Summary
Ribose 2′-O-methylation is involved in critical biological processes, but its biological functions and significance in mRNAs remain underexplored. We have developed NJU-seq, a sensitive method for unbiased 2′-O-methylation (Nm) profiling, and Nm-VAQ, a site-specific quantification tool. Using these tools in tandem, we identified thousands of Nm sites on mRNAs of human and mouse cell lines, of which 68 of 84 selected sites were further validated to be more than 1% 2′-O-methylated. Unlike rRNA, most mRNA Nm sites were from 1% to 30% methylated. In addition, mRNA Nm was dynamic, changing according to the circumstance. Furthermore, we show that fibrillarin is involved as a methyltransferase. By mimicking the detected Nm sites and the context sequence, the RNA fragments could be 2′-O-methylated and demonstrated higher stability but lower translation efficiency. Last, profiling of Nm sites in lung surgery samples revealed common signatures of lung cancer pathogenesis, providing potential new diagnostic markers.
Keywords: 2-O-methylation, epigenetics, RNA modification, virus response, mRNA regulation, alternative splicing, translation regulation, fibrillarin methyltransferase, diagnose marker
Graphical abstract

Highlights
-
•
A platform for high-throughput and site-specific detection of RNA 2′-O-methylation (Nm)
-
•
Nominates thousands of mRNA Nm sites, with methylation ratios ranging from 1% to 30%
-
•
Demonstrates dynamic response of Nm to viral infection in cells
-
•
Profiling of Nm sites in patient samples reveals signatures of lung cancer pathogenesis
Motivation
2′-O-methylation (Nm) modification is a widely distributed and abundant modification, playing crucial roles in RNA structural stability, protein translation rates, and alternative splicing types. However, research on the location information, catalytic mechanisms, and functions of Nm on mRNAs would benefit from the development of effective and reliable detection tools. In addition, it is also difficult for existing methods to provide an accurate measure of the proportion of modification at the Nm site. To address these gaps, we have developed an integrated methodological strategy to screen and validate Nm sites, especially on mRNA.
Tang et al. present an integrative platform for detection of RNA 2′-O-methylation (Nm), including high-throughput and site-specific Nm detection technologies. Using these methods in tandem, they identify precise mRNA Nm sites and ratios and explore molecular mechanisms and disease-related occurrences.
Introduction
More than 100 RNA modifications have been identified, constituting the epi-transcriptome code and affecting RNA functions.1 Among these RNA modifications, 2′-O-methylation (otherwise known as Nm), whereby ribose is methylated at the 2′ hydroxyl position without limitation of the base identity, is a widespread RNA modification phenomenon including multiple RNA types and species.1,2,3,4,5 Furthermore, several sporadic published studies have demonstrated the importance of Nm in multiple biological processes as an essential signaling regulator. For example, Nm stabilizes the RNA structure by maintaining the ribose C3′-endo conformation, resisting nucleophilic attack, and avoiding 3′–5′ degradation triggered by uridylation.6,7,8 In addition, a single Nm modification as a molecular tag distinguishes between internal and external RNAs, generates abnormal alternative splicing, and inhibits translation.5,9,10,11,12,13,14,15 However, despite its potential wide-ranging cellular activities, an in-depth exploration of Nm biological functions is still limited due to the lack of screening tools to detect Nm sites.
Four high-throughput Nm detection methods have been developed in the past few years: 2′OMe-seq,16 RiboMethSeq,7 Nm-seq,17,18 and Nm-mut-seq.19 These methods can reliably detect rRNA Nm modifications for an abundant amount of rRNA and a high modification ratio. In addition, liquid chromatography-mass spectrometry (LC-MS) and reverse transcription at low deoxy-ribonucleoside triphosphate (dNTP) concentrations followed by polymerase chain reaction (RTL-P) have also been used to validate rRNA site-specific Nm status.20,21 However, the small amount, complex sequence context, and low methylation ratio of mRNA present barriers to the practical application of those methods in mRNA. To address this problem, we established an accurate high-throughput single-base Nm-site screening method, NJU-seq, and a site-specific Nm quantification tool, Nm-VAQ. With the help of these two methods, we efficiently revealed the broad distribution of Nm sites on mRNAs and observed that most validated Nm sites were methylated at ratios from 1% to 30%.
In the previous studies, rRNA was methylated by a small nucleolar ribonucleoprotein (snoRNP) complex, which contained fibrillarin (FBL) as the methyltransferase, NOP56/58 and SNU13 as co-factors, and a partial complementary small nucleolar RNA (snoRNA) with conserved C/D box as the guide RNA.22 Although a particular Nm site on mRNA was reported to be methylated by the same mechanism,12 how the rest of the Nm sites on mRNA were methylated remained unknown. On the other hand, viruses such as HIV were reported to be 2′-O-methylated by human FTSJ3, which acts as a methyltransferase, in the host cell.23 However, further exploration of the whole mechanism relies on accurate Nm site information and methylation ratio evaluation.
Results
Establishment of NJU-seq
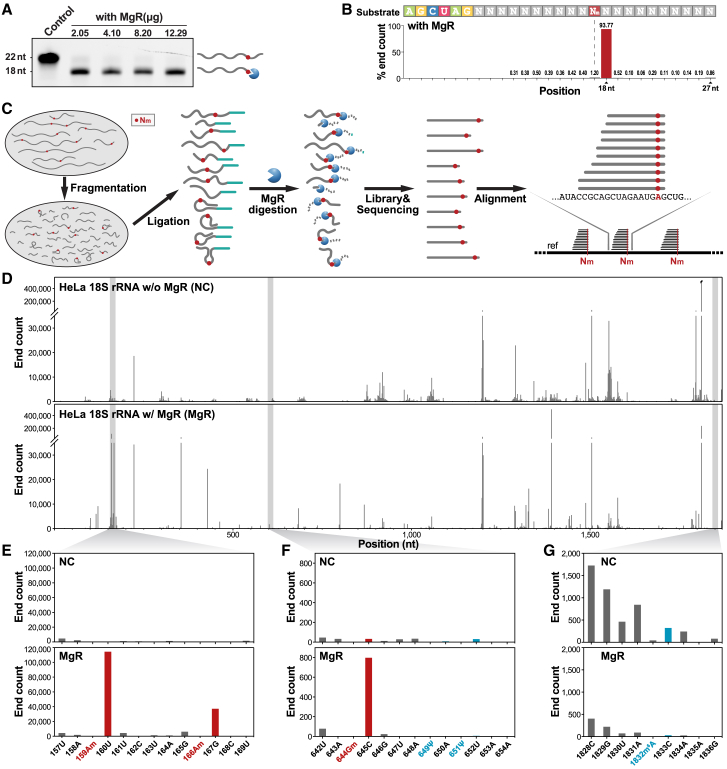
Based on the characteristics of Nm, our detection strategy was to take advantage of an Nm-sensitive RNase. Therefore, the newly established NJU-seq relied on helicase and hydrolase activities of RNase R from Mycoplasma genitalium (MgR) on single-stranded RNA (ssRNA) from the 3′ to the 5′ direction, which produced the digested end products at every Nm + 1 site.13 First, a synthesized 22 nt ssRNA substrate carrying one mixed Nm site, NmNN (N = A/G/C/U), was incubated with MgR to evaluate its sensitivity to Nm sites (Figures 1A, S1A, and S1B). As expected, the 18 nt ssRNA products accumulated with increasing MgR concentration, confirming the enzyme activity’s versatility. In addition, we also performed a reaction of MgR with the synthesized 40 nt ssRNA substrate specifically carrying only the Am site at position 28 to demonstrate the exact breakpoint of MgR activity, and the products were TA cloned and sequenced (Figure S1C). Importantly, we discovered that all ssRNA products ended at the Am + 1 position (20/20), supporting the detection of Nm sites by the accumulated 3′ ends of MgR reaction products. To confirm that the digestion pause was stable in most contexts and may not be affected by certain secondary structures, we incubated an ssRNA with Nm at the 17th position among mixed ribonucleotides (N) from the 7th to the 17th position (−10 to 10 position relative to the Nm site) with MgR, and the products were sequenced by next-generation sequencing (NGS). As shown in Figure 1B, more than 93% of products ended at the Nm + 1 position, with almost equal ribonucleotide distribution from −10 to −1. In addition, we used multiple ssRNAs with an extended stem region as substrates. As expected, MgR digested all the ssRNA to the Nm + 1 position successfully (Figures S1E and S1F). Furthermore, an ssRNA substrate with m6A and m5C sites downstream of the Am site was incubated with MgR, demonstrating clear products ending at the Nm + 1 site but nothing at other modification sites (Figure S1D). All the above data indicated that MgR digestion was a great solution to create RNA fragments that ended at the Nm + 1 position stably and precisely with no ribonucleotide, context, or secondary structure bias.
Figure 1.
Establishment of a high-throughput method, NJU-seq, for RNA 2′-O-methylation
(A) In vitro ssRNA hydrolysis assay. The ssRNA substrate (5′-6-Fam-UAACCUAUGA AGNNNUNmNNC UC-3′, N represents the mix of A/G/C/U) was reacted with varying MgR amounts. The left electrophoresis shows the digestion products of MgR, and a schematic is shown on the right.
(B) High-throughput sequencing of MgR-hydrolyzed ssRNA products. The ssRNA substrate (5′-AGCUA GNNNN NNNNN NNmNNN NNNNN NN-3′) was incubated with MgR protein, followed by library construction and NGS. All reads shorter than 27 nt were counted, and the ratios are presented.
(C) Schematic workflow of NJU-seq. (1) Total RNA fragmentation; (2) 3′ tail ligation; (3) RNase R from Mycoplasma genitalium (MgR) digestion; (4) NGS library preparation and high-throughput sequencing; (5) alignment and analysis.
(D) Plots showing read 3′ end counts on HeLa 18S rRNA with (MgR, bottom) and without (NC, top) MgR digestion.
(E–G) Plots showing read 3′ end counts of regions 157–169, 642–654, and 1,828–1,836 on 18S rRNA, without and with MgR digestion. Sites marked with red were previously reported as 2′-O-methylated, and those with blue were previously reported as m6A or pseudouridine.
We combined MgR with NGS to develop a new high-throughput Nm screening method named NJU-seq (Nm-judged-universally sequencing). As detailed in the STAR Methods, there are five main steps in NJU-seq (Figure 1C). A sample prepared with the same process without MgR treatment is used as a negative control (NC) group to filter the false-positive outcomes caused by RNA fragmentation bias. First, we tested NJU-seq on total RNA from HeLa cells and analyzed the rRNA results. The sequencing data were aligned to a reference sequence, and the 3′ ends of the reads were counted (Figure 1D). Comparing rRNA alignments after MgR digestion with the NC group, 3′ end enrichments corresponded to potential Nm sites. For example, between positions 157 and 169 in 18S rRNA, we found that the reads ending at positions 160 and 167 were significantly accumulated, indicating two possible Am sites at positions 159 and 166 (Figure 1E). To further evaluate whether other modifications on RNA would affect the activity of MgR, the sites’ read distributions around all reported sites with eight other epigenetic RNA modifications were browsed: m6A, pseudouridine (ψ), ac4C, m62A, m1A, m7G, m5C, and m3U (Figures S2 and S3).24 Based on the results, none of these eight epigenetic modifications caused significant enrichment at 1 nt downstream of or surrounding the position compared with the NC group, while Nm sites demonstrated notable enrichment at 1 nt downstream, as expected.
We scored each site with the difference of reads ending at 1 nt downstream divided by reads ending just at the target site between the MgR treatment and the NC group to set a quantified standard (Figure S5A). The score was mainly correlated with the copy number of fragments with Nm modification at the Nm site (such as in Nm-seq) and not the methylation ratio (such as in RiboMethSeq). Subsequently, considering the enrichment of specific sites by RNA fragmentation bias and other unknown factors, candidate Nm sites were further filtered based on whether reads ending at 1 nt downstream of the MgR treatment group were significantly more abundant than in the NC group to exclude false-positive results. We defined the filtering parameter as FoldChange, which was calculated by dividing the reads from the treatment group at position N + 1 by the reads from the NC group. The details of the scoring and filtering steps and some examples are also described in the STAR Methods.
