuals that may not have been detected by creatinine and enhanced eGFR-based risk stratification (25). However, it has also been argued that the available literature does not encourage the use of cystatin C or cystatin C-based equations to estimate the GFR in PLWH (26). This is compounded by the lack of consideration for other hypotheses regarding the potential detrimental effects of certain ART regimens on the kidney function in PLWH (27, 28). Thus, this aspect indicates the necessity for further research to scrutinize the relevance of using cystatin C or its relation to other biomarkers of kidney injury, such as creatinine, in PLWH on ART. Therefore, this report aimed to understand whether serum levels of cystatin C may be potentially used as a plausible biomarker to assess the renal function through the GFR. The current study, therefore, makes use of a systematic approach to identify and discuss clinical studies that provide information on the potential use of cystatin C as a biomarker for kidney disease or a risk thereof in PLWH. The analysis and discussion extend to evaluating whether ART has a negative or positive impact on the circulating levels of this protein in PLWH.
2. Methodology
2.1. Literature search strategy
This study was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (29), although a meta-analysis was not conducted for this particular study (Supplementary File 1). Briefly, a systematic literature search was conducted using major online databases, such as PubMed/MEDLINE, Scopus, and Google Scholar, to identify clinical studies reporting on any association between cystatin C and kidney disease in PLWH on HARRT. The search strategy was compiled using a combination of the following keywords or Medical Subject Headings (MeSH); “cystatin C,” “ART,” “glomerular filtration rate,” “creatinine,” and “HIV” including the most relevant synonyms (Supplementary File 2). All the retrieved references were reviewed for additional relevant studies. This literature search was run without limitation until December 2023, for an adequate update on the literature. While this protocol was not registered with PROSPERO (International Prospective Register for Systematic Reviews), we conducted thorough searches of this database and any other relevant registries to ensure that our study did not duplicate existing research.
2.2. Study selection by inclusion and exclusion criteria
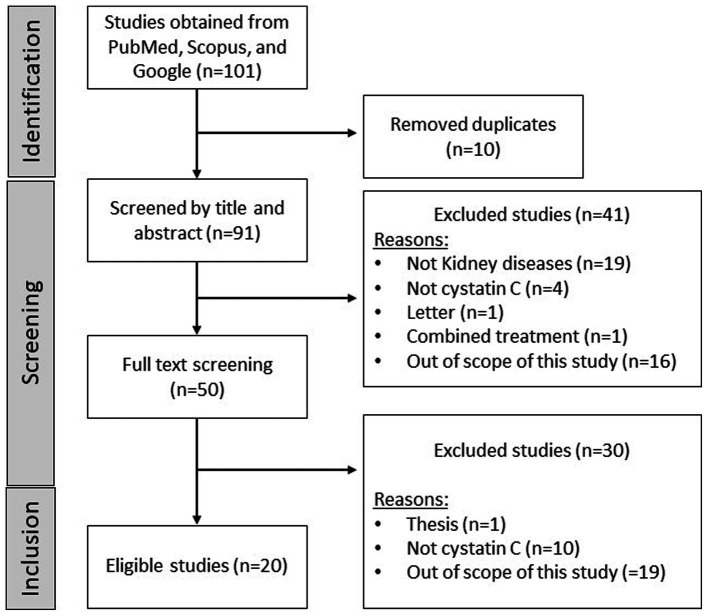
Study selection was primarily conducted by two independent authors, focusing only on clinical articles meeting the criteria. Briefly, the inclusion criteria extend to articles that presented clinical research on kidney diseases, gray literature, pre-prints, and index journal publications, focusing on cystatin C and kidney disease in PLWH on ART. Exclusions were reviews, letters, editorials, notes, non-human studies, incomplete or unpublished studies, and those irrelevant to the scope (Figure 1). The following populations, interventions/exposures, comparators, outcomes (PICO/PECO) were used:
Figure 1.
Flow diagram of the study selection process. Briefly, a systematic search of major electron databases retrieved 80 records, of which 81 were excluded for being duplicates or for not meeting the inclusion criteria. Notably, 20 studies (n = 20) were included and discussed within the manuscript, reporting on cystatin C and kidney disease in people living with HIV (PLWH) on antiretroviral therapy. Studies were mainly excluded because they were out of scope, one is a thesis or not reporting on the modulation of cystatin C.
P: PLWH on ART
I/E: Levels of cystatin C (in PLWH on ART)
C: PLWH not on ART or treatment naive individuals
O: Markers/indicators of renal function.
2.3. Data extraction and quality assessment
Data extraction was independently conducted by two authors, with a primary focus on eligible studies reporting any association between cystatin C and kidney disease in PLWH on ART. The extracted items included author details, the year the study was published, the age of participants, the type of ART regimen, and the main findings reported in each report. Data reporting on other factors, such as ethnicity and existing chronic comorbidities, were also extracted, if available. The quality of included studies was also independently assessed by two authors, using the Downs and Black Checklist (30, 31). The checklist comprises 27 questions and 4 domains, allowing studies to be assessed as either good or excellent (29, 30, 32–35); of these, 12 received a score of good (26, 28, 31, 36–44) and 3 were rated as fair (25, 27, 45). Supplementary File 3 shows that included studies had low reporting bias with a mean score of 10 out of a possible score of 11 (overall agreement 85.67%, kappa = 0.71), excellent external validity with a mean score of 3 out of 3 (overall agreement 39.13%, kappa = 0.13), good internal validity with a mean score of 5 out of 7 (overall agreement 65.22%, kappa = 0.34), and low risk selection bias with a mean score of 4 out of possible 6 (overall agreement 56.51%, kappa = 0.19).
