Abstract
D-dimer, a soluble fibrin degradation product that originates from plasmin-induced degradation of cross-linked fibrin, is an important biomarker of coagulation activation and secondary fibrinolysis that is routinely used to rule out venous thromboembolism (VTE), and to evaluate the risk of VTE recurrence, as well as the optimal duration of anticoagulant therapy. Besides VTE, D-dimer may be high due to physiologic conditions, including aging, pregnancy, and strenuous physical activity. In addition, several disorders have been associated with increased D-dimer levels, ranging from disseminated intravascular coagulation to infectious diseases and cancers. Thus, it is far from unusual for hematologists to have to deal with ambulatory individuals with increased D-dimer without signs or symptoms of thrombus formation. This narrative review is dedicated to the management of these cases by the hematologist.
Introduction
Hemostasis can be schematically represented as a constant, delicate equilibrium between anticoagulant and procoagulant strengths in order to maintain the blood in physiological conditions of fluidity. The primary function of hemostasis is the generation of a stable clot in the event of vascular injury, thereby preventing excessive blood loss.1 While the formation of a clot, composed essentially of fibrin and blood cells (i.e., erythrocytes, leukocytes, and platelets), is essential to stop bleeding, its timely removal by the fibrinolytic system is also needed to restore blood flow within the repaired blood vessel.2 Clot lysis, made by plasmin and other proteases, is accompanied by the generation of fibrin degradation products. Among the laboratory biomarkers of fibrinolysis, D-dimer is currently considered the gold standard, not only because of its high sensitivity, but also rapid results, widespread availability, and relatively low cost of the assays.3-5 Besides thromboembolic diseases, several physiological and pathological conditions (i.e., aging, pregnancy, cancer, inflammation, infection), not necessarily characterized by thrombus formation, have been associated with increased D-dimer.6
In this narrative review aimed at the hematologist, we first present a typical clinical case and describe the physiology and physiopathology of D-dimer formation. This is followed by a summary of the main conditions associated with increased D-dimer and we propose an approach to their management, based on evidence in the literature and personal experiences. COVID-19-associated increased D-dimer levels will be not discussed here, since they have already been extensively addressed elsewhere.7-9 It is, however, undeniable that during the 3-year pandemic, an overuse of D-dimer testing was performed in many patients infected by (or recovering from) SARS-CoV-2 infection, thereby creating a generalized and often unjustified alarm among patients and medical teams.10 Accordingly, hematologists have frequently met cases of isolated increased D-dimer levels with no apparent thrombotic process, and have thus been called upon to make important decisions as to their management.
Methods
For this narrative review, we examined the medical literature for complete published studies on the management of patients with increased D-dimer. A literature search of the PubMed (through Medline) electronic database was carried out without time limits. Only studies published in English were considered. The Medical Subject Heading (MeSH) and keywords used were: “D-dimer”, “thrombosis”, “venous thromboembolism”, “pulmonary embolism”, “deep vein thrombosis”, “cancer”, “management”, “treatment”, “inflammation”, “disseminated intravascular coagulation”, “trauma”, “surgery”, “infection”, “sepsis”, “pregnancy”, “joint arthroplasty”, “cardiovascular disease”, “acute aortic dissection”, and “coronary artery disease”. We also screened the reference lists of the most relevant articles for further studies not captured in the initial literature search.
Clinical case
In order to show that D-dimer testing is often misused (which can lead to inappropriate diagnosis and therapies), we present here a typical case involving a 76-year old woman referred to us three years after an unprovoked deep vein thrombosis (DVT) of the popliteal vein of the left leg. All the screenings for thrombophilia or para-neoplastic syndrome were negative. After six months of antithrombotic therapy with a direct oral anticoagulant (DOAC) and negative venous ultrasonography she started a risk stratification protocol measuring D-dimer twice: first, at the time of stopping anticoagulant therapy and then one month later. Since both results were negative (below the fixed cutoff value of 500 µg/L), it was decided to stop the anticoagulation therapy.
After a few months, her general practitioner, prompted by non-specific complaints of a “swollen and heavy leg”, prescribed D-dimer testing. This showed increased levels (1,350 µg/L) so that the patient was instructed to resume anticoagulation with a DOAC. During the last two years, anticoagulation has been stopped and started again several times due to the patient’s concerns about a possible hemorrhage and the fluctuation of her D-dimer levels. Finally, the general practitioner referred the case to us. Medical history and physical examination revealed that in the last five years she had been suffering from severe coxarthrosis and gonarthrosis of the left leg. Surgery had been postponed and the patient made frequent use of anti-inflammatory drugs when pain was unbearable (the cause of her fear of a possible hemorrhage if she received anticoagulants). A recent venous ultrasonography was negative. Because we observed that D-dimer levels fluctuated according to the degree of pain and inflammation, she has been instructed not to resume the anticoagulant therapy, to stop random D-dimer measurement, and to refer to an orthopedic surgeon to tackle her joint problems.
What is D-dimer?