Establishment of Nm-VAQ as a site-specific validation and quantification tool of Nm status
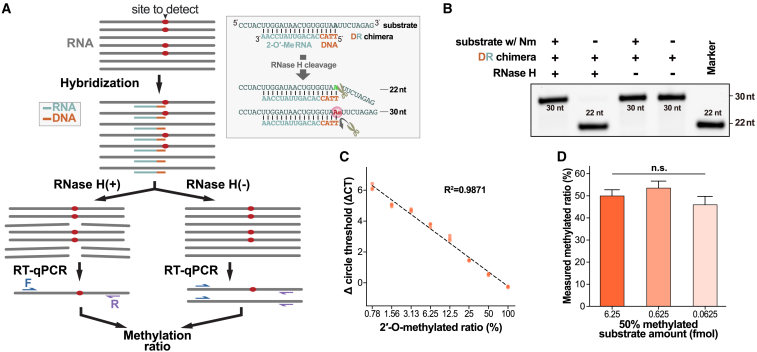
In previous studies, RTL-P was widely used as a semi-quantification method that could validate sites with a high Nm ratio.21 However, RTL-P had several disadvantages, such as it being hard to validate one site specifically or measure the methylation level accurately, results varying due to the number of targets, or demonstrating potential false-positive or -negative results.21 Here, we hoped to develop a method to validate the Nm status of a specific site and acquire the methylation ratio. Hence, we established the Nm-VAQ (an RNase H-based Nm validation and absolute quantification tool) with inspiration from previous related studies.25 Briefly, Nm-VAQ was based on selective cleavage of RNase H and the guidance of a DNA-RNA hybrid probe to target the cleavage site (Figure 2B). With several tests of multiple designs of hybrid probes, we anchored the RNase H cleavage site to the target site with a probe containing 4 DNAs and 13 RNAs, as shown (Figures 2B and S4A–S4G). Combined with qPCR, Nm-VAQ quantified the mixed substrates with Nm gradients accurately with much lower methylation ratio (Figure 2C), while RTL-P could detect only Nm sites with modifications higher than 40%21 and presented significantly different results under different conditions (Figures S4H and S4I). In addition, the tests of Nm-VAQ with 50% 2′-O-methylated substrates at 6.25, 0.625, and 0.0625 fmol demonstrated highly consistent results, proving its evaluation capability in low-amount RNA studies (Figure 2D). To date, Nm-VAQ has allowed the study of Nm to the absolute quantification stage.
Figure 2.
Establishment of a low-throughput Nm validation tool, Nm-VAQ
(A) Schematic workflow of Nm-VAQ. (1) The hybridization of RNA and chimera probe. In the substrate, the red site indicates that the target site is 2′-O-methylated. In the chimera probe, the reddish-brown region shows the DNA, and the light green region shows the 2′-O-methylated RNA; (2) with/without RNase H cleavage; (3) RT-qPCR. The methylation ratio is from the ΔCT (cycle threshold) of RNase H cleavage and control sample. The box on the right shows an example of RNase H cleavage directed by the chimera probe in (B). Hybrid schemes of RNA substrates (upper sequences) and chimera probes (lower sequences) are shown.
(B) The RNase H reaction products are presented by electrophoresis. The numbers present the length of the FAM-labeled cleavage products.
(C) Correlation between the 2′-O-methylated ratio of the substrate and ΔCT (cycle threshold) for the Nm-VAQ assay. Substrate (6.25 × 10−2 pmol) with and without Nm was mixed to obtain a known Nm ratio, and ΔCT was obtained from the RNase-H-treated and control samples.
(D) Measurement of three different substrate concentrations with an Nm ratio of 50%. Nm ratio was calculated with ΔCT. n.s., not significant, by ordinary one-way ANOVA.
Mapping of Nm sites of human cell lines
Although our main goal was to identify Nm sites on mRNA, we started with rRNA to evaluate the results. Briefly, based on Score(average) ≥30 and FoldChange(average) ≥3, we identified 54, 39, and 1 potential Nm sites in 28S, 18S, and 5.8S rRNA, of which 46, 34, and 1 site were previously reported Nm sites (Figure S7A; Table S1). For these three rRNAs, the true-positive rate was ∼66.7%, 81.0%, and 50.0%, which means ∼2/3 of the known Nm sites were screened out. Meanwhile, the positive predictive value was ∼85.2%, 87.2%, and 100.0%, which means more than 4/5 potential Nm sites screened out were reported to be true positive. To have a side-by-side comparison of this result with other methods, we ranked the scores based on their respective algorithms and selected the top 10, 20, and 30, up to the top 39 for 18S rRNAs and up to the top 54 for 28S rRNAs, and counted true-positive Nm sites, which demonstrated comparable results (Table S1). These findings highlighted the reliability and promise of NJU-seq in challenging Nm identification on mRNA.
We next focused on the exploration of Nm sites on mRNAs from HeLa cell lines. With rRNA elimination, the proportion of mRNA in the substrate was substantially increased before MgR treatment. The same identification criteria as for rRNA, with Score(average) ≥30 and FoldChange(average) ≥3, were applied to identify the potential Nm sites on mRNA, which amounted to 4,074 in HeLa cell lines, as detailed in the STAR Methods (Figure 3A; Table S2).
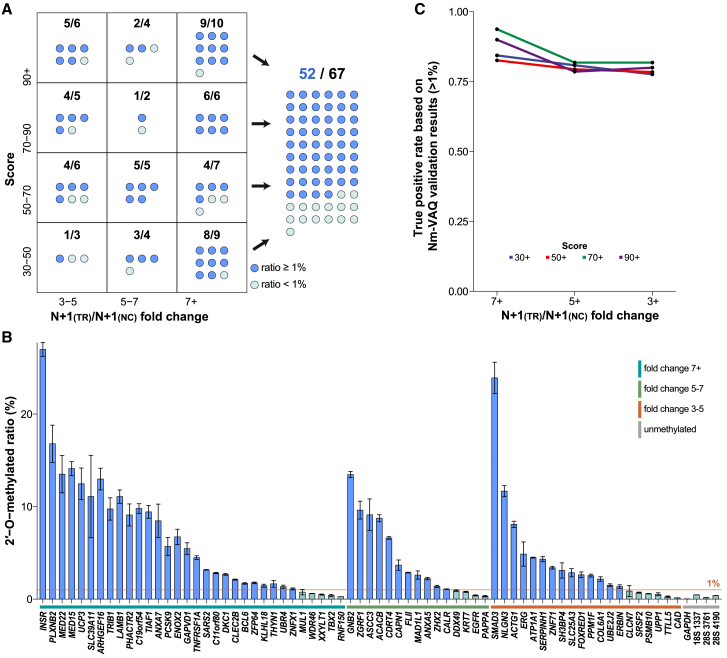
Figure 3.
Validation of HeLa mRNA Nm sites by Nm-VAQ
(A) The distribution of HeLa mRNA Nm sites validated by Nm-VAQ. Sixty-seven mRNA Nm sites were divided into 12 regions based on Score(average) and FoldChange(average) from NJU-seq. Each box represents the number of Nm sites detected with (blue dots) and without (light green dots) an Nm ratio ≥1%. Overall, among the detected 67 mRNA Nm sites, 52 exhibited a modification ratio ≥1%.
(B) The methylation ratio in HeLa mRNA Nm sites detected by Nm-VAQ. The Nm sites are divided into three ranges of FoldChange(average) (3–5, 5–7, 7+) and ranked from highest to lowest. The unmethylated sites on GAPDH mRNA (12:6,537,190, G) and 28S 4,190A and 3,761C and 18S 1,337C, which were reported as m6A, m5C, and ac4C in previous studies, were selected as the negative controls. The sites in blue had 2′-O-methylation ratios higher than 1%, while the cyan sites were less methylated. The complete information on all selected sites can be found in Table S6. Error bars show the SEM for three technical replicates.
(C) True-positive rates based on Nm-VAQ validation results (>1%) in different FoldChange(average) ranges and Score(average) ranges.
Before moving forward to further analysis and statistics of newly identified sites, we wanted to assess the rationality of the standard we set and the approximate true-positive rate of the 2′-O-methylated sites we selected. Generally, all 4,074 identified mRNA sites of the HeLa cell lines were separated into 12 regions within the matrix of the Score(average) (30/50/70/90) and FoldChange(average) (3/5/7) (Figures 3A and S8B). Sixty-seven sites were selected from the boundary regions of each region as the candidates to be further validated by Nm-VAQ (Figure 3A; Table S2). As shown in Figure 3B, 52 of 67 sites demonstrated Nm ratios higher than 1%, while four NCs, unmethylated or methylated by other modification sites, demonstrated no positive results by Nm-VAQ. Meanwhile, the in vitro-transcribed RNA fragments were used to evaluate the potential 2′-O-methylated status of corresponding sites on BCL6 (3, 187,721,452, Gm) and PLXNB2 (22, 50,290,219, Gm) by Nm-VAQ as an NC, which did not produce any false-positive signal (Figure S8A). In addition, the in vitro transcriptome, which is similar in amounts and composition of mRNA but with almost no modification, served as an NC in RNA m6A modification detection tools to confirm the specificity of the identified modification sites.26 Here, the mRNA site scores of in vitro transcriptomes were significantly lower than those identified Nm sites of the template, indicating the high correlation of NJU-seq with Nm modification (Figure S9). The methylation ratios of all selected sites were lower than 30%, much lower than sites in rRNA24 (Figures S7C and 3B). By calculating the proportion of sites with methylated ratios of each region and different standards, the true-positive rate remained higher than 75% no matter which Score or FoldChange threshold we used (Figure 3C). Hence, NJU-seq was confirmed to be a reliable method to screen potential Nm sites with pretty high accuracy.
We also selected five Nm sites on HeLa mRNA with high scores as targets for RTL-P validation. Only three showed positive results compared with GAPDH as an NC (Figure S10A). Since the mRNA Nm status of most sites may be much lower than rRNA, and RTL-P was limited by false-positive, false-negative, semi-quantitative, and non-single-nucleotide resolution defects, Nm-VAQ was more appropriate in the following study than RTL-P.
We next applied NJU-seq on the other two cell lines, HEK293T and A549, and acquired 2,059/2,367 mRNA Nm sites. One thousand forty-four mRNA Nm sites were shared among all three cell lines (Figure 4A). Three Nm sites shared by three cell lines were selected for further validation: BCL6 (3, 187,721,452, Gm), PLXNB2 (22, 50,290,219, Gm), and TBX2 (17, 61,401,930, Cm). Nm-VAQ confirmed their methylation and evaluated the Nm ratios as 0.4%–18% (Figure 4B). All validated sites demonstrated clear Nm status but were much lower than Nm sites on rRNA. In addition, we performed gene ontology (GO) enrichment analysis of genes with three-cell-line-shared Nm sites and could see their extensive involvement in various biological processes (Figure S13B).
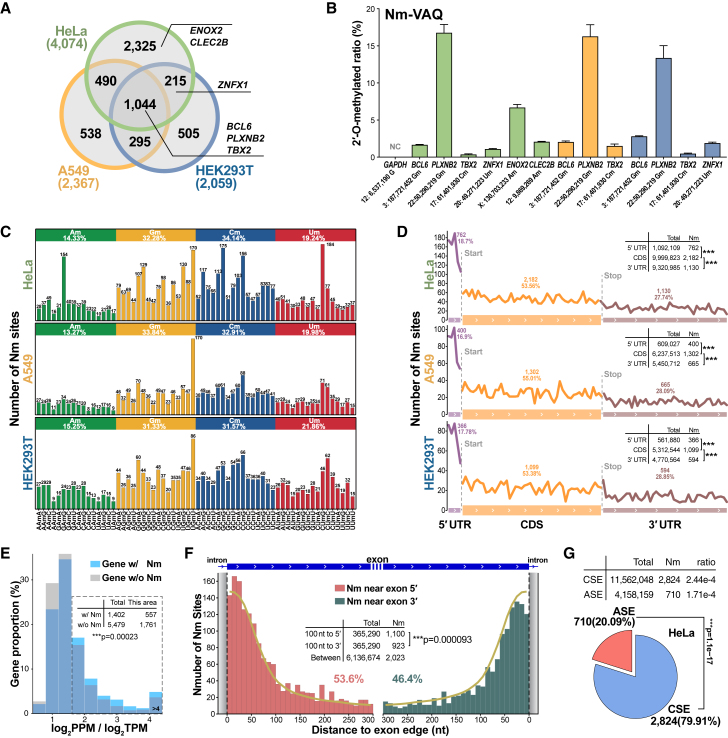
Figure 4.
mRNA Nm sites in three human cell lines
(A) Overlap of mRNA Nm sites among the three cell lines by NJU-seq.
(B) The methylation status in mRNA Nm sites detected by Nm-VAQ. The Nm sites of HeLa, HEK293T, and A549 cells are presented as green, blue, and orange sites, respectively. The HeLa Nm sites’ ratio data are the same as in Figure 3B. Error bars show the SEM for three technical replicates.
(C) Methylated nucleotide frequency distribution of HeLa mRNA. Nm − 1, Nm, and Nm + 1 sites were considered tri-ribonucleotide units.
(D) Distribution of HeLa Nm sites on a length-normalized mRNA transcript (see STAR Methods). ∗∗∗p < 0.001 (Pearson’s chi-squared test).
(E) Translation efficiency of genes with and without Nm site(s) in HeLa. The ratio of protein abundance and RNA expression (log2 PPM/log2 TPM) represents translation efficiency (PPM, parts per million, describing each protein to the entire expressed proteome; TPM, transcripts per kilobase of exon model per million mapped reads).∗∗∗p < 0.001 (Pearson’s chi-squared test).
(F) Distribution of Nm sites near the exon 5′ (left) and exon 3′ (right) boundaries. All Nm sites were counted and divided into these two parts. The yellow line represents the simulation of an even distribution. ∗∗∗p < 0.001 (Pearson’s chi-squared test).
(G) Distribution of HeLa Nm sites in alternatively spliced exon (ASE) and constitutively spliced exon (CSE) regions. The density of Nm is calculated by dividing the number of positions by the length. ∗∗∗p < 0.001 (Pearson’s chi-squared test).
