Abstract
During secondary immune responses to influenza virus, virus-specific T memory cells are a major source of gamma interferon (IFN-γ). We assessed the contribution of IFN-γ to heterologous protection against the A/WSN/33 (H1N1) virus of wild-type and IFN-γ−/− mice previously immunized with the A/HK/68 (H3N2) virus. The IFN-γ−/− mice displayed significantly reduced survival rates subsequent to a challenge with various doses of the A/WSN/33 virus. This was associated with an impaired ability of the IFN-γ−/− mice to completely clear the pulmonary virus by day 7 after the challenge, although significant reduction of the virus titers was noted. However, the IFN-γ−/− mice developed type A influenza virus cross-reactive cytotoxic T lymphocytes (CTLs) similar to the wild-type mice, as demonstrated by both cytotoxicity and a limiting-dilution assay for the estimation of CTL precursor frequency. The pulmonary recruitment of T cells in IFN-γ−/− mice was not dramatically affected, and the percentage of CD4+ and CD8+ T cells was similar to that of wild-type mice. The T cells from IFN-γ−/− mice did not display a significant switch toward a Th2 profile. Furthermore, the IFN-γ−/− mice retained the ability to mount significant titers of WSN and HK virus-specific hemagglutination-inhibiting antibodies. Together, these results are consistent with a protective role of IFN-γ during the heterologous response against influenza virus independently of the generation and local recruitment of cross-reactive CTLs.
Gamma interferon (IFN-γ) is a cytokine produced by NK cells, CD4+ Th1 cells, and a subset of CD8+ T cells, and it is thought to be a major defense arm during the immune response against intracellular bacteria, certain parasites, and viruses (2). IFN-γ exerts two major effects, directly inhibiting the ability of some microbes to multiply and stimulating the cellular immune response. The direct effect of IFN-γ is mediated by the induction of cellular products that interfere with the microbial metabolism (19) or promote the apoptosis of infected cells (8, 17). The indirect effects of IFN-γ on the generation and function of specific immune effectors is very complex and range from upregulation of antigen processing (11) and presentation in the context of major histocompatibility complex (MHC) class I (39) and II (30, 34) molecules to modulation of the priming (10), recruitment (36), and death of activated T lymphocytes (25). Furthermore, IFN-γ exerts stimulatory effects on the function of macrophages and NK cells (7).
Previous studies revealed protective roles for IFN-γ in animal models of infection with herpes simplex virus (33), cytomegalovirus (16), murine hepatitis virus 3 (26), lymphocytic choriomeningitis virus (22), and adenovirus (39). The generation of mice lacking functional IFN-γ genes (7) allowed the assessment of the role of this cytokine during primary infection with influenza virus (12). Surprisingly, mice lacking IFN-γ did not display a reduced ability to recover from primary infection with the A/JAP/57 (H2N2) strain of influenza virus and mounted cytotoxic T-lymphocyte (CTL) activity comparable to that of their wild-type counterparts. Furthermore, CTL clones obtained from IFN-γ−/− mice, adoptively transferred into wild-type recipients previously challenged with influenza virus, mediated effective recovery from infection (12). However, no data were available regarding a protective role of IFN-γ during the secondary response to influenza virus.
Most cases of influenza virus infection throughout the human population are, in fact, reinfections with drift or shift variants, and only a minority of them are primary infections. There is ongoing emergence of new shift variants subsequent to gene reassortment among strains of different subtypes. Consequently, influenza virus poses a continuous danger of morbidity and mortality for primed and naive human populations, since the immune memory is limited to cross-reactive T-cell epitopes located on the more conserved internal proteins.
Since a major source of IFN-γ during the immune response to shift variants of influenza virus are the memory T cells specific for cross-reactive epitopes, we studied the secondary response to the A/WSN/33 (H1N1) virus strain of IFN-γ−/− mice previously immunized with the A/HK/68 (H3N2) virus strain. Besides the fact that the WSN virus is of a different subtype, it bears certain mutations in neuraminidase that are responsible for its increased replication ability and virulence (23). In the present report, we show that IFN-γ plays a protective role during the memory response to a virulent strain of influenza virus of a subtype that is different from that of the strain used for priming. However, this role is independent of the generation and local recruitment of effector T cells specific for epitopes conserved among influenza viruses of different subtypes.
MATERIALS AND METHODS
Mice.
Mice bearing nonfunctional IFN-γ genes (IFN-γ−/−), obtained by gene targeting (7) and bred into a BALB/c genetic background, were purchased from The Jackson Laboratory (Bar Harbor, Maine). The BALB/c genotype and potential genetic contamination were routinely monitored during breeding at The Jackson Laboratory by assessing phenotypic, biochemical, and serological markers. As a control, we used haplotype- and age-matched BALB/c animals. Mice were housed in the Mount Sinai Animal Facility and immunized at 8 to 12 weeks of age.
Viruses and immunization.
Influenza viruses A/PR/8/34 (H1N1) and A/HK/68 (H3N2) were grown in 10-day-old embryonated hen eggs incubated at 37°C. The allantoic fluid was harvested 48 h later and stored at −80°C. Influenza virus A/WSN/33 (H1N1) was grown on MDBK cells in Dulbecco modified Eagle medium (DMEM) supplemented with 1% bovine serum albumin (BSA) at 37°C in a humidified atmosphere in the presence of 7% CO2. The supernatant was harvested 2 days later. The virus concentration was measured on MDCK cells, and the titers were expressed as 50% tissue culture-infective doses (TCID50) per milliliter.
Wild-type and IFN-γ−/− mice were immunized with the live HK virus by intraperitoneal inoculation of 106 TCID50 in 200 μl of saline. Similar groups of mice were immunized intramuscularly three times at 3-week intervals with 30 μg of a plasmid (NPV1) expressing the nucleoprotein (NP) of the A/PR/8/34 virus, which was previously shown to induce cross-reactive CTLs against type A influenza viruses (38).
Infection and measurement of pulmonary virus titers.
Mice were challenged via the aerosol route with 100% lethal doses of the WSN or PR8 virus in the form of supernatant or allantoic fluid diluted in 10 ml of saline. The infection was carried out for 30 min in an aerosol chamber to which a nebulizer (Ace Glass Inc., Long Island, N.Y.) was attached, connected to a vacuum-pressure system pump. The nebulizer was loaded with 1.5 × 107, 2.25 × 107, or 3 × 107 TCID50 of the WSN virus or 1.5 × 105 TCID50 of the PR8 virus. Control and IFN-γ−/− mice were simultaneously infected. Mice were observed daily after infection for up to 20 days, and their clinical status, namely, respiratory pattern and weight loss, was assessed. At least seven mice per group were infected for survival rate analysis. The surviving mice were sacrificed, and their pulmonary virus titers were measured.
Three mice from each group were sacrificed at days 3 and 7 after challenge for measurement of pulmonary virus titers. The lungs were harvested and homogenized in 1.8 ml of phosphate-buffered saline (PBS)–1% gelatin. Log10 dilutions of lung homogenates were incubated for 1 h with trypsinized MDCK cells, in 25 μl of DMEM–1% BSA at 37°C. The medium was supplemented with 175 μl of DMEM–10% fetal calf serum (FCS), and after 48 h, the supernatants were harvested. Virus presence in the supernatants was assessed by hemagglutination with 0.5% chicken erythrocytes by a previously described method (18). Virus titers were determined by interpolation of the dilution that showed hemagglutination in 50% of the wells.