Coagulation is the physiological process that leads to blood “solidification” through the conversion of soluble fibrinogen into insoluble fibrin through the enzymatic action of the thrombin, derived from conversion from its zymogen prothrombin. Once the coagulation mechanisms have started, there is the secondary activation of fibrinolysis meant to prevent the uncontrolled propagation of fibrin formation and facilitate the repair of the lesion.1
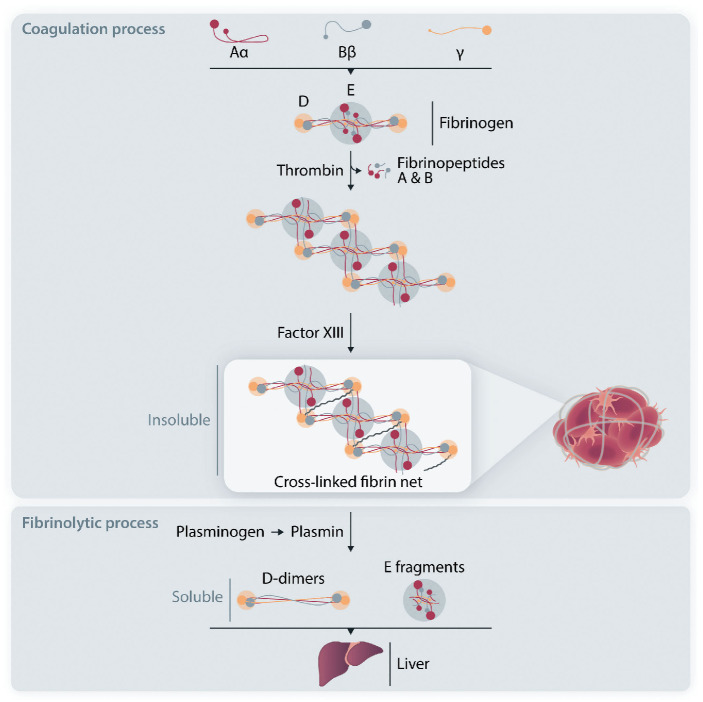
However, to better understand D-dimer formation, we need to examine more deeply the molecular mechanisms underlying the physiological coagulation process. Circulating fibrinogen is made of three paired protein chains: Aα, Bb and g. When needed at the vascular injury site, thrombin cleaves fibrinopeptides A and B from fibrinogen to form fibrin monomers that polymerize in the frame of a non-enzymatic process. Finally, Factor XIII provides monomer stabilization through covalent binding involving the g chains, so that the insoluble fibrin net is ready to act as the main backbone of the coagulation process and related repair of the vascular lesion. Then the fibrinolytic process begins locally and converts insoluble fibrin to soluble products that can be cleared from the bloodstream by the liver. This process depends on the enzyme plasmin, derived from activation from its zymogen plasminogen that circulates in the blood and is localized inside the forming clot together with fibrinogen.1 The plasminogen to plasmin activation and its progressive action upon the fibrin net produces a number of soluble products among which there are the D-dimer fragments (Figure 1) which are present only if there is a stabilized fibrin net but are not formed with fibrinogen alone.11,12
The presence of D-dimer in the bloodstream is physiological, representing the continuous balance between coagulation and fibrinolysis. Only when D-dimer exceeds the cutoff value is it considered pathologic. Cutoff values, earlier considered fixed and universal, are now considered variable and age-dependent, perhaps reflecting the physiological changes of the homeostatic system with the increased prevalence with aging of inflammatory -coagulative - reparative processes. From the time of its formation, D-dimer begins to be detectable in blood within approximately two hours, with a half-life of approximately six hours. After surgery, D-dimer increases, peaking at about one week and then decreasing by 5-10% daily; it is detectable for at least one month.13-15
Numerous assays are commercially available for the laboratory measurement of D-dimer. Most of them rely on monoclonal antibodies and are typically enzyme-linked immunosorbent assays (ELISA). But other methods use microparticles covered with monoclonal antibodies that bind D-dimer and cause agglutination, detected and quantified by nephelometry or turbidometry. There are point of care (POC) assays that produce both qualitative and quantitative results, and provide faster results in emergency care settings. Quantitative ELISA have a high sensitivity (>95%) but a low specificity for VTE and thus are principally used for exclusion purposes. For this reason, establishing a cutoff value is very important; but these values differ greatly among the various assays used, perhaps due to the different specificity and epitope affinity of the different monoclonal antibodies. Thus, it is of paramount importance to use an assay method for which the cutoff value has been determined by association of the D-dimer results with the clinical and instrumental diagnostic findings of VTE.3-5 D-dimer results can be expressed in two different units: FEU (Fibrinogen Equivalent Units) that correlate the mass of the D-dimer to that of fibrinogen, or DDU (D-dimer Units) that relate to the mass of the D-dimer itself. It is important not to switch or confuse a result expressed in FEU with one expressed in DDU, because they differ by a factor of 1.75.5
Figure 1.
Mechanism of D-dimer formation. (Top; coagulation process) Circulating human fibrinogen is made up of three pairs of polypeptide chains, designated Aα, Bb and g. Thrombin converts plasma fibrinogen to fibrin monomers by cleaving fibrinopeptides A and B. Polymerized fibrin monomers are stabilized by Factor XIII (activated by thrombin) into an insoluble cross-linked fibrin net. (Bottom; fibrinolytic process) Plasminogen is activated to plasmin, which releases soluble D-dimers and other fragments, including E fragments, from fibrin polymers. Finally, these soluble products are cleared from the bloodstream by the liver.
Conditions associated with increased D-dimer
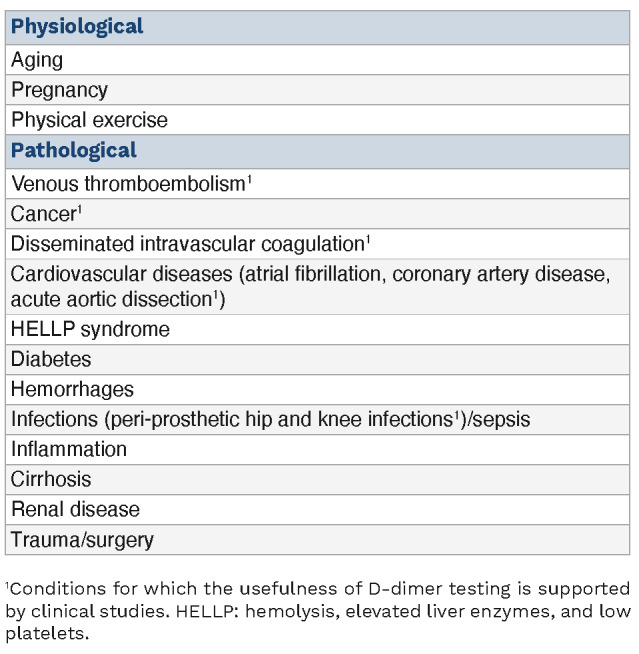
As mentioned above, D-dimer is used to guide the diagnostic procedure of VTE, which includes DVT and pulmonary embolism. However, while patients with pulmonary embolism or DVT in the acute phase usually have high D-dimer levels, individuals with high D-dimer do not necessarily have an underlying thromboembolic disease, but they may have several other pathological and non-pathological conditions (Table 1).