To identify the overall distribution of Nm, we analyzed the sequence composition around the Nm sites. We found that Nm modification occurred in a distinct pattern on different ribonucleotides but was similar among the three cell lines (Figure 4C). Although A on mRNA may act as a branchpoint in the intron part and regulate alternative splicing by its Nm status,11 more Nms were modified on C and G than on A and U. We found high GC content around the Nm sites in the three cell lines, especially G, which might indicate modification bias (Figure S10B). When we further calculated Nm − 1 and Nm + 1 ribonucleotides with Nm together as a tri-ribonucleotide unit, the results showed an apparent uneven distribution with a clear enrichment of UGmU among all three cell lines (Figure 4C).
2′-O-methylation is involved in mRNA alternative splicing events and translation
To identify the potential functions of Nm modifications, we analyzed Nm’s distribution patterns in different functional mRNA regions. By exploring the Nm distribution patterns on mRNA transcripts, we found that Nm numbers were coding sequence (CDS) > 3′ untranslated region (UTR) > 5′ UTR in the three cell lines, while the 5′ UTR demonstrated the highest density (Figure 4D). To further investigate the Nm site distribution, we focused on the start and stop codons (Figures S10C and S10D). We selected 3,479 HeLa Nm sites on mRNAs that could cover −50 to +500 nt around the start codon to count the distribution in 50-nt-wide windows. Similarly, 3,074 HeLa Nm sites were selected to evaluate the stop codon’s −500 to +500 nt regions. Interestingly, both areas demonstrated apparent downward trends of Nm sites from the 5′ to the 3′ direction. Such a pattern was also seen in the other two cell lines. Such results implied that there were relatively stable patterns of Nm distribution in the functional regions of mRNA.
Next, we compared the protein abundance of genes with and without Nm (Figure 4E). The correlation was assessed by protein abundance/mRNA abundance. Within the entire mRNA population, Nm modifications tended to be more enriched in mRNAs with higher protein abundance level. This observation strongly implies a correlation between Nm modification and protein scale regulation, thus affirming the essential role of Nm in regulating biological processes linked to proteins, including translation/degradation and export. Interestingly, such enrichments were observed in genes with Nm sites located in the CDS and 3′ UTR, but not in the 5′ UTR (Figures S10I–S10K). In 2019, Elliott et al. demonstrated translation inhibition by Nm modification.12 Here, Nm may play a similar role in regulating genes with higher protein abundances as a negative feedback regulator.
Previous studies elucidated that pre-mRNA splicing of ACT1 was affected by Am modification, so we explored whether Nm sites were extensively involved in mRNA splicing.11 Interestingly, there were more Nm sites closer to the 5′ exon boundary than to the 3′ exon boundary, significant in the 0–100 window from the boundary (Figures 4F, S10E, and S10G). Such discoveries demonstrated a potential correlation between Nm and splicing. To further demonstrate the effects of splicing, we assigned mRNA Nm sites as constitutively spliced exons (CSEs) or alternatively spliced exons (ASEs).10,27 The total length of ASEs and CSEs with modifications was counted, and the density of Nm sites was obtained to eliminate the impact of length differences. We found that higher Nm ratios were significantly located more in the CSEs than in the ASEs by chi-squared test (Figures 4G, S10F, and S10H). Such results suggested the importance of Nm in the occurrence and regulation of splicing during mRNA maturation.
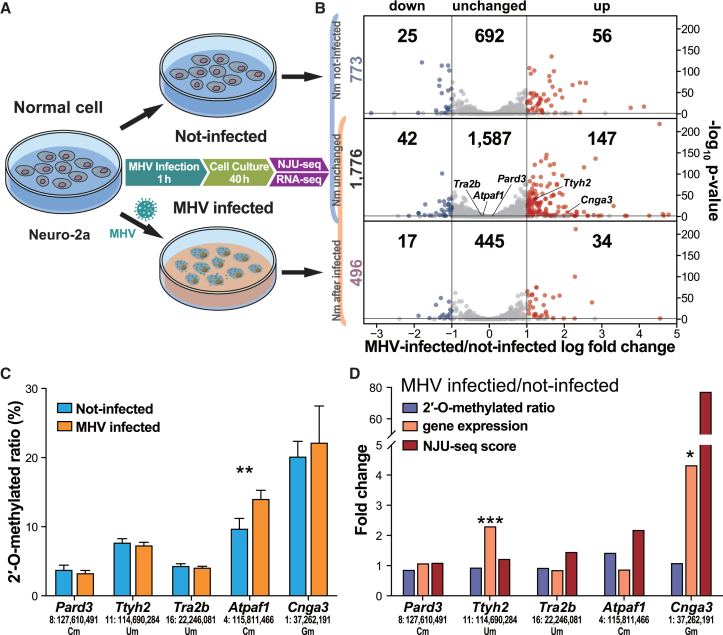
2′-O-methylation dynamic response to virus infection
As Nm sites were associated with gene functions, it was interesting to explore whether mRNA Nm participates in gene regulation in response to external stimuli. Therefore, we designed virus infection experiments to observe the dynamic change in Nm sites. We first selected the mouse neuroblastoma (Neuro-2a) cell line as a target to explore mRNA Nm changes before and after mouse hepatitis virus (MHV) A59 infection as a model of viral infection response (Figure 5A). Based on the same identification criteria as the previous parts (Score(average) ≥ 30 and FoldChange(average) ≥ 3), 2,272 and 2,549 Nm sites were identified from Neuro-2a cell lines with or without MHV infection, among which 496 Nm sites specifically appeared in the infection group (Figure 5B; Table S3).
Figure 5.
Dynamic 2′-O-methylation response to virus infection
(A) mRNA Nm sites changed in the Neuro-2a cell line after MHV infection. The flow of MHV infection is shown. Each of three MHV-infected and uninfected samples was assessed with NJU-seq and RNA-seq.
(B) As shown on the left, 773 uninfected-specific, 1,776 shared, and 496 MHV-infected-specific Nm sites were detected. Genes containing these Nm sites were identified as downregulated, unchanged, or upregulated according to RNA-seq. The x axis shows the expression level of MHV infection compared with the uninfected group, while the y axis shows significance tested by DESeq2.
(C) The 2′-O-methylated ratio of shared Nm sites detected by Nm-VAQ. Error bars show the SEM for three biological replicates. ∗∗p < 0.01 (t test).
(D) Fold change in the shared Nm sites’ 2′-O-methylated ratio, NJU-seq score, and Nm-site-located gene expression after MHV infection. ∗p < 0.05, ∗∗∗p < 0.001 (t test).
Together with the results of RNA sequencing (RNA-seq), all sites and the relative mRNA expression were divided into nine groups of MHV-infected specific/shared/uninfected specific and expression downregulated/unchanged/upregulated as shown (Figures 5B, S13C, and S13D). Five sites were selected to evaluate their Nm status and expression change further. Among them, the methylation ratios of Pard3 (8:127,610,491, Cm), Ttyh2 (11:114,690,284, Um), Tra2b (16:22,246,081, Um), and Cnga3 (1:37262191, Gm) showed no obvious change, while the methylation ratio of Atpaf1 (4:115,811,466, Cm) increased from 9.8% to 14.1% (Figures 5C and 5D).
Based on the principle of NJU-seq, the results were affected by both methylation status and expression change. For example, the expression level of Atpaf1 showed no apparent change, while the methylation ratio increased by about 4%, which led to a significant increase in the NJU-seq score. On the other hand, the methylated ratio of Cnga3 remained at approximately 20%, but the score by NJU-seq increased dramatically due to four times more upregulated mRNA expression (Figure 5D).
Notably, 284 sites methylated after MHV infection were located on the 3′ UTR, of which 264 were located on the microRNA (miRNA)/RNA-induced silencing complex (RISC) binding region (Table S3). For example, Gm (15:5,097,989) of Card6 mRNA was located upstream of the cleavage site precisely in the target area of mmu-miR-3073a-3p (Figures S11A and S11C). Based on the limited results, it was interesting that Nm might affect the interaction between mRNA molecules and proteins by affecting binding. According to previous studies, Nm could affect RNA-protein interactions by changing the binding surface and space.2,5 We mimicked the Nm on the target mRNA of RISC based on the RISC-mRNA crystal structure (PDB: 6MDZ), and the distances between the relative amino acid and the modified ribonucleotides of positions 5, 12, and 20 on mRNA were too short, which may interfere with RISC binding (Figure S11B). In addition, other biological processes relying on RNase-H-like RNA endoribonuclease activity may also be inhibited by Nm at particular sites, such as Prp8 in alternative splicing, Dicer in miRNA maturation, and RNase L in RNA cleavage of the immune response.28,29,30 Therefore, Nm modifications may be involved in the regulation of miRNA/RISC to modulate the expression of immune-related genes in response to viral stimuli, which is worthwhile to investigate further in subsequent studies.
Exploration of the 2′-O-methylation mechanism on mRNA
In the previous studies, the methylated complex of rRNA Nm sites was well studied; it contained the methyltransferase FBL; companion proteins SNU13, NOP56, and NOP58; and a snoRNA with conserved C-and-D-box motif as a guide to binding to the complementary target.31 Although this complex was reported to modify some mRNA sites and virus RNA sites12,32 and a similar complex may also respond to other mRNA Nm sites, it was hard to believe there were thousands of “undiscovered” snoRNAs that regulated the huge number of mRNA Nm sites. Therefore, in this part, we tried to explore the Nm mechanism of mRNA. On the other hand, unlike m6A, m1A, or m5C, none of the specific motifs or regions were discovered to be Nm enriched, which suggested the methylation process was unlikely to be accomplished by direct recognition of methyltransferase.
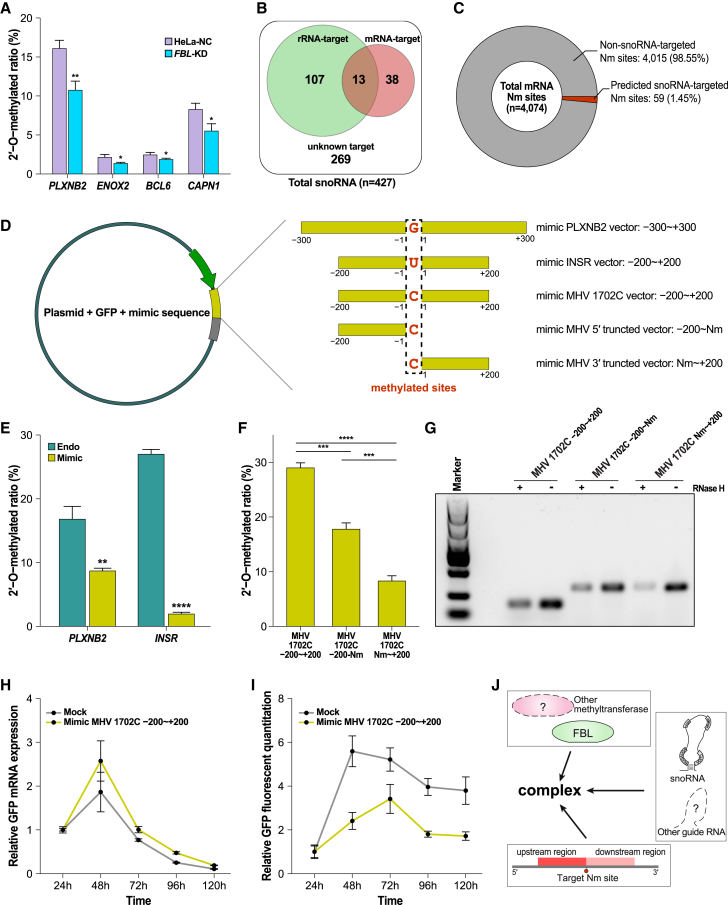
We first confirmed whether FBL was involved in mRNA methylation, since previous studies reported its potential role in mRNA Nm. Based on the proteomic data pulled down by mRNA from previous studies and by ourselves, FBL/NOP56/NOP58 but not SNU13 were found in both lists, implying the potential interaction of FBL with mRNA33 (Table S7). To further evaluate the role of FBL, we selected four sites and used Nm-VAQ to measure the methylation ratios in both groups (Figure 6A). The methylation ratios of all four sites in the small interfering RNA (siRNA) treatment group decreased by approximately one-third compared with the NC group. These results highly suggested that FBL participates in the Nm of mRNA as well as implying the existence of other methyltransferases.
Figure 6.
Exploration of the occurrence and function of Nm modification
(A) The methylation ratio of selected mRNA Nm sites in wild-type and the FBL-KD group HeLa cell line. Error bars show the SEM for three technical replicates. ∗p < 0.05, ∗∗p < 0.01 (unpaired t test).
(B) snoRNA target analysis. All identified snoRNA data and analysis of snoRNA-targeted C/D-box mRNA Nm sites are detailed in the STAR Methods.
(C) The proportion of mRNA Nm sites with or without predicted snoRNA-targeted C/D-box.
(D) Plasmid design of the mimic mRNA sequence to generate Nm. Three intracellular Nm-modified gene fragments were inserted into the 3′ UTR of GFP. Three hundred nucleotides upstream and downstream of the mRNA Nm site PLXNB2 (22:50290219, Gm), 200 nt upstream and downstream of INSR (19:7116934, Um), and 200 nt upstream and downstream of the MHV 1,702 (Cm) site were inserted into plasmids. Similar plasmids with the 1,502–1,702 and 1,702–1,902 regions of the MHV 1,702 insertion were also constructed. The red sites correspond to the original 2′-O-methylated sites.
