Abstract
Interferon regulatory factor 8 (IRF8), a transcription factor expressed in immune cells, functions as a negative regulator of osteoclasts and helps maintain dental and skeletal homeostasis. Previously, we reported that a novel mutation in the IRF8 gene increases susceptibility to multiple idiopathic cervical root resorption (MICRR), a form of tooth root resorption mediated by increased osteoclast activity. The IRF8 G388S variant in the highly conserved C-terminal motif is predicted to alter the protein structure, likely impairing IRF8 function. To investigate the molecular basis of MICRR and IRF8 function in osteoclastogenesis, we generated Irf8 knock-in (KI) mice using CRISPR/Cas9 technique modeling the human IRF8 G388S mutation. The heterozygous (Het) and homozygous (Homo) Irf8 KI mice showed no gross morphological defects, and the development of hematopoietic cells was unaffected and similar to wild-type (WT) mice. The Irf8 KI Het and Homo mice showed no difference in macrophage gene signatures important for antimicrobial defenses and inflammatory cytokine production. Consistent with the phenotype observed in MICRR patients, Irf8 KI Het and Homo mice demonstrated significantly increased osteoclast formation and resorption activity in vivo and in vitro when compared to WT mice. The oral ligature–inserted Het and Homo mice displayed significantly increased root resorption and osteoclast-mediated alveolar bone loss compared to WT mice. The increased osteoclastogenesis noted in KI mice is due to the inability of IRF8 G388S mutation to inhibit NFATc1-dependent transcriptional activation and downstream osteoclast specific transcripts, as well as its impact on autophagy-related pathways of osteoclast differentiation. This translational study delineates the IRF8 domain important for osteoclast function and provides novel insights into the IRF8 mutation associated with MICRR. IRF8 G388S mutation mainly affects osteoclastogenesis while sparing immune cell development and function. These insights extend beyond oral health and significantly advance our understanding of skeletal disorders mediated by increased osteoclast activity and IRF8’s role in osteoclastogenesis.
Keywords: genetic mutation, osteoclasts, dental biology, genetic animal models, human association studies, NFATC Transcription Factors
Introduction
Tooth root resorption mediated by osteoclasts/odontoclasts is a normal physiologic process required for exfoliation of primary teeth to make space for the eruption of permanent teeth (Harokopakis-Hajishengallis 2007). However, root resorption of permanent teeth is largely pathological (Ne et al. 1999; Fuss et al. 2003; Darcey and Qualtrough 2013). Multiple idiopathic cervical root resorption (MICRR) is an aggressive form of pathologic root resorption that affects multiple teeth at the cementoenamel junction within the permanent dentition (Liang et al. 2003; Iwamatsu-Kobayashi et al. 2005; Neely and Gordon 2007; Jiang, Lin, et al. 2014; Neely et al. 2016; Wu et al. 2016). MICRR lesions are often asymptomatic, are noncarious, and lack overt gingival inflammation, deeper pocket depth, or tooth mobility that are associated with classical cases of periodontitis (Liang et al. 2003; Iwamatsu-Kobayashi et al. 2005; Neely and Gordon 2007; Jiang, Lin, et al. 2014; Neely et al. 2016; Wu et al. 2016). MICRR lesions are often detected as incidental findings during routine dental examination and are resistant to interventions, resulting in tooth loss (Neely and Gordon 2007). To date, the etiology of MICRR remains largely speculative (Chu et al. 2021).
Previously, by examining a rare cohort of family members afflicted with MICRR, we identified a novel heterozygous interferon regulatory factor 8 (IRF8) (G388S) mutation associated with increased osteoclast activity and susceptibility to MICRR (Thumbigere-Math et al. 2019). IRF8 is a member of the IRF family of transcription factors and plays an important role in myeloid cell differentiation, immune response, and transcription of type I interferon (IFN) and IFN-inducible genes (Tamura et al. 2000; Tamura and Ozato 2002; Turcotte et al. 2007). Additionally, IRF8 functions as a negative regulator of osteoclasts by inhibiting NFATc1, a master regulator of osteoclastogenesis (Zhao et al. 2009; Thumbigere-Math et al. 2019; Das et al. 2021). The identified C-terminal mutation within exon 9 of the IRF8 gene is immediately adjacent to the IRF association domain (IAD), a critical regulatory domain that mediates homo- and heterodimeric interactions among IRFs and between IRFs and other transcriptional comodulators (Sharf et al. 1995; Jiang, Wei, et al. 2014). The IRF8 G388 residue is highly conserved among mammalian species (Thumbigere-Math et al. 2019). Conformational and bioinformatics analyses indicated that the G388S substitution could alter overall protein structure, likely impairing IRF8 heterodimerization with other transcription factors, including ETS, IRF, and NFAT family members, ultimately affecting IRF8 function (Thumbigere-Math et al. 2019).
To establish the molecular basis of increased osteoclastogenesis and MICRR, we generated Irf8 knock-in (KI) mice modeling the human IRF8 G388S mutation. Consistent with the human findings and bioinformatics prediction, the Irf8 KI heterozygous (Het) and homozygous (Homo) mice recapitulated increased osteoclast formation and root resorption activity when compared to the wild-type (WT) mice. The IRF8 G388S mutation may exert specific influence on the canonical and autophagy-related pathways of osteoclast differentiation, promoting increased osteoclastogenesis. This comparative translational study delineates the IRF8 domain important for osteoclast function and provides novel insights into the Irf8G388S mutation associated with MICRR. The Irf8 KI mouse model presents a valuable translational tool for further investigations into the etiology and potential therapies for MICRR.
Materials and Methods
Detailed materials and methods are provided in the Appendix.