Venous thromboembolism
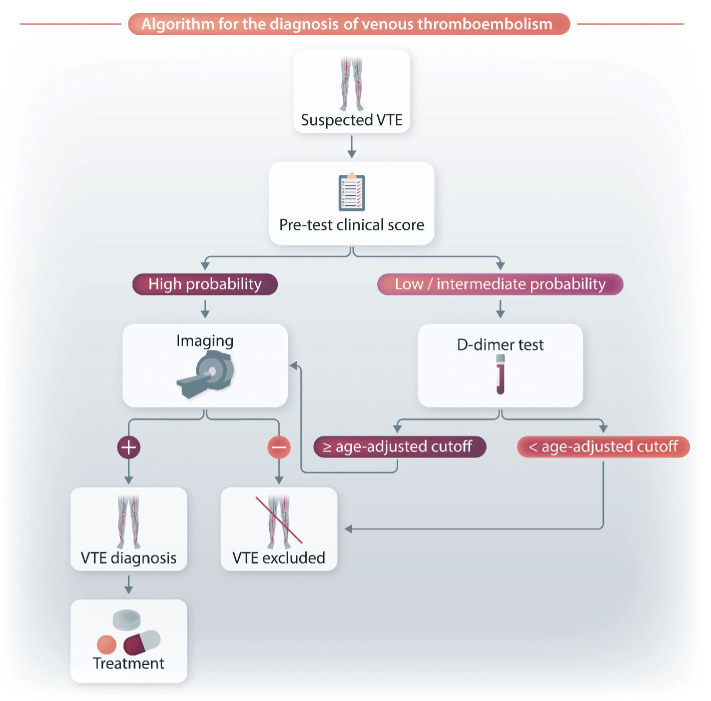
To begin, we provide a concise description of the D-dimer-based diagnostic workup of this thrombotic disorder. VTE affects nearly 10 million people every year worldwide, with an annual incidence of 1-2 cases per 1,000 population. It is the third most frequent cardiovascular disease.16,17 Although its incidence increases with age both in men and women, women have a higher risk of developing VTE at the young age of 20-40 years due to hormonal-related factors (i.e., pregnancy or oral contraceptive use).16,17 Owing to the substantial global burden of VTE, suspected VTE requires a timely and preferably non-invasive diagnostic tool. In this context, thanks to the high sensitivity (almost 100%) of quantitative ELISA, D-dimer measurement plays a key role in the diagnostic workup. However, due to its low specificity (high negative and low positive predictive value), the test should only be used to exclude VTE.18-20 This means that a D-dimer negative result (i.e., <500 µg/L) categorizes patients as having a low to moderate clinical pre-test probability of VTE.
Several scores have been developed in order to assess the clinical probability of VTE. The most used are the Wells and Geneva scores.21,22 In addition, a simplified and validated version of the Geneva score has been developed to widen its adoption in clinical practice.23,24 The combination of negative D-dimer ELISA and low-intermediate pre-test clinical probability excludes DVT or pulmonary embolism in approximately 30% of the cases with suspected VTE with no need for imaging (i.e., venous ultrasonography for a suspected DVT and computed tomography [CT] pulmonary angiogram for a suspected pulmonary embolism).25 Conversely, D-dimer is not recommended in patients with a high clinical probability, because a normal result does not exclude VTE, even when a highly sensitive assay is used; these cases should directly undergo imaging without D-dimer testing (Figure 2).26 Notably, as D-dimer levels increase with age, an age-adjusted (age x 10 µg/L in patients aged >50 years) rather than a fixed cutoff (< 500 µg/L) is warranted to exclude VTE in patients with low or intermediate pre-test clinical probability.27 Although impaired renal function has been suggested to affect D-dimer levels in individuals suspected of having VTE,28 its influence seems to be less important than that of age.29
D-dimer testing in combination with a clinical pre-test probability assessment (YEARS criteria or revised GENEVA score) has also been used for the exclusion of pulmonary embolism in pregnant women with the aim of avoiding unnecessary imaging.30,31 In addition, D-dimer testing provides important information not only for the diagnosis of VTE, but also for the anticoagulant management of VTE. Current evidence indicates that a positive D-dimer after completion of three months of anticoagulant therapy in patients with a first unprovoked VTE is associated with a 2-fold higher risk of VTE recurrence than a negative test.4 Thus, following the demonstration of the strong association between D-dimer and VTE recurrence,32 D-dimer testing is often included in the prediction models designed to guide decisions for stopping anticoagulant therapy.33,34 On the other hand, persistently high D-dimer after stopping a 3-month anticoagulant treatment argues in favor of therapy prolongation in cases with unprovoked VTE.35
The role of D-dimer for the diagnosis of cerebral vein thrombosis (CVT) has been the object of research in the last 20 years. A systematic review and meta-analysis by
Table 1.
Pathological and non-pathological conditions associated with high D-dimer levels.
Dentali and colleagues (14 studies including 1,134 patients) found high sensitivity and specificity for D-dimer (93.9% and 89.7%, respectively) in patients with suspected CVT.36 Another meta-analysis performed by Alons et al. (8 studies including 636 patients) showed that D-dimer had a high negative predictive value for CVT in low-risk patients (defined as those with normal neurological examination, normal standard head CT scan, and absence of such risk factors as puerperium and pregnancy) with isolated headache.37 A recent prospective study proposed a new score that, combining clinical data with normal D-dimer (i.e., < 500 µg/L), was very effective in predicting CVT, thus reducing unnecessary neuroimaging.38 Finally, the current view is that D-dimer testing should not be recommended as a first-line diagnostic tool for the diagnosis of venous thrombosis in unusual sites (upper limbs, retinal veins, abdominal veins).39
Physiological conditions
It has previously been pointed out that the detection of increased D-dimer does not necessarily imply the presence of an underlying disease because there are a number of physiological conditions that are accompanied by abnormal values (Table 1).40 D-dimer increases with age, thus leading to a greater proportion of older patients (> 50%) with values higher than the conventional cutoff of 500 µg/L.41 The lower specificity of D-dimer for VTE in older compared to younger people implies that the former are unlikely to have negative D-dimer results even when VTE is absent, with a related high rate of unnecessary use of imaging tests.42 For this reason, an age-adjusted cutoff has been validated and recommended in the diagnostic workup of VTE.43 However, in patients >80 years of age, the use of D-dimer measurement is not recommended for the exclusion of VTE, because high baseline levels lead to unacceptably high false positive results in cases with no VTE.44
Figure 2.
Proposed algorithm for the diagnostic evaluation of venous thromboembolism. VTE: venous thromboembolism.