Isolation of lymphocytes from spleens and lungs.
The mice were sacrificed by injection with anesthetics (ketamine and xylazine), followed by bleeding of the axillar arteries. Spleens were aseptically removed, and after fine mincing, the fragments were passed through cell strainers. The erythrocytes were lysed by hypotonic shock. Lung tissue fragments were pretreated with 8 U of collagenase per ml in RPMI medium–1% BSA for 90 min at 37°C, in accordance with a previously described method (35). The fragments were passes through cell strainers, and the erythrocytes were lysed by hypotonic shock. The resulting cells were incubated in petri dishes at 37°C for 30 min to remove the plastic-adherent cells. The nonadherent cells were further used in functional assays or for staining. Bronchoalveolar lavage (BAL) lymphocytes were obtained by tracheal cannulation and three consecutive washings with 1 ml of saline, in accordance with a previously reported method (1). The BAL lymphocytes from three mice were pooled and used for functional assays.
Cytotoxicity assay and estimation of pCTL frequency.
Primary CTL assays were carried out by incubating various numbers of freshly harvested effector cells with 5 × 103 51Cr-labelled P815 cells in 96-well U-bottom plates. The target cells were previously infected with influenza viruses (106 TCID50/105 cells) for 1 h at 37°C in FCS-free medium supplemented with 1% BSA. In parallel, target cells were coated with an NP147-155 synthetic peptide, which corresponds to the major CTL epitope of the PR8 virus, presented in the context of Kd molecules. As a negative control, we used a Db-restricted peptide derived from NP of the PR8 virus. After 4 h of incubation at 37°C in 5% CO2, the plates were centrifuged and the supernatants were harvested and counted in a gamma counter (Automatic/Wallac-Finland). For secondary CTL assays, mixed lymphocyte cultures were prepared and incubated for 3 to 5 days in RPMI medium supplemented with 10% FCS, 50 mM 2-mercaptoethanol, 1% nonessential amino acids (Gibco BRL), and 2% HEPES buffer (Gibco BRL) at a density of 4 × 106 cells/ml with a responder-to-stimulator cell ratio of 1. Before incubation, the stimulator cells were irradiated and infected with the A/PR/8/34 or A/HK/68 virus, both of which share the dominant class I-restricted epitopes with the A/WSN/33 virus. The PR8 virus was used instead of the WSN virus, since the latter productively infects and kills a broad range of cells, including lymphocytes (23). In vitro stimulation was performed by using stimulator cells from naive animals of a similar strain, i.e., IFN-γ−/− for responder cells harvested from IFN-γ−/− mice. Before incubation with target cells, effector cells were separated on Histopaque-1083 (Sigma, St. Louis, Mo.). The results were expressed as the mean percent specific lysis of triplicates ± the standard deviation (SD) after subtraction of the background, that is, the percent lysis of noninfected, noncoated P815 target cells.
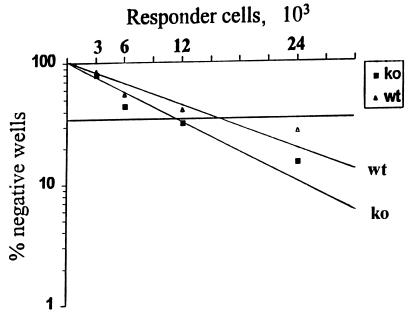
To estimate the frequency of specific precursor CTLs (pCTLs) in the spleen, single cell suspensions were prepared and twofold dilutions of responder cells were incubated in 24 parallel wells of 96-well flat-bottom plates. Irradiated and PR8 virus-infected stimulator cells of the same strain of mice were added at 2 × 105/well. Micromixed lymphocyte cultures were incubated for 5 days in complete RPMI medium, and individual cultures were tested in a 51Cr release assay on MHC-matched P815 target cells that were infected with the WSN virus or noninfected. Positive wells were considered those that displayed higher 51Cr release than the matched background + 3 SDs. The percentage of cultures that were negative was logarithmically plotted against the number of responder cells per well for each dilution. After linear regression by the least-squares method, the frequency of pCTLs was read as corresponding to 37% negative wells.
Measurement of cytokine production.
Lymphocytes (2 × 105) were incubated with the same number of stimulator cells that had previously been irradiated and pulsed for 30 min with the sucrose-purified live PR8 or HK virus (50 μg of virus/200 μl of DMEM–1% BSA/106 stimulator cells harvested from the same mouse strain as the responder cells). In parallel, 2 × 105 responder cells were coincubated with 1-μg/ml concanavalin A (ConA). After 3 days of incubation in RPMI medium supplemented with 10% FCS, the supernatants were harvested and the concentrations of interleukin 2 (IL-2), IL-4, and IFN-γ were measured by sandwich enzyme-linked immunosorbent assay (ELISA) with anti-mouse cytokine specific reagents (Biosource International, Camarillo, Calif.). Samples with known concentrations were included in order to plot standard curves. The concentration of the samples was interpolated from the standard curve. The results were expressed as the mean of triplicates ± SD (picograms per milliliter). Values lower than the background plus 3 SDs were considered to be below the limit of detection and assigned a value of 0. Generally, the sensitivity of the method was below 5 pg/ml.
FACS analysis.
Lymphocytes from three mice in each group were isolated by collagenase treatment of lung tissue and analyzed in three-color mode on an EPICS-Coulter fluorescence-activated cell sorter (FACS; Coulter Corporation, Hialeah, Fla.) equipped with a 488-nm argon laser. The cells were washed with cold PBS–2% BSA and stained for 30 min on ice with the following antibodies: 29B anti-mouse CD3 (Quantum Red), H129.19 anti-mouse CD4 (phycoerythrin), and 53-6.7 anti-mouse CD8 (fluorescein isothiocyanate) purchased from Sigma. The cell samples were run after fixation in PBS–1% paraformaldehyde supplemented with 0.1% sodium azide. The number of T cells in each subset was individually estimated, taking into consideration the total number of cells separated from the lung tissue by collagenase digestion. The results were expressed as the mean cell number in a particular subset ± the standard error of the mean (SEM).
Measurement of virus-specific HI antibodies.
The hemagglutination inhibition (HI) assay was carried out after treatment of sera with receptor-destroying enzyme (RDE/neuraminidase; Sigma) overnight at 37°C to remove the nonspecific activity due to serum sialoproteins. The RDE was inactivated by incubation with 2.5% sodium citrate at 56°C for 30 min. Twofold serial dilutions of RDE-treated sera were incubated with a 0.5% human erythrocyte saline suspension in the presence of agglutinating titers of the WSN or HK virus. After 45 min of incubation in 96-well round-bottom flexible plates (Falcon) at room temperature, the results were read and expressed as the log2 of the last inhibitory dilution. Negative controls (blank sera) and positive controls (hemagglutinin [HA]-specific monoclonal antibodies) were included in the experiment. The results were expressed as geometric mean of individual HI titers ± the standard error (SE).
Statistical analysis.
We performed statistical analysis of samples obeying binomial distribution, namely, the survival rates. The P values were computed by Fisher’s exact test. For estimation of pCTL frequency by limiting-dilution analysis, we used linear interpolation by the least-squares method.