Generation of Irf8 KI Mice
The Irf8 G388S KI mouse model was generated using CRISPR/Cas9 technology (Wang et al. 2013) on a C57BL/6 background. Briefly, a sgRNA (GCGGGCAAGAGCTGCGGTGC) was designed and synthesized using ThermoFisher’s Custom In Vitro Transcription Service. A single-strand donor oligonucleotide containing the desired mutation (GCAGGTAGAGCA GCTGTATGCCAGGCAGCTGGTGGAGGAAGCGGGCA AGAGCTGCGGTGC CTCT TCCCTGATGCCAGCCCTG GAGGAGCCCCAGCCGGACCAGGCTTCCGCA TGTTTCCGGAT; the 4 bold and underlined nucleotides were changed from the wild-type sequence for introducing the true and silent mutations) was ordered from IDT (https://www.idtdna.com/pages). The single-guide RNA (sgRNA) (20 ng/µL) and donor oligonucleotides (100 ng/µL) were co-microinjected with Cas9 mRNA (20 ng/µL, purchased from Trilink Biotechnologies) into the cytoplasm of zygotes collected from C57BL/6N mice (Charles River Laboratory). Injected embryos were cultured in M16 medium (MilliporeSigma) overnight in a 37°C incubator with 6% CO2. The next morning, embryos that had reached 2-cell stage of development were implanted into the oviducts of pseudopregnant surrogate mothers. The offspring born to the foster mothers were screened by polymerase chain reaction (PCR) genotyping and Sanger sequencing, and founder mice were established. The founder mice were subsequently bred with WT C57BL/6 mice for at least 2 generations to dilute any potential off-target mutations and then used for the current study. Heterozygous mice with the same modification were mated to generate homozygous offspring.
Results
Generation of Irf8 KI Mice
To establish the molecular basis of increased osteoclast regulation and MICRR, we generated Irf8 KI mice using CRISPR/Cas9 modeling the human IRF8 G388S mutation (Fig. 1A). The knock-in allele was validated by Sanger sequencing (Fig. 1B) and PCR-based genotyping (Fig. 1C). The Irf8 KI Het and Homo mice showed no evidence of gross developmental or morphological abnormalities compared to WT mice, in contrast to the Irf8 global knockout (gKO) mice that are known to exhibit splenomegaly (Fig. 1D, E). Irf8 KI Het and Homo mice were healthy, viable, and fertile and exhibited no deviation from expected Mendelian ratios. The G388S mutation did not affect Irf8 expression in KI Het and Homo mice when compared to WT mice (Fig. 1F).
Figure 1.
Generation of Irf8 knock-in (KI) mice. (A) Schematic illustration of the G388S mutation in the mouse Irf8 gene. Displayed at the top is the structure of the mouse Irf8 gene, with introns represented by lines and the direction of transcription indicated by arrows. Exons are depicted as solid boxes, with coding regions as wide boxes and untranslated regions (UTRs) as narrow boxes. Directly beneath the gene structure is a diagram illustrating important domains of the IRF8 protein. The wild-type (WT) and mutant DNA sequences are listed at the bottom, with the CRISPR single-guide RNA (sgRNA) binding region underlined and the PAM shown in bold. The altered amino acid and its codon are shown in red, while a silent mutation is shown in blue. The silent mutation does not result in amino acid change, but it can facilitate the use of polymerase chain reaction (PCR) to differentiate the WT and KI alleles. (B) Sanger sequencing validated G388S knock-in allele in F2 mice. Left panel shows chromatograms of WT sequence. Right panel shows mutant reads. Gray shaded region indicates amino acid(s) at position 388 for each mouse. Arrow indicates desired knock-in mutation (G388S). (C) PCR of genomic DNA with primers flanking exons 2 and 3 shows that WT mice have 1 WT band (365-bp PCR product), heterozygous mice carry a WT band (365 bp) and 2 mutant bands (205 and 160 bp), and homozygous-null mice carry 2 mutant bands (205 and 160 bp). (D) Photographic images of representative WT, Irf8 Het, and Homo mice aged 9 wk. (E) Photographic images for spleen from Irf8 KI WT, Het, Homo, and global knockout mice. (F) Quantitative reverse transcription PCR analysis of Irf8 gene expression in bone marrow macrophages. The data are presented as the mean ± SD, and Het and Homo mice were compared against WT mice. One-way analysis of variance and post hoc Tukey’s test was used for comparisons among groups.
Irf8 KI Mice Exhibit Unaltered Hematopoiesis
IRF8 is known to enforce monocyte development by opposing neutrophil lineage (Kurotaki et al. 2014) and additionally promoting the differentiation of common lymphoid progenitors (CLPs) to B cells (Wang et al. 2008), driving effector differentiation of CD8 T cells (Miyagawa et al. 2012), and regulating the development of type 1 conventional DCs (cDC1s) and plasmacytoid DCs (pDCs) (Schiavoni et al. 2002; Tsujimura et al. 2003). Consequently, humans and mice harboring mutations within the IRF8 DNA-binding domain and IAD domain exhibit reduced monocytes, B cells, T cells, cDCs, and pDCs and are immunodeficient (Schiavoni et al. 2002; Tsujimura et al. 2003; Hambleton et al. 2011; Bigley et al. 2017; Kurotaki et al. 2019). The development of myeloid and lymphoid lineage cells in Irf8 KI Het and Homo mice was examined by flow cytometric analysis by comparing bone marrow (BM), blood, and spleen cells for expression of cell lineage markers. In contrast to the abovementioned findings, we noted that the development of monocytes, neutrophils, B cells, and T cells was normal in Irf8 KI Het and Homo mice when compared to WT mice (Appendix Fig. 1A–C). These results are consistent with MICRR patients harboring the IRF8 G388S mutation (Thumbigere-Math et al. 2019) who showed no deficiencies in immune cells, suggesting that the G388S variant does not negatively impact hematopoietic cell development.