3. Results
3.1. General characteristics and quality of included studies
Briefly, a systematic search yielded nine qualifying clinical studies, published between 2008 and 2023 (Table 1). In terms of regional distribution, China contributed two qualifying studies, while evidence from other countries was broadly spread; the majority of studies were from the United States (n = 5), followed by Germany (n = 2), Nigeria (n = 2), Italy (n = 2), and China (n = 2), while other countries presented with one report, including France, Indonesia, Japan, Serbia, Spain, Poland, and the United Kingdom (Table 1). The study design included four randomized controlled trials, nine observational studies, four cross-sectional studies, one case–control, and two retrospective designs (Table 1). The study population involved PLWH on ART, with a sample size ranging from as low as n = 15 to as high as n = 670. Most studies (n = 10) encompassed adults with mean ages ranging from 34 to 53 years, one study involved young adults with a mean age of 17 years, and one study reported on children with a mean age of 12 years (Table 1). The study duration ranged from as low as 12 weeks to a maximum of 8 years of ART monitoring. However, it was also clear that most studies (n = 4) had a duration of approximately 48 weeks (Table 1). The quality of the included studies (n = 20) was assessed using the Downs and Black Checklist. Briefly, most of the studies (n = 15) were rated as having good quality of evidence, with two studies classified as having excellent quality evidence, scoring 25 and 27 out of 28 total scores (32, 45). Three of the 20 included studies were rated as fair quality studies, with scores of 17, 18, and 19 out of 28 possible scores (39, 43, 50). Based on the different domains, overall, there was an excellent reporting bias as indicated by a mean score of 10 (9–11) out of 11 possible scores, with a slight rater agreement between the two independent reviewers as indicated by a Cohen’s Kappa value (K) of 0.020. Furthermore, the studies exhibited overall good external validity with a mean score of 3 (0–3) out of 3 total scores and a fair rater agreement value of K = 0.30, and they also demonstrated overall good internal validity with a mean score of 5 (4–6) out of 7 total scores and a moderate rater agreement value of K = 0.42. The majority of the studies (n = 17) had good power (≥90%), indicating that these studies had no type 2 error, with a median score of 0.85 (0–1) (Supplementary File 3).
Table 1.
Overview of clinical evidence linking cystatin C and kidney disease in people living with human immunodeficiency virus (PLWH).
| References | Country | Type of study | Study population, including age | Intervention, including ART regimen and treatment duration | Main findings |
|---|---|---|---|---|---|
| Jones et al. (32) | United States | Cross-sectional study | PLWH on ART (n = 250), with a mean age of 41 years | Received ART for at least 1 year | Approximately 2.4% of the participants showed lower eGFRscr compared to approximately 15% showing low estimated glomerular filtration rate from creatinine (eGFRcystC) |
| Mauss et al. (33) | Germany | Observational study | PLWH on ART (n = 92), with a mean age of 37 years | Received ART for 3 years | Cystatin C correlated with HIV RNA and CD4+ T-cell count, but was suppressed after initiation of ART |
| Falasca et al. (34, 35) | Italy | Observational study | PLWH on ART (n = 15) with higher cardiovascular disease risk, with a mean age of 51 years | Received different types of ART for ≥12 months | Raised plasma levels of cystatin C were consistent with increased concentrations of leptin, interleukin-6/-18, and hypoadiponectinemia |
| Inker et al. (36) | United States | Observational study | PLWH on ART (n = 200), with a mean age of 48 years | Received ART with or without tenofovir, within 6 months after confirmation of HIV status | Cystatin C equation was not more accurate than the creatinine equation. The creatinine–cystatin C equation was more accurate than the cystatin C equation |
| Overton et al. (37) | United States | Observational study | PLWH on ART (n = 670) with renal dysfunction, with a mean age of 41 years | Received ART-containing tenofovir or ritonavir or both | Totally, 40% of subjects had renal dysfunction; 3.3% had chronic kidney disease. Elevated cystatin C was present in 18% of subjects |
| Bhasin et al. (38) | United States | Observational study | PLWH on ART (n = 187), with a mean age of 49 years | Received ART-containing tenofovir or cobicistat | eGFRCystC bias and accuracy were strongly associated with the use of ART, HIV RNA suppression, and percentages of activated CD4 or CD8 T-cells |
| Yoshino et al. (39) | Japan | Retrospective design | PLWH on ART (n = 18), with a mean age of 44 years | Received ART-containing dolutegravir for ≥12 months | While the level of eGFRCystC was not changed, that of the estimated glomerular filtration rate from creatinine (eGFRscr) fluctuated |
| Dragović et al. (40) | Serbia | Cross-sectional study | PLWH on ART (n = 33) with metabolic syndrome, with a mean age of 46 years | Received ART for at least 6 months | There was a positive correlation of cystatin C and C-reactive protein in PLWH with metabolic syndrome, compared to those without the metabolic syndrome |
| Szymczak et al. (41) | Poland | Observational study | PLWH on ART (n = 119) without a history of kidney dysfunction, with a mean age of 40 years | Received tenofovir disoproxil fumarate, protease inhibitors or 3 years | Low current CD4+ cell count was consistent with raised levels of cystatin C level. Use of tenofovir or other potentially nephrotoxic antiretroviral drugs did not have any impact on urinary cystatin C levels |
| Casado et al. (24) | Spain | Observational study | PLWH (n = 288) on ART, with a mean age of 50 years | Received dual regimens that included (dolutegravir + rilpivirine, 92; dolutegravir + darunavir/cobicistat, 23; dolutegravir, 26; cobicistat, 19; control group, 128) for 48 weeks | eGFRscr was reduced in PLWH taking two transporter inhibitors. This was similar to those taking dolutegravir or cobicistat. Similarly, the evolution of proteinuria and tubular dysfunction was apparent in all the groups, albeit no significant changes in eGFRCystC |