RESULTS
Survival of immunized IFN-γ−/− mice subsequent to heterologous and homologous infections.
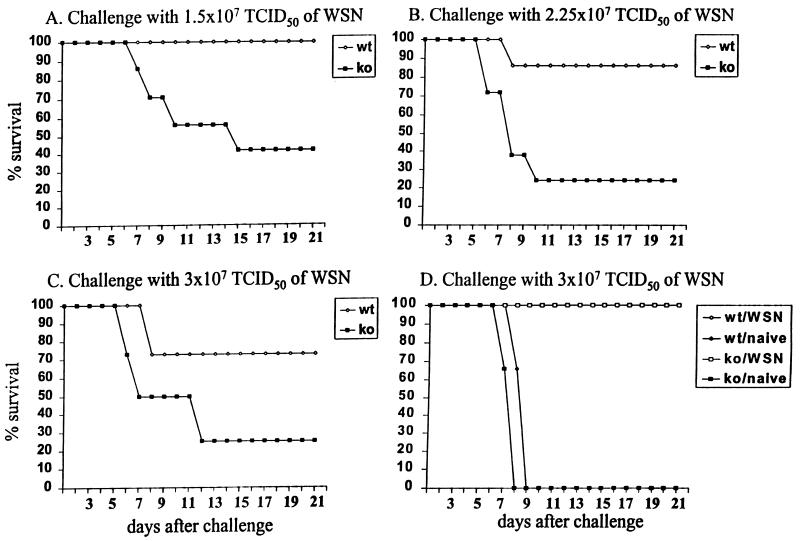
We have studied the protection from heterologous challenge conferred by live-virus immunization in the absence of IFN-γ. IFN-γ−/− and wild-type BALB/c mice were immunized intraperitoneally with the live HK (H3N2) virus and challenged 1 month later with various 100% lethal doses of the WSN (H1N1) virus. In all cases, the IFN-γ−/− mice displayed lower survival rates than their wild-type counterparts subsequent to the heterologous challenge (Fig. 1A to C). The difference in the survival rates was statistically significant in the case of a challenge with a dose of 1.5 × 107 or 2.25 × 107 TCID50 (P values of 0.038 and 0.009, respectively). In contrast, both IFN-γ−/− and BALB/c WSN-immunized mice challenged with the homologous virus displayed complete protection in terms of survival rates (Fig. 1D).
FIG. 1.
Survival profiles of wild-type (wt) and IFN-γ−/− (ko) mice infected with various doses of the WSN virus. Mice were immunized with the live HK (H3N2) virus and challenged 1 month later with the WSN virus (A to C). As controls, we included naive mice and mice immunized with the WSN virus (D). The mice were observed during a period of 20 days following the challenge, and the recovery of survivors was documented by the absence of infectious virus in the lungs. Daily results are expressed as percent survival.
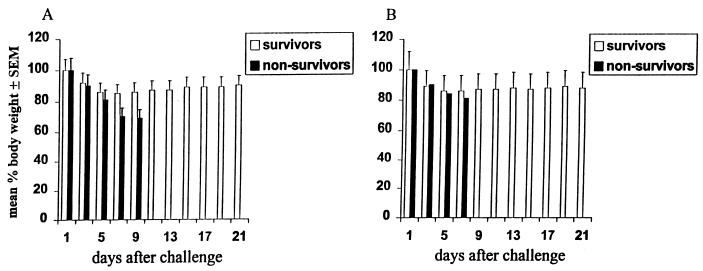
Mice infected with influenza virus display extensive pneumonia associated with weight loss. In the absence of IFN-γ, most of the mice immunized with the HK virus and challenged with the WSN virus rapidly lost approximately one-third of their weight and died at around day 7 after challenge (Fig. 2A). In contrast, most of the wild-type mice and a few of the IFN-γ−/− mice immunized with the HK virus and challenged with the WSN virus exhibited a smaller decrease in body weight and total or partial recovery of their initial weight (Fig. 2).
FIG. 2.
Body weight loss of IFN-γ−/− (A) and wild-type (B) mice infected with the WSN virus. Seven mice in each group were immunized with the live HK virus and challenged 1 month later with a dose of 2.25 × 107 TCID50 of the WSN virus. Body weight was individually recorded every 2 days. The results are expressed separately for mice that recovered or did not survive as mean percent body weight loss ± SEM.
Together, these data strongly suggest that IFN-γ plays an important protective role during recall responses to shift variants of influenza virus, although in the absence of IFN-γ, mice can recover from lethal heterologous infections. In contrast, the absence of IFN-γ did not impair a protective memory response to a homologous challenge.
Clearance of pulmonary virus in the absence of IFN-γ.
To address the question of whether the decreased survival of HK-immunized IFN-γ−/− mice challenged with the WSN virus was due to defective clearance of the pulmonary virus, we measured virus titers in the lungs at 3 and 7 days after infection. Wild-type and IFN-γ−/− naive mice displayed significant pulmonary virus titers at days 3 and 7 after infection (Table 1). Wild-type mice immunized with the live HK virus cleared the pulmonary virus by day 7 after the heterologous challenge. In contrast, HK-immunized IFN-γ−/− mice did not clear the virus, although they displayed significantly decreased titers at day 7 compared to naive wild-type and IFN-γ−/− mice challenged with the WSN virus (Table 1). Furthermore, at day 3 after the heterologous challenge, the HK-immunized IFN-γ−/− mice displayed significantly higher titers of virus than their wild-type counterparts.
TABLE 1.
Pulmonary virus titers of wild-type and IFN-γ−/− mice immunized with the HK virus and challenged with the WSN virus
| Mouse group | Immunization with HK virus | Pulmonary virus titera
|
|
|---|---|---|---|
| Day 3 | Day 7 | ||
| Wild type | − | 6.7 ± 1.4 | 4.7 |
| Wild type | + | 2.6 ± 1.6 | 0b |
| IFN-γ−/− | − | 6.8 ± 0.1 | 4.8 ± 0.1 |
| IFN-γ−/− | + | 6.4 ± 0.5 | 1.9 ± 1.9 |
Pulmonary virus titers were measured individually at 3 and 7 days after the challenge in groups of at least three mice. Results are expressed as the geometric mean log10 TCID50 ± SE.
The virus was not detectable by the technique used.
Thus, the decreased protection of influenza virus-immunized IFN-γ−/− mice against a heterologous challenge was associated with an impaired ability to completely clear the pulmonary virus. However, in the absence of IFN-γ, the immune mechanisms mediated a significant reduction of pulmonary virus titers by day 7 after the heterologous challenge.
Induction of cross-reactive CTLs against type A influenza viruses in the absence of IFN-γ.