Irf8 KI Mice Exhibit Normal Macrophage Function
IRF8, along with IRF1 and PU.1, is known to regulate the expression of genes important for macrophage function (Dror et al. 2007; Mancino et al. 2015; Langlais et al. 2016). Hence, IRF8 deficiency renders macrophages hypofunctional in their response to IFN-γ (Hu and Ivashkiv 2009), production of inflammatory cytokines (Dror et al. 2007), and defense against intracellular infections (Langlais et al. 2016). In order to determine the effects of IRF8 G388S mutation on macrophage function, we performed quantitative reverse transcription PCR (RT-qPCR) analysis on resting and IFN-γ + Lipopolysaccharide (LPS)–activated macrophages. IFN-γ caused upregulation of several macrophage-related genes, which were further amplified upon LPS stimulation (Appendix Fig. 2), but there were no significant differences between Irf8 KI Het and Homo mice versus WT mice. These findings suggest that the IRF8 G388S variant has minimal impact on macrophage gene signatures that are important for antimicrobial defenses and inflammatory cytokine production.
Irf8 KI Mice Exhibit Increased Osteoclastogenesis
IRF8 deficiency is known to promote increased osteoclastogenesis (Zhao et al. 2009; Thumbigere-Math et al. 2019; Das et al. 2021). Hence, to determine the effects of IRF8 G388S mutation on osteoclast regulation, we examined Irf8 KI Het and Homo mice for abnormal bone and OC phenotypes. We noted that Irf8 KI Het and Homo mice respectively displayed reduced bone mass accompanied by dramatic decreases in trabecular bone mineral density (29% and 28%), cortical thickness (16% and 18%), and cortical area fraction (12% and 13%) when compared to WT mice (Fig. 2A). Histological analyses of femurs by tartrate-resistant acid phosphatase (TRAP) staining showed a substantial increase in osteoclast numbers and resorption activity in Irf8 KI Het and Homo mice compared to WT mice (Fig. 2B). To measure osteoclast formation and its activity in vitro, we cultured bone marrow macrophages (BMMs) from WT and Irf8 KI mice with Macrophage Colony-Stimulating Factor (M-CSF) and Receptor Activator of Nuclear factor κB Ligand (RANKL) for 6 to 8 d. Approximately 1.5- to 2-fold increased number of osteoclasts formed in Irf8 KI Het and Homo cultures with cell size ~1.5-fold larger than WT cells, which was appended by a 2- to 3-fold increase in resorption activity when compared to WT cells (Fig. 3A). When normalized by osteoclast number, the resorption activity per osteoclast did not differ between groups, indicating a dominant effect of cell number in increasing total resorption in Irf8 KI Het and Homo groups (Fig. 3A). Correspondingly, the messenger RNA (mRNA) and protein expression of NFATc1 and its downstream osteoclast-related genes such as Ctsk, Acp5, and Dcstamp were significantly upregulated in both Irf8 KI Het and Homo osteoclasts when compared to WT osteoclasts (Fig. 3B, C). Taken together, these results establish that the loss of IRF8 regulatory function in Irf8 KI mice due to G388S mutation promotes increased osteoclastogenesis.
Figure 2.
IRF8 G388S promotes increased osteoclast activity in femurs. (A) Micro–computed tomography analysis of femurs (9-wk-old mice). Top, longitudinal view; middle, axial view of the cortical bone in midshaft; bottom, axial view of the trabecular bone in metaphysis. Bar graphs show bone morphometric analysis of femurs. Ct.Ar/Tt.Ar, cortical area fraction; Ct. Th, cortical thickness; Tb. BMD, trabecular bone mineral density. (B) Histological analysis of femurs (8- to 10-wk-old mice) by tartrate-resistant acid phosphatase (TRAP) staining. Bar graph shows quantified osteoclast numbers. Panel A includes n = 4 to 6 mice per genotype (wild type [WT]: 3 males, 3 females; Het: 2 males, 2 females; Homo: 2 males, 3 females; circle denotes male mice, and triangle denotes female mice). Panel B includes n = 4 mice per genotype (2 males and 2 females in each group). The data are presented as the mean ± SD, and Het and Homo mice were compared against WT mice. One-way analysis of variance and post hoc Tukey’s test was used for comparisons among groups.
Figure 3.
IRF8 G388S promotes increased in vitro osteoclastogenesis. (A) In the top panel, tartrate-resistant acid phosphatase (TRAP)–stained cells show osteoclast (OC) formation. Bottom panel shows pit formation ability of OCs. Scale bar: 1,000 µm. Bar graphs show quantified number of TRAP+ cells, average cell size of TRAP+ cells, number of resorption pits, and percentage of resorption normalized to osteoclast number in each group. (B) Quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis of OC-specific genes in bone marrow macrophages (BMMs) and OCs. (C) Immunoblot analysis of OC-specific proteins. Bar graph shows quantified results of protein expression. The Western blot band intensities were measured with ImageJ software. Day 0 = BMMs and day 6 = OCs. (D) RT-qPCR analysis of genes involved in the canonical-, noncanonical-, and autophagy-related pathways in osteoclastogenesis. (A–C) Data are representative of at least 4 independent experiments, each performed in triplicate, with equal representation of male (n = 2) and female (n = 2) mice in each genotype. The data from male mice are presented. The data are presented as the mean ± SD, and Het and Homo mice were compared against WT mice. One-way analysis of variance and post hoc Tukey’s test was used for comparisons among groups.