| Rashbaum et al. (45) | United States | Randomized controlled trial | PLWH (n = 725) on ART, with a mean age of 34 years | Received single-tablet regimen of darunavir/cobicistat/emtricitabine/tenofovir vs. darunavir/cobicistat + emtricitabine/tenofovir disoproxil fumarate (control) alafenamide for 48 weeks | Estimated glomerular filtration rate from serum cystatin C (eGFRCystC) remained unchanged between treatment groups. This was consistent with bone mineral density |
| Hamzah et al. (42) | United Kingdom | Randomized controlled trial | PLWH (n = 31) with a history of tenofovir disoproxil fumarate-associated proximal renal tubulopathy on ART, with a mean age of 53 years | Received 1:1 to continue current antiretroviral therapy or initiate emtricitabine/ tenofovir alafenamide for 12 weeks | eGFRCystC and eGFRscr were not affected, including other makers such as albuminuria, proteinuria, renal phosphate or urea handling, (fasting) urine osmolality, parathyroid hormone, and bone turnover markers |
| John et al. (43) | Nigeria | Randomized controlled trial | PLWH on ART (n = 200), with a mean age of 27 years | Received ART-containing tenofovir or ritonavir or both | There was a significant difference between the values of creatinine, cystatin c, and urea recorded in PLWH on treatment |
| Priscilla et al. (44) | Nigeria | Case–control study | PLWH (n = 100) on ART, with a mean age of 37 years | Received lamivudine, stavudine, and nevirapine and monitored for no longer than 5 years | Serum cystatin C levels were elevated in men, regardless of ART status. This was correlated with serum creatinine levels |
| Zhao et al. (46) | China | Cross-sectional study | PLWH (n = 172) on ART, with a mean age of 40 years | Received tenofovir disoproxil fumarate + lamivudine + efavirenz, tenofovir disoproxil fumarate + plus lamivudine + ritonavir-boosted lopinavir, tenofovir disoproxil fumarate + lamivudine plus dolutegravir, or elvitegravir, cobicistat, emtricitabine and tenofovir alafenamide fumarate, and monitored for 1 year | eGFRscr alone was higher than eGFR calculated by the combination of serum creatinine and cystatin C, while that of eGFRCystC was lower than eGFR calculated from both markers. This indicates that eGFR calculated by the combination of serum creatinine and cystatin C is more accurate, than each marker alone |
| Hikasa et al. (47) | Japan | Observational study | PLWH (n = 63) on ART, with a mean age of 39 years | Switched the antiretroviral drug from tenofovir disoproxil fumarate to tenofovir alafenamide, and exposures were 1.6 and 1.5 years, respectively | Switching from tenofovir disoproxil fumarate to tenofovir alafenamide was an independent predictor of improved eGFRCystC slope |
| Lu et al. (48) | China | Retrospective design | PLWH (n = 138), over the mean age of 36 years | Received dolutegravir + tenofovir disoproxil fumarate, dolutegravir only, or tenofovir disoproxil fumarate for 48 weeks at most | Serum creatinine was significantly elevated in PLWH receiving dolutegravir + tenofovir disoproxil fumarate. Serum cystatin C and eGFRCystC in those receiving dolutegravir were not affected, while eGFRcr was significantly higher in those receiving dolutegravir |
| Monin et al. (49) | Germany | Randomized controlled trial | PLWH (n = 263) on ART, with a mean age of 17 years | Switched to dolutegravir + boosted darunavir (with ritonavir) or continued with two nucleoside reverse transcriptase inhibitors in combination with ritonavir-boosted darunavir once daily for 48 weeks | eGFRscr was reduced while serum levels of cystatin C were not affected. Meanwhile, the low-density lipoprotein fraction was improved |
| Mondesert et al. (23) | France | Observational study | PLWH on ART (n = 262) with kidney dysfunction | Received cobicistat +elvitegravir, ritonavir + protease inhibitor, dolutegravir, dolutegravir +rilpivirine, rilpivirine, raltegravir, bictegravir, and other antiretroviral drugs | Mean eGFRcystC was higher than mean eGFRscr |
| Rostania et al. (50) | Indonesia | Cross-sectional study | PLWH (n = 60) on ART PLWH with a mean age of 12 years | Received a combination of tenofovir, stavudine, lamivudine, zidovudine, abacavir, nevirapine, efavirenz, lopinavir/ for 8 years, at least | The serum cystatin C levels were high and correlated with high viral load in PLWH on ART. However, CD4+ count had no association with the serum levels of cystatin C |
4. Discussion
4.1. Clinical evidence on the potential use of cystatin C to monitor kidney disease in PLWH on ART
Approximately nine studies reported on the use of cystatin C, together with the creatinine-based GFR, to monitor the kidney function in PLWH on ART (Table 1). However, nearly half of the studies included in this study supported the use of estimated GFR (from creatinine) as the preferred biomarker to assess or monitor the kidney function in PLWH on ART (42, 45, 46, 49). Interestingly, in addition to using GFR-based creatinine to monitor kidney disease, most of these studies favored the use of additional biomarkers to assess the kidney function in PLWH (Table 1). These biomarkers include albuminuria, proteinuria, and renal phosphate or urea handling, although cystatin C was predominantly used (Table 1). In terms of reliability between both creatinine- and cystatin-based GFRs, it was evident that the reported information was inconsistent between the studied subjects. For example, in some studies, it was shown that while the creatinine-based GFR was fluctuating, the levels of cystatin C remained unchanged and could be reliably used to monitor the kidney function in PLWH (24, 49), while other studies indicated that using both creatinine- and cystatin C-based GFRs could even be more reliable than using either marker alone in monitoring the kidney function in PLWH (36, 43, 46). Nonetheless, other studies indicated that cystatin C use alone could also be effectively used to detect the kidney function or kidney disease in PLWH (45), even correlating cystatin C levels with high viral loads in some participants (50). In other studies (34, 35, 40), it was also evident that elevated levels of cystatin C were consistent with increased levels of pro-inflammatory markers such as C-reactive protein, leptin, and interleukin-6/−18, possibly indicating other underlying conditions such as metabolic disease, which could contribute to the progression of kidney dysfunction in PLWH. Interestingly, in recent years, the rapid growth in the prevalence of metabolic diseases that is accompanied by abnormally high levels of inflammation has significantly affected PLWH (51–54), possibly contributing to the high burden of disease in many developing and developed countries.