Similarly, D-dimer levels increase physiologically during pregnancy and in the post-partum period.45,46 D-dimer levels are also higher in women taking oral contraceptives than in non-users, perhaps reflecting their hypercoagulable state.47
Exercise has effects on hemostasis that are dependent on the duration and intensity of the physical activity.48 D-dimer levels significantly increase following short-duration strenuous exercise,49 and exercise-induced fibrinolytic activity in healthy subjects is proportional to the amount of exercise taken.10 Changes in cardiac and hemostatic biomarkers (including D-dimer) after strenuous endurance exercise can mimic pulmonary embolism and myocardial injury in individuals who are not actually at risk.50
Disseminated intravascular coagulation
Disseminated intravascular coagulation (DIC) is a severe, often life-threatening, syndrome characterized by diffuse and persistent activation of the hemostatic system with intravascular thrombin generation, and fibrin formation and degradation.51 Early recognition of DIC is crucial to allow prompt treatment, the primary aim of which is to eliminate the underlying condition (i.e., sepsis, malignancy, trauma, obstetric diseases).48 D-dimer has become a cornerstone in the diagnosis of DIC because, thanks to its excellent negative predictive value, the use of D-dimer measurement in this context has been endorsed by all national and international guidelines.52 For example, the International Society of Thrombosis and Hemostasis (ISTH) not only validated a scoring system (including prothrombin time, platelet count, fibrinogen and D-dimer levels) for its diagnostic and prognostic value, but also recommended sequential D-dimer measurements to monitor DIC evolution, and to guide clinical and therapeutic management.53,54
Cancer, inflammation, and infection
The activation of coagulation is a common finding in malignant tumors, being associated with growth and progression.55 As a consequence, up to 20% of cancer patients develop VTE, which is the second leading cause of death.56 D-dimer is over-produced in the presence of active malignancy and levels are increased in a variety of tumors.57 However, the common finding of high D-dimer in cancer even in the absence of thrombosis limits the diagnostic usefulness of this test. Nevertheless, incorporating D-dimer measurement in appropriate scoring systems including other biomarkers may help to identify cancer patients at increased risk of developing VTE who are thus candidates for primary thromboprophylaxis.58-62 In addition, although the value of screening patients with increased D-dimer for occult cancer is still a subject of debate, an occult cancer may be considered as the potential source of a substantial rise in D-dimer levels when other physiological or pathological causes have been ruled out.48 The usefulness of this approach has been highlighted in a clinical study in patients with unprovoked VTE, demonstrating that extremely high D-dimer (>4,000 µg/L) is independently associated with the likelihood of an occult cancer.63
There is a close link between fibrinogen, thrombus formation, fibrinolysis and the inflammatory process.10 Fibrinogen, an acute phase reactant, leaks out of the vasculature contributing to the inflammatory process.64 The involvement of clotting factors (i.e., FVIII and others) helps to form fibrin in the extravascular space, which functions as a scaffold for the inflammatory cells of the immune system to exert their functions.64 As a result of fibrin formation, secondary fibrinolysis occurs and this generates D-dimer, with levels paralleling the degree of inflammation.65 Several diseases are characterized by low-grade chronic inflammation and thus high D-dimer; the most common are autoimmune diseases and diabetes. Although D-dimer is considered a marker of inflammation (the so call “D-dimeritis”), high values do not necessarily imply the presence of an increased thrombotic risk10 because D-dimer increases exponentially in parallel with C-reactive protein.66
Inflammation also provides the molecular basis to explain the detection of high D-dimer during infections. The best example is sepsis, a life-threatening systemic infection-related condition associated with defects in hemostasis and, as mentioned above, with DIC.67 Marked elevations of D-dimer have been observed in sepsis, demonstrating the prognostic importance of D-dimer in relation to the severity of the patient’s condition.68,69 Besides SARS-CoV-2 infection, several viral infections (i.e., Ebola virus, influenza A virus, human immunodeficiency virus [HIV], hepatitis C virus, Coxsackievirus, herpes simplex virus and parvovirus B19) are characterized by a marked rise in plasma levels of hypercoagulability markers, such as D-dimer and thrombin-antithrombin complex.70 The endothelial dysfunction and the inflammatory state associated with infections drive patients towards a hypercoagulable condition, that translates into an increased thromboembolic and cardiovascular risk.66 This is particularly evident in HIV-infected patients.71 In addition to chronic, low-grade inflammation (an important mechanism for their enhanced risk of cardiovascular disease), people living with HIV have increased D-dimer that correlate with VTE and mortality risks.72
The role of D-dimer in the diagnosis of peri-prosthetic infections merits special mention.73,74 A systematic review and meta-analysis based on 12 studies including a total of 1,818 patients concluded that D-dimer has sufficient diagnostic accuracy to exclude peri-prosthetic joint infection.75 In addition, the updated 2018 Musculoskeletal Infection Society criteria validated a score for the diagnosis of peri-prosthetic hip and knee infections which included D-dimer levels >860 µg/L.76
Other conditions
Several other disorders have been associated with abnormal D-dimer.77 Among cardiovascular diseases, atrial fibrillation is accompanied by high D-dimer levels, which decrease during anticoagulant treatment or after successful cardio-version.78,79 Because D-dimer is higher in atrial fibrillation patients with such additional risk factors for stroke as hypertension, diabetes or heart failure, its measurement has been proposed as a biomarker for predicting the risk of stroke.80 Notably, a recent study demonstrated the efficacy of an age-adjusted D-dimer cutoff to exclude the presence of left atrial thrombi in patients with atrial fibrillation.81 However, predictive value is less powerful than that of other biomarkers (i.e., troponin, N-terminal pro-B-type natriuretic peptide), which are preferentially incorporated into the available risk prediction models.82 Prospective studies have also evaluated the utility of D-dimer measurement to predict other cardiovascular adverse events (i.e., myocardial infarction and cardiovascular death), but with conflicting results.77 Likewise, in patients with pre-existing coronary artery disease, D-dimer is of uncertain value for the prediction of incident cardiovascular events.82 A separate mention should be made of acute aortic dissection, an extremely severe condition for which a rapid diagnosis is essential. As D-dimer levels are high in the presence of acute aortic dissection and low in its absence, a normal D-dimer helps to exclude this diagnosis.83 The diagnostic value of this measurement was recently assessed by a systematic review and meta-analysis including 16 clinical studies and 1,135 patients. This concluded that a D-dimer <500 µg/L had excellent exclusion diagnostic sensitivity (96%), thus playing a crucial role in the differential diagnosis of acute aortic dissection.84
The role of D-dimer for the diagnosis of acute intestinal ischemia has also been acknowledged in the literature.85 A systematic review and meta-analysis including 12 studies with 1,300 patients calculated a sensitivity of 94%, suggesting a potential diagnostic role for this biomarker.86
Beside these thrombotic or pre-thrombotic conditions, the clinical usefulness of D-dimer has also been studied in hemorrhagic conditions, including intracerebral and subarachnoid hemorrhages. A retrospective study of 1,332 consecutive cases with spontaneous intracerebral hemorrhage found that high D-dimer (>550 µg/L) was an independent predictor of poor functional outcome and mortality.87 Similar results were obtained by a retrospective study of 2,056 patients with aneurysmal subarachnoid hemorrhage.88 High D-dimer have also been reported in HELLP (hemolysis, elevated liver enzymes, low platelets) syndrome, a rare complication of pregnancy considered a variant of pre-eclampsia.48 This biomarker has, therefore, been proposed for the early identification of patients with pre-eclampsia at risk of developing a severe HELLP syndrome, although its clinical utility is questionable due to increased D-dimer during physiological pregnancy (see above). D-dimer is also increased in liver cirrhosis, and since it has been positively associated with the severity of liver dysfunction and the presence of portal vein thrombosis, it has been proposed as a predictor of adverse outcomes in these patients.89 Finally, inflammation-related increased D-dimer levels have been reported in other conditions, including pancreatitis and diabetes.5 For example, the marker has been recently proposed for cardiovascular risk stratification in patients with type 2 diabetes.90
Proposed management of ambulatory individuals with high D-dimer
While there is little doubt of the role of D-dimer in the diagnostic workup of patients affected by acute illnesses such as VTE, DIC and sepsis (Table 1), a challenge is represented by the management of ambulatory individuals characterized by the detection of high D-dimer levels but no evidence of thrombosis. This issue has received greater attention given the generalized and indiscriminate dispensation of D-dimer testing during and following the COVID-19 pandemic. When an ostensibly healthy and asymptomatic person is referred for persistently high D-dimer, they should first be reassured by explaining all the possible reasons other than disease that may underly this abnormality. On the other hand, an abnormal D-dimer should not be overlooked in otherwise asymptomatic subjects. Thus, they should have access to differential diagnostic procedures, and all possible conditions associated with increased D-dimer should be taken into consideration in the process.