IRF8 G388S Mutation Affects Canonical- and Autophagy-Related Pathways of Osteoclast Differentiation
The IRF8 IAD domain (IAD1: N/200-377aa) is known to physically interact with the TAD-A domain of NFATc1 (N/1-205aa) to inhibit NFATc1 nuclear translocation and activation of downstream target genes (Jiang, Wei, et al. 2014). The identified G388S mutation is 11 amino acids downstream of the IAD domain. We previously showed that the mutant IRF8 G388S isoform fails to physically interact with NFATc1 and inhibit NFATc1-dependent transcriptional activation, thus leading to increased osteoclastogenesis (Thumbigere-Math et al. 2019). However, the specific influences of the G388S mutation on the canonical and noncanonical pathways, as well as on autophagy-related mechanisms within osteoclast differentiation, remain to be clarified.
To explore these effects, we cultured BMMs from WT and Irf8 KI mice with M-CSF and RANKL for 3 to 4 d to generate pre-OCs. RT-qPCR analysis of these cultures revealed that genes integral to the canonical osteoclastogenesis pathway (Nfatc1, c-Fos, and Traf6) and those related to autophagy (Lc3 and Atg5) were upregulated in Irf8 KI Het and Homo cultures compared to WT pre-OCs (Fig. 3D). In contrast, genes related to the tumor necrosis factor α (TNF-α)–driven noncanonical pathway (Tnfr and TNF-α) showed no significant difference between the groups. These results point to a specific influence of the IRF8 G388S mutation on the canonical- and autophagy-related pathways of osteoclast differentiation, warranting further investigation.
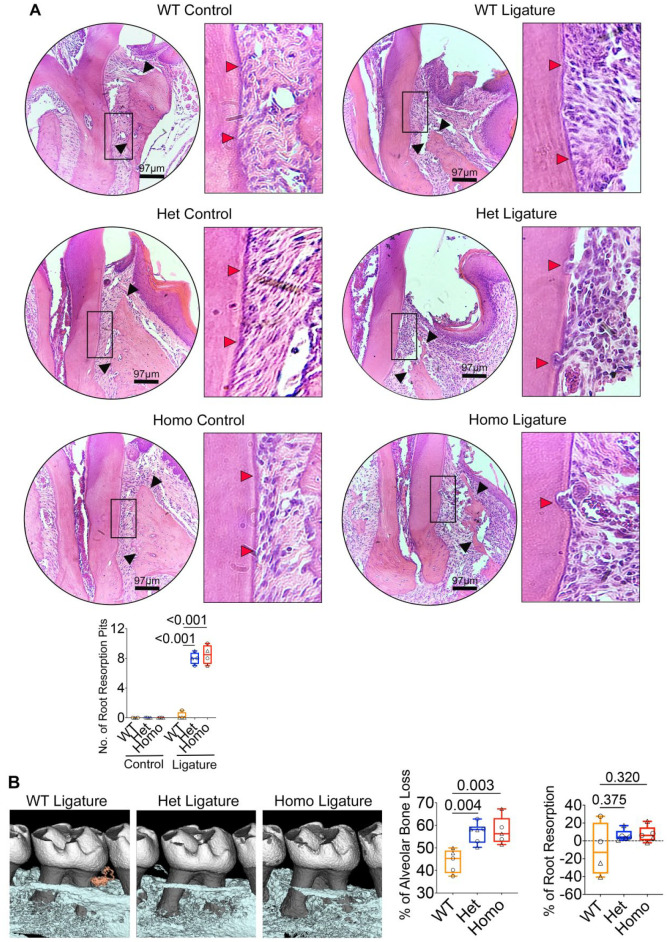
Oral Ligature–Inserted Irf8 KI Mice Exhibit Increased Root Resorption and Alveolar Bone Loss
To investigate the effects of G388S mutation on in vivo osteoclast activity in the dentoalveolar region, we used the oral ligature model that is known to promote oral bacteria–mediated inflammation (Abe and Hajishengallis 2013). At a steady state, we noted no root resorption activity in Irf8 KI Het and Homo mice when compared to WT mice (Figs. 4A and 5). Following oral ligature placement around the maxillary second molar for 5 d, histological analyses of maxillae showed significantly increased numbers of CTSK+ osteoclasts lining the root surface and alveolar bone (surrounding second molar teeth) in Irf8 KI Het and Homo mice when compared to WT mice (Figs. 4A and 5). Increased osteoclast numbers were consequently associated with significantly increased root resorption and alveolar bone loss in Irf8 KI Het and Homo mice when compared to WT mice (Figs. 4A and 5). Micro–computed tomography (CT) analysis further corroborated increased alveolar bone loss and a trend toward increased root resorption in Irf8 KI Het and Homo mice when compared to WT mice (Fig. 4B). The increased osteoclast activity in the dentoalveolar region is directly correlated with increased NFATc1 expression in Irf8-deficient mice (Thumbigere-Math et al. 2019). Taken together, these results suggest that the oral microbiota and loss of Irf8 function collectively contribute to increased osteoclast activity, alveolar bone loss, and root resorption in Irf8 KI Het and Homo mice, which supports an important parallel to our patients with heterozygous IRF8 G388S mutation and increased frequency of osteoclast/odontoclast activity in the jaw bones.
Figure 4.
IRF8 G388S promotes increased tooth root resorption and alveolar bone loss. (A) Histological analyses of maxillae (9-wk-old mice) by hematoxylin and eosin staining demonstrate increased tooth root resorption (red arrows) and alveolar bone loss (black arrows) in Irf8 KI Het and Homo mice compared to WT mice. Bar graph shows quantified root resorption pits. (B) Micro–computed tomography analyses of maxillae. Two-dimensional cut planes in sagittal orientation show alveolar bone loss around the ligated maxillary second molar. Scale bar: 0.5 mm. Bar graphs show quantified results for percentage of alveolar bone loss and tooth root resorption around the maxillary second molar in each group. Positive values indicate a loss in root structure/root resorption, while negative values will indicate minimal change or potentially an increase in root structure of ligated teeth. Panel A includes n = 4 mice per genotype (2 males and 2 females in each group; circle denotes male mice, and triangle denotes female mice). Panel B includes n = 5 to 6 mice per genotype (wild type [WT]: 3 males, 2 females; Het: 3 males, 3 females; Homo: 2 males, 3 females). The data are presented as the mean ± SD, and Het and Homo mice were compared against WT mice. One-way analysis of variance and post hoc Tukey’s test was used for comparisons among groups.