4.2. Impact of ART on serum levels of cystatin C and other biomarkers of kidney disease in PLWH
It is important to note that the initial use of the zidovudine drug, which was approved in 1987 by the United States Food and Drug Administration, was instrumental in initiating the effective management of PLWH (55). Currently, many developments have been made in terms of managing PLWH, especially through the use of ART to suppress or reduce the viral load (56). In fact, the published literature predominately reports on recent ART combinations, especially protease inhibitors or nucleoside reverse transcriptase inhibitors, which are potentially linked with the development of non-communicable diseases, including kidney disease-associated risk factors (57–59). Notably, the sustained use of a tenofovir disoproxil fumarate-containing ART regimen has been linked with the detrimental effects of oxidative stress and inflammation, driving pathological abnormalities in renal cells (60, 61). This could be observed independently through the fluctuations in cystatin C levels in PLWH on ART (41). However, it was evident that the effective use of ART and suppression of CD4+ cell count were associated with low cystatin C levels in some patients (32, 33, 41). Thus, it remains important to understand the relationship between serum cystatin C and eGFR to monitor the kidney function in PLWH on ART. In this study, evidence indicated that the creatinine-based GFR increased in PLWH taking tenofovir disoproxil fumarate-containing ART (42, 45), but this was not observed in other studies (46, 48). Moreover, switching to a dolutegravir-based ART regimen could improve the kidney function in PLWH, as measured using both creatinine- and cystatin C-based GFRs (Table 1).
4.3. Summary and future perspective
Kidney injury is common in PLWH and has been associated with an increased risk of morbidity and mortality (62). Therefore, it has become imperative to routinely monitor biomarkers of kidney disease in PLWH. This aspect affirms existing literature on the necessity for screening, early diagnosis, and prediction of kidney disease in PLWH, especially those from low- and middle-income countries (63, 64). Although serum creatinine is considered an indirect marker for the renal GFR, it lacks specificity to detect damage to kidney tissue, and its relatively delayed response to injury could hinder early detection of acute kidney injury (65). Furthermore, studies have reported that serum creatinine levels are elevated with increasing age, body weight, and grip strength in some PLWH (66). This information renders this biomarker potentially unreliable to efficiently monitor the kidney function or the development of kidney disease. Clinical evidence from the current review indicates that while the measurement of creatinine-based GFR can fluctuate, cystatin C is likely to remain consistent in PLWH on ART. The use of both creatinine- and cystatin C-based GRF remains important and is recommended for monitoring the kidney function in PLWH on ART. However, the costs associated with the routine use of cystatin C together with creatinine to monitor the kidney function should be considered. In terms of the type of ART regimen, the use of tenofovir-containing ART may be potentially detrimental by increasing creatinine-based GFR as an indicator of kidney dysfunction in PLWH, while that of dolutegravir-based ART could be less toxic to the kidney. Evidence already supports the beneficial or superior effects of dolutegravir in combination with nucleoside reverse transcriptase inhibitors for suppressing HIV in PLWH (67, 68). This aspect underscores the need for more evidence to confirm these effects, especially since the cystatin C-based GFR was not affected. Further research is still required to provide a clear description of the modulation of these biomarkers to describe the relationship between both acute and chronic kidney dysfunction in PLWH on ART. Importantly, additional clinical evidence is required to determine the impact of different ART regimens on kidney injury in PLWH.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SH: Conceptualization, Funding acquisition, Investigation, Writing – original draft, Writing – review & editing. JC: Conceptualization, Writing – original draft, Writing – review & editing. HM: Writing – review & editing. SM: Funding acquisition, Writing – review & editing. ZM: Writing – review & editing. MM: Writing – review & editing, Data curation. NM: Writing – review & editing, Data curation. BN: Writing – review & editing. DN: Writing – review & editing. UN: Writing – review & editing. AK: Writing – review & editing. PD: Conceptualization, Funding acquisition, Writing – original draft, Writing – review & editing, Methodology, Supervision.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. SH was funded by the National Research Foundation (NRF) (grant number: TTK2204082828). PD was supported in part by the NRF (grant numbers: 117829 and 141929). The study by SM, reported herein, was made possible through partial funding by the South African Medical Research Council through its Division of Research Capacity Development under the Researcher Development Award Program. NM acknowledges funding from the NRF.