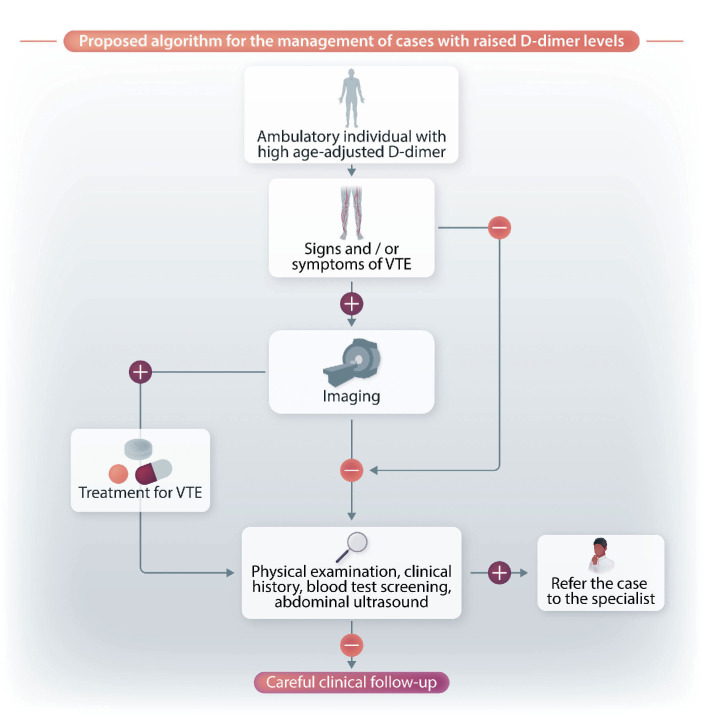
Figure 3 shows a flow chart with a summary of the evaluation processes to be carried out by hematologists in ambulatory cases with increased D-dimer levels following age adjustment. This flow-chart is based mostly on our personal experience rather than on the scarse data available in the literature. First, and most importantly, physical examination is crucial for excluding the presence of signs and/or symptoms related to VTE. It is also essential to rule out not only current symptoms, but also those that arose in the previous few days. For example, we recently observed an asymptomatic patient with increased D-dimer who reported that in the previous few days he had had some respiratory symptoms that had gradually disappeared. A precautionary CT pulmonary angiogram was performed and a pulmonary embolism was diagnosed.
In cases with clinical signs/symptoms potentially suggestive of VTE, we suggest performing a leg venous ultrasonography for the detection of occult DVT, plus, when pulmonary embolism is suspected, a CT angiogram (see the flow chart in Figure 3). If negative, the individual should then undergo blood screening to identify conditions possibly associated with increased D-dimer. Again, it is essential to check the individual’s case history and carry out a physical examination to restrict the range of blood tests to be performed. The individual should also be asked about the presence of autoimmune or rheumatic disorders, diabetes, chronic inflammatory disorders, renal disease, active or previous cancer, chronic infection, liver disease, and cardiovascular disorders. All physiological causes of high D-dimer (ongoing or recent pregnancies, strenuous physical exercise) should also be ruled out. Blood tests should, therefore, be focused on those able to confirm or diagnose an underlying disorder. In case of identification of a pathological condition, the patient should be referred to the specialist for the most appropriate care. In addition to physical examination, we suggest performing an abdominal ultrasound in order to exclude aortic dissection. If no underlying condition is identified, the individual should be reassured about their health status and a clinical follow-up set in place.
Conclusions
D-dimer testing is unequivocally a useful tool for the diagnosis of VTE. However, owing to its intrinsic poor positive predictive value, D-dimer is not specific for thromboembolic disease because increased levels are also observed in many other conditions, ranging from DIC to infections and malignant neoplasms. In other words, increased D-dimer levels may be encountered as part of an inflammatory state or cancer without being the sign of intravascular thrombus formation. Thus, while D-dimer measurement must be incorporated in multi-test algorithms for the evaluation of patients with suspected VTE, this measurement has limited clinical utility in unselected ambulatory cases and should, therefore, only be performed in specified clinical situations. However, the detection of increased D-dimer cannot be ignored and warrants a series of diagnostic procedures aimed at the proper management of these cases. This means, on the one hand, the exclusion of any associated thromboembolic complication and, on the other hand, the identification or exclusion of conditions associated with increased D-dimer. When the latter remains the only abnormality, individuals should be reassured about their health status and encouraged to avoid the repeated, obsessive measurement of D-dimer.
Figure 3.
Proposed algorithm for the management of cases with increased D-dimer levels. VTE: venous thromboembolism.