Figure 5.
IRF8 G388S promotes increased osteoclast activity and tooth root resorption in the dentoalveolar region. Histological analyses of maxillae by immunohistochemical (IHC) staining for CTSK demonstrate increased osteoclast activity (arrows indicate osteoclasts) and root resorption in oral ligature–inserted Irf8 KI Het and Homo mice compared to wild-type (WT) mice. Bar graph shows quantified CTSK+ osteoclast numbers. n = 5 mice per genotype (2 males and 3 females in each group; circle denotes male mice, and triangle denotes female mice). The data are presented as the mean ± SD, and Het and Homo mice were compared against WT mice. One-way analysis of variance and post hoc Tukey’s test was used for comparisons among groups.
Discussion
The IRF8 G388S KI mice generated in this study provide a unique translational model to study 1) the etiopathology of MICRR, 2) the critical role of IRF8 in osteoclast regulation, 3) the IRF8 domain important for osteoclast function, and 4) the development of targeted therapies for MICRR and other skeletal disorders mediated by increased osteoclast activity.
To date, several IRF8 mutations have been identified in humans that cause a range of immune cell phenotypes leading to increased susceptibility to infectious diseases (Hambleton et al. 2011; Bigley et al. 2017; Mace et al. 2017). The homozygous K108E variant, located in the DNA-binding domain, causes severe immunodeficiency and a complete lack of monocytes, DCs, IL-12, IFN-γ, and TNF-α production (Hambleton et al. 2011). On the other hand, the heterozygous T80A variant, located in the key DNA-binding helix, is associated with milder immunodeficiency and selective depletion of the CD1c+ compartment of CD11c+ circulating DCs (Hambleton et al. 2011). The compound heterozygous mutations R83C/R291Q cause a complex immunodeficiency syndrome characterized by DC and monocyte deficiency (Bigley et al. 2017). The biallelic A201V/P224L mutation within the IAD domain leads to natural killer (NK) cell deficiency, decreased NK cell number, and CD56dim subset (Mace et al. 2017). However, unlike the K108E mutation, the A201V/P224L variant can activate IRF1- and PU.1-dependent transcription normally, which results in a subtle DC deficiency and normal production of IFN-γ and TNF-α. Together, these findings highlight the varying impact of different IRF8 mutations on immune cells. However, there are no reports of detailed dental or skeletal phenotypes in patients with these mutations, except for a history of oral candidiasis and normal osteoclast activity in a 10-wk-old infant carrying the K108E variant (Hambleton et al. 2011).
In contrast to these previously reported mutations, we show that the IRF8 G388S mutation mainly affects osteoclastogenesis, sparing immune cell development and function. The mechanisms underlying the differential impact of IRF8 mutations on immune cells versus osteoclasts remain unclear. IRF8 by itself possesses weak transcriptional activity. However, it can function as both a transcriptional activator and a repressor by forming different DNA-binding heterocomplexes with multiple partners, including IRF, ETS, and NFAT family members (Sharf et al. 1995; Jiang, Wei, et al. 2014). It is plausible that variants in the DNA-binding domain (DBD) versus IRF association domain (IAD) may differentially affect IRF8 heterodimerization with other partners, leading to differing phenotypes. The IRF8 IAD domain is known to physically interact with the TAD-A domain of NFATc1 to inhibit NFATc1 nuclear translocation and activation of downstream target genes (Jiang, Wei, et al. 2014). We have shown that the mutant IRF8 G388S isoform fails to physically interact with NFATc1 and inhibit NFATc1-dependent transcriptional activation, thus leading to increased osteoclastogenesis (Thumbigere-Math et al. 2019). Conversely, the G388S mutation may not impede IRF8 interaction with other immune regulatory members such as IRF1, STAT1, and PU.1, thereby preserving immune cell functionality. Nevertheless, this hypothesis requires comprehensive validation through further empirical studies.
The canonical pathway for osteoclast differentiation involves RANKL-RANK signaling, which triggers a cascade of intracellular signaling events, leading to the activation of NF-κB and other signaling molecules such as Mitogen-Activated Protein Kinases (MAPKs), NFATc1, and AP-1 (Knowles and Athanasou 2009; Park et al. 2017). The increased osteoclastogenesis noted in Irf8 KI Het and Homo mice could be partially attributed to this pathway. Furthermore, autophagy, a process integral to cellular maintenance through degradation and recycling, is significantly implicated in osteoclast differentiation (Montaseri et al. 2020). IRF8 is known to activate many genes involved in various steps of autophagy, promoting autophagosome formation and lysosomal fusion (Gupta et al. 2015). However, the role of IRF8 in autophagy-mediated osteoclast differentiation is not well understood. Our results highlight that the G388S mutation promotes the upregulation of critical autophagic genes such as Lc3 and Atg5, which are essential in osteoclast function, particularly in facilitating lysosomal secretion into the extracellular matrix for bone resorption (DeSelm et al. 2011). In summary, our results demonstrate that the G388S mutation has specific influence on the canonical- and autophagy-related pathways of osteoclast differentiation.