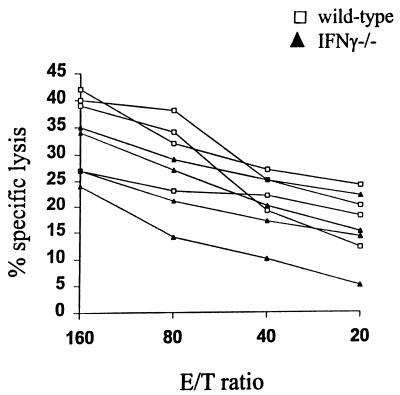
We took advantage of the fact that the WSN strain of influenza virus, due to a mutation in neuraminidase (23), exhibits high-level and promiscuous replication associated with the ability to induce enhanced levels of virus-specific cytotoxicity that are detectable in primary CTL assays without secondary in vitro stimulation. Thus, we have studied the in vivo priming of WSN-specific CTL responses in BALB/c and IFN-γ−/− mice infected with the WSN virus. Freshly harvested splenocytes from wild-type and IFN-γ−/− mice infected with the WSN virus 7 days previously displayed significant CTL activity against virus-infected target cells (Fig. 3). The IFN-γ−/− mice exhibited slightly lower CTL activities that were not significantly different from those of their wild-type counterparts. Thus, the ability to mount primary cytotoxicity was not impaired in the absence of IFN-γ.
FIG. 3.
Cytotoxicity of splenocytes from wild-type and IFN-γ−/− mice immunized with the WSN virus. Mice were immunized with the live WSN virus 7 days before sacrifice. Splenocytes were harvested and tested in a standard 51Cr release assay against infected and noninfected target cells. The results are expressed as mean percent specific lysis at various effector-to-target cell (E/T) ratios. The experiment was carried out in triplicate wells. SEs were less than 25% of the means. Four mice in each group were included in the experiment.
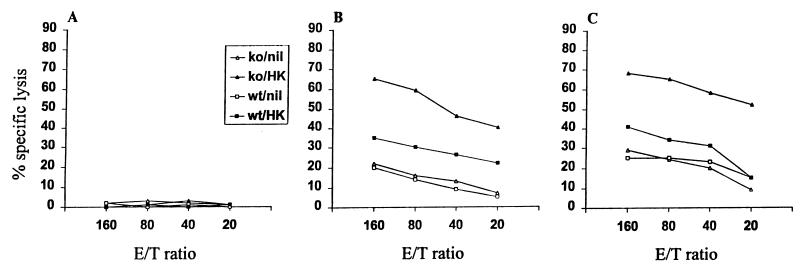
We have studied the induction of memory CTL responses in the absence of IFN-γ by measuring the cytotoxicity of freshly harvested splenocytes from wild-type and IFN-γ−/− mice infected with the WSN (H1N1) virus that had been immunized 1 month previously with the live HK (H3N2) virus. Both the IFN-γ−/− mice and their wild-type counterparts developed significant secondary CTL activities against the WSN virus, as well as the dominant NP-specific CTL epitope that is Kd restricted and conserved among the subtypes of type A influenza virus, namely, NP147-155 (Fig. 4). In contrast, we measured no significant CTL activity against another NP peptide that is recognized by CTLs from C57BL/6 mice. Surprisingly, wild-type mice immunized with the HK virus and challenged with the WSN virus displayed slightly lower levels of cytotoxicity than IFN-γ−/− mice. To address this point in a quantitative manner, we measured the frequency of virus-specific pCTLs in spleens by limiting-dilution analysis. As shown in Fig. 5, the frequency of the pCTLs induced by HK immunization followed by WSN challenge was slightly higher in IFN-γ−/− mice than their wild-type counterparts (1 in 12 × 103 versus 1 in 15.5 × 103). Thus, both the primary and secondary CTL responses were not decreased in the absence of IFN-γ.
FIG. 4.
Recall responses of CTLs from IFN-γ−/− (ko) and wild-type (wt) mice previously immunized with the live HK virus. As controls, we included IFN-γ−/− and wild-type mice not immunized with the HK virus. Seven days after infection with the WSN virus, freshly harvested splenocytes pooled from three mice in each group were tested in a standard 51Cr release assay against target cells coated with a control NP-Db peptide (A) or an NP-Kd peptide that is a dominant CTL epitope (B) or infected with the WSN virus (C). Results are expressed as mean percent specific lysis (after subtraction of percent lysis against noninfected, noncoated target cells) at various effector-to-target cell (E/T) ratios. The experiment was carried out in triplicate wells, and the SDs were less than 25% of the means.
FIG. 5.
Estimation of pCTL frequency in IFN-γ−/− (ko) and wild-type (wt) mice by limiting-dilution analysis. Mice were immunized with the live HK virus and, after 1 month, infected with the WSN virus. Seven days after infection, the splenocytes from three mice in each group were pooled and restimulated in a limiting-dilution manner. The results of a standard 51Cr release assay are expressed as percent negative wells versus number of responder cells per well. The pCTL frequency was estimated after linear interpolation as the number of responder cells per well corresponding to 37% negative wells.
Local recruitment of virus-specific effector cells in mice that lack IFN-γ.
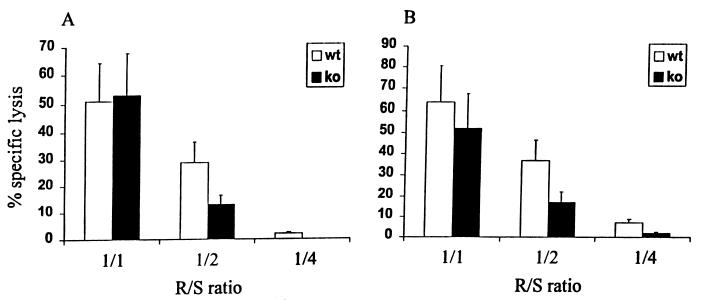
The impaired clearance of pulmonary virus by IFN-γ−/− mice immunized with the HK virus and challenged with the WSN virus may have been due to a reduction in the local recruitment of virus-specific T cells. Previous studies showed a correlation between the rapid recruitment of virus-specific CTLs into the lungs of infected mice and complete clearance of the pulmonary virus by day 7 after challenge (6). We studied the local recruitment of the effector cells by measuring the virus-specific CTL activity of nonadherent cells harvested from collagenase-digested lungs 3 days after a challenge with the WSN virus. After in vitro expansion with virus-infected splenocytes, effector cells derived from both IFN-γ−/− and wild-type mice displayed significant and comparable CTL activities (Fig. 6). Thus, the absence of IFN-γ did not impair the early recruitment of virus-specific pCTLs, which are thought to play the major protective role during the immune response to a shift variant.
FIG. 6.
Local recruitment of CTLs in lungs of IFN-γ−/− (ko) and wild-type (wt) mice immunized with the live HK virus and then infected with the WSN virus. Lymphocytes from lung tissue pooled from three mice in each group were isolated and stimulated in vitro with the PR8 (A) or HK (B) virus. Cytotoxicity was tested by a standard 51Cr release assay against WSN virus-infected target cells. Results are expressed as mean percent specific lysis ± SE at various responder/stimulator (R/S) ratios.
Local recruitment of T cells was assessed by another means, namely, cytokine production by interstitial cells from IFN-γ−/− and wild-type mice immunized with the HK virus and challenged with the WSN virus. After in vitro stimulation in the presence of ConA or antigen-presenting cells (APC) pulsed with live virus, the T cells from IFN-γ−/− mice and their wild-type counterparts produced similar amounts of IL-2 (Table 2). Interestingly, besides the lack of IFN-γ, the knockout mouse T cells isolated from lungs produced significantly smaller amounts of IL-4 after in vitro stimulation with either ConA or live viruses. However, 7 days after infection, the T cells isolated from lungs of IFN-γ−/− mice displayed enhanced production of IL-4 following in vitro stimulation with ConA (data not shown).