References
- 1.Lippi G, Favaloro EJ. Laboratory hemostasis: from biology to the bench. Clin Chem Lab Med. 2018;56(7):1035-1045. [DOI] [PubMed] [Google Scholar]
- 2.Franchini M, Mannucci PM. Primary hyperfibrinolysis: facts and fancies. Thromb Res. 2018;166:71-75. [DOI] [PubMed] [Google Scholar]
- 3.Riley RS, Gilbert AR, Dalton JB, Pai S, McPherson RA. Widely used types and clinical applications of D-dimer assay. Lab Med. 2016;47(2):90-102. [DOI] [PubMed] [Google Scholar]
- 4.Linkins LA, Takach Lapner S. Review of D-dimer testing: good, bad, and ugly. Int J Lab Hematol. 2017;39(Suppl 1):98-103. [DOI] [PubMed] [Google Scholar]
- 5.Johnson ED, Schell JC, Rodgers GM. The D-dimer assay. Am J Hematol. 2019;94(7):833-839. [DOI] [PubMed] [Google Scholar]
- 6.Lippi G, Mullier F, Favaloro EJ. D-dimer: old dogmas, new (COVID-19) tricks. Clin Chem Lab Med. 2022;61(5):841-850. [DOI] [PubMed] [Google Scholar]
- 7.Semiz S. COVID19 biomarkers: what did we learn from systematic reviews? Front Cell Infect Microbiol. 2022;12:1038908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iba T, Levy JH, Levi M, Thachil J. Coagulopathy in COVID-19. J Thromb Haemost. 2020;18(9):2103-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franchini M, Marano G, Cruciani M, et al. COVID-19-associated coagulopathy. Diagnosis (Berl). 2020;7(4):357-363. [DOI] [PubMed] [Google Scholar]
- 10.Thachil J, Favaloro EJ, Lippi G. D-dimers-”normal” levels versus elevated levels due to a range of conditions, including “D-dimeritis”, inflammation, thromboembolism, disseminated intravascular coagulation, and COVID-19. Semin Thromb Hemost. 2022;48(6):672-679. [DOI] [PubMed] [Google Scholar]
- 11.Doolittle RE. Structural aspects of the fibrinogen to fibrin conversion. Adv Protein Chem. 1973;27:1-109. [DOI] [PubMed] [Google Scholar]
- 12.McKee PA, Rogers LA, Marler E, et al. The subunit polypeptides of human fibrinogen. Arch Biochem Biophys. 1966;116(1):271. [DOI] [PubMed] [Google Scholar]
- 13.Mager JJ, Schutgens RE, Haas FJ, et al. The early course of D-dimer concentration following pulmonary artery embolisation, Thromb Haemost. 2001;86(6):1578-1579. [PubMed] [Google Scholar]
- 14.Chandler WL, Velan T. Plasmin generation and D-dimer formation during cardiopulmonary bypass. Blood Coagul Fibrinolysis. 2004;15(7):583-591. [DOI] [PubMed] [Google Scholar]
- 15.Dindo D, Breitenstein S, Hahnloser D, et al. Kinetics of D-dimer after general surgery. Blood Coagul Fibrinolysis. 2009;20(5):347-352. [DOI] [PubMed] [Google Scholar]
- 16.Khan F, Tritschler T, Kahn SR, Rodger MA. Venous thromboembolism. Lancet. 2021;398(10294):64-77. [DOI] [PubMed] [Google Scholar]
- 17.Freund Y, Cohen-Aubart F, Bloom B. Acute pulmonary embolism. JAMA. 2022;328(13):1336-1345. [DOI] [PubMed] [Google Scholar]
- 18.Di Nisio M, Squizzato A, Rutjes AW, Büller HR, Zwinderman AH, Bossuyt PM. Diagnostic accuracy of D-dimer test for exclusion of venous thromboembolism: a systematic review. J Thromb Haemost. 2007;5(2):296-304. [DOI] [PubMed] [Google Scholar]
- 19.Konstantinides SV, Meyer G, Becattini C, et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS). Eur Heart J. 2020;41(4):543-603. [DOI] [PubMed] [Google Scholar]
- 20.Crawford F, Andras A, Welch K, Sheares K, Keeling D, Chappell FM. D-dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Syst Rev. 2016;2016(8):CD010864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wells PS, Anderson DR, Rodger M, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349(13):1227-1235. [DOI] [PubMed] [Google Scholar]
- 22.Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144(3):165-171. [DOI] [PubMed] [Google Scholar]
- 23.Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83(3):416-420. [PubMed] [Google Scholar]
- 24.Klok FA, Mos IC, Nijkeuter M, et al. Simplification of the revised Geneva score for assessing clinical probability of pulmonary embolism. Arch Intern Med. 2008;168(19):2131-2136. [DOI] [PubMed] [Google Scholar]
- 25.Wells PS, Anderson DR, Rodger M, et al. Excluding pulmonary embolism at the bedside without diagnostic imaging: management of patients with suspected pulmonary embolism presenting to the emergency department by using a simple clinical model and D-dimer. Ann Intern Med. 2001;135(2):98-107. [DOI] [PubMed] [Google Scholar]
- 26.Duffett L, Castellucci LA, Forgie MA. Pulmonary embolism: update on management and controversies. BMJ. 2020;370:m2177. [DOI] [PubMed] [Google Scholar]
- 27.Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study. JAMA. 2014;311(11):1117-1124. [DOI] [PubMed] [Google Scholar]
- 28.Robert-Ebadi H, Bertoletti L, Combescure C, Le Gal G, Bounameaux H, Righini M. Effects of impaired renal function on levels and performance of D-dimer in patients with suspected pulmonary embolism. Thromb Haemost. 2014;112(3):614-620. [DOI] [PubMed] [Google Scholar]
- 29.Ten Cate V, Nagler M, Panova-Noeva M, et al. The diagnostic performance of renal function-adjusted testing in individuals suspected of having venous thromboembolism. Haematologica. 2019;104(9):e424-e427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Righini M, Robert-Ebadi H, Elias A, et al. CT-PE-Pregnancy Group. Diagnosis of pulmonary embolism during pregnancy: a multicenter prospective management outcome study. Ann Intern Med. 2018;169(11):766-773. [DOI] [PubMed] [Google Scholar]
- 31.van der Pol LM, Tromeur C, Bistervels IM, et al. Artemis Study Investigators. Pregnancy-adapted YEARS algorithm for diagnosis of suspected pulmonary embolism. N Engl J Med. 2019;380(12):1139-1149. [DOI] [PubMed] [Google Scholar]
- 32.Legnani C, Palareti G, Cosmi B, Cini M, Tosetto A, Tripodi A. Different cut-off values of quantitative D-dimer methods to predict the risk of venous thromboembolism recurrence: a post-hoc analysis of the PROLONG study. Haematologica. 2008;93(6):900-907. [DOI] [PubMed] [Google Scholar]
- 33.Rodger MA, Le Gal G, Langlois NJ, et al. REVERSE II investigators. “HERDOO2” clinical decision rule to guide duration of anticoagulation in women with unprovoked venous thromboembolism. Can I use any D-dimer? Thromb Res. 2018;169:82-86. [DOI] [PubMed] [Google Scholar]