Currently, the only other mutation associated with MICRR is a missense mutation c.5630 C > T in the filamin A (FLNA) gene located on the chromosome X, suggesting the possibility of sex-linked recessive inheritance (Qin et al. 2022). A 19-yr-old man inherited the mutated X chromosome from his mother and reported noncontributory medical and dental history with laboratory results within normal ranges (Qin et al. 2022). The FLNA gene encodes for actin-binding protein or filamin that crosslinks actin filaments into orthogonal networks in the cortical cytoplasm and plays a role in anchoring membrane proteins to the actin cytoskeleton (Stossel et al. 2001; Razinia et al. 2012). FLNA is required for monocyte migration during osteoclastogenesis via its role in regulating Rho GTPase-mediated actin remodeling (Leung et al. 2010; Goldberg et al. 2015). FLNA also interacts with low-density lipoprotein receptor-related protein 6 (LRP6) to inhibit β-catenin expression and enhances NFATc1-dependent osteoclastogenic gene expression to inhibit osteogenesis and promote osteoclastogenesis (Yang et al. 2022). Based on these findings and previously reported “gain-of-function” FLNA mutations that are known to cause congenital malformations affecting craniofacial and skeletal structures, the authors speculate that the FLNA c.5630 C > T mutation possibly leads to augmented odontoclastic/osteoclastic activity (Qin et al. 2022). However, no functional assays were performed to validate this hypothesis. Empirical observations suggest that MICRR may not be a monogenic disease, and other regulators upstream or downstream of the osteoclast signaling pathway could potentially contribute to root resorption.
In our study, the Irf8 KI Het and Homo mice exhibited increased root resorption and alveolar bone loss following insertion of oral ligature. At a steady state, these animals exhibited no evidence of tooth root resorption. These findings suggest that IRF8 dysfunction, increased osteoclast activity, and oral microbiota collectively contribute to the development of root resorption in mice. Similarly, in humans with IRF8 mutation, increased osteoclast activity alone would not be sufficient to promote MICRR. Patient-specific factors such as developmental tooth defects, oral microbiome, trauma, bruxism, and environmental exposure are needed to collectively increase susceptibility to MICRR. MICRR is not always noted in patients with overactive osteoclast-related disorders, further supporting that MICRR is a multifactorial disease and involves etiological factors specific to the oral cavity. This notion further helps explain why patients with the IRF8 G388S mutation and FLNA c.5630 C > T mutation mainly exhibited dental problems and no other obvious systemic bone disorders. Future studies should explore whether orthodontic treatment similarly induces root resorption in these mice in the absence of oral inflammation.
In summary, this translational study delineates the IRF8 domain important for osteoclast function and provides novel insights into the etiopathology of MICRR and IRF8 mutation associated with MICRR. The IRF8 IAD domain appears to be more important for osteoclast function versus immune cell function. Dysregulation of osteoclast differentiation/activity appears to be the “common denominator” or “central triggering issue” for MICRR, further complemented by animal/patient-specific factors. Agents that modulate osteoclast activity, such as bisphosphonates and RANKL antibodies, may prove beneficial in preventing and treating MICRR, although the risks of medication-related osteonecrosis of the jaw associated with these antiresorptive medications must be carefully considered and investigated further. These insights extend beyond oral health and significantly advance our understanding of skeletal disorders mediated by increased osteoclast activity and IRF8’s role in negatively regulating osteoclasts. Targeting the IRF8 IAD domain could help in developing novel therapies for skeletal disorders, including MICRR.
Author Contributions
A. Das, S.K. Yesupatham, D. Allison, H. Tanwar, J. Gnanasekaran, B. Kear, X. Wang, S. Wang, C. Zachariadou, Y. Abbasi, M.K. Chung, K. Ozato, C. Liu, B.L. Foster, contributed to conception, design, data analysis, critically revised the manuscript; V. Thumbigere-Math, contributed to conception, design, data analysis, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, sj-docx-1-jdr-10.1177_00220345231222173 for Murine IRF8 Mutation Offers New Insight into Osteoclast and Root Resorption by A. Das, S.K. Yesupatham, D. Allison, H. Tanwar, J. Gnanasekaran, B. Kear, X. Wang, S. Wang, C. Zachariadou, Y. Abbasi, M.K. Chung, K. Ozato, C. Liu, B.L. Foster and V. Thumbigere-Math in Journal of Dental Research
Acknowledgments
The authors thank Dr. Anthony Neely from University of Detroit–Mercy for data related to human research subjects and Dr. Xiaoxuan Fan from the University of Maryland for assistance with flow cytometry.
Footnotes
A supplemental appendix to this article is available online.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: R00DE028439, R03DE029258, and R56DK131277 to V. Thumbigere-Math; startup funds from the University of Maryland School of Dentistry to V. Thumbigere-Math; University of Maryland Baltimore Institute of Clinical & Translational Research (ICTR) grant to V. Thumbigere-Math; R01DE027639 to B.L. Foster; R35 DE030045 to M.K. Chung; and intramural funding to K. Ozato from the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health.