TABLE 2.
Cytokine production by T cells recruited into the lungs of wild-type and IFN-γ−/− mice infected with the WSN virusa
| Mouse strain and in vitro stimulation | Cytokine production (pg/ml)b
|
||
|---|---|---|---|
| IFN-γ | IL-4 | IL-2 | |
| BALB/c | |||
| ConA | 245 ± 42 | 127 ± 25 | 181 ± 39 |
| PR8 (H1N1) virus | 82 ± 3 | 21 ± 6 | 41 ± 4 |
| HK (H3N2) virus | 106 ± 20 | 49 ± 11 | 31 ± 5 |
| IFN-γ−/− | |||
| ConA | 0 | 22 ± 8 | 259 ± 10 |
| PR8 (H1N1) virus | 0 | 9 ± 1 | 25 ± 5 |
| HK (H3N2) virus | 0 | 8 ± 3 | 30 ± 4 |
Effector cells obtained by collagenase digestion of lungs harvested at day 3 after infection with the WSN virus were depleted of adherent cells and stimulated in vitro with live viruses or ConA (see Materials and Methods). The cells from three mice in each group were pooled. Cytokines were measured by ELISA after 4 days of incubation.
Results are expressed as means ± SE of triplicates.
Local recruitment of T cells was further assessed by flow cytometry with anti-CD3, anti-CD4, and anti-CD8 monoclonal antibodies. There was no striking difference in the number of total CD3+ T cells or the major subsets between IFN-γ−/− and wild-type mice at day 3 after the heterologous infection (Table 3). Similarly, no significant differences in the percentage of the major T-cell subsets were noted in the cell populations harvested by collagenase treatment or BAL from IFN-γ−/− and wild-type mice at day 3 after the heterologous infection (data not shown).
TABLE 3.
Pulmonary recruitment of T cells in IFN-γ−/− mice infected with the WSN virus
| Phenotype | No. of cells/lung (105)a
|
|
|---|---|---|
| IFN-γ−/− mice | Wild-type mice | |
| Total CD3+ | 21.5 ± 6.4 | 18.6 ± 4.9 |
| CD3+ CD4+ CD8− | 6.2 ± 2.4 | 7.0 ± 1.9 |
| CD3+ CD8+ CD4− | 8.8 ± 2.9 | 7.3 ± 2.0 |
| CD3+ CD4− CD8− | 6.5 ± 1.8 | 4.3 ± 0.9 |
Cells were harvested from the lungs of immunized mice at day 3 after the heterologous challenge, depleted of plastic-adherent cells, and stained for CD3, CD4, and CD8 antigens. The cell number for each subset was estimated in groups of three animals while taking into account the total number of cells separated per lung and the percentage of the particular subpopulation. Results are expressed as means ± SEM.
Together, these results indicate that in the absence of IFN-γ, the recruitment of functional virus-specific T cells in the lungs of influenza virus-infected mice is not impaired.
Induction of influenza virus-neutralizing antibodies in the absence of IFN-γ.
Previous studies suggested that T-dependent virus-neutralizing antibodies may participate in the clearance of influenza virus (28, 32). We tested the ability of IFN-γ−/− mice to mount protective HI antibodies, although the design of the experiment limited their eventual protective role, since the mice were immunized with an H3N2 strain and subsequently infected with an H1N1 strain of influenza virus. IFN-γ−/− and wild-type mice immunized with the live HK virus mounted significant and comparable titers of HI antibodies specific for the homologous strain and lacking cross-reactivity for the heterologous strain, namely the WSN virus (Table 4). Mice immunized with the HK virus and challenged with the WSN virus developed similar primary responses in terms of HI antibodies specific for the WSN virus, independently of the presence of a functional IFN-γ gene. No boost effect was noted regarding the HK-specific HI antibodies subsequent to infection with the WSN virus (Table 4). Thus, neither the primary nor the secondary humoral response, in terms of virus-neutralizing antibodies, was significantly impaired in the absence of IFN-γ.
TABLE 4.
Induction of influenza virus-neutralizing antibodies in IFN-γ−/− mice infected with the WSN virus
| Mouse strain | Immunization with HK virus | HI antibody titera
|
|||
|---|---|---|---|---|---|
| WSN specific
|
HK specific
|
||||
| Day 3 | Day 7 | Day 3 | Day 7 | ||
| BALB/c | − | 0b | 2.0 | 0 | 0 |
| BALB/c | + | 0 | 1.6 ± 0.5 | 5.0 ± 1.4 | 6.0 ± 1.0 |
| IFN-γ−/− | − | 0 | 3.0 ± 1.4 | 0 | 0 |
| IFN-γ−/− | + | 0 | 2.6 ± 1.9 | 4.0 | 4.0 ± 1.9 |
Blood was harvested at days 3 and 7 after the heterologous infection. The titers of WSN- and HK-specific HI antibodies were estimated individually, and the results are expressed as the geometric mean log2 HI antibody titers of groups of at least five mice ± SE.
HI antibody titer of less than 1/40.
Protection conferred by DNA immunization of IFN-γ−/− mice.
Whereas the memory CTLs specific for epitopes on internal proteins are thought to play the major protective role during the response to a heterologous challenge, T cells that are specific for HA epitopes that are conserved among certain variants of different subtypes can play a role as well. We addressed the role of IFN-γ in the absence of T memory cells specific for HA. It was previously shown that DNA immunization of BALB/c mice with a plasmid (NPV1) expressing NP of influenza virus PR8 elicited cross-reactive CTL immunity that was protective against a lethal challenge (4, 38).
We immunized IFN-γ−/− and wild-type mice with NPV1 and challenged them at 1 month after the completion of immunization with lethal doses of the PR8 virus. As shown in Table 5, the wild-type mice completely cleared the virus by day 7 after the challenge. In contrast, the IFN-γ−/− mice did not completely clear the virus, although they displayed 100 times lower titers than did naive BALB/c mice infected with similar doses of the PR8 virus (4, 6). Again, wild-type BALB/c and IFN-γ−/− mice mounted comparable CTL responses (Table 5). Furthermore, except for the lack of IFN-γ secretion, T cells obtained by BAL and those harvested from the spleens of IFN-γ−/− mice produced similar amounts of IL-2 and IL-4 compared with those obtained from wild-type mice (Table 5). Thus, in the absence of IFN-γ, mice immunized with NPV1 did not completely clear the pulmonary virus after a challenge with the PR8 virus.
TABLE 5.
T-cell response of IFN-γ−/− mice immunized with plasmid NPV1 and challenged with the PR8 virus
| Strain | Pulmonary virus titera | Cytotoxic activityb | Cytokine productionc
|
|||||
|---|---|---|---|---|---|---|---|---|
| Splenocytes
|
BAL cells
|
|||||||
| IFN-γ | IL-4 | IL-2 | IFN-γ | IL-4 | IL-2 | |||
| BALB/c | 0 | 24 ± 2 | 155 ± 21 | 6 ± 3 | 42 ± 12 | 27 ± 7 | 8 ± 3 | 34 ± 10 |
| IFN-γ−/− | 1.7 ± 1.5 | 29 ± 3 | 0 | 12 ± 4 | 60 ± 5 | 0 | 6 ± 5 | 36 ± 5 |
Pulmonary virus titers were measured 7 days after infection and the results are expressed as geometric means for groups of three mice ± SE. A value of 0 was given to nondetectable virus titers.