(E) The methylation ratios of mRNA Nm sites generated by a plasmid with mimic sequence and genome. Error bars show the SEM for three technical replicates. ∗∗p < 0.01, ∗∗∗∗p < 0.0001 (unpaired t test).
(F) The methylation ratios of MHV 1,702C and two truncated sequences generated by the plasmid. Error bars show the SEM for three technical replicates. ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 (unpaired t test).
(G) PCR amplification bands of mRNA with/without RNase H cleavage at potential 2′-O-methylated sites (which are presented in (F)).
(H) The relative GFP mRNA expression. The plasmids with (mimic) or without (mock) fragment insertion were transfected into N2A cells. The cells were subsequently collected at the indicated time points. The GFP mRNA expression levels of each time point were corrected by the relative expression at 24 h after transfection. Error bars show the SEM for three technical replicates.
(I) The relative GFP fluorescence quantitation. The GFP fluorescence of each indicated time point was calculated by ImageJ and corrected by the relative expression at 24 h after transfection. Error bars show the SEM for three technical replicates.
(J) The potential mRNA Nm complex consists of methyltransferase, guide RNA, and target RNA: (1) FBL and another methyltransferase catalyze Nm of mRNA sites. (2) snoRNA and another RNA serve as guides to target the potential Nm sites. (3) Both upstream and downstream context sequences are necessary for the determination of Nm sites.
Based on their unique and highly conserved sequence and secondary structure, most snoRNAs were well identified in the previous studies.16 Among 427 reported snoRNAs in the HeLa cell line, 107 were believed to target specific sites on rRNA. Meanwhile, 51 of them could target the newly identified mRNA Nm sites we reported, with 13 overlapping with rRNA (Figure 6B; Table S8). On the other hand, only 59 mRNA Nm sites were predicted to be targeted by known snoRNAs, accounting for less than 2% of total Nm sites (Figure 6C). This discovery suggests that the potential guide RNA of mRNA Nm may not follow the same rules as snoRNA.
Since the Nm methylation sites were quite specific, we tried to explore whether we could induce 2′-methylation sites by mimicking the sequence of known Nm sites. Briefly, we constructed PX330 plasmids with GFP and introduced a sequence into the 3′ UTR to mimic the context sequence of HeLa cell line PLXNB2 Gm, INSR Um, and MHV 1,702 Cm as described (Figure 6D). As described in the STAR Methods, the plasmids were transferred to relative cell lines, and the RNA was harvested after 24 h. Based on the results, all three complete mimic sequences with upstream and downstream fragments were 2′-O-methylated successfully but with a lower methylation ratio than the relative original sites generated in the genome (Figure 6E). To our surprise, only the mRNA with the mimic upstream or downstream 200 nt sequence of MHV 1,702 Cm could also be 2′-O-methylated, but with only approximately 60% and 20% ratio compared with the full context (Figure 6F). Furthermore, the amplification products before and after RNase H site-specific cleavage of three groups demonstrated the different amounts of methylated fragments (Figure 6G). These results implied that both upstream and downstream context sequences were necessary for Nm target determination, while sequences upstream of the Nm site may play a more important role. Meanwhile, the mRNA seemed to accumulate more in the Nm mimic sequence group than in the mock group (Figure 6H). However, Nm on the 3′ UTR strongly inhibited GFP expression, leading to significantly lower GFP fluorescence (Figures 6I and S13A).
In general, FBL and snoRNAs may be involved in the occurrence of mRNA Nm. However, the remaining pieces of the whole mRNA Nm mechanism needed further exploration (Figure 6J).
Nm demonstrated significantly different patterns in normal and lung cancer tissues
Since viral infection is a transient change, we further explored the dynamics of Nm in chronic diseases. Here, we used eight clinical lung cancer samples (six lung adenocarcinoma [LUAD] and two lung squamous cell carcinoma [LUSC]) as a chronic-disease-response model. NJU-seq was performed to study Nm site differences in lung cancer tissues compared with distant normal lung tissues. As indicated, normal tissue-specific Nm sites were more often present in only one to three samples, while cancer samples had more shared Nm sites, implying the commonness of lung cancer pathogenesis (Figures 7A and S13E). Such cancerous-specific mRNA Nm sites are ideal candidates as diagnostic markers and are essential for future cancer studies (Figures 7B and S12D–S12I; Table S4). For instance, CDH22 encodes the transmembrane protein cadherin-like protein 22, crucial in metastasis or the spread of cancer.34,35 Cm (20:46,174,611) located in the CDH22 CDS was detected in all eight cancer samples but not in distant normal samples with no correlation to mRNA expression differences.
Figure 7.
Significantly different Nm patterns in normal and lung cancer tissues
(A) Sharing of specific Nm sites detected in lung cancer or normal tissue samples is shown. Eight pairs of tumor tissues and distant normal tissues from different lung cancer patients were sequenced and detected. Cancer-specific Nm sites occurred in at least one cancer tissue but were not detected in any normal tissue. The opposite is true for normal-specific Nm sites. The numbers of sites specific in more than one cancer or normal tissue were subjected to the chi-squared test. ∗∗∗p < 0.001 (Pearson's chi-squared test).
(B) Specific mRNA Nm sites that appear in more than five lung cancer samples are listed.
(C) The Um site (11:113,263,177) on NCAM1 was located at the 3′ end of transcript NCAM1-211. The green or red arrows indicate the primers for six transcripts (NCAM1-207, 211, 214, 228, 229, and 230) or five transcripts (without NCAM1-211) in RT-qPCR amplification.
(D) Percentage of transcripts except for NCAM1-211. The proportion of these five transcripts to the six transcripts covering the Nm site is shown. Red means Nm-detected, while gray means undetected.
Further analysis of cancer-specific Nm sites revealed that Nm sites might regulate the molecular function of cancer gene mRNAs in multiple ways. As mentioned above, Nm is closely related to splicing, so we focused on regulating the splicing complex. Interestingly, several Nm sites located upstream of the alternative splicing site can be found, regulating alternative splicing products. For instance, the NCAM1 gene is involved in cell growth, differentiation, and migration.36 Um (11:113,263,177) on NCAM1 mRNA was detected in four LUAD and two LUSC samples located on the 3′ boundary of transcript NCAM1-211 (Figure 7C). We examined all six transcripts covering this site (NCAM1-207, 211, 214, 228, 229, and 230) to verify the association between the Um site and splicing. Among six LUAD samples, the expression ratio of the five longer transcripts was lower in the four LUAD samples with Nm than in the two samples without Nm, suggesting that Nm modification was associated with alternative splicing to regulate the mRNA products (Figures 7D and S12J–S12M).
Discussion
The Nm modification of RNA ribose affects diverse biological processes. Thus, high-throughput identification for comprehensive and accurate profiling of Nm sites is vital for further research on cellular regulation and functions of Nm. Four high-throughput Nm detection methods have been developed in the past few years: 2′OMe-seq,16 RiboMethSeq,7,37,38 Nm-seq,17,18 and Nm-mut-seq19 (Table S1). Based on the above principle, 2′OMe-seq and RiboMethSeq can identify Nm sites with abundant amounts and high methylation ratios with very good sensitivity, such as most Nm sites on rRNA, but with various and inconsistent results on Nm sites with lower methylation ratios. For example, 18S 354U was ∼20% 2′-O-methylated and was identified as methylated in 2016 by 2′OMe-seq, but the opposite by RiboMethSeq.7,16 Such challenge was also seen on 18S 1,440 Cm, which was not reported until 2020.39 Based on our results, the methylation ratio of almost all tested mRNA Nm sites was lower than 30%, which indicated that most Nm sites on mRNAs were unable to be detected by 2′OMe-seq and RiboMethSeq. On the other hand, the Nm-seq method and Nm-mut-seq demonstrated improved mRNA Nm-site detection by direct targeting to mRNA molecules with Nm.17,18,19 However, two main problems of Nm-seq remained and were solved by NJU-seq: (1) Nm-seq is based on multiple cycles of OED (oxidation-elimination-dephosphorylation) + RNA purification to enrich the Nm signal, which results in a massive loss of RNA content and takes at least 16 h. With NJU-seq, enrichment of the Nm + 1 site can be accomplished with only 30 min MgR digestion for only one reaction with much less RNA sample. (2) Nm-seq has a ligation bias of fragments. After Nm-seq treatment, the 3′ end of the RNA is 2′-O-methylated, resulting in a much lower ligation efficiency than for fragments without the oxidation process.40 Thus, some Nm sites are insufficient to obtain more sequencing reads than surrounding non-modified sites. The RNA fragment in NJU-seq ends at the Nm + 1 site without Nm, which has no bias in the ligation step. On the other hand, Nm-mut-seq has two remaining significant deficiencies: (1) Nm-mut-seq cannot detect Um modifications, which account for roughly 20% of the mRNA Nm sites. In contrast, NJU-seq demonstrates excellent capability in identifying Um modifications. (2) The Nm-mut-seq samples among three replicate samples exhibited poor stability, with a replication rate of less than 10% observed in both HeLa and HepG2 cells across three replicate samples. These shortages may limit the use of Nm-seq and Nm-mut-seq in subsequent studies.
To observe the specificity and sensitivity of NJU-seq, we knocked down the known rRNA 2′-O-methyltransferase FBL with siRNA in the HeLa cell line as reported in the previous study.12,37 Although FBL knockout was proven to be lethal, siRNA could lower the expression of FBL, which led to a small decrease in rRNA Nm.37 As expected, FBL mRNA expression was significantly decreased as confirmed by RT-qPCR (Figure S14A). After normalization to total rRNA-aligned reads, fragments ending 1 nt downstream of reported Nm sites in the FBL-knockdown (KD) group were significantly fewer than those in the untreated group (Figures S14B and S14C). Similar decreases in read accumulations have also been observed in some mRNA Nm sites of the FBL-KD group (Figure S14D). Such results supported that NJU-seq can reflect the copy number of fragments with Nm. In addition, the methylation levels of several selected sites previously reported as Nm sites between the HeLa NC and the FBL-KD groups decreased by 8%–20%, as quantified by Nm-VAQ (Figure S14D).
The site-specific Nm validation and quantification method Nm-VAQ is another new tool established in this study to fulfill the Nm quantification gap in the study of Nm. Several previously developed low-throughput Nm detection methods, including LC-MS, RTL-P, and DNA polymerase, have various defects.20,21,41 LC-MS is labor-intensive and difficult for mRNA Nm detection due to the requirement for a large number of RNA molecules.20 Both RTL-P and DNA polymerase rely on Nm blocking on reverse transcription, which can be called RT-based methods.21,41 Any Nm site between the amplification products will generate a methylation signal, and thus, these two detections are non-site-specific methods. Meanwhile, although both RTL-P and DNA polymerase methods could acquire linear results correlated with the methylation ratio with synthetic RNA, the results varied with different amounts of target RNA. As we observed in the evaluation of 18S 1,391Cm, the results were significantly different in three RNA amounts, making it difficult to accurately evaluate the Nm ratio of the actual target (Figure S4I). In addition, the original study of RTL-P mentioned false-positive and -negative results, which may be caused by RNA secondary structures that can occur on several rRNA sites.21 Nm-VAQ demonstrated apparent advantages compared with those methods, as listed in Table S6. Nm-VAQ anchored the cleavage position to the target site directed by the chimera and discriminated 2′-O-methylated RNA from unmethylated RNA molecules by RNase H. This method simultaneously acquired the absolute amount of accurate Nm of the target site. In addition, Nm-VAQ showed its capability to consistently evaluate targets with low amounts or low methylation ratios, demonstrating its capability to allow study of the status of specific Nm sites and their dynamic changes. NJU-seq reflected the methylated fragments, and Nm-VAQ reflected the Nm ratio. For different sites on the same RNA molecule, the higher the score, the higher the level of modification. For Nm sites occurring on different genes, it is not possible to simply compare the two modifications by their scores. Gene A expresses a total of 50 RNA molecules, of which 10 contain Nm modification. Gene B expresses a total of 10 RNA molecules, of which 2 contain Nm modification. After NJU-seq treatment, the fractions of site A and site B were 10 and 2, respectively. Although mRNA of both genes was 20% 2′-O-methylated, gene A presented a higher score than gene B. Therefore, for different sites, the expression of the gene itself should be taken into account in addition to the reference score.
In general, we performed the following three designs to avoid false positives: (1) we set up a control sample (NC) that was not treated with MgR, presenting the original fragmentation state of RNA. Comparing the treated group with the NC group can largely exclude the influence of FoldChange of factors such as RNA structure on the reverse transcription and library-building process. (2) We set up an in vitro transcriptome control sample of MgR treatment, presenting the effect of factors other than Nm modification on MgR and the whole NJU-seq process, which can further exclude the false positives caused by other factors. (3) To further rule out other possibilities, we introduced the Nm-VAQ method for site-specific precise quantification. Together, these methods comprise a platform that can detect Nm in the transcriptome.