ORCID iDs: M.K. Chung  https://orcid.org/0000-0001-7637-1148
https://orcid.org/0000-0001-7637-1148
B.L. Foster  https://orcid.org/0000-0003-3444-0576
https://orcid.org/0000-0003-3444-0576
References
- Abe T, Hajishengallis G. 2013. Optimization of the ligature-induced periodontitis model in mice. J Immunol Methods. 394(1–2):49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigley V, Maisuria S, Cytlak U, Jardine L, Care MA, Green K, Gunawan M, Milne P, Dickinson R, Wiscombe S, et al. 2017. Biallelic interferon regulatory factor 8 mutation: a complex immunodeficiency syndrome with dendritic cell deficiency, monocytopenia, and immune dysregulation. J Allergy Clin Immunol. 141(6):2234–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu EY, Deeb JG, Foster BL, Hajishengallis E, Somerman MJ, Thumbigere-Math V. 2021. Multiple idiopathic cervical root resorption: a challenge for a transdisciplinary medical-dental team. Front Dent Med. 2:652605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darcey J, Qualtrough A. 2013. Resorption: part 1. Pathology, classification and aetiology. Br Dent J. 214(9):439–451. [DOI] [PubMed] [Google Scholar]
- Das A, Wang X, Kang J, Coulter A, Shetty AC, Bachu M, Brooks SR, Dell’Orso S, Foster BL, Fan X, et al. 2021. Monocyte subsets with high osteoclastogenic potential and their epigenetic regulation orchestrated by IRF8. J Bone Miner Res. 36(1):199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeSelm CJ, Miller BC, Zou W, Beatty WL, van Meel E, Takahata Y, Klumperman J, Tooze SA, Teitelbaum SL, Virgin HW. 2011. Autophagy proteins regulate the secretory component of osteoclastic bone resorption. Dev Cell. 21(5):966–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror N, Alter-Koltunoff M, Azriel A, Amariglio N, Jacob-Hirsch J, Zeligson S, Morgenstern A, Tamura T, Hauser H, Rechavi G, et al. 2007. Identification of IRF-8 and IRF-1 target genes in activated macrophages. Mol Immunol. 44(4):338–346. [DOI] [PubMed] [Google Scholar]
- Fuss Z, Tsesis I, Lin S. 2003. Root resorption—diagnosis, classification and treatment choices based on stimulation factors. Dent Traumatol. 19(4):175–182. [DOI] [PubMed] [Google Scholar]
- Goldberg S, Glogauer J, Grynpas MD, Glogauer M. 2015. Deletion of filamin A in monocytes protects cortical and trabecular bone from post-menopausal changes in bone microarchitecture. Calcif Tissue Int. 97(2):113–124. [DOI] [PubMed] [Google Scholar]
- Gupta M, Shin DM, Ramakrishna L, Goussetis DJ, Platanias LC, Xiong H, Morse HC, III, Ozato K. 2015. IRF8 directs stress-induced autophagy in macrophages and promotes clearance of Listeria monocytogenes. Nat Commun. 6:6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hambleton S, Salem S, Bustamante J, Bigley V, Boisson-Dupuis S, Azevedo J, Fortin A, Haniffa M, Ceron-Gutierrez L, Bacon CM, et al. 2011. IRF8 mutations and human dendritic-cell immunodeficiency. N Engl J Med. 365(2):127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harokopakis-Hajishengallis E. 2007. Physiologic root resorption in primary teeth: molecular and histological events. J Oral Sci. 49(1):1–12. [DOI] [PubMed] [Google Scholar]
- Hu X, Ivashkiv LB. 2009. Cross-regulation of signaling pathways by interferon-gamma: implications for immune responses and autoimmune diseases. Immunity. 31(4):539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwamatsu-Kobayashi Y, Satoh-Kuriwada S, Yamamoto T, Hirata M, Toyoda J, Endo H, Kindaichi K, Komatsu M. 2005. A case of multiple idiopathic external root resorption: a 6-year follow-up study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 100(6):772–779. [DOI] [PubMed] [Google Scholar]
- Jiang DS, Wei X, Zhang XF, Liu Y, Zhang Y, Chen K, Gao L, Zhou H, Zhu XH, Liu PP, et al. 2014. IRF8 suppresses pathological cardiac remodelling by inhibiting calcineurin signalling. Nat Commun. 5:3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang YH, Lin Y, Ge J, Zheng JW, Zhang L, Zhang CY. 2014. Multiple idiopathic cervical root resorptions: Report of one case with 8 teeth involved successively. Int J Clin Exp Med. 7(4):1155–1159. [PMC free article] [PubMed] [Google Scholar]
- Knowles HJ, Athanasou NA. 2009. Canonical and non-canonical pathways of osteoclast formation. Histol Histopathol. 24(3):337–346. [DOI] [PubMed] [Google Scholar]
- Kurotaki D, Kawase W, Sasaki H, Nakabayashi J, Nishiyama A, Morse HC, III, Ozato K, Suzuki Y, Tamura T. 2019. Epigenetic control of early dendritic cell lineage specification by the transcription factor IRF8 in mice. Blood. 133(17):1803–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurotaki D, Yamamoto M, Nishiyama A, Uno K, Ban T, Ichino M, Sasaki H, Matsunaga S, Yoshinari M, Ryo A, et al. 2014. IRF8 inhibits C/EBPα activity to restrain mononuclear phagocyte progenitors from differentiating into neutrophils. Nat Commun. 5:4978. [DOI] [PubMed] [Google Scholar]
- Langlais D, Barreiro LB, Gros P. 2016. The macrophage IRF8/IRF1 regulome is required for protection against infections and is associated with chronic inflammation. J Exp Med. 213(4):585–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung R, Wang Y, Cuddy K, Sun C, Magalhaes J, Grynpas M, Glogauer M. 2010. Filamin A regulates monocyte migration through Rho small GTPases during osteoclastogenesis. J Bone Miner Res. 25(5):1077–1091. [DOI] [PubMed] [Google Scholar]
- Liang H, Burkes EJ, Frederiksen NL. 2003. Multiple idiopathic cervical root resorption: systematic review and report of four cases. Dentomaxillofac Radiol. 32(3):150–155. [DOI] [PubMed] [Google Scholar]