The CTL activity of splenocytes against PR8-infected target cells was estimated after in vitro stimulation with virus-infected APC at a responder/stimulator ratio of 1:1. The results were expressed as mean percent specific lysis of triplicates ± SE.
Cells harvested from spleens or bronchoalveolar tracts were restimulated in vitro with APC pulsed with the PR8 virus. The concentration of cytokines was estimated by ELISA. The results are expressed as means of duplicates ± SE (picograms per milliliter).
DISCUSSION
In this investigation, we have studied the requirement for IFN-γ during the secondary immune response of influenza virus-immunized mice against a strain of a different subtype. We found that both survival and the ability to completely clear the pulmonary virus were impaired in the absence of IFN-γ. However, the immunized mice lacking functional IFN-γ genes displayed significantly decreased pulmonary virus titers after a heterologous challenge, compared to their nonimmunized counterparts. The generation and pulmonary recruitment of virus-specific CTLs and the induction of antibody responses were not impaired in the absence of IFN-γ. Besides the lack of IFN-γ production, the T cells isolated from various compartments of IFN-γ−/− mice did not display dramatic differences in the ability to produce IL-2 or IL-4. Finally, in the absence of IFN-γ, the clearance of pulmonary virus in mice previously immunized with a plasmid expressing NP was also impaired.
The T-cell immunity against class I- and class II-restricted epitopes that are conserved among drift and shift variants of influenza virus plays an important role in the recovery from lung infection, since the B-cell epitopes exhibit a high natural genetic variation. The role of memory T cells during the recall response to influenza virus was previously suggested by two types of data: first, adoptive transfer experiments with T-cell lines or clones demonstrated the ability of virus-specific T cells to mediate clearance of pulmonary virus (24, 40). Secondly, experiments carried out with B-cell-deficient mice showed that in the absence of antibodies, the cellular immunity conferred significant protection during secondary responses to influenza virus (3). However, the antibodies were essential for the recovery from primary infection (14) and greatly enhanced the protection during the secondary response against homologous strains (3).
The role of T-cell immunity in protection against a heterologous challenge with strains of a different subtype is strongly supported by our data regarding the survival (Fig. 1) and virus clearance (Table 1) of wild-type mice immunized with the HK virus and challenged with lethal doses of the WSN virus (Fig. 1). The mechanisms by which CD8+ and CD4+ T cells specific for influenza virus epitopes contribute to the clearance of pulmonary virus are thought to be different. Whereas CD8+ T cells are endowed with ability to directly lyse infected cells and are thought to play the major role in the clearance of influenza virus (1), CD4+ T cells exert their protective effects in a pleiotropic manner. Previous reports showed that the generation of neutralizing antibodies is a process that depends on T help (32), and a recent study demonstrated the requirement for B cells in protection conferred by adoptive transfer of virus-specific CD4+ T cells (14). It has been previously shown that MHC class II-restricted T cells, in certain transgenic models, are endowed with the ability to directly lyse virus-infected cells (20). However, the in vivo protective role of such class II-restricted cytotoxicity has been challenged (5, 37). The virus-specific CD4+ T cells were shown to up-regulate the induction and local recruitment of CD8+ T cells during primary infection in transgenic mice expressing a T-cell receptor specific for an HA peptide (5). The role of proinflammatory cytokines produced by CD4+ and CD8+ T cells during infection with influenza virus is not clear. A previous study showed that Th1, but not Th2, clones mediated effective clearance of influenza virus from infected lungs, indirectly suggesting a beneficial role for IFN-γ (13). The study of mice lacking functional IFN-γ genes did not reveal a protective role for IFN-γ during the primary response to the A/JAP/57 strain of influenza virus (12).
Our results suggest that IFN-γ exerts protective roles during a secondary response to influenza virus strains of different subtypes. Decreased survival rates of IFN-γ−/− mice previously immunized with the HK virus after a challenge with three different doses of the WSN virus (Fig. 1) were associated with impaired clearance of pulmonary virus (Table 1). This result indicates that the lack of IFN-γ does not merely exacerbate the viral pneumonia but primarily prevents complete viral clearance by day 7 after challenge. However, even in the absence of IFN-γ, there was a significant reduction of virus lung titers in the immunized versus nonimmunized animals (Table 1), as well as clinical recovery in some mice (Fig. 1 and 2). Since the antibody response plays an unlikely role in the protection against a heterologous challenge (Table 4), these results suggest that T-cell immune mechanisms are operational during secondary responses to influenza virus in the absence of IFN-γ. Indeed, IFN-γ−/− mice mounted not only primary CTL responses, as shown by the presence of effector cells among freshly harvested splenocytes (Fig. 3), but secondary CTL responses as well against a cross-reactive dominant epitope that is presented in the context of Kd class I molecules (Fig. 4). Furthermore, data depicted in Fig. 4 and an estimation of the frequency of virus-specific pCTLs (Fig. 5) indicate a slightly enhanced CTL response in the absence of IFN-γ. This can be due to the prolonged presence of virus or, alternatively, to a previously speculated role of IFN-γ in down-regulating T-cell responses (25). Although our results are concordant with a previous report showing increased in vitro generation of alloreactive cytotoxicity in the absence of IFN-γ (7), we did not address these slight differences by performing subsequent experiments.
The inability of HK-immunized IFN-γ−/− mice to completely clear the pulmonary virus upon a heterologous challenge with the WSN virus was not due to defective recruitment of virus-specific CTLs into the infected lungs (Fig. 6). This result correlated with the comparable presence of CD8+ together with CD4+ T cells in the lungs of IFN-γ−/− and wild-type mice challenged with the WSN virus (Table 3). Similar results were obtained by staining the T cells from BAL, although slightly reduced numbers of CD4+ T cells with an activated phenotype were noted in IFN-γ−/− compared to wild-type mice (data not shown). However, our data cannot not rule out the possibility that the access of CTLs to certain compartments or cells of lung tissue is impaired in the absence of IFN-γ. Interestingly, the absence of IFN-γ did not lead to increased IL-4 production by locally recruited T cells (Table 2). This result, which is concordant with a previous study (31) but discordant with another report (12), indicates that the inability of IFN-γ−/− mice to completely clear the virus and recover from the infection was not due to exacerbated Th2 activity. However, we cannot exclude the possibility of slight increases in the frequency of IL-4-producing Th cells, which are difficult to assess by measuring IL-4 in cell culture supernatants. Furthermore, the absence of IFN-γ did not significantly impair a humoral response in terms of neutralizing antibodies (Table 4). This is further substantiated by the complete protection of immunized IFN-γ−/− mice during a challenge with a homologous strain (Fig. 1).
The protective role of IFN-γ was further assessed in a model lacking the involvement of Th memory cells against the dominant class II-restricted epitopes on HA. The results shown in Table 5 indicate the inability of CTLs primed by a plasmid expressing NP to completely clear the pulmonary virus in the absence of IFN-γ. Our results are not concordant with a previous report showing that influenza virus-specific CTL clones from IFN-γ−/− mice could clear the pulmonary virus following adoptive cell transfer (12). A possible cause for this discrepancy is the fact that the recipient mice used in that particular protocol were wild-type mice bearing functional IFN-γ genes (12).