In the data process, we set two parameters, Score(average) and FoldChange(average), as 30+ and 3+ with a higher standard to maintain a high true-positive rate of the screened Nm sites. More than 80% specificity was obtained in rRNA, and more than 75% of the sites in mRNA were confirmed to have modification by Nm-VAQ. Due to the huge differences between rRNA and mRNA in terms of content, structure, density of Nm-modified sites, and modification ratios, it is not possible to compare or speculate on the result accuracy of the two targets. This is why many methods are able to achieve accurate detection of rRNA modification sites, but not mRNA. Predictably, more restricted parameters would theoretically result in higher specificity, which means both parameters could be adjusted according to the purpose of the study. Although some screened sites were proved to be methylated at lower than 1%, the amount of methylated fragments was not negligible, considering their high expression level (Figure S8C), which might play essential roles in cell metabolism. Furthermore, NJU-seq achieved high consistency in mRNA duplicate samples (Tables S2 and S3). These results indicated the stable broad-spectrum applicability of NJU-seq in detecting Nm modification in various RNA molecules with high complexity and low content. On the other hand, sequencing coverage is also often an important influence on NGS-dependent assays. To explore this factor, we analyzed the detection effect by randomly extracting 1 million, 2 million, and 3 million reads from the sequencing samples of HeLa (corresponding to 5×, 10×, and 15×, respectively) (Figure S8D). The overlap between the three independent assays was stable with increasing sequencing depth over 5×. On the other hand, the number of measured Nm sites increased with increasing sequencing depth (Figure S8D). These results imply that, for researchers, the sequencing depth can be adjusted according to their purposes, such as increasing the sequencing depth if more potential Nm sites are needed or using other higher Nm sensitivity RNase R. 42
In this study, we identified approximately 3/4 previously reported as well as two new Nm sites on rRNA of a HeLa cell line. 18S 354U and 1,440C were further validated to be 2′-O-methylated with ratios lower than 50%, which may be hard to detect consistently by RiboMethSeq and LC-MS (Figure S7). By screening the snoRNA database, 18S 1,320G and 1,784G could be targeted by snoRNA117 and snoRNA116-4, which needs further validation in future studies (Figure S7C). As regards mRNA, thousands of new Nm sites were uncovered on mRNAs from humans and mice, installing multiple areas (UTR/CDS, CSE/ASE), revealing its broad distribution. Unlike Nm sites on rRNA, all tested Nm sites on mRNA were 2′-O-methylated at less than 30%. Among them, 5.9% (4/68) exhibited a methylation ratio between 20% and 30%, 17.6% (12/68) had a methylation ratio between 10% and 20%, and the majority, 76.5% (52/68), displayed a methylation ratio between 1% and 10% (Figure 4B). It was hard to answer whether such low methylation ratios could regulate biological processes widely. However, based on previous studies, m6A was proved to be modified with a median modification ratio of ∼40%, which was enough to regulate the function of mRNA.26,43 On the other hand, when NJU-seq and Nm-VAQ were used to study a group of cells from cell lines or tissues, it was unclear whether all cells demonstrated such low modification or some cells were highly methylated while others were not, which required other methods to explore further. A similar distribution pattern of Nm was shared in different cell lines. The non-random distribution of Nm sites and the occurrence of Nm were tightly regulated in critical functional regions, suggesting that Nm might be an essential means of regulation. Thus, Nm modifications played an essential role in mRNA metabolism. In contrast to m1A with enrichment in the start codon or m6A with accumulation around the stop codon,44,45,46 the Nm distribution pattern was more complicated and may possess broader biological functions.
Consistent with Nm-seq and Nm-mut-seq results, we did not discover any conserved motif for mRNA Nm.17,18 Such results implied that mRNA Nm went through a different pathway than sequence-specific Nm on rRNA by FBL/snoRNA or structure-specific Nm on tRNA/miRNA/piRNA by FTSJ and HENMT1.47,48 It was interesting to see that the well-understood rRNA methyltransferase FBL, NOP56, and NOP58 participated in mRNA methylation as well. However, the absence of SNU13 implied a potential difference in methylation mechanism of different RNA types, which needs further exploration in future studies. Based on our mimic plasmid results, it was clear that Nm on the 3′ UTR increased mRNA’s stability but slowed down the translation process, which was consistent with the discovery made by Elliott et al.12 Combined with the discovery that Nm modifications are more clustered on mRNAs with higher translational efficiency (Figure 4E), Nm may exercise a more refined regulation on a large number of more translationally efficient mRNAs to limit a potentially too-fast translation efficiency. Such discovery may help us to understand mRNA regulation from a new aspect. In addition, we succeeded in generating Nm sites at locations we designed on the inserted fraction, which confirmed the accuracy of NJU-seq from another aspect and deserves further investigation in future studies.
With the help of NJU-seq and Nm-VAQ, we hope that the study of Nm modifications will expand from the current partial RNAs to most types of RNAs and refine from the presence or absence of modifications to the precise proportion of modifications. For example, NJU-seq identified Cm31 and Um38 tRNACys(GCA) and Um54 tRNALys(UUU), which turned out to be consistent with sites reported in previous studies,47 which led to significant fragment accumulation ending at the 32th, 39th, and 55th positions (Figure S6). Further studies on the function, modification/demodification/regulation of Nm could be extended. On the other hand, Nm has demonstrated a critical function in environmental response and great potential in disease study, diagnosis, and drug discovery.
Limitations of the study
As newly developed tools, there are some limitations that need to be further improved in follow-up studies. First, for the screening and final quantification of modification sites, we need to rely on the combination of NJU-seq and Nm-VAQ, which increases the workload. Although NJU-seq itself demonstrated a good performance compared with existing methods, we calculated a false discovery rate of around 12% and false-positive rate around 0.2% based on rRNA. Precisely extrapolating these estimates to mRNA is difficult, due to the huge differences in their RNA amounts, modification ratios, and distribution densities. However, given also the massive size of the mRNA transcriptome, these percentages could still translate to a non-negligible, absolute number of false-positive sites for a screening experiment. Nm-VAQ will be an important validation tool if follow-up studies are to be carried out on specific Nm sites. Further, the score of NJU-seq is not only related to the modification ratio of the site, it is also related to the expression amount of the corresponding mRNA and other factors, and thus it is not possible to select highly modified modification sites directly from Score.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and virus strains | ||
| E. coli DH5α | Vazyme | C502 |
| BL21(DE3) Chemically Competent Cell | Transgen Biotech | CD601 |
| Mouse hepatitis virus-A59 | This study | N/A |
| Biological samples | ||
| Clinical samples | This study | Ethical number: 2022-168-01 (Nanjing Drum Tower Hospital) |
| Chemicals, peptides, and recombinant proteins | ||
| IPTG | Sangon Biotech Co. | A100487 |
| TCEP | Sangon Biotech Co. | A600974 |
| LB Broth | Sangon Biotech Co. | A507002 |
| High Affinity Ni-NTA Resin | GenScript Biotech Co. | L00250 |
| imidazole | Sangon Biotech Co. | A600277 |
| RNA Clean & Concentrator-5 kit | Zymo Research | R1015 |
| VAHTS DNA Clean Beads | Vazyme Biotech Co., Ltd. | N411 |
| Ribo-off rRNA Depletion Kit (Human/Mouse/Rat) | Vazyme Biotech Co., Ltd. | N406 |
| VAHTS RNA Clean Beads | Vazyme Biotech Co., Ltd. | N412 |
| RNase H | New England BioLabs | M0297 |
| DNase I | New England BioLabs | M0303 |
| T4 RNA Ligase 1 (ssRNA Ligase) | New England BioLabs | M0204 |
| T4 Polynucleotide Kinase (3' phosphatase minus) | New England BioLabs | M0236 |
| NEBNext Multiplex Small RNA Library Prep Set for Illumina | New England BioLabs | E7580 |
| RNA Fragmentation Reagents | Thermo Fischer Scientific | AM8740 |
| Dulbecco's modified Eagle’s medium | Gibco | 11995065 |
| QIAzol Lysis Reagent | Qiagen | 79306 |
| Superior quality fetal bovine serum | Wisten | 086 |
| Agarose | Sangon Biotech Co. | A620014 |
| Lipofectamine™ RNAiMAX Transfection Reagent | Invitrogen | 13778100 |
| ChamQ Universal SYBR qPCR Master Mix | Vazyme Biotech Co., Ltd. | Q711 |
| HiScript II 1st Strand cDNA Synthesis Kit (+gDNA wiper) | Vazyme Biotech Co., Ltd. | R212 |
| M-MLV (H-) Reverse Transcriptase | Vazyme Biotech Co., Ltd. | R021 |
| RNase inhibitor | Vazyme Biotech Co., Ltd. | R301 |
| dNTP Mix | Vazyme Biotech Co., Ltd. | P032 |
| RNA Loading Dye (2X) | New England BioLabs | N0362 |
| T7 High Yield RNA Transcription kit instructions | Vazyme Biotech Co., Ltd. | TR101 |
| ClonExpress Ultra One Step Cloning Kit | Vazyme Biotech Co., Ltd. | C115 |
| TRIzol Reagent | Invitrogen | 15596026 |
| IPTG | Sangon Biotech Co. | A100487 |
| TCEP | Sangon Biotech Co. | A600974 |
| LB Broth | Sangon Biotech Co. | A507002 |
| High Affinity Ni-NTA Resin | GenScript Biotech Co. | L00250 |
| imidazole | Sangon Biotech Co. | A600277 |
| RNA Clean & Concentrator-5 kit | Zymo Research | R1015 |
| VAHTS DNA Clean Beads | Vazyme Biotech Co., Ltd. | N411 |
| Deposited data | ||
| NJU-seq for rRNA sites | This paper | SRR13333771, SRR13333776, SRR13333787 to SRR13333788 |
| NJU-seq for mRNA sites | This paper | SRR13333772 to SRR13333775, SRR13333777 to SRR13333784, SRR13314941 to SRR13314946, SRR13314948, and SRR13314949 |
| RNA-seq | This paper | SRR27559296 to SRR27559301 |
| HeLa protein abundance data | PaxDb | Experiment ID 182 |
| Experimental models: Cell lines | ||
| HeLa | Shanghai Institute of Cell Biology, Chinese Academy of Sciences | SCSP-504 |
| HEK293T | Shanghai Institute of Cell Biology, Chinese Academy of Sciences | SCSP-502 |
| A549 | Shanghai Institute of Cell Biology, Chinese Academy of Sciences | SCSP-503 |
| Neuro-2a | Shanghai Institute of Cell Biology, Chinese Academy of Sciences | SCSP-5035 |
| Oligonucleotides | ||
| See Tables S5 and S6 | This study | N/A |
| Software and algorithms | ||
| Cutadapt 2.10 | Kechin et al.49 | https://cutadapt.readthedocs.io/en/stable/ |
| Bowtie 2 2.3.4 | Langmead et al.50 | https://bowtie-bio.sourceforge.net/bowtie2/index.shtml |
| Perl scripts | This paper | https://github.com/IvanWoo22/NJU_seq (https://doi.org/10.5281/zenodo.10572326) |
| R scripts | This paper | https://github.com/IvanWoo22/NJU_seq (https://doi.org/10.5281/zenodo.10572326) |
| ggseqlogo | Wagih et al.51 | https://omarwagih.github.io/ggseqlogo/ |
| PLEXY | Kehr et al.52 | http://www.bioinf.uni-leipzig.de/Software/PLEXY/ |
| DESeq2 1.30.1 | Love et al.53 | https://github.com/thelovelab/DESeq2 |
| Image J 1.54h | Schneider et al.54 | https://imagej.net/ij/ |
| MaxQuant 1.6.1.0 | Cox and Mann55 | https://www.maxquant.org |
| PyMOL 2.4 | Schrödinger et al.56 | https://pymol.org/2/ |
Resource availability
Lead contact
Further information and requests can be directed to lead contact, Qihan Chen (lyonchen@um.edu.mo).
Materials availability
Plasmids and strains created in this paper are available from lead contact upon request.
Data and code availability
-
•
Sequencing data is available in the National Center for Biotechnology Information (accession PRJNA685323). Additional data from this study are provided in the main text or supplemental information.
-
•
Original code generated for this study is available at https://github.com/IvanWoo22/NJU_seq. An archival DOI is listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper be obtained by contacting lead contact upon request.
Experimental model and study participant details
Cell culture, tissue, and RNA extraction
HeLa cells, HEK293T cells, A549 cells, and Neuron-2a cells were purchased from the Shanghai Institute of Cell Biology, Chinese Academy of Sciences (Shanghai, China). All cells were grown in Dulbecco's modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 units/ml penicillin, and 100 μg/ml streptomycin at 37 °C with 5% CO2.
Following relevant guidelines and regulations, cancerous and distal noncancerous lung tissue samples were obtained from newly diagnosed lung cancer patients. Nanjing Drum Tower Hospital approved the study, and written consent was obtained from all patients. Detailed information on clinical patients is shown on Table S4. Briefly, the sample contains 3 males and 5 females ranging from 52 to 75 in age, including 2 LUSC and 6 LUAD patients.
Total RNA from cells and tissues was extracted by the QIAzol Lysis Reagent (QIAGEN). Extracted total RNA was confirmed by Nanodrop (Thermo Fisher) and 1% agarose gel electrophoresis (Sangon Biotech).