Abbreviations
ART, Antiretroviral therapy; HIV, Human immunodeficiency virus; LDL, Low-density lipoprotein; PECO, Population, Exposure, Comparison, Outcome; PLWH, People living with human immunodeficiency virus; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PROSPERO, International Prospective Register for Systematic Reviews.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Author disclaimer
The content hereof is the sole responsibility of the authors and does not necessarily represent the official views of the NRF.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2024.1295217/full#supplementary-material
References
- 1.Kovesdy CP. Epidemiology of chronic kidney disease: an update 2022. Kidney Int Suppl. (2022) 12:7–11. doi: 10.1016/j.kisu.2021.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hariparshad S, Bhimma R, Nandlal L, Jembere E, Naicker S, Assounga A. The prevalence of chronic kidney disease in South Africa—limitations of studies comparing prevalence with sub-Saharan Africa, Africa, and globally. BMC Nephrol. (2023) 24:62. doi: 10.1186/s12882-023-03109-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfano G, Cappelli G, Fontana F, di Lullo L, di Iorio B, Bellasi A, et al. Kidney disease in HIV infection. J Clin Med. (2019) 8:1254. doi: 10.3390/jcm8081254, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferenbach DA, Bonventre JV. Acute kidney injury and chronic kidney disease: from the laboratory to the clinic. Nephrol Ther. (2016) 12:S41–8. doi: 10.1016/j.nephro.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Makris K, Spanou L. Acute kidney injury: definition, pathophysiology and clinical phenotypes. Clin Biochem Rev. (2016) 37:85–98. PMID: [PMC free article] [PubMed] [Google Scholar]
- 6.Fisher MC, Fazzari MJ, Hanna DB, Patel VV, Felsen UR, Alahiri E, et al. Brief report: acute kidney injury in people living with HIV hospitalized with coronavirus disease 2019: clinical characteristics and outcomes. J Acquir Immune Defic Syndr. (2021) 87:1167–72. doi: 10.1097/QAI.0000000000002698, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Y, Shlipak MG, Grunfeld C, Choi AI. Incidence and risk factors for acute kidney injury in HIV infection. Am J Nephrol. (2012) 35:327–34. doi: 10.1159/000337151, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.George C, Hill J, Nqebelele U, Peer N, Kengne AP. Leveraging the south African diabetes prevention Programme to screen for chronic kidney disease: an observational study. BMJ Open. (2023) 13:e068672. doi: 10.1136/bmjopen-2022-068672, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montessori V, Press N, Harris M, Akagi L, Montaner JS. Adverse effects of antiretroviral therapy for HIV infection. CMAJ. (2004) 170:229–38. PMID: [PMC free article] [PubMed] [Google Scholar]
- 10.Silva BF, Peixoto G, da Luz SR, de Moraes S, Peres SB. Adverse effects of chronic treatment with the Main subclasses of highly active antiretroviral therapy: a systematic review. HIV Med. (2019) 20:429–38. doi: 10.1111/hiv.12733 [DOI] [PubMed] [Google Scholar]
- 11.Wyatt CM. Kidney disease and HIV infection. Top Antivir Med. (2017) 25:13–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Kaboré NF, Poda A, Zoungrana J, da O, Ciaffi L, Semdé A, et al. Chronic kidney disease and HIV in the era of antiretroviral treatment: findings from a 10-year cohort study in a west African setting. BMC Nephrol. (2019) 20:155. doi: 10.1186/s12882-019-1335-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swanepoel CR, Atta MG, D'Agati VD, Estrella MM, Fogo AB, Naicker S, et al. Kidney disease in the setting of HIV infection: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Kidney Int. (2018) 93:545–59. doi: 10.1016/j.kint.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oguntibeju OO. Quality of life of people living with HIV and AIDS and antiretroviral therapy. HIV AIDS. (2012) 4:117–24. doi: 10.2147/HIV.S32321, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diana NE, Davies M, Mosiane P, Vermeulen A, Naicker S. Clinicopathological correlation of kidney disease in HIV infection pre- and post-ART rollout. PLoS One. (2022) 17:e0269260. doi: 10.1371/journal.pone.0269260, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lucas GM, Cozzi-Lepri A, Wyatt CM, Post FA, Bormann AM, Crum-Cianflone NF, et al. Glomerular filtration rate estimated using creatinine, cystatin C or both markers and the risk of clinical events in HIV-infected individuals. HIV Med. (2014) 15:116–23. doi: 10.1111/hiv.12087, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yukawa S, Watanabe D, Uehira T, Shirasaka T. Clinical