Together, our results suggest that IFN-γ exerts its protective effect during the secondary response against heterologous strains of influenza virus in a manner that is independent of the generation and local recruitment of virus-specific effector T cells. Furthermore, our data do not support the model in which IFN-γ mediates the up-regulation of cytotoxic activity by T helper cells, despite the fact that a previous study associated the activation of CD4+ T cells with the availability of IFN-γ (31). There are at least two potential ways for IFN-γ to mediate its protective effects: (i) by up-regulating the presentation of viral peptides in the context of class I molecules of infected cells, thus allowing virus clearance by CTLs, and (ii) by inhibiting virus replication in infected cells. A few previous studies carried out in other experimental models support the first mechanism. Thus, it was shown that IFN-γ contributed to the clearance of recombinant adenovirus constructs by up-regulating the expression of peptide-class I complexes on infected hepatocytes (39). Another report showed that IFN-γ was required for optimal processing and presentation of foreign peptides in the context of class I molecules on nonprofessional APC (11). A very recent study demonstrated the production of IFN-γ by neurons and attributed the up-regulation of MHC class I expression on these cells to an autocrine regulatory loop involving IFN-γ (27). The WSN virus, which is a neurovirulent strain endowed with the ability to replicate in cells that do not express furin (23), may continue to multiply in certain cells without being detected by CTLs in the absence of IFN-γ. Thus, IFN-γ may facilitate the recognition by influenza virus-specific CTLs of certain permissive cells that are, in the absence of IFN-γ, endowed with a low ability to process and present foreign peptides in the context of class I molecules.
Another possibility is that IFN-γ acts in a manner independent of the lysis of infected cells by CD8+ CTLs. A previous study showed that β2-microglobulin-deficient mice that lack CD8+ T cells, when treated with anti-IFN-γ antibodies, displayed delayed clearance of influenza virus (31). Numerous reports support the concept that IFN-γ may act through other mechanisms that lead to inhibition of virus replication or death of infected cells (reviewed in reference 2). Thus, IFN-γ up-regulates the expression of ICAM-1 (9) and FcγRI (15, 29), possibly facilitating the lysis of infected cells by CTLs in a manner dependent or not dependent on virus-specific antibodies. Other reports described the apoptosis of bystander cells mediated by nitric oxide produced subsequent to IFN-γ-dependent activation of macrophages (17). Some studies suggested that IFN-γ up-regulates the production of certain Mx-like factors (i.e., Mg-21), although their function in directly inhibiting the replication of influenza virus is still uncertain (21).
Since our model employs transgenic mice, it is theoretically possible that genetic contamination from strains that were originally used to construct the knockout might contribute to the susceptibility of IFN-γ−/− mice to secondary infection with influenza virus. However, this possibility seems remote since both C57BL/6 and 129/Sv mice mount effective secondary immune responses against influenza virus, even in the absence of B cells (3). Furthermore, C57BL/6 mice mount protective responses against primary infection with influenza virus, even in the absence of IFN-γ (12).
Thus, multiple mechanisms may be responsible for the protective effect exerted by IFN-γ during recall responses to influenza viruses of different subtypes. Further work is required to pinpoint the most important ones, depending on the particular virus strains and the genetic background of the animals used.
ACKNOWLEDGMENTS
This work was supported by grant 5PO1AI24671 from the National Institutes of Health.
The HK virus and NPV1 plasmid were kindly donated by Margaret A. Liu (Chiron Vaccines, Emeryville, Calif.). The WSN virus was kindly donated by Peter Palese (Mount Sinai School of Medicine, New York, N.Y.). We are thankful to Sofia Casares and George Italas (Mount Sinai School of Medicine, New York, N.Y.) for technical help regarding FACS analysis.
REFERENCES
- 1.Allan W, Tabi Z, Clearly A, Doherty P C. Cellular events in the lymph node and lung of mice infected with influenza. Consequences of depleting CD4+ T cells. J Immunol. 1990;144:3980–3986. [PubMed] [Google Scholar]
- 2.Billiau A. Interferon-γ: biology and role in pathogenesis. Adv Immunol. 1996;62:61–130. doi: 10.1016/s0065-2776(08)60428-9. [DOI] [PubMed] [Google Scholar]
- 3.Bot A, Reichlin A, Isobe H, Bot S, Schulman J, Yokoyama W M, Bona C. Cellular mechanisms involved in protection and recovery from influenza virus infection in immunodeficient mice. J Virol. 1996;70:5668–5672. doi: 10.1128/jvi.70.8.5668-5672.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bot A, Bot S, Garcia-Sastre A, Bona C. DNA immunization of newborn mice with a plasmid expressing nucleoprotein of influenza virus. Viral Immunol. 1996;9:207–210. doi: 10.1089/vim.1996.9.207. [DOI] [PubMed] [Google Scholar]
- 5.Bot A, Casares S, Bot S, von Boehmer H, Bona C. Cellular mechanisms involved in protection against influenza virus infection of transgenic mice expressing a TCR receptor specific for class II-hemagglutinin peptide in CD4+ and CD8+ T cells. J Immunol. 1998;160:4500–4507. [PubMed] [Google Scholar]
- 6.Bot, A., S. Bot, A. Garcia-Sastre, and C. Bona. Protective cellular immunity against influenza virus induced by plasmid inoculation of newborn mice. Dev. Immunol., in press. [DOI] [PMC free article] [PubMed]
- 7.Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- 8.Drapier J-C, Hibbs J B. Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells resulting in l-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988;140:2829–2838. [PubMed] [Google Scholar]
- 9.Dustin M L, Rothlein R, Bhan A K, Dinarello C E, Springer T A. Tissue distribution, biochemistry and function of a natural adherence molecule (ICAM-1). Induction by IL-1α and interferon-γ. J Immunol. 1993;137:245–254. [PubMed] [Google Scholar]
- 10.Freedman A S, Freeman G J, Rhynhart K, Nadler L M. Selective induction of B7/BB-1 on interferon-γ stimulated monocytes: a potential mechanism for amplification of T cell activation through the CD28 pathway. Cell Immunol. 1991;137:429–437. doi: 10.1016/0008-8749(91)90091-o. [DOI] [PubMed] [Google Scholar]
- 11.Geginat G, Ruppert T, Hengel H, Holtappels R, Koszinowski U H. IFN-γ is a prerequisite for optimal antigen processing of viral peptides in vivo. J Immunol. 1997;158:3303–3310. [PubMed] [Google Scholar]