Method details
Cloning, expression, and purification
The RNase R sequence from Mycoplasma genitalium (accession number: WP_009885662.1) was synthesized by GenScript Biotech Co. and inserted into the pET28a vector (+) with digestion and ligation at the NdeI and BamHI sites, followed by confirmation by Sanger sequencing. The plasmid was transferred into E. Coli. BL21 (DE3) strain. Cells were grown in 1 L LB media under 37 °C. IPTG (0.3 mM) was added to every 1 L medium in a shaker incubator to induce protein expression at 16 °C for 18 h. Cell pellets were harvested and lysed by French press with lysis buffer (50 mM Tris-HCl at pH 8.0, 250 mM NaCl, 0.5 mM TCEP), followed by centrifuging at 10000 rpm for 1 h. MgR protein was purified by passing through a column Nickle resin (GenScript Biotech Co.) and washed by using 3 column volumes of wash buffer (50 mM Tris-HCl at pH 8.0, 250 mM NaCl, 20 mM imidazole, 0.5 mM TCEP), then eluted with elution buffer (50 mM Tris-HCl at pH 8.0, 250 mM NaCl, 100 mM imidazole, 0.5 mM TCEP). The elution was concentrated and aliquoted into multiple small tubes and stored at −80 °C for further experiments. Protein purity was confirmed by SDS–PAGE gel, and its concentration was determined by standard BSA protein.
In vitro assay of RNA hydrolysis
Nm-modified ssRNA substrate was synthesized by GenScript Biotech Co. (5′6-Fam-UAACC UAUGA AGNNN UNmNNC UC-3′). Briefly, various purified proteins were reacted with 600 ng ssRNA substrate in reaction buffer (20 mM Tris-HCl pH 8.0, 100 mM KCl, 0.01 mM ZnCl2) at 37 °C for 30 mins. The reaction was terminated by heating to 85 °C for 10 mins. The products were separated by 20% urea-PAGE, visualized with a ChemiDoc XRS+\UnUniversal alHoodII gel imaging system (Bio-Rad) or Tanon 3500, and quantified with ImageJ software. A series of reactions with 400 ng ssRNA were designed to test the range of pH and reaction duration. Subsequently, multiple ssRNA substrates were incubated with 18 μg MgR in a 10 μl reaction (20 mM Tris-HCl pH 8.0, 100 mM KCl, 0.01 mM ZnCl2) at 37 °C for 30 mins to test MgR properties. The ssRNA sequences were as follows: ssRNA substrate with multiple modifications (5′6-Fam-AUACC GCAGC UAGAA UGAmGC Um5CAGA UGm6AAA-3′) and ssRNA substrate with hairpin structure (5′6-Fam-UAGCU CUGGA AAUUC UAGAmG CUAAU ACAAA-3′). Another Nm-modified ssRNA substrate was synthesized by GenScript Biotech Co. (5′-AGCCG CCUGG AUACC GCAGC UAGAA UGAmGC UGAGA UGAAA-3′) to test the consistency of hydrolysis. After reacting with MgR, reaction products were purified by an RNA Clean & Concentrator-5 kit (Zymo Research), followed by 5′ phosphorylation (New England BioLabs), repurification, and adaptor ligation (New England BioLabs). The final products were cloned by T vector and analyzed by Sanger sequencing. An Nm-modified ssRNA substrate was also synthesized by GenScript Biotech Co. (5′-AGCUA GNNNN NNNNN NNmNNN NNNNN NN-3′) and treated with the above protocol. The final products were high throughput sequencing.
Knockdown of FBL in HeLa cell line
For siRNA experiments, siRNA duplexes were used for fibrillarin silencing: 5′-GUCUU CAUUU GUCGA GGAA-AdTdT-3′ (sense sequence). Control siRNA does not target any human sequence (RiboBio). HeLa cells were transfected using Lipofectamine™ RNAiMAX Transfection Reagent (Invitrogen) according to the manufacturer’s instructions and collected after 48 h. FBL mRNA expression was quantified by a HiScript II 1st Strand cDNA Synthesis Kit (+gDNA wiper) and ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd.) according to instructions. The primers were listed in Table S5.
Viral infection
Neuro-2a cells were infected with MHV strain A59 at a multiplicity of 2 plaque-forming units (p.f.u.) per cell. The infected cells were cultured for 1 h and washed three times with PBS to remove the cell-free virus. The infected cells were harvested after 40h.
rRNA elimination
Except for HeLa, HEK293T, and A549 cells, which used 15 μg total RNA, all the other samples were processed with 10 μg. The RNA was diluted to 29 μl, and 1 μl rRNA probe (H/M/R) was added (Vazyme Biotech Co., Ltd.). RNA was hybridized with the probe and heated to 95 °C for 2 min, then gradually cooled to 22 °C at 0.1 °C/s and maintained for 5 min. Next, the rRNA was eliminated by adding 1 μl RNase H (New England Biolabs), 4 μl 10X RNase H buffer, 4 μl RNase-free water and incubating at 37 °C for 30 min. Subsequently, the hybridization and RNase H reaction were repeated 5/3 times to eliminate the rRNA fragment as much as possible. Finally, the probe was digested by 2 units of DNaseI (New England Biolabs) at 37 °C for 30 min. According to the manufacturer's protocol, the RNA product was purified using VAHTS RNA Clean Beads (Vazyme Biotech Co., Ltd.).
NJU-seq
RNA was fragmented by an RNA fragmentation reagents kit (Thermo Fisher) at 95 °C for 10 mins and then purified by 10 μl 3-M NaOAc (pH 5.2), 24 μl 5-μg/μl glycogen, and 372 μl 96% cold ethanol. For facilitating MgR binding of fragments with complex secondary structures after solubilization in water, every 1 μg RNA solution was ligated with 3 μl 25-μM poly(A)-ssRNA adaptor (pAGCUAAAAAAAAAAAAp, synthesized by GenScript Biotech Co.) at 16 °C overnight. The products were then reacted with 30 μg MgR in a 60 μl final volume with 20 mM Tris-Cl (pH 8.0), 100 mM KCl, and 0.01 mM ZnCl2 at 37 °C for 30 min. Next, the products were purified by an RNA Clean & Concentration-5 kit (Zymo Research), followed by 5′ phosphorylation (39 μl RNA, 5 μl (10X T4 PNK Reaction Buffer), 5 μl ATP (10 mM), 1 μl T4 PNK (3′ phosphatase minus, New England BioLabs)). Finally, we constructed the NGS library using the products from the last step following the NEBNext Small RNA Library Prep Set for Illumina (New England BioLabs) protocol. Fragments smaller than 50 nt were extracted from a 6% PAGE gel and sent for NGS using Illumina X-Ten. The exact process prepared the NC sample (negative control group) without MgR protein.
NGS data analysis and statistics
Nm site detection and various statistical analyses were performed using homemade Perl and R scripts and open-source bioinformatics tools. Adaptors were filtered from raw reads using Cutadapt (Version 2.10). Reads were mapped the reference sequences by Bowtie 2 50 (Version 2.3.4). rRNA reference sequences are shown in Table S1, and the transcript reference sequences were from GENCODE release 34 and M25 57. Only protein-coding transcripts with 'BASIC' tags and without 'cds_start_NF' or 'cds_end_NF' tags were retained. Perl scripts handled alignment results, including filtering low-quality or multiple-hit alignments and calculating each site's reads' end count. The likelihood of 2′-O-methylation modification at each site was judged based on the following scoring system.
Identification of Nm sites
As shown in Figure S5, we counted the stacking position of the 3′ end of the reads for the treatment group with MgR digestion (TR) and the control group without digestion (NC). First, Score was performed for each site on the rRNA and transcriptome reference based on the number of reads ending at the current position (N) and the latter (N+1). The NJU-seq score algorithm was as follows.
After getting a score for each repeat sample, the average score among the three replicates is further calculated. Subsequently, we took Score(average) ≥30 to identify candidate Nm sites. Then, to prevent some points with a similar high N+1 enrichment in NC from being misclassified, we also required a parameter for FoldChange. After normalization correction was performed according to the total aligned reads, FoldChange was as follows: . At this point, we finished processing the NJU-seq data and acquiring the Nm sites for subsequent analysis.
Here, we have outlined the calculations involved for the positive site 18S 601Gm and the negative site 18S 1056Ψ as examples to further demonstrate the calculation process (Figures S5B and S5C).
The reads in the NC group with 18S 601G and 602G as 3 end-stop points were 1 and 30, 19 and 2920 for treatment group 1, 4 and 1244 for treatment group 2, and 3 and 1316 for treatment group 3. In order to obtain comparable scores, the points with reads of 0 were calculated uniformly with 1 as a substitute. According to , , and so on to get , and Final , for potential Nm site. Subsequently calibrated with the total number of reads in the NC group, 18S 602G for 3 end-stop reads 3692, 2001, and 1680, respectively. Based on , . Therefore, 18S 601G has been identified as an Nm site (Figures S5B and S5C).
Meanwhile, we take 18S 1056Ψ as an example to show the process of determining non-Nm sites and the rationality of Score and Foldchange in excluding the enrichment phenomenon of reads due to other non-Nm factors. The reads with 18S 1056Ψ and 1057C as 3-terminal stops in the NC group are 252 and 9700, 59 and 3285, 51 and 4846 for treatment group 1, 2, and 40 and 6864 for treatment group 3. According to , , and so on to obtain and so , a potential Nm site with enrichment. Subsequently calibrated with the total number of reads in the NC group, 1057C for the 3 end-stop reads 4153, 7792, and 8762, respectively. , so it was inferred to be a non-Nm site. From the result of 18S 1056Ψ, the enrichment of reads may be caused by other reasons, which can be further excluded (Figures S5B and S5C).
Statistics on genomic features
We mainly used Perl and R scripts to perform tri-ribonucleotide unit statistics, presentation of distribution positions, and alternative splicing region statistics on all Nm sites.
Sequence probability logo plots for Nm sites were drawn by the R package ggseqlogo51. The length-normalized mRNA transcript was obtained by averaging all Nm-located transcripts’ 5′UTR/CDS/3′UTR length of each sample. Equal-length bins of three regions were taken for Nm site counting in each standard transcript. We counted the distance from the Nm site position to the start and stop codon. ASE and CSE were obtained from the statistics of all alternative splicing events provided in the SpliceSeq database 27.
Quantitation of RNA Nm status by RTL-P method
Two sets of primers were designed for the target site, with the FU forward primer upstream of the Nm site and the FD forward primer downstream of the Nm site (Table S5). For rRNA Nm detection, the high dNTP concentration reaction mixture consisted of 5× RT Buffer 4 μl, M-MLV (H-) Reverse Transcriptase (Vazyme Biotech Co., Ltd., 200 U/μL) 1 μl, RNase inhibitor (40 U/μL) 1 μl, RNA 100 ng, RT primer (10 μM) 1 μl, RNase-free H2O 5 μl, dNTPs (1 mM each) 1 μl. The low dNTP concentration reaction mixture was replaced by dNTPs (2 μM each). Then, the mixtures were incubated at 45 °C for 1 hour and 85 °C for 2 min. The cDNA was diluted 100-fold and subsequently subjected to qPCR, with both FU and FD products amplified for each cDNA. For mRNA Nm detection, 1 μg of total RNA was used for further RTL-P tests. RT efficiency and RT fold change were calculated according to the following strategy. RT efficiency=template amount measured by FU and R/template amount measured by FD and R. RT fold change=RT efficiency with low dNTPs/RT efficiency with high dNTPs 21.
The assay of RNase H cleavage
GenScript Biotech Co synthesized RNA oligonucleotides and chimera probes. Briefly, 12.5 pmol 5′-FAM-labeled RNA oligonucleotides were mixed with a 75 pmol chimera probe, heated to 95 °C for 2 min, cooled to 22 °C at 0.1 °C/s, and maintained for 5 min. Next, the hybrid was reacted with 1 μl RNase H (New England BioLabs) at 37 °C for 30 min and then heated to 90 °C for 10 min to terminate the reaction. The cleavage products were added to RNA Loading Dye (2X) (New England BioLabs), analyzed by 20% UERA-PAGE, and visualized by a ChemiDoc XRS+\UnUniversal alHoodII gel imaging system (Bio-Rad).
Nm-VAQ assay of RNA 2′-O-methylation ratio
Synthetic RNA oligonucleotides with/without Nm were obtained from GenScript Biotech Co., and Sangon Biotech Co. synthesized qRT–PCR primers. All sequences were listed in Table S5. The oligonucleotides with/without Nm were mixed to obtain gradient 2′-O-methylation ratio substrates. Then, 6.25∗10-2 pmol substrate was added to 6.25 pmol D(4)R(13) chimera probe at 95 °C for 2 min, followed by cooling to 22 °C at 0.1 °C/s and 22 °C for 5 min. The products were divided into 2 parts for the following reaction. One mixture contained 5 μl previous product, 1 μl RNase H (New England BioLabs), 1 μl 10X RNase H Reaction Buffer, and 3 μl RNase-free H2O. RNase H storage buffer was substituted for RNase H to form the other mixture to serve as a blank control, followed by 30 min at 37 °C and 10 min at 90 °C. The products were diluted 50-fold and then used to obtain cDNA by a HiScript II 1st Strand cDNA Synthesis Kit (Vazyme Biotech Co., Ltd.). cDNA was diluted 100-fold, and then qPCR was conducted in a 20 μl reaction mixture containing 10 μl ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd.), 0.4 μl F primer, 0.4 μl R primer, 2 μl cDNA, and 7.2 μl H2O, following the protocol: 30 s at 95 °C, then 40 cycles of 95 °C for 10 s and 60 °C for 30 s. Each cDNA was analyzed in 3 replicates. ΔCT (cycle threshold) = Ct value of RNase H reaction- Ct value of control. The linear relationship between the Nm ratio and ΔCT was obtained by Excel. The additional 6.25 pmol, 0.625 pmol, and 0.0625 pmol substrates with 50% Nm ratio were also assayed (cDNA did not dilute), and ΔCT was obtained. The measured modification ratios were calculated by the above linear relationship.