benefits of using inulin clearance and cystatin C for determining glomerular filtration rate in HIV-1-infected individuals treated with dolutegravir. J Infect Chemother. (2018) 24:199–205. doi: 10.1016/j.jiac.2017.10.015, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Heron JE, Bagnis CI, Gracey DM. Contemporary issues and new challenges in chronic kidney disease amongst people living with HIV. AIDS Res Ther. (2020) 17:11. doi: 10.1186/s12981-020-00266-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Murty MS, Sharma UK, Pandey VB, Kankare SB. Serum cystatin C as a marker of renal function in detection of early acute kidney injury. Indian J Nephrol. (2013) 23:180–3. doi: 10.4103/0971-4065.111840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. (2008) 3:348–54. doi: 10.2215/CJN.02870707, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaitonde DY, Cook DL, Rivera IM. Chronic kidney disease: detection and evaluation. Am Fam Physician. (2017) 96:776–83. [PubMed] [Google Scholar]
- 22.Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol Hypertens. (2015) 24:295–300. doi: 10.1097/MNH.0000000000000115 [DOI] [PubMed] [Google Scholar]
- 23.Mondesert E, Reynes J, Makinson A, Bargnoux AS, Plawecki M, Morquin D, et al. Cystatin C in addition to creatinine for better assessment of glomerular renal function decline in people with HIV receiving antiretroviral therapy. AIDS. (2023) 37:447–54. doi: 10.1097/QAD.0000000000003434, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Casado JL, Monsalvo M, Vizcarra P, Fontecha M, Serrano-Villar S, Moreno S. Evaluation of kidney function in HIV-infected patients receiving an antiretroviral regimen containing one or two inhibitors of the tubular secretion of creatinine. HIV Med. (2019) 20:648–56. doi: 10.1111/hiv.12784 [DOI] [PubMed] [Google Scholar]
- 25.Chen DC, Lees JS, Lu K, Scherzer R, Rutherford E, Mark PB, et al. Differential associations of cystatin C versus creatinine-based kidney function with risks of cardiovascular event and mortality among south Asian individuals in the UK biobank. J Am Heart Assoc. (2023) 12:e027079. doi: 10.1161/JAHA.122.027079, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gagneux-Brunon A, Mariat C, Delanaye P. Cystatin C in HIV-infected patients: promising but not yet ready for prime time. Nephrol Dial Transplant. (2012) 27:1305–13. doi: 10.1093/ndt/gfs001 [DOI] [PubMed] [Google Scholar]
- 27.Wondifraw Baynes H, Tegene B, Gebremichael M, Birhane G, Kedir W, Biadgo B. Assessment of the effect of antiretroviral therapy on renal and liver functions among HIV-infected patients: a retrospective study. HIV AIDS. (2017) 9:1–7. doi: 10.2147/HIV.S120979, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Röling J, Schmid H, Fischereder M, Draenert R, Goebel FD. HIV-associated renal diseases and highly active antiretroviral therapy-induced nephropathy. Clin Infect Dis. (2006) 42:1488–95. doi: 10.1086/503566, PMID: [DOI] [PubMed] [Google Scholar]
- 29.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JPT, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. (2019) 10:Ed000142. doi: 10.1002/14651858.ED000142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health. (1998) 52:377–84. doi: 10.1136/jech.52.6.377, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Connor SR, Tully MA, Ryan B, Bradley JM, Baxter GD, McDonough SM. Failure of a numerical quality assessment scale to identify potential risk of bias in a systematic review: a comparison study. BMC Res Notes. (2015) 8:224. doi: 10.1186/s13104-015-1181-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones CY, Jones CA, Wilson IB, Knox TA, Levey AS, Spiegelman D, et al. Cystatin C and creatinine in an HIV cohort: the nutrition for healthy living study. Am J Kidney Dis. (2008) 51:914–24. doi: 10.1053/j.ajkd.2008.01.027, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mauss S, Berger F, Kuschak D, Henke J, Hegener P, Wolf E, et al. Cystatin C as a marker of renal function is affected by HIV replication leading to an underestimation of kidney function in HIV patients. Antivir Ther. (2008) 13:1091–5. doi: 10.1177/135965350801300810, PMID: [DOI] [PubMed] [Google Scholar]
- 34.Falasca K, Ucciferri C, Mancino P, di Iorio A, Vignale F, Pizzigallo E, et al. Cystatin C, adipokines and cardiovascular risk in HIV infected patients. Curr HIV Res. (2010) 8:405–10. doi: 10.2174/157016210791330365, PMID: [DOI] [PubMed] [Google Scholar]
- 35.Falasca K, Ucciferri C, Mancino P, Vignale F, Vecchiet J. Cystatin C and cardiovascular risk in HIV infected patients. Retrovirology. (2010) 7:P62. doi: 10.1186/1742-4690-7-S1-P62 [DOI] [PubMed] [Google Scholar]