- 34.Eichinger S, Heinze G, Kyrle PA. D-dimer levels over time and the risk of recurrent venous thromboembolism: an update of the Vienna prediction model. J Am Heart Assoc. 2014;3(1):e000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevens SM, Woller SC, Kreuziger LB, et al. Antithrombotic therapy for VTE disease: second update of the CHEST Guideline and Expert Panel Report. Chest. 2021;160(6):e545-e608. [DOI] [PubMed] [Google Scholar]
- 36.Dentali F, Squizzato A, Marchesi C, Bonzini M, Ferro JM, Ageno W. D-dimer testing in the diagnosis of cerebral vein thrombosis: a systematic review and a meta-analysis of the literature. J Thromb Haemost. 2012;10(4):582-589. [DOI] [PubMed] [Google Scholar]
- 37.Alons IM, Jellema K, Wermer MJ, et al. D-dimer for the exclusion of cerebral venous thrombosis: a meta-analysis of low risk patients with isolated headache. BMC Neurol. 2015;15:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heldner MR, Zuurbier SM, Li B, et al. Prediction of cerebral venous thrombosis with a new clinical score and D-dimer levels. Neurology. 2020;95(7):e898-e909. [DOI] [PubMed] [Google Scholar]
- 39.Ordieres-Ortega L, Demelo-Rodríguez P, Galeano-Valle F, Kremers BMM, ten Cate-Hoek AJ, ten Cate H. Predictive value of D-dimer testing for the diagnosis of venous thrombosis in unusual locations: a systematic review. Thromb Res. 2020;189:5-12. [DOI] [PubMed] [Google Scholar]
- 40.Favresse J, Lippi G, Roy PM, et al. D-dimer: preanalytical, analytical, postanalytical variables, and clinical applications. Crit Rev Clin Lab Sci. 2018;55(8):548-577. [DOI] [PubMed] [Google Scholar]
- 41.Haase C, Joergensen M, Ellervik C, Joergensen MK, Bathum L. Age- and sex-dependent reference intervals for D-dimer: evidence for a marked increase by age. Thromb Res. 2013;132(6):676-680. [DOI] [PubMed] [Google Scholar]
- 42.Schouten HJ, Geersing GJ, Koek HL, et al. Diagnostic accuracy of conventional or age adjusted D-dimer cut-off values in older patients with suspected venous thromboembolism: systematic review and meta-analysis. BMJ. 2013;346:f2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Pooter N, Brionne-François M, Smahi M, Abecassis L, Toulon P. Age-adjusted D-dimer cut-off levels to rule out venous thromboembolism in patients with non-high pre-test probability: clinical performance and cost-effectiveness analysis. J Thromb Haemost. 2021;19(5):1271-1282. [DOI] [PubMed] [Google Scholar]
- 44.Olson JD. D-dimer: an overview of hemostasis and fibrinolysis, assays, and clinical applications. Adv Clin Chem. 2015;69:1-46. [DOI] [PubMed] [Google Scholar]
- 45.Eichinger S. D-dimer testing in pregnancy. Pathophysiol Haemost Thromb. 2003;33(5-6):327-329. [DOI] [PubMed] [Google Scholar]
- 46.Chabloz P, Reber G, Boehlen F, Hohlfeld P, de Moerloose P. TAFI antigen and D-dimer levels during normal pregnancy and at delivery. Br J Haematol. 2001;115(1):150-152. [DOI] [PubMed] [Google Scholar]
- 47.Tekle E, Gelaw Y, Asrie F. Hematological profile changes among oral contraceptive users: a narrative review. J Blood Med. 2022;13:525-536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lippi G, Franchini M, Targher G, Favaloro EJ. Help me, Doctor! My D-dimer is raised. Ann Med. 2008;40(8):594-605. [DOI] [PubMed] [Google Scholar]
- 49.Zadow EK, Kitic CM, Wu SSX, Fell JW, Adams MJ. Time of day and short-duration high-intensity exercise influences on coagulation and fibrinolysis. Eur J Sport Sci. 2018;18(3):367-375. [DOI] [PubMed] [Google Scholar]
- 50.Sedaghat-Hamedani F, Kayvanpour E, Frankenstein L, et al. Biomarker changes after strenuous exercise can mimic pulmonary embolism and cardiac injury-a meta-analysis of 45 studies. Clin Chem. 2015;61(10):1246-1255. [DOI] [PubMed] [Google Scholar]
- 51.Favaloro EJ. Laboratory testing in disseminated intravascular coagulation. Semin Thromb Hemost. 2010;36(4):458-467. [DOI] [PubMed] [Google Scholar]
- 52.Wada H, Matsumoto T, Yamashita Y. Diagnosis and treatment of disseminated intravascular coagulation (DIC) according to four DIC guidelines. J Intensive Care. 2014;2(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Toh CH, Hoots WK. SSC on disseminated intravascular coagulation of the ISTH. The scoring system of the Scientific and Standardisation Committee on Disseminated Intravascular Coagulation of the International Society on Thrombosis and Haemostasis: a 5-year overview. J Thromb Haemost. 2007;5(3):604-606. [DOI] [PubMed] [Google Scholar]
- 54.Suzuki K, Wada H, Imai H, et al. Subcommittee on Disseminated Intravascular Coagulation. A re-evaluation of the D-dimer cut-off value for making a diagnosis according to the ISTH overt-DIC diagnostic criteria: communication from the SSC of the ISTH. J Thromb Haemost. 2018;16(7):1442-1444. [DOI] [PubMed] [Google Scholar]
- 55.Franchini M, Montagnana M, Targher G, Manzato F, Lippi G. Pathogenesis, clinical and laboratory aspects of thrombosis in cancer. J Thromb Thrombolysis. 2007;24(1):29-38. [DOI] [PubMed] [Google Scholar]
- 56.Khorana AA, Francis CW, Culakova E, Kuderer NM, Lyman GH. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632-634. [DOI] [PubMed] [Google Scholar]
- 57.Ma M, Cao R, Wang W, et al. The D-dimer level predicts the prognosis in patients with lung cancer: a systematic review and meta-analysis. J Cardiothorac Surg. 2021;16(1):243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116(24):5377-5382. [DOI] [PubMed] [Google Scholar]
- 59.Pabinger I, van Es N, Heinze G, et al. A clinical prediction model for cancer-associated venous thromboembolism: a development and validation study in two independent prospective cohorts. Lancet Haematol. 2018;5(7):e289-e298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumar V, Shaw JR, Key NS, et al. D-Dimer enhances risk-targeted thromboprophylaxis in ambulatory patients with cancer. Oncologist. 2020;25(12):1075-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Falanga A, Ay C, Di Nisio M, et al. ; ESMO Guidelines Committee. Venous thromboembolism in cancer patients: ESMO Clinical Practice Guideline. Ann Oncol. 2023;34(5):452-467. [DOI] [PubMed] [Google Scholar]