Quantitation of RNA Nm status by Nm-VAQ method
All primers were obtained from Sangon Biotech Co., and the sequences are shown in Table S6. For rRNA Nm detection, 100 ng total RNA and 6.25 pmol chimera probes (molar ratio of rRNA molecules to chimera probes was ∼1:200) were hybridized, followed by RNase H cleavage, reverse transcription, and qPCR (cDNA was diluted to 100-fold) according to Nm-VAQ protocol. ΔCT was used to calculate the ratio of modification. In addition, some treatments were adjusted to perform mRNA Nm detection. 2-10 μg of total RNA was used for hybrids, and 4 μl of cDNA was used for the qPCR assay.
In vitro transcription
The Nm-VAQ detection DNA fragment of PLXNB2 (22, 50,290,219, Gm) and BCL6 (3, 187,721,452, Gm) were obtained by qRT-PCR. The T7 primer (TAATA CGACT CACTA TAGGG) was then added to the DNA fragment by PCR. The DNA fragments were as templates to produce RNA fragments without modifications following the T7 High Yield RNA Transcription kit instructions (Vazyme Biotech Co., Ltd.). 1ng of in vitro transcribed RNA was mixed with 1μg total Arabidopsis RNA, followed by hybridization, RNase H cleavage, and reverse transcription according to the Nm-VAQ. The cDNA was amplified by PCR and then the product was detected by electrophoresis.
The in vitro transcriptome total HeLa mRNA was generated as described in the Zhang et al., study 58. The modification-free mRNAs were then performed NJU-seq process and the score.
The snoRNA target prediction
The C/D box snoRNAs were downloaded from snoPY (http://snoopy.med.miyazaki-u.ac.jp). All 427 C/D box snoRNA were obtained. The efficient target prediction for box C/D snoRNAs was followed by PLEXY (http://www.bioinf.uni-leipzig.de/Software/PLEXY/).
qRT–PCR and calculation of expression of NCAM1 transcripts
1 μg of total RNA was used to obtain cDNA with a HiScript II 1st Strand cDNA Synthesis Kit (Vazyme Biotech Co., Ltd.). cDNA was added to ChamQ Universal SYBR qPCR Master Mix (Vazyme Biotech Co., Ltd.), following the process: 95 °C for 30 s, 40 cycles of 95 °C for 10S and 60 °C for 30 s. Each cDNA was assayed in 2 replicates. The primer oligos were as follows: (NCAM1-all-F: CAACC TTGGG AGGCA ATTCT), (NCAM1-all-R: ACTGC CATTA AAAAG GGGGC), (NCAM1-others-F: ACCTT GGGAG GCAAT TCTGC), and (NCAM1-others-R: GCAGA AACTT CTCTG TAAAT CTAGC). The standard curve was completed as above.
Standards of qRT–PCR amplification products were subjected to gradient dilution. qPCR was completed under standard protocols based on the above reagents and procedures.
RNA-seq
We depleted rRNA from total RNA, fragmented it into ∼300 bp fragments, and prepared the library for RNA-seq. After library preparation and pooling of different samples, the samples were subjected to PE150 sequencing on the Illumina NovaSeq 6000 platform. Paired-end reads were aligned to the GENCODE M25 genome using Bowtie2. Gene expression statistics were performed using HTSeq 59 (Version 1.99.2), and differentially expressed genes were obtained using DESeq2 53 (Version 1.30.1).
Mimic plasmid construction
300 nt upstream and downstream of the mRNA Nm site PLXNB2 (22: 50,290,219, Gm), 200 nt upstream and downstream of INSR (19: 7,116,934, Um), and 200 nt upstream and downstream of the MHV 1702 (Cm) were inserted into the 3′UTR of px330 plasmid GFP region by homologous recombination method following the ClonExpress Ultra One Step Cloning Kit (Vazyme Biotech Co., Ltd.) as shown in Figure 6D. All sequences were confirmed by Sanger sequencing.
Evaluation of mRNA and fluorescence of GFP
4 μg Plasmids were transfected into HeLa or N2A cells, and the RNA was collected after 24, 48, 72, 96, and 120 hours with TRIzol (Invitrogen). 2 μg total RNA from each time point was digested with DNase I (New England BioLabs) to remove the potential remained plasmid and quantified by qRT-PCR with the primer (Table S5). Each cDNA was assayed in 3 replicates. The GFP mRNA expression was quantified with the relative expression of GAPDH. Photomicrographs were taken on the inverted microscope (Olympus, Tokyo, Japan) and further processed by Image J software to quantify signal intensity.
Identification of mRNA-interacting protein
The capture and identification of mRNA-interacting proteins were carried out following Alexander et al 's experimental protocol with minor modification 33. HeLa cells were cultured in DMEM medium supplemented with 4-thiouridine and 6-thioguanosine, and exposed to 365 nm UV light for cross-linking. Then, the cells were collected and lysed, resulting in release of cellular contents. The magnetic beads coupled with Oligo(dT) were added to the cell lysate and incubated at room temperature. The mRNA-interacting proteins were eluted from beads by washing buffer. Subsequently, the mRNAs from the mRNA-protein complex were removed by RNase. The obtained protein products were used for LC-MS analysis. MaxQuant software (version 1.6.1.0) was used to process the MS data. The data were searched against the UniProtKB Homo sapiens database. Trypsin was employed as the digestion enzyme. The search parameters included a maximum of two missed sites, a mass tolerance of 4.5 ppm for precursor ions and 20 ppm for fragment ions. The database search results were refined with a false discovery rate of less than 1% at both the peptide-spectrum-matched and protein levels.
Protein abundance
The HeLa protein abundance data were downloaded from the Protein Abundances Across Organisms (PaxDb, Experiment ID 182) 60,61. We used transcriptome expression data from the ENCODE 62 portal with the following identifiers: ENCFF796REI and ENCFF846THO, and only kept genes with TPM > 0.2. The 6,881 genes with both protein abundance and mRNA expression were used for subsequent analysis. Among them, the number of Nm-located genes was 1,397.
Gene ontology (GO) enrichment
For functional enrichment analysis, genes containing Nm sites were uploaded to DAVID Bioinformatics Resources (http://david.abcc.ncifcrf.gov). Enriched GO terms were restricted to P ≤ 0.05.
Simulation of structural models
The influence of 2′-O-methylation on RNA-RISC binding was simulated by PyMOL (Version 2.4). We used the molecular structure of Argonaute2-miR-122 bound to the target RNA (PDB ID: 6MDZ) 63 for observation and further simulated the modification at some target RNA sites. The methyl group bond angle was strictly controlled to match the real situation, and the distance to the surrounding amino acids before and after binding was measured.
Quantification and statistical analysis
All quantification statistical analysis details are described in the figure legends or main text, including significance criteria, sample size, and p-values. No samples were excluded.
Acknowledgments
We are grateful to the High-Performance Computing Center of Nanjing University for performing the numerical calculations in this paper on its blade cluster system. In addition, we acknowledge all members of Dr. Jian-Qun Chen’s lab, Dr. Shanqing Li, and Dr. Naixin Liang for comments and discussions. We also gratefully acknowledge Dr. Robin Chan, Dr. Hui Zhang, and Dr. Li Zhou for editing the paper. In addition, we appreciate Dr. Yaping Liu’s efforts in the peer evaluation of this work. This work was supported by the Youth Program of the National Natural Science Foundation of China (31801065), the National Key R&D Program of China (2021YFC2701603), the Fundamental Research Funds for the Central Universities (021414380507), and the National Natural Science Foundation of China (32170218).
Author contributions
Q.C., Q.W., and J.-Q.C. designed this research; Y.T., S.W., X.L., X.G., Y.L., F.Y., R.X., Yan Wu, and L.L. performed this research; Q.C., Q.W., Y.T., and Yifan Wu wrote the manuscript; Yifan Wu and Q.W. analyzed the data; T.W. and Z.J. provided the clinical samples and pretreatment.
Declaration of interests
The authors declare no competing interests.
Published: March 6, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.crmeth.2024.100721.
Contributor Information
Jian-Qun Chen, Email: chenjq@nju.edu.cn.
Qiang Wang, Email: wangq@nju.edu.cn.
Qihan Chen, Email: lyonchen@um.edu.mo.
Supplemental information
References
- 1.Motorin Y., Helm M. RNA Nucleotide Methylation. Wiley Interdiscip Rev RNA. 2011;2:611–631. doi: 10.1002/wrna.79. [DOI] [PubMed] [Google Scholar]
- 2.Jöckel S., Nees G., Sommer R., Zhao Y., Cherkasov D., Hori H., Ehm G., Schnare M., Nain M., Kaufmann A., Bauer S. The 2'-O-methylation status of a single guanosine controls transfer RNA-mediated Toll-like receptor 7 activation or inhibition. J. Exp. Med. 2012;209:235–241. doi: 10.1084/jem.20111075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deryusheva S., Choleza M., Barbarossa A., Gall J.G., Bordonné R. Post-transcriptional modification of spliceosomal RNAs is normal in SMN-deficient cells. RNA. 2012;18:31–36. doi: 10.1261/rna.030106.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tycowski K.T., Smith C.M., Shu M.D., Steitz J.A. A small nucleolar RNA requirement for site-specific ribose methylation of rRNA in Xenopus. Proc. Natl. Acad. Sci. USA. 1996;93:14480–14485. doi: 10.1073/pnas.93.25.14480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Züst R., Cervantes-Barragan L., Habjan M., Maier R., Neuman B.W., Ziebuhr J., Szretter K.J., Baker S.C., Barchet W., Diamond M.S., et al. Ribose 2'-O-methylation provides a molecular signature for the distinction of self and non-self mRNA dependent on the RNA sensor Mda5. Nat. Immunol. 2011;12:137–143. doi: 10.1038/ni.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawai G., Yamamoto Y., Kamimura T., Masegi T., Sekine M., Hata T., Iimori T., Watanabe T., Miyazawa T., Yokoyama S. Conformational rigidity of specific pyrimidine residues in tRNA arises from posttranscriptional modifications that enhance steric interaction between the base and the 2'-hydroxyl group. Biochemistry. 1992;31:1040–1046. doi: 10.1021/bi00119a012. [DOI] [PubMed] [Google Scholar]
- 7.Krogh N., Jansson M.D., Häfner S.J., Tehler D., Birkedal U., Christensen-Dalsgaard M., Lund A.H., Nielsen H. Profiling of 2'-O-Me in human rRNA reveals a subset of fractionally modified positions and provides evidence for ribosome heterogeneity. Nucleic Acids Res. 2016;44:7884–7895. doi: 10.1093/nar/gkw482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ji L., Chen X. Regulation of small RNA stability: methylation and beyond. Cell Res. 2012;22:624–636. doi: 10.1038/cr.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Y., Wei Y., Zhang X., Zhang Y., Cai H., Zhu Y., Shilo K., Oglesbee M., Krakowka S., Whelan S.P.J., Li J. mRNA cap methylation influences pathogenesis of vesicular stomatitis virus in vivo. J. Virol. 2014;88:2913–2926. doi: 10.1128/JVI.03420-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge J., Liu H., Yu Y.T. Regulation of pre-mRNA splicing in Xenopus oocytes by targeted 2'-O-methylation. RNA. 2010;16:1078–1085. doi: 10.1261/rna.2060210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao X., Yu Y.T. Targeted pre-mRNA modification for gene silencing and regulation. Nat. Methods. 2008;5:95–100. doi: 10.1038/nmeth1142. [DOI] [PubMed] [Google Scholar]