- 36.Inker LA, Wyatt C, Creamer R, Hellinger J, Hotta M, Leppo M, et al. Performance of creatinine and cystatin C GFR estimating equations in an HIV-positive population on antiretrovirals. J Acquir Immune Defic Syndr. (2012) 61:302–9. doi: 10.1097/QAI.0b013e31826a6c4f, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Overton ET, Patel P, Mondy K, Bush T, Conley L, Rhame F, et al. Cystatin C and baseline renal function among HIV-infected persons in the SUN study. AIDS Res Hum Retrovir. (2012) 28:148–55. doi: 10.1089/aid.2011.0018, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Bhasin B, Lau B, Atta MG, Fine DM, Estrella MM, Schwartz GJ, et al. HIV viremia and T-cell activation differentially affect the performance of glomerular filtration rate equations based on creatinine and cystatin C. PLoS One. (2013) 8:e82028. doi: 10.1371/journal.pone.0082028, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoshino Y, Koga I, Seo K, Kitazawa T, Ota Y. Short communication: the clinical value of cystatin C as a marker of renal function in HIV patients receiving Dolutegravir. AIDS Res Hum Retrovir. (2017) 33:1080–2. doi: 10.1089/aid.2017.0074, PMID: [DOI] [PubMed] [Google Scholar]
- 40.Dragović G, Srdić D, al Musalhi K, Soldatović I, Kušić J, Jevtović D, et al. Higher levels of cystatin C in HIV/AIDS patients with metabolic syndrome. Basic Clin Pharmacol Toxicol. (2018) 122:396–401. doi: 10.1111/bcpt.12919, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Szymczak A, Szymanek-Pasternak A, Zalewska M, Małyszczak K, Rymer W, Knysz B. Assessment of urinary cystatin C levels in HIV-1-infected patients with preserved kidney function. HIV AIDS Rev. (2018) 17:236–42. doi: 10.5114/hivar.2018.80254 [DOI] [Google Scholar]
- 42.Hamzah L, Williams D, Bailey AC, Jones R, Ibrahim F, Musso CG, et al. Early safety of tenofovir alafenamide in patients with a history of tubulopathy on tenofovir disoproxil fumarate: a randomized controlled clinical trial. HIV Med. (2020) 21:198–203. doi: 10.1111/hiv.12819, PMID: [DOI] [PubMed] [Google Scholar]
- 43.Ikpeama Osita John OPA, Mariam ON, Anthonia IC, Joy IC, Osazuwa IO, Andrew IE, et al. Study of cystatin C in early detection of renal impairment in patient with HIV/AIDS. South Asian Res J Agri Fisher. (2020) 2:3. doi: 10.36346/sarjaf.2020.v02i04.003 [DOI] [Google Scholar]
- 44.Ezeugwunne Ifeoma Priscilla AC, Chukwuemeka MS, Adamma AR, Chinonso NJ, Nwabunwanne OV, Onyeka IEC, et al. Evaluation of microalbumin, cystatin c, creatinine and uric acid levels in HIV patients in Nnamdi Azikiwe university teaching hospital, Nnewi. J Commun Health Manage. (2021) 8:132–42. doi: 10.18231/j.jchm.2021.030 [DOI] [Google Scholar]
- 45.Rashbaum B, Spinner CD, McDonald C, Mussini C, Jezorwski J, Luo D, et al. Darunavir/cobicistat/emtricitabine/tenofovir alafenamide in treatment-naïve patients with HIV-1: subgroup analyses of the phase 3 AMBER study. HIV Res Clin Pract. (2019) 20:24–33. doi: 10.1080/15284336.2019.1608714, PMID: [DOI] [PubMed] [Google Scholar]
- 46.Zhao N, Zeng Z, Liang H, Wang F, Yang D, Xiao J, et al. Estimation of renal function by three CKD-EPI equations in Chinese HIV/AIDS patients: a STROBE-compliant article. Medicine. (2021) 100:e26003. doi: 10.1097/MD.0000000000026003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hikasa S, Shimabukuro S, Hideta K, Higasa S, Sawada A, Tokugawa T, et al. Effect of switching from tenofovir disoproxil fumarate to tenofovir alafenamide on estimated glomerular filtration rate slope in patients with HIV: a retrospective observational study. J Infect Chemother. (2022) 28:396–400. doi: 10.1016/j.jiac.2021.11.016 [DOI] [PubMed] [Google Scholar]
- 48.Lu L, Li X, Liu X, Han Y, Qiu Z, Song X, et al. Comparison of renal function biomarkers of serum creatinine and cystatin C in HIV-infected people on Dolutegravir-containing therapy. Infect Drug Resist. (2022) 15:1695–706. doi: 10.2147/IDR.S347054, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Monin M, Kümmerle T, Schneider J, Cordes C, Heiken H, Stellbrink HJ, et al. Switching to a NRTI-free 2 drug regimen (2DR) -a sub-analysis of the 48 weeks DUALIS study on metabolic and renal changes. HIV Res Clin Pract. (2021) 23:15–21. PMID: [PubMed] [Google Scholar]
- 50.Wita Rostania AA, Hilmanto D. Association of CD4 cell counts and viral load with cystatin C level in children with human immunodeficiency virus (HIV) infection. Paediatr Indones. (2023) 63:88–95. doi: 10.14238/pi63.2.2023.88-95 [DOI] [Google Scholar]