- Mace EM, Bigley V, Gunesch JT, Chinn IK, Angelo LS, Care MA, Maisuria S, Keller MD, Togi S, Watkin LB, et al. 2017. Biallelic mutations in IRF8 impair human NK cell maturation and function. J Clin Invest. 127(1):306–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancino A, Termanini A, Barozzi I, Ghisletti S, Ostuni R, Prosperini E, Ozato K, Natoli G. 2015. A dual cis-regulatory code links IRF8 to constitutive and inducible gene expression in macrophages. Genes Dev. 29(4):394–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa F, Zhang H, Terunuma A, Ozato K, Tagaya Y, Katz SI. 2012. Interferon regulatory factor 8 integrates T-cell receptor and cytokine-signaling pathways and drives effector differentiation of CD8 T cells. Proc Natl Acad Sci U S A. 109(30):12123–12128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montaseri A, Giampietri C, Rossi M, Riccioli A, Del Fattore A, Filippini A. 2020. The role of autophagy in osteoclast differentiation and bone resorption function. Biomolecules. 10(10):1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ne RF, Witherspoon DE, Gutmann JL. 1999. Tooth resorption. Quintessence Int. 30(1):9–25. [PubMed] [Google Scholar]
- Neely AL, Gordon SC. 2007. A familial pattern of multiple idiopathic cervical root resorption in a father and son: a 22-year follow-up. J Periodontol. 78(2):367–371. [DOI] [PubMed] [Google Scholar]
- Neely AL, Thumbigere-Math V, Somerman MJ, Foster BL. 2016. A familial pattern of multiple idiopathic cervical root resorption with a 30-year follow-up. J Periodontol. 87(4):426–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Lee NK, Lee SY. 2017. Current understanding of rank signaling in osteoclast differentiation and maturation. Mol Cells. 40(10):706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Gao J, Ma S, Wang Y, Li DM, Jiang WK, Chen F, Tay F, Niu LN. 2022. Multiple cervical root resorption involving 22 teeth: a case with potential genetic predisposition. J Endod. 48(12):1526–1532. [DOI] [PubMed] [Google Scholar]
- Razinia Z, Makela T, Ylanne J, Calderwood DA. 2012. Filamins in mechanosensing and signaling. Annu Rev Biophys. 41:227–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavoni G, Mattei F, Sestili P, Borghi P, Venditti M, Morse HC, III, Belardelli F, Gabriele L. 2002. ICSBP is essential for the development of mouse type I interferon-producing cells and for the generation and activation of CD8α+ dendritic cells. J Exp Med. 196(11):1415–1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharf R, Azriel A, Lejbkowicz F, Winograd SS, Ehrlich R, Levi BZ. 1995. Functional domain analysis of interferon consensus sequence binding protein (ICSBP) and its association with interferon regulatory factors. J Biol Chem. 270(22):13063–13069. [DOI] [PubMed] [Google Scholar]
- Stossel TP, Condeelis J, Cooley L, Hartwig JH, Noegel A, Schleicher M, Shapiro SS. 2001. Filamins as integrators of cell mechanics and signalling. Nat Rev Mol Cell Biol. 2(2):138–145. [DOI] [PubMed] [Google Scholar]
- Tamura T, Nagamura-Inoue T, Shmeltzer Z, Kuwata T, Ozato K. 2000. ICSBP directs bipotential myeloid progenitor cells to differentiate into mature macrophages. Immunity. 13(2):155–165. [DOI] [PubMed] [Google Scholar]
- Tamura T, Ozato K. 2002. ICSBP/IRF-8: its regulatory roles in the development of myeloid cells. J Interferon Cytokine Res. 22(1):145–152. [DOI] [PubMed] [Google Scholar]
- Thumbigere-Math V, Foster BL, Bachu M, Yoshii H, Brooks SR, Coulter A, Chavez MB, Togi S, Neely AL, Deng Z, et al. 2019. Inactivating mutation in IRF8 promotes osteoclast transcriptional programs and increases susceptibility to tooth root resorption. J Bone Miner Res. 34(6):1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsujimura H, Tamura T, Ozato K. 2003. Cutting edge: IFN consensus sequence binding protein/IFN regulatory factor 8 drives the development of type I IFN-producing plasmacytoid dendritic cells. J Immunol. 170(3):1131–1135. [DOI] [PubMed] [Google Scholar]
- Turcotte K, Gauthier S, Malo D, Tam M, Stevenson MM, Gros P. 2007. Icsbp1/IRF-8 is required for innate and adaptive immune responses against intracellular pathogens. J Immunol. 179(4):2467–2476. [DOI] [PubMed] [Google Scholar]
- Wang H, Lee CH, Qi C, Tailor P, Feng J, Abbasi S, Atsumi T, Morse HC, III. 2008. IRF8 regulates B-cell lineage specification, commitment, and differentiation. Blood. 112(10):4028–4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Yang H, Shivalila CS, Dawlaty MM, Cheng AW, Zhang F, Jaenisch R. 2013. One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell. 153(4):910–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lin LY, Yang J, Chen XF, Ge JY, Wu JR, Sun WB. 2016. Multiple idiopathic cervical root resorption: a case report. Int Endod J. 49(2):189–202. [DOI] [PubMed] [Google Scholar]
- Yang C, Yang P, Liu P, Wang H, Ke E, Li K, Yan H. 2022. Targeting Filamin A alleviates ovariectomy-induced bone loss in mice via the WNT/β-catenin signaling pathway. Cell Signal. 90:110191. [DOI] [PubMed] [Google Scholar]
- Zhao B, Takami M, Yamada A, Wang X, Koga T, Hu X, Tamura T, Ozato K, Choi Y, Ivashkiv LB, et al. 2009. Interferon regulatory factor-8 regulates bone metabolism by suppressing osteoclastogenesis. Nat Med. 15(9):1066–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jdr-10.1177_00220345231222173 for Murine IRF8 Mutation Offers New Insight into Osteoclast and Root Resorption by A. Das, S.K. Yesupatham, D. Allison, H. Tanwar, J. Gnanasekaran, B. Kear, X. Wang, S. Wang, C. Zachariadou, Y. Abbasi, M.K. Chung, K. Ozato, C. Liu, B.L. Foster and V. Thumbigere-Math in Journal of Dental Research