- 12.Elliott B.A., Ho H.T., Ranganathan S.V., Vangaveti S., Ilkayeva O., Abou Assi H., Choi A.K., Agris P.F., Holley C.L. Modification of messenger RNA by 2'-O-methylation regulates gene expression in vivo. Nat. Commun. 2019;10:3401. doi: 10.1038/s41467-019-11375-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lalonde M.S., Zuo Y., Zhang J., Gong X., Wu S., Malhotra A., Li Z. Exoribonuclease R in Mycoplasma genitalium can carry out both RNA processing and degradative functions and is sensitive to RNA ribose methylation. RNA. 2007;13:1957–1968. doi: 10.1261/rna.706207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayadi L., Galvanin A., Pichot F., Marchand V., Motorin Y. RNA ribose methylation (2'-O-methylation): Occurrence, biosynthesis and biological functions. Biochim. Biophys. Acta. Gene Regul. Mech. 2019;1862:253–269. doi: 10.1016/j.bbagrm.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 15.Daffis S., Szretter K.J., Schriewer J., Li J., Youn S., Errett J., Lin T.Y., Schneller S., Zust R., Dong H., et al. 2'-O methylation of the viral mRNA cap evades host restriction by IFIT family members. Nature. 2010;468:452–456. doi: 10.1038/nature09489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Incarnato D., Anselmi F., Morandi E., Neri F., Maldotti M., Rapelli S., Parlato C., Basile G., Oliviero S. High-throughput single-base resolution mapping of RNA 2-O-methylated residues. Nucleic Acids Res. 2017;45:1433–1441. doi: 10.1093/nar/gkw810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dai Q., Moshitch-Moshkovitz S., Han D., Kol N., Amariglio N., Rechavi G., Dominissini D., He C. Nm-seq maps 2'-O-methylation sites in human mRNA with base precision. Nat. Methods. 2017;14:695–698. doi: 10.1038/nmeth.4294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dai Q., Moshitch-Moshkovitz S., Han D., Kol N., Amariglio N., Rechavi G., Dominissini D., He C. Corrigendum: Nm-seq maps 2'-O-methylation sites in human mRNA with base precision. Nat. Methods. 2018;15:226–227. doi: 10.1038/nmeth0318-226c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen L., Zhang L.S., Ye C., Zhou H., Liu B., Gao B., Deng Z., Zhao C., He C., Dickinson B.C. Nm-Mut-seq: a base-resolution quantitative method for mapping transcriptome-wide 2'-O-methylation. Cell Res. 2023;33:727–730. doi: 10.1038/s41422-023-00836-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Douthwaite S., Kirpekar F. Identifying modifications in RNA by MALDI mass spectrometry. Methods Enzymol. 2007;425:3–20. doi: 10.1016/S0076-6879(07)25001-3. [DOI] [PubMed] [Google Scholar]
- 21.Dong Z.W., Shao P., Diao L.T., Zhou H., Yu C.H., Qu L.H. RTL-P: a sensitive approach for detecting sites of 2'-O-methylation in RNA molecules. Nucleic Acids Res. 2012;40:e157. doi: 10.1093/nar/gks698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yi Y., Li Y., Meng Q., Li Q., Li F., Lu B., Shen J., Fazli L., Zhao D., Li C., et al. A PRC2-independent function for EZH2 in regulating rRNA 2'-O methylation and IRES-dependent translation. Nat. Cell Biol. 2021;23:341–354. doi: 10.1038/s41556-021-00653-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ringeard M., Marchand V., Decroly E., Motorin Y., Bennasser Y. FTSJ3 is an RNA 2'-O-methyltransferase recruited by HIV to avoid innate immune sensing. Nature. 2019;565:500–504. doi: 10.1038/s41586-018-0841-4. [DOI] [PubMed] [Google Scholar]
- 24.Taoka M., Nobe Y., Yamaki Y., Sato K., Ishikawa H., Izumikawa K., Yamauchi Y., Hirota K., Nakayama H., Takahashi N., Isobe T. Landscape of the complete RNA chemical modifications in the human 80S ribosome. Nucleic Acids Res. 2018;46:9289–9298. doi: 10.1093/nar/gky811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Y.T., Shu M.D., Steitz J.A. A new method for detecting sites of 2'-O-methylation in RNA molecules. RNA. 1997;3:324–331. [PMC free article] [PubMed] [Google Scholar]
- 26.Liu C., Sun H., Yi Y., Shen W., Li K., Xiao Y., Li F., Li Y., Hou Y., Lu B., et al. Absolute quantification of single-base m(6)A methylation in the mammalian transcriptome using GLORI. Nat. Biotechnol. 2023;41:355–366. doi: 10.1038/s41587-022-01487-9. [DOI] [PubMed] [Google Scholar]
- 27.Ryan M.C., Cleland J., Kim R., Wong W.C., Weinstein J.N. SpliceSeq: a resource for analysis and visualization of RNA-Seq data on alternative splicing and its functional impacts. Bioinformatics. 2012;28:2385–2387. doi: 10.1093/bioinformatics/bts452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fica S.M., Oubridge C., Galej W.P., Wilkinson M.E., Bai X.C., Newman A.J., Nagai K. Structure of a spliceosome remodelled for exon ligation. Nature. 2017;542:377–380. doi: 10.1038/nature21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ha M., Kim V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 30.Burke J.M., Moon S.L., Matheny T., Parker R. RNase L Reprograms Translation by Widespread mRNA Turnover Escaped by Antiviral mRNAs. Mol. Cell. 2019;75:1203–1217.e5. doi: 10.1016/j.molcel.2019.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nostramo R.T., Hopper A.K. Beyond rRNA and snRNA: tRNA as a 2'-O-methylation target for nucleolar and Cajal body box C/D RNPs. Genes Dev. 2019;33:739–740. doi: 10.1101/gad.328443.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang S.L., DeFalco L., Anderson D.E., Zhang Y., Aw J.G.A., Lim S.Y., Lim X.N., Tan K.Y., Zhang T., Chawla T., et al. Comprehensive mapping of SARS-CoV-2 interactions in vivo reveals functional virus-host interactions. Nat. Commun. 2021;12:5113. doi: 10.1038/s41467-021-25357-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baltz A.G., Munschauer M., Schwanhäusser B., Vasile A., Murakawa Y., Schueler M., Youngs N., Penfold-Brown D., Drew K., Milek M., et al. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol. Cell. 2012;46:674–690. doi: 10.1016/j.molcel.2012.05.021. [DOI] [PubMed] [Google Scholar]
- 34.Aydın M., Aydın E.B., Sezgintürk M.K. Electrochemical immunosensor for CDH22 biomarker based on benzaldehyde substituted poly(phosphazene) modified disposable ITO electrode: A new fabrication strategy for biosensors. Biosens. Bioelectron. 2019;126:230–239. doi: 10.1016/j.bios.2018.10.051. [DOI] [PubMed] [Google Scholar]
- 35.Piche B., Khosravi S., Martinka M., Ho V., Li G. CDH22 expression is reduced in metastatic melanoma. Am. J. Cancer Res. 2011;1:233–239. [PMC free article] [PubMed] [Google Scholar]
- 36.Sasca D., Szybinski J., Schüler A., Shah V., Heidelberger J., Haehnel P.S., Dolnik A., Kriege O., Fehr E.M., Gebhardt W.H., et al. NCAM1 (CD56) promotes leukemogenesis and confers drug resistance in AML. Blood. 2019;133:2305–2319. doi: 10.1182/blood-2018-12-889725. [DOI] [PubMed] [Google Scholar]
- 37.Erales J., Marchand V., Panthu B., Gillot S., Belin S., Ghayad S.E., Garcia M., Laforêts F., Marcel V., Baudin-Baillieu A., et al. Evidence for rRNA 2'-O-methylation plasticity: Control of intrinsic translational capabilities of human ribosomes. Proc. Natl. Acad. Sci. USA. 2017;114:12934–12939. doi: 10.1073/pnas.1707674114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Birkedal U., Christensen-Dalsgaard M., Krogh N., Sabarinathan R., Gorodkin J., Nielsen H. Profiling of ribose methylations in RNA by high-throughput sequencing. Angew. Chem. Int. Ed. Engl. 2015;54:451–455. doi: 10.1002/anie.201408362. [DOI] [PubMed] [Google Scholar]
- 39.Krogh N., Asmar F., Côme C., Munch-Petersen H.F., Grønbæk K., Nielsen H. Profiling of ribose methylations in ribosomal RNA from diffuse large B-cell lymphoma patients for evaluation of ribosomes as drug targets. NAR Cancer. 2020;2 doi: 10.1093/narcan/zcaa035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saikia M., Dai Q., Decatur W.A., Fournier M.J., Piccirilli J.A., Pan T. A systematic, ligation-based approach to study RNA modifications. RNA. 2006;12:2025–2033. doi: 10.1261/rna.208906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aschenbrenner J., Marx A. Direct and site-specific quantification of RNA 2'-O-methylation by PCR with an engineered DNA polymerase. Nucleic Acids Res. 2016;44:3495–3502. doi: 10.1093/nar/gkw200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu X., Gu X., Li Y., Wu Y., Wang Q., Yu W., Chen Q. Biochemical characterization of RNase R 2’-O-methylation sensitivity. Biochimie. 2023;212:106–113. doi: 10.1016/j.biochi.2023.04.016. [DOI] [PubMed] [Google Scholar]
- 43.Liu N., Parisien M., Dai Q., Zheng G., He C., Pan T. Probing N6-methyladenosine RNA modification status at single nucleotide resolution in mRNA and long noncoding RNA. RNA. 2013;19:1848–1856. doi: 10.1261/rna.041178.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dominissini D., Nachtergaele S., Moshitch-Moshkovitz S., Peer E., Kol N., Ben-Haim M.S., Dai Q., Di Segni A., Salmon-Divon M., Clark W.C., et al. The dynamic N(1)-methyladenosine methylome in eukaryotic messenger RNA. Nature. 2016;530:441–446. doi: 10.1038/nature16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meyer K.D., Saletore Y., Zumbo P., Elemento O., Mason C.E., Jaffrey S.R. Comprehensive analysis of mRNA methylation reveals enrichment in 3' UTRs and near stop codons. Cell. 2012;149:1635–1646. doi: 10.1016/j.cell.2012.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li X., Xiong X., Wang K., Wang L., Shu X., Ma S., Yi C. Transcriptome-wide mapping reveals reversible and dynamic N(1)-methyladenosine methylome. Nat. Chem. Biol. 2016;12:311–316. doi: 10.1038/nchembio.2040. [DOI] [PubMed] [Google Scholar]
- 47.Marchand V., Pichot F., Thüring K., Ayadi L., Freund I., Dalpke A., Helm M., Motorin Y. Next-Generation Sequencing-Based RiboMethSeq Protocol for Analysis of tRNA 2'-O-Methylation. Biomolecules. 2017;7 doi: 10.3390/biom7010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liang H., Jiao Z., Rong W., Qu S., Liao Z., Sun X., Wei Y., Zhao Q., Wang J., Liu Y., et al. 3'-Terminal 2'-O-methylation of lung cancer miR-21-5p enhances its stability and association with Argonaute 2. Nucleic Acids Res. 2020;48:7027–7040. doi: 10.1093/nar/gkaa504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kechin A., Boyarskikh U., Kel A., Filipenko M. cutPrimers: A New Tool for Accurate Cutting of Primers from Reads of Targeted Next Generation Sequencing. J. Comput. Biol. 2017;24:1138–1143. doi: 10.1089/cmb.2017.0096. [DOI] [PubMed] [Google Scholar]
- 50.Langmead B., Salzberg S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wagih O. ggseqlogo: a versatile R package for drawing sequence logos. Bioinformatics. 2017;33:3645–3647. doi: 10.1093/bioinformatics/btx469. [DOI] [PubMed] [Google Scholar]
- 52.Kehr S., Bartschat S., Stadler P.F., Tafer H. PLEXY: efficient target prediction for box C/D snoRNAs. Bioinformatics. 2011;27:279–280. doi: 10.1093/bioinformatics/btq642. [DOI] [PubMed] [Google Scholar]
- 53.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schneider C.A., Rasband W.S., Eliceiri K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cox J., Mann M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008;26:1367–1372. doi: 10.1038/nbt.1511. [DOI] [PubMed] [Google Scholar]
- 56.Schrödinger . The PyMol Molecular Graphics System Version 1.8. Schrödinger LCC; 2015. [Google Scholar]
- 57.Frankish A., Diekhans M., Ferreira A.M., Johnson R., Jungreis I., Loveland J., Mudge J.M., Sisu C., Wright J., Armstrong J., et al. GENCODE reference annotation for the human and mouse genomes. Nucleic Acids Res. 2019;47:D766–D773. doi: 10.1093/nar/gky955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Z., Chen T., Chen H.X., Xie Y.Y., Chen L.Q., Zhao Y.L., Liu B.D., Jin L., Zhang W., Liu C., et al. Systematic calibration of epitranscriptomic maps using a synthetic modification-free RNA library. Nat. Methods. 2021;18:1213–1222. doi: 10.1038/s41592-021-01280-7. [DOI] [PubMed] [Google Scholar]
- 59.Anders S., Pyl P.T., Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–169. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang M., Herrmann C.J., Simonovic M., Szklarczyk D., von Mering C. Version 4.0 of PaxDb: Protein abundance data, integrated across model organisms, tissues, and cell-lines. Proteomics. 2015;15:3163–3168. doi: 10.1002/pmic.201400441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Geiger T., Wehner A., Schaab C., Cox J., Mann M. Comparative proteomic analysis of eleven common cell lines reveals ubiquitous but varying expression of most proteins. Mol. Cell. Proteomics. 2012;11 doi: 10.1074/mcp.M111.014050. M111.014050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Davis C.A., Hitz B.C., Sloan C.A., Chan E.T., Davidson J.M., Gabdank I., Hilton J.A., Jain K., Baymuradov U.K., Narayanan A.K., et al. The Encyclopedia of DNA elements (ENCODE): data portal update. Nucleic Acids Res. 2018;46:D794–D801. doi: 10.1093/nar/gkx1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sheu-Gruttadauria J., Pawlica P., Klum S.M., Wang S., Yario T.A., Schirle Oakdale N.T., Steitz J.A., MacRae I.J. Structural Basis for Target-Directed MicroRNA Degradation. Mol. Cell. 2019;75:1243–1255.e7. doi: 10.1016/j.molcel.2019.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Sequencing data is available in the National Center for Biotechnology Information (accession PRJNA685323). Additional data from this study are provided in the main text or supplemental information.
-
•
Original code generated for this study is available at https://github.com/IvanWoo22/NJU_seq. An archival DOI is listed in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper be obtained by contacting lead contact upon request.