- 12.Graham B M, Dalton D K, Giltinan D, Braciale V L, Stewart T A, Braciale T J. Response to influenza infection in mice with a targeted disruption in the interferon γ gene. J Exp Med. 1993;178:1725–1732. doi: 10.1084/jem.178.5.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham M B, Braciale V L, Braciale T J. Influenza virus specific CD4+ T helper type 2 T lymphocytes do not promote recovery from experimental virus infection. J Exp Med. 1994;180:1273–1282. doi: 10.1084/jem.180.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graham M B, Braciale T J. Resistance to and recovery from lethal influenza virus infection in B lymphocyte deficient mice. J Exp Med. 1997;186:2063–2068. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guyre P M, Morganelli P M, Miller R. Recombinant immune interferon increases immunoglobulin G Fc receptors on cultured human mononuclear phagocytes. J Clin Invest. 1983;72:393–397. doi: 10.1172/JCI110980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heise M T, Virgin H W., IV The T-cell-independent role of gamma interferon and tumor necrosis factor alpha in macrophage activation during murine cytomegalovirus and herpes simplex virus infections. J Virol. 1995;69:904–909. doi: 10.1128/jvi.69.2.904-909.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henry Y, Lepoivre M, Drapier J-C, Ducrocq C, Boucher J-L, Guissani A. EPR characterization of molecular targets for NO in mammalian cells and organelles. FASEB J. 1993;7:1124–1134. doi: 10.1096/fasebj.7.12.8397130. [DOI] [PubMed] [Google Scholar]
- 18.Isobe H, Alt F W, Bona C A, Schulman J. Intact anti-influenza virus immune response in targeted k-deficient mice. Viral Immunol. 1994;7:25–30. doi: 10.1089/vim.1994.7.25. [DOI] [PubMed] [Google Scholar]
- 19.Karupiah G, Xie Q, Buller R M L, Nathan C, Duarte C, MacMicking J D. Inhibition of viral replication by interferon-γ induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 20.Kirberg J, Baron A, Jakob S, Rolink A, Karjalainen K, von Boehmer H. Thymic selection of CD8 single positive cells with a class II MHC-restricted receptor. J Exp Med. 1994;180:25–34. doi: 10.1084/jem.180.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lafuse W P, Brown D, Castle L, Zwilling B S. Cloning and characterization of a novel cDNA that is IFN-gamma-induced in mouse peritoneal macrophages and encodes a putative GTP-binding protein. J Leukocyte Biol. 1995;57:477–483. doi: 10.1002/jlb.57.3.477. [DOI] [PubMed] [Google Scholar]
- 22.Leist T P, Eppler M, Zinkernagel R M. Enhanced virus replication and inhibition of lymphocytic choriomeningitis virus disease in anti-gamma interferon-treated mice. J Virol. 1989;63:2813–2819. doi: 10.1128/jvi.63.6.2813-2819.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Schulman J, Itamura S, Palese P. Glycosylation of neuraminidase determines the neurovirulence of influenza A/WSN/33 virus. J Virol. 1993;67:6667–6673. doi: 10.1128/jvi.67.11.6667-6673.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y-L, Askonas B A. Biological properties of an influenza A virus-specific killer T cell clone. Inhibition of virus replication in vivo and induction of delayed-type hypersensitivity reactions. J Exp Med. 1981;154:225–231. doi: 10.1084/jem.154.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Janeway C A J. Interferon γ plays a critical role in induced cell death of effector T cell: a possible third mechanism of self tolerance. J Exp Med. 1990;172:1735–1739. doi: 10.1084/jem.172.6.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lucchiari M A, Modolell M, Eichmann K, Pereira C A. In vivo depletion of interferon-gamma leads to susceptibility of A/J mice to mouse hepatitis virus 3 infection. Immunobiology. 1992;185:475–482. doi: 10.1016/S0171-2985(11)80089-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann H, Schmidt H, Wilharm E, Behrens L, Wekerle H. Interferon g gene expression in sensory neurons: evidence for autocrine gene regulation. J Exp Med. 1997;186:2023–2031. doi: 10.1084/jem.186.12.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Palladino G, Mozdzanowska K, Washko G, Gerhard W. Virus-neutralizing antibodies of immunoglobulin G (IgG) but not of IgM or IgA isotypes can cure influenza virus pneumonia in SCID mice. J Virol. 1995;69:2075–2081. doi: 10.1128/jvi.69.4.2075-2081.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petroni K, Shen L, Guyre P M. Modulation of human polymorphonuclear leukocyte IgG Fc receptors and Fc-receptor mediated functions by IFN-γ and glucocorticoids. J Immunol. 1988;140:3467–3472. [PubMed] [Google Scholar]
- 30.Pujol-Borrell R, Todd I, Doshi M, Botazzo G F, Sutton D, Gray D, Adolf G R, Feldman M. HLA class II induction in human islet cells by interferon-γ plus tumor necrosis factor or lymphotoxin. Nature. 1987;326:304–306. doi: 10.1038/326304a0. [DOI] [PubMed] [Google Scholar]
- 31.Sarawar S, Sangster M, Coffman R L, Doherty P C. Administration of anti-IFN-γ antibody to β2-microglobulin deficient mice delays influenza virus clearance but does not switch the response to a T helper cell 2 phenotype. J Immunol. 1994;153:1246–1253. [PubMed] [Google Scholar]
- 32.Scherle P A, Gerhard W. Functional analysis of influenza-specific helper T cell clones in vivo. T cells specific for internal viral proteins provide cognate help for B cell responses to hemagglutinin. J Exp Med. 1986;164:1114–1128. doi: 10.1084/jem.164.4.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanton G J, Jordan C, Hart A, Heard H, Langford M P, Baron S. Nondetectable levels of interferon-gamma is a critical host defense during the first day of herpes simplex virus infection. Microb Pathog. 1995;3:179–183. doi: 10.1016/0882-4010(87)90094-5. [DOI] [PubMed] [Google Scholar]
- 34.Steiniger B, Falk P, Van der Meide P. Interferon-g in vivo. Induction and loss of MHC class II antigens and immature myelomonocytic cells in rat organs. Eur J Immunol. 1988;18:661–669. doi: 10.1002/eji.1830180502. [DOI] [PubMed] [Google Scholar]
- 35.Stein-Streilein J, Bennet M, Mann D, Kumar V. NK cells in mouse lung: surface, phenotype, target preference and response to local influenza virus infection. J Immunol. 1983;131:2699–2704. [PubMed] [Google Scholar]
- 36.Taub D D, Lloyd A R, Conlon K, Wang J M, Ortaldo J R, Harada A, Matsushima K, Kelvin J, Oppenheim J J. Recombinant human interferon-inducible protein 10 is a chemoattractant for human monocytes and lymphocytes and promotes T cell adhesion to endothelial cells. J Exp Med. 1993;177:1809–1818. doi: 10.1084/jem.177.6.1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topham D J, Tripp R A, Sarawar S R, Sangster M Y, Doherty P C. Immune CD4+ T cells promote the clearance of influenza virus from major histocompatibility complex class II −/− respiratory epithelium. J Virol. 1996;70:1288–1291. doi: 10.1128/jvi.70.2.1288-1291.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Felgner P L, Dwarki V J, Gromkowski S H, Randall Reck R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Heterologous protection against influenza virus infection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Xiang Z, Ertl H C, Wilson J M. Upregulation of class I major histocompatibility complex antigens by interferon-gamma is necessary for T cell mediated elimination of recombinant adenovirus infected hepatocytes in vivo. Proc Natl Acad Sci USA. 1995;92:7257–7261. doi: 10.1073/pnas.92.16.7257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yap K L, Ada G L. The recovery of mice from influenza virus infection: adoptive transfer of immunity with immune T lymphocytes. Scand J Immunol. 1978;7:389–395. doi: 10.1111/j.1365-3083.1978.tb00469.x. [DOI] [PubMed] [Google Scholar]