- 51.Todowede OO, Mianda SZ, Sartorius B. Prevalence of metabolic syndrome among HIV-positive and HIV-negative populations in sub-Saharan Africa-a systematic review and meta-analysis. Syst Rev. (2019) 8:4. doi: 10.1186/s13643-018-0927-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ojong E, Iya B, Djeufouata J, Ndeh F, Nsonwu A, Njongang V, et al. Metabolic syndrome and its components among HIV/AIDS patients on antiretroviral therapy and ART-Naïve patients at the University of Calabar Teaching Hospital, Calabar, Nigeria. Afr Health Sci. (2022) 22:410–7. doi: 10.4314/ahs.v22i1.50, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nyambuya TM, Dludla PV, Mxinwa V, Nkambule BB. The effect of successful antiretroviral therapy on immune activation and reconstitution in HIV infected adults: a systematic review and Meta-analysis. AIDS Rev. (2020) 23:1–12. doi: 10.24875/AIDSRev.20000039, PMID: [DOI] [PubMed] [Google Scholar]
- 54.Nkambule BB, Mxinwa V, Mkandla Z, Mutize T, Mokgalaboni K, Nyambuya TM, et al. Platelet activation in adult HIV-infected patients on antiretroviral therapy: a systematic review and meta-analysis. BMC Med. (2020) 18:357. doi: 10.1186/s12916-020-01801-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sperling R. Zidovudine. Infect Dis Obstet Gynecol. (1998) 6:197–203. doi: 10.1155/S1064744998000404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ebrahim O, Mazanderani AH. Recent developments in hiv treatment and their dissemination in poor countries. Infect Dis Rep. (2013) 5:e2. doi: 10.4081/idr.2013.s1.e2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kajogoo VD, Gorret Atim M, Amare D, Geleta M, Muchie Y, Tesfahunei HA, et al. HIV protease inhibitors and insulin sensitivity: a systematic review and Meta-analysis of randomized controlled trials. Front Pharmacol. (2021) 12:635089. doi: 10.3389/fphar.2021.635089, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Malindisa E, Balandya E, Njelekela M, Kidenya BR, Francis F, Mmbaga BT, et al. Metabolic syndrome among people living with HIV on antiretroviral therapy in Mwanza, Tanzania. BMC Endocr Disord. (2023) 23:88. doi: 10.1186/s12902-023-01340-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gallant JE, Moore RD. Renal function with use of a tenofovir-containing initial antiretroviral regimen. AIDS. (2009) 23:1971–5. doi: 10.1097/QAD.0b013e32832c96e9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tourret J, Deray G, Isnard-Bagnis C. Tenofovir effect on the kidneys of HIV-infected patients: a double-edged sword? J Am Soc Nephrol. (2013) 24:1519–27. doi: 10.1681/ASN.2012080857, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Venter WDF, Fabian J, Feldman C. An overview of tenofovir and renal disease for the HIV-treating clinician. South Afr J HIV Med. (2018) 19:817. doi: 10.4102/sajhivmed.v19i1.817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kalim S, Szczech LA, Wyatt CM. Acute kidney injury in HIV-infected patients. Semin Nephrol. (2008) 28:556–62. doi: 10.1016/j.semnephrol.2008.08.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.George C, Echouffo-Tcheugui JB, Jaar BG, Okpechi IG, Kengne AP. The need for screening, early diagnosis, and prediction of chronic kidney disease in people with diabetes in low- and middle-income countries-a review of the current literature. BMC Med. (2022) 20:247. doi: 10.1186/s12916-022-02438-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.George C, Mogueo A, Okpechi I, Echouffo-Tcheugui JB, Kengne AP. Chronic kidney disease in low-income to middle-income countries: the case for increased screening. BMJ Glob Health. (2017) 2:e000256. doi: 10.1136/bmjgh-2016-000256, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Duijl TT, Ruhaak LR, de Fijter JW, Cobbaert CM. Kidney injury biomarkers in an academic hospital setting: where are we now? Clin Biochem Rev. (2019) 40:79–97. doi: 10.33176/AACB-18-00017, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yilma D, Abdissa A, Kæstel P, Tesfaye M, Olsen MF, Girma T, et al. Serum creatinine and estimated glomerular filtration rates in HIV positive and negative adults in Ethiopia. PLoS One. (2019) 14:e0211630. doi: 10.1371/journal.pone.0211630, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Paton NI, Musaazi J, Kityo C, Walimbwa S, Hoppe A, Balyegisawa A, et al. Dolutegravir or Darunavir in combination with zidovudine or Tenofovir to treat HIV. N Engl J Med. (2021) 385:330–41. doi: 10.1056/NEJMoa2101609 [DOI] [PubMed] [Google Scholar]
- 68.Fantauzzi A, Mezzaroma I. Dolutegravir: clinical efficacy and role in HIV therapy. Ther Adv Chronic Dis. (2014) 5:164–77. doi: 10.1177/2040622314530461, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.