- 62.Verzeroli C, Giaccherini C, Russo L, et al. HYPERCAN Investigators. Utility of the Khorana and the new-Vienna CATS prediction scores in cancer patients of the HYPERCAN cohort. J Thromb Haemost. 2023;21(7):1869-1881. [DOI] [PubMed] [Google Scholar]
- 63.Han D, Hartaigh B, Lee JH, et al. Impact of D-Dimer for prediction of incident occult cancer in patients with unprovoked venous thromboembolism. PLoS One. 2016;11(4):e0153514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Clemmensen I, Andersen RB. The fibrinolytic system and its relation to inflammatory diseases. Semin Arthritis Rheum. 1982;11(4):390-398. [DOI] [PubMed] [Google Scholar]
- 65.Shorr AF, Thomas SJ, Alkins SA, Fitzpatrick TM, Ling GS. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest. 2002;121(4):1262-1268. [DOI] [PubMed] [Google Scholar]
- 66.Steeghs N, Goekoop RJ, Niessen RW, Jonkers GJ, Dik H, Huisman MV. C-reactive protein and D-dimer with clinical probability score in the exclusion of pulmonary embolism. Br J Haematol. 2005;130(4):614-619. [DOI] [PubMed] [Google Scholar]
- 67.Prucha M, Bellingan G, Zazula R. Sepsis biomarkers. Clin Chim Acta. 2015;440:97-103. [DOI] [PubMed] [Google Scholar]
- 68.Kinasewitz GT, Yan SB, Basson B, et al. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism. Crit Care. 2004;8(2):R82-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iba T, Gando S, Murata A, et al. Predicting the severity of systemic inflammatory response syndrome (SIRS)-associated coagulopathy with hemostatic molecular markers and vascular endothelial injury markers. J Trauma. 2007;63(5):1093-1098. [DOI] [PubMed] [Google Scholar]
- 70.Subramaniam S, Scharrer I. Procoagulant activity during viral infections. Front Biosci (Landmark Ed). 2018;23(6):1060-1081. [DOI] [PubMed] [Google Scholar]
- 71.Vos AG, Idris NS, Barth RE, Klipstein-Grobusch K, Grobbee DE. Pro-inflammatory markers in relation to cardiovascular disease in HIV infection. A systematic review. PLoS One. 2016;11(1):e0147484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Perkins MV, Joseph SB, Dittmer DP, Mackman N. Cardiovascular disease and thrombosis in HIV infection. Arterioscler Thromb Vasc Biol. 2023;43(2):175-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang R, Zhang H, Ding P, Jiao Q. The accuracy of D-dimer in the diagnosis of periprosthetic infections: a systematic review and meta-analysis. J Orthop Surg Res. 2022;17(1):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cutter B, Lum ZC, Giordani M, Meehan JP. Utility of D-dimer in total joint arthroplasty. World J Orthop. 2023;14(3):90-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balato G, De Franco C, Balboni F, et al. The role of D-dimer in periprosthetic joint infection: a systematic review and meta-analysis. Diagnosis (Berl). 2021;9(1):3-10. [DOI] [PubMed] [Google Scholar]
- 76.Parvizi J, Tan TL Goswami K, et al. The 2018 definition of periprosthetic hip and knee infection: an evidence-based and validated criteria. J Arthroplasty. 2018;33(5):1309-1314.e2. [DOI] [PubMed] [Google Scholar]
- 77.Weitz JI, Fredenburgh JC, Eikelboom JW. A test in context: D-dimer. J Am Coll Cardiol. 2017;70(19):2411-2420. [DOI] [PubMed] [Google Scholar]
- 78.Hijazi Z, Oldgren J, Siegbahn A, Wallentin L. Application of biomarkers for risk stratification in patients with atrial fibrillation. Clin Chem. 2017;63(1):152-164. [DOI] [PubMed] [Google Scholar]
- 79.Pezzo MP, Tufano A, Franchini M. Role of new potential biomarkers in the risk of thromboembolism in atrial fibrillation. J Clin Med. 2022;11(4):915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Danese E, Montagnana M, Cervellin G, Lippi G. Hypercoagulability, D-dimer and atrial fibrillation: an overview of biological and clinical evidence. Ann Med. 2014;46(6):364-371. [DOI] [PubMed] [Google Scholar]
- 81.Almorad A, Ohanyan A, Pintea Bentea G, et al. D-dimer blood concentrations to exclude left atrial thrombus in patients with atrial fibrillation. Heart. 2021;107(3):195-200. [DOI] [PubMed] [Google Scholar]
- 82.Danesh J, Whincup P, Walker M, et al. Fibrin D-dimer and coronary heart disease: prospective study and meta-analysis. Circulation. 2001;103(19):2323-2327. [DOI] [PubMed] [Google Scholar]
- 83.Cui JS, Jing ZP, Zhuang SJ, et al. D-dimer as a biomarker for acute aortic dissection: a systematic review and meta-analysis. Medicine (Baltimore). 2015;94(4):e471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yao J, Bai T, Yang B, Sun L. The diagnostic value of D-dimer in acute aortic dissection: a meta-analysis. J Cardiothorac Surg. 2021;16(1):343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Derikx JP, Schellekens DH, Acosta S. Serological markers for human intestinal ischemia: a systematic review. Best Pract Res Clin Gastroenterol. 2017;31(1):69-74. [DOI] [PubMed] [Google Scholar]
- 86.Sun DL, Li SM, Cen YY, et al. Accuracy of using serum D-dimer for diagnosis of acute intestinal ischemia: a meta-analysis. Medicine (Baltimore). 2017;96(13):e6380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhou Q, Zhang D, Chen X, et al. Plasma D-dimer predicts poor outcome and mortality after spontaneous intracerebral hemorrhage. Brain Behav. 2021;11(1):462-468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fang F, Wang P, Yao W, et al. Association between D-dimer levels and long-term mortality in patients with aneurysmal subarachnoid hemorrhage. Neurosurg Focus. 2022;52(3):E8. [DOI] [PubMed] [Google Scholar]
- 89.Li Y, Qi X, Li H, et al. D-dimer level for predicting the in-hospital mortality in liver cirrhosis: a retrospective study. Exp Ther Med. 2017;13(1):285-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cheng L, Fu Q, Zhou L, et al. D-dimer as a predictor of cardiovascular outcomes in patients with diabetes mellitus. BMC Cardiovasc Disord. 2022